
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Behav. Neurosci. , 14 March 2014
Sec. Motivation and Reward
Volume 8 - 2014 | https://doi.org/10.3389/fnbeh.2014.00086
This article is part of the Research Topic Neuronal and Psychological Underpinnings of Pathological Gambling View all 15 articles
 Andrew D. Lawrence1*
Andrew D. Lawrence1* David J. Brooks2,3
David J. Brooks2,3Pathological gambling, alongside addictive and antisocial disorders, forms part of a broad psychopathological spectrum of externalizing disorders, which share an underlying genetic vulnerability. The shared externalizing propensity is a highly heritable, continuously varying trait. Disinhibitory personality traits such as impulsivity and novelty seeking (NS) function as indicators of this broad shared externalizing tendency, which may reflect, at the neurobiological level, variation in the reactivity of dopaminergic (DAergic) brain reward systems centered on the ventral striatum (VS). Here, we examined whether individual differences in ventral striatal dopamine (DA) synthesis capacity were associated with individual variation in disinhibitory personality traits. Twelve healthy male volunteers underwent 6-[18F]Fluoro-L-DOPA (FDOPA) positron emission tomography (PET) scanning to measure striatal DA synthesis capacity, and completed a measure of disinhibited personality (NS). We found that levels of ventral, but not dorsal, striatal DA synthesis capacity were significantly correlated with inter-individual variation in disinhibitory personality traits, particularly a propensity for financial extravagance and irresponsibility. Our results are consistent with preclinical models of behavioral disinhibition and addiction proneness, and provide novel insights into the neurobiology of personality based vulnerability to pathological gambling and other externalizing disorders.
Patterns of systematic co-occurrence (“comorbidity”) between substance misuse and antisocial disorders are best accounted for by a model positing a shared underlying genetic vulnerability, known as externalizing (Krueger et al., 2002, 2007). This broad externalizing vulnerability is a highly heritable, continuously varying dimension of risk (Krueger et al., 2007). Pathological gambling [now called gambling disorder] systematically co-occurs with both substance misuse and antisocial disorders (Kessler et al., 2008; Oleski et al., 2011) and this co-variation likewise reflects a shared genetic vulnerability (Slutske et al., 2001, 2013; Blanco et al., 2012). Thus, pathological gambling can be considered one variant of an externalizing spectrum of disorders.
The broad personality trait of disinhibition reflects individual differences in the tendency to behave in a disinhibited vs. controlled fashion (Dindo et al., 2009). Disinhibitory personality traits are strongly linked with externalizing disorders (Ruiz et al., 2008), including pathological gambling (MacLaren et al., 2011). Importantly, a shared genetic diathesis underlies the associations between trait disinhibition and externalizing disorders (Krueger et al., 2002; Hicks et al., 2011).
Furthermore, prospective studies suggest that trait disinhibition, measured early in life, predates and predicts the emergence of externalizing pathology, including pathological gambling (Elkins et al., 2006; Slutske et al., 2012) and mediates the co-variation between externalizing disorders (Ruiz et al., 2008). Thus the antecedent trait of disinhibition provides the temperamental core of the externalizing disorders, and disinhibitory personality traits such as impulsivity and sensation seeking function as indicators of the general externalizing propensity (Krueger et al., 2002, 2007).
The genetic liability to externalizing may be related, at the neurobiological level, to brain mechanisms underpinning sensitivity to reward (Iacono et al., 2008). Brain dopamine (DA) systems have long been hypothesized to underlie individual variation in reward sensitivity. According to Gray (1987), individual differences in trait impulsivity reflect individual variation in the reactivity of a neural “behavioral activation system” (BAS), centred on the ventral striatum (VS) and its dopaminergic (DAergic) irrigation, which is triggered by cues for reward. Likewise, in Cloninger’s (1986) model of temperament, novelty-seeking (NS) tendencies reflect genetically determined variation in reward-seeking behaviors, mediated by DAergic modulation of the BAS. When activated, the BAS functions as an impulsive “go” motivational system, and variation in BAS reactivity is potentially a potent source of inter-individual variation in behavioral disinhibition (Newman and Wallace, 1993).
Recent research highlights that genetic variation in DA synthesis pathways may play a key role in the etiology of externalizing liability. DA synthesis occurs within DA neurons. Tyrosine is transported into the cell via amino acid carriers in the blood-brain barrier and cell membranes. Once in the intracellular space it is hydroxylated to L-3,4-dihydroxiphenylalanine (L-DOPA) by tyrosine hydroxylase (TH). L-DOPA is then decarboxylated by aromatic L-amino acid decarboxylase (AADC) (also called dopa decarboxylase, DDC) to DA (Elsworth and Roth, 2009). In an important study, Derringer et al. (2010) found that a combination of multiple common variants (single nucleotide polymorphisms, SNPs) in the DDC gene predicted individual variation in sensation seeking traits, suggesting that genetic variation in DA synthesis contributes to the broad externalizing liability, of which sensation seeking functions as one indicator (Krueger et al., 2007).
