- 1Department of Adult Habilitation, Akershus University Hospital, Lørenskog, Norway
- 2Institute of Clinical Medicine, Ahus Campus University of Oslo, Oslo, Norway
- 3Institute of Clinical Medicine, University of Oslo, Oslo, Norway
- 4Department of Research and Education, Institution of Oslo University Hospital, Oslo, Norway
- 5Centre of Anatomy, Institute of Cell Biology and Neurobiology, Charite Universitätsmedizin Berlin, Berlin, Germany
Schizophrenia is a highly heritable disorder with diverse mental and somatic symptoms. The molecular mechanisms leading from genes to disease pathology in schizophrenia remain largely unknown. Genome-wide association studies (GWASs) have shown that common single-nucleotide polymorphisms associated with specific diseases are enriched in the recognition sequences of transcription factors that regulate physiological processes relevant to the disease. We have used a “bottom-up” approach and tracked a developmental trajectory from embryology to physiological processes and behavior and recognized that the transcription factor NK2 homeobox 1 (NKX2-1) possesses properties of particular interest for schizophrenia. NKX2-1 is selectively expressed from prenatal development to adulthood in the brain, thyroid gland, parathyroid gland, lungs, skin, and enteric ganglia, and has key functions at the interface of the brain, the endocrine-, and the immune system. In the developing brain, NKX2-1-expressing progenitor cells differentiate into distinct subclasses of forebrain GABAergic and cholinergic neurons, astrocytes, and oligodendrocytes. The transcription factor is highly expressed in mature limbic circuits related to context-dependent goal-directed patterns of behavior, social interaction and reproduction, fear responses, responses to light, and other homeostatic processes. It is essential for development and mature function of the thyroid gland and the respiratory system, and is involved in calcium metabolism and immune responses. NKX2-1 interacts with a number of genes identified as susceptibility genes for schizophrenia. We suggest that NKX2-1 may lie at the core of several dose dependent pathways that are dysregulated in schizophrenia. We correlate the symptoms seen in schizophrenia with the temporal and spatial activities of NKX2-1 in order to highlight promising future research areas.
Introduction
Schizophrenia is a complex disorder that affects approximately 1% of the world's population over the age of 18. Although its heritability is estimated to be as high as 0.7–0.9 (Mulle, 2012), environmental and/or stochastic influences can still influence the pathogenesis of this disorder. The two-hit hypothesis, which has gained considerable support, suggests that an underlying genetic susceptibility, combined with a distinct developmental insult, i.e., an infectious or inflammatory episode, can prime an individual for a later event that ultimately leads to the onset of the full clinical syndrome (Bayer and Altman, 2009; Feigenson et al., 2014).
One of the fundamental goals in understanding schizophrenia is to link the observable symptoms to the underlying unobservable pathophysiology. The hallmark symptom is psychosis, with positive, negative, and cognitive manifestations (Andreasen et al., 1990). However, the majority of patients with schizophrenia receive neuroleptic treatment at an early stage of their illness. Accordingly, the fully evolved phenotype, as seen in untreated patients prior to the neuroleptic era, is seldom witnessed today. While being a welcome development for the patient, this can hamper our understanding of the key pathophysiological processes involved in schizophrenia. In this respect, a review of the remarkably detailed phenomenological studies of schizophrenic patients, conducted prior to the introduction of neuroleptics, is of particular value.
The most detailed observations of the evolving symptomatology in schizophrenia were made almost 100 years ago (Kraepelin, 1919). Kraepelin observed a number of non-psychiatric symptoms in his patients, indicating that several organ systems and physiological processes were affected.
Neurologic symptoms with spasmodic phenomena, reminiscent of choreic patients, were one of the observed dysfunctions. Later studies confirmed that children at high risk of developing schizophrenia often display neurological symptoms, including choreatic movements (Walker et al., 1994; McNeil et al., 2003). The presence and characteristics of these involuntary movements for patients with untreated psychosis, which disappear on treatment, indicate that these symptoms are an intrinsic feature of the disease process. Further, these involuntary movements imply a fundamental dysfunction in the cortical-basal ganglia-cortical circuitry (Whitty et al., 2009).
Frequent thyroid dysfunction, including an enlarged gland, rapid changes in size during progression of the illness, occasional exophthalmia, tremor, and myxoedema, have also been described (Kraepelin, 1919). A high incidence of thyroid pathology has been a consistent feature in reports of schizophrenia, with the available evidence supporting a role for thyroid hormone deregulation in the illness. The implication of thyroid hormone homeostasis in the fine-tuning of crucial brain networks would appear to warrant further research (Santos et al., 2012; Jose et al., 2015; Labad et al., 2016).
Furthermore, patients with schizophrenia present with somewhat accelerated and an excessively deep respiration, with several anomalies, especially for expiration (Kraepelin, 1919). Subsequently, it has been confirmed that patients with schizophrenia have an increased risk of lung disease, with a smoking-independent increase in lung disease-related mortality (Carney et al., 2006; Copeland et al., 2007).
Other symptoms and physiologic abnormalities initially described by Kraepelin, and subsequently confirmed by others, includes the following; impaired immunity (Anders and Kinney, 2015; Müller et al., 2015), enteric dysfunction (Peupelmann et al., 2009), altered calcium homeostasis (Bojarski et al., 2010), metabolic irregularities (Mitchell et al., 2013), skin abnormalities (Kamolz et al., 2003; Smesny et al., 2007); altered sleep patterns (Bromundt et al., 2011), an abnormal pupillary light reflex (Bar et al., 2008), altered thermoregulation (Chong and Castle, 2004), and menstrual patterns (Malik et al., 2011). Taken together, these observations indicate that, in addition to being a neurodevelopmental disorder, schizophrenia affects multiple physiologic processes that may lead to thyroid-, parathyroid- gastrointestinal and immune system dysfunction, respiratory symptoms, and skin abnormalities.
Although genetic findings consistently point to a role for genes involved in transcription/gene expression, neural development, neurotransmission, and immune function in schizophrenia, the vast majority of heritability still remains unexplained. However, an interesting insight has come from analyses of genome-wide association studies (GWASs) for complex disorders. Common single-nucleotide polymorphisms appear to be systematically enriched in transcription factor binding sites, particularly those active during fetal development (Maurano et al., 2012). Interestingly, the transcription factors whose activities may be altered, govern those physiologic processes relevant to the disease or trait under study.
In aiming to link the observable symptoms to the underlying unobservable pathophysiology of schizophrenia, we believe that a “bottom-up” approach where we track a developmental trajectory from embryology, to physiologic process and behavior, may be of value. In this fashion, we have come to recognize that the highly conserved transcription factor NK2 homeobox 1 (NKX2-1, encoded by NKX2-1; GENE ID 7080; chromosome 14q13), also called thyroid transcription factor 1, or thyroid-specific enhancer binding protein, possesses properties of particular interest for schizophrenia. Contrary to many transcription factors that are ubiquitously expressed, NKX2-1's expression is restricted to specific parts of the brain, the thyroid- and parathyroid glands, the skin, lungs, and enteric ganglia (Bingle, 1997; Suzuki et al., 1998a; Garcia-Barcelo et al., 2005; Boggaram, 2009; Germain et al., 2013; Fernandez et al., 2015). NKX2-1 governs multiple physiologic processes relevant to those somatic symptoms commonly seen in schizophrenia (as described). NKX2-1 also plays a central role in neurodevelopment and is essential for the formation and function of subgroups of neurons, glia, and functional neural networks that are affected in schizophrenia. This transcription factor also interacts with several susceptibility genes for schizophrenia, and is involved in gene-environment interactions with neurodevelopmental implications.
Further indications of the possible role of NKX2-1 in the pathophysiology of schizophrenia came from studies of families affected by inactivating mutations in NKX2-1. These result in a syndrome called brain-lung-thyroid disease, or benign hereditary chorea, which presents with impaired coordination, delayed speech development, neonatal pulmonary distress, and congenital hypothyroidism (OMIM#118700) (Krude et al., 2002; Glik et al., 2008; Yamada et al., 2009; Vloet et al., 2010; Monti et al., 2015; Peall and Kurian, 2015). Schizophrenia, psychosis, and behavioral disturbances, have all been reported in several of the reported pedigrees, suggesting that the association is not merely coincidental.
The scientific literature gives no indication that a large proportion of people with schizophrenia could carry a mutation or copy number variation in the NKX2-1 gene. However, the influence of a transcription factor on disease processes may not necessarily be directly linked to a mutation in the transcription factor itself. Dysregulated pathway activity, as a result of genetic or epigenetic alterations in downstream target genes, upstream regulators, transcriptional co-activators, or suppressors, may lead to genetic and phenotypic heterogeneity that ultimately manifests as a disorder. Accordingly, NKX2-1 may lie at the core of several dose dependent pathways that are dysregulated in schizophrenia. To further explore the possible role of NKX2-1 in schizophrenia, we correlated the symptoms seen in schizophrenia with the temporal and spatial activities of NKX2-1, in order to highlight promising future research areas.
Neurodevelopment and Brain Function
The neurodevelopmental hypothesis of schizophrenia proposes that pathological neurodevelopmental processes begin as early as the first and second trimester, and result in neuronal circuits that are primed to generate psychotic symptoms during adolescence or in young adults. In line with this, Nkx2-1 expression in the mouse brain commences at embryonic day 9 (comparable to the third gestational week in humans), when Nkx2-1 becomes detectable in a longitudinal band in the ventral forebrain that encompasses the medial ganglionic eminence (MGE), the preoptic area, and the ventral hypothalamus (Rakic and Zecevic, 2003a; Xu et al., 2008; Pauly et al., 2013) (Figure 1). Nkx2-1 participates in key early developmental events such as brain parcellation and cellular-fate specification in the embryonic forebrain (Sussel et al., 1999; Marin et al., 2000; Flames et al., 2007; Garcia-Lopez et al., 2008). Fate-mapping studies have shown that the progeny of Nkx2-1 expressing progenitor cells give rise to subclasses of neurons, astrocytes, and oligodendrocytes (Marshall and Goldman, 2002; Chojnacki and Weiss, 2004; Xu et al., 2008) while neurons derived from Nkx2-1-expressing progenitors are incorporated into specific neural circuits and networks, astrocytes and oligodendrocytes appear to be more widely distributed, and are found throughout the forebrain by the time of birth (Marshall and Goldman, 2002; Chojnacki and Weiss, 2004; Torigoe et al., 2015). Ultimately, in mice, the majority of Nkx2-1-expressing oligodendrocytes will be replaced by other populations of oligodendrocytes in the adult brain (Kessaris et al., 2006).
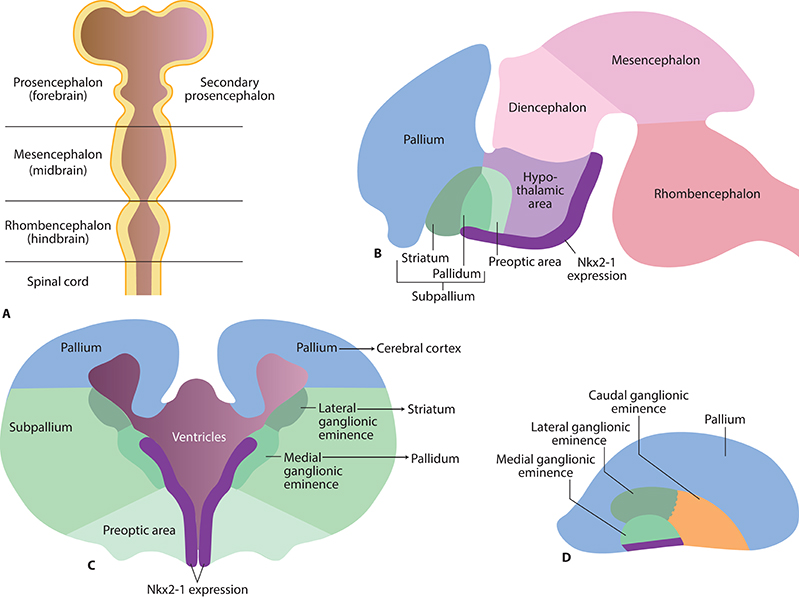
Figure 1. Early brain development and Nkx2-1 expression in the early embryonic brain. (A) Brain vesicles and divisions in the mammalian brain corresponding to gestational day 33 in humans. (B) Nkx2-1 is expressed as a longitudinal band in the ventral secondary prosencephalon from the rostral pallidum to the caudal hypothalamic area (rodent brain E15, corresponding to gestational day 49 in humans). (C) Future basal ganglia develop from subcortical parts of the telencephalon (“subpallium”). The striatum develops from the lateral ganglionic eminence, and pallidum from the medial ganglionic eminence. (D) Side view illustrating the medial, lateral, and caudal ganglionic eminences.
Cortical Gabaergic Inhibition
Deficits in cortical GABA neurotransmission are among the most consistent findings in schizophrenia research (Inan et al., 2013). It has been suggested that this deficit results from dysfunctional GABAergic interneurons that share a common developmental origin, and possibly the same aberrant precursor pool (Fung et al., 2010). We would suggest that this population represents the progeny of NKX2-1-expressing progenitors, derived from the MGE, that then develop into specific subgroups of cortical GABAergic interneurons.
The GABAergic fate of Nkx2-1 expressing progenitor cells is determined by downstream activation of the transcription factor Lim homeobox 6 (Lhx6) (Du et al., 2008; Flandin et al., 2011). The continuous expression of Lhx6 in post-mitotic cortical interneurons allows us to trace their ancestry, even if Nkx2-1 expression is down regulated. Interestingly, a deficit in cortical LHX6 mRNA expression has been reported in schizophrenic patients (Volk et al., 2012, 2014). In line with our suggestions, the authors propose that their finding implies a dysfunction in a GABAergic interneuron pool in schizophrenia.
Depending on temporal and spatial factors, Nkx2-1 derived, Lhx6 expressing GABAergic neuronal progenitors in the MGE may develop into subclasses of cortical GABAergic interneurons containing reelin, parvalbumin (PV+), somatostatin (SST+), calbindin (CB+), neuropeptide Y (NPY+), and nitric oxide synthase (NOS+) (Chojnacki and Weiss, 2004; Xu et al., 2004; Fogarty et al., 2007; Butt et al., 2008).
In rodents, early Nkx2-1-expressing progenitors in the MGE develop into a subpopulation of GABAergic reelin-containing Cajal-Retzius cells that will populate the (future) cortical layer I (Lavdas et al., 1999). Whether these will ultimately become reelin secreting cells is, as yet, unknown. Indirect evidence for dysfunctional reelin-containing cells in schizophrenia comes from studies of reeler mice, which are haploinsufficient for reelin, and share several biochemical and behavioral similarities with schizophrenic patients (Nullmeier et al., 2011). Among other abnormalities, reeler mice display impaired cortical lamination (Dekimoto et al., 2010). In line with this observation, patients with schizophrenia harbor an aberrant distribution of Cajal-Retzius cells and display a reduced expression of reelin in cortical layer I (Kalus et al., 1997; Ruzicka et al., 2007).
Some of the Nkx2-1 derived, reelin-secreting interneurons, also accumulate as interstitial cells in the medial regions of the subcortical white matter, at the midline in the corpus callosum, and in the hippocampus (DeDiego et al., 1994; Lavdas et al., 1999). In patients with schizophrenia, an aberrant distribution of interstitial cells and reduced reelin mRNA levels have been detected in the prefrontal cortex and in the hippocampal formation (Akbarian et al., 1996; Kirkpatrick et al., 2003; Eastwood and Harrison, 2006).
Although these findings may suggest that NKX2-1-derived Cajal-Retzius and interstitial cells are dysfunctional in patients with schizophrenia, it is important to bear in mind that these cell populations are heterogeneous, and have diverse molecular origins (Rakic and Zecevic, 2003b).
In mice, Nkx2-1-expressing precursors in the ventral MGE develop into PV+ interneurons that populate the cerebral cortex and the striatum. In the cerebral cortex, PV+ interneurons occur as basket and chandelier cells (Chojnacki and Weiss, 2004; Xu et al., 2004); approximately 90 and 68% of PV+ interneurons in the deep and superficial cortical layers, respectively, were co-labeled with reporter genes for Nkx2-1 (Xu et al., 2008).
Human and animal studies suggest that compromised cortical PV+ interneuron function is a major hallmark of schizophrenia (Reynolds et al., 2004; Behrens and Sejnowski, 2009; Curley et al., 2011; Nullmeier et al., 2011; Nakazawa et al., 2012; Volk et al., 2012; Jiang et al., 2013; Glausier et al., 2014; Yanagi et al., 2014; Brisch et al., 2015; Fujihara et al., 2015; Gonzalez-Burgos et al., 2015). Although most studies report functional impairment in cortical PV+ interneurons, some studies have also detected structural alterations (Konradi et al., 2011; Wang A. Y. et al., 2011).
In mice, most cortical SST+ interneurons develop from Nkx2-1-expressing progenitors in the MGE (Xu et al., 2004; Fogarty et al., 2007; Tricoire et al., 2011; Cai et al., 2013). In the neocortex, SST+ interneurons occur mainly as small basket cells in layers IV and V, and Martinotti cells in layers II-III and V-VI (Viollet et al., 2008). SST+ interneurons constitute 30–50% of all interneurons in the hippocampus, including bistratified, axo-axonic, and oriens-lacunosum-moleculare cells (Lawrence, 2008).
The cortical SST+ interneurons have been investigated less extensively than PV+ interneurons, but evidence is accumulating to show that their functions are also compromised in patients with schizophrenia (Hashimoto et al., 2008; Lewis et al., 2008; Morris et al., 2008; Fung et al., 2010; Beneyto et al., 2012). For example, the number of SST+ interneurons, as well as levels of SST mRNA, are both reduced in the hippocampus of schizophrenic patients (Konradi et al., 2011).
In mice, Nkx2-1-expressing progenitors in the preoptic area give rise to cortical CB+ interneurons (Fogarty et al., 2007). In the neocortex, most of this interneuron class occurs in the upper layers, often at the boundary between layers I and II (Gelman et al., 2009). Several studies have reported a reduction in the density of CB+ interneurons in the upper cortical levels in patients with schizophrenia (Beasley et al., 2002; Cotter et al., 2002; Reynolds et al., 2004; Chance et al., 2005; Sakai et al., 2008). Contradicting this, a 40% increase in the density of CB+ interneurons expressing the NR2A subunit of the N-methyl-D-aspartate receptor was reported for layer II of the anterior cingulate cortex in schizophrenic patients (Woo et al., 2008).
In rodents, cortical calretinin-containing interneurons do not develop from Nkx2-1-expressing progenitors. In humans, the NKX2-1 derived cortical calretinin-containing interneurons would appear to be a relatively minor subpopulation (Yu and Zecevic, 2011). Thus, far, the majority of studies have failed to identify any abnormality in calretinin-containing interneurons in schizophrenia (Beasley et al., 2002; Tooney and Chahl, 2004). However, decreased expression of calretinin mRNA in the dorsolateral prefrontal cortex of schizophrenia patients has been detected in a single study (Fung et al., 2010).
Integration of Information in Cortical-Basal Ganglia-Thalamocortical Neural Circuits
The basal ganglia receive afferent inputs from different areas of the cortex and send projections back to the cortex via the thalamus. This neuronal loop serves as the basis for various functions, including motor functions, cognitive control, motivational, and emotional processing. Early observations of involuntary movements in schizophrenic patients led researchers to suggest that the cortical-basal ganglia-thalamocortical circuits might be, in some way, dysfunctional (Whitty et al., 2009). It is now widely acknowledged that this dysfunction is actually widespread and contributes substantially to the pathophysiology of schizophrenia (Siegel et al., 1993; Ellison-Wright et al., 2008; Yoon et al., 2013; Cordon et al., 2015; Duan et al., 2015).
The progeny of Nkx2-1 expressing progenitors are widely distributed in cortical-basal ganglia-thalamocortical circuits. During early development, Nkx2-1 is expressed in both the dorsal and ventral parts of the striatum and pallidum. However, persistent expression into adulthood, is most prominent in the dorsal striatum and globus pallidus (Marin et al., 2000; Nobrega-Pereira et al., 2010).
The majority of GABAergic and cholinergic interneurons in the dorsal striatum in rodents are derived from the Nkx2-expressing progenitors in the MGE and preoptic area (Marin et al., 2000; Fragkouli et al., 2009; Magno et al., 2011). GABAergic interneurons belong to different subclasses containing PV, SST, nitric oxide synthase, neuropeptide Y, or calretinin (Marin et al., 2000), and modulate the output of neurons in the striatum (the medium spiny neurons) in a complex and highly orchestrated manner.
In humans, a major proportion of the NKX2-1 expressing striatal GABAergic interneurons contain PV (Magno et al., 2011). These constitute the major component of a powerful feed-forward inhibition that focuses cortical excitation, and synchronizes the activity of medium spiny neurons in order to regulate striatal output (Tepper et al., 2008). Although we have limited knowledge about the role of striatal GABAergic interneurons in schizophrenia, striatal PV+ interneurons have been suggested to play an important role in the behavioral effects mediated by antipsychotic drugs (Wiltschko et al., 2010). Indirect indicators of the influence of striatal PV+ interneurons in schizophrenia have also come from studies of reeler mice (Marrone et al., 2006; Ammassari-Teule et al., 2009). These reported a reduced number of striatal PV+ interneurons in all striatal sub regions, together with behavioral deficits such as fear extinction and latent inhibition.
In mice, at least 80% of striatal cholinergic interneurons develop from Nkx2-1-expressing progenitors (Fragkouli et al., 2009; Magno et al., 2009). Similarly, in the human brain, striatal cholinergic neurons are NKX2-1-immunoreactive (Magno et al., 2011). Although little is known about this interneuron group in schizophrenia, Holt et al. (1999, 2005) found that the mean density of cholinergic interneurons in the ventral striatum was reduced to just over a quarter (26%) of that seen in controls.
The globus pallidus is a rather homogenous structure composed of a network of inhibitory GABA-containing projection neurons that comprise the final pathway of the cortical output apparatus. In mice, approximately 75% of neurons in the globus pallidus develop from Nkx2-1-expressing progenitors that maintain their expression of Nkx2-1 into adulthood (Flandin et al., 2010; Nobrega-Pereira et al., 2010; Abdi et al., 2015; Dodson et al., 2015). These neurons are PV+ and display a spontaneous high-frequency discharge. The Nkx2-1 expressing neurons project through the direct and indirect pathway to the thalamus and the subthalamic nucleus. Their dendrites often cross functional borders within the pallidum to receive and integrate inputs from all parts of the pallidal complex (Bolam et al., 2000). In contrast, neurons that do not express Nkx2-1 send their projections back to striatum (Abdi et al., 2015).
To our knowledge, the projection neurons of the globus pallidus have not been directly studied in patients with schizophrenia. However, studies in an animal model based on the immunoinflammatory hypothesis of schizophrenia suggest that the globus pallidus may play crucial roles in the behavioral deficits seen in schizophrenia (Sotoyama et al., 2011). These include abnormal sensorimotor gating, latent inhibition, social interaction, working memory, and behavioral sensitization to dopamine and methamphetamine. In particular, the globus pallidus neurons in the lateral area display an increased firing rate that was ameliorated by treatment with risperidone (Sotoyama et al., 2013). Morphological and functional alterations of the globus pallidus have also been observed in patients with schizophrenia. Volumetric analyses, diffusion tensor imaging, and positron emission tomography, have revealed microstructural and functional alterations (Velakoulis et al., 2002; Galeno et al., 2004; Spaniel et al., 2005; Spinks et al., 2005; Hashimoto et al., 2009). There is also a strong positive correlation between markers of inflammatory and endothelial activation, and the volume of the globus pallidus in patients with schizophrenia (Dieset et al., 2015).
A Putative Special Role for PV+ Neurons in Data Integration
The classic view of the organization of the basal ganglia is that functionally diverse information from the cerebral cortex is processed in the striatum and subsequent divisions of the basal ganglia by parallel and segregated circuits; these are organized in a dorsolateral to ventromedial gradient, from motor to limbic functions (Alexander et al., 1986) (Figure 2). However, context-dependent goal-directed patterns of behavior are increasingly recognized to be dependent on the temporal integration of limbic, associative, and motor information, rather than relying on spatial segregation (Bolam et al., 2000; Tisch et al., 2004).
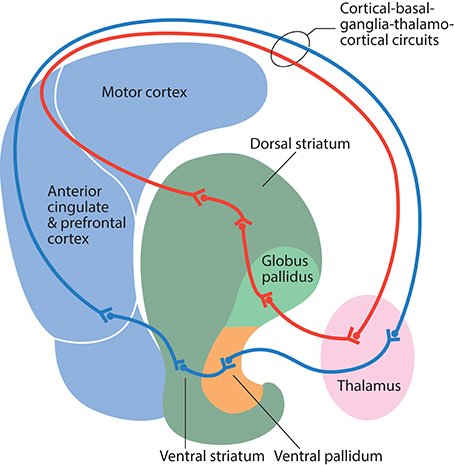
Figure 2. Schematic illustration of information flow through the direct pathway in cortical-basal ganglia-thalamocortical circuits. Information from the motor cortex passes to the dorsal striatum, globus pallidus, ventral lateral thalamus, and back to the motor cortex (red line). Information from the anterior cingulate and the prefrontal cortex passes to the ventral striatum, ventral pallidum, mediodorsal thalamus, and back to the anterior cingulate and prefrontal cortex (blue line).
We would suggest that the morphological organization and functional role of Nkx2-1-derived PV+ neurons in the rodent cortex, striatum, and pallidum, indicates that these neurons play a special role in data integration. Cortical PV+ interneurons receive their primary inputs from thalamocortical afferents, and integrate signals that converge at various parts of the cortical apparatus via their interneuron network. They mediate widespread perisomatic feed-forward inhibition to principal cortical neurons, and participate in the control of their output to the striatum (Mallet et al., 2005). Striatal PV+ interneurons receive convergent inputs from functionally different cortical territories and transmit integrated cortical information to the principal striatal medium spiny neurons (Ramanathan et al., 2002; Gage et al., 2010). PV+ projection neurons in the globus pallidus receive convergent inputs from all regions of the pallidal complex and integrate motor, associative, and limbic information (Bolam et al., 2000). The sum of their firing rate (which fluctuates) modulates the output of the basal ganglia (Elias et al., 2008) and thalamic activity. The thalamus completes the circuit by sending robust projections back to cortical PV+ interneurons and principal cells.
