- 1NIHR Nottingham Biomedical Research Centre, Hearing Sciences, Mental Health and Clinical Neurosciences, School of Medicine, University of Nottingham, Nottingham, United Kingdom
- 2Department of Psychology, University of Sheffield, Sheffield, United Kingdom
- 3National Institute for Health and Care Research (NIHR) Coordinating Centre, School of Healthcare Enterprise and Innovation, University of Southampton, Southampton, United Kingdom
- 4Bolton NHS Foundation Trust, Bolton, United Kingdom
- 5Nottingham University Hospitals NHS Trust, Nottingham, United Kingdom
- 6University College London Hospitals NHS Foundation Trust, London, United Kingdom
- 7School of Clinical Therapies, College of Medicine and Health, University College Cork, Cork, Ireland
Introduction: Tinnitus is a common disorder of the auditory system. Questionnaires are essential tools for clinical assessment and research. Whilst many questionnaires are available to measure different aspects of tinnitus complaint in adults, there is currently no self-report questionnaire measure of tinnitus that has been developed for or is suitable for use with children. This study describes the development of the first self-report measure of tinnitus impact for children aged 8–16 years old.
Methods: Two phases of questionnaire development were conducted. In Phase 1 children's tinnitus-related problems were elicited from interviews with children with tinnitus (n = 11; aged 9–16 years old), parents (n = 5), and clinicians (n = 8). Interview transcripts were analyzed using qualitative content analysis. Findings were combined with problems identified by the clinical co-authors, researchers, and clinicians in a conference workshop, and those previously reported in service evaluation of UK National Health Service pediatric tinnitus services and in a scoping review. From this, a conceptual framework of tinnitus impact on health-related quality of life in children was developed. Based on the conceptual framework, a 38-item pilot questionnaire was drafted. In Phase 2, content validity of the pilot questionnaire was assessed in cognitive interviews with six children who had tinnitus (aged 8–15 years old) and an online survey with clinicians working in pediatric tinnitus services (n = 8 services and 28 clinicians). Finally, readability assessments were conducted. Feedback led to iterative revisions to the questionnaire. The final questionnaire was named the Impact of Tinnitus in Children Questionnaire (iTICQ).
Results: The iTICQ contains three scene setting (non-scoring) items, and 33 scoring items covering six domains of tinnitus impact: Sleep and Feeling Tired, Learning, Emotional Health, Hearing and Listening, Taking Part, and Relationships.
Conclusions: The iTICQ is a new self-report measure of tinnitus impact that can be self-completed by children aged 8–16 years old. It shows good content validity and can be used to measure problem severity across the domains of core relevance to children with tinnitus. Further validation studies and translations of the iTICQ are indicated to determine its psychometric properties in different child populations and to make it widely accessible.
1 Introduction
Tinnitus is a complex percept that can cause various degrees of distress and detriment to an individual's quality of life. Although it is often considered a problem associated with adulthood, it is often observed in children and young people (Humphriss et al., 2016; Rosing et al., 2016; Raj-Koziak et al., 2020; Jacquemin et al., 2023). The prevalence figures for tinnitus in children are widely contrasting, with the prevalence of “clinically significant” tinnitus ranging from 3.1 to 15.6% (Humphriss et al., 2016; Nemholt et al., 2020). However, unlike adults, few children spontaneously discuss their tinnitus with adults (Savastano, 2007; Kentish et al., 2015). These linguistic and communication factors present a potential barrier to others becoming aware of the child's tinnitus, recognizing it as significant, and the child going on to access health services. Furthermore, in contrast to treatments and clinical services for adults with tinnitus, treatments and services for children with tinnitus are less established (Smith et al., 2020). Several treatment approaches for children have found their way into clinical practice such as advice and information giving, hearing aids, sound therapy, sleep hygiene, relaxation techniques, mindfulness techniques, cognitive behavioral therapy (CBT), or narrative therapy. However, there has been little research to evaluate their effectiveness (Kentish et al., 2015; Tegg-Quinn et al., 2023).
Common to both adults and children, there is currently no objective way to detect tinnitus, measure its severity, or assess whether it has changed after treatment (McFerran et al., 2019). Clinically meaningful assessment of tinnitus therefore relies on the individual reporting their symptoms and the impact of tinnitus on their life. A recent review concluded that measures used to assess tinnitus severity and/or evaluate tinnitus interventions for children were not fit for purpose (Tegg-Quinn et al., 2023). The measures used were not developed for use with children, nor were they validated in child populations (Tegg-Quinn et al., 2023). In order to measure the impact of tinnitus, discriminate between different levels of tinnitus severity, and assess the impact of clinical intervention, it is necessary to have a valid, tinnitus-specific self-report measure that is suitable for use with children. Indeed, the need to “identify a clinical and cost-effective tinnitus questionnaire for use with children and young people” was highlighted in the 2020 UK National Institute for Health and Care Excellence guideline on tinnitus assessment and management (NICE, 2020).
Patient Reported Outcome Measures (PROMs) are standardized questionnaires used directly with the patient to measure the impact of a health condition [Food and Drug Administration (FDA), 2009]. The recent proliferation of PROM development and use has largely been driven by a step-change to include patient reported outcomes as endpoints in clinical trials (Ediebah et al., 2018). In addition, PROMs are increasingly used in clinical practice and have been argued to facilitate health-care decision making at micro (individual), meso (within defined groups and services), and macro (population) levels (Lipscomb et al., 2004; Williams, 2018; Wong et al., 2021). Child PROMs may be self-reported (completed by the child), or informant reported (e.g., completed by a parent, teacher, or clinician). PROMs can be categorized as generic, disease, or domain-specific and can be designed to measure various conceptualisations of health status. Health-Related Quality of Life (HRQoL), defined by Haverman et al. (2017) as a measure of “physical symptoms, functional status and disease impact on psychological and social functioning” (p. 393), is the focus of many PROMs (Janssens et al., 2015; Comins et al., 2021). Generic examples include the Pediatric Quality of Life Inventory (PedsQL; Varni et al., 1999) or the Child Health Questionnaire (CHQ; Landgraf et al., 1998). Disease-specific examples include questionnaire measures of Chronic Fatigue Syndrome (CFS)/myalgic encephalopathy (ME; Parslow et al., 2020), asthma (Juniper et al., 1996), and diabetes (Skinner et al., 2003).
The growth of PROMs has led to best practice guidance to support development of robust measurement tools [Food and Drug Administration (FDA), 2009; Rothrock et al., 2011; Mokkink et al., 2019]. Broadly, the initial process of PROM development involves devising a conceptual framework that indicates the relationships between the overarching construct, the hypothesized domains, and candidate questionnaire items (Patrick et al., 2011a). Beyond initial development, evidencing the validity of a novel measure is an ongoing and iterative process (Rothrock et al., 2011).
