- 1Zoology Department, Rajiv Gandhi University, Doimukh, Arunachal Pradesh, India
- 2National Centre for Biological Sciences, Tata Institute of Fundamental Research, Bangalore, Karnataka, India
- 3School of Environmental Sciences, University of Guelph, Guelph, ON, Canada
- 4Institute of Bee Health, Vetsuisse Faculty, University of Bern and Agroscope, Bern, Switzerland
Introduction: The taxonomy and phylogeny of the giant honey bees (Apis; subgenus Megapis) remain controversial and unresolved. The species boundaries within the subgenus are unclear, and some species that are recognized on the basis of genetic differences lack supporting morphological characteristics. Two species are now well accepted: Apis dorsata Fabricius, 1793, of tropical regions of Asia, and Apis laboriosa Moore et al., 1871, an inhabitant of the foothills of the Himalayas and neighboring mountain ranges. In addition, researchers have suggested that the two allopatric populations of giant honey bees that inhabit Sulawesi, Indonesia (Apis binghami Cockerell, 1906) and the oceanic Philippine islands (Apis breviligula Maa, 1953), as well as the South Indian form also deserve species status. We evaluated morphological characters of all of these taxa in order to revise the taxonomy of the subgenus.
Methods: We conducted a taxonomic study based on morphological characters of Megapis from throughout Asia. In addition, we created taxonomic keys to workers and drones for the giant honey bee species that we recognize.
Results: Our study confirms that A. laboriosa is a distinct species based on numerous morphological characters. Moreover, A. dorsata of mainland Asia differs from the two island taxa based on coloration, ocellus size, and the spacing of compound eyes and ocelli. We found no evidence that breviligula of the Philippines has a distinctively short tongue. Moreover, we detected only one minor character (the shape of sternum 5) that differed statistically between bees from Sulawesi and the Philippines. We conclude that the bees from these islands represent a single morphological species, A. binghami, with two subspecies, A. b. binghami and A. b. breviligula. A. dorsata from the Andaman Islands are smaller than but conspecific with dorsata of mainland Asia. We found no morphological autapomorphies in the giant honey bees of southern India that are known to differ in mtDNA from A. dorsata from elsewhere in mainland Asia.
Discussion: Our morphological examination of Megapis specimens firmly supports three species of giant honey bees: A. laboriosa, A. dorsata, and A. binghami. More detailed examination of specimens is required to reconcile our three morphological species with the five clades that have been identified with genetic analyses.
1 Introduction
The taxonomy and phylogeny of the giant honey bees (Apis L.; subgenus Megapis Ashmead) are still controversial and poorly resolved, partly due to a paucity of studies. In his seminal review of the honey bees, Maa (1953) recognized four species of giant honey bees within the genus Megapis, viz., dorsata Fabricius, 1793, laboriosa Smith, 1871 (Moore et al., 1871), binghami Cockerell, 1906, and breviligula Maa, 1953. However, his classification was criticized by many in the research community for the excessive splitting of the genus Apis into three genera and 24 species (including 12 species within the taxon currently recognized as Apis mellifera) (Ruttner, 1988; Engel, 1999). Rather than rejecting the systematic revision by Maa (1953), Sakagami et al. (1980) examined in detail the morphological differences between workers of the two mainland Asian forms, dorsata and laboriosa. Examining more than a hundred morphological features, they found many discrete characters that distinguish laboriosa from dorsata, including the color of the thoracic hairs and metasoma, relative widths of the head to both the meso- and metathorax, ocellar size and shape, malar length, and number of sting barbs. Unfortunately, they had only two specimens of breviligula and none of binghami to examine and, therefore, they did not comment on their status.
In 1991, Alexander had the opportunity to examine both workers and drones of all four giant honey bee taxa (Alexander, 1991). He examined 18 morphological and two behavioral traits to determine the cladistic relationships of the species within Apis. Unfortunately, none of the characters he assessed resolved the phylogenetic relationships among the giant honey bees. Therefore, he treated dorsata, laboriosa, binghami, and breviligula as the “dorsata group”.
Despite Sakagami et al. (1980) recognizing several diagnostic characters that differed strikingly between dorsata and laboriosa, most taxonomists who have studied bee taxonomy (e.g., Ruttner, 1988; Alexander, 1991; Engel, 1999) remained unconvinced that laboriosa deserved species status and adopted a conservative view that there is a single giant honey bee species, namely, Apis dorsata. They considered that laboriosa, binghami, and breviligula are distinctive populations within A. dorsata that occur in circumscribed geographical areas. Specifically, dorsata is broadly distributed throughout Southeast Asia and the Indian subcontinent, from as far west as Afghanistan to Timor and possibly the Kei Islands of Indonesia in the east (Maa, 1953). Laboriosa prevails across relatively high elevations of the pan-Himalaya region and smaller mountain ranges extending eastward into China, Laos, and Vietnam, southward into central Myanmar, and westward into Pakistan (Kitnya et al., 2020; Otis et al., 2024). Binghami is found only on the island of Sulawesi and nearby islands of Indonesia, and breviligula occurs on the islands of the Philippines, excluding Palawan (Smith, 2021; Bhatta et al., 2024).
The phylogenetic relationships resulting from genetic studies are suggestive of four, possibly five, species of the giant honey bees, although tree topologies differ among the studies. Most phylogenetic relationships inferred from molecular analyses (based on mitochondrial genes ND2, coxII, nad2, and rrnL and nuclear genes EF-1α and itpr) have reported laboriosa as sister to the other taxa (Arias and Sheppard, 2005; Raffiudin and Crozier, 2007; Wang et al., 2018). However, Lo et al. (2010), based on analyses of rrnL and coxII mtDNA sequences and a nuclear DNA (itpr) sequence, reported that breviligula of the Philippines diverged prior to splits among the other taxa. Their results showed laboriosa (with low support value) closer to dorsata than breviligula, while binghami was not clearly resolved from either dorsata or laboriosa. In contrast, the phylogenetic inferences of Raffiudin and Crozier (2007) (based on three mitochondrial genes—rrnL, coxII, and nad2—and one nuclear gene, itpr), and of Smith (2021) based on single-nucleotide polymorphisms (SNPs) showed dorsata and binghami as highly resolved clades with high support values. Most recently, Bhatta et al. (2024), in their analyses of mitochondrial coxI and coxII gene sequences, detected five well-resolved taxa, the four named species mentioned above as well as the South India form, but failed to resolve their phylogenetic relationships. The topological differences within phylogenetic trees result from different analytical methods and portions of the genome examined, as well as the specific analyses utilized to determine the relationships among taxa.
Lo et al. (2010) and Bhatta et al. (2024) are the only researchers who have included all of the giant honeybee taxa in their analyses, unlike others who omitted one or more taxa. Lo et al. reported that dorsata from Tamil Nadu and Karnataka in India and Sri Lanka had diverged from the common ancestor of dorsata of Southeast Asia and formed a distinct clade within their phylogenies, suggesting that the giant honey bees of South India and Sri Lanka may deserve species status, a result first proposed by Smith (1991) and echoed in the results of Arias and Sheppard (2005). Our recent study based on coxI mtDNA (Kitnya et al., 2022) also showed two distinct clades of dorsata from Karnataka (South India) and Arunachal Pradesh (North East India). In the most complete analysis of mtDNA sequences to date, Bhatta et al. (2024) provided support for laboriosa as well as four species within the dorsata complex: dorsata, binghami, breviligula, and the South Indian form.
Morphological reanalysis of specimens of Megapis taxa throughout their ranges will yield significant information to help resolve the taxonomy of the giant honey bees. Some of the species recognized on the basis of genetic differences (Smith, 1991; Arias and Sheppard, 2005; Raffiudin and Crozier, 2007; Lo et al., 2010; Cao et al., 2012; Smith, 2021) lack data for previously recognized morphological characters (Maa, 1953; Sakagami et al., 1980; Trung et al., 1996). Without that supporting evidence, elevating taxa to species status is somewhat controversial on the basis of genetic differences alone, especially when the phylogenies based on those genetic analyses are not congruent.
In an attempt to resolve the species boundaries within Megapis bees, we examined specimens from across the ranges of all the taxa (Figure 1) and quantified numerous characters. Here, we describe those characters and create taxonomic keys for the identification of both workers and drones. Hereafter, for the sake of convenience, while describing each taxon within Megapis, Maa’s (1953) names—dorsata, laboriosa, binghami, and breviligula—will be used, though the study will reassess the validity of these names based on present research. Fortunately, we had specimens of both workers and drones from many localities, unlike the limited series of worker specimens only on which Maa (1953) and Sakagami et al. (1980) based their taxonomic evaluations.
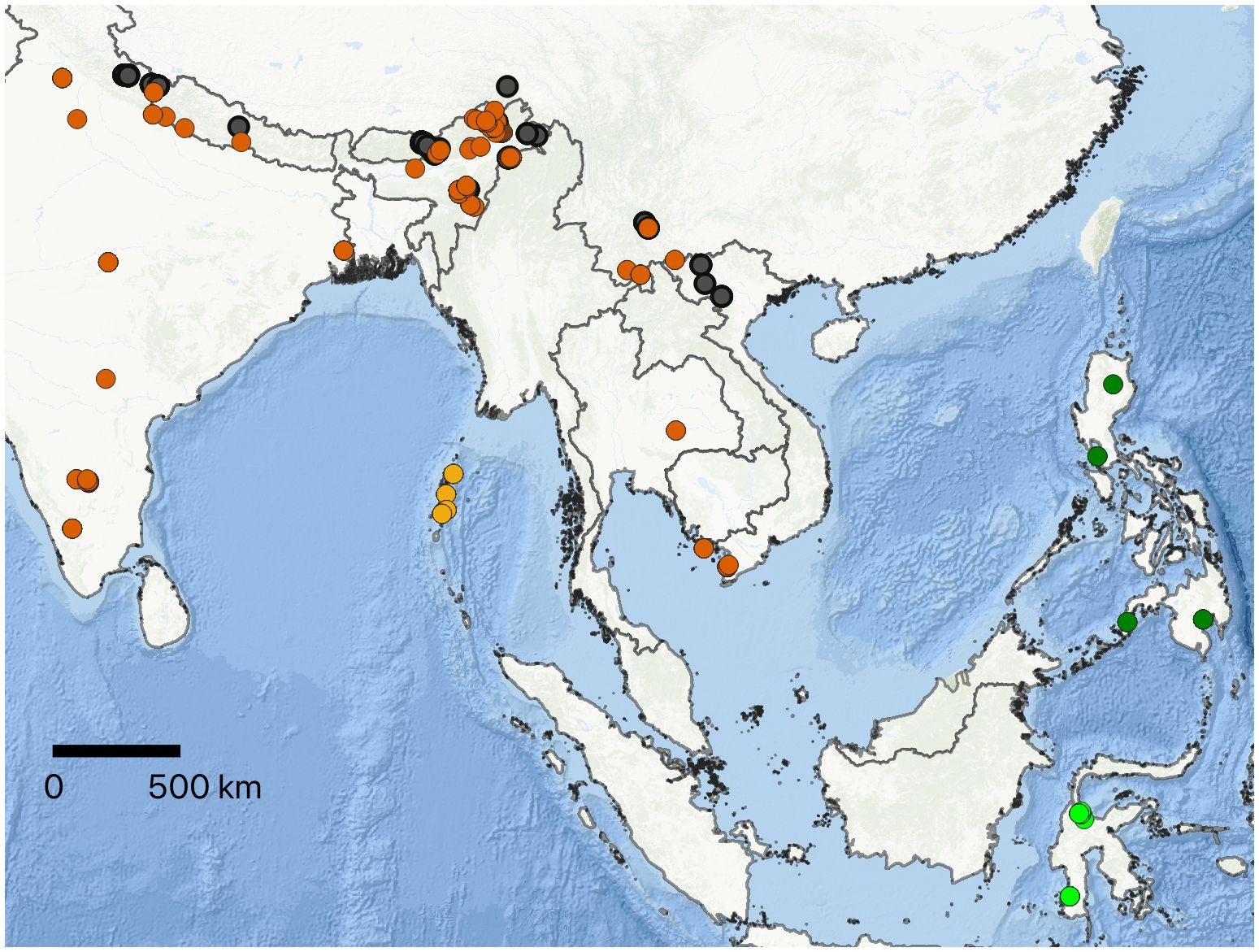
Figure 1 Map showing collection localities of Megapis examined in this study. Neon green circles, binghami; dark green circles, breviligula; orange circles, dorsata (mainland); mustard yellow circles, dorsata (Andaman Island); black circles, laboriosa. Color codes are consistent throughout the manuscript.
