- 1Department of Medicine, University of Connecticut Health Center (UCONN Health), Farmington, CT, United States
- 2Mamome Inc., Brooklyn, NY, United States
Gestational diabetes mellitus (GDM) represents a significant health concern during pregnancy, impacting both maternal and fetal well-being. While conventional diagnostic protocols typically rely on blood glucose levels in the latter stages of pregnancy, there is a pressing need for early detection methods to mitigate potential risks. A plethora of glucose-based or non-glucose-based biomarkers have been investigated for their potential to predict GDM in early pregnancy. Though specific biomarkers showed promise in predicting GDM, their clinical usage has been constrained by the lack of validation and limitation in translating them into routine clinical use. This review aims to highlight and discuss the potential and practical utility of existing biomarkers and emergent biomarkers, such as microbiomes, in diagnosing GDM. A comprehensive analysis of recent studies reveals significant alterations in the composition and diversity of microbiota among women with GDM, suggesting their potential utility as predictive markers for this condition. For instance, distinct microbial profiles characterized by an increased abundance of Eisenbergiella, Tyzzerella 4, and Lachnospiraceae NK4A136, alongside decreased levels of Parabacteroides, Parasutterella, and Ruminococcaceae UCG 002, correlated with fasting blood glucose levels, hinting at their relevance in early GDM detection. Furthermore, proposed microbiota-targeted panels demonstrated promising predictive accuracy. Beyond gut microbiota, recent investigations have also explored the potential of oral microbiota as predictive biomarkers for GDM. Studies have highlighted the discriminatory capacity of specific oral microbes, such as Streptococcus in saliva and Leptotrichia in dental plaque, in distinguishing GDM from healthy pregnancies. Moreover, the examination of gut microbiota-derived metabolites has shown promising results in serum-based GDM prediction. These findings collectively underscore the potential of microbiota and its metabolites as valuable biomarkers for the early detection of GDM. However, further research is warranted to elucidate the mechanistic links between microbial dysbiosis and GDM pathogenesis, ultimately facilitating the development of targeted therapeutic interventions and personalized management strategies.
1 Introduction
Gestational Diabetes Mellitus (GDM) is characterized by varying degrees of severe glucose intolerance with onset or first seen during pregnancy (American Diabetes Association, 2020b). GDM often develops between the 24th and 28th week of gestation. In middle and later pregnancy, GDM is one of the most prevalent maternal complications (Guariguata et al., 2014; Simjak et al., 2018; Kim et al., 2021). GDM is estimated to affect 10%–25% of all pregnancies globally, and its prevalence depends on diagnostic guidelines (Lauring et al., 2018), screening procedures (Nguyen et al., 2018), and obesity (Huvinen et al., 2018). The prevalence of GDM has risen in recent decades in parallel with Westernized living and conceiving at an older age, accompanied by an economic boom (Simjak et al., 2018). Specific racial and ethnic groups, including Hispanics, Asians, and Black women, reported higher odds of developing GDM than non-Hispanic Whites (CDC, 2020; Gardner et al., 2022). GDM is typically asymptomatic but is associated with adverse maternal outcomes like preeclampsia (Yogev et al., 2010), postpartum hemorrhage, and increased cesarean deliveries (O’Sullivan et al., 2011). It also leads to undesirable infant outcomes such as macrosomia, neonatal hypoglycemia (HAPO Study Cooperative Research Group et al., 2008), perinatal mortality (Wendland et al., 2012), and large gestational age (Saravanan et al., 2020). GDM can lead to long-term consequences in mothers, such as an increased risk of type 2 diabetes (Hakkarainen et al., 2016), metabolic syndrome (Hakkarainen et al., 2018), and cardiovascular diseases (Fraser et al., 2012). Additionally, there is a significant risk of recurrent GDM in subsequent pregnancies, with estimates ranging from 29–80% (Schwartz et al., 2016). The children of mothers with GDM also face long-term health risks, including a higher incidence of obesity, type 2 diabetes, and neurodevelopmental disorders (Farahvar et al., 2019). These consequences highlight the importance of early detection and proper management of GDM to minimize health impacts on both mother and child.
Various countries use different diagnostic approaches to assess the prevalence of GDM due to the lack of uniform criteria for its diagnosis. Currently, most GDM diagnoses are made in the later stages of the second trimester (Rani and Begum, 2016), putting the vulnerable fetus at risk for intrauterine metabolic abnormalities and epigenetic modifications (Elliott et al., 2019; Chu and Godfrey, 2020). Hence, early prediction and diagnosis of GDM, preferably prior to the onset of elevated blood sugar levels, including during pre-pregnancy, are crucial. This timely identification would facilitate proactive measures to mitigate complications and health hazards linked with GDM. The identification of GDM biomarkers during early pregnancy and even pre-conception could enhance existing clinical risk assessments, pinpointing high-risk individuals who could benefit from tailored strategies to mitigate GDM development. Advancements in metagenomics have allowed the study of the human microbiota, providing valuable insights into its diagnostic and therapeutic potential (Martin et al., 2014; Malla et al., 2018). Quantitative analysis of biological samples using next-generation sequencing methods has yielded extensive data on the role of microbiota as a diagnostic tool across various diseases (Qin et al., 2012; Yu et al., 2017). Exploring microbiota as a novel biomarker holds promise for the future diagnosis of GDM. This review aims to discuss and evaluate existing screening and diagnostic strategies for GDM. Additionally, we provide insights into emerging biomarkers, with a particular focus on microbiota, that exhibit potential for predicting GDM.
1.1 Screening strategies of GDM
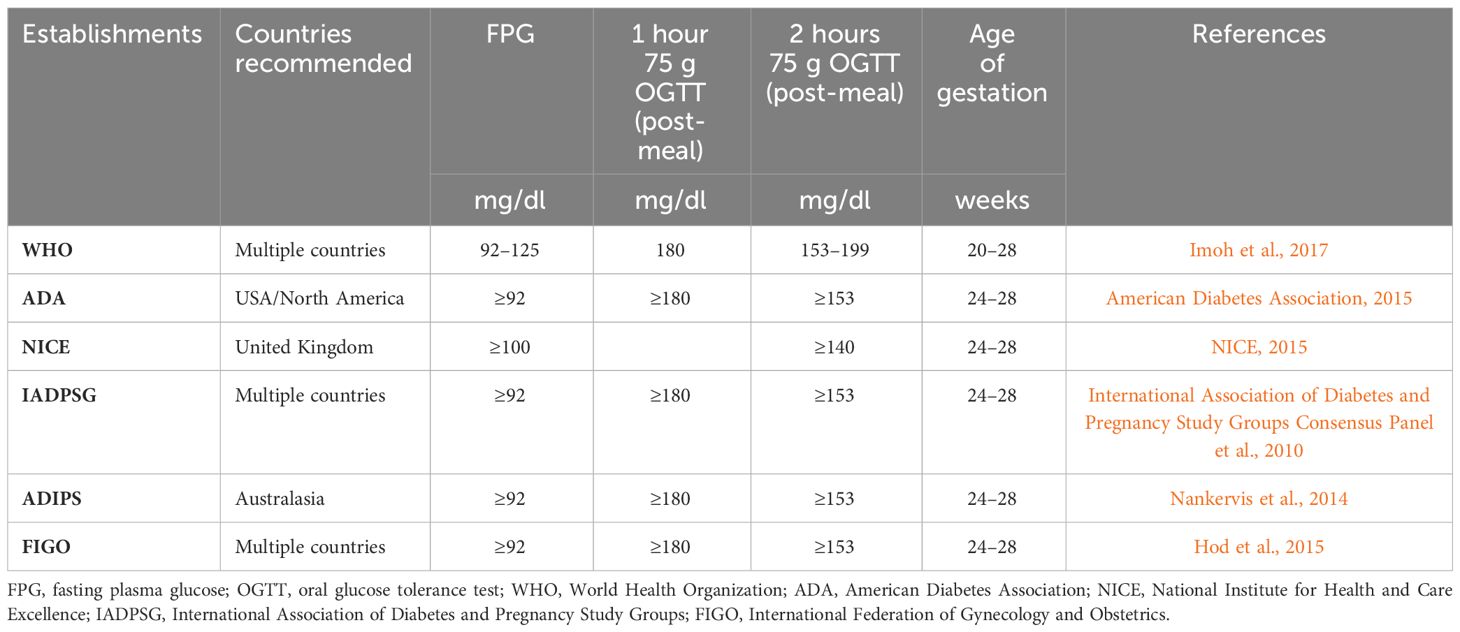
The detection of GDM involves a screening process followed by necessary diagnostic measures, with methods and diagnostic thresholds varying among countries (International Diabetes Federation, 2019; American Diabetes Association, 2020a). Three main approaches to GDM screening exist: universal, two-step, and selective. Universal screening involves testing all pregnant women, regardless of symptoms or glycemic status, with a non-fasting 50 g glucose challenge test (GCT) to assess the likelihood of GDM (Tieu et al.). Two-step screening includes the 50 g GCT followed by a 3-hour 100g oral glucose tolerance test (OGTT) for those exceeding the GCT threshold (Vandorsten et al., 2013; Cundy et al., 2014). Selective screening examines women with specific risk factors, such as high body mass index (BMI), family history of GDM, smoking history, and age at pregnancy (Tieu et al.). In 2010, the International Association of Diabetes and Pregnancy Study Groups (IADPSG) proposed a one-step approach using a 2-hour, 75 g OGTT for all pregnant women at 24–28 weeks (International Association of Diabetes and Pregnancy Study Groups Consensus Panel et al., 2010). This approach has gained acceptance by organizations like the World Health Organization (WHO), the American Diabetes Association (ADA), the International Federation of Gynecology and Obstetrics (FIGO), and the Australasian Diabetes in Pregnancy Society (ADIPS). However, not all major health organizations worldwide, including the American College of Obstetricians and Gynecologists (ACOG) and the National Institute for Health and Care Excellence (NICE), have endorsed the IADPSG screening and diagnostic approach. Due to the array of recommendations available, there is a lack of consensus on the screening and diagnosis of GDM. The decision of to screen for GDM or not, as well as the choice of screening approach, continues to be a subject of controversy.