Critically, questionnaire items must have content validity. Content must be comprehensive and relevant to the intended construct, the target clinical population (who will complete the questionnaire), and the context of use. Thus, the content of child PROMs should reflect the way in which children experience, understand, and discuss the construct (Patrick et al., 2011a). In the development of child-specific PROMs, it is recommended that content validity is established using qualitative methods with children as informants. Measures can also be informed by input from parents and clinicians; however, the perspectives of children should be prioritized (Matza et al., 2013).
The aim of this study was to develop a disease-specific questionnaire to measure self-reported tinnitus impact on children's HRQoL. The questionnaire was designed to be completed independently by children with tinnitus aged 8–16 years old, for use in research and clinical practice. This study was conducted in two phases with objectives to (1) develop a conceptual framework of tinnitus impact on HRQoL in children through interviews with children with tinnitus, parents, and clinicians and previous research (this conceptual framework informed the design of the questionnaire), and (2) assess the content validity of the questionnaire through cognitive interviews with children with tinnitus, a survey with clinicians who care for children with tinnitus, and readability assessment.
2 Materials and methods
The two study phases received University of Nottingham Internal Review Board approval (IRB 18076), NHS Research Ethics Committee (18/EE/0396) and Health Research Authority approval (IRAS 253103). Parental consent, and child assent, was obtained for children aged 8–15 years old. Informed consent was obtained from parents, clinicians and children aged 16 years old. The study was informed by standards for content validity outlined in the “COSMIN methodology for assessing the content validity of PROMs” (Terwee et al., 2018) and the International Society for Pharmoeconomics Outcome Research (IPSOR) guidance for establishing content validity in PROMs (Patrick et al., 2011a,b; Matza et al., 2013). Three clinical coauthors (VK, CB, and LP) advised the research team throughout the questionnaire development processes. These clinicians were experienced in the management of tinnitus in children, and represented clinical specialties in Audiovestibular Medicine, Audiology, and Clinical Psychology.
2.1 Phase 1: development of the conceptual framework
2.1.1 Participants
Children were eligible to take part if they were aged 8–16 years old, had experienced tinnitus for 3 months or more, had experienced tinnitus-related problems, and had seen a health professional about their tinnitus. Parents were eligible if they had a child who met the aforementioned child criteria. Clinicians were eligible to take part if they were responsible for the management of children with tinnitus.
Children and parents were recruited via UK NHS pediatric tinnitus services, research databases, and social media and children were offered a voucher for taking part. Clinicians were recruited via professional networks.
Nine children were interviewed in individual sessions (mean age: 12 years; SD: 1 year; age range: 9–16 years; female: 7; male: 2) and two children were interviewed in a group session (ages 11 and 13 years; both male). Five parents (child mean age: 11 years; SD: 2 years; child age range: 9–15 years; female: 1; male: 4) were interviewed individually. Eight clinicians were interviewed individually. The professional roles of the clinicians interviewed included Head of Audiology/ Chief Audiologist (n = 4), Senior Pediatric Audiologist (n = 1), Lead Clinical Scientist (n = 1), Audiologist (n = 1), and Hearing Therapist (n = 1).
2.1.2 Procedure
2.1.2.1 Child individual interviews
Single interviews of 30–60 min in length took place at three UK NHS pediatric tinnitus services. The interviewer, HS (white, female) used a semi-structured interview guide (Supplementary Figure 1) to explore the child's experience of tinnitus and tinnitus-related problems. HS was experienced in qualitative methods, had attended training on the clinical management of tinnitus in children, and had observed pediatric tinnitus appointments. An interview topic guide was developed to include open-ended questions about tinnitus impact. Prompts were included to cover broad areas of tinnitus impact identified in our previous studies and ensure that all relevant areas of tinnitus impact were discussed (Smith et al., 2019, 2020). The guide was piloted with two children without tinnitus aged 8 and 12 years old. Individual interviews with children under 13 years of age used interactive activities including (i) an activity card “icebreaker” whereby children chose their most and least favorite activity from a selection of picture cards, (ii) “drawing tinnitus” where children were invited to draw what their tinnitus sounds like using pictures and/or words, and (iii) the Ida institute My World tool (Ida Institute, 2021) where, using a board game-like tool, children created scenes to represent situations where tinnitus was a problem for them. Detailed questioning explored each situation. For children aged 13–16 years old, interactive activities were not used as children were sufficiently forthcoming in describing their tinnitus and its impact.
2.1.2.2 Child group interview
A single group interview of 80 min took place at one of the NHS sites. The interviewer (HS) used a semi-structured interview guide (Supplementary Figure 2) to explore the children's experience of tinnitus and tinnitus-related problems. The interview guide was piloted in a group interview format with two adults. The group interview also included, (i) the activity card “ice breaker,” (ii) a “problem generation” activity where children were asked to suggest problems that tinnitus can cause, (iii) a “problem prioritization” activity where children were asked to prioritize the most difficult problems, (iv) a “persona exercise” where children were asked to create a character similar to their age with tinnitus and suggest the problems they may experience, and (v) a “role play” exercise where children were asked to imagine they were a health care person and suggest the most important questions to ask a child with tinnitus.
2.1.2.3 Parent and clinician interviews
Interviews were 30–60 min long and conducted over the telephone. The interviewer used a semi-structured interview guide (Supplementary Figures 3, 4) to explore parent/clinician reports of children's experience of tinnitus and tinnitus-related problems.
2.1.3 Analysis of interview data
Interviews were audio recorded and transcribed verbatim. Photographs capturing the outputs of the interactive activities (where used) supported data analysis and interpretation. Analysis was conducted in concurrence with data collection. First, transcripts were compared with audio recordings to assess accuracy. The primary coder (HS) used the steps of inductive, manifest qualitative content analysis (Elo and Kyngäs, 2008) to develop codes and categories from the data. This was completed using NVivo software (Version 12.4.0). Where possible, codes were labeled using the language used by participants, prioritizing the language used by children. Codes and categories were then reviewed and appraised by a second reviewer (DJH) and differences in opinion in labeling or grouping of codes were resolved through discussion. Data saturation was assessed after the analysis of every three interviews and was defined a priori as the point at which analysis of three further transcripts yielded no new codes.
2.1.4 Clinical advisor and conference delegate problem list
Clinical coauthors jointly produced an exhaustive list of children's tinnitus-related problems recalled from cases seen in clinic. Advisors were asked to “list ALL tinnitus-related problems you recall reported by or observed in children, ideally just considering those aged 8–16 years old.” In addition, delegates (researchers and clinicians) to a conference workshop delivered by HS (Smith, 2018) were asked to write down 2–3 important tinnitus-related problems experienced by children. The written problems were compiled into a list.
