- 1Audiology, The University of Auckland, Auckland, New Zealand
- 2Eisdell Moore Centre, The University of Auckland, Auckland, New Zealand
- 3School of Psychology, Speech and Hearing, University of Canterbury, Christchurch, New Zealand
- 4New Zealand Institute of Language, Brain and Behaviour, University of Canterbury, Christchurch, New Zealand
Background: Untreated hearing loss is a risk factor for age-related cognitive decline and hearing aids have been shown to slow cognitive decline in a population at risk for dementia. This double-blind multiple site randomized trial tested the hypothesis that for older adults with below-average cognition, a “Simple” hearing aid fitting strategy (based on linear amplification with output limiting compression signal processing) would improve hearing and cognition more than a “Standard” approach (adaptive compression-based processing).
Methods: Two hundred and fifty-six adults aged over 65 were screened for cognitive function using the NIH toolbox cognitive battery. Participants with below median age-adjusted fluid composite cognitive scores (<100) were eligible to participate (n = 104). Sixty-seven eligible participants proceeded to trial and were randomized 1:1 to a simple or standard hearing aid fitting. Participants in the Standard group were fitted with hearing aids matched to non-linear real-ear prescription targets (either NAL-NL1 or NL2), while participants in the Simple group were fitted with hearing aids matched to linear prescription targets (NAL-R). Participants and researchers not fitting the hearing aids were blinded to allocation.
Results: Forty-eight participants completed assessments in 12 months. The Standard hearing aid group improved on measures of fluid cognition and hearing. There was a statistically significant difference in fluid cognition scores between groups. The fluid cognition composite score for participants receiving the Simple fitting changed by 3.5 points. Those with the Standard fitting improved by 10.3 points. Hearing outcomes for each group were improved by the same amount.
Conclusion: This is the first study to show that hearing aid fitting strategies using markedly different signal processing result in significantly different cognitive outcomes after 12 months of use. The Standard fitting resulted in greater improvement in cognition than the Simpler fitting which was the opposite result to what had been hypothesized. The results reinforce findings indicating hearing aid benefits for the elderly and that they improve cognition.
1 Introduction
Worldwide, hearing loss affects over 466 million people (World Health Organization, 2021), and 50 million people have dementia (Patterson, 2018). As populations age globally, there will be an increased burden of both age-related hearing loss (World Health Organization, 2021) and dementia (Dawes et al., 2019). Physiological and functional declines in hearing and some aspects of cognition are a normal part of the aging process, but the rate and degree of decline can vary greatly between individuals (Dawes et al., 2019). Cognition can be classified as fluid and crystallized (Salthouse, 2000; Heaton et al., 2014). Dimensions of fluid cognitive performance such as working memory, processing speed, and auditory attention decline with age (Smith, 2016). Crystallized abilities such as general knowledge and vocabulary are preserved and paradoxically may improve with age, possibly due to cultural experience and education (Laumann, 1999; Anstey and Low, 2004; Ben-David et al., 2015; Kavé and Halamish, 2015). If cognitive functioning declines to the extent that it affects daily life, it is defined as dementia. A Lancet Commission report identified hearing loss as the largest (8.2%) of 12 modifiable risk factors for dementia (Livingston et al., 2020). The risk of dementia might be reduced with early intervention for hearing loss (Mulrow et al., 1990; Amieva et al., 2015; Dawes et al., 2015; Nguyen et al., 2017; Mamo et al., 2018; Sanders et al., 2021).
Although studies have shown decreased progression of cognitive decline or improved cognitive performance with amplification (Mulrow et al., 1990; Amieva et al., 2015; Dawes et al., 2015; Nguyen et al., 2017; Mamo et al., 2018; Sanders et al., 2021), others have not (Tesch-Römer, 1997; van Hooren et al., 2005). A meta-analysis found hearing aids were associated with a 19% decrease in hazards of long-term cognitive decline and a 3% improvement in short-term cognitive test scores, but it was concluded that further randomized trials were needed (Yeo et al., 2022). Since that review was published, the results of the ACHIEVE multicenter controlled trial comparing health education to hearing aids have been published (Lin et al., 2023). The results suggest that hearing aid intervention reduced cognitive change in a group with normal cognition at risk for dementia (atherosclerosis) but not in a healthy volunteer group (Lin et al., 2023).
Hearing aids, selected and prescribed by audiologists, use different signal-processing strategies. The simplest approach is linear processing in which the amount of amplification is the same for different levels of sound. Although simple linear processing is available as a programmable option, modern hearing aids default to non-linear processing that differs according to the incoming signal level. There are multiple hearing aid prescriptive formulae that account differently for audibility and comfort of speech. These prescriptions were developed with different goals and reflect the hearing aid signal processing used at the time (Dillon, 2012). The National Acoustics Laboratory—Revised (NAL-R) prescription was developed for use with linear hearing aids (Byrne and Dillon, 1986). Non-linear prescriptions such as NAL Non-linear 1 and 2 [NAL-NL 1 (Dillon, 1999) and NAL-NL2 (Keidser et al., 2011)] were designed for compression processing. The amplification type prescribed may impact cognitive outcomes because of the degree of change to the processed signal, particularly the compression of peaks and troughs of speech to match the dynamic range of the listener. Most hearing aid users appear to have sufficient redundancy in auditory processing to profit from complex processing that compresses signals, but those with cognitive difficulties may not be able to adequately process the modified signals; they may instead experience distortion (Agnew, 1998; Lunner and Sundewall-Thorén, 2007; Ng and Rönnberg, 2020). In older laboratory studies, listeners with poorer cognition seem less able to use the audibility afforded by fast-acting compression across a wide dynamic range than persons with good cognition (Gatehouse et al., 2006; Lunner and Sundewall-Thorén, 2007). In a recent study (Sarant et al., 2020), speech recognition tests were not significantly different between persons with good and low cognitive abilities, but persons with good cognitive abilities performed better when using faster processing (compression release times) on auditory feature and speech in noise tests than those with low cognitive abilities. It has been reported that in background noise, as little as 5% of the variance in hearing aid success may be explained by hearing test results, while 40% may be explained by cognitive test results (Lunner and Sundewall-Thorén, 2007). None of the studies undertaken investigating the longer-term effects of hearing aids on cognition have, thus far, considered the benefit of different types of amplification.
Although not definitive, much of the available evidence reviewed, albeit older, suggests that in the short-term, persons with poorer cognitive function benefit less from complex signal processing such as fast-acting wide-dynamic-range-compression, but long-term, the greater audibility that compression processing provides may result in greater benefit (Souza et al., 2015). Modern adaptive processing combines slow and fast compression of the signal while acting in a linear manner at times, the goal being to balance audibility, intelligibility, comfort, and sound quality (Simonsen and Behrens, 2009). Other complex processing such as frequency lowering and digital noise reduction may also be impacted by cognition (Souza et al., 2015). To test the effects of signal processing type on cognition, we compared Standard adaptive compression-based fittings using non-linear prescriptions (NAL-NL1 or NL2) to a simple linear-based strategy fit to the linear NAL-R prescription (without frequency compression and digital noise reduction only used for comfort). To avoid a ceiling effect in any improvements, the trial was undertaken in older adults with below-average cognition. This is the first study, that we are aware of, to examine the longitudinal effects of different hearing aid amplification strategies on cognitive outcomes.
2 Methods
The methods described were approved by the University of Auckland and the University of Canterbury Human Participants Ethics Committees (reference number 019538). This study was registered as a clinical trial on the Australian New Zealand Clinical Trials Registry (Trial id ACTRN12617001155381, Universal Trial Number U1111-1198-6847).
2.1 Study design
The study was a double-blinded randomized trial with groups randomly assigned 1:1.
