
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Behav. Neurosci. , 27 August 2018
Sec. Learning and Memory
Volume 12 - 2018 | https://doi.org/10.3389/fnbeh.2018.00189
This article is part of the Research Topic From Stimulus to Behavioral Decision-Making View all 12 articles
Cydia pomonella (Lepidoptera: Tortricidae) is a major pest of apple, pear and walnuts. For its control, alternative strategies targeting the olfactory system, like mating disruption, have been combined with insecticide applications. The efficacy of these strategies headed the direction of efforts for the functional characterization of codling moth chemosensory receptors to implement further control methods based on chemical sensing. With the advent of transcriptomic analysis, partial and full-length coding sequences of chemosensory receptors have been identified in antennal transcriptomes of C. pomonella. Extension of partial coding sequences to full-length by polymerase chain reaction (PCR)-based techniques and heterologous expression in empty neurons of Drosophila melanogaster and in Human Embryonic Kidney cells allowed functional studies to investigate receptor activation and ligand binding modalities (deorphanization). Among different classes of antennal receptors, several odorant receptors of C. pomonella (CpomORs) have been characterized as binding kairomones (CpomOR3), pheromones (CpomOR6a) and compounds emitted by non-host plants (CpomOR19). Physiological and pharmacological studies of these receptors demonstrated their ionotropic properties, by forming functional channels with the co-receptor subunit of CpomOrco. Further investigations reported a novel insect transient receptor potential (TRPA5) expressed in antennae and other body parts of C. pomonella as a complex pattern of ribonucleic acid (RNA) splice-forms, with a possible involvement in sensing chemical stimuli and temperature. Investigation on chemosensory mechanisms in the codling moth has practical outcomes for the development of control strategies and it inspired novel trends to control this pest by integrating alternative methods to interfere with insect chemosensory communication.
The codling moth Cydia pomonella (Lepidoptera: Trotricidae) is a major pest insect of commercial crops such as apple, pear and walnuts of Palearctic and Nearctic regions (Witzgall et al., 2008).
Integrated with insecticides, alternative methods are commonly used to control this insect (Starà et al., 2008; Odendaal et al., 2015; Arnault et al., 2016; Iraqui and Hmimina, 2016). Among these methods, mating disruption, which targets the olfactory system of C. pomonella males through the use of female sex pheromones, demonstrated efficient results to limit crop infestation (Hathaway et al., 1974; Ridgway et al., 1990; Light et al., 2001; Light, 2016). Furthermore, odors emitted by host-plants (kairomones), are combined with pheromones to enhance male attraction for the codling moth (Knight and Light, 2001; Light et al., 2001; Witzgall et al., 2001, 2005; Yang et al., 2004).
In insects, odors such as pheromones and kairomones are detected by olfactory sensory neurons (OSNs) that innervate specialized sensilla on their antennae (Buck and Axel, 1991; Chess et al., 1992; Vosshall et al., 2000; Carlson, 2001; Kurtovic et al., 2007). On the dendritic membrane of OSNs, odorants and pheromones mostly bind a class of seven-transmembrane proteins known as odorant receptors (ORs; Clyne et al., 1999). Deciphering mechanisms of receptor/ligand interactions and understanding pharmacological, kinetic properties and activation modalities of OR proteins, unveil promising aspects to improve strategies for the control of pest insects (Jones et al., 2011; Pask et al., 2011, 2013; Röllecke et al., 2013; Bobkov et al., 2014). Identification of ligands for specific ORs (deorphanization) among odors emitted from females and plant-hosts of the codling moth facilitates our understanding of the neurobiological and behavioral aspects at the base of the chemical ecology of C. pomonella. This contributes to possible application of novel ligands for semiochemical-based control strategies.
This mini-review reports the state of the art of current findings on the functional characterization of codling moth ORs as well as findings of a novel transient receptor potential (TRP) channel expressed in the olfactory system of C. pomonella. This contribution introduces ongoing studies on the molecular aspects of chemical sensing of the codling moth and their possible application to current control strategies targeting the olfactory system of the insect.
By means of a polymerase chain reaction (PCR)-based technique, the 3’ end of gene transcripts encoding putative members of C. pomonella ORs (CpomORs) have been initially identified from total ribonucleic acid (RNA) samples extracted from antennae (Garczynski et al., 2012). In this study, a similar method described by Buck and Axel (1991) was used to design degenerate forward primers based on polypeptide sequence alignments of the C-terminus of 12 members of the pheromone receptor subfamily of Bombyx mori (Lepidoptera: Bombycidae) and Heliothis virescens (Lepidoptera: Noctuidae). Forward primers were used to amplify partial 3’-ends starting from retro-transcribed 3’-cDNA templates generated by SMART™ kits (Clontech, Mountain View, CA, USA). Among amplified 3’-ends, the first set of CpomORs were identified. This method represented the first effort in the isolation of CpomORs from antennal RNA samples, leading optimization of further RACE-PCR approaches to amplify the full-length coding sequences of other chemosensory genes of this insect, aimed to address their phylogenetic and functional characterization.
With the advent of transcriptomic analysis, a wider investigation was conducted by the use of 454-next generation sequencings (NGS) of antennal RNA-samples (Bengtsson et al., 2012). For the first time, a wide asset of assembled fragments of gene coding sequences was identified, revealing 14 candidate ionotropic receptors (IRs), one candidate gustatory receptor (GR) and 43 candidate ORs. Among these, five ORs were members of the putative pheromone receptors (PRs) subfamily: a monophyletic clade in Lepidopteran insect OR phylogeny, with receptors that predominantly respond to odors emitted by females (Jacquin-Joly and Merlin, 2004; Ihara et al., 2013; Leal, 2013). Among the five candidate PRs reported by Bengtsson et al. (2012), two PRs represented some of the same ORs identified in the previous work of Garczynski et al. (2012). With the aim to complement these studies, using Illumina-based RNA-sequencing, assembly of a transcriptome from male, female and larval olfactory tissues of the codling moth, a more complete list of chemosensory receptors of C. pomonella was updated to 21 IRs, 20 GRs and 58 putative ORs, among which, 11 represented members of the PR-clade (Walker et al., 2016; Table 1). Identification of IRs and GRs in antennal transcriptomes of the codling moth was in accordance with the reported findings of their functional importance in insect chemosensation (Clyne et al., 2000; Robertson et al., 2003; Benton et al., 2009; Montell, 2009; Ai et al., 2010; Silbering et al., 2011; Rytz et al., 2013; Missbach et al., 2014; Sanchez-Alcaniz et al., 2018). Despite their importance, most of the efforts to functionally characterize chemosensory receptors of the codling moth targeted ORs, with particular focus on members of the PR-subfamily.