2.1.5 Conceptual framework
Categories and codes elicited from the interviews were pooled with data from the (i) clinical advisor problem list, (ii) conference delegates problem list, (iii) service evaluation (Smith et al., 2020), and (iv) scoping review (Smith et al., 2019) to create a single dataset. This dataset was reviewed independently by all authors, and then collectively in an in-person workshop to develop the conceptual framework. The inclusion of concepts was guided by what was considered by the research team and clinical advisors as within the scope of tinnitus impact on HRQoL. Concepts considered outside of scope were removed. The concepts reported directly by children, and across multiple sources, were interpreted as pervasive aspects of tinnitus and were prioritized for inclusion. Key aspects of the PROM design (e.g., recall period, response scale) were also discussed and agreed in the workshop.
2.1.6 Drafting the pilot questionnaire
Based on the conceptual framework, candidate items for the questionnaire were drafted, where possible using the language used by children during the interview study. Through a process of iterative review and revision, completed by all members of the research team and clinical advisors, a pilot questionnaire was drafted.
2.2 Phase 2: assessing content validity
Content validity of the pilot questionnaire was assessed via cognitive interviews with children with tinnitus. Concurrently, the questionnaire was reviewed by clinicians working within NHS pediatric tinnitus services in an online survey. Finally, a readability assessment was completed.
2.2.1 Participants
2.2.1.1 Cognitive interviews
To be eligible to participate, children had to have experienced tinnitus for 3 months or more, had experienced tinnitus-related problems, had seen a health professional about their tinnitus, and were able to read English. Children were recruited via three specialist NHS pediatric tinnitus services, via research databases, and via social media. Cognitive interviews were conducted with six children (mean age: 11 years; SD: 3 years; age range: 8 – 15 years; female: 2; male: 4).
2.2.1.2 Clinician survey
Eight pediatric tinnitus service departments were surveyed from eight individual NHS Trusts, representing views from 28 clinicians. Members of each service were approached via the authors' professional network.
2.2.1.3 Readability assessment
Item readability was assessed by two children aged 8 years old and three children aged 9 years old with normal hearing (no tinnitus). Children were invited via their parents who were personal contacts of HS.
2.2.2 Procedure
2.2.2.1 Cognitive interviews
The questionnaire was tested in pen and paper format with four children (aged 8, 9, 10, and 15 years old) and in digital format using surveys.ac.uk with two children (aged 12 and 13 years old). HS conducted the interviews using a semi-structured interview guide (Supplementary Figure 5). The approach drew from the “retrospective verbal probing” cognitive interviewing technique described by Willis (2005) whereby the child was instructed to complete the questionnaire independently and ask for help only when needed. Following the completion of the questionnaire children were asked to give their initial feedback (likes, dislikes, item relevance, comprehensiveness, and comprehensibility) and feedback about the questionnaire instructions, recall period, response options, overall format, and length. This enabled a realistic assessment of the child's independent completion of the PROM.
2.2.2.2 Clinician survey
The clinician survey was administered online using surveys.ac.uk (Supplementary Figure 6). The staff member who was contacted about the study (lead reviewer) was instructed to download the pilot questionnaire, share it with members of their department and collect feedback. The lead reviewer was then asked to use the joint feedback to complete the survey questions. The survey prompted feedback regarding the suitability of the instruction text, recall period, response scale, and the suitability and comprehensiveness of the items.
The pilot questionnaire was revised iteratively between rounds of cognitive interviews and the clinician feedback (Figure 1).
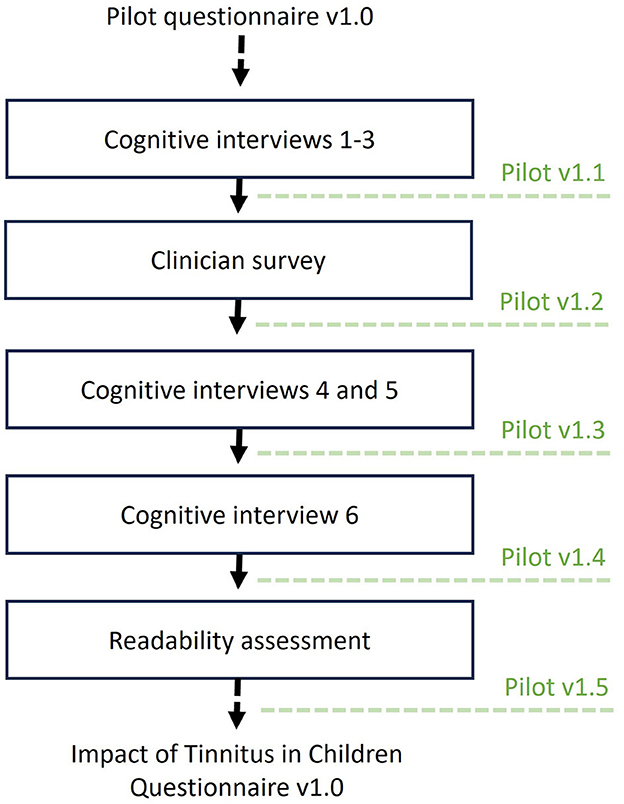
Figure 1. Overview of the stages of assessing content validity of the pilot questionnaire. Modifications to the questionnaire were made iteratively, at several time points (i.e., in between cognitive interviews, following the clinician survey, and readability assessment). Dotted lines indicate when the pilot questionnaire was updated.
2.2.2.3 Readability assessment
Readability assessment was performed on the item text using an online readability calculator (ReadabilityFormulas.com, 2023). The calculator analyzed the text based on seven commonly used readability formulae (The Flesch Reading Ease formula, The Flesch-Kincaid Grade Level, The Fog Scale, The SMOG Index, The Coleman-Liau Index, Automated Readability Index, and Linsear Write Formula) and provided a consensus output regarding the reading age required to read the text. Possible outputs ranged from no age recommendation (very, very, easy to read) to Grade Level 12 (children aged 17–18 years old). Additionally, five children (n = 1 aged 8 years, n = 3 aged 9 years) with normal hearing (no tinnitus) completed readability questions about the pilot questionnaire. For each item, they were asked to select one of the following options: “easy to read and understand,” “hard to read and understand,” or “in-between (not easy or hard)” and to circle any words that they did not understand. An optional open-text box was provided so that they could write their thoughts about the items or task in general.
2.2.3 Analysis of content validity
2.2.3.1 Cognitive interviews
Audio recordings were used to make detailed feedback notes for each interview in Microsoft Word. These notes were collated into a single document, and all comments were grouped relating to specific aspects of the questionnaire or each item. Pilot questionnaire scores were analyzed in Microsoft Excel to identify any items showing floor or ceiling effects, defined as more than 50% selecting “Strongly disagree/None of the time” (floor effect) or more than 50% selecting “Strongly Agree/All of the time” (ceiling effect). Feedback was discussed with the research team before each modification was made.