2 Biomarkers for GDM screening
Multiple laboratory assays have been utilized for screening GDM, examining both direct and indirect hyperglycemic markers. Direct hyperglycemic biomarkers include fasting plasma glucose (FPG), GCT, or OGTT. In addition, indirect hyperglycemic biomarkers include glycosylated hemoglobin A1c (HbA1c) and more current biomarkers, many of which have been explored through proteomic or metabolomic studies. Nonetheless, the optimal approach for GDM detection has not been definitively established.
2.1 Glucose markers
Glucose serves as a critical biomarker in the screening, diagnosing, monitoring, and management of GDM. Over the past decade, several guidelines have been proposed or updated to improve diagnostic accuracy and global consistency. WHO guidelines emphasized predictive accuracy rather than endorsing a specific gold standard. Typically, GDM diagnosis is confirmed through a 75 g or 100 g OGTT, with most guidelines recommending testing between 24 and 28 weeks of gestation (International Association of Diabetes and Pregnancy Study Groups Consensus Panel et al., 2010; Nankervis et al., 2014; American Diabetes Association, 2015; Imoh et al., 2017) (Table 1). However, the exact association between various OGTT parameters and pregnancy outcomes is still unknown (Feng et al., 2017) and may vary among populations (Farrar et al., 2016). Furthermore, the OGTT has limitations such as poor reproducibility (Davidson, 2002; Munang et al., 2017), dependence on ethnicity (Bonongwe et al., 2015), and time-intensive nature, as it necessitates fasting and a commitment of 2 hours to complete the test (Buckley et al., 2012). Moreover, the OGTT does not account for maternal BMI when determining the glucose load to administer (Bonongwe et al., 2015). Side effects such as vomiting, nausea, and diarrhea can also occur following OGTT administration (ACOG Practice Bulletin No. 180, 2017).
Compared to GCT and OGTT, FPG testing was reported to be more patient-friendly, rapid, economical, and reproducible (Agarwal and Dhatt, 2007). Several countries have supported using an FPG concentration of 92 mg/dL as a cut-off for increased blood sugar during the first trimester (International Association of Diabetes and Pregnancy Study Groups Consensus Panel et al., 2010; American Diabetes Association, 2015; Imoh et al., 2017). ADA and IADPSG recommended an FPG concentration ≥ 92 mg/dl, while NICE and WHO suggested an FPG concentration ≥ 100 mg/dl and 92–125 mg/dl, respectively, for diagnosing GDM (International Association of Diabetes and Pregnancy Study Groups Consensus Panel et al., 2010; NICE, 2015; American Diabetes Association, 2015; Imoh et al., 2017). However, researchers have highlighted the need for a precise cut-off of FPG for the first trimester (Sacks et al., 2002) as the current cut-offs are not relevant at earlier gestational stages (Sacks et al., 2018). In addition, the sensitivity and specificity of FPG for screening GDM showed significant regional variations (Agarwal et al., 2011; Zhu et al., 2013a; Trujillo et al., 2014), and its low specificity makes it unsuitable as a standalone screening test for early pregnancy (Riskin-Mashiah et al., 2010; Zhu et al., 2013b).
In addition to OGTT and FPG, HbA1c is also an acceptable method for early pregnancy diabetes screening, as recommended by the ADA and IADPSG (International Association of Diabetes and Pregnancy Study Groups Consensus Panel et al., 2010; American Diabetes Association, 2016). However, the American College of Obstetricians and Gynecologists disapproves of HbA1c as a standalone screening test due to its low sensitivity in identifying early hyperglycemia (ACOG Practice Bulletin No. 190, 2018). While HbA1c is considered precise and accurate in non-pregnant women (Jia, 2016), studies have shown that it is subpar as a screening test for GDM (O’Connor et al., 2012). Furthermore, the diagnostic potential of HbA1c in early pregnancy and its interpretation are still uncertain, as pregnancy-specific thresholds for identifying early GDM have not been established (Göbl et al., 2014; Shinar and Berger, 2018). Hence, the validity of HbA1c during pregnancy as a diagnostic tool for early GDM remains debatable.
2.2 Non-glucose biomarkers
Despite advancements in healthcare and updated screening approaches, the diagnosis of GDM is still arguable. Challenges such as limited sensitivity, specificity, invasiveness, racial/ethnic variations, and testing costs have hindered the prediction of GDM. To address these challenges, numerous studies have investigated non-glucose biomarkers as potential diagnostic tools for GDM. These biomarkers include inflammatory markers like C-reactive protein (CRP), tumor necrosis factor-alpha (TNF-α), and interleukin-6 (IL-6), as well as adipocyte-derived markers such as adiponectin, leptin, and visfatin. Additionally, other biomarkers such as pregnancy-associated plasma protein A (PAPP-A), placental growth factor (PLGF), insulin-resistant markers, urinary markers like alanine, L-tryptophan, and serotonin, genetic markers, growth factors, glycoproteins, hormones, microRNAs, single nucleotide polymorphisms (SNPs), and DNA methylation have been explored.
However, the clinical utility of these biomarkers is still under investigation. Inflammatory biomarkers, such as CRP, are affected by factors such as limited clinical data and variations in ethnicity and immunological assays (Amirian et al., 2020). Adiponectin has shown a moderate correlation with predicting GDM, while visfatin and leptin levels have produced inconsistent results (Iliodromiti et al., 2016). Low levels of PAPP-A have been suggested as a link to GDM and insulin resistance (Ramezani et al., 2020), although conflicting findings exist (Savvidou et al., 2012; Syngelaki et al., 2015). PLGF, despite some studies indicating its potential as predicting GDM during the first trimester of pregnancy (Eleftheriades et al., 2014), lacks consistent evidence to validate its use as a dependable biomarker (Mosimann et al., 2016; Maymon et al., 2019). Reduced levels of sex hormone-binding globulin have shown associations with obesity and insulin resistance, hinting at its potential as a GDM biomarker in the first trimester (Zhang T. et al., 2018); however, its diagnostic significance is influenced by factors such as BMI, ethnic disparities, and family history (Corcoran et al., 2018).
Furthermore, urinary markers like 3-hydroxybutanoic acid, and specific amino acids, such as serotonin, valine amino acid, and L-tryptophan, have been found to increase in GDM cases during the 12th and 26th weeks of gestation (Leitner et al., 2017). Yet, further evaluation is warranted to determine their precise predictive and clinical significance. Genetic markers such as microRNAs, SNPs, and DNA methylation have also shown promise (Yahaya et al., 2020); however, contradictory reports (Jamalpour et al., 2018; Tagoma et al., 2018) highlight the need for further studies to determine their clinical usefulness. It is also important to note that most biomarkers have been evaluated in limited case-control studies without additional prospective confirmation, emphasizing the need for more extensive research.