Rodent Nkx2-1 expression is developmentally regulated; correct spatial and temporal expression is required for the development and mature function of cortical-basal ganglia-thalamocortical circuits. Impaired Nkx2-1 expression in murine basal ganglia leads to substantial changes in coordinated movement (Magno et al., 2011). For normal mature function, rodent PV+ interneurons must down-regulate their expression of Nkx2-1 in the cortex, while achieving a mix of down-regulation and sustained expression in the allocortex. Conversely, continual expression of Nkx2-1 is required for the normal function of striatal PV+ interneurons and pallidal PV+ projection neurons in the basal ganglia (Xu et al., 2008; Magno et al., 2009).
Accordingly, any aberration in the developmental regulation of Nkx2-1 expression in PV+ neurons would alter information processing and integration in cortical-basal ganglia-thalamocortical circuits, with potentially far-reaching consequences for context-dependent goal-directed patterns of behavior. Such behavioral disturbances in patients with schizophrenia are well-known, having first been described in the pre-neuroleptic area (Kraepelin, 1919).
Neural Oscillations and Neurogenesis
The functions most affected in schizophrenia require the large-scale integration of subsystems that use neural oscillations; these are thought to require a synchronization that enables flexible communication both within, and between, cortical areas. Impaired synchrony has emerged as a potentially fundamental pathophysiological mechanism in schizophrenia. Aberrant neural oscillations and their synchronization may account for enduring deficits in cognition, and some of the psychotic symptoms of the disorder (Uhlhaas and Singer, 2011). This idea is supported by accumulating evidence of abnormal theta and gamma activity in patients with schizophrenia (Schmiedt et al., 2005; Basar-Eroglu et al., 2008; Haenschel et al., 2009; Hamm et al., 2011; Gonzalez-Burgos et al., 2015).
Nkx2-1 expressing GABAergic and cholinergic neuronal clusters in the septal area, the diagonal band of Broca, the ventral pallidum, and the preoptic area play key roles in the generation of oscillatory activity (Morris and Henderson, 2000; Siapas et al., 2005; Hangya et al., 2009; Magno et al., 2009, 2011; Brockmann et al., 2011; McDonald et al., 2011; Griguoli and Cherubini, 2012). These clusters demonstrate topographically organized connections to various areas of the brain in order to influence arousal, sensory processing, emotion, motivation, learning, memory, and motor functions (Semba, 2000; Zaborszky et al., 2008).
The NKX2-1 expressing GABAergic basal forebrain neurons in humans and rodents have been identified as fast-spiking PV+ projection neurons (Freund, 1989; Magno et al., 2009). They exhibit burst firing at theta frequency and serve as pacemakers for the generation of cortical low-frequency theta oscillations (6–10 Hz; Morris and Henderson, 2000; Hangya et al., 2009; McDonald et al., 2011). In rats, hippocampal theta-burst activity, originating in the medial septum and ventral diagonal band, is known to drive oscillatory activity in the prefrontal cortex (Siapas et al., 2005; Brockmann et al., 2011), and is implicated in episodic and working memory, and the top-down control of cognitive functions (Uhlhaas et al., 2008). PV+ projection neurons in the basal forebrain can also regulate higher-frequency gamma-band oscillations (30–80 Hz), which are involved in higher cognitive functions such as feature binding, attention, and memory (Kim et al., 2015). The PV+ projection neurons in the medial septum and ventral diagonal band are also important for regulating hippocampal neurogenesis. For example, partial septohippocampal GABAergic denervation has been shown to reduce the survival of newly generated hippocampal neurons by approximately 40% in rats (Van der Borght et al., 2005). Collectively, data from human and animal research supports a link between schizophrenia and decreased hippocampal neurogenesis (Reif et al., 2006; DeCarolis and Eisch, 2010; Allen et al., 2015).
Activation of the Nkx2-1 expressing cholinergic projection neurons may change the direction of information flow within cortical circuits, and it has been suggested that the cholinergic projections support a common electrophysiological function in cortical target areas by increasing the amplitude and signal-to-noise ratio of sensory responses, while enhancing response selectivity (Castillo et al., 1999; Eggermann and Feldmeyer, 2009). This function has different effects on psychological processes depending on the neural network operations within the various cortical domains (Everitt and Robbins, 1997). The Nkx2-1 expressing cholinergic projection neurons in the rodent basal forebrain enhance GABAergic transmission in cortical interneurons, with a concomitant decrease in the firing of principal cells, thereby contributing to the promotion of low-frequency theta oscillations (Griguoli and Cherubini, 2012). In addition, cholinergic neurons recruit noncholinergic neurons that are required for hippocampal theta synchronization (Dannenberg et al., 2015).
The notion that basal forebrain cholinergic projection neurons may be dysfunctional in schizophrenia is supported by the finding that schizophrenic patients show less stable cortical signals with a lack of stimulus-related phase-synchronization of electromagnetic activity after stimulus presentation (Winterer et al., 2000). Patients tend to increase noise instead of building up a signal, thus generating a reduced signal-to-noise ratio during information processing. In support of this, a study of event-related blood-oxygen-dependent responses showed that noise variance strongly correlated with psychotic symptoms (Winterer et al., 2006).
The cholinergic neurons also play a role in spatial learning, which is impaired in mice after postnatal deletion of Nkx2-1-expressing cholinergic neurons in the basal forebrain (Magno et al., 2011). Spatial learning is known to be impaired among individuals with schizophrenia (Wilkins et al., 2013).
Social Processes and Reproduction; A Neuroendocrine Network
Social recognition, affiliation, and attachment are all adversely affected in schizophrenia. Not surprisingly, the typical post pubertal onset of schizophrenia has stimulated interest in the potential contribution of neuroendocrine mechanisms and gonadal hormones (Walker and Bollini, 2002; Markham, 2012; Trotman et al., 2013).
Nkx2-1 displays a prominent lifelong expression in the highly interconnected neural neuroendocrine network that links sensory, hormonal, and homeostatic signals to social recognition and affiliation, reproduction, and parenting behaviors (Magno et al., 2009). This network includes subnuclei and cell groups in the amygdala, the lateral septum, the islands of Calleja, the hypothalamus, and the pituitary (Figure 3). As this network has been neatly delineated, it will be discussed in some detail.
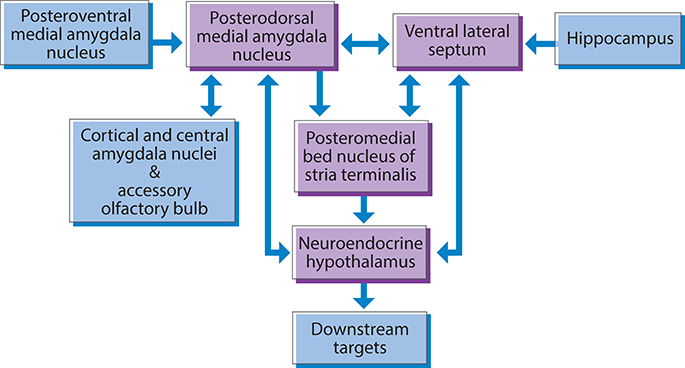
Figure 3. Nkx2-1 is expressed in a subcortical neuroendocrine network involved in social behavior and reproduction in the rodent brain. The posterodorsal medial amygdala, ventral lateral septum, and posteromedial bed nucleus of the stria terminalis, provide major inputs to the hypothalamic neuroendocrine effector system. Neuroendocrine hypothalamic nuclei provide feedback to the posterodorsal medial amygdala, and the ventral lateral septum.
Cell groups that express Nkx2-1 are present in all parts of the amygdala complex during pre- and postnatal mouse development. In adults, Nkx2-1 expression is restricted to a small population of subnuclei. Its most pronounced expression is seen in subnuclei involved in the processing of olfactory and steroid hormone information into socio-sexual, neuroendocrine, and reproductive responses; these include the posterodorsal medial amygdala nucleus, the posteromedial bed nucleus of the stria terminalis, and the ventral anterior amygdala (Garcia-Lopez et al., 2008) (Figure 4). These subnuclei represent a pallidal-like output apparatus with projections to downstream targets in the neuroendocrine hypothalamus.
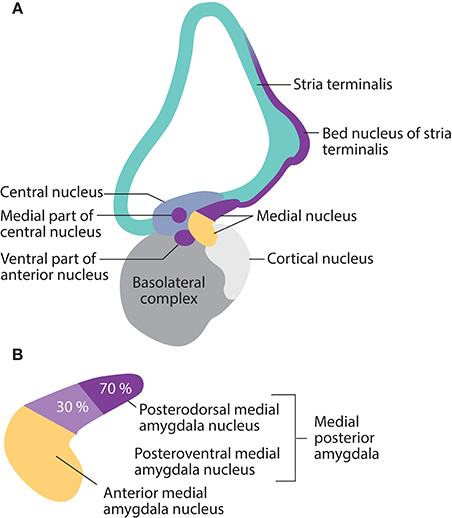
Figure 4. Nkx2-1 expression in mature amygdala nuclei, and the stria terminalis in the rodent brain is restricted to subnuclei involved in social, reproductive, and fear-defense responses. (A) Expression occurs in the posterodorsal and posteroventral medial nuclei, the ventral part of the anterior nucleus, the medial part of the central nucleus, the medial extended amygdala, and the posteromedial bed nucleus of the stria terminalis. (B) Details of the medial amygdala nucleus. Nkx2-1 is expressed by 70% of neurons in the posterodorsal medial nuclei (social-reproductive) and 30% of neurons in the posteroventral medial nuclei (fear-defense), suggesting different roles for NKX2-1 in the two sets of responses.
The lateral septum is a node responsible for integrating the cognitive and affective information that control behavioral responses to particular environmental stimuli (D'Anna and Gammie, 2009; Freiria-Oliveira et al., 2009; Lee and Gammie, 2009). Nkx2-1 is expressed in several proliferating domains in the rodent lateral septum during early development (Flames et al., 2007). In adult rodents, conspicuous expression of Nkx2-1 was detected in a group of large CB+ neurons in the ventral part of the lateral septum that projects to neuroendocrine hypothalamic subnuclei (Garcia-Lopez et al., 2008; Xu et al., 2008; Magno et al., 2009).
The islands of Calleja in the ventral striatum/pallidum constitute another olfactory and chemosensory neuroendocrine striato-pallidal system which impacts social affiliation, pair bonding, and reproduction. In rodents, some neuronal clusters persistently express Nkx2-1 (Fallon et al., 1983; Magno et al., 2009; Novejarque et al., 2011).
Neuroendocrine hypothalamic subnuclei involved in mating, fertility, reproduction, and the integration of reproduction with other homeostatic and environmental signals include the central part of the medial preoptic nucleus, the ventrolateral part of the ventromedial hypothalamic nucleus, the anteroventral periventricular nucleus, and the ventral premammillary nucleus (Lee et al., 2001; Magno et al., 2009; Matagne et al., 2012). In rodents, Nkx2-1 is highly expressed in the neuroendocrine hypothalamus. Nkx2-1 contributes to the control of sexual maturation in mice, with expression increasing abruptly at puberty in order to repress the transcription of genes that inhibit puberty (e.g., proenkephalin; Lee et al., 2001) and to activate genes that drive puberty (such as gonadotropin-releasing hormone) (Matagne et al., 2012). Nkx2-1 also participates in the maintenance of the female reproductive cycle, which necessitates changes in gonadotropin-releasing hormone (Ojeda et al., 2000, 2006; Mastronardi et al., 2006; Provenzano et al., 2010).
In the rat anterior pituitary, Nkx2-1 mRNA is expressed in growth hormone/prolactin-containing neurons, where Nkx2-1 inhibits transcription of the gene encoding growth hormone, while activating transcription of the gene encoding prolactin. These observations suggest that Nkx2-1 plays a role in regulating the trans-differentiation of neurons containing these two hormones (Lee et al., 2007). In the posterior pituitary in rodents, Nkx2-1 expression is localized to specialized astrocytes, called pituicytes, which are involved in regulating the secretion of oxytocin and vasopressin into general circulation (Theodosis, 2002; Magno et al., 2009; Rosso and Mienville, 2009).
The neuroendocrine neural network is sexually dimorphic. It is enriched in gonadal steroid hormone receptors (Coolen and Wood, 1998; Spiteri et al., 2010; Griffin and Flanagan-Cato, 2011) as well as the neuropeptides vasopressin and oxytocin, which are important modulators of network activity (Gabor et al., 2012; Gur et al., 2014). The functional connectivity of this network has been characterized in sheep. During parturition, levels of oxytocin mRNA increase in the islands of Calleja, the medial amygdala, the posteromedial bed nucleus of the stria terminalis, the lateral septum, and the neuroendocrine hypothalamic nuclei (Broad et al., 1999).
Several lines of evidence indicate that the neuroendocrine network that links sensory, hormonal, and homeostatic signals to a broad range of social behaviors is adversely affected in schizophrenia. Developmental changes in both positive and negative schizophrenic symptoms increase exponentially as the individual passes through adolescence and approaches early adulthood. The age at which positive symptoms first appear differs between males and females, which may be due to later puberty and associated maturational processes in boys (Galdos and van Os, 1995). A negative correlation between menarche age and schizophrenia onset has also been reported by some investigators (Cohen et al., 1999), although these findings have yet to be replicated by others (Ruiz et al., 2000; Hochman and Lewine, 2004). However, higher negative-symptom scores and greater functional impairment have been associated with subjects who reported a later age at menarche (Hochman and Lewine, 2004).
Schizophrenia is also associated with gonadal and sexual dysfunction, as emphasized in a report describing sexual function in first-episode schizophrenia patients (Malik et al., 2011). Menstrual irregularities and amenorrhea associated with psychosis were described long before the introduction of neuroleptic drugs (Kohen and Wildgust, 2008). Elevated basal levels of prolactin and growth hormone, aberrations in neuroendocrine-stimulation tests, and abolished diurnal hormonal variation also occurs in neuroleptic-naive schizophrenic patients (Kahn et al., 1992; Warner et al., 1993; Rao et al., 1994; Muller-Spahn et al., 1998).
Investigations into the circulating levels of vasopressin and oxytocin in patients with schizophrenia have returned inconsistent results (Raskind et al., 1987; Legros et al., 1992). Plasma levels of oxytocin in patients with schizophrenia appear to be lower than in healthy individuals, particularly in patients exhibiting hyponatremic polydipsia and emotional deficit (Goldman et al., 2008). Lower endogenous oxytocin levels have consistently been associated with impaired social cognition, especially for lower-level processes such as facial-affect perception (Strauss et al., 2015).
Parenting behaviors, which are also mediated through the Nkx2-1-expressing neuroendocrine social-reproductive network in rodents, have been a major concern for female schizophrenia patients after giving birth. Mothers with schizophrenia ordinarily have difficulty with the practical aspects of caregiving and tend to interact less sensitively with their infants (Abel et al., 2005). Fathers with schizophrenia also experience difficulty with their parental role (Jungbauer et al., 2010).
The social-neuroendocrine network is also of interest with respect to pharmacological interventions in schizophrenia. Intranasal administration of oxytocin may improve social cognition and social skills in patients (Gibson et al., 2014; Mercedes Perez-Rodriguez et al., 2015; Rich and Caldwell, 2015). The islands of Calleja have received particular attention due to their dense innervation by dopaminergic projections and their high expression of dopamine D3 receptors. These receptors have been suggested to mediate the unique antipsychotic effects of clozapine and are currently a major target for drug-discovery programs related to schizophrenia (Guo et al., 1998; Davoodi et al., 2014). Abnormal hormone responses elicited by gonadotropin-releasing hormone have been detected in schizophrenic patients (Brambilla et al., 1976; Ferrier et al., 1983; Cantalamessa et al., 1985). Beneficial effects on positive and negative symptoms, as well as the exacerbation of schizoaffective psychosis, have also been described in case reports following treatment with gonadotropin-releasing hormone analogs (Soreni et al., 2004; Abu-Tair et al., 2007).
In summary, multiple lines of evidence indicate a disrupted social-neuroendocrine network in schizophrenia. Nkx2-1's prominent expression in this network indicates that this transcription factor may be an important modulator of network activity, and, as a result, could influence the physiological processes and social behaviors that are disturbed in patients with schizophrenia.
Fear and Defensive Responses
Individuals who suffer from schizophrenia often exhibit marked and stable deficits in fear perception and recognition; it has been suggested that schizophrenic patients suffer from a general disconnect in the processing of danger signals between the central and autonomic systems (Williams et al., 2007). Although the most pronounced expression of Nkx2-1 is seen in the social neuroendocrine neural network, it is also highly expressed, in rodents, in the core of a subcortical neural network, which is involved in fear perception, defense, and escape responses. This network comprises the posteroventral medial amygdala, the anteromedial and posteromedial bed nucleus of the stria terminalis, some neuronal groups in the medial part of the ventral anterior, the medial central amygdala nucleus, and the hypothalamic dorsal premammillary nucleus (Choi et al., 2005; Garcia-Lopez et al., 2008; Magno et al., 2009; Carney et al., 2010) (Figure 4). Electrical stimulation of the hypothalamic part of this network elicits somatomotor and autonomic responses that resemble the behavior of animals facing natural threats. This fear network has been shown to be critical for the defensive responses (flight or freezing) elicited in animals in the presence of a predator (Canteras, 2002).
The investigation of fear-related behavior in patients with schizophrenia indicates that their fear system is hyper-activated. In a study of 1030 patients, 27% were found to exhibit “flight” behavior (Wang et al., 1997). In another study, of auditory hallucination, patients reported that they experienced these voices as powerful, dominating, and controlling; these provoked subordinate defensive responses, especially the fight/flight response (Gilbert et al., 2001). The initial “flight” behavior of patients in medical consultations, which is so frequently seen, has been suggested to be specific for schizophrenia, and is associated with the symptoms of the illness (Dimic et al., 2010).
In rats, the fear circuitry that we've just described mediates the freezing behavior that dominates fear responses to dominant males, and the risk assessment behaviors elicited by contextual fear of social defeat (Faturi et al., 2014). This is highly relevant for the social defeat hypothesis for schizophrenia, that posits that social defeat (i.e., the negative experience of being excluded from the majority group) is the common denominator of five major schizophrenia risk factors: urban upbringing, migration, childhood trauma, low intelligence, and drug abuse (Selten and Cantor-Graae, 2007; Selten et al., 2013).
The Nkx2-1 expressing, fear-defense related network, is so intimately connected, anatomically and functionally, with the social-neuroendocrine network, that activation of the fear network inhibits the social-neuroendocrine network. In a behavioral context this means that, upon the appearance of a threatening stimulus, a “gate control” mechanism ensures the rapid shut-down of the social-neuroendocrine network and associated behaviors in order to aid survival (Choi et al., 2005; Carney et al., 2010). Accordingly, a permanent hyper activation of the fear-defense network may have substantial implications for pro-social behaviors.
Regulatory and Homeostatic Systems
Circadian Rhythms, Sleep and Other Effects of Light
The influence of light on physiology and behavior (its so called masking behavior), extends to melatonin synthesis, daily activity, circadian rhythm, and sleep. Studies indicate that photic neurotransmission and photosensitive gene regulation are significant in schizophrenia and that the deregulation of masking behaviors are a common finding (Miller, 2013).
Melanopsin containing, adenylate cyclase-activating polypeptide producing intrinsically photosensitive retinal ganglion cells (ipRGCs), play a central role in transmitting photic information to non-visual target areas in the brain (Schmidt et al., 2011). Nkx2-1 is highly expressed in ipRGCs, where it is thought to regulate the transcription of adenylate cyclase-activating polypeptide, which is essential for the maintenance of photic sensitivity (Son et al., 2007; Kawaguchi et al., 2010).
The ipRGCs transmit daytime light information to the hypothalamic suprachiasmatic nucleus (SCN), and in doing so, contribute to circadian rhythms (Hattar et al., 2006). They also innervate several hypothalamic and pretectal areas involved in the control of masking behavior, regulation of the sleep-wake state, control of the pupillary light reflex, regulation of pineal melatonin levels, circadian oscillation of core body temperature, and neuroendocrine processes related to reproductive function (Hannibal, 2006; Hattar et al., 2006; Bailes and Lucas, 2010).
Nkx2-1 is also expressed in many of the down-stream targets that receive projections from ipRGCs, including the hypothalamic nuclei involved in the regulation of circadian rhythms: the dorsomedial hypothalamic nucleus and the SCN (Nakamura et al., 2001; Son et al., 2009). The dorsomedial hypothalamic nucleus is the key output nucleus of the brain's circadian system, as it is involved in circadian activation of a wide range of behavioral and endocrine functions (Chou et al., 2003). The SCN contains the circadian master clock. In the murine SCN, Nkx2-1 is expressed in neurons that express the protein, period-2 (Kim et al., 2002). In the rat SCN, period-1 and -2, additively stimulate the transcription of angiotensinogen, which integrates changes in the light-cycle with circadian variation in blood pressure and heart rate (Son et al., 2009). Essential elements of the master clock, such as the circadian locomotor output cycles kaput protein, and the aryl hydrocarbon receptor nuclear translocator-like protein, suppress Nkx2-1 expression (Kim et al., 2002). There is also evidence that Nkx2-1 is involved in generating a daily rhythm via its regulation of hypothalamic pituitary adenylate cyclase-activating polypeptide expression in rats (Kim et al., 2002). This process is required for the normal integration of the phase-advancing light signal (i.e., light induced changes to the circadian rhythm) by the SCN, and may be involved in synaptic plasticity in the hypothalamus (Gasperini et al., 2012).
The wide range of masking behaviors that are affected in schizophrenia indicate that the Nkx2-1 expressing neural network may be dysfunctional. Disturbances in circadian rhythm and the regulation of the sleep-wake state are generally observed and often appear as prodromal signs prior to the first and subsequently recurring psychotic episodes (Nishino et al., 2002; Karatsoreos, 2014; Manoach et al., 2014). Alteration in the synthesis and circadian rhythm of melatonin has also been reported (Monteleone et al., 1992, 1997; Rao et al., 1994). Compared to controls, patients with schizophrenia generally exhibit a deregulated body temperature, including a different baseline temperature, an abnormal daily temperature range, and an impaired ability to compensate for temperature stress (Chong and Castle, 2004; Shiloh et al., 2009). Significant alterations in pupillomotor control, with abnormal latencies and decreased light reaction, have also been reported (Bar et al., 2008). For example, a 10-fold higher stimulus intensity was required to induce a pupillary light reaction in patients with schizophrenia compared to controls (Rubin and Barry, 1976).
Although the major function of ipRGCs is to support non-image forming behaviors, their innervation pattern to the superior colliculus, the intergeniculate leaflet, and lateral geniculate nucleus, suggests that they relay inputs from the visual system to centers and circuits involved in the orientation of the head and eyes to sensory stimuli, image-forming vision, luminance, and spatial information (Ecker et al., 2010; Schmidt et al., 2011). This opens the possibility that abnormalities seen in early visual processing in schizophrenia might be influenced by Nkx2-1 expressing ipRGCs (Koychev et al., 2011; Khosravani and Goodarzi, 2013; Nunez et al., 2014; Lee et al., 2016).
Regulation of Food Intake
Long before the discovery of the neuroleptics, the altered consumption of food was noted in patients with schizophrenia, with substantial variations in food intake and large weight fluctuations over comparatively short periods of time (Kraepelin, 1919). Modern studies have also revealed that schizophrenia is often associated with eating disorders (Kouidrat et al., 2014). Anorexia nervosa affects between 1 and 4% of patients with schizophrenia. In addition, binge eating- and night eating disorders have an average prevalence of 5–20% in the schizophrenic population, which is approximately five fold that of the general population (Kouidrat et al., 2014). The mechanisms behind these altered eating behaviors are poorly understood.
One of the hypothalamic nuclei with a high continuous expression of Nkx2-1 is the arcuate nucleus, the master hypothalamic center for feeding control (Kim et al., 2002, 2006, 2011; Yee et al., 2009; Kaji and Nonogaki, 2013). In rodents, Nkx2-1 is selectively expressed in the two main centrally projecting cell populations involved in the regulation of feeding behavior; neurons containing the agouti-related protein and neuropeptide Y, and neurons containing peptide products of pro-opiomelanocortin and the cocaine and amphetamine-regulating transcript (Kim et al., 2006; Yee et al., 2009; Kaji and Nonogaki, 2013). Nkx2-1 stimulates transcription of agouti-related protein and inhibits transcription of the gene encoding pro-opiomelanocortin; up-regulation of Nkx2-1 has also been shown to increase appetite and eating (Kim et al., 2011).
Neuroleptic drugs are associated with varying degrees of weight gain, and direct or indirect effects on the hypothalamic neuronal circuits that control food intake and satiety represent plausible causal mechanisms for these side-effects (Kouidrat et al., 2014). Sub-chronic exposure to olanzapine, which leads to hyperphagia and weight gain in rats, is associated with the up-regulation of agouti-related protein/neuropeptide Y, and down-regulation of the pro-opiomelanocortin/cocaine and amphetamine-regulating transcript in the arcuate nucleus (Ferno et al., 2011; Weston-Green et al., 2012). Given that Nkx2-1 ordinarily regulates the balance between these two proteins, the possibility exists that olanzapine's side effects related to hyperphagia and weight gain may be mediated by up-regulated Nkx2-1. As yet, this possibility has not been explored.
