Division of Biochemistry, Department of Medicine, University of Fribourg, Fribourg, Switzerland
Brain-specific neural-zinc-finger transcription factor-2b (NZF2b/7ZFMyt1) is induced in the mesolimbic dopaminergic region after chronic cocaine exposure and lentiviral-mediated expression of NZF2b/7ZFMyt1 in the nucleus accumbens results in decreased locomotor activity (Chandrasekar and Dreyer, 2010
). In this study the role of NZF2b/7ZFMyt1 in active cocaine seeking and of its interaction with histone deacetylase on the altered behavior has been observed. Localized expression of NZF2b/7ZFMyt1 in the nucleus accumbens resulted in attenuated cocaine self-administration, whereas silencing this transcription factor with lentiviruses expressing siRNAs increased the animal′s motivation to self-infuse cocaine. Low doses of sodium butyrate, a potent inhibitor of histone deacetylase, were sufficient to reverse the NZF2b/7ZFMyt1-mediated decrease in cocaine self-administration. NZF2b/7ZFMyt1 expression resulted in strong induction of transcription factors REST1 and NAC1 and of the dopamine D2 receptor, with concomitant inhibition of BDNF and its receptor TrkB. We show that NZF2b/7ZFMyt1 colocalizes with histone deacetylase-2 (HDAC2), probably overcoming the suppression of transcriptional activity caused by Lingo1. These findings show that molecular adaptations mediated by NZF2b/7ZFMyt1 expression possibly lead to decreased responsiveness to the reinforcing properties of cocaine and play a prominent role in affecting the behavioral changes induced by the drug.
Drugs of abuse induce long-term synaptic changes in the brain’s reward system. Synaptic plasticity directly underlies addictive behaviors after drug exposure (Nestler, 2000
; Conrad et al., 2008
; Lüscher and Bellone, 2008
), but the mechanisms involved in these processes, and how drug-induced neural plasticity is mediated by transcriptional regulation, are still poorly known. Changes in synaptic transmission and neuronal morphology results from altered gene expression and epigenetic mechanisms, and are thus the emerging focus for recent investigations on addiction (Nestler, 2000
; Tsankova et al., 2004
, 2007
; Kumar et al., 2005
; Renthal et al., 2007
). These studies support the key role of cocaine-induced transcription factors and their implication in chromatin remodeling for drug mediated altered gene expression (Berke and Hyman, 2000
; Nestler, 2001
). Transcription factors regulate target genes by recruiting histone modifying enzymes, eg., histone acetyl transferases [HATs] and histone deactylases [HDACs] (Brami-Cherrier et al., 2005
; Kumar et al., 2005
; Levine et al., 2005
). In adult neurons this may be associated with learning and memory and in response to psychotropic drugs (Guan et al., 2002
; Alarcón et al., 2004
; Korzus et al., 2004
; Levenson et al., 2004
; Li et al., 2004
; Renthal et al., 2007
).
In the previous studies we have shown that the brain-specific transcription factor NZF2b/7ZFMyt1 (Neural Zinc Finger Transcription Factor-2b/7-Zinc Finger Myelin Transcription Factor-1) is induced significantly in the brain reward pathway after chronic cocaine administration and may play a key role in regulating the locomotor effects of cocaine (Chandrasekar and Dreyer, 2010
). Expressed predominantly in the developing CNS, neural precursors and some subpopulations of mature neurons (Kim and Hudson, 1992
; Bellefroid et al., 1996
; Kim et al., 1997
; Matsushita et al., 2002
) NZF-2b/7ZFMyt1 is also expressed in developing oligodendrocytes and tightly regulates expression of several myelin-specific gene products, such as PLP1, MAL2, etc. (Armstrong et al., 1995
; Nielsen et al., 2004
). NZF2b/7ZFMyt1 interacts with the co-repressor Sin3B and actively recruits HDAC1 and HDAC2 to specific genes (Romm et al., 2005
).
To further understand the role of NZF2b/7ZFMyt1, we investigated its effects on modulating cocaine self-infusion in rats. For this purpose, we manipulated NZF2b/7ZFMyt1 expression levels in the nucleus accumbens (NAc) using lentivirus-mediated overexpression or silencing. First we studied the effects of this manipulation on cocaine self-administration. Second, we observed that this altered behavior is mediated by histone deacetylase (HDAC) that is recruited by NZF2b/7ZFMyt1 for suppression of gene expression (Romm et al., 2005
). Third we found differential expression changes in several downstream target genes after NZF2b/7ZFMyt1 regulation, a possible result from epigenetic modification after cocaine administration. Together these results point to the potential role of NZF2b/7ZFMyt1 in regulation of gene expression in cocaine-induced neural plasticity.
Animal Handling and Surgical Procedures
All animal experiments were carried out in accordance with guidelines and regulations for Animal Experimentation, BAG, Bern, Switzerland. Male Wistar rats weighing 250–300 g (BRL, Fillingsdorf), Switzerland) were used for all the experiments. The animals were housed in groups (four per cage) in clear plastic cages with wire grid lids. The animals were kept on a 12-h light/dark cycle (lights off at 07.00 h), with access to food and water ad libitum. For doxycycline treatment, animals were maintained with 0.02% doxycycline (Sigma, Steinheim, Germany) and 5% sucrose supplemented in water. During the gene over expression period, animals were maintained only with 5% sucrose.
Intravenous Catheter Implantation Surgery
Male Wistar rats (250–280 g) were individually housed under a constant 12 h light:dark cycle (lights off at 07.00 h) with free access to food and water. Rats were anaesthetized with a cocktail mixture of ketamine (10 mg/kg) and xylazine HCl (0.1 mg/kg) administered i.p. with a volume of 1 mL/kg. The surgical procedures were performed according to previous publications (Boyer and Dreyer, 2007
) and consisted of making an opening between the breastbone and the clavicle to expose the jugular vein and a SILASTIC catheter (15-μL dead volume) was inserted into the external jugular vein by a discrete incision. The heparinized catheter was inserted 3 cm into the jugular vein and secured with sutures. The catheter was then passed under the skin and fixed in the mid-scapular region. The catheter, which consisted of a SILASTIC tubing 14 cm long (Instech Laboratories, Paolo Alto, USA), was fitted to a 22 gauge guide cannula (Instech Laboratories) that was custom bent and embedded in dental cement on a circular 3-cm mercylene mesh base. For the first 3 days after surgery, the catheters were flushed daily with 0.1 mL of saline mixed with heparin (100 U/mL) and gentamicin (1 mg/kg, Sigma, Steinheim, Germany). Thereafter, the catheters were flushed with a saline–heparin mixture after each session to prevent clotting.
Stereotaxic surgery
Stereotaxic surgery and lentiviral injection were performed according to previous publications (Bahi et al., 2004
; Boyer and Dreyer, 2007
). Rats were anesthetized with ketamine-xylazine (10 mg/kg and 0.1 mg/kg, Streuli and Co. AG) administered i.p. Animals were bilaterally injected into the NAc with 2 μL of concentrated lentiviral expression system stock (0.2 mg/mL of p24, corresponding to 8 × 109 IU/mL). The injections were performed bilaterally at the following coordinates, as calculated from bregma and the dura mera: anterior +1.4; lateral ±1.2; ventral −6.8 (Paxinos and Watson, 1998
).
