- 1Soundfair Australia Ltd, Mount Waverley, VIC, Australia
- 2Department of Audiology and Speech Pathology, University of Melbourne, Parkville, VIC, Australia
- 3Brain and Hearing, Ear Science Institute Australia, Subiaco, WA, Australia
- 4Curtin Medical School, Curtin University, Perth, WA, Australia
- 5Medical School, The University of Western Australia, Perth, WA, Australia
- 6Curtin School of Allied Health, Curtin University, Perth, WA, Australia
Introduction: The use of telehealth for cochlear implant (CI) and hearing aid service provision has the potential to provide efficient, effective, and equitable services to users. However, clinicians require evidence that remote technologies provide care that is equal, or superior to, standard delivery. There are many outcome measures used across audiology, however there is little consensus for a standardized approach to assessment. This systematic review aims to identify the outcome measures to assess remote technologies for CI and hearing aid users, as a first step in a larger project to develop a core outcome set for remote technologies in CI users.
Methods: A systematic search of seven electronic databases was conducted using a search strategy defined by PICOTS for the research question. Eligible studies were in English and published in 2012 onwards. Search strategy, selection, and data collection followed PRISMA 2020 guidelines.
Results: This systematic literature review of 49 articles revealed over 250 discrete outcomes. Outcome measures were grouped into core areas, outcome domains, and outcome sub-domains. Studies assessing remote technology for CI users assessed significantly more outcomes in the ear and labyrinth domain (43% vs. 10%) and studies assessing remote technology in hearing aid users assessed significantly more outcomes in the cognitive (28% vs. 5%) and emotional (35% vs. 10%) functioning domains. Outcome measures within the auditory functioning domain were also significantly different, with CI studies utilizing more speech perception measures (95% vs. 21%) and hearing aid studies utilizing significantly more self-reported outcome measures (73% vs. 19%).
Discussion: The inclusion of hearing aid studies was to ensure that all key outcome domains used within remote hearing rehabilitation were captured, as well as to compare differences in outcome domains between the two user groups. There were significant differences between studies of remote technologies for CI and hearing aid users. These results will inform the ongoing development of a core outcome set for remote technologies in CI users.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=370171, identifier: CRD42022370171.
1 Introduction
Cochlear implant (CI) care has traditionally been provided face-to-face, often in centralized specialist locations via a fixed schedule of appointments with little opportunity for personalization. Over the last 5 years there has been an increase in digital technology options to deliver hearing services, including CI services (Kim et al., 2021; Muñoz et al., 2021). CI service options include synchronous telehealth options, including intraoperative CI telemetry, implant programming, assessment of speech recognition and electrode-specific measures (Bush et al., 2016), and asynchronous technology to support CI users with regard to management and review (Bush et al., 2016; Philips et al., 2018; Maruthurkkara et al., 2022). The use of telehealth for CI service provision has the potential to significantly improve the ability for CI services to provide efficient, effective, and equitable services to CI users in a personalized manner. However, despite its potential, the use of telehealth to provide CI services remains limited (Ravi et al., 2018; Fletcher et al., 2019; Bennett et al., 2023a; Chong-White et al., 2023).
The move of telehealth services from research to routine practice is often hampered by limited ability to integrate current clinical practices with telehealth provision, both in terms of clinical practice and funding (Morgan et al., 2022). As part of the translation to clinical practice, it is essential to demonstrate that remote service provision provides equivalent, if not superior, CI service compared to the current standard of face-to-face clinical care (D'Onofrio and Zeng, 2021). This is necessary both for regulatory purposes, as well as to ensure that CI service providers and users have sufficient trust and confidence in the outcomes of the telehealth service to consider using it (Bennett et al., 2024). The use of sensitive and clinically relevant outcome measures to evaluate CI service provision and CI performance delivered via remote technologies is vital to facilitate the provision of evidence-based health care services, allowing stakeholders to make informed decisions about how to best care for their patients (Woods and Burgess, 2021).
Within audiology, there are large numbers of outcome measures used to assess the effectiveness of CIs and hearing aids (Granberg et al., 2014; Akeroyd et al., 2015; Boisvert et al., 2020; Neal et al., 2022). For example, a recent review reported 6,714 discrete assessment measures for hearing ability alone, of which 139 were self-report measures (Neal et al., 2022). Yet there is little consensus among research and clinical professionals on which outcome domains should be assessed, let alone which measures should be used (PriceWaterhouseCoopers, 2017; Schaefer et al., 2017; Woods and Burgess, 2021; Allen et al., 2022). This non-standardized approach makes it difficult to compare results across different studies and services, for example in systematic reviews with meta-analyses.
Over the last decade there has been increasing development and use of core outcome sets (COSs; Clarke and Williamson, 2016). A COS is an agreed standardized set of outcome that should be measured and reported as a minimum dataset for a specific condition, ideally with input from end-users including patients, clinicians, and industry (COMET, 2022). There are several benefits of using a COS in addition to standardizing outcomes for systematic review, which include reducing outcome reporting bias, enhancing transparency and accountability in research, and in clinical decision-making including the development of national or international guidelines. Although a core outcome domain set (CODS) has been defined for hearing aids, which was developed with significant input from clients and consumers (Allen et al., 2022) and based on best practice guidelines (Hall et al., 2015), there is nothing similar for CIs.
The overall aim of this research was to develop a COS to evaluate remote technologies delivered within CI services. This was informed by a systematic approach used in the “roadmap” described by Hall et al. (2015), and successfully used by Allen et al. (2022) to develop a CODS for hearing aids. An important first step in this process is to synthesize the current evidence-base. Here, we present a systematic review for remote digital technologies and the associated outcome measures for both CI and hearing aid clients and services. Hearing aid studies were also included within this systematic review to ensure that all key outcome domains used within remote hearing rehabilitation were captured and considered. It was also deemed important to identify and understand any differences in outcome domains between hearing aid and cochlear implant remote technology.