Osmoregulation, Water Balance, and Sympathetic Outflow
Patients with schizophrenia often display alterations both in central and peripheral water balance. Multiple studies of the brains of patients with schizophrenia have uncovered abnormalities in their volume of cerebrospinal fluid. In a meta-analysis, ventricular cerebrospinal fluid volume was 20–30% higher in schizophrenic patients (Wright et al., 2000). A smaller increase of 7% was reported in a large Finnish cohort study which took into account both the ventricular and external volumes of cerebrospinal fluid (Tanskanen et al., 2009). In rats, Nkx2-1 has a regulatory role in the formation of cerebrospinal fluid. Nkx2-1 is expressed in the apical membrane of the ventricular choroid plexus, where it regulates expression of the water-channel protein aquaporin-1(Kim et al., 2007). This protein plays an essential role in the homeostasis of intracellular and extracellular water in the brain, and blockade of Nkx2-1 decreases cerebrospinal fluid formation by approximately 20% (Kim et al., 2007).
Deregulation of body-fluid homeostasis is also a well-known phenomenon in patients with schizophrenia. Primary polydipsia is the most common physiologic abnormality and may be present in more than 20% of chronic inpatients (de Leon et al., 1994). Increased sensitivity to osmotic stress and water intoxication due to polydipsia have often been described (Hundt et al., 2001; Bralet et al., 2007; Goldman et al., 2007; Satoh et al., 2007; Siegel, 2008; Goldman, 2009). In rats, Nkx2-1 influences water intake (Son et al., 2003). Nkx2-1 is expressed in the circumventricular organs; the organum vasculosum of the lamina terminalis, and the subfornical organ (Kim et al., 2008). Nkx2-1 activates transcription of the gene encoding angiotensinogen after water deprivation or dehydration, which influences drinking behavior.
Patients with schizophrenia regularly exhibit altered regulation of sympathetic outflow (Bar et al., 2007; Chang et al., 2009). Nkx2-1 is indirectly involved in autonomic regulation, as a major central target for angiotensinogen from the subfornical organ is the hypothalamic paraventricular nucleus, where it is converted to angiotensin II. Angiotensin II in the paraventricular nucleus increases the activity of the brain renin-angiotensin system and contributes to autonomic output and sympathoexcitation (Ferguson and Bains, 1997; Kang et al., 2009). Angiotensinogen from the subfornical organ also influences neuroendocrine secretion of adrenocorticotropic hormone, oxytocin, and vasopressin from the pituitary gland (Bartanusz and Jezova, 1994; Macova et al., 2009).
Integration of Trophic and Metabolic Processes and the Regulation of Brain-Body Homeostasis
In mice, Nkx2-1 demonstrates a high, lifelong expression in tanycytes, although, to our knowledge, the role of Nkx2-1 in these cells has not yet been explored. Tanycytes are specialized astrocytes located in the periventricular area that bridges the lumen of the third ventricle with the blood vessels of the medial basal hypothalamus (Lee et al., 2001). Their strategic proximity to, and relationship with, fenestrated capillaries, the blood brain barrier, axonal nerve terminals, and hypothalamic nuclei that regulate appetite/energy expenditure, places them in a privileged position with which to integrate multiple inputs and regulate homeostasis (Goodman and Hajihosseini, 2015). Although much is still unknown about the specific roles of tanycytes, they are known to regulate the release of gonadotropin-releasing hormone and to participate in the regulation of the hypothalamo-pituitary-gonadal axis (Fekete and Lechan, 2014). They may also act as glucosensors and participate in the control of insulin secretion (Frayling et al., 2011). Tanycytes function as gatekeepers of thyroid-hormone level in the hypothalamus and regulate the hypothalamic-pituitary-thyroid axis during fasting and infection (Lechan and Fekete, 2007; Herwig et al., 2008; Fonseca et al., 2013; Fekete and Lechan, 2014). Currently, there are no reports about tanycytes in schizophrenia. However, their role in body-brain communication and homeostatic regulation should make them of interest in schizophrenia research.
Thyroid Hormone Homeostasis
The available evidence supports thyroid hormone deregulation as a common feature in schizophrenia (Santos et al., 2012). In humans, NKX2-1 is necessary for organogenesis and the development of the thyroid gland (Kimura, 1996; Suzuki et al., 1998a; Damante et al., 2001). NKX2-1 makes major contributions to the maintenance of normal thyroid-hormone homeostasis by regulating expression of the thyrotropin-stimulating hormone receptor, thyroglobulin, thyroid peroxidase, the sodium/iodide symporter, and type II iodothyronine deiodinase (Berg et al., 1996; Endo et al., 1997; Suzuki et al., 1999; Damante et al., 2001; Gereben et al., 2001; Moeller et al., 2003).
Thyroid Hormones and the Brain
Thyroid hormones exert profound neurodevelopmental effects; even modest disruption during critical periods of fetal development can influence mature brain function (Zoeller and Rovet, 2004; Oerbeck et al., 2007; Flamant et al., 2015). Triiodothyronine depletion results in the delayed expression of oligodendrocyte-specific markers, fewer oligodendrocyte cell bodies in the main white-matter tracts, delayed expression of genes encoding structural myelin proteins, fewer myelinated axons, and a lower myelin content (Valcana et al., 1975; Ibarrola and Rodriguez-Pena, 1997; Mohacsik et al., 2011). The maturation of GABAergic PV+ neurons depends heavily on thyroid hormones; deficiency during the early postnatal period in rats (corresponding to 5–6 months of gestation in humans) reduced PV immunoreactivity in GABAergic interneurons in the adult neocortex and hippocampus, leading to a compromised inhibitory function (Gilbert et al., 2007). Adult neural stem cell cycling and the maintenance of hippocampal pyramidal neuron populations depend on the modulation of specific cell-cycle regulators by thyroid hormones (Lemkine et al., 2005; Alva-Sanchez et al., 2009). Thyroid hormones also affect synaptic proteins (Yang et al., 2012) and glucose metabolism (Bauer et al., 2009), and protect the brain from oxidative stress by maintaining glutathione homeostasis (Dasgupta et al., 2007).
Brains from patients with schizophrenia display alterations that are compatible with the pre- and postnatal depletion of thyroid hormones. These alterations include oligodendrocyte abnormalities, defects in white matter tracts, and the dysfunction of PV+ GABAergic interneurons (Behrens and Sejnowski, 2009; Melicher et al., 2015). There are also findings suggestive of reduced neural stem-cell proliferation (Reif et al., 2006), presynaptic dysfunction (Castillo et al., 2010), and deficits in glucose metabolism and glutathione homeostasis (Katz et al., 1996; Gysin et al., 2007; Do et al., 2009; Yao and Keshavan, 2011).
Thyroid Autoimmune Disease
Data from Danish registries show that the incidence of Graves' disease and autoimmune thyroiditis is increased in patients with schizophrenia and their parents, and that a history of any autoimmune disease is associated with a 45% increase in the risk of schizophrenia (Eaton et al., 2006; Benros et al., 2014). Autoimmune thyroid disease can be associated with other autoimmune endocrine failures or non-endocrine diseases (vitiligo, pernicious anemia, myasthenia gravis, autoimmune gastritis, celiac disease, hepatitis; Wemeau et al., 2013). Several of these disorders have also been associated with schizophrenia (Zoabi et al., 2012; Benros et al., 2014).
Autoimmune thyroid disease is provoked by a loss of self-tolerance to the autoantigens thyroid peroxidase thyroglobulin, and thyroid stimulating hormone receptor, which leads to a destructive immune infiltration of the gland. NKX2-1 promotes the expression of these thyroid autoantigens, and the up-regulation of NKX2-1 results in a concomitant increase in human leukocyte antigen (or major histocompatibility complex (MHC) class I expression, together with the thyroid autoantigens. This may constitute the pathologic state that leads to thyroid autoimmune disease (Huang et al., 2011). Bacterial lipopolysaccharide, which has been proposed to be another etiopathogenic agent in autoimmune disease, also up-regulates the expression of thyroid antigens by increasing Nkx2-1 expression in rats (Velez et al., 2006). It has recently been shown that the strong genetic association between variation in the MHC locus and schizophrenia arises, in part, from the many structurally diverse alleles of the complement component 4 (C4) gene (Sekar et al., 2016). Autoimmune thyroid disease can also be mediated by direct binding of complement C4 to thyroid peroxidase (Blanchin et al., 2003). Whether NKX2-1 is directly involved in this process has yet to be explored.
Calcium Homeostasis and Bone Cell Metabolism
Research has demonstrated that impaired calcium homeostasis and signaling is common to many schizophrenia-related processes, including dysregulated dopamine and glutamate neurotransmission (Bergson et al., 2003; Martins-de-Souza et al., 2009a,b; Bojarski et al., 2010). Platelets from drug-free schizophrenia patients harbor significantly higher levels of cytosolic calcium than healthy controls (Ripova et al., 1997); data from a recent GWAS also supports the involvement of calcium-signaling in schizophrenia (Hertzberg et al., 2015).
Chief cells in the parathyroid gland coordinate with thyroid neuroendocrine C cells to maintain calcium and phosphorus levels in the blood, as well as bone-cell metabolism throughout the body. C cells secrete calcitonin, and in rats, Nkx2-1 activates transcription of calcitonin (Suzuki et al., 1998b, 2007). Calcitonin decreases serum calcium levels, increases calcium levels in cerebrospinal fluid, increases serum concentrations of 1,25-dihydroxyvitamin D, inhibits bone resorption, and regulates phosphorus metabolism (Carmen and Wyatt, 1977; Felsenfeld and Levine, 2015). In patients with schizophrenia, injections with synthetic salmon calcitonin have been shown to decrease agitation, and improve malignant catatonia (Carmen and Wyatt, 1977; Carman and Wyatt, 1979a, Carman and Wyatt, 1979b).
Nkx2-1 is also expressed in rat chief parathyroid cells (Suzuki et al., 1998a), with binding sites for Nkx2-1 present within genes encoding the calcium sensor receptor and calmodulin. Nkx2-1 may therefore act as an important calcium-sensing factor that responds to altered intracellular calcium levels in order to regulate the gene expression required to maintain calcium homeostasis (Suzuki et al., 1998b). Animals with abnormal calcium homeostasis, after removal of the parathyroid gland, exhibit changes in basal dopamine and noradrenaline levels in limbic structures, abnormalities in conditioned reflex activity, and the acquisition of adaptive behavioral strategies (Sashkov et al., 2007).
Regulation of bone formation and turnover is a main function of chief and parafollicular cells. Bone metabolism has been shown to be disturbed in patients with schizophrenia and decreased bone mineral density and osteoporosis are common in these patients. Schizophrenia has been found to be an independent determinant of a poor skeletal status in women after controlling for common risk factors such as osteoporosis, vitamin D status, and medication (Partti et al., 2010). From a young age, both men and women with schizophrenia present a lower bone mass than the general population (Renn et al., 2009).
The Respiratory System
Patients with schizophrenia have significantly lower lung-function values than control subjects after adjustment for smoking and other potential confounders (Partti et al., 2015). They experience a higher incidence of lung disease such as asthma, chronic obstructive pulmonary disease, and pneumonia (Carney et al., 2006; Filik et al., 2006; Copeland et al., 2007; Chen et al., 2009). Schizophrenic patients aged 10–39 years and 40–49 years are eight times and five times more likely, respectively, to die from respiratory causes (Partti et al., 2015).
NKX2-1 is highly expressed in the lungs, where it regulates the expression of several genes critical to lung development and mature function (Bohinski et al., 1994; Li et al., 2000, 2002; Naltner et al., 2000; Yuan et al., 2000; Berhane and Boggaram, 2001; Zhou et al., 2001; Reynolds et al., 2003; Zhu et al., 2004; Reynolds and Hoidal, 2005; Sparkman et al., 2006; Besnard et al., 2007; Spiteri et al., 2007; Minoo et al., 2008; Boggaram, 2009; Kolla et al., 2009; Tagne et al., 2012; Li C. et al., 2013; Zscheppang et al., 2013). In the adult mouse lung, Nkx2-1 is expressed predominantly in two special cell types, the alveolar type II epithelial cell, and Clara cells (Boggaram, 2009).
Alveolar type II cells are responsible for the production of surfactant; in rodents, rabbits and humans, Nkx2-1/NKX2-1 positively regulates the expression of surfactant proteins A, B, and C (Margana et al., 2000; Yi et al., 2002; Alcorn et al., 2004; Liu et al., 2008; Cao et al., 2010). Surfactant protein A interacts with a wide range of pathogens to suppress microbial growth, damage bacterial membranes, modulate macrophage phagocytosis, clear lipopolysaccharide, and regulate complement activation (Kishore et al., 2006). Surfactant proteins B and C are critical in lowering the surface tension at the air/water interface, and for airway host defense (Chroneos et al., 2010). Abnormal expression and activity of surfactant proteins have been suggested to underlie the pathogenesis of a variety of lung inflammatory diseases, including asthma and chronic obstructive pulmonary disease (Kishore et al., 2006; Chroneos et al., 2010). Consistent with this hypothesis, patients with heterozygous loss-of-function mutations in NKX2-1 are predisposed to neonatal respiratory distress and pulmonary infections due to reduced expression of surfactant proteins (Krude et al., 2002).
Clara cells located in the bronchioles secrete surfactant protein B and Clara cell secretory protein (CCSP), both of which are transcriptional targets of NKX2-1 (Ray et al., 1996; Cassel et al., 2000; Margana et al., 2000). CCSP is a natural immuno-suppressant and anti-inflammatory secretory protein that exhibits several immunomodulatory features, including helper T-cell regulation (Dierynck et al., 1996; Hung et al., 2004; Johansson et al., 2007). Reduced plasma CCSP levels have been reported in patients with schizophrenia, and it has been suggested that inflammatory responses in schizophrenia may be causally related to lower serum CCSP levels, which may represent a biomarker for the disorder (Maes et al., 1996a,b; Lin et al., 1998).
The Enteric Nervous System
Gastric dysmotility and enteric nervous-system dysfunction are common in schizophrenic patients (Peupelmann et al., 2009; Berger et al., 2010). In humans, NKX2-1 is expressed in mature enteric ganglia, where it regulates expression of the ret proto-oncogene by interacting with the transcription factors paired-like homeobox 2B, and sex-determining region Y-box 10 (Leon et al., 2009). Both of these are genes have been associated with susceptibility to schizophrenia (Ide et al., 2005; Maeno et al., 2007b; Glessner et al., 2010).
The Skin
Patients with schizophrenia exhibit skin abnormalities such as reduced wound healing and an attenuated response to inflammatory stimuli in the niacin skin-flushing test (Kamolz et al., 2003; Smesny et al., 2007). In rats, Nkx2-1 is expressed in keratinocytes and dermal fibroblasts (Suzuki et al., 1998a). While targets for Nkx2-1 in the skin have yet to be explored, genes related to thyroid hormones, inflammation, and calcium homeostasis, that are regulated by Nkx2-1 in other tissues, are also expressed in keratinocytes and dermal fibroblasts (Cianfarani et al., 2010; Yun et al., 2011). Skin keratinocytes and fibroblasts are also generally considered to be a useful model system for studying vulnerability factors and the pathophysiology of schizophrenia (Ramchand et al., 1995; Catts et al., 2006; Olsson et al., 2006; Gysin et al., 2009; Wang et al., 2010).
Discussion
NKX2-1 and Candidate Schizophrenia Genes
A review of the publically available online database for genes associated with Schizophrenia (SchizophreniaGene) in December 2015, revealed in excess of 1000 possible candidate genes; this database was, until Dec. 2011, regularly updated (Allen et al., 2008). Some of these candidates were identified during the pre-GWAS period, with others based on GWAS studies. NKX2-1 is known to interact with a number of these genes (Thome et al., 1996; Perrone et al., 1999; Stober et al., 1999; Marin et al., 2000; Naltner et al., 2000; Ojeda et al., 2000; Stoykova et al., 2000; Kim et al., 2002; Leonard et al., 2002; Yi et al., 2002; Chojnacki and Weiss, 2004; Cui et al., 2005; Ide et al., 2005; Reynolds and Hoidal, 2005; Aghajani et al., 2006; Sanjuan et al., 2006; Takahashi et al., 2006; Benzel et al., 2007; Hashimoto et al., 2007; Son et al., 2007; Takao et al., 2007; Kahler et al., 2008; Zhou et al., 2008; Leon et al., 2009; Carney et al., 2010; Waddington et al., 2010; Goulburn et al., 2011; Zhang et al., 2011; Crisafulli et al., 2012; Matagne et al., 2012; Quednow et al., 2012; Li T. et al., 2013; Trotman et al., 2013; Zscheppang et al., 2013; Luo et al., 2014) (Table 1).
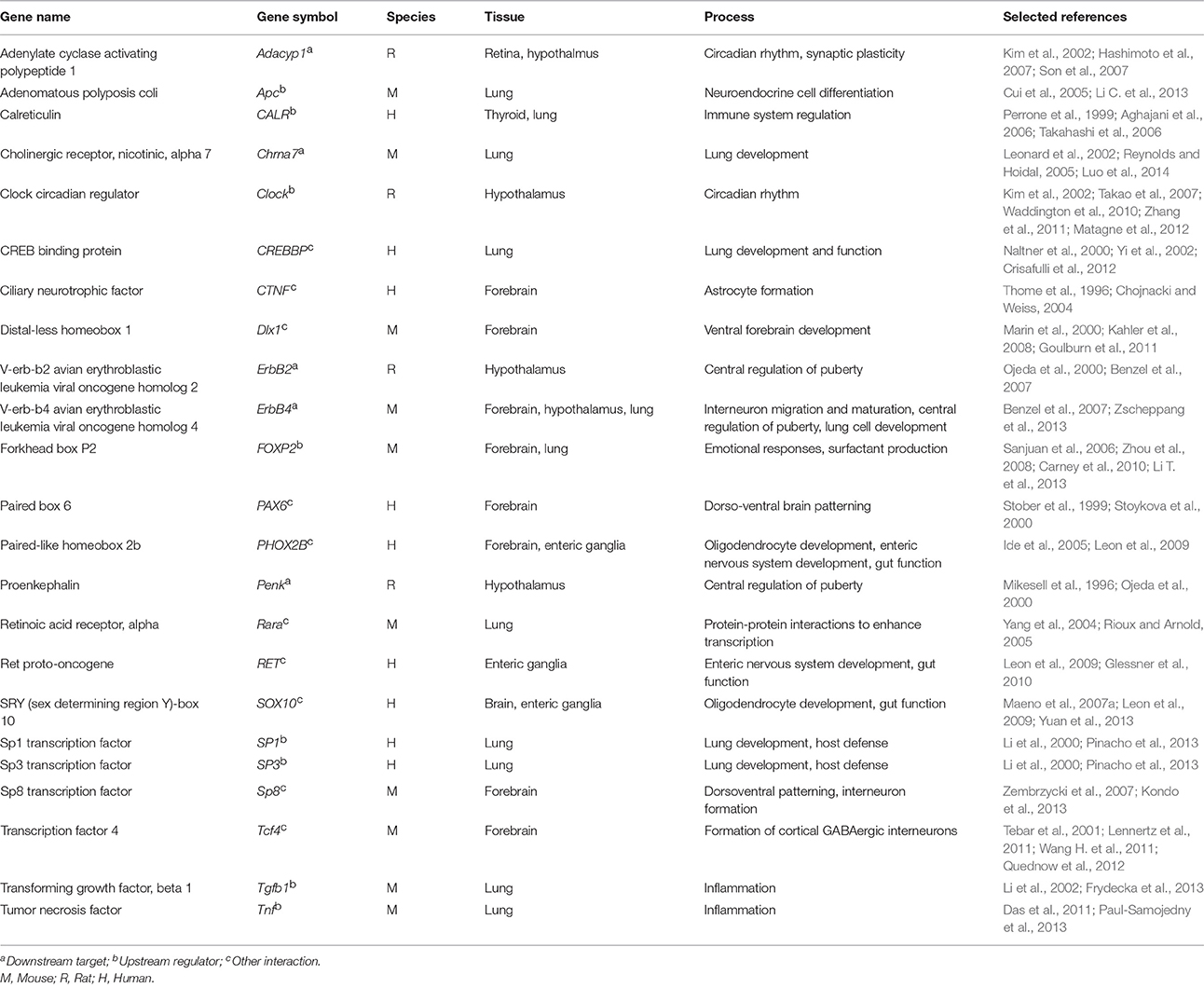
Table 1. Genes identified to interact with NKX2-1/Nkx2-1 in human or rodent studies that have also been associated with susceptibility for development of schizophrenia or with the course of illness.
In a recent publication, Farrell et al. (2015) presented genomic evidence for 25 candidate schizophrenia genes identified during the pre-GWAS period, including Disrupted in schizophrenia 1, Neuregulin 1, Nicotinic cholinergic receptor α7, and Receptor-erb-b4 avian erythroblastic leukemia viral oncogene homolog. Several of these genes, and their gene products, influence or are influenced by, Nkx2-1. Disrupted in schizophrenia 1 is necessary for the migration of Nkx2-1-derived interneurons to the cortex (Steinecke et al., 2014), and for mature function of the PV+ subgroup (Sauer et al., 2015). Disrupted in schizophrenia 1 is also involved in adult neurogenesis (Jun et al., 2012), a process in which Nkx2-1-expressing GABAergic PV+ projection neurons in the medial septum/ventral diagonal band play a key role (Van der Borght et al., 2005). Neuregulin 1, brain-derived neurotrophic factor, and dystrobrevin binding protein 1, are also of critical importance for the maturation and inhibitory function of Nkx2-1-derived PV+ neurons (Hashimoto et al., 2005; Grabert and Wahle, 2008; Fazzari et al., 2010; Carlson et al., 2011; Yin et al., 2013). Neuregulin 1 and nicotinic cholinergic receptor α7 are involved in the modulation of Nkx2-1-dependent hippocampal gamma oscillations (Fisahn et al., 2009; Lu and Henderson, 2010), as well as regulation of plasticity of the airway epithelium (Maouche et al., 2009). In the lungs of mice, signaling via neuregulin 1, and its receptor-erb-b4 avian erythroblastic leukemia viral oncogene homolog, activates Nkx2-1 expression (Zscheppang et al., 2013), while Nkx2-1 activates nicotinic cholinergic receptor α7 transcription (Reynolds and Hoidal, 2005).
Multiple GWASs have identified a relatively large number of single-nucleotide polymorphisms, each contributing a small risk for the disorder. A meta-analysis of 18 GWASs for schizophrenia supported involvement of the MHC region, TCF4, POM121L2, NOTCH4, AS3MT, CNNM2, and NT5C2 (Aberg et al., 2013). Transcription factor 4 (TCF4) is implicated in Wnt signaling, which is critical for the maintenance the proliferation of Nkx2-1-derived GABAergic interneurons in the mouse forebrain (Gulacsi and Anderson, 2008) and for lung development (Tebar et al., 2001). Signaling through the Notch receptor may be a common regulator of neuronal differentiation within the developing forebrain (Faux et al., 2001). The Notch receptor ligand NOTCH4 is involved in lung function and remodeling as well as in protection against innate immune inflammation in the lung, where it acts on tumor necrosis factor, an upstream suppressor of NKX2-1 transcription (Boggaram, 2009; Das et al., 2011; Li C. et al., 2013). NKX2-1 in the human thyroid gland is involved in the regulation of MHC class I genes (Huang et al., 2011).
Although Nkx2-1 has been shown to interact with a number of susceptibility genes for schizophrenia, most of these studies were performed in rodents and species-related differences may occur. Many of these gene-gene interactions are also described in tissues other than the brain. However, although transcriptional targets are often tissue-specific, a significant overlap exists between transcriptional targets in different tissues. For example, Spiteri et al. (2007) reported overlaps of 47% and 37% for genes identified as forkhead box P2 (FOXP2) targets in human lung and basal ganglia, and in the lung and inferior frontal cortex, respectively. Interestingly, FOXP2, a candidate gene for schizophrenia, interacts with NKX2-1 in the human lung, and is co-expressed with Nkx2-1 in the posterodorsal medial amygdala nucleus in mice (Carney et al., 2010).
NKX2-1 and the Two Hit Hypothesis
The possible involvement of NKX2-1 in the pathogenesis and pathophysiology of schizophrenia is fully compatible with the two-hit hypothesis, underscoring the importance of environmental factors in this disease (Bayer et al., 1999; Feigenson et al., 2014). Research using transgenic mice has determined that early prenatal stress results in significant fluctuations in the expression of Nkx2-1 and its downstream targets that affect the distribution of MGE-derived interneurons in the cortex (Stevens et al., 2013). Immune activation in the fetus as a result of inflammatory stimuli emanating from the mother is proposed to represent an important environmental susceptibility factor for schizophrenia (Brown, 2011; Wischhof et al., 2015). Nkx2-1-expressing periventricular tanycytes and other cortical astrocytes in rodents respond to the inflammatory stimulus provided by lipopolysaccharide. The effects of lipopolysaccharide are at least partly mediated through regulation of the availability of triiodothyronine in the brain parenchyma, followed by changes in triiodothyronine-mediated gene expression involved in neurodevelopment and oxidative stress (Klecha et al., 2006). Prenatal administration of lipopolysaccharide is known to induce sex-dependent changes in glutamic acid decarboxylase and PV in the adult rat brain (Basta-Kaim et al., 2015). The effect of gene-environment interactions have been demonstrated in transgenic mice expressing a dominant-negative schizophrenia 1 gene; these mice display histologic and behavioral endophenotypes that are relevant to schizophrenia. When exposed to an immune challenge corresponding to viral infection during the neonatal period, the transgenic mice exhibited impaired Nkx2-1-associated behaviors, such as fear memory, social recognition, and social interaction (Ibi et al., 2010). Additive effects of the disrupted in schizophrenia 1 genotype and immune challenge were reflected in a marked decrease in the number of PV+ interneurons in the medial prefrontal cortex.
Are Dysregulated NKX2-1 Associated Processes Specific to Schizophrenia?