2.2 Participants
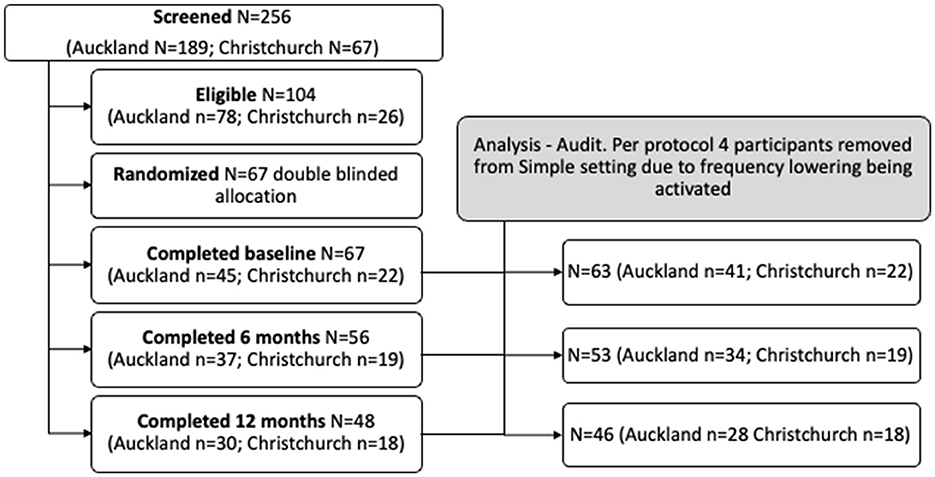
Participants in the trial were aged over 65 years old and attended one of two New Zealand (NZ) University Audiology clinics (University of Auckland, Auckland and University of Canterbury, Christchurch) for their first hearing aids. Participants were recruited through advertisements in community newsletters (print and online). Potential participants using hearing aids within the year prior to baseline testing were excluded. Persons with fluctuating hearing loss or fast-progressing ongoing otologic disease or requiring referrals to psychiatric or mental health services were excluded. Persons with severe, profound hearing loss sufficient to make auditory cognition test materials inaudible were not included. Persons unable to manually adjust aids using controls on the aid or remote control were also excluded. A priori analysis for sample size was calculated at n = 218 (109 per group) to achieve a 7-point difference in NIH toolbox composite cognitive scores between groups, SD 15, 90% power, and a two-sided level of significance of 0.05 (allowing for a drop-out rate of 10%; anticipated n = 22 drop-outs). It was estimated that to identify 218 participants with below median NIH score, 436 adults would need to be screened. Two hundred and fifty-six participants were screened for study eligibility using the National Institutes of Health (NIH). Toolbox age-corrected fluid cognition composite score (Slotkin et al., 2012). Persons with median and greater NIH toolbox composite cognition scores (score >100) were excluded from the trial (n = 152). One hundred and four participants had below median composite cognition scores on the NIH Toolbox application and so were eligible. Sixty-seven participants electing to undertake hearing aid fitting were enrolled and randomly assigned (1:1) to one of two treatment groups (Simple n = 34, Standard n = 33).
2.3 Retention and trial completion
This research was undertaken from July 2018 to December 2021. During this period, Auckland and Christchurch researchers experienced various levels of restricted access to research institutions or lockdowns under NZ Government COVID-19 pandemic regulations. The Auckland site was unable to see research participants for periods between March to May 2020, August to September 2020, February to March 2021, and August to December 2021. The Christchurch site was unable to see research participants from March to May 2020 and ceased seeing participants at the end of May 2021. The restrictions limited fitting to 67 participants.
Individual participant hearing aid fitting files were audited for compliance, and four participants in the simple fitting group were excluded for a per-protocol analysis as frequency lowering was activated during the 12-month trial. Twenty-six per-protocol individuals in the Simple and 27 in the Standard groups attended the 6-month follow-up, and 24 individuals in the Simple and 22 in the Standard groups attended the 12-month follow-up. Participants flow through the study are shown in Figure 1.
2.4 Study settings
The primary settings for the study were the clinics of the two audiology training programs in NZ. These University Clinics provide evidence-based clinical services to the local community through a fee for services like non-university private clinics. The clinics are also a resource for the development and translation of new clinical processes and technologies into practice. In NZ, the vast majority of hearing aid fitting is undertaken in private clinics with patients purchasing the hearing aids and paying consultation fees. Many participants were eligible for some government subsidy toward costs, but most of the cost sat with the individual. Participants in the study purchased the hearing aids, subsidized according to eligibility for government funding. The clinics' normal consultation fees were waived as compensation for participation in the research (an approximate discount of 10–20%).
2.5 Assessments
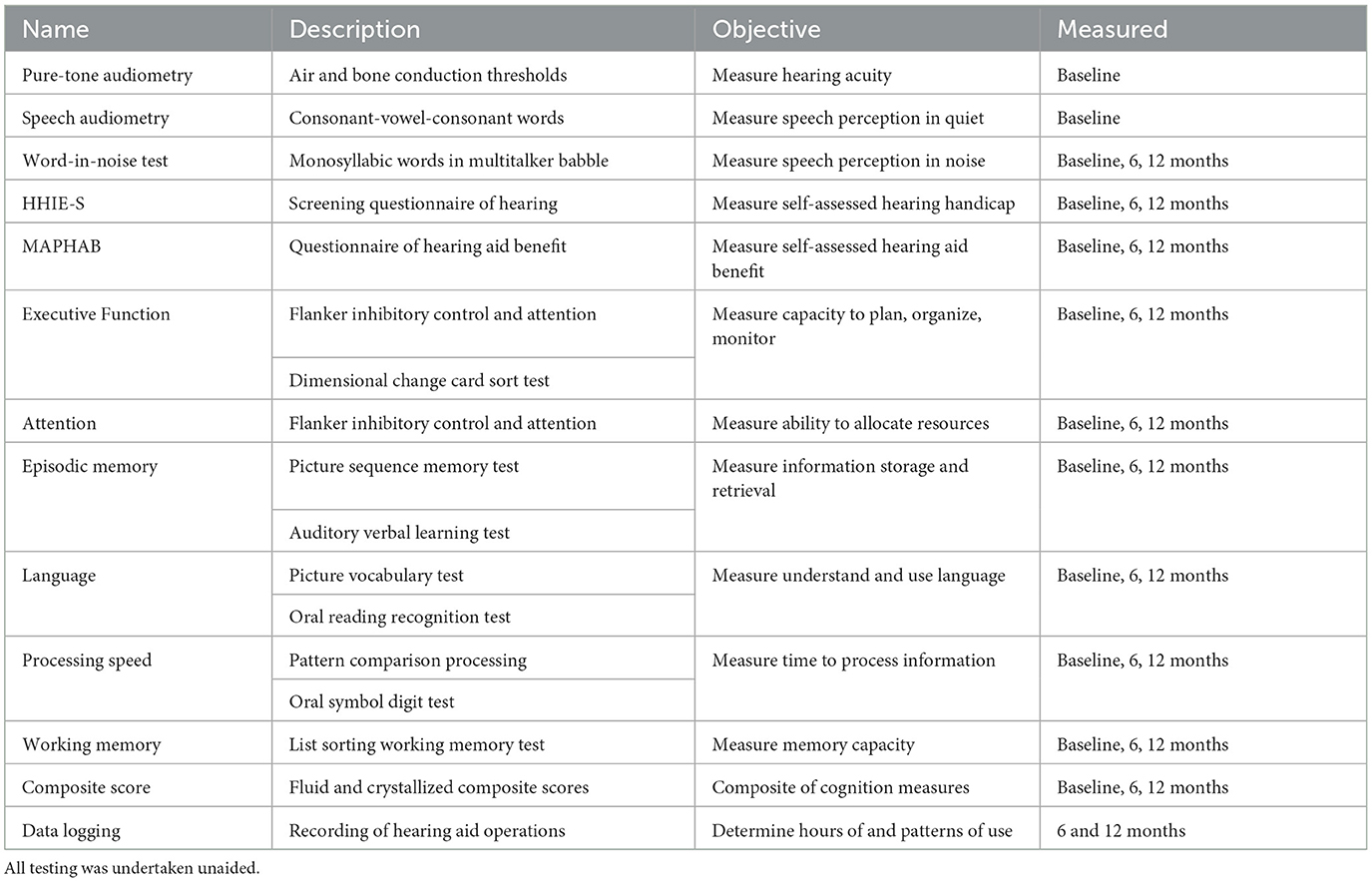
The study assessment measures are outlined in Table 1 and described in the following text.
