
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Bee Sci., 13 March 2025
Sec. Bee Genetics
Volume 3 - 2025 | https://doi.org/10.3389/frbee.2025.1498092
Introduction: In beekeeping, queen cell size is a critical factor influencing the growth and development of queen bees. It was hypothesized that larger queen cells would produce queens with greater weight, enhanced resilience to heat stress, and higher expression of heat shock proteins (HSP90 and HSC70), leading to improved survival under environmental stress.
Materials and methods: This study, conducted in Shiraz Province, Iran, in 2023, aimed to test this hypothesis. A total of 270 queens were divided into three groups based on queen cell size: large (10–9.5 mm), medium (9–8.5 mm), and small (7.5–7 mm). The queens were reared using three different methods: 1) simultaneous starter_finisher colonies, 2) separate starter_finisher colonies, and 3) rearing in the presence of the queen. Since there were three different cell types, this resulted in a total of 90 cells for each rearing method. Each group of 90 cells consisted of three subsets of 30 cells: large, small, and medium. From 270 cells, 176 survived and 94 queens died.
Results and discussion: We conducted a stepwise procedure using a logistic model, and the results indicated that the model, which included cell type, rearing method, and birth weight, showed the best predictive performance. This was evidenced by the lowest Akaike information criterion value. Then, from rearing method 2, we placed 12 queens of each cell type in two groups of six each subjected to two different stress levels: a low temperature of 4°C and a high temperature above 40°C. A total of 36 frozen queen samples with six replicates for each treatment combination were used for molecular testing. Gene expression analysis was conducted using real-time PCR to evaluate HSP90 and HSC70 gene expression. Results showed that queens produced in larger cells had significantly higher weight, enhanced resilience to heat stress, and higher gene expression of HSP90. These queens demonstrated superior survival rates under high-temperature conditions compared to queens from smaller cells.
Conclusions: The findings support the hypothesis that optimizing queen cell size can enhance queen performance and colony resilience. Our results suggest that larger cells promote improved development, heat stress resilience, and higher survival rates, ultimately improving colony health and productivity.
Iran’s growing population and increasing demand for beekeeping jobs have driven a significant transformation in industrial beekeeping practices. Queen bee breeding technology plays a pivotal role in this transformation, improving beekeeping and achieving optimal management. However, successful breeding requires specialized skills to ensure desirable physical traits, such as high egg-laying capacity and resilient colonies for overall productivity (Amiri et al., 2020). The breeding of honeybee queens (Apis mellifera) is central to achieving these improvements. Modern beekeepers prioritize the selection of queen bees from high-performing breeding lines, with an emphasis on producing strong colonies with larger populations to maximize honey production (Morse, 1994). Additionally, the ability of queens to adapt to diverse climates is crucial for the sustainable production of honey in various regions, and it is a key consideration in queen selection and breeding (Cao et al., 2016). The breeding practices used vary depending on factors like local environmental conditions and specific traits desired in the colony, such as honey production capacity, disease resistance, and behavioral traits (Tlak Gajger and Mutinelli, 2024).
Morphologically, queen bees are distinguished by their size and physical characteristics, including body weight, head size, chest muscle development, and reproductive organs, particularly the ovaries and spermatheca (Facchini et al., 2021). Queen quality is heavily influenced by these physical traits, which are important indicators of reproductive success (Ozbakir, 2021; Büchler et al., 2013). For instance, larger queens often have better egg-laying capacity, and their development is influenced by factors such as age, genetics, breeding season, and nutrition. Furthermore, the number of transplants, which refers to the process of moving queen bees from one colony to another, can also influence queen development and overall colony health (Ozbakir, 2021).
Gene expression plays a crucial role in the physiology and development of queen bees, especially under environmental stressors. For example, the HSP90 and HSC70 genes are involved in the molecular response to temperature stress, and their expression can vary significantly depending on the queen’s size and resilience (Alqarni et al., 2019; Abou-Shaara, 2024). Heat stress, in particular, can negatively impact sperm viability within the queen’s spermatheca, leading to reduced fertility and reproductive success (Abou-Shaara, 2014). The relationship between queen cell size and queen development has been a subject of recent research, with studies by Wu et al. (2018) and Shi et al. (2011) suggesting that queen cell size plays a critical role in the development of queen morphometric characteristics, influencing overall reproductive success and fitness. Wu et al. (2018) specifically highlighted how queen cell size impacts the development of young queens, and Shi et al. (2011) demonstrated that diet and cell size influence queen–worker differentiation through DNA methylation. These studies emphasize the significant impact of queen cell size on queen quality and adaptability.
