- 1Department of Psychology, State University of New York at Stony Brook, Stony Brook, NY, USA
- 2Department of Physiology & Pharmacology, School of Medicine, Oregon Health & Science University, Portland, OR, USA
- 3Laboratory of Neuroimaging, National Institute on Alcohol Abuse and Alcoholism, Bethesda, MD, USA
Studies have shown that exposure to chronic mild stress decreases ethanol intake and preference in dopamine D2 receptor wild-type mice (Drd2+/+), while it increases intake in heterozygous (Drd2+/−) and knockout (Drd2−/−) mice. Dopaminergic neurotransmission in the basal forebrain plays a major role in the reinforcing actions of ethanol as well as in brain responses to stress. In order to identify neurochemical changes associated with the regulation of ethanol intake, we used in vitro receptor autoradiography to measure the levels and distribution of dopamine D1 and D2 receptors and dopamine transporters (DAT). Receptor levels were measured in the basal forebrain of Drd2+/+, Drd2+/−, and Drd2−/− mice belonging to one of four groups: control (C), ethanol intake (E), chronic mild stress exposure (S), and ethanol intake under chronic mild stress (ES). D2 receptor levels were higher in the lateral and medial striatum of Drd2+/+ ES mice, compared with Drd2+/+ E mice. Ethanol intake in Drd2+/+ mice was negatively correlated with striatal D2 receptor levels. D2 receptor levels in Drd2+/− mice were the same among the four treatment groups. DAT levels were lower in Drd2+/− C and Drd2−/− C mice, compared with Drd2+/+ C mice. Among Drd2+/− mice, S and ES groups had higher DAT levels compared with C and E groups in most regions examined. In Drd2−/− mice, ethanol intake was positively correlated with DAT levels in all regions studied. D1 receptor levels were lower in Drd2+/− and Drd2−/− mice, compared with Drd2+/+, in all regions examined and remained unaffected by all treatments. The results suggest that in normal mice, ethanol intake is associated with D2 receptor-mediated neurotransmission, which exerts a protective effect against ethanol overconsumption under stress. In mice with low Drd2 expression, where DRD2 levels are not further modulated, ethanol intake is associated with DAT function which is upregulated under stress leading to ethanol overconsumption.
Introduction
Research shows that alcohol addiction has a strong genetic component shaped by many genes (for review see Enoch, 2014). Drd2 encodes for the dopamine D2 receptor protein and is one of these genes with a strong regulatory role on alcohol intake. In vivo, in vitro, preclinical, and animal studies have shown that alcoholism and ethanol (ETOH) consumption are negatively modulated by dopamine D2 receptors (Blum et al., 1990; Stefanini et al., 1992; McBride et al., 1993; Volkow et al., 1996, 2006; Thanos et al., 2001, 2005; Tupala et al., 2001). Due to the comorbid nature of the disease, alcohol-related studies often investigate the role of stress in the development of alcoholism either through direct, and reciprocal, interactions between stress experience and levels of alcohol intake (Anthenelli and Grandison, 2012) or through more complex interactions among stress, ETOH consumption, and specific genetic factors (Anthenelli, 2012).
We have previously shown that when normal, Drd2+/+, mice are exposed to chronic mild stress (CMS) they decrease their ETOH intake and preference. In contrast, Drd2+/− and Drd2−/− mice exposed to the same CMS protocol increase their ETOH intake and preference (Delis et al., 2013). Here we sought to study responses of the brain dopamine system relating to CMS and ETOH intake, as a function of Drd2 expression. To this purpose, we studied dopamine D1 and D2 receptor and dopamine transporter (DAT) levels in the basal forebrain of Drd2+/+, Drd2+/−, and Drd2−/− mice that were exposed to CMS, ETOH, or their combination. Our study showed that lower ETOH intake under CMS in Drd2+/+ mice was associated with increased D2 receptor levels in the striatum, in agreement with previous findings. In contrast, higher ETOH intake under CMS in Drd2+/− and Drd2−/− mice was associated with higher DAT levels in the striatum and the n. accumbens.
Materials and Methods
Animals
Eighty-six male Drd2+/+, Drd2+/−, and Drd2−/− mice were used in this study. The mice were originally obtained from the laboratory of Dr. David Grandy and bred accordingly as previously described (Kelly et al., 1998) from Drd2+/− breeders congenic on C57Bl6 strain. After weaning, the mice were tattooed on the tail and a 1 mm long tailsnip was also obtained and used for genotyping (Transnetyx, Cordova, TN). All animals were individually housed and kept on a 12:12 L/D reverse cycle with lights off at 07:00. Food and water were provided ad lib. The Institutional Animal Care and Use Committee (IACUC) of Stony Brook University approved this work in accordance with the guidelines established by the National Institutes of Health in “The Guide for Care and Use of Laboratory Animals.”
Chronic Mild Stress and Ethanol Treatments
The animals were randomly assigned to one of the four following groups: control, chronic mild stress (CMS) only, ETOH only, CMS + ETOH, as described in detail in our previous publication (Delis et al., 2013). The CMS protocol was adapted from previous studies (Muscat et al., 1992) but did not include food and water deprivation. All mice had access to two bottles of water starting 1 week prior to the beginning of the behavioral experiments. Mice in the two groups that were given ETOH (E and ES) had continuous access to both 5% (v/v) ETOH and water starting from the beginning of Week 2. Mice not in an ETOH group continued to have access to two bottles of water throughout the study. The position of the bottles was switched daily to prevent a position preference bias.
In vitro Receptor Autoradiography
Twenty four hours after the end of the experiment, between 09:00 and 12:00, mice were anesthetized with isoflurane and decapitated; brains were rapidly extracted, flash-frozen in methyl butane, and stored at −80°C. Fifteen micrometer-thick coronal brain sections were cut with the use of a cryostat, thaw-mounted on glass slides, and stored at −20°C in tightly sealed slide boxes until the day of the receptor binding experiment.
Dopamine Transporter (DAT) binding was assayed according to a previously established protocol (Hebert et al., 1999) using 3.5 nM [3H]WIN35428 (Specific activity 64Ci/mmol, PerkinElmer, Waltham, MA) as the radioligand. Non-specific binding was determined in the presence of 30 μM cocaine (Sigma-Aldrich, USA). The total sample size was 86, with 6–9 mice per group.
D1 dopamine receptor binding was performed according to a previously established protocol (Tarazi et al., 1997) using 2.5 nM [3H]SCH 23390 (Specific activity 85Ci/mmol, PerkinElmer, Waltham, MA) and 40 nM ketanserin to block 5HT2a binding sites. Non-specific binding was determined in the presence of 1 μM cis-flupenthixol (Sigma Aldrich, USA). The total sample size was 81, with 6–8 mice per group. Slides or sections from 5 mice were damaged during the assay and were not analyzed.
D2 dopamine receptor binding was performed according to a previously established protocol (Tarazi et al., 1997) using 2 nM [3H]raclopride (70Ci/mmol, PerkinElmer, Waltham, MA). Non-specific binding was determined in the presence of 10 μM sulpiride (Sigma Aldrich, USA). Binding was performed on tissue from Drd2+/+ and Drd2+/− mice and the total sample size was 60, with 6–7 mice per group.
Quantification
The slides were exposed to 3H-sensitive film (BiomaxMR, Kodak, USA) for 4–12 weeks along with [3H] microscales (ARC, St. Louis, MO, USA). The films were developed in Kodak D19 developer and scanned under constant conditions. Optical densities and bound radioactivity were measured with Image J software. The main body of the striatum was divided in quadrants [dorsolateral (DL), dorsomedial (DM), ventrolateral (VL), ventromedial (VM)], and the caudal striatal sections (the tail of the striatum) in two parts (dorsal, ventral).
Statistical Analysis
Receptor autoradiography measurements were analyzed using a three-way ANOVA, with Genotype (3 levels for DAT and D1 binding; 2 levels for D2 binding), Ethanol (2 levels, H2O/ETOH) and Stress (2 levels, NO/CMS) as between-subjects factors. When appropriate, the Tukey post-hoc test was applied to determine the significant pairwise differences. Overall threshold of significance was set at p < 0.05. All data are presented as mean ± standard error of the mean.
