- 1Department of Otolaryngology-Head and Neck Surgery, Johns Hopkins Hospital, Baltimore, MD, United States
- 2Department of Otolaryngology, University of Iowa College of Medicine, Iowa City, IA, United States
Background: Perilymph fistula (PLF) can cause symptoms of dizziness, vertigo, and fluctuating hearing. We hypothesized that publications on PLF have decreased in recent years relative to other inner ear disorders with overlapping symptoms.
Methods: We performed a Scopus search using the terms “perilymphatic fistula” OR “perilymph fistula,” limited to original studies or reviews involving human subjects published in English before 2022. We noted the senior author's institution, country affiliation, and publishing journal. The Kendall rank correlation coefficient test was used to analyze the trend of each variable over the past 30 years. Using the same search strategy, we compared these data to those from vestibular neuritis (VN), Meniere's disease (MD), superior semicircular canal dehiscence (SSCD), and benign paroxysmal positional vertigo (BPPV).
Results: Six hundred and ninety-eight PLF publications were returned. The top publishing country was the United States (n = 334), the top publishing journal was the American Journal of Otology/Otology and Neurotology (n = 68), and the top publishing institution was the University of Pittsburgh (n = 15). In the past 30 years (n = 501), there was no trend in the frequency of annual PLF publications (τ = −0.150, p = 0.265). Still there were positive trends in publications on vestibular neuritis (τ = 0.724, p < 0.001), Meniere's disease (τ = 0.587, p < 0.001), superior canal dehiscence syndrome (τ = 0.840, p < 0.001), and benign paroxysmal positional vertigo (τ = 0.882, p < 0.001) 5,398 PLF cases were identified, of which 4,356 specified the etiology; the majority identified an inciting insult (74.8%, n = 3,257).
Conclusions: The absence of diagnostic criteria for PLF may hinder its appeal to researchers as similar syndromes have had diagnostic consensus documents set forth. Given the greater number of cases with inciting insults, efforts to define a diagnostic criterion should focus on this subtype.
1 Introduction
Within the realm of uncommon vestibular disorders like Meniere's disease, vestibular migraine, and benign paroxysmal positional vertigo (BPPV), the emergence of the perilymphatic fistula in 1962 marked a new addition to the spectrum of causes of vertigo (Schuknecht, 1962). A perilymph fistula (PLF, sometimes referred to as perilymphatic fistula) occurs when there is communication between the perilymphatic space of the inner ear and the middle ear or mastoid. In 2013, its incidence was estimated at 1.5/100,000, similar to the incidence of vestibular schwannoma (1.3/100,000; Fiedler et al., 2013; Marinelli et al., 2021). Reports of PLF have been stratified into those with an identified insult and spontaneous cases. The former encompasses cases preceded by trauma, such as barotrauma in divers, and iatrogenic sequelae, such as stapes surgery and cochlear implantation (Sarna et al., 2020). The latter encompass idiopathic cases without an identified inciting cause and presumed congenital etiologies (Sarna et al., 2020).
Patients with PLF may present with vague symptoms of dizziness, vertigo, or fluctuating hearing loss (Sarna et al., 2020). In rare cases, PLF is diagnosed radiographically in the setting of pneumolabyrinth in patients with acute vestibular syndrome or after temporal bone trauma. Without clear findings on exam or imaging, PLF is often a diagnosis of exclusion for symptoms that do not meet the established criteria of another diagnosis. The vague symptomatology and diagnostic criteria make PLF a controversial topic in neurotology.
Efforts are underway by the Bárány Society's Committee for Classification of Vestibular Disorders to establish consensus diagnostic criteria for vestibular disorders (Bisdorff et al., 2009). Diagnostic criteria for other diagnoses that were once considered vague or controversial, such as Meniere's Disease (MD), vestibular migraine (VM), and Persistent Postural-Perceptual Dizziness (PPPD), have been published (Lempert et al., 2022; Staab et al., 2017; Lopez-Escamez et al., 2015). There is growing interest in characterizing the publication trends and quality of research in neurotology (Boerner et al., 2018; O'Byrne et al., 2022; Zou et al., 2022; Zhou et al., 2023). Still, no publication trend study has examined PLF. Bibliometric reports like these are particularly valuable in understanding the development of rare diagnoses like PLF as they help to uncover the historical context surrounding the evolution of diagnostic frameworks, changes in treatment trends, and gaps in epistemology.
Here, we seek to characterize the publication trends of PLF in medical and scientific literature to allow a historical assessment of this topic's evolution in medicine.
2 Materials and methods
A Scopus search was performed on August 15, 2022, using the search terms “perilymphatic fistula” OR “perilymph fistula” with Scopus filters to limit results to human studies, articles, or reviews published in English before the year 2022. The authors performed a secondary review to remove publications included erroneously and not within our scope. The results were tabulated per year for the total number of publications per country, journal, and institution. The senior author's affiliation determined the country and institution of origin. The Kendall rank correlation coefficient test was used to analyze the trend of each category over the past 30 years (1992–2022), and during the first 30 years of publications (1962–1991). We compared these results to those from analyses of vestibular neuritis (VN), Meniere's disease (MD), superior semicircular canal dehiscence (SSCD), and benign paroxysmal positional vertigo (BPPV) using the same search strategy.
To better characterize trends in PLF publications, articles returned using our PLF query for which we could locate a full-text manuscript in English were further analyzed. Publications were categorized by study type, including case reports, case series, retrospective reviews, literature reviews, etc. We noted the total number of patients in the study and the number of distinct PLF cases. We also noted whether the report included patients with spontaneous/idiopathic PLF (category 1), cases with an identified inciting insult (category 2), or both (category 3). Publications included in category 3 reported cases of both spontaneous etiology and those with an inciting insult in their case series or when discussing PLF. Cases series listed as a category 3 publication were further stratified into spontaneous and incited cases when analyzing the number of PLF cases by etiology over time. If a report did not specify the type of PLF or discussed the topic broadly without mentioning a specific case, it was categorized as unspecified or not applicable (category 0).
The Kendall rank correlation coefficient test was used to analyze the trend of publication frequencies since 1962, the first 30 years (1962–1991), and the past 30 years (1992–2022), measuring the strength and direction of the association between time and the number of publications for each inner ear disorder. A positive tau value of 1 indicates a perfect correlation, helping to identify significant trends in research.
