- 1Soundwave Hearing, LLC, Oakbrook, IL, United States
- 2Evanston Audiology, PLLC, Evanston, IL, United States
- 3Department of Otolaryngology, Northwestern University, Chicago, IL, United States
- 4Department of Biomedical Engineering, Northwestern University, Evanston, IL, United States
- 5Roxelyn and Richard Pepper Department of Communication Sciences and Disorders, School of Communication, Northwestern University, Evanston, IL, United States
- 6The Hugh Knowles Center for Clinical and Basic Science in Hearing and its Disorders, Northwestern University, Evanston, IL, United States
Introduction: To provide better access to hearing aids and lower the devices' costs for patients with mild to moderate hearing loss, the Food and Drug Administration (FDA) changed its rules for hearing aid distribution, making them available to consumers over-the-counter without the supervision, involvement, or prescription of a licensed health care professional. While this regulation provides some patients direct access to hearing aids, the hearing aid fitting creates challenges for the patients and the hearing aid providers. OTC hearing aids should be programmable outside of a clinical setting. This study demonstrates that the self-fitting of hearing aids does not differ significantly from and is non-inferior to the fitting of the device by a licensed audiologist.
Method: Hearing aid and patient performance after fitting the device by the patient (self-fitting) and a certified audiologist (clinical fitting) were compared in a laboratory setting and a field trial. The laboratory session used a repeated-measures design to assess the reliability and validity of the self-fitting method. A 7–14 days of wear time was used for the field study. The outcome measures for the laboratory session were the differences in acoustical real-ear aided response (REAR). For the wear-time trial, the outcome was the clinical self-report measure of benefit (Abbreviated Profile of Hearing Aid Benefit, APHAB). The benefit of the hearing aid was tested after the self-fitting and the clinical fitting of the hearing aid with a speech-in-noise test (QucikSIN).
Results: The test outcomes showed no statistically significant differences between repeated self-fitting and clinical fitting of the hearing aids. The hearing aid self-fitting was non-inferior to the clinical fitting.
Discussion: It is important to emphasize that the results and conclusion obtained in this study strictly relate to the specific self-fitting process using the Gaussian Process. Many other potential methods for fitting hearing aids exist and future field studies are required to compare the efficacy of the self-fitting methods.
1 Introduction
Hearing loss is a global health crisis. According to the World Health Organization (WHO), over 1.5 billion people globally suffer from hearing loss, 466 million of them disabling (Olusanya et al., 2019; WHO, 2021). The same reports suggest that the numbers will almost double by 2050 (Olusanya et al., 2019; WHO, 2021). Unaddressed hearing loss costs the global economy ~US$ 980 billion annually (WHO, 2021). Furthermore, a recent meta-analysis suggested that hearing loss is a modifiable factor for dementia (Lin and Black, 2017; Loughrey, 2022). Untreated hearing loss correlates with accelerated cognitive decline, anxiety, and depression (Lin et al., 2011; Gallacher et al., 2012; Mener et al., 2013; Chung, 2015; Hopper et al., 2016; Keidser and Convery, 2016; Livingston et al., 2017, 2020). It has been suggested that treating hearing loss will decrease the risk of long-term cognitive decline by 19% (Yeo et al., 2023).
To provide better access to hearing aids and lower the devices' costs for patients with perceived mild to moderate hearing loss, on August 16, 2022, the Food and Drug Administration (FDA) established new rules for the distribution of hearing aids. They can now be distributed to adults “over the counter” (OTC) without the written statement signed by a licensed physician that the patient's hearing has been medically evaluated and the identified hearing loss makes the patient eligible for a hearing aid. While this regulatory change provides some patients direct access to hearing aids, the hearing aid fitting creates challenges for both the patient and the hearing aid providers. OTC hearing aids should be programmable outside of a clinical setting because patients are no longer required to visit or consult an audiologist or physician. In other words, it must be possible to “self-fit” the devices.
Hearing aid fitting typically starts by assessing the patient's audiogram (Hughson and Westlake, 1944; ANSI, 1978; ASHA, 1978; Ravn and Preves, 2015), which provides the base for the hearing aid amplification settings. The settings are assigned using well-known prescriptive standards such as National Acoustic Laboratories (NAL), Desired Sensation Level (DSL), or others using the patient's audiogram (Byrne et al., 2001; Keidser and Convery, 2018). In other words, the audiogram is the starting point for the fitting process. In the following steps, each patient optimizes the hearing aid's settings. Since the fitting is an iterative process that requires the patient's feedback, it is not crucial how well the starting point matches in a hearing aid self-fitting or clinical fitting procedure. The alignment between the audiogram obtained by the audiologist or obtained by the self-fitting procedure is primarily to satisfy the need for documentation. Following this logic, no audiogram would be required for the fitting process. The fitting could start with an arbitrary audiogram, aligning somewhat with the patient's hearing ability. Using such an approach, the fitting will likely take more iterations. We suggest that a good alignment of the results from the self-fitting will optimize the fitting procedure.
Self-fitting of hearing aids is not a novel concept and has been explored under clinical supervision (Convery et al., 2015; Keidser and Convery, 2016, 2018). In this context, a fitting procedure using the Gaussian Process Classification has been proposed to obtain continuous pure-tone threshold curves (Yang et al., 2016; Cox and De Vries, 2021; Boven et al., 2023). The procedure differs from the established hearing aid fitting in a clinical setting, where the hearing aid is adjusted step-by-step, following well-described procedures based on the audiogram. The new procedure combines in-situ pure-tone audiometry with Bayesian statistical inference. Our recent study verified that differences in hearing obtained with pure tone audiometry and the Gaussian Process implemented on a hearing aid are within 3 dB of the standard audiogram (Boven et al., 2023). In this study, the pure tone audiometry that was used as a reference for the self-administered hearing test was given by a licensed audiologist.
This clinical study built on and expanded our previously published results (Boven et al., 2023). It was an effort to validate our method for patients to fit their hearing aids outside of a clinical setting using their hearing aids. The study had an in-lab session and a wear-time trial. The in-lab session used a repeated-measures design to assess the reliability and validity of the self-fitting method. The gold-standard measure for the acoustical function of hearing aids was used to assess the reliability of the method; differences in acoustical real-ear aided response (REAR) between two replications of self-fitting the hearing aids (SF) for the robustness and the differences between the SF and the outcomes after fitting the hearing aids by a licensed clinician (CF) to validate the self-fitting of the hearing aid. Differences were tested for significance and the non-inferiority of the self-fitting. The field trial included 7–14 days of wear time. For the wear-time trial, the primary outcome measure is a widely used clinical self-report measure of benefit (Abbreviated Profile of Hearing Aid Benefit, APHAB). For both the laboratory testing and the field trial, patient performance was quantified by the QuickSIN test, a standardized measure of speech communication in noise.
2 Methods
This study tested the hypothesis that a self-fitting procedure of hearing aids, based on the GP, is non-inferior to the hearing aid fitting by a licensed audiologist. The performance was tested in an over-the-counter hearing aid (Sontro OTC Hearing Aid) in adults with mild to moderate sensorineural hearing loss. The reliability and validity of the self-fitting method were examined in an in-lab session. A single-blinded, counter-balanced wear-time field trial evaluated the validity of the self-fitting method.
2.1 Study participants
2.1.1 Subject number justification and target group
The number of test subjects was estimated before starting the study with G*Power using the median values and the variability obtained from published results. The primary outcome measure of the wear-time field trial, the APHAB, powered the sample size. It is the most variable of the outcome measures across all components of this study. Enrollment targets were set to include an equal number of male and female subjects, a representative balance of race and ethnicity, an age group of 18-75 years (primarily 50–70 years, with an average age of ~60 years), and a mix of prior hearing-aid use (aiming for 70–80% persons with no prior hearing aid use). Reading and comprehending English and providing informed written consent was another inclusion criterion.
2.1.2 Subject recruitment and inclusion criteria
An initial telephone or internet screening of interested persons took place. During this remote screening, prospective subjects provided their age (subject to verification at Visit 1) and answered a Yes/No question about whether they have difficulties in hearing in noise. Those who responded with “No” were not considered for enrollment. Those who answered with “Yes” were asked to describe their perceived hearing loss on a 4-point scale (no difficulties, little difficulties, a lot of difficulties, and they cannot hear). Prospects who answered at the two extremes were not considered for this study. Those who responded with “little difficulties” or “a lot of difficulties” were invited to the in-person screen that immediately preceded the hearing aid fittings during visit 1. A licensed audiologist assessed the hearing threshold for each participant before the study to determine whether to include a prospect. The testing equipment was a standard audiometer at the clinic. The study inclusion criterion was mild-to-moderate bilateral sensorineural hearing loss, with hearing threshold elevations >20 dB at least at one frequency ranging from 250 to 8,000 Hz. Hearing thresholds at 500, 1,000, 2,000, and 4,000 Hz must be ≤ 65 dB hearing level (HL), respectively.
2.1.3 Exclusion criteria
Vulnerable patients were not enrolled in this study. Other exclusion criteria included hearing outside of the limits noted above. Subjects were excluded upon self-reported ear-related pathology, including previous middle ear surgery, head trauma/injury, a family history of non-age-related hearing loss, sudden hearing loss, fluctuating hearing loss, active discharge from the ear, pain, fullness, and history of Ménière's disease. Patients were also excluded during the otoscopy evaluation if excessive ear wax completely covered the tympanic membrane, drainage, tympanic membrane perforation, presence of a foreign body, and infections.
2.1.4 Compensation
For participating in the study, the individuals either received a $500 gift card or could keep the pair of hearing aids used during the study.
2.2 Sequence of study events and data acquisition
2.2.1 Procedures during visit 1 (session 1)
After the patients arrived at the clinic following the telephone or internet screening and invitation to the study, they completed a nine-step study protocol.
