
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
GENERAL COMMENTARY article
Front. Behav. Neurosci., 19 May 2017
Sec. Pathological Conditions
Volume 11 - 2017 | https://doi.org/10.3389/fnbeh.2017.00093
A commentary on
Adenosine A2A Receptor Blockade Prevents Rotenone-Induced Motor Impairment in a Rat Model of Parkinsonism
by Fathalla, A. M., Soliman, A. M., Ali, M. H., and Moustafa, A. A. (2016). Front. Behav. Neurosci. 10:35. doi: 10.3389/fnbeh.2016.00035
A recently published paper by Fathalla et al. (2016), demonstrated a rotenone induced possible protective effect of ZM241385 (a selective A2A receptor antagonist), but not of 8-cyclopentyl-1,3-dipropylxanthine (a selective A1 receptor antagonist), in a rat model of Parkinson's disease (PD). In the present paper, the discussion is short and presents few details. This commentary aimed to emphasize certain fundamental issues involving the rotenone model, the neuroprotective capacity of A2A receptor antagonists and compensatory mechanism of the non-dopaminergic approach for the treatment of PD.
Rotenone is the most potent member of the Rotenoids, a family of a natural flavonoids obtained from roots of tropical and subtropical plants belonging to the genus Lonchocarpus and Derris (Alam and Schmidt, 2002). Despite some limitations regarding variability and reproducibility seen in the animal model of PD induced by rotenone (Cannon et al., 2009), this model seems to replicate many hallmarks of illness including α-synuclein aggregation and Lewy body formation (Martinez and Greenamyre, 2012). Rotenone has lipophilic nature, and this feature induces nigrostriatal degeneration because rotenone inhibits complex I of the mitochondrial electron transport chain, decreasing ATP production, which can form reactive oxygen species such as superoxide, and reduced glutathione levels cause oxidative stress and cell death (Duty and Jenner, 2011; Johnson and Bobrovskaya, 2015). Fathalla et al. (2016) showed a progressive model of PD induced by six subcutaneous injections of rotenone. In this model, animals exhibited motor deficits as well as reduced level of dopamine in the midbrain.
To date, there is no efficient strategy to block or prevent the PD progression. It is expected that new drugs would stop the disease by a neuroprotective action. Thereby, A2A receptor antagonists represent a new way forward in the treatment of pathology (Roshan et al., 2016). All subtypes of adenosine receptors have been found in the central nervous system. The adenosine A2A receptors are abundant in striatum as well as in nucleus acumbens, where they are always co-localized with the dopaminergic D2 receptors (Perez-Lloret and Merello, 2014). In striatum, A2A receptors are localized mainly postsynaptically, but also presynaptically, on the neuron body and on glia cells (Cieślak et al., 2008). The adenosine was identified as a modulator of dopaminergic neurotransmission, based on studies with adenosine receptor antagonists in the rat models of hemiparkinsonism (Navarro et al., 2016). These receptors also regulate the release of other neurotransmitters such as noradrenaline, glutamate, acetylcholine, and gamma-aminobutyric acid (GABA; Cieślak et al., 2008). Studies suggest that the mechanism of interaction between the dopamine and adenosine receptors leads to changes in the affinity and coupling of G proteins, modulating the receptor efficacy. Thus, on stimulation of the adenosine receptor, the affinity between the dopaminergic agonists and DA receptors is reduced; hence, the adenosine agonists have similar effects to that of the dopaminergic antagonists. Alternatively, the effects of adenosine antagonists are similar to those produced by the dopaminergic agonists (Fuxe et al., 1998; Prediger et al., 2005).
In this study, Fathalla et al. demonstrated that A2A receptor antagonist attenuated the motor impairments (assessed by stride length and grid walking test) induced by rotenone, whereas A1 receptor antagonists did not show significant effect. Besides, the authors showed that A2A receptor antagonists might prevent the dopaminergic neuronal loss in striatum. We agree with the authors when they affirm that multiple mechanisms may be involved in this process. The neuroprotective capacity of A2A receptor antagonists could be linked to the action of microglial and astroglial cells in striatum as well as to the cytokines release (TNF-α, IL-1β) (Daré et al., 2007). It is important to emphasize that A2A antagonist attenuated the motor alterations after the last injection of rotenone even with a reduced level of dopamine in this group. Previous studies have shown that the decreased level of dopamine in striatum leads to enhanced dopamine receptor density (Takahashi et al., 2016). We assume that the attenuation of motor impairments by A2A antagonists can be related to compensatory mechanism in dopaminergic receptor density. Moreover, the A2A receptors modulate the indirect basal ganglia pathway due their co-localization with the dopaminergic D2 receptors (A2A–D2). Thus, the effect of adenosinergic antagonists on this dopaminergic pathway is significantly increased due the enhanced A2A–D2 receptor density (Figure 1).