3 Results
3.1 Quantitative analysis
The search returned 698 PLF publications beginning in 1962 (Figure 1). The top three countries contributing to these publications were the USA (n = 334), Japan (n = 70), and the UK (n = 45). The top three journals were the American Journal of Otology (AJO)/Otology and Neurotology (ON; n = 68), Laryngoscope (n = 65), and Otolaryngology-Head and Neck Surgery (OHNS; n = 41). Of note, the American Journal of Otology (AJO), was renamed Otology and Neurotology (ON) in 2001; results for AJO and ON were combined. The top three affiliations were the University of Pittsburgh (n = 15), Massachusetts Eye and Ear Infirmary (n = 13), and Wake Forest University (n = 13).
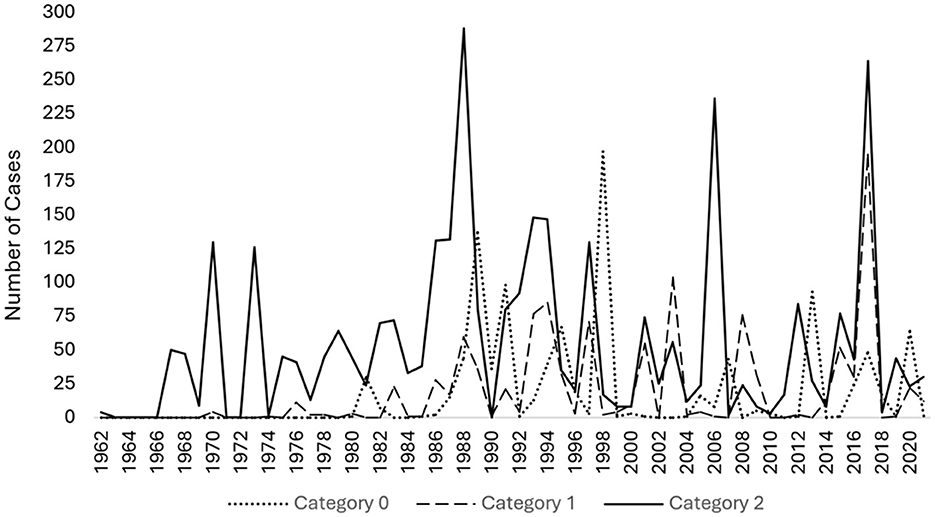
Figure 1. Line graph of publications per year on perilymphatic fistula (PLF) from 1962 to 2021. Our query returned 698 PLF publications starting from 1962.
In the past 30 years (1992–2022, n = 501), there was no significant trend in PLF publications (τ = −0.150, p = 0.265) but there were positive trends in publications on VN (τ = 0.724, p < 0.001), MD (τ = 0.587, p < 0.001), SSCD (τ = 0.840, p < 0.001), and BPPV (τ = 0.882, p < 0.001; Figure 2). Publications from the USA showed a negative trend (n = 209, τ = −0.448, p < 0.001, while there was no significant trend for Japan (n = 59, τ = −0.011, p = 0.93) or the UK (n = 29, τ = −0.052, p = 0.720). There was no trend in publications from Laryngoscope (n = 28, τ = −0.105, p = 0.482), but there were negative trends from OHNS (n = 20, τ = −0.317, p < 0.05) and AJO/ON (n = 43, τ = −0.266, p = 0.056), although the latter did not reach significance.
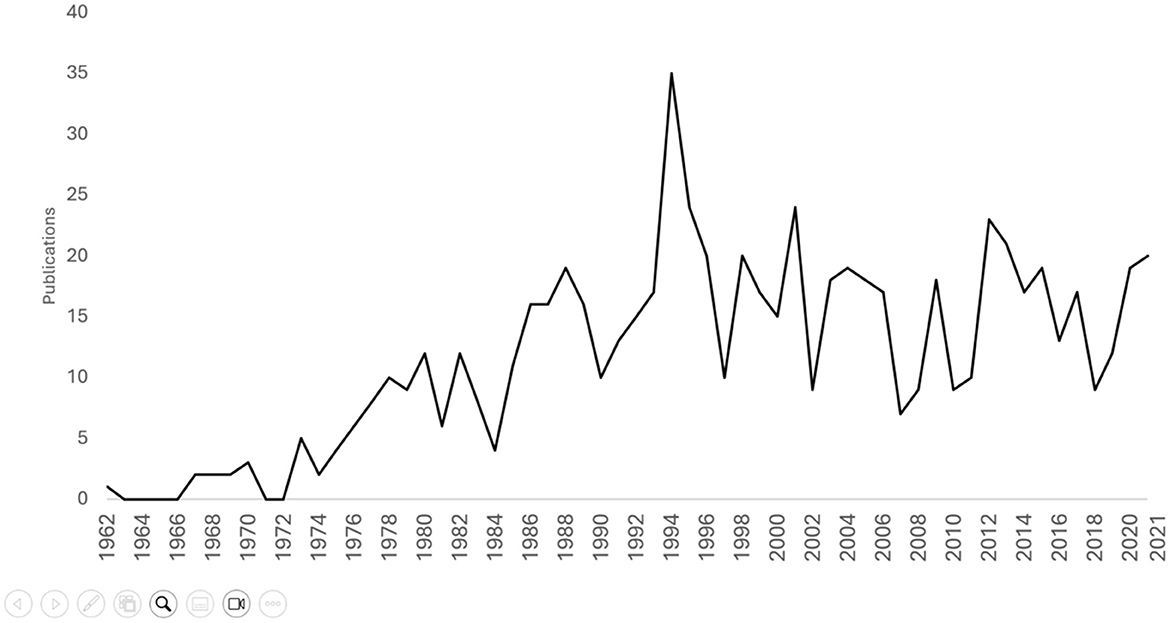
Figure 2. Line graph of publications per year from 1962 to 2021 for common vestibular disorders over the past 30 years (1992–2021). The disorders include PLF (Perilymphatic Fistula), MD (Meniere's Disease), VN (Vestibular Neuritis), SSCD (Superior Canal Dehiscence Syndrome), and BPPV (Benign Paroxysmal Positional Vertigo). There was no significant trend in PLF publications (τ = −0.150, p = 0.265), but there were positive trends in VN (τ = 0.724, p < 0.001), MD (τ = 0.587, p < 0.001), SSCD (τ = 0.840, p < 0.001), and BPPV (τ = 0.882, p < 0.001). The Bárány Society released diagnostic criteria consensus for these disorders, including MD in 2015, BPPV in 2017, SSCD in 2021, and VN in 2022 (Staab et al., 2017; Lopez-Escamez et al., 2015; Strupp et al., 2022; Ward et al., 2021).