Step 1: The inclusion criteria were validated by taking the case history, the ability to read and comprehend English, the patients' willingness to provide written informed consent, and were 18 years old or older.
Step 2: The study and its procedures were explained to the patient, questions by the patient were answered, and written informed consent was obtained. The subjects' payment forms were completed, and relevant demographic information, such as age and gender, was collected.
Step 3: The best receiver in canal (RIC) and open ear dome size were selected by the patient under the guidance of the audiologist to comfortably fit the Sontro® Hearing Aid to the subjects' ears.
Step 4: All subjects completed an unaided (no hearing aids) Abbreviated Profile of Hearing Aid Benefit (APHAB) test using the Harlmemphis.org APHAB program on a tablet.
Step 5: In a sound-reduced enclosure, the AVANT (Stealth) Audiometer (MedRx, Inc., Largo, FL) was used to assess the patients' hearing and to confirm their eligibility for the study. The audiogram also served the audiologist to fit the hearing aids (CF). Thresholds at 3 and 6 kHz were included for the fitting by the audiologist.
Step 6: All subjects completed two practice lists of the QuickSIN test (unaided; lists 1 and 2) using the QuickSIN module of the audiometer, followed by two lists in the unaided condition.
Step 7: All subjects placed the first set of Sontro® Hearing Aids into their ears, connected them to the app on the phone provided, and repeated the hearing test with the hearing aids. The resulting audiogram was stored and used to complete the self-fit prescription called SFA. The procedure was repeated with the second set of Sontro® Hearing Aids. The audiogram was stored and used to complete the self-fit prescription called SFB. The clinician entered the data from each audiogram into the audiometer's Real Ear measurement module to generate upper gain targets at 90 dB HL and lower gain targets at 50 dB HL for all three fitting conditions. These target gains were generated using the second-generation prescription procedures from the National Acoustics Laboratories (NAL) for fitting hearing aids (NAL/NL2).
Step 8: After completion of each fitting condition, the aided QuickSIN scores were determined in the sound-reduced enclosure using two lists, out of all available lists, as directed in a randomization spreadsheet. Half of the subjects wore the devices fitted with the settings obtained by the SFA procedure, and half of the subjects after the device was fitted with the SFB procedure. The other subjects wear the pair of hearing aids fitted by the clinician, the CF procedure. Subjects were blinded to the setting for the field trial. The field trial setting was specified in a randomization spreadsheet.
Step 9: After Visit 1, each subject was provided with one set of hearing aids, a pack of 312 batteries, and a copy of the hearing aid's Quick Start Guide (“QSG”). Questions regarding the QSG were addressed before the patient left for the field trial. Furthermore, each subject demonstrated that they could insert and remove the battery, adjust the volume with the rocker switch on the device, and power on and off the device. Subjects could only fine-tune the adjustments to the hearing aid during the field trial for volume. Upon returning to the clinic for visit two, the hearing aid settings were recorded as “after trial fit”. For the wear-time field trial, subjects were asked to wear the devices a minimum of 2 h per day in a variety of listening situations during the trial, including (1) while listening to music, (2) while watching TV, (3) while using the telephone, (4) while visiting noisy environments such as a restaurant, (5) while talking with a group of people of two or more.
2.2.2 Procedures during visit 2 (session 2)
After the one to two-week wear-time trial, the subjects returned to measure the REAR, completed the aided APHAB, and aided QuickSIN tests.
2.3 Study procedures
2.3.1 Real-ear aided response (REAR)
2.3.1.1 Description of the test
The real-ear-aided response (REAR) is a method to verify the hearing aid's output within 5 mm of the tympanic membrane (Mueller, 2001; Sinclair et al., 2001). During the real-ear measurements, a thin probe microphone was inserted into the ear canal alongside the hearing aid to measure the sound pressure level SPL in dB (re 20 μPa), as a function of frequency, at the specified measurement point in the ear canal, for a specified sound field, with the hearing aid (and its acoustic coupling) in place and turned on. The audiologist recorded the sound levels the user received from the hearing aid. In the clinical setting, the audiologist adjusted the sound levels to match target amplification levels based on the hearing aid user's hearing loss across the speech frequencies.
2.3.1.2 Implementation of the REAR in the study, data analysis, and statistical testing
In this study, probe-tube microphone measures of the REAR were obtained for @65 dB SPL speech input using the real-ear measurement module of the audiometer. Pure-tone levels at 500, 1,000, 2,000, and 4,000 Hz were averaged, and averages were compared between the different experimental groups. To determine the robustness of the self-fitting procedures, REAR values were measured twice after self-fitting the hearing aid, trial A (SFA) and trial B (SFB). Differences between REAR values after the self-fitting (REARSFA-REARSFB) were averaged, and the corresponding standard deviations, standard errors, and 95%-confidence intervals were calculated. The results were tested for normal distribution using the Jarque-Bera test, [h,p] = jbtest(x) (MATLAB, R2022b). The test provides a decision [h, with h = 1, indicating that the data (x) are not normally distributed] and the corresponding probability (p). The significance level was 0.05. The Mann-Whitney U Test (Wilcoxon rank sum test), [p,h] = ranksum(x,y), (MATLAB, R2022b), was used to test the null hypothesis that data in x and y are samples from continuous distributions with equal medians, against the alternative that they are not. Again, the test provided a decision [h, with h = 1 rejecting the null hypothesis] and the corresponding probability (p) for the decision. For the self-fitting procedure's non-inferiority (NI) testing, the NI margins (M) were M1 = −5 dB and M2 = 5 dB. Non-inferiority was established for M1 ≤ 95% CI lower bound and 95% CI upper bound ≤ M2. Significance levels for the 95% CI calculations were adjusted for multiple tests on the dependent variable by applying the Bonferroni method.
The REAR values were also determined after a licensed audiologist (CF) fitted the hearing aid. The average of the differences and corresponding standard deviations, standard errors, and 95%-confidence intervals between the clinical and self-fitting procedures in session 1 (REARCF-(REARSFA+REARSFB)/2). For the wear-time field study, the REAR values obtained in session 1 (S1) during visit 1 were compared with the REAR values in session 2 (S2) during visit 2. Since not every participant had the same procedure, the differences in the averages, mean (REARSF) – mean (REARCF), and the corresponding pooled standard deviations, standard errors, and 95%-confidence intervals were calculated. Results were tested for normal distribution using the Jarque-Bera test with a significance level of 0.05. The Mann-Whitney U Test (Wilcoxon rank sum test) was used to compare differences between the groups with a significance level of 0.05. For the self-fitting procedure's non-inferiority (NI) testing, the NI margins (M) were M1 = −5 dB and M2 = 5 dB. Non-inferiority was established for M1 ≤ 95% CI lower bound and 95% CI upper bound ≤ M2. The significance levels for the 95% CI calculations were adjusted for multiple tests on the dependent variable by applying the Bonferroni method.
The sequence of the hearing aid fitting was randomized for the patients: (1) SFA, SFB, CF; (2) SFA, CF, SFB; and (3) CF, SFA, SFB. An equal number of subjects received each sequence at each site.
2.3.2 Abbreviated profile of hearing aid benefit (APHAB)
2.3.2.1 Description of the test
The APHAB is a 24-item self-assessment inventory. Patients report their difficulties with communication or noises in various everyday situations. The benefit is calculated for each patient by comparing the reported difficulty in the unaided (no hearing aid) with the difficulty in the aided condition (using amplification). The APHAB produces scores for the Ease of Communication (EC), Reverberation (RV), Background Noise (BN), and Aversiveness (AV). The APHAB-global score (GLB), based on all 24 items, increases the reliability of the test.
2.3.2.2 Implementation in the study, data analysis, and statistical testing
Although it is possible to collect unaided and aided scores at the same time by asking the subject to reflect on unaided listening, we obtained unaided APHAB scores during Visit 1 before the initiation of the wear-time trial and determination of the aided APHAB scores during (Visit 2).
The differences in the APHAB scores, aided vs. unaided, provided the benefit (APHABbenefit) of using the hearing aid. They were determined by a licensed audiologist during visit 2 after the field trial that followed the fitting of the hearing aid with the self-fitting procedures SFA and SFB and clinical fitting procedure CF. The differences between average APHABbenefit scores, the corresponding pooled standard deviation, pooled standard errors, and 95%-confidence intervals after the clinical-fitting and self-fitting [mean (APHABbenefit_CF) – mean (APHABbenefit_SF)] served to test for equivalence and non-inferiority of the self-fitting procedure. The NI margin for the differences between the benefits was ≤ 8.4. Results were tested for normal distribution using the Jarque-Bera test with a significance level of 0.05. The Mann-Whitney U Test (Wilcoxon rank sum test) was used to compare differences between the groups with a significance level of 0.05. For the self-fitting procedure's non-inferiority (NI) testing, the NI-margin (M) was M = 8.4. Non-inferiority was established for the 95% CI upper bound ≤ 8.4. Note that significance levels for the 95% CI calculations were adjusted for multiple testing on the dependent variable by applying the Bonferroni method.
2.3.3 QuickSIN
2.3.3.1 Description of the test
The QuickSIN speech-in-noise test provides 12 lists of six sentences to test the ability to understand speech in background noise at six signal-to-noise ratios (SNRs), 25, 20, 15, 10, 5, and 0 dB. Performance is scored using 5 keywords per sentence, resulting in 30 keywords scored per list. The signal-to-noise ratio (SNR) for which 50% of the presented words are intelligible is calculated by subtracting the number of correct words (out of 30) for a given list from 25.5 (Killion et al., 2004). The test is time efficient; administering a single list takes ~1 min. The standard deviation for an SNR estimate using a single list is 1.4 dB. Averaging multiple lists results in a lower standard deviation (Killion et al., 2004).