2.5.1 History and demographics
A clinical history questionnaire was undertaken that identified important baseline attributes of the population being tested (e.g., age, gender, ethnicity, and education).
2.5.2 Audiometry
Pure-tone air conduction audiometry was performed according to the New Zealand Audiological Society's (2016) Best Practice Guidelines. Air conduction thresholds were determined bilaterally from 250 to 8 kHz, with bone conduction as needed. Thresholds were obtained using the modified Hughson-Westlake technique (Carhart and Jerger, 1959), with participants in a sound-proof booth. The Consonant-Vowel-Consonant (CVC) word lists from the New Zealand National Audiology Center Millennium recording were used to obtain performance intensity function discrimination percentages for the left and right ears. Tympanometry and acoustic reflex testing were undertaken as needed. The complete evaluation took ~30 min.
2.5.3 Modified Abbreviated Profile of Hearing Aid Benefit
A Modified Abbreviated Profile of Hearing Aid Benefit (MAPHAB) was used as a hearing-related outcome measure. The MAPHAB (Hill and Purdy, 2011) is a New Zealand-verified version of the Abbreviated Profile of Hearing Aid Benefit (Cox and Alexander, 1995). The MAPHAB used consisted of 24 items with a 0–100 response scale (Hill and Purdy, 2011). The questionnaire took ~5 min to complete. Participants provided responses on a printed form.
2.5.4 Hearing Handicap Inventory for the elderly
The Hearing Handicap Inventory for the elderly-screening version (HHIE-S) questionnaire contained 10 items designed to measure the self-perceived handicap related to the social and emotional aspects of having a hearing loss (Ventry and Weinstein, 1983). The participants could answer “Yes” (0), “Sometimes” (2), and “No” (4) for each question on a printed form. An overall score of 40 represented the highest score/greatest self-perceived handicap, and 0 reflected no perceived handicap. The questionnaire took ~5 min to complete. Participants provided responses on a printed form.
2.5.5 Words-in-noise test (WIN)
This test measured a person's ability to recognize monosyllabic words in seven signal-to-noise ratios of multitalker babble (Wilson et al., 2007). The test was administered through the NIH toolbox iPad application using a speaker in a sound-treated room. A recorded voice instructed the participant to listen to and then repeat words. The task became increasingly difficult with the reduction of the signal-to-noise ratio. The researcher scored the participant's responses as correct or incorrect (a maximum of 35 points). The WIN took ~6 min to administer.
2.5.6 Cognitive measures
To assess cognitive performance, the NIH Toolbox of Cognition was administered (Slotkin et al., 2012). The cognitive tests were administered using the NIH toolbox iPad application. The iPad was set up in a consistent manner between the participants. It was placed in front of the participant, at a specified distance where the bottom of the iPad lined up with the bottom of a printed gray rectangle described as the “home base.” The home base ensured that the iPad was at a comfortable and reachable distance from the participant's hands. Each participant was entered as a new applicant under a unique code. Instructions were presented visually on the screen and, for some tests, verbally (in English). Participants were advised to adjust the volume to a comfortable listening level. A Touch Screen Tutorial 3+ was also administered to help participants become familiar with the iPad touch screen. Most responses were generated by the participant, with some responses needing to be entered by the researcher via a wireless keyboard that was paired with the iPad prior to the appointment. The cognitive testing took ~40 min. Throughout the testing, participants were given the opportunity to take a break as needed.
The toolbox contained a range of standardized tests that explored aspects of attention and executive function, memory, processing speed, and language (Slotkin et al., 2012).
2.5.6.1 Attention and executive function
2.5.6.1.1 Dimensional change card sort test
The Dimensional change card sort test measured executive function. In this task, participants were required to identify the target image from two response options that differed in shape and/or color. Images were first presented along with the color and then the shape dimension. Subsequently, the target dimensions were mixed in random order, with a star indicating whether to match by shape or color. Scoring was based on both accuracy and reaction time. Reaction time was recorded as the time it took for a participant to respond by pressing a button on the screen, with their hand starting on the printed home base reference position. The computed score ranged from 0 to 10, but if the score was between 0 and 5, the participant did not score high enough in accuracy for the reaction time to count. The test took ~4 min to administer.
2.5.6.1.2 Flanker inhibitory control test
The Flanker task assessed visual attention and inhibitory control. In this task, participants were required to focus on a specific stimulus (middle arrow) while disregarding peripheral stimuli (two left and two right arrows). Participants were then required to select the response that corresponded to the direction that the middle arrow was pointing. Scoring was based on accuracy and reaction time. Reaction time was recorded as the time it took for a participant to respond by pressing a button on the screen, with their hand starting on the printed home base reference position. The computed score ranges from 0 to 10. The test duration was ~3 min.
2.5.6.2 Episodic memory
2.5.6.2.1 Picture sequence memory test
The picture sequence memory test measured episodic memory. In this task, participants were required to recall a sequence of items in the same order in which they had been presented on the screen. Items shared a common theme, e.g., “how to play in the park.” Images were presented serially, first appearing in the center of the screen supplemented by audio narration, then moved to a fixed position. After all the images were presented, they returned to the center in a random manner, at which time the participant was required to recall the spatial order in which the images had originally appeared. The score was based on the number of correct adjacent pairs over 2–3 trials. The test took ~10 min to administer.
2.5.6.2.2 Auditory verbal learning test (Rey)
The auditory verbal learning test (Rey) is a word-list learning task in which 15 unrelated words were presented auditorily over three consecutive trials. After each presentation, the participant was asked to recall as many of the words as they could. The test was scored as the sum of the number of words recalled across all trials (possible range of 0–45 words). The test duration was ~3 min.
2.5.6.3 Language
2.5.6.3.1 Picture vocabulary test
The picture vocabulary test assessed receptive vocabulary. For this task, participants heard one word, paired with four images on the screen. They were asked to select the image that best matched the meaning of the word. There were two practice items and the experimental items varied depending on the participant's responses. Scores range from 200 to 2000. The test duration was ~5 min.
2.5.6.3.2 Oral reading recognition test
The oral reading recognition test assessed the pronunciation of single words and provided a measurement of language (English). When a word was presented, the participant was required to read it aloud. Accuracy was scored by the researcher. Based on an algorithm, the presentation of words was designed to adapt and not be repeated. The word bank consisted of ~250 items; 30–40 were presented, depending on participant performance. The computed score could range from 500 to 2,500. The test took ~4 min.
2.5.6.4 Processing speed
2.5.6.4.1 Pattern comparison processing test
The pattern comparison processing test is a measure of processing speed. Participants were required to categorize whether the two images presented adjacently were the same or different. The overall score reflected the total correct score out of a maximum of 130, in a 90 s period. The test took ~3 min to administer.
2.5.6.4.2 Oral symbol digit test
In the oral symbol digit test, nine abstract symbols were presented paired with a number between 1 and 9. Participants were asked to orally indicate which numbers went with symbols presented in a long string. The participant was given 120 s to call out as many numbers corresponding to symbols as possible without skipping any. The Oral Symbol Digit Test was scored as the number of items answered correctly in 120 s (possible range of 0–144). The test took ~3 min to administer.
2.5.6.5 Working memory
2.5.6.5.1 List sorting working memory test
The list sorting working memory test was a measure of working memory. Pictures of food and animals were presented in succession, with the image displayed simultaneously with its corresponding audio recording. Participants were required to order the items in size from smallest to largest. The first list was presented within a single dimension (either all food or all animals), then in both dimensions (both food and animals). In the latter condition, participants were then required to recall the sequence in size from smallest to largest for the food first and then the animals. The task was scored by summing the total number of items correctly recalled and sequenced and could range from 0 to 26. The test duration was ~7 min.