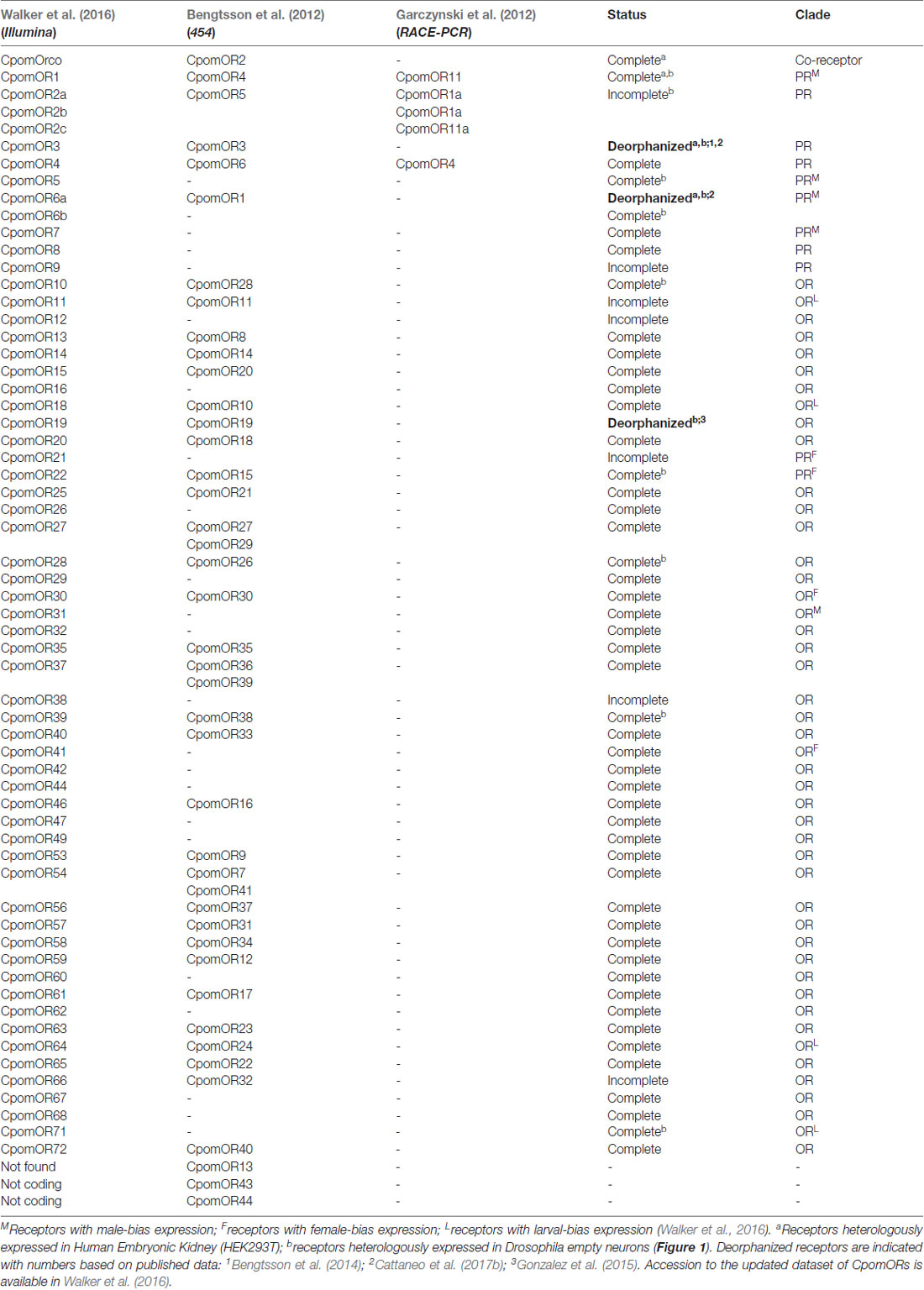
Table 1. Updated list of Cydia pomonella odorant receptors (CpomORs) in comparison among results from Garczynski et al. (2012); Bengtsson et al. (2012) and Walker et al. (2016), based on their techniques (brackets).
CpomOR3 represents the first OR of the codling moth that was isolated, heterologously expressed and functionally characterized (Bengtsson et al., 2014). Expression of this receptor was conducted in Drosophila melanogaster ab3A (Dobritsa et al., 2003; Gonzalez et al., 2016) and aT1 (Kurtovic et al., 2007; Montagné et al., 2012) empty neurons, to screen a panel of ligands among pheromones, synergists and antagonists known for their activation of the olfactory system of C. pomonella. Activation of CpomOR3 was demonstrated for the plant volatile ethyl-E,Z-2,4-decadienoate, commonly known as pear ester (Jennings et al., 1964; Berger and Drawert, 1984; Light et al., 2001; Yang et al., 2004; Knight et al., 2005; Willner et al., 2013). Phylogenetic analysis demonstrated CpomOR3 to be a PR-candidate. Activation of a putative PR to the synergist pear ester was in accordance with neurological effects identified when this compound was tested with the primary sex pheromone component of the codling moth (codlemone) on AL-glomeruli of the insect (Trona et al., 2010, 2013). CpomOR3 response to pear ester gave further support to the role of this compound as a kairomone, already known to enhance male attraction in orchards when combined with female pheromones (Light et al., 2001; Yang et al., 2004). These findings suggest a possible role of kairomones in the mate-choice behavior and in the reproductive isolation of tortricids (Trona et al., 2013; Bengtsson et al., 2014).
To better elucidate mechanisms involving pear ester sensing for CpomOR3, functional characterization experiments based on Drosophila empty neurons have been implemented through the heterologous expression of this receptor in Human Embryonic Kidney (HEK293T) cells (Cattaneo et al., 2017b). The use of an in vitro method represented an alternative to the common approaches for the functional characterization of insect ORs based on Drosophila empty neurons. The choice of this method was supported by successful attempts on the expression of PR-candidates from moths belonging to Bombycidae (Grosse-Wilde et al., 2006), Noctuidae (Grosse-Wilde et al., 2007), Saturniidae (Forstner et al., 2009) and Tortricidae (Steinwender et al., 2015). Comparison of heterologous expression between Drosophila OSNs and HEK293T is shown in Figure 1.
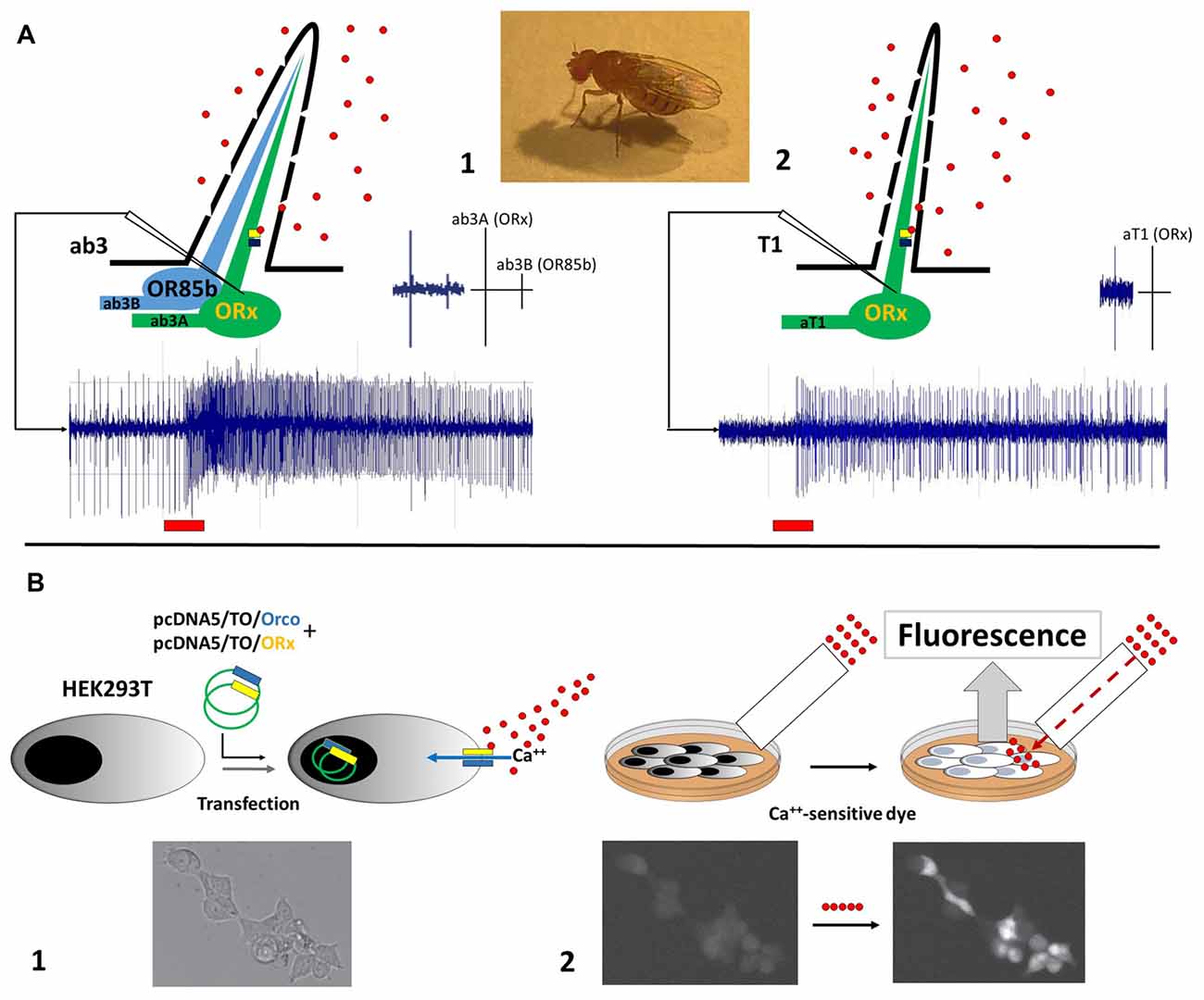
Figure 1. Illustration of heterologous expression methods adopted to functionally characterize odorant receptors (ORs) of Cydia pomonella. (A) Functional expression in empty neurons of D. melanogaster, between ab3A (1) and aT1 (2) neurons. Spike trains example are provided below; red bars indicate stimulation period. Spike scales between ab3A/B and aT1 are shown on the top-right. Recording electrodes are shown as white triangles. (B) Functional expression in Human Embryonic Kidney (HEK293T) cells: (1) transfection of HEK293T cells with plasmids carrying coding sequences of Orco (blue) and ORx (yellow): activation of the Orco+ORx cation channel by ligand-binding is represented; calcium-influx in response to Orco+ORx activation is indicated with a blue arrow. (2) Incubation of HEK cells with a Ca++-sensitive dye: fluorescent response elicited by calcium-influx through the cation channel is represented. Perfusion system is shown as a white rectangle. Ligands are represented as red circles. Orco+ORx is represented as blue/yellow bars. Images of HEK293T cells at the bottom are part of our published data (Cattaneo et al., 2017b). Licence to the use of the material reported in the aforementioned publication is available at the following link: http://creativecommons.org/licenses/by/4.0/.