2.2.3.2 Clinician survey
Survey data were exported and analyzed in Microsoft Excel. Feedback was grouped for each questionnaire aspect or item. Data for each aspect/item were reviewed and discussed among the research team and revisions were made based on feedback. Anonymised respondent quotes are used to provide examples of feedback in this report. For example, COG4M12 refers to cognitive interview number 4, male, aged 12. CLNS5 refers to clinician survey number 5.
2.2.3.3 Readability assessment
Based on the answers from the child reviews and the output from the readability calculator, items and questionnaire text were modified. Modifications aimed to meet the reading level of the youngest users in the questionnaire age range, Grade Level 3 (children aged 8–9 years).
3 Results
3.1 Phase 1: development of conceptual framework of tinnitus impact on HRQoL in children
Data saturation was confirmed after the analysis of 21 interviews (i.e., no new codes were identified across the final three interviews (Supplementary Table 1). Through content analysis of the interview transcripts, 14 categories of tinnitus impact were developed which were used to inform the conceptual framework: Sleep, Physical health, School/learning, Cognitive impact, Hearing and Listening, Activities, Emotions, Concerns and questions, Behavior, Negative coping, Social impact, Perceptions of others, Knowledge and resources, and Exacerbating factors. These categories were pooled with problems identified by clinical advisors and conference delegates, and in the service evaluation (Smith et al., 2020) and scoping review (Smith et al., 2019). Following review of the pooled dataset, the final conceptual framework was developed.
Problems were excluded from the conceptual framework where they were deemed by the authors as external to, or not directly indicative of tinnitus impact on HRQoL. These included problems relating to the child's perception of their tinnitus sound (e.g., change in loudness), problems relating to tinnitus knowledge or resources (e.g., lack of professional help), associated physical problems (e.g., ear pain), or exacerbating factors (e.g., tinnitus is worse when stressed). Other problems were excluded if they were only or mainly reported by adults (parents and clinicians) and therefore had limited salience to children. For example, problems relating to behavior (e.g., disruptive behavior), negative coping (e.g., physical responses to tinnitus), or parental anxiety were excluded. In addition, rarely reported problems relating to self-harm and suicidal thoughts were excluded as they were considered inappropriate to include in a self-report measure for children. The review of the pooled dataset also led to the merging or regrouping of some problems. For example, problems under the category of “cognitive impact” (identified in the interview data) were grouped under “learning.” The final conceptual framework included six broad domains of tinnitus impact on HRQoL; Sleep and Feeling Tired, Learning, Emotional Health, Hearing and Listening, Taking Part, and Relationships (Figure 2).
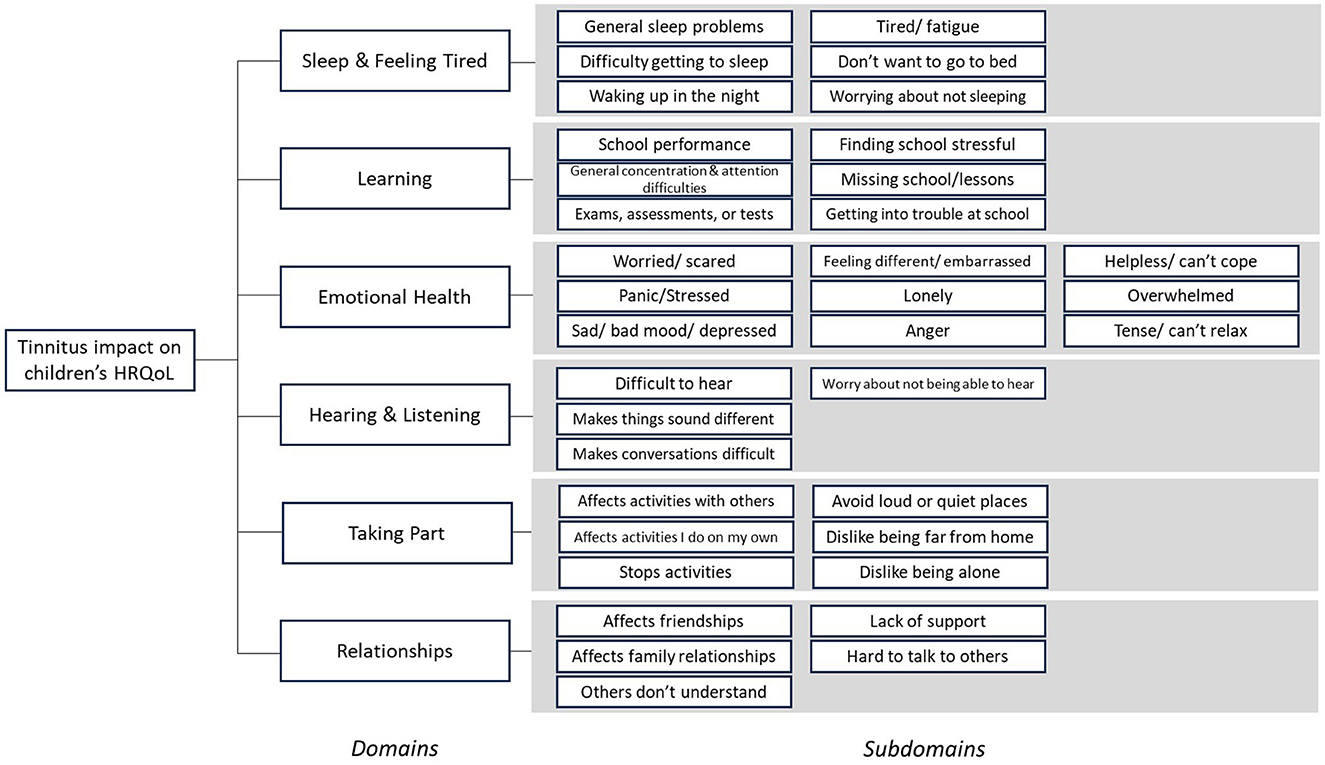
Figure 2. Conceptual framework of tinnitus impact on children's Health Related Quality of Life (HRQoL). The impact of tinnitus on children was conceptualized to be represented by six distinct domains, each of which may contribute equally to the overall impact. Domains were further explained by at least four distinct subdomains.
3.1.1 The pilot questionnaire development
A pilot questionnaire was drafted to assess the domains and subdomains included in the conceptual framework. Supplementary Table 2 lists the domains and subdomains of the conceptual framework, the corresponding 38 items in the pilot questionnaire (not including “scene setting” items), and example quotes from the interviews that informed the development of the items.