Cocaine operant self-administration
Apparatus. Standard operant chambers (Coulbourn Instruments, Allentown, CA, USA) housed in ventilated sound-attenuating cubicles (Coulbourn Instruments) with fans to mask outside noise were utilized. A single channel fluid swivel (Instech Laboratories; Plymouth Meeting, CA, USA) was mounted on a balanced arm above each chamber. These chambers were equipped with syringe pump systems, which consisted of an infusion pump (Razel model A-99; Stamford, CT, USA) with a 10-mL plastic syringe connected by a single-channel 22-gauge swivel (Instech Laboratories) with Teflon tubing. The tubing could be connected to the animal’s catheter system in order to deliver cocaine solution. Two nose poke holes with a 3-cm diameter each and located 4 cm above the floor were situated on the same wall of each chamber. One of these was selected as the active hole for delivering the reinforcer and the other as the inactive hole. Nose pokes into both holes were recorded. When the number of programmed nose pokes into the active hole had been reached, a 40 μl volume of cocaine solution was delivered per active-nose poking over a period of 2 s, followed by a 40-s time out. Inactive-nose pokes were recorded as a measure of general activity. Three ‘cue’ lights above the active hole were paired contingently with delivery of cocaine. Active-nose pokes that were performed during the infusion time or during the time out period were tabulated but did not result in any further cocaine delivery. During infusion of cocaine, the house lights were turned off, and the cue lights over the active nose poke were illuminated. During the time out period house lights were turned on and the cue lights were switch off. Close to the nose poke was a recessed food receptacle into which food pellets (45 mg each; Bio-Serv, Frenchtown, NJ, USA) could be dispensed from a pellet dispenser. A house light was also located within the chamber. Graphic State Notation (Coulbourn Instruments, Allentown, CA, USA) was used to program experimental parameters (e.g. schedules of reinforcements, time periods, etc.), and tabulate and store session data.
Drug self-administration paradigm. Three groups of animals (n = 7) were prepared and were stereotaxically injected with either LV-7ZFMyt1, a mix of three LV-7ZFMyt1-shRNAs silencers or LV-GFP (control) as described above. After 1 week of recovery, rats were returned to the operant chambers and were allowed to self-administer cocaine on a FR1 schedule of reinforcement in a daily 1-h session. Testing started 2 h after lights off. The animals, self-administered cocaine (0.7 mg/kg) per injection. The criteria for acquisition of cocaine self-administration were defined by a constant number of self-infusions over a period of at least three consecutive sessions (±20%) (Boyer and Dreyer, 2007
). After establishment of cocaine induced stable behavior (self-infusion) under the FR1 schedule of reinforcement, the fixed ratio requirements were raised to FR2, FR3 and then finally to FR5, which was maintained for the remaining of the experiment. During this whole procedure animals were fed with doxycycline (0.02% in 5% sucrose solution) to block ectopic gene expression during the operant conditioning training period. Once the operant conditioning session is stabilized in FR5 for five consecutive days, the animals were fed without doxycycline for 7 days and recording was performed 48 h after regimen switch (Session A); thereafter animals were again fed doxycycline for 7 days and recording was performed 48 h after the switch (Session B) and then fed again without doxycycline for another 7 days, starting the recordings 48 h after regimen switch (Session C). At the end of this period animals remained fed without doxycycline for 6 days and pharmacologically treated over three consecutive days with a single daily injection of sodium butyrate (NaB) (100 mg/kg, i.p.) a histone deacetylase (HDAC) inhibitor (Sigma, Steinheim, Germany), and self-administration was recorded 30 min after the i.p. injection. After this schedule, one-third of each group of the animals were killed by decapitation 24 h after the last cocaine self-administration session (under sodium butyrate treatment); half of the remaining animals from each groups were fed doxycycline and the other half without doxycycline for 5 days (to remove the NaB from the system) before being sacrificed. NAc from brain tissues were dissected and used either for Western blot or quantitative real-time PCR analysis. Sections from frozen brains were used for immunohistochemistry.
Preparation of Lentiviruses
LV-7ZFMyt1 lentiviral expression constructs
The lentiviral expression construct of the Myelin transcription factor 1 (MYT1) gene was generated from the MYT1 plasmids kindly provided by Prof. Armstrong′s lab (Uniformed Services University of the Health Sciences, Bethesda). The 7 zinc finger MYT1 gene coding region along with the Myc tag (∼3.9 kb) from pMycMyt1-7zf-IRES/RED expression vector was first digested with EcoR I and sub-cloned into pDRIVE vector (QIAGEN). MYT1 gene ORF was then digested with SnaB I and Xho I from pDRIVE and cloned using Hpa I and Xho I sites into the lentiviral vector pTK-431 that expresses the gene of interest under control of a Tet-Off promotor. The clones were verified by conventional methods. The green fluorescent protein (GFP) expressing lentiviral vector pTK433 (Bahi et al., 2004
; Boyer and Dreyer, 2007
) was used as a control vector.
LV-7ZFMyt1-siRNA lentiviral expression constructs
Three different siRNAs, targeting different regions of the MYT1 mRNA sequence, were designed to silence NZF-2b/7ZFMyt1 expression in vitro and in vivo. The following targets within the MYT1 mRNA sequence were selected, based on Hannon’s design criterion
1
: first target: bp 1127–1145; second target: bp 2239–2268 and third target: bp 3487–3507. To each oligo, a Xho-I restriction site was added at 3’, and a U6-3’-specific 10mer at 5’. Using the pSilencer 1.0-U6 (Ambion, UK) as a template and a U6 promoter-specific forward primer containing BamH-I restriction site (5’-GCG GAT CCC GCT CTA GAA CTA GTG C-3’), each siRNA target was added to the mouse U6 promoter by PCR, using the following PCR program: 120 s at 93°C (initial denaturation) followed by 35 cycles (45 s at 92°C, 45 s at 64°C and 45 s at 72°C) in 5% dimethyl sulphoxide (Sigma, Lucern, Switzerland). The PCR product was directly ligated into pDRIVE vector from QIAGEN PCR Cloning Kit (QIAGEN, Basel, Switzerland). The shRNA insert along with the U6 promoter was then digested with BamH-I and Xho-I, and sub-cloned into similar sites in the lentiviral vector pTK-431, and sequenced to verify the integrity of each construct.
Lentivirus production
The lentiviral vector expression plasmids (either pTK431-7ZFMyt1, pTK433-GFP or pTK431-U6-7ZFMyt1-siRNAs), along with the packaging construct plasmid pΔNRF and the envelope plasmid pMDG-VSV-G were co-transfected into human embryonic kidney (HEK) 293T cells to produce the viral particles (Bahi et al., 2004
; Boyer and Dreyer, 2007
). The viral titers were determined by p24 antigen measurements (KPL, USA). For the in vivo experiments, the different viral stocks were matched for viral particle content and used at 0.2 mg/mL of p24.
Molecular Analysis
RNA isolation and real-time quantitative PCR (qRT-PCR)
Post behavioral experiments, animals were sacrificed and the NAc regions were dissected out from 1-mm thin slice sections using rat brain matrix (RBM-3000, ASI instruments, inc, USA), for both RNA and protein extraction. Rat brain NAc region (micro-dissected from lentivirus treated animals) or HEK-293 cells infected with lentiviruses for gene expression were homogenized in TRIzol reagent (Invitrogen, Basel, Switzerland), according to manufacturers protocol. After being washed with 70% and 100% (v/v) ethanol, RNA pellets were dissolved in DEPC-treated H2O. Quality and integrity of the RNA was analyzed and quantified accurately by a bioanalyzer, and 2%agarose gel. All RNA samples were stored at −70°C. The cDNAs were prepared using 4 μg of RNA from each sample reverse transcribed at 42°C for 30 min with 1 μg of Oligo(dT) or random hexamers, 5X first strand buffer, 100-mM DTT, 10-mM dNTP, Rnasin (Invitrogen, Basel, Switzerland) and M-MLV reverse transcriptase (Promega, Wallisellen, Switzerland). Primer sets were designed to amplify 100–200 bp products, using PRIMER3 software
2
. PCR reaction mixtures included cDNAs in optimum dilution, the SYBR-Green qPCR Master mix (BioRad Reinach, Switzerland), 10-μM primers, in a total reaction volume of 20 μl. Expression profiling was done with dissociation curves using iCycler (BioRad). Cycling parameters were 95°C for 4 min followed by 40 cycles of 20°C/s temperature transition rate up to 95°C (30 s), 62°C (45 s), followed by melting curve analysis. All reactions were performed in triplicates with reference dye normalization (β-actin or Cyclophilin) and the median Ct (Cycle threshold) value was used for analysis. To determine the linearity and detection limit of the assay, cDNA samples were amplified for successive 10-fold dilutions in a series of real-time PCRs, using a duplicate assay on each dilution, so that the correlation coefficient could be calculated from the standard curve of Ct values. The ΔCt for each candidate was calculated as: ΔCt = [Ct (candidate) − Ct (Cyclophilin or β-actin)]. The relative abundance of each target can be calculated as the ratio between treated and untreated samples (Bahi et al., 2004
; Mühlbauer et al., 2004
; Boyer and Dreyer, 2007
). The PCR reaction was evaluated by melting curve analysis and by checking the PCR products on 2% agarose gel. Comparisons were made between cocaine and saline groups, and significance was calculated using two-way anova followed by Bonferroni post hoc tests and the level of statistical significance was set at P < 0.001. Data were expressed as means ± SEM.