In search of other possible ligands for CpomOR3, screening of a compound library on HEK293T cells validated activation of the receptor to both pear ester and the analogous methyl-(E, Z)-2, 4-decadienoate. Sensing of an analogous methyl-ester for the codling moth was reported for the first time by demonstrating larval attraction from emissions of ripe Bartlett pear (Knight and Light, 2001), although origins of methyl ester as a plant-emitted odorant are still debated. Indeed, aside from emission by Bartlett pear, methyl ester was found in the head, thoraxes and fecal pellets of the bark beetle Pityogenes chalcographus (Coleoptera: Curculionidae; Birgersson et al., 1990). In addition, methyl ester was also found in emissions from stink bugs of the genus Euschistus (Heteroptera: Pentatomidae; Aldrich et al., 1991; Tognon et al., 2016).
A remaining question is if interaction of the analogous methyl ester with the same receptor of ethyl-(E,Z)-2,4-decadienoate may result in a similar effect in the antennal lobe as an evidence of its synergism with codlemone (Trona et al., 2010, 2013).
Heterologous expression of codling moth receptors in HEK293T cells also deorphanized the PR candidate CpomOR6a as responsive to (E, E)-8–12-dodecadien-1-yl acetate (Codelmone acetate; Cattaneo et al., 2017b). Combining heterologous expression in Drosophila aT1, (E,Z)- and (Z,Z)-geometric isomers of codlemone acetate were also identified as partial ligands of the receptor. Together with these ligands, CpomOR6a sensed (E)-10-dodecadien-1-yl acetate and, although with less specificity, (Z,E)-8–12-dodecadien-1-yl acetate.
Codlemone acetates are main pheromone components emitted by female moths closely related to C. pomonella (Frerot et al., 1979; Roelofs and Brown, 1982; Davis et al., 1984; Witzgall et al., 1996; Chambers et al., 2011). Although receptors of codlemone acetates of these species have not been isolated and deorphanized yet, overall sequence similarities and relatively high expression in C. nigricana and Hedya nubiferana, suggested the gene locus OR6 to express a conserved receptor between C. pomonella and these tortricid species (Gonzalez et al., 2017). While speculative, a possible explanation of the existence of the codlemone acetate receptor in C. pomonella may be as a remnant of the former ancestor of the insect. However, conserving a receptor dedicated to detect other species may be important for reproductive isolation of the codling moth. Otherwise, since moths emitting codlemone acetates share the same host range with C. pomonella, detection of codlemone acetates may facilitate host finding for the codling moth. The evolution of a receptor specialized for the detection of a main pheromone compound, like codlemone, may likely represent a step towards allopatric speciation of C. pomonella.
Among candidate PRs of the codling moth (Table 1), the most likely sensor for codlemone is CpomOR1 given its abundant expression in OSNs of male moths (Bengtsson et al., 2014; Walker et al., 2016). Although heterologous expression methods in HEK cells and Drosophila empty neurons were unable to demonstrate CpomOR1 responsiveness to codlemone (Cattaneo et al., 2017b), future deorphanization attempts will unveil if this prediction holds true. Another remaining question is whether the transcript variant CpomOR6b has the same response spectrum as CpomOR6a and what might be its relevance, especially considering the lack of knowledge on alternative splicing in lepidopteran PRs (Garczynski and Leal, 2015).
Although CpomOR19 is not a PR-candidate in C. pomonella, testing heterologous expression of this receptor in Drosophila ab3As is part of documented deorphanizations of chemosensory receptors of the codling moth (Gonzalez et al., 2015).
CpomOR19 is responsive to 1-indanone; several analogs of this compound (2-methyl-1-indanone, 2-ethyl-1-indanone and 3-methyl-1-indanone) elicit responses of different sensitivities by this receptor. These compounds are renowned for their “non-host” origins (Klein et al., 1990; Anderson et al., 1993; Nagle et al., 2000; Okpekon et al., 2009; Rukachaisirikul et al., 2013). CpomOR19 binding to indanes represented the first deorphanization of a receptor of the codling moth to compounds emitted by non-hosts. Interestingly, different sensitivities between 1-indanone and its analogs for the CpomOR19 binding is consistent with observations reported between pear and methy esters on CpomOR3, where one carbon of the alkyl group may determine different binding affinity, perhaps due to differences in the polarity of the compounds (Cattaneo et al., 2017b).
By use of the same method, activation of the ortholog SlitOR19 of the African cotton-leaf worm Spodoptera littoralis (Lepidoptera: Noctuidae) demonstrated conservation in binding 1-indanone and analogous. When compared, ab3A expressing CpomOR19 and SlitOR19 showed increased response to indanes, when substituted with alkyl groups at position two and three of the five-membered ring. On the contrary, indanes provided with methyl substituents on the benzene ring largely did not activate these receptors. Furthermore, indanes provided with alcohols, hydrocarbons and amine groups also did not activate any of the two receptors, which suggested a conserved function for CpomOR19 and SlitOR19 orthologs, despite the phylogenetical and ecological distance of their respective moths. A recent report on Spodoptera ORs provides a blueprint for prediction of SlitOR ligands based on the interaction of phylogeny and chemical structure (de Fouchier et al., 2017). Given evidences of conserved function between CpomOR19 and SlitOR19, prediction of SlitOR ligands may benefit future studies on deorphanization of CpomORs.
