- Laboratory of Regulation of Function of Brain Neurons, Pavlov Institute of Physiology RAS, St. Petersburg, Russia
Regulation of gene expression is an essential step during long-term memory formation. Recently, the involvement of DNA-binding transcription factors and chromatin remodeling in synaptic plasticity have been intensively studied. The process of learning was shown to be associated with chromatin remodeling through histone modifications such as acetylation and phosphorylation. We have previously shown that the MAPK/ERK (mitogen-activated protein kinase/extracellular signal-regulated kinase) regulatory cascade plays a key role in the food aversion conditioning in the mollusk Helix. Specifically, command neurons of withdrawal behavior exhibit a learning-dependent asymmetry (left–right) in MAPK/ERK activation. Here, we expanded our molecular studies by focusing on a potential MAPK/ERK target – histone H3. We studied whether there is a learning-induced MAPK/ERK-dependent acetylation of histone H3 in command neurons RPa(2/3) and LPa(2/3) of the right and left parietal ganglia and whether it is asymmetrical. We found a significant learning-dependent increase in histone H3 acetylation in RPa(2/3) neurons but not in LPa(2/3) neurons. Such an increase in right command neurons depended on MAPK/ERK activation and correlated with a lateralized avoidance movement to the right visible 48 h after training. The molecular changes found in a selective set of neurons could thus represent a lateralized memory process, which may lead to consistent turning in one direction when avoiding a food that has been paired with an aversive stimulus.
Introduction
Long-term memory formation requires gene expression regulation, which occurs through the chromatin remodeling and regulation of DNA-binding transcription factors (TFs; Reul and Chandramohan, 2007). Histone modifications such as acetylation, phosphorylation, and DNA methylation lead to chromatin remodeling upon learning (Wood et al., 2006; Sweatt, 2009).
Histone acetylation is associated with activation of transcription (Peterson and Laniel, 2004). The amount of histone acetylation is controlled by histone acetyltransferases (HATs) and histone deacetylases (HDACs). Importantly, defects in long-term memory dependent on acetylation are compensated by injection of HDAC inhibitors (Alarson et al., 2004; Korzus et al., 2004; Wood et al., 2006; Fischer et al., 2007; Abel and Zukin, 2008).
Prior investigations have demonstrated that histone phosphorylation, followed by acetylation, may be induced via the MAPK/ERK (mitogen-activated protein kinase/extracellular signal-regulated kinase)-dependent pathway (Levenson et al., 2004; Chwang et al., 2006; Sweatt, 2009) during long-term memory formation. This regulatory cascade has been intensively studied in the last decade. The MAPK/ERK pathway plays a fundamental role in adaptive processes both in vertebrates and invertebrates. Its activation pattern determines cellular survival or apoptosis, effectiveness of pre-existing synapses or growth of new synaptic connections (Kaplan and Miller, 2000; Thomas and Huganir, 2004). It is also an essential step during long-term memory formation (Martin et al., 1997; Atkins et al., 1998; Crow et al., 2001; Sananbenesi et al., 2003; Sharma and Carew, 2004; Feld et al., 2005; Ribeiro et al., 2005).
Mollusks have played a key role in these studies due to the relative simplicity of their central nervous system (CNS) and their stereotyped behavior, which exhibits nevertheless different levels of plasticity (Kandel, 2001). For many years we have been using the terrestrial mollusk Helix lucorum and its food aversion conditional reflex to investigate long-term memory formation (Grinkevich, 1994; Grinkevich and Vasil’ev, 2000; Grinkevich et al., 2003, 2007, 2008). Several forms of conditioned avoidance reflex have been reported for this snail (Stepanov et al., 1988; Grinkevich and Vasil’ev, 2000; Balaban, 2002). In one paradigm this mollusk can be trained to avoid a piece of food (the conditioned stimulus, CS; e.g., carrot) if it is appropriately paired with an electric shock (the unconditioned stimulus, US). Neuronal networks underlying feeding behavior and withdrawal in Helix have been determined and neural correlates of withdrawal behavior have been described in detail (Balaban, 2002).
We have previously demonstrated that MAPK/ERK, as well as its downstream targets, such as TFs controlling gene expression via CRE, SRE, and AP-1 elements, are involved in the regulation of food aversion learning in adult Helix. Moreover MAPK/ERK activation is serotonin-dependent (Grinkevich and Vasil’ev, 2000; Grinkevich et al., 2003, 2007, 2008). In contrast to adults, juvenile Helix snails, which possess immature mechanisms of sensitization and undeveloped conditioned avoidance responses, do not exhibit MAPK/ERK activation in the CNS after training (Grinkevich et al., 2008).These snails differ from the adults in the spectrum of TFs that bind to regulatory elements SRE and AP-1 (Grinkevich and Vasil’ev, 2000; Grinkevich et al., 2003). In addition, we demonstrated that a significant MAPK/ERK-dependent increase in histone H3 acetylation occurs in adult animals after learning, whereas no increase in histone H3 acetylation was observed in juveniles. The injection of sodium butyrate, an inhibitor of HDAC, prior to training led to induction in histone H3 acetylation and significantly ameliorated long-term memory formation in juvenile snails.
Recently, we have studied molecular processes underlying learning in command neurons RPa(2/3) and LPa(2/3) controlling withdrawal behavior of adult snails. Such neurons constitute the plastic link of food aversion reflex and might be responsible for unilateral right [RPa(2/3)] or left [LPa(2/3)] turning when withdrawal or escape responses are initiated. Balaban (1979) reported that RPa(2/3) and LPa(2/3) neurons are responsible for producing contractions of ipsilateral body walls so that they may not be involved in the production of bilateral movements of the foot, which are mediated by ipsilateral populations of motor neurons. We focused on left and right command neurons and showed that serotonin-dependent MAPK/ERK activation is involved in the formation of the withdrawal reflex; moreover we found that following learning, there is an asymmetry of MAPK/ERK activation in the left and right command neurons, which could result in the lateralization of molecular memory processes (Kharchenko et al., 2010). Specifically, we found that after food aversion learning phospho-ERK levels increased significantly in RPa(2/3) command neurons but no increase was found in LPa(2/3) command neurons. We concluded that learning involves synchronous and asymmetric serotonin-dependent MAPK/ERK activation and that such an asymmetry may reflect lateralization of memory processes in the mollusk brain. Here we expanded our molecular analyses of command neurons in the framework of food aversion learning in Helix, and focused on histone H3 acetylation, a process so far unexplored in this experimental context. We aimed at understanding whether histone H3 acetylation is induced in RPa(2/3) and LPa(2/3) command neurons after learning and whether it is MAPK/ERK-dependent. We analyzed if, consistently with MAPK/ERK activation observed in our previous work (Kharchenko et al., 2010), learning-dependent induction of histone H3 acetylation is also asymmetrical between the left and right command neurons. Our results show that food aversion learning in Helix induces a significant learning-dependent increase in histone H3 acetylation in command neurons of the right parietal ganglion RPa(2/3) but not in the symmetrical command neurons of the left parietal ganglion LPa(2/3). Moreover histone H3 acetylation in command neurons RPa(2/3) was MAPK/ERK-dependent. We suggest that these unilateral molecular changes in command neurons during learning lead to consistent turning in one direction when avoiding a food stimulus that has been paired with an aversive stimulus.
