
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Behav. Neurosci. , 03 November 2014
Sec. Motivation and Reward
Volume 8 - 2014 | https://doi.org/10.3389/fnbeh.2014.00378
Motivation is a key neurobehavioral concept underlying adaptive responses to environmental incentives and threats. As such, dysregulation of motivational processes may be critical in the formation of abnormal behavioral patterns/tendencies. According to the long standing model of the Reinforcement Sensitivity Theory (RST), motivation behaviors are driven by three neurobehavioral systems mediating the sensitivity to punishment, reward or goal-conflict. Corresponding to current neurobehavioral theories in psychiatry, this theory links abnormal motivational drives to abnormal behavior; viewing depression and mania as two abnormal extremes of reward driven processes leading to either under or over approach tendencies, respectively. We revisit the RST framework in the context of bipolar disorder (BD) and challenge this concept by suggesting that dysregulated interactions of both punishment and reward related processes better account for the psychological and neural abnormalities observed in BD. We further present an integrative model positing that the three parallel motivation systems currently proposed by the RST model, can be viewed as subsystems in a large-scale neurobehavioral network of motivational decision making.
The term motivation as used in neuroscience refers to the processes which modulate the organism’s responses to environmental reinforcing cues, according to their perceived value (i.e., reward/punishment) (Smillie, 2008). As it is a major determinant of adaptive goal-directed behavior, it may be useful to look at human psychopathological conditions in terms of aberrant neuro-behavioral functioning of motivational processes. Accordingly, in this perspective paper we revisit a long-standing framework stemmed from rodent research, the Reinforcement Sensitivity Theory (RST; Gray, 1982), which defines sensitivity to reward and punishment as the main underlying forces of goal-directed behavior. Specifically, we inspect the neural organization proposed by the RST with respect to its possible involvement in a specific mood dysregulation condition- Bipolar Disorder (BD). We briefly review previous attempts to formulate a conceptual model linking motivation-related abnormalities and BD, and suggest an alternative perspective regarding the compound interactions between the different neural systems underlying motivational processes.
The basic concept regarding the consequential effects of stimuli on behavior, depending on their incentive values for the organism (reward or punishment) originated with the seminal work of Pavlov (Pavlov, 1927). Essentially, animals tend to approach rewarding and avoid punishing cues, creating the idea of sensitivity to reward and punishment as the main underlying forces of goal-oriented behavior (Corr and Perkins, 2006). Following this view the RST (Gray, 1982) assigned theses sensitivities to three specific neural systems mediating different motivational processes: (1) The “Fight, Flight, Freeze System” (FFFS), sensitive to punishment stimuli and facilitates behavioral responses via activation of the periaquaductal Gray (PAG), medial hypothalamus, central amygdala, and subgenual anterior cingulate cortex (sgACC); (2) The “Behavioral Activation System” (BAS), sensitive to reward stimuli and facilitates behavioral responses via the ventral tegmental area (VTA), nucleus accumbens (NAcc) and dorsomedial prefrontal cortex (dmPFC); and (3) The “Behavioral Inhibition System” (BIS), sensitive to goal-conflict situations (i.e., stimuli of mixed value) and thus triggered when the other two systems are simultaneously activated, relying mainly on the septo-hippocampal system (SHS) and the ventromedial prefrontal cortex (vmPFC) recursive circuit, along with the Anterior Cingulate Cortex (ACC) and entorhinal cortex (Gray and McNaughton, 2000). Figure 1 depicts the detailed neuroanatomy of these systems. Research on the neural dynamics and interactions of these proposed motivation systems has been mainly carried out in animals (rodents), with translation of the experimental paradigms to the complexity of human behavior somewhat lacking (Avila et al., 2008; Barrós-Loscertales et al., 2010; Costumero et al., 2013). With this in mind, in a recent fMRI study we used an interactive computer game and Dynamic Causal Model analysis to demonstrate the effective connectivity and stimulus specificity in similar neural networks in humans as described in the RST model (Gonen et al., 2012). This finding has encouraged us to revisit RST in light of current evidence on the relevance of motivation systems to psychopathology.
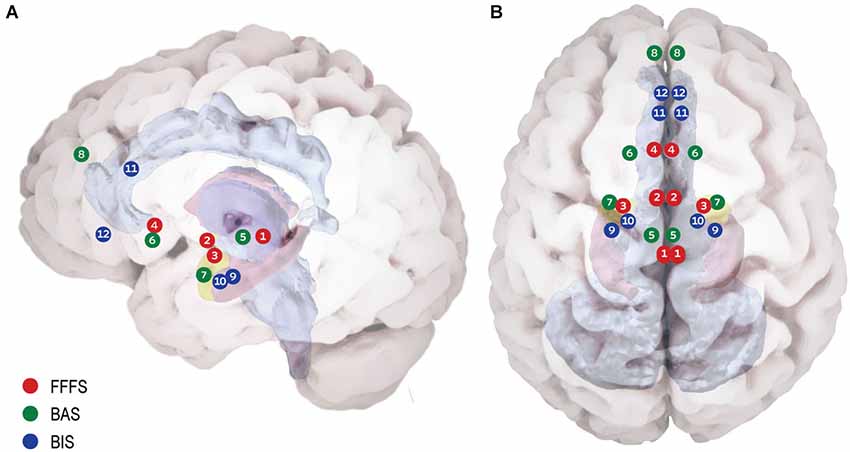
Figure 1. Proposed neuroanatomy of the RST motivational systems. (A) Lateral view. (B) Superior view. According to RST three bio-behavioral systems participate in reinforcement modulation of goal directed behavior: (1) The Fight Flight Freeze System (FFFS) is activated by all punishment stimuli (shown in red). The emotional consequence of its activation is fear, and the motivational consequence is defensive approach (i.e., fight) or defensive avoidance (i.e., flight/freeze). Anatomically, this system includes the Peri-Aqueductal Gray (1), Medial Hypothalamus (2), central Amygdala (3) and subgenual Anterior Cingulate Cortex (4). (2) The Behavioral Activation System (BAS) believed to underlie reward (and non-punishment) sensitivity (shown in green). When detecting reward, the system enhances incentive motivation, which facilitates approach. The system relies on Ventral Tegmental Area dopamine phasic activity (5) to Nucleus Accumbens (NAc) (6) in response to reward, signaling its salience. Information regarding the classical stimulus-reinforcement conditioning, along with integrative stimulus-reward associations is projected to the NAc from the basolateral amygdala (7). The medial pre-frontal cortex carries integrative representation of complex reinforcement associations with both stimuli and responses, is suggested to control and modulate incentive motivation and approach behavior (8). (3) Behavioral Inhibition System (BIS) underlying goal-conflict situations (shown in blue). The BIS is activated by stimuli of mixed valence (i.e., both BAS and FFFS are already activated), causing conflict between differing goals. The system functions as a comparator between the current state, previous knowledge and expected consequences, for the sake of adaptive behavioral selection. BIS consists of two neural foci: the Septo-Hippocampal System (SHS) (9) is informed comprehensively regarding possible behavioral plans for the current situation and their consequences by the entorhinal cortex (10) and cingulate cortex (11). The SHS is further modulated by information from the FFFS’s central amygdala, signaling the valance and importance of the stimuli. The ventro-medial pre-frontal cortex (12) is considered as a behavioral control modulator.