This systematic review goes beyond the CODS developed by Allen et al. (2022) by also including outcome measures. Therefore, the aim of this systematic review is to identify the outcome measures that have been used to evaluate remote technologies for both cochlear implant and hearing aid users, as well as service outcomes for remote technologies.
2 Methods
This systematic review was prospectively registered with The International Prospective Register of Systematic Reviews (PROSPERO; ID: CRD42022370171). The method was guided by The Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement 2020 (Page et al., 2021). See Supplementary material 1.
2.1 Eligibility criteria
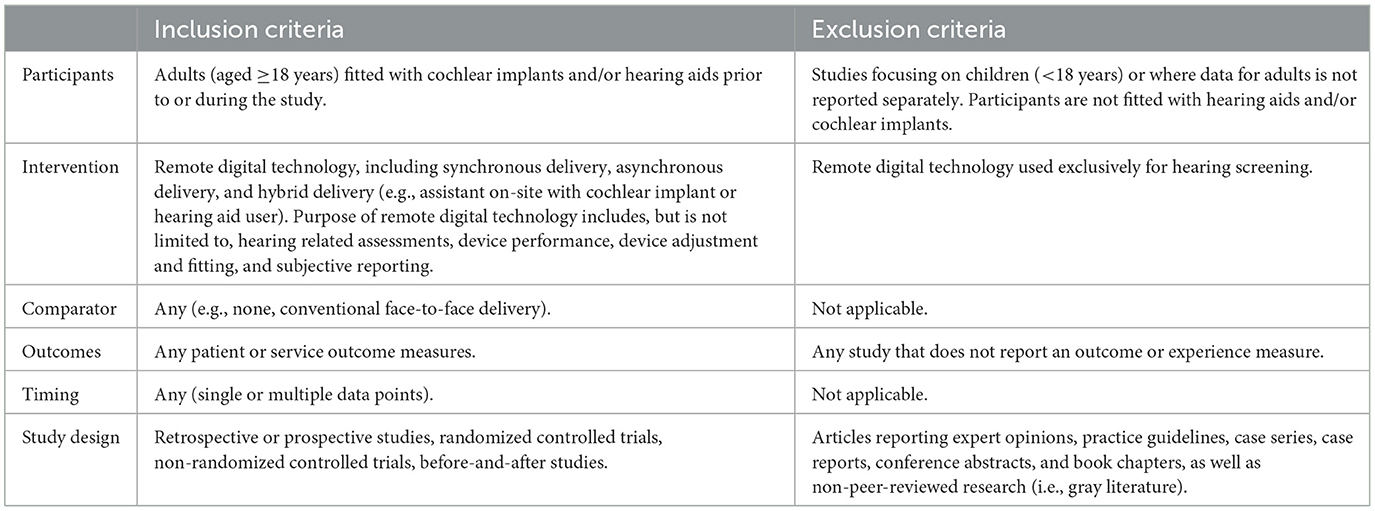
The inclusion and exclusion criteria were selected to answer the research question in relation to participants, intervention, comparator, outcome, timing and study design (PICOTS; Samson and Schoelles, 2012), as shown in Table 1. Additionally, eligible studies were English-language manuscripts published in 2012 onwards in peer-reviewed academic journals.
2.2 Information sources
An initial systematic literature search was conducted on 24th October 2022 by KN via seven electronic databases: MEDLINE, Embase, CINAHL, PsycINFO, Web of Science, Scopus, and The Cochrane Library. Additional publications were sought by KN and EL via expert consultation, reference lists of relevant publications and citation tracking in Google Scholar. Automated search alerts were set up to capture new publications up until 30th January 2024.
2.3 Search strategy
Medical subject headings (MeSH) and manual search terms were used in conjunction with Boolean operators adapted to the requirements of each electronic database, with advice from an academic librarian at the University of Western Australia. Publications were limited to English and the date range was restricted to 2012 onwards. For the search strategy used in each database, see Supplementary material 2.
2.4 Selection process
Search results were exported from each electronic database to an Endnote library where duplicate publications were removed. Publications were imported into Covidence where two authors (KN and EL) independently performed title and abstract screening against inclusion and exclusion criteria (Table 1). Subsequent full text screening was independently performed by KN and EL with an interviewer agreement of Cohen's κ = 0.577 (95% CI: 0.377–0.777) indicating moderate agreement. Disparities (n = 12) were discussed and resolved with additional reviewers (MF and CS).
2.5 Data collection process
A data extraction template was created in Covidence Review Management Software based on the PICOTS for the study question. Two reviewers (KN and EL) independently extracted data from the included studies. One reviewer (EL) used the cross-checking function in Covidence to check for any discrepancies in the extracted data, of which there were only formatting differences found.
2.6 Data items
Items extracted following the PICOTS framework, including participant characteristics (age, gender, pre/post-lingual hearing loss, duration of hearing loss, hearing device, duration of device use, type of fitting e.g., unilateral, bilateral, and bimodal), intervention characteristics (service and delivery information such as description of remote digital technology, synchronous or asynchronous delivery, hybrid, clinician or client-led services, details of intervention purpose e.g., assessment, monitoring, validating, adjusting, and reporting), comparator characteristics (service and delivery information), outcome characteristics (definition and measurement scales for patient or service outcomes), timing (duration of follow-up), and study characteristics (study design, country, year, and purpose). It was planned that missing data would be requested from study investigators, however, this was not necessary.