2.3.3.2 Implementation in the study, data analysis, and statistical testing
At the end of the initial screening during patient visit 1, the SNR loss was determined using two lists presented as practice lists (lists 1 and 2). Out of the remaining 8 lists (lists 3–10), two were randomly assigned to each of the following conditions: unaided (during initial screen; to permit measures of relative benefit), in-lab final SFA, in-lab final SFB, in-lab final CF, and at the end, the one-to-two-week field-trial wear period (Visit 2; either SF or CF). The condition, order, and list pair assigned for each condition were randomized for each subject. Each QuickSIN score was based on two lists, 60 keywords. No list was repeated for a given subject.
All QuickSIN measures, unaided and aided, were binaural. The patients were sitting in a chair in the center of the sound-reduced enclosure facing the speaker, from which the test materials were played (0 degrees azimuth, 1-meter distance). The level was chosen to approximate a typical conversational level (60–65 dB SPL) and match the speech input level used for the REAR measures (65 dB SPL). The level of the co-located background four-talker babble increased across the six sentences for signal-to-noise ratios (SNRs) ranging from +25 to 0 dB SNR (in steps of 5 dB). The subjects were asked to repeat each sentence. The audiologist scored whether the subjects correctly repeated the predetermined keywords in each sentence. The resulting scores were interpreted as an SNR loss where a value near 0 indicates better hearing and larger values indicate more difficulty listening in noise.
The QuickSIN test was given for four conditions: unaided, with the aid of the self-fitted hearing, QSINSFA, and QSINSFB, and after the hearing aid fitting by an audiologist, QSINCF. The results were tested for normal distribution using the Jarque-Bera test with a significance level of 0.05. The Mann-Whitney U Test (Wilcoxon rank sum test) was used to compare differences between the groups with a significance level of 0.05. Non-inferiority of the self-fitting (SFA or SFB) vs. the clinical fitting (CF) procedure was tested after the field trial (QSINCF-QSINSF). For the self-fitting procedure's non-inferiority (NI) testing, the NI-margin (M) was M = −1.5. Non-inferiority was established for the 95% CI lower bound ≥−1.5. The significance levels for the 95% CI calculations were adjusted for multiple tests on the dependent variable by applying the Bonferroni method.
2.4 Study endpoints
The two primary endpoints to test for non-inferiority of the self-fitting procedure vs. the clinical fitting were the outcomes of the REAR measurements and the APHAB score. The three secondary endpoints were the performance on the QuickSIN test. The robustness of the self-fitting procedure was tested using the results from the REAR measurements, the APHAB, and QuickSIN tests.
2.5 The hearing aid
Sontro® Hearing Aids have been used for the clinical study. The device has been designed for users 18 years and older to treat their perceived mild to moderate hearing loss through sound amplification. To meet their hearing needs, hearing aid users can adjust the device's settings without the aid of a hearing care professional. The fitting of the hearing aids is done with an app called otoTune®, installed on the patient's smartphone. The app instructs installing the batteries into the hearing aid battery door. Closing the door activates the Hearing Aid. After the left and the right Hearing Aids were placed into the user's ear canals, the user paired them with the smartphone. If the Hearing Aids were powered on for the first time, they started with basic settings and a small linear gain of < 15 dB. User controls were limited until the self-fitting process with the dedicated fitting feature on the otoTune® app was completed.
During the fitting procedure, the hearing aid presented the users with a series of tones. The user taped the app on the smartphone screen to indicate when or if a tone was heard. Based on the user's responses to these tones, initial gain settings were applied according to the NAL/NL2 fitting algorithm. This self-assessment of hearing loss, described in detail in a previous publication (Boven et al., 2023), does not provide the user with feedback about the accuracy of their responses, nor does it give the user a diagnosis or information about their hearing loss. The information obtained during this process is used internally to fit the device to the NAL/NL2 prescribed gain by frequency in each ear. An important element of the Sontro® Hearing Aid is the possibility of fine-tuning the devices for volume via the rocker switches after the initial fitting. During the self-fitting, the Hearing Aids monitor the broadband background noise level. If the noise level was too loud during the hearing assessment and self-fitting, the user was instructed to repeat the self-fitting in a quieter environment.
2.6 Ethics declaration
All experimental procedures with human subjects followed ethical standards and the 1964 Helsinki Declaration and its later amendments. The study was submitted and approved by BRANY IRB (BRANY File # 22-02-771-1327). Each subject gave informed written consent before participating in this study.
3 Results
3.1 Test subjects
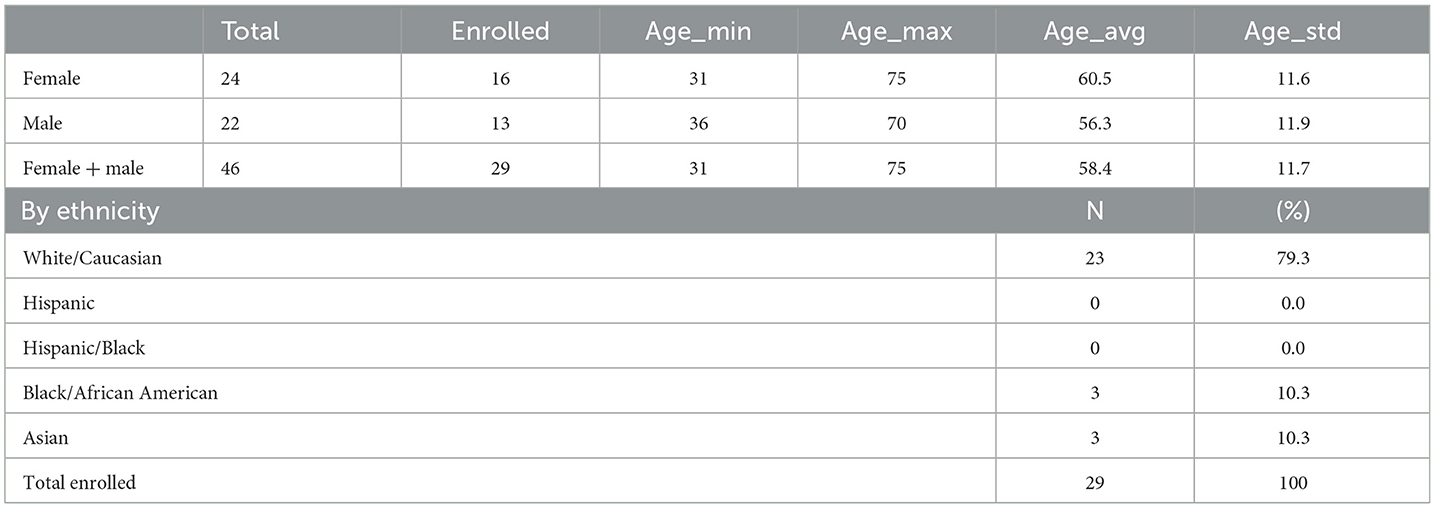
Forty-six potential patients were screened. Twenty-nine, 13 men and 16 women fulfilled the inclusion criteria and enrolled in the study. By ethnicity, 79.3% (N = 23) were White/Caucasian, 10.3% (N = 3) Black/African American, and 10.3% (N = 3) Asian (Table 1). All patients who were enrolled completed the study. The age of the patients ranged from 31 to 75 years, on average 58.9 ± 11.7.
The educational level was a different demographic obtained during enrollment. Participants had a Doctoral degree (N = 1, 3%), a Master's degree (N = 5, 17%), a Bachelor's degree (N = 12, 41%), an Associate degree (N = 2, 7%), and some college education (N = 9, 31%).
The inclusion criterion required at least one hearing threshold >20 dB HL. This selection criterion bears the possibility that for the frequency range between 250 and 8,000 Hz, many individuals may have normal hearing at most or nearly all audiometric frequencies. The audiograms of the left and right ears are shown in Figure 1. With an evident hearing loss of more than 20 dB for frequencies above 1,000 Hz, the device must provide amplification, and the setting of the hearing aid gain through self- vs. clinical fitting is crucial.
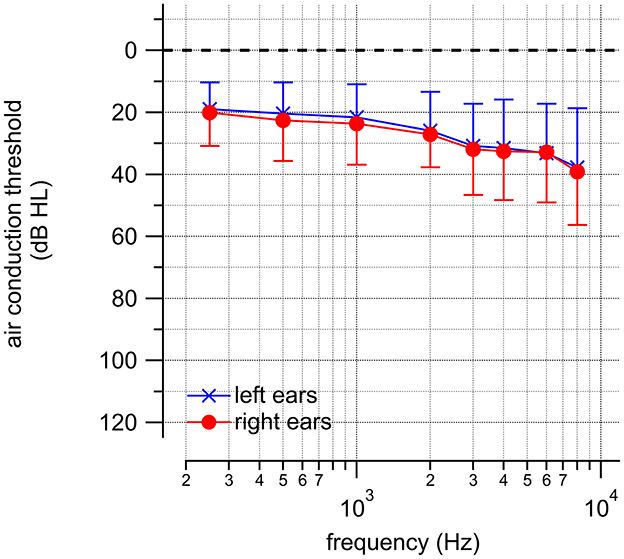
Figure 1. The audiograms obtained from the included participants' left and right ears. The averages ± one standard deviation are shown.
3.2 Real-ear-aided response (REAR)
The sound pressure level in dB relative to 20 μPa (SPL) was determined as a function of frequency at a specific point in the ear canal with the hearing aid in place and turned on. Measurements were completed after the hearing aids were fitted using the SFA, SFB, and CF procedures. They are shown in Figure 2. The results demonstrate that little amplification is required at 500 and 1,000 Hz. Different for 2,000 and 4,000 Hz. REAR outcomes following the self-fitting procedure, trial A and trial B, and the fitting by a licensed audiologist are similar at the selected frequencies. The confidence intervals of the REAR differences after self- and clinical fitting of the hearing aids were within −5 and 5 dB SPL in each frequency band.