We hypothesized that the four Megapis species recognized by Maa would be upheld. The striking and distinctive morphological characteristics of laboriosa reported in previous studies (Maa, 1953; Sakagami et al., 1980; Trung et al., 1996; Kitnya et al., 2022) suggested that it deserved distinct species status from dorsata of mainland Asia. The genetic and morphometric analyses of sympatric laboriosa and dorsata of Kitnya et al. (2022) further demonstrated the species status of laboriosa. The giant honey bees, binghami and breviligula, from the islands of Sulawesi, Indonesia, and the Philippines, respectively, are similar in appearance to each other. As described by Maa (1953), these black island giant honey bees superficially resemble laboriosa with which they were earlier sometimes confused (e.g., Cockerell, 1914). Based on the distinctive short tongue and other characteristics of breviligula reported by Maa (1953), we hypothesized that the black island taxa represent two separate species. We were unsure what our results may reveal about the South Indian population, given that dorsata of South India and dorsata of Southeast Asia are very similar in external appearance. However, as reviewed above, they form well-resolved clades in molecular phylogenies (Smith, 1991; Lo et al., 2010; Smith, 2021; Kitnya et al., 2022; Bhatta et al., 2024).
2 Materials and methods
2.1 Collection and processing of specimens
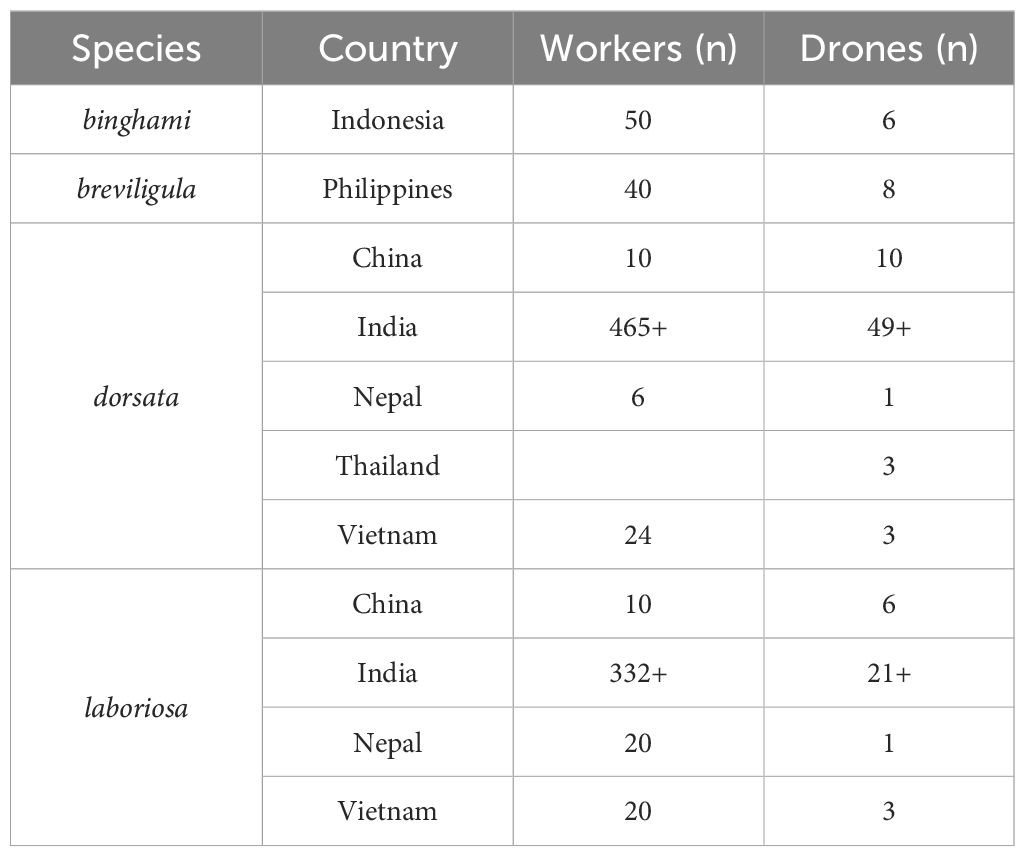
Collections of giant honey bees were carried out for 4 years (October 2017 to October 2021) by author N. Kitnya and her colleagues in different parts of India: Andaman Islands, Arunachal Pradesh, Delhi, Karnataka, Madhya Pradesh, Manipur, Nagaland, Punjab, Tamil Nadu, Telangana, Uttarakhand, and West Bengal. Additionally, we examined specimens from China, Nepal, Thailand, Vietnam, Indonesia, and the Philippines. The difficulties of collecting Apis laboriosa, particularly drones that must be collected from colonies or combs, and of sharing museum specimens with researchers in India resulted in the sample sizes of specimens differing greatly among regions, a situation that is typical in taxonomic revisions. Refer to Supplementary File 1 for the locations of the specimens we examined and the names of collectors.
During field trips, bees were collected using an aerial insect net while they foraged on flowers. Generally, they were killed in a tube containing a few drops of ethyl acetate on a ball of cotton; however, some bees killed by being placed directly into 70% ethanol were also included. Dead bees with locality and date information were transferred to packets made from butter paper (transparent paper) for temporary storage in the field. All bees collected were pinned with number 2 insect pins and labeled with collection details. These specimens were then dried in a warm oven at 35°C for 72 hours and deposited in an insect cabinet in the NCBS-TIFR Research Collection Facility for further study. Photographs of each specimen along with label details were taken to create a digital database, available from the NCBS Research Collection Facility.
The specimens studied include bees of all four named taxa as well as the South Indian form. These include laboriosa from India (Arunachal Pradesh, Nagaland, and Uttarakhand), Nepal, China, and Vietnam; dorsata from India (Andaman Islands, Arunachal Pradesh, Delhi, Karnataka, Madhya Pradesh, Manipur, Nagaland, Punjab, Tamil Nadu, Telangana, Uttarakhand, West Bengal), China, Nepal, Thailand, and Vietnam; binghami from Central and South Sulawesi in Indonesia; and breviligula from Luzon and Mindanao islands in the Philippines (refer to Figure 1; Supplementary File 1 for collection details). The numbers of specimens examined and their provenance are given in Table 1. The map was prepared in QGIS (Version 3.32.2-Lima) a free, publically available software program. The shape file for Southeast Asia was obtained from DIVA-GIS (https://diva-gis.org/). The base map was obtained through QuickMapServices, a function available in QGIS, and is available to anyone to use. The coordinates of collection localities were plotted on this map.
2.2 Dissection of specimens
For assessment of characters that required dissection, both the dried pinned specimens and specimens preserved in 70% ethanol were placed in a humidification chamber for 24–48 hours to relax the tissues. To soften the tissues and dissect the delicate body parts like mouthparts and spiracular plates without damaging them, they were treated with 10% KOH. For mouthparts, whole heads were boiled in 10% KOH for 4–5 min and then transferred to a dissection glass block containing water. The mouthparts were carefully dissected with the help of fine forceps and placed on a microscope slide. They were carefully spread, a few drops of glycerine were added, and a second slide was set over it to avoid any folding or distortion before imaging. Likewise, for metasomal sterna, abdomens were boiled in 10% KOH for 4–5 min or kept overnight in 10% KOH. Then, the metasomal terga were separated from the metasomal sterna. Soft tissues were removed with the help of a soft brush, and the sterna were carefully pulled apart and washed with distilled water. The dissected metasomal sterna 2–6, spiracular plates (seventh hemitergite), were mounted on microscope slides as described above for mouthparts and examined under a dissecting microscope at various magnifications (sterna 2–5 at 1.6×; seventh hemitergite at 4×, and mouthparts at 1.6×). Simultaneously, images were captured using a Leica M125 C stereomicroscope, with a dedicated Leica MC 190 HD camera and LAS V4.12 software to process images. While taking the images, another slide was placed over the body part so that it remained in a horizontal plane and properly spread. The body parts were stored in glycerine along with the respective voucher code in the NCBS-TIFR Research Collection Facility. The specimens of bees we dissected were a small subset of the total specimens available for study.
2.3 Terminology used in the study
The morphological terminology used in this study mostly follows Michener (1944, 2007) and Maa (1953). The abbreviations used were as follows: MOD = median ocellus diameter, IOD = interocellar distance, OOD = ocellocular distance, UOD = upper ocular distance, HW = head width, ML = malar length, MW = malar width, i-V = indica vein, L1 = tongue length (prementum + glossa), S5L = sternum 5 length, S5W = sternum 5 width, Sn = metasomal sternum number n, and Tn = metasoamal tergum number n. Note that length and width of sternum 5 refer to only the darkened portion posterior to the wax mirrors. All measurements, in mm, were taken using ImageJ (http://imagej.nih.gov/ij).
2.4 Statistical analysis
The data set for each morphological trait was evaluated for normality with Shapiro-Wilk and Kolmogorov–Smirnov tests along with visual inspection of density and Q–Q plots. Sample means and standard deviations were computed for each morphological character. Statistical analyses of the data included one-way ANOVA and post-hoc Tukey’s pairwise tests. Statistical analyses were performed in R version 4.1.2 (http://www.R-project.org/), using the RStudio IDE (http://www.rstudio.com). We used the following R base functions: ggdensity() and ggqqplot() to check normality visually, shapiro.test() and ks.test() to perform the Shapiro–Wilk tests and Kolmogorov–Smirnov tests of normality for each trait, aov() to conduct the one-way ANOVAs, and pairwise.t.test() to detect significant differences between taxa for each characteristic. The packages ggplot2 (Wickham, 2016) and tidyr (Wickham, 2021) were used to visualize the data and results.