Of the 698 publications, 669 had full-text manuscripts available in English, and were further investigated for the type of PLF. Within these publications, a total of 5,398 unique cases of PLF were identified. The majority of cases had an identified inciting event (n=3,257, 60%, category 2), followed by spontaneous/idiopathic cases (n = 1,099, 20%, category 1) and unspecified cases (n = 1,042, 19.3%, category 0). Publications most commonly reported cases with an inciting insult only (n = 326, 48.7%, category 2), followed by unspecified cases (n = 175, 26.2%, category 0), those containing both spontaneous/idiopathic cases and those with an inciting event (n = 92, 13.8%, category 3), and only spontaneous/idiopathic cases (n = 76, 11.4%, category 1; Figure 3). Considering only cases with a clear etiology, namely idiopathic/spontaneous and those with inciting insults (n = 4,356), the majority are cases with an identifiable event suspected as the cause of PLF (74.8%, n = 3,257).
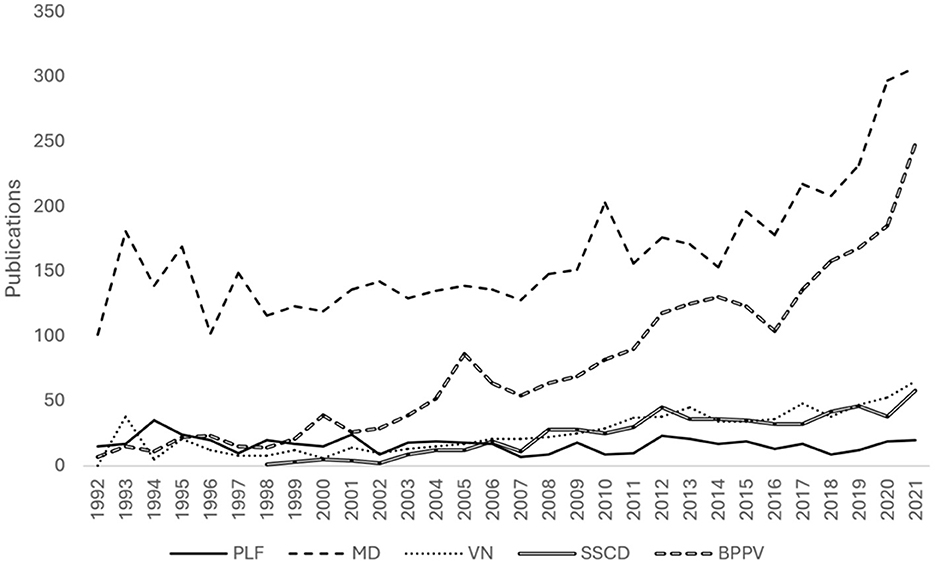
Figure 3. Scatterplot of case report publications per year between 1962 and 2021, categorized by perilymphatic fistula (PLF) type. Category 1 includes publications with spontaneous or idiopathic PLF. Category 2 includes PLF cases with an identified inciting insult. Category 3 includes articles mentioning both Categories 1 and 2. Reports that did not specify the type of PLF or discussed the topic broadly without mentioning a specific case were categorized as “Undeclared/NA” (Category 0).
In the first 30 years of publications from 1962 to 1991, there was a positive trend in the number of unspecified or unclassified cases (τ = 0.6355, p < 0.001, category 0), a mild positive trend in idiopathic cases (τ = 0.5487, p < 0.001, category 1), and a positive trend in cases with an identified inciting insult (τ = 0.4279, p < 0.001, category 2). In the past 30 years, from 1992 to 2022, there was no increase or decrease in the frequency of perilymph fistula being reported, by origin. The analysis revealed no significant trend in the number of unspecified or unclassified cases (τ = −0.1301, p = 0.3384, category 0), spontaneous/idiopathic cases (τ = −0.1695, p = 0.2129, category 1), or cases with an inciting event (τ = −0.0846, p = 0.5351, category 2, Figure 4).
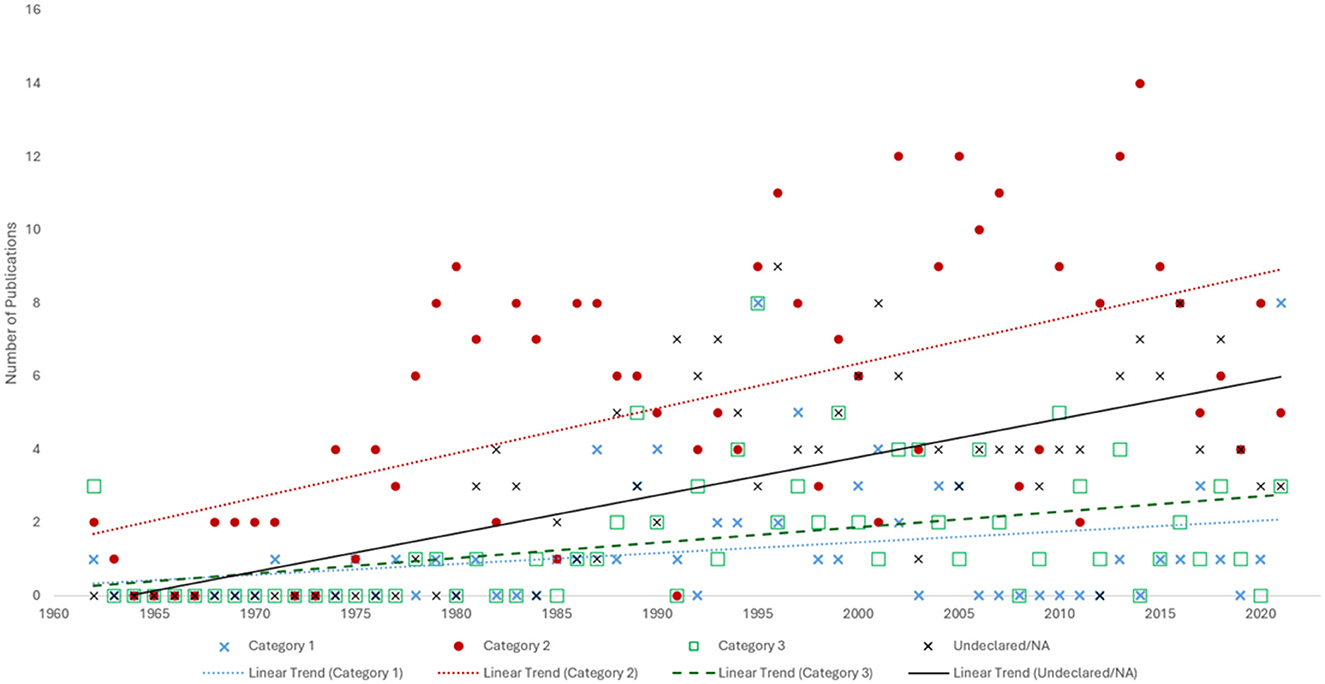
Figure 4. Line graph showing the number of cases per year between 1962 and 2021, categorized by perilymphatic fistula type. Category 0 includes unspecified or unclassified cases. Category 1 comprises idiopathic cases. Category 2 consists of cases with an inciting insult, such as prior stapes surgery, barotrauma, excessive straining, etc.