The following primers sets were used for amplification: NZF-2b/7ZFMyt1: forward 5′-GCA GAC CTC AGT TGT CCT ACC-3′ and reverse 5′-CTT GGA TAC CAG GTG CTC AG-3′; Cyclophilin: forward 5′-GTG AGA AGG GCT TTG GCT AC-3′ and reverse 5′-TTC TCG TCA GGA AAG CGG-3′; β-actin: forward 5′-AGC CAT GTA CGT AGC CAT CC-3′ and reverse 5′-CTC TCA GCT GTG GTG GTG AA-3′; BDNF: forward 5′-GGT TCG AGA GGT CTG ACG AC-3′ and reverse 5′- CAA AGG CAC TTG ACT GCT GA-3′; TrkB forward 5′- CCT CGT CGG AGA AGA TCA AG -3′ and reverse 5′- CGT GGT ACT CCG TGT GAT GT-3′; REST1: forward 5′- CGA GTT GAT GCC TGT TGG AGA C-3′ and reverse 5′- TGC TTC AAA TAC GGG CTG GG-3′; NAC1: forward 5′- GCT CTT CCT GAG CAG GTC GT-3′ and reverse 5′- GTG CCT GTC ACA AGC TCC AG-3′. Urokinase: forward 5′- CAG ATC CGA TGC TCT TAG CC -3′ and reverse 5′- TAG AGC CTT CTG GCC ACA CT-3′; Dopamine transporter: forward 5′- GTT CTA CGG CGT CCA GCA -3′ and reverse 5′- TGA CCA CGA CCA CAT ACA GG-3′; CD81: forward 5′- TGA TCC TGT TTG CCT GTG AG -3′ and reverse 5′- CAG TTG AGC GTC TCA TGG AA-3′; Dopamine D2 receptor: forward 5′- CAT TGT CTG GGT CCT GTT CCT -3′ and reverse 5′- GAC CAG CAG AGT GAC GAT GA-3′; Dopamine D3 receptor: forward 5′- GGG GTG ACT GTC CTG GTC TA -3′ and reverse 5′- TGG CCC TTA TTG AAA ACT GC-3′.
Immunohistochemistry
To check the expression of NZF-2b/7ZFMyt1 after lentiviral injections, rats were decapitated, brains were quickly removed, immediately frozen in isopentane (at −30°C for 3 min) and kept at −25°C. Coronal sections were cut at 14 μm in a cryostat and placed on gelatinized glass slides, air−dried at room temperature for 20 min and kept at −25°C until further processing. Brain sections were fixed for 10 min in cold acetone and washed three times in 1 × phosphate-buffered saline (PBS). Non-specific binding sites were blocked by incubating slices for 1 h in 1 × PBS containing 1% bovine serum albumin, 1% Triton X-100 and 3% normal goat serum. Sections were then incubated overnight at 4°C with primary antibodies specifically against either endogenous NZF-2b/7ZFMyt1 (rabbit polyclonal anti-MYT1 antibody; 1:15, Sigma prestige antibodies-HPA006303) or against Myc for ectopic Myc-tagged protein (rabbit polyclonal anti-Myc-tag antibody; 1:1000, Abcam-ab9106), for neuronal marker, NeuN (mouse monoclonal anti-neuronal-nuclei; 1:1000, Chemicon-MAB 377), for HDAC2 (mouse monoclonal to Histone deacetylase-2; 1:1000, Abcam-ab12169), for dopaminergic neuronal marker, TH (rabbit polyclonal to Tyrosine hydroxylase protein; 1:750; Abcam, ab112-100), for oligodendrocyte marker, Oligo2 (rabbit polyclonal; 1:500, Abcam, ab33427), for Lingo1 (rabbit polyclonal to LINGO-1; 1:500, Abcam-ab23631). Antibodies were diluted in 1 × PBS containing 0.1% Triton X-100 and 1% normal goat serum. Thereafter, slices were washed three times in 1 × PBS, and incubated for 2 h in the secondary antibody (Texas red-conjugated goat anti-rabbit immunoglobulin G, 1:5000) or (FITC-conjugated goat anti-mouse IgG, 1:5000, Invitrogen 65-6411) in 1 × PBS containing 0.1% Triton X-100. The sections were cover-slipped with a medium containing glycerol in PBS (AF1 mounting solution, Citifluor Ltd., London). For double and triple staining experiments the nuclei were stained by 30-min incubation with Hoechst 33342 (1:1500, Sigma, Steinheim, Germany). Negative controls included omission or substitution of primary antibodies.
Fluorescence microscopy was performed to observe the stained sections using a multi-fluorescence microscope (Axioplan 2 imaging; Zeiss). Fluorophores (FITC and Texas red) used were detected with the appropriate detecting systems (HAL 100). FITC was excited at 495 nm and was detected through a light path ranging 510–550 nm. Texas red was exited at 570 nm and was detected through a light path ranging 600–660 nm. Stained sections were visualized, photographed using a multichannel camera (Axiocam, Zeiss) combined with acquisition software (Axiovision system 3.1) and recorded on CD (Boyer and Dreyer, 2007
).
Protein isolation and western blotting
Microdissected NAc regions from lentiviral injected rats (150–200 mg) or transfected HEK293T cells were homogenized in buffer (Tris-HCl 50 mm, pH 7.5; NaCl 120 mm; CaCl2 1.5 mm; MgCl2 5 mm; KCl 5 mm; EDTA 5 mm) with a protease inhibitor mixture (Sigma, St Louis, MO, USA; 1 mL/20 g of tissue). The homogenates were then solubilized with 1% digitonin, followed by the addition of secondary solubilization buffer [Tris-HCl 50 mm, pH 7.6; NaCl 150 mm; 1% Nonidet-P40; 0.5% sodium deoxycholate; EDTA 2 mm; sodium ortho-vanadate 1 mm; phenylmethylsulphonyl fluoride (PMSF) 1 mm; 1% Triton X-100]. Protein concentration was determined by the DC protein assay method (Bio-Rad) using BSA as a standard. The protein extracts (20 μg) were subjected to SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred into Immobilon PVDF membranes (Millipore). Blots were blocked with 5% non-fat dried milk dissolved in TBST (Tris 10 mm; NaCl 150 mm; 0.1% Tween-20) for 1 h at room temperature. The blots were then incubated with primary antibodies for either Myc tag (rabbit polyclonal; 1:1000; Abcam, ab9106) or MYT1 (rabbit polyclonal; 1:15; Sigma prestige antibodies HPA006303) overnight at 4°C. Blots were washed three times with TBST and incubated for 1 h with the peroxidase-conjugated secondary antibody solution. Proteins were then visualized using enhanced chemiluminescence (Millipore, Basel, Switzerland). For house keeping gene, loading control measurements, membranes were washed for 30 min in TBST and placed in stripping solution (glycine-HCl 25 mm, pH 2.0, 1% SDS) for 30 min and were blocked and put for antibody binding as described above for β-actin detection (1:4000; Sigma) using the same membrane.
Statistical Analysis
For statistical analysis two-way repeated-measures analysis of variance Anova was used to compare both lentiviral groups and drug treatment in the presence or absence of doxycycline, using GraphPad PRISM (V3.0, GraphPad, San Diego, CA, USA). For self-administration two-way Anova with time as the within-subject factors and lentiviral gene expression as the between-subject factors followed by Bonferroni post hoc comparison was used. Effects of the HDAC inhibitor during the totality of the FR sessions were analyzed by using treatment and session as factors of variation, between groups of rats. qPCR results were analyzed by two-way Anova, with drug treatment (presence or absence of doxycycline) as the within-subject factors and lentiviral-mediated gene expression as the between-subject factors followed by Bonferroni post hoc comparison. Statistical significance was set at P ≤ 0.05.