The relevance of motivation systems to abnormal mood conditions is supported by recent studies focusing mainly on reward processing. This has reflected developments in the investigation of the neural underpinnings of reward processes in terms of their neurochemistry (Berridge and Kringelbach, 2013) and mechanistic circuitry (Haber and Knutson, 2009; Miller et al., 2013). Furthermore, various aspects of motivation induced behaviors, such as reward learning (Pizzagalli et al., 2008; Dayan and Berridge, 2014), reward prediction (Dowd and Barch, 2012) or aversion processing (Hayes and Northoff, 2011) were proposed as related to psychopathological conditions (Phillips and Swartz, 2014). For example, altered effort-based motivational decision making has been demonstrated in Major Depressive Disorder (MDD) patients, who were not only willing to devote less effort for rewards than healthy controls, but were also less efficient in using information regarding the magnitude and probability of the rewards to guide effort based motivational decision making (Treadway et al., 2012). Such effort based decision making has been shown to rely on striatal dopamine transmission (Salamone and Correa, 2012) and its interaction with Ach muscarinic function, mostly in the NAcc core (Nunes et al., 2013). These findings have led to the idea that poor DA-ACh regulation within the NAcc may underlie common depressive symptoms such as anhedonia, fatigue or psychomotor slowness (Treadway and Zald, 2011). In the framework of the RST, these recent mechanistic evidence may provide new neurobiological support for involvement of the BAS in pathological affective states, since NAcc is a core region of this system. The contribution of abnormal motivational processes to human psychopathology in terms of the RST model has been formulated by two central models thus far. The first and most prominent is the “Neuropsychology Theory of Anxiety” (Gray and McNaughton, 2000), and the second is the “BAS Dysregulation Theory” (Depue and Iacono, 1989; Johnson et al., 2012). The first model posits that hyperactive nodes of FFFS, as well as BIS underlie different anxiety disorders (e.g., generalized anxiety, phobias etc.). A thorough discussion of the relation of RST to anxiety disorders is beyond the scope of this paper and can be found elsewhere (McNaughton and Corr, 2008). In the following section we will re-visit the BAS dysregulation theory and its limitations in thoroughly elucidating the underlying neural pathology in BD.
The “BAS Dysregulation Theory” suggests that it is BAS hyper- or hypo-activation that underlies BD manic or depressive states, respectively. Indeed, the model seems compatible with the nature of the symptoms often observed in BD patients. For example, decreased energy in depression or increased engagement in goal-directed activities in mania, support the consideration of motivational abnormalities when modeling these illnesses. Therefore, it seems quite straightforward that extreme over- and/or under- sensitivity to reward (manifested as over and under activation of the BAS) underlies mania or depression, respectively. Accordingly, numerous self-report studies using RST based questionnaires have shown higher BAS sensitivity in bipolar patients (Salavert et al., 2007) and also found it to be related to vulnerability to manic episodes (Meyer et al., 2007; Alloy et al., 2008). However, some have found relation of BIS sensitivity (measured by the self-report BIS/BAS scales) to depressive symptoms. For example, in a longitudinal study, within-patient fluctuations in depressive symptoms were correlated to changes in BIS levels (Meyer et al., 2001). Highly sensitive BIS was found related to proneness to- or concurrent depressive symptoms as well (Alloy et al., 2006). This inspires a reconsideration of the RST relevance to BD in a broader perspective of two processes, reward and punishment dysregulation.
In this context we suggest that dysregulation of the BIS system may result in insensitivity to costs and efforts, a symptomatic phenomenology of the manic state in BD. Recent evidence considering the inter-regional regulation of effort based behavioral choice via NAcc and ACC may support this notion. The ACC has been implicated in evaluating the costs and benefits and comparing between current and future costs /benefits of optional behavioral plans (Phillips and Vieta, 2007). Of note, the ACC has been shown to be involved in effort based decision processs only in cases where there were more than one potential reward (Schweimer et al., 2005), supporting its role in the RST’s BIS as evaluating optional behavioral plans under a goal conflict. However, while the RST postulated that motivational behavioral decisions are guided by the septo-hippocampal-vmPFC recursive signaling, with the ACC signaling information to the hippocampus; more recent models show in addition direct connections from the ACC to the NAC (Knutson and Gibbs, 2007; Phillips and Vieta, 2007). Lesions to the ACC or its afferent connections with NAcc have been shown to diminish the effort an animal is willing to invest in a reward (Schweimer et al., 2005; Phillips et al., 2007; Treadway and Zald, 2011).