2.3 Microbiota in GDM
Recent research indicates that the gut microbiota plays a significant role in the etiology of metabolic illnesses, such as diabetes (Brunkwall and Orho-Melander, 2017), cardiovascular disease (Tang et al., 2017), and obesity (DiBaise et al., 2008). Microbiota often plays a pivotal role in developing and shaping the immune and endocrine systems (Mohajeri et al., 2018; Rastelli et al., 2019). Advancements in metagenomics have provided a deeper understanding of the human microbiome, which comprises bacteria, archaea, and eukaryotes (Marchesi, 2010; Rajilić-Stojanović and De Vos, 2014). Alterations in microbiota composition have been observed in women with GDM throughout pregnancy (Liu et al., 2017) (Table 2). Specifically, the gut microbiota exhibits increased α diversity and reduced β diversity in the first trimester, while this pattern reverses in the third trimester, characterized by reduced α diversity and increased β diversity. The α diversity measures species richness and evenness within a single sample, β diversity quantifies differences in species composition between different samples or communities (Ionescu et al., 2022; Tang et al., 2022). These measures help in identifying potential alterations in microbial communities associated with GDM.
The dysbiosis or imbalance in gut microbiota diversity starts early in pregnancy, with decreased diversity observed in women with GDM. Reduced gut microbiota abundance in GDM has been associated with insulin resistance and inflammation (Le Chatelier et al., 2013). Numerous investigations have provided insights into the significant alterations in microbiome diversity in women with GDM. For example, Kuang et al. (2017) observed a higher abundance of Parabacteroides distasonis and Klebsiella variicola in GDM. They also reported a strong correlation between blood glucose tolerance levels and the ratio of overall abundances of GDM-enriched to control-enriched metagenome linkage groups. These findings suggest that the alteration of the microbiome may be directly linked to the pathogenesis of GDM.
Furthermore, Crusell et al. (2018) reported a higher abundance of phylum Actinobacteria and specific genera such as Collinsella, Rothia, and Desulfovibrio in the GDM women during the third trimester. These women also exhibited increased enrichment in Faecalibacterium and Anaerotruncus species, while species annotated to Clostridium sensu stricto and Veillonella showed reduced enrichment. Certain operational taxonomic units (OTUs), like Christensenella, have been linked to higher glucose levels (Crusell et al., 2018), whereas Akkermansia has demonstrated potential in enhancing insulin sensitivity (Crusell et al., 2018) and regulating metabolic syndromes (Christiansen, 2013; Dao et al., 2016). Remarkably, GDM women maintained these altered microbiota months after postpartum (Crusell et al., 2018), indicating a potential long-term impact on metabolic health. Additional studies have shown an increased abundance of Collinsella and Blautia and decreased abundance of Sutterella in GDM women (Crusell et al., 2018; Cortez et al., 2019; Li G. et al., 2021). Moreover, the ratio of Firmicutes/Bacteroidetes was reported to be higher in GDM women (Li G. et al., 2021). These findings underscore the potential impact of gut microbiota on GDM pathogenesis and highlight the need for further investigation in this field. Furthermore, Cortez et al. (2019) revealed an increased abundance of Ruminococcus, Eubacterium, and Prevotella genera, along with a reduced abundance of Akkermansia, Bacteroides, Parabacteroides, Roseburia, and Dialister in the third trimester of GDM women. Wei et al. (2022) found an increased abundance of Ruminococcus bromii, Clostridium colinum, and Streptococcus infantis in GDM women at 24–28 weeks of gestational age. Notably, S.infantis was associated with a higher risk of GDM (Wei et al., 2022). Moreover, GDM women exhibited a reduced abundance of genera such as Megasphaera, Barnesiella, and Blautia in both the first and third trimesters. Conversely, the genera Acidaminococcus, Clostridium, and Allisonella showed higher abundance in GDM women during these trimesters (Abdullah et al., 2022). Ye et al. (2023) demonstrated that microbiota and its short-chain acids play a role in the development of GDM, with reduced abundance of several genera (Faecalibacterium, Prevotella, and Streptococcus) and species (Bacteroides coprophilus, Eubacterium siraeum, Faecalibacterium prausnitzii, Prevotella copri, and Prevotella stercorea) observed in GDM women. Furthermore, Xu et al. (2020) found that during the third trimester of pregnancy, the gut microbiome of GDM women had higher β-diversity and an increased abundance of Gammaproteobacteria and Haemophilus. Additionally, they observed reduced α-diversity, elevated levels of Selenomonas and Bifidobacterium, and decreased levels of Fusobacteria and Leptotrichia. They further identified a significant correlation between maternal blood sugar levels and the oral microbiome, particularly in relation to Streptococcus, Leptotrichia, and Veillonella, suggesting a potential link between the oral microbiome and glucose tolerance in later stages of pregnancy (Xu et al., 2020).
Wang et al. (2018) reported significant oral microbiome alteration in GDM, with increased Proteobacteria but reduced Firmicutes and Leptotrichia (Wang et al., 2018). Moreover, Hu et al. (2021) noted a higher prevalence of Enterobacteriaceae, Ruminococcaceae spp., and Veillonellaceae spp. very early in pregnancy (at 6–15 weeks of gestation) of GDM women. Majority of the research findings highlight an increased abundance of Proteobacteria, Neisseria (Wang et al., 2018; Li X. et al., 2021), Prevotella (Ganiger et al., 2019; Crusell et al., 2020), and Capnocytophaga (Yao et al., 2019; Li X. et al., 2021) in GDM. In contrast, a reduced abundance of Streptococcus (Yao et al., 2019; Crusell et al., 2020; Li X. et al., 2021), Firmicutes and Leptotricia (Wang et al., 2018; Li X. et al., 2021) is consistently observed across multiple studies investigating GDM women. Inconsistent findings were also noted in the study conducted by Xu et al. (2020) where they reported a reduced abundance of Neisseria in GDM. GDM is often associated with adverse perinatal outcomes as well as dysbiosis in vaginal flora (Hirji et al., 2012; Goncalves et al., 2016). It is widely accepted that pregnant women with GDM have an increased susceptibility to Candida colonization in the vagina (Guggenheimer et al., 2000; Hirji et al., 2012). Zhang X. et al. (2018) identified Lactobacillus listeri, Lactobacillus amylovorus, and Lactobacillus fructivorans as abundant in GDM but absent in healthy pregnant women. They further reported Lactobacillus acidophilus being the most abundant among Lactobacillus, followed by Lactobacillus crispatus and Lactobacillus inersclone in GDM women compared to healthy pregnant women (Zhang X. et al., 2018). Nevertheless, regardless of whether women had GDM or were healthy during pregnancy, they did not notice significant differences in the abundance and diversity of vaginal flora between the 28–30 weeks and 37–40 weeks gestation periods. Notably, Lactobacillus emerged as the dominant bacteria during both stages of pregnancy (Zhang X. et al., 2018). Cortez et al. (2019) reported an increased abundance of vaginal microbiomes such as Bacteroides, Veillonella, Klebsiella, Escherichia-Shigella, Enterococcus, and Enterobacter in GDM women (Cortez et al., 2019). The studies highlighted above emphasize the substantial role of microbiota in GDM, showcasing altered diversity and composition that impact metabolic health during pregnancy. These alterations, occurring notably in the first and third trimesters, may influence perinatal outcomes. Further investigation is needed to comprehend these alterations fully, offering insights for diagnostic and therapeutic strategies aimed at managing GDM and enhancing maternal and fetal outcomes. Collectively, these findings contribute to our understanding of GDM pathogenesis, suggesting a potential link between gut, oral, and vaginal microbiota and the development of GDM.