Mattiello et al. (2022) also observed that larger queen cells result in queens with greater body mass and enhanced reproductive organs. This is consistent with the findings of Adgaba et al. (2019), who studied various artificial queen-rearing techniques and noted that queen cell size significantly affects the resulting queen’s development and performance within the colony. In terms of environmental stress, Bordier et al. (2017) explored how stress responses in honeybees are linked to changes in task-related physiology and energetic metabolism, showing that colonies under stress exhibit altered metabolic activity, which affects both the bees and colony health. Furthermore, McKinstry et al. (2017) highlighted the interaction between the heat shock response and immune responses, noting their antagonistic effects under extreme heat stress.
These findings underline the importance of queen cell size in enhancing queen development and resilience to environmental stressors. Abou-Shaara (2024) further explored the response of heat shock proteins in honeybees to various abiotic and biotic stressors, such as temperature changes. The expression of heat shock proteins like HSP90 and HSC70 is vital for maintaining colony stability and individual resilience under stress conditions, thereby directly impacting queen survival, reproductive success, and overall colony health (Stillman, 2019).
This study aims to assess how queen size—specifically large, medium, and small queen cells—affects queen weight, gene expression, and resilience to heat stress while exploring the molecular responses of the HSP90 and HSC70 genes under stress conditions. By investigating these factors, we can better understand the complex relationship between queen quality, environmental stress, and the ability of bees to adapt and thrive in different conditions.
This study was conducted in Shiraz Province, Iran (29.6°N, 52.5°E), in May 2023, during the initial phases of queen breeding. Shiraz has a semiarid climate characterized by hot summers, mild winters, and an annual average temperature of 18.5°C. Most of the 300-mm annual rainfall occurs between November and April, while summers remain mostly dry. The region also experiences moderate humidity (average 38%) and over 3,000 h of sunshine annually.
Following the completion of the first phase of queen rearing and one cycle of queen spawning, the queens were transferred for monitoring to the University of Tehran’s Central Laboratory of the Faculty of Agricultural Technology, Iran, on 29 October 2023.
Prior to the experiment, colonies were checked for varroa mite infestation level, and colonies that had mites below threshold level were selected for this experiment. Colonies were also determined free of symptomatic diseases (European foulbrood, American foulbrood, and chalkbrood) based on thorough visual inspection.
We began by preparing 90 large (10–9.5 mm), medium (9–8.5 mm), and small cells (7.5–7 mm), resulting in 270 cells. Next, we arranged the nurse colonies in three different ways: 1) starter_finisher colonies simultaneously, 2) separate starter_finisher colonies, and 3) rearing in the presence of the queen. Then, we placed 30 cells of each type of transplanted cell into each of the colonies. Since there were three different types of cells, this resulted in a total of 90 cells for each rearing method. Each group of 90 cells consisted of three subsets of 30 cells: large, small, and medium. In the starting and ending colonies method, queen cells were present in the colonies from the beginning until the end of the closing period. This approach was used for limited queen production. For large-scale queen production, it is important to separate the starter and finisher colonies. The difference was that in the latest method, we moved the transplanted young larvae after being accepted in the hive, and when they gradually became older, we moved them to the side parts of the hive. Then from the method of separate starter_finisher colonies, which was the best type of breeding, we placed 12 queens of each cell type in two groups of six each subjected to two different stress levels: a low temperature of 4°C and a high temperature above 40°C. Therefore, six groups of six queens, totaling 36, were used for molecular testing. A completely randomized design was implemented, considering queen size, breeding method, and stress level, with each factor replicated six times.
Virgin queen honeybees (Apis mellifera) were divided into various postnatal treatment groups and subsequent temperature stress exposures to investigate the link between pre-existing morphological variations and environmental stress tolerance. The starter and termination phases of these treatments occurred within large double-transplant cells.
To investigate the effect of queen size and environmental stress on the expression of genes related to morphological traits (HSC70 and HSP90 activity), real-time PCR was conducted.
We started our phenotypical data collection with 270 queens, and among them, 176 queens survived to collect more phenotypical analysis. The queens were then divided into two groups of six and then subjected to heat stress. The instrument was stabilized with physiological serum with 7% formalin and for morphological study in liquid nitrogen at −70°C.
Molecular analysis to measure the gene expression was performed for six queens/treatment. A total of 36 frozen queens’ samples were used from six treatments with two replications and three queens per replication. Samples were cooled with liquid nitrogen in an autoclaved paw. Then, the samples were quickly crushed and transferred to precooled 2 ml microtubes using a sterile spatula. Total RNA extraction was performed using Biozol from Bioflux (Japan).