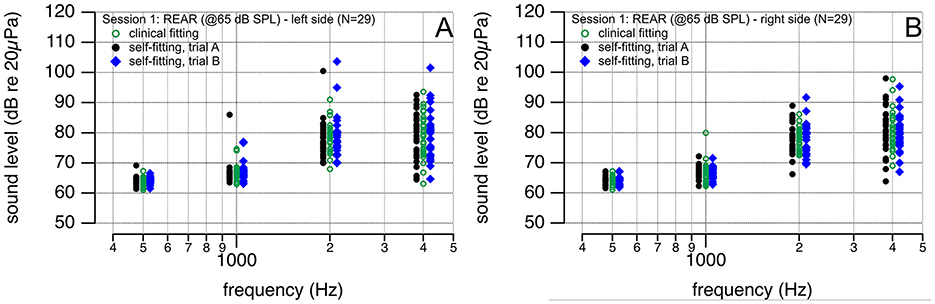
Figure 2. (A,B) REAR (@65 dB SPL) after the hearing aid was self-fitted by the participant (black circles and blue diamonds) and fitted by a licensed audiologist (green circles). Outcomes are similar.
In addition to sound levels at individual frequencies, the average sound levels were then calculated using the results at 500, 1,000, 2,000, and 4,000 Hz. In Figure 3A, the outcomes after fitting the hearing aid using the self-fitting procedures SFA and SFB were compared to document the robustness of the procedure. Figure 3B shows the results following the self-fitting, SF (SF = (SFA + SFB)/2), and the clinical fitting CF procedures. Figure 3C shows the REAR levels after the self- and clinical fitting of the hearing aid during session 1 and the clinic fitting in session 2. The REAR values are comparable for all three conditions after the self- and clinical fitting and for the left and right sides (Figure 3).
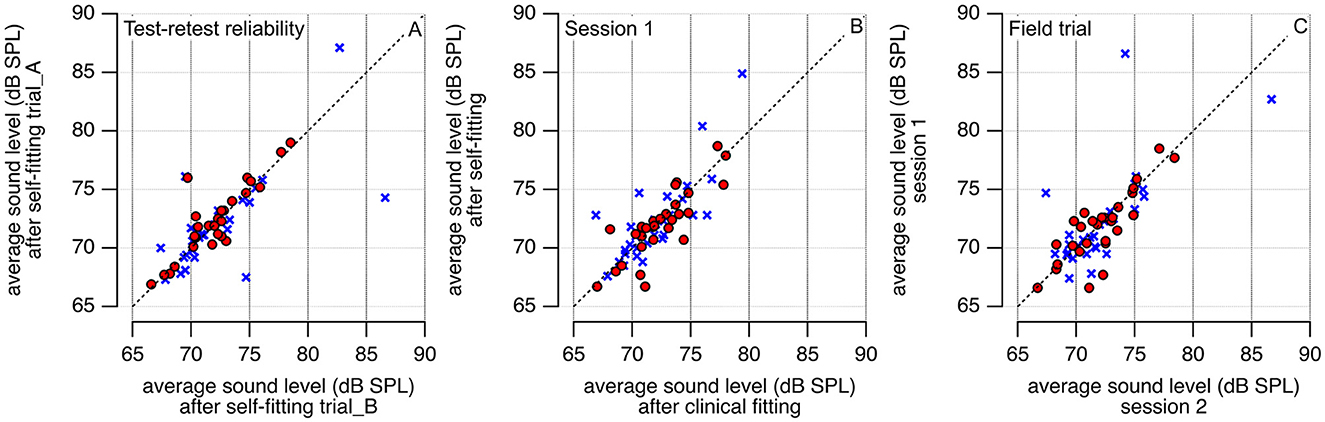
Figure 3. REAR measurement results (@65 dB SPL) after the hearing aid was self-fitted by the participant and fitted by a licensed audiologist. Sound levels at 500, 1,000, 2,000, and 4,000 Hz are averaged. In (A) the participants' test-retest reliability is shown; in (B) the averaged participants' REAR levels after the self-fitting procedure are compared with the results after the clinical fitting. (C) Compares the results from session 1 (S1) with those obtained in session 2 (S2). Red circles are data from the right and blue crosses from the left ear.
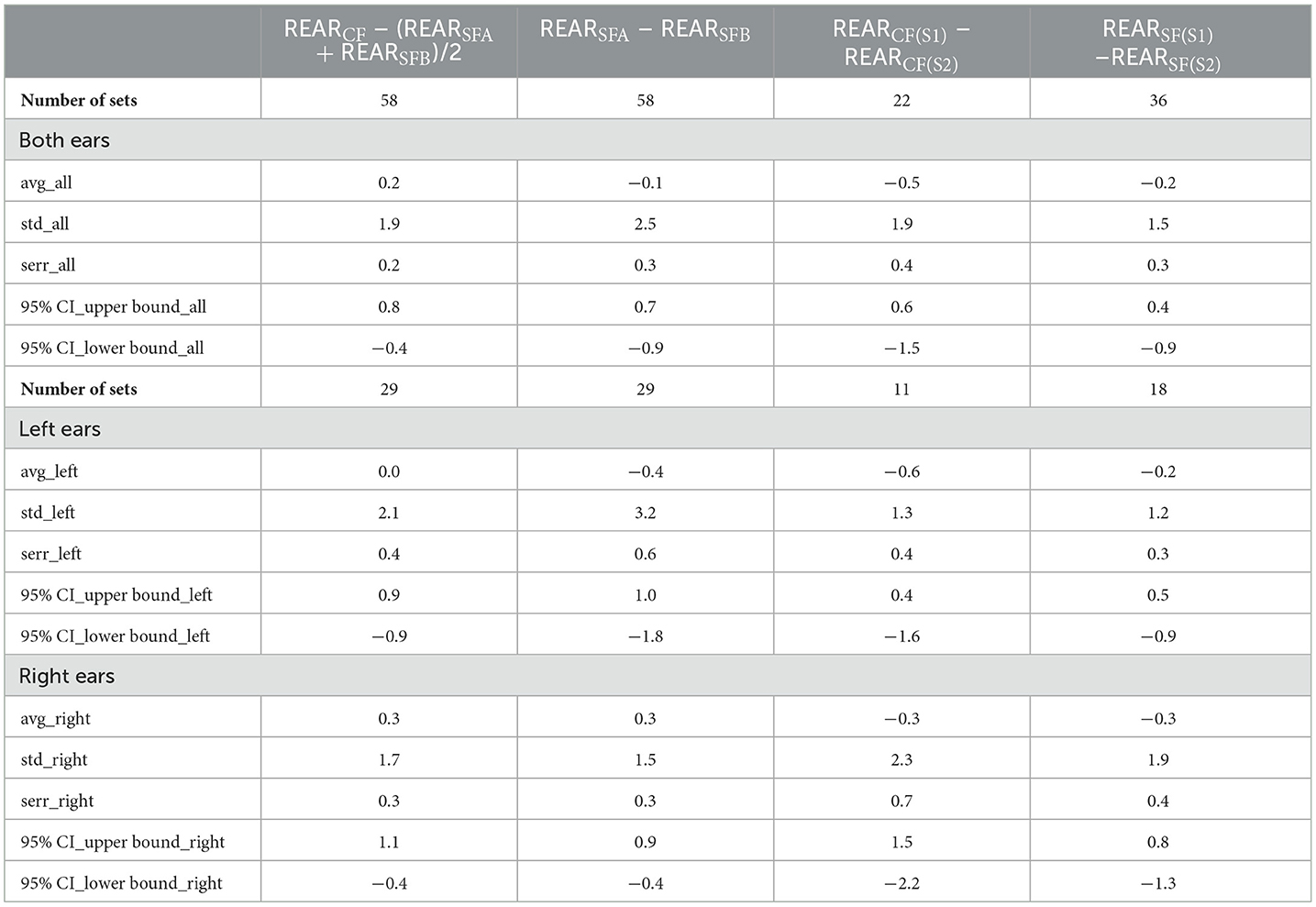
Table 2 shows the averages in sound levels for those frequencies, the differences between the average sound levels following SFA and SFB (REARSFA-REARSFB), and the difference in average sound levels after the SF and the CF in session 1 (REARCF-(REARSFA+REARSFB)/2), and the SF and CF procedure after the field trial (before-after). The 95% confidence intervals (CI) were calculated for both ears and the right and left ears separately.
The Jarque-Bera test showed that all REAR values from the left ear and the combined left and right ear data are not normally distributed. The two-sided Wilcoxon rank sum test was used to test the null hypothesis that data are samples from continuous distributions with equal medians against the alternative that they are not. Results for the different conditions tested are shown in Table 3.