Similarly, among publications on PLF, there was a positive trend from 1962 to 1992 in the number of publications not specifying the etiology (τ = 0.6703, p < 0.001, category 0), spontaneous/idiopathic (τ = 0.5062, p < 0.001, category 1), and combined spontaneous/idiopathic and inciting event (τ = 0.5802, p < 0.001, category 3) publications, as well as a mild positive trend in publications reporting PLF with an inciting event (τ = 0.4156, p = 0.0017, category 2). In the most recent 30 years studied (1993–2022), there was no significant trend in the number of unclassified (τ = −0.1872, p = 0.1757, category 0), spontaneous/idiopathic (τ = −0.1851, p = 0.1887, category 1) or inciting event (τ = −0.0522, p = 0.7063, category 2) publications, and a small negative trend in those containing both spontaneous/idiopathic cases and those with an inciting event (τ = −0.2777, p = 0.0438, category 3), suggesting that in more recent years publications are tending to report either spontaneous or idiopathic etiologies, and not combining them in a single publication.
3.2 Historical review
Following the first publication describing a perilymphatic fistula in 1962, reports steadily increased, with a notable surge in the 1980–90's (Schuknecht, 1962; Figure 1). This rise in reporting coincided with the resurgence of stapes surgery in 1958 when John Shea published a case series introducing the removal of the stapes crura and fenestration of the footplate for severe conductive hearing loss secondary to otosclerosis, replaced with a vein graft connected to the incus (Shea, 1958). Shea's procedure was the early version of modern stapedectomy (Shea, 1958; Meltzer et al., 1956). In the broader realm of stapes surgery, many innovators preceded him, including physicians in the late nineteenth century (Gibson, 1980). In 1876, Professor Johannes Kessel removed the tympanic membrane, malleus, and incus in a failed effort to restore hearing for a man with chronic otitis media. He discussed several methods of restoring mobility to the stapes, including advocating for stapedectomy (Kessel, 1877). Following an initial surge in interest, the early 1900's saw a decline after prominent physician William Politzer reported severe complications with the operation, including meningitis and death at the 6th International Otological Congress in 1899 (Baber and Cresswell, 1900). Rosen reignited interest in stapes surgery in the 1950's a direct fenestration at the oval window for otosclerosis, achieving notable improvements in hearing up to 45 dB and no fatalities (Rosen, 1956, 1953). While previous approaches relied on direct mobilization of the stapes and were marred by mortality, Rosen's operation was minimally invasive, and safer. By creating a half-millimeter fenestration at the footplate, the passage of sound from the oval window to the cochlea was restored (Rosen, 1956).
As these operations became more commonplace, other post-operative complications were increasingly recognized. In 1962, Schuknecht reported on 750 stapedectomies performed from 1959 to 1961 alone, involving the removal of the stapes and replacement with an adipose graft and a steal strut prosthesis (Schuknecht, 1962). He observed sensorineural hearing loss in 2.66% (n = 20) of cases, most often presenting shortly after the surgery. During reoperation in a few cases, he observed a soft tissue mass at the oval window extending into the vestibule, along with partial resorption of the adipose graft. Microscopic examination revealed serofibrinous labyrinthitis with proliferative fibrous tissue, vascular stroma and polymorphonuclear lymphocytes. These changes were identical to those noted in cat ears when a perilymphatic fistula was intentionally created at the oval window, or when the soft tissue graft was only partly effective in closing the opening, leaving a small fistula (Schuknecht, 1962; Harrison et al., 1967). A history of surgical difficulties was noted in all patients with sensorineural hearing loss, with extensive drilling required in 11 of the 20 cases. Other contributing factors included hemotympanum, instrumental trauma to the membranous labyrinth, and prior trauma from stapes mobilization (Schuknecht, 1962).
Other surgeons reported similar complications and additional symptoms such as vertigo, tinnitus, postural imbalance, and aural fullness (Harrison et al., 1967). Thus, the earliest conceptualization of PLF was as an iatrogenic sequela. It is worth noting that although Rosen reports the escape of perilymphatic fluid in certain cases immediately after oval window fenestration, this opening was small and unlikely to have triggered the inflammatory changes seen after stapedectomy. Furthermore, patients had no associated hearing deficits, and symptoms, if present, were limited to transient post-operative vertigo (Rosen, 1956).
Reports in later years associated several activities with PLF, including forceful Valsalva maneuvers, childbirth, heavy lifting, diving, and aviation, which were thought to exert increased pressure on gases in closed spaces like the middle ear, leading to membrane rupture (Armstrong, 1967; Macfie, 1962). In 1970, the first publication of congenital PLF was released, linking recurring meningitis or otorrhea as possible indications of PLF in children (Stroud and Calcaterra, 1970). A round window fistula was incidentally found in one such case with a history of bilateral otorrhea in the setting of a left cholesteatoma and right-sided otitis media refractory to medical management; both ears were initially managed with a modified radical mastoidectomy. Years later, the child was laughing and heard “something pop,” which was followed by vertigo and right-sided tinnitus; reoperation reportedly revealed a fistula at the stapes footplate (Stroud and Calcaterra, 1970).