At this stage, the electrophoresis process was applied by using a 1% agarose gel in TBE buffer. Next, we mixed 5 μl of RNA with 2 μl of DYE and injected the solution into the wells of gel (see Supplementary File in Supplementary Material). Finally, we connected the electrophoresis equipment to a power supply set at 100 V for 30 min. The extracted RNAs were quantified by NanoDrop, and the results of optical density were recorded (see Supplementary File in Supplementary Material).
We designed forward and reverse primers for the ACT, CAT, HSP90, and HSC70 genes by using the Primer3 software (Untergasser et al., 2012) (see Supplementary File in Supplementary Material). Then, the designed primers were blasted to align with the reference gene in NCBI to investigate a 100% match. The OligoAnalyzer software was used to identify the characteristics of the designed primers.
Initially, the base solution was prepared according to the number of reactions desired. The cDNA synthesis reaction was performed using a Pars Toos kit (see Supplementary File in Supplementary Material). For each reaction, 2 μl of enzyme and 10 μl of buffer mixture were added to the base solution. Then, 12 μl of the origin solution was poured into each tube. Then, the appropriate amount of RNA was calculated based on the initial RNA concentration. At the end, nuclease-free water was added to adjust the final volume of the reaction to 20 μl. The samples were then placed in a thermocycler device and incubated at 25°C for 10 min, followed by 47°C for 60 min, to allow cDNA formation. Enzyme inactivation was performed at 85°C for 5 min and finally cooled to 4°C (see Supplementary File in Supplementary Material). The composition of the real-time PCR reaction mixture is shown in the Supplementary File in Supplementary Material. To correct for differences in the amount of DNA/RNA added for each sample and to reduce variations due to the PCR setup and cycling process, internal control (NTC) and reference genes (ACT and CAT housekeeping genes) were used to normalize the PCRs (Vandesompele et al., 2002). Housekeeping genes are commonly used as reference genes because their expression levels remain relatively constant in response to each treatment (Jeon et al., 2020). Real-time PCR results showed that actin and CAT genes did not exhibit significant differences in expression under heat and cold stress conditions, indicating that the use of these two genes as reference genes for normalization of the expression data of other genes is valid.
The relative mRNA expression was determined using the ΔΔCT method (Pfaffl, 2001). For the 2−ΔΔCT method, the first ΔCT in the 2−ΔΔCT method is the difference in the threshold cycle between the target gene and the reference gene.
We carefully measured and recorded body weight, head dimensions (including width and depth if applicable), chest dimensions (including width and depth if applicable), and body length to assess morphological differences. To examine the relationship between queen cell size and rearing method on queen survival, a logistic regression analysis was performed using the R (R 4.3.0) program. Unlike a linear relationship between dependent and independent variables, logistic regression employs a function that constrains predicted values between 0 and 1, making it suitable for modeling probabilities. The logit transformation method was applied to enable accurate parameter estimation during the subsequent statistical analysis. This approach leverages the concept of odds ratios, providing a robust framework for interpreting the relationship between predictors and the likelihood of queen survival.
where β0, β1, β2, and β3 are model parameters.
We conducted a stepwise procedure using a logistic model, and the results indicated that the model, which included cell type, rearing method, and birth weight, showed the best predictive performance. This was evidenced by its lowest Akaike information criterion (AIC) value, which was 317.66, indicating that it provided the best balance between predictive accuracy and model complexity (Aho et al., 2014). AIC assesses models by determining how well they explain the data while penalizing the inclusion of unnecessary parameters.
This criterion is widely used in statistical modeling to identify the most efficient model that avoids overfitting, making it ideal for selecting the best-fit model in this study. The inclusion of critical variables such as cell type, rearing method, and birth weight was essential for capturing the factors influencing survival outcomes, and the model’s low AIC suggests that it achieved an optimal balance between explaining the data and maintaining simplicity (Symonds and Moussalli, 2011).
To determine differences in gene expression levels between small and medium queens, ‘statistical analysis was performed using the Student’s t-test in GraphPad Prism software (GraphPad Software, 2020).
Table 1 shows that the survival rate for the entire dataset is divided into two outcomes: 94 samples did not survive (death), while 176 samples survived (live). Those 94 queens died after emergence. A queen emergence rate of 65% for queens is acceptable. This distribution reflects the overall survival trends without distinguishing survival rates within individual groups. The data indicate that most of the samples survived, suggesting favorable conditions for survival across all groups.
We conducted a stepwise procedure using a logistic model, and the results indicated that the model, which included cell type, rearing method, and birth weight, showed the best predictive performance (Table 2). This was evidenced by its lowest AIC value, which was 317.66.