Expression of CpomOR genes in HEK cells was undertaken by co-transfecting the CpomOrco co-receptor subunit of the codling moth (Figure 1B). Functional studies of CpomOrco demonstrated heteromeric complexes of the co-receptor with OR subunits being more sensitive than homomeric co-receptor complexes, as previously demonstrated for Orco-based channels of other insects (Jones et al., 2011; Pask et al., 2011; Kumar et al., 2013; Turner et al., 2014). By the use of the main ligand VUAA (Jones et al., 2011), calcium response was characterized by faster activation/deactivation kinetics for CpomORco+OR than CpomORco alone. Testing inhibitors like amiloride derivatives (ADs; Pask et al., 2013; Röllecke et al., 2013) demonstrated similar effects for both homomeric and heteromeric complexes. When HEK cells were tested by whole-cell and outside-out patch-clamp recordings, activation of CpomOrco+OR complexes resembled modalities of ligand-gated cation channels: responses to multiple stimulations were characterized by constant amplitudes and stable kinetic parameters, which is indicative of the ionotropic nature of insect OR receptors (Sato et al., 2008).
Despite that the molecular mechanisms at the base of signal transduction of insect olfactory systems still remain unknown (Krieger and Breer, 1999; Jacquin-Joly and Merlin, 2004; Sakurai et al., 2014), results on the functional characterization of C. pomonella ORs are consistent with the idea that all insect or, perhaps, even all arthropod chemosensory receptor channels (among ORs and IRs) can be characterized by somewhat common pharmacology (Bobkov and Ache, 2007; Abuin et al., 2011; Bobkov et al., 2014). Although, this might be called into question given some evidence pointing towards metabotropic signaling modalities for insect ORs (Sargsyan et al., 2011; Getahun et al., 2013; Ignatious Raja et al., 2014).
A second analysis of sequencing data from Bengtsson et al. (2012), unveiled further transcripts related with ligand-gated cation channels belonging to the class of TRP. In several organisms, TRP-channels enable sensing of multiple stimuli from the environment (Liedtke and Heller, 2007). Among chemical stimuli, several compounds commonly found in food plants and spices are reported to activate TRPs (Caterina et al., 1997; Jordt et al., 2004; Xu et al., 2006; Bautista et al., 2007). Interestingly, TRP-active compounds are reported for their ability to repel insects (Leung and Foster, 1996; Barnard, 1999) and, in particular, to activate the olfactory system of tortricid and noctuid moths (Cattaneo et al., 2014; Wei et al., 2015).
In C. pomonella, five TRPs have been found in the antennae belonging to the TRPC (TRP, TRPC) and the TRPA subfamily (Pyrexia, water witch, TRPA5; Cattaneo et al., 2016). Up to now, CpomTRPA5 is the only TRP of the codling moth that has been extended to the full length. Interestingly, five variants of the spliced-coding sequence have been found, demonstrating different expression patterns among body parts of the codling moth. Analysis of the CpomTRPA5 mRNA sequence demonstrated the transcript undergoing to mRNA editing by insertion of 15 additional nucleotides within the third exon of the full-length sequence, which is a mechanism occurring for K+ channels of multiple organisms, including insects (Holmgren and Rosenthal, 2015). Evolutionary studies suggested the relatedness of TRPA5 gene to the thermal sensor Pyrexia (Peng et al., 2015), which has also been descripted as a thermal-gated K+-channel of insect (Lee et al., 2005).
Identification of TRPs in C. pomonella represented the first documented finding within this species for this particular class of chemoreceptors. Identification of CpomTRPA5 and its spliceforms is among the first documented existences of this particular subunit for arthropod TRPAs (Peng et al., 2015). Relatedness of CpomTRPA5 with Pyrexia suggested a possible role of the CpomTRPA5 receptor as a thermal sensor, which is consistent with behavioral evidences for the codling moth of odor-guided responses in relation with temperature (Witzgall et al., 1999).
By means of methods adopted to test activation of mammalian TRPAs expressed in HEK cells (Bassoli et al., 2009, 2013; Cattaneo et al., 2017a), functional characterization studies of CpomTRPA5 may be conducted to better elucidate possible roles of this receptor in chemical and physical sensing modalities of the codling moth.
The technologies adapted to the setup of transcriptomic and heterologous expression studies for the functional characterization of chemosensory receptors of C. pomonella, may offer new opportunities to address longstanding questions in the field of insect ecology, with a practical outcome for the implementation of its control strategies.
Two out of the three codling moth ORs that have been deorphanized, belong to the clade of putative Pheromone Receptors. Although attempted, the receptor for the main pheromone codlemone has not been functionally characterized. To validate a possible role of CpomOR1 as a main candidate sensor (Bengtsson et al., 2012; Walker et al., 2016; Cattaneo et al., 2017b), future experiments will verify if co-expression of CpomOR1 with CpomOR6a in Drosophila aT1 neurons is sensitive to codlemone. This approach is supported by evidences of response to codlemone acetates from OSNs of C. pomonella responding to codlemone (Bäckman et al., 2000), which may suggest a possible role of the CpomOR6a subunit to sense this pheromone. In addition, studies on several insects demonstrated co-expression of different OR subunits in the same OSN (Couto et al., 2005; Fishilevich and Vosshall, 2005; Goldman et al., 2005; Ray et al., 2007; Koutroumpa et al., 2014; Karner et al., 2015; Lebreton et al., 2017), and stoichiometry of OR heteromers is still debated (Larsson et al., 2004; Benton et al., 2006; Wicher, 2018).
In support of the control of the codling moth with mating disruption, novel trends are leading the direction of studies to integrate targeting of sensing modalities of codling moth females. Indeed, methods based on mating disruption demonstrated inefficacy to the control of the codling moth at high population in the orchards, as well as on the top of tree branches, where the pheromone cloud is limited (Witzgall et al., 1999). Identification of CpomORs with a female-biased expression (Bengtsson et al., 2012; Walker et al., 2016) motivates the use of heterologous methods to address their functional characterization (Swedish Research Council Formas, Project Reg. No. 2016-01281 “Control of Apple Pest Insects with Fruit and Yeast Odorants”). This approach may identify novel ligands active on female olfactory systems. Among these ligands, odors emitted by fruits and their associated microbes may be tested, given the importance of yeasts for attractiveness of egg-lying females (Witzgall et al., 2012).
Recent studies based on CRISPR/Cas9 editing of the codling moth, demonstrated efficacy of this method to address knockdown of functional OR proteins, which resulted in affection of fecundity and fertility, with edited females producing nonviable eggs (Garczynski et al., 2017). Future targets may combine heterologous expression methods, with the use of CRISPR/Cas9 to generate OR-edited insects, as a complementary approach to address the functional characterization of codling moth receptors.
Future trends integrating research on the olfactory system of C. pomonella may target larval chemical sensing as complementary to the current approaches addressing the functional characterization of adult ORs (Formas Mobility Starting Grant Reg. No. 2018-00891 “Control of Fruit Pests by Targeting Larval Chemical Sensing,” submitted). Indeed, chemosensory mechanisms at the base of larval behavior are long renowned for the codling moth (Knight and Light, 2001; Jumean et al., 2005) and expression of CpomORs with a larval-bias has been reported (Walker et al., 2016).
Broader discoveries on the molecular bases of the olfactory mechanisms of C. pomonella will enhance current control strategies interfering with the insect’s chemosensory communication. Development of novel methods targeting olfaction may help limit the use of insecticides, with beneficial effects on the quality of life for apple growers, consumers, as well as public living around the orchard areas, reducing further the conflict between agricultural and urban worlds.
AC wrote the manuscript.
The financial support for this study has been provided by the Craaford Foundation (Ref. No: 20170728), integrated with the Formas Project Reg. No.: 2016-01281 “Control of Apple Pest Insects with Fruit and Yeast odorants”—Swedish Research Council. Costs for submission will be covered by the Martha och Dagny Larssons fond—Swedish University of Agricultural Sciences (Protokoll 172-174, Sammanträdesdatum 2018-04-24).
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author acknowledges Dr. William B. Walker III and Prof. Peter Witzgall for general discussion, personal communications and availability in phase of writing this contribution. Language editing has been courtesy performed by Dr. William Walker.