Results
Ethanol Intake
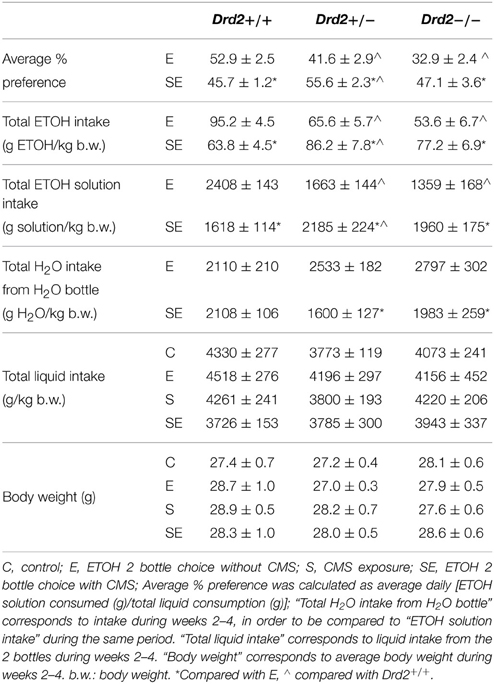
We previously showed that CMS decreased ETOH intake and preference in Drd2+/+ mice and increased it in Drd2+/− and Drd2−/− mice (Delis et al., 2013). These results are presented in Table 1 to help the reader follow the results and discussion of the current study. CMS-induced decrease in Drd2+/+ ETOH intake/preference was driven by their lower ETOH consumption, while the respective increases in Drd2+/− and Drd2−/− were driven by their higher ETOH consumption as well as their lower water intake (Table 1) (Two-way ANOVA for water intake, with Genotype and Stress as between subjects factors: Genotype × Stress F(2, 74) = 3.22, p = 0.041, Drd2+/+: No Stress vs. CMS p = 0.99, Drd2+/−: No Stress vs. CMS p = 0.011, Drd2−/−: No Stress vs. CMS p = 0.041). Body weights and total liquid intake were not affected by the treatments.
DAT Binding
We performed an in vitro quantitative autoradiographic study of [3H]WIN35428 specific binding to determine changes in the levels and distribution of dopamine transporters associated with Drd2 expression, with CMS exposure, and with ETOH intake (Figure 1). Three-way ANOVA for [3H]WIN35428 specific binding showed significant main effects of Genotype and Stress and a significant Genotype × Stress interaction in most regions studied. Post-hoc pairwise comparisons with Tukey test showed that non-stressed (C and E) Drd2+/+ mice had significantly higher DAT binding levels compared with non-stressed Drd2+/− and non-stressed Drd2−/− mice in the four quadrants of the striatum. The results also showed that stressed Drd2+/− mice (S and SE) had significantly higher DAT binding compared with non-stressed mice (C and E) of the same genotype in the four quadrants and the tail of the striatum and in the n. accumbens (DL striatum: Genotype F(2, 74) = 20.99, p < 0.001, Stress F(1, 74) = 17.86, p < 0.001, Genotype × Stress F(2, 74) = 3.08, p = 0.041; No Stress: Drd2+/+ vs. Drd2+/−p = 0.012, Drd2+/+ vs. Drd2−/−p = 0.002; CMS: Drd2+/+ vs. Drd2−/−p < 0.001, Drd2+/− vs. Drd2−/−p = 0.001; Drd2+/−: CMS vs. No Stress: p < 0.001. VL striatum: Genotype F(2, 74) = 21.76, p < 0.001, Stress F(1, 74) = 15.59, p < 0.001, Genotype × Stress F(2, 74) = 3.22, p = 0.045; No Stress: Drd2+/+ vs. Drd2+/−p = 0.016, Drd2+/+ vs. Drd2−/−p = 0.001; CMS: Drd2+/+ vs. Drd2−/−p < 0.001, Drd2+/− vs. Drd2−/−p = 0.003; Drd2+/−: CMS vs. No Stress: p = 0.002. DM striatum: Genotype F(2, 74) = 14.14, p < 0.001, Stress F(1, 74) = 12.36, p < 0.001, Genotype × Stress F(2, 74) = 4.87, p = 0.010; No Stress: Drd2+/+ vs. Drd2+/−p = 0.007, Drd2+/+ vs. Drd2−/−p = 0.013; CMS: Drd2+/+ vs. Drd2−/−p = 0.006, Drd2+/− vs. Drd2−/−p = 0.003; Drd2+/−: CMS vs. No Stress: p < 0.001. VM striatum: Genotype F(2, 74) = 19.45, p < 0.001, Stress F(1, 74) = 15.21, p < 0.001, Genotype × Stress F(2, 74) = 4.01, p = 0.022; No Stress: Drd2+/+ vs. Drd2+/−p = 0.005, Drd2+/+ vs. Drd2−/−p = 0.002; CMS: Drd2+/+ vs. Drd2−/−p < 0.001, Drd2+/− vs. Drd2−/−p = 0.002; Drd2+/−: CMS vs. No Stress: p < 0.001. Nucleus Accumbens: Genotype F(2, 74) = 5.54, p = 0.005, Stress F(1, 74) = 14.17, p < 0.001, Genotype × Stress F(2, 74) = 4.49, p = 0.014; CMS: Drd2+/− vs. Drd2−/−p = 0.04; Drd2+/−: CMS vs. No Stress: p < 0.001. Olfactory tubercle: Genotype F(2, 74) = 6.74, p = 0.002, Stress F(1, 74) = 9.46, p = 0.003. D tail: Genotype F(2, 74) = 10.45, p < 0.001, Stress F(1, 74) = 15.22, p < 0.001, Genotype × Stress F(2, 74) = 3.47, p = 0.036; CMS: Drd2+/+ vs. Drd2−/−p = 0.023, Drd2+/− vs. Drd2−/−p = 0.001; Drd2+/−: CMS vs. No Stress: p < 0.001. V tail: Genotype F(2, 74) = 17.99, p < 0.001, Stress F(1, 74) = 19.04, p < 0.001, Genotype × Stress F(2, 74) = 4.47, p = 0.036; CMS: Drd2+/+ vs. Drd2−/−p = 0.006, Drd2+/− vs. Drd2−/−p < 0.001; Drd2+/−: CMS vs. No Stress: p = 0.001.).
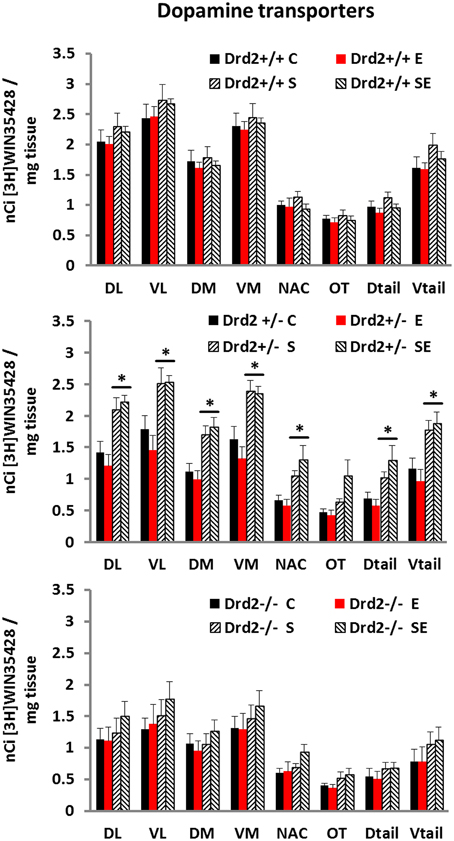
Figure 1. Specific [3H]WIN35428 binding in the basal forebrain of Drd2+/+, Drd2+/−, andDrd2−/−mice. The bars represent mean + S.E.M., C, control; E, ethanol; S, chronic mild stress (CMS); SE CMS + ETOH; *compared with C and E; Drd2−/− and non-stressed Drd2+/− measures are significantly different from the respective Drd2+/+ measures (for details please see Results).