Table 3 shows the odds ratios pairwise comparison for rearing type and cell type, where the reference (death) is set to 0. The odds ratios in Table 3 help us understand how different factors influence the chance of survival. The intercept, which represents the basic chance of survival, is not statistically significant (P > 0.05), suggesting that other factors might play a more significant role in affecting survival rates.
The lower AIC value indicates that this model provides the best balance between goodness-of-fit and model complexity compared to alternative models. Rearing method 2 has an odds ratio of 1.77, indicating a tendency for a higher chance of survival compared to the reference category.
After modifying our reference treatment to include rearing method 2 and cell type 2, our analysis revealed significant disparities between rearing method 3 and rearing method 2 (P < 0.02).
The results depicted in Figure 1 highlight the significant influence of rearing method, cell type, birth weight, and mating weight on survival residuals, demonstrating that these factors collectively contribute to variations in survival outcomes.
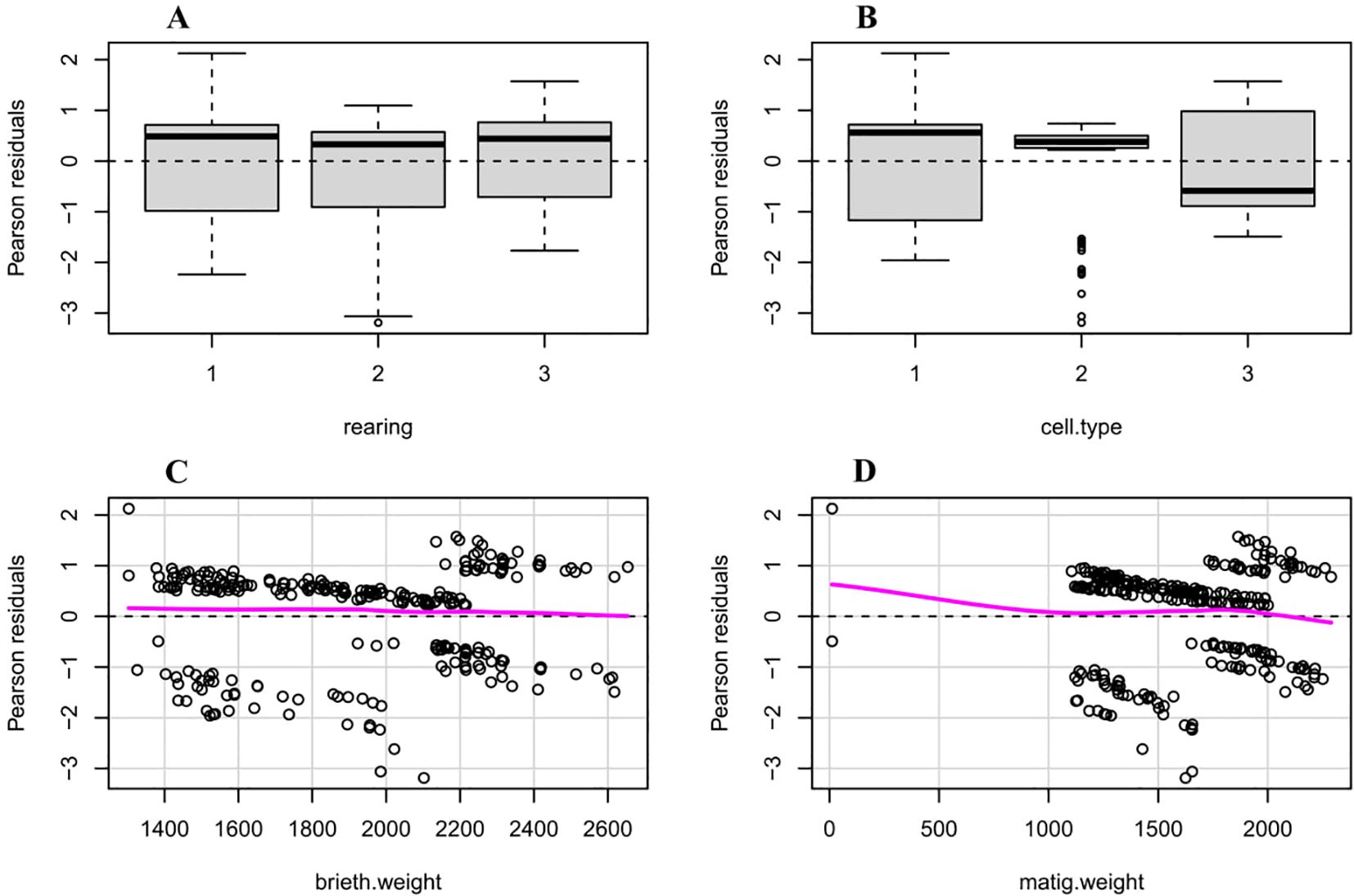
Figure 1. The figure illustrates the impact of rearing type, cell type, birth weight, and mating weight on the survival of honeybee queens. Box plots (A, B) and scatter plots with linear regression lines (C, D) visualize the distribution and relationships between these variables and survival. The data were analyzed using a linear mixed model, and the results indicated that rearing type and birth weight had a significant impact on queen survival (P < 0.05). These findings suggest that optimizing rearing conditions and selecting queens with higher birth weights can improve colony health and productivity.