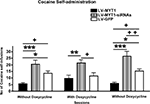
Lentiviral-Mediated NZF-2b/7ZFMyt1 Expression in the NAc Inhibits Cocaine Self-Administration
The effects of NZF-2b/7ZFMyt1 overexpression or silencing in the NAc on cocaine self-administration were tested under FR5 schedule. Three groups of animals (n = 7) were used for the study. Stereotaxical injections were performed into the NAc shell; one group was injected LV-7ZFMyt1 (a doxycycline-regulatable lentiviral vector), the second group LV-7ZFMyt-shRNAs (a mixture of three non-regulatable lentiviral vectors expressing shRNAs targeted against NZF-2b/7ZFMyt1) and the third group the regulatable LV-GFP (control group). LV-7ZFMyt1 expression and specific silencing efficiency with LV-7ZFMyt1-siRNAs have been confirmed in vitro (Chandrasekar and Dreyer, 2010
). After intravenous catheterization, training and acquisition of cocaine SA under FR5, the effects of LV-mediated gene expression were assessed over a period of 4–5 weeks. During the training period all animals were fed doxycycline to block ectopic gene expression in the NAc (see Materials and Methods). Once animals have been stabilized under FR5 for five consecutive days, they were fed without doxycycline for another 5 days (Session A). Under these conditions the control LV-GFP group exhibited strong self-administration with a mean of 13.7 infusions per session. The LV-7ZFMyt1 group, overexpressing NZF-2b/7ZFMyt1 in the NAc, displayed a significant 2.4-fold decrease in cocaine self-administration compared to the control LV-GFP group, with a mean of 5.7 infusions per session (Figure 1
, F2,36 = 37.70, P < 0.0001). By contrast, in the third group, silencing of NZF-2b/7ZFMyt1 in the NAc yielded a 1.5 fold increase (P > 0.05) in self-administration over the LV-GFP group and a very significant 3.6-fold increase over the LV-7ZFMyt1 group (P < 0.001).
Figure 1. Differential expression of NZF-2b/7ZFMyt1 in the Nucleus Accumbens (NAc) alters cocaine self-administration. Animals injected with regulatable LV-7ZFMyt1, LV-GFP and non-regulatable LV-7ZFMyt1-siRNAs were used. After surgery, recovery and training, rats (n = 7) were subjected to cocaine self-administration (1 h/day) under FR5 schedule over the three consecutive sessions. Session A: animals were fed without doxycycline for 6 days; Session B: then animals were fed doxycycline in the drinking water for 6 days; Session C: finally animals were switched back and fed again without doxycycline (see Materials and Methods). Session A: Ectopic expression of NZF-2b/7ZFMyt1 in the NAc inhibits cocaine self-administration, whereas local knockdown of the NZF-2b/7ZFMyt1 promotes self-infusion. Session B: doxycycline-mediated inhibition of ectopic NZF-2b/7ZFMyt1 expression (black bar) leads to reversal of the cocaine self-administration suppression (compare sessions A with B, black bars), and self-infusion is comparable to the control LV-GFP level. Session C: removal of doxycycline reverses to the initial behaviors observed in session A. Data shows the means ± SEM of self-infusions of cocaine in the three sessions. *P < 0.05, **P < 0.01 and ***P < 0.001, represent values significantly different from Lenti-7ZFMyt1 group;+P < 0.05,++P < 0.01 and +++P < 0.001 represent values significantly different from Lenti-GFP group, two-way ANOVA, Bonferroni post hoc tests.
Thereafter all animals were fed doxycycline for 7 days and self-administration was recorded 48 h after switching regimen, once full switch-off in gene expression has been established (Session B). Under these conditions, the LV-GFP group showed no significant change in cocaine SA with a mean of 11.7 infusions per session, in agreement with previous studies showing that GFP overexpression in the NAc does not affect behavior (Bahi et al., 2004
, 2005
; Bahi and Dreyer, 2005
; Boyer and Dreyer, 2007
). Under this regimen, ectopic expression of NZF-2b/7ZFMyt1 is repressed in the LV-7ZFMyt1 group, which showed a significant 1.7 fold increase in self-administration compared to the same group in Session A, with a mean of 9.8 infusions per session (Figure 1
, F2,36 = 1.18, P = 0.3189) and no significant change compared to control LV-GFP group (P > 0.05). As expected self-administration of the LV-7ZFMyt1-siRNA group was not affected by regimen switch, with a mean at 21.4 self-infusions per session, resulting in a 1.8-fold (P < 0.05) and 2.2-fold (P < 0.01) increase compared to the LV-GFP and the LV-7ZFMyt1 group respectively.
Finally doxycycline was again removed and cocaine self-administration was recorded 48 h after regimen change for five consecutive days (Session C). Under these conditions, which recapitulate gene expression of Session A, self-administration in the LV-GFP group (control) was unchanged, with a mean of 15.4 infusions per session, whereas ectopic expression of NZF-2b/7ZFMyt1 in the NAc in the LV-7ZFMyt group induced a significant 2.6-fold drop compared to the LV-GFP group, with a mean of 6 infusions per session (F2,36 = 37.70, P < 0.0001). In the LV-7ZFMyt1-siRNA group, a mean at 26.6 self-infusions per session were recorded, i.e. a 1.7 (P < 0.01) and 4.4-fold (P < 0.001) increase over the LV-GFP and the LV-7ZFMyt1 group, respectively.
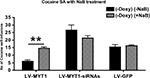
HDAC Inhibition by Sodium Butyrate Blocks NZF-2b/7ZFMyt1-Mediated Suppression of Cocaine Self-Administration
NZF-2b/7ZFMyt1 regulates neural transcription partly via its interaction with co-repressors Sin3B and recruitment of histone deacetylases (HDACs) for suppression of gene expression (Romm et al., 2005
). HDAC inhibition affects behavior by augmenting cocaine-mediated histone modifications (Kumar et al., 2005
; Renthal et al., 2007
). To test whether the decrease in cocaine self-administration observed in the LV-7ZFMyt1 group is mediated by NZF-2b/7ZFMyt1-HDAC interaction, we used sodium butyrate (NaB), a widely used non-specific inhibitor of HDACs which promotes cocaine-induced behavior (Kumar et al., 2005
; Sun et al., 2008
). At the end of Session C animals received a daily dose of 100 mg/kg of NaB for three consecutive days, 30 mins before the cocaine self-administration session (see Materials and Methods). NaB treatment induced a significant 2.4-fold increase (P < 0.01) in the LV-7ZFMyt1 group compared to the same group in the Session C (i.e. without doxycycline and NaB) with a mean of 14.5 self-infusions of cocaine per session (Figure 2
, F2,24 = 31.27, P < 0.0001). NaB treatment in the LV-7ZFMyt1 group brought back cocaine self-administration to the level observed in the control LV-GFP group. The two other groups (LV-GFP or LV-7ZFMyt1-siRNA groups) displayed no significant behavioral changes upon NaB treatment (F1,24 = 0.96, P = 0.3359) with a mean of 16.3 and 21.4 self-infusions, respectively.
Figure 2. The HDAC inhibitor Sodium Butyrate (NaB) reverses inhibition of self-administration mediated by NZF-2b/7ZFMyt1. At the end of Session C (Figure 1
), animals fed without doxycycline were administered NaB (100 mg/kg, i.p.) 30 min prior to each cocaine self-administration tests, for three consecutive days. Values represent means ± SEM of self-infusions of cocaine in the three LV-treated groups (n = 7). NaB treatment reverted the inhibition of cocaine self-administration observed upon 7ZFMyt1 overexpression in the NAc to levels comparable to the control LV-GFP group. Neither LV-GFP nor LV-7ZFMyt1-siRNA group showed any statistically significant change after NaB treatments. *P < 0.05, **P < 0.01 and ***P < 0.001, represents values significantly different for the same groups compared between session C (no NaB) and session D (with NaB), two-way ANOVA, Bonferroni post hoc tests.