3 Results
3.1 Species accounts
The general habitus of Megapis workers and drones of four taxa are displayed in Figure 2. The sample sizes along with countries from where specimens were collected are given in Table 1. The mean, SD, range of measurement values, and sample size for each of the characters are given in Table 2.

Figure 2 Dorsal habitus of Megapis (Ashmead, 1904) taxa (workers on left, drones on right). (A) binghami [(A-1) NRC-AA-3057, Palolo Valley, Rahmat, Sigi Regency, Central Sulawesi, Indonesia, 19ix1998, collector: G. W. Otis; (A-2) NRC-AA-3070, Forestry Centre, Tabo Tabo, South Sulawesi, Indonesia, 3vi1989, collector: G. W. Otis]. (B) breviligula [(B-1) NRC-AA-7668, Bulano ERTC, Philippines, July 2008, collector: Y.C. Su; (B-2) NRC-AA-7665, Alfonso, Province of Cavite, Luzon, Philippines, 12–20vii2008, collector: Y. C. Su]. (C) dorsata [(C-1) NRC-AA-7260, NCBS Campus, Bangalore, Karnataka, India, 27iii2023, collector: N. Kitnya; (C-2) NRC-AA-7256, NCBS Campus, Bangalore, Karnataka, India, 28iii2023, collector: N. Kitnya]. (D) laboriosa [(D-1) NRC-AA-2862, Xinping County, Sa Town, Yuxi City, Yunnan, China, 29iv2019, collector: Q. Lifeu; (D-2) NRC-AA-2918, Kaski, Gandaki, Nepal, 8v1984, collector: B. A. Underwood].

Table 2 Means (mm), standard deviations and sample sizes, and ranges of values of the characters measured.
3.1.1 Apis binghami Cockerell, 1906
A. zonata Smith, 1859: 8 (junior homonym) [binghami Cockerell]
M. zonata (Smith); Ashmead, 1904: 121 [binghami Cockerell]
A. dorsata binghami Cockerell, 1906: 166. Replacement name for A. zonata Smith, 1859 [binghami Cockerell]
M. binghami (Cockerell); Maa, 1953: 564 [binghami Cockerell]
A. dorsata binghami (Cockerell); Engel, 1999: 185 [binghami Cockerell]
3.1.1.1 Description of worker A. binghami
1. a) Integument: generally black, except edge of labrum, mandibles, antennae, and legs, which are iridescent brown.
2. b) Pubescence: Pubescence on head predominantly black except for short, whitish decumbent hairs on the paraocular area, and mixture of yellow-brown hairs on the genal area. Black-brown hairs on mesosoma (scutum and scutellum) except pale yellow hair on pronotum, lateral mesosoma, metanotum, and propodeum. Distinct patch of brown hairs on mesopleuron. Pale-yellow hairs on the propodeum continue to the base of tergum 1 (T1). However, black or sooty brown hairs covering a major portion of T1 continue to the end of the metasomal terga. Yellowish brown hairs on sternum 1 (S1), coxae, trochanters, femurs, and tibiae. Visible band of short, decumbent whitish hairs at the anterior bases of T3, T4, and T5.
3. There is some slight variation of color pattern among the individuals as described here. For example, in some specimens, such as those collected in ethanol (or with compressed metasoma after being killed), the bands of white hairs are not visible. This applies to the descriptions of other taxa as well.
4. c) Structures:
I. Head: Ocelli arise from a prominently raised pedestal. Lateral ocelli much bulged and tilted toward compound eyes (Supplementary File S2: A). Median ocellus, 0.460–0.517 mm in diameter (Figure 3A). Interocellar distance (IOD) is wider than ocellocular distance (OOD) (IOD = 0.359–0.531 mm; OOD = 0.269–0.351 mm). Ratio of IOD/OOD, 1.280–1.959 (Figure 3B). Malar length (0.503–0.611 mm) slightly shorter than malar width (0.588–0.751 mm). Ratio of upper ocular distance (UOD) and head width (HW), 0.358–0.408 (Figure 3C). Tongue length (prementum + glossa), 6.156–6.491 mm (Figure 4A ; Table 2).
II. Metasomal abdomen: Anteglandulus of sternum 2 medially angulated, not arcuate (Figure 5: A-1). Glandulus at the midpoint of sterna 3–5 curved posteriorly (see sternum 3 in Figure 5: A-2). Ratio of length and width of sternum 5, 0.551–0.604. On seventh hemitergite, posterior laminal spiracularis not invaginated (Figure 5: A-3). The number of pairs of barbs on the sting, 10–11.
III. Wing: indica vein on hind wing short, 0.324–0.613 mm.

Figure 3 Comparisons of head characteristics of Megapis workers. (A) Median ocellus diameter (MOD) in mm. (B) Ratio of interocellar distance (IOD) and ocellocular distance (OOD). (C) Ratio of upper ocular distance (UOD) and head width (HW). (D) Malar Area (Malar Width/Malar Lenght × 100). Significance of Tukey’s pairwise test: ***p ≤ 0.001; ****p ≤ 0.0001; ns: non-significant (p>0.05). Refer to Supplementary File 2, Supplementary Figures S2: A–: C for comparative images of the traits mentioned here.
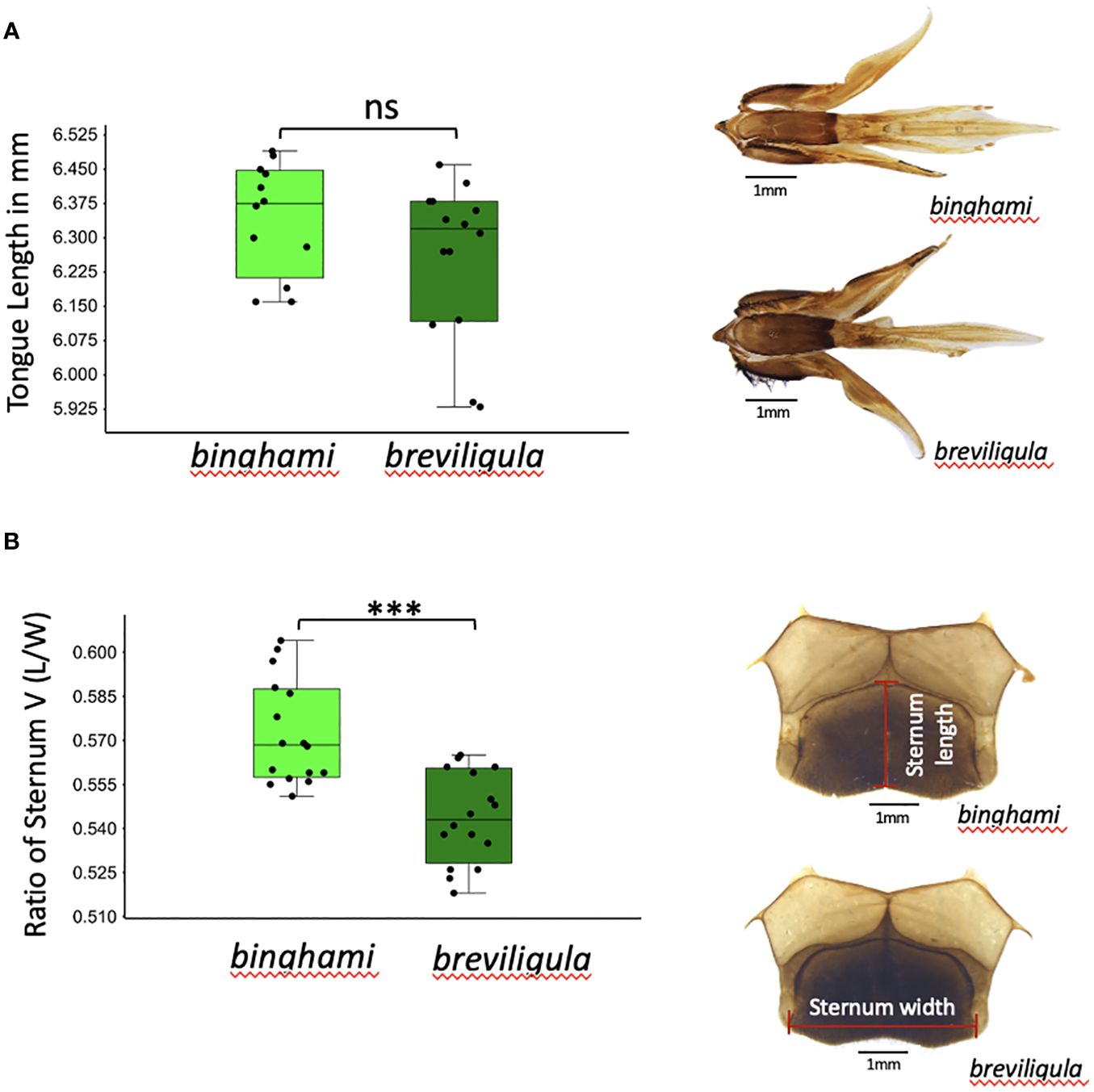
Figure 4 Tongue length and proportions of sternum 5 of binghami and breviligula workers. (A) Tongue lengths (mm). (B) Ratio of sternum 5 length to width. Significance of t-tests: ns, non-significant (p > 0.05); ***p ≤ 0.001.