Abuin, L., Bargeton, B., Ulbrich, M. H., Isacoff, E. Y., Kellenberger, S., and Benton, R. (2011). Functional architecture of olfactory ionotropic glutamate receptors. Neuron 69, 44–60. doi: 10.1016/j.neuron.2010.11.042
Ai, M., Min, S., Grosjean, Y., Leblanc, C., Bell, R., Benton, R., et al. (2010). Acid sensing by the Drosophila olfactory system. Nature 468, 691–695. doi: 10.1038/nature09537
Aldrich, J. R., Hoffmann, M. P., Kochansky, J. P., Lusby, W. R., Eger, J. E., and Payne, J. A. (1991). Identification and attractiveness of a major pheromone component for Nearctic Euschistus spp. stink bugs (Heteroptera: Pentatomidae). Environ. Entomol. 20, 477–483. doi: 10.1093/ee/20.2.477
Anderson, P., Hilker, M., Hansson, B., Bombosch, S., Klein, B., and Schildknecht, H. (1993). Oviposition deterring components in larval frass of Spodoptera littoralis (Boisd.) (Lepidoptera: Noctuidae): a behavioral and electrophysiological evaluation. J. Insect Physiol. 39, 129–137. doi: 10.1016/0022-1910(93)90104-y
Arnault, I., Lombarkia, N., Joy-Ondet, S., Romet, L., Brahim, I., Meradi, R., et al. (2016). Foliar application of microdoses of sucrose to reduce codling moth Cydia pomonella L. (Lepidoptera: Tortricidae) damage to apple trees. Pest Manag. Sci. 72, 1901–1909. doi: 10.1002/ps.4228
Bäckman, A. C., Anderson, P., Bengtsson, M., Löfqvist, J., Unelius, C. R., and Witzgall, P. (2000). Antennal response of codling moth males, Cydia pomonella L. (Lepidoptera: Tortricidae), to the geometric isomers of codlemone and codlemone acetate. J. Comp. Physiol. A 186, 513–519. doi: 10.1007/s003590000101
Barnard, D. R. (1999). Repellency of essential oils to mosquitoes (Diptera: Culicidae). J. Med. Entomol. 36, 625–629. doi: 10.1093/jmedent/36.5.625
Bassoli, A., Borgonovo, G., Caimi, S., Scaglioni, L., Morini, G., Moriello, A. S., et al. (2009). Taste-guided identication of high potency TRPA1 agonists from Perilla frutescens. Bioorg. Med. Chem. 17, 1636–1639. doi: 10.1016/j.bmc.2008.12.057
Bassoli, A., Borgonovo, G., Morini, G., De Petrocellis, L., Schiano Moriello, A., and Di Marzo, V. (2013). Analogues of perillaketone has highly potent agonists of TRPA1 channel. Food Chem. 141, 2044–2051. doi: 10.1016/j.foodchem.2013.05.063
Bautista, D. M., Siemens, J., Glazer, J. M., Tsuruda, P. R., Basbaum, A. I., Stucky, C. L., et al. (2007). The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 448, 204–208. doi: 10.1038/nature05910
Bengtsson, J. M., Gonzalez, F., Cattaneo, A. M., Montagné, N., Walker, W. B. III., Bengtsson, M., et al. (2014). A predicted sex pheromone receptor of codling moth Cydia pomonella detects the plant volatile pear ester. Front. Ecol. Evol. 2:33. doi: 10.3389/fevo.2014.00033
Bengtsson, J. M., Trona, F., Montagné, N., Anfora, G., Ignell, R., Witzgall, P., et al. (2012). Putative chemosensory receptors of the codling moth, Cydia pomonella, identified by antennal transcriptome analysis. PLoS One 7:e31620. doi: 10.1371/journal.pone.0031620
Benton, R., Sachse, S., Michnick, S. W., and Vosshall, L. B. (2006). A typical membrane topology and heteromeric function of Drosophila odorant receptors in vivo. PLoS Biol. 4:e20. doi: 10.1371/journal.pbio.0040020
Benton, R., Vannice, K. S., Gomez-Diaz, C., and Vosshall, L. B. (2009). Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell 136, 149–162. doi: 10.1016/j.cell.2008.12.001
Berger, R. G., and Drawert, F. (1984). Changes in the composition of volatiles by post-harvest application of alcohol stored delicious apples. J. Sci. Food Agric. 35, 1318–1325. doi: 10.1002/jsfa.2740351208
Birgersson, J., Byers, J. A., Bergström, G., and Löfqvist, J. (1990). Production of pheromone components, chalcogran and methyl (E,Z)-2,4-decadienoate, in the spruce engraver Pityogenes chalcographus. J. Ins. Physiol. 36, 391–395. doi: 10.1016/0022-1910(90)90056-l
Bobkov, Y. V., and Ache, B. W. (2007). Block by amiloride derivatives of odor-evoked discharge in lobster olfactory receptor neurons through action on a presumptive TRP channel. Chem. Senses 32, 149–159. doi: 10.1093/chemse/bjl041
Bobkov, Y. V., Corey, E. A., and Ache, B. W. (2014). An inhibitor of Na+/Ca2+ exchange blocks activation of insect olfactory receptors. Biochem. Biophys. Res. Commun. 450, 1104–1109. doi: 10.1016/j.bbrc.2014.06.120
Buck, L., and Axel, R. (1991). A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell 65, 175–187. doi: 10.1016/0092-8674(91)90418-x
Carlson, J. R. (2001). Functional expression of a Drosophila odor receptor. Proc. Natl. Acad. Sci. U S A 98, 8936–8937. doi: 10.1073/pnas.171311198
Caterina, M. J., Schumacher, M. A., Tominaga, M., Rosen, T. A., Levine, J. D., and Julius, D. (1997). The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389, 816–824. doi: 10.1038/39807
Cattaneo, A. M., Bengtsson, J. M., Borgonovo, G., Bassoli, A., and Anfora, G. (2014). Response of the European grapevine moth Lobesia botrana to somatosensory-active volatiles emitted by the non-host plant Perilla frutescens. Physiol. Entomol. 39, 229–236. doi: 10.1111/phen.12067
Cattaneo, A. M., Bengtsson, J. M., Montagné, N., Jacquin-Joly, E., Rota-Stabelli, O., Salvagnin, U., et al. (2016). TRPA5, an ankyrin subfamily insect TRP channel, is expressed in antennae of Cydia pomonella (Lepidoptera: Tortricidae) in multiple splice variants. J. Insect Sci. 16:83. doi: 10.1093/jisesa/iew072
Cattaneo, A. M., Bobkov, Y. V., Corey, E. A., Borgonovo, G., and Bassoli, A. (2017a). Perilla derived compounds mediate human TRPA1 channel activity. Med. Aromat. Plants. 6:283. doi: 10.4172/2167-0412.1000283
Cattaneo, A. M., Gonzalez, F., Bengtsson, J. M., Corey, E. A., Jacquin-Joly, E., Montagné, N., et al. (2017b). Candidate pheromone receptors of codling moth Cydia pomonella respond to pheromones and kairomones. Sci. Rep. 7:41105. doi: 10.1038/srep41105
Chambers, U., Walton, V. M., and Mehlenbacher, S. A. (2011). Susceptibility of hazelnut cultivars to filbertworm, Cydia latiferreana. Hort. Science 46, 1377–1380.