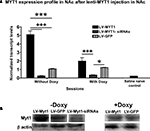
Quantification of NZF-2b/7ZFMyt1 Levels After in vivo LV-7ZFMyt1 Expression in the NAc
RNA from the NAc was isolated at the end of the behavioral experiments (see Materials and Methods) and NZF-2b/7ZFMyt1 mRNA levels were measured by qRT-PCR. As shown in Figure 3
, animals injected with LV-7ZFMyt1 displayed a ∼5-fold increase in expression of NZF-2b/7ZFMyt1 in the NAc, compared to LV-GFP control (endogenous expression) in the absence of doxycycline (Figure 3
A, F2,12 = 96.05, P < 0.0001). When fed doxycycline (shutting off expression of ectopic LV-NZF-2b/7ZFMyt1 in the NAc) the expression decreased to a mere ∼1.9-fold compared to LV-GFP control (F1,12 = 22.85, P = 0.0004). Control genes, e.g. cyclophilin or β-actin mRNA levels, remained unchanged. Animals injected with LV-7ZFMyt1-siRNAs in the NAc displayed a ∼4.3-fold decrease in NZF-2b/7ZFMyt1 mRNA in the NAc, compared to controls under all regimens (F2,12 = 29.78, P < 0.0001). Doxycycline blocks ectopic NZF-2b/7ZFMYT1 expression but not endogenously expressed NZF-2b/7ZFMYT1, while the LV-7ZFMyt1 silencers block both ectopic and endogenous NZF-2b/7ZFMYT1 expression. Cocaine self-administration induced a 5.6-fold increase in NZF-2b/7ZFMYT1 expression in the control animals (LV-GFP) over naive animals (saline controls), correlating with our earlier observation (Chandrasekar and Dreyer, 2010
). Western blot analysis of protein expression confirmed the expression changes observed at the mRNA levels (Figure 3
B). Together these data show that behavioral changes observed in cocaine self-administration is clearly related to expression changes of NZF-2b/7ZFMyt1.
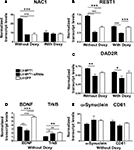
Figure 3. NZF-2b/7ZFMyt1 expression in the NAc after cocaine self-administration. (A) NAc samples were retrieved after the end of the behavioral studies (cocaine self-administration) from animals fed either with or without doxycycline. NZF-2b/7ZFMyt1 mRNA expression levels were determined by qRT-PCR relative to Cyclophilin. LV-7ZFMyt1 group displayed increased expression of NZF-2b/7ZFMyt1 compared to LV-GFP control, whereas in LV-7ZFMyt1-siRNA group a significant decrease in NZF-2b/7ZFMyt1 expression compared to LV-GFP was observed. Doxycycline regimen that inhibits ectopic expression resulted in a significant decrease in NZF-2b/7ZFMyt1 levels in the LV-7ZFMyt1 group. *P < 0.05; **P < 0.01; ***P < 0.001 represents values significantly different from LV-GFP group, and +P < 0.05; ++P < 0.01; +++P < 0.001 represents values significantly different from LV-7ZFMyt1 group, by two way ANOVA, Bonferroni post hoc tests. (B) Western blot analysis of NZF-2b/7ZFMyt1 protein levels in the NAc of the LV-treated animals after cocaine self-administration (see Materials and Methods). Western blots from gels loaded with 20 μg protein/lane were analyzed with antibody against NZF-2b/7ZFMyt1. LV-7ZFMyt1 group displays high protein expression compared to GFP control, whereas LV-7ZFMyt1-siRNA group shows a strong inhibition in the NZF-2b/7ZFMyt1 levels, correlating with the mRNA levels observed. The membranes were reprobed with antibodies against β-actin to serve as standard protein control. As with mRNA profile, doxycycline inhibits the ectopic NZF-2b/7ZFMyt1 expression level to that of the control GFP group.
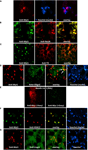
NZF-2b/7ZFMyt1 Recruits HDAC2 and Colocalizes in the Nuclei of the Neurons after Lentiviral-Mediated Overexpression in the NAc
Our previous studies showed that endogenous NZF-2b/7ZFMyt1 protein level does not change between sessions in the control LV-GFP group and that overexpression in the LV-7ZFMyt1 group results in nuclear localization of NZF-2b/7ZFMyt1, predominantly in neurons (Chandrasekar and Dreyer, 2010
). These observations were further analyzed after self-administration (Figure 4
). LV-7ZFMyt1 group without doxycycline displayed strong expression of NZF-2b/7ZFMyt1 when stained with anti-Myt1 antibody (Figures 4
A–E). Double-staining with Hoechst (staining all cell nuclei) showed co-localization of NZF-2b/7ZFMyt1 protein with some, but not all nuclei, suggesting a cell-type specific localization (Figure 4
A). Double immuno-staining with the neuronal nuclei-specific marker NeuN shows that NZF-2b/7ZFMyt1 is predominantly expressed in the neuronal nuclei and co-localizes with NeuN (Figure 4
B), with spatial segregation into discrete nuclear domains similar to the sites of active transcription. Double-staining with anti-TH (Tyrosine Hydroxylase, a dopaminergic neuron specific marker) showed that NZF-2b/7ZFMyt1 expression is strongly co-localized with nuclei of the TH-immunoreactive projection areas in the NAc (Figure 4
C). Furthermore, triple-staining with anti-Oligo2 (an oligodendrocytic nuclei marker) along with Hoechst (staining all cell nuclei), showed co-localization of NZF-2b/7ZFMyt1 protein with a few Oligo2 positive nuclei, indicating a limited expression in oligodendrocyte lineage cells (Figure 4
D) In addition, previous studies have shown that NZF-2b/7ZFMyt1 is not colocalized in GFAP-positive glial cells (Chandrasekar and Dreyer, 2010
). It should be noted that the lentiviral-mediated, ectopic expression of NZF-2b/7ZFMyt1 in the NAc is clearly very restricted along the needle tract, as observed using anti-Myc antibody, and treatment with doxycycline induced a potent inhibition (>85%) of ectopic expression in the target area (Figure 4
E); this shows the tight regulation of NZF-2b/7ZFMyt1 protein levels mediated by doxycycline-switch in LV-7ZFMyt1 group.
Figure 4. Lentivirus-mediated expression of NZF-2b/7ZFMyt1 recruits and co-localizes with HDAC2 in neuronal nuclei in the NAc. Animals were sacrificed at the end of cocaine self-administration behavioral tests, either in the presence or absence of doxycycline. Brains were dissected out and processed for immuno-histochemistry (see Materials and Methods). (Scale bar: 20 μm). (A) Double immunostaining with anti-Myt1 antibody (red) and Hoechst nuclei staining (blue) (100× magnification); merged image shows that the NZF-2b/7ZFMyt1 expression is localized in most if not all cell nuclei in the NAc. (B) Double immunostaining with anti-Myt1 antibodies for detecting NZF-2b/7ZFMyt1 protein (green) and anti-NeuN (a neuronal nuclei marker; red) (100× magnification); merged image shows that NZF-2b/7ZFMyt1 predominantly co-localizes (yellow) with NeuN within the nuclei. (C) Double immunostaining with anti-Myt1 (green) and anti-TH (Tyrosine hydroxylase; red) antibodies (63× magnification); merged image shows that most of the NZF-2b/7ZFMyt1 expression is localized in TH-immunoreactive projection areas (yellow) in the NAc. (D) Triple-staining with anti-Myt1 (red), Hoechst (nuclei staining; blue) and anti-Oligo2 (oligodendrocytic marker; green) antibodies (63× magnification). While Hoechst (blue) stains all nuclei non-specifically, regardless of the cell type, the oligodendrocytic marker Oligo2 shows a distinct profile compared to Myt1. Merged image shows that only few cells (arrows) display colocalization of Oligo2 and NZF-2b/7ZFMyt1 (yellow) in the NAc. (E) Low-magnification (20×) of ectopic NZF-2b/7ZFMyt1 labeling with anti-Myc in the needle tract of the NAc either in the presence (red) or absence (green) of doxycycline, showing a tightly regulatable expression of ectopic, Myc-tagged NZF-2b/7ZFMyt1. (F) Triple immunostaining with anti-Myt1 (green) and anti-HDAC2 (red) antibodies (63× magnification); merged image shows co-localization of NZF-2b/7ZFMyt1 with HDAC2 in the nuclei of the neurons. (G) Triple-staining with anti-Myt1 (red), Hoechst (nuclei staining; blue) and anti-Lingo1 (component of the Nogo-receptor complex; green) antibodies (63× magnification). Lingo1 is restricted to the cytoplasm whereas NZF-2b/7ZFMyt1 is localized to the nuclei of the neurons as evident from the Hoechst nuclei staining (blue).