2.4 Microbiota as predictors of GDM
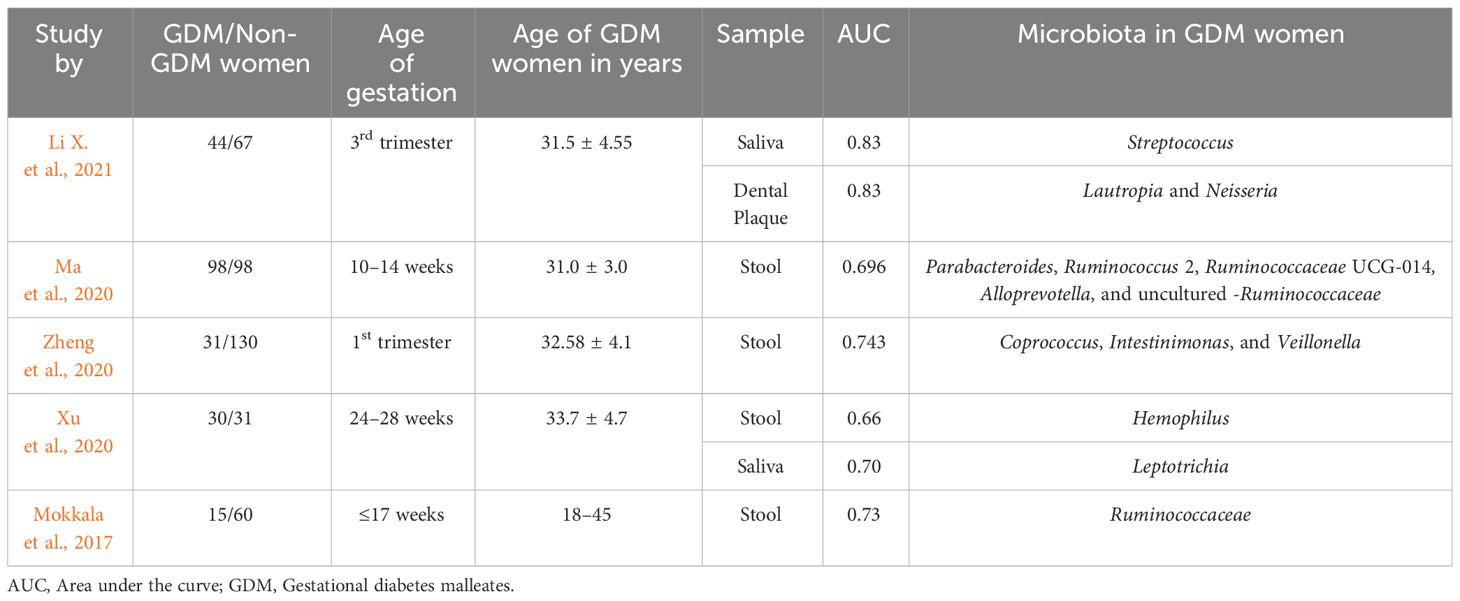
Various studies have revealed significant alterations in the composition and diversity of microbiota, suggesting their promising role as predictors of GDM (Table 3). Ma et al. (2020) observed an increased relative abundance of Eisenbergiella, Tyzzerella 4, and Lachnospiraceae NK4A136 in women with GDM. Conversely, their microbiota was predominantly dominated by Parabacteroides, Megasphaera, and Eubacterium eligens. Furthermore, fasting blood glucose levels were positively correlated with an increased abundance of Eisenbergiella and Tyzzerella 4, while Parabacteroides, Parasutterella, and Ruminococcaceae UCG 002 showed the opposite trend. The authors suggested that dysbiosis in early pregnancy might be associated with the development of GDM. Their study identified five microbiota-targeted panels (Parabacteroides, Ruminococcus 2, Ruminococcaceae UCG-014, Alloprevotella, and uncultured-Ruminococcaceae) that could potentially serve as predictors of GDM, with an area under the curve (AUC) of 0.696 (Ma et al., 2020). Zheng Wei et al. (2020) identified a moderately good performance of a three-bacteria component consisting of Coprococcus, Intestinimonas, and Veillonella in predicting GDM in the first trimester. Additionally, they proposed a potential association between the reduced abundance of Coprococcus (a butyrate producer) and Streptococcus (a lactate producer) and the development of insulin resistance and GDM during both the first and second trimesters. Furthermore, they hypothesized that the progression of GDM might be facilitated by a stable gut microbiota composition or a lack of typical dynamic changes (Zheng et al., 2020). Sililas et al. (2021) found that the Firmicutes/Bacteroidetes ratio serves as a more sensitive marker of severe GDM than a specific microbe. On the other hand, Mokkala et al. (2017) observed that an increased relative abundance of the Ruminococcaceae family correlated with higher odds of a positive GDM diagnosis, along with a significant positive correlation between Ruminococcaceae and OGTT results. They noted that the Ruminococcaceae family exhibited an AUC of 0.73 for predicting GDM, suggesting that alterations in gut microbiota composition may influence the onset of GDM by affecting glucose metabolism through inflammatory mechanisms (Mokkala et al., 2017).
Apart from gut microbiota, recent studies have also implicated oral microbiota as potential predictors of GDM. Li X. et al. (2021) demonstrated that Streptococcus in saliva (AUC=0.83) and Lautropia and Neisseria in dental plaque (AUC=0.83) could efficiently distinguish both GDM and healthy pregnant women, highlighting their potential as predictors of GDM.
Xu Yajuan et al. (2020) reported that oral microbes such as Leptotrichia (AUC=0.70) or gut microbes such as Haemophilus (AUC=0.66) could serve as potential biomarkers to predict GDM status. In a separate investigation conducted by Gao et al. (2022), it was found that gut microbiota-derived metabolites hold promise as potential serum biomarkers for predicting GDM. Valeric acid, in particular, demonstrated the highest predictive potential with an AUC of 0.831 for GDM diagnosis. A combination of metabolites panel (isobutyrate, isovalerate, valerate, caproic acid, GUDCA, THDCA + TUDCA, and LCA-3S) could distinguish GDM patients from healthy women with a highest AUC of 0.890.
These findings collectively highlight the potential of microbiota and its metabolites as non-invasive biomarkers for early GDM diagnosis. Future research is needed with focus on longitudinal studies to elucidate microbiota dynamics during pregnancy. Additionally, mechanistic investigations are crucial to uncover the underlying pathways linking microbiota alterations and GDM development. Validation studies are also necessary to confirm the reliability of microbiota-based biomarkers. Furthermore, integrating these biomarkers with clinical parameters, conducting large-scale clinical trials, and exploring broader clinical applications are essential steps to fully leverage microbiome potential in improving GDM prediction, management, and maternal–child health outcomes.
2.5 Microbiota as a dietary intervention in GDM
Dietary interventions, including probiotics, prebiotics, and synbiotics, present a promising avenue for altering the diversity and richness of the gut microbiota. (Zheng et al., 2020) and potentially reducing the risk of GDM. Microbial metabolites play a significant role in glucose regulation through intestinal gluconeogenesis (De Vadder et al., 2016). In women with GDM who followed dietary recommendations, there was a decrease in the abundance of Bacteroides species along with improved glycemic control (Ferrocino et al., 2018). In a clinical trial, the administration of daily supplements containing Lactobacillus rhamnosus HN001, at a dosage of 6 x 109 colony forming units (CFU), between weeks 14 to 16 of pregnancy has demonstrated correlations with reduced incidence and recurrence rates of GDM. This effect appears to be particularly notable among older pregnant women and those with a history of GDM (Wickens et al., 2017). Additionally, a clinical trial conducted by Kijmanawat et al. (2019) found that GDM women who took probiotics containing Lactobacillus and Bifidobacteria in the second trimester demonstrated advantages for glucose metabolism, fasting plasma glucose, insulin fasting, and insulin resistance. Moreover, the administration of a combination of probiotics, including Lactobacillus acidophilus LA-5, Bifidobacterium BB-12, Streptococcus thermophilus STY-31, and Lactobacillus delbrueckii bulgaricus LBY-27, led to a reduction in fasting blood glucose levels (Dolatkhah et al., 2015).
Furthermore, studies on symbiotic supplements containing various strains of bacteria (such as Lactobacillus acidophilus, Lactobacillus casei, and Bifidobacterium bifidum) along with inulin have demonstrated improvements in very-low-density lipoprotein cholesterol and insulin sensitivity among GDM women (Ahmadi et al., 2016). However, some reports have indicated inconsistency in the efficacy of dietary interventions involving microbiota (Pellonperä et al., 2019; Shahriari et al., 2021). Further research is warranted to fully explore the potential of dietary supplement interventions in modulating gastrointestinal microecology during pregnancy. The field is still in its early stages and requires additional investigation.
3 Conclusion
GDM impacts a significant percentage of women during pregnancy, and the incidence of GDM is anticipated to increase as obesity prevalence rises worldwide. If accurately predicted in the early stages of pregnancy and effective measures implemented, the clinical course of GDM with its accompanying gestational complications and long-term maternal and fetal health risks could be mitigated. Although there have been advancements in the study of accurate biomarkers for diagnosing GDM, none have proven high clinical utility and validity. The microbiome provides an exciting insight into the biology of metabolic disorders, increasing the likelihood of novel biomarkers for clinical application in GDM and their possible use as targeted therapeutics. The processing of enormous metagenomics datasets has the potential to provide more accurate models of GDM, even in the earliest stages of pregnancy. In addition, diagnosing GDM via microbiota assessment for dysbiosis is simple and pragmatic. Obtaining stool and salivary samples from pregnant women is more manageable than blood collection via venipuncture after an oral glucose challenge load. Doctors, nurses, and pregnant women can collect oral and stool samples. In several trials, it is encouraging to see that a small number of bacteria can produce an acceptable level of discrimination, allowing for the development specialized bacterial probes for GDM detection.