Taken together, we feel that the BAS dysregulation model does not adequately capture the complex dysfunction of motivational processes in BD and that a conceptual revision is needed to better account for both phenomenological and neuroscientific observations in BD. Our premise echoes recent advances in neuroscientific research which repeatedly point to large-scale neural networks, rather than localized regions, as the underpinnings of cognitive and emotional processes (e.g., Bressler and Menon, 2010). We propose to regard the three motivational RST systems as functionally specialized subsystems of one larger system, interacting together in order to mediate motivational behavior. Thus, ineffective compound interactions between the three subsystems, rather than one subsystem’s abnormal activity, may underlie the different abnormal behaviors in mood disorders such as BD (see Figure 2). To establish this view, the following sections present a conceptual framework alongside supporting evidence from structural as well as functional neuroanatomical studies.
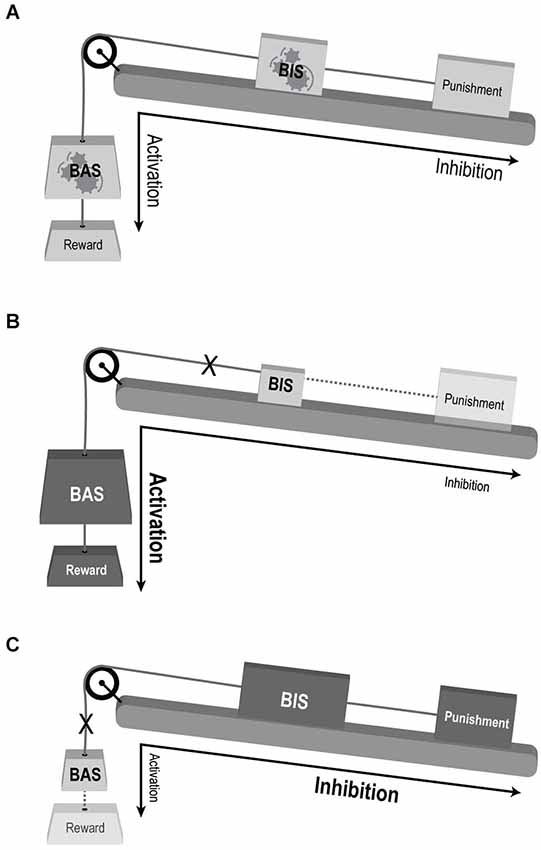
Figure 2. Conceptual illustration of the suggested model for the involvement of motivational systems in mood disorders. (A) Normal interaction. BAS mediating reward driven approach behavior and BIS mediating behavioral response to complex valence stimuli. Both sub-systems’ response and influence on behavioral output are balanced. The large-scale system is flexible and adaptive to changing motivational cues (denoted be gears within the “BAS” and “BIS” weights). (B) BAS over-activation enhancing reward sensitivity and approach behavior, and BIS under-activation reducing punishment sensitivity and avoidance—compatible with manic state. (C) BAS under-activation with reduced reward driven approach behavior, and BIS over-activation enhancing punishment sensitivity and avoidance—compatible with depression. Weight’s size and shading denotes activation levels: larger and darker weight indicate over activation, while smaller and lighter weights indicate under-activation. Dotted lines decipher weak impact of the reinforcer (punishment or reward) on the relevant system (BIS and BAS, respectively). X denoted disconnection of the sub-system from the large-scale motivational system.
The clinical presentation of the manic state includes by definition, among other symptoms, an increase in goal-directed activity and excessive indulgence in pleasurable activities that have an increased potential for painful consequences; whereas depression is characterized by symptoms including anhedonia and fatigue or loss of energy. It is thus rather intuitive that extreme over- and/or under- reactivity of the BAS may underlie mania or depression, respectively, as suggested by the “BAS dysregulation theory” (Depue and Iacono, 1989; Johnson et al., 2012). However, there seems to be an important role for abnormal punishment sensitivity as well. Manic patients lack accurate perception of possible aversive consequences of their behaviors, especially the concept of punishment and its related results (Diekhof et al., 2008), leading to damaging interactions or consequences (Malhi et al., 2004a). Manic patients have also been shown to display positive cognitive bias (e.g., remembering more positive self-descriptive words than healthy controls (Pavlickova et al., 2013)). Nevertheless their behavior seems to be guided more by sensitivity to potential rewards than by avoiding dangers (Swann et al., 2004). We suggest that this complex positive cognitive-behavioral bias may reflect impaired generation, or resolution, of goal-conflict processes, which according to RST depends on normal activation of the BIS. Thus, in the search for the motivational processes underlying the manic state, BIS dysregulation should also be considered (as demonstrated in Figure 2B).
A parallel argument applies to the depressive state in BD patients in whom lack of incentive motivation is a prominent feature: depressed BD patients exhibit decreased reward seeking behavior and a reduced ability to experience reward, even in the absence of acute stressors, resulting in dominance of persistent dysphoric emotions and thoughts (Drevets, 2001), together corresponding to a hypoactive BAS. Other characteristics of the depressive state, such as the well-established negative cognitive bias, may hint at the involvement of a dysregulated FFFS/BIS (Leppänen, 2006; Haas and Canli, 2008; Zinbarg and Yoon, 2008; Figure 2C). Altogether it seems that one RST system’s abnormality is not sufficient to account for the complex behavioral abnormalities observed in BD. Rather, imbalanced interactions of both BAS and FFFS/BIS, resulting from over/under activation of one or both systems or from abnormal inter-system connectivity, can account for what seems to be both incentive and regulatory dysfunction. From this perspective, mania and depression can be viewed as two separate entities, rather than opposite ends of the same continuum along the BAS axis, a view that may also better account for their co-occurrence during mixed episodes (i.e., simultaneous episodes of mania and depression) (Cuellar et al., 2005).