2.5.6.6 Composite scores
The NIH toolbox provides three composite cognition scores:
1. Fluid cognition composite score. This score includes the results from Flanker, Dimensional Change Card Sort, Picture Sequence Memory, List Sorting, and Pattern Comparison measures.
2. Crystallized cognition composite score. This composite score includes the Picture Vocabulary and Reading Test results.
3. Total cognition composite score which is an average of the combination of 1 and 2 above.
Higher scores indicated higher levels of cognitive functioning. A standard score near 100 indicated ability that was average compared with normative data. The fluid age-corrected cognition composite score was used to determine study eligibility as it represented developmentally expected levels of performance (Casaletto et al., 2015). Uncorrected scores were used to determine the effects of intervention as they do not change if an individual's birthday occurs during the trial.
2.5.7 Intervention randomization and blinding
Participants were randomly assigned 1:1 to Simple or Standard groups. The randomization was blocked and stratified to ensure that ethnicity and age-adjusted cognition scores were balanced in the two groups. Protocols ensured adherence to allocation concealment. Participants, principal investigators, research assistants, and statisticians were blinded to allocation until study completion. The hearing aids in the study were fitted by three experienced audiologists [GC, AS (University of Auckland), and JG (University of Canterbury) all full members of the NZ Audiological Society]. The audiologists were blinded at the time the aids were selected but could not be blinded at the fitting as they had to manually set hearing aids to suit participants according to protocols. The audiologists were blinded to the outcomes of the participants' hearing and cognitive assessments.
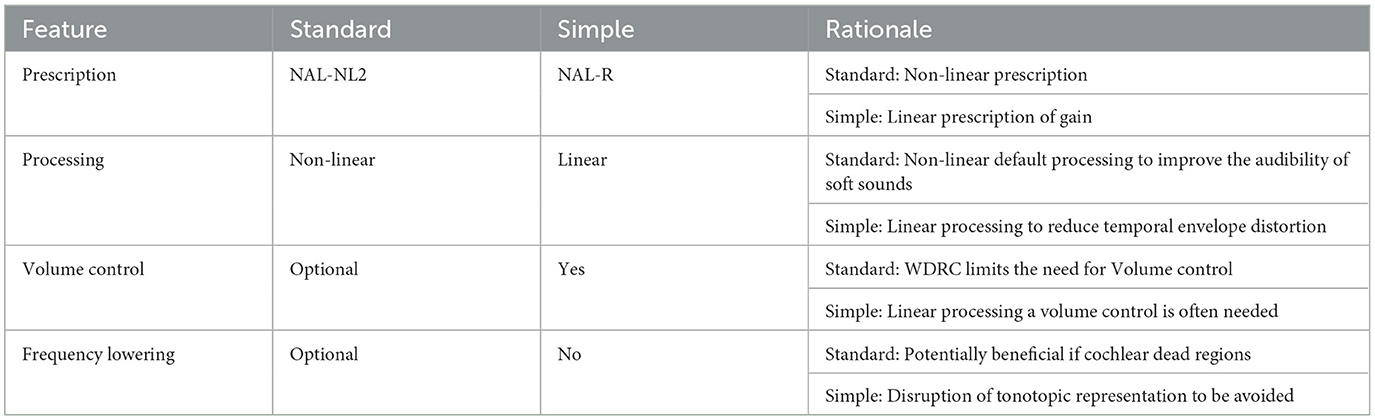
2.5.8 Hearing aid intervention
The participant and clinician decided together on the brand (from Bernafon, Oticon, Phonak, ReSound, Signia, Starkey, Unitron, and Widex), model, and technology level (cost) of the HA based on a hearing needs assessment. Participants were randomly assigned to one of two groups: Standard or Simple processing. Consensus settings for persons with poorer than average cognition were agreed upon for use in this study through two focus group discussions with audiologists and a review of existing evidence in application with options available between and within manufacturers' software (the core Standard and Simple settings are summarized in Table 2). The Simple processing consisted of settings proposed to be most suitable for slower processing ability (e.g., linear input-output processing, output limiting compression, and no frequency lowering). The aids were programmed and matched to NAL-R linear hearing aid targets (Byrne et al., 1990). Audiologist option selection at fine-tuning appointments placed an emphasis on simple solutions to maintain the fidelity of the processed signal, e.g., if acoustic feedback occurred, the ear-coupling system used (domes, ear molds) was modified for acoustic seal before digital feedback reduction methods were employed. The Standard group received current best-practice hearing aid fitting using default signal processing. The selected aids Digital Signal Processing (DSP) emphasized the audibility of sound using adaptive signal processing proprietary to the different manufacturers. The hearing aids were programmed to the NAL-NL2 prescription procedure (Keidser et al., 2011).
For both the Standard and Simple groups, real-ear probe microphone measures were undertaken using calibrated real-ear measurement systems to match outputs to prescribed targets. Following the fitting, fine tuning based on real-world patient self-report experience was undertaken as needed within the principles for each fitting strategy (Table 2). Counseling on the use of aids was provided. No auditory training was undertaken.
2.5.9 Adverse events
Adverse events (AE) were considered as any harmful or unintended medical, clinical, or health outcome occurring during or following the fitting of the hearing aids. An internal Data Safety and Monitoring Committee was established for the trial. There were no serious or moderate adverse events. Minor adverse effects (skin irritation due to poor hearing aid tubing fit) were managed as part of the normal hearing aid fitting process. All AEs were followed to resolution or stabilization as a continuation of normal clinical practice.
2.6 Analysis
Details of the individual's hearing loss, hearing aids, and data logging with participant details were entered into “NOAH” databases (https://www.himsa.com/) at the participating clinics. The NIH toolbox generated a spreadsheet of data for each test undertaken. These data were duplicated and, along with other test results and observations, securely imported into a RedCap database (https://www.project-redcap.org).
Data were plotted and analyzed using Prism (version 9.3.1). The data were normally distributed according to the one-sample Kolmogorov-Smirnov test. A mixed model ANOVA with Greenhouse-Geisser sphericity correction for repeated measures was used to examine group-by-time effects for fluid cognition, crystallized cognition, MAPHAB, HHIE, and WIN results. Multiple comparisons were undertaken using the Bonferroni correction. Cohens effect size for fluid cognition change baseline to 12 months was calculated for each group.
2.6.1 Primary outcome
NIH toolbox fluid composite cognitive score at 6 and 12 months.
2.6.2 Secondary outcomes
NIH toolbox crystallized composite cognitive score at 6 and 12 months.
Changes in hearing aid HHIE-S and MAPHAB questionnaire scores at 6 and 12 months.
Changes in Word-in-Noise test (WIN) scores at 6 and 12 months.
Hearing aid use between Simple and Standard groups was examined by comparing hours of use recorded in the data logging feature of the hearing devices.
Primary hypothesis: A simplified hearing aid processing strategy will result in greater improvement in NIH toolbox composite fluid cognition score after 12 months of hearing aid use than a standard hearing aid fitting in older adults with below-average cognition.