Positron Emission Tomography (PET) can be used to study the activity of AADC in pre-synaptic DA terminals in the living brain. The PET tracer 6-[18F]fluoro-L-DOPA (FDOPA), a radioactive analog of L-DOPA, the precursor of DA, is taken up by pre-synaptic DAergic neurons and is metabolized by AADC to 18F-DA, which is trapped and stored within vesicles in the nerve terminals (Kumakura and Cumming, 2009). FDOPA uptake, quantified as the influx constant Ki, can be used as a measure of AADC activity and vesicular storage capacity (Brown et al., 1999). High values for FDOPA Ki are observed in areas of dense DA nerve terminal innervation, such as the striatum (Kumakura and Cumming, 2009).
Consistent with the notion that externalizing propensity reflects, neuro-biologically, inter-individual variation in DAergic modulation of the BAS, we recently found, in a group of Parkinson’s disease patients, that individual differences in ventral striatal FDOPA Ki values were related to individual differences in disinhibitory personality traits, particularly a propensity for financial extravagance (Lawrence et al., 2013). The patients in that study, were however, being treated with DA agonist medication, which could potentially have influenced levels of both striatal DA synthesis (Rowlett et al., 1993) and behavioral disinhibition (Lawrence et al., 2003). Thus, it is important to ascertain whether the relationship between ventral striatal DA synthesis capacity and disinhibitory personality traits holds in a sample of healthy, medication-free individuals. Based on our previous findings, we predicted that increased FDOPA uptake in ventral, but not dorsal, striatum would be related to increased levels of trait disinhibition, in particular propensities for financial extravagance and irresponsibility.
Twelve right-handed healthy male volunteers (mean age 38 years, SD ± 7 years, range 29–49 years) participated, all with a normal neurological history and examination. A trained psychiatrist assessed participants and current and past psychiatric morbidity, including alcohol or drug dependency, was excluded by routine psychiatric interview and the General Health Questionnaire (Jackson, 2006) with a cut-off of 5 points or fewer.
The study was limited to men as there are gender differences in the prevalence and clinical presentation of gambling disorder and its relation to the externalizing spectrum (Blanco et al., 2006; Oleski et al., 2011) and in DA synthesis capacity (Laakso et al., 2002). Additionally, fMRI studies suggest a stronger relationship between ventral striatal activity to reward cues and impulsivity in men than women (Lahey et al., 2012).
Permission to undertake the study was granted by the Hammersmith Hospitals Research Ethics Committee and all participants gave written informed consent following a full explanation of the procedure. The Administration of Radioactive Substances Advisory Committee (ARSAC) of the UK approved radioisotope use.
Our measure of trait behavioral disinhibition was based on NS from Cloninger’s Tri-dimensional Personality Questionnaire (TPQ; Cloninger, 1987). The version of the TPQ used here was a 100-item, self-administered, true-false instrument. The questionnaire is scored so that higher scores reflect greater NS tendencies.
As originally constructed (Cloninger, 1987) TPQ-NS comprised four narrow facet-level scales: Exploratory Excitability vs. Stoic Rigidity (NS1), Impulsiveness vs. Reflection (NS2), Extravagance vs. Reserve (NS3), and Disorderliness vs. Regimentation (NS4). When Ando et al. (2004), however, examined the genetic and environmental factor structure of NS, factor analysis of the genetic inter-correlations yielded factors that did not fully resemble the phenotypic structure of NS as proposed by Cloninger (1987). NS was revised (r-NS) to consist of Impulsiveness vs. Reflection (NS2), Extravagance vs. Reserve (NS3) and Disorderliness vs. Regimentation (NS4), excluding Exploratory Excitability vs. Stoic Rigidity (NS1). Further, Flory and Manuck (2009), using factor analysis in a large normative sample of adults, found Impulsiveness vs. Reflection (NS2) and Extravagance vs. Reserve (NS3) to have high loadings on a “disinhibition” factor, along with the Barratt Impulsiveness Scale (BIS), whereas Exploratory Excitability vs. Stoic Rigidity (NS1) and Disorderliness vs. Regimentation (NS4) loaded on a distinct “Experience seeking” factor.
Hence, in the current study, we focused on those r-NS facets most strongly linked to trait disinhibition: Impulsiveness (vs. Reflection) (NS2) (8 items) and Extravagance (vs. Reserve) (NS3) (7 items). Sample items include “I often follow my instincts, hunches, or intuition without thinking through all the details” (Impulsivity, NS2) and “I often spend money until I run out of cash or get into debt from using too much credit” (Extravagance, NS3).
In addition to NS, we also measured Harm Avoidance (HA) traits using the TPQ. We calculated a total HA score based on the sum of the four individual HA facet-level scales, as Ando et al. (2004) confirmed Cloninger’s (1987) claim that the subscales used to define HA share a common genetic basis. According to Cloninger (1986), although NS and HA are genetically independent traits, at the phenotypic level high levels of HA should inhibit the expression of NS tendencies, since activation of the HA system results in a “reflexive” or “reactive” form of behavioral inhibition (Carver, 2008)—dampening the expression of appetitive approach behavior and NS, given cues of potential punishment (Newman and Wallace, 1993; Nikolova and Hariri, 2012). Indeed, meta-analysis reveals a consistent strong negative correlation between NS and HA (Miettunen et al., 2008; here the relation between HA and NS3 for example was r = −0.44), a relationship that is environmentally (i.e., through experience) and not genetically mediated (Ando et al., 2002). Hence, we controlled for the influence of HA when examining the relation between striatal FDOPA Ki values and NS traits.