D2 Receptor Binding
We also studied changes in the basal forebrain D2 receptor levels, as determined with [3H]raclopride specific binding, associated with Drd2 expression, with CMS exposure, and with ETOH intake (Figure 2). Three-way ANOVA for [3H]raclopride specific binding showed a significant main effect of Genotype in all regions studied and a Genotype × Stress × Ethanol interaction in the DL, VL, and DM quadrants of the striatum. Tukey post-hoc tests showed that Drd2+/+ mice had significantly higher D2 dopamine receptor levels compared with Drd2+/− mice in all regions studied. The results also showed that Drd2+/+ ES mice had significantly higher D2 receptor levels than Drd2+/+ E mice in the DL, VL, and DM quadrants of the striatum (DL striatum: Genotype F(1, 52) = 342.71, p < 0.001, Genotype × Stress × Ethanol F(1, 52) = 4.34, p = 0.042; Genotype: Drd2+/+ C vs. Drd2+/− C p < 0.001, Drd2+/+E vs. Drd2+/− E p < 0.001, Drd2+/+S vs. Drd2+/− S p < 0.001, Drd2+/+ES vs. Drd2+/− ES p < 0.001; Drd2+/+: E vs. ES p < 0.001. VL striatum: Genotype F(1, 52) = 360.73, p < 0.001, Genotype × Stress × Ethanol F(1, 52) = 4.30, p = 0.043; Genotype: Drd2+/+ C vs. Drd2+/− C p < 0.001, Drd2+/+E vs. Drd2+/− E p < 0.001, Drd2+/+S vs. Drd2+/− S p < 0.001, Drd2+/+ES vs. Drd2+/− ES p < 0.001; Drd2+/+: E vs. ES p = 0.002. DM striatum: Genotype F(1, 52) = 485.75, p < 0.001, Genotype × Stress × Ethanol F(1, 52) = 4.03, p = 0.047; Genotype: Drd2+/+ vs. Drd2+/− p < 0.001, Drd2+/+E vs. Drd2+/− E p < 0.001, Drd2+/+S vs. Drd2+/− S p < 0.001, Drd2+/+ES vs. Drd2+/− ES p < 0.001; Drd2+/+: E vs. ES p = 0.001. VM striatum: Genotype F(1, 52) = 467.42, p < 0.001; Drd2+/+ vs. Drd2+/− p < 0.001. Nucleus accumbens core: Genotype F(1, 52) = 191.51, p < 0.001; Drd2+/+ vs. Drd2+/− p < 0.001. Nucleus accumbens shell: Genotype F(1, 52) = 170.40, p < 0.001; Drd2+/+ vs. Drd2+/− p < 0.001. Olfactory tubercle: Genotype F(1, 52) = 109.67, p < 0.001; Drd2+/+ vs. Drd2+/− p < 0.001. Dtail: Genotype F(1, 52) = 200.53, p < 0.001; Drd2+/+ vs. Drd2+/− p < 0.001. Dtail: Genotype F(1, 52) = 192.29, p < 0.001; Drd2+/+ vs. Drd2+/− p < 0.001).
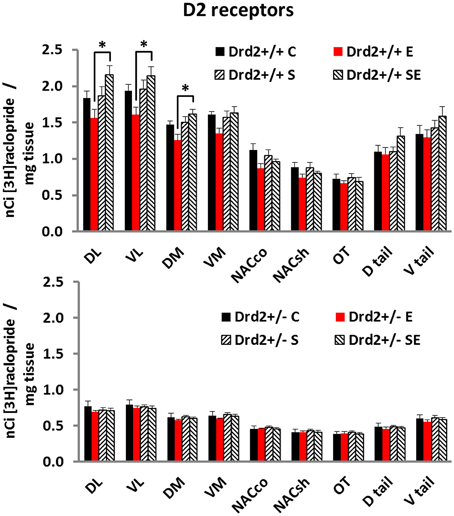
Figure 2. [3H]Raclopride specific binding in the basal forebrain of Drd2+/+ and Drd2+/−mice. The bars represent mean + S.E.M.; C, control; E, ethanol; S, chronic mild stress; SE, chronic mild stress and ethanol; Drd2+/− measures are significantly different from the respective Drd2+/+ measures (for details please see Results).
D1 Receptor Binding
The levels and distribution of D1 dopamine receptors in the basal forebrain, associated with Drd2 expression, with exposure to CMS, and with ETOH intake, were studied with in vitro [3H]SCH23390 autoradiography (Figure 3). D1 dopamine receptor levels were lower in Drd2+/− and Drd2−/− mice, compared with Drd2+/+, in all regions examined (Three-way ANOVA, DL striatum: Genotype F(2, 69) = 15.24, p < 0.001; Drd2+/+ vs. Drd2+/−p < 0.001, Drd2+/+ vs. Drd2−/−p < 0.001). VL striatum: Genotype F(2, 69) = 13.36, p < 0.001; Drd2+/+ vs. Drd2+/−p < 0.001, Drd2+/+ vs. Drd2−/−p < 0.001). DM striatum: Genotype F(2, 69) = 11.50, p < 0.001; Drd2+/+ vs. Drd2+/−p < 0.001, Drd2+/+ vs. Drd2−/−p = 0.002). VM striatum: Genotype F(2, 69) = 8.16, p < 0.001; Drd2+/+ vs. Drd2+/−p = 0.001, Drd2+/+ vs. Drd2−/−p = 0.015). Nucleus accumbens core: Genotype F(2, 69) = 6.64, p = 0.001; Drd2+/+ vs. Drd2+/−p = 0.002, Drd2+/+ vs. Drd2−/−p = 0.035). Nucleus accumbens shell: Genotype F(2, 69) = 5.344, p = 0.007; Drd2+/+ vs. Drd2+/−p = 0.007, Drd2+/+ vs. Drd2−/−p = 0.039). Olfactory tubercle: Genotype F(2, 69) = 5.325, p = 0.007; Drd2+/+ vs. Drd2+/−p = 0.007, Drd2+/+ vs. Drd2−/−p = 0.031). Dtail: Genotype F(2, 69) = 21.75, p < 0.001; Drd2+/+ vs. Drd2+/−p = 0.007, Drd2+/+ vs. Drd2−/−p < 0.001). Vtail: Genotype F(2, 69) = 13.96, p < 0.001; Drd2+/+ vs. Drd2+/−p < 0.001, Drd2+/+ vs. Drd2−/−p = 0.017).
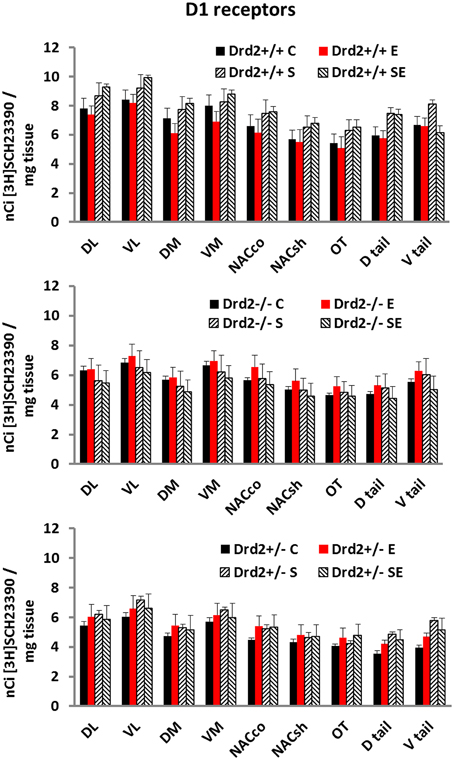
Figure 3. [3H]SCH23390 specific binding in the basal forebrain of Drd2+/+, Drd2+/−, and Drd2−/−mice. The bars represent mean + S.E.M.; C control, E ethanol, S chronic mild stress, SE chronic mild stress and ethanol; Drd2+/− and Drd2−/− measures are significantly different from the respective Drd2+/+ measures (for details please see Results).