A recall period of 2 weeks was selected as this was considered relevant in terms of capturing the full range of symptom or treatment experience. Based on published evidence to suggest that Likert scales are appropriate for use with children aged 8 years and older (Matza et al., 2013), items were scored on a 5-point Likert scale of agreement (Strongly disagree, disagree, I don't agree or disagree, agree, or strongly agree). Here, an agreement scale was favored over a frequency scale as children are more likely to use the full extent of the agreement scale, thus optimizing scale sensitivity. For example, in instances of severe levels of worry, for item “My tinnitus makes me feel worried,” a child would more likely select “strongly agree” than “all of the time.” Individual item scores would be summed to produce both subscale scores and a global index with higher scores indicating greater tinnitus impact on HRQoL.
Items with positive valence were included to reflect the positive aspects of wellbeing included in HRQoL. Positive items can also enhance the questionnaire's ability to discriminate between groups insufficiently examined by negative items alone and can improve the detection of positive treatment outcome (Healthy People, 2020, 2010). Furthermore, a reverse in scale polarity provides a tool for the administrator to check the consistency of the rater's responses (Streiner et al., 2015).
Finally, input from clinical advisors led to the inclusion of three additional scene setting items at the beginning of the questionnaire to measure overall tinnitus awareness, annoyance, and impact. These general concepts were not considered to provide additional understanding of the impact of tinnitus on HRQoL beyond the other included items but were considered clinically useful to aid the overall interpretation of questionnaire scores. It was therefore decided that the three scene setting items would not contribute to the questionnaire scores. Scene setting items would be scored on a scale of frequency (Never, almost never, some of the time, often, all of the time) to provide an overall indication of how much time the child is aware of/affected by their tinnitus.
3.2 Phase 2: assessing content validity
The pilot questionnaire was revised based on cognitive interviews, clinician survey, and readability assessments (Supplementary Tables 3–5 outline changes made between the initial pilot and final questionnaire). This involved formatting adjustments in the paper version of the questionnaire, amendments to language (instruction text and items), a change of response scale, the removal of eight items, and the addition of three new items. Following these modifications, the pilot questionnaire, which initially included three scene setting items and 38 scoring items was revised to three scene setting items and 33 scoring items.
3.2.1 Formatting and language
Overall feedback from children and clinicians was positive and children found both paper and online versions of the iTICQ easy to complete. Feedback from children and clinicians led to several formatting improvements to the paper version. For example, improvements were made to the spacing of items, row sizing, tick box size, and use of color. Adaptations to language were made to replace certain words or phrases with more child-friendly alternatives, or to improve consistency of language.
3.2.2 Item refinement
A minority of items were removed because they either overlapped with other items, were overly complex, or lacked relevance to children. For example, children and clinicians suggested that the item “I struggle with how tinnitus makes me feel” was too complex. One child said, “It's tricky and confusing, I don't understand the question. In my opinion it doesn't make much sense” COG4M12. Similarly, a clinician said “‘Struggle' feels like a grown-up concept which some may find challenging” CLNS5. As a result, this item was removed.
Eight additional items were suggested by clinicians, all for the Emotional Health subscale. Of these, three new items were included; “My tinnitus makes me feel sad,” “My tinnitus makes me feel scared,” and “I prefer to spend time on my own because of my tinnitus.” These items were considered not to be covered by items that were already in the Emotional Health subscale. Suggested items that were not included concerned specific types of worry about tinnitus (e.g., “I worry that the tinnitus will get worse,” “I worry that the tinnitus will affect my future”). It was believed by the authors that tinnitus-related worry would be assessed by the existing “I worry about my tinnitus” item. Additionally, it would not be possible to include items for all worries experienced by children. Specific worries could instead be identified in follow-up discussion with the child. Children and clinicians also suggested the addition of open-ended questions about the child's tinnitus or related conditions (e.g., “What has helped your tinnitus?”, “How does your tinnitus change?”, “Do you have any co-occurring neurodevelopmental conditions?”). These questions were considered out of scope of a child self-report measure of tinnitus impact on HRQoL. Children indicated that the final questionnaire items were relevant and comprehensive of tinnitus impact on HRQoL.
3.2.3 Response scale
In the first three cognitive interviews it was clear that children were using the middle option of the agreement scale (I don't agree or disagree) inconsistently. Sometimes this option was selected when a problem was not experienced by the child, and other times this option was selected when a problem affected the child occasionally. Thus, after the third cognitive interview, response options were changed from an agreement scale (Strongly disagree, disagree, I don't agree or disagree, agree, strongly agree) to a frequency scale (None of the time, A little of the time, Some of the time, A lot of the time, All of the time) on the basis that a frequency scale would be used more consistently. This also meant that the same scale could be used across all items in the questionnaire. In the later three cognitive interviews, the frequency scale was received positively. For example, one child said; “It was good…I like none because you can be none, or just have a little and not a lot. There was always an answer to choose.” COG5F9.
3.2.4 Recall period
There were mixed views about the 2-week recall period. While children indicated this was challenging to think about, they also suggested this was an appropriate length of time to use in order to capture fluctuations in tinnitus severity and impact. Several children indicated a tendency to broaden the timeframe they were thinking about but would generally consider recent times. For example, one child said:
“Two weeks is easy. I get it every other day. People may generalise from the past month or so…as sometimes it is hard to remember exactly…it's not like you make a record of when it happens. I was thinking about recently, over the past month, but not really sticking to the past 2 weeks.” COG3M13
While some clinicians said the 2-week recall period was suitable, there were also suggestions to lengthen or to shorten the recall period. As children's feedback suggested overall that this timeframe was acceptable, the 2-week recall period was retained.
3.2.5 Readability
In the cognitive interviews children aged 10–15 years old found most questionnaire items easy to read and understand, with only minor suggestions for improvement. However, when prompted, children aged 8 and 9 expressed difficulty in explaining the meaning of some words and phrases to the interviewer. It was difficult to establish if children found the process of explaining the meaning of items to the interviewer difficult or if the words and phrases were difficult for the child to read and understand. In addition, several clinicians expressed concerns about the suitability of the questionnaire for the reading age of younger children.
Readability was further assessed using (ReadabilityFormulas.com, 2023) An initial output suggested that the items were “fairly easy to read” and suitable for Grade Level 5 (children aged 10–11 years). This score was driven by the inclusion of words with greater than two syllables (e.g., tinnitus, difficult, activities, interferes). Therefore, three-syllable words were substituted for two-syllable words where a suitable alternative was available. For example, “difficult” with “hard,” doing “activities” with doing “things,” “interferes” with “gets in the way of.” Commonly used three/four-syllable words such as “concentrate,” “family,” and “conversations,” were not substituted. Whilst “tinnitus” is also a three-syllable word, the essential nature of its inclusion and the presence of a definition of tinnitus within the questionnaire instruction text supported the decision for it to remain. Following these adjustments, the output for the revised 36 items was “easy to read,” suitable for Grade Level 4 (children aged 9–10 years). For the purpose of assessment only, “tinnitus” was substituted with single-syllable word “noise” to test the readability of items in its absence. With this substitution the output was “very easy to read,” suitable for Grade Level 3 (children aged 8–9 years).