The years that followed saw an increase in reports on spontaneous fistulas (Supplementary Figure 1). However, the consensus was that “the diagnosis of a perilymph fistula, regardless of its etiology, remains the major problem” (Stroud and Calcaterra, 1970). The presentation of such cases was at times indistinguishable from the vertigo, tinnitus, and hearing loss in MD and other otologic conditions (Stroud and Calcaterra, 1970). The distinction might be made with a detailed history, which may reveal an inciting traumatic event. Generally, PLF was reserved as a clinical diagnosis for cases of sudden hearing loss following an identified potential cause of otologic trauma.
While previous case reports had introduced an approach to a diagnosis, no systematic report had been produced until 1978, when Singleton published a detailed case-control study investigating up to 59 variables in each case, including demographics, history, quality of nystagmus, and audiogram findings (Singleton et al., 1978). The interest in standardizing the approach to a diagnosis was met with the timely introduction of novel radiologic technologies like magnetic resonance imaging (MRI) and computed tomography (CT) in the late 1970's-early 1980's (Ambrose, 1973; Lauterbur, 1973). These became readily integrated in PLF research, given their potential role in identifying and diagnosing structural causes of otologic pathologies (Kaseff, 1979; Lipkin et al., 1985; Weissman et al., 1994). While small fistulas were not readily identified, abnormalities in the cochlear aqueduct or other structures believed to predispose to PLF could be observed. At other times, the technology could also exclude other pathologies that could cause similar symptoms, like vestibular schwannoma or SSCD (Silver et al., 1987; Swartz and Harnsberger, 1990; Sood et al., 2017).
Different forms of nystagmus have been identified in patients with perilymph fistula, including spontaneous nystagmus, pressure-induced nystagmus, and positional nystagmus (Choi et al., 2017). Presumably, these can be caused by a sudden change in resting vestibular tone on one side in the case of spontaneous nystagmus, by coupling of pressure to the inner ear in the case of pressure-induced nystagmus, or by free air within the inner ear in the case of positional nystagmus. A thorough history of the presentation of vertigo is valuable in distinguishing PLF from conditions such as third window syndrome in which loud sounds or pressure can provoke symptoms, or MD which often presents with classic paroxysms of hearing loss, vertigo, and tinnitus (Sarna et al., 2020).
Several tests have been proposed to aid in the diagnosis of PLF. One of the earliest suggested methods included Hennebert's sign, which was first described in 1911 in a case of a patient with syphilis and now involves applying positive and negative pressure to the ear canal using a pneumatic otoscope to induce nystagmus (Hennebert, 1911). If present, this was believed to indicate a possible connection between the middle and inner ear. However, it is non-specific and can be positive in conditions like MD and SSCD (Hain, 1997). In 1935, sound evoked vestibular responses were introduced and in 1964 this response was measured in the form of myogenic electrical evoked potentials (Cal and Bahmad, 2009). We now refer to these as vestibular evoked myogenic potentials (VEMPs), and their mention in the literature has gained significant traction in the last two decades. In Modugno et al. (2006) published a report of four cases with reduced stimuli thresholds at 500 Hz, attributed to a suspected PLF whose opening reduced inner ear impedance. Exploratory tympanotomy in three of the cases revealed a PLF in two; the fourth case had spontaneous resolution of his symptoms and did not undergo intervention. Other reports on PLF have noted reduced VEMPs, however, this has also been established in SSCD and is part of the diagnostic work-up (Colebatch et al., 1998; Matsuda et al., 2023). Although a reduced unilateral VEMP may not be a unique sign to PLF, it could help increase suspicion for the condition particularly if other diagnoses have been ruled out.
Middle ear exploration or tympanotomy had been regarded as a means of verifying the diagnosis, but critics claim that the perilymph leak observed could be mistaken for washback from the injected anesthetic (Hornibrook, 2012; Black, 1986). Despite this, even in patients with no visible perilymph leakage upon exploration, if a surgeon felt the symptoms were likely PLF based on patient report, perhaps one with a leak too small to be detected, the supposed fistula was sealed and many patients reported improvement of symptoms after the operation (Black, 1986). As with many surgical interventions, it is impossible to distinguish the surgery results from natural history or placebo effects without a placebo-controlled study.
Beginning in the mid-1990's, several biomarkers were studied that hold promise in increasing the likelihood of identifying a PLF but have limitations. Beta-2 transferrin is an asialo-fraction of transferrin and can be found in cerebrospinal fluid (CSF) and perilymph, but not serum (Delaroche et al., 1996). It has been demonstrated as detectable in washings of the middle ear, but a CSF leak can confound the results. Beta-trace protein (β-TP, also called prostaglandin D synthase) has demonstrated high specificity but variable sensitivity, depending on the detection method (Michel et al., 2005; Bachmann-Harildstad et al., 2011). Cochlin-tomoprotein (CTP) has shown more success as a perilymph-specific protein and, therefore, a potential biomarker of PLF. Ikezono et al. (2009) demonstrated that CTP is selectively expressed in perilymph, not other body fluids. This specificity was supported in a subsequent study showing that the CTP detection test could identify a traumatic PLF in cases where other methods, such as high-resolution CT scans, failed (Ikezono et al., 2009). The test's high specificity (98.2%) and stability under various conditions further support its utility in clinical settings (Ikezono et al., 2010; Kubota et al., 2024). CTP may be a reliable marker for diagnosing PLF and aiding in patient selection for surgery, but it is currently available only in Japan.