The expression of the HSP90 gene demonstrated a direct link to the queen’s size, with large queens showing higher expression intensity when exposed to heat stress (Figures 2A, B S2 in Supplementary Material). No significant differences in gene expression levels were found between small and medium queens. Under cold stress, no significant differences in HSP90 expression were observed between large (S2) and small (control) queens (Figures 2A, B). However, medium queens (S1) exhibited significant differences compared to small queens (P < 0.05).
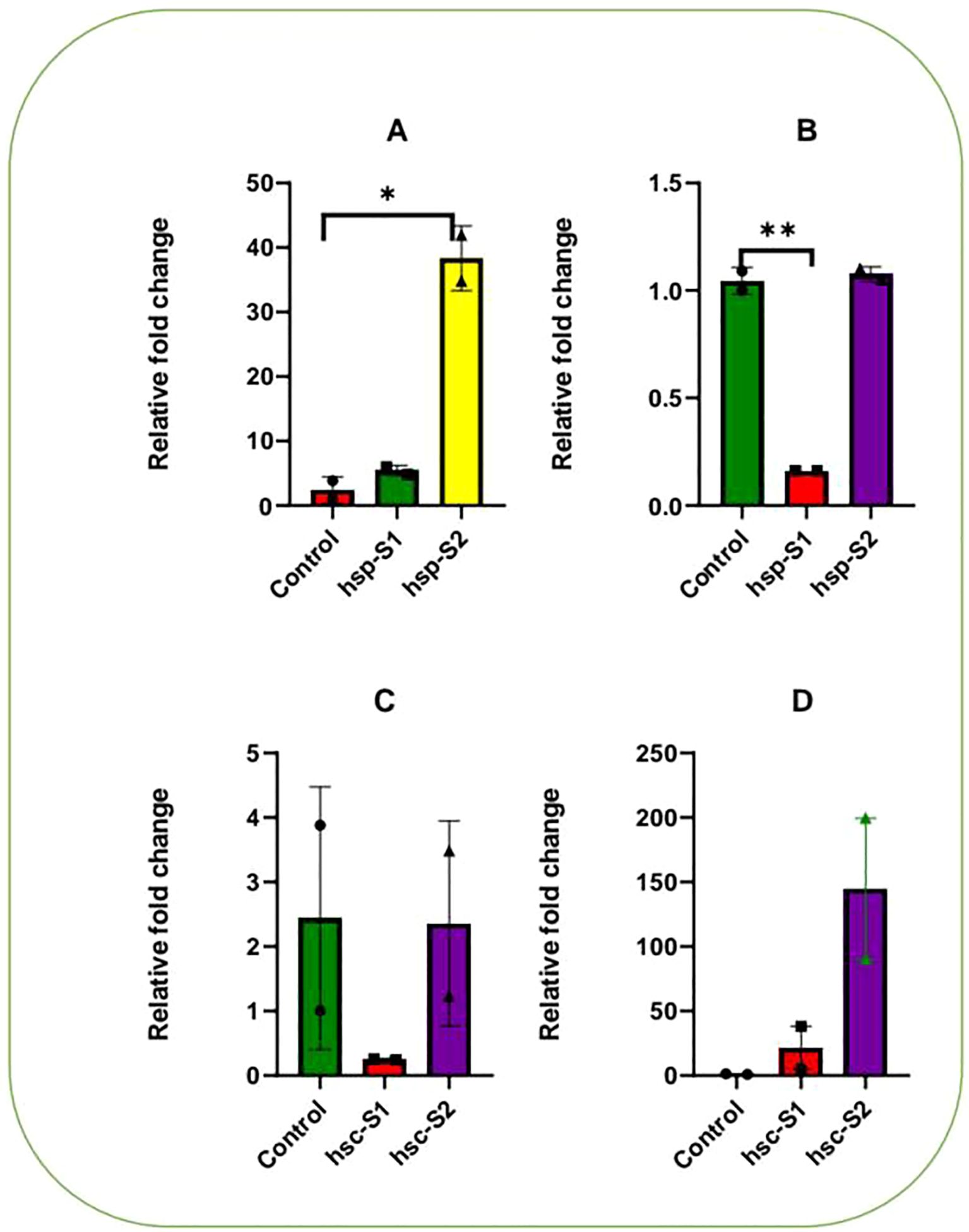
Figure 2. Relative variations in HSP90 and HSC70 gene expression for three queens with large (S2), medium (s1), and small (control) queens. HSP90: (A) under heat stress at 40°C and (B) cold stress at 4°C. HSC70: (C) under heat stress at 40°C and (D) cold stress at 4°C.