A central question is whether the disruption of NKX2-1associated networks and processes should be considered to be specific to the diagnostic category schizophrenia, or instead could be of broader relevance to neurodevelopmental disorders. For example, many genes identified as susceptibility genes for schizophrenia are also associated with other mental disorders (e.g., autism spectrum disorders and bipolar disorder). Overlapping symptoms are also commonly found. Psychotic symptoms can arise in bipolar disorder, and persistent social dysfunction is a prominent feature of autism. NKX2-1 expression patterns have not been explored either in schizophrenia or in any other neurodevelopmental or psychiatric disorder. However, patients with schizophrenia certainly have brain dysfunctions that involve the progeny of NKX2-1 expressing progenitor cells. Susceptibility to the somatic symptoms related to the dysfunction of physiological processes regulated by NKX2-1 are also well documented. To date, we are not aware of a similar cluster of mental and somatic dysfunction in any other mental illness, other than schizophrenia. Therefore, we would propose that aberrant NKX2-1 function could result in a wide-ranging, multi-organ disorder that is the hallmark of classic untreated “Kraepelinian” schizophrenia. However, until results of research into the role of NKX2-1 in schizophrenia, including comparisons with other highly heritable disorders are published, our proposal must be regarded as an educated hypothesis.
Implications for Research
In the past, most schizophrenia research has focused on the brain or blood. NKX2-1 is not known to be expressed in blood cells, and analyses performed on blood samples may not be feasible. However, several alternative ways of studying the role of NKX2-1 are available. Studies of genetically-engineered, experimental animals, with variable age- and tissue-specific expression of Nkx2-1, or its downstream targets, may shed light on relevant behavioral phenotypes (Fragkouli et al., 2005; Magno et al., 2011). Studies of the interactions between identified susceptibility genes and Nkx2-1 may also increase our knowledge about the mechanisms that underlie altered neurotransmission and other physiologic processes. Genes that interact with NKX2-1 also represent a new, and as yet unidentified cohort of susceptibility genes. A recent interesting GWAS identified 42 sets of single-nucleotide polymorphisms associated with a 70% or greater risk of schizophrenia (Arnedo et al., 2015). Molecular pathway analyses then indicated that processes related to neurodevelopment, immunity, neurotransmission, gene expression, and oxidative stress were all involved in the pathogenesis of schizophrenia; these are all strikingly similar to those processes involving NKX2-1. Follow-up work to assess if those single-nucleotide polymorphisms are related to NKX2-1 is now needed.
Post-mortem studies in patients with schizophrenia can be performed to study cell populations developed from NKX2-1-expressing progenitors. Analyses of the activities of NKX2-1-related gene pathways in skin biopsies or other tissues can be correlated with the symptoms and various manifestations of schizophrenia. Human pluripotent stem cells are a powerful tool with which to model brain development and disease, and it is feasible to derive cortical interneurons that express NKX2-1 in order to investigate their function under diverse experimental conditions (Maroof et al., 2013). The generation of induced pluripotent stem cells (iPSCs) from patients with schizophrenia would also enable in vitro electrophysiological studies of synaptic function to be conducted for iPSC-derived neurons (Bellin et al., 2012; Brennand et al., 2012). Such electrophysiological methods will enable us to study how NKX2-1 impacts synaptic function in humans, both at the single-neuron level (in vitro), and non-invasively, in vivo (in the brain). These techniques may be complemented by novel computational, in silico modeling of synaptic function. This unique approach has the potential to provide new, groundbreaking knowledge about disease mechanisms that could, potentially, inform clinical practice, and enable the personalized medicine that could improve outcomes in schizophrenia.
By linking NKX2-1 findings to electrophysiology, such studies may contribute to an improved understanding of brain oscillations in schizophrenia. Clinical studies focusing on the relationships among photic transmission, neuroendocrine anomalies, and social and emotional deficits and their association with NKX2-1, should also be performed. The hallmark mental symptoms of schizophrenia can be further investigated in the context of immune, thyroid, parathyroid, lung, skin, and gut pathology. New imaging techniques allow us to explore the impact of thyroid hormones on brain function and metabolism (Bauer et al., 2009; Pilhatsch et al., 2011), as well as visualization of distinct neural circuits (Granziera et al., 2011). New imaging techniques currently under development will also allow us to study small structures, deep in the human brain, such as the septal and amygdala subnuclei, and the hypothalamus (Prestia et al., 2015).
Although speculative, a possible implication of our emphasis on the role of the NKX2-1 transcription factor, not only for brain development, but also in relation to other physiologic defects with embryonic origins (e.g., gut, respiratory system), is that new therapies for diseases associated with those physiological defects may also be of value to patients with schizophrenia. Our hypothesis would be markedly strengthened by studies of how new drug treatments interact with NKX2-1.
Conclusion
NKX2-1 may be involved in core, dose-sensitive, molecular pathways that are deregulated in schizophrenia. This deregulation may result from the mutation of genes that directly or indirectly interact with NKX2-1, and/or from environmental factors that influence epigenetic gene regulation. Deregulated pathways may involve both under- and over-expression, which might activate non-physiologic targets, or repress true targets. Cell type- and tissue-specific factors, together with the timing and magnitude of dysfunction, could also contribute to the phenotypic variability of schizophrenia. NKX2-1 conducts key functions at the interface of the brain, the neuroendocrine system, and the immune system, and may be an important mediator of aberrant cross-communication among these systems in schizophrenia. Knowledge of the processes that involve NKX2-1 may be of value in devising future treatment strategies for schizophrenia, including pharmacologic approaches, deep brain stimulation, and, potentially, stem cell-based treatments.
Author Contributions
EM, KJ, UM, and TN made substantial contributions to the concept and design of this work. EM drafted this manuscript. KJ, UM, and TN critically reviewed the manuscript and gave final approval of the submitted version. EM, KJ, UM, and TN are accountable for all aspects of this work and confirm that any questions related to the accuracy or integrity of any part of the work will be appropriately investigated and resolved.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Abdi, A., Mallet, N., Mohamed, F. Y., Sharott, A., Dodson, P. D., Nakamura, K. C., et al. (2015). Prototypic and arkypallidal neurons in the dopamine-intact external globus pallidus. J. Neurosci. 35, 6667–6688. doi: 10.1523/JNEUROSCI.4662-14.2015
Abel, K. M., Webb, R. T., Salmon, M. P., Wan, M. W., and Appleby, L. (2005). Prevalence and predictors of parenting outcomes in a cohort of mothers with schizophrenia admitted for joint mother and baby psychiatric care in England. J. Clin. Psychiatry 66, 781–789. doi: 10.4088/JCP.v66n0618
Aberg, K. A., Liu, Y., Bukszar, J., McClay, J. L., Khachane, A. N., Andreassen, O. A., et al. (2013). A comprehensive family-based replication study of schizophrenia genes. JAMA Psychiatry 70, 573–581. doi: 10.1001/jamapsychiatry.2013.288
Abu-Tair, F., Strowitzki, T., and Bergemann, N. (2007). [Exacerbation of a schizoaffective psychosis after in vitro fertilization with leuproreline acetate]. Nervenarzt 78, 691–695. doi: 10.1007/s00115-007-2276-2
Aghajani, A., Rahimi, A., Fadai, F., Ebrahimi, A., Najmabadi, H., and Ohadi, M. (2006). A point mutation at the calreticulin gene core promoter conserved sequence in a case of schizophrenia. Am. J. Med. Genet. B Neuropsychiatr. Genet. 141B, 294–295. doi: 10.1002/ajmg.b.30300
Akbarian, S., Kim, J. J., Potkin, S. G., Hetrick, W. P., Bunney, W. E. Jr., and Jones, E. G. (1996). Maldistribution of interstitial neurons in prefrontal white matter of the brains of schizophrenic patients. Arch Gen. Psychiatry 53, 425–436. doi: 10.1001/archpsyc.1996.01830050061010
Alcorn, J. L., Islam, K. N., Young, P. P., and Mendelson, C. R. (2004). Glucocorticoid inhibition of SP-A gene expression in lung type II cells is mediated via the TTF-1-binding element. Am. J. Physiol. Lung Cell. Mol. Physiol. 286, L767–L776. doi: 10.1152/ajplung.00280.2003
Alexander, G. E., DeLong, M. R., and Strick, P. L. (1986). Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu. Rev. Neurosci. 9, 357–381. doi: 10.1146/annurev.ne.09.030186.002041
Allen, K. M., Fung, S. J., and Shannon Weickert, C. (2015). Cell proliferation is reduced in the hippocampus in schizophrenia. Aust. N. Z. J. Psychiatry. 1–8. doi: 10.1177/0004867415589793
Allen, N. C., Bagade, S., McQueen, M. B., Ioannidis, J. P., Kavvoura, F. K., Khoury, M. J., et al. (2008). Systematic meta-analyses and field synopsis of genetic association studies in schizophrenia: the SzGene database. Nat. Genet. 40, 827–834. doi: 10.1038/ng.171
Alva-Sanchez, C., Sanchez-Huerta, K., Arroyo-Helguera, O., Anguiano, B., Aceves, C., and Pacheco-Rosado, J. (2009). The maintenance of hippocampal pyramidal neuron populations is dependent on the modulation of specific cell cycle regulators by thyroid hormones. Brain Res. 1271, 27–35. doi: 10.1016/j.brainres.2009.02.043
Ammassari-Teule, M., Sgobio, C., Biamonte, F., Marrone, C., Mercuri, N. B., and Keller, F. (2009). Reelin haploinsufficiency reduces the density of PV+ neurons in circumscribed regions of the striatum and selectively alters striatal-based behaviors. Psychopharmacology 204, 511–521. doi: 10.1007/s00213-009-1483-x
Anders, S., and Kinney, D. K. (2015). Abnormal immune system development and function in schizophrenia helps reconcile diverse findings and suggests new treatment and prevention strategies. Brain Res. 1617, 93–112. doi: 10.1016/j.brainres.2015.02.043
Andreasen, N. C., Flaum, M., Swayze, V. W. II., Tyrrell, G., and Arndt, S. (1990). Positive and negative symptoms in schizophrenia. A critical reappraisal. Arch. Gen. Psychiatry 47, 615–621. doi: 10.1001/archpsyc.1990.01810190015002
Arnedo, J., Svrakic, D. M., Del Val, C., Romero-Zaliz, R., Hernandez-Cuervo, H., Consortium Molecular Genetics of Schizophrenia Fanous, et al. (2015). Uncovering the hidden risk architecture of the schizophrenias: confirmation in three independent genome-wide association studies. Am. J. Psychiatry 172, 139–153. doi: 10.1176/appi.ajp.2014.14040435
Bailes, H. J., and Lucas, R. J. (2010). Melanopsin and inner retinal photoreception. Cell. Mol. Life Sci. 67, 99–111. doi: 10.1007/s00018-009-0155-7
Bar, K. J., Boettger, M. K., Berger, S., Baier, V., Sauer, H., Yeragani, V. K., et al. (2007). Decreased baroreflex sensitivity in acute schizophrenia. J. Appl. Physiol. 102, 1051–1056. doi: 10.1152/japplphysiol.00811.2006
Bar, K. J., Boettger, M. K., Schulz, S., Harzendorf, C., Agelink, M. W., Yeragani, V. K., et al. (2008). The interaction between pupil function and cardiovascular regulation in patients with acute schizophrenia. Clin. Neurophysiol. 119, 2209–2213. doi: 10.1016/j.clinph.2008.06.012
Bartanusz, V., and Jezova, D. (1994). Angiotensin II induces reduced oxytocin but normal corticotropin release in rats with lesions of the subfornical organ. Fundam. Clin. Pharmacol. 8, 539–545. doi: 10.1111/j.1472-8206.1994.tb00835.x
Basar-Eroglu, C., Schmiedt-Fehr, C., Marbach, S., Brand, A., and Mathes, B. (2008). Altered oscillatory alpha and theta networks in schizophrenia. Brain Res. 1235, 143–152. doi: 10.1016/j.brainres.2008.06.114
Basta-Kaim, A., Fijal, K., Slusarczyk, J., Trojan, E., Glombik, K., Budziszewska, B., et al. (2015). Prenatal administration of lipopolysaccharide induces sex-dependent changes in glutamic acid decarboxylase and parvalbumin in the adult rat brain. Neuroscience 287, 78–92. doi: 10.1016/j.neuroscience.2014.12.013
Bauer, M., Silverman, D. H., Schlagenhauf, F., London, E. D., Geist, C. L., van Herle, K., et al. (2009). Brain glucose metabolism in hypothyroidism: a positron emission tomography study before and after thyroid hormone replacement therapy. J. Clin. Endocrinol. Metab. 94, 2922–2929. doi: 10.1210/jc.2008-2235
Bayer, S. A., and Altman, J. (2009). The Human Brain During the Early First Trimester. Boca Raton, FL: CRC Press.
Bayer, T. A., Falkai, P., and Maier, W. (1999). Genetic and non-genetic vulnerability factors in schizophrenia: the basis of the “two hit hypothesis.” J. Psychiatr. Res. 33, 543–548. doi: 10.1016/S0022-3956(99)00039-4
Beasley, C. L., Zhang, Z. J., Patten, I., and Reynolds, G. P. (2002). Selective deficits in prefrontal cortical GABAergic neurons in schizophrenia defined by the presence of calcium-binding proteins. Biol. Psychiatry 52, 708–715. doi: 10.1016/S0006-3223(02)01360-4
Behrens, M. M., and Sejnowski, T. J. (2009). Does schizophrenia arise from oxidative dysregulation of parvalbumin-interneurons in the developing cortex? Neuropharmacology 57, 193–200. doi: 10.1016/j.neuropharm.2009.06.002
Bellin, M., Marchetto, M. C., Gage, F. H., and Mummery, C. L. (2012). Induced pluripotent stem cells: the new patient? Nat. Rev. Mol. Cell Biol. 13, 713–726. doi: 10.1038/nrm3448
Beneyto, M., Morris, H. M., Rovensky, K. C., and Lewis, D. A. (2012). Lamina- and cell-specific alterations in cortical somatostatin receptor 2 mRNA expression in schizophrenia. Neuropharmacology 62, 1598–1605. doi: 10.1016/j.neuropharm.2010.12.029
Benros, M. E., Pedersen, M. G., Rasmussen, H., Eaton, W. W., Nordentoft, M., and Mortensen, P. B. (2014). A nationwide study on the risk of autoimmune diseases in individuals with a personal or a family history of schizophrenia and related psychosis. Am. J. Psychiatry 171, 218–226. doi: 10.1176/appi.ajp.2013.13010086
Benzel, I., Bansal, A., Browning, B. L., Galwey, N. W., Maycox, P. R., McGinnis, R., et al. (2007). Interactions among genes in the ErbB-Neuregulin signalling network are associated with increased susceptibility to schizophrenia. Behav. Brain Funct. 3:31. doi: 10.1186/1744-9081-3-31
Berg, V., Vassart, G., and Christophe, D. (1996). Identification of a thyroid-specific and cAMP-responsive enhancer in the upstream sequences of the human thyroglobulin promoter. Biochim. Biophys. Acta 1307, 35–38. doi: 10.1016/0167-4781(96)00044-9
Berger, S., Hocke, M., and Bar, K. J. (2010). Gastric dysmotility in healthy first-degree relatives of patients with schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 34, 1294–1299. doi: 10.1016/j.pnpbp.2010.07.013
Bergson, C., Robert, L., Goldman-Rakic, P. S., and Lidow, M. S. (2003). Dopamine receptor-interacting proteins: the Ca2+ connection in dopamine signaling. Trends Pharmacol. Sci. 24, 486–492. doi: 10.1016/S0165-6147(03)00232-3
Berhane, K., and Boggaram, V. (2001). Identification of a novel DNA regulatory element in the rabbit surfactant protein B (SP-B) promoter that is a target for ATF/CREB and AP-1 transcription factors. Gene 268, 141–151. doi: 10.1016/S0378-1119(01)00417-6
Besnard, V., Xu, Y., and Whitsett, J. A. (2007). Sterol response element binding protein and thyroid transcription factor-1 (Nkx2.1) regulate Abca3 gene expression. Am. J. Physiol. Lung Cell. Mol. Physiol. 293, L1395–L1405. doi: 10.1152/ajplung.00275.2007
Bingle, C. D. (1997). Thyroid transcription factor-1. Int. J. Biochem. Cell Biol. 29, 1471–1473. doi: 10.1016/S1357-2725(97)00007-1
Blanchin, S., Estienne, V., Durand-Gorde, J. M., Carayon, P., and Ruf, J. (2003). Complement activation by direct C4 binding to thyroperoxidase in Hashimoto's thyroiditis. Endocrinology 144, 5422–5429. doi: 10.1210/en.2003-0918
Boggaram, V. (2009). Thyroid transcription factor-1 (TTF-1/Nkx2.1/TITF1) gene regulation in the lung. Clin. Sci. (Lond). 116, 27–35. doi: 10.1042/CS20080068
Bohinski, R. J., Lauro Di, R., and Whitsett, J. A. (1994). The lung-specific surfactant protein B gene promoter is a target for thyroid transcription factor 1 and hepatocyte nuclear factor 3, indicating common factors for organ-specific gene expression along the foregut axis. Mol. Cell. Biol. 14, 5671–5681. doi: 10.1128/MCB.14.9.5671
Bojarski, L., Debowska, K., and Wojda, U. (2010). In vitro findings of alterations in intracellular calcium homeostasis in schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry. 34, 1367–1374. doi: 10.1016/j.pnpbp.2010.08.020
Bolam, J. P., Hanley, J. J., Booth, P. A., and Bevan, M. D. (2000). Synaptic organisation of the basal ganglia. J. Anat. 196(Pt 4), 527–542. doi: 10.1046/j.1469-7580.2000.19640527.x
Bralet, M. C., Ton, T., and Falissard, B. (2007). Schizophrenic patients with polydipsia and water intoxication more often have a form of schizophrenia first described by Kraepelin. Psychiatry Res. 152, 267–271. doi: 10.1016/j.psychres.2006.11.009
Brambilla, F., Rovere, C., Guastalla, A., Guerrini, A., and Riggi, F. (1976). Gonadotropin response to synthetic gonadotropin hormone-releasing hormone (GnRH) in chronic schizophrenia. Acta Psychiatr. Scand. 54, 131–145. doi: 10.1111/j.1600-0447.1976.tb00105.x
Brennand, K. J., Simone, A., Tran, N., and Gage, F. H. (2012). Modeling psychiatric disorders at the cellular and network levels. Mol. Psychiatry 17, 1239–1253. doi: 10.1038/mp.2012.20
Brisch, R., Bielau, H., Saniotis, A., Wolf, R., Bogerts, B., Krell, D., et al. (2015). Calretinin and parvalbumin in schizophrenia and affective disorders: a mini-review, a perspective on the evolutionary role of calretinin in schizophrenia, and a preliminary post-mortem study of calretinin in the septal nuclei. Front. Cell. Neurosci. 9:393. doi: 10.3389/fncel.2015.00393
Broad, K. D., Levy, F., Evans, G., Kimura, T., Keverne, E. B., and Kendrick, K. M. (1999). Previous maternal experience potentiates the effect of parturition on oxytocin receptor mRNA expression in the paraventricular nucleus. Eur. J. Neurosci. 11, 3725–3737. doi: 10.1046/j.1460-9568.1999.00782.x
Brockmann, M. D., Poschel, B., Cichon, N., and Hanganu-Opatz, I. L. (2011). Coupled oscillations mediate directed interactions between prefrontal cortex and hippocampus of the neonatal rat. Neuron 71, 332–347. doi: 10.1016/j.neuron.2011.05.041
Bromundt, V., Koster, M., Georgiev-Kill, A., Opwis, K., Wirz-Justice, A., Stoppe, G., et al. (2011). Sleep-wake cycles and cognitive functioning in schizophrenia. Br. J. Psychiatry. 198, 269–276. doi: 10.1192/bjp.bp.110.078022
Brown, A. S. (2011). Exposure to prenatal infection and risk of schizophrenia. Front. Psychiatry 2:63. doi: 10.3389/fpsyt.2011.00063
Butt, S. J., Sousa, V. H., Fuccillo, M. V., Hjerling-Leffler, J., Miyoshi, G., Kimura, S., et al. (2008). The requirement of Nkx2-1 in the temporal specification of cortical interneuron subtypes. Neuron 59, 722–732. doi: 10.1016/j.neuron.2008.07.031
Cai, Y., Zhang, Q., Wang, C., Zhang, Y., Ma, T., Zhou, X., et al. (2013). Nuclear receptor COUP-TFII-expressing neocortical interneurons are derived from the medial and lateral/caudal ganglionic eminence and define specific subsets of mature interneurons. J. Comp. Neurol. 521, 479–497. doi: 10.1002/cne.23186
Cantalamessa, L., Catania, A., Silva, A., Orsatti, A., Motta, P., and Cazzullo, C. L. (1985). Gonadotropin releasing hormone elicits abnormal hormone responses in schizophrenia. Psychoneuroendocrinology 10, 481–484. doi: 10.1016/0306-4530(85)90088-5
Canteras, N. S. (2002). The medial hypothalamic defensive system: hodological organization and functional implications. Pharmacol. Biochem. Behav. 71, 481–491. doi: 10.1016/S0091-3057(01)00685-2
Cao, Y., Vo, T., Millien, G., Tagne, J. B., Kotton, D., Mason, R. J., et al. (2010). Epigenetic mechanisms modulate thyroid transcription factor 1-mediated transcription of the surfactant protein B gene. J. Biol. Chem. 285, 2152–2164. doi: 10.1074/jbc.M109.039172
Carlson, G. C., Talbot, K., Halene, T. B., Gandal, M. J., Kazi, H. A., Schlosser, L., et al. (2011). From the cover: Dysbindin-1 mutant mice implicate reduced fast-phasic inhibition as a final common disease mechanism in schizophrenia. Proc. Natl. Acad. Sci. U.S.A. 108, E962–E970. doi: 10.1073/pnas.1109625108
Carman, J. S., and Wyatt, R. J. (1979a). Calcium: bivalent cation in the bivalent psychoses. Biol. Psychiatry 14, 295–336.
Carman, J. S., and Wyatt, R. J. (1979b). Calcium: pacesetting the periodic psychoses. Am. J. Psychiatry 136, 1035–1039. doi: 10.1176/ajp.136.8.1035
Carmen, J. S., and Wyatt, R. J. (1977). Calcium and malignant catatonia. Lancet 2, 1124–1125. doi: 10.1016/S0140-6736(77)90561-X
Carney, C. P., Jones, L., and Woolson, R. F. (2006). Medical comorbidity in women and men with schizophrenia: a population-based controlled study. J. Gen. Intern. Med. 21, 1133–1137. doi: 10.1111/j.1525-1497.2006.00563.x
Carney, R. S., Mangin, J. M., Hayes, L., Mansfield, K., Sousa, V. H., Fishell, G., et al. (2010). Sonic hedgehog expressing and responding cells generate neuronal diversity in the medial amygdala. Neural Dev. 5:14. doi: 10.1186/1749-8104-5-14
Cassel, T. N., Suske, G., and Nord, M. (2000). C/EBP alpha and TTF-1 synergistically transactivate the Clara cell secretory protein gene. Ann. N. Y. Acad. Sci. 923, 300–302. doi: 10.1111/j.1749-6632.2000.tb05537.x
Castillo, M. A., Ghose, S., Tamminga, C. A., and Ulery-Reynolds, P. G. (2010). Deficits in syntaxin 1 phosphorylation in schizophrenia prefrontal cortex. Biol. Psychiatry 67, 208–216. doi: 10.1016/j.biopsych.2009.07.029
Castillo, P. E., Carleton, A., Vincent, J. D., and Lledo, P. M. (1999). Multiple and opposing roles of cholinergic transmission in the main olfactory bulb. J. Neurosci. 19, 9180–9191.
Catts, V. S., Catts, S. V., McGrath, J. J., Feron, F., McLean, D., Coulson, E. J., et al. (2006). Apoptosis and schizophrenia: a pilot study based on dermal fibroblast cell lines. Schizophr. Res. 84, 20–28. doi: 10.1016/j.schres.2006.03.016
Chance, S. A., Walker, M., and Crow, T. J. (2005). Reduced density of calbindin-immunoreactive interneurons in the planum temporale in schizophrenia. Brain Res. 1046, 32–37. doi: 10.1016/j.brainres.2005.03.045
Chang, J. S., Yoo, C. S., Yi, S. H., Hong, K. H., Oh, H. S., Hwang, J. Y., et al. (2009). Differential pattern of heart rate variability in patients with schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 33, 991–995. doi: 10.1016/j.pnpbp.2009.05.004
Chen, Y. H., Lee, H. C., and Lin, H. C. (2009). Prevalence and risk of atopic disorders among schizophrenia patients: a nationwide population based study. Schizophr. Res. 108, 191–196. doi: 10.1016/j.schres.2008.12.021
Choi, G. B., Dong, H. W., Murphy, A. J., Valenzuela, D. M., Yancopoulos, G. D., Swanson, L. W., et al. (2005). Lhx6 delineates a pathway mediating innate reproductive behaviors from the amygdala to the hypothalamus. Neuron 46, 647–660. doi: 10.1016/j.neuron.2005.04.011
Chojnacki, A., and Weiss, S. (2004). Isolation of a novel platelet-derived growth factor-responsive precursor from the embryonic ventral forebrain. J. Neurosci. 24, 10888–10899. doi: 10.1523/JNEUROSCI.3302-04.2004
Chong, T. W., and Castle, D. J. (2004). Layer upon layer: thermoregulation in schizophrenia. Schizophr. Res. 69, 149–157. doi: 10.1016/S0920-9964(03)00222-6
Chou, T. C., Scammell, T. E., Gooley, J. J., Gaus, S. E., Saper, C. B., and Lu, J. (2003). Critical role of dorsomedial hypothalamic nucleus in a wide range of behavioral circadian rhythms. J. Neurosci. 23, 10691–10702. doi: 10.1038/nn1651
Chroneos, Z. C., Sever-Chroneos, Z., and Shepherd, V. L. (2010). Pulmonary surfactant: an immunological perspective. Cell. Physiol. Biochem. 25, 13–26. doi: 10.1159/000272047
Cianfarani, F., Baldini, E., Cavalli, A., Marchioni, E., Lembo, L., Teson, M., et al. (2010). TSH receptor and thyroid-specific gene expression in human skin. J. Invest. Dermatol. 130, 93–101. doi: 10.1038/jid.2009.180
Cohen, R. Z., Seeman, M. V., Gotowiec, A., and Kopala, L. (1999). Earlier puberty as a predictor of later onset of schizophrenia in women. Am. J. Psychiatry 156, 1059–1064.