2.7 Outcome taxonomy
Study outcome details, including the outcome measure, purpose, description, and unit of measurement, were extracted from all studies, and collated. Similar outcome measures were inductively grouped together (e.g., all measures of speech perception), and then classified deductively into the five core areas (death, physiological/clinical, life impact, resource use, and adverse events) and 38 outcome domains as described in the outcome taxonomy developed by COMET (Core Outcome Measures in Effectiveness Trials) and endorsed by Cochrane (Dodd et al., 2018). The proportion of outcomes from studies of hearing aid and cochlear implant remote digital technology were compared in each outcome domain using the “N-1” chi-squared test (Campbell, 2007). Given the heterogeneity of study outcomes and the purpose of the systematic review, further quantitative analysis and meta-analysis were not performed.
2.8 Study risk of bias assessment
Individual study quality was assessed using the Downs and Black Checklist for Measuring Study Quality that contains 27 questions assessing six domains: study quality, external validity, study bias, confounding and selection bias, and power of the study (Downs and Black, 1998). Twenty-six items are answered yes (assigning a score of 1) or no/unable to determine (assigning a score of 0), and one item is scored yes (2), partially (1), or no (0), resulting in a global score range of 0–28. In line with other systematic reviews, quality categories have been described as excellent (26–28), good (20–25), fair (15–19), and poor ( ≤ 14) (Hooper et al., 2008; Silverman et al., 2012). The checklist has shown good internal consistency (KR-20 = 0.89), test-retest reliability (r = 0.88), inter-rater reliability (r = 0.75) and criterion validity (r = 0.90; Downs and Black, 1998). The risk of bias assessment was performed by EL and 10.2% (n = 5) quality assessments were cross checked by MF.
3 Results
3.1 Study selection
The systematic search yielded 214 publications following the removal of 165 duplicate records (Figure 1). Title and abstract screening removed a further 153 studies, with 61 publications undergoing full-text screening against inclusion and exclusion criteria. Twenty-three studies were excluded for incorrect publication type or language (n = 8), inappropriate intervention characteristics (n = 6), lack of study outcomes (n = 2), incorrect participant sample (n = 5) and duplication (n = 2). Expert identification, searching reference lists and automated search alerts yielded a further 17 studies, of which three were subsequently excluded for not including hearing devices and three were excluded for using incorrect interventions. A total of 49 records were included in qualitative synthesis.
3.2 Study characteristics
Twenty-eight studies assessed the outcomes of remote digital technologies for hearing aids, 20 for cochlear implants and one study included both hearing aid and cochlear implant users. The CI and hearing aid cohorts were analyzed separately for this study, giving a total of N = 50 studies used in analysis. Together the studies included 2,724 participants fitted with hearing aids and 624 recipients of cochlear implants. Study publication years ranged from 2012 to 2024 and were conducted in the UK (n = 11), USA (n = 9), Australia (n = 6), Netherlands (n = 6), South Africa (n = 4), Brazil (n = 3), Sweden (n = 2), Turkiye (n = 2), Germany (n = 1), Belgium (n = 1), France (n = 1), Finland (n = 1), Israel (n = 1), Greece (n = 1), Italy (n = 1), and Denmark (n = 1). Study designs included randomized controlled trials (n = 14), pseudo- or non-randomized experimental designs (n = 9), prospective, within-subject, repeated measures studies (n = 12), prospective mixed-method studies (n = 3), qualitative studies (n = 4), a descriptive, cross-sectional study (n = 1), a retrospective cohort study (n = 1), process evaluation (n = 1), and a prospective (n = 1) and retrospective (n = 2) case-control study.
The purpose of the remote digital technologies included hearing aid fitting and programming (n = 15), cochlear implant processor mapping (n = 6), education and communication programs (n = 11), auditory training (n = 4), cognitive training (n = 2), and monitoring of hearing and speech perception (n = 21). Further descriptions of the study sample, participants, remote digital technology, and outcomes are provided in Supplementary material 3.
Together, the 49 studies reported over 250 outcomes which were deductively classified using the COMET taxonomy (Dodd et al., 2018) into core areas (n = 4), and outcome domains (n = 10). The number and percentage of hearing aid and cochlear implant studies are presented for each core area, outcome domain, and outcome sub-domain in Table 2. There were ten studies that reported qualitative results, all of which were categorized into the outcome domains of delivery of care, emotional functioning, and cognitive functioning. Studies of CI remote technologies were present in each of the outcome domains, and hearing aid studies were present in all but the adverse events outcome domain.