Correlations between Receptor Binding and Ethanol Intake
ETOH intake in Drd2+/+ mice was negatively correlated with [3H]raclopride specific binding in the VM striatum (r = –0.54, p = 0.03) and the DL striatum (r = −0.5, p = 0.04) (Figure 4). Ethanol intake in Drd2−/−mice was positively correlated with [3H]WIN35428 specific binding in all regions studied (DL r = 0.73, p = 0.004; VL r = 0.76, p = 0.003; DM r = 0.657, p = 0.014; VM r = 0.722, p = 0.005; NAC r = 0.791, p = 0.001; OT r = 0.680, p = 0.011; Dtail r = 0.671, p = 0.012; Vtail r = 0.698, p = 0.008) (Figure 5).
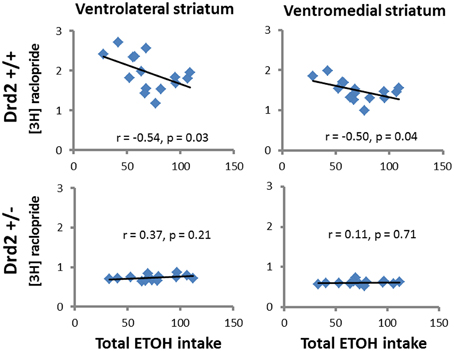
Figure 4. Significant correlations between D2 receptor levels ([3H]raclopride specific binding, nCi/mg tissue) and total ethanol intake (g ETOH/kg b.w.) in Drd2+/+ and Drd2+/− mice in the ventrolateral and ventromedial striatum.
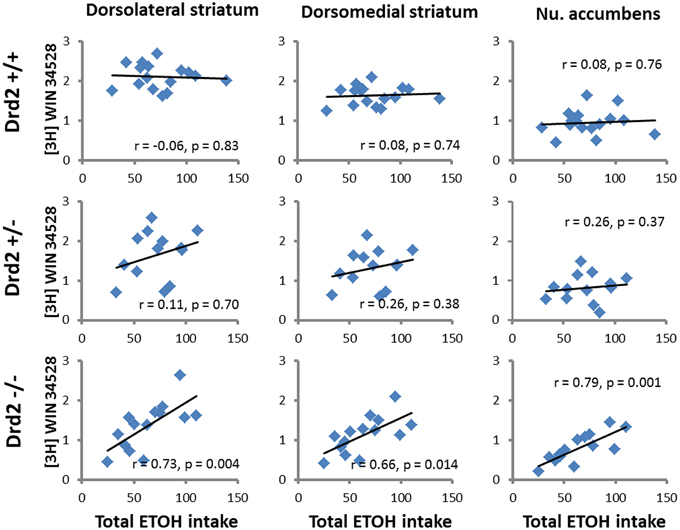
Figure 5. Significant correlations between DAT levels ([3H]WIN35428 specific binding, nCi/mg tissue) and total ethanol intake (g ETOH/kg b.w.) in Drd2+/+, Drd2+/−, and Drd2−/− mice in representative motor (dorsolateral striatum), cognitive (dorsomedial striatum), and limbic (nucleus accumbens) regions of the dopaminergic basal forebrain.
Discussion
Overall, the current and our previous study (Delis et al., 2013) show that lower ETOH intake in Drd2+/+ mice exposed to 4 weeks of CMS is associated with higher D2 receptor levels in the striatum and that ETOH intake is negatively correlated with D2 receptor levels. In Drd2+/− mice, D2 receptor levels are not affected by any treatment; perhaps their low expression levels do not allow for a modulatory role. In these mice, CMS exposure leads to higher ETOH intake and to higher DAT levels in the striatum and the n. accumbens. In Drd2−/− mice, DAT levels do not change in response to the applied treatments, but they are positively correlated with ETOH intake. In addition, we show a significant decrease in D1 dopamine receptor levels throughout the dopaminergic basal forebrain of Drd2+/− and Drd2−/− mice, which is not affected by the applied treatments. Our findings on the regulation of ethanol consumption by CMS, Drd2 expression, and markers of dopamine neurotransmission are schematically presented in Figure 6.
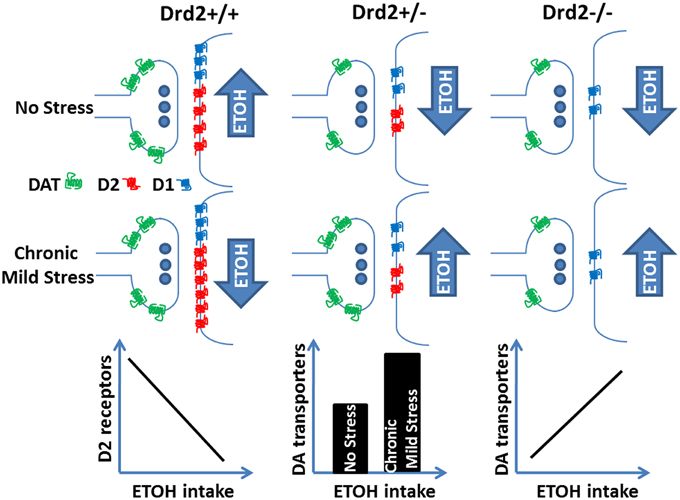
Figure 6. Ethanol intake regulation by Drd2 expression, dopamine receptor and transporter changes, and exposure to chronic mild stress.
Drd2 Knockout Decreases DAT and Dopamine D1 Receptor Levels
Drd2+/− and −/− mice have significantly lower DAT levels in the dopaminergic basal forebrain, compared with Drd2+/+ mice. Previous studies have shown that the lack of dopamine D2 receptors, which include the presynaptic D2 autoreceptors and the post-synaptic D2 receptors, leads to increased stimulus-induced DA release, increased extracellular DA half-life, lower DA clearance, and lower DA uptake (Dickinson et al., 1999; Rouge-Pont et al., 2002; Benoit-Marand et al., 2011). Our finding of lower DAT levels in Drd2−/− mice suggests that the lack of Drd2 expression leads to slower clearance and lower DA uptake in vivo, not only because of disinhibited DA release, but also because of a decrease in DAT levels which, in turn, could be a direct result of the disrupted DAT - D2 autoreceptor interaction (Lee et al., 2007).
Our finding of lower D1 receptor levels in Drd2+/− and Drd2−/− mice, compared with Drd2+/+, is in agreement with previous receptor binding and early gene expression studies showing lower D1 receptor binding (Short et al., 2006) and blunted D1-mediated c-fos expression in Drd2−/− mice (Schmauss et al., 2002).
Lower Dopamine D1 Receptor and DAT Levels are Associated with Lower ETOH Preference
Studies show that ETOH intake/preference is negatively associated with dopamine D2 receptor levels (Stefanini et al., 1992; McBride et al., 1993; Volkow et al., 1996; Thanos et al., 2001, 2004, 2005), which predicts that Drd2+/− and Drd2−/− mice would have higher ethanol intake/preference, compared with Drd2+/+. This, however, is not the case (Phillips et al., 1998; Delis et al., 2013). The paradoxical finding of lower ETOH intake and preference in Drd2+/− and Drd2−/− mice, compared with Drd2+/+, has been explained by the lack of reward-mediating D2 receptors. An additional plausible explanation is suggested by the current study and involves the lower D1 receptor levels in Drd2+/− and Drd2−/− C mice. D1 receptor antagonism prevents the reward potentiating effects of alcohol in the intracranial self-stimulation paradigm (Fish et al., 2014) and decreases ETOH seeking as well as ETOH conditioned place preference (Liu and Weiss, 2002; Hamlin et al., 2007; Chaudhri et al., 2009; Bahi and Dreyer, 2012; Pina and Cunningham, 2014; Sciascia et al., 2014; Young et al., 2014), while D1 agonism increases ETOH self-administration (D−souza et al., 2003) and ethanol-induced motor sensitization (Abrahao et al., 2011). Since the rewarding properties of ETOH are decreased by D1 receptor antagonism, we conclude that the lower D1 striatal receptor levels in Drd2+/− and Drd2−/− C mice contribute to their lower ETOH intake/preference, compared with Drd2+/+ mice.