Considerable evidence from morphometric MRI and post-mortem histological studies in BD patients demonstrate abnormalities in brain structures related to the three RST systems, thereby supporting the involvement of multiple dysregulated motivation systems and our integrative model (Diekhof et al., 2008; Savitz and Drevets, 2009). For example, the common finding of reduced gray matter volumes of the ACC (Phillips and Vieta, 2007; Strakowski et al., 2012) is compatible with disrupted function within a major BIS area. To note, these abnormalities have mostly been described in the sub-genual ACC, which is regarded as a central node of the FFFS (Pearlson, 1999; Fountoulakis, 2008; Sanches et al., 2008). In a similar manner, reduced gray matter volumes have been found in BD in several prefrontal cortices (PFC), spanning more than one motivation subsystem, including ventro-medial PFC for BIS (Doris et al., 2004; Lyoo et al., 2004), dorso-medial PFC for BAS (Locke and Braver, 2008) and Orbito Frontal Cortex (OFC; Haznedar et al., 2005; Lyoo et al., 2006), a region which some relate to BAS (Depue and Collins, 1999). Interestingly, Diffusion Tensor Imaging (DTI) analysis further demonstrated reduced integrity of white matter tracts in OFC (Beyer et al., 2005). Although the inter-segregation of the PFC is somewhat simplistic, given the multiple different processes these regions are involved in, these findings suggest possible deficits in PFC regulation and control functions affecting both BAS and BIS. To sum up the structural findings in the PFC—abnormalities were found in the sgACC related to the FFFS, the dmPFC and OFC related to BAS and in the vmPFC and ACC related to BIS.
Structural variations in amygdala and hippocampal volume are also common in BD (Strakowski et al., 2012; Phillips and Swartz, 2014), suggesting the involvement of BAS, FFFS and BIS. Yet, results in these regions vary with respect to the type of abnormality (e.g., Pearlson et al., 1997; Altshuler et al., 1998), possibly due to inter-study variations in medication regimes and comorbidities.
Several functional imaging studies using functional MRI (fMRI) indicate combined system involvement in BD (see summary in Table 1). Together these finding suggest two patterns of RST activations depending on BD states: hyperactivation of BAS along with hypoactivation in the FFFS/BIS in mania and vice versa depression.
This pattern has been convincingly demonstrated in the study by Blumberg et al. (2003) who investigated three BD patient groups: hypomanic, euthymic and depressed, while performing a Stroop task. Intriguingly, the hypomanic group showed decreased activity in the sgACC compared to the euthymic group, contrary to depressed patients who showed increased sgACC activity compared with the euthymic group, suggesting that FFFS, a punishment sensitive system, is under-activated in the manic state and over-activated in depression. In addition, the depressed group showed decreased dorsal and increased ventral activity in mPFC but the hypomanic group showed increased dorsal and decreased ventral activity in mPFC. These findings are compatible with decreased BAS and increased BIS activity in depression and vice versa in the manic state. This study thus points to deficits in prefrontal signaling in BD, possibly resulting from reduced FFFS-related punishment alerts, coupled with a diminished regulatory control of the BIS in manic state and vice versa in the depressed state. Further supporting this view is evidence from Positron Emission Tomography (PET) studies, showing increased sgACC, ACC and hippocampal metabolism in depressed BD patients during the resting state (Bauer et al., 2005; Mah et al., 2007).
Altogether, imaging findings strongly support our proposal for the involvement of several RST systems in BD, suggesting that rather than a deficit in BAS alone, the coupled interaction of BAS with BIS/FFFS is impaired (Figure 2). To note, this systems imbalance shows opposite directions for manic vs. depressed states, which may reflect a more general rule for explaining swings in mood states in other psychiatric conditions such as personality disorders.
We argue here for considering abnormal motivational processes in the pathophysiological study of mental illnesses, driven by neural system sensitive to reward, punishment or their regulation during goal-conflict. Based on a theoretical framework and converging empirical evidence, we suggest that although the BAS dysregulation theory has indeed provided a parsimonious and intuitive model for the involvement of motivational processes in BD, a conceptual revision is needed to further adapt these insights to the complexity of human psychopathology. Our new model maintains that since combined activity of the three motivation systems is responsible for the varied encounters with the external environment, BD is probably underpinned by compound motivational abnormalities: depression involves a hyperactive BIS and a hypoactive BAS, while mania results from a hyperactive BAS coupled with a hypoactive BIS (as illustrated in Figure 2). Notably, while it is possible that abnormal activity in BIS stems entirely from primary system abnormalities, deficits in FFFS activation may also be a contributing factor.
Thinking along this theoretical framework may provide useful insights into other psychiatric disorders as well. For example, similar abnormalities in motivation may also exist in unipolar depression MDD (Kasch et al., 2002; Kircanski et al., 2013), as was previously suggested (Eshel and Roiser, 2010).
Such a complex large-scale network model has recently become a common conceptual framework in neuroscientific research (Bressler and Menon, 2010) and especially with regard to emotional processing (Raz et al., 2012). Recent findings indeed point to abnormalities in large-scale network configuration in various psychopathologic states (e.g., Bassett and Bullmore, 2009), as we have recently discussed for the case of OCD (Hendler et al., 2014). By delineating the neurobiological underpinnings of basic psychological processes such as motivation and their dysfunction, this mechanistic approach may offer substantial progress in understanding the clinical presentation of mental illnesses and in treating them. This idea echoes the growing interest in the new approach termed Research Domain Criteria (RDoC; Sanislow et al., 2010) advanced by the NIMH, which aims to classify mental disorders based on dimensions of observable behavior and neurobiological measures. Indeed, within the framework of this approach motivational mechanisms were formulated into two RDoC domains: the “positive valence system” domain is related to approach motivation and reward processing, whereas the “negative valence system” domain is related to threat, loss and frustrative non-reward processing. The centrality of motivational processes in normal behavior attests to their probable involvement in pathological behavior as well. Inversely, looking at the complex abnormalities in motivation systems in human psychopathology may help determine the relations between these different constructs of motivation in normal behavior. Similar to studies in classic “lesion-neurology”, which advanced our understanding of normal brain function by exploring patients with focal brain lesions, the study of abnormalities of motivation as expressed across a range of pathological mental states may yield a deeper understanding of this primal yet complex neurobehavioral process.