Furthermore, well-designed prospective clinical trials are necessary to define and develop the ideal biomarkers. Thanks to these novel biomarkers, our understanding of the pathophysiology and progression of GDM will expand and deepen. The obvious and necessary goal is improved outcomes in maternal–fetal health on a global scale.
Author contributions
SB: Writing – original draft, Writing – review & editing. SM: Writing – review & editing. YZ: Writing – review & editing. NF: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
Authors NF and SM were employed by the company Mamome Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdullah B., Daud S., Aazmi M. S., Idorus M. Y., Mahamooth M. I. J. (2022). Gut microbiota in pregnant Malaysian women: a comparison between trimesters, body mass index and gestational diabetes status. BMC Pregnancy Childbirth 22, 1–15. doi: 10.1186/s12884-022-04472-x
ACOG Practice Bulletin No. 190. (2018). ACOG practice bulletin no. 190: gestational diabetes mellitus. Obstet. Gynecol. 131 (2), e49–e64. doi: 10.1097/AOG.0000000000002501
ACOG Practice Bulletin No. 180. (2017). Practice bulletin no. 180 summary: gestational diabetes mellitus. Obstet. Gynecol. 130 (1), e17–e37. doi: 10.1097/AOG.0000000000002159
Agarwal M. M., Dhatt G. S. (2007). Fasting plasma glucose as a screening test for gestational diabetes mellitus. Arch. Gynecol. Obstet. 275, 81–87. doi: 10.1007/s00404-006-0245-9
Agarwal M. M., Weigl B., Hod M. (2011). Gestational diabetes screening: the low-cost algorithm. Int. J. Gynecol. Obstet. 115, S30–SS3. doi: 10.1016/S0020-7292(11)60009-X
Ahmadi S., Jamilian M., Tajabadi-Ebrahimi M., Jafari P., Asemi Z. (2016). The effects of synbiotic supplementation on markers of insulin metabolism and lipid profiles in gestational diabetes: a randomised, double-blind, placebo-controlled trial. Br. J. Nutr. 116, 1394–1401. doi: 10.1017/S0007114516003457
American Diabetes Association. (2015). (2) Classification and diagnosis of diabetes. Diabetes Care 38 Suppl, S8–S16. doi: 10.2337/dc15-S005
American Diabetes Association. (2020a). 14. Management of diabetes in pregnancy: standards of medical care in diabetes-2020. Diabetes Care 43, S183–SS92. doi: 10.2337/dc20-S014
American Diabetes Association. (2016). 2. Classification and diagnosis of diabetes. Diabetes Care 40, S11–S24. doi: 10.2337/dc17-S005doi: 10.2337/dc17-S005
American Diabetes Association. (2020b). 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes—2020. Diabetes Care 43, S14–S31. doi: 10.2337/dc20-S002
Amirian A., Rahnemaei F. A., Abdi F. (2020). Role of C-reactive Protein (CRP) or high-sensitivity CRP in predicting gestational diabetes Mellitus: Systematic review. Diabetes Metab. Syndrome: Clin. Res. Rev. 14, 229–236. doi: 10.1016/j.dsx.2020.02.004
Bonongwe P., Lindow S. W., Coetzee E. J. (2015). Reproducibility of a 75G oral glucose tolerance test in pregnant women. J. Perinatal Med. 43, 333–338. doi: 10.1515/jpm-2014-0208
Brunkwall L., Orho-Melander M. (2017). The gut microbiome as a target for prevention and treatment of hyperglycaemia in type 2 diabetes: from current human evidence to future possibilities. Diabetologia 60, 943–951. doi: 10.1007/s00125-017-4278-3
Buckley B. S., Harreiter J., Damm P., Corcoy R., Chico A., Simmons D., et al. (2012). Gestational diabetes mellitus in Europe: prevalence, current screening practice and barriers to screening. A review. Diabetic Med. 29, 844–854. doi: 10.1111/j.1464-5491.2011.03541.x
CDC. (2020). National Diabetes Statistics Report, 2020 (Atlanta, GA: Centers for Disease Control and Prevention, US Dept of Health and Human Services).
Christiansen O. B. (2013). Reproductive immunology. Mol. Immunol. 55, 8–15. doi: 10.1016/j.molimm.2012.08.025
Chu A. H. Y., Godfrey K. M. (2020). Gestational diabetes mellitus and developmental programming. Ann. Nutr. Metab. 76 Suppl 3, 4–15. doi: 10.1159/000509902
Corcoran S. M., Achamallah N., Loughlin J. O., Stafford P., Dicker P., Malone F. D., et al. (2018). First trimester serum biomarkers to predict gestational diabetes in a high-risk cohort: Striving for clinically useful thresholds. Eur. J. Obstet. Gynecol. Reprod. Biol. 222, 7–12. doi: 10.1016/j.ejogrb.2017.12.051
Cortez R. V., Taddei C. R., Sparvoli L. G., Ângelo A. G., Padilha M., Mattar R., et al. (2019). Microbiome and its relation to gestational diabetes. Endocrine 64, 254–264. doi: 10.1007/s12020-018-1813-z
Crusell M. K. W., Brink L. R., Nielsen T., Allin K. H., Hansen T., Damm P., et al. (2020). Gestational diabetes and the human salivary microbiota: a longitudinal study during pregnancy and postpartum. BMC Pregnancy Childbirth 20, 69. doi: 10.1186/s12884-020-2764-y
Crusell M. K. W., Hansen T. H., Nielsen T., Allin K. H., Rühlemann M. C., Damm P., et al. (2018). Gestational diabetes is associated with change in the gut microbiota composition in third trimester of pregnancy and postpartum. Microbiome 6, 1–19. doi: 10.1186/s40168-018-0472-x
Cundy T., Ackermann E., Ryan E. A. (2014). Gestational diabetes: new criteria may triple the prevalence but effect on outcomes is unclear. Bmj 348. doi: 10.1136/bmj.g1567
Dao M. C., Everard A., Aron-Wisnewsky J., Sokolovska N., Prifti E., Verger E. O., et al. (2016). Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut 65, 426–436. doi: 10.1136/gutjnl-2014-308778
Davidson M. B. (2002). Counterpoint: the oral glucose tolerance test is superfluous. Diabetes Care 25, 1883–1885. doi: 10.2337/diacare.25.10.1883
De Vadder F., Kovatcheva-Datchary P., Zitoun C., Duchampt A., Bäckhed F., Mithieux G. (2016). Microbiota-produced succinate improves glucose homeostasis via intestinal gluconeogenesis. Cell Metab. 24, 151–157. doi: 10.1016/j.cmet.2016.06.013
DiBaise J. K., Zhang H., Crowell M. D., Krajmalnik-Brown R., Decker G. A., Rittmann B. E. (2008). Gut microbiota and its possible relationship with obesity. Mayo Clinic Proc. 83, 460–469. doi: 10.4065/83.4.460
Dolatkhah N., Hajifaraji M., Abbasalizadeh F., Aghamohammadzadeh N., Mehrabi Y., Mesgari Abbasi M. (2015). Is there a value for probiotic supplements in gestational diabetes mellitus? A randomized clinical trial. J. Health Popul. Nutr. 33, 1–8. doi: 10.1186/s41043-015-0034-9
Eleftheriades M., Papastefanou I., Lambrinoudaki I., Kappou D., Lavranos D., Akalestos A., et al. (2014). Elevated placental growth factor concentrations at 11–14 weeks of gestation to predict gestational diabetes mellitus. Metabolism 63, 1419–1425. doi: 10.1016/j.metabol.2014.07.016