3 Results
3.1 Demographic characteristics
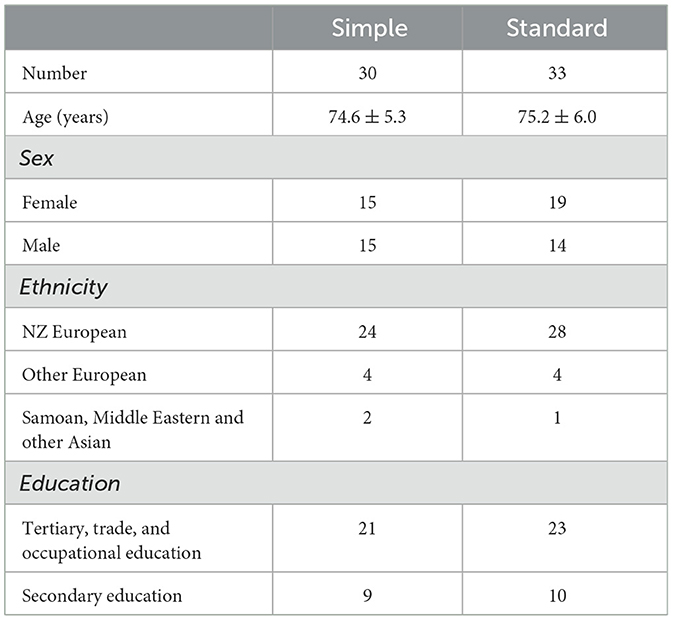
Two hundred and fifty-six patients were screened, and 104 patients were eligible to participate in the study. A total of 67 patients were included and randomly assigned into either the Simple group (n = 34) or the Standard group (n = 33). Four participants were removed from the Simple group for per-protocol analysis because they received a hearing aid setting that deviated from the protocol (frequency lowering was activated). This resulted in 30 participants in the Simple group and 33 in the Standard group. The mean ages of the Simple and Standard groups were compared using independent-sample t-tests and were not statistically different. Cross-tabulation and z-testing revealed that the groups did not significantly differ in proportions of sex and ethnicity. The demographic characteristics of participants at baseline are presented in Table 3.
3.1.1 Audiogram
There were no statistically significant differences in three frequencies (500, 1,000, and 2,000 Hz) pure tone average audiograms at baseline: best ear (Simple mean 38.79, SD 8.05 dB HL; Standard mean 35.61, SD 14.02 dB HL).
3.2 Intervention outcomes
The primary and secondary results are summarized in Figure 2 and Table 4. Two-way mixed-measures ANOVA tests were undertaken to examine whether time had a significant effect on the outcome measures. For the primary outcome of the uncorrected fluid cognition composite, the scores differed between the two groups [F(1, 61) = 5.909, p < 0.05], and there was a significant time effect [F(1.84, 87.48) = 11.04, p < 0.0001]. A post-hoc pairwise comparison using the Bonferroni correction found a significantly increased score for the Standard group from baseline to 6-month follow-up (difference 6.54 p < 0.01) and from baseline to 12-month follow-up (difference 10.32, p < 0.01). No significant difference was found for the Simple group across time: 6-month follow-up (difference 2.20) and from baseline to 12-month follow-up (difference 3.48). The group-by-time interaction was not significant [F(2, 95) = 3.03, p = 0.053]. The effect size was calculated for the per-protocol analysis comparing baseline to 12 months for the two interventions, and Cohens' d for the Standard fitting was 1.3 (very large effect), and for the Simple fitting, it was 0.4 (small-medium effect). The crystallized composite score did not differ between groups or across time. The mean HHIE-S scores differed significantly across three time points [F(1.80, 82.00) = 53.31, p < 0.0001] but not between groups, and there was no time-by-group interaction. The mean MAPHAB scores also differed significantly across the three time points [F(1.79, 82.39) = 14.43, p < 0.0001] but not between groups and there was no time-by-group interaction. The WIN test scores did not differ between groups or across time.
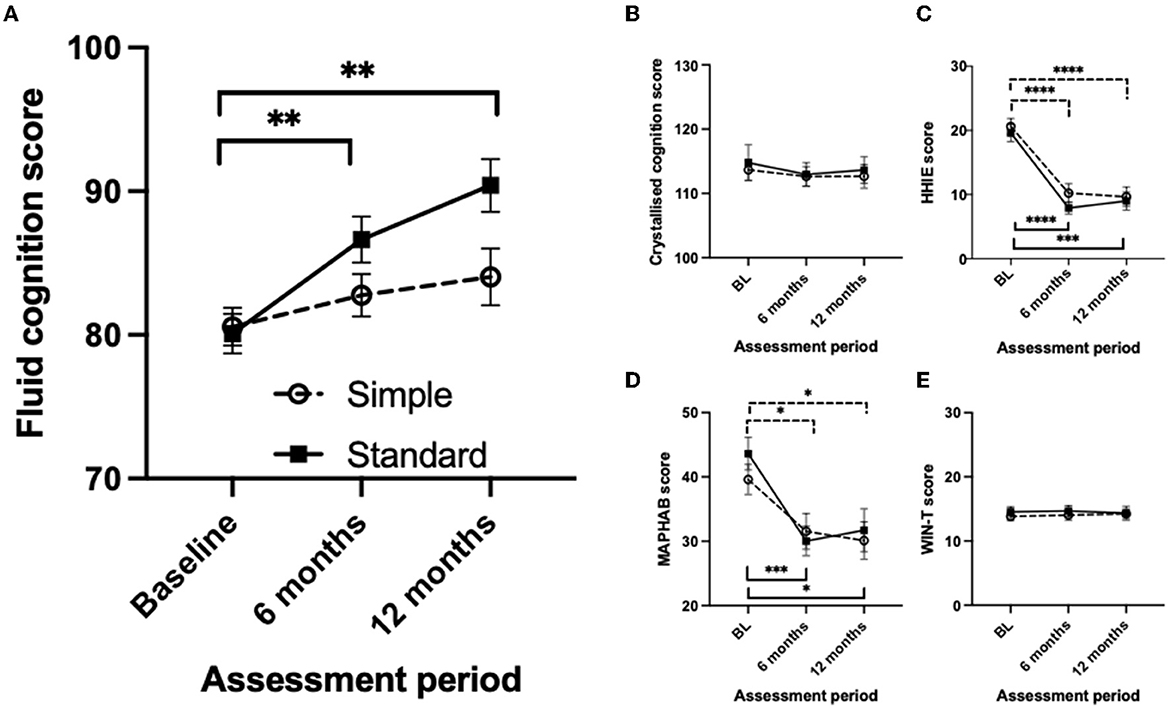
Figure 2. Comparison of the measurement scores at baseline, 6- and 12-month follow-up visits between the simple and standard hearing aid groups for Intention to Treat analysis. (A) Uncorrected fluid cognition composite score. (B) Uncorrected crystallized cognition composite score. (C) Hearing Handicap Inventory for Elderly score. (D) Modified Abbreviated Profile of Hearing Aid benefit score. (E) Word-in-Noise Test score. Mean values are shown with the standard error of the mean error bars. Significant differences are shown by horizontal bars (dashed – Simple; solid – Standard) *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
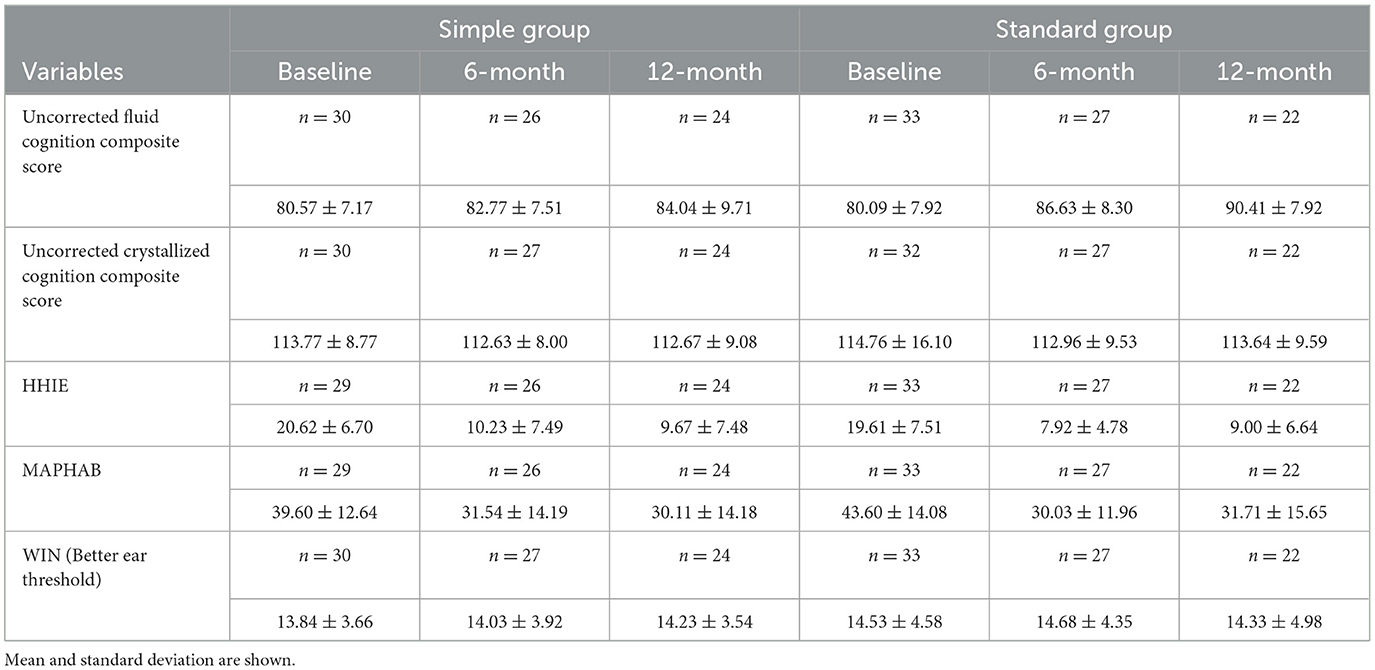
Table 4. Trial scores at baseline, 6- and 12-month follow-up visits between simple and standard HA groups, intention to treat and per protocol analysis.