To further investigate the core features of our model, such as dysfunction within and between motivational networks, future studies may explore the dynamics in functional-connectivity of the different systems’ key nodes using, for example, the network cohesion index (NCI; Raz et al., 2012), to reveal their ongoing interactions throughout performance in motivational tasks. Additionally, DTI studies may add the structural substrate to FC analyses in this effort. A causal role for different motivational circuits may also be demonstrated using interventional approaches. Recent developments in neurofeedback-based Brain Computer Interface (NF-BCI) using EEG and real-time fMRI methods aimed at modulating the activity in major nodes of different motivational systems may offer unique opportunities to track and validate the role of brain abnormalities in BD (Keedwell and Linden, 2013). Assessing the clinical efficacy of modulating a single vs. multiple motivation systems would further help to elucidate the underlying pathophysiology, and thus, may provide evidence regarding the suggested integrated model of motivational processes in the healthy brain as well.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Eyal Soreq for his substantial help with the graphical artwork in this paper.
Alloy, L. B., Abramson, L. Y., Walshaw, P. D., Cogswell, A., Grandin, L. D., Hughes, M. E., et al. (2008). Behavioral approach system and behavioral inhibition system sensitivities and bipolar spectrum disorders: prospective prediction of bipolar mood episodes. Bipolar Disorder. 10, 310–322. doi: 10.1111/j.1399-5618.2007.00547.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Alloy, L. B., Abramson, L. Y., Walshaw, P. D., Cogswell, A., Smith, J. M., Neeren, A. M., et al. (2006). Behavioral approach system (BAS) sensitivity and bipolar spectrum disorders: a retrospective and concurrent behavioral high-risk design. Motiv. Emot. 30, 143–155. doi: 10.1007/s11031-006-9003-3
Altshuler, L., Bartzokis, G., Grieder, T., Curran, J., and Mintz, J. (1998). Amygdala enlargement in bipolar disorder and hippocampal reduction in schizophrenia: an MRI study demonstrating neuroanatomic specificity. Arch. Gen. Psychiatry 55, 663–664.
Altshuler, L., Bookheimer, S., Proenza, M. A., Townsend, J., Sabb, F., Firestine, A., et al. (2005). Increased amygdala activation during mania: a functional magnetic resonance imaging study. Am. J. Psychiatry 162, 1211–1213. doi: 10.1176/appi.ajp.162.6.1211
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Avila, C., Parcet, M. A., and Barrós-Loscertales, A. (2008). A cognitive neuroscience approach to individual differences in sensitivity to reward. Neurotox. Res. 14, 191–203. doi: 10.1007/bf03033810
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Barrós-Loscertales, A., Ventura-Campos, N., Sanjuán-Tomás, A., Belloch, V., Parcet, M.-A., and Ávila, C. (2010). Behavioral activation system modulation on brain activation during appetitive and aversive stimulus processing. Soc. Cogn. Affect. Neurosci. 5, 18–28. doi: 10.1093/scan/nsq012
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Bassett, D. S., and Bullmore, E. T. (2009). Human brain networks in health and disease. Curr. Opin. Neurol. 22, 340–347. doi: 10.1097/wco.0b013e32832d93dd
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Bauer, M., London, E. D., Rasgon, N., Berman, S. M., Frye, M. A., Altshuler, L. L., et al. (2005). Supraphysiological doses of levothyroxine alter regional cerebral metabolism and improve mood in bipolar depression. Mol. Psychiatry 10, 456–469. doi: 10.1038/sj.mp.4001647
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Bermpohl, F., Kahnt, T., Dalanay, U., Hägele, C., Sajonz, B., Wegner, T., et al. (2010). Altered representation of expected value in the orbitofrontal cortex in mania. Hum. Brain Mapp. 31, 958–969. doi: 10.1002/hbm.20909
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Berridge, K. C., and Kringelbach, M. L. (2013). Neuroscience of affect: brain mechanisms of pleasure and displeasure. Curr. Opin. Neurobiol. 23, 294–303. doi: 10.1016/j.conb.2013.01.017
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Beyer, J. L., Taylor, W. D., Macfall, J. R., Kuchibhatla, M., Payne, M. E., Provenzale, J. M., et al. (2005). Cortical white matter microstructural abnormalities in bipolar disorder. Neuropsychopharmacology 30, 2225–2229. doi: 10.1038/sj.npp.1300802
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Blumberg, H., Leung, H. C., Skudlarski, P., Lacadie, C. M., Fredericks, C. A., Harris, B. C., et al. (2003). A functional magnetic resonance imaging study of bipolar disorder: state—and trait—related dysfunction in ventral prefrontal cortices. Arch. Gen. Psychiatry 60, 601–609. doi: 10.1001/archpsyc.60.6.601
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Bressler, S. L., and Menon, V. (2010). Large-scale brain networks in cognition: emerging methods and principles. Trends Cogn. Sci. 14, 277–290. doi: 10.1016/j.tics.2010.04.004
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Chen, P. S., Mcquoid, D. R., Payne, M. E., and Steffens, D. C. (2006). White matter and subcortical gray matter lesion volume changes and late-life depression outcome: a 4-year magnetic resonance imaging study. Int. Psychogeriatr. 18, 445–456. doi: 10.1017/s1041610205002796
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Corr, P. J., and Perkins, A. M. (2006). The role of theory in the psychophysiology of personality: from Ivan Pavlov to Jeffrey Gray. Int. J. Psychophysiol. 62, 367–376. doi: 10.1016/j.ijpsycho.2006.01.005