Participants were pre-treated with 150 mg carbidopa and 400 mg entacapone 1 h prior to radioisotope administration (to block peripheral metabolism of FDOPA and so enhance specific signal detection) and underwent three-dimensional FDOPA PET using an ECAT EXACT HR++ (CTI/Siemens 966) camera, which covers an axial field of view of 23.4 cm and provides 95 transaxial planes. The tomograph has a spatial resolution of 4.8 + 0.2 mm FWHM (transaxial, 1 cm off axis) and 5.6 mm + 0.5 mm (axial, on axis) after image reconstruction (Spinks et al., 2000). A transmission scan, which corrects for attenuation of emitted radiation by skull and tissues, was acquired using a single rotating photon point source of 150 MBq of 137Cs. 30 s after the start of the emission scan, 110 (range 102–135) MBq of FDOPA in 10 ml normal saline was infused intravenously over 30 s. Three-dimensional sinograms of emission data were then acquired over 90 min as 26 time frames. Participants were placed in the scanner with the orbito-meatel line parallel to the transaxial plane of the tomograph. Head position was monitored via laser crosshairs and video camera.
Parametric images of specific FDOPA influx constants (Ki maps) were created at a voxel level for the whole brain using linear graphical analysis (Patlak and Blasberg, 1985) of time activity curves with an occipital cortex (Brown et al., 1979) non-specific reference input function. Qualitative summated ADD images created from the dynamic FDOPA time series by integrating all 26 frames of the dynamic image were also produced and then transformed into standard stereotaxic (Montreal Neurological Institute, MNI) space using an FDOPA template created in-house from a healthy volunteer database. These ADD images contain both tracer delivery and specific uptake information and provide adequate anatomical detail to allow them to be stereotaxically normalized into standard MNI space. Subsequently, the Ki maps were individually normalized to MNI stereotaxic space by applying the transformation parameters defined during the normalization of their respective ADD images. This spatial transformation of parametric images made it possible to perform a region of interest (ROI) analysis as described below.
Standard ROI object maps sampling the ventral and dorsal striatum were defined on the MNI single-subject ROI in stereotaxic space. For our striatal ROIs, the volume was subdivided as follows: all planes containing striatal structures below the anterior commissure-posterior commissure plane were operationally defined as the ventral striatum (VS) ROI, and all planes above the anterior commissure-posterior commissure plane containing striatal structures formed the dorsal striatum (DS) ROI. The standard object map was applied to the transformed Ki maps and values of FDOPA Ki (units: ml • g−1 • min−1) were obtained for the two striatal ROIs for each individual (McGowan et al., 2004). When performing our ROI analysis a manual correction for head movement was applied as previously described (Whone et al., 2003).
We used Pearson partial correlations to examine the relationships between striatal FDOPA Ki values and disinhibitory NS traits, controlling for relevant nuisance variables (Spector and Brannick, 2011). Statistical significance was set at a Bonferroni-corrected P < 0.0125 (i.e., 0.05/4).
Mean ± SD scores in our sample for NS2 (Impulsivity) and NS3 (Extravagance) were 3.5 ± 2.5 and 4.3 ± 1.1, respectively. These results are comparable to those obtained in a normative sample of 106 UK men (mean age 31, SD ± 11.5) by Otter et al. (1995) (NS2 mean 3.1, SD ± 2.2; NS3 mean 3.8, SD ± 2.0). HA scores (HA mean 8.1, SD ± 4.7) were somewhat lower than those reported by Otter et al. (HA mean 10.7 ± 6.2), perhaps reflecting self-selection bias in individuals who volunteer for PET scanning (Oswald et al., 2013).
Mean ±SD FDOPA Ki values for the VS and DS ROIs were 0.0131 ± 0.001 and 0.0125 ± 0.002 ml • g−1 • min−1 respectively.
Since, in adults, NS shows a significant decrease with increasing age (Otter et al., 1995), we controlled for the effects of age when examining the relationship between striatal FDOPA Ki and disinhibitory NS traits (Impulsivity and Extravagance). Furthermore, for the reasons outlined above, we additionally controlled for HA scores.
When controlling for the influence of age and HA there was a significant relationship between VS FDOPA Ki and NS3 (Extravagance) (r = 0.78, bootstrap 95% CI 0.52–0.98, P = 0.008), but not between VS FDOPA Ki and NS2 (Impulsivity) (r = 0.44, P = 0.2). There were no significant relations between DS FDOPA Ki and either NS3 (r = 0.28, P = 0.40) or NS2 (r = 0.003, P = 0.99) when controlling for age and HA (see Figure 1). Examination of Figure 1 suggests that one individual data point may be an outlier. When this data point was removed, however, the relationship between VS FDOPA Ki and NS3, controlling for age and HA, remained significant (r = 0.71, bootstrap 95% CI 0.51–0.92, P = 0.014). We found identical results when using a Spearman partial correlation (Schemper, 1991).