Chess, A., Buck, L., Dowling, M. M., Axel, R., and Ngai, J. (1992). Molecular biology of smell: expression of the multigene family encoding putative odorant receptors. Cold. Spring Harb. Symp. Quant. Biol. 57, 505–516. doi: 10.1101/sqb.1992.057.01.056
Clyne, P. J., Warr, C. G., and Carlson, J. R. (2000). Candidate taste receptors in Drosophila. Science 287, 1830–1834. doi: 10.1126/science.287.5459.1830
Clyne, P. J., Warr, C. G., Freeman, M. R., Lessing, D., Kim, J., and Carlson, J. R. (1999). A novel family of divergent seven-transmembrane proteins: candidate odorant receptors in Drosophila. Neuron 22, 327–338. doi: 10.1016/S0896-6273(00)81093-4
Couto, A., Alenius, M., and Dickson, B. J. (2005). Molecular, anatomical and functional organization of the Drosophila olfactory system. Curr. Biol. 15, 1535–1547. doi: 10.1016/j.cub.2005.07.034
Davis, H. G., McDonough, L. M., Burditt, A. K., and Bieri-Leonhardt, B. A. (1984). Filbertworm sex pheromone. Identification and field tests of (E,E)- and (E,Z)- 8,10 dodecadien-1-ol acetates. J. Chem. Ecol. 10, 53–61. doi: 10.1007/bf00987643
de Fouchier, A., Walker, W. B. III., Montagné, N., Steiner, C., Binyameen, M., Schlyter, F., et al. (2017). Functional evolution of Lepidoptera olfactory receptors revealed by deorphanization of a moth repertoire. Nat. Comm. 8:15709. doi: 10.1038/ncomms15709
Dobritsa, A. A., van der Goes van Naters, W., Warr, C. G., Steinbrecht, R. A., and Carlson, J. R. (2003). Integrating the molecular and cellular basis of odor coding in the Drosophila antenna. Neuron 37, 827–841. doi: 10.1016/s0896-6273(03)00094-1
Fishilevich, E., and Vosshall, L. B. (2005). Genetic and functional subdivision of the Drosophila antennal lobe. Curr. Biol. 15, 1548–1553. doi: 10.1016/j.cub.2005.07.066
Forstner, M., Breer, H., and Krieger, J. (2009). A receptor and binding protein interplay in the detection of a distinct pheromone component in the silkmoth Antheraea polyphemus. Int. J. Biol. Sci. 5, 745–757. doi: 10.7150/ijbs.5.745
Frerot, B., Priesner, E., and Gallois, M. A. (1979). Sex attractant for the green budworm moth, Hedya nubiferana. Z. Naturforsch. C 34, 1248–1252. doi: 10.1515/znc-1979-1229
Garczynski, S. F., and Leal, W. S. (2015). Alternative splicing produces two transcripts encoding female-biased pheromone subfamily receptors in the navel orangeworm, Amyelois transitella. Front. Ecol. Evol. 3:115. doi: 10.3389/fevo.2015.00115
Garczynski, S. F., Martin, J. A., Griset, M., Willett, L. S., Cooper, W. R., Swisher, K. D., et al. (2017). CRISPR/Cas9 editing of the codling moth (Lepidoptera: Tortricidae) CpomOR1 Gene affects egg production and viability. J. Econ. Entomol. 110, 1847–1855. doi: 10.1093/jee/tox166
Garczynski, S. F., Wanner, K. W., and Unruh, T. R. (2012). Identification and initial characterization of the 3′ end of gene transcripts encoding putative members of the pheromone receptor subfamily in Lepidoptera. Ins. Sci. 19, 64–74. doi: 10.1111/j.1744-7917.2011.01423.x
Getahun, M. N., Olsson, S. B., Lavista-llanos, S., Hansson, B. S., and Wicher, D. (2013). Insect odorant response sensitivity is tuned by metabotropically autoregulated olfactory receptors. PLoS One 8:e58889. doi: 10.1371/journal.pone.0058889
Goldman, A. L., van Naters, W. V., Lessing, D., Warr, C. G., and Carlson, J. R. (2005). Coexpression of two functional odor receptors in one neuron. Neuron 45, 661–666. doi: 10.1016/j.neuron.2005.01.025
Gonzalez, F., Bengtsson, J. M., Walker, W. B. III., Sousa, M., Cattaneo, A. M., Montagné, N., et al. (2015). A conserved odorant receptor detects the same substituted indan compounds in a totricid and a noctuid moth. Front. Ecol. Evol. 3:131. doi: 10.3389/fevo.2015.00131
Gonzalez, F., Witzgall, P., and Walker, W. B. III. (2016). Protocol for heterologous expression of insect odourant receptors in Drosophila. Front. Ecol. Evol. 4:24. doi: 10.3389/fevo.2016.00024
Gonzalez, F., Witzgall, P., and Walker, W. B. III. (2017). Antennal transcriptomes of three tortricid moths reveal putative conserved chemosensory receptors for social and habitat olfactory cues. Sci. Rep. 7:41829. doi: 10.1038/srep41829
Grosse-Wilde, E., Gohl, T., Bouche, E., Breer, H., and Krieger, J. (2007). Candidate pheromone receptors provide the basis for the response of distinct antennal neurons to pheromonal compounds. Eur. J. Neurosci. 25, 2364–2373. doi: 10.1111/j.1460-9568.2007.05512.x
Grosse-Wilde, E., Svatos, A., and Krieger, J. (2006). A pheromone-binding protein mediates the bombykol-induced activation of a pheromone receptor in vitro. Chem. Senses 31, 547–555. doi: 10.1093/chemse/bjj059
Hathaway, D. O., McGovern, T. P., Beroza, M., Moffitt, H. R., McDonough, L. M., and Buit, B. A. (1974). An inhibitor of sexual attraction of male codling moths to a synthetic sex pheromone and virgin females in traps. Environ. Entomol. 3, 522–524. doi: 10.1093/ee/3.3.522
Holmgren, M., and Rosenthal, J. J. (2015). Regulation of ion channel and transporter function through RNA editing. Curr. Issues Mol. Biol. 17, 23–36. doi: 10.21775/cimb.017.023
Ignatious Raja, J. S., Katanayeva, N., Katanaev, V. L., and Galizia, C. G. (2014). Role of Go/i subgroup of G proteins in olfactory signaling of Drosophila melanogaster. Eur. J. Neurosci 39, 1245–1255. doi: 10.1111/ejn.12481
Ihara, S., Yoshikawa, K., and Touhara, K. (2013). Chemosensory signals and their receptors in the olfactory neural system. Neuroscience 254, 45–60. doi: 10.1016/j.neuroscience.2013.08.063
Iraqui, S., and Hmimina, M. (2016). Assessment of control strategies against Cydia pomonella (L.) in Morocco. J. Plant Prot. Res. 56, 82–88. doi: 10.1515/jppr-2016-0012
Jacquin-Joly, E., and Merlin, C. (2004). Insect olfactory receptors: contributions of molecular biology to chemical ecology. J. Chem. Ecol. 30, 2359–2397. doi: 10.1007/s10886-004-7941-3
Jennings, W. G., Creveling, R. K., and Heinz, D. E. (1964). Volatile esters of Bartlett pear. IV. Esters of trans-2-cis-4-decadienoic acid. J. Food Sci. 29, 730–734. doi: 10.1111/j.1365-2621.1964.tb00439.x
Jones, P. L., Paska, G. M., Rinkerb, D. C., and Zwiebel, L. J. (2011). Functional agonism of insect odorant receptor ion channels. Proc. Natl. Acad. Sci. U S A 108, 8821–8825. doi: 10.1073/pnas.1102425108