Previous studies have shown that NZF-2b/7ZFMyt1 interacts with Sin3B and that, for its transcriptional suppressor function, it actively recruit histone deacetylases-1 and -2 (HDACs) (Romm et al., 2005
), which are histone modifying enzymes involved in maintaining suppressive chromatin structure for the target genes. To check whether NZF-2b/7ZFMyt1 interacts with HDAC2 for exerting its suppressive function, – correlating with the behavioral studies with HDAC inhibitors, – triple-staining with anti-HDAC2 antibodies were performed. The results showed that NZF-2b/7ZFMyt1 is strongly co-localized with HDAC2, and together is localized to the neuronal nuclei (Figure 4
F).
In addition, Myt1 interacts with Lingo1 which regulates its transcriptional activity based on recruitment to cytoplasm (Llorens et al., 2008
). In our previous studies we proposed a model wherein ectopic expression of NZF-2b/7ZFMyt1 results probably in saturating this negative regulation, resulting in the availability of NZF-2b/7ZFMyt1 for transcriptional regulation of its target genes. In order to check whether this holds true in the self-administration paradigm, we performed immuno-staining of the NZF-2b/7ZFMyt1 overexpression group NAc region with Myt1 and Lingo1 antibodies. The results show that Lingo1 staining is restricted to the cytoplasm, whereas NZF-2b/7ZFMyt1 expression is predominantly localized to the nucleus, as evidenced from the triple staining with Hoechst (nuclei staining) (Figure 4
G), suggesting that ectopic expression of NZF-2b/7ZFMyt1 relieves it from the negative regulatory effect caused by Lingo1.
NZF-2b/7ZFMyt1-Mediated Behavioral Changes Correlate with Down-Stream Molecular Adaptations in the NAc
In our previous studies we have shown that NZF-2b/7ZFMyt1 expression leads to induction of REST1 and NAC1 and inhibition of BDNF (Chandrasekar and Dreyer, 2010
). Expression of some downstream signaling genes with putative binding sites for NZF-2b/7ZFMyt1 were further tested upon self-administration paradigm (Figure 5
).
Figure 5. NZF-2b/7ZFMyt1 in the NAc specifically alters expression of several reward-related genes. qRT-PCR quantification of: NAC1 (A); REST1 (B); the dopamine receptor DAD2R (C); BDNF and its receptor TrkB (D), α-Synuclein and the tetraspanin CD81 (E). qRT-PCR was performed from NAc tissue of rats (n = 7) bilaterally injected into the NAc with either LV-7ZFMyt1 or the LV-7ZFMyt1-siRNAs or LV-GFP (see Materials and Methods). Expression levels were calculated relative to cyclophilin. Ectopic expression of NZF-2b/7ZFMyt1 led to increased expression of NAC1 (A) REST1 (B), DAD2R (C) and decreased expression of BDNF and TrkB (D), whereas it did not significantly modify the expression of α-Synuclein and the tetraspanin CD81 (E) and few other genes with binding domains for NZF-2b/7ZFMyt1 (data not shown). Doxycycline regimen that inhibits ectopic NZF-2b/7ZFMyt1 expression results in a significant decrease in the NAC-1 (A) REST1 (B) and DAD2R (C) levels. *P < 0.05; **P < 0.01; ***P < 0.001 represents values significantly different from lenti-GFP injected rats, and +P < 0.05; ++P < 0.01; +++P < 0.001 represents values significantly different from LV-7ZFMyt1 injected rats, by two way ANOVA, Bonferroni post hoc tests.
NZF-2b/7ZFMyt1 expression strongly induces NAC1 in the NAc (Figure 5
A, F2,12 = 30.88, P < 0.0001). LV-7ZFMyt1 group displays a ∼3-fold increase in NAC1 expression compared to LV-GFP-treated controls (Figure 5
A, F2,12 = 42.64, P < 0.0001). Doxycycline that suppresses local ectopic expression of NZF-2b/7ZFMyt1 did not affect NAC1 expression profile in LV-GFP-treated control animals, but in LV-7ZFMyt1 treated animals it induced a ∼2.5-fold suppression of NAC-1 (Figure 5
A, F1,12 = 23.90, P = 0.0004). No significant change in expression was observed in LV-7ZFMyt1-siRNAs group compared to LV-GFP group under the same conditions.
NZF-2b/7ZFMyt1 displays 13 putative conserved binding site modules in the BDNF promoter and it down-regulates its expression (Chandrasekar and Dreyer, 2010
). Further confirmation was achieved under self-administration paradigm, where LV-7ZFMyt1-treated animals showed a 1.6 fold inhibition of BDNF mRNA levels compared to LV-GFP group (Figure 5
B, F2,12 = 70.65, P < 0.0001). Suppression of NZF-2b/7ZFMyt1 (in animals treated with LV-7ZFMyt1-siRNAs) also resulted in 2.2-fold (P < 0.001) decreased expression of BDNF compared to LV-GFP group (F1,12 = 280.94, P < 0.0001). Under these conditions TrkB showed a ∼2-fold inhibition in the LV-7ZFMyt1 group compared to the LV-GFP group (P < 0.01) and ∼1.6-fold compared to the LV-7ZFMyt1-siRNA group (P < 0.05) (Figure 5
B, F2,12 = 70.65, P < 0.0001). No significant difference was observed between LV-GFP and LV-7ZFMyt1-siRNA group.
NZF-2b/7ZFMyt1 can also indirectly regulate BDNF expression through repression of MeCP2 (Liu et al., 2006
), which in turn regulates REST1 and CoREST expression leading to inhibition of BDNF expression (Abuhatzira et al., 2007
). qRT-PCR analysis of REST1 levels displays significant expression changes that correlate with expression changes of NZF-2b/7ZFMyt1 (Figure 5
C). The different lentivirus-treated animal groups and doxycycline regimen that modified NZF-2b/7ZFMyt1 expression also altered REST1 expression (Figure 5
C, F2,12 = 33.65, P < 0.0001). In the absence of doxycycline a ∼1.8-fold induction of REST1 was observed in LV-7ZFMyt1-treated animals (Figure 5
C, F2,12 = 128.92, P < 0.0001) compared to LV-GFP control animals, where it was unaltered by doxycycline. Silencing NZF-2b/7ZFMyt1 expression resulted in ∼1.5-fold decrease in REST1 level compared to LV-GFP-treated animals and this level was comparable to saline-treated naive animals (Chandrasekar and Dreyer, 2010
). Under doxycycline regimen the levels of REST1 expression in the LV-7ZFMyt1 group dropped significantly and was comparable to LV-GFP controls (F1,12 = 42.99, P < 0.0001).
Expression changes of the dopamine D2 receptor (DAD2R) in the NAc were analyzed. No significant changes were observed in DAD2R levels when LV-7ZFMyt1 or LV-7ZFMyt1-siRNAs groups were compared to the control LV-GFP group. In the absence of doxycycline a ∼1.4-fold (P < 0.01) induction of DAD2R was observed in LV-7ZFMyt1 treated animals compared to LV-7ZFMyt1-siRNA treated animals (Figure 5
D, F2,12 = 11.02, P = 0.0019), whereas under doxycycline the expression levels dropped significantly in the LV-7ZFMyt1 group (∼1.3-fold compared to LV-7ZFMyt1-siRNA group, F1,12 = 3.00, P = 0.1089). Doxycycline regimen did not alter the DAD2R expression profile of the LV-7ZFMyt1-siRNA or the LV-GFP group.