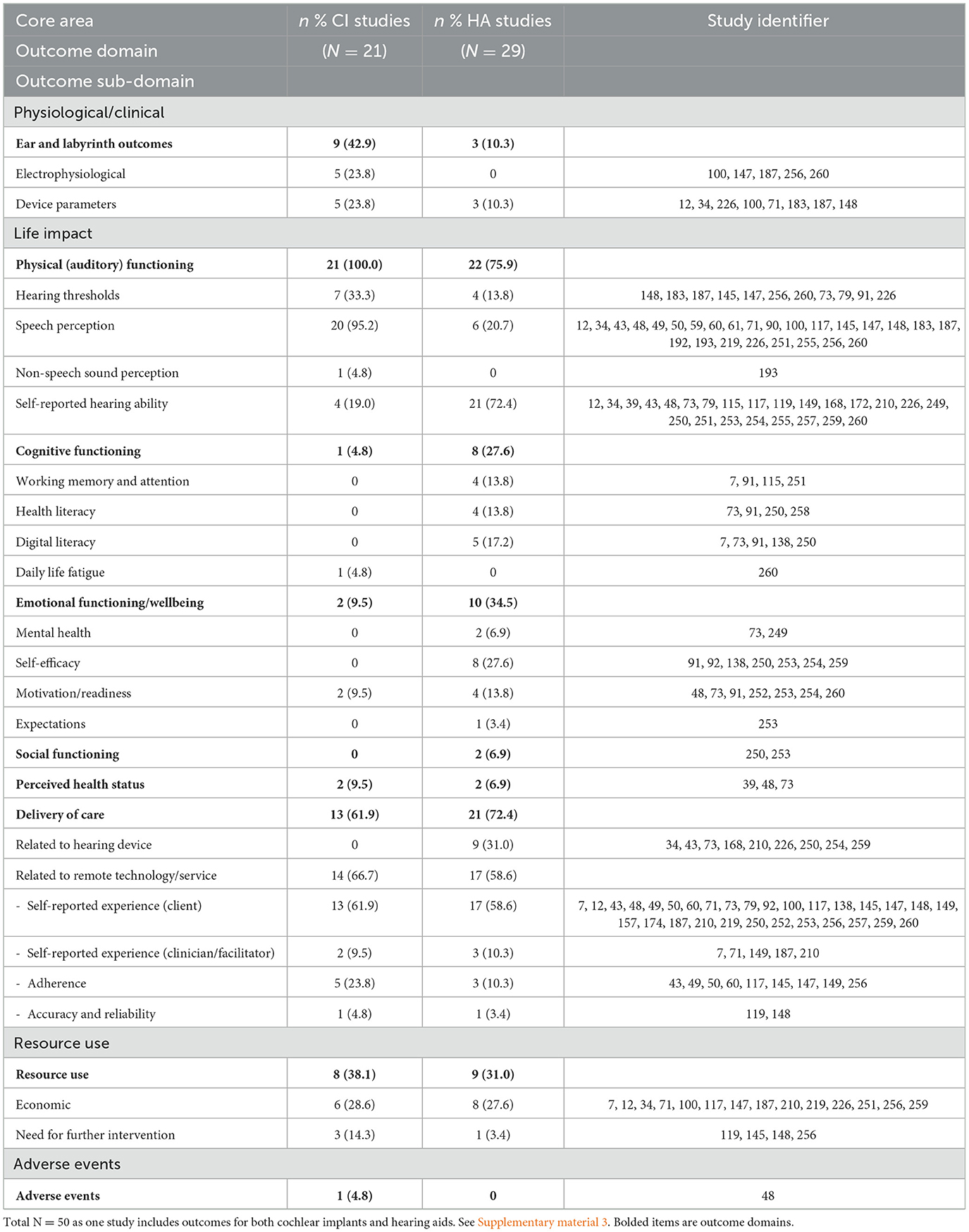
Table 2. Cochlear implant and hearing aid remote technology and service outcomes grouped into core areas, outcome domains and outcome sub-domains.
The proportion of outcomes in each domain and sub-domain varied between studies of remote technologies for CI users and hearing aid users. Compared to studies with hearing aid users, there were significantly more CI studies that assessed outcomes in the ear and labyrinth outcome domain [X2 (1, N = 50) = 6.96, p = 0.008], and auditory functioning domain [X2 (1, N = 50) = 5.77, p =0.016]. Conversely, there were significantly more hearing aid studies that assessed outcomes in the cognitive functioning domain [X2 (1, N = 50) = 4.20 p = 0.040], and the emotional functioning domain [X2 (1, N = 50) = 4.09, p = 0.043]. There were no significant differences in the proportion of outcomes in the general physiological/clinical domain [X2 (1, N = 50) = 1.39, p = 0.238], delivery of care domain [X2 (1, N = 50) = 0.61, p = 0.437], social functioning domain [X2 (1, N = 50) = 1.48, p = 0.224], perceived health status domain [X2 (1, N = 50) = 0.11, p = 0.740], resource use domain [X2 (1, N = 50) = 0.27, p = 0.605], and adverse events domain [X2 (1, N = 50) = 1.39, p = 0.238].
3.3 Risk of bias in studies
The Downs and Black Checklist for Measuring Study Quality was used to investigate individual risk of bias and study quality for the included publications (Downs and Black, 1998). Ten studies were rated as good quality (Ferguson et al., 2016; Cullington et al., 2018; Gomez and Ferguson, 2020; Tao et al., 2020; Venail et al., 2021; Henshaw et al., 2022; Brewer et al., 2023; Coco et al., 2023; Maidment et al., 2023; Malmberg and Hagberg, 2023), seven as poor quality (de Graaff et al., 2016, 2018; Maidment et al., 2019; Schepers et al., 2019; Ratanjee-Vanmali et al., 2020; Carner et al., 2023; Çelikgün and Büyükkal, 2023) and the remaining 32 studies were rated as fair quality. The Downs and Black results are available in Supplementary material 4.
4 Discussion
This systematic review reported the range and type of outcome domains of remote digital technology for CI and hearing aid users. The findings presented here contribute toward a larger project to develop a COS for remote technologies for CI users. The extensive outcome measures from the 49 studies were consistent with other reviews reporting large outcome diversity (Boisvert et al., 2020; Neal et al., 2022). Outcomes were classified using the COMET taxonomy (Dodd et al., 2018) into core areas, outcome domains, and outcome sub-domains. The proportion of studies in each outcome domain were compared to understand the differences between assessment in remote technology for CI and hearing aid users. Even though CI remote technology is the focus of the ongoing larger COS project, it was important to also gather information about hearing aid outcomes to understand any gaps in outcome assessment in this relatively new area of technology. This ensures that all potential outcome domains have been considered for the future COS development.
Notably, there were several differences in the types of outcomes that were assessed for users of these hearing devices. Within the auditory functioning domain, 95% of studies assessing remote CI technology reported speech perception outcome measures, compared to only 20% of hearing aid remote technology studies. However, when auditory functioning was assessed via self-reported outcome measures, there were a greater number of hearing aid studies assessing these outcomes compared to CI studies (73% and 19%, respectively). This is not particularly surprising as traditionally, CI outcomes have focused on the domains of speech perception. However, there is growing evidence that self-report measures offer a more functional real-world outcome, tapping into different mechanisms of benefit to speech perception outcomes (Dietz et al., 2022).