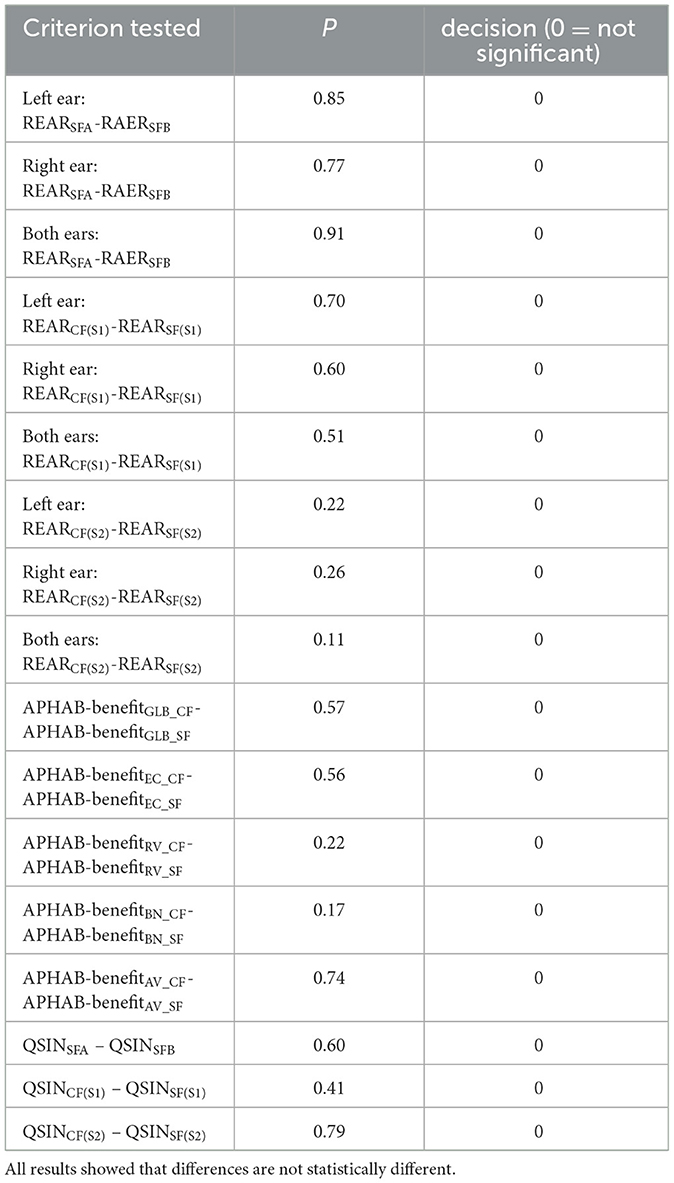
Table 3. Results from the two-sided Wilcoxon rank sum test to test the null hypothesis that data are samples from continuous distributions with equal medians, against the alternative that they are not.
A non-inferiority analysis was conducted to further compare the outcomes of the self-fitting and clinical fitting procedures. The REAR results showed that the self-fitting procedure was non-inferior to the clinical-fitting procedure (Figure 4). For the calculations of the confidence intervals, the significance level was adjusted for the number of analyses on the dependent variable, two primary endpoints, using the Bonferroni method (Table 4).
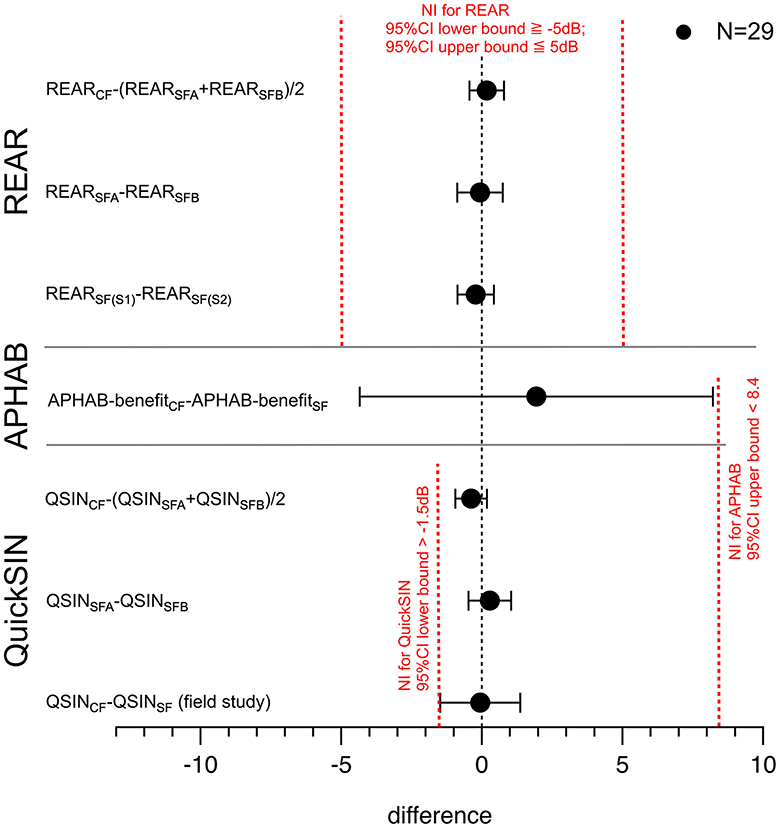
Figure 4. The point estimate of the REAR values (both ears) for the self-fitting procedure is 1.9%, favoring the clinical fit. The figure shows the difference between the means, lower, and upper bound of the 95%-confidence interval. The point estimate of the REAR measurements REARCF-(REARSFA+REARSFB)/2 demonstrates non-inferiority. The upper bound of the 95% CI for clinical-fitting benefit—clinical-fitting benefit is <M, and the lower bound is <0, demonstrating non-inferiority.
S1–S2 shows the difference between the average performance on the QuickSIN test after the clinical fitting and the clinical fitting. Bars show the confidence interval, 95% CI [−1.3, 1.5]. SFA-SFB shows the difference between the average performance on the QuickSIN test after the clinical-fitting trial A (SFA) and the self-fit trial B (SFB). Bars show the confidence interval, 95% CI [−1.1, 1.3]. CF-SF shows the difference between the average performance on the QuickSIN test for the field study. One group had the clinical fitting, and the other had the self-fitting procedure for the hearing aids. Bars show the confidence interval, 95% CI [−1.5, 0.8].
3.3 Abbreviated profile of hearing aid benefit (APHAB)
The APHAB is a self-assessment inventory for patients to rate their challenges with communication or noises in various everyday situations. The scores and benefits of the hearing aid for the two approaches, clinical-fitting and self-fitting, are shown and quantified (Figure 5). Both approaches show an improvement in the APHAB scores for the global (GLB), ease of communication (EC), reverberation (RV), and background noise (BN). The aversion (AV) increases after using the hearing aid for the clinical fitting and the self-fit group. Scores during session 2 (S2) were lower than during session 1 (S1), demonstrating a perceived benefit of the hearing aid use. Scores between the self-fitting and the clinical fitting groups compare, being higher in the clinical-fitting group. Session 2 reflects the self-assessment after 1 week of hearing aid use.
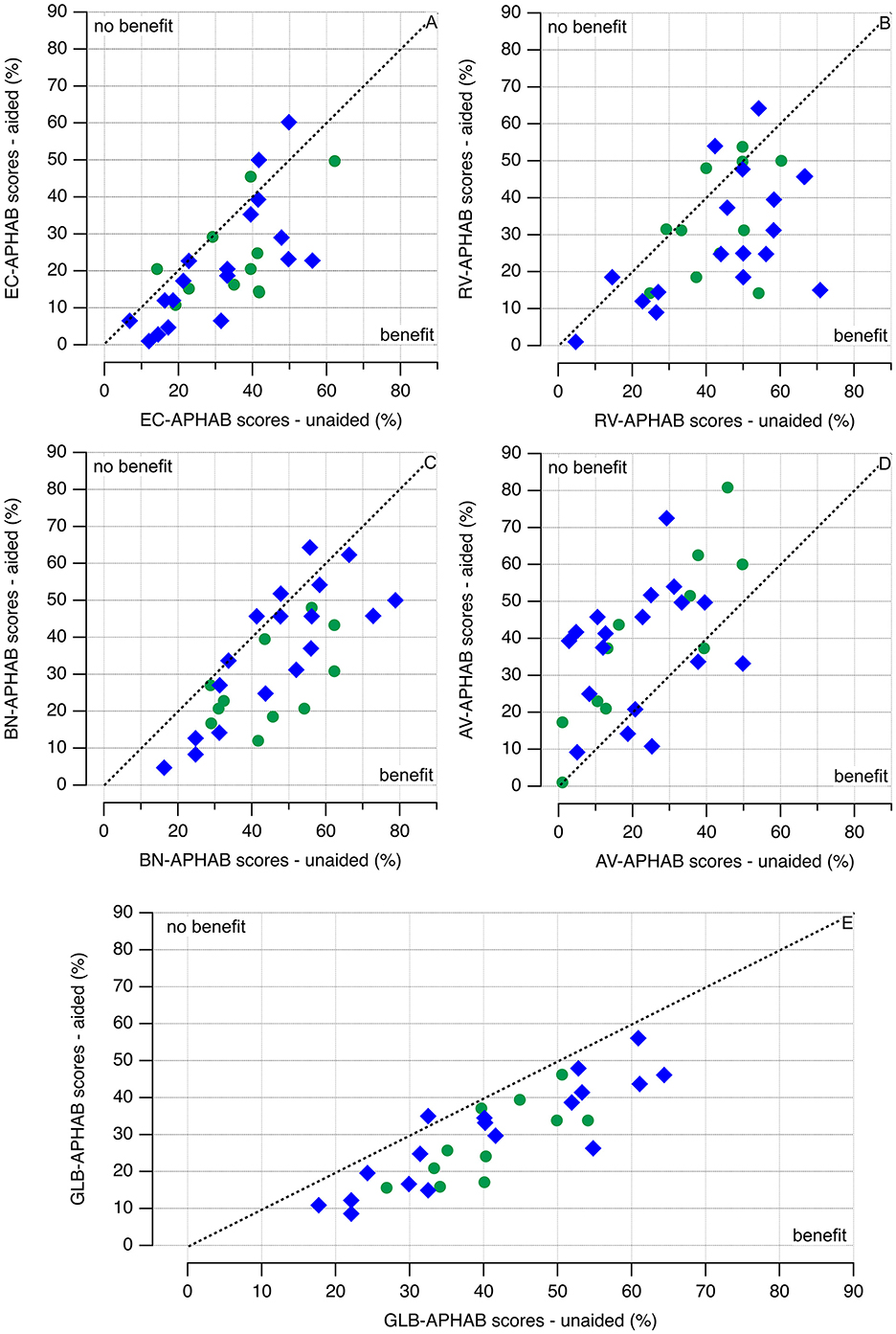
Figure 5. (A–E) APHAB test scores. Blue diamonds indicate that the hearing aid was self-fitted, and green circles indicate the outcomes of a healthcare professional fitting the hearing aid. The APHAB benefit is calculated by subtracting the patient's reported scores (difficulty) in the aided condition from their scores (difficulty) in the unaided condition. The hearing aids benefit if the data points are below the broken line (APHAB scores unaided > APHAB scores aided). While the data are variable, no clear difference between clinical fitting and self-fitting of the hearing aids can be seen from the plots.