Interestingly, small queens exhibited a higher rate of HSC70 expression than large and medium queens under heat stress (Figures 2C, D). These results support the view that larger queens generally exhibit better functioning.
The findings of this study highlight the intricate relationships among queen body size, survival rates, and genetic factors such as HSP90 and HSC70 gene expressions. Larger queens consistently exhibited superior survival outcomes and enhanced heat tolerance, likely due to their greater physiological capacities. This aligns with established biological principles correlating larger body size with higher fecundity, longevity, and fitness. Honěk (1993) and Wilson (1985) have shown that larger queens in bigger colonies tended to exhibit greater productivity and enhanced colony performance due to superior reproductive capacity, energy reserves, and thermoregulation capabilities.
Morphologically, queen bees’ size and physical characteristics, including body weight and reproductive organs, are critical determinants of reproductive success and colony health. Larger queens not only possess enhanced egg-laying capacity but are also more resilient to environmental stressors. This is consistent with the studies by Mattiello et al. (2022) and Adgaba et al. (2019), who demonstrated that larger queen cells result in queens with greater body mass and superior reproductive organ development. Similarly, Wu et al. (2018) and Shi et al. (2011) emphasized the influence of cell size on queen–worker differentiation and morphometric characteristics, driven by DNA methylation and nutritional factors.
In our study, rearing method 2 showed a tendency toward higher survival odds. This highlights the nuanced role of breeding strategies in queen survival and productivity. Queen bee breeding technology plays a pivotal role in improving beekeeping practices, particularly in regions like Iran, where a growing demand has driven a transformation in industrial beekeeping (Amiri et al., 2020). Selection of queens from high-performing breeding lines with traits such as egg-laying capacity, disease resistance, and climate adaptability is critical for optimizing colony health and productivity (Morse, 1994; Cao et al., 2016).
Additionally, our results confirmed the significant impact of cell type on larval production, with larger cell types yielding more larvae, corroborating findings by Tarpy et al. (2011) and Ozbakir (2021).
Moreover, the advanced logistic models employed in our analysis revealed critical disparities between rearing methods and cell types, with rearing method 3 showing slightly lower odds of survival. These insights underscore the need for targeted interventions in breeding practices to enhance queen quality, colony health, and adaptation to diverse environmental conditions.
Queens are naturally shielded from temperature stress through the thermoregulation of the hive; however, queens remain at risk of extreme temperature exposure, as the ambient temperature still influences internal hive temperatures (Fahrenholz et al., 1989; Bordier et al., 2017; McAfee et al., 2020). During extreme heat waves, queens could become exposed to hot temperatures within small colonies (such as mating nucs) which have poor thermoregulatory capacity, or queen banks, in which queens are immobile and cannot relocate away from hot parts of the colony (e.g., near the top of the hive). Moreover, queens in queen banks may become abandoned by workers during heat-induced reorganization, in which workers tend to move away from brood frames and toward peripheral areas (Jhawar et al., 2023). We expect that the risk of heat stress will increase in the future as heat waves become more intense and more common. Beekeepers, especially queen producers, may need to breed heat-resistant queens and adopt heat management strategies (e.g., shade nets, insulation, ventilation) even in historically cooler regions. Queens, often shipped long distances in small cages or packages, face temperature stress during transport, which can harm their fertility (Strange et al., 2008; Pettis et al., 2016).
Gene expression analysis further revealed critical insights into physiological resilience under stress. Larger queens exhibited upregulation of HSP90 under heat stress, highlighting their superior adaptability, while smaller queens relied on compensatory mechanisms through HSC70 expression. This aligns with the studies by Bordier et al. (2017) and Abou-Shaara (2024), who emphasized the vital role of heat shock proteins in maintaining colony stability under abiotic stressors. Heat stress, in particular, negatively impacts queen fertility by reducing sperm viability in the spermatheca, underscoring the importance of thermal tolerance for reproductive success (Abou-Shaara, 2014).
Our findings challenge the conventional belief that birth weight and weaning weight are significant predictors of queen survival. Instead, it seems that genetic and environmental factors play a more crucial role. This perspective aligns with the research of Wu et al. (2018) and Büchler et al. (2013).