3.2.1 Duration of use
There were no statistically significant differences in valid data logging recordings of daily hours of use: Per protocol at 6 months (Simple mean 8.76, SD 3.82; Standard mean 8.60, SD 4.66) and 12 months (Simple mean 8.23, SD 3.71; Standard mean 7.75, SD 4.63).
4 Discussion
In this study, we compared a “Standard” adaptive compression-based hearing aid fitting strategy to a “Simple” strategy using linear amplification across 12 months of hearing aid use. Participants who received a “Standard” fitting had a 10.3-point improvement in the fluid cognition composite score, compared to a 3.5-point improvement with a “Simple” fitting. Measures of hearing (HHIE, MAPHAB) improved equally for both groups. Crystallized cognition and WIN test results did not change with time for either group.
We had hypothesized that over 1 year, fluid cognition and hearing would improve more with a “Simple” hearing aid processing strategy than a “Standard” strategy. The hypothesis was disproven. Hearing-related outcomes were equivalent and the more complex fitting based on compression processing resulted in superior cognitive outcomes. As expected, crystallized cognition did not change. Given that the hypothesis of the superiority of simple processing for cognition was disproven, we might have then expected better hearing outcomes for the standard fitting; this was not the case. The finding that linear and compression fittings had equivalent hearing results may be surprising to clinicians, as compression over a wide dynamic range is the default signal processing method used by manufacturers, having supplanted linear (with output compression limiting) processing as the predominant processing several decades ago. However, clinical trials at the time that compression was being introduced did not overwhelmingly endorse compression across a wide dynamic range as providing superior hearing outcomes to linear amplification (Larson et al., 2002) and many studies had significant limitations (Metselaar et al., 2008). There have not been recent trials comparing linear processing against modern adaptive digital processing. Modern adaptive processing combines slow and fast compression of the signal while acting in a linear manner at times (Simonsen and Behrens, 2009). The equivalency of hearing outcome results for the Simple and Standard processing in this study was not anticipated and should be investigated further. The selection of linear processing may have affected DSP linked to the aid's compression algorithms. Given the absence of differences in hearing outcomes, the clear difference in cognitive outcomes from the Standard fitting suggests that either improvement in cognition with hearing aids is independent of improvement in the measures of hearing aid benefit used or another factor not captured by our measurements may be responsible. We hypothesize that the increased audibility of soft sounds enabled by non-linear amplification would have created more ongoing neural activity, requiring more interpretation and filtering across time (i.e., greater listening effort) than linear amplification. This additional demand on cognitive resources could act overtime to passively improve auditory processing and attention. These skills may have transferred to the processes tested by the cognitive test battery used. Improved attention from “being forced” to filter greater information available from the Standard compared to Simple fitting may explain some of the findings. Future studies are necessary to evaluate this idea or alternative hypotheses for the dichotomy in cognitive outcomes with the two different approaches to amplification.
4.1 Strengths of the study
Several factors did not contribute to the difference in fluid cognitive scores between groups. The groups did not differ significantly in demographics or hours of hearing aid use. Neither group showed a change in crystallized cognition, which is consistent with the known stability of this form of cognition over time. There was no evidence that procedural learning had a significant impact on testing through repeated measurement, as crystallized cognition did not change, and both fitting groups were tested equivalently. There are more general reasons to have confidence in differences in cognitive outcome due to the hearing aid settings including rigorous vetting of methods by a multidisciplinary research team, employment of a multi-center design, and independence from potential industry conflicts of interest. Participants were all new hearing aid users and had met strict screening criteria. Validated, generic, prescriptive procedures were used alongside real-ear measurement verification to ensure consistent application of fitting methods. Clinic files were audited for adherence to agreed practice at trial end.
The population was deliberately sampled so that only individuals with below median cognition were eligible. This was because we believed that persons with reduced cognitive ability would be more likely to show improved cognition with aiding. A risk of recruiting persons with scores below the mean is that the following measurement scores regress to the mean. Our results were not extreme relative to the expected population average; regression to the mean is most likely to occur when scores are extremely different from the mean. The criteria for eligibility were an age-corrected fluid cognition score below 100; the mean baseline score for both groups was ~80, and scores below 70 were considered significant cognitive impairment. Further reasons that regression to the mean is unlikely to be the cause for improvement is the effect size difference in results for the two groups and the progressive improvement from baseline to 6 months and then from 6 to 12 months following hearing aid fitting.
4.2 Limitations
The study had some limitations. The study population was homogenous, consisting of primarily New Zealand European participants. In the context of New Zealand, Māori and people of other ethnicities were underrepresented. New Zealand research based on the Lancet Commission on Dementia report identifies that hearing loss is an important modifiable risk factor for dementia in Māori, Pacific, and Asian communities, so further research on hearing aid use in these communities is needed (Ma'u et al., 2021). The use of a research population could affect generalizability to the typical clinic population; however, the hearing aids were not provided for free, at least limiting the effects of inducement and distortion of technology selection due to cost. The technology used between participants differed. Pinpointing the exact processing responsible for the effects seen is not possible; however, the use of multiple manufacturers and technology levels is representative of real-world practice.
The test battery used established tools; however, there are always risks that an important attribute will not be measured, or the methods may not be ideal for the population. The population did not have dementia, they had below-average cognition, and we have no evidence that they did not understand the tasks or could not complete questionnaires. The participants had hearing loss, which can affect auditory-based cognitive assessments. The test material was checked to ensure audibility. If these factors did affect participants' scores, the effects on the study are largely controlled for by the repeated measures design. However, future studies may be advised to include an additional measure of cognition that does not use any auditory instruction, for example, the Montreal cognitive assessment for people with hearing impairment (Dawes et al., 2023).