Our finding of lower DAT levels in the dopaminergic basal forebrain of the non-preferring Drd2+/− and Drd2−/− C mice is in agreement with the lower ETOH intake levels in DAT−/− mice, compared with DAT+/+ (Savelieva et al., 2002; Mittleman et al., 2011) (but see Hall et al., 2003) and complementary to findings of higher dopamine uptake and higher DAT levels in alcohol preferring HAD rats (Carroll et al., 2006) and ethanol preferring monkeys (Mash et al., 1996). On the other hand, studies show that ethanol preferring Sardinian rats have lower DAT levels (Casu et al., 2002) and that DAT levels fluctuate periodically as a function of the duration of ETOH exposure (Hamdi and Prasad, 1991). Studies in humans suggest that alcoholism, particularly Cloninger type I, is associated with lower DAT levels in the dorsal rather than the ventral striatum (Dobashi et al., 1997; Repo et al., 1999; Tupala et al., 2001), although other studies are often inconclusive (Xu and Lin, 2011) or show DAT associations with other aspects of alcohol addiction, such as visual and somatosensory hallucinations (Limosin et al., 2004; Huber et al., 2007), the severity of withdrawal (Schmidt et al., 1998; Gorwood et al., 2003), and novelty seeking (Bau et al., 2001; Laine et al., 2001) but not ETOH intake per se. It is obvious that the role of DAT in the regulation of ETOH preference remains inconclusive, particularly in humans. The findings of this study are in line with a majority of preclinical studies and suggest that in the Drd2 genetic model of ETOH intake, lower DAT levels contribute to lower levels of ETOH intake.
ETOH Intake in CMS-Exposed Normal Mice is Moderated by D2 Receptors
In this study we show that among ETOH consuming Drd2+/+ mice, those exposed to CMS have significantly higher D2 receptor levels in regions of the motor (lateral) and cognitive (dorsomedial) striatum. CMS-exposed Drd2+/+ mice have lower ETOH intake compared to non-CMS exposed Drd2+/+ mice (Delis et al., 2013), which suggests that high D2 receptor levels moderate ETOH intake, in agreement with previous studies in rodents and humans. Ethanol preferring rats express low D2 receptor levels (Stefanini et al., 1992; McBride et al., 1993; Thanos et al., 2001) in agreement with studies in humans showing that alcoholism is associated with lower post mortem and in vivo D2 receptors (Blum et al., 1990; Volkow et al., 1996; Tupala et al., 2001). In addition, Drd2 overexpression decreases ETOH intake and preference in ETOH preferring and non-preferring rodents (Thanos et al., 2001, 2004, 2005), which is in agreement with studies in humans showing higher D2 receptor levels in unaffected members of families with alcoholics (Volkow et al., 2006). Our finding of a negative correlation between ETOH intake and D2 receptor levels in all ETOH consuming Drd2+/+ mice is in agreement with the aforementioned studies and supports the protective role of D2 receptors against ETOH overconsumption.
The lower ETOH intake levels in stressed Drd2+/+ mice were, perhaps, the most intriguing finding of our previous study (Delis et al., 2013). Instead of increasing ETOH intake after CMS exposure, as would be suggested if ethanol consumption was a self-medication strategy to counteract stress, the mice decreased consumption. Similar findings of lower ethanol consumption when stress and ethanol are experienced together or in proximity have been previously presented (Rockman and Glavin, 1986; van Erp and Miczek, 2001; van Erp et al., 2001; Chester et al., 2004; Clark et al., 2007; Deehan et al., 2011; Norman et al., 2014). A likely explanation of our result is that the stressors, in spite of being chronic and incontrollable, were presented to the mice in the same order each week, which may have allowed them to cope with stress (Miller, 1981; Koolhaas et al., 2011; Herman, 2013; Lucas et al., 2014). This would also explain the lack of effect of CMS on D2 receptors in Drd2+/+ mice, in agreement with studies showing normal D2 receptor levels in CMS-resilient rats (Zurawek et al., 2013). In agreement with this hypothesis, DRD2 antagonism or knockout impair cognitive function (Glickstein et al., 2002; Tillerson et al., 2006; Watson et al., 2012), which could account for the presumed difficulty of the Drd2+/− and Drd2−/− mice to predict the CMS stressors.
DAT Levels Increase after CMS and Remain High in ETOH-Consuming CMS-Exposed Drd2+/− Mice
In this study we observe a significant increase in DAT levels in CMS-exposed Drd2+/− mice (S and SE), in most regions of the dopaminergic basal forebrain studied, compared with Drd2+/− animals that were not exposed to CMS (C and E). Our finding is in agreement with previous preclinical studies showing higher DAT levels and function after exposure to various types of stress. DAT levels/function increase after chronic restraint stress (Copeland et al., 2005), immobilization stress (Lucas et al., 2007), social defeat (Novick et al., 2011), variable stress (Kohut et al., 2012), prenatal stress (Converse et al., 2013), and isolation rearing (Yorgason et al., 2013). In humans, the DAT gene contains a 40 nucleotide repeat polymorphism in the 3′ non-coding region that modulates the expression of the gene. This polymorphism may present with a variable number of repeats (R), with the 9R and 10R being the most frequent. The 9R polymorphism is particularly interesting in humans since its presence is associated with increased DAT expression levels (Michelhaugh et al., 2001; van de Giessen et al., 2009; Faraone et al., 2014) as well as post-traumatic stress disorder (Fuke et al., 2001; Segman et al., 2002; Valente et al., 2011; Hoexter et al., 2012). Therefore, preclinical and clinical findings converge to the suggestion that exposure to stress, including chronic mild stress, leads to increased DAT levels in the dopaminergic basal forebrain, in agreement with our findings of higher DAT levels in Drd2+/− mice exposed to CMS. In addition, in humans, the high DAT expression levels induced by the 9R polymorphism are associated with novelty seeking in alcoholics (Bau et al., 2001; Laine et al., 2001) and with severe alcohol withdrawal symptomatology (Schmidt et al., 1998; Gorwood et al., 2003). In animals, high DAT levels are associated with chronic ethanol consumption in HAD rats (Carroll et al., 2006) and are markers of high ethanol preference in primates (Mash et al., 1996). Based on the above, we postulate that CMS-induced increases in DAT levels contribute to the higher ETOH intake observed in Drd2+/− SE mice. Finally, although DAT levels do not change significantly between treatment groups in Drd2−/− mice, they are positively correlated with ETOH intake, which is in the same line with our findings in Drd2+/− mice and suggests that in the absence of Drd2 expression, ETOH intake is primarily and positively regulated by DAT expression levels.
Conclusions
The present study shows that in normal mice, ETOH intake is primarily, and negatively, regulated by dopamine D2 receptors. ETOH overconsumption under CMS is prevented in normal mice expressing high dopamine D2 receptor levels. The study also suggests that in mice with limited Drd2 expression, ETOH overconsumption is prevented by lower D1 receptor and DAT levels. In these mice, ETOH intake under CMS is positively regulated by DAT. The existence of a regulatory role of DAT on ETOH intake is also evident in mice with no Drd2 expression, in which DAT levels are positively correlated with ETOH intake. These findings illustrate the significant interaction between an environmental factor -stress- and Drd2 and Dat, two fundamental genetic factors in the regulation of reward perception and handling (Comings and Blum, 2000; Blum et al., 2011, 2014). Our study is in agreement with previous findings on the role of D2 receptors in ETOH intake and reveals new mechanisms of ETOH intake regulation by D1 and DAT. Studies of neurotransmitter systems that interact with the dopaminergic system and that are involved in ETOH reward and addiction, such as the GABAergic and endocannabinoid systems, are necessary in order to further comprehend the biological mechanisms underlying the complex interactions between stress, brain, and ETOH consumption.
Author Contribution
All authors have contributed in the design, execution, analysis, and writing of this study.
Funding
(NIAA)AA11034, AA07574, AA07611.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Abrahao, K. P., Quadros, I. M., and Souza-Formigoni, M. L. (2011). Nucleus accumbens dopamine D(1) receptors regulate the expression of ethanol-induced behavioural sensitization. Int. J. Neuropsychopharmacol. 14, 175–185. doi: 10.1017/S1461145710000441
Anthenelli, R., and Grandison, L. (2012). Effects of stress on alcohol consumption. Alcohol Res. 34, 381–382.