Coolen, L. M., and Wood, R. I. (1998). Bidirectional connections of the medial amygdaloid nucleus in the Syrian hamster brain: simultaneous anterograde and retrograde tract tracing. J. Comp. Neurol. 399, 189–209.
Copeland, L. A., Mortensen, E. M., Zeber, J. E., Pugh, M. J., Restrepo, M. I., and Dalack, G. W. (2007). Pulmonary disease among inpatient decedents: Impact of schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 31, 720–726. doi: 10.1016/j.pnpbp.2007.01.008
Cordon, I., Nicolas, M. J., Arrieta, S., Lopetegui, E., Lopez-Azcarate, J., Alegre, M., et al. (2015). Coupling in the cortico-basal ganglia circuit is aberrant in the ketamine model of schizophrenia. Eur. Neuropsychopharmacol. 25, 1375–1387. doi: 10.1016/j.euroneuro.2015.04.004
Cotter, D., Landau, S., Beasley, C., Stevenson, R., Chana, G., MacMillan, L., et al. (2002). The density and spatial distribution of GABAergic neurons, labelled using calcium binding proteins, in the anterior cingulate cortex in major depressive disorder, bipolar disorder, and schizophrenia. Biol. Psychiatry 51, 377–386. doi: 10.1016/S0006-3223(01)01243-4
Crisafulli, C., Chiesa, A., Han, C., Lee, S. J., Shim, D. S., Balzarro, B., et al. (2012). Possible influence of CREB1, CREBBP and CREM variants on diagnosis and treatment outcome in patients with schizophrenia. Neurosci. Lett. 508, 37–41. doi: 10.1016/j.neulet.2011.12.013
Cui, D. H., Jiang, K. D., Jiang, S. D., Xu, Y. F., and Yao, H. (2005). The tumor suppressor adenomatous polyposis coli gene is associated with susceptibility to schizophrenia. Mol. Psychiatry 10, 669–677. doi: 10.1038/sj.mp.4001653
Curley, A. A., Arion, D., Volk, D. W., Asafu-Adjei, J. K., Sampson, A. R., Lewis, D. A. et al. (2011). Cortical deficits of glutamic acid decarboxylase 67 expression in schizophrenia: clinical, protein, and cell type-specific features. Am. J. Psychiatry 168, 921–929. doi: 10.1176/appi.ajp.2011.11010052
Damante, G., Tell, G., and Lauro Di, R. (2001). A unique combination of transcription factors controls differentiation of thyroid cells. Prog. Nucleic Acid Res. Mol. Biol. 66, 307–356. doi: 10.1016/S0079-6603(00)66033-6
D'Anna, K. L., and Gammie, S. C. (2009). Activation of corticotropin-releasing factor receptor 2 in lateral septum negatively regulates maternal defense. Behav. Neurosci. 123, 356–368. doi: 10.1037/a0014987
Dannenberg, H., Pabst, M., Braganza, O., Schoch, S., Niediek, J., Bayraktar, M., et al. (2015). Synergy of direct and indirect cholinergic septo-hippocampal pathways coordinates firing in hippocampal networks. J. Neurosci. 35, 8394–8410. doi: 10.1523/JNEUROSCI.4460-14.2015
Das, A., Acharya, S., Gottipati, K. R., McKnight, J. B., Chandru, H., Alcorn, J. L., et al. (2011). Thyroid transcription factor-1 (TTF-1) gene: identification of ZBP-89, Sp1, and TTF-1 sites in the promoter and regulation by TNF-alpha in lung epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 301, L427–L440. doi: 10.1152/ajplung.00090.2011
Dasgupta, A., Das, S., and Sarkar, P. K. (2007). Thyroid hormone promotes glutathione synthesis in astrocytes by up regulation of glutamate cysteine ligase through differential stimulation of its catalytic and modulator subunit mRNAs. Free Radic. Biol. Med. 42, 617–626. doi: 10.1016/j.freeradbiomed.2006.11.030
Davoodi, N., te Riele, P., and Langlois, X. (2014). Examining dopamine D3 receptor occupancy by antipsychotic drugs via [3H]7-OH-DPAT ex vivo autoradiography and its cross-validation via c-fos immunohistochemistry in the rat brain. Eur. J. Pharmacol. 740, 669–675. doi: 10.1016/j.ejphar.2014.06.011
de Leon, J., Verghese, C., Tracy, J. I., Josiassen, R. C., and Simpson, G. M. (1994). Polydipsia and water intoxication in psychiatric patients: a review of the epidemiological literature. Biol. Psychiatry 35, 408–419. doi: 10.1016/0006-3223(94)90008-6
DeCarolis, N. A., and Eisch, A. J. (2010). Hippocampal neurogenesis as a target for the treatment of mental illness: a critical evaluation. Neuropharmacology 58, 884–893. doi: 10.1016/j.neuropharm.2009.12.013
DeDiego, I., Smith-Fernandez, A., and Fairen, A. (1994). Cortical cells that migrate beyond area boundaries: characterization of an early neuronal population in the lower intermediate zone of prenatal rats. Eur. J. Neurosci. 6, 983–997. doi: 10.1111/j.1460-9568.1994.tb00593.x
Dekimoto, H., Terashima, T., and Katsuyama, Y. (2010). Dispersion of the neurons expressing layer specific markers in the reeler brain. Dev. Growth Differ. 52, 181–193. doi: 10.1111/j.1440-169X.2009.01153.x
Dierynck, I., Bernard, A., Roels, H., and Ley De, M. (1996). The human Clara cell protein: biochemical and biological characterisation of a natural immunosuppressor. Mult. Scler. 1, 385–387.
Dieset, I., Haukvik, U. K., Melle, I., Rossberg, J. I., Ueland, T., Hope, S., et al. (2015). Association between altered brain morphology and elevated peripheral endothelial markers–implications for psychotic disorders. Schizophr. Res. 161, 222–228. doi: 10.1016/j.schres.2014.11.006
Dimic, S., Wildgrube, C., McCabe, R., Hassan, I., Barnes, T. R., and Priebe, S. (2010). Non-verbal behaviour of patients with schizophrenia in medical consultations–a comparison with depressed patients and association with symptom levels. Psychopathology 43, 216–222. doi: 10.1159/000313519
Do, K. Q., Cabungcal, J. H., Frank, A., Steullet, P., and Cuenod, M. (2009). Redox dysregulation, neurodevelopment, and schizophrenia. Curr. Opin. Neurobiol. 19, 220–230. doi: 10.1016/j.conb.2009.05.001
Dodson, P. D., Larvin, J. T., Duffell, J. M., Garas, F. N., Doig, N. M., Kessaris, N., et al. (2015). Distinct developmental origins manifest in the specialized encoding of movement by adult neurons of the external globus pallidus. Neuron 86, 501–513. doi: 10.1016/j.neuron.2015.03.007
Du, T., Xu, Q., Ocbina, P. J., and Anderson, S. A. (2008). NKX2.1 specifies cortical interneuron fate by activating Lhx6. Development 135, 1559–1567. doi: 10.1242/dev.015123
Duan, M., Chen, X., He, H., Jiang, Y., Jiang, S., Xie, Q., et al. (2015). Altered basal ganglia network integration in schizophrenia. Front. Hum. Neurosci. 9:561. doi: 10.3389/fnhum.2015.00561
Eastwood, S. L., and Harrison, P. J. (2006). Cellular basis of reduced cortical reelin expression in schizophrenia. Am. J. Psychiatry 163, 540–542. doi: 10.1176/appi.ajp.163.3.540
Eaton, W. W., Byrne, M., Ewald, H., Mors, O., Chen, C. Y., Agerbo, E., et al. (2006). Association of schizophrenia and autoimmune diseases: linkage of Danish national registers. Am. J. Psychiatry 163, 521–528. doi: 10.1176/appi.ajp.163.3.521
Ecker, J. L., Dumitrescu, O. N., Wong, K. Y., Alam, N. M., Chen, S. K., LeGates, T., et al. (2010). Melanopsin-expressing retinal ganglion-cell photoreceptors: cellular diversity and role in pattern vision. Neuron 67, 49–60. doi: 10.1016/j.neuron.2010.05.023
Eggermann, E., and Feldmeyer, D. (2009). Cholinergic filtering in the recurrent excitatory microcircuit of cortical layer 4. Proc. Natl. Acad. Sci. U.S.A. 106, 11753–11758. doi: 10.1073/pnas.0810062106
Elias, S., Ritov, Y., and Bergman, H. (2008). Balance of increases and decreases in firing rate of the spontaneous activity of basal ganglia high-frequency discharge neurons. J. Neurophysiol. 100, 3086–3104. doi: 10.1152/jn.90714.2008
Ellison-Wright, I., Glahn, D. C., Laird, A. R., Thelen, S. M., and Bullmore, E. (2008). The anatomy of first-episode and chronic schizophrenia: an anatomical likelihood estimation meta-analysis. Am. J. Psychiatry. 8, 1015–1023. doi: 10.1176/appi.ajp.2008.07101562
Endo, T., Kaneshige, M., Nakazato, M., Ohmori, M., Harii, N., and Onaya, T. (1997). Thyroid transcription factor-1 activates the promoter activity of rat thyroid Na+/I- symporter gene. Mol. Endocrinol. 11, 1747–1755.
Everitt, B. J., and Robbins, T. W. (1997). Central cholinergic systems and cognition. Annu. Rev. Psychol. 48, 649–684. doi: 10.1146/annurev.psych.48.1.649
Fallon, J. H., Loughlin, S. E., and Ribak, C. E. (1983). The islands of Calleja complex of rat basal forebrain. III. Histochemical evidence for a striatopallidal system. J. Comp. Neurol. 218, 91–120. doi: 10.1002/cne.902180106
Farrell, M. S., Werge, T., Sklar, P., Owen, M. J., Ophoff, R. A., O'Donovan, M. C., et al. (2015). Evaluating historical candidate genes for schizophrenia. Mol. Psychiatry 20, 555–562. doi: 10.1038/mp.2015.16
Faturi, C. B., Rangel, M. J. Jr., Baldo, M. V., and Canteras, N. S. (2014). Functional mapping of the circuits involved in the expression of contextual fear responses in socially defeated animals. Brain Struct. Funct. 219, 931–946. doi: 10.1007/s00429-013-0544-4
Faux, C. H., Turnley, A. M., Epa, R., Cappai, R., and Bartlett, P. F. (2001). Interactions between fibroblast growth factors and Notch regulate neuronal differentiation. J. Neurosci. 21, 5587–5596. doi: 10.1016/j.neulet.2007.05.020
Fazzari, P., Paternain, A. V., Valiente, M., Pla, R., Lujan, R., Lloyd, K., et al. (2010). Control of cortical GABA circuitry development by Nrg1 and ErbB4 signalling. Nature 464, 1376–1380. doi: 10.1038/nature08928
Feigenson, K. A., Kusnecov, A. W., and Silverstein, S. M. (2014). Inflammation and the two-hit hypothesis of schizophrenia. Neurosci. Biobehav. Rev. 38, 72–93. doi: 10.1016/j.neubiorev.2013.11.006
Fekete, C., and Lechan, R. M. (2014). Central regulation of hypothalamic-pituitary-thyroid axis under physiological and pathophysiological conditions. Endocr. Rev. 35, 159–194. doi: 10.1210/er.2013-1087
Felsenfeld, A. J., and Levine, B. S. (2015). Calcitonin, the forgotten hormone: does it deserve to be forgotten? Clin. Kidney J. 8, 180–187. doi: 10.1093/ckj/sfv011
Ferguson, A. V., and Bains, J. S. (1997). Actions of angiotensin in the subfornical organ and area postrema: implications for long term control of autonomic output. Clin. Exp. Pharmacol. Physiol. 24, 96–101. doi: 10.1111/j.1440-1681.1997.tb01790.x
Fernandez, L. P., Lopez-Marquez, A., and Santisteban, P. (2015). Thyroid transcription factors in development, differentiation and disease. Nat. Rev. Endocrinol. 11, 29–42. doi: 10.1038/nrendo.2014.186
Ferno, J., Varela, L., Skrede, S., Vazquez, M. J., Nogueiras, R., Dieguez, C., et al. (2011). Olanzapine-induced hyperphagia and weight gain associate with orexigenic hypothalamic neuropeptide signaling without concomitant AMPK phosphorylation. PLoS ONE 6:e20571. doi: 10.1371/journal.pone.0020571
Ferrier, I. N., Johnstone, E. C., Crow, T. J., and Rincon-Rodriguez, I. (1983). Anterior pituitary hormone secretion in chronic schizophrenics. Arch. Gen. Psychiatry 40, 755–761. doi: 10.1001/archpsyc.1983.01790060053007
Filik, R., Sipos, A., Kehoe, P. G., Burns, T., Cooper, S. J., Stevens, H., et al. (2006). The cardiovascular and respiratory health of people with schizophrenia. Acta Psychiatr. Scand. 113, 298–305. doi: 10.1111/j.1600-0447.2006.00768.x
Fisahn, A., Neddens, J., Yan, L., and Buonanno, A. (2009). Neuregulin-1 modulates hippocampal gamma oscillations: implications for schizophrenia. Cereb. Cortex 19, 612–618. doi: 10.1093/cercor/bhn107
Flamant, F., Koibuchi, N., and Bernal, J. (2015). Editorial: thyroid hormone in brain and brain cells. Front. Endocrinol. 6:99. doi: 10.3389/fendo.2015.00099
Flames, N., Pla, R., Gelman, D. M., Rubenstein, J. L., Puelles, L., and Marin, O. (2007). Delineation of multiple subpallial progenitor domains by the combinatorial expression of transcriptional codes. J. Neurosci. 27, 9682–9695. doi: 10.1523/JNEUROSCI.2750-07.2007
Flandin, P., Kimura, S., and Rubenstein, J. L. (2010). The progenitor zone of the ventral medial ganglionic eminence requires Nkx2-1 to generate most of the globus pallidus but few neocortical interneurons. J. Neurosci. 30, 2812–2823. doi: 10.1523/JNEUROSCI.4228-09.2010
Flandin, P., Zhao, Y., Vogt, D., Jeong, J., Long, J., Potter, G., et al. (2011). Lhx6 and Lhx8 coordinately induce neuronal expression of Shh that controls the generation of interneuron progenitors. Neuron 70, 939–950. doi: 10.1016/j.neuron.2011.04.020
Fogarty, M., Grist, M., Gelman, D., Marin, O., Pachnis, V., and Kessaris, N. (2007). Spatial genetic patterning of the embryonic neuroepithelium generates GABAergic interneuron diversity in the adult cortex. J. Neurosci. 27, 10935–10946. doi: 10.1523/JNEUROSCI.1629-07.2007
Fonseca, T. L., Correa-Medina, M., Campos, M. P., Wittmann, G., Werneck-de-Castro, J. P., Arrojo e Drigo, R., et al. (2013). Coordination of hypothalamic and pituitary T3 production regulates TSH expression. J. Clin. Invest. 123, 1492–1500. doi: 10.1172/JCI61231
Fragkouli, A., Hearn, C., Errington, M., Cooke, S., Grigoriou, M., Bliss, T., et al. (2005). Loss of forebrain cholinergic neurons and impairment in spatial learning and memory in LHX7-deficient mice. Eur. J. Neurosci. 21, 2923–2938. doi: 10.1111/j.1460-9568.2005.04141.x
Fragkouli, A., van Wijk, N. V., Lopes, R., Kessaris, N., and Pachnis, V. (2009). LIM homeodomain transcription factor-dependent specification of bipotential MGE progenitors into cholinergic and GABAergic striatal interneurons. Development 136, 3841–3851. doi: 10.1242/dev.038083
Frayling, C., Britton, R., and Dale, N. (2011). ATP-mediated glucosensing by hypothalamic tanycytes. J. Physiol. 589(Pt 9), 2275–2286. doi: 10.1113/jphysiol.2010.202051
Freiria-Oliveira, A. H., Blanch, G. T., Camargo, L. A., Menani, J. V., and Saad, W. A. (2009). Involvement of the intermediate nucleus of the lateral septal area on angiotensin II-induced dipsogenic and pressor responses. Regul. Pept. 157, 14–18. doi: 10.1016/j.regpep.2009.07.002
Freund, T. F. (1989). GABAergic septohippocampal neurons contain parvalbumin. Brain Res. 478, 375–381. doi: 10.1016/0006-8993(89)91520-5
Frydecka, D., Misiak, B., Beszlej, J. A., Karabon, L., Pawlak-Adamska, E., Tomkiewicz, A., et al. (2013). Genetic variants in transforming growth factor-beta gene (TGFB1) affect susceptibility to schizophrenia. Mol. Biol. Rep. 40, 5607–5614. doi: 10.1007/s11033-013-2662-8
Fujihara, K., Miwa, H., Kakizaki, T., Kaneko, R., Mikuni, M., Tanahira, C., et al. (2015). Glutamate decarboxylase 67 deficiency in a subset of GABAergic neurons induces schizophrenia-related phenotypes. Neuropsychopharmacology 40, 2475–2486. doi: 10.1038/npp.2015.117
Fung, S. J., Webster, M. J., Sivagnanasundaram, S., Duncan, C., Elashoff, M., and Weickert, C. S. (2010). Expression of interneuron markers in the dorsolateral prefrontal cortex of the developing human and in schizophrenia. Am. J. Psychiatry 167, 1479–1488. doi: 10.1176/appi.ajp.2010.09060784
Gabor, C. S., Phan, A., Clipperton-Allen, A. E., Kavaliers, M., and Choleris, E. (2012). Interplay of oxytocin, vasopressin, and sex hormones in the regulation of social recognition. Behav. Neurosci. 126, 97–109. doi: 10.1037/a0026464
Gage, G. J., Stoetzner, C. R., Wiltschko, A. B., and Berke, J. D. (2010). Selective activation of striatal fast-spiking interneurons during choice execution. Neuron 67, 466–479. doi: 10.1016/j.neuron.2010.06.034
Galdos, P., and van Os, J. (1995). Gender, psychopathology, and development: from puberty to early adulthood. Schizophr. Res. 14, 105–112. doi: 10.1016/0920-9964(94)00020-9
Galeno, R., Molina, M., Guirao, M., and Isoardi, R. (2004). Severity of negative symptoms in schizophrenia correlated to hyperactivity of the left globus pallidus and the right claustrum. A PET study. World J. Biol. Psychiatry 5, 20–25. doi: 10.1080/15622970410029903
Garcia-Barcelo, M., Ganster, R. W., Lui, V. C., Leon, T. Y., So, M. T., Lau, A. M., et al. (2005). TTF-1 and RET promoter SNPs: regulation of RET transcription in Hirschsprung's disease. Hum. Mol. Genet. 14, 191–204. doi: 10.1093/hmg/ddi015
Garcia-Lopez, M., Abellan, A., Legaz, I., Rubenstein, J. L., Puelles, L., and Medina, L. (2008). Histogenetic compartments of the mouse centromedial and extended amygdala based on gene expression patterns during development. J. Comp. Neurol. 506, 46–74. doi: 10.1002/cne.21524
Gasperini, L., Piubelli, C., and Carboni, L. (2012). Proteomics of rat hypothalamus, hippocampus and pre-frontal/frontal cortex after central administration of the neuropeptide PACAP. Mol. Biol. Rep. 39, 2921–2935. doi: 10.1007/s11033-011-1054-1
Gelman, D. M., Martini, F. J., Nobrega-Pereira, S., Pierani, A., Kessaris, N., and Marin, O. (2009). The embryonic preoptic area is a novel source of cortical GABAergic interneurons. J. Neurosci. 29, 9380–9389. doi: 10.1523/JNEUROSCI.0604-09.2009
Gereben, B., Salvatore, D., Harney, J. W., Tu, H. M., and Larsen, P. R. (2001). The human, but not rat, dio2 gene is stimulated by thyroid transcription factor-1 (TTF-1). Mol. Endocrinol. 15, 112–124. doi: 10.1210/mend.15.1.0579
Germain, N. D., Banda, E. C., Becker, S., Naegele, J. R., and Grabel, L. B. (2013). Derivation and isolation of NKX2.1-positive basal forebrain progenitors from human embryonic stem cells. Stem Cells Dev. 22, 1477–1489. doi: 10.1089/scd.2012.0264
Gibson, C. M., Penn, D. L., Smedley, K. L., Leserman, J., Elliott, T., and Pedersen, C. A. (2014). A pilot six-week randomized controlled trial of oxytocin on social cognition and social skills in schizophrenia. Schizophr. Res. 156, 261–265. doi: 10.1016/j.schres.2014.04.009
Gilbert, M. E., Sui, L., Walker, M. J., Anderson, W., Thomas, S., Smoller, S. N., et al. (2007). Thyroid hormone insufficiency during brain development reduces parvalbumin immunoreactivity and inhibitory function in the hippocampus. Endocrinology 148, 92–102. doi: 10.1210/en.2006-0164
Gilbert, P., Birchwood, M., Gilbert, J., Trower, P., Hay, J., Murray, B., et al. (2001). An exploration of evolved mental mechanisms for dominant and subordinate behaviour in relation to auditory hallucinations in schizophrenia and critical thoughts in depression. Psychol. Med. 31, 1117–1127. doi: 10.1017/s0033291701004093
Glausier, J. R., Fish, K. N., and Lewis, D. A. (2014). Altered parvalbumin basket cell inputs in the dorsolateral prefrontal cortex of schizophrenia subjects. Mol. Psychiatry 19, 30–36. doi: 10.1038/mp.2013.152
Glessner, J. T., Reilly, M. P., Kim, C. E., Takahashi, N., Albano, A., Hou, C., et al. (2010). Strong synaptic transmission impact by copy number variations in schizophrenia. Proc. Natl. Acad. Sci. U.S.A. 107, 10584–10589. doi: 10.1073/pnas.1000274107
Glik, A., Vuillaume, I., Devos, D., and Inzelberg, R. (2008). Psychosis, short stature in benign hereditary chorea: a novel thyroid transcription factor-1 mutation. Mov. Disord. 23, 1744–1747. doi: 10.1002/mds.22215
Goldman, M. B. (2009). The mechanism of life-threatening water imbalance in schizophrenia and its relationship to the underlying psychiatric illness. Brain Res. Rev. 61, 210–220. doi: 10.1016/j.brainresrev.2009.06.004
Goldman, M. B., Torres, I. J., Keedy, S., Marlow-O'Connor, M., Beenken, B., and Pilla, R. (2007). Reduced anterior hippocampal formation volume in hyponatremic schizophrenic patients. Hippocampus 17, 554–562. doi: 10.1002/hipo.20292
Goldman, M., Marlow-O'Connor, M., Torres, I., and Carter, C. S. (2008). Diminished plasma oxytocin in schizophrenic patients with neuroendocrine dysfunction and emotional deficits. Schizophr. Res. 98, 247–255. doi: 10.1016/j.schres.2007.09.019
Gonzalez-Burgos, G., Cho, R. Y., and Lewis, D. A. (2015). Alterations in cortical network oscillations and parvalbumin neurons in schizophrenia. Biol. Psychiatry 77, 1031–1040. doi: 10.1016/j.biopsych.2015.03.010
Goodman, T., and Hajihosseini, M. K. (2015). Hypothalamic tanycytes-masters and servants of metabolic, neuroendocrine, and neurogenic functions. Front. Neurosci. 9:387. doi: 10.3389/fnins.2015.00387
Goulburn, A. L., Alden, D., Davis, R. P., Micallef, S. J., Ng, E. S., Yu, Q. C., et al. (2011). A targeted NKX2.1 human embryonic stem cell reporter line enables identification of human basal forebrain derivatives. Stem Cells 29, 462–473. doi: 10.1002/stem.587
Grabert, J., and Wahle, P. (2008). Neuronal activity and TrkB ligands influence Kv3.1b and Kv3.2 expression in developing cortical interneurons. Neuroscience 156, 618–629. doi: 10.1016/j.neuroscience.2008.08.008
Granziera, C., Hadjikhani, N., Arzy, S., Seeck, M., Meuli, R., and Krueger, G. (2011). In-vivo magnetic resonance imaging of the structural core of the Papez circuit in humans. Neuroreport 22, 227–231. doi: 10.1097/WNR.0b013e328344f75f
Griffin, G. D., and Flanagan-Cato, L. M. (2011). Ovarian hormone action in the hypothalamic ventromedial nucleus: remodelling to regulate reproduction. J. Neuroendocrinol. 23, 465–471. doi: 10.1111/j.1365-2826.2011.02143.x
Griguoli, M., and Cherubini, E. (2012). Regulation of hippocampal inhibitory circuits by nicotinic acetylcholine receptors. J. Physiol. 590(Pt 4), 655–666. doi: 10.1113/jphysiol.2011.220095
Gulacsi, A. A., and Anderson, S. A. (2008). Beta-catenin-mediated Wnt signaling regulates neurogenesis in the ventral telencephalon. Nat. Neurosci. 11, 1383–1391. doi: 10.1038/nn.2226
Guo, N., Vincent, S. R., and Fibiger, H. C. (1998). Phenotypic characterization of neuroleptic-sensitive neurons in the forebrain: contrasting targets of haloperidol and clozapine. Neuropsychopharmacology 19, 133–145. doi: 10.1016/S0893-133X(97)00202-9
Gur, R., Tendler, A., and Wagner, S. (2014). Long-term social recognition memory is mediated by oxytocin-dependent synaptic plasticity in the medial amygdala. Biol. Psychiatry 76, 377–386. doi: 10.1016/j.biopsych.2014.03.022
Gysin, R., Kraftsik, R., Sandell, J., Bovet, P., Chappuis, C., Conus, P., et al. (2007). Impaired glutathione synthesis in schizophrenia: convergent genetic and functional evidence. Proc. Natl. Acad. Sci. U.S.A. 104, 16621–16626. doi: 10.1073/pnas.0706778104
Gysin, R., Riederer, I. M., Cuenod, M., Do, K. Q., and Riederer, B. M. (2009). Skin fibroblast model to study an impaired glutathione synthesis: consequences of a genetic polymorphism on the proteome. Brain Res. Bull. 79, 46–52. doi: 10.1016/j.brainresbull.2008.10.015