Materials and Methods
Conditioned Reflex Formation
Experiments were carried out on adult (20–25 g) snails H. lucorum. Animals were trained to associate a piece of carrot as the CS with an electric shock as the US. Conditioned food aversion is established in this protocol, following the procedure established by Balaban (2002). Specifically, a piece of carrot was placed at a distance of 1 cm from the head of a snail freely moving on a metal plate (serving as one of stimulating electrodes). When the snail began to eat the carrot, another stimulating electrode was manually placed on the snail’s head, and an electric shock (DC, 5 mA, 0.5 s) was applied. Food and the shock US were presented to the midline. If the snail did not contact the carrot during 2 min, a piece of carrot was placed close to its mouth, and the electric shock was applied. Thus, all trained snails received equal amount of CS and US stimulation. The training procedure consisted of eight CS–US pairings applied at 15 min interval (four treatments per day). Animals were deprived of food during 3 days before the experiments. Naive animals were used as control group.
Central Nervous System
Prior to the isolation of the CNS, animals were anesthetized with ice-cold saline supplemented by the injection of isotonic solution of MgCl2. In the case of animals that were previously trained, the subesophageal complex of ganglia was quickly removed from the head 10 min after training and placed into a camera containing saline solution (80 mM NaCl; 4 mM KCl; 7 mM CaCl2; 5 mM MgCl2; 5 mM TRIS–HCl; pH = 7,8). In order to quantify H3 histone in specific subsets of neurons, the ganglia were delicately opened under microscope using cutters and tweezers. Identified neurons or groups of neurons were then quickly dissected and suctioned into a pipette, and transferred to the extraction buffer. Command neurons RPa2 and RPa3 or LPa2 and LPa3 from three individual animals were combined for analysis. All procedures were performed at 4°C.
Drugs and Injection Procedure
The MEK1 inhibitor PD98059 (Cell Signaling) was freshly dissolved in dimethyl sulfoxide (DMSO) at the concentration of 20 mM. Then 6 μl of PD98059 or vehicle were injected into the cephalopedal sinus 30 min prior to conditioning. The total volume of adult Helix hemolymph was estimated at 3 ml resulting in an approximate 500-fold dilution of the drug in hemolymph and a final concentration of PD98059 in hemolymph of around 40 μM.
Histone Extraction and Immunoblotting
To identify histone acetylation status, CNS were homogenized in extraction buffer: 10 mM Tris–HCl pH 7.5, 1 mM EDTA, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM, 0.2 mM PMSF, 1% protease inhibitor cocktail (Sigma), 0.1 mM Na3VO4, and 1% Igepal CA-630. Histones were extracted according to Levenson et al. (2004). All procedures were performed on ice. Tissue homogenates were centrifuged at 7,700×g for 5 min (4°C). The pellet was resuspended in 1 ml of 0.4 N H2SO4 (30 min histone extraction) and was centrifuged at 14,000×g for 10 min (4°C). The supernatant was transferred to a fresh tube, and proteins were precipitated with the addition of 250 μl of 100% trichloroacetic acid containing 4 mg/ml deoxycholic acid (Na+ salt, Helicon) for 30 min and then centrifuged at 14,000×g for 30 min (4°C). The supernatant was discarded, and the protein pellet was washed with 1 ml of acidified acetone (0.1% HCl) followed by 1 ml of acetone for 5 min each. Protein precipitates were collected by centrifugation (14,000×g, 5 min, 4°C) and were then resuspended in 10 mM Tris (pH 8) and stored at −80°C. Protein concentration was measured by Bradford assay. Samples were boiled with loading buffer and equal amount of protein was loaded into the 14% SDS-PAGE. Protein markers were from Fermentas (Lithuania). Separated proteins were transferred to a nitrocellulose membrane (Schleicher and Schuell). Ponceau S staining was used to check transfer quality. Membranes were incubated in Tris-buffered saline with 0.1% Tween 20 (TBS-T) containing 5% non-fat dry milk for 1 h at 4°C to block non-specific binding. Following this blots were incubated with primary Acetylated-H3-Histone antibodies (4°C overnight) and with secondary antibodies conjugated with HRP (horseradish peroxidase) for 1 h. Immunolabeling was detected by enhanced chemoluminescence using ECL system (standard protocol and components from Amersham Pharmacia Biotech). Subsequently, blots were stripped (glycine-HCl, pH 2.8, two times for 20 min each at 55°C), saturated 1 h in 5% non-fat dry milk and incubated with antibodies against total form of histone H3. After exposure of membranes, films were scanned and amount of protein was quantified using Gel Pro Anal computer program.
The amount of acetylated histone H3 was normalized to total histone H3 whose level remains stable with respect of learning. To visualize H3-histone acetylation polyclonal antibodies against Acetylated Lysine 14-H3-histone (Upstate Biotechnology, Millipore Corporation) were used. Polyclonal antibodies against total histone H3 (Upstate Biotechnology, Millipore Corporation) were used for analysis of H3 content. Antibodies against Acetylated-H3-histone and total histone H3 were diluted 1:1,000 and secondary antibodies (Amersham) were diluted 1:1,500–1:2,500.
Data Analysis
For statistical analyses we used ANOVA followed by Fisher’s and Tukey’s tests for post hoc comparisons. Binomial tests were used for comparing laterality of behavior. Significance of results was accepted at p ≤ 0.05. Results are presented as mean ± SEM. All analyses were carried out with SPSS statistical package.