Anthenelli, R. M. (2012). Overview: stress and alcohol use disorders revisited. Alcohol Res. 34, 386–390.
Bahi, A., and Dreyer, J. L. (2012). Involvement of nucleus accumbens dopamine D1 receptors in ethanol drinking, ethanol-induced conditioned place preference, and ethanol-induced psychomotor sensitization in mice. Psychopharmacology (Berl) 222, 141–153. doi: 10.1007/s00213-011-2630-8
Bau, C. H., Almeida, S., Costa, F. T., Garcia, C. E., Elias, E. P., Ponso, A. C., et al. (2001). DRD4 and DAT1 as modifying genes in alcoholism: interaction with novelty seeking on level of alcohol consumption. Mol. Psychiatry 6, 7–9. doi: 10.1038/sj.mp.4000819
Benoit-Marand, M., Ballion, B., Borrelli, E., Boraud, T., and Gonon, F. (2011). Inhibition of dopamine uptake by D2 antagonists: an in vivo study. J. Neurochem. 116, 449–458. doi: 10.1111/j.1471-4159.2010.07125.x
Blum, K., Chen, A. L., Oscar-Berman, M., Chen, T. J., Lubar, J., White, N., et al. (2011). Generational association studies of dopaminergic genes in reward deficiency syndrome (RDS) subjects: selecting appropriate phenotypes for reward dependence behaviors. Int. J. Environ. Res. Public Health 8, 4425–4459. doi: 10.3390/ijerph8124425
Blum, K., Noble, E. P., Sheridan, P. J., Montgomery, A., Ritchie, T., Jagadeeswaran, P., et al. (1990). Allelic association of human dopamine D2 receptor gene in alcoholism. JAMA 263, 2055–2060. doi: 10.1001/jama.1990.03440150063027
Blum, K., Thanos, P. K., and Gold, M. S. (2014). Dopamine and glucose, obesity, and reward deficiency syndrome. Front. Psychol. 5:919. doi: 10.3389/fpsyg.2014.00919
Carroll, M. R., Rodd, Z. A., Murphy, J. M., and Simon, J. R. (2006). Chronic ethanol consumption increases dopamine uptake in the nucleus accumbens of high alcohol drinking rats. Alcohol 40, 103–109. doi: 10.1016/j.alcohol.2006.10.003
Casu, M. A., Dinucci, D., Colombo, G., Gessa, G. L., and Pani, L. (2002). Reduced DAT- and DBH-immunostaining in the limbic system of Sardinian alcohol-preferring rats. Brain Res. 948, 192–202. doi: 10.1016/S0006-8993(02)03220-1
Chaudhri, N., Sahuque, L. L., and Janak, P. H. (2009). Ethanol seeking triggered by environmental context is attenuated by blocking dopamine D1 receptors in the nucleus accumbens core and shell in rats. Psychopharmacology (Berl) 207, 303–314. doi: 10.1007/s00213-009-1657-6
Chester, J. A., Blose, A. M., Zweifel, M., and Froehlich, J. C. (2004). Effects of stress on alcohol consumption in rats selectively bred for high or low alcohol drinking. Alcohol. Clin. Exp. Res. 28, 385–393. doi: 10.1097/01.ALC.0000117830.54371.7A
Clark, J. W., Fixaris, M. C., Belanger, G. V., and Rosenwasser, A. M. (2007). Repeated light-dark phase shifts modulate voluntary ethanol intake in male and female high alcohol-drinking (HAD1) rats. Alcohol. Clin. Exp. Res. 31, 1699–1706. doi: 10.1111/j.1530-0277.2007.00476.x
Comings, D. E., and Blum, K. (2000). Reward deficiency syndrome: genetic aspects of behavioral disorders. Prog. Brain Res. 126, 325–341. doi: 10.1016/S0079-6123(00)26022-6
Converse, A. K., Moore, C. F., Moirano, J. M., Ahlers, E. O., Larson, J. A., Engle, J. W., et al. (2013). Prenatal stress induces increased striatal dopamine transporter binding in adult nonhuman primates. Biol. Psychiatry 74, 502–510. doi: 10.1016/j.biopsych.2013.04.023
Copeland, B. J., Neff, N. H., and Hadjiconstantinou, M. (2005). Enhanced dopamine uptake in the striatum following repeated restraint stress. Synapse 57, 167–174. doi: 10.1002/syn.20169
Deehan, G. A. Jr. Palmatier, M. I., Cain, M. E., and Kiefer, S. W. (2011). Differential rearing conditions and alcohol-preferring rats: consumption of and operant responding for ethanol. Behav. Neurosci. 125, 184–193. doi: 10.1037/a0022627
Delis, F., Thanos, P. K., Rombola, C., Rosko, L., Grandy, D., Wang, G. J., et al. (2013). Chronic mild stress increases alcohol intake in mice with low dopamine D2 receptor levels. Behav. Neurosci. 127, 95–105. doi: 10.1037/a0030750
Dickinson, S. D., Sabeti, J., Larson, G. A., Giardina, K., Rubinstein, M., Kelly, M. A., et al. (1999). Dopamine D2 receptor-deficient mice exhibit decreased dopamine transporter function but no changes in dopamine release in dorsal striatum. J. Neurochem. 72, 148–156. doi: 10.1046/j.1471-4159.1999.0720148.x
Dobashi, I., Inada, T., and Hadano, K. (1997). Alcoholism and gene polymorphisms related to central dopaminergic transmission in the Japanese population. Psychiatr. Genet. 7, 87–91. doi: 10.1097/00041444-199722000-00006
D'souza, M. S., Ikegami, A., Olsen, C. M., and Duvauchelle, C. L. (2003). Chronic D1 agonist and ethanol coadministration facilitate ethanol-mediated behaviors. Pharmacol. Biochem. Behav. 76, 335–342. doi: 10.1016/j.pbb.2003.08.004
Enoch, M. A. (2014). Genetic influences on response to alcohol and response to pharmacotherapies for alcoholism. Pharmacol. Biochem. Behav. 123, 17–24. doi: 10.1016/j.pbb.2013.11.001
Faraone, S. V., Spencer, T. J., Madras, B. K., Zhang-James, Y., and Biederman, J. (2014). Functional effects of dopamine transporter gene genotypes on in vivo dopamine transporter functioning: a meta-analysis. Mol. Psychiatry 19, 880–889. doi: 10.1038/mp.2013.126
Fish, E. W., Diberto, J. F., Krouse, M. C., Robinson, J. E., and Malanga, C. J. (2014). Different contributions of dopamine D1 and D2 receptor activity to alcohol potentiation of brain stimulation reward in C57BL/6J and DBA/2J mice. J. Pharmacol. Exp. Ther. 350, 322–329. doi: 10.1124/jpet.114.216135
Fuke, S., Suo, S., Takahashi, N., Koike, H., Sasagawa, N., and Ishiura, S. (2001). The VNTR polymorphism of the human dopamine transporter (DAT1) gene affects gene expression. Pharmacogenomics J. 1, 152–156. doi: 10.1038/sj.tpj.6500026
Glickstein, S. B., Hof, P. R., and Schmauss, C. (2002). Mice lacking dopamine D2 and D3 receptors have spatial working memory deficits. J. Neurosci. 22, 5619–5629.
Gorwood, P., Limosin, F., Batel, P., Hamon, M., Ades, J., and Boni, C. (2003). The A9 allele of the dopamine transporter gene is associated with delirium tremens and alcohol-withdrawal seizure. Biol. Psychiatry 53, 85–92. doi: 10.1016/S0006-3223(02)01440-3
Hall, F. S., Sora, I., and Uhl, G. R. (2003). Sex-dependent modulation of ethanol consumption in vesicular monoamine transporter 2 (VMAT2) and dopamine transporter (DAT) knockout mice. Neuropsychopharmacology 28, 620–628. doi: 10.1038/sj.npp.1300070
Hamdi, A., and Prasad, C. (1991). Attenuation of pulsatile changes in the density of striatal [3H]GBR-12935 binding sites during chronic ethanol consumption. Brain Res. 567, 71–75. doi: 10.1016/0006-8993(91)91437-6
Hamlin, A. S., Newby, J., and McNally, G. P. (2007). The neural correlates and role of D1 dopamine receptors in renewal of extinguished alcohol-seeking. Neuroscience 146, 525–536. doi: 10.1016/j.neuroscience.2007.01.063
Hebert, M. A., Larson, G. A., Zahniser, N. R., and Gerhardt, G. A. (1999). Age-related reductions in [3H]WIN 35,428 binding to the dopamine transporter in nigrostriatal and mesolimbic brain regions of the fischer 344 rat. J. Pharmacol. Exp. Ther. 288, 1334–1339.