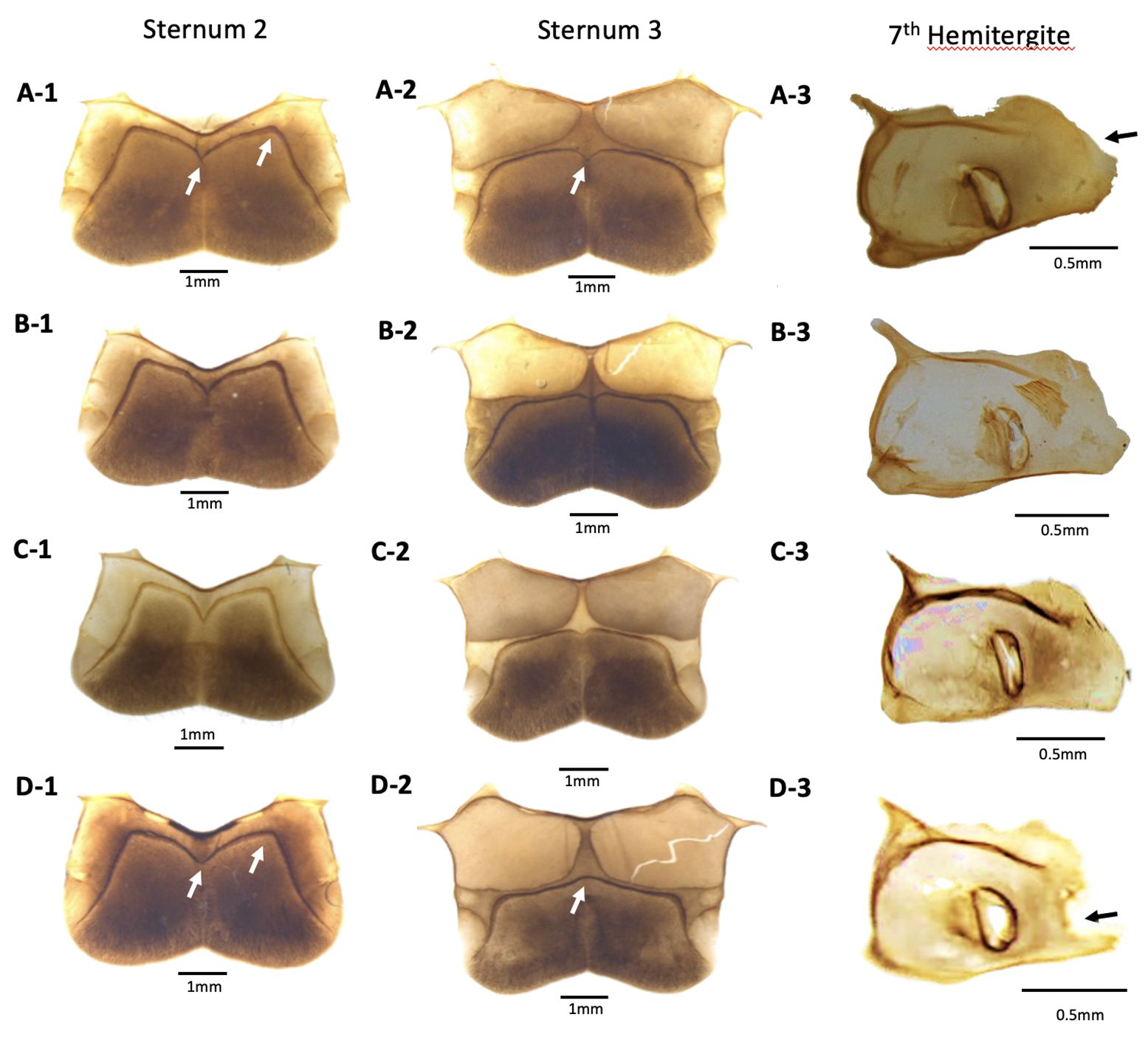
Figure 5 Abdominal morphology of Megapis workers: (A) binghami, (B) breviligula, (C) dorsata, and (D) laboriosa for (1) sternum 2, (2) sternum 3, and (3) 7th Hemitergite. Arrows on sternum 2 indicate locations of differences in anteglandular structure. Arrows on sternum 3 indicate differences at mid-point of glandulus. Arrow on seventh hemitergite indicates invagination of spiracular lamella at posterior edge in laboriosa that is absent in the other taxa.
Callow individuals with gray integument and pale whitish hairs.
3.1.1.2 Description of drone A. binghami
a) Integument: head, antennae, mesosoma, and metasoma iridescent brown; antennae and legs brown.
b) Pubescence: Pale yellow hairs on face, eyes, gena, scutum, scutellum, lateral mesosoma, metasomal tergum 1, ventral mesosoma, sterna, and legs. Brown hairs on metasomal tergum 2 to apex of metasoma; long, stiff, brown hairs on metasoma from tergum 5 onward to apex of metasoma. Clumps of light brown hairs on basitarsus III.
c) Structures: Ocellar triangle prominently raised (Figure 6: A-1, A-2); in fact, it is the most prominently raised among the giant honey bee taxa. Lateral ocelli close together, with almost no space between lateral ocelli and compound eyes (Figure 6: A-2). Ocelli protrude strongly; lateral ocelli angled toward compound eyes. In dorsal view, ocelli extend prominently beyond the plane of compound eyes (Figure 6: A-3).

Figure 6 Head morphology of Megapis drones: (A) binghami, (B) breviligula, (C) dorsata, and (D) laboriosa: (1) frontal view of head; (2) SEM of ocelli; (3) dorsal view of head. White arrows identify the relatively flat ocelli and unraised ocellar platform of laboriosa compared to the other three taxa.
Queen: Unknown.
Specimens examined: INDONESIA: Central Sulawesi: Kech. Lore Utara, Wuasa, Kab. Poso, 9 XI 1996 (GWO) 1 worker; Sigi Regency, Palolo Valley, Rahmat, 19 IX 1998 (GWO), 13 workers; Sigi Regency, Palolo Valley, Kamarora, 9 IX 1989 (GWO), 4 workers; 9 XI 1995 (GWO), 1 worker; Sigi Regency, Palolo Valley, Kamarora, Phpaposton Minlosa, 22 IX 1996 (GWO), 1 worker; South Sulawesi: Tabo Tabo, Forestry Center, 3 VI 1989 (GWO), 22 workers, and 4 drones.
3.1.1.3 Distribution
Indonesia: Sulawesi and nearby islands (e.g., Buton Is., Kabaena Is., and Taliabu Is.) of Indonesia (Smith, 2021).
3.1.1.4 Remarks
This taxon of giant honey bee has a strongly raised ocellar triangle, and the ocelli are larger on average than in the other taxa in both workers and drones, possibly suggesting that it is more nocturnal in its foraging than other Megapis species (Figures 3, 6; Supplementary File S2: A). The indica vein on the worker’s hind wing is shorter than in laboriosa but does not differ from that of dorsata or breviligula. On average, sternum 5 is proportionately longer (i.e., ratio of length/width is greater) than that of breviligula workers, similar to the report by Maa (1953, Figure 3.9).
3.1.2 Apis breviligula Maa, 1953
Megapis breviligula Maa, 1953: 563 [breviligula Maa]
A. dorsata breviligula (Maa); Engel, 1999: 186 [breviligula Maa]
3.1.2.1 Description of worker A. breviligula
a) Integument and pubescence: Integument of adult worker black over entire body. Bronzy iridescence on the edge of the labrum, mandibles, and legs. Pattern of pubescence coloration not different from that of binghami. Callow individuals pale gray, as in binghami.
b) Structures:
I. Head: Ocellar triangle raised (Supplementary File S2: A). Ocelli domed. Lateral ocelli angled toward compound eyes. Diameter of median ocellus, 0.451–0.483 mm. IOD 0.380–0.479 mm; OOD, 0.268–0.338 mm; ratio of IOD/OOD, 1.193–1.520 (Figure 3B). Malar length and width, 0.522–0.663 mm and 0.640–0.722 mm, respectively. Ratio of upper ocular distance and head width (UOD/HW), 0.377–0.408. Tongue, 5.933–6.456 mm long (Figure 4A).
II. Metasomal abdomen: Anteglandulus of sternum 2 angulated at the midpoint (Figure 5: B-1). Glandulus on sterna 3–5 curved posteriorly at the midpoint (Figure 5: B-2, showing sternum 3). Ratio of length to width of sternum 5, 0.518–0.565. Posterior laminal spiracularis on seventh hemitergite not invaginated. On sting, the number of pairs of barbs ranges from 10 to 11.
III. Wing: indica vein on hind wing 0.327–0.617 mm long.
3.1.2.2 Description of drone A. breviligula
a) Integument: head, antennae, mesosoma, legs, and metasoma iridescent brown.
b) Pubescence: Brown hairs on head, mesosoma, and metasoma with pale yellow to brown hairs from ventral view. Mesosoma fully covered with long, pale yellow to brown hairs. Clumps of brown hairs on basitarsus III.
c) Structure: Ocellar triangle prominently raised. Ocelli protrude strongly, angled toward compound eyes (Figure 6: B-1). Lateral ocelli seem to meet compound eyes with no space between them (Figure 6: B-2). In dorsal view, ocelli extend beyond the plane of compound eyes (Figure 6: B-3).
Queen: Unknown.
Specimens examined: PHILIPPINES: Luzon: Cavite Province, Alfonso, Upli, 4 III 2004 (DRS), 1 worker, 5 drones; Los Baños, College Villa, 2008 (DRS), 7 workers; (SR) 11 workers. Mindanao: Bulano-Ecological Research and Training Centre (ERTC), Zamboanga, 7 XII 2008 (YCS), 4 workers; Bulano Pasan, Zamboanga, 7 XII 2008 (YCS), 1 worker; Davao City, Davao del Sur, 30 IX 1998 (YCS), 1 worker, 30 IX 1998 (YCS), 16 workers, 1 drone.
3.1.2.3 Distribution
Throughout the Philippine Islands, excluding Palawan (Smith, 2021).
3.1.2.4 Remarks
Workers of breviligula are very similar to those of binghami in their overall morphological appearance (Figure 2). Maa (1953) described breviligula as a species based in part on its short tongue length. However, in the fully extended tongues measured in the present study, this was not the case: tongue lengths overlapped broadly, and mean length did not differ statistically between binghami and breviligula (Figure 4A). This study confirmed the less prominently raised ocellar triangle of breviligula compared to binghami (Maa, 1953), and its proportionately shorter sternum 5 (Figure 4B). Furthermore, the lateral ocelli bulge outward toward the compound eyes but to a lesser extent than in binghami (Figure 3B; Supplementary File S2: A). Diameter of the median ocellus is similar to that in binghami but larger than in dorsata and laboriosa (Figure 3A). Values for the ratio of upper ocular distance to head width (UOD/HW) are very similar between breviligula and binghami but smaller than those of dorsata and laboriosa (Figure 3C). The ratio of malar width to length is greatest in binghami, approximately the same in breviligula and dorsata, and smallest in laboriosa (Figure 3D). No distinct differences between binghami and breviligula drones were observed. However, they both have prominently raised ocelli that are larger than in dorsata and very unlike laboriosa, which has relatively unraised ocelli and ocellar triangle.
3.1.3 Apis dorsata Fabricius, 1793
A. dorsata Fabricius, 1793: 328 [dorsata Fabricius]
A. nigripennis Latreille, 1804: 170 [dorsata Fabricius]
A. bicolor Klug, 1807: 264. Preoccupied (nec Fabricius 1781, Villers 1789) [dorsata Fabricius]
A. testacea Smith, 1858: 49 [dorsata Fabricius]
A. testaccea Moore et al., 1871: 396 Lapsus calami [dorsata Fabricius]
M. dorsata (Smith); Ashmead, 1904: 121 [dorsata Fabricius]
A. dorsata Baldensperger, 1928: 173 L. calami [dorsata Fabricius]
M. dorsata (Fabricius), Maa, 1953: 566 [dorsata Fabricius]
A. dorsatao Ruttner, 1988: 118 Lapsus calami [dorsata Fabricius]
A. dorsata dorsata (Fabricius), Engel, 1999: 186 [dorsata Fabricius]
3.1.3.1 Description of worker A. dorsata
a) Integument: head and antennae shiny black except for the apex of the mandibles and labrum, which are reddish brown. Legs black, excluding the margins and ventral surface of basitarsus III, which are reddish brown. Mesosoma integument black. Metasomal terga 1–3 and sterna 1–2 yellow to brown; other terga and sterna black toward apex of metasoma (Figure 2: C-1). Extent of bicolorism varies, with yellow integument extending to tergum 4 or 5 in some individuals. Young callow individuals with pale yellowish integument. Specimens collected from southern India and Sri Lanka not noticeably different from those of Southeast Asia.
b) Pubescence:
I. Head: Yellowish and brown hairs on labrum; short, decumbent white hairs on clypeus, paraocular area, and adjacent antennal suture; stiff, medium to long black and brown hairs on frons, ocellar triangle and vertex of head; pale yellow and brown hairs on compound eyes; short black hairs on antennal scape, pedicel and first two segments of flagellum, followed by short decumbent pale yellow hairs on the remaining flagellar segments; pale yellow hairs on genal area.