Whilst auditory functioning outcomes were present in all studies of remote technology for CI users, there was a marked reduction of outcomes across emotional functioning and wellbeing (9.5%) and cognitive functioning (4.8%) with no studies in the social functioning domain. These results are consistent with a scoping review of CI outcomes in adults (Boisvert et al., 2020) in which self-reported measures were dominated by measures of auditory ability. Studies have demonstrated the importance of social, emotional, and cognitive functioning in hearing loss and rehabilitation in general (Kricos, 2000; Knudsen et al., 2010; Barnett et al., 2017) so it is important that these psychosocial and cognitive factors are considered going forward. There was only one study (Cullington et al., 2018) that reported adverse event outcomes for CI remote technology, and none in studies of remote hearing aid technology. This may be due to an absence of adverse events occurring, however, there is a dearth of reporting adverse events in the hearing aid literature, identified in Cochrane reviews where both primary and secondary adverse events are required outcome domains (Barker et al., 2016; Ferguson et al., 2017). Adverse event reporting is more prominent in CI research (Crowson et al., 2020; Jinka et al., 2023), therefore it was surprising that this was not reported more frequently in the studies of remote technologies. Therefore, it is not possible to comment on the safety of a remote technology service delivery, and this may act as a barrier to implementation.
Given the nature of remote technology and services, there may be a need to focus on outcomes that are of specific importance in remote delivery. For example, empowerment has recently emerged as feature of remote technologies, given the additional demands on client technological literacy and self-management (Maidment et al., 2019, 2020; Gomez et al., 2022). As with all new emerging concepts, these outcomes also need to be developed and measured. For example, empowerment for hearing health has been recently conceptualized (Gotowiec et al., 2022). This has led to a new patient reported outcome measure on empowerment (EmpAQ), developed according to best practice COSMIN principles (Prinsen et al., 2018) with hearing aid users (Bennett et al., 2023b). As remote technology becomes more prevalent, there may be other outcomes, such as empowerment, that are revealed to be crucial in understanding the benefits of remote CI services. Therefore, the outcomes and outcome domains presented in this review are limited to those that have been measured to date.
Furthermore, there are other considerations specific to the service delivery of remote technologies that are of particular importance during a time when remote technology and service uptake remain low (D'Onofrio and Zeng, 2021). Service outcomes, collated in the delivery of care domain, can give providers assurance of remote technology efficacy, accuracy, and reliability, and may demonstrate benefits to both clients and services, such as reduced time, convenience, and costs. Some recent studies indicate that audiologists are largely positive toward remote technology within service provision for hearing aids, and that the low uptake is a result of system barriers such as infrastructure, funding, and privacy regulations (D'Onofrio and Zeng, 2021; Bennett et al., 2023a). This positivity from audiologists is not reflected in CI clinics, where there is more ambivalence, although there are similar barriers regarding infrastructure (Ferguson et al., 2023). These barriers sit within the resource use domain, which was one of the few outcome domains that had similar representation in both groups, albeit in only around a third of studies of both CI and hearing aid remote technology (31 and 38%, respectively). It is likely then that there may be a need to continue to assess resource use outcomes until more is known about infrastructure and funding requirements to support remote technologies.
Grouping outcomes using the COMET taxonomy (Dodd et al., 2018) allowed for an initial understanding of what type of client and service information is being assessed for remote technology. A strength of using this taxonomy is the ability to compare outcomes across audiology and wider health fields. A weakness of this method, and of domain classification more widely, is that many outcomes measures do not assess isolated domains. For example, the International Outcome Inventory for Hearing Aids (IOI-HA; Cox and Alexander, 2002) was categorized within the physical (auditory) functioning domain in this systematic review. However, there are items within this measure that are also applicable to the delivery of care domain, perceived health status domain and emotional functioning domain. It is important to therefore consider these domain classifications as “best fit” rather than strict categorizations.
5 Conclusion
In conclusion, there are a wide variety of outcomes that have been identified for digital technologies and services for CI and hearing aid users. These outcomes sit within the physiological and clinical domain, functioning (auditory, cognitive, emotional, and social) domain, perceived health status domain, delivery of care domain, resource use domain, and adverse events domain. There are differences in the way outcomes are measured in studies of CI and hearing aid remote technology and services. Finally, the results from this study will inform next steps in the development of a COS for remote technology and services for CI users.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
EL: Data curation, Formal analysis, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing, Visualization. CS: Conceptualization, Funding acquisition, Methodology, Validation, Writing – review & editing. KN: Data curation, Formal analysis, Investigation, Methodology, Validation, Writing – review & editing. MF: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was funded by Cochlear Limited (APAC AU IIR 22001/CTR-17855). The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of the article or the decision to submit it for publication. None of the authors received personal payment from Cochlear Limited.
Conflict of interest
The organizations employing MF and CS have received research funding from Cochlear Limited, Widex A/S, and Sonova. EL was employed by Soundfair Australia Ltd.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fauot.2024.1403814/full#supplementary-material
Supplementary material 1. PRISMA 2020 checklist.
Supplementary material 2. Search strategy.
Supplementary material 3. Summary of studies.
Supplementary material 4. Risk of bias.