A graphic representation of the data is given in Figures 5, 6. Green markers indicate the data obtained from the patients using the clinical fitting, and the red circles show the data from the patients with the self-fitting procedure. All hearing aid fitting procedures show an improvement of the APHAB scores for the global (GLB), ease of communication (EC), reverberation (RV), and background noise (BN). The aversion (AV) increases after using the hearing aid for the clinical-fitting and the clinical-fitting groups. The raw data, averages, and standard deviations are shown in Figure 6. The red markers show the data for the clinical fitting, circles for the raw data, circles with black lines for averages ± one standard deviation, the green markers show the data for the clinical fitting, circles for the raw data, and circles with black lines for averages ± one standard deviation. The cyan diamonds indicate the differences in benefits of using a hearing aid for clinical fitting and self-fitting procedures. Differences >0 favor the clinical fitting, and differences < 0 favor the self-fitting; for differences =0, none of the conditions is favored.
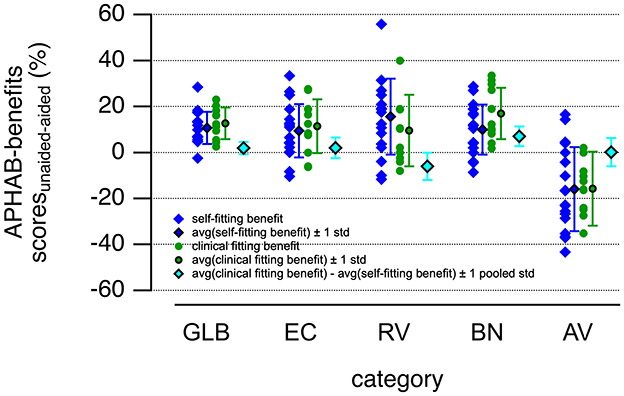
Figure 6. The figure shows the hearing aid benefits obtained by subtracting the APHAB scores for the aided from the unaided condition. The benefits for the clinical-fitting group (green circles) and the self-fitting group (blue diamonds) in sessions 1 and 2 are shown. The averaged benefits for the clinical-fitting group (green circles with black lines) and the self-fitting group (blue diamonds with black lines) in sessions 1 and 2 are plotted next to the raw data. Error bars equal ± one standard deviation. The differences in hearing aid benefits for the self- and clinical fitting procedures were calculated by subtracting the average of the APHAB scores after self-fitting the hearing aids from those obtained after the clinical fitting procedure (cyan diamonds with black lines). The error bars show the pooled standard deviations for the differences. Abbreviations for the sub-categories of the APHAB are global (GLB), ease of communication (EC), reverberation (RV), background noise (BN), and aversion (AV). Averages and standard deviations were calculated.
To test for non-inferiority in hearing aid benefits using the self-fitting and the clinical-fitting procedures, we calculated the averages ± one standard deviation of the benefits determined by the APHAB for the two conditions (Figure 4). The average differences of befit for the hearing aid use are 1.9 ± 6.9, 2.0 ± 11.7, −6.0 ± 15.6, 7.0 ± 11.2, and 0.2 ± 16.1 for GLB, EC, RV, BN, and AV, respectively. Note that the standard deviations reflect the pooled standard deviations for the two groups. For the non-inferiority testing, the confidence intervals are calculated; the upper and lower bounds are shown in Figure 4. Since the sample size in any of the groups is below 30, the value for t-critical was taken from the t-distribution table of critical values with a degree of freedom of 27 (n1 + n2 – 2), with n1 the number of patients in the clinical-fitting group, and n2 the number of patients in the CF group.
The GLB was one of the primary endpoints for our study, with a non-inferiority margin of 8.4%. The average difference for the hearing aid benefits (clinical-fitting minus self-fitting) determined by the global results of the APHAB is 1.9%, with a 95% CI [−4.35, 8.21]. The point estimate of the clinical-fitting benefit—clinical-fitting benefit is 1.9%, favoring the clinical fit. The lower bound, −4.35%, is below 0, and the upper bound of the 95% CI for clinical-fitting benefit–clinical-fitting benefit is 8.21% < M, demonstrating non-inferiority (Figure 4).
3.4 Quick speech-in-noise (QuickSIN) test
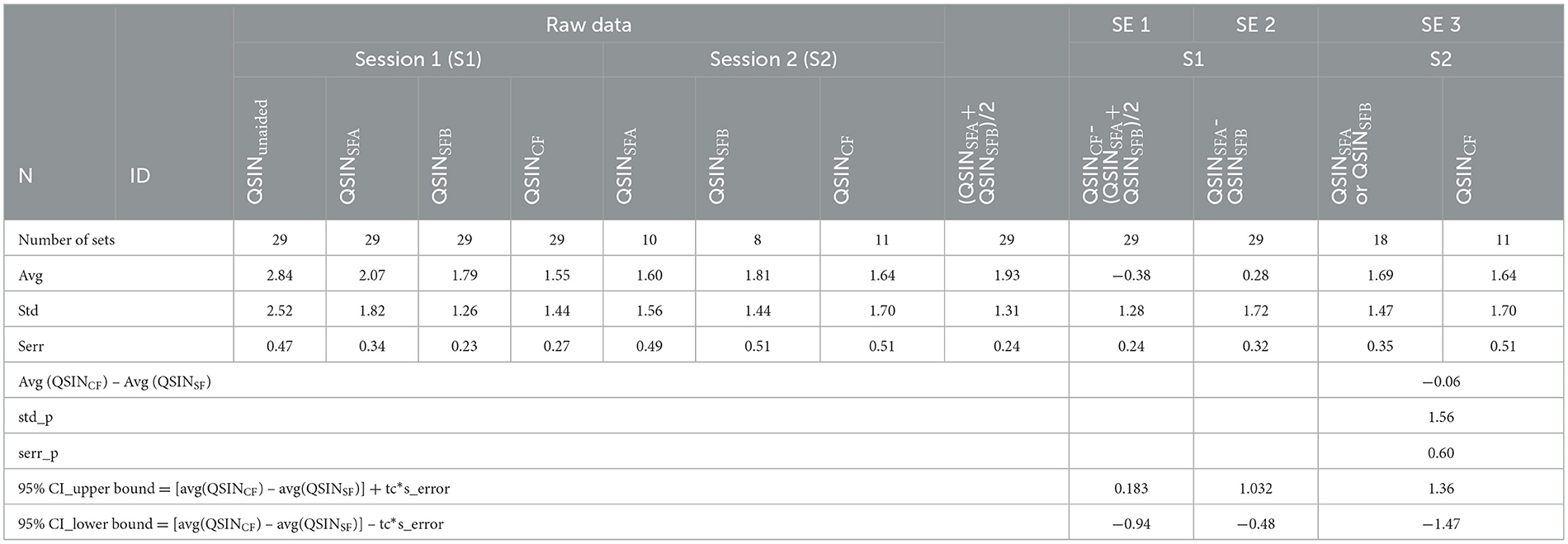
The SNR losses in dB for the subjects participating in the study are shown in Figure 7. For all patients, the performance on the QuickSIN test was first obtained without a hearing aid (QSINunaided). It was then repeated with a hearing aid after fitting it with the self-fitting (QSINSFA and QSINSFB) and the clinical fitting (QSINCF) procedure. The plots in Figure 7A show that hearing aids improve performance for participants with hearing loss. Figure 7B shows the results after the two trials of self-fitting the hearing aids; red circles show the values obtained after SFA, and the blue circles after SFB. Figure 7C compares the performance after using the clinical fitting and self-fitting procedure following the one-week field trial. The results for the three secondary endpoints (SE1 to SE3) are tabulated in Table 4.
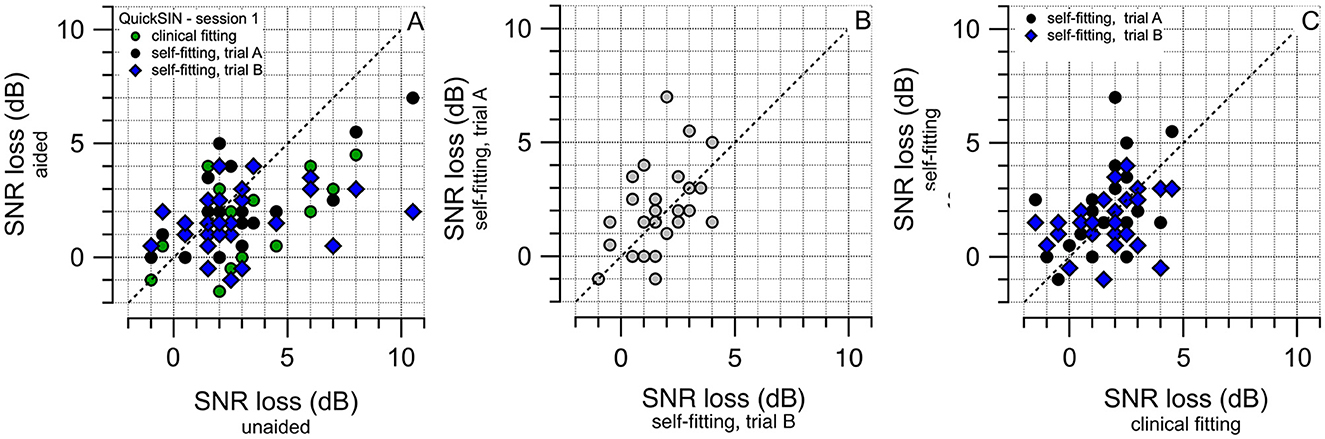
Figure 7. The results of the QuickSIN test after the hearing aid fitting during session 1 are shown. The fitting procedures are the self-fitting, SFA and SFB, and the clinical fitting (CF). (A) Shows the changes before or after the fitting of the hearing aids. The color of the circles indicates the fitting method, SFA (first trial) by the black circles, SFB (second trial) by the blue diamonds, and clinical fitting shown by the green circles. For participants, changes in the aided condition appear favorable over the unaided. (B) Shows the outcomes of the QuickSIN test after the two SF procedures, SFA and SFB. Outcomes are comparable, confirming the test-retest reliability. (C) Shows the outcomes of the QuickSIN test for the field trial (one week of use of the fitted hearing aid). Outcomes between the self-fitting and the clinical fitting are comparable.