II. Mesosoma: Long black hairs cover pronotum and scutum; long brown to yellow hairs on scutellum, metanotum, and propodeum that continue onto metasomal abdominal segments. Brown hairs on ventral mesosoma. Patch of brown hairs present at the mesopleuron. Long, dense, yellow hairs on coxae and trochanters; long, scattered, yellow hairs on femurs; and stiff brown hairs on basitarsi and tibiae.
III. Metasoma: Yellow hairs on terga 1–2 (T1–T2); mixture of pale yellow and black hairs laterally on T3 and T4; yellow hairs on the anterior margin of T5 and T6. Black hairs cover majority of T5 and T6 (Figure 2: C-1). From ventral view, yellow to brown hairs on sterna 1 and 2. Transverse band of short, decumbent, whitish hairs on anterior bases of sterna 3–5. The extent of whitish hairs visible varies depending on how bees died and the extension of the metasoma of the dead bees; e.g., the whitish or yellow hairs seem to be absent or dull in specimens killed in ethanol.
c) Structures:
I. Head: Ocelli arise from a raised pedestal (Supplementary File S2: A). Lateral ocelli much bulged and tilted toward compound eyes. Median ocellus diameter, 0.399–0.439 mm (Figure 3A). IOD approximately equal to the distance between lateral ocelli and compound eyes (OOD) (IOD = 0.370–0.510 mm; OOD = 0.380–510 mm). Ratio of IOD/OOD, 0.813–1.159 (Figure 3B). Malar length (0.523–0.662 mm) equal to or slightly shorter than malar width (0.593–0.797 mm). Ratio of UOD to HW, 0.420–0.459 (Figure 3C).
II. Metasomal abdomen: Anteglandulus medially angulated, not arcuate (Figure 5: C-1). Glandulus at the midpoint of sterna 3–5 curved posteriorly. Absence of invaginated posterior laminal spiracularis on seventh hemitergite; 10–11 pairs of barbs on sting.
III. Wing: indica vein on hind wing, 0.264–0.728 mm.
A. dorsata workers from across its range have similar morphological appearance. The South India and North India groups, as indicated by molecular data (Smith, 1991; Lo et al., 2010; Smith, 2021; Kitnya et al., 2022; Bhatta et al., 2024), are not differentiated by the morphological characters we examined. However, worker specimens from the Andaman Islands are smaller in overall size (for example, wing length and width, two traits that serve as proxies for body size), though color pattern and other morphological features do not distinguish them from mainland dorsata.
3.1.3.2 Description of drone, A. dorsata
a) Integument: head, scape, and pedicel brown; flagellum orange-brown. Mesosoma black. Metasomal terga 2 and 3 orange-brown (tergum 1 cannot be viewed, fully covered with dense hairs); posterior portion of terga 2 and 3 light brown. Terga 3–7 chestnut brown. Legs brown, with last tarsi of hind legs orange.
b) Pubescence: Brown hairs on face, eyes, and gena; long, dense, brown hairs fully covering the mesosoma and continuing onto metasomal tergum 1 and sternum 1. Short brown hairs on terga 2 and 3; long, stiff, brown hairs on terga 4–7. Pale yellow hairs on sterna 2–7. Long, brown hairs on coxae, trochanters, and femurs; nearly bare tibiae; clumps of pale-yellow hairs on ventral basitarsus III.
c) Structures: Ocellar triangle visibly raised. Ocelli protruded, strongly domed, and tilted toward compound eyes (Figure 6: C-1, C-2); extend beyond compound eyes in dorsal view (Figure 6: C-3). We were unable to examine drones from Andaman Island due to lack of specimens.
Queen: Unknown
Specimens examined: CHINA: Yunnan: Mandian Village, Dragon fruit land, Xishuangbanna, 30 IV 2019 (QL), 2 workers; Xishuangbanna Botanical Garden—National Museum, Xishuangbanna, 1 V 2019 (QL), 6 workers, 10 drones; INDIA: Andaman and Nicobar Islands: South Andaman Island, Bambooflat, 19 I 2018 (PMV), 2 workers; Haddo, 19 I 2018 (PMV, VH), 20 workers; Mohanpur Terminal, 19 I 2018 (PMV, VH), 16 workers; Diglipur, 19 I 2018 (PMV, VH), 5 workers; Rangat, 19 I 2018 (PMV, VH), 8 workers; Arunachal Pradesh: East Siang, Menekrong, 18 V 2019 (NK), 16 workers; East Siang, Pasighat, 18 V 2019 (NK), 10 workers; Papum Pare, RGU Campus, 29 III 2019 (NK), 2 workers; Papum Pare, Rono Hills, 4 XII 2017 (NK), 1 worker; Siang, Modi, 28 III 2019 (NK, KM, GWO), 6 workers; Tirap, Kala Pahar, 13 X 2017 (NK), 2 workers; Tirap, Thungjang, 12 XI 2017 (NK), 7 workers; Tirap, Tutnyu, 13 X 2017 (NK), 7 workers, 28 IV 2018 (NK), 10 workers; Upper Siang, Kuging village, 28 VII 2017 (RGU, UW), 2 workers; Upper Siang, Pangkang, 28 III 2019 (NK, KM, GWO), 41 workers; West Kameng, Nag Mandir, 28 X 2017 (PMV, NK), 9 workers; West Kameng, Sessa, 20 VI 2018 (NK), 2 workers; West Siang, Amtung, 21 IV 2018 (NK, KM), 15 workers; West Siang, Kaying, (NK), 13 workers; West Siang, Mechuka, 5–10 VI 2016 (RGU, UW), 7 workers; West Siang, Parong, 27 III 2019 (NK), 3 workers; West Siang, Songum, 9 V 2019 (NK), 12 workers; West Siang, Tumbin, 16 V 2019 (NK), 22 workers, 16 drones; Assam: Kamrup Metropolitan, Airport (Lokpriya Gopinath Bordoloi International Airport), 14 VI 2023 (NK, AJ), 1 worker; Gogamukh, 12 VI 2016 (RGU, UW), 2 workers; Karnataka: Bangalore, GKVK Campus, 28 II 2023 (BK), 1 drone; Bangalore, ICTS Campus, 16 II 2018 (GGT), 2 workers, 27 II 2018 (GGT), 5 workers; 9 workers; Bangalore, NCBS Campus, 12, 13 I 2018 (NK), 11 workers, 14 II 2023 (NK), 13 workers, 29 III 2023 (NK) 10 workers; Bangalore, near NCBS Campus, Canara Bank Layout, 18 IV 2021 (NK), 3 drones, 15 II 2023 (BK, RS), 12 workers, 18 and 20 I 2022 (NK), 2 workers; Bangalore, Palace ground, 9 II 2014 (Dudde), 3 drones; Bangalore, Peenya, 27 II 2018 (GGT) 4 workers; Bangalore, Sahakara Nagar, 6 II 2020 (NK), 2 drones; Bangalore, Yelangka, NCBS Mandara Campus, 28 II 2023 (NK), 7 drones, 28 III 2023 (NK), 4 workers; Manipur: Imphal West, Ema Market, 24 IV 2018 (JN), 12 workers; Imphal West, Imphal Hotel, 24 IV 2018 (JN), 3 workers; Nagaland: Dimapur, Dimapur, 5 III 2018 (NK), 17 workers; Peren, Hainikal, 1 V 2018 (NK), 22 workers, 16 drones; Peren, Heningkunglwa, 15 VI 2019 (NK), 14 workers; Peren, Lamhai Dungki, 24 V 2019 (NK), 10 workers; Punjab: Chandigarh, Punjab University Campus, (AB), 18 workers; Tamil Nadu: Tiruppur, Coimbatore (CGR Farm, Indira Puram, Amaravathi Nagar), 3 III 2023 (KA), 14 workers; Telangana: Ranga Reddy, University of Hyderabad, Life Science Building, 17 IV 2023 (SC), 20 workers; Madhya Pradesh: Narmadapuram, Pachmarhi, 15 III 2023 (SC), 9 workers; West Bengal: Kolkata, IISER Kolkata Campus, 15 IV 2019 (PS), 12 workers; Uttarakhand: Pithoragarh, Jauljibi, 28 IV 2019 (PMV), 10 workers; NEPAL: Lumbini province: Banke, Kohalpur, (CPB), 1 worker; Sudurpashchim Province: Kailali, (CPB), 1 worker; Kanchanpur, (CPB), 3 workers; Kawasoti, (CPB), 1 worker; THAILAND: Chanthaburi, Phuang subdist, Khao Khitchakut, 6 II 1999 (NW), 3 drones; VIETNAM: Ca Mau, 21 I 2018 (BR) 2 workers; Ca Mau, U Minh, 8 VIII 2001 (PHT and DVS), 6 workers, 1 drone; Kien Giang, Phu Quoc, Mekong Delta, 21 II 2018 (BR), 10 workers; Lai Chau, Moung Te, 17 V 2007 (PHT), 3 workers; Minh Hải, U Minh, U Minh, 1994 (PHT), 5 workers.
3.1.3.3 Distribution
Afghanistan, Pakistan, India, Nepal, Bhutan, Bangladesh, China, Myanmar, Thailand, Cambodia, Laos, Vietnam, Malaysia, Singapore, Indonesia (excluding Sulawesi and neighboring islands), Philippines (Palawan only), and Sri Lanka (Smith, 2021).
3.1.3.4 Remarks
This is the most widely distributed taxon of the giant honey bees. In workers, the mesosoma has brown to black hairs except for the posterior and lateral edges of the scutum with pale yellow hairs. The metasomal abdomen is bicolored in foragers (Figure 2: C-1) and uniformly yellow in callow workers. The legs are paler than in the other Megapis taxa. The lateral marginal area of sterna 2–5 is smallest in dorsata. Our casual observations in the present study indicated that dorsata has the smallest wax plates, in contrast to Maa’s (1953) report that the wax plates of binghami are the smallest. Ratio of upper ocular distance to head width is smaller than in laboriosa but larger than in binghami and breviligula (Figure 3C). Also, the median ocellus diameter is intermediate between laboriosa and binghami/breviligula (Figure 3A).
3.1.4 Apis laboriosa Smith, 1871
A. laboriosa Smith in Moore et al., 1871: 249 [laboriosa Smith]
A. himalayana Maa, 1944: 4 (nomen nudum) [laboriosa Smith]
M. laboriosa (Smith), Maa, 1953: 570 [laboriosa Smith]
A. labortiosa Willis et al., 1992: 169 Lapsus calami [laboriosa Smith]
A. dorsata laboriosa (Smith), Engel, 1999: 186 [laboriosa Smith]
3.1.4.1 Description of worker A. laboriosa
a) Integument: Uniformly black over the entire body, except the apical edge of mandibles, labrum, antennae, and legs are reddish brown.
b) Pubescence:
I. Head: Mixture of short decumbent pale yellow, brown, and black hairs on mandibles, labrum, clypeus, and paraocular area; slightly longer pale yellow hairs around antennal suture; yellow and brown hairs on frons; long, dense, brown, and golden hairs cover the vertex; long, yellow, and brown hairs on compound eyes; short, decumbent, black hairs on the scape, pedicel, and first two flagellomeres of antennae while short, decumbent pale yellow hairs cover the flagellum from segment 3 onward to tip of antennae; long, dense golden-yellow hairs on genal area.