The study did not recruit as many participants as intended; the smaller sample limits generalization to the wider population. This research took place during the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic when New Zealand was subject to various lockdowns that restricted recruitment. In addition, the drop-out rate was higher than anticipated, with many eligible participants electing not to proceed with a hearing aid fitting following the initial consultation. It was expected that through the deliberate sampling of individuals considering obtaining hearing aids, along with advertised statements regarding access to hearing aids for purchase (subject to eligibility), most eligible participants would proceed to hearing aid fitting. This was not the case. Although eligible, many participants who met the criteria following testing did not proceed to trialing aids. Informal feedback was that the timing of the study and the costs involved (cost of both aids and travel) alongside stigma were barriers to participation. Some participants appeared to be primarily motivated by the opportunity of having their cognition tested. The possibility that hearing aids may benefit cognition was insufficient motivation for some participants to proceed to hearing aids. In terms of interpreting statistics, Type II error (insufficient power) is associated with samples that are too small. The statistically significant difference between the Standard and Simple groups is robust and group effects were large. So, the primary finding is reliable. A risk is that non-significant differences found might be significant with a larger sample size. While we cannot discount this for hearing outcomes, it is unlikely given the small difference in means obtained.
4.3 Clinical ramifications
This study has several important ramifications for hearing care service delivery. Willingness to participate in cognitive testing was higher than enrolment for hearing aids. Cognitive screening should be available through primary care doctors or other healthcare practitioners including audiologists, and people should be made more aware of its availability. This requires the establishment of referral pathways for further assessment and appropriate education, including within professional training programs. The result adds weight to arguments that early intervention with hearing aids may reduce the burden of cognitive decline. The cost-benefit of early access to hearing aids relative to potential reductions in the cost of dementia care needs to be ascertained as it will indicate potential funding priorities for the best use of limited healthcare funding. The clear difference in cognitive outcomes between the two hearing aid strategies in this study suggests that the selection of amplification can have a strong impact on cognitive outcomes. The participants had below-average cognition; we cannot conclude that the results would hold for the population as a whole, but can, with some confidence, state that hearing aids are beneficial for persons with lower-than-average cognition. The differences between the Standard and Simple fittings used in this study provide preliminary evidence that the selection of markedly different signal processing matters for cognitive outcomes. Audiologists should be cognizant that signal-processing strategies may affect cognitive outcomes as well as hearing outcomes.
Work is underway to develop protocols for the fitting of hearing aids in older adults experiencing changes in auditory processing (Windle et al., 2023). Windle et al.'s (2023) recommendations to avoid hearing aid settings that introduce distortion to the speech envelope need to be reviewed in light of the findings reported here. Our study found superior results with Standard fittings compared to a setting that should not distort the speech envelope. The effects of hearing aids on cognition and how they should be fit and used promises to be a topic requiring ongoing research for some time.
The mechanisms for these observations should also be researched.
5 Conclusions
The hearing aid signal processing prescribed affected cognitive outcomes over a year of use in persons with below-average cognitive ability. Standard hearing aid processing improved cognitive outcomes more than a Simple fitting. The findings of different cognitive outcomes with equivalent improvement in hearing suggest that the mechanisms underlying benefit in cognition from hearing aids are complex. These findings, comparing large differences in DSP between groups, require replication and further study. Ideally, the short and long-term effects of all hearing aid DSP features on cognition should be evaluated and published.
Data availability statement
The datasets presented in this article are not readily available because, individual data are not publicly available due to data sovereignty rights. Requests to access the datasets should be directed to Zy5zZWFyY2hmaWVsZEBhdWNrbGFuZC5hYy5ueg==.
Ethics statement
The studies involving humans were approved by the University of Auckland and University of Canterbury Human Participants Ethics Committees (reference number 019538). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
GS: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. MM: Conceptualization, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing. CF: Data curation, Formal analysis, Investigation, Methodology, Project administration, Validation, Visualization, Writing – original draft. TK: Data curation, Formal analysis, Investigation, Validation, Visualization, Writing – original draft, Writing – review & editing. EW: Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. LB-H: Formal analysis, Validation, Writing – review & editing. GC: Investigation, Methodology, Writing – review & editing. JG: Investigation, Methodology, Validation, Writing – review & editing. AS: Investigation, Methodology, Writing – review & editing. DV: Data curation, Investigation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by Health Research Council of NZ Health Delivery Project grant 17/164. HRC id: 21983.
Acknowledgments
The authors express their sincere gratitude to the participants of this study and the organizations that participated in public consultation and advertising the research including the National Foundation for the Deaf and Hearing Association of NZ.
Conflict of interest
GS has undertaken tinnitus research funded by hearing aid manufacturers.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Agnew, J. (1998). The causes and effects of distortion and internal noise in hearing aids. Trends Amplificat. 3, 82–118. doi: 10.1177/108471389800300302
Amieva, H., Ouvrard, C., Giulioli, C., Meillon, C., Rullier, L., and Dartigues, J. F. (2015). Self-reported hearing loss, hearing aids, and cognitive decline in elderly adults: a 25-year study. J. Am. Geriatr. Soc. 63, 2099–2104. doi: 10.1111/jgs.13649
Anstey, K. J., and Low, L.-F. (2004). Normal cognitive changes in aging. Austr. Fam. Physician 33, 783–787.
Ben-David, B. M., Erel, H., Goy, H., and Schneider, B. A. (2015). “Older is always better”: age-related differences in vocabulary scores across 16 years. Psychol. Aging 30, 856. doi: 10.1037/pag0000051
Byrne, D., and Dillon, H. (1986). The National Acoustic Laboratories'(NAL) new procedure for selecting the gain and frequency response of a hearing aid. Ear Hear. 7, 257–265. doi: 10.1097/00003446-198608000-00007
Byrne, D., Parkinson, A., and Newall, P. (1990). Hearing aid gain and frequency response requirements for the severely/profoundly hearing impaired. Ear Hear. 11, 40–49. doi: 10.1097/00003446-199002000-00009
Carhart, R., and Jerger, J. F. (1959). Preferred method for clinical determination of pure-tone thresholds. J. Speech Hear. Disord. 24, 330–345. doi: 10.1044/jshd.2404.330
Casaletto, K. B., Umlauf, A., Beaumont, J., Gershon, R., Slotkin, J., Akshoomoff, N., et al. (2015). Demographically corrected normative standards for the English version of the NIH Toolbox Cognition Battery. J. Int. Neuropsychol. Soc. 21, 378–391. doi: 10.1017/S1355617715000351
Cox, R. M., and Alexander, G. C. (1995). The abbreviated profile of hearing aid benefit. Ear Hear. 16, 176–186. doi: 10.1097/00003446-199504000-00005
Dawes, P., Emsley, R., Cruickshanks, K. J., Moore, D. R., Fortnum, H., Edmondson-Jones, M., et al. (2015). Hearing loss and cognition: the role of hearing AIDS, social isolation and depression. PLoS ONE 10:e0119616. doi: 10.1371/journal.pone.0119616
Dawes, P., Reeves, D., Yeung, W. K., Holland, F., Charalambous, A. P., Côté, M., et al. (2023). Development and validation of the Montreal cognitive assessment for people with hearing impairment (MoCA-H). J. Am. Geriatr. Soc. 2023:18241. doi: 10.1111/jgs.18241
Dawes, P., Wolski, L., Himmelsbach, I., Regan, J., and Leroi, I. (2019). Interventions for hearing and vision impairment to improve outcomes for people with dementia: a scoping review. Int. Psychogeriatr. 31, 203–221. doi: 10.1017/S1041610218000728
Dillon, H. (1999). NAL-NL1: a new procedure for fitting non-linear hearing aids. Hear. J. 52, 10–12. doi: 10.1097/00025572-199904000-00002
Gatehouse, S., Naylor, G., and Elberling, C. (2006). Linear and nonlinear hearing aid fittings-2. Patterns of candidature: Adaptación de auxiliares auditivos lineales y no lineales-2. Patrones de selección de candidatos. Int. J. Audiol. 45, 153–171. doi: 10.1080/14992020500429484
Heaton, R. K., Akshoomoff, N., Tulsky, D., Mungas, D., Weintraub, S., Dikmen, S., et al. (2014). Reliability and validity of composite scores from the NIH Toolbox Cognition Battery in adults. J. Int. Neuropsychol. Soc. 20, 588–598. doi: 10.1017/S1355617714000241
Hill, J. M., and Purdy, S. C. (2011). Predictors of bilateral hearing aid success in older adults with hearing impairment. N. Zeal. Audiol. Soc. Bullet. 21, 17–51.