Jordt, S. E., Bautista, D. M., Chuang, H. H., McKemy, D. D., Zygmunt, P. M., Högestätt, E. D., et al. (2004). Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 427, 260–265. doi: 10.1038/nature02282
Jumean, Z., Lafontaine, J. P., Wood, C., Judd, G. J. R., and Gries, G. (2005). Pheromone-based trapping of larval codling moth, Cydia pomonella, in apple orchards. J. Chem. Ecol. 31, 911–924. doi: 10.1007/s10886-005-3552-x
Karner, T., Schneider, I., Schultze, A., Breer, H., and Krieger, J. (2015). Co-expression of six tightly clustered odorant receptor genes in the antenna of the malaria mosquito. Front. Ecol. Evol. 3:26. doi: 10.3389/fevo.2015.00026
Klein, B., Schildknecht, H., Hilker, M., and Bombosch, S. (1990). Eiablagehemmende wirkstoffe aus dem Larvenkot von Spodoptera littoralis (Boisd.). Z. Naturforsch. C 45, 895–901.doi: 10.1515/znc-1990-7-823
Knight, A., Hilton, R., and Light, D. (2005). Monitoring codling moth (Lepidoptera: Tortricidae) in apple with blends of ethyl (E, Z)-2, 4-decadienoate and codlemone. Environ. Entomol. 34, 598–603. doi: 10.1603/0046-225x-34.3.598
Knight, A. L., and Light, D. M. (2001). Attractants from Bartlett pear for codling moth, Cydia pomonella (L.), larvae. Naturwissenschaften 88, 339–342. doi: 10.1007/s001140100244
Koutroumpa, F. A., Kárpáti, Z., Monsempes, C., Hill, S. R., Hansson, B. S., Jacquin-Joly, E., et al. (2014). Shifts in sensory neuron identity parallel differences in pheromone preference in the European corn borer. Front. Ecol. Evol. 2:65. doi: 10.3389/fevo.2014.00065
Krieger, J., and Breer, H. (1999). Olfactory reception in invertebrates. Science 286, 720–723. doi: 10.1126/science.286.5440.720
Kumar, B. N., Taylor, R. W., Pask, G. M., Zwiebel, L. J., Newcomb, R. D., and Christie, D. L. A. (2013). A conserved aspartic acid is important for agonist (VUAA1) and odorant/tuning receptor-dependent activation of the insect odorant co-receptor (Orco). PLoS One 8:e70218. doi: 10.1371/journal.pone.0070218
Kurtovic, A., Widmer, A., and Dickson, B. J. (2007). A single class of olfactory neurons mediates behavioural responses to a Drosophila sex pheromone. Nature 446, 542–546. doi: 10.1038/nature05672
Larsson, M. C., Domingos, A. I., Jones, W. D., Chiappe, M. E., Amrein, H., and Vosshall, L. B. (2004). Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron 43, 703–714. doi: 10.1016/j.neuron.2004.08.019
Leal, W. S. (2013). Odorant reception in insects: roles of receptors, binding proteins and degrading enzymes. Annu. Rev. Entomol. 58, 373–391. doi: 10.1146/annurev-ento-120811-153635
Lebreton, S., Borrero-Echeverry, F., Gonzalez, F., Solum, M., Wallin, E. A., Hedenström, E., et al. (2017). A Drosophila female pheromone elicits species-specific long-range attraction via an olfactory channel with dual specificity for sex and food. BMC Biol. 15:88. doi: 10.1186/s12915-017-0427-x
Lee, Y., Lee, J., Bang, S., Hyun, S., Kang, J., Hong, S. T., et al. (2005). Pyrexia is a new thermal transient receptor potential channel endowing tolerance to high temperatures in Drosophila melanogaster. Nat. Genet. 37, 305–310. doi: 10.1038/ng1513
Leung, A. Y., and Foster, S. (1996). Encyclopedia of Common Natural Ingredients Used in Food, Drugs and Cosmetics. 2nd Edn. New York: John Wiley and Sons Inc.
Liedtke, W. B., and Heller, S. (Eds). (2007). “Trp channels: diversity of form and function,” in Frontiers in Neuroscience (Boca Raton, FL: CRC Press/Taylor & Francis). Available online at: https://www.ncbi.nlm.nih.gov/books/NBK1856/.
Light, D. M. (2016). Control and monitoring of codling moth (Lepidoptera: Tortricidae) in walnut orchards treated with novel high-load, low-density “meso” dispensers of sex pheromone and pear ester. Environ. Entomol. 45, 700–707. doi: 10.1093/ee/nvw017
Light, D. M., Knight, A. L., Henrick, C. A., Rajapaska, D., Lingren, B., Dickens, J. C., et al. (2001). A pear-derived kairomone with pheromonal potency that attracts male and female codling moth, Cydia pomonella (L.). Naturwissenschaften 88, 333–338. doi: 10.1007/s001140100243
Missbach, C., Dweck, H. K. M., Vogel, H., Vilcinskas, A., Stensmyr, M. C., Hansson, B. S., et al. (2014). Evolution of insect olfactory receptors. Elife 3:e05087. doi: 10.7554/eLife.02115
Montagné, N., Chertemps, T., Brigaud, I., François, A., François, M. C., de Fouchier, A., et al. (2012). Functional characterization of a sex pheromone receptor in the pest moth Spodoptera littoralis by heterologous expression in Drosophila. Eur. J. Neurosci. 36, 2588–2596. doi: 10.1111/j.1460-9568.2012.08183.x
Montell, C. (2009). A taste of the Drosophila gustatory receptors. Curr. Opin. Neurobiol. 19, 345–353. doi: 10.1016/j.conb.2009.07.001
Nagle, D. G., Zhou, Y.-D., Park, P. U., Paul, V. J., Rajbhandari, I., Duncan, C. J., et al. (2000). A new indanone from the marine cyanobacterium Lyngbya majuscula that inhibits hypoxia-induced activation of the VEGF promoter in Hep3B cells. J. Nat. Prod. 63, 1431–1433. doi: 10.1021/np000216e
Odendaal, D., Addison, M. F., and Malan, A. P. (2015). Control of codling moth (Cydia pomonella) (Lepidoptera: Tortricidae) in South Africa with special emphasis on using entomopathogenic nematodes. Afr. Entomol. 23, 259–274. doi: 10.4001/003.023.0224
Okpekon, T., Millot, M., Champy, P., Gleye, C., Yolou, S., Bories, C., et al. (2009). A novel 1-indanone isolated from Uvaria afzelii roots. Nat. Prod. Res. 23, 909–915. doi: 10.1080/14786410802497240
Pask, G. M., Bobkov, Y. V., Corey, E. A., Ache, B. W., and Zwiebe, L. J. (2013). Blockade of insect odorant receptor currents by amiloride derivatives. Chem. Senses 38, 221–229. doi: 10.1093/chemse/bjs100
Pask, G. M., Jones, P. L., Rützler, M., Rinker, D. C., and Zwiebel, L. J. (2011). Heteromeric anopheline odorant receptors exhibit distinct channel properties. PLoS One 6:e28774. doi: 10.1371/journal.pone.0028774
Peng, G., Xiao, S., and Kadowaki, T. (2015). Evolution of TRP channels inferred by their classification in diverse animal species. Mol. Phylogenet. Evol. 84, 145–157. doi: 10.1016/j.ympev.2014.06.016
Ray, A., van Naters, W. G., Shiraiwa, T., and Carlson, J. R. (2007). Mechanisms of odor receptor gene choice in Drosophila. Neuron 53, 353–369. doi: 10.1016/j.neuron.2006.12.010
Ridgway, M., Silverstein, R. L., and Inscoe, M. N. (1990). Behavior-Modifying Chemicals for Insect Management: Applications of Pheromones And Other Attractants. New York, NY: Marcel Dekker.