II. Mesosoma: Long, dense, golden yellow hairs on pronotum; long, dense, brown hairs cover center of scutum, while dense, long, golden yellow hairs cover rest of the mesosoma, a distinctive characteristic of A. laboriosa (Figure 2: D-1). Unlike other taxa of giant honey bees, patch of brown hairs at the center of mesopleuron absent. Long, golden-yellow hairs on coxae, trochanters, and femurs; stiff, brown to black hairs completely cover tibiae and basitarsi.
III. Metasoma: Dorsal view: golden-yellow hairs completely cover tergum 1 (T1) and continue to the basal margin of T2 (Figure 2: D-1); short black hairs on terga 2–6; thin bands of white hairs along bases of T1–T5 (some of the bands of white hairs may become removed during aging of the bee, collection, or storage; therefore, in some specimens bands are absent); no band of white hairs at basal margin of T6. Ventral view: short, decumbent black hairs with strips of white hairs at anterior bases of sterna 3–5. However, the presence of white hairs on terga varies between bees; also, they are not clearly visible if the specimens were collected in ethanol or if the bee died with compressed metasoma.
c) Structures:
I. Head: Region where ocelli occur is flat or only slightly raised (Supplementary File S2: A). Lateral ocelli do not tilt toward compound eyes (Supplementary File S2: B). Median ocellus width, 0.338–0.391 mm (Figure 3A). Distance between lateral ocelli and compound eyes distinctly wide as compared to other three taxa: OOD = 0.550–0.740 mm, interocellar distance: IOD = 0.300–0.470 mm (Table 2). Ratio of interocellar distance to ocellocular distance (IOD/OOD), 0.500–0.712 (Figure 3B; Table 2). Malar area, distinctly long, ML = 0.753–0.957 mm (Figure 3D; Supplementary File S2: C). Ratio of UOD to HW, 0.480–0.519 mm.
II. Metasomal abdomen: Anteglandulus of sternum 2 medially arcuate (Figure 5: D-1). Lateral marginal area of sterna 2–5 widest as compared to other three taxa. On sterna 3–5, glandulus at midpoint curved toward base (anteriorly) (Figure 5: D-2: sternum 3). Posteror laminal spiracularis (seventh hemitergite) distinctly invaginated (Figure 5: D-3); 13–14 pairs of barbs on sting.
III. Wing: indica vein on hind wing relatively long, 0.534–1.282 mm.
3.1.4.2 Description of drone A. laboriosa
a) Integument: head, antennae, mesosoma, legs, and metasomal abdomen are uniformly black.
b) Pubescence: Brown to black hairs on head; black hairs on mesosoma; golden yellow hairs at the posterior scutum and scutellum and lateral mesosoma that continue onto metasomal tergum 1. Black branched hairs on metasomal tergum 2–4; black, long, unbranched, and stiff hairs on terga 5–6. Long yellow hairs on coxae, trochanters, and femurs. Stiff, black hairs on basitarsi. Clumps of coppery brown hairs on ventral basitarsus III.
c) Structures: Ocellar triangle nearly flat (i.e., only slightly raised). Space between lateral ocelli is distinctly wide compared to that of all other Megapis taxa (Figure 6: D-1, D-2). Ocelli not visible beyond the plane of compound eyes from dorsal view (Figure 6: D-3).
Queen: Unknown
Specimens examined: CHINA: Yunnan: Yuxi City, Xinping County-Sa, 29 IV 2019 (QL), 6 workers; Yuxi City, Xinping County-Street Town, 14 IV 2019 (QL), 5 workers, 6 drones; INDIA: Arunachal Pradesh: Anjaw, Hawai, 18 V 2018 (KM), 1 worker; Anjaw, Tidding, 18 V 2018 (KM), 4 workers; East Siang, Pangin, 11 VI 2016 (NK), 4 workers; East Siang, Yembo, 26 III 2019 (NK, KM, GWO), 2 workers; Siang, Modi, 28 III 2019 (NK, KM, GWO), 7 workers; Tawang, Bomdir, 7 IV 2018 (PMV), 27 workers; Tawang, Jang, 27, 30 V 2016 (RGU, UW), 4 workers; Tawang, Karpo, 29 V 2016 (RGU, UW), 1 worker; Tawang, P.T. So Lake, 5 VII 2018 (NK), 8 workers; Tawang, Padma, 6 VII 2018 (NK), 3 workers; Tawang, Pane Lake, 5 VII 2018 (NK), 9 workers; Tawang, Psangpong, 4 VII 2018 (NK), 4 workers; Tawang, Tawang, 28 V 2016 (RGU, UW), 6 workers; Tawang, Urgelling, 4–5 VII 2018 (NK), 18 workers; Tawang, WRD Colony Tawang, 3–5 VII 2018 (NK), 20 workers; Tirap, Kala Pahar, 12, 13 X 2017 (NK), 11 workers; Tirap, Tutnyu-Nakaang, 10 VI 2023 (NK, TS, AJ), 19 workers, 17 drones; Tirap, Tutnyu, 12 X 2017, 28 IV 2018 (NK), 25 workers, 4 drones, West Kameng, Bomdila, 26 V 2016 (RGU, UW), 3 workers; West Kameng, Dirang, 26 V 2016 (RGU, UW), 6 X 2017 (NK), 6 workers; West Kameng, Nag Mandir, 28 X 2017, 28 X 2021 (NK), 7 workers; West Kameng, New Kaspi, 7 IX 2017 (NK), 1 worker; West Kameng, Old Dirang, Riverbed, 26 V 2016 (RGU, UW), 5 workers; West Kameng, Photin, 4 IV 2019 (NK, KM, GWO), 11 workers; West Kameng, Senge, 31 V 2016 (RGU, UW), 1 worker; West Kameng, Tenzing Gaon, 6 VII 2018 (NK), 5 workers; West Siang, Tumbin, 16 V 2019 (NK), 11 workers; Nagaland: Peren, Benrue, 6 IV 2018 (NK), 13 workers; Peren, Sengkui, 6 IV 2018 (NK), 10 workers; Uttarakhand: Chamoli, Attri, 2 V 2019 (PMV), 10 workers; Chamoli, Mandal, 2 V 2019 (PMV), 19 workers; Kanchyoti, 30 VI 2018 (PMV), 14 workers; Pithoragarh, Munsyari, 31 V 2018 (PMV), 10 workers; Rudraprayag, Kaakad, 2 V 2019 (PMV), 13 workers; NEPAL: Kaski, Gandaki Province, 8 V 1984 (BAU), 1 drone; (CPB), 18 workers; VIETNAM: Son La, Mộc Châu, VII 2001 (PHT), 3 workers, 2 drones; Son La, Mộc Châu, 7 VI 1996 (LQT), 8 workers; Sơn La (SL), Mộc Châu, Moung La, 30 XI 2019, 1 worker (THP); Lao Cai, Sapa, 12 IV 2019 (THP), 3 workers, 1 drone; Hoa Binh, Mai Chau, 1996, 5 workers.
3.1.4.3 Distribution
Pakistan (Azad Jammu and Kashmir), India (Uttarakhand, Sikkim, West Bengal, and states comprising North East India), Nepal, Bhutan, southern China, Myanmar, northern Thailand, northern Laos, and northern Vietnam (Kitnya et al., 2020; Smith, 2021; Otis et al., 2024).
3.1.4.4 Remarks
Among Megapis, this taxon is the most distinctive. A. laboriosa workers can be easily distinguished from other taxa by their long, golden-yellow thoracic hair (Figure 2: D-1), flat ocellar triangle, barely projecting ocelli (Supplementary File S2: A), much wider distance between lateral ocelli and compound eye (OOD), distinctly longer malar area (Figure 3D), wider lateral marginal area on S2–5, larger wax plates, uniquely arcuate midpoint of glandulus of sternum 2 (Figure 5: D-1), distinctly anteriorly curved midpoint of glandulus of sternum 3, shape of the posterior end of the lamella on the seventh hemitergite (Figure 5: D-3), and greater number of barbs on the sting. Drone ocelli are distinctly unraised and do not extend beyond the plane of compound eyes from dorsal view (Figure 6: D-2, D-3). The drone metatarsus is narrower and longer in laboriosa than that of the other three giant honey bee taxa (Figure 7).
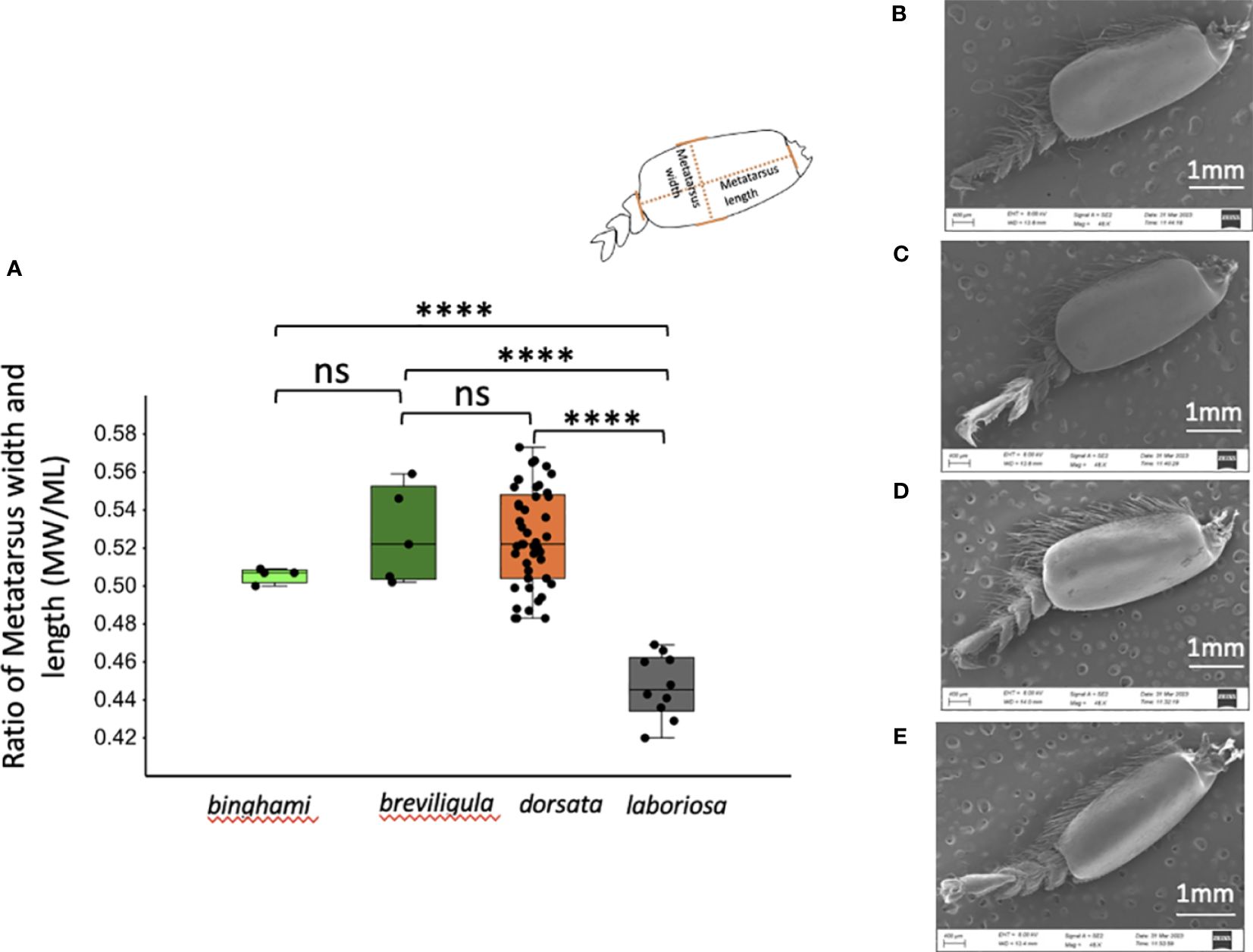
Figure 7 Leg morphology of Megapis drones. (A) Ratio of metatarsus width to length. (B–E) Photographs of drone metatarsi: (B) binghami, (C) breviligula, (D) dorsata, and (E) laboriosa. Metatarsus of laboriosa is narrower. Significance of t-tests: ns, non-significant (p > 0.05); ****p ≤ 0.0001.