Results
Histone H3 Acetylation in Command Neurons Controlling Withdrawal Behavior Upon Food Aversion Learning
To study the involvement of histone H3 acetylation in conditioned food aversion in Helix, we quantified histone H3 acetylation in identified command neurons (premotor withdrawal interneurons) of the food aversion network following learning. These neurons constitute the main plastic element in the network controlling withdrawal behavior of Helix upon electric shock stimulation and are involved, therefore, in US processing (Balaban, 2002). For this purpose, we designed a micro variant of western blot analysis, which allowed us to detect proteins purified from single neurons. We analyzed and compared histone H3 acetylation in the left (L) and right (R) command neurons of the parietal ganglia (Pa). Specifically, we analyzed LPa2 and LPa3 [LPa(2/3)], RPa2 and RPa3 [RPa(2/3)] command neurons. These are giant neurons (about 250 microns) symmetrically located in the left and right parietal ganglia, respectively, which can be easily visualized and isolated (Figure 1). As a control, we analyzed neurons belonging to the D-group, which do not participate in the food aversion network and are located on the right parietal ganglia (Maksimova and Balaban, 1983).
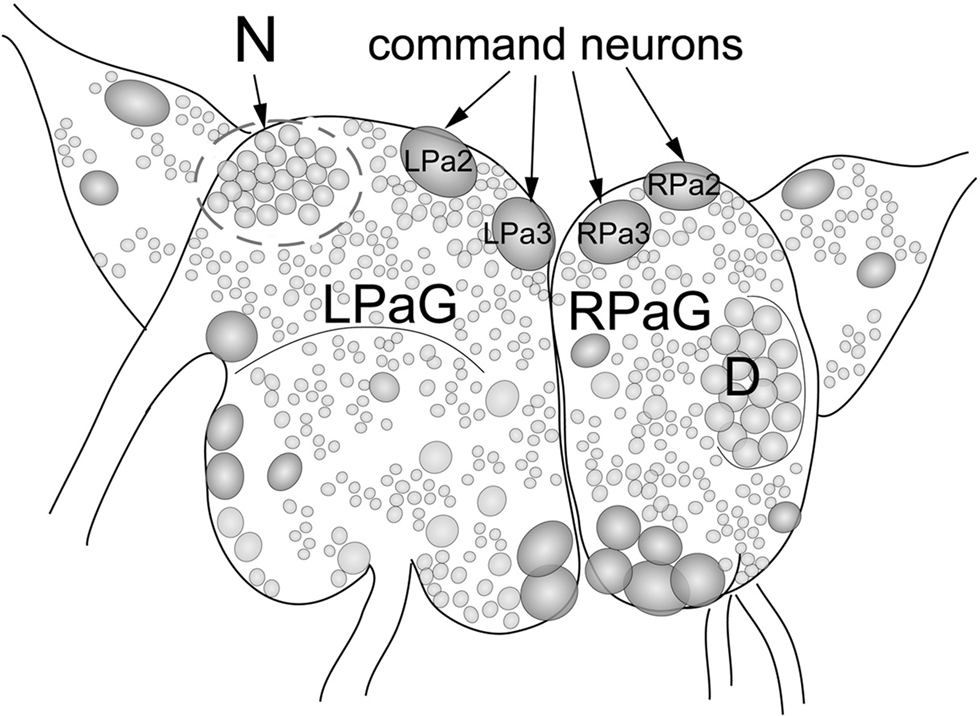
Figure 1. Location of large identified neurons and neuronal clusters in the CNS of Helix lucorum. The figure shows the left and right parietal ganglia (LPaG, RPaG). Numbers designate individual identified neurons: giant neurons (2 and 3) symmetrically located in the left and right parietal ganglia, correspond to the giant interneurons (command neurons) of withdrawal behavior LPa2, LPa3 [LPa(2/3)] and RPa2, RPa3 [RPa(2/3)]. Outline areas indicate the region containing neurons belonging to the D-group and the N-group. Command neurons LPa(2/3), RPa(2/3), and neurons of the D-group were used for experiments.
Groups of three snails were conditioned and were then sacrificed 15 min after training. Command neurons of the right and the left parietal ganglia were separately combined (RPa2 and RPa3 together, and LPa2 and LPa3 together) and a comparative analysis of H3 acetylation involving D-group neurons was performed. Three groups of animals were analyzed: control naïve animals pretreated with the vehicle, trained animals pretreated with the vehicle and trained animals pretreated with the MEK inhibitor PD98059 (40 μM) dissolved in vehicle.
Fifteen minutes after training, acetylation of histone H3 increased in command neurons RPa(2/3) of the right parietal ganglion (Figure 2). Specifically, the increase in histone H3 acetylation was detected in RPa(2/3) command neurons (ANOVA: F1,11 = 6.034, p < 0.032, learning vs control). In contrast, no difference in histone H3 acetylation was found in command neurons of the left parietal ganglion LPa(2/3) (F1,9 = 0.07, p = 0.8, learning vs control). Thus, after food aversion learning, induction of histone H3 acetylation takes place only in the right parietal ganglion. D-group neurons, which do not belong to the network controlling withdrawal behavior of Helix and which were thus used as a within-subject control, did not exhibit significant changes in histone H3 acetylation (F1,6 = 0.13, p = 0.73; Figure 2). Their total level of histone H3 did not change after training. To test whether the increase in histone H3 acetylation in RPa(2/3) neurons was MAPK/ERK-dependent, we injected animals with the MEK kinase inhibitor PD98059 30 min prior to training. We compared control vehicle-injected, trained vehicle-injected, and trained PD98059-injected animals. Figure 2 shows that PD98059 injection inhibited the increase in histone H3 acetylation induced by learning in RPa(2/3) command neurons (F2,13 = 4.01, p < 0.04). As expected (see above), trained animals pretreated with vehicle exhibited a significantly higher level of histone H3 acetylation than control, untrained animals; p < 0.03 (post hoc Fisher test), thus confirming the asymmetric effect of training on histone H3 acetylation as a consequence of conditioning (see above). Similarly, a comparison between trained, vehicle-injected animals and trained, PD98059-injected animals was also significant (post hoc Fisher test: p < 0.04) as the latter did not exhibit a significant increase of H3 acetylation in RPa(2/3) neurons. Consequently, there was no difference between vehicle-injected untrained animals and trained, PD98059-injected animals (post hoc Fisher test: p = 0.67) in histone H3 acetylation in RPa(2/3) neurons. These results show that the increase in histone H3 acetylation detected in RPa(2/3) neurons is learning- and MAPK/ERK-dependent.