Herman, J. P. (2013). Neural control of chronic stress adaptation. Front. Behav. Neurosci. 7:61. doi: 10.3389/fnbeh.2013.00061
Hoexter, M. Q., Fadel, G., Felicio, A. C., Calzavara, M. B., Batista, I. R., Reis, M. A., et al. (2012). Higher striatal dopamine transporter density in PTSD: an in vivo SPECT study with [(99m)Tc]TRODAT-1. Psychopharmacology (Berl) 224, 337–345. doi: 10.1007/s00213-012-2755-4
Huber, M., Kirchler, E., Karner, M., and Pycha, R. (2007). Delusional parasitosis and the dopamine transporter. A new insight of etiology? Med. Hypotheses 68, 1351–1358. doi: 10.1016/j.mehy.2006.07.061
Kelly, M. A., Rubinstein, M., Phillips, T. J., Lessov, C. N., Burkhart-Kasch, S., Zhang, G., et al. (1998). Locomotor activity in D2 dopamine receptor-deficient mice is determined by gene dosage, genetic background, and developmental adaptations. J. Neurosci. 18, 3470–3479.
Kohut, S. J., Decicco-Skinner, K. L., Johari, S., Hurwitz, Z. E., Baumann, M. H., and Riley, A. L. (2012). Differential modulation of cocaine's discriminative cue by repeated and variable stress exposure: relation to monoamine transporter levels. Neuropharmacology 63, 330–337. doi: 10.1016/j.neuropharm.2012.03.012
Koolhaas, J. M., Bartolomucci, A., Buwalda, B., De Boer, S. F., Flugge, G., Korte, S. M., et al. (2011). Stress revisited: a critical evaluation of the stress concept. Neurosci. Biobehav. Rev. 35, 1291–1301. doi: 10.1016/j.neubiorev.2011.02.003
Laine, T. P., Ahonen, A., Rasanen, P., and Tiihonen, J. (2001). Dopamine transporter density and novelty seeking among alcoholics. J. Addict. Dis. 20, 91–96. doi: 10.1300/J069v20n04_08
Lee, F. J., Pei, L., Moszczynska, A., Vukusic, B., Fletcher, P. J., and Liu, F. (2007). Dopamine transporter cell surface localization facilitated by a direct interaction with the dopamine D2 receptor. EMBO J. 26, 2127–2136. doi: 10.1038/sj.emboj.7601656
Limosin, F., Loze, J. Y., Boni, C., Fedeli, L. P., Hamon, M., Rouillon, F., et al. (2004). The A9 allele of the dopamine transporter gene increases the risk of visual hallucinations during alcohol withdrawal in alcohol-dependent women. Neurosci. Lett. 362, 91–94. doi: 10.1016/j.neulet.2004.02.065
Liu, X., and Weiss, F. (2002). Reversal of ethanol-seeking behavior by D1 and D2 antagonists in an animal model of relapse: differences in antagonist potency in previously ethanol-dependent versus nondependent rats. J. Pharmacol. Exp. Ther. 300, 882–889. doi: 10.1124/jpet.300.3.882
Lucas, L. R., Wang, C. J., McCall, T. J., and McEwen, B. S. (2007). Effects of immobilization stress on neurochemical markers in the motivational system of the male rat. Brain Res. 1155, 108–115. doi: 10.1016/j.brainres.2007.04.063
Lucas, M., Ilin, Y., Anunu, R., Kehat, O., Xu, L., Desmedt, A., et al. (2014). Long-term effects of controllability or the lack of it on coping abilities and stress resilience in the rat. Stress 17, 423–430. doi: 10.3109/10253890.2014.930430
Mash, D. C., Staley, J. K., Doepel, F. M., Young, S. N., Ervin, F. R., and Palmour, R. M. (1996). Altered dopamine transporter densities in alcohol-preferring vervet monkeys. Neuroreport 7, 457–462. doi: 10.1097/00001756-199601310-00020
McBride, W. J., Chernet, E., Dyr, W., Lumeng, L., and Li, T. K. (1993). Densities of dopamine D2 receptors are reduced in CNS regions of alcohol-preferring P rats. Alcohol 10, 387–390. doi: 10.1016/0741-8329(93)90025-J
Michelhaugh, S. K., Fiskerstrand, C., Lovejoy, E., Bannon, M. J., and Quinn, J. P. (2001). The dopamine transporter gene (SLC6A3) variable number of tandem repeats domain enhances transcription in dopamine neurons. J. Neurochem. 79, 1033–1038. doi: 10.1046/j.1471-4159.2001.00647.x
Mittleman, G., Call, S. B., Cockroft, J. L., Goldowitz, D., Matthews, D. B., and Blaha, C. D. (2011). Dopamine dynamics associated with, and resulting from, schedule-induced alcohol self-administration: analyses in dopamine transporter knockout mice. Alcohol 45, 325–339. doi: 10.1016/j.alcohol.2010.12.006
Muscat, R., Papp, M., and Willner, P. (1992). Antidepressant-like effects of dopamine agonists in an animal model of depression. Biol. Psychiatry 31, 937–946. doi: 10.1016/0006-3223(92)90119-K
Norman, K. J., Seiden, J. A., Klickstein, J. A., Han, X., Hwa, L. S., Debold, J. F., et al. (2014). Social stress and escalated drug self-administration in mice I. Alcohol and corticosterone. Psychopharmacology (Berl) 232, 991–1001. doi: 10.1007/s00213-014-3733-9
Novick, A. M., Forster, G. L., Tejani-Butt, S. M., and Watt, M. J. (2011). Adolescent social defeat alters markers of adult dopaminergic function. Brain Res. Bull. 86, 123–128. doi: 10.1016/j.brainresbull.2011.06.009
Phillips, T. J., Brown, K. J., Burkhart-Kasch, S., Wenger, C. D., Kelly, M. A., Rubinstein, M., et al. (1998). Alcohol preference and sensitivity are markedly reduced in mice lacking dopamine D2 receptors. Nat. Neurosci. 1, 610–615. doi: 10.1038/2843
Pina, M. M., and Cunningham, C. L. (2014). Effects of dopamine receptor antagonists on the acquisition of ethanol-induced conditioned place preference in mice. Psychopharmacology (Berl) 231, 459–468. doi: 10.1007/s00213-013-3252-0
Repo, E., Kuikka, J. T., Bergstrom, K. A., Karhu, J., Hiltunen, J., and Tiihonen, J. (1999). Dopamine transporter and D2-receptor density in late-onset alcoholism. Psychopharmacology (Berl) 147, 314–318. doi: 10.1007/s002130051173
Rockman, G. E., and Glavin, G. B. (1986). Activity stress effects on voluntary ethanol consumption, mortality and ulcer development in rats. Pharmacol. Biochem. Behav. 24, 869–873. doi: 10.1016/0091-3057(86)90428-4
Rouge-Pont, F., Usiello, A., Benoit-Marand, M., Gonon, F., Piazza, P. V., and Borrelli, E. (2002). Changes in extracellular dopamine induced by morphine and cocaine: crucial control by D2 receptors. J. Neurosci. 22, 3293–3301.