Finally, children with normal hearing (no tinnitus) reviewed the revised items. Three children (n = 1 aged 8, n = 2 aged 9) indicated that all items were easy to read and understand and two children (n = 1 aged 8 years, n = 1 aged 9 years) found that most items were easy to read and understand and a few items were in-between (not easy or hard). No items were considered hard to read and understand.
The final questionnaire was named the Impact of Tinnitus in Children Questionnaire (iTICQ) to reflect both the self-complete nature and the construct of interest (Supplementary File Impact of Tinnitus in Children Questionnaire (iTICQ)_v1.0).
4 Discussion
This study developed a conceptual framework of the impact of tinnitus on HRQoL in children. The framework was developed from multiple data sources including direct input from children with tinnitus, parents of children with tinnitus, and from clinicians experienced in the management of children with tinnitus. Tinnitus impact on HRQoL in children was found to comprise six domains, being Sleep and Feeling Tired, Learning, Emotional Health, Hearing and Listening, Taking part, and Relationships. This conceptual framework informed the design of a pilot questionnaire, refined through cognitive interviews with children with tinnitus, feedback from clinicians working in pediatric tinnitus services, and readability assessment. This final questionnaire contains three scene setting items and 33 scoring items and was named Impact of Tinnitus in Children Questionnaire (iTICQ). Overall, evaluation indicated suitability for self-completion by children aged 8–16 years old.
The domains of tinnitus impact identified in the present study align with a study by Tegg-Quinn et al. (2021) which investigated the reflections of adults who had experienced tinnitus during childhood, and the perspectives of primary carers of children/ adolescents with tinnitus, and clinicians. Those authors used hierarchical cluster analysis to group participant statements about tinnitus impact in childhood, finding four clusters of impact; emotional wellbeing, academic performance, social/relations, and auditory/cognitive processing. Likewise, domains of emotional impact, academic impact, social/relational impact, and auditory/cognitive impact were identified in the present study. Our study provides additional evidence that these domains are relevant to the experiences of children with tinnitus. A major difference between the findings of Tegg-Quinn and colleagues and the present study is that sleep difficulty, social/ relationships impact, and impact on taking part were all conceptualized as distinct domains in the present study and not grouped together. Tegg-Quinn et al. (2021) acknowledged their participants tended to group statements relating to sleep difficulty, conversation, and attention and concentration into more than one of the four clusters, suggesting the diffuse nature of tinnitus and its impact in children. These differences could have been because the present study did not use quantitative methods to identify areas of impact but rather developed domains of impact via iterative stages of analysis and pooling of qualitative data. Furthermore, the inclusion of direct accounts of current tinnitus in childhood in the present study may have driven the differences in grouping. Future validation studies, inspecting the factor structure of the iTICQ will provide greater understanding of the robustness of these distinct domains of tinnitus impact.
The iTICQ's subscales; Sleep and Feeling Tired, Hearing and Listening, and Emotional Health, are comparable with subscales included in commonly used adult tinnitus questionnaires such as the Tinnitus Functional Index (TFI; Meikle et al., 2012), Tinnitus Handicap Questionnaire (THQ; Kuk et al., 1990), Tinnitus Handicap Inventory (THI; Newman et al., 1996), and Tinnitus Questionnaire (TQ; Hiller and Goebel, 2004). However, the Learning, Taking Part, and Relationships subscales are unique to the iTICQ and are not present in adult questionnaires. The prominence of social, contextual domains in the iTICQ reflects the inclusion of similar domains in many other general and disease-specific measures of children's HRQoL (Eiser and Morse, 2001; Janssens et al., 2015). When assessing the health status of children, understanding the social contexts in which the child is embedded (i.e., family, friendships, school, and community) is essential. These contexts are likely to contribute to the child's HRQoL and can mediate the impact of disease on the child's life and the effectiveness of treatment (Matza et al., 2004). Furthermore, mitigation of disease impact in these contexts is highly important as the child's impaired participation can have long-term consequences on their cognitive, social, and emotional development. For example, interruptions in learning may lead to negative academic outcomes or limited life chances. Children also have less power than adults to make changes to their context. An adult may have the power to improve a challenging work environment, or even leave their job, whereas a child does not have the ability to make similar changes at school. In sum, the impact of tinnitus on social context is likely to play a more important role for children with tinnitus compared to adults and it is important for the assessment of tinnitus in children to consider these aspects.
Children who participated in this study were extremely forthcoming in sharing their experiences and gave detailed accounts of how tinnitus had affected their life. As well as providing a measure of impact, the iTICQ may facilitate discussions with children about tinnitus, prompting more thorough and detailed accounts of their experience. In addition, analysis of problem data revealed some differences in the problems reported by children and parents. This supports the idea that seeking independent perspectives from children and parents is essential when assessing a child's tinnitus. PROM studies in other disease areas have found poor agreement between scores on child self-report and parent proxy-report measures (Davis et al., 2007; Vetter et al., 2012; Eiser and Varni, 2013; Janssens et al., 2015), particularly where measures concern children's feelings or internalizing symptoms. Thus, it will be important for clinicians to be mindful of potential differences in child and parent perspectives when assessing a child with tinnitus. Given that children are less likely to spontaneously report tinnitus and related problems, parents may be the first to raise these in an appointment. Use of the iTICQ as a discussion tool may bring to light important differences in parent and child perspectives of the child's tinnitus, with implications for the choice of tinnitus treatment.
The iTICQ was designed to be used in both clinical research and practice. The proposed scoring structure provides both a global index of tinnitus impact on HRQoL and a profile assessment of tinnitus impact on discrete domains (subscales). Findings from the qualitative interviews indicated the heterogeneous impact of tinnitus on domains of children's HRQoL. For example, for some children, tinnitus would have a notable impact on sleep whereas for other children sleep would be unaffected. This heterogeneity has also been highlighted in adult tinnitus research (Gu et al., 2022). In line with this, many tinnitus treatment approaches used with children target specific domain areas, e.g., sleep hygiene for sleep difficulty, hearing aids for tinnitus with hearing loss, referral to specialist mental health services for significant emotional impact (Kentish et al., 2015). As such, compared to single visual analog scales and open interview questions, the function of the iTICQ in providing a profile assessment of tinnitus impact is likely to be beneficial in identifying important problem areas and informing discussions and decisions about treatment. The global index of tinnitus impact can provide an additional, overall measure of tinnitus impact that will be particularly useful for research and the evaluation of clinical services.