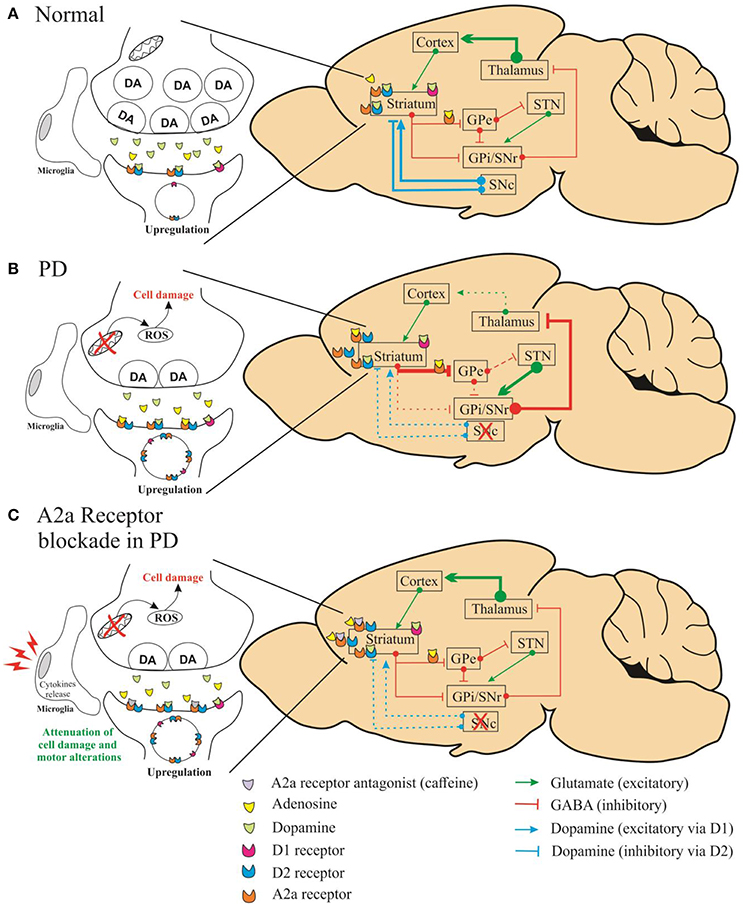
Figure 1. Schematic representation of simplified diagram demonstrating the connections within the basal circuitry, and changes in the activity of basal ganglia nuclei associated with the mechanism of symptomatic anti-Parkinsonian activity of A2A receptor antagonists. The striatum is connected to the thalamus through two pathways: indirect (striatum/globus pallidus pars externa-GPe/subthalamic-STN/nigral) and direct (globus pallidus pars interna complex-GPi/substantia nigra pars reticulate-SNr) pathways. In the normal state (A), dopamine of the substantia nigra pars compacta (SNc) acts on inhibitory D2 receptors of the indirect pathway and on stimulatory D1 receptors of the direct pathway. In (B), altered pathways are seen in Parkinson's disease caused by depletion of dopamine. In (C), the A2A receptors modulate the indirect basal ganglia pathway due the co-localization with the dopaminergic D2 receptors (A2A–D2).
The neuroprotective effect of adenosinergic antagonists was demonstrated by other studies. Soliman et al. (2016) showed that the treatment with caffeine (adenosinergic antagonist) ameliorates the neuron loss in the substantia nigra pars compacta (SNpc), induced by rotenone. Besides, this study demonstrated that caffeine has a dose-dependent neuroprotective effect. Randomized controlled trial showed that caffeine may to represent a promising therapeutic tool in PD (Postuma et al., 2012); however, there are some limitations related to the atypical dose of caffeine used to improve the motor symptoms. High caffeine intake causes hyperactivity, which affects the basic and fundamental human process. Furthermore, researches are needed to elucidate the underlying mechanism related to the neuroprotective potential of adenosinergic antagonists for the treatment of PD.
All authors listed, have made substantial, direct and intellectual contribution to the work, and approved it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This research was supported by fellowships from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Apoio à Pesquisa e à Inovação Tecnológica do Estado de Sergipe (FAPITEC), and Pró-reitoria de Pesquisa da Universidade Federal de Sergipe (POSGRAP/UFS).