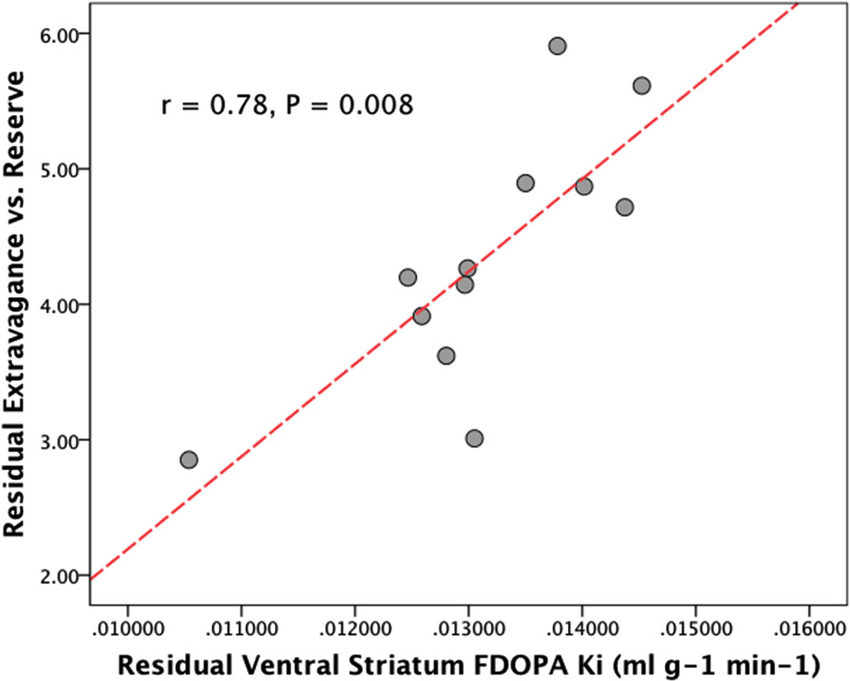
Figure 1. Plot showing the partial correlation between ventral striatal dopamine synthesis capacity (FDOPA Ki), and disinhibitory personality traits (NS3, Extravagance vs. reserve) controlling for age and Harm Avoidance.
Consistent with our hypothesis, we found that, controlling for the effects of age and HA, variation in trait disinhibition was associated with levels of striatal DA synthesis capacity. Individuals with greater levels of trait disinhibition, in particular, tendencies to financial irresponsibility and extravagance, had greater DA synthesis capacity, as indexed by FDOPA Ki values, in the ventral but not dorsal striatum.
We (Lawrence et al., 2013) recently found that individual differences in behavioral disinhibition (using the same personality trait measure as used here) were similarly related to individual differences in ventral striatal DA synthesis capacity in individuals with Parkinson’s disease. Those, individuals, were however, being treated with DA agonist medication, which could potentially have influenced both striatal DA synthesis (Rowlett et al., 1993) and externalizing behaviors, including pathological gambling (Weintraub et al., 2006). The current results importantly extend our earlier findings to healthy, non-medicated individuals, showing a relationship between disinhibitory traits and ventral striatal DA synthesis capacity in the absence of potential DAergic drug-induced effects. Taken together with the finding that genetic variation in DDC activity predicts disinhibitory sensation seeking tendencies in healthy individuals (Derringer et al., 2010), our results suggest that the link between behavioral disinhibition and ventral striatal DA synthesis capacity is likely to be, to a significant extent, genetically mediated. At the same time, we acknowledge that there are substantial (potentially shared) environmental influences on ventral striatal DA synthesis capacity (Stokes et al., 2013), behavioral disinhibition (Lomanowska et al., 2011) and externalizing (Hicks et al., 2013).
As in our earlier study of Parkinson’s disease, here we found that only the r-NS facet-level scale NS3 (Extravagance vs. Reserve) was related to ventral striatal DA synthesis capacity. There was no significant relation with the NS2 subscale (Impulsivity vs. Reflection). The reasons for this are unclear. It is notable, however, that, of the NS facet-level scales, NS3 shows the strongest relation to both pathological gambling (Kim and Grant, 2001; Nordin and Nylander, 2007) and substance abuse (Etter et al., 2003). It may be that, of the disinhibitory NS facets, NS3 most closely indexes those traits (irresponsibility, problematic impulsivity) that lie at the core of the broad externalizing factor (Krueger et al., 2007).
Consistent with the proposal that externalizing vulnerability reflects, at least in part, individual differences in reward sensitivity (Iacono et al., 2008); the influence of variation in ventral striatal DA synthesis capacity on externalizing propensity likely reflects DA’s role in one particular aspect of reward processing, namely the attribution of incentive salience (Berridge, 2012). Incentive salience is a motivational component of reward, one that transforms sensory information about rewards and reward cues into attractive, “wanted” incentives, motivating pursuit (Berridge, 2012). Notably, Flagel et al. (2010) found in rats that incentive salience attribution and behavioral disinhibition are genetically influenced, correlated traits. Available data suggest that animals prone to attribute incentive salience to reward cues have a more active DA system than those who do not (Flagel et al., 2010). In humans, VS FDOPA Ki values have been found to positively correlate with BOLD-fMRI activity to reward cues in limbic brain regions linked to incentive salience attribution (Siessmeier et al., 2006), and limbic BOLD-fMRI responses to reward cues are correlated with both disinhibitory personality traits (Beaver et al., 2006; Buckholtz et al., 2010) and externalizing symptomatology (Bjork et al., 2010). One possibility is that individuals high on externalizing risk show exaggerated phasic DA release to reward cues, resulting from a larger releasable pool of DA generated by increased DA synthesis capacity (Bello et al., 2011; Anzalone et al., 2012), triggering excessive attribution of incentive salience to environmental cues and their associated rewards, leading to behavioral disinhibition (Flagel et al., 2010; Lovic et al., 2011) (but see Huys et al., 2014 for an alternative proposal).