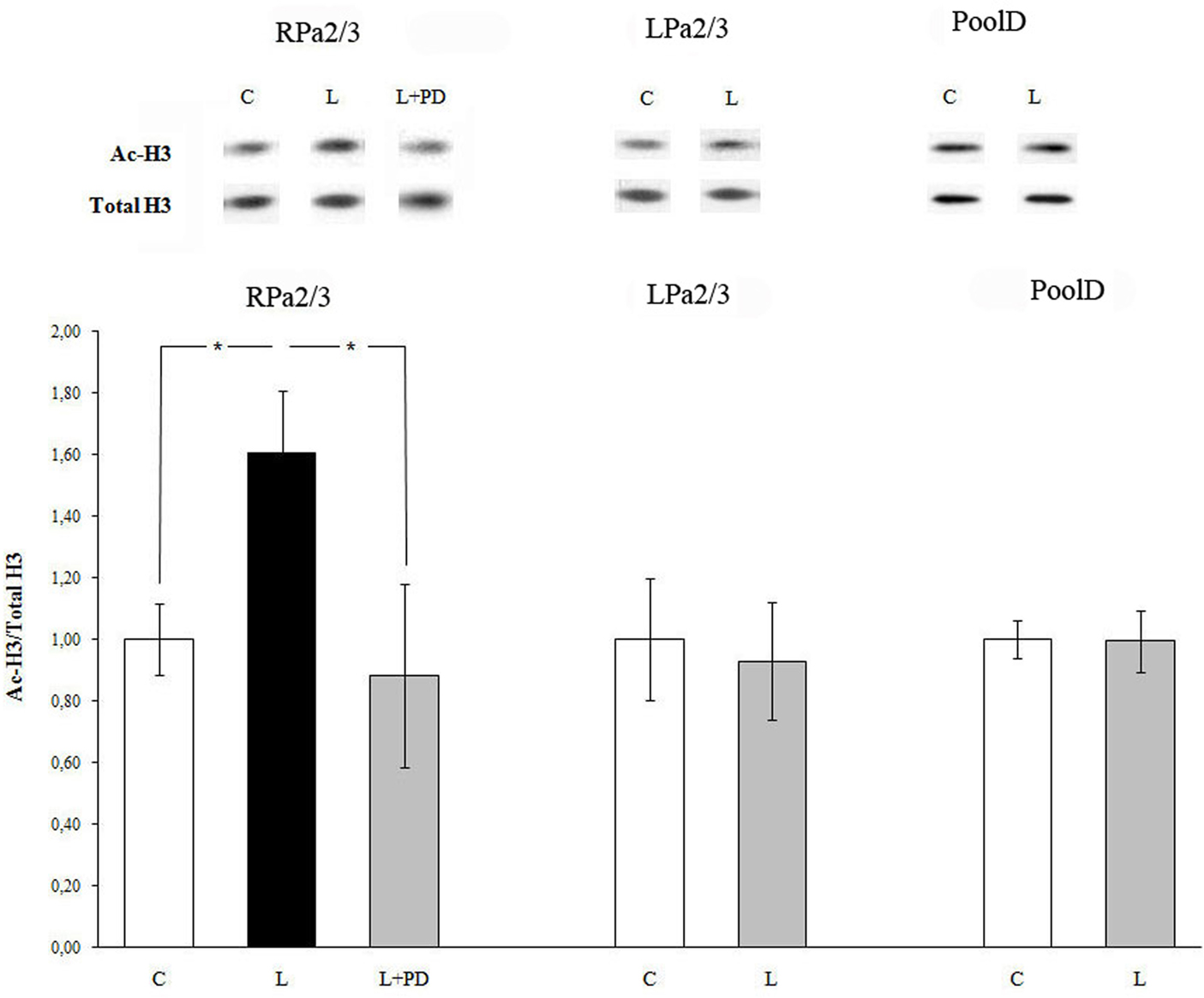
Figure 2. Food aversion learning induces histone H3 acetylation in identified neurons of the CNS of Helix lucorum. Increased amount of H3 histone acetylation was detected 15 min after learning in command neurons of withdrawal behavior RPa(2/3) in comparison to naïve controls. The selective MEK inhibitor PD98059 abolished ERK activation. C, control naïve animals pretreated with the vehicle; L, trained animals pretreated with the vehicle; L + PD, trained animals pretreated with the MEK inhibitor PD98059 (40 μM). Data shown are mean ± SEM normalized ratios of ac-H3. Number of independent experiments: command neurons RPa 2/3 (C, n = 6; L, n = 7; L + PD, n = 3), LPa 2/3 (C, n = 6; L, n = 5); *p < 0.04; D-group (C, n = 4; L, n = 4). Upper panel – representative western blot.
Lateralization of Avoidance Movement During Food Aversion Reflex Formation in Helix Lucorum
Studies performed by Salimova et al. (1984) showed that the capacity of mollusks to turn to the right or to the left sides differ in their latent periods. Asymmetric movements of mollusks could be related to different activities of the serotoninergic and dopaminergic systems underlying left-hand and right-hand movement (Salimova et al., 1984). In particular, they could be related to RPa(2/3) and LPa(2/3) neurons which are thought to be involved in the control of ipsi (unilateral) but not bilateral movements (Balaban, 1979). Given the asymmetry in terms of learning-dependent molecular processes between left and right unilateral command neurons, we reasoned that such asymmetry may result in snails learning to move away in an asymmetric way (i.e., to the right or to the left) from the piece of carrot they avoid. We thus analyzed whether Helix snails have a preferred direction of turning upon and after food avoidance learning. During training we did not observe any lateralization of avoidance movement (p > 0.2 Binomial test, n = 18). No direction preference was observed while testing the animals 24 h after training, either (p > 0.2 Binominal test, n = 18). But, interestingly 48 h after learning all animals demonstrated lateralization of avoidance movement direction (p < 0.001 Binominal test). All of the eighteen snails moved to the right while avoiding carrot (Figure 3). These results indicate that behavioral lateralization is established only after consolidation of the conditional reflex. Taken together our data demonstrate a correlation between a lateralized increase in histone H3 acetylation in RPa(2/3) neurons and a lateralized avoidance to the right 48 h after learning.