Elliott H. R., Sharp G. C., Relton C. L., Lawlor D. A. (2019). Epigenetics and gestational diabetes: a review of epigenetic epidemiology studies and their use to explore epigenetic mediation and improve prediction. Diabetologia 62, 2171–2178. doi: 10.1007/s00125-019-05011-8
Farahvar S., Walfisch A., Sheiner E. (2019). Gestational diabetes risk factors and long-term consequences for both mother and offspring: a literature review. Expert Rev. Endocrinol. Metab. 14, 63–74. doi: 10.1080/17446651.2018.1476135
Farrar D., Simmonds M., Bryant M., Sheldon T. A., Tuffnell D., Golder S., et al. (2016). Hyperglycaemia and risk of adverse perinatal outcomes: systematic review and meta-analysis. bmj 354. doi: 10.1136/bmj.i4694
Feng H., Zhu W.-W., Yang H.-X., Wei Y.-M., Wang C., Su R.-N., et al. (2017). Relationship between oral glucose tolerance test characteristics and adverse pregnancy outcomes among women with gestational diabetes mellitus. Chin. Med. J. 130, 1012–1018. doi: 10.4103/0366-6999.204928
Ferrocino I., Ponzo V., Gambino R., Zarovska A., Leone F., Monzeglio C., et al. (2018). Changes in the gut microbiota composition during pregnancy in patients with gestational diabetes mellitus (GDM). Sci. Rep. 8, 1–13. doi: 10.1038/s41598-018-30735-9
Fraser A., Nelson S. M., Macdonald-Wallis C., Cherry L., Butler E., Sattar N., et al. (2012). Associations of pregnancy complications with calculated cardiovascular disease risk and cardiovascular risk factors in middle age: the Avon Longitudinal Study of Parents and Children. Circulation 125, 1367–1380. doi: 10.1161/CIRCULATIONAHA.111.044784
Ganiger K., Sridharan S., Rahul A., Satyanarayana A. (2019). Quantitative analysis of key periodontopathic bacteria in gestational diabetic and non-diabetic women. J. Diabetes Metab. Disord. 18, 363–369. doi: 10.1007/s40200-019-00420-3
Gao Y., Chen H., Li J., Ren S., Yang Z., Zhou Y., et al. (2022). Alterations of gut microbiota-derived metabolites in gestational diabetes mellitus and clinical significance. J. Clin. Lab. Anal. 36, e24333. doi: 10.1002/jcla.24333
Gardner A. B., Champion M. L., Battarbee A. N. (2022). Exploring racial/ethnic groups at risk for gestational diabetes mellitus: genetic differences or effects of racism? Am. J. Obstet. Gynecol. 226, S616–S6S7. doi: 10.1016/j.ajog.2021.11.1015
Göbl C. S., Bozkurt L., Yarragudi R., Tura A., Pacini G., Kautzky-Willer A. (2014). Is early postpartum HbA1c an appropriate risk predictor after pregnancy with gestational diabetes mellitus? Acta Diabetol. 51, 715–722. doi: 10.1007/s00592-014-0574-2
Goncalves B., Ferreira C., Alves C. T., Henriques M., Azeredo J., Silva S. (2016). Vulvovaginal candidiasis: Epidemiology, microbiology and risk factors. Crit. Rev. Microbiol. 42, 905–927. doi: 10.3109/1040841X.2015.1091805
Guariguata L., Linnenkamp U., Beagley J., Whiting D. R., Cho N. H. (2014). Global estimates of the prevalence of hyperglycaemia in pregnancy. Diabetes Res. Clin. Pract. 103, 176–185. doi: 10.1016/j.diabres.2013.11.003
Guggenheimer J., Moore P. A., Rossie K., Myers D., Mongelluzzo M. B., Block H. M., et al. (2000). Insulin-dependent diabetes mellitus and oral soft tissue pathologies: II. Prevalence and characteristics of Candida and Candidal lesions. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endodontics 89, 570–576. doi: 10.1067/moe.2000.104477
Hakkarainen H., Huopio H., Cederberg H., Paakkonen M., Voutilainen R., Heinonen S. (2016). The risk of metabolic syndrome in women with previous GDM in a long-term follow-up. Gynecol. Endocrinol. 32, 920–925. doi: 10.1080/09513590.2016.1198764
Hakkarainen H., Huopio H., Cederberg H., Voutilainen R., Heinonen S. (2018). Future risk of metabolic syndrome in women with a previous LGA delivery stratified by gestational glucose tolerance: a prospective cohort study. BMC Pregnancy Childbirth 18, 326. doi: 10.1186/s12884-018-1958-z
HAPO Study Cooperative Research Group, Metzger B. E., Lowe L. P., Dyer A. R., Trimble E. R., Chaovarindr U., et al. (2008). Hyperglycemia and adverse pregnancy outcomes. N Engl. J. Med. 358, 1991–2002. doi: 10.1056/NEJMoa0707943
Hirji I., Andersson S. W., Guo Z., Hammar N., Gomez-Caminero A. (2012). Incidence of genital infection among patients with type 2 diabetes in the UK General Practice Research Database. J. Diabetes Complicat 26, 501–505. doi: 10.1016/j.jdiacomp.2012.06.012
Hod M., Kapur A., Sacks D. A., Hadar E., Agarwal M., Di Renzo G. C., et al. (2015). The International Federation of Gynecology and Obstetrics (FIGO) Initiative on gestational diabetes mellitus: A pragmatic guide for diagnosis, management, and care(). Int. J. Gynaecol Obstet. 131 Suppl 3, S173–S211. doi: 10.1016/S0020-7292(15)30033-3
Hu P., Chen X., Chu X., Fan M., Ye Y., Wang Y., et al. (2021). Association of gut microbiota during early pregnancy with risk of incident gestational diabetes mellitus. J. Clin. Endocrinol. Metab. 106, e4128–e4e41. doi: 10.1210/clinem/dgab346
Huvinen E., Eriksson J. G., Koivusalo S. B., Grotenfelt N., Tiitinen A., Stach-Lempinen B., et al. (2018). Heterogeneity of gestational diabetes (GDM) and long-term risk of diabetes and metabolic syndrome: findings from the RADIEL study follow-up. Acta Diabetol 55, 493–501. doi: 10.1007/s00592-018-1118-y
Iliodromiti S., Sassarini J., Kelsey T. W., Lindsay R. S., Sattar N., Nelson S. M. (2016). Accuracy of circulating adiponectin for predicting gestational diabetes: a systematic review and meta-analysis. Diabetologia 59, 692–699. doi: 10.1007/s00125-015-3855-6
Imoh L. C., Asorose A., Odo A. I., Aina D. O., Abu A. O., Ocheke A. N. (2017). Modification of WHO diagnostic criteria for gestational diabetes: implications for classification of hyperglycemia in pregnancy. Int. J. Reprod. Contracept Obstet. Gynecol. 6, 2716–2723. doi: 10.18203/2320-1770.ijrcog20172900
International Association of Diabetes and Pregnancy Study Groups Consensus Panel, Metzger B. E., Gabbe S. G., Persson B., Buchanan T. A., Catalano P. A., et al. (2010). International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 33, 676–682. doi: 10.2337/dc09-1848
International Diabetes Federation (2019). IDF diabetes atlas, ninth edition Brussels, Belgium: International Diabetes Federation.