After its original description by Smith in 1871, most honey bee researchers ignored the large, dark honey bees of Himalaya. In 1944, Maa referred to it as A. himalayana. Later, in 1953, he correctly referred to it as Apis laboriosa and described it as a distinct species, as confirmed by Sakagami et al. (1980). However, later researchers considered it to be a high-elevation form of A. dorsata (Ruttner, 1988; Engel, 1999). Given the large number of autapomorphies of both drone and worker, it is remarkable that A. laboriosa was not widely accepted as a species long ago.
3.2 Taxonomic keys
3.2.1 Taxonomic key for Megapis workers
1. Ocelli slightly domed, positioned on nearly flat platform; IOD (<0.48 mm) shorter than OOD (>0.54 mm); median ocellus is <0.395 mm wide; UOD/HW is large (>0.47); malar space longer than broad, with ML >0.70 mm; glandulus of sternum 2 weekly curved at the mid and antero-lateral area; antegradular area of sternum 2 very wide; glandulus of sternum 3 curved anteriorly at the midpoint; posterior laminal spiracularis distinctly invaginated; 13–14 pairs of sting barbs; mesopleural patch at lateral mesosoma absent; dorsal mesosoma covered with long golden-yellow hairs.
………………. A. laboriosa
- Ocelli strongly domed, positioned on a prominent pedestal; IOD wider or equal to OOD; ratio of UOD/HW <0.47; malar length <0.70 mm and shorter or equal to malar width; median ocellus >0.395 mm wide; glandulus of sternum 2 sharply curved at the mid and antero-lateral area; antegradular area of sternum 2 not wide; glandulus at sternum 3 curved posteriorly at the midpoint; posterior laminal spiracularis not invaginated; 10–11 pairs of sting barbs; mesopleural patch of hairs at the lateral mesosoma present; black or brown hairs on dorsal mesosoma.
……………………. 2
2. IOD approximately equal to OOD; UOD/HW is >0.410; median ocellus <0.440 mm wide; integument of abdominal tergites is bicolored with anterior terga yellow or orange; lateral mesosoma with distinct black patch of hairs at the center.
………………. A. dorsata
- IOD distinctly greater than OOD; UOD/HW <0.410; median ocellus is >0.440 mm; integument of abdominal tergites entirely black; lateral mesosoma with very small black patch of hairs at the center.
……………. A. binghami
3.2.2 Taxonomic key for Megapis drones
1. Ocelli small and slightly convex and enveloped by long hairs; lateral ocelli are widely separated and do not overlap compound eyes; the ocellar triangle is nearly flat; ocelli do not protrude beyond the plane of compound eyes from dorsal view; integument of head, mesosoma, metasoma, and legs black; scutellum and metasomal tergum 1 covered with long, golden-yellow hairs that continue to the anterior base of metasomal tergum 2; metasomal terga 2–4 covered with short black hairs; tergum 5 to apex of metasoma covered with very long, stiff, unbranched, black hairs.
………………. A. laboriosa
- Ocelli large, strongly convex (domed); lateral ocelli touch the compound eyes; the ocelli are positioned on a raised platform; the distance between lateral ocelli is narrow; the ocelli extend prominently beyond the plane of compound eyes in dorsal view; integument of head, antennae, mesosoma, metasoma range in color from orange to iridescent brown; scutellum and metasomal tergum 1 covered with long, pale yellow to brown hairs; metasomal terga 2–4 covered with short brown hairs; tergum 5 to apex of metasoma covered with very long, stiff, unbranched brown hairs.
.……………………. 2
2. Integument of basal metasomal terga orange to light orange brown; metasomal tergum 4 onward with apical edge chestnut brown (bicolored metasoma); ocellar triangle visibly raised; callow drones have completely yellowish metasomal abdomen.
………………. A. dorsata
- Integument of all metasomal terga iridescent to dark brown (mono-colored); ocellar triangle prominently raised; callow drones have completely gray metasomal abdomen.
………………. A. binghami
4 Discussion
Our detailed examination of numerous morphological characters confirms the status of A. laboriosa as a distinct species, with several characteristics of both workers and drones separating it from the other taxa of giant honey bees. Several autapomorphies of A. laboriosa noted in the comparative study of A. laboriosa and A. dorsata from sites of co-occurrence (Kitnya et al., 2022) were consistent throughout their distributions. Proportions of head characters, median ocellus diameter, malar length, sternal glandulus shape, and spiracular plate (seventh hemitergite) shape are some of characters that allow one to easily distinguish workers of A. laboriosa from the other taxa of giant honey bees, in addition to the very obvious and discrete differences in the color of thoracic hairs and abdominal tergites (for bees of mainland Asia) noted by Sakagami et al. (1980) and confirmed here. [For additional photos of A. dorsata and A. laboriosa, refer to iNaturalist (2024a; b) respectively.] The more numerous barbs on the lancets of the sting (13–14) are also diagnostic of A. laboriosa. Our results agree with and extend the previous reports of Maa (1953), Sakagami et al. (1980), and Trung et al. (1996).
Our results also indicate that the widespread mainland A. dorsata is a species distinct from the giant honey bees of the islands of the oceanic Philippines and Sulawesi, Indonesia. Bicolored yellow/orange and black metasomal tergites, width of the median ocellus, and ratio of UOD/HW consistently separate workers of A. dorsata from bees of those two island populations that exhibit black gasters with white bands. [Refer to iNaturalist (2024c; d) for photographs of binghami and breviligula, respectively.] Additionally, we detected no distinct morphological characters between the two genetic groups found in mainland India. This was surprising given the considerable genetic distance between the clades that inhabit southern portions of the Indian subcontinent and the rest of mainland Asia (Smith, 1991; Lo et al., 2010; Smith, 2021; Bhatta et al., 2024). Additional sampling and analyses of both nuclear and mitochondrial DNA should resolve the extent of gene flow between these two groups and whether they represent cryptic species or simply divergent populations of A. dorsata.
Worker bees from the Andaman islands are generally smaller in size than mainland Asia A. dorsata. For example, wing length and width, two traits that are proxies for overall body size, are notably smaller (mean and standard deviation: forewing length: mainland Asia = 13.11 ± 0.098 mm; Andaman Island = 12.08 ± 0.073 mm; forewing width: mainland Asia = 4.39 ± 0.098 mm; Andaman Island = 4.07 ± 0.047 mm). Worker bees from Andaman Island also have a significantly (p < 0.0001) smaller median ocellus (Figure 3A). However, the absence of non-size-related traits that differentiate these populations along with a lack of distinctive genetic differences (Bhatta et al., 2024) suggest that the giant honey bees of the Andaman Islands are in the early stages of divergence from the population on the mainland. During the Last Glacial Maximum, 26.5–19.0 ka, the sea level was 120–130 m lower than it is today (Clark et al., 2009). At that time, the water gap between the Andaman Islands and mainland Asia was only ~40–50 km wide, as inferred from the estimated shorelines when sea levels were lower (Voris, 2000; Ali, 2018). Swarms of bees may have flown between these land masses, allowing for gene exchange that would have slowed divergence. More detailed examination may lead to the discovery of morphological autapomorphies that distinguish these lineages.
We are the first to explore in detail the morphology of giant honey bees of the Philippines and Sulawesi, Indonesia. Maa (1953) indicated the giant honey bee of the Philippines, A. breviligula—the “short-tongued honeybee”—is a species distinct from binghami of Sulawesi from which it “can be readily distinguished by its uniquely short glossa, longer malar areas, less strongly raised ocellar triangle and much shorter but broader abdominal sternum 5” (Maa, 1953, p. 564). In contrast to that statement, our measurements of tongue lengths did not differ significantly between these two geographic forms (Figure 4A). Additional research on tongue lengths of specimens stored in identical conditions is recommended. The distributions of malar width/malar length of the two forms, although differing significantly, have overlapping distributions (Figure 3D). Differences in the ocellar region and proportions of sternum 5 between binghami and breviligula are relatively minor and not diagnostic. These differences are similar in degree to differences between subspecies of A. mellifera and A. cerana. Therefore, on the basis of morphological characteristics, we consider breviligula and binghami to be conspecific. As such, according to the rules of priority in taxonomic nomenclature, the giant honey bees of the Philippines would be recognized as A. binghami breviligula due to the earlier description of A. binghami from Sulawesi.
Our morphological examination of Megapis bees firmly supports three species of giant honey bees: A. laboriosa, A. dorsata, and A. binghami. In contrast, phylogenic analyses of DNA sequences provide evidence for four or five species depending on the sequences examined: A. laboriosa, A. dorsata, A. binghami, A. breviligula, and the South Indian form (Arias and Sheppard, 2005; Raffiudin and Crozier, 2007; Lo et al., 2010; Smith, 2021; Bhatta et al., 2024). A comprehensive genetic analysis (e.g., based on ultra-conserved elements or whole genome sequencing) coupled with further morphological comparisons will help to further resolve the phylogeny and taxonomy of Megapis.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
NK: Data curation, Formal analysis, Writing – original draft, Methodology, Visualization, Writing – review & editing. AB: Conceptualization, Funding acquisition, Investigation, Supervision, Writing – review & editing. GO: Conceptualization, Investigation, Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. NK was supported by a National Fellowship for Higher Education of ST Students (NFST), UGC, Government of India, and the Ministry of Tribal Affairs, Government of India, during the project (Award No.: 201920-NFST-ARU-02200). AB supported some of the fieldwork and provided the facilities (museum and imaging) charges from his NCBS-TIFR institutional funds (No. 12P4167) and the Department of Atomic Energy, Government of India (No. 12-R&D-TFR-5.04–0800 and 12-R&D-TFR-5.04–0900).