Haenschel, C., Bittner, R. A., Waltz, J., Haertling, F., Wibral, M., Singer, W., et al. (2009). Cortical oscillatory activity is critical for working memory as revealed by deficits in early-onset schizophrenia. J. Neurosci. 29, 9481–9489. doi: 10.1523/JNEUROSCI.1428-09.2009
Hamm, J. P., Gilmore, C. S., Picchetti, N. A., Sponheim, S. R., and Clementz, B. A. (2011). Abnormalities of neuronal oscillations and temporal integration to low- and high-frequency auditory stimulation in schizophrenia. Biol. Psychiatry 69, 989–996. doi: 10.1016/j.biopsych.2010.11.021
Hangya, B., Borhegyi, Z., Szilagyi, N., Freund, T. F., and Varga, V. (2009). GABAergic neurons of the medial septum lead the hippocampal network during theta activity. J. Neurosci. 29, 8094–8102. doi: 10.1523/JNEUROSCI.5665-08.2009
Hannibal, J. (2006). Roles of PACAP-containing retinal ganglion cells in circadian timing. Int. Rev. Cytol. 251, 1–39. doi: 10.1016/S0074-7696(06)51001-0
Hashimoto, R., Hashimoto, H., Shintani, N., Chiba, S., Hattori, S., Okada, T., et al. (2007). Pituitary adenylate cyclase-activating polypeptide is associated with schizophrenia. Mol. Psychiatry 12, 1026–1032. doi: 10.1038/sj.mp.4001982
Hashimoto, R., Mori, T., Nemoto, K., Moriguchi, Y., Noguchi, H., Nakabayashi, T., et al. (2009). Abnormal microstructures of the basal ganglia in schizophrenia revealed by diffusion tensor imaging. World J. Biol. Psychiatry 10, 65–69. doi: 10.1080/15622970701762536
Hashimoto, T., Arion, D., Unger, T., Maldonado-Aviles, J. G., Morris, H. M., Volk, D. W., et al. (2008). Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol. Psychiatry 13, 147–161. doi: 10.1038/sj.mp.4002011
Hashimoto, T., Bergen, S. E., Nguyen, Q. L., Xu, B., Monteggia, L. M., Pierri, J. N., et al. (2005). Relationship of brain-derived neurotrophic factor and its receptor TrkB to altered inhibitory prefrontal circuitry in schizophrenia. J. Neurosci. 25, 372–383. doi: 10.1523/JNEUROSCI.4035-04.2005
Hattar, S., Kumar, M., Park, A., Tong, P., Tung, J., Yau, K. W., et al. (2006). Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J. Comp. Neurol. 497, 326–349. doi: 10.1002/cne.20970
Hertzberg, L., Katsel, P., Roussos, P., Haroutunian, V., and Domany, E. (2015). Integration of gene expression and GWAS results supports involvement of calcium signaling in Schizophrenia. Schizophr. Res. 164, 92–99. doi: 10.1016/j.schres.2015.02.001
Herwig, A., Ross, A. W., Nilaweera, K. N., Morgan, P. J., and Barrett, P. (2008). Hypothalamic thyroid hormone in energy balance regulation. Obes. Facts 1, 71–79. doi: 10.1159/000123428
Hochman, K. M., and Lewine, R. R. (2004). Age of menarche and schizophrenia onset in women. Schizophr. Res. 69, 183–188. doi: 10.1016/S0920-9964(03)00176-2
Holt, D. J., Bachus, S. E., Hyde, T. M., Wittie, M., Herman, M. M., Vangel, M., et al. (2005). Reduced density of cholinergic interneurons in the ventral striatum in schizophrenia: an in situ hybridization study. Biol. Psychiatry 58, 408–416. doi: 10.1016/j.biopsych.2005.04.007
Holt, D. J., Herman, M. M., Hyde, T. M., Kleinman, J. E., Sinton, C. M., German, D. C., et al. (1999). Evidence for a deficit in cholinergic interneurons in the striatum in schizophrenia. Neuroscience 94, 21–31. doi: 10.1016/S0306-4522(99)00279-1
Huang, H., Li, X., Lin, L., Shi, Y., Lin, X., Li, L., et al. (2011). Upregulation of thyroid transcription factor-1 and human leukocyte antigen class I in Hashimoto's disease providing a clinical evidence for possible triggering autoimmune reaction. Eur. J. Endocrinol. 164, 795–800. doi: 10.1530/EJE-10-0960
Hundt, W., Kellner, M., and Wiedemann, K. (2001). Neuroendocrine effects of a short-term osmotic stimulus in patients with chronic schizophrenia. World J. Biol. Psychiatry 2, 27–33. doi: 10.3109/15622970109039981
Hung, C. H., Chen, L. C., Zhang, Z., Chowdhury, B., Lee, W. L., Plunkett, B., et al. (2004). Regulation of TH2 responses by the pulmonary Clara cell secretory 10-kd protein. J. Allergy Clin. Immunol. 114, 664–670. doi: 10.1016/j.jaci.2004.05.042
Ibarrola, N., and Rodriguez-Pena, A. (1997). Hypothyroidism coordinately and transiently affects myelin protein gene expression in most rat brain regions during postnatal development. Brain Res. 752, 285–293. doi: 10.1016/S0006-8993(96)01480-1
Ibi, D., Nagai, T., Koike, H., Kitahara, Y., Mizoguchi, H., Niwa, M., et al. (2010). Combined effect of neonatal immune activation and mutant DISC1 on phenotypic changes in adulthood. Behav Brain Res. 206, 32–37. doi: 10.1016/j.bbr.2009.08.027
Ide, M., Yamada, K., Toyota, T., Iwayama, Y., Ishitsuka, Y., Minabe, Y., et al. (2005). Genetic association analyses of PHOX2B and ASCL1 in neuropsychiatric disorders: evidence for association of ASCL1 with Parkinson's disease. Hum. Genet. 117, 520–527. doi: 10.1007/s00439-005-1342-8
Inan, M., Petros, T. J., and Anderson, S. A. (2013). Losing your inhibition: linking cortical GABAergic interneurons to schizophrenia. Neurobiol. Dis. 53, 36–48. doi: 10.1016/j.nbd.2012.11.013
Jiang, Z., Cowell, R. M., and Nakazawa, K. (2013). Convergence of genetic and environmental factors on parvalbumin-positive interneurons in schizophrenia. Front. Behav. Neurosci. 7:116. doi: 10.3389/fnbeh.2013.00116
Johansson, S., Wennergren, G., Aberg, N., and Rudin, A. (2007). Clara cell 16-kd protein downregulates T(H)2 differentiation of human naive neonatal T cells. J. Allergy Clin. Immunol. 120, 308–314. doi: 10.1016/j.jaci.2007.03.021
Jose, J., Nandeesha, H., Kattimani, S., Meiyappan, K., Sarkar, S., and Sivasankar, D. (2015). Association between prolactin and thyroid hormones with severity of psychopathology and suicide risk in drug free male schizophrenia. Clin. Chim Acta 444, 78–80. doi: 10.1016/j.cca.2015.02.003
Jun, H., Mohammed Qasim Hussaini, S., Rigby, M. J., and Jang, M. H. (2012). Functional role of adult hippocampal neurogenesis as a therapeutic strategy for mental disorders. Neural Plast. 2012:854285. doi: 10.1155/2012/854285
Jungbauer, J., Stelling, K., Kuhn, J., and Lenz, A. (2010). [How do mothers and fathers suffering from schizophrenia experience their parenthood? Results from an in-depth interview study]. Psychiatr. Prax. 37, 233–239. doi: 10.1055/s-0029-1223535
Kahler, A. K., Djurovic, S., Kulle, B., Jonsson, E. G., Agartz, I., Hall, H., et al. (2008). Association analysis of schizophrenia on 18 genes involved in neuronal migration: MDGA1 as a new susceptibility gene. Am. J. Med. Genet. B Neuropsychiatr. Genet. 147b, 1089–1100. doi: 10.1002/ajmg.b.30726
Kahn, R. S., Davidson, M., Hirschowitz, J., Stern, R. G., Davis, B. M., Gabriel, S., et al. (1992). Nocturnal growth hormone secretion in schizophrenic patients and healthy subjects. Psychiatry Res. 41, 155–161. doi: 10.1016/0165-1781(92)90107-E
Kaji, T., and Nonogaki, K. (2013). Role of homeobox genes in the hypothalamic development and energy balance. Front. Biosci. 18, 740–747. doi: 10.2741/4136
Kalus, P., Senitz, D., and Beckmann, H. (1997). Cortical layer I changes in schizophrenia: a marker for impaired brain development? J. Neural Transm. 104, 549–559. doi: 10.1007/BF01277671
Kamolz, L. P., Andel, H., Schmidtke, A., Valentini, D., Meissl, G., and Frey, M. (2003). Treatment of patients with severe burn injuries: the impact of schizophrenia. Burns 29, 49–53. doi: 10.1016/S0305-4179(02)00207-3
Kang, Y. M., Ma, Y., Zheng, J. P., Elks, C., Sriramula, S., Yang, Z. M., et al. (2009). Brain nuclear factor-kappa B activation contributes to neurohumoral excitation in angiotensin II-induced hypertension. Cardiovasc. Res. 82, 503–512. doi: 10.1093/cvr/cvp073
Karatsoreos, I. N. (2014). Links between circadian rhythms and psychiatric disease. Front. Behav. Neurosci. 8:162. doi: 10.3389/fnbeh.2014.00162
Katz, M., Buchsbaum, M. S., Siegel, B. V. Jr., Wu, J., Haier, R. J., and Bunney, W. E. Jr. (1996). Correlational patterns of cerebral glucose metabolism in never-medicated schizophrenics. Neuropsychobiology 33, 1–11. doi: 10.1159/000119241
Kawaguchi, C., Isojima, Y., Shintani, N., Hatanaka, M., Guo, X., Okumura, N., et al. (2010). PACAP-deficient mice exhibit light parameter-dependent abnormalities on nonvisual photoreception and early activity onset. PLoS ONE 5:e9286. doi: 10.1371/journal.pone.0009286
Kessaris, N., Fogarty, M., Iannarelli, P., Grist, M., Wegner, M., and Richardson, W. D. (2006). Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat. Neurosci. 9, 173–179. doi: 10.1038/nn1620
Khosravani, N., and Goodarzi, M. A. (2013). Patients with schizophrenia show deficits on spatial frequency doubling. Vision Res. 93, 49–53. doi: 10.1016/j.visres.2013.10.007
Kim, J. G., Bae, K. D., Yun, C. H., Im, H. L., Park, J. W., Nam-Goong, I. S., et al. (2008). Thyroid transcription factor-1 exhibits osmosensitive transcription in brain-derived cell lines. Biochem. Biophys. Res. Commun. 370, 468–472. doi: 10.1016/j.bbrc.2008.03.125
Kim, J. G., Nam-Goong, I. S., Yun, C. H., Jeong, J. K., Kim, E. S., Park, J. J., et al. (2006). TTF-1, a homeodomain-containing transcription factor, regulates feeding behavior in the rat hypothalamus. Biochem. Biophys. Res. Commun. 349, 969–975. doi: 10.1016/j.bbrc.2006.08.147
Kim, J. G., Park, B. S., Yun, C. H., Kim, H. J., Kang, S. S., D'Elia, A. V., et al. (2011). Thyroid transcription factor 1 regulates feeding behavior via melanocortin pathway in the hypothalamus. Diabetes. 60, 710–719. doi: 10.2337/db10-0183
Kim, J. G., Son, Y. J., Yun, C. H., Kim, Y. I., Nam-Goong, I. S., Park, J. H., et al. (2007). Thyroid transcription factor-1 facilitates cerebrospinal fluid formation by regulating aquaporin-1 synthesis in the brain. J. Biol. Chem. 282, 14923–14931. doi: 10.1074/jbc.M701411200
Kim, M. J., Lee, J. Y., Stanley, E. G., Elefanty, A. G., and Petratos, S. (2015). Efficient derivation of oligodendrocyte progenitors from nkx2.1-gfp human embryonic stem cell reporter line. Int. J. Dev. Neurosci. 47(Pt A), 69. doi: 10.1016/j.ijdevneu.2015.04.190
Kim, M. S., Hur, M. K., Son, Y. J., Park, J. I., Chun, S. Y., D'Elia, A. V., et al. (2002). Regulation of pituitary adenylate cyclase-activating polypeptide gene transcription by TTF-1, a homeodomain-containing transcription factor. J. Biol. Chem. 277, 36863–36871. doi: 10.1074/jbc.M206443200
Kimura, S. (1996). Thyroid-specific enhancer-binding protein Role in thyroid function and organogenesis. Trends Endocrinol. Metab 7, 247–252. doi: 10.1016/S1043-2760(96)00115-4
Kirkpatrick, B., Messias, N. C., Conley, R. R., and Roberts, R. C. (2003). Interstitial cells of the white matter in the dorsolateral prefrontal cortex in deficit and nondeficit schizophrenia. J. Nerv. Ment. Dis. 191, 563–567. doi: 10.1097/01.nmd.0000087181.61164.e1
Kishore, U., Greenhough, T. J., Waters, P., Shrive, A. K., Ghai, R., Kamran, M. F., et al. (2006). Surfactant proteins SP-A and SP-D: structure, function and receptors. Mol. Immunol. 43, 1293–1315. doi: 10.1016/j.molimm.2005.08.004
Klecha, A. J., Genaro, A. M., Gorelik, G., Barreiro Arcos, M. L., Silberman, D. M., Schuman, M., et al. (2006). Integrative study of hypothalamus-pituitary-thyroid-immune system interaction: thyroid hormone-mediated modulation of lymphocyte activity through the protein kinase C signaling pathway. J. Endocrinol. 189, 45–55. doi: 10.1677/joe.1.06137
Kohen, D., and Wildgust, H. J. (2008). The evolution of hyperprolactinaemia as an entity in psychiatric patients. J. Psychopharmacol. 22(2 Suppl.), 6–11. doi: 10.1177/0269216307087147
Kolla, V., Gonzales, L. W., Bailey, N. A., Wang, P., Angampalli, S., Godinez, M. H., et al. (2009). Carcinoembryonic cell adhesion molecule 6 in human lung: regulated expression of a multifunctional type II cell protein. Am. J. Physiol. Lung Cell. Mol. Physiol. 296, L1019–L1030. doi: 10.1152/ajplung.90596.2008
Kondo, K., Ikeda, M., Kajio, Y., Saito, T., Iwayama, Y., Aleksic, B., et al. (2013). Genetic variants on 3q21 and in the Sp8 transcription factor gene (SP8) as susceptibility loci for psychotic disorders: a genetic association study. PLoS ONE 8:e70964. doi: 10.1371/journal.pone.0070964
Konradi, C., Yang, C. K., Zimmerman, E. I., Lohmann, K. M., Gresch, P., Pantazopoulos, H., et al. (2011). Hippocampal interneurons are abnormal in schizophrenia. Schizophr. Res. 131, 165–173. doi: 10.1016/j.schres.2011.06.007
Kouidrat, Y., Amad, A., Lalau, J. D., and Loas, G. (2014). Eating disorders in schizophrenia: implications for research and management. Schizophr. Res. Treat. 2014:791573. doi: 10.1155/2014/791573
Koychev, I., El-Deredy, W., and Deakin, J. F. (2011). New visual information processing abnormality biomarker for the diagnosis of Schizophrenia. Expert Opin. Med. Diagn. 5, 357–368. doi: 10.1517/17530059.2011.586029
Krude, H., Schutz, B., Biebermann, H., von Moers, A., Schnabel, D., Neitzel, H., et al. (2002). Choreoathetosis, hypothyroidism, and pulmonary alterations due to human NKX2-1 haploinsufficiency. J. Clin. Invest. 109, 475–480. doi: 10.1172/JCI0214341
Labad, J., Barbero, J. D., Gutierrez-Zotes, A., Montalvo, I., Creus, M., Cabezas, A., et al. (2016). Free thyroxine levels are associated with cognitive changes in individuals with a first episode of psychosis: a prospective 1-year follow-up study. Schizophr. Res. 171, 182–186. doi: 10.1016/j.schres.2016.01.036
Lavdas, A. A., Grigoriou, M., Pachnis, V., and Parnavelas, J. G. (1999). The medial ganglionic eminence gives rise to a population of early neurons in the developing cerebral cortex. J. Neurosci. 19, 7881–7888.
Lawrence, J. J. (2008). Cholinergic control of GABA release: emerging parallels between neocortex and hippocampus. Trends Neurosci. 31, 317–327. doi: 10.1016/j.tins.2008.03.008
Lechan, R. M., and Fekete, C. (2007). Infundibular tanycytes as modulators of neuroendocrine function: hypothetical role in the regulation of the thyroid and gonadal axis. Acta Biomed. 78(Suppl. 1), 84–98.
Lee, B. J., Cho, G. J., Norgren, R. B. Jr., Junier, M. P., Hill, D. F., Tapia, V., et al. (2001). TTF-1, a homeodomain gene required for diencephalic morphogenesis, is postnatally expressed in the neuroendocrine brain in a developmentally regulated and cell-specific fashion. Mol. Cell. Neurosci. 17, 107–126. doi: 10.1006/mcne.2000.0933
Lee, G., and Gammie, S. C. (2009). GABA(A) receptor signaling in the lateral septum regulates maternal aggression in mice. Behav. Neurosci. 123, 1169–1177. doi: 10.1037/a0017535
Lee, J. S., Park, G., Song, M. J., Choi, K. H., and Lee, S. H. (2016). Early visual processing for low spatial frequency fearful face is correlated with cortical volume in patients with schizophrenia. Neuropsychiatr. Dis. Treat. 12, 1–14. doi: 10.2147/NDT.S97089
Lee, N. O., Son, Y. J., Kim, J. G., Ha, C. M., Yun, C. H., Lim, H. L., et al. (2007). TTF-1 regulates growth hormone and prolactin transcription in the anterior pituitary gland. Biochem. Biophys. Res. Commun. 362, 193–199. doi: 10.1016/j.bbrc.2007.08.009
Legros, J. J., Gazzotti, C., Carvelli, T., Franchimont, P., Timsit-Berthier, M., von Frenckell, R., et al. (1992). Apomorphine stimulation of vasopressin- and oxytocin-neurophysins. Evidence for increased oxytocinergic and decreased vasopressinergic function in schizophrenics. Psychoneuroendocrinology 17, 611–617. doi: 10.1016/0306-4530(92)90019-4
Lemkine, G. F., Raj, A., Alfama, G., Turque, N., Hassani, Z., Alegria-Prevot, O., et al. (2005). Adult neural stem cell cycling in vivo requires thyroid hormone and its alpha receptor. FASEB J. 19, 863–865. doi: 10.1096/fj.04-2916fje
Lennertz, L., Quednow, B. B., Benninghoff, J., Wagner, M., Maier, W., and Mossner, R. (2011). Impact of TCF4 on the genetics of schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 261(Suppl. 2), S161–S165. doi: 10.1007/s00406-011-0256-9
Leon, T. Y., Ngan, E. S., Poon, H. C., So, M. T., Lui, V. C., Tam, P. K., et al. (2009). Transcriptional regulation of RET by Nkx2-1, Phox2b, Sox10, and Pax3. J. Pediatr. Surg. 44, 1904–1912. doi: 10.1016/j.jpedsurg.2008.11.055
Leonard, S., Gault, J., Hopkins, J., Logel, J., Vianzon, R., Short, M., et al. (2002). Association of promoter variants in the alpha7 nicotinic acetylcholine receptor subunit gene with an inhibitory deficit found in schizophrenia. Arch. Gen. Psychiatry 59, 1085–1096. doi: 10.1001/archpsyc.59.12.1085
Lewis, D. A., Hashimoto, T., and Morris, H. M. (2008). Cell and receptor type-specific alterations in markers of GABA neurotransmission in the prefrontal cortex of subjects with schizophrenia. Neurotox. Res. 14, 237–248. doi: 10.1007/BF03033813
Li, C., Li, A., Xing, Y., Li, M., Chan, B., Ouyang, R., et al. (2013). Apc deficiency alters pulmonary epithelial cell fate and inhibits Nkx2.1 via triggering TGF-beta signaling. Dev. Biol. 378, 13–24. doi: 10.1016/j.ydbio.2013.03.018
Li, C., Ling, X., Yuan, B., and Minoo, P. (2000). A novel DNA element mediates transcription of Nkx2.1 by Sp1 and Sp3 in pulmonary epithelial cells. Biochim. Biophys. Acta 1490, 213–224. doi: 10.1016/S0167-4781(99)00183-9
Li, C., Zhu, N. L., Tan, R. C., Ballard, P. L., Derynck, R., and Minoo, P. (2002). Transforming growth factor-beta inhibits pulmonary surfactant protein B gene transcription through SMAD3 interactions with NKX2.1 and HNF-3 transcription factors. J. Biol. Chem. 277, 38399–38408. doi: 10.1074/jbc.M203188200
Li, T., Zeng, Z., Zhao, Q., Wang, T., Huang, K., Li, J., et al. (2013). FoxP2 is significantly associated with schizophrenia and major depression in the Chinese Han population. World J. Biol. Psychiatry 14, 146–150. doi: 10.3109/15622975.2011.615860
Lin, A., Kenis, G., Bignotti, S., Tura, G. J., Jong De, R., Bosmans, E., et al. (1998). The inflammatory response system in treatment-resistant schizophrenia: increased serum interleukin-6. Schizophr. Res. 32, 9–15. doi: 10.1016/S0920-9964(98)00034-6
Liu, D., Yi, M., Smith, M., and Mendelson, C. R. (2008). TTF-1 response element is critical for temporal and spatial regulation and necessary for hormonal regulation of human surfactant protein-A2 promoter activity. Am. J. Physiol. Lung Cell Mol. Physiol. 295, L264–L271. doi: 10.1152/ajplung.00069.2008
Lu, C. B., and Henderson, Z. (2010). Nicotine induction of theta frequency oscillations in rodent hippocampus in vitro. Neuroscience 166, 84–93. doi: 10.1016/j.neuroscience.2009.11.072
Luo, X., Huang, L., Han, L., Luo, Z., Hu, F., Tieu, R., et al. (2014). Systematic prioritization and integrative analysis of copy number variations in schizophrenia reveal key schizophrenia susceptibility genes. Schizophr. Bull. 40, 1285–1299. doi: 10.1093/schbul/sbu045
Macova, M., Pavel, J., and Saavedra, J. M. (2009). A peripherally administered, centrally acting angiotensin II AT2 antagonist selectively increases brain AT1 receptors and decreases brain tyrosine hydroxylase transcription, pituitary vasopressin and ACTH. Brain Res. 1250, 130–140. doi: 10.1016/j.brainres.2008.11.006
Maeno, N., Takahashi, N., Saito, S., Ji, X., Branko, A., Ishihara, R., et al. (2007a). Association study between the transferrin gene and schizophrenia in the Japanese population. Neuroreport 18, 517–520. doi: 10.1097/WNR.0b013e3280586890
Maeno, N., Takahashi, N., Saito, S., Ji, X., Ishihara, R., Aoyama, N., et al. (2007b). Association of SOX10 with schizophrenia in the Japanese population. Psychiatr. Genet. 17, 227–231. doi: 10.1097/YPG.0b013e3280ae6cd8
Maes, M., Bosmans, E., Ranjan, R., Vandoolaeghe, E., Meltzer, H. Y., De Ley, M., et al. (1996a). Lower plasma CC16, a natural anti-inflammatory protein, and increased plasma interleukin-1 receptor antagonist in schizophrenia: effects of antipsychotic drugs. Schizophr. Res. 21, 39–50. doi: 10.1016/0920-9964(96)00029-1
Maes, M., De Meester, I., Scharpe, S., Desnyder, R., Ranjan, R., and Meltzer, H. Y. (1996b). Alterations in plasma dipeptidyl peptidase IV enzyme activity in depression and schizophrenia: effects of antidepressants and antipsychotic drugs. Acta Psychiatr. Scand. 93, 1–8. doi: 10.1111/j.1600-0447.1996.tb10612.x
Magno, L., Catanzariti, V., Nitsch, R., Krude, H., and Naumann, T. (2009). Ongoing expression of Nkx2.1 in the postnatal mouse forebrain: potential for understanding NKX2.1 haploinsufficiency in humans? Brain Res. 1304, 164–186. doi: 10.1016/j.brainres.2009.09.050
Magno, L., Kretz, O., Bert, B., Ersozlu, S., Vogt, J., Fink, H., et al. (2011). The integrity of cholinergic basal forebrain neurons depends on expression of Nkx2-1. Eur. J. Neurosci. 34, 1767–1782. doi: 10.1111/j.1460-9568.2011.07890.x
Malik, P., Kemmler, G., Hummer, M., Riecher-Roessler, A., Kahn, R. S., and Fleischhacker, W. W. (2011). Sexual dysfunction in first-episode schizophrenia patients: results from European first episode schizophrenia trial. J. Clin. Psychopharmacol. 31, 274–280. doi: 10.1097/JCP.0b013e3182199bcc
Mallet, N., Le Moine, C., Charpier, S., and Gonon, F. (2005). Feedforward inhibition of projection neurons by fast-spiking GABA interneurons in the rat striatum in vivo. J. Neurosci. 25, 3857–3869. doi: 10.1523/JNEUROSCI.5027-04.2005
Manoach, D. S., Demanuele, C., Wamsley, E. J., Vangel, M., Montrose, D. M., Miewald, J., et al. (2014). Sleep spindle deficits in antipsychotic-naïve early course schizophrenia and in non-psychotic first-degree relatives. Front. Hum. Neurosci. 8:762. doi: 10.3389/fnhum.2014.00762
Maouche, K., Polette, M., Jolly, T., Medjber, K., Cloez-Tayarani, I., Changeux, J. P., et al. (2009). {alpha}7 nicotinic acetylcholine receptor regulates airway epithelium differentiation by controlling basal cell proliferation. Am. J. Pathol. 175, 1868–1882. doi: 10.2353/ajpath.2009.090212
Margana, R., Berhane, K., Alam, M. N., and Boggaram, V. (2000). Identification of functional TTF-1 and Sp1/Sp3 sites in the upstream promoter region of rabbit SP-B gene. Am. J. Physiol. Lung Cell Mol. Physiol. 278, L477–L484.