Ionescu R. F., Enache R. M., Cretoiu S. M., Gaspar B. S. (2022). Gut microbiome changes in gestational diabetes. Int. J. Mol. Sci. 23, 12839. doi: 10.3390/ijms232112839
Jamalpour S., Zain S. M., Mosavat M., Mohamed Z., Omar S. Z. (2018). A case-control study and meta-analysis confirm glucokinase regulatory gene rs780094 is a risk factor for gestational diabetes mellitus. Gene 650, 34–40. doi: 10.1016/j.gene.2018.01.091
Kijmanawat A., Panburana P., Reutrakul S., Tangshewinsirikul C. (2019). Effects of probiotic supplements on insulin resistance in gestational diabetes mellitus: A double-blind randomized controlled trial. J. Diabetes Invest. 10, 163–170. doi: 10.1111/jdi.12863
Kim K. S., Hong S., Han K., Park C. Y. (2021). The clinical characteristics of gestational diabetes mellitus in Korea: A national health information database study. Endocrinol. Metab. 36, 628–636. doi: 10.3803/EnM.2020.948
Kuang Y.-S., Lu J.-H., Li S.-H., Li J.-H., Yuan M.-Y., He J.-R., et al. (2017). Connections between the human gut microbiome and gestational diabetes mellitus. Gigascience 6, gix058. doi: 10.1093/gigascience/gix058
Lauring J. R., Kunselman A. R., Pauli J. M., Repke J. T., Ural S. H. (2018). Comparison of healthcare utilization and outcomes by gestational diabetes diagnostic criteria. J. Perinatal Med. 46, 401–409. doi: 10.1515/jpm-2017-0076
Le Chatelier E., Nielsen T., Qin J., Prifti E., Hildebrand F., Falony G., et al. (2013). Richness of human gut microbiome correlates with metabolic markers. Nature 500, 541–546. doi: 10.1038/nature12506
Leitner M., Fragner L., Danner S., Holeschofsky N., Leitner K., Tischler S., et al. (2017). Combined metabolomic analysis of plasma and urine reveals AHBA, tryptophan and serotonin metabolism as potential risk factors in gestational diabetes mellitus (GDM). Front. Mol. Biosci. 4, 84. doi: 10.3389/fmolb.2017.00084
Li G., Yin P., Chu S., Gao W., Cui S., Guo S., et al. (2021). Correlation analysis between GDM and gut microbial composition in late pregnancy. J. Diabetes Res. 2021, 1–7. doi: 10.1155/2021/8892849
Li X., Zheng J., Ma X., Zhang B., Zhang J., Wang W., et al. (2021). The oral microbiome of pregnant women facilitates gestational diabetes discrimination. J. Genet. Genomics = Yi Chuan xue bao 48, 32–39. doi: 10.1016/j.jgg.2020.11.006
Liu R., Hong J., Xu X., Feng Q., Zhang D., Gu Y., et al. (2017). Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nat. Med. 23, 859–868. doi: 10.1038/nm.4358
Ma S., You Y., Huang L., Long S., Zhang J., Guo C., et al. (2020). Alterations in gut microbiota of gestational diabetes patients during the first trimester of pregnancy. Front. Cell. Infect. Microbiol. 10, 58. doi: 10.3389/fcimb.2020.00058
Malla M. A., Dubey A., Kumar A., Yadav S., Hashem A., Abd Allah E. F. (2018). Exploring the human microbiome: the potential future role of next-generation sequencing in disease diagnosis and treatment. Front. Immunol. 9, 2868. doi: 10.3389/fimmu.2018.02868
Marchesi J. R. (2010). Prokaryotic and eukaryotic diversity of the human gut. Adv. Appl. Microbiol. 72, 43–62. doi: 10.1016/S0065-2164(10)72002-5
Martin R., Miquel S., Langella P., Bermudez-Humaran L. G. (2014). The role of metagenomics in understanding the human microbiome in health and disease. Virulence 5, 413–423. doi: 10.4161/viru.27864
Maymon R., Meiri H., Svirski R., Weiner E., Cuckle H. (2019). Maternal serum screening marker levels in twin pregnancies affected by gestational diabetes. Arch. Gynecol. Obstet. 299, 655–663. doi: 10.1007/s00404-018-5010-3
Mohajeri M. H., Brummer R. J. M., Rastall R. A., Weersma R. K., Harmsen H. J. M., Faas M., et al. (2018). The role of the microbiome for human health: from basic science to clinical applications. Eur. J. Nutr. 57, 1–14. doi: 10.1007/s00394-018-1703-4
Mokkala K., Houttu N., Vahlberg T., Munukka E., Rönnemaa T., Laitinen K. (2017). Gut microbiota aberrations precede diagnosis of gestational diabetes mellitus. Acta Diabetol 54, 1147–1149. doi: 10.1007/s00592-017-1056-0
Mosimann B., Amylidi S., Risch L., Wiedemann U., Surbek D., Baumann M., et al. (2016). First-trimester placental growth factor in screening for gestational diabetes. Fetal Diagnosis Ther. 39, 287–291. doi: 10.1159/000441027
Munang Y. N., Noubiap J. J., Danwang C., Sama J. D., Azabji-Kenfack M., Mbanya J. C., et al. (2017). Reproducibility of the 75 g oral glucose tolerance test for the diagnosis of gestational diabetes mellitus in a sub-Saharan African population. BMC Res. Notes 10, 1–6. doi: 10.1186/s13104-017-2944-7
Nankervis A., McIntyre H., Moses R., Ross G., Callaway L., Porter C., et al. (2014). ADIPS consensus guidelines for the testing and diagnosis of gestational diabetes mellitus in Australia. Australia: Australasian Diabetes in Pregnancy Society (ADIPS). 1–8.
Nguyen C. L., Pham N. M., Binns C. W., Duong D. V., Lee A. H. (2018). Prevalence of gestational diabetes mellitus in eastern and Southeastern Asia: A systematic review and meta-analysis. J. Diabetes Res. 2018, 6536974. doi: 10.1155/2018/6536974
NICE. (2015). Diabetes in pregnancy: management of diabetes and its complications from preconception to the postnatal period (London: National Institute for Health and Care Excellence: Clinical Guidelines).
O’Connor C., O’Shea P. M., Owens L. A., Carmody L., Avalos G., Nestor L., et al. (2012). Trimester-specific reference intervals for haemoglobin A1c (HbA1c) in pregnancy. Clin. Chem. Lab. Med. 50, 905–909. doi: 10.1515/cclm.2011.397
O’Sullivan E. P., Avalos G., O’Reilly M., Dennedy M. C., Gaffney G., Dunne F., et al. (2011). Atlantic Diabetes in Pregnancy (DIP): the prevalence and outcomes of gestational diabetes mellitus using new diagnostic criteria. Diabetologia 54, 1670–1675. doi: 10.1007/s00125-011-2150-4
Pellonperä O., Mokkala K., Houttu N., Vahlberg T., Koivuniemi E., Tertti K., et al. (2019). Efficacy of fish oil and/or probiotic intervention on the incidence of gestational diabetes mellitus in an at-risk group of overweight and obese women: a randomized, placebo-controlled, double-blind clinical trial. Diabetes Care 42, 1009–1017. doi: 10.2337/dc18-2591
Pinto Y., Frishman S., Turjeman S., Eshel A., Nuriel-Ohayon M., Shrossel O., et al. (2023). Gestational diabetes is driven by microbiota-induced inflammation months before diagnosis. Gut 72, 918-928. doi: 10.1136/gutjnl-2022-328406
Qin J., Li Y., Cai Z., Li S., Zhu J., Zhang F., et al. (2012). A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 490, 55–60. doi: 10.1038/nature11450
Rajilić-Stojanović M., De Vos W. M. (2014). The first 1000 cultured species of the human gastrointestinal microbiota. FEMS Microbiol. Rev. 38, 996–1047. doi: 10.1111/1574-6976.12075
Ramezani S., Doulabi M. A., Saqhafi H., Alipoor M. (2020). Prediction of gestational diabetes by measuring the levels of pregnancy associated plasma protein-A (PAPP-A) during gestation weeks 11–14. J. Reprod. Infertil. 21, 130.