Kavé, G., and Halamish, V. (2015). Doubly blessed: older adults know more vocabulary and know better what they know. Psychol. Aging 30:68. doi: 10.1037/a0038669
Keidser, G., Dillon, H., Flax, M., Ching, T., and Brewer, S. (2011). The NAL-NL2 prescription procedure. Audiol. Res. 1:e24. doi: 10.4081/audiores.2011.e24
Larson, V. D., Williams, D. W., Henderson, W. G., Luethke, L. E., Beck, L. B., Noffsinger, D., et al. (2002). A multi-center, double blind clinical trial comparing benefit from three commonly used hearing aid circuits. Ear Hear. 23, 269–276. doi: 10.1097/00003446-200208000-00001
Laumann, L. L. (1999). Adult Age Differences in Vocabulary Acquisition as a Function of Individual Differences in Working Memory and Prior Knowledge. Morgantown: West Virginia University.
Lin, F. R., Pike, J. R., Albert, M. S., Arnold, M., Burgard, S., Chisolm, T., et al. (2023). Hearing intervention versus health education control to reduce cognitive decline in older adults with hearing loss in the USA (ACHIEVE): a multicentre, randomised controlled trial. Lancet 2023:1406. doi: 10.1016/S0140-6736(23)01406-X
Livingston, G., Huntley, J., Sommerlad, A., Ames, D., Ballard, C., Banerjee, S., et al. (2020). Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 396, 413–446. doi: 10.1016/S0140-6736(20)30367-6
Lunner, T., and Sundewall-Thorén, E. (2007). Interactions between cognition, compression, and listening conditions: effects on speech-in-noise performance in a two-channel hearing aid. J. Am. Acade. Audiol. 18, 604–617. doi: 10.3766/jaaa.18.7.7
Mamo, S. K., Reed, N. S., Price, C., Occhipinti, D., Pletnikova, A., Lin, F. R., et al. (2018). Hearing loss treatment in older adults with cognitive impairment: a systematic review. J. Speech Lang. Hear. Res. 61, 2589–2603. doi: 10.1044/2018_JSLHR-H-18-0077
Ma'u, E., Cullum, S., Cheung, G., Livingston, G., and Mukadam, N. (2021). Differences in the potential for dementia prevention between major ethnic groups within one country: a cross sectional analysis of population attributable fraction of potentially modifiable risk factors in New Zealand. Lancet Region. Health Western Pacific 13:100191. doi: 10.1016/j.lanwpc.2021.100191
Metselaar, M., Maat, B., Verschuure, H., Dreschler, W. A., and Feenstra, L. (2008). Comparative studies on hearing aid selection and fitting procedures: a review of the literature. Eur. Archiv. Oto-Rhino-Laryngol. 265, 21–29. doi: 10.1007/s00405-007-0494-7
Mulrow, C. D., Aguilar, C., Endicott, J. E., Tuley, M. R., Velez, R., Charlip, W. S., et al. (1990). Quality-of-life changes and hearing impairment: a randomized trial. Ann. Intern. Med. 113, 188–194. doi: 10.7326/0003-4819-113-3-188
New Zealand Audiological Society (2016). Best Practice Guidelines. New Zealand Audiological Society, New Zealand.
Ng, E. H. N., and Rönnberg, J. (2020). Hearing aid experience and background noise affect the robust relationship between working memory and speech recognition in noise. Int. J. Audiol. 59, 208–218. doi: 10.1080/14992027.2019.1677951
Nguyen, M.-F., Bonnefoy, M., Adrait, A., Gueugnon, M., Petitot, C., Collet, L., et al. (2017). Efficacy of hearing aids on the cognitive status of patients with Alzheimer's disease and hearing loss: a multicenter controlled randomized trial. J. Alzheimer's Dis. 58, 123–137. doi: 10.3233/JAD-160793
Salthouse, T. A. (2000). “Steps toward the explanation of adult age differences in cognition,” in Models of Cognitive Aging, eds T. J. Perfect and E. A. Maylor (Oxford University Press), 19–49.
Sanders, M. E., Kant, E., Smit, A. L., and Stegeman, I. (2021). The effect of hearing aids on cognitive function: a systematic review. PLoS ONE 16:e0261207. doi: 10.1371/journal.pone.0261207
Sarant, J., Harris, D., Busby, P., Maruff, P., Schembri, A., Lemke, U., et al. (2020). The effect of hearing aid use on cognition in older adults: can we delay decline or even improve cognitive function? J. Clin. Med. 9:254. doi: 10.3390/jcm9010254
Simonsen, C., and Behrens, T. (2009). A new compression strategy based on a guided level estimator. Hear. Rev. 16, 26–31.
Slotkin, J., Kallen, M., Griffith, J., Magasi, S., Salsman, J., and Nowinski, C. (2012). NIH Toolbox. Technical Manual. National Institutes of Health and Northwestern University, USA.
Smith, G. E. (2016). Healthy cognitive aging and dementia prevention. Am. Psychol. 71:268. doi: 10.1037/a0040250
Souza, P., Arehart, K., and Neher, T. (2015). Working memory and hearing aid processing: literature findings, future directions, and clinical applications. Front. Psychol. 6:1894. doi: 10.3389/fpsyg.2015.01894
Tesch-Römer, C. (1997). Psychological effects of hearing aid use in older adults. J. Gerontol. Ser. B 52, 127–138. doi: 10.1093/geronb/52B.3.P127
van Hooren, S. A., Anteunis, L. J., Valentijn, S. A., Bosma, H., Ponds, R., Jolles, J., et al. (2005). Does cognitive function in older adults with hearing impairment improve by hearing aid use? Int. J. Audiol. 44, 265–271. doi: 10.1080/14992020500060370
Ventry, I. M., and Weinstein, B. E. (1983). Identification of elderly people with hearing problems. Asha 25, 37–42.
Wilson, R. H., Carnell, C. S., and Cleghorn, A. L. (2007). The Words-in-Noise (WIN) test with multitalker babble and speech-spectrum noise maskers. J. Am. Acad. Audiol. 18, 522–529. doi: 10.3766/jaaa.18.6.7
Windle, R., Dillon, H., and Heinrich, A. (2023). A review of auditory processing and cognitive change during normal ageing, and the implications for setting hearing aids for older adults. Front. Neurol. 14:1122420. doi: 10.3389/fneur.2023.1122420
Keywords: cognition, hearing, geriatrics, aging, trial, hearing aids
Citation: Searchfield GD, McAuliffe MJ, Fok C, Kyaw TA, Williams E, Burton-Harris L, Coad G, Grady J, Smith A and Vajsakovic D (2024) The CogniAid trial. The impact of two hearing aid signal processing strategies on cognition. Front. Audiol. Otol. 2:1285496. doi: 10.3389/fauot.2024.1285496
Received: 30 August 2023; Accepted: 10 January 2024;
Published: 25 January 2024.
Edited by:
Martin Skoglund, Linköping University, SwedenReviewed by:
Niels Henrik Pontoppidan, Eriksholm Research Centre, DenmarkMaren Stropahl, Sonova, Switzerland
Robert Eikelboom, Ear Science Institute Australia, Australia
Copyright © 2024 Searchfield, McAuliffe, Fok, Kyaw, Williams, Burton-Harris, Coad, Grady, Smith and Vajsakovic. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Grant Donald Searchfield, Zy5zZWFyY2hmaWVsZEBhdWNrbGFuZC5hYy5ueg==
 Grant Donald Searchfield
Grant Donald Searchfield Megan J. McAuliffe
Megan J. McAuliffe Christine Fok
Christine Fok Tin Aung Kyaw
Tin Aung Kyaw Eric Williams3
Eric Williams3 Lisa Burton-Harris
Lisa Burton-Harris Jonny Grady
Jonny Grady