Marin, O., Anderson, S. A., and Rubenstein, J. L. (2000). Origin and molecular specification of striatal interneurons. J. Neurosci. 20, 6063–6076.
Markham, J. A. (2012). Sex steroids and schizophrenia. Rev. Endocr. Metab. Disord. 13, 187–207. doi: 10.1007/s11154-011-9184-2
Maroof, A. M., Keros, S., Tyson, J. A., Ying, S. W., Ganat, Y. M., Merkle, F. T., et al. (2013). Directed differentiation and functional maturation of cortical interneurons from human embryonic stem cells. Cell Stem Cell 12, 559–572. doi: 10.1016/j.stem.2013.04.008
Marrone, M. C., Marinelli, S., Biamonte, F., Keller, F., Sgobio, C. A., Ammassari-Teule, M., et al. (2006). Altered cortico-striatal synaptic plasticity and related behavioural impairments in reeler mice. Eur. J. Neurosci. 24, 2061–2070. doi: 10.1111/j.1460-9568.2006.05083.x
Marshall, C. A., and Goldman, J. E. (2002). Subpallial dlx2-expressing cells give rise to astrocytes and oligodendrocytes in the cerebral cortex and white matter. J. Neurosci. 22, 9821–9830.
Martins-de-Souza, D., Gattaz, W. F., Schmitt, A., Rewerts, C., Maccarrone, G., Dias-Neto, E., et al. (2009a). Prefrontal cortex shotgun proteome analysis reveals altered calcium homeostasis and immune system imbalance in schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 259, 151–163. doi: 10.1007/s00406-008-0847-2
Martins-de-Souza, D., Gattaz, W. F., Schmitt, A., Rewerts, C., Marangoni, S., Novello, J. C., et al. (2009b). Alterations in oligodendrocyte proteins, calcium homeostasis and new potential markers in schizophrenia anterior temporal lobe are revealed by shotgun proteome analysis. J. Neural Transm. 116, 275–289. doi: 10.1007/s00702-008-0156-y
Mastronardi, C., Smiley, G. G., Raber, J., Kusakabe, T., Kawaguchi, A., Matagne, V., et al. (2006). Deletion of the Ttf1 gene in differentiated neurons disrupts female reproduction without impairing basal ganglia function. J. Neurosci. 26, 13167–13179. doi: 10.1523/JNEUROSCI.4238-06.2006
Matagne, V., Kim, J. G., Ryu, B. J., Hur, M. K., Kim, M. S., Kim, K., et al. (2012). Thyroid transcription factor 1, a homeodomain containing transcription factor, contributes to regulating periodic oscillations in GnRH gene expression. J. Neuroendocrinol. 24, 916–929. doi: 10.1111/j.1365-2826.2012.02302.x
Maurano, M. T., Humbert, R., Rynes, E., Thurman, R. E., Haugen, E., Wang, H., et al. (2012). Systematic localization of common disease-associated variation in regulatory DNA. Science 337, 1190–1195. doi: 10.1126/science.1222794
McDonald, A. J., Muller, J. F., and Mascagni, F. (2011). Postsynaptic targets of GABAergic basal forebrain projections to the basolateral amygdala. Neuroscience 183:144–159. doi: 10.1016/j.neuroscience.2011.03.027
McNeil, T. F., Cantor-Graae, E., and Blennow, G. (2003). Mental correlates of neuromotoric deviation in 6-year-olds at heightened risk for schizophrenia. Schizophr. Res. 60, 219–228. doi: 10.1016/S0920-9964(02)00235-9
Melicher, T., Horacek, J., Hlinka, J., Spaniel, F., Tintera, J., Ibrahim, I., et al. (2015). White matter changes in first episode psychosis and their relation to the size of sample studied: a DTI study. Schizophr. Res. 162, 22–28. doi: 10.1016/j.schres.2015.01.029
Mercedes Perez-Rodriguez, M., Mahon, K., Russo, M., Ungar, A. K., and Burdick, K. E. (2015). Oxytocin and social cognition in affective and psychotic disorders. Eur. Neuropsychopharmacol. 25, 265–282. doi: 10.1016/j.euroneuro.2014.07.012
Mikesell, M. J., Sobell, J. L., Sommer, S. S., and McMurray, C. T. (1996). Identification of a missense mutation and several polymorphisms in the proenkephalin A gene of schizophrenic patients. Am. J. Med. Genet. 67, 459–467.
Miller, C. L. (2013). Evidence for phenotypic plasticity in response to photic cues and the connection with genes of risk in schizophrenia. Front. Behav. Neurosci. 7:82. doi: 10.3389/fnbeh.2013.00082
Minoo, P., Hu, L., Zhu, N., Borok, Z., Bellusci, S., Groffen, J., et al. (2008). SMAD3 prevents binding of NKX2.1 and FOXA1 to the SpB promoter through its MH1 and MH2 domains. Nucleic Acids Res. 36, 179–188. doi: 10.1093/nar/gkm871
Mitchell, A. J., Vancampfort, D., Sweers, K., van Winkel, R., Yu, W., and De Hert, M. (2013). Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders–a systematic review and meta-analysis. Schizophr. Bull. 39, 306–318. doi: 10.1093/schbul/sbr148
Moeller, L. C., Kimura, S., Kusakabe, T., Liao, X. H., Van Sande, J., and Refetoff, S. (2003). Hypothyroidism in thyroid transcription factor 1 haploinsufficiency is caused by reduced expression of the thyroid-stimulating hormone receptor. Mol. Endocrinol. 17, 2295–2302. doi: 10.1210/me.2003-0175
Mohacsik, P., Zeold, A., Bianco, A. C., and Gereben, B. (2011). Thyroid hormone and the neuroglia: both source and target. J. Thyroid Res. 2011:215718. doi: 10.4061/2011/215718
Monteleone, P., Maj, M., Fusco, M., Kemali, D., and Reiter, R. J. (1992). Depressed nocturnal plasma melatonin levels in drug-free paranoid schizophrenics. Schizophr. Res. 7, 77–84. doi: 10.1016/0920-9964(92)90077-I
Monteleone, P., Natale, M., La Rocca, A., and Maj, M. (1997). Decreased nocturnal secretion of melatonin in drug-free schizophrenics: no change after subchronic treatment with antipsychotics. Neuropsychobiology 36, 159–163. doi: 10.1159/000119377
Monti, S., Nicoletti, A., Cantasano, A., Krude, H., and Cassio, A. (2015). NKX2.1-Related Disorders: a novel mutation with mild clinical presentation. Ital. J. Pediatr. 41:45. doi: 10.1186/s13052-015-0150-6
Morris, H. M., Hashimoto, T., and Lewis, D. A. (2008). Alterations in somatostatin mRNA expression in the dorsolateral prefrontal cortex of subjects with schizophrenia or schizoaffective disorder. Cereb. Cortex 18, 1575–1587. doi: 10.1093/cercor/bhm186
Morris, N. P., and Henderson, Z. (2000). Perineuronal nets ensheath fast spiking, parvalbumin-immunoreactive neurons in the medial septum/diagonal band complex. Eur. J. Neurosci. 12, 828–838. doi: 10.1046/j.1460-9568.2000.00970.x
Mulle, J. G. (2012). Schizophrenia genetics: progress, at last. Curr. Opin. Genet. Dev. 22, 238–244. doi: 10.1016/j.gde.2012.02.011
Müller, N., Weidinger, E., Leitner, B., and Schwarz, M. J. (2015). The role of inflammation in schizophrenia. Front. Neurosci. 9:372. doi: 10.3389/fnins.2015.00372
Muller-Spahn, F., Modell, S., Ackenheil, M., Brachner, A., and Kurtz, G. (1998). Elevated response of growth hormone to graded doses of apomorphine in schizophrenic patients. J. Psychiatr. Res. 32, 265–271. doi: 10.1016/S0022-3956(98)00005-3
Nakamura, K., Kimura, S., Yamazaki, M., Kawaguchi, A., Inoue, K., and Sakai, T. (2001). Immunohistochemical analyses of thyroid-specific enhancer-binding protein in the fetal and adult rat hypothalami and pituitary glands. Brain Res. Dev. Brain Res. 130, 159–166. doi: 10.1016/S0165-3806(01)00226-7
Nakazawa, K., Zsiros, V., Jiang, Z., Nakao, K., Kolata, S., Zhang, S., et al. (2012). GABAergic interneuron origin of schizophrenia pathophysiology. Neuropharmacology 62, 1574–1583. doi: 10.1016/j.neuropharm.2011.01.022
Naltner, A., Ghaffari, M., Whitsett, J. A., and Yan, C. (2000). Retinoic acid stimulation of the human surfactant protein B promoter is thyroid transcription factor 1 site-dependent. J. Biol. Chem. 275, 56–62. doi: 10.1074/jbc.275.1.56
Nishino, S., Ripley, B., Mignot, E., Benson, K. L., and Zarcone, V. P. (2002). CSF hypocretin-1 levels in schizophrenics and controls: relationship to sleep architecture. Psychiatry Res. 110, 1–7. doi: 10.1016/S0165-1781(02)00032-X
Nobrega-Pereira, S., Gelman, D., Bartolini, G., Pla, R., Pierani, A., and Marin, O. (2010). Origin and molecular specification of globus pallidus neurons. J. Neurosci. 30, 2824–2834. doi: 10.1523/JNEUROSCI.4023-09.2010
Novejarque, A., Gutierrez-Castellanos, N., Lanuza, E., and Martinez-Garcia, F. (2011). Amygdaloid projections to the ventral striatum in mice: direct and indirect chemosensory inputs to the brain reward system. Front. Neuroanat. 5:54. doi: 10.3389/fnana.2011.00054
Nullmeier, S., Panther, P., Dobrowolny, H., Frotscher, M., Zhao, S., Schwegler, H., et al. (2011). Region-specific alteration of GABAergic markers in the brain of heterozygous reeler mice. Eur. J. Neurosci. 33, 689–698. doi: 10.1111/j.1460-9568.2010.07563.x
Nunez, D., Oelkers-Ax, R., de Haan, S., Ludwig, M., Sattel, H., Resch, F., et al. (2014). Do deficits in the magnocellular priming underlie visual derealization phenomena? Preliminary neurophysiological and self-report results in first-episode schizophrenia patients. Schizophr. Res. 159, 441–449. doi: 10.1016/j.schres.2014.08.019
Oerbeck, B., Reinvang, I., Sundet, K., and Heyerdahl, S. (2007). Young adults with severe congenital hypothyroidism: cognitive event related potentials (ERPs) and the significance of an early start of thyroxine treatment. Scand. J. Psychol. 48, 61–67. doi: 10.1111/j.1467-9450.2006.00545.x
Ojeda, S. R., Ma, Y. J., Lee, B. J., and Prevot, V. (2000). Glia-to-neuron signaling and the neuroendocrine control of female puberty. Recent Prog. Horm. Res. 55:197–223. doi: 10.1080/07853890310005164
Ojeda, S. R., Roth, C., Mungenast, A., Heger, S., Mastronardi, C., Parent, A. S., et al. (2006). Neuroendocrine mechanisms controlling female puberty: new approaches, new concepts. Int. J. Androl 29, 256–263. doi: 10.1111/j.1365-2605.2005.00619.x
Olsson, E., Wiesel, F. A., Bjerkenstedt, L., and Venizelos, N. (2006). Tyrosine transport in fibroblasts from healthy volunteers and patients with schizophrenia. Neurosci. Lett. 393, 211–215. doi: 10.1016/j.neulet.2005.09.070
Partti, K., Heliovaara, M., Impivaara, O., Perala, J., Saarni, S. I., Lönnqvist, J., et al. (2010). Skeletal status in psychotic disorders: a population-based study. Psychosom. Med. 72, 933–940. doi: 10.1097/PSY.0b013e3181f7abd3
Partti, K., Vasankari, T., Kanervisto, M., Perala, J., Saarni, S. I., Jousilahti, P., et al. (2015). Lung function and respiratory diseases in people with psychosis: population-based study. Br. J. Psychiatry 207, 37–45. doi: 10.1192/bjp.bp.113.141937
Paul-Samojedny, M., Owczarek, A., Kowalczyk, M., Suchanek, R., Palacz, M., Kucia, K., et al. (2013). Association of interleukin 2 (IL-2), interleukin 6 (IL-6), and TNF-alpha (TNFalpha) gene polymorphisms with paranoid schizophrenia in a Polish population. J. Neuropsychiatry Clin. Neurosci. 25, 72–82. doi: 10.1176/appi.neuropsych.12020021
Pauly, M. C., Dobrossy, M. D., Nikkhah, G., Winkler, C., and Piroth, T. (2013). Organization of the human fetal subpallium. Front. Neuroanat. 7:54. doi: 10.3389/fnana.2013.00054
Peall, K. J., and Kurian, M. A. (2015). Benign hereditary chorea: an update. Tremor Other Hyperkinet. Mov. (N.Y). 5:314. doi: 10.7916/D8RJ4HM5
Perrone, L., Tell, G., and Lauro Di, R. (1999). Calreticulin enhances the transcriptional activity of thyroid transcription factor-1 by binding to its homeodomain. J. Biol. Chem. 274, 4640–4645. doi: 10.1074/jbc.274.8.4640
Peupelmann, J., Quick, C., Berger, S., Hocke, M., Tancer, M. E., Yeragani, V. K., et al. (2009). Linear and non-linear measures indicate gastric dysmotility in patients suffering from acute schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 33, 1236–1240. doi: 10.1016/j.pnpbp.2009.07.007
Pilhatsch, M., Marxen, M., Winter, C., Smolka, M. N., and Bauer, M. (2011). Hypothyroidism and mood disorders: integrating novel insights from brain imaging techniques. Thyroid Res. 4(Suppl. 1):S3. doi: 10.1186/1756-6614-4-S1-S3
Pinacho, R., Villalmanzo, N., Roca, M., Iniesta, R., Monje, A., Haro, J., et al. (2013). Analysis of Sp transcription factors in the postmortem brain of chronic schizophrenia: a pilot study of relationship to negative symptoms. J. Psychiatr. Res. 47, 926–934. doi: 10.1016/j.jpsychires.2013.03.004
Prestia, A., Cavedo, E., Boccardi, M., Muscio, C., Adorni, A., Geroldi, C., et al. (2015). Hippocampal and amygdalar local structural differences in elderly patients with schizophrenia. Am. J. Geriatr. Psychiatry 23, 47–58. doi: 10.1016/j.jagp.2014.01.006
Provenzano, C., Pascucci, B., Lupari, E., and Civitareale, D. (2010). Large scale analysis of transcription factor TTF-1/NKX2.1 target genes in GnRH secreting cell line GT1-7. Mol. Cell Endocrinol. 323, 215–223. doi: 10.1016/j.mce.2010.02.038
Quednow, B. B., Brinkmeyer, J., Mobascher, A., Nothnagel, M., Musso, F., Grunder, G., et al. (2012). Schizophrenia risk polymorphisms in the TCF4 gene interact with smoking in the modulation of auditory sensory gating. Proc. Natl. Acad. Sci. U.S.A. 109, 6271–6276. doi: 10.1073/pnas.1118051109
Rakic, S., and Zecevic, N. (2003a). Early oligodendrocyte progenitor cells in the human fetal telencephalon. Glia 41, 117–127. doi: 10.1002/glia.10140
Rakic, S., and Zecevic, N. (2003b). Emerging complexity of layer I in human cerebral cortex. Cereb. Cortex 13, 1072–1083. doi: 10.1093/cercor/13.10.1072
Ramanathan, S., Hanley, J. J., Deniau, J. M., and Bolam, J. P. (2002). Synaptic convergence of motor and somatosensory cortical afferents onto GABAergic interneurons in the rat striatum. J. Neurosci. 22, 8158–8169.
Ramchand, C. N., Clark, A. E., Ramchand, R., and Hemmings, G. P. (1995). Cultured human keratinocytes as a model for studying the dopamine metabolism in schizophrenia. Med. Hypotheses 44, 53–57. doi: 10.1016/0306-9877(95)90302-X
Rao, M. L., Gross, G., Strebel, B., Halaris, A., Huber, G., Braunig, P., et al. (1994). Circadian rhythm of tryptophan, serotonin, melatonin, and pituitary hormones in schizophrenia. Biol. Psychiatry 35, 151–163. doi: 10.1016/0006-3223(94)91147-9
Raskind, M. A., Courtney, N., Murburg, M. M., Backus, F. I., Bokan, J. A., Ries, R. K., et al. (1987). Antipsychotic drugs and plasma vasopressin in normals and acute schizophrenic patients. Biol. Psychiatry 22, 453–462. doi: 10.1016/0006-3223(87)90167-3
Ray, M. K., Chen, C. Y., Schwartz, R. J., and DeMayo, F. J. (1996). Transcriptional regulation of a mouse Clara cell-specific protein (mCC10) gene by the NKx transcription factor family members thyroid transciption factor 1 and cardiac muscle-specific homeobox protein (CSX). Mol. Cell. Biol. 16, 2056–2064. doi: 10.1128/MCB.16.5.2056
Reif, A., Herterich, S., Strobel, A., Ehlis, A. C., Saur, D., Jacob, C. P., et al. (2006). A neuronal nitric oxide synthase (NOS-I) haplotype associated with schizophrenia modifies prefrontal cortex function. Mol. Psychiatry 11, 286–300. doi: 10.1038/sj.mp.4001779
Renn, J. H., Yang, N. P., Chueh, C. M., Lin, C. Y., Lan, T. H., and Chou, P. (2009). Bone mass in schizophrenia and normal populations across different decades of life. BMC. Musculoskelet. Disord. 10:1. doi: 10.1186/1471-2474-10-1
Reynolds, G. P., Abdul-Monim, Z., Neill, J. C., and Zhang, Z. J. (2004). Calcium binding protein markers of GABA deficits in schizophrenia–postmortem studies and animal models. Neurotox. Res. 6, 57–61. doi: 10.1007/BF03033297
Reynolds, P. R., and Hoidal, J. R. (2005). Temporal-spatial expression and transcriptional regulation of alpha7 nicotinic acetylcholine receptor by thyroid transcription factor-1 and early growth response factor-1 during murine lung development. J. Biol. Chem. 280, 32548–32554. doi: 10.1074/jbc.M502231200
Reynolds, P. R., Mucenski, M. L., and Whitsett, J. A. (2003). Thyroid transcription factor (TTF) -1 regulates the expression of midkine (MK) during lung morphogenesis. Dev. Dyn. 227, 227–237. doi: 10.1002/dvdy.10304
Rich, M. E., and Caldwell, H. K. (2015). A role for oxytocin in the etiology and treatment of schizophrenia. Front. Endocrinol. 6:90. doi: 10.3389/fendo.2015.00090
Rioux, L., and Arnold, S. E. (2005). The expression of retinoic acid receptor alpha is increased in the granule cells of the dentate gyrus in schizophrenia. Psychiatry Res. 133, 13–21. doi: 10.1016/j.psychres.2004.11.003
Ripova, D., Strunecka, A., Nemcova, V., and Farska, I. (1997). Phospholipids and calcium alterations in platelets of schizophrenic patients. Physiol. Res. 46, 59–68.
Rosso, L., and Mienville, J. M. (2009). Pituicyte modulation of neurohormone output. Glia 57, 235–243. doi: 10.1002/glia.20760
Rubin, L. S., and Barry, T. J. (1976). Amplitude of pupillary contraction as a function of intensity of illumination in schizophrenia. Biol. Psychiatry 11, 267–282.
Ruiz, A., Blanco, R., Santander, J., and Miranda, E. (2000). Relationship between sex differences in onset of schizophrenia and puberty. J. Psychiatr. Res. 34, 349–353. doi: 10.1016/S0022-3956(00)00030-3
Ruzicka, W. B., Zhubi, A., Veldic, M., Grayson, D. R., Costa, E., and Guidotti, A. (2007). Selective epigenetic alteration of layer I GABAergic neurons isolated from prefrontal cortex of schizophrenia patients using laser-assisted microdissection. Mol. Psychiatry 12, 385–397. doi: 10.1038/sj.mp.4001954
Sakai, T., Oshima, A., Nozaki, Y., Ida, I., Haga, C., Akiyama, H., et al. (2008). Changes in density of calcium-binding-protein-immunoreactive GABAergic neurons in prefrontal cortex in schizophrenia and bipolar disorder. Neuropathology 28, 143–150. doi: 10.1111/j.1440-1789.2007.00867.x
Sanjuan, J., Tolosa, A., Gonzalez, J. C., Aguilar, E. J., Perez-Tur, J., Najera, C., et al. (2006). Association between FOXP2 polymorphisms and schizophrenia with auditory hallucinations. Psychiatr. Genet. 16, 67–72. doi: 10.1097/01.ypg.0000185029.35558.bb
Santos, N. C., Costa, P., Ruano, D., Macedo, A., Soares, M. J., Valente, J., et al. (2012). Revisiting thyroid hormones in schizophrenia. J. Thyroid Res. 2012:569147. doi: 10.1155/2012/569147
Sashkov, V. A., Dzhandarova, T. I., and Sel'verova, N. B. (2007). Interaction of total blood calcium levels and brain catecholamines during the formation and reinforcement of memory traces in hypoparathyroidism. Neurosci. Behav. Physiol. 37, 623–629. doi: 10.1007/s11055-007-0061-0
Satoh, M., Sakuda, H., Kobayashi, T., Kataoka, T., Nakao, F., and Turale, S. (2007). Comparison of the body fluid levels in healthy individuals and those with schizophrenia in Japan: using the bioelectrical impedance method. Nurs. Health Sci. 9, 177–184. doi: 10.1111/j.1442-2018.2007.00322.x
Sauer, J. F., Struber, M., and Bartos, M. (2015). Impaired fast-spiking interneuron function in a genetic mouse model of depression. Elife 4, 1–20. doi: 10.7554/eLife.04979
Schmidt, T. M., Chen, S. K., and Hattar, S. (2011). Intrinsically photosensitive retinal ganglion cells: many subtypes, diverse functions. Trends Neurosci. 34, 572–580. doi: 10.1016/j.tins.2011.07.001
Schmiedt, C., Brand, A., Hildebrandt, H., and Basar-Eroglu, C. (2005). Event-related theta oscillations during working memory tasks in patients with schizophrenia and healthy controls. Brain Res. Cogn. Brain Res. 25, 936–947. doi: 10.1016/j.cogbrainres.2005.09.015
Sekar, A., Bialas, A. R., de Rivera, H., Davis, A., Hammond, T. R., Kamitaki, N., et al. (2016). Schizophrenia risk from complex variation of complement component 4. Nature 530, 177–183. doi: 10.1038/nature16549
Selten, J. P., and Cantor-Graae, E. (2007). Hypothesis: social defeat is a risk factor for schizophrenia? Br. J. Psychiatry Suppl. 51, s9–s12. doi: 10.1192/bjp.191.51.s9
Selten, J. P., van der Ven, E., Rutten, B. P., and Cantor-Graae, E. (2013). The social defeat hypothesis of schizophrenia: an update. Schizophr. Bull. 39, 1180–1186. doi: 10.1093/schbul/sbt134
Semba, K. (2000). Multiple output pathways of the basal forebrain: organization, chemical heterogeneity, and roles in vigilance. Behav. Brain Res. 115, 117–141. doi: 10.1016/S0166-4328(00)00254-0
Shiloh, R., Weizman, A., Stryjer, R., Kahan, N., and Waitman, D. A. (2009). Altered thermoregulation in ambulatory schizophrenia patients: a naturalistic study. World J. Biol. Psychiatry 10, 163–170. doi: 10.1080/15622970701413833
Siapas, A. G., Lubenov, E. V., and Wilson, M. A. (2005). Prefrontal phase locking to hippocampal theta oscillations. Neuron 46, 141–151. doi: 10.1016/j.neuron.2005.02.028
Siegel, A. J. (2008). Hyponatremia in psychiatric patients: update on evaluation and management. Harv. Rev. Psychiatry 16, 13–24. doi: 10.1080/10673220801924308
Siegel, B. V. Jr., Buchsbaum, M. S., Bunney, W. E. Jr., Gottschalk, L. A., Haier, R. J., Lohr, J. B., et al. (1993). Cortical-striatal-thalamic circuits and brain glucose metabolic activity in 70 unmedicated male schizophrenic patients. Am. J. Psychiatry 150, 1325–1336. doi: 10.1176/ajp.150.9.1325
Smesny, S., Klemm, S., Stockebrand, M., Grunwald, S., Gerhard, U. J., Rosburg, T., et al. (2007). Endophenotype properties of niacin sensitivity as marker of impaired prostaglandin signalling in schizophrenia. Prostaglandins Leukot. Essent. Fatty Acids 77, 79–85. doi: 10.1016/j.plefa.2007.08.006
Son, Y. J., Hur, M. K., Ryu, B. J., Park, S. K., Damante, G., D'Elia, A. V., et al. (2003). TTF-1, a homeodomain-containing transcription factor, participates in the control of body fluid homeostasis by regulating angiotensinogen gene transcription in the rat subfornical organ. J. Biol. Chem. 278, 27043–27052. doi: 10.1074/jbc.M303157200
Son, Y. J., Park, J. W., and Lee, B. J. (2007). TTF-1 expression in PACAP-expressing retinal ganglion cells. Mol. Cells 23, 215–219.