Findings from the cognitive interviews identified the inadequacy of the agreement scale. While “Neither agree nor disagree” has been recommended as an appropriate midpoint response option (Streiner et al., 2015), in the present study “I don't agree or disagree” was eliciting mixed responses from children, being selected where the item affected the child occasionally and where the item was not relevant to the child at all. Several researchers have highlighted such inconsistent use of neutral midpoints within Likert scales by Nadler et al. (2015) and Chyung et al. (2017). However, discussion of the pitfalls of the Likert midpoint is not prominent in the children's PROM development literature. The findings from this study suggest that a Likert scale of agreement with a neutral midpoint is not appropriate for use in a child PROM. If used, PROM developers should provide clear guidance to children about how to use the scale and children's interpretation of the scale should be thoroughly tested.
Feedback from the cognitive interviews and the clinician survey indicated that younger children found some of the words or phrases in the pilot version of the iTICQ difficult to understand. It is possible that some of the questions used to assess comprehension (e.g., “question paraphrasing”- asking children to explain the meaning of a word or phrase in their own words) may have been too cognitively demanding for the youngest children interviewed (Willis, 2005). As a result, some of the feedback may not have accurately represented children's true level of understanding. Following simplifications to the item text, subsequent stages of readability assessment indicated that the iTICQ is suitable for the reading ability of children aged 8 years and older.
In line with the FDA [Food and Drug Administration (FDA), 2009] and ISPOR (Patrick et al., 2011a) guidance, the development of the iTICQ included input from members of the target population for which the PROM is intended to establish content validity. While the sample of children included had some diversity in terms of age, gender, and geographic location, the diversity of the sample could not be fully characterized as details of socioeconomic status, cognitive ability, reading level, and hearing ability were not recorded. Future studies assessing the validity of the iTICQ should ensure the inclusion of diverse groups of children with tinnitus. Questionnaire adaptations may be necessary to establish content validity in certain groups [e.g., children with intellectual disability Kooijmans et al., 2022].
Although the sample required for the current study was small it was important to recruit via multiple sources given children experiencing tinnitus can present to different clinical specialties. There was no selection bias as all children with tinnitus within the study age range were invited to participate. As children do not regularly present to clinical services reporting tinnitus, databases and social media were used to supplement recruitment from clinical services. Restricting some stages of recruitment to only children who had previously discussed their tinnitus with a clinician may have excluded the experiences of children with less troublesome tinnitus and children who were unable to access clinical services. Although the sample size for children was small for the interviews, the conceptual framework and items were based on evidence from clinical advisors and tinnitus conference delegates, a service evaluation (Smith et al., 2020), a scoping review (Smith et al., 2019). and semi-structured interviews/focus groups. The approach taken therefore was exhaustive of sources of evidence, and this was considered necessary to create a valid and representative questionnaire.
Overall, the iTICQ has good content validity. Concept elicitation with input from target clinical population ensured that items included in the pilot questionnaire were relevant to the construct of tinnitus impact on HRQoL. Feedback from cognitive interviews, the clinician survey, and readability assessment further indicated the relevance, comprehensiveness, and comprehensibility of the questionnaire. Further evaluation and adaptations are necessary to ensure the iTICQ has content validity in wider groups of children with tinnitus. The next stage is extensive assessment of the iTICQ's psychometric properties (validity, reliability, and responsiveness). The iTICQ offers a tool for use in clinical practice and research to measure the impact of tinnitus on children's HRQoL. Use of the iTICQ will also support clinical practice by enabling differentiating between children of varying tinnitus severity, facilitating discussions with children and parents in clinical appointments, and informing decisions regarding the most appropriate treatment.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by NHS Research Ethics Committee (18/EE/0396). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin.
Author contributions
HS: Data curation, Formal analysis, Investigation, Methodology, Project administration, Writing—original draft, Writing—review & editing. KF: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Supervision, Writing—review & editing. VK: Conceptualization, Data curation, Funding acquisition, Methodology, Writing—review & editing. JB: Conceptualization, Funding acquisition, Methodology, Supervision, Writing—review & editing. CB: Data curation, Writing—review & editing. LP: Data curation, Writing—review & editing. DJH: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Supervision, Writing—review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by Tinnitus UK (previously British Tinnitus Association) (C000I and C9999S). DJH was funded by the National Institute for Health and Care Research (NIHR) Biomedical Research programme. KF was funded by National Institute for Health Research (NIHR Post-Doctoral Fellowship, PDF-2018-11-ST2-003) at the time of completing this work.
Acknowledgments
The authors would like to thank the contributions of all children, parents, and clinicians who participated in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
The views expressed are those of the authors and not necessarily those of the NIHR, the NHS, or the Department of Health and Social Care.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fauot.2023.1323864/full#supplementary-material
References
Chyung, S. Y. Y., Roberts, K., Swanson, I., and Hankinson, A. (2017). Evidence-based survey design: the use of a midpoint on the likert scale. Perform. Improv. 56, 15–23. doi: 10.1002/pfi.21727
Comins, J. D., Brodersen, J., Siersma, V., Jensen, J., Hansen, C. F., Krogsgaard, M. R., et al. (2021). How to develop a condition-specific PROM. Scand. J. Medi. Sci. Sports 31, 1216–1224. doi: 10.1111/sms.13868
Davis, E., Nicolas, C., Waters, E., Cook, K., Gibbs, L., Gosch, A., et al. (2007). Parent-proxy and child self-reported health-related quality of life: using qualitative methods to explain the discordance. Qual. Life Res. 16, 863–871. doi: 10.1007/s11136-007-9187-3
Ediebah, D. E., Quinten, C., Coens, C., Ringash, J., Dancey, J., Zikos, E., et al. (2018). Quality of life as a prognostic indicator of survival: a pooled analysis of individual patient data from canadian cancer trials group clinical trials. Cancer 124, 3409–3416. doi: 10.1002/cncr.31556
Eiser, C., and Morse, R. (2001). Quality of life measures in chronic diseases of childhood. Health Technol. Assess. 5, 1–157. doi: 10.3310/hta5040
Eiser, C., and Varni, J. W. (2013). Health-related quality of life and symptom reporting: similarities and differences between children and their parents. Eur. J. Pediatr. 172, 1299–1304. doi: 10.1007/s00431-013-2049-9
Elo, S., and Kyngäs, H. (2008). The qualitative content analysis process. J. Adv. Nurs. 62, 107–115. doi: 10.1111/j.1365-2648.2007.04569.x
Food and Drug Administration (FDA) (2009). Guidance for Industry: Patient- Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims. Rome: FDA.