While S2-S1(CF) provides the difference between the performance on the QuickSIN test in session 1 and session 2 after the field trial with the clinical fit of the hearing aid, S2-S1(SF) provides the difference after the field trial with a clinical fitting of the hearing aid. The columns with the header SFA-SFB provide the performance in session 1 on the QuickSIN test after the clinical-fitting trials, QSINSFA, and QSINSFB. The columns with the header CF-SF provide the performance in session 1 on the QuickSIN test after the self-fitting (QSINSF) and the clinical fitting (QSINCF) procedure.
The QuickSIN SNR losses in Figure 7A appear considerably lower and more homogeneous than those previously reported in other extensive studies (Fitzgerald et al., 2023; Smith et al., 2024). This may indicate that most of the patients had normal or near-normal hearing. Figure 8 shows QuickSIN SNR losses in our study. They are in agreement with the reported values in the literature. For example, Fitzgerald et al. (2023) demonstrated the relation between the high-frequency pure tone average (HFA), an average of the audiogram at 1,000, 2,000, and 4,000 Hz, and the SNR loss. An HFA < 15 dB HL (normal hearing) has a mean QuickSIN SNR loss of 2.16 dB (range: −3.5 to 13.5 dB), an HFA of 16–25 dB HL (normal hearing) has a mean QuickSIN SNR loss of 3.14 dB (range: −3.5 to 16.5 dB), an HFA of 26–40 dB HL (mild hearing loss) has a mean QuickSIN SNR loss of 5.09 dB (range: −3 to 25.5 dB), and an HFA of 41–55 dB HL (moderate hearing loss) has a mean QuickSIN SNR loss of 8.21 dB (range: −3.5 to 23.5 dB). For the right ears, in our study, the QuickSIN SNR loss was 2.5 dB (HFA: < 15 dB HL), 1.86 dB (HFA: 16–15 dB HL), 3 dB (HFA: 26–40 dB HL), and 7.5 (HFA: 41–55 dB HL); For the left ears it was 2.0 dB (HFA: < 15 dB HL), 1.79 dB (HFA: 16–15 dB HL), 3.14 dB (HFA: 26–40 dB HL), and 6.83 (HFA: 41–55 dB HL).
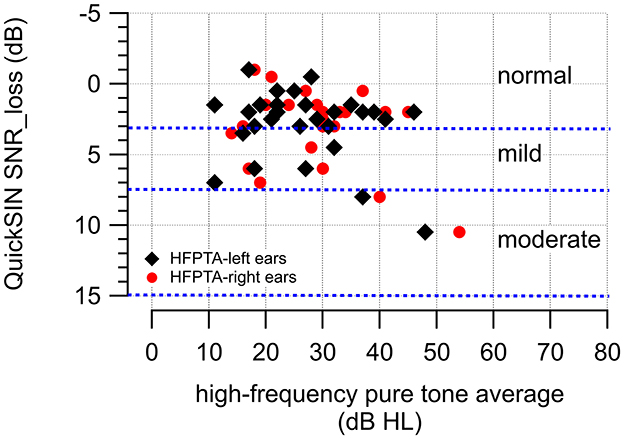
Figure 8. The figure shows the high-frequency pure tone averages obtained for the left and right ears of the participants and the corresponding QuickSIN SNR losses in dB.
To test the non-inferiority of the clinical fitting procedure, the difference in performance on the QuickSIN test was compared with the results after the clinical fitting. The difference in the averages on the QuickSIN was calculated to compare the two methods, including the corresponding pooled standard deviations and confidence intervals (Table 4). The data showed non-inferiority for the clinical-fitting procedure compared to the clinical-fitting procedure (Figure 4).
4 Discussion
The study aimed to validate that the self-fitting of hearing aids is non-inferior to the clinical fitting of the devices. The study results include the REAR values, outcomes from the APHAB test, and the results from the QuickSIN test. All primary and secondary endpoints were reached. The results showed good test-retest reliability and that the self-fitting of the OTC hearing aids is non-inferior to fitting the same hearing aids by a licensed audiologist. OTC hearing aids constitute a viable option in treating mild-to-moderate hearing loss in adults, with the added benefit of lower costs to the patients and the ability to treat in locations with limited access to health care providers. Noteworthy is that the patient's audiogram, which serves as the starting point for the fitting process, was obtained with the hearing aids and the Gaussian Process as described before (Boven et al., 2023).
Hearing aid fitting by a certified health professional starts with establishing a starting condition, typically a patient's behavioral hearing threshold (audiogram). From this initial information, amplification parameters for the hearing aid are deduced. The patient's hearing experience is then adjusted through fine-tuning. For over-the-counter hearing aids, the device fitting process needs the patient, who will accomplish the fitting process. Hereby, the level of the participants' education is a factor that can affect the outcomes of the self-fitting procedure. This study has a significant number of participants with some college education (N = 9, 31%) and a Bachelor's degree (N = 12, 41%). The distribution of the level of education and the relatively small number of study subjects do not allow a decision on how much education affects the outcomes of the self-fitting procedure.
The idea of self-fitting hearing aids is not novel (Köpke et al., 1984). However, barriers to the first concepts included missing sound sources, training algorithms, and additional user controls to further fine-tune the device (Dillion et al., 2006). Hearing aid self-fitting procedures include steps like those of an audiologist. The procedures enable users to perform threshold measurements, leading to a prescribed hearing aid setting and fine-tuning. No audiological support or access to other equipment is required for this procedure (Köpke et al., 1984; Convery et al., 2011a,b,c; Keidser and Convery, 2016, 2018). While self-fitting hearing aids have been commercially available for some time, the challenge for the devices is the simplicity and robustness of the fitting process. Previously published results demonstrated that under controlled conditions, in a sound-reduced environment, the Gaussian process constitutes a fast and robust method to determine a patient's audiogram (Cox and De Vries, 2016, 2021). The audiogram is converted into amplification settings of the hearing aid and serves as the starting point for fine-tuning the hearing aid fitting. We expanded on the concept and have shown that similar results can be achieved in a “field setting” with a patient's hearing aid (Boven et al., 2023).
This study confirmed that self-fitting the hearing aid is non-inferior to an audiologist's fitting of the devices. While the results are reassuring, one must be aware of the limitations of purchasing hearing aids without the involvement of a physician or audiologist. The devices are for treating perceived mild to moderate hearing loss. The patient makes this decision without direct feedback or reports from a professional healthcare provider. Therefore, patients with normal hearing thresholds may use a hearing aid. The device fitting under those conditions, when little to no amplification is required, is difficult, and the benefit of a hearing aid can be limited. Our REAR measurements and the APHAB results demonstrate that the hearing aid can be self-fitted and benefit the patient.
According to large-scale studies, high-frequency pure tone averages (HFPTA) and the performance on a speech-in-noise test (QuickSIN) or word recognition test correlate (Fitzgerald et al., 2023; Smith et al., 2024). While the correlation is obvious, the variability of the results is still large. For example, patients with close to normal HFPTA can have a range of QuickSIN SNR losses found in normal hearing patients and SNR losses in patients with moderate hearing loss. In both scenarios, the patient may decide to use a hearing aid. Even if the patient has normal hearing, it is important that the self-fitting procedure does not overamplify the sound. In this study we tested, by measuring the REAR, the acoustic output of the hearing aid after self-fitting and fitting by an audiologist. We also verified that the results obtained by each patient are repeatable.
An important question is whether a selection bias in the testing procedures and materials exist and might have affected the study outcomes. The QuickSIN test includes 18 lists. The equivalency of the lists was determined by the mean recognition performance of normal hearing- and hearing-impaired listeners (McArdle and Wilson, 2006). Their data showed that nine lists provide homogenous results for normal and hearing-impaired listeners. The performance by normal-hearing listeners was 2.8–4.3 dB SNR and 10–14.3 dB SNR by hearing-impaired listeners. Individual performance for lists 4, 5, 13, and 16 showed high-performance variability for the hearing impaired but not for normal hearing listeners. Consequently, listeners with hearing loss require a more favorable SNR to obtain equal performance. In response to McArdle and Wilson (2006) and Killion et al. (2006), argued that including the non-homogenous lists should not influence the results. For our study, we did not distinguish between homogenous and non-homogenous lists during the testing.
Whether a subjective description of the patient's hearing abilities is sufficient for the hearing aid fitting or if the assessment of the patient's hearing is essential remains to be discussed. Important remains the fine-tuning, where the patients adjust the amplification parameters of the hearing aids for user satisfaction (Dillion et al., 2006).