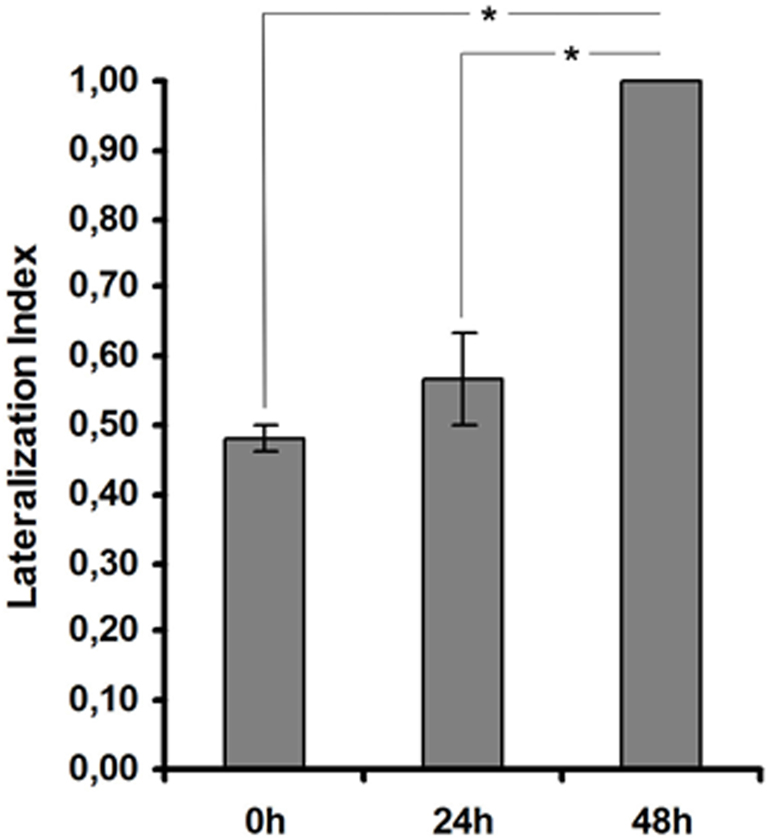
Figure 3. Lateralization of avoidance movement during food aversion reflex formation in Helix lucorum. Forty-eight hours after training, Helix demonstrates lateralization of avoidance movement. An index to quantify lateralization of avoidance movement was calculated using R/(L + R), where R represents the number of animals, which moved to the right while avoiding carrot, and L the number of animals, which moved to the left while avoiding carrot. n = 18 (three series, six animals in every series) for all groups. *p < 0.001. Errors bars = SEM.
Discussion
Our work shows that histone H3 acetylation is selectively increased in identified neurons of the CNS of H. lucorum upon food aversion learning. Such an increase was found in the command neurons of the right parietal ganglion RPa(2/3) but not in the symmetrical neurons of the left parietal ganglion LPa(2/3). The D-group neurons, which do not belong to the food aversion network, did not show an increase in histone H3 acetylation. Injection of the MAPK/ERK pathway inhibitor PD98059 prior to training prevented learning-dependent histone H3 acetylation in RPa(2/3) neurons, thus showing that acetylation is related to MAPK/ERK activity. We have previously shown that blocking MAPK/ERK activity via pretreatment with PD98059 impairs food avoidance learning in Helix (Grinkevich et al., 2008).
Our experiments suggest that changes in histone H3 acetylation in command neurons of withdrawal behavior are required for learning and are regulated by MAPK/ERK. Our data support findings obtained in other animals, showing the important role of acetylation during long-term memory formation (Kandel, 2001; Guan et al., 2002; Levenson and Sweatt, 2006).
Recently MAPK/ERK was reported to be involved in the regulation of histone acetylation in a number of studies carried out in vertebrates (Levenson et al., 2004; Chwang et al., 2006, 2007; Sweatt, 2009). It is supposed that MAPK/ERK-dependent acetylation of histones could be mediated by the CREB-binding protein (CBP), a known MAPK/ERK target and transcription activator, which possesses endogenous HATs activity (Alarson et al., 2004; Korzus et al., 2004; Wood et al., 2005). MAPK/ERK-dependent acetylation is mediated by RSK and MSK protein-kinases (Chwang et al., 2007). HDCAs play an important role in the regulation of histone acetylation. Injection of HDACs inhibitors was shown to improve long-term memory formation both in wild-type animals and mutants with dysfunctional CBP (Alarson et al., 2004; Korzus et al., 2004; Wood et al., 2005). Moreover, in the last years, the possibility of memory amelioration through HDACs inhibition even in animals with neurodegeneration has been suggested (Fischer et al., 2007; Abel and Zukin, 2008).
In addition, the central role of MAPK/ERK-dependent histone H3 acetylation during food aversion learning is supported by our research on juvenile snails. Juvenile animals, which possess immature mechanisms of long-term plasticity of avoidance behavior, in contrast to adults do not exhibit changes in histone H3 acetylation upon conditioning. This result is related with our previous findings, which demonstrated both a lack of MAPK/ERK activation and a difference in the spectrum of TFs binding DNA regulatory elements SRE and AP-1 the juvenile animals (Grinkevich et al., 2003, 2008). Thereby dysfunction of MAPK/ERK activation during training may result in a deficit in histone H3 acetylation in juvenile snails. Taken together, our data confirm the essential role of MAPK/ERK-dependent histone H3 acetylation in food aversion learning in Helix.
We suggest that sensory stimulation does not have a significant effect on H3 acetylation as after learning we observed an increase in H3 acetylation in RPa(2/3) neurons only, although left and right command neurons from parietal ganglia have common sensory fields (Balaban, 2002). Also, histone H3 acetylation induced by learning is due to sensitization underlying the formation of conditioned food aversion in Helix. It should be noted that similar biochemical alterations occur at the cellular level during the formation of both sensitization and conditioned defensive responses. These effects only differ in their magnitude and duration (Abrams et al., 1991; Grinkevich, 1994; Antonov et al., 2001). Moreover, our recent findings (Kharchenko et al., 2010) support the idea of a significant role of sensitization in the molecular processes underlying withdrawal reflex formation. We have shown asymmetrical activation of MAPK/ERK in RPa(2/3) neurons not only after learning but also after incubation in serotonin, the neurotransmitter which mediates the effect of the US and stimulates sensitization.