Acknowledgments
The authors are thankful to the Research and Collection Facility, Imaging Facility of NCBS-TIFR, Bangalore, for the space and support to carry out the work. The authors also acknowledge various people who helped in the collection or contributed specimens used in the study: Bharath Kumar (BK), Benjamin Rutschmann (BR), Chet Prasad Bhatta (CPB), Deborah R Smith (DRS), Geetha GT (GGT), Jaya Narah (JN), Joby Joseph (JJ), Karsing Megu (KM), Krishnaswamy A (KA), Le Quang Trung (LQT), Natapot Warrit (NW), Pham Hong Thai (PHT), Prabhudev MV (PMV), Prateek Sahu (PS), Rajath S (RS), Savita Chib (SC), Sivaraju C (SC), Stefan Reyes (SR), Tarun Karmakar (TK), Thomas Schmitt (TS), Viney Hedge (VH), Yong-Chao Su (YCS), and Yeshwant HM (YHM). Comments from two reviewers improved the quality of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frbee.2024.1379952/full#supplementary-material
Supplementary File 1 | (in excel spreadsheet) Details of specimens examined: collection locality, date, preservation method, collector, and voucher codes.
References
Alexander B. A. (1991). Phylogenetic analysis of the genus Apis (Hymenoptera: Apidae). Ann. Entomol. Soc Am. 84, 137–149. doi: 10.1093/aesa/84.2.137
Ali J. R. (2018). Islands as biological substrates: continental. J. Biogeogr. 45, 1003–1018. doi: 10.1111/jbi.13186
Arias M. C., Sheppard W. S. (2005). Phylogenetic relationships of honey bees (Hymenoptera: Apinae: Apini) inferred from nuclear and mitochondrial DNA sequence data. Mol. Phylogenet. Evol. 37, 25–35. doi: 10.1016/j.ympev.2005.02.017
Ashmead W. H. (1904). Remarks on honey bees. Proc. Entomol. Soc Wash. 6, 120–122. Available at: https://repository.si.edu/handle/10088/68135.
Baldensperger P. J. (1928). Bees in their natural state and with beekeepers. Bee World. 9, 173–174. doi: 10.1080/0005772X.1928.11096277
Bhatta C. P, Zajonz S. C., Smith D. R. (2024). Phylogeography of the giant honey bees based on mitochondrial gene sequences. Front. Bee Sci. 2, 1401851. doi: 10.3389/frbee.2024.1379952
Cao L., Zheng H., Hu C., He S., Kuang H., Hu F. (2012). Phylogeography of Apis dorsata (Hymenoptera: Apidae) from China and neighboring Asian areas. Ann. Entomol. Soc Am. 105, 298–304. doi: 10.1603/AN11104
Clark P. U., Dyke A. S., Shakun J. D., Carlson A. E., Clark J., Wohlfarth B., et al. (2009). The last glacial maximum. Science 325, 710–714. doi: 10.1126/science.1172873
Cockerell T. D. A. (1906). New Rocky Mountain bees, and other notes. Can. Entomol. 38, 160–166. doi: 10.4039/Ent38160-5
Cockerell T. D. A. (1914). Description and records of bees, LX. Ann. Mag. Nat. Hist. 14, 1–13. Available at: https://digitalcommons.usu.edu/bee_lab_co/522/.
Engel M. S. (1999). The taxonomy of fossil and recent honey bees (Hymenoptera: Apidae; Apis). J. Hymenopt. Res. 8, 165–196. Available at: http://hdl.handle.net/1808/16476.
Fabricius J. C. (1793). Entomologia Systematica Emendata et Aucta. Secundum Classes, Ordines, Genera, Species adiectis Synonymis, Locis, Observationibus, Descriptionibus Vol. 2 (Hafniae, Denmark: Proft). Available at: https://www.biodiversitylibrary.org/bibliography/125869.
iNaturalist (2024a) Apis dorsata. Available online at: https://www.inaturalist.org/taxa/245955-Apis-dorsata (Accessed 22 May, 2024).
iNaturalist (2024b) Apis laboriosa. Available online at: https://www.inaturalist.org/taxa/574869-Apis-laboriosa (Accessed 22 May, 2024).
iNaturalist (2024c) Apis binghami. Available online at: https://www.inaturalist.org/taxa/574879-Apis-dorsata-binghami (Accessed 22 May, 2024).
iNaturalist (2024d) Apis breviligula. Available online at: https://www.inaturalist.org/taxa/545800-Apis-dorsata-breviligula (Accessed 22 May, 2024).
Kitnya N., Otis G. W., Chakravorty J., Smith D. R., Brockmann A. (2022). Apis laboriosa confirmed by morphometric and genetic analyses of giant honey bees (Hymenoptera, Apidae) from sites of sympatry in Arunachal Pradesh, North East India. Apidologie 53, 47. doi: 10.1007/s13592-022-00956-z
Kitnya N., Prabhudev M. V., Bhatta C. P., Pham T. H., Nidup T., Megu K., et al. (2020). Geographical distribution of the giant honey bee Apis laboriosa Smith 1871 (Hymenoptera, Apidae, Apis). ZooKeys 951, 67–81. doi: 10.3897/zookeys.951.49855
Klug J. C. F. (1807). Species Apiariarum familie novas descripsit; generumque characters adjecit. Sitzungsberichte der Gesellschaft Naturforschender Freunde zu Berlin 1, 263–265.
Latreille P. A. (1804). Notice des Espèces d’Abeilles vivant en grande Société, ou Abeillies proprement dites, et Description d’Espèces Nouvelles. Ann. Mus. Hist. Nat. Paris 5, 161–178. Available at: https://www.biodiversitylibrary.org/item/23266#page/195/mode/1up.
Lo N., Gloag R. S., Anderson D. L., Oldroyd B. P. (2010). A molecular phylogeny of the genus Apis suggests that the giant honey bee of the Philippines, A. breviligula Maa, and the plains honey bee of southern India, A. indica Fabricius, are valid species. Syst. Entomol. 35, 226–233. doi: 10.1111/j.1365-3113.2009.00504.x
Maa T. C. (1944). On the classification and phylogeny of the Chinese honeybees. Entomology Shaowuana 1, 4–5.
Maa T. C. (1953). An inquiry into the systematics of the tribus Apidini or honeybees (Hym.). Treubia 21, 525–640. Available at: https://archive.org/details/treubia-v21i3-2669.
Michener C. D. (1944). Comparative external morphology, phylogeny, and a classification of the bees (Hymenoptera). Bull. Am. Mus. Nat. Hist. 82, 151–326. Available at: http://hdl.handle.net/2246/1272.
Michener C. D. (2007). The bees of the World. 2nd Edition (Baltimore: Johns Hopkins University Press). doi: 10.56021/9780801885730
Moore F., Walker F., Smith F. (1871). Descriptions of some new insects collected by Dr. Anderson during the expedition to Yunnan. Proc. Zool. Soc Lond. 1871, 244–249. Available at: https://www.biodiversitylibrary.org/item/90542#page/16/mode/1up.
Otis G. W., Huang M. -J., Kitnya N., Sheikh U. A. A., Faiz A. H., Phung C. H., et al. (2024). The distribution of Apis laboriosa revisited: range extensions, biogeographic affinities, and species distribution modeling. Front. Bee Sci. 2, 1374852. doi: 10.3389/frbee.2004.1374852
Raffiudin R., Crozier R. H. (2007). Phylogenetic analysis of honey bee behavioral evolution. Mol. Phylogenet. Evol. 43, 543–552. doi: 10.1016/j.ympev.2006.10.013
Ruttner F. (1988). Biogeography and taxonomy of honey bees (Berlin, Germany: Springer). doi: 10.1007/978-3-642-72649-1
Sakagami S. F., Matsumura T., Ito K. (1980). Apis laboriosa in Himalaya, the little known world largest honeybee (Hymenoptera: Apidae). Insecta Matsumurana 19, 47–77. Available at: https://eprints.lib.hokudai.ac.jp/dspace/bitstream/2115/9801/1/19_p47-77.pdf.
Smith F. (1858). Catalogue of hymenopterous insects collected at Sarawak, Borneo; Mount Ophir, Malacca; and at Singapore, by Mr. A. R. Wallace. Proc. Linn. Soc. Lond. 2, 42–130. Available at: https://archive.org/details/ants_02588/2588/page/n1/mode/2up.
Smith F. (1859). Catalogue of hymenopterous insects collected at Celebes by Mr. A. R. Wallace. Proc. Linn. Soc Lond. 3, 4–27. doi: 10.1111/j.1096-3642.1858.tb02506.x
Smith F. (1871). A catalogue of the aculeate Hymenoptera and Ichneumonidae of India and the eastern Archipelago. J. Linn. Soc. Lond. 11, 285–415. doi: 10.1111/j.1096-3642.1871.tb02225.x
Smith D. R. (1991). “Mitochondrial DNA and honey bee biogeography,” in Diversity of the genus Apis. Ed. Smith D. R. (Westview Press, Boulder, USA), 131–176.
Smith D. R. (2021). “Biogeography of honey bees,” in Encyclopedia of social insects. Ed. Starr C. K. (Springer, Cham, Switzerland). doi: 10.1007/978–3-319–90306-4_60–1
Trung L. Q., Dung P. X., Ngan T. X. (1996). A scientific note on the first report of Apis laboriosa F. Smith 1871 in Vietnam. Apidologie 27, 487–488. doi: 10.1051/apido:19960608
Voris H. K. (2000). Maps of Pleistocene sea levels in Southeast Asia: shorelines, river systems and time durations. J. Biogeogr. 27, 1153–1167. doi: 10.1046/j.1365-2699.2000.00489.x
Wang A. R., Kim J. S., Kim J. M., Kim H., Choi Y. S., Kim I. (2018). Comparative description of mitochondrial genomes of the honey bee Apis (Hymenoptera: Apidae): four new genome sequences and Apis phylogeny using whole genomes and individual genes. J. Apic. Res. 4, 484–503. doi: 10.1080/00218839.2018.1494885
Wickham H. (2016). ggplot2: Elegant graphics for data analysis, 2nd ed. Cham, Switzerland: Springer Nature. doi: 10.1007/978-3-319-24277-4
Wickham H. (2021). tidyr: Tidy messy data. R package version 1.1.3. https://CRAN.R-project.org/package=tidyr.
Keywords: Megapis, morphology, taxomonic key, distribution, species, worker bee, drone bee
Citation: Kitnya N, Brockmann A and Otis GW (2024) Taxonomic revision and identification keys for the giant honey bees. Front. Bee Sci. 2:1379952. doi: 10.3389/frbee.2024.1379952
Received: 31 January 2024; Accepted: 06 June 2024;
Published: 26 July 2024.
Edited by:
Xesús Feás, Academy of Veterinary Sciences of Galicia, SpainReviewed by:
Petar Hristov, Bulgarian Academy of Sciences, BulgariaChristian W. W. Pirk, University of Pretoria, South Africa
Copyright © 2024 Kitnya, Brockmann and Otis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nyaton Kitnya, TnlhdG9ua2l0bnlhQGdtYWlsLmNvbQ==
†Present address: Nyaton Kitnya, Trivedi School of Biosciences, Ashoka University, Sonipat, Haryana, India
 Nyaton Kitnya
Nyaton Kitnya Axel Brockmann
Axel Brockmann Gard W. Otis
Gard W. Otis