Robertson, H. M., Warr, C. G., and Carlson, J. R. (2003). Molecular evolution of the insect chemoreceptor gene superfamily in Drosophila melanogaster. Proc. Natl. Acad. Sci. U S A 100, 14537–14542. doi: 10.1073/pnas.2335847100
Roelofs, W. L., and Brown, R. L. (1982). Pheromones and evolutionary relationships of Tortricidae. Ann. Rev. Ecol. Syst. 13, 395–422. doi: 10.1146/annurev.es.13.110182.002143
Röllecke, K., Werner, M., Ziemba, P. M., Neuhaus, E. M., Hatt, H., and Gisselmann, G. (2013). Amiloride derivatives are effective blockers of insect odorant receptors. Chem. Senses 38, 231–236. doi: 10.1093/chemse/bjs140
Rukachaisirikul, V., Buadam, S., Sukpondma, Y., Phongpaichit, S., Sakayaroj, J., and Hutadilok-Towatana, N. (2013). Indanone and mellein derivatives from the Garcinia-derived fungus Xylaria sp. PSU-G12. Phytochem. Lett. 6, 135–138. doi: 10.1016/j.phytol.2012.11.007
Rytz, R., Croset, V., and Benton, R. (2013). Ionotropic receptors (IRs): chemosensory ionotropic glutamate receptors in Drosophila and beyond. Insect Biochem. Mol. Biol. 43, 888–897. doi: 10.1016/j.ibmb.2013.02.007
Sakurai, T., Namiki, S., and Kanzaki, R. (2014). Molecular and neural mechanisms of sex pheromone reception and processing in the silkmoth Bombyx mori. Front. Physiol. 5:125. doi: 10.3389/fphys.2014.00125
Sanchez-Alcaniz, J. A., Silbering, A. F., Croset, V., Zappia, G., Sivasubramaniam, A. K., Abuin, L., et al. (2018). An expression atlas of chemosensory ionotropic glutamate receptors identifies a molecular basis of carbonation detection. arXiv:1101/278804 [Preprint]. doi: 10.1101/278804
Sargsyan, V., Getahun, M. N., Llanos, S. L., Olsson, S. B., Hansson, B. S., and Wicher, D. (2011). Phosphorylation via PKC regulates the function of the Drosophila odorant co-receptor. Front. Cell. Neurosci. 5:5. doi: 10.3389/fncel.2011.00005
Sato, K., Pellegrino, M., Nakagawa, T., Nakagawa, T., Vosshall, L. B., and Touhara, K. (2008). Insect olfactory receptors are heteromeric ligand-gated ion channels. Nature 452, 1002–1006. doi: 10.1038/nature06850
Silbering, A. F., Rytz, R., Grosjean, Y., Abuin, L., Ramdya, P., Jefferis, G. S., et al. (2011). Complementary function and integrated wiring of the evolutionarily distinct Drosophila olfactory subsystems. J. Neurosci. 31, 13357–13375. doi: 10.1523/JNEUROSCI.2360-11.2011
Starà, J., Kocourek, F., and Falta, V. (2008). Control of codling moth (Cydia pomonella L., Lepidoptera: Tortricidae) by the “attract and kill” strategy. J. Plant Dis. Prot. 115, 75–79. doi: 10.1007/BF03356242
Steinwender, B., Thrimawithana, A. H., Crowhurst, R. N., and Newcomb, R. D. (2015). Pheromone receptor evolution in the cryptic leafroller species, Ctenopseustis obliquana and C. herana. J. Mol. Evol. 80, 42–56. doi: 10.1007/s00239-014-9650-z
Tognon, R., Sant’Ana, J., Zhang, Q.-H., Millar, J. G., Aldrich, J. R., and Zalom, F. G. (2016). Volatiles mediating parasitism of Euschistus conspersus and Halyomorpha halys eggs by Telenomus podisi and Trissolcus erugatus. J. Chem. Ecol. 42, 1016–1027. doi: 10.1007/s10886-016-0754-3
Trona, F., Anfora, G., Balkenius, A., Bengtsson, M., Tasin, M., Knight, A., et al. (2013). Neural coding merges sex and habitat chemosensory signals in an insect herbivore. Proc. Biol. Sci. 280:20130267. doi: 10.1098/rspb.2013.0267
Trona, F., Anfora, G., Bengtsson, M., Witzgall, P., and Ignell, R. (2010). Coding and interaction of sex pheromone and plant volatile signals in the antennal lobe of the codling moth Cydia pomonella. J. Exp. Biol. 213, 4291–4303. doi: 10.1242/jeb.047365
Turner, R. M., Derryberry, S. L., Kumar, B. N., Brittain, T., Zwiebel, L. J., Newcomb, R. D., et al. (2014). Mutational analysis of cysteine residues of the insect odorant co-receptor (Orco) from Drosophila melanogaster reveals differential effects on agonist- and odorant-tuning receptor-dependent activation. J. Biol. Chem. 289, 31837–31845. doi: 10.1074/jbc.M114.603993
Vosshall, L. B., Wong, A. M., and Axel, R. (2000). An olfactory sensory map in the fly brain. Cell 102, 147–159. doi: 10.1016/s0092-8674(00)00021-0
Walker, W. B. III., Gonzalez, F., Garczynski, S. F., and Witzgall, P. (2016). The chemosensory receptors of codling moth Cydia pomonella-expression in larvae and adults. Sci. Rep. 6:23518. doi: 10.1038/srep23518
Wei, J. J., Fu, T., Yang, T., Liu, Y., and Wang, G. R. (2015). A TRPA1 channel that senses thermal stimulus and irritating chemicals in Helicoverpa armigera. Insect Mol. Biol. 24, 412–421. doi: 10.1111/imb.12168
Wicher, D. (2018). Tuning insect odorant receptors. Front. Cell. Neurosci. 12:94. doi: 10.3389/fncel.2018.00094
Willner, B., Granvogl, M., and Schieberle, P. (2013). Characterization of the key aroma compounds in Bartlett pear brandies by means of the sensomic concept. J. Agric. Food Chem. 61, 9583–9593. doi: 10.1021/jf403024t
Witzgall, P., Ansebo, L., Yang, Z., Angeli, G., Sauphanor, B., and Bengtsson, M. (2005). Plant volatiles affect oviposition by codling moths. Chemoecology 15, 77–83. doi: 10.1007/s00049-005-0295-7
Witzgall, P., Bäckman, A., Svensson, M., Koch, U., Rama, F., El-Sayed, A., et al. (1999). Behavioral observations of codling moth, Cydia pomonella, in orchards permeated with synthetic pheromone. BioControl 44, 211–237. doi: 10.1023/A:1009976600272
Witzgall, P., Bengtsson, M., Rauscher, S., Liblikas, I., Bäckman, A.-C., Coracini, M., et al. (2001). Identification of further sex pheromone synergists in the codling moth, Cydia pomonella. Entomol. Exp. Appl. 101, 131–141. doi: 10.1046/j.1570-7458.2001.00898.x
Witzgall, P., Chambon, J.-P., Bengtsson, M., Unelius, C. R., Appelgren, M., Makranczy, G., et al. (1996). Sex pheromones and attractants in the Eucosmini and Grapholitini (Lepidoptera, Tortricidae). Chemoecology 7, 13–23. doi: 10.1007/bf01240633
Witzgall, P., Proffit, M., Rozpedowska, E., Becher, P. G., Andreadis, S., Coracini, M., et al. (2012). “This is not an apple”-yeast mutualism in codling moth. J. Chem. Ecol. 38, 949–957. doi: 10.1007/s10886-012-0158-y
Witzgall, P., Stelinski, L., Gut, L., and Thomson, D. (2008). Codling moth management and chemical ecology. Annu. Rev. Entomol. 53, 503–522. doi: 10.1146/annurev.ento.53.103106.093323
Xu, H., Delling, M., Jun, J. C., and Clapham, D. E. (2006). Oregano, thyme and clove derived flavors and skin sensitizers activate specific TRP channels. Nat. Neurosci 9, 628–635. doi: 10.1038/nn1692
Keywords: Cydia pomonella, chemosensory receptors, functional characterization, Drosophila empty neuron system, human embryonic kidney (HEK293T) cells
Citation: Cattaneo AM (2018) Current Status on the Functional Characterization of Chemosensory Receptors of Cydia pomonella (Lepidoptera: Tortricidae). Front. Behav. Neurosci. 12:189. doi: 10.3389/fnbeh.2018.00189
Received: 28 June 2018; Accepted: 06 August 2018;
Published: 27 August 2018.
Edited by:
Gérard Manière, Université de Bourgogne, FranceReviewed by:
Ryuichi Okada, Kobe University, JapanCopyright © 2018 Cattaneo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alberto Maria Cattaneo, YWxiZXJ0b21hcmlhLmNhdHRhbmVvQHNsdS5zZQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.