It has been previously shown that all command neurons, RPa(2/3) and LPa(2/3), trigger the withdrawal responses and are involved in habituation, sensitization, and aversive conditioning (Balaban, 2002). Command neurons of the right and left parietal ganglia constitute the plastic link of food aversion reflex and might be responsible for unilateral right [RPa(2/3)] or left [LPa(2/3)] turning when withdrawal or escape responses are initiated. Morphological and functional differences have been described for RPa(2/3) and LPa(2/3) neurons. Firstly, every command neuron has its own specific non-habituating area of the receptive field. RPa(2/3) and LPa(2/3) neurons have specific receptive fields, which are predominantly located ipsilaterally on the poda. Furthermore, there is a difference in the organization of the motor fields of these neurons (Bravarenko et al., 1982). Balaban (1979) reported that RPa(2/3) and LPa(2/3) neurons are responsible for producing contractions of ipsilateral body walls. These contractions may be related to the presence of inhibitory synaptic connections from command neurons onto ipsilateral neurons of pedal ganglion, participating in the locomotory control. Activation of the command neurons in the right parietal ganglion may result in the contraction of the ipsilateral muscles via pedal neurons, which in turn may lead to the movement to the right (Ierusalimsky and Zakharov, 1994; Ierusalimsky et al., 1994; Zakharov et al., 1995).
Due to their ipsilaterality, command neurons of parietal ganglia may not be involved in the production of bilateral movements of the foot, which are mediated by other populations of motor neurons. Secondly, serotonin (5-HT) has opposite effects on acetylcholine (Ach)-dependent responses of LPa3 and RPa3; 5-HT increases Ach-dependent responses in RPa3 while it decreases them in LPa3. This fact is connected with differences in the Ca-systems of these neurons (Dyatlov, 1988). Moreover, the amount of brain specific proteins differs between command neurons of the right and the left parietal ganglia after avoidance learning (Shtark et al., 1982; Grinkevich, 1994). Additionally, a recent transcriptomic analysis from single neurons of Aplysia showed a significant heterogeneity of gene expression in neurons that seemed to be functionally similar (Moroz et al., 2006). Thus, our data suggest that command neurons located in the right and left parietal ganglia of Helix play different roles in food aversion learning. In particular we suggest that the molecular processes occurring in an increased way in right command neurons RPa(2/3), and which may reflect a lateralized memory upon food aversion learning, are related with unilateral turning to the right, visible 48 h after training. In other words, the unilateral command properties of these neurons would provide the substrate to generate a lateralized behavior established upon food aversion learning via increased and lateralized molecular processes.
Evidence for lateralization of the invertebrate nervous system has been recently reported for nematodes and insects. In the nematode C. elegans asymmetric expression of olfactory and taste receptors was shown in symmetrically located cells (AWCL/AWCR and ASEL/ASER), despite the fact that cells are morphologically and anatomically identical. Mutants symmetrically expressing these receptors have impaired odor and taste recognition (Hobert et al., 2002). Unilateral activation experiments indicate that the asymmetry extends to the level of behavioral output: ASEL lengthens bouts of forward locomotion (runs) whereas ASER promotes direction changes (turns) (Suzuki et al., 2008). In insects, anatomical and functional asymmetries of the nervous system have also been described in the fruit fly Drosophila melanogaster. Flies presenting an asymmetrical brain structure (“asymmetrical body”) establish long-term memory after aversive conditioning (odor–shock associations), while those with symmetrical brains do not (Pascual et al., 2004). Honeybees (Apis mellifera) learn to associate odor delivered to their antennae and sugar reward delivered to antennae and proboscis (Giurfa, 2007) and different olfactory retention performances have been reported depending on which antenna, left or right is used (Letzkus et al., 2006). Using the same protocol, Rogers and Vallortigara (2008) showed a lateral shift of olfactory recall from the right to the left antennae 6–8 h after training, so that memory can now be recalled mainly when the left antenna is in use. Visual learning in bees consisting of color–sucrose associations (Giurfa, 2004) is also lateralized as bees learn a color stimulus better with their right eye (Letzkus et al., 2008). Our investigation shows that locomotion is also lateralized in snails after training. While no preference for a given movement direction was observed during training and 24 h after it, all tested animals moved to right while exhibiting avoidance of carrot 48 h after training. Behavioral lateralization occurs only after the final consolidation of the conditional reflex (48 h after learning). Whether such a movement lateralization is a cause or a consequence of the lateralization in MAPK/ERK activation and H3 acetylation, as observed in command neurons of the right parietal ganglion, remains an open question.
Long-term memory formation in Helix is associated with selective activation of the MAPK/ERK pathway and action on downstream targets, such as histone H3, in command neurons located in the right parietal ganglion. The asymmetry in MAPK/ERK activation and histone acetylation between right and left command neurons controlling withdrawal behavior suggests lateralization of a long-term memory trace in mollusk. The main question is why should the memory trace be asymmetrical in Helix? One possible explanation might be related to the developmental processes that build up a gastropod. Gastropods are different from their primitive mollusk ancestors in having an enlarged head and visceral mass, in most cases a logarithmically spiraled shell, and a visceral mass that has undergone a 180 rotation during development (torsion). This results in an asymmetrical development with the majority of growth occurring on the left or right side. On the other hand, as in C. elegans (Hobert et al., 2002) this asymmetry might be determined by the difference of the intracellular regulatory systems and TFs, specific for every cell. Therefore, the asymmetry would be genetically determined and learning and memory formation would build up on a pre-existing lateralized substrate, thereby leading to an increase in lateralization.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are thankful to Olga Vorobjova for technical assistance, to Vera Grinkevich for editing the manuscript, and Igor Stepanov for performing the statistical analysis. This work was supported by the grant RFBR No. 08-04-01325.
References
Abel, T., and Zukin R. S. (2008). Epigenetic targets of HDAC inhibitor in neurodegenerative and psychiatric disorders. Curr. Opin. Pharmacol. 8, 57–64.