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Costumero, V., Barrós-Loscertales, A., Bustamante, J. C., Ventura-Campos, N., Fuentes, P., and Ávila, C. (2013). Reward sensitivity modulates connectivity among reward brain areas during processing of anticipatory reward cues. Eur. J. Neurosci. 38, 2399–2407. doi: 10.1111/ejn.12234
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Cuellar, A., Johnson, S. L., and Winters, R. (2005). Distinctions between bipolar and unipolar depression. Clin. Psychol. Rev. 25, 307–339. doi: 10.1016/j.cpr.2004.12.002
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Dayan, P., and Berridge, K. C. (2014). Model-based and model-free Pavlovian reward learning: revaluation, revision and revelation. Cogn. Affect. Behav. Neurosci. 14, 473–492. doi: 10.3758/s13415-014-0277-8
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Deckersbach, T., Rauch, S. L., Buhlmann, U., Ostacher, M. J., Beucke, J. C., Nierenberg, A. A., et al. (2008). An fMRI investigation of working memory and sadness in females with bipolar disorder: a brief report. Bipolar Disord. 10, 928–942. doi: 10.1111/j.1399-5618.2008.00633.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Depue, R. A., and Collins, P. F. (1999). Neurobiology of the structure of personality: dopamine, facilitation of incentive motivation and extraversion. Behav. Brain Sci. 22, 491–517. doi: 10.1017/s0140525x99002046
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Depue, R. A., and Iacono, W. G. (1989). Neurobehavioral aspects of affective disorders. Annu. Rev. Psychol. 40, 457–492. doi: 10.1146/annurev.psych.40.1.457
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Diekhof, E. K., Falkai, P., and Gruber, O. (2008). Functional neuroimaging of reward processing and decision-making: a review of aberrant motivational and affective processing in addiction and mood disorders. Brain Res. Rev. 59, 164–184. doi: 10.1016/j.brainresrev.2008.07.004
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Doris, A., Belton, E., Ebmeier, K. P., Glabus, M. F., and Marshall, I. (2004). Reduction of cingulate gray matter density in poor outcome bipolar illness. Psychiatry Res. 130, 153–159. doi: 10.1016/j.pscychresns.2003.09.002
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Dowd, E. C., and Barch, D. M. (2012). Pavlovian reward prediction and receipt in schizophrenia: relationship to anhedonia. Plos One 7:e35622. doi: 10.1371/journal.pone.0035622
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Drevets, W. C. (2001). Neuroimaging and neuropathological studies of depression: implications for the cognitive-emotional features of mood disorders. Curr. Opin. Neurobiol. 11, 240–249. doi: 10.1016/s0959-4388(00)00203-8
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Elliott, R., Ogilvie, A., Rubinsztein, J. S., Calderon, G., Dolan, R. J., and Sahakian, B. J. (2004). Abnormal ventral frontal response during performance of an affective go/no go task in patients with mania. Biol. Psychiatry 55, 1163–1170. doi: 10.1016/j.biopsych.2004.03.007
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Eshel, N., and Roiser, J. P. (2010). Reward and punishment processing in depression. Biol. Psychiatry 68, 118–124. doi: 10.1016/j.biopsych.2010.01.027
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Fountoulakis, K. (2008). Assessing the role of cingulate cortex in bipolar disorder: neuropathological, structural and functional imaging data. Brain Res. Rev. 59, 9–21. doi: 10.1016/j.brainresrev.2008.04.005
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Gonen, T., Admon, R., Podlipsky, I., and Hendler, T. (2012). From animal model to human brain networking: dynamic causal modeling of motivational systems. J. Neurosci. 32, 7218–7224. doi: 10.1523/jneurosci.6188-11.2012
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Gray, J. (1982). The Neuropsychology of Anxiety: An Enquiry into the Functions of the Septo—Hippocampal. Oxford: Oxford University Press.
Gray, J. A., and McNaughton, N. (2000). The Neuropsychology of Anxiety: An Enquiry in to the Functions of the Septo-hippocampal System. Oxford: Oxford University Press.
Haas, B., and Canli, T. (2008). Emotional memory function, personality structure and psychopathology: a neural system approach to the identification of vulnerability markers. Brain Res. Rev. 58, 71–84. doi: 10.1016/j.brainresrev.2007.10.014
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Haber, S. N., and Knutson, B. (2009). The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology 35, 4–26. doi: 10.1038/npp.2009.129
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hayes, D. J., and Northoff, G. (2011). Identifying a network of brain regions involved in aversion-related processing: a cross-species translational investigation. Front. Integr. Neurosci. 5:49. doi: 10.3389/fnint.2011.00049
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Haznedar, M. M., Roversi, F., Pallanti, S., Baldini-Rossi, N., Schnur, D. B., Licalzi, E. M., et al. (2005). Fronto-thalamo-striatal gray and white matter volumes and anisotropy of their connections in bipolar spectrum illnesses. Biol. Psychiatry 57, 733–742. doi: 10.1016/j.biopsych.2005.01.002
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hendler, T., Gonen, T., Harel, E. V., and Sharon, H. (2014). From circuit activity to network connectivity and back: the case of obsessive-compulsive disorder. Biol. Psychiatry 75, 590–592. doi: 10.1016/j.biopsych.2014.02.017
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Johnson, S. L., Edge, M. D., Holmes, M. K., and Carver, C. S. (2012). The behavioral activation system and mania. Annu. Rev. Clin. Psychol. 8, 243–267. doi: 10.1146/annurev-clinpsy-032511-143148