In the past 30 years, interest in PLF seems to be waning (Figure 1). There has not been a significant change in annual publications, which has stabilized around 15–20 articles per year. Meanwhile, annual publications on MD and BPPV have more than doubled (Figure 2). During this time, the Bárány Society released diagnostic criteria consensus for these disorders, including for MD in 2015, BPPV in 2017, SSCD in 2021, and VN in 2022 (Staab et al., 2017; Lopez-Escamez et al., 2015; Strupp et al., 2022; Ward et al., 2021). These documents aided to better identify and distinguish patients who may have previously been erroneously diagnosed with PLF from those with similar conditions. No consensus has been reached regarding a diagnostic schema for PLF. Moreover, the diagnosis is often questioned, given the lack of reliable symptoms, and the research landscape remains one of controversy (Hornibrook, 2012).
Without clear diagnostic guidelines, the burden of proof required to diagnose and operate is variable and specific to the surgeon considering a particular case. Clinical counseling is also inconsistent, for while some practitioners deny the existence of PLF altogether, others are as cautious as to advise patients against flying after stapedectomy or with the common cold due to increased susceptibility to pressure changes (Friedland and Wackym, 1999; Meyerhoff, 1993; Klokker and Vesterhauge, 2005; Akhal and Bassim, 2023). Overall, the range of opinions and seemingly unavailable means of proving or disproving PLF may be contributing to the decreased interest in publishing related papers, which could reflect a shift in regarding the condition as an entity studied and reported on in academic circles to a diagnosis of exclusion that lies more so in the hands of individual practitioners.
4 Discussion
The definition of PLF has changed over time and remains contentious. While PLF was initially introduced as a complication of stapes surgery, over time, the term evolved into a comprehensive diagnosis for a variety of inner ear symptoms, such as sudden sensorineural hearing loss, episodic vertigo, and tinnitus following various forms of trauma. Refinements in the diagnostic criteria for similarly presenting conditions like MD and SSCD, as well as advances in imaging technology and otologic testing, may have removed a subset of cases that could have been diagnosed as PLFs in the past. Other otologic pathologies seem to have captured the interest of academics and researchers in favor of PLF, perhaps due to gains in refining the diagnoses.
Since the height of PLF publications in the late 1990's, the downward and relatively stable trend in recent decades reflects a shifting interest in studying this condition. Advances in neurotology research and introducing diagnostic criteria for other inner ear conditions have helped distinguish these from PLF better. Once a diagnosis of PLF is suspected, the decision to operate is contentious, particularly in spontaneous cases, because there is no clear test apart from surgical exploration to confirm the presence of a PLF. Surgery is also the means of sealing the defect. Although the operation carries minimal risk, the procedure is nevertheless invasive and unnecessary if performed in a case misdiagnosed as having a PLF. For cases with a highly suggestive iatrogenic cause, such as after stapes surgery, the index of suspicion for PLF is higher, increasing the likelihood of finding a PLF during surgery that can then be repaired.
In recent decades, there was no significant trend in the number of PLF cases by category. However, the correlation coefficient was negative across all categories, which may reflect a waning interest in the topic. In the past 30 years, the number of case reports mentioning spontaneous PLF has decreased (Supplementary Figure 1), while those referencing iatrogenic cases or those with an identified inciting insult has increased (Supplementary Figure 2). Furthermore, for cases identified in the literature for which there is a detailed patient history, the majority (74.8%) identify an inciting event, including prior stapes surgery, barotrauma, and other causes of increased pressures. This may reflect a shift toward diagnosing PLF as a complication of otologic surgery and rare trauma to the ear rather than as a spontaneous condition describing cases of vertigo, ear fullness and hearing fluctuation. The spontaneous PLF cases which were more common in the late 1990's and are now seldom seen may have been individuals with other diagnoses for which there are now clearer diagnostic criteria.
Overall, the number of reported PLF cases with an inciting event (category 2, n = 3,257) far outweighs the number of spontaneous/idiopathic cases (category 1, n = 1,099). This may reflect a greater incidence of iatrogenic/incited PLF compared to idiopathic, or the reality that it is easier to diagnose PLF as an iatrogenic sequela compared to those cases in which it is a diagnosis of exclusion and termed “idiopathic” or “spontaneous” PLF. Given the rarity of spontaneous or idiopathic PLF cases, efforts to develop diagnostic criteria might be constrained to cases with an inciting insult or with suspected iatrogenic causes. In addition, while the role of VEMPs as a diagnostic tool is promising, there are no case-control studies assessing the specificity of a reduced VEMP for PLF compared to similar conditions such as SSCD. Clarifying the specificity of reduced VEMPs for PLF would help to inform the function of this test in future diagnostic guidelines.
If a diagnostic consensus is reached and diagnostic criteria are set forth for PLF, the condition may see newfound interest from academics and another surge in publications. Furthermore, consensus adds validity to the disorder. Without clear criteria, the appropriateness of operating on those cases where the diagnosis is established as one of exclusion remains contentious. As with other uncommon vestibular disorders, creating standardized criteria will likely aid in distinguishing PLF from similar conditions and guide appropriate management.
Author contributions
DP: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. WS: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. RS: Data curation, Investigation, Writing – original draft, Writing – review & editing. DS: Conceptualization, Writing – review & editing. JC: Conceptualization, Supervision, Writing – review & editing. BW: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The project was partially supported by K23 DC018302.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fauot.2024.1479628/full#supplementary-material
References
Akhal, T., and Bassim, M. (2023). Flight after stapes surgery: an evidence-based recommendation. OTO Open 7:e65. doi: 10.1002/oto2.65
Ambrose, J. (1973). Computerized transverse axial scanning (tomography): part 2. Clinical application. Br. J. Radiol. 46, 1023–1047. doi: 10.1259/0007-1285-46-552-1023
Armstrong, B. W. (1967). Sudden deafness in the unoperated ear after stapedectomy. Arch. Otolaryngol. 86, 156–157. doi: 10.1001/archotol.1967.00760050158006
Baber, L., and Cresswell, E. (1900). Sixth International Otological Congress. London: The Southern Publishing Company, Limited.