At first glance, our findings seem inconsistent with an earlier study of detoxified alcoholics, which found no differences in ventral striatal DA synthesis capacity relative to a healthy control group (Heinz et al., 2005). Alcohol misuse, however, is multiply determined, and influenced to a greater extent by factors unique to alcohol, than by the general tendency to externalizing (Krueger et al., 2007). Further, it is possible that chronic alcohol use may produce potentially neurotoxic effects on DA neurons (Gilman et al., 1998), obscuring any pre-morbid trait influence on DA synthesis capacity.
It is important to note that FDOPA is not a specific ligand for DA neurons but rather is metabolized by all neurons that contain AADC (Brown et al., 1999). Hence, it is a marker for all tissues that take up and store monoamines, including serotonin (5-hydroxytryptamine, 5-HT) as well as DA neurons (Hashemi et al., 2012). 5-HT has been implicated in various aspects of impulsivity (Carver et al., 2008; Cools et al., 2008). Notably, Jupp et al. (2013) however, failed to find a relationship between levels of trait impulsivity (defined by premature responding on a 5-choice serial reaction time task) and levels of accumbens 5-HT in rats. It is likely, therefore, that individual differences in trait disinhibition are primarily related to individual differences in ventral striatal DA synthesis capacity.
In conclusion, we have found that personality based vulnerability to externalizing problems, including pathological gambling, is related to relatively increased DA synthesis capacity in the ventral, but not dorsal, striatum in a sample of healthy men. Our results are consistent with preclinical models of behavioral disinhibition and addiction proneness, and may prove informative in understanding the neurobiological and psychological mechanisms underlying personality risk for phenotypically diverse forms of disinhibitory psychopathology.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The UK Medical Research Council funded this research. We are grateful to the technical PET staff for their assistance and to all the volunteers who participated in this study. This paper is dedicated to the memories of Dr Stephen McGowan and Dr Andy Calder.
Ando, J., Ono, Y., Yoshimura, K., Onoda, N., Shinohara, M., Kanba, S., et al. (2002). The genetic structure of Cloninger’s seven-factor model of temperament and character in a Japanese sample. J. Pers. 70, 583–609. doi: 10.1111/1467-6494.05018
Ando, J., Suzuki, A., Yamagata, S., Kijima, N., Maekawa, H., Ono, Y., et al. (2004). Genetic and environmental structure of Cloninger’s temperament and character dimensions. J. Pers. Disord. 18, 379–393. doi: 10.1521/pedi.2004.18.4.379
Anzalone, A., Lizardi-Ortiz, J. E., Ramos, M., De Mei, C., Hopf, F. W., Iaccarino, C., et al. (2012). Dual control of dopamine synthesis and release by presynaptic and postsynaptic dopamine D2 receptors. J. Neurosci. 32, 9023–9034. doi: 10.1523/jneurosci.0918-12.2012
Beaver, J. D., Lawrence, A. D., van Ditzhuijzen, J., Davis, M. H., Woods, A., and Calder, A. J. (2006). Individual differences in reward drive predict neural responses to images of food. J. Neurosci. 26, 5160–5166. doi: 10.1523/jneurosci.0350-06.2006
Bello, E. P., Mateo, Y., Gelman, D. M., Noaín, D., Shin, J. H., Low, M. J., et al. (2011). Cocaine supersensitivity and enhanced motivation for reward in mice lacking dopamine D2 autoreceptors. Nat. Neurosci. 14, 1033–1038. doi: 10.1038/nn.2862
Berridge, K. C. (2012). From prediction error to incentive salience: mesolimbic computation of reward motivation. Eur. J. Neurosci. 35, 1124–1143. doi: 10.1111/j.1460-9568.2012.07990.x
Bjork, J. M., Chen, G., Smith, A. R., and Hommer, D. W. (2010). Incentive-elicited mesolimbic activation and externalizing symptomatology in adolescents. J. Child Psychol. Psychiatry 51, 827–837. doi: 10.1111/j.1469-7610.2009.02201.x
Blanco, C., Hasin, D. S., Petry, N., Stinson, F. S., and Grant, B. F. (2006). Sex differences in subclinical and DSM-IV pathological gambling: results from the National Epidemiological Survey on Alcohol and Related Conditions. Psychol. Med. 36, 943–953. doi: 10.1017/s0033291706007410
Blanco, C., Myers, J., and Kendler, K. S. (2012). Gambling, disordered gambling and their association with major depression and substance use: a web-based cohort and twin-sibling study. Psychol. Med. 42, 497–508. doi: 10.1017/s0033291711001401
Brown, R. M., Crane, A. M., and Goldman, P. S. (1979). Regional distribution of monoamines in the cerebral cortex and subcortical structures of the rhesus monkey: concentrations and in vivo synthesis rates. Brain Res. 168, 133–150. doi: 10.1016/0006-8993(79)90132-x
Brown, W. D., Taylor, M. D., Roberts, A. D., Oakes, T. R., Schueller, M. J., Holden, J. E., et al. (1999). FluoroDOPA PET shows the nondopaminergic as well as dopaminergic destinations of levodopa. Neurology 53, 1212–1218. doi: 10.1212/wnl.53.6.1212
Buckholtz, J. W., Treadway, M. T., Cowan, R. L., Woodward, N. D., Benning, S. D., Li, R., et al. (2010). Mesolimbic dopamine reward system hypersensitivity in individuals with psychopathic traits. Nat. Neurosci. 13, 419–421. doi: 10.1038/nn.2510
Carver, C. S., Johnson, S. L., and Joormann, J. (2008). Serotonergic function, two-mode models of self-regulation, and vulnerability to depression: what depression has in common with impulsive aggression. Psychol. Bull. 134, 912–943. doi: 10.1037/a0013740
Carver, C. S. (2008). Two distinct bases of inhibition of behaviour: viewing biological phenomena through the lens of psychological theory. Eur. J. Pers. 22, 388–390. doi: 10.1002/per
Cloninger, C. R. (1986). A unified biosocial theory of personality and its role in the development of anxiety states. Psychiatric Dev. 4, 167–226.