References
Akeroyd, M. A., Wright-Whyte, K., Holman, J. A., and Whitmer, W. M. (2015). A comprehensive survey of hearing questionnaires: how many are there, what do they measure, and how have they been validated? Trials 16, 1–1. doi: 10.1186/1745-6215-16-S1-P26
Allen, D., Hickson, L., and Ferguson, M. (2022). Defining a patient-centred core outcome domain set for the assessment of hearing rehabilitation with clients and professionals. Front. Neurosci. 16:787607. doi: 10.3389/fnins.2022.787607
Barker, F., Mackenzie, E., Elliott, L., Jones, S., and de Lusignan, S. (2016). Interventions to improve hearing aid use in adult auditory rehabilitation. Cochr. Datab. Systemat. Rev. 8:CD010342. doi: 10.1002/14651858.CD010342.pub3
Barnett, M., Hixon, B., Okwiri, N., Irungu, C., Ayugi, J., Thompson, R., et al. (2017). Factors involved in access and utilization of adult hearing healthcare: a systematic review. Laryngoscope 127, 1187–1194. doi: 10.1002/lary.26234
Bennett, B., Laird, E., Timmer, B., and Campbel, E. (2024). Development and implementation of national teleaudiology guidelines. Hear. J. 77, 23–27. doi: 10.1097/01.HJ.0001006568.83628.45
Bennett, R. J., Kelsall-Foreman, I., Barr, C., Campbell, E., Coles, T., Paton, M., et al. (2023a). Barriers and facilitators to tele-audiology service delivery in Australia during the COVID-19 pandemic: perspectives of hearing healthcare clinicians. Int. J. Audiol. 62, 1145–1154. doi: 10.1080/14992027.2022.2128446
Bennett, R. J., Larsson, J., Gotowiec, S., and Ferguson, M. (2023b). Refinement and validation of the empowerment audiology questionnaire: rasch analysis and traditional psychometric evaluation. Ear Hear 2023:1449. doi: 10.1097/AUD.0000000000001449
Boisvert, I., Reis, M., Au, A., Cowan, R., and Dowell, R. C. (2020). Cochlear implantation outcomes in adults: a scoping review. PLoS ONE 15:e0232421. doi: 10.1371/journal.pone.0232421
Brewer, D. M., Bernstein, C. M., Calandrillo, D., Muscato, N., Introcaso, K., Bosworth, C., et al. (2023). Teledelivery of aural rehabilitation to improve cochlear implant outcomes. Laryngoscope 2023:31031. doi: 10.1002/lary.31031
Bush, M. L., Thompson, R., Irungu, C., and Ayugi, J. (2016). The role of telemedicine in auditory rehabilitation: a systematic review. Otol. Neurotol. 37:1466. doi: 10.1097/MAO.0000000000001236
Campbell, I. (2007). Chi-squared and Fisher-Irwin tests of two-by-two tables with small sample recommendations. Stat. Med. 26, 3661–3675. doi: 10.1002/sim.2832
Carner, M., Bianconi, L., Fulco, G., Confuorto, G., Soloperto, D., Molteni, G., et al. (2023). Personal experience with the remote check telehealth in cochlear implant users: from COVID-19 emergency to routine service. Eur. Arch. Otorhinolaryngol. 280, 5293–5298. doi: 10.1007/s00405-023-08045-2
Çelikgün, B., and Büyükkal, F. (2023). The effect of remote fitting technology on hearing aid satisfaction. Egypt. J. Otolaryngol. 39:7. doi: 10.1186/s43163-023-00447-7
Chong-White, N., Incerti, P., Poulos, M., and Tagudin, J. (2023). Exploring teleaudiology adoption, perceptions and challenges among audiologists before and during the COVID-19 pandemic. BMC Digit. Health 24:1. doi: 10.1186/s44247-023-00024-1
Clarke, M., and Williamson, P. R. (2016). Core outcome sets and systematic reviews. Systemat. Rev. 5, 1–4. doi: 10.1186/s13643-016-0188-6
Coco, L., Carvajal, S., Navarro, C., Piper, R., and Marrone, N. (2023). Community health workers as patient-site facilitators in adult hearing aid services via synchronous teleaudiology: feasibility results from the conexiones randomized controlled trial. Ear Hear 44, 28–42. doi: 10.1097/AUD.0000000000001281
COMET (2022). Core Outcome Measures in Effectiveness Trials. Available online at: https://www.comet-initiative.org/ (accessed February 24, 2023).
Cox, R. M., and Alexander, G. C. (2002). The International Outcome Inventory for Hearing Aids (IOI-HA): psychometric properties of the English version. Int. J. Audiol. 41, 30–35. doi: 10.3109/14992020209101309
Crowson, M. G., Hamour, A., Lin, V., Chen, J. M., and Chan, T. C. Y. (2020). Machine learning for pattern detection in cochlear implant FDA adverse event reports. Cochl. Implant. Int. 21, 313–322. doi: 10.1080/14670100.2020.1784569
Cullington, H., Kitterick, P., Weal, M., and Margol-Gromada, M. (2018). Feasibility of personalised remote long-term follow-up of people with cochlear implants: a randomised controlled trial. Br. Med. J. Open 8:19640. doi: 10.1136/bmjopen-2017-019640
de Graaff, F., Huysmans, E., Merkus, P., Goverts, S. T., and Smits, C. (2018). Assessment of speech recognition abilities in quiet and in noise: a comparison between self-administered home testing and testing in the clinic for adult cochlear implant users. Int. J. Audiol. 57, 872–880. doi: 10.1080/14992027.2018.1506168
de Graaff, F., Huysmans, E., Qazi, O. U. R., Vanpoucke, F. J., Merkus, P., Goverts, S. T., et al. (2016). The development of remote speech recognition tests for adult cochlear implant users: the effect of presentation mode of the noise and a reliable method to deliver sound in home environments. Audiol. Neuro-Otol. 21, 48–54. doi: 10.1159/000448355