NZF-2b/7ZFMyt1 expression did not cause any significant change in the expression of the α-Synuclein and the tetraspanin, CD81 (Figure 5
E, F2,12 = 0.16, P = 0.8550) albeit having putative binding sites in their respective promoters. We restricted our gene expression analysis with a few genes based on in silico promoter analysis data, as analyzing of all the possible genes modulated upon NZF-2b/7ZFMyt1 overexpression is beyond the scope of this study. Further analysis of genes with putative binding site modules for NZF-2b/7ZFMyt1 was nevertheless tested (dopamine transporter, dopamine receptor DAD3R, Sema4C), but interestingly they displayed no significant expression changes in the mRNA level, among the different groups and doxycycline treatments (data not shown).
We have found previously that NZF-2b/7ZFMyt1 is significantly induced in the NAc and in the ventral tegmental area after chronic cocaine administration and that its expression changes in the NAc significantly affects cocaine-mediated locomotor activity (Chandrasekar and Dreyer, 2010
). Here we show that NZF-2b/7ZFMyt1 overexpression in the NAc significantly inhibits self-administration of cocaine, whereas silencing NZF-2b/7ZFMyt1 increases the motivation to self-administer the drug. Both such gain of function and loss of function studies show that NZF-2b/7ZFMyt1 alters responsiveness to cocaine and is one of the factors mediating behavioral response to cocaine. The behavioral effects observed strongly correlate with mRNA and protein expression of NZF-2b/7ZFMyt1 in the NAc. Immuno-histochemistry data show that ectopic NZF-2b/7ZFMyt1 expression in the NAc is predominantly localized to the nuclei of neurons, and to a larger extent to TH-positive dopaminergic projections areas in the NAc and is also observed in a few nuclei of oligodendrocytes. These results suggests that the behavioral effects are manifestations of the dynamic transcriptional activity of NZF-2b/7ZFMyt1 in the NAc.
Ectopic NZF-2b/7ZFMyt1 expression resulted in active recruitment and colocalization of HDAC2. NZF-2b/7ZFMyt1 interacts with Sin3B and with histone deacetylases HDAC1 and HDAC2 (Romm et al., 2005
) to mediate transcriptional repression. Our study shows that NZF-2b/7ZFMyt1-induced behavior alteration is dependent on its interaction with HDACs, since sodium butyrate (NaB) treatment – a potent non-specific HDAC inhibitor – strongly affected only the LV-Myt1 treated animals, but had no effect on LV-GFP and LV-Myt1-siRNAs groups. HDAC inhibition increases histone acetylation in the NAc after cocaine administration and increases cocaine-mediated locomotor activity (Kumar et al., 2005
) and cocaine conditioned place preference (Renthal et al., 2007
). Systemic administration of low doses (100 mg/kg) of NaB enhances cocaine hyperlocomotor activity (Kumar et al., 2005
), whereas cocaine self-administration is not affected at such low doses. Only at higher doses (400 mg/kg) of NaB augmentation of self-administration was observed (Sun et al., 2008
). Nevertheless our results show that even at 100 mg/kg NaB relieves the suppression of self-administration caused by ectopic NZF-2b/7ZFMyt1 in LV-7ZFMyt1 group and brings it back to the control levels. At this dose NaB treatment did not affect self-administration in the control LV-GFP or in the LV-7ZFMyt1-siRNA groups, which clearly suggests that NZF-2b/7ZFMyt1 effects are mediated by interaction with HDACs and that this interaction is sensitive to very lose doses of HDAC inhibitors. Our double-immunoflourescence studies support the view that ectopic NZF-2b/7ZFMyt1 expression resulted in HDAC2 recruitment to the neuronal nuclei, possibly leading to transcriptional suppression of target genes. Histone hyperacetylation induced by HDAC inhibition in the presence of cocaine might elicits enhanced gene expression specific for addiction-like state. NaB inhibits most class I and class II histone deacetylases (even at the millimolar range) and has been used at much higher doses in other studies (1200 mg/kg in Huntington′s model, Ferrante et al., 2003
; and 630 mg/kg in studying the effects of amphetamine-induced behavioral effects, Kalda et al., 2007
).
We also show evidence supporting our model (Chandrasekar and Dreyer, 2010
) that ectopic expression of NZF-2b/7ZFMyt1 might overcome the cytoplasmic recruitment of Lingo1 and its transcriptional inhibition, probably by saturating the inhibitor complex and thereby causing nuclear localization of NZF-2b/7ZFMyt1 for transcriptional regulation of its target genes. The precise mechanism behind this effect needs further investigation. Lingo-1 is an essential component of the Nogo receptor complex and is present in neurons and immature oligodendrocytes during brain development and in adult brain (Llorens et al., 2008
). It mediates signaling during critical developmental periods and is required for acute growth cone collapse, acting on the neuronal cytoskeleton mainly via Rho kinase (Chivatakarn et al., 2007
; Llorens et al., 2008
). Interaction of NZF-2b/7ZFMyt1 with Lingo1 is responsible for the intracellular localization and regulation of transcriptional activity of NZF-2b/7ZFMyt1, whereby Lingo1 causes cytoplasmic localization of NZF-2b/7ZFMyt1 and transcriptional inactivation (Llorens et al., 2008
). Interestingly BDNF, a target of NZF-2b/7ZFMyt1, induces expression of Lingo 1 (Trifunovski et al., 2004
). and a dynamic regulation between NZF-2b/7ZFMyt1 and BDNF might determine key events in the reward pathway.
From our study, NZF-2b/7ZFMyt1 expression in the NAc results in significant expression changes of a number of important target genes. Interestingly we found no significant change in the mRNA levels of many NZF-2b/7ZFMyt1 putative targets like Sema-4C, α-synuclein, the tetraspanin CD81, the dopamine transporter and the dopamine receptor DAD3R (that contain 6, 18, 7, 3 and 12 putative NZF-2b/7ZFMyt1 binding modules, respectively; Chandrasekar and Dreyer, 2010
), which display no significant change after NZF-2b/7ZFMyt1 expression. On the other hand, we found significant differential expression of NAC1, BDNF, REST1 and DAD2R. This underlies the high specificity of the NZF-2b/7ZFMyt1 action in this cocaine self-administration paradigm. NAC1 is a transcriptional repressor whose expression is increased in the NAc after prolonged exposure to cocaine; it decreases cocaine-induced behavioral sensitization and interacts with HDAC3, HDAC4, along with Co-REST and mSin3a (Mackler et al., 2000
; Korutla et al., 2002
, 2005a
,b
, 2007
; Wang et al., 2003
). In our study NAC1 was strongly induced by ectopic NZF-2b/7ZFMyt1 while doxycycline treatment and silencing NZF-2b/7ZFMyt1 brought NAC1 levels down to the LV-GFP group, correlating the specificity of the NAC1 induction with NZF-2b/7ZFMyt1 levels. Recent reports have shown that NAC1 itself lacks DNA binding ability, but acts as a co-repressor (Korutla et al., 2009
). Elevated levels of NAC1 in the NAc, weeks after cocaine administration, may influence expression of addicted behaviors, such as sensitization, craving and paranoia (Everitt and Wolf, 2002
). Based on the above findings and our results, we propose that induction of NAC1 might be one of the key events by which NZF-2b/7ZFMyt1 exerts the observed behavioral effects, probably by maintaining a repressed chromatin state for certain genes.
LV-7ZFMyt1 group also showed significant induction of REST1 in the NAc compared to the LV-GFP control group, whereas the LV-7ZFMyt1-siRNA group showed a significant decrease in REST1 levels. REST1 is a transcriptional repressor required for the acquisition of the differentiated functional neuronal phenotype during early development (Armisen et al., 2002
). REST1 interacts with Co-REST, Sin3A, HDAC1 and HDAC2 to maintain a repressive chromatin environment in neural stem cells (Belyaev et al., 2004
; Greenway et al., 2006
). This suggests that induction of REST1 following NZF-2b/7ZFMyt1 expression after cocaine self-administration probably is important for the observed behavioral alterations. Indeed cocaine regulation of chromatin-modifying enzymes is currently considered to play a central function in shaping the addicted state (Tsankova et al., 2007
).