In summary, it is important to emphasize that the results and conclusion obtained in this study strictly relate to the specific self-fitting process using the Gaussian Process. This method was implemented in the Sontro® Hearing Aids. Since many other potential methods for fitting hearing aids exist, the proposed method should not be extended to a general class called self-fitting or OTC hearing aids. Future field studies are required to compare the efficacy of the self-fitting methods.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were submitted and approved by BRANY IRB (BRANY File # 22-02-771-1327). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
CB: Resources, Project administration, Funding acquisition, Writing – review & editing, Supervision, Software, Methodology, Investigation, Conceptualization. JT: Validation, Resources, Writing – review & editing, Supervision, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. KD: Writing – review & editing, Investigation. C-PR: Conceptualization, Writing – review & editing, Writing – original draft, Methodology, Investigation, Formal analysis, Data curation.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Soundwave Hearing provided funding for the study.
Conflict of interest
CB and JT are members of the Soundwave Hearing technical team. KD is employed at Evanston Audiology, PLLC. C-PR is employed at Northwestern University and served as a consultant to Soundwave Hearing.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
ANSI (1978). Methods for Manual Pure-tone Threshold Audiometry (New York: American National Standards Institute).
ASHA (1978). Guidelines for manual pure-tone audiometry. Am. Speech-Lang.-Hear. Assoc. ASHA 20, 297–301.
Boven, C., Roberts, R., Biggus, J., Patel, M., Matsuoka, A. J., and Richter, C. P. (2023). In-situ hearing threshold estimation using Gaussian process classification. Sci. Rep. 13:14667. doi: 10.1038/s41598-023-40495-w
Byrne, D., Dillon, H., Ching, T., Katsch, R., and Keidser, G. (2001). NAL-NL1 procedure for fitting nonlinear hearing aids: characteristics and comparisons with other procedures. J. Am. Acad. Audiol. 12, 37–51. doi: 10.1055/s-0041-1741117
Chung, S. (2015). Association between sudden sensorineural hearing loss and anxiety disorder: a population-based study. Eur. Arch. Oto-Rhino-Laryngol. 272, 2673–2678. doi: 10.1007/s00405-014-3235-8
Convery, E., Keidser, G., Dillon, H., and Hartley, L. (2011a). A self-fitting hearing aid: need and concept. Trends Amplif. 15, 157–166. doi: 10.1177/1084713811427707
Convery, E., Keidser, G., and Hartley, L. (2011b). Perception of a self-fitting hearing aid among urban-dwelling hearing-impaired adults in a developed country. Trends Amplif. 15, 175–183. doi: 10.1177/1084713811424886
Convery, E., Keidser, G., Hartley, L., Caposecco, A., Hickson, L., and Meyer, C. (2011c). Management of hearing aid assembly by urban-dwelling hearing-impaired adults in a developed country: implications for a self-fitting hearing aid. Trends Amplif. 15, 196–208. doi: 10.1177/1084713811431694
Convery, E., Keidser, G., Seeto, M., Yeend, I., and Freeston, K. (2015). Factors affecting reliability and validity of self-directed automatic in situ audiometry: implications for self-fitting hearing AIDS. J. Am. Acad. Audiol. 26, 5–18. doi: 10.3766/jaaa.26.1.2
Cox, M., and De Vries, B. (2016). A Bayesian binary classification approach to pure tone audiometry. arXiv [preprint] arXiv:1511.08670v2. doi: 10.48550/arXiv.1511.08670
Cox, M., and De Vries, B. (2021). Bayesian pure-tone audiometry through active learning under informed priors. Front Digit Health 3, 723348. doi: 10.3389/fdgth.2021.723348
Dillion, H., Zakis, J. A., Mcdermott, H., Keidser, G., Dreschler, W., and Convery, E. (2006). The trainable hearing aid: What will it do for clients and clinicians? Hear. J. 59, 30–36. doi: 10.1097/01.HJ.0000286694.20964.4a
Fitzgerald, M. B., Gianakas, S. P., Qian, Z. J., Losorelli, S., and Swanson, A. C. (2023). Preliminary guidelines for replacing word-recognition in quiet with speech in noise assessment in the routine audiologic test battery. Ear Hear. 44, 1548–1561. doi: 10.1097/AUD.0000000000001409
Gallacher, J., Ilubaera, V., Ben-Shlomo, Y., Bayer, A., Fish, M., Babisch, W., et al. (2012). Auditory threshold, phonologic demand, and incident dementia. Neurology 79, 1583–1590. doi: 10.1212/WNL.0b013e31826e263d
Hopper, T., Slaughter, S. E., Hodgetts, B., Ostevik, A., and Ickert, C. (2016). Hearing loss and cognitive-communication test performance of long-term care residents with dementia: effects of amplification. J. Speech Lang. Hear. Res. 59, 1533–1542. doi: 10.1044/2016_JSLHR-H-15-0135
Hughson, W., and Westlake, H. (1944). Manual for program outline for rehabilitation of aural casualties both military and civilian. Trans. Am. Acad. Ophthalmol. Otolaryngol. 48(Suppl.), 1–15.
Keidser, G., and Convery, E. (2016). Self-fitting hearing aids: status quo and future predictions. Trends Hear. 20:2331216516643284. doi: 10.1177/2331216516643284
Keidser, G., and Convery, E. (2018). Outcomes with a self-fitting hearing aid. Trends Hear. 22:2331216518768958. doi: 10.1177/2331216518768958
Killion, M. C., Niquette, P. A., and Gudmundsen, G. I. (2006). Homogeneity of the 18 QuickSIN lists. J. Am. Acad. Audiol. 17, 617–618. doi: 10.3766/jaaa.17.8.8
Killion, M. C., Niquette, P. A., Gudmundsen, G. I., Revit, L. J., and Banerjee, S. (2004). Development of a quick speech-in-noise test for measuring signal-to-noise ratio loss in normal-hearing and hearing-impaired listeners. J. Acoust. Soc. Am. 116, 2395–2405. doi: 10.1121/1.1784440
Köpke, W., Wiener, P., Maas, R., Eggert, A., and Götze, G. W. (1984). Digital Hearing aid and Method. U.S. Patent No. 4,471,171.
Lin, F. R., Metter, E. J., O'brien, R. J., Resnick, S. M., Zonderman, A. B., and Ferrucci, L. (2011). Hearing loss and incident dementia. Arch. Neurol. 68, 214–220. doi: 10.1001/archneurol.2010.362
Lin, V. Y. W., and Black, S. E. (2017). Linking deafness and dementia: challenges and opportunities. Otol. Neurotol. 38, e237–e239. doi: 10.1097/MAO.0000000000001408
Livingston, G., Huntley, J., Sommerlad, A., Ames, D., Ballard, C., Banerjee, S., et al. (2020). Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 396, 413–446. doi: 10.1016/S0140-6736(20)30367-6
Livingston, G., Sommerlad, A., Orgeta, V., Costafreda, S. G., Huntley, J., Ames, D., et al. (2017). Dementia prevention, intervention, and care. Lancet 390, 2673–2734. doi: 10.1016/S0140-6736(17)31363-6
Loughrey, D. G. (2022). Is age-related hearing loss a potentially modifiable risk factor for dementia? Lancet Healthy Longev 3, e805–e806. doi: 10.1016/S2666-7568(22)00252-5
McArdle, R. A., and Wilson, R. H. (2006). Homogeneity of the 18 QuickSIN lists. J. Am. Acad. Audiol. 17, 157–167. doi: 10.3766/jaaa.17.3.2
Mener, D. J., Betz, J., Genther, D. J., Chen, D., and Lin, F. R. (2013). Hearing loss and depression in older adults. J. Am. Geriatr. Soc. 61, 1627–1629. doi: 10.1111/jgs.12429
Mueller, H. G. (2001). Probe microphone measurements: 20 years of progress. Trends Amplif. 5, 35–68. doi: 10.1177/108471380100500202
Olusanya, B. O., Davis, A. C., and Hoffman, H. J. (2019). Hearing loss: rising prevalence and impact. Bull World Health Organ 97, 646-646A. doi: 10.2471/BLT.19.224683
Ravn, G., and Preves, D. (2015). Hearing aid-related standards and test systems. Semin. Hear. 36, 29–48. doi: 10.1055/s-0034-1396925
Sinclair, S., Cole, W., and Pumford, J. (2001). The Audioscan(R) RM500 real-ear hearing aid analyzer: measuring for a successful fit. Trends Amplif. 5, 81–90. doi: 10.1177/108471380100500206
Smith, M. L., Winn, M. B., and Fitzgerald, M. B. (2024). A large-scale study of the relationship between degree and type of hearing loss and recognition of speech in quiet and noise. Ear Hear. 45, 915–928. doi: 10.1097/AUD.0000000000001484
Yang, J., Zhu, H., Choi, T., and Cox, D. D. (2016). Smoothing and mean-covariance estimation of functional data with a bayesian hierarchical model. Bayesian Anal. 11, 649–670. doi: 10.1214/15-BA967
Keywords: over-the-counter, hearing aid, Gaussian Process, self-fitting, hearing loss
Citation: Boven C, Turek J, Dunckley K and Richter C-P (2024) Over the counter hearing aids self-fitting using the Gaussian Process Classification. Front. Audiol. Otol. 2:1437469. doi: 10.3389/fauot.2024.1437469
Received: 23 May 2024; Accepted: 07 October 2024;
Published: 30 October 2024.
Edited by:
Ilona Anderson, MED-EL, AustriaReviewed by:
Sten Olof Martin Hellström, Karolinska Institutet (KI), SwedenMatthew Fitzgerald, Stanford University, United States
Copyright © 2024 Boven, Turek, Dunckley and Richter. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Claus-Peter Richter, Y3JpNTI5QG5vcnRod2VzdGVybi5lZHU=
 Christopher Boven
Christopher Boven Joseph Turek1
Joseph Turek1 Claus-Peter Richter
Claus-Peter Richter