Dietz, A., Heinrich, A., Törmakangas, T., Iso-Mustajärvi, M., Miettinen, P., Willberg, T., et al. (2022). The effectiveness of cochlear implantation on performance-based and patient-reported outcome measures in Finnish recipients. Front. Neurosci. 2022:786939. doi: 10.3389/fnins.2022.786939
Dodd, S., Clarke, M., Becker, L., Mavergames, C., Fish, R., and Williamson, P. R. (2018). A taxonomy has been developed for outcomes in medical research to help improve knowledge discovery. J. Clin. Epidemiol. 96, 84–92. doi: 10.1016/j.jclinepi.2017.12.020
D'Onofrio, K. L., and Zeng, F. G. (2021). Tele-audiology: current state and future directions. Front. Digit. Health 3:788103. doi: 10.3389/fdgth.2021.788103
Downs, S. H., and Black, N. (1998). The feasibility of creating a checklist for the assessment of the methodological quality both of randomized and non-randomized studies of health care interventions. J. Epidemiol. Commun. Health 52, 377–384. doi: 10.1136/jech.52.6.377
Ferguson, M., Brandreth, M., Brassington, W., Leighton, P., and Wharrad, H. (2016). A randomized controlled trial to evaluate the benefits of a multimedia educational program for first-time hearing aid users. Ear Hear. 37, 123–136. doi: 10.1097/AUD.0000000000000237
Ferguson, M. A., Eikelboom, R. H., Sucher, C. M., Maidment, D. W., and Bennett, R. J. (2023). Remote technologies to enhance service delivery for adults: clinical research perspectives. Semin. Hear. 44, 328–350. doi: 10.1055/s-0043-1769742
Ferguson, M. A., Kitterick, P. T., Chong, L. Y., Edmondson-Jones, M., Barker, F., and Hoare, D. J. (2017). Hearing aids for mild to moderate hearing loss in adults. Cochr. Datab. Systemat. Rev. 9:CD012023. doi: 10.1002/14651858.CD012023.pub2
Fletcher, K. T., Dicken, F. W., Adkins, M. M., Cline, T. A., McNulty, B. N., Shinn, J. B., et al. (2019). Audiology telemedicine evaluations: potential expanded applications. Otolaryngol. Head Neck Surg. 161, 63–66. doi: 10.1177/0194599819835541
Gomez, R., and Ferguson, M. (2020). Improving self-efficacy for hearing aid self-management: the early delivery of a multimedia-based education programme in first-time hearing aid users. Int. J. Audiol. 59, 272–281. doi: 10.1080/14992027.2019.1677953
Gomez, R., Habib, A., Maidment, D. W., and Ferguson, M. A. (2022). Smartphone-connected hearing aids enable and empower self-management of hearing loss: a qualitative interview study underpinned by the behavior change wheel. Ear Hear 43, 921–932. doi: 10.1097/AUD.0000000000001143
Gotowiec, S., Larsson, J., Incerti, P., Young, T., Smeds, K., Wolters, F., et al. (2022). Understanding patient empowerment along the hearing health journey. Int. J. Audiol. 61, 148–158. doi: 10.1080/14992027.2021.1915509
Granberg, S., Dahlström, J., Möller, C., Kähäri, K., and Danermark, B. (2014). The ICF Core Sets for hearing loss-researcher perspective. Part I: systematic review of outcome measures identified in audiological research. Int. J. Audiol. 53, 65–76. doi: 10.3109/14992027.2013.851799
Hall, D. A., Haider, H., Kikidis, D., Mielczarek, M., Mazurek, B., Szczepek, A. J., et al. (2015). Toward a global consensus on outcome measures for clinical trials in tinnitus: report from the first international meeting of the COMiT Initiative, November 14, 2014, Amsterdam, The Netherlands. Trends Hear. 19:2331216515580272. doi: 10.1177/2331216515580272
Henshaw, H., Heinrich, A., Tittle, A., and Ferguson, M. (2022). Cogmed training does not generalize to real-world benefits for adult hearing aid users: results of a blinded, active-controlled randomized trial. Ear Hear 43, 741–763. doi: 10.1097/AUD.0000000000001096
Hooper, P., Jutai, J. W., Strong, G., and Russell-Minda, E. (2008). Age-related macular degeneration and low-vision rehabilitation: a systematic review. Can. J. Ophthalmol. 43, 180–187. doi: 10.3129/i08-001
Jinka, S., Wase, S., and Jeyakumar, A. (2023). Complications of cochlear implants: a MAUDE database study. J. Laryngol. Otol. 137, 1267–1271. doi: 10.1017/S0022215123000828
Kim, J., Jeon, S., Kim, D., and Shin, Y. (2021). A review of contemporary teleaudiology: literature review, technology, and considerations for practicing. J. Audiol. Otol. 25:1. doi: 10.7874/jao.2020.00500
Knudsen, L. V., Oberg, M., Nielsen, C., Naylor, G., and Kramer, S. E. (2010). Factors influencing help seeking, hearing aid uptake, hearing aid use and satisfaction with hearing aids: a review of the literature. Trends Amplif. 14, 127–154. doi: 10.1177/1084713810385712
Kricos, P. B. (2000). The influence of nonaudiological variables on audiological rehabilitation outcomes. Ear Hear. 21, 7S−14S. doi: 10.1097/00003446-200008001-00003
Maidment, D., Heyes, R., Gomez, R., Coulson, N. S., Wharrad, H., and Ferguson, M. A. (2020). Evaluating a theoretically informed and co-created mHealth educational intervention for first-time hearing aid users: a qualitative interview study. J. Med. Internet Res. 8:e17193. doi: 10.2196/17193
Maidment, D. W., Ali, Y. H., and Ferguson, M. A. (2019). Applying the COM-B model to assess the usability of smartphone-connected listening devices in adults with hearing loss. J. Am. Acad. Audiol. 30, 417–430. doi: 10.3766/jaaa.18061