Bachmann-Harildstad, G., Stenklev, N. C., Myrvoll, E., and Jablonski, G. (2011). β-Trace protein as a diagnostic marker for perilymphatic fluid fistula: a prospective controlled pilot study to test a sample collection technique. Otol. Neurotol. 32, 7–10. doi: 10.1097/MAO.0b013e3181fc74d0
Bisdorff, A., Von Brevern, M., Lempert, T., and Newman-Toker, D. E. (2009). Classification of vestibular symptoms: towards an international classification of vestibular disorders. J. Vestib. Res. 19, 1–13. doi: 10.3233/VES-2009-0343
Black, F. (1986). Quantitative diagnostic tests for perilymph fistulas. Arch. Otolaryngol. Head Neck Surg. 112:257. doi: 10.1001/archotol.1986.03780030021005
Boerner, R., Hatch, J. L., Harruff, E., Nguyen, S. A., Rizk, H. G., Meyer, T. A., et al. (2018). Publishing trends in otology and neurotology. Otol. Neurotol. 39, 127–132. doi: 10.1097/MAO.0000000000001637
Cal, R., and Bahmad, F. Jr. (2009). Vestibular evoked myogenic potentials: an overview. Braz. J. Otorhinolaryngol. 75, 456–462. doi: 10.1590/S1808-86942009000300023
Choi, J. E., Moon, I. J., Kim, H., Lee, K., Cho, Y. S., Chung, W. H., et al. (2017). Diagnostic criteria of barotraumatic perilymph fistula based on clinical manifestations. Acta Otolaryngol. 137, 16–22. doi: 10.1080/00016489.2016.1213419
Colebatch, J. G., Day, B. L., Bronstein, A. M., Davies, R. A., Gresty, M. A., Luxon, L. M., et al. (1998). Vestibular hypersensitivity to clicks is characteristic of the Tullio phenomenon. J. Neurol. Neurosurg. Psychiat. 65, 670–678. doi: 10.1136/jnnp.65.5.670
Delaroche, O., Bordureb, P., and Lippert, E. (1996). Perilymph detection by β2-transferrin immunoblotting assay. Application to the diagnosis of perilymphatic fistulae. Clinica Chimica Acta 245, 93–104. doi: 10.1016/0009-8981(95)06177-0
Fiedler, T., Boeger, D., Buentzel, J., Esser, D., Hoffmann, K., Jecker, P., et al. (2013). Middle ear surgery in thuringia, Germany: a population-based regional study on epidemiology and outcome. Otol. Neurotol. 34, 890–897. doi: 10.1097/MAO.0b013e318280dc55
Friedland, D. R., and Wackym, P. A. A. (1999). critical appraisal of spontaneous perilymphatic fistulas of the inner ear. Am. J. Otol. 20, 261–276.
Gibson, W. P. (1980). The operating microscope and the development of ear surgery. J. R Soc. Med. 73, 53–55. doi: 10.1177/014107688007300112
Hain, T. C. (1997). Limits of normal for pressure sensitivity in the fistula test. Audiol. Neurotol. 2, 384–390. doi: 10.1159/000259263
Harrison, W. H., Shambaugh, G. E., Derlacki, E. L., and Clemis, J. D. (1967). Perilymph fistula in stapes surgery. Laryngoscope 77, 836–849. doi: 10.1288/00005537-196705000-00011
Hennebert, C. A. (1911). new syndrome in her editary syphilis of the labyrinth. Press. Med. Belg. Brux. 63, 467–470.
Hornibrook, J. (2012). Perilymph fistula: fifty years of controversy. ISRN Otolaryngol. 2012:281248. doi: 10.5402/2012/281248
Ikezono, T., Shindo, S., Sekiguchi, S., Hanprasertpong, C., Li, L., Pawankar, R., et al. (2009). Cochlin-tomoprotein: a novel perilymph-specific protein and a potential marker for the diagnosis of perilymphatic fistula. Audiol. Neurotol. 14, 338–344. doi: 10.1159/000212113
Ikezono, T., Shindo, S., Sekiguchi, S., Morizane, T., Pawankar, R., Watanabe, A., et al. (2010). The performance of Cochlin-tomoprotein detection test in the diagnosis of perilymphatic fistula. Audiol. Neurotol. 15, 168–174. doi: 10.1159/000241097
Kessel, J. (1877). Ueber das Mobilisiren des Steigbügels durch Ausschneiden des Trommelfelles, Hammers und Ambosses bei Undurchgängigkeit der Tuba. Archiv f. Ohrenheilkunde 13, 69–88. doi: 10.1007/BF01810218
Klokker, M., and Vesterhauge, S. (2005). Perilymphatic fistula in cabin attendants: an incapacitating consequence of flying with common cold. Aviat. Space Environ. Med. 76, 66–68.
Kubota, T., Ito, T., Furukawa, T., Matsui, H., Goto, T., Shinkawa, C., et al. (2024). Clinical course of five patients definitively diagnosed with idiopathic perilymphatic fistula treated with transcanal endoscopic ear surgery. Front. Neurol. 15:1376949. doi: 10.3389/fneur.2024.1376949
Lauterbur, P. C. (1973). Image formation by induced local interactions: examples employing nuclear magnetic resonance. Nature 242, 190–191. doi: 10.1038/242190a0
Lempert, T., Olesen, J., Furman, J., Waterston, J., Seemungal, B., Carey, J., et al. (2022). Vestibular migraine: diagnostic criteria. J. Vestib. Res. 32, 1–6. doi: 10.3233/VES-201644
Lipkin, A. F., Bryan, R. N., and Jenkins, H. A. (1985). Pneumolabyrinth after temporal bone fracture: documentation by high-resolution CT. Am. J. Neuroradiol. 6:294.
Lopez-Escamez, J. A., Carey, J., Chung, W. H., Goebel, J. A., Magnusson, M., Mandalà, M., et al. (2015). Diagnostic criteria for Menière's disease. J. Vestib. Res. 25, 1–7. doi: 10.3233/VES-150549
Macfie, D. D. (1962). Perceptive deafness sustained while diving: report of a case. Med. Serv. J. Can. 18, 383–387.
Marinelli, J. P., Nassiri, A. M., Habermann, E. B., Lohse, C. M., Holton, S. J., Carlson, M. L., et al. (2021). Underreporting of vestibular schwannoma incidence within national brain tumor and cancer registries in the United States. Otol. Neurotol. 42, e758–e763. doi: 10.1097/MAO.0000000000003049
Matsuda, H., Hornibrook, J., and Ikezono, T. (2023). Assessing the efficacy of perilymphatic fistula repair surgery in alleviating vestibular symptoms and associated auditory impairments. Front. Neurol. 14:1269298. doi: 10.3389/fneur.2023.1269298
Meltzer, P. E., Lindsay, J. R., Goodhill, V., Shambaugh, G. H. Jr., Kos, C. M., and Fowler, E. P. Jr. (1956). Symposium. The operation for the mobilization of the stapes in otosclerotic deafness. Laryngoscope 66, 729–784.