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kasch, K., Rottenberg, J., Arnow, B., and Gotlib, I. (2002). Behavioral activation and inhibition systems and the severity and course of depression. J. Abnorm. Psychol. 111, 589–597. doi: 10.1037//0021-843x.111.4.589
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Keedwell, P. A., and Linden, D. E. (2013). Integrative neuroimaging in mood disorders. Curr. Opin. Psychiatry 26, 27–32. doi: 10.1097/yco.0b013e32835a0b63
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Killgore, W. D. S., Gruber, S. A., and Yurgelun-Todd, D. A. (2008). Abnormal corticostriatal activity during fear perception in bipolar disorder. Neuroreport 19, 1523–1527. doi: 10.1097/wnr.0b013e328310af58
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kircanski, K., Mazur, H., and Gotlib, I. H. (2013). Behavioral activation system moderates self-referent processing following recovery from depression. Psychol. Med. 43, 1909–1919. doi: 10.1017/s0033291712002851
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Knutson, B., Bhanji, J. P., Cooney, R. E., Atlas, L. Y., and Gotlib, I. H. (2008). Neural responses to monetary incentives in major depression. Biol. Psychiatry 63, 686–692. doi: 10.1016/j.biopsych.2007.07.023
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Knutson, B., and Gibbs, S. E. B. (2007). Linking nucleus accumbens dopamine and blood oxygenation. Psychopharmacology (Berl) 191, 813–822. doi: 10.1007/s00213-006-0686-7
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Lawrence, N. S., Williams, A. M., Surguladze, S., Giampietro, V., Brammer, M. J., Andrew, C., et al. (2004). Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biol. Psychiatry 55, 578–587. doi: 10.1016/j.biopsych.2003.11.017
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Leppänen, J. (2006). Emotional information processing in mood disorders: a review of behavioral and neuroimaging findings. Curr. Opin. Psychiatry 19, 34–39. doi: 10.1097/01.yco.0000191500.46411.00
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Locke, H. S., and Braver, T. S. (2008). Motivational influences on cognitive control: behavior, brain activation and individual differences. Cogn. Affect. Behav. Neurosci. 8, 99–112. doi: 10.3758/cabn.8.1.99
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Lyoo, I. K., Kim, M. J., Stoll, A. L., Demopulos, C. M., Parow, A. M., Dager, S. R., et al. (2004). Frontal lobe gray matter density decreases in bipolar I disorder. Biol. Psychiatry 55, 648–651. doi: 10.1016/j.biopsych.2003.10.017
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Lyoo, I. K., Sung, Y. H., Dager, S. R., Friedman, S. D., Lee, J. Y., Kim, S. J., et al. (2006). Regional cerebral cortical thinning in bipolar disorder. Bipolar Disord. 8, 65–74. doi: 10.1111/j.1399-5618.2006.00284.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Mah, L., Zarate, C. A. Jr., Singh, J., Duan, Y. F., Luckenbaugh, D. A., Manji, H. K., et al. (2007). Regional cerebral glucose metabolic abnormalities in bipolar II depression. Biol. Psychiatry 61, 765–775. doi: 10.1016/j.biopsych.2006.06.009
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Malhi, G. S., Lagopoulos, J., Sachdev, P., Mitchell, P. B., Ivanovski, B., and Parker, G. B. (2004a). Cognitive generation of affect in hypomania: an fMRI study. Bipolar Disorder. 6, 271–285. doi: 10.1111/j.1399-5618.2004.00123.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Malhi, G. S., Lagopoulos, J., Ward, P. B., Kumari, V., Mitchell, P. B., Parker, G. B., et al. (2004b). Cognitive generation of affect in bipolar depression: an fMRI study. Eur. J. Neurosci. 19, 741–754. doi: 10.1111/j.0953-816x.2003.03159.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
McNaughton, N., and Corr, P. J. (2008). “The neuropsychology of fear and anxiety: a foundation for reinforcement sensitivity theory,” in The Reinforcement Sensitivity Theory of Personality, ed P. J. Corr (Cambridge: Cambridge University Press), 44–94.
Meyer, B., Beevers, C. G., Johnson, S. L., and Simmons, E. (2007). Unique association of approach motivation and mania vulnerability. Cogn. Emot. 21, 1647–1668. doi: 10.1080/02699930701252686
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Meyer, B., Johnson, S. L., and Winters, R. (2001). Responsiveness to threat and incentive in bipolar disorder: relations of the BIS/BAS scales with symptoms. J. Psychopathol. Behav. Assess. 23, 133–143. doi: 10.1023/A:1010929402770
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Miller, E. M., Shankar, M. U., Knutson, B., and McClure, S. M. (2013). Dissociating motivation from reward in human striatal activity. J. Cogn. Neurosci. 26, 1075–1084. doi: 10.1162/jocn_a_00535
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Nunes, E. J., Randall, P. A., Podurgiel, S., Correa, M., and Salamone, J. D. (2013). Nucleus accumbens neurotransmission and effort-related choice behavior in food motivation: effects of drugs acting on dopamine, adenosine and muscarinic acetylcholine receptors. Neurosci. Biobehav. Rev. 37, 2015–2025. doi: 10.1016/j.neubiorev.2013.04.002
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Pavlickova, H., Varese, F., Turnbull, O., Scott, J., Morriss, R., Kinderman, P., et al. (2013). Symptom-specific self-referential cognitive processes in bipolar disorder: a longitudinal analysis. Psychol. Med. 43, 1895–1907. doi: 10.1017/s0033291712002711
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Pavlov, I. P. (1927). Conditioned reflexes: An Investigation of the Physiological Activity of the Cerebral Cortex. London: Oxford University Press.