Integrating molecular markers such as HSP90 and HSC70 into breeding strategies could significantly enhance colony resilience and productivity in the face of climate change.
This study highlights the importance of breeding methods and queen bee characteristics in optimizing queen quality. We found that rearing and mating queens in separate colonies led to higher-quality queens, with queen cell size being a key factor influencing queen size and health. Queen size positively correlated with heat tolerance, though it had minimal impact on cold tolerance. These findings emphasize the need to consider breeding methods and queen traits to improve colony resilience, particularly in response to environmental stressors. Future research should focus on understanding the genetic mechanisms behind queen size and its role in stress adaptation.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
AS: Methodology, Project administration, Supervision, Writing – review & editing, Conceptualization, Validation, Visualization. AD: Data curation, Formal analysis, Funding acquisition, Investigation, Writing – original draft, Project administration, Resources. EA: Conceptualization, Supervision, Writing – review & editing, Methodology. MB: Formal analysis, Software, Visualization, Writing – review & editing, Methodology.
The author(s) declare that no financial support was received for the research and/or publication of this article.
We express our appreciation for the assistance provided by the Faculty of Agricultural Technology at the University of Tehran for facilitating this research.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frbee.2025.1498092/full#supplementary-material
Abou-Shaara H. F. (2014). The foraging behaviour of honey bees, Apis mellifera: a review. Vet. Med. 59 (1), 1–10. doi: 10.17221/7240-VETMED
Abou-Shaara H. F. (2024). The response of heat shock proteins in honey bees to abiotic and biotic stressors. J. Therm. Biol. 119, 103784. doi: 10.1016/j.jtherbio.2024.103784
Adgaba N., Al-Ghamdi A., Tadesse Y., Alsarhan R., Single A., Mohammed S. E., et al. (2019). The responses of Apis mellifera jemenitica to different artificial queen rearing techniques. Saudi J. Biol. Sci. 26, 1649–1654. doi: 10.1016/j.sjbs.2018.08.028
Aho K., Derryberry D., Peterson T. (2014). Model selection for ecologists: the worldviews of AIC and BIC. Ecology 95, 631–636. doi: 10.1890/13-1452.1
Alqarni A. S., Ali H., Iqbal J., Owayss A. A., Smith B. H. (2019). Expression of heat shock proteins in adult honey bee (Apis mellifera L.) workers under hot-arid subtropical ecosystems. Saudi J. Biol. Sci. 26, 1372–1376. doi: 10.1016/j.sjbs.2019.08.017
Amiri E., Le K., Melendez C. V., Strand M. K., Tarpy D. R., Rueppell O. (2020). Egg-size plasticity in Apis mellifera: Honey bee queens alter egg size in response to both genetic and environmental factors. J. Evol. Biol. 33, 534–543. doi: 10.1111/jeb.13589
Bordier C., Suchail S., Pioz M., Devaud J. M., Collet C., Charreton M., et al. (2017). Stress response in honeybees is associated with changes in task-related physiology and energetic metabolism. J. Insect Physiol. 98, 47–54. doi: 10.1016/j.jinsphys.2016.11.013
Büchler R., Andonov S., Bienefeld K., Costa C., Hatjina F., Kezic N., et al. (2013). Standard methods for rearing and selection of Apis mellifera queens. J. Apic. Res. 52, 1–30. doi: 10.3896/IBRA.1.52.1.07
Cao L. F., Zheng H. Q., Pirk C. W., Hu F. L., Xu Z. W. (2016). High royal jelly-producing honeybees (Apis mellifera ligustica) (Hymenoptera: Apidae) in China. J. Econ. Entomol. 109, 510–514. doi: 10.1093/jee/tow013
Facchini E., De Iorio M. G., Turri F., Pizzi F., Laurino D., Porporato M., et al. (2021). Investigating genetic and phenotypic variability of queen bees: Morphological and reproductive traits. Animals 11, 3054. doi: 10.3390/ani11113054
Fahrenholz L., Lamprecht I., Schricker B. (1989). Thermal investigations of a honey bee colony: thermoregulation of the hive during summer and winter and heat production of members of different bee castes. J. Comp. Physiol. B 159, 551–560. doi: 10.1007/BF00694379
GraphPad Software (2020). GraphPad Prism version 9.0 for Windows (San Diego, California USA: GraphPad Software, Inc). Available at: https://www.graphpad.com.