Alam, M., and Schmidt, W. J. (2002). Rotenone destroys dopaminergic neurons and induces parkinsonian symptoms in rats. Behav. Brain Res. 136, 317–324. doi: 10.1016/s0166-4328(02)00180-8
Cannon, J. R., Tapias, V. M., Na, H. M., Honick, A. S., Drolet, R. E., and Greenamyre, J. T. (2009). A highly reproducible rotenone model of Parkinson's disease. Neurobiol. Dis. 34, 279–290. doi: 10.1016/j.nbd.2009.01.016
Cieślak, M., Komoszyński, M., and Wojtczak, A. (2008). Adenosine A2A receptors in Parkinson's disease treatment. Purinergic Signal. 4, 305–312. doi: 10.1007/s11302-008-9100-8
Daré, E., Schulte, G., Karovic, O., Hammarberg, C., and Fredholm, B. B. (2007). Modulation of glial cell functions by adenosine receptors. Physiol. Behav. 92, 15–20. doi: 10.1016/j.physbeh.2007.05.031
Duty, S., and Jenner, P. (2011). Animal models of Parkinson's disease: a source of novel treatments and clues to the cause of the disease. Br. J. Pharmacol. 164, 1357–1391. doi: 10.1111/j.1476-5381.2011.01426.x
Fathalla, A. M., Soliman, A. M., Ali, M. H., and Moustafa, A. A. (2016). Adenosine A2A Receptor Blockade Prevents Rotenone-Induced Motor Impairment in a Rat Model of Parkinsonism. Front. Behav. Neurosci. 10:35. doi: 10.3389/fnbeh.2016.00035
Fuxe, K., Ferré, S., Zoli, M., and Agnati, L. F. (1998). Integrated events in central dopamine transmission as analyzed at multiple levels. Evidence for intramembrane adenosine A2A/dopamine D2 and adenosine A1/dopamine D1 receptor interactions in the basal ganglia. Brain Res. Rev. 26, 258–273. doi: 10.1016/S0165-0173(97)00049-0
Johnson, M. E., and Bobrovskaya, L. (2015). An update on the rotenone models of Parkinson's disease: their ability to reproduce the features of clinical disease and model gene environment interactions. Neurotoxicology 46, 101–116. doi: 10.1016/j.neuro.2014.12.002
Martinez, T. N., and Greenamyre, J. T. (2012). Toxin models of mitochondrial dysfunction in Parkinson's disease. Antioxid. Redox Signal. 16, 920–934. doi: 10.1089/ars.2011.4033
Navarro, G., Borroto-Escuela, D. O., Fuxe, K., and Franco, R. (2016). Purinergic signaling in Parkinson's disease. Relevance for treatment. Neuropharmacology 104, 161–168. doi: 10.1016/j.neuropharm.2015.07.024
Perez-Lloret, S., and Merello, M. (2014). Two new adenosine receptor antagonists for the treatment of Parkinson's Disease: istradefylline versus tozadenant. Exp. Opin. Pharm. 15, 1097–1107. doi: 10.1517/14656566.2014.903924
Postuma, R. B., Lang, A. E., Munhoz, R. P., Charland, K., Pelletier, A., Moscovich, M., et al. (2012). Caffeine for treatment of Parkinson disease: a randomized controlled trial. Neurology 79, 651–658. doi: 10.1212/WNL.0b013e318263570d
Prediger, R. D. S., Da Cunha, C., and Takahashi, R. N. (2005). Antagonistic interaction between adenosine A2A and dopamine D2 receptors modulates the social recognition memory in reserpine-treated rats. Behav. Pharmacol. 16, 209–218. doi: 10.1097/01.fbp.0000166825.62130.9a
Roshan, M. H. K., Tambo, A., and Pace, N. P. (2016). Potential role of caffeine in the treatment of Parkinson's disease. Open Neurol. J. 10, 42–58. doi: 10.2174/1874205X01610010042
Soliman, A. M., Fathalla, A. M., and Moustafa, A. A. (2016). Dose-dependent neuroprotective effect of caffeine on a rotenone induced rat model of parkinsonism: a histological study. Neurosci. Lett. 623, 63–70. doi: 10.1016/j.neulet.2016.04.057
Takahashi, K., Nakagawasai, O., Nemoto, W., Nakajima, T., Arai, Y., Hisamitsu, T., et al. (2016). Alterations in behavioral responses to dopamine agonists in olfactory bulbectomized mice: relationship to changes in the striatal dopaminergic system. Psychopharmacology 233, 1311–1322. doi: 10.1007/s00213-016-4224-y
Keywords: Parkinson disease, adenosine, neuroprotection, caffeine, dopamine
Citation: de Souza MF, Bispo JMM, Leal PC, de Gois AM and dos Santos JR (2017) Commentary: Adenosine A2A Receptor Blockade Prevents Rotenone-Induced Motor Impairment in a Rat Model of Parkinsonism. Front. Behav. Neurosci. 11:93. doi: 10.3389/fnbeh.2017.00093
Received: 03 March 2017; Accepted: 02 May 2017;
Published: 19 May 2017.
Edited by:
Bruno Poucet, Centre National de la Recherche Scientifique, FranceReviewed by:
Micaela Morelli, University of Cagliari, ItalyCopyright © 2017 de Souza, Bispo, Leal, de Gois and dos Santos. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: José R. dos Santos, am9zZXJvbmFsZG9zYW50b3NAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.