Meyerhoff, W. L. (1993). Spontaneous perilymphatic fistula: myth or fact. Am. J. Otol. 14, 478–481. doi: 10.1097/00129492-199309000-00012
Michel, O., Petereit, H., Klemm, E., and Walther, L. E. (2005). First clinical experience with β-trace protein (prostaglandin D synthase) as a marker for perilymphatic fistula. J. Laryngol. Otol. 119, 765–769. doi: 10.1258/002221505774481228
Modugno, G. C., Magnani, G., Brandolini, C., Savastio, G., and Pirodda, A. (2006). Could vestibular evoked myogenic potentials (VEMPs) also be useful in the diagnosis of perilymphatic fistula? Eur. Arch. Otorhinolaryngol. 263, 552–555. doi: 10.1007/s00405-006-0008-z
O'Byrne, L., Copperthwaite, A., Rente, M., Fenton, J. E., and Coelho, D. H. (2022). The quality of otology and neurotology research in otology journals. Otol. Neurotol. 43, 153–158. doi: 10.1097/MAO.0000000000003425
Rosen, S. (1953). Mobilization of the stapes to restore hearing in otosclerosis. N. Y. State J. Med. 53, 2650–2653.
Rosen, S. (1956). Fenestra ovalis for otosclerotic deafness; an adjunct to stapes mobilization. Am. Med. Assoc. Arch. Otolaryngol. 64, 227–237. doi: 10.1001/archotol.1956.03830150057011
Sarna, B., Abouzari, M., Merna, C., Jamshidi, S., Saber, T., Djalilian, H. R., et al. (2020). Perilymphatic fistula: a review of classification, etiology, diagnosis, and treatment. Front. Neurol. 11:1046. doi: 10.3389/fneur.2020.01046
Schuknecht, H. F. (1962). Sensorineural hearing loss following stapedectomy. Acta Otolaryngol. 54, 336–348. doi: 10.3109/00016486209126953
Shea Jr, J. J. (1958). LXV III fenestration of the oval window. Ann. Otol. Rhinol. Laryngol. 67, 932–951. doi: 10.1177/000348945806700403
Silver, A. J., Janecka, I., Wazen, J., Hilal, S. K., and Rutledge, J. N. (1987). Complicated cholesteatomas: CT findings in inner ear complications of middle ear cholesteatomas. Radiology 164, 47–51. doi: 10.1148/radiology.164.1.3588926
Singleton, G. T., Post, K. N., Karlan, M. S., and Bock, D. G. (1978). Perilymph fistulas: diagnostic criteria and therapy. Ann. Otol. Rhinol. Laryngol. 87, 797–803. doi: 10.1177/000348947808700606
Sood, D., Rana, L., Chauhan, R., Shukla, R., and Nandolia, K. (2017). Superior semicircular canal dehiscence: a new perspective. Eur. J. Radiol. Open 4, 144–146. doi: 10.1016/j.ejro.2017.10.003
Staab, J. P., Eckhardt-Henn, A., Horii, A., Jacob, R., Strupp, M., Brandt, T., et al. (2017). Diagnostic criteria for persistent postural-perceptual dizziness (PPPD): consensus document of the committee for the Classification of Vestibular Disorders of the Bárány Society. J. Vestib. Res. 27, 191–208. doi: 10.3233/VES-170622
Stroud, M. H., and Calcaterra, T. C. (1970). Spontaneous perilymph fistulas. Laryngoscope 80, 479–487. doi: 10.1288/00005537-197003000-00012
Strupp, M., Bisdorff, A., Furman, J., Hornibrook, J., Jahn, K., Maire, R., et al. (2022). Acute unilateral vestibulopathy/vestibular neuritis: diagnostic criteria. J. Vestib. Res. 32, 389–406. doi: 10.3233/VES-220201
Swartz, J. D., and Harnsberger, H. R. (1990). The temporal bone: magnetic resonance imaging. Top. Magnet. Reson. Imag. 2, 1–6. doi: 10.1097/00002142-199009000-00004
Ward, B. K., van de Berg, R., van Rompaey, V., Bisdorff, A., Hullar, T. E., Welgampola, M. S., et al. (2021). Superior semicircular canal dehiscence syndrome: diagnostic criteria consensus document of the committee for the classification of vestibular disorders of the Bárány Society. J. Vestib. Res. 31, 131–141. doi: 10.3233/VES-200004
Weissman, J. L., Weber, P. C., and Bluestone, C. D. (1994). Congenital perilymphatic fistula: computed tomography appearance of middle ear and inner ear anomalies. Otolaryngol. Head Neck Surg. 111, 243–249. doi: 10.1177/01945998941113P113
Zhou, F., Yu, B., Ma, Y., Zhang, T., Luo, J., Li, J., et al. (2023). A bibliometric and visualization analysis of global research on vestibular schwannoma. Am. J. Transl. Res. 15, 755–778.
Keywords: perilymph fistula, vertigo, publication trends, hearing loss, spontaneous vertigo
Citation: Perdomo D, Schoo WW, Stemme R, Schoo DP, Carey JP and Ward BK (2024) Perilymphatic fistula: a historical overview of publication trends. Front. Audiol. Otol. 2:1479628. doi: 10.3389/fauot.2024.1479628
Received: 12 August 2024; Accepted: 27 September 2024;
Published: 09 October 2024.
Edited by:
Angela Schell, University of Heidelberg, GermanyReviewed by:
Gianluca Piras, Gruppo Otologico, ItalyMadalina Georgescu, Carol Davila University of Medicine and Pharmacy, Romania
Copyright © 2024 Perdomo, Schoo, Stemme, Schoo, Carey and Ward. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dianela Perdomo, ZHBlcmRvbTFAamguZWR1
†These authors have contributed equally to this work and share first authorship
 Dianela Perdomo
Dianela Perdomo Wesley W. Schoo
Wesley W. Schoo Rachel Stemme1
Rachel Stemme1 Bryan K. Ward
Bryan K. Ward