Cloninger, C. R. (1987). A systematic method for clinical description and classification of personality variants. Arch. Gen. Psychiatry 44, 573–588. doi: 10.1001/archpsyc.1987.01800180093014
Cools, R., Roberts, A. C., and Robbins, T. W. (2008). Serotoninergic regulation of emotional and behavioural control processes. Trends Cogn. Sci. 12, 31–40. doi: 10.1016/j.tics.2007.10.011
Derringer, J., Krueger, R. F., Dick, D. M., Saccone, S., Grucza, R. A., Agrawal, A., et al. (2010). Predicting sensation seeking from dopamine genes: a candidate-system approach. Psychol. Sci. 21, 1282–1290. doi: 10.1177/0956797610380699
Dindo, L., McDade-Montez, E., Sharma, L., Watson, D., and Clark, L. A. (2009). Development and initial validation of the disinhibition inventory: a multifaceted measure of disinhibition. Assessment 16, 274–291. doi: 10.1177/1073191108328890
Elkins, I. J., King, S. M., McGue, M., and Iacono, W. G. (2006). Personality traits and the development of nicotine, alcohol and illicit drug disorders: prospective links from adolescence to young adulthood. J. Abnorm. Psychol. 115, 26–39. doi: 10.1037/0021-843x.115.1.26
Elsworth, J. D., and Roth, R. H. (2009). “Dopamine,” in Encyclopaedia of Neuroscience, ed L. R. Squire (Amsterdam: Elsevier), 539–547.
Etter, J. F., Pélissolo, A., Pomerleau, C., and De Saint-Hilaire, Z. (2003). Associations between smoking and heritable temperament traits. Nicotine Tob. Res. 5, 401–409. doi: 10.1080/1462220031000094240
Flagel, S. B., Robinson, T. E., Clark, J. J., Clinton, S. M., Watson, S. J., Seeman, P., et al. (2010). An animal model of genetic vulnerability to behavioral disinhibition and responsiveness to reward-related cues: implications for addiction. Neuropsychopharmacology 35, 388–400. doi: 10.1038/npp.2009.142
Flory, J. D., and Manuck, S. B. (2009). Impulsiveness and cigarette smoking. Psychosom. Med. 71, 431–437. doi: 10.1097/psy.0b013e3181988c2d
Gilman, S., Koeppe, R. A., Adams, K. M., Junck, L., Kluin, K. J., Johnson-Greene, D., et al. (1998). Decreased striatal monoaminergic terminals in severe chronic alcoholism demonstrated with (+)[11C]dihydrotetrabenazine and positron emission tomography. Ann. Neurol. 44, 326–333. doi: 10.1002/ana.410440307
Gray, J. A. (1987). Perspectives on anxiety and impulsivity: a commentary. J. Res. Pers. 21, 493–509. doi: 10.1016/0092-6566(87)90036-5
Hashemi, P., Dankoski, E. C., Lama, R., Wood, K. M., Takmakov, P., and Wightman, R. M. (2012). Brain dopamine and serotonin differ in regulation and its consequences. Proc. Natl. Acad. Sci. U S A 109, 11510–11515. doi: 10.1073/pnas.1201547109
Heinz, A., Siessmeier, T., Wrase, J., Buchholz, H. G., Gründer, G., Kumakura, Y., et al. (2005). Correlation of alcohol craving with striatal dopamine synthesis capacity and D2/3 receptor availability: a combined [18F]DOPA and [18F]DMFP PET study in detoxified alcoholic patients. Am. J. Psychiatry 162, 1515–1520. doi: 10.1176/appi.ajp.162.8.1515
Hicks, B. M., Foster, K. T., Iacono, W. G., and McGue, M. (2013). Genetic and environmental influences on the familial transmission of externalizing disorders in adoptive and twin offspring. JAMA Psychiatry 70, 1076–1083. doi: 10.1001/jamapsychiatry.2013.258
Hicks, B. M., Schalet, B. D., Malone, S. M., Iacono, W. G., and McGue, M. (2011). Psychometric and genetic architecture of substance use disorder and behavioral disinhibition measures for gene association studies. Behav. Genet. 41, 459–475. doi: 10.1007/s10519-010-9417-2
Huys, Q. J. M., Tobler, P. T., Hasler, G., and Flagel, S. B. (2014). The role of learning-related dopamine signals in addiction vulnerability. Prog. Neurobiol. in press.