Son, Y. J., Yun, C. H., Kim, J. G., Park, J. W., Kim, J. H., Kang, S. G., et al. (2009). Expression and role of TTF-1 in the rat suprachiasmatic nucleus. Biochem. Biophys. Res. Commun. 380, 559–563. doi: 10.1016/j.bbrc.2009.01.119
Soreni, N., Weizman, A., and Weiss, M. (2004). Beneficial effects of gonadotropin-releasing hormone analogue treatment on positive and negative symptoms of schizophrenia: a case report. J. Clin. Psychiatry 65, 1020–1021. doi: 10.4088/JCP.v65n0720f
Sotoyama, H., Namba, H., Chiken, S., Nambu, A., and Nawa, H. (2013). Exposure to the cytokine EGF leads to abnormal hyperactivity of pallidal GABA neurons: implications for schizophrenia and its modeling. J. Neurochem. 126, 518–528. doi: 10.1111/jnc.12223
Sotoyama, H., Zheng, Y., Iwakura, Y., Mizuno, M., Aizawa, M., Shcherbakova, K., et al. (2011). Pallidal hyperdopaminergic innervation underlying D2 receptor-dependent behavioral deficits in the schizophrenia animal model established by EGF. PLoS ONE 6:e25831. doi: 10.1371/journal.pone.0025831
Spaniel, F., Herynek, V., Hajek, T., Dezortova, M., Horacek, J., Hajek, M., et al. (2005). Magnetic resonance relaxometry in monozygotic twins discordant and concordant for schizophrenia. Eur. Psychiatry 20, 41–44. doi: 10.1016/j.eurpsy.2004.11.004
Sparkman, L., Chandru, H., and Boggaram, V. (2006). Ceramide decreases surfactant protein B gene expression via downregulation of TTF-1 DNA binding activity. Am. J. Physiol. Lung Cell. Mol. Physiol. 290, L351–L358. doi: 10.1152/ajplung.00275.2005
Spinks, R., Nopoulos, P., Ward, J., Fuller, R., Magnotta, V. A., and Andreasen, N. C. (2005). Globus pallidus volume is related to symptom severity in neuroleptic naive patients with schizophrenia. Schizophr. Res. 73, 229–233. doi: 10.1016/j.schres.2004.05.020
Spiteri, E., Konopka, G., Coppola, G., Bomar, J., Oldham, M., Ou, J., et al. (2007). Identification of the transcriptional targets of FOXP2, a gene linked to speech and language, in developing human brain. Am. J. Hum. Genet. 81, 1144–1157. doi: 10.1086/522237
Spiteri, T., Musatov, S., Ogawa, S., Ribeiro, A., Pfaff, D. W., and Agmo, A. (2010). The role of the estrogen receptor alpha in the medial amygdala and ventromedial nucleus of the hypothalamus in social recognition, anxiety and aggression. Behav. Brain Res. 210, 211–220. doi: 10.1016/j.bbr.2010.02.033
Steinecke, A., Gampe, C., Nitzsche, F., and Bolz, J. (2014). DISC1 knockdown impairs the tangential migration of cortical interneurons by affecting the actin cytoskeleton. Front. Cell. Neurosci. 8:190. doi: 10.3389/fncel.2014.00190
Stevens, H. E., Su, T., Yanagawa, Y., and Vaccarino, F. M. (2013). Prenatal stress delays inhibitory neuron progenitor migration in the developing neocortex. Psychoneuroendocrinology 38, 509–521. doi: 10.1016/j.psyneuen.2012.07.011
Stober, G., Syagailo, Y. V., Okladnova, O., Jungkunz, G., Knapp, M., Beckmann, H., et al. (1999). Functional PAX-6 gene-linked polymorphic region: potential association with paranoid schizophrenia. Biol. Psychiatry 45, 1585–1591. doi: 10.1016/S0006-3223(99)00024-4
Stoykova, A., Treichel, D., Hallonet, M., and Gruss, P. (2000). Pax6 modulates the dorsoventral patterning of the mammalian telencephalon. J. Neurosci. 20, 8042–8050.
Strauss, G. P., Keller, W. R., Koenig, J. I., Gold, J. M., Frost, K. H., and Buchanan, R. W. (2015). Plasma oxytocin levels predict social cue recognition in individuals with schizophrenia. Schizophr. Res. 162, 47–51. doi: 10.1016/j.schres.2015.01.034
Sussel, L., Marin, O., Kimura, S., and Rubenstein, J. L. (1999). Loss of Nkx2.1 homeobox gene function results in a ventral to dorsal molecular respecification within the basal telencephalon: evidence for a transformation of the pallidum into the striatum. Development 126, 3359–3370.
Suzuki, K., Kobayashi, Y., Katoh, R., Kohn, L. D., and Kawaoi, A. (1998a). Identification of thyroid transcription factor-1 in C cells and parathyroid cells. Endocrinology 139, 3014–3017. doi: 10.1210/endo.139.6.6126
Suzuki, K., Lavaroni, S., Mori, A., Okajima, F., Kimura, S., Katoh, R., et al. (1998b). Thyroid transcription factor 1 is calcium modulated and coordinately regulates genes involved in calcium homeostasis in C cells. Mol. Cell. Biol. 18, 7410–7422. doi: 10.1128/MCB.18.12.7410
Suzuki, K., Mori, A., Lavaroni, S., Ulianich, L., Miyagi, E., Saito, J., et al. (1999). Thyroglobulin regulates follicular function and heterogeneity by suppressing thyroid-specific gene expression. Biochimie 81, 329–340. doi: 10.1016/S0300-9084(99)80078-9
Suzuki, M., Katagiri, N., Ueda, M., and Tanaka, S. (2007). Functional analysis of Nkx2.1 and Pax9 for calcitonin gene transcription. Gen. Comp. Endocrinol. 152, 259–266. doi: 10.1016/j.ygcen.2007.02.017
Tagne, J. B., Gupta, S., Gower, A. C., Shen, S. S., Varma, S., Lakshminarayanan, M., et al. (2012). Genome-wide analyses of Nkx2-1 binding to transcriptional target genes uncover novel regulatory patterns conserved in lung development and tumors. PLoS ONE 7:e29907. doi: 10.1371/journal.pone.0029907
Takahashi, H., Sano, H., Chiba, H., and Kuroki, Y. (2006). Pulmonary surfactant proteins A and D: innate immune functions and biomarkers for lung diseases. Curr. Pharm. Des. 12, 589–598. doi: 10.2174/138161206775474387
Takao, T., Tachikawa, H., Kawanishi, Y., Mizukami, K., and Asada, T. (2007). CLOCK gene T3111C polymorphism is associated with Japanese schizophrenics: a preliminary study. Eur. Neuropsychopharmacol. 17, 273–276. doi: 10.1016/j.euroneuro.2006.09.002
Tanskanen, P., Haapea, M., Veijola, J., Miettunen, J., Jarvelin, M. R., Pyhtinen, J., et al. (2009). Volumes of brain, grey and white matter and cerebrospinal fluid in schizophrenia in the Northern Finland 1966 Birth Cohort: an epidemiological approach to analysis. Psychiatry Res. 174, 116–120. doi: 10.1016/j.pscychresns.2009.04.009
Tebar, M., Destree, O., de Vree, W. J., and Ten Have-Opbroek, A. A. (2001). Expression of Tcf/Lef and sFrp and localization of beta-catenin in the developing mouse lung. Mech. Dev. 109, 437–440. doi: 10.1016/S0925-4773(01)00556-1
Tepper, J. M., Wilson, C. J., and Koos, T. (2008). Feedforward and feedback inhibition in neostriatal GABAergic spiny neurons. Brain Res. Rev. 58, 272–281. doi: 10.1016/j.brainresrev.2007.10.008
Theodosis, D. T. (2002). Oxytocin-secreting neurons: a physiological model of morphological neuronal and glial plasticity in the adult hypothalamus. Front. Neuroendocrinol. 23, 101–135. doi: 10.1006/frne.2001.0226
Thome, J., Durany, N., Harsanyi, A., Foley, P., Palomo, A., Kornhuber, J., et al. (1996). A null mutation allele in the CNTF gene and schizophrenic psychoses. Neuroreport 7, 1413–1416. doi: 10.1097/00001756-199605310-00018
Tisch, S., Silberstein, P., Limousin-Dowsey, P., and Jahanshahi, M. (2004). The basal ganglia: anatomy, physiology, and pharmacology. Psychiatr. Clin. North Am. 27, 757–799. doi: 10.1016/j.psc.2004.06.004
Tooney, P. A., and Chahl, L. A. (2004). Neurons expressing calcium-binding proteins in the prefrontal cortex in schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 28, 273–278. doi: 10.1016/j.pnpbp.2003.10.004
Torigoe, M., Yamauchi, K., Zhu, Y., Kobayashi, H., and Murakami, F. (2015). Association of astrocytes with neurons and astrocytes derived from distinct progenitor domains in the subpallium. Sci. Rep. 5:12258. doi: 10.1038/srep12258
Tricoire, L., Pelkey, K. A., Erkkila, B. E., Jeffries, B. W., Yuan, X., and McBain, C. J. (2011). A blueprint for the spatiotemporal origins of mouse hippocampal interneuron diversity. J. Neurosci. 31, 10948–10970. doi: 10.1523/JNEUROSCI.0323-11.2011
Trotman, H. D., Holtzman, C. W., Ryan, A. T., Shapiro, D. I., Macdonald, A. N., Goulding, S. M., et al. (2013). The development of psychotic disorders in adolescence: a potential role for hormones. Horm. Behav. 64, 411–419. doi: 10.1016/j.yhbeh.2013.02.018
Uhlhaas, P. J., and Singer, W. (2011). The development of neural synchrony and large-scale cortical networks during adolescence: relevance for the pathophysiology of schizophrenia and neurodevelopmental hypothesis. Schizophr. Bull. 37, 514–523. doi: 10.1093/schbul/sbr034
Uhlhaas, P. J., Haenschel, C., Nikolic, D., and Singer, W. (2008). The role of oscillations and synchrony in cortical networks and their putative relevance for the pathophysiology of schizophrenia. Schizophr. Bull. 34, 927–943. doi: 10.1093/schbul/sbn062
Valcana, T., Einstein, E. R., Csejtey, J., Dalal, K. B., and Timiras, P. S. (1975). Influence of thyroid hormones on myelin proteins in the developing rat brain. J. Neurol. Sci. 25, 19–27. doi: 10.1016/0022-510X(75)90183-5
Van der Borght, K., Mulder, J., Keijser, J. N., Eggen, B. J., Luiten, P. G., and Van der Zee, E. A. (2005). Input from the medial septum regulates adult hippocampal neurogenesis. Brain Res. Bull. 67, 117–125. doi: 10.1016/j.brainresbull.2005.06.018
Velakoulis, D., Wood, S. J., Smith, D. J., Soulsby, B., Brewer, W., Leeton, L., et al. (2002). Increased duration of illness is associated with reduced volume in right medial temporal/anterior cingulate grey matter in patients with chronic schizophrenia. Schizophr. Res. 57, 43–49. doi: 10.1016/S0920-9964(01)00307-3
Velez, M. L., Costamagna, E., Kimura, E. T., Fozzatti, L., Pellizas, C. G., Montesinos, M. M., et al. (2006). Bacterial lipopolysaccharide stimulates the thyrotropin-dependent thyroglobulin gene expression at the transcriptional level by involving the transcription factors thyroid transcription factor-1 and paired box domain transcription factor 8. Endocrinology 147, 3260–3275. doi: 10.1210/en.2005-0789
Viollet, C., Lepousez, G., Loudes, C., Videau, C., Simon, A., and Epelbaum, J. (2008). Somatostatinergic systems in brain: networks and functions. Mol. Cell. Endocrinol. 286, 75–87. doi: 10.1016/j.mce.2007.09.007
Vloet, J. A., Herpertz-Dahlmann, B., Hahn, F., Hausler, M., and Holtkamp, K. (2010). [Schizophreniform symptoms in Chorea Minor–case report and literature review]. Z. Kinder Jugendpsychiatr. Psychother. 38, 161–168. doi: 10.1024/1422-4917/a000029
Volk, D. W., Edelson, J. R., and Lewis, D. A. (2014). Cortical inhibitory neuron disturbances in schizophrenia: role of the ontogenetic transcription factor Lhx6. Schizophr Bull. 40, 1053–1061. doi: 10.1093/schbul/sbu068
Volk, D. W., Matsubara, T., Li, S., Sengupta, E. J., Georgiev, D., Minabe, Y., et al. (2012). Deficits in transcriptional regulators of cortical parvalbumin neurons in schizophrenia. Am. J. Psychiatry 169, 1082–1091. doi: 10.1176/appi.ajp.2012.12030305
Waddington, L. E., Coutu, D. L., Cermakian, N., and Boivin, D. B. (2010). Circadian rhythms and clock genes in psychotic disorders. Isr. J. Psychiatry Relat. Sci. 47, 27–35. doi: 10.1254/jphs.fmj06003x5
Walker, E. F., Savoie, T., and Davis, D. (1994). Neuromotor precursors of schizophrenia. Schizophr. Bull. 20, 441–451. doi: 10.1093/schbul/20.3.441
Walker, E., and Bollini, A. M. (2002). Pubertal neurodevelopment and the emergence of psychotic symptoms. Schizophr. Res. 54, 17–23. doi: 10.1016/S0920-9964(01)00347-4
Wang, A. Y., Lohmann, K. M., Yang, C. K., Zimmerman, E. I., Pantazopoulos, H., Herring, N., et al. (2011). Bipolar disorder type 1 and schizophrenia are accompanied by decreased density of parvalbumin- and somatostatin-positive interneurons in the parahippocampal region. Acta Neuropathol. 122, 615–626. doi: 10.1007/s00401-011-0881-4
Wang, H., Lei, Q., Oosterveen, T., Ericson, J., and Matise, M. P. (2011). Tcf/Lef repressors differentially regulate Shh-Gli target gene activation thresholds to generate progenitor patterning in the developing CNS. Development 138, 3711–3721. doi: 10.1242/dev.068270
Wang, L., Lockstone, H. E., Guest, P. C., Levin, Y., Palotas, A., Pietsch, S., et al. (2010). Expression profiling of fibroblasts identifies cell cycle abnormalities in schizophrenia. J. Proteome Res. 9, 521–527. doi: 10.1021/pr900867x
Wang, R. Y., Chen, Y. F., and Yuan, J. L. (1997). [The analysis of schizophrenic inpatients' escape behavior]. Zhonghua Hu Li Za Zhi. 32, 630–632.
Warner, M. D., Sinha, Y. N., and Peabody, C. A. (1993). Potential abnormalities in molecular forms of growth hormone in schizophrenia: a pilot study. Biol. Psychiatry 34, 487–491. doi: 10.1016/0006-3223(93)90240-E
Wemeau, J. L., Proust-Lemoine, E., Ryndak, A., and Vanhove, L. (2013). Thyroid autoimmunity and polyglandular endocrine syndromes. Hormones.(Athens.) 12, 39–45.
Weston-Green, K., Huang, X. F., Lian, J., and Deng, C. (2012). Effects of olanzapine on muscarinic M3 receptor binding density in the brain relates to weight gain, plasma insulin and metabolic hormone levels. Eur. Neuropsychopharmacol. 22, 364–373. doi: 10.1016/j.euroneuro.2011.09.003
Whitty, P. F., Owoeye, O., and Waddington, J. L. (2009). Neurological signs and involuntary movements in schizophrenia: intrinsic to and informative on systems pathobiology. Schizophr. Bull. 35, 415–424. doi: 10.1093/schbul/sbn126
Wilkins, L. K., Girard, T. A., Konishi, K., King, M., Herdman, K. A., King, J., et al. (2013). Selective deficit in spatial memory strategies contrast to intact response strategies in patients with schizophrenia spectrum disorders tested in a virtual navigation task. Hippocampus. 23, 1015–1024. doi: 10.1002/hipo.22189
Williams, L. M., Das, P., Liddell, B. J., Olivieri, G., Peduto, A. S., David, A. S., et al. (2007). Fronto-limbic and autonomic disjunctions to negative emotion distinguish schizophrenia subtypes. Psychiatry Res. 155, 29–44. doi: 10.1016/j.pscychresns.2006.12.018
Wiltschko, A. B., Pettibone, J. R., and Berke, J. D. (2010). Opposite effects of stimulant and antipsychotic drugs on striatal fast-spiking interneurons. Neuropsychopharmacology 35, 1261–1270. doi: 10.1038/npp.2009.226
Winterer, G., Musso, F., Beckmann, C., Mattay, V., Egan, M. F., Jones, D. W., et al. (2006). Instability of prefrontal signal processing in schizophrenia. Am. J. Psychiatry 163, 1960–1968. doi: 10.1176/ajp.2006.163.11.1960
Winterer, G., Ziller, M., Dorn, H., Frick, K., Mulert, C., Wuebben, Y., et al. (2000). Schizophrenia: reduced signal-to-noise ratio and impaired phase-locking during information processing. Clin. Neurophysiol. 111, 837–849. doi: 10.1016/S1388-2457(99)00322-3
Wischhof, L., Irrsack, E., Osorio, C., and Koch, M. (2015). Prenatal LPS-exposure–a neurodevelopmental rat model of schizophrenia–differentially affects cognitive functions, myelination and parvalbumin expression in male and female offspring. Prog. Neuropsychopharmacol. Biol. Psychiatry 57, 17–30. doi: 10.1016/j.pnpbp.2014.10.004
Woo, T. U., Shrestha, K., Lamb, D., Minns, M. M., and Benes, F. M. (2008). N-methyl-D-aspartate receptor and calbindin-containing neurons in the anterior cingulate cortex in schizophrenia and bipolar disorder. Biol. Psychiatry 64, 803–809. doi: 10.1016/j.biopsych.2008.04.034
Wright, I. C., Rabe-Hesketh, S., Woodruff, P. W., David, A. S., Murray, R. M., and Bullmore, E. T. (2000). Meta-analysis of regional brain volumes in schizophrenia. Am. J. Psychiatry 157, 16–25. doi: 10.1176/ajp.157.1.16
Xu, Q., Inma, C., De La Cruz, E., Rubenstein, J. L., and Anderson, S. A. (2004). Origins of cortical interneuron subtypes. J. Neurosci. 24, 2612–2622. doi: 10.1523/JNEUROSCI.5667-03.2004
Xu, Q., Tam, M., and Anderson, S. A. (2008). Fate mapping Nkx2.1-lineage cells in the mouse telencephalon. J. Comp. Neurol. 506, 16–29. doi: 10.1002/cne.21529
Yamada, H., Ohji, T., Sakurai, S., Yamaguchi, E., Uchimura, N., Morita, K., et al. (2009). Chorea-acanthocytosis presenting with schizophrenia symptoms as first symptoms. Psychiatry Clin. Neurosci. 63, 253–254. doi: 10.1111/j.1440-1819.2008.01915.x
Yanagi, M., Joho, R. H., Southcott, S. A., Shukla, A. A., Ghose, S., and Tamminga, C. A. (2014). Kv3.1-containing K(+) channels are reduced in untreated schizophrenia and normalized with antipsychotic drugs. Mol. Psychiatry 19, 573–579. doi: 10.1038/mp.2013.49
Yang, H. Y., Sun, C. P., Jia, X. M., Gui, L., Zhu, D. F., and Ma, W. Q. (2012). Effect of thyroxine on SNARE complex and synaptotagmin-1 expression in the prefrontal cortex of rats with adult-onset hypothyroidism. J. Endocrinol. Invest. 35, 312–316. doi: 10.3275/7767
Yang, L., Yan, D., Bruggeman, M., Du, H., and Yan, C. (2004). Mutation of a lysine residue in a homeodomain generates dominant negative thyroid transcription factor 1. Biochemistry 43, 12489–12497. doi: 10.1021/bi049283o
Yao, J. K., and Keshavan, M. S. (2011). Antioxidants, redox signaling, and pathophysiology in schizophrenia: an integrative view. Antioxid. Redox Signal. 15, 2011–2035. doi: 10.1089/ars.2010.3603
Yee, C. L., Wang, Y., Anderson, S., Ekker, M., and Rubenstein, J. L. (2009). Arcuate nucleus expression of NKX2.1 and DLX and lineages expressing these transcription factors in neuropeptide Y(+), proopiomelanocortin(+), and tyrosine hydroxylase(+) neurons in neonatal and adult mice. J. Comp. Neurol. 517, 37–50. doi: 10.1002/cne.22132
Yi, M., Tong, G., X., Murry, B., and Mendelson, C. R. (2002). Role of CBP/p300 and SRC-1 in transcriptional regulation of the pulmonary surfactant protein-A (SP-A) gene by thyroid transcription factor-1 (TTF-1). J. Biol. Chem. 277, 2997–3005. doi: 10.1074/jbc.M109793200
Yin, D. M., Sun, X. D., Bean, J. C., Lin, T. W., Sathyamurthy, A., Xiong, W. C., et al. (2013). Regulation of spine formation by ErbB4 in PV-positive interneurons. J. Neurosci. 33, 19295–19303. doi: 10.1523/JNEUROSCI.2090-13.2013
Yoon, J. H., Minzenberg, M. J., Raouf, S., D'Esposito, M., and Carter, C. S. (2013). Impaired prefrontal-basal ganglia functional connectivity and substantia nigra hyperactivity in schizophrenia. Biol. Psychiatry 74, 122–129. doi: 10.1016/j.biopsych.2012.11.018
Yu, X., and Zecevic, N. (2011). Dorsal radial glial cells have the potential to generate cortical interneurons in human but not in mouse brain. J. Neurosci. 31, 2413–2420. doi: 10.1523/JNEUROSCI.5249-10.2011
Yuan, A., Li, W., Yu, T., Zhang, C., Wang, D., Liu, D., et al. (2013). SOX10 rs139883 polymorphism is associated with the age of onset in schizophrenia. J. Mol. Neurosci. 50, 333–338. doi: 10.1007/s12031-013-9982-y
Yuan, B., Li, C., Kimura, S., Engelhardt, R. T., Smith, B. R., and Minoo, P. (2000). Inhibition of distal lung morphogenesis in Nkx2.1(-/-) embryos. Dev. Dyn. 217, 180–190.
Yun, C. H., Kim, J. G., Park, B. S., Lee, H. M., Kim, D. H., Kim, E. O., et al. (2011). TTF-1 Action on the Transcriptional Regulation of Cyclooxygenase-2 Gene in the Rat Brain. PLoS ONE 6:e28959. doi: 10.1371/journal.pone.0028959
Zaborszky, L., Hoemke, L., Mohlberg, H., Schleicher, A., Amunts, K., and Zilles, K. (2008). Stereotaxic probabilistic maps of the magnocellular cell groups in human basal forebrain. Neuroimage 42, 1127–1141. doi: 10.1016/j.neuroimage.2008.05.055
Zembrzycki, A., Griesel, G., Stoykova, A., and Mansouri, A. (2007). Genetic interplay between the transcription factors Sp8 and Emx2 in the patterning of the forebrain. Neural Dev. 2:8. doi: 10.1186/1749-8104-2-8
Zhang, H., Fan, Y., Xia, F., Geng, C., Mao, C., Jiang, S., et al. (2011). Prenatal water deprivation alters brain angiotensin system and dipsogenic changes in the offspring. Brain Res. 1382, 128–136. doi: 10.1016/j.brainres.2011.01.031
Zhou, B., Zhong, Q., Minoo, P., Li, C., Ann, D. K., Frenkel, B., et al. (2008). Foxp2 inhibits Nkx2.1-mediated transcription of SP-C via interactions with the Nkx2.1 homeodomain. Am. J. Respir. Cell Mol. Biol. 38, 750–758. doi: 10.1165/rcmb.2007-0350OC
Zhou, H., Morotti, R. A., Profitt, S. A., Langston, C., Wert, S. E., Whitsett, J. A., et al. (2001). Expression of thyroid transcription factor-1, surfactant proteins, type I cell-associated antigen, and Clara cell secretory protein in pulmonary hypoplasia. Pediatr. Dev. Pathol. 4, 364–371. doi: 10.1007/s10024001-0002-9
Zhu, N. L., Li, C., Xiao, J., and Minoo, P. (2004). NKX2.1 regulates transcription of the gene for human bone morphogenetic protein-4 in lung epithelial cells. Gene 327, 25–36. doi: 10.1016/j.gene.2003.11.013
Zoabi, A., Ziv, M., Rozenman, D., and Lovoshitski, R. (2012). [Prevalence of thyroid abnormalities among psoriatic patients]. Harefuah 151, 566–566.
Zoeller, R. T., and Rovet, J. (2004). Timing of thyroid hormone action in the developing brain: clinical observations and experimental findings. J. Neuroendocrinol. 16, 809–818. doi: 10.1111/j.1365-2826.2004.01243.x
Keywords: brain development, GABA, NKX2-1, Schizophrenia, thyroid hormones, calcium, immune system, brain
Citation: Malt EA, Juhasz K, Malt UF and Naumann T (2016) A Role for the Transcription Factor Nk2 Homeobox 1 in Schizophrenia: Convergent Evidence from Animal and Human Studies. Front. Behav. Neurosci. 10:59. doi: 10.3389/fnbeh.2016.00059
Received: 17 December 2015; Accepted: 11 March 2016;
Published: 30 March 2016.
Edited by:
Allan V. Kalueff, ZENEREI Institute, USA, Guangdong Ocean University, China, St Petersburg State University, RussiaReviewed by:
Charles Patrick Gilman, Ripened Agrotech Inc., USAEvan Joseph Kyzar, University of Illinois at Chicago, USA
Copyright © 2016 Malt, Juhasz, Malt and Naumann. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eva A. Malt, ZW1hbEBhaHVzLm5v
 Eva A. Malt
Eva A. Malt Katalin Juhasz1
Katalin Juhasz1 Ulrik F. Malt
Ulrik F. Malt Thomas Naumann
Thomas Naumann