Honěk A. (1993). Intraspecific variation in body size and fecundity in insects: a general relationship. Oikos 67, 483–492. doi: 10.2307/3544943
Jeon J. H., Moon K., Kim Y., Kim Y. H. (2020). Reference gene selection for qRT-PCR analysis of season-and tissue-specific gene expression profiles in the honey bee Apis mellifera. Sci. Rep. 10, 13935. doi: 10.1038/s41598-020-70965-4
Jhawar J., Davidson J. D., Weidenmüller A., Wild B., Dormagen D. M., Landgraf T., et al. (2023). How honeybees respond to heat stress from the individual to colony level. J. R. Soc Interface 20, 20230290. doi: 10.1098/rsif.2023.0290
Mattiello S., Rizzi R., Cattaneo M., Martino P. A., Mortarino M. (2022). Effect of queen cell size on morphometric characteristics of queen honey bees (Apis mellifera ligustica). Ital. J. Anim. Sci. 21, 532–538. doi: 10.1080/1828051X.2022.2043790
McAfee A., Chapman A., Higo H., Underwood R., Milone J., Foster L. J., et al. (2020). Vulnerability of honey bee queens to heat-induced loss of fertility. Nat. Sustain. 3, 367–376. doi: 10.1038/s41893-020-0493-x
McKinstry M., Chung C., Truong H., Johnston B. A., Snow J. W. (2017). The heat shock response and humoral immune response are mutually antagonistic in honey bees. Sci. Rep. 7, 8850. doi: 10.1038/s41598-017-09159-4
Ozbakir G. O. (2021). Effects of rearing method on some morphological and reproductive organ characteristics of queen honey bees (Apis mellifera L.). Med. Weter. 77, 89–94. doi: 10.21521/mw.6496
Pettis J. S., Rice N., Joselow K., vanEngelsdorp D., Chaimanee V. (2016). Colony failure linked to low sperm viability in honey bee (Apis mellifera) queens and an exploration of potential causative factors. PloS One 11, e0147220. doi: 10.1371/journal.pone.0147220
Pfaffl M. W. (2001). A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 29, e45. doi: 10.1093/nar/29.9.e45
Shi Y. Y., Huang Z. Y., Zeng Z. J., Wang Z. L., Wu X. B., Yan W. Y. (2011). Diet and cell size both affect queen-worker differentiation through DNA methylation in honey bees (Apis mellifera, Apidae). PloS One 6, e18808. doi: 10.1371/journal.pone.0018808
Stillman J. H. (2019). Heat waves, the new normal: summertime temperature extremes will impact animals, ecosystems, and human communities. Physiology 34, 86–100. doi: 10.1152/physiol.00040.2018
Strange J. P., Garnery L., Sheppard W. S. (2008). Morphological and molecular characterization of the Landes honey bee (Apis mellifera L.) ecotype for genetic conservation. J. Insect Conserv. 12, 527–537. doi: 10.1007/s10841-007-9093-6
Symonds M. R., Moussalli A. (2011). A brief guide to model selection, multimodel inference and model averaging in behavioural ecology using Akaike’s information criterion. Behav. Ecol. Sociobiol. 65, 13–21. doi: 10.1007/s00265-010-1037-6
Tarpy D. R., Keller J. J., Caren J. R., Delaney D. A. (2011). Experimentally induced variation in the physical reproductive potential and mating success in honey bee queens. Insectes Soc 58, 569–574. doi: 10.1007/s00040-011-0180-z
Tlak Gajger I., Mutinelli F. (2024). Impact of environmental factors and management practices on bee health. Insects 15, 996. doi: 10.3390/insects15120996
Untergasser A., Cutcutache I., Koressaar T., Ye J., Faircloth B. C., Remm M., et al. (2012). Primer3—new capabilities and interfaces. Nucleic Acids Res. 40, e115. doi: 10.1093/nar/gks596
Vandesompele J., De Preter K., Pattyn F., Poppe B., Van Roy N., De Paepe A., et al. (2002). Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3, 1–12. doi: 10.1186/gb-2002-3-7-research0034
Wilson E. O. (1985). The sociogenesis of insect colonies. Science 228, 1489–1495. doi: 10.1126/science.228.4707.1489
Keywords: queen, cell size, heat shock protein, qPCR, beekeeping practices
Citation: Derafsh A, Salehi A, Amiri E and Bakhtiarizadeh MR (2025) Effect of honeybee queen size and HSP90 and HSC70 gene expression on thermal stress resistance. Front. Bee Sci. 3:1498092. doi: 10.3389/frbee.2025.1498092
Received: 18 September 2024; Accepted: 17 February 2025;
Published: 13 March 2025.
Edited by:
Andrea Galimberti, University of Milano - Bicocca, ItalyCopyright © 2025 Derafsh, Salehi, Amiri and Bakhtiarizadeh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abdolreza Salehi, YXJzYWxlaGlAdXQuYWMuaXI=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.