Iacono, W. G., Malone, S. M., and McGue, M. (2008). Behavioral disinhibition and the development of early-onset addiction: common and specific influences. Annu. Rev. Clin. Psychol. 4, 325–348. doi: 10.1146/annurev.clinpsy.4.022007.141157
Jackson, C. (2006). The general health questionnaire. Occup. Med. 57, 79. doi: 10.1093/occmed/kql169
Jupp, B., Caprioli, D., Saigal, N., Reverte, I., Shrestha, S., Cumming, P., et al. (2013). Dopaminergic and GABA-ergic markers of impulsivity in rats: evidence for anatomical localisation in ventral striatum and prefrontal cortex. Eur. J. Neurosci. 37, 1519–1528. doi: 10.1111/ejn.12146
Kessler, R. C., Hwang, I., LaBrie, R., Petukhova, M., Sampson, N. A., Winters, K. C., et al. (2008). DSM-IV pathological gambling in the national comorbidity survey replication. Psychol. Med. 38, 1351–1360. doi: 10.1017/s0033291708002900
Kim, S. W., and Grant, J. E. (2001). Personality dimensions in pathological gambling disorder and obsessive-compulsive disorder. Psychiatry Res. 104, 205–212. doi: 10.1016/s0165-1781(01)00327-4
Krueger, R. F., Hicks, B. M., Patrick, C. J., Carlson, S. R., Iacono, W. G., and McGue, M. (2002). Etiologic connections among substance dependence, antisocial behavior and personality: modeling the externalizing spectrum. J. Abnorm. Psychol. 111, 411–424. doi: 10.1037/0021-843X.111.3.411
Krueger, R. F., Markon, K. E., Patrick, C. J., Benning, S. D., and Kramer, M. D. (2007). Linking antisocial behavior, substance use and personality: an integrative quantitative model of the adult externalizing spectrum. J. Abnorm. Psychol. 116, 645–666. doi: 10.1037/0021-843x.116.4.645
Kumakura, Y., and Cumming, P. (2009). PET studies of cerebral levodopa metabolism: a review of clinical findings and modelling approaches. Neuroscientist 15, 635–650. doi: 10.1177/1073858409338217
Laakso, A., Vilkman, H., Bergman, J., Haaparanta, M., Solin, O., Syvälahti, E., et al. (2002). Sex differences in striatal presynaptic dopamine synthesis capacity in healthy subjects. Biol. Psychiatry 52, 759–763. doi: 10.1016/s0006-3223(02)01369-0
Lahey, B. B., McNealy, K., Knodt, A., Zald, D. H., Sporns, O., Manuck, S. B., et al. (2012). Using confirmatory factor analysis to measure contemporaneous activation of defined neuronal networks in functional magnetic resonance imaging. Neuroimage 60, 1982–1991. doi: 10.1016/j.neuroimage.2012.02.002
Lawrence, A. D., Brooks, D. J., and Whone, A. L. (2013). Ventral striatal dopamine synthesis capacity predicts financial extravagance in Parkinson’s disease. Front. Psychol. 4:90. doi: 10.3389/fpsyg.2013.00090
Lawrence, A. D., Evans, A. H., and Lees, A. J. (2003). Compulsive use of dopamine replacement therapy in Parkinson’s disease: reward systems gone awry? Lancet Neurol. 2, 595–604. doi: 10.1016/s1474-4422(03)00529-5
Lomanowska, A. M., Lovic, V., Rankine, M. J., Mooney, S. J., Robinson, T. E., and Kraemer, G. W. (2011). Inadequate early social experience increases the incentive salience of reward-related cues in adulthood. Behav. Brain Res. 220, 91–99. doi: 10.1016/j.bbr.2011.01.033
Lovic, V., Saunders, B. T., Yager, L. M., and Robinson, T. E. (2011). Rats prone to attribute incentive salience to reward cues are also prone to impulsive action. Behav. Brain Res. 223, 255–261. doi: 10.1016/j.bbr.2011.04.006
MacLaren, V. V., Fugelsang, J. A., Harrigan, K. A., and Dixon, M. J. (2011). The personality of pathological gamblers: a meta-analysis. Clin. Psychol. Rev. 31, 1057–1067. doi: 10.1016/j.cpr.2011.02.002
McGowan, S., Lawrence, A. D., Sales, T., Quested, D., and Grasby, P. (2004). Presynaptic dopaminergic dysfunction in schizophrenia: a positron emission tomographic [18F]fluorodopa study. Arch. Gen. Psychiatry 61, 134–142. doi: 10.1001/archpsyc.61.2.134
Miettunen, J., Lauronen, E., Kantojärvi, L., Veijola, J., and Joukamaa, M. (2008). Inter-correlations between Cloninger’s; temperament dimensions – a meta-analysis. Psychiatr. Res. 160, 106–114. doi: 10.1016/j.psychres.2007.05.003
Newman, J. P., and Wallace, J. F. (1993). Diverse pathways to deficient self-regulation: implications for disinhibitory psychopathology in children. Clin. Psychol. Rev. 13, 699–720. doi: 10.1016/s0272-7358(05)80002-9
Nikolova, Y. S., and Hariri, A. R. (2012). Neural responses to threat and reward interact to predict stress-related problem drinking: a novel protective role of the amygdala. Biol. Mood Anxiety Disord. 2, 19. doi: 10.1186/2045-5380-2-19