Furthermore we also observed DAD2R induction specific to NZF-2b/7ZFMyt1 expression. Doxycycline treatment significantly reduces DAD2R levels only in the LV-7ZFMyt1 group and silencing NZF-2b/7ZFMyt1 did not significantly change the DAD2R levels compared to the control group. DAD2R has both presynaptic and postsynaptic localization and function; its activation decreases PKA-dependent phosphorylation of DARPP-32 at Thr34 (Nishi et al., 1997
; De Mei et al., 2009
). Studies have shown that D2-like receptors are important in mediating the abuse-related effects of cocaine and that DAD2R–/– mice display increased cocaine self-administration over wt mice (Caine et al., 2002
). Although DAD2R receptor is not necessary for cocaine self-administration, it is involved in mechanisms that limit rates of high-dose infusion (Caine et al., 2002
). Our results suggest that induction of DAD2R by NZF-1b/7ZFMyt1 might be a counteractive mechanism to inhibit firing and DA discharge activity, resulting in the attenuation of cocaine self-administration.
Studies have shown that BDNF mRNA is absent (Castren et al., 1995
; Altar et al., 1997
; Conner et al., 1997
) or expressed at relatively low levels (Hofer et al., 1990
; Graham et al., 2007
) in dopamine terminal regions such as the NAc under normal conditions, but it is induced markedly following cocaine exposure (Filip et al., 2006
; Liu et al., 2006
) together with its receptor TrkB (Bahi et al., 2008
). Cocaine self-administration increases BDNF protein levels in the VTA, amygdala and accumbens after 30 and 90 days (but not 1 day) of withdrawal (Grimm et al., 2003
). There is evidence that BDNF contributes significantly to cocaine behavioural effects (Schoenbaum et al., 2007
). This appears essential for cocaine-induced behavioral changes (Nestler, 2000
; Bahi et al., 2008
; Kalivas and O’Brien, 2008
; Thomas et al. 2008
). In our study, NZF-2b/7ZFMyt1 expression results in downregulation of BDNF. This probably occurs via induction of REST1 (Chandrasekar and Dreyer, 2010
), since BDNF expression is affected by REST1 through specific interaction with BDNF-RE-1. Decreased levels of REST1 is needed for activity-dependent gene transcription to occur more easily in neuronal cells (Hara et al., 2009
). Our present finding that NZF-2b/7ZFMyt1 expression leads to BDNF suppression (probably by induction of REST1) supports the above studies. Furthermore NZF-2b/7ZFMyt1 expression also resulted in the significant decrease in TrkB, which suggests an inhibition of the BDNF-TrkB signaling cascade in the NAc, leading to a decrease in cocaine induced activity dependent transcription.
According to current views, compulsive cocaine use and cocaine relapse is due to drug-induced neuroadaptations in reward-related learning and memory processes, which cause hypersensitivity to cocaine-associated cues, impulsive decision making and abnormal habit-like learned behaviours that are insensitive to adverse conséquences (Thomas and Malenka, 2003
; Thomas et al., 2008
). The transient enhancement in synaptic strength after normal reward learning may transform neutral stimuli into reward-predictive stimuli, whereas the rescaling of synaptic strength after learning would allow for the formation of future cue-reward associations (Stuber et al., 2008
). Exposure to the drug activates selected signalling cascades, growth factors and physiological processes implicated in neuroplasticity, that include the extracellular signal-regulated kinase (ERK) signalling pathway, BDNF, as well as glutamate transmission (Thomas et al., 2008
). These neuro-adaptations have been hypothesized to cause hyper-sensitivity to cocaine-associated cues (Di Chiara, 1998
; Everitt and Wolf, 2002
) and impulsive decision making (Volkow and Fowler, 2000
). As we show, NZF-2b/7ZFMyt1 through its action on REST1, DAD2R, BDNF HDAC2 and other downstream targets, plays a critical role in these processes. Although analysis of all the genes regulated by NZF-2b/7ZFMyt1 and the possible structural or synaptic strength modifications by NZF-2b/7ZFMyt1 is beyond the scope of the present study, the fewer genes analyzed shows the dynamic regulation of target genes by NZF-2b/7ZFMyt1 expression, responsible for the attenuation of cocaine self-administration observed. Histone modifications are associated with different transcriptional regulatory mechanisms after cocaine administration and specific combinations of histone modifications correspond to various states of remodeled chromatin (activation or repression of distinct sets of genes) (Kumar et al., 2005
). Our data suggest that NZF-2b/7ZFMyt1 is involved in titrating behavioral responses to psycho-stimulants through activating critical upstream events governing multiple pathways that differentially contribute to the manifestation of the addictive behavior. Such regulation provides a new layer of complexity, at the molecular level, through which cocaine produces neural and behavioral plasticity. Our observation thus firmly consolidates the current notion that induction of transcriptional events plays a major role in cocaine-induced plasticity (Kumar et al., 2005
). Clearly NZF-2b/7ZFMyt1 mediates some of the local compensatory mechanisms following drug injections. In addition NZF2b/7ZFMyt1 so far has been implicated during development in the differentiation of myelin cells (mainly oligodendrocytes); it controls myelin-expressing genes and maturation of oligodendrocytes which in the nucleus accumbens are known to express dopaminergic D3R receptors. Together our data may thus also explain many observations describing that altered myelin structure and integrity is part of the phenotypic changes induced by chronic cocaine abuse in humans and may integrate many epigenetic hypotheses in relation to addiction. Therefore a better understanding of this transcription factor, also of these transcriptional events and the epigenetic mechanisms involved in drug administration will facilitate the development of more successful drug treatment strategies.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors are grateful to Dr F. Boyer for critical comments and to M. Guillaume Murat and Mrs C. Deforel-Poncet for skillful assistance.
This study was supported by Swiss National Foundation grants 3100-059350 and 3100AO-100686 (Jean-Luc Dreyer). The SNF had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Jean-Luc Dreyer designed the study. Vijay Chandrasekar performed the analyses. Vijay Chandrasekar and Jean-Luc Dreyer undertook the data analysis. Vijay Chandrasekar wrote the first draft of the manuscript. Jean-Luc Dreyer wrote the final version of the manuscript. All authors contributed to and have approved the final manuscript.
Ferrante, R. J., Kubilus, J. K., Lee, J., Ryu, H., Beesen, A., Zucker, B., Smith, K., Kowall, N. W., Ratan, R. R., Luthi-Carter, R., and Hersch, S. M. (2003). Histone deacetylase inhibition by sodium butyrate chemotherapy ameliorates the neurodegenerative phenotype in Huntington’s disease mice. J. Neurosci. 23, 9418–9427.
Grimm, J. W., Lu, L., Hayashi, T., Hope, B. T., Su, T. P., and Shaham, Y. (2003). Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving. J. Neurosci. 23, 742–747.
Kim, J. G., Armstrong, R. C., v Agoston, D., Robinsky, A., Wiese, C., Nagle, J., and Hudson, L. D. (1997). Myelin transcription factor 1 (Myt1) of the oligodendrocyte lineage, along with a closely related CCHC zinc finger, is expressed in developing neurons in the mammalian central nervous system. J. Neurosci. Res. 50, 272–290.
Kumar, A., Choi, K. H., Renthal, W., Tsankova, N. M., Theobald, D. E., Truong, H. T., Russo, S. J., Laplant, Q., Sasaki, T. S., Whistler, K. N., Neve, R. L., Self, D. W., and Nestler, E. J. (2005). Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron 48, 303–314.
Mühlbauer, M., Allard, B., Bosserhoff, A. K., Kiessling, S., Herfarth, H., Rogler, G., Schölmerich, J., Jobin, C., and Hellerbrand, C. (2004). Differential effects of deoxycholic acid and taurodeoxycholic acid on NF{kappa}B signal transduction and IL-8 gene expression in colonic epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 286, G1000–G1008.
Renthal, W., Maze, I., Krishnan, V., Covington, H. E. 3rd., Xiao, G., Kumar, A., Russo, S. J., Graham, A., Tsankova, N., Kippin, T. E., Kerstetter, K. A., Neve, R. L., Haggarty, S. J., McKinsey, T. A., Bassel-Duby, R., Olson, E. N., and Nestler, E. J. (2007). Histone deacetylase 5 epigenetically controls behavioral adaptations to chronic emotional stimuli. Neuron 56, 517–529.