Abrams, T. W., Kevin, A. K., and Kandel, E. R. (1991). Biochemical studies of stimulus convergence during classical conditioning in Aplysia: dual regulation of adenylate cyclase by Ca2+/calmodulin and transmitter. J. Neurosci. 11, 2655–2665.
Alarson, J. M., Mallaretet, G., Touzani, K., Vronskaya, S., Ishii, S., Kandel, E. R., and Barco, A. (2004). Chromatin acetylation, memory, and LTP are impaired in CBP+/− mice: a model for the cognitive deficit in Rubinstein–Taybi syndrome and its amelioration. Neuron 42, 947–959.
Antonov, I., Antonova, I., Kandel, E. R., and Hawkins, R. D. (2001). The contribution of activity-dependent synaptic plasticity to classical conditioning in Aplysia. J. Neurosci. 21, 6413–6422.
Atkins, C. M., Selcher, J. S., Petraitis, J. J., Trzaskos, J. M., and Sweatt, J. D. (1998). The MAPK cascade is required for mammalian associative learning. Nat. Neurosci. 1, 602–609.
Balaban, P. M. (1979). A system of command neurons in snail’s escape behavior. Acta Neurobiol. Exp. (Wars) 39, 97–107.
Balaban, P. M. (2002). Cellular mechanisms of behavioral plasticity in terrestrial snail. Neurosci. Biobehav. Rev. 26, 597–630.
Bravarenko, N. I., Balaban, P. M., and Sokolov, E. N. (1982). Organization of sensory input to a system of command neurones. Zh. Vyssh. Nerv. Deiat. Im. I P Pavlova 32, 94–99.
Chwang, W. B., Arthur, J. S., Schumacher, A., and Sweatt, J. D. (2007). The nuclear kinase mitogen- and stress-activated protein kinase 1 regulates hippocampal chromatin remodeling in memory formation. J. Neurosci. 27, 12732–12742.
Chwang, W. B., O’Riordan, K. J., Levenson, J. M., and Sweatt, J. D. (2006). ERK/MAPK regulates hippocampal histone phosphorylation following contextual fear conditioning. Learn. Mem. 13, 322–328.
Crow, T., Xue-Bian, J.-J., Siddiqi, V., and Neary, J. T. (2001). Serotonin activation of ERK pathway in Hermissenda: contribution of calcium-dependent protein kinase C. J. Neurosci. 78, 358–364.
Dyatlov, V. A. (1988). Role of calcium ions in processes of serotonin modulation of Helix pomatia neurons responses to acetylcholine application. Neurophysiology 5, 489–492.
Feld, V., Dimant, B., Delorenzi, A., Coso, O., and Romano, A. (2005). Phosphorylation of extranuclear ERK/MAPK is required for long-term memory consolidation in the crab Chasmagnathus. Behav. Brain Res. 158, 251–261.
Fischer, A., Sananbenesi, F., Wang, X., Dobbin, M., and Tsai, L. H. (2007). Recovery of learning and memory is associated with chromatin remodeling. Nature 447, 178–182.
Giurfa, M. (2004). Conditioning procedure and color discrimination in the honeybee Apis mellifera. Naturwissenschaften 91, 228–231.
Giurfa, M. (2007). Behavioral and neural analysis of associative learning in the honeybee: a taste from the magic well. J. Comp. Physiol. 193, 801–824.
Grinkevich, L. N. (1994). Protein metabolism in the formation of the conditioned avoidance reflex of mollusks. Neurosci. Behav. Physiol. 24, 105–110.
Grinkevich, L. N., Lisachev, P. D., Baranova, K. A., and Kharchenko, O. A. (2007). Comparative analysis of the activation of MAP/ERK kinases in the CNS of animals with different learning abilities. Neurosci. Behav. Physiol. 37, 715–720.
Grinkevich, L. N., Lisachev, P. D., Kharchenko, O. A., and Vasil’ev, G. V. (2008). Expression of MAP/ERK kinase cascade corresponds to the ability to develop food aversion in terrestrial snail at different stages of ontogenesis. Brain Res. 1187, 12–19.
Grinkevich, L. N., Lisachev, P. D., and Merkulova, T. I. (2003). Formation of AP-1 transcription factors during learning in Helix. Neurosci. Behav. Physiol. 33, 39–47.
Grinkevich, L. N., and Vasil’ev, G. V. (2000). Possible molecular-cellular mechanisms of the regulation of gene expression during learning. Neurosci. Behav. Physiol. 30, 277–292.
Guan, Z., Giustetto, M., Lomvardas, S., Kim, J.-H., Miniaci, M. C., Schwartz, J. H., Thanos, D., and Kandel, E. R. (2002). Integration of long-term-memory-related synaptic plasticity involves bidirectional regulation of gene expression and chromatin structure. Cell 111, 483–493.
Hobert, O., Johnston, R. J., and Chang, S. (2002). Left–right asymmetry in the nervous system: Caenorhabditis elegans model. Nat. Rev. Neurosci. 3, 629–640.
Ierusalimsky, V. N., and Zakharov, I. S. (1994). Mapping of neurons participating in the innervation of the body wall of the snail. Neurosci. Behav. Physiol. 24, 33–39.
Ierusalimsky, V. N., Zakharov, I. S., Palikhova, T. A., and Balaban, P. M. (1994). Nervous system and neural maps in gastropod Helix lucorum L. Neurosci. Behav. Physiol. 24, 13–22.
Kandel, E. R. (2001). The molecular biology of memory storage: a dialogue between genes and synapses. Science 294, 1030–1038.
Kaplan, D. R., and Miller, F. D. (2000). Neurotrophins signal transduction in the nervous system. Curr. Opin. Neurobiol. 10, 381–391.
Kharchenko, O. A., Grinkevich, V. V., Vorobiova, O. V., and Grinkevich, L. N. (2010). Learning-induced lateralized activation of the MAPK/ERK cascade in identified neurons of the food aversion network in the mollusk Helix lucorum. Neurobiol. Learn. Mem. 94, 158–166.
Korzus, E., Rosenfeld, M. G., and Mayford, M. (2004). CBP histone acetyltransferase activity is a critical component of memory consolidation. Neuron 42, 961–972.
Letzkus, P., Boeddeker, N., Wood, J. T., Zhang, S. W., and Srinivasan, M. (2008). Lateralization of visual learning in the honeybee. Biol. Lett. 4, 16–18.