Pearlson, G. D. (1999). Structural and functional brain changes in bipolar disorder: a selective review. Schizophr. Res. 39, 133–140. doi: 10.1016/s0920-9964(99)00112-7
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Pearlson, G. D., Barta, P. E., Powers, R. E., Menon, R. R., Richards, S. S., Aylward, E. H., et al. (1997). Medial and superior temporal gyral volumes and cerebral asymmetry in schizophrenia versus bipolar disorder. Biol. Psychiatry 41, 1–14. doi: 10.1016/s0006-3223(96)00373-3
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Phillips, M. L., and Swartz, H. A. (2014). A critical appraisal of neuroimaging studies of bipolar disorder: toward a new conceptualization of underlying neural circuitry and a road map for future research. Am. J. Psychiatry 171, 829–843. doi: 10.1176/appi.ajp.2014.13081008
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Phillips, M. L., and Vieta, E. (2007). Identifying functional neuroimaging biomarkers of bipolar disorder: toward DSM-V. Schizophr. Bull. 33, 893–904. doi: 10.1093/schbul/sbm060
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Phillips, P. E., Walton, M. E., and Jhou, T. C. (2007). Calculating utility: preclinical evidence for cost-benefit analysis by mesolimbic dopamine. Psychopharmacology (Berl) 191, 483–495. doi: 10.1007/s00213-006-0626-6
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Pizzagalli, D. A., Goetz, E., Ostacher, M., Iosifescu, D. V., and Perlis, R. H. (2008). Euthymic patients with bipolar disorder show decreased reward learning in a probabilistic reward task. Biol. Psychiatry 64, 162–168. doi: 10.1016/j.biopsych.2007.12.001
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Raz, G., Winetraub, Y., Jacob, Y., Kinreich, S., Maron-Katz, A., Shaham, G., et al. (2012). Portraying emotions at their unfolding: a multilayered approach for probing dynamics of neural networks. Neuroimage 60, 1448–1461. doi: 10.1016/j.neuroimage.2011.12.084
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Salamone, J. D., and Correa, M. (2012). The mysterious motivational functions of mesolimbic dopamine. Neuron 76, 470–485. doi: 10.1016/j.neuron.2012.10.021
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Salavert, J., Caseras, X., Torrubia, R., Furest, S., Arranz, B., Dueñas, R., et al. (2007). The functioning of the behavioral activation and inhibition systems in bipolar I euthymic patients and its influence in subsequent episodes over an eighteen-month period. Pers. Individ. Dif. 42, 1323–1331. doi: 10.1016/j.paid.2006.10.010
Sanches, M., Keshavan, M. S., Brambilla, P., and Soares, J. C. (2008). Neurodevelopmental basis of bipolar disorder: a critical appraisal. Prog. Neuropsychopharmacol. Biol. Psychiatry 32, 1617–1627. doi: 10.1016/j.pnpbp.2008.04.017
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Sanislow, C. A., Pine, D. S., Quinn, K. J., Kozak, M. J., Garvey, M. A., Heinssen, R. K., et al. (2010). Developing constructs for psychopathology research: research domain criteria. J. Abnorm. Psychol. 119, 631–639. doi: 10.1037/a0020909
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Savitz, J., and Drevets, W. C. (2009). Bipolar and major depressive disorder: neuroimaging the develop mental-degenerative divide. Neurosci. Biobehav. Rev. 33, 699–771. doi: 10.1016/j.neubiorev.2009.01.004
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Schweimer, J., Saft, S., and Hauber, W. (2005). Involvement of catecholamine neurotransmission in the rat anterior cingulate in effort-related decision making. Behav. Neurosci. 119, 1687–1692. doi: 10.1037/0735-7044.119.6.1687
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Smillie, L. D. (2008). What is reinforcement sensitivity? Neuroscience paradigms for approach-avoidance process theories of personality. Eur. J. Pers. 22, 359–384. doi: 10.1002/per.674
Strakowski, S. M., Adler, C. M., Almeida, J., Altshuler, L. L., Blumberg, H. P., Chang, K. D., et al. (2012). The functional neuroanatomy of bipolar disorder: a consensus model. Bipolar Disorder. 14, 313–325. doi: 10.1111/j.1399-5618.2012.01022.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Swann, A. C., Dougherty, D. M., Pazzaglia, P. J., Pham, M., and Moeller, F. G. (2004). Impulsivity: a link between bipolar disorder and substance abuse. Bipolar Disorder. 6, 204–212. doi: 10.1111/j.1399-5618.2004.00110.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Treadway, M. T., Bossaller, N. A., Shelton, R. C., and Zald, D. H. (2012). Effort-based decision-making in major depressive disorder: a translational model of motivational anhedonia. J. Abnorm. Psychol. 121, 553–558. doi: 10.1037/a0028813
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Treadway, M. T., and Zald, D. H. (2011). Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci. Biobehav. Rev. 35, 537–555. doi: 10.1016/j.neubiorev.2010.06.006
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Wessa, M., Houenou, J., Paillère-Martinot, M. L., Berthoz, S., Artiges, E., Leboyer, M., et al. (2007). Fronto-striatal overactivation in euthymic bipolar patients during an emotional go/nogo task. Am. J. Psychiatry 164, 638–646. doi: 10.1176/appi.ajp.164.4.638
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Yurgelun-Todd, D. A., Gruber, S. A., Kanayama, G., Killgore, W. D., Baird, A. A., and Young, A. D. (2000). fMRI during affect discrimination in bipolar affective disorder. Bipolar Disord. 2, 237–248. doi: 10.1034/j.1399-5618.2000.20304.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Keywords: functional neuroimaging, motivation and affectives process, bipolar disorder, depression, system neuroscience
Citation: Gonen T, Sharon H, Pearlson G and Hendler T (2014) Moods as ups and downs of the motivation pendulum: revisiting reinforcement sensitivity theory (RST) in bipolar disorder. Front. Behav. Neurosci. 8:378. doi: 10.3389/fnbeh.2014.00378
Received: 30 March 2014; Accepted: 13 October 2014;
Published online: 03 November 2014.
Edited by:
John D. Salamone, University of Connecticut, USAReviewed by:
Therese A. Kosten, Baylor College of Medicine, USACopyright © 2014 Gonen, Sharon, Pearlson and Hendler. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution and reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Talma Hendler, Functional Brain Center, Wohl Institute for Advanced Imaging, Tel Aviv Medical Center, Weizmann 6, Tel Aviv, Israel e-mail:dGFsbWFAdGx2bWMuZ292Lmls
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.