Savelieva, K. V., Caudle, W. M., Findlay, G. S., Caron, M. G., and Miller, G. W. (2002). Decreased ethanol preference and consumption in dopamine transporter female knock-out mice. Alcohol. Clin. Exp. Res. 26, 758–764. doi: 10.1111/j.1530-0277.2002.tb02602.x
Schmauss, C., Glickstein, S. B., Adlersberg, M., Hsiung, S. C., and Tamir, H. (2002). A single dose of methamphetamine rescues the blunted dopamine D(1)-receptor activity in the neocortex of D(2)- and D(3)-receptor knockout mice. Ann. N.Y. Acad. Sci. 965, 21–27. doi: 10.1111/j.1749-6632.2002.tb04148.x
Schmidt, L. G., Harms, H., Kuhn, S., Rommelspacher, H., and Sander, T. (1998). Modification of alcohol withdrawal by the A9 allele of the dopamine transporter gene. Am. J. Psychiatry 155, 474–478. doi: 10.1176/ajp.155.4.474
Sciascia, J. M., Mendoza, J., and Chaudhri, N. (2014). Blocking dopamine d1-like receptors attenuates context-induced renewal of pavlovian-conditioned alcohol-seeking in rats. Alcohol. Clin. Exp. Res. 38, 418–427. doi: 10.1111/acer.12262
Segman, R. H., Cooper-Kazaz, R., Macciardi, F., Goltser, T., Halfon, Y., Dobroborski, T., et al. (2002). Association between the dopamine transporter gene and posttraumatic stress disorder. Mol. Psychiatry 7, 903–907. doi: 10.1038/sj.mp.4001085
Short, J. L., Ledent, C., Borrelli, E., Drago, J., and Lawrence, A. J. (2006). Genetic interdependence of adenosine and dopamine receptors: evidence from receptor knockout mice. Neuroscience 139, 661–670. doi: 10.1016/j.neuroscience.2005.12.052
Stefanini, E., Frau, M., Garau, M. G., Garau, B., Fadda, F., and Gessa, G. L. (1992). Alcohol-preferring rats have fewer dopamine D2 receptors in the limbic system. Alcohol Alcohol. 27, 127–130.
Tarazi, F. I., Florijn, W. J., and Creese, I. (1997). Differential regulation of dopamine receptors after chronic typical and atypical antipsychotic drug treatment. Neuroscience 78, 985–996. doi: 10.1016/S0306-4522(96)00631-8
Thanos, P. K., Rivera, S. N., Weaver, K., Grandy, D. K., Rubinstein, M., Umegaki, H., et al. (2005). Dopamine D2R DNA transfer in dopamine D2 receptor-deficient mice: effects on ethanol drinking. Life Sci. 77, 130–139. doi: 10.1016/j.lfs.2004.10.061
Thanos, P. K., Taintor, N. B., Rivera, S. N., Umegaki, H., Ikari, H., Roth, G., et al. (2004). DRD2 gene transfer into the nucleus accumbens core of the alcohol preferring and nonpreferring rats attenuates alcohol drinking. Alcohol. Clin. Exp. Res. 28, 720–728. doi: 10.1097/01.ALC.0000125270.30501.08
Thanos, P. K., Volkow, N. D., Freimuth, P., Umegaki, H., Ikari, H., Roth, G., et al. (2001). Overexpression of dopamine D2 receptors reduces alcohol self-administration. J. Neurochem. 78, 1094–1103. doi: 10.1046/j.1471-4159.2001.00492.x
Tillerson, J. L., Caudle, W. M., Parent, J. M., Gong, C., Schallert, T., and Miller, G. W. (2006). Olfactory discrimination deficits in mice lacking the dopamine transporter or the D2 dopamine receptor. Behav. Brain Res. 172, 97–105. doi: 10.1016/j.bbr.2006.04.025
Tupala, E., Kuikka, J. T., Hall, H., Bergstrom, K., Sarkioja, T., Rasanen, P., et al. (2001). Measurement of the striatal dopamine transporter density and heterogeneity in type 1 alcoholics using human whole hemisphere autoradiography. Neuroimage 14, 87–94. doi: 10.1006/nimg.2001.0793
Valente, N. L., Vallada, H., Cordeiro, Q., Miguita, K., Bressan, R. A., Andreoli, S. B., et al. (2011). Candidate-gene approach in posttraumatic stress disorder after urban violence: association analysis of the genes encoding serotonin transporter, dopamine transporter, and BDNF. J. Mol. Neurosci. 44, 59–67. doi: 10.1007/s12031-011-9513-7
van de Giessen, E., de Win, M. M., Tanck, M. W., van den Brink, W., Baas, F., and Booij, J. (2009). Striatal dopamine transporter availability associated with polymorphisms in the dopamine transporter gene SLC6A3. J. Nucl. Med. 50, 45–52. doi: 10.2967/jnumed.108.053652
van Erp, A. M., and Miczek, K. A. (2001). Persistent suppression of ethanol self-administration by brief social stress in rats and increased startle response as index of withdrawal. Physiol. Behav. 73, 301–311. doi: 10.1016/S0031-9384(01)00458-9
van Erp, A. M., Tachi, N., and Miczek, K. A. (2001). Short or continuous social stress: suppression of continuously available ethanol intake in subordinate rats. Behav. Pharmacol. 12, 335–342. doi: 10.1097/00008877-200109000-00004
Volkow, N. D., Wang, G. J., Begleiter, H., Porjesz, B., Fowler, J. S., Telang, F., et al. (2006). High levels of dopamine D2 receptors in unaffected members of alcoholic families: possible protective factors. Arch. Gen. Psychiatry 63, 999–1008. doi: 10.1001/archpsyc.63.9.999
Volkow, N. D., Wang, G. J., Fowler, J. S., Logan, J., Hitzemann, R., Ding, Y. S., et al. (1996). Decreases in dopamine receptors but not in dopamine transporters in alcoholics. Alcohol. Clin. Exp. Res. 20, 1594–1598. doi: 10.1111/j.1530-0277.1996.tb05936.x
Watson, D. J., Loiseau, F., Ingallinesi, M., Millan, M. J., Marsden, C. A., and Fone, K. C. (2012). Selective blockade of dopamine D3 receptors enhances while D2 receptor antagonism impairs social novelty discrimination and novel object recognition in rats: a key role for the prefrontal cortex. Neuropsychopharmacology 37, 770–786. doi: 10.1038/npp.2011.254
Xu, M., and Lin, Z. (2011). Genetic influences of dopamine transport gene on alcohol dependence: a pooled analysis of 13 studies with 2483 cases and 1753 controls. Prog. Neuropsychopharmacol. Biol. Psychiatry 35, 1255–1260. doi: 10.1016/j.pnpbp.2010.11.001
Yorgason, J. T., Espana, R. A., Konstantopoulos, J. K., Weiner, J. L., and Jones, S. R. (2013). Enduring increases in anxiety-like behavior and rapid nucleus accumbens dopamine signaling in socially isolated rats. Eur. J. Neurosci. 37, 1022–1031. doi: 10.1111/ejn.12113
Young, E. A., Dreumont, S. E., and Cunningham, C. L. (2014). Role of nucleus accumbens dopamine receptor subtypes in the learning and expression of alcohol-seeking behavior. Neurobiol. Learn. Mem. 108, 28–37. doi: 10.1016/j.nlm.2013.05.004
Keywords: dopamine transporter, D2 receptor, D1 receptor, chronic mild stress, ethanol
Citation: Delis F, Rombola C, Bellezza R, Rosko L, Grandy DK, Volkow ND and Thanos PK (2015) Regulation of ethanol intake under chronic mild stress: roles of dopamine receptors and transporters. Front. Behav. Neurosci. 9:118. doi: 10.3389/fnbeh.2015.00118
Received: 11 March 2015; Accepted: 24 April 2015;
Published: 12 May 2015.
Edited by:
Agnes Gruart, University Pablo de Olavide, Seville, SpainReviewed by:
Gregg Stanwood, Florida State University, USAChristina Dalla, University of Athens, Greece
Copyright © 2015 Delis, Rombola, Bellezza, Rosko, Grandy, Volkow and Thanos. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Panayotis K. Thanos, Department of Psychology, State University of New York at Stony Brook, 131 Psychology B, Stony Brook, NY 11794-2500, USA,cGV0ZXIudGhhbm9zQHN0b255YnJvb2suZWR1
 Foteini Delis
Foteini Delis Christina Rombola
Christina Rombola Robert Bellezza1
Robert Bellezza1 Lauren Rosko
Lauren Rosko David K. Grandy
David K. Grandy Panayotis K. Thanos
Panayotis K. Thanos