Maidment, D. W., Heffernan, E., and Ferguson, M. A. (2023). A randomised controlled clinical trial to assess the benefits of a telecare tool delivered prior to the initial hearing assessment. Int. J. Audiol. 62, 400–409. doi: 10.1080/14992027.2022.2059713
Malmberg, M., and Hagberg, J. (2023). Synchronous remote fine-tuning and follow-up within aural rehabilitation: a randomised controlled trial. Int. J. Audiol. 2023, 1–9. doi: 10.1080/14992027.2023.2188437
Maruthurkkara, S., Case, S., and Rottier, R. (2022). Evaluation of remote check: a clinical tool for asynchronous monitoring and triage of cochlear implant recipients. Ear Hear. 43, 495–506. doi: 10.1097/AUD.0000000000001106
Morgan, S. D., Zeng, F. G., and Clark, J. (2022). Adopting change and incorporating technological advancements in audiology education, research, and clinical practice. Am. J. Audiol. 31, 1052–1058. doi: 10.1044/2022_AJA-21-00215
Muñoz, K., Nagaraj, N. K., and Nichols, N. (2021). Applied tele-audiology research in clinical practice during the past decade: a scoping review. Int. J. Audiol. 60, S4–S12. doi: 10.1080/14992027.2020.1817994
Neal, K., McMahon, C. M., Hughes, S. E., and Boisvert, I. (2022). Listening-based communication ability in adults with hearing loss: a scoping review of existing measures. Front. Psychol. 13:786347. doi: 10.3389/fpsyg.2022.786347
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst. Rev. 10:89. doi: 10.1186/s13643-021-01626-4
Philips, B., Smits, C., Govaerts, P. J., Doorn, I., and Vanpoucke, F. (2018). Empowering senior cochlear implant users at home via a tablet computer application. Am. J. Audiol. 27, 417–430. doi: 10.1044/2018_AJA-IMIA3-18-0014
PriceWaterhouseCoopers (2017). Review of Services and Technology Supply in the Hearing Service Program: Final Report. Adelaide, SA: ProceWaterhouseCoopers Australia.
Prinsen, C. A. C., Mokkink, L. B., Bouter, L. M., Alonso, J., Patrick, D. L., de Vet, H. C. W., et al. (2018). COSMIN guideline for systematic reviews of patient-reported outcome measures. Qual. Life Res. 27, 1147–1157. doi: 10.1007/s11136-018-1798-3
Ratanjee-Vanmali, H., Swanepoel, W., and Laplante-Levesque, A. (2020). Patient uptake, experience, and satisfaction using web-based and face-to-face hearing health services: process evaluation study. J. Med. Internet Res. 22:e15875. doi: 10.2196/15875
Ravi, R., Gunjawate, D. R., Yerraguntla, K., and Driscoll, C. (2018). Knowledge and perceptions of teleaudiology among audiologists: a systematic review. J. Audiol. Otol. 22, 120–127. doi: 10.7874/jao.2017.00353
Samson, D., and Schoelles, K. M. (2012). Chapter 2: medical tests guidance (2) developing the topic and structuring systematic reviews of medical tests: utility of PICOTS, analytic frameworks, decision trees, and other frameworks. J. Gen. Intern. Med. 27(Suppl.)1, S11–S19. doi: 10.1007/s11606-012-2007-7
Schaefer, S., Henderson, L., Graham, J., Broomfield, S., Cullington, H., Schramm, D., et al. (2017). Review of outcomes and measurement instruments in cochlear implantation studies. Cochl. Implant. Int. 18, 237–239. doi: 10.1080/14670100.2017.1353761
Schepers, K., Steinhoff, H. J., Ebenhoch, H., Böck, K., Bauer, K., Rupprecht, L., et al. (2019). Remote programming of cochlear implants in users of all ages. Acta Oto-Laryngol. 139, 251–257. doi: 10.1080/00016489.2018.1554264
Silverman, S. R., Schertz, L. A., Yuen, H. K., Lowman, J. D., and Bickel, C. S. (2012). Systematic review of the methodological quality and outcome measures utilized in exercise interventions for adults with spinal cord injury. Spinal Cord 50, 718–727. doi: 10.1038/sc.2012.78
Tao, K. F. M., Moreira, T. D., Jayakody, D. M. P., Swanepoel, D., Brennan-Jones, C. G., Coetzee, L., et al. (2020). Teleaudiology hearing aid fitting follow-up consultations for adults: single blinded crossover randomised control trial and cohort studies. Int. J. Audiol. 60, S49–S60. doi: 10.1080/14992027.2020.1805804
Venail, F., Picot, M. C., Marin, G., Falinower, S., Samson, J., Cizeron, G., et al. (2021). Speech perception, real-ear measurements and self-perceived hearing impairment after remote and face-to-face programming of hearing aids: a randomized single-blind agreement study. J. Telemed. Telecare 27, 409–423. doi: 10.1177/1357633X19883543
Keywords: cochlear implants, hearing aids, remote technology, outcomes, teleaudiology
Citation: Laird E, Sucher C, Nakano K and Ferguson M (2024) Systematic review of patient and service outcome measures of remote digital technologies for cochlear implant and hearing aid users. Front. Audiol. Otol. 2:1403814. doi: 10.3389/fauot.2024.1403814
Received: 19 March 2024; Accepted: 17 April 2024;
Published: 07 May 2024.
Edited by:
Dayse Tavora-Vieira, Fiona Stanley Hospital, AustraliaReviewed by:
Luis Lassaletta, Madrid Health Service, SpainSten Olof Martin Hellström, Karolinska Institutet (KI), Sweden
Copyright © 2024 Laird, Sucher, Nakano and Ferguson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Melanie Ferguson, bWVsYW5pZS5mZXJndXNvbkBjdXJ0aW4uZWR1LmF1
 Emma Laird
Emma Laird Cathy Sucher3,4,5
Cathy Sucher3,4,5 Melanie Ferguson
Melanie Ferguson