Letzkus, P., Ribi, A. W., Wood, J. T., Zhu, H., Zhang, S.-W., and Srinivasan, M. V. (2006). Lateralization of olfaction in the honeybee Apis mellifera. Curr. Biol. 16, 1471–1476.
Levenson, J. M., O’Riordan, K. J., Brown, K. D., Trinh, M. A., Molfese, D. L., and Sweatt, J. D. (2004). Regulation of histone acetylation during memory formation in the hippocampus. J. Biol. Chem. 279, 40545–40559.
Levenson, J. M., and Sweatt, J. D. (2006). Epigenetic mechanisms: a common theme in vertebrate and invertebrate memory formation. Cell. Mol. Life Sci. 63, 1009–1016.
Maksimova, O. A., and Balaban, P. M. (1983). Neural Mechanisms of Behavioral Plasticity. Moscow: Nauka (in Russian).
Martin, K. C., Michael, D., Rose, J. C., Barad, M., Casadio, A., Zhu, H., and Kandel, E. R. (1997). MAP kinase translocates into the nucleus of the presynaptic cell and is required for long-term facilitation in Aplysia. Neuron 18, 899–912.
Moroz, L. L., Edwards, J. R., Puthanveettil, S. V., Kohn, A. B., Ha, T., Heyland, A., Knudsen, B., Sahni, A., Yu, F., Liu, L., Jezzini, S., Lovell, P., Iannucculli, W., Chen, M., Nguyen, T., Sheng, H., Shaw, R., Kalachikov, S., Panchin, Y. V., Farmerie, W., Russo, J. J., Ju, J., and Kandel, E. R. (2006). Neuronal transcriptome of Aplysia: neuronal compartments and circuitry. Cell 127, 1453–1467.
Pascual, A., Huang, K. L., Neveu, J., and Preat, T. (2004). Brain asymmetry and long-term memory. Nature 427, 605–606.
Peterson, C. L., and Laniel, M. A. (2004). Histones and histone modification. Curr. Biol. 14, R546–R551.
Reul, J. M., and Chandramohan, Y. (2007). Epigenetic mechanisms in stress-related memory formation. Psychoneuroendocrinology 32, S21–S25.
Ribeiro, M. J., Shofield, M. J., Kemenes, I., O’Shea, M., Kemenes, J., and Benjamin, P. R. (2005). Activation of MAPK is necessary for long-term memory consolidation following food-reward conditioning. Learn. Mem. 12, 538–545.
Rogers, L. J., and Vallortigara, G. (2008). From antenna to antenna: lateral shift of olfactory memory recall by honeybees. PLoS ONE 3, e2340. doi: 10.1371/journal.pone.0002340.
Salimova, N. B., Miloshevich, I., and Salimov, R. M. (1984). The effects of 5,6-dihydroxytryptamine and 6-hydroxydopamine on maze choice behavior in snail (Helix lucorum). Zh. Vyssh. Nerv. Deiat. Im. I P Pavlova 34, 941–947.
Sananbenesi, F., Fischer, A., Schrick, C., Spiess, J., and Radulovic, J. (2003). Mitogen-activated protein kinase signaling in the hippocampus and its modulation by corticotropin-releasing factor receptor 2: a possible link between stress and fear memory. J. Neurosci. 23, 11436–11443.
Sharma, S. K., and Carew T. J. (2004). The roles of MAPK cascades in synaptic plasticity and memory in Aplysia: facilitatory effects and inhibitory constraints. Learn. Mem. 11, 373–378.
Shtark, M. B., Grinkevich, L. N., Deriy, B. N., Zapara, T. A., and Tretyakov, V. P. (1982). “On the plasticity of a simple nervous system,” in Neuronal Plasticity and Memory Formation, eds A. Marsan and H. Matthies (New York: Raven Press), 271–283.
Stepanov, I. I., Lokvov, M. I., Satarov, A. S., Kunsevich, S. V., and Vartainyan, G. A. (1988). Humoral link in the mechanism of formation of the food refusal conditioned response in the snail. Neurosci. Behav. Physiol. 18, 257–264.
Suzuki, H., Thiele, T. R., Faumot, S., Ezcurra, M., Lockery, S. R., and Schafer, W. R. (2008). Functional asymmetry in Caenorhabditis elegans taste neurons and its computational role in chemotaxis. Nature 454, 114–118.
Sweatt, J. D. (2009). Experience-dependent epigenetic modifications in the central nervous system. Biol. Psychiatry 65, 191–197.
Thomas, G. M., and Huganir, R. L. (2004). MAPK cascade signalling and synaptic plasticity. Nat. Rev. Neurosci. 5, 173–183.
Wood, M. A., Hawk, J. D., and Abel, T. (2006). Combinatorial chromatin modifications and memory storage: a code for memory. Learn. Mem. 13, 241–244.
Wood, M. A., Kaplan M. P., Park A., Blanchard E. G., Olivera A. M., Lombardi T. L., and Abel T. (2005). Transgenic mice expressing a truncated form of CREB-binding protein (CBP) exhibit deficits in hippocampal synaptic plasticity and memory storage. Learn. Mem. 12, 11–119.
Keywords: learning, epigenetics, histone H3 acetylation, MAPK/ERK, neuronal networks, lateralization, Helix, chromatin remodeling
Citation: Danilova AB, Kharchenko OA, Shevchenko KG and Grinkevich LN (2010) Histone H3 acetylation is asymmetrically induced upon learning in identified neurons of the food aversion network in the mollusk Helix lucorum. Front. Behav. Neurosci. 4:180. doi: 10.3389/fnbeh.2010.00180
Received: 01 June 2010;
Accepted: 11 November 2010;
Published online: 25 November 2010.
Edited by:
Martin Giurfa, Université Paul Sabatier, FranceReviewed by:
Martin Giurfa, Université Paul Sabatier, FranceCopyright: © 2010 Danilova, Kharchenko, Shevchenko and Grinkevich. This is an open-access article subject to an exclusive license agreement between the authors and the Frontiers Research Foundation, which permits unrestricted use, distribution, and reproduction in any medium, provided the original authors and source are credited.
*Correspondence: Larisa N. Grinkevich, Laboratory of Regulation of Function of Brain Neurons, Pavlov Institute of Physiology RAS, 199034, Nab. Makarova 6, St. Petersburg, Russia. e-mail: larisa_gr_spb@mail.ru