Nordin, C., and Nylander, P.-O. (2007). Temperament and character in pathological gambling. J. Gambl. Stud. 23, 113–120. doi: 10.1007/s10899-006-9049-x
Oleski, J., Cox, B. J., Clara, I., and Hills, A. (2011). Pathological gambling and the structure of common mental disorders. J. Nerv. Ment. Dis. 199, 956–960. doi: 10.1097/nmd.0b013e3182392931
Oswald, L. M., Wand, G. S., Zhu, S., and Selby, V. (2013). Volunteerism and self-selection in human positron emission tomography neuroimaging research. Brain Imaging Behav. 7, 163–176. doi: 10.1007/s11682-012-9210-3
Otter, C., Huber, J., and Bonner, A. (1995). Cloninger’s tridimensional personality questionnaire: reliability in an English sample. Pers. Individ. Dif. 18, 471–480. doi: 10.1016/0191-8869(94)00199-3
Patlak, C. S., and Blasberg, R. G. (1985). Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. Generalizations. J. Cereb. Blood Flow Metab. 5, 584–590. doi: 10.1038/jcbfm.1985.87
Rowlett, J. K., Mattingly, B. A., and Bardo, M. T. (1993). Neurochemical correlates of behavioral sensitization following repeated apomorphine treatment: assessment of the role of D1 dopamine receptor stimulation. Synapse 14, 160–168. doi: 10.1002/syn.890140209
Ruiz, M. A., Pincus, A. L., and Schinka, J. A. (2008). Externalizing pathology and the five-factor model: a meta-analysis of personality traits associated with antisocial personality disorder, substance use disorder and their co-occurrence. J. Pers. Disord. 22, 365–388. doi: 10.1521/pedi.2008.22.4.365
Schemper, M. (1991). Non-parametric partial association revisited. Statistician 40, 73–76. doi: 10.2307/2348226
Siessmeier, T., Kienast, T., Wrase, J., Larsen, J. L., Braus, D. F., Smolka, M. N., et al. (2006). Net influx of plasma 6-[18F]fluoro-L-DOPA (FDOPA) to the ventral striatum correlates with prefrontal processing of affective stimuli. Eur. J. Neurosci. 24, 305–313. doi: 10.1111/j.1460-9568.2006.04903.x
Slutske, W. S., Eisen, S., Xian, H., True, W. R., Lyons, M. J., Goldberg, J., et al. (2001). A twin study of the association between pathological gambling and antisocial personality disorder. J. Abnorm. Psychol. 110, 297–308. doi: 10.1037/0021-843x.110.2.297
Slutske, W. S., Ellingson, J. M., Richmond-Rakerd, L. S., Zhu, G., and Martin, N. G. (2013). Shared genetic vulnerability for disordered gambling and alcohol use disorder in men and women: evidence from a national community-based Australian Twin Study. Twin Res. Hum. Genet. 16, 525–534. doi: 10.1017/thg.2013.11
Slutske, W. S., Moffitt, T. E., Poulton, R., and Caspi, A. (2012). Undercontrolled temperament at age 3 predicts disordered gambling at age 32: a longitudinal study of a complete birth cohort. Psychol. Sci. 23, 510–516. doi: 10.1177/0956797611429708
Spector, P. E., and Brannick, M. T. (2011). Methodological urban legends: the misuse of statistical control variables. Organ. Res. Methods 14, 287–305. doi: 10.1177/1094428110369842
Spinks, T. J., Jones, T., Bloomfield, P. M., Bailey, D. L., Miller, M., Hogg, D., et al. (2000). Physical characteristics of the ECAT EXACT 3D positron tomograph. Phys. Med. Biol. 45, 2601–2618. doi: 10.1088/0031-9155/45/9/313
Stokes, P. R., Shotbolt, P., Mehta, M. A., Turkheimer, E., Benecke, A., Copeland, C., et al. (2013). Nature or nurture? Determining the heritability of human striatal dopamine function: an [18F]-DOPA PET study. Neuropsychopharmacology 38, 485–491. doi: 10.1038/npp.2012.207
Weintraub, D., Siderowf, A. D., Potenza, M. N., Goveas, J., Morales, K. H., Duda, J. E., et al. (2006). Association of dopamine agonist use with impulse control disorders in Parkinson disease. Arch. Neurol. 63, 969–973. doi: 10.1001/archneur.63.7.969
Keywords: addiction, dopamine, externalizing, impulsivity, positron emission tomography, pathological gambling, reward, ventral striatum
Citation: Lawrence AD and Brooks DJ (2014) Ventral striatal dopamine synthesis capacity is associated with individual differences in behavioral disinhibition. Front. Behav. Neurosci. 8:86. doi: 10.3389/fnbeh.2014.00086
Received: 04 February 2014; Accepted: 28 February 2014;
Published online: 14 March 2014.
Edited by:
Mike James Ferrar Robinson, Wesleyan University, USACopyright © 2014 Lawrence and Brooks. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrew D. Lawrence, School of Psychology, Cardiff University, Tower Building, 70 Park Place, CF10 3AT Cardiff, UK e-mail:bGF3cmVuY2VhZEBjYXJkaWZmLmFjLnVr
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.