Rani P. R., Begum J. (2016). Screening and diagnosis of gestational diabetes mellitus, where do we stand. J. Clin. Diagn. Res.: JCDR 10, QE01–QE04. doi: 10.7860/JCDR/2016/17588.7689
Rastelli M., Cani P. D., Knauf C. (2019). The gut microbiome influences host endocrine functions. Endocr. Rev. 40, 1271–1284. doi: 10.1210/er.2018-00280
Riskin-Mashiah S., Damti A., Younes G., Auslender R. (2010). First trimester fasting hyperglycemia as a predictor for the development of gestational diabetes mellitus. Eur. J. Obstet. Gynecol. Reprod. Biol. 152, 163–167. doi: 10.1016/j.ejogrb.2010.05.036
Sacks D. B., Bruns D. E., Goldstein D. E., Maclaren N. K., McDonald J. M., Parrott M. (2002). Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin. Chem. 48, 436–472. doi: 10.1093/clinchem/48.3.436
Sacks D. B., Coustan D. R., Cundy T., Donovan L., Hod M. (2018). Gestational diabetes mellitus: why the controversy? Clin. Chem. 64, 431–438. doi: 10.1373/clinchem.2016.266577
Saravanan P., Diabetes in Pregnancy Working G, Maternal Medicine Clinical Study G, Royal College of O, Gynaecologists UK (2020). Gestational diabetes: opportunities for improving maternal and child health. Lancet Diabetes Endocrinol. 8, 793–800. doi: 10.1016/S2213-8587(20)30161-3
Savvidou M., Syngelaki A., Muhaisen M., Emelyanenko E., Nicolaides K. (2012). First trimester maternal serum free β-human chorionic gonadotropin and pregnancy-associated plasma protein A in pregnancies complicated by diabetes mellitus. BJOG: Int. J. Obstet. Gynaecol. 119, 410–416. doi: 10.1111/j.1471-0528.2011.03253.x
Schwartz N., Nachum Z., Green M. S. (2016). Risk factors of gestational diabetes mellitus recurrence: a meta-analysis. Endocrine 53, 662–671. doi: 10.1007/s12020-016-0922-9
Shahriari A., Karimi E., Shahriari M., Aslani N., Arab A. (2021). The effect of probiotic supplementation on the risk of gestational diabetes mellitus among high-risk pregnant women: A parallel double-blind, randomized, placebo-controlled clinical trial. Biomed. Pharmacother. 141, 111915. doi: 10.1016/j.biopha.2021.111915
Shinar S., Berger H. (2018). Early diabetes screening in pregnancy. Int. J. Gynecol. Obstet. 142, 1–8. doi: 10.1002/ijgo.12484
Sililas P., Huang L., Thonusin C., Luewan S., Chattipakorn N., Chattipakorn S., et al. (2021). Association between gut microbiota and development of gestational diabetes mellitus. Microorganisms 9, 1686. doi: 10.3390/microorganisms9081686
Simjak P., Cinkajzlova A., Anderlova K., Parizek A., Mraz M., Krsek M., et al. (2018). The role of obesity and adipose tissue dysfunction in gestational diabetes mellitus. J. Endocrinol. 238, R63–R77. doi: 10.1530/JOE-18-0032
Syngelaki A., Kotecha R., Pastides A., Wright A., Nicolaides K. H. (2015). First-trimester biochemical markers of placentation in screening for gestational diabetes mellitus. Metabolism 64, 1485–1489. doi: 10.1016/j.metabol.2015.07.015
Tagoma A., Alnek K., Kirss A., Uibo R., Haller-Kikkatalo K. (2018). MicroRNA profiling of second trimester maternal plasma shows upregulation of miR-195-5p in patients with gestational diabetes. Gene 672, 137–142. doi: 10.1016/j.gene.2018.06.004
Tang W. H., Kitai T., Hazen S. L. (2017). Gut microbiota in cardiovascular health and disease. Circ. Res. 120, 1183–1196. doi: 10.1161/CIRCRESAHA.117.309715
Tang M., Weaver N. E., Frank D. N., Ir D., Robertson C. E., Kemp J. F., et al. (2022). Longitudinal reduction in diversity of maternal gut microbiota during pregnancy is observed in multiple low-resource settings: results from the women first trial. Front. Microbiol. 13, 823757. doi: 10.3389/fmicb.2022.823757
Tieu J., McPhee A. J., Crowther C. A., Middleton P. (2014). Screening and subsequent management for gestational diabetes for improving maternal and infant health. Cochrane Database System. Rev. 2014, CD007222. doi: 10.1002/14651858.CD007222.pub3
Trujillo J., Vigo A., Reichelt A., Duncan B., Schmidt M. (2014). Fasting plasma glucose to avoid a full OGTT in the diagnosis of gestational diabetes. Diabetes Res. Clin. Pract. 105, 322–326. doi: 10.1016/j.diabres.2014.06.001
Vandorsten J. P., Dodson W. C., Espeland M. A., Grobman W. A., Guise J. M., Mercer B. M., et al. (2013) in NIH consensus development conference: diagnosing gestational diabetes mellitus. NIH consensus and state-of-the-science statements, NIH Consensus State Sci Statements. Vol. 29. 1–31.
Wang J., Zheng J., Shi W., Du N., Xu X., Zhang Y., et al. (2018). Dysbiosis of maternal and neonatal microbiota associated with gestational diabetes mellitus. Gut 67, 1614–1625. doi: 10.1136/gutjnl-2018-315988
Wei J., Qing Y., Zhou H., Liu J., Qi C., Gao J. (2022). 16S rRNA gene amplicon sequencing of gut microbiota in gestational diabetes mellitus and their correlation with disease risk factors. J. Endocrinol. Invest. 45, 279–289. doi: 10.1007/s40618-021-01595-4
Wendland E. M., Torloni M. R., Falavigna M., Trujillo J., Dode M. A., Campos M. A., et al. (2012). Gestational diabetes and pregnancy outcomes–a systematic review of the World Health Organization (WHO) and the International Association of Diabetes in Pregnancy Study Groups (IADPSG) diagnostic criteria. BMC Pregnancy Childbirth 12, 23. doi: 10.1186/1471-2393-12-23
Wickens K. L., Barthow C. A., Murphy R., Abels P. R., Maude R. M., Stone P. R., et al. (2017). Early pregnancy probiotic supplementation with Lactobacillus rhamnosus HN001 may reduce the prevalence of gestational diabetes mellitus: a randomised controlled trial. Br. J. Nutr. 117, 804–813. doi: 10.1017/S0007114517000289
Xu Y., Zhang M., Zhang J., Sun Z., Ran L., Ban Y., et al. (2020). Differential intestinal and oral microbiota features associated with gestational diabetes and maternal inflammation. Am. J. Physiol. Endocrinol. Metab. 319, E247–EE53. doi: 10.1152/ajpendo.00266.2019
Yahaya T. O., Salisu T., Abdulrahman Y. B., Umar A. K. (2020). Update on the genetic and epigenetic etiology of gestational diabetes mellitus: a review. Egyptian J. Med. Hum. Genet. 21, 1–13. doi: 10.1186/s43042-020-00054-8
Yao H., Xu D., Zhu Z., Wang G. (2019). Gestational diabetes mellitus increases the detection rate and the number of oral bacteria in pregnant women. Medicine 98, e14903. doi: 10.1097/MD.0000000000014903
Ye D., Huang J., Wu J., Xie K., Gao X., Yan K., et al. (2023). Integrative metagenomic and metabolomic analyses reveal gut microbiota-derived multiple hits connected to development of gestational diabetes mellitus in humans. Gut Microbes 15, 2154552. doi: 10.1080/19490976.2022.2154552
Yogev, Chen, Hod, Coustan, Oats, McIntyre, et al. (2010). Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study: preeclampsia. Am. J. Obstet. Gynecol. 202, 255 e1–255 e7. doi: 10.1016/j.ajog.2010.01.024
Yu J., Feng Q., Wong S. H., Zhang D., yi Liang Q., Qin Y., et al. (2017). Metagenomic analysis of faecal microbiome as a tool towards targeted non-invasive biomarkers for colorectal cancer. Gut 66, 70–78. doi: 10.1136/gutjnl-2015-309800
Zhang T., Du T., Li W., Yang S., Liang W. (2018). Sex hormone-binding globulin levels during the first trimester may predict gestational diabetes mellitus development. Biomarkers Med. 12, 239–244. doi: 10.2217/bmm-2016-0030
Zhang X., Liao Q., Wang F., Li D. (2018). Association of gestational diabetes mellitus and abnormal vaginal flora with adverse pregnancy outcomes. Medicine 97, e11891. doi: 10.1097/MD.0000000000011891
Zheng W., Xu Q., Huang W., Yan Q., Chen Y., Zhang L., et al. (2020). Gestational diabetes mellitus is associated with reduced dynamics of gut microbiota during the first half of pregnancy. mSystems 5, e00109–e00120. doi: 10.1128/mSystems.00109-20
Zhu W.-W., Fan L., Yang H.-X., Kong L.-Y., Su S.-P., Wang Z.-L., et al. (2013a). Fasting plasma glucose at 24–28 weeks to screen for gestational diabetes mellitus: new evidence from China. Diabetes Care 36, 2038–2040. doi: 10.2337/dc12-2465
Keywords: fasting plasma glucose (FPG), oral glucose tolerance test (OGTT), World Health Organization (WHO), American Diabetes Association (ADA), National Institute for Health and Care Excellence (NICE), International Association of Diabetes and Pregnancy Study Groups (IADPSG), International Federation of Gynecology and Obstetrics (FIGO)
Citation: Bokoliya S, McClellan S, Zhou Y and Fan N (2024) Exploring the influence of microbiota on gestational diabetes and its potential as a biomarker. Front. Bacteriol. 3:1352227. doi: 10.3389/fbrio.2024.1352227
Received: 07 December 2023; Accepted: 15 March 2024;
Published: 09 April 2024.
Edited by:
Darina Cejkova, Brno University of Technology, CzechiaReviewed by:
Itziar Chapartegui-González, Karolinska Institutet (KI), SwedenMojtaba Akbari, Isfahan University of Medical Sciences, Iran
Copyright © 2024 Bokoliya, McClellan, Zhou and Fan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nini Fan, bmluaWZhbjdAZ21haWwuY29t
 Suresh Bokoliya
Suresh Bokoliya Stephanie McClellan2
Stephanie McClellan2 Yanjiao Zhou
Yanjiao Zhou Nini Fan
Nini Fan