Gu, H., Kong, W., Yin, H., and Zheng, Y. (2022). Prevalence of sleep impairment in patients with tinnitus: a systematic review and single-arm meta-analysis. Eur. Arch. Otorhinolaryngol. 279, 2211–2221. doi: 10.1007/s00405-021-07092-x
Haverman, L., Limperg, P. F., Young, N. L., Grootenhuis, M. A., and Klaassen, R. J. (2017). Paediatric health-related quality of life: what is it and why should we measure it? Arch. Dis. Child. 102, 393–400. doi: 10.1136/archdischild-2015-310068
Healthy People (2010). In Foundation Health Measure Report (Issue November). Available online at: http://www.healthypeople.gov/sites/default/files/HRQoLWBFullReport.pdf
Healthy People (2020). Foundation Health Measure Report: Health-Related Quality of Life and Well-Being. Available online at: http://www.healthypeople.gov/sites/default/files/HRQoLWBFullReport.pdf
Hiller, W., and Goebel, G. (2004). Rapid assessment of tinnitus-related psychological distress using the mini-TQ. Int. J. Audiol. 43, 600–604. doi: 10.1080/14992020400050077
Humphriss, R., Hall, A. J., and Baguley, D. M. (2016). Prevalence and characteristics of spontaneous tinnitus in 11-year-old children. Int. J. Audiol. 55, 142–148. doi: 10.3109/14992027.2015.1120890
Ida Institute (2021). Ida Institute My World. Availabe online at: https://idainstitute.com/tools/my%7B%5C_%7Dworld/ (accessed February 9, 2021).
Jacquemin, L., van der Poel, N., Biot, L., Schollaert, J., Bonné, F., Vanderveken, O. M., et al. (2023). Prevalence of tinnitus and hyperacusis in 9–12-year-old children. Eur. Arch. Otorhinolaryngol. 280, 4819–4825. doi: 10.1007/s00405-023-07995-x
Janssens, A., Thompson Coon, J., Rogers, M., Allen, K., Green, C., Jenkinson, C., et al. (2015). A systematic review of generic multidimensional patient-reported outcome measures for children, part I: descriptive characteristics. Value Health 18, 315–333. doi: 10.1016/j.jval.2014.12.006
Juniper, E. F., Guyatt, G. H., Feeny, D. H., Ferrie, P. J., Griffith, L. E., Townsend, M., et al. (1996). Measuring quality of life in children with asthma. Qual. Life Res. 5, 35–46. doi: 10.1007/BF00435967
Kentish, R., Benton, C., Kennedy, V., Munro, C., Philips, J., Rogers, C., et al (2015). Tinnitus in Children Practice Guidance. Available online at: https://www.maryhare.org.uk/sites/maryhare.org.uk/files/17.%20Tinnitus%20in%20children%20practice%20guidance.pdf
Kooijmans, R., Mercera, G., Langdon, P. E., and Moonen, X. (2022). The adaptation of self-report measures to the needs of people with intellectual disabilities: a systematic review. Clin. Psychol.: Sci. Pract. 29, 250–271. doi: 10.1037/cps0000058
Kuk, F. K., Tyler, R. S., Russell, D., and Jordan, H. (1990). The psychometric properties of a tinnitus handicap questionnaire. Ear Hear. 11, 434–445. doi: 10.1097/00003446-199012000-00005
Landgraf, J. M., Maunsell, E., Nixon Speechley, K., Bullinger, M., Campbell, S., Abetz, L., et al. (1998). Canadian-French, German and UK versions of the child health questionnaire: methodology and preliminary item scaling results. Qual. Life Res. 7, 433–445. doi: 10.1023/A:1008810004694
Lipscomb, J., Donaldson, M. S., and Hiatt, R. A. (2004). Cancer outcomes research and the arenas of application. J. Natl. Cancer Inst. Monographs 20892, 1–7. doi: 10.1093/jncimonographs/lgh038
Matza, L. S., Patrick, D. L., Riley, A. W., Alexander, J. J., Rajmil, L., Pleil, A. M., et al. (2013). Pediatric patient-reported outcome instruments for research to support medical product labeling : report of the ISPOR PRO good research practices for the assessment of children and adolescents task force. Value Health 16 461–479. doi: 10.1016/j.jval.2013.04.004
Matza, L. S., Swensen, A. R., Flood, E. M., Secnik, K., and Leidy, N. K. (2004). Assessment of health-related quality of life in children: a review of conceptual, methodological, and regulatory issues. Value Health 7, 79–92. doi: 10.1111/j.1524-4733.2004.71273.x
McFerran, D. J., Stockdale, D., Holme, R., Large, C. H., and Baguley, D. M. (2019). Why is there no cure for tinnitus? Front. Neurosci. 13, 802. doi: 10.3389/fnins.2019.00802
Meikle, M. B., Henry, J. A., Griest, S. E., Stewart, B. J., Abrams, H. B., McArdle, R., et al. (2012). The tinnitus functional index: development of a new clinical measure for chronic, intrusive tinnitus. Ear Hear. 32, 153–176. doi: 10.1097/AUD.0b013e31822f67c0
Mokkink, L. B., Princen, C. A. C., Patrick, D. L., Alonso, J., Bouter, L. M., De Vet, H. C. W., et al (2019). COSMIN Study Design Checklist for Patient-Reported Outcome Measurement Instruments. Available online at: www.cosmin.nl (accessed December 27, 2023).
Nadler, J. T., Weston, R., and Voyles, E. C. (2015). Stuck in the middle: the use and interpretation of mid-points in items on questionnaires. J. Gen. Psychol. 142, 71–89. doi: 10.1080/00221309.2014.994590
Nemholt, S., Schmidt, J. H., Wedderkopp, N., and Baguley, D. M. (2020). A cross-sectional study of the prevalence and factors associated with tinnitus and/or hyperacusis in children. Ear Hear. 41, 344–355. doi: 10.1097/AUD.0000000000000759
Newman, C. W., Jacobson, G. P., and Spitzer, J. B. (1996). Development of the tinnitus handicap inventory. Arch. Otolaryngol. Head Neck Surg. 122, 143–148. doi: 10.1001/archotol.1996.01890140029007
NICE (2020). Tinnitus: Assessment and Management NICE guideline. Available online at: https://www.nice.org.uk/guidance/ng155
Parslow, R. M., Anderson, N., Byrne, D., Haywood, K. L., Shaw, A., Crawley, E., et al. (2020). Development of a conceptual framework to underpin a health-related quality of life outcome measure in paediatric chronic fatigue syndrome/myalgic encephalopathy (CFS/ME): prioritisation through card ranking. Qual. Life Res. 29, 1169–1181. doi: 10.1007/s11136-019-02399-z
Patrick, D. L., Burke, L. B., Gwaltney, C. J., Leidy, N. K., Martin, M. L., Molsen, E., et al. (2011a). Content Validity — establishing and reporting the evidence in newly developed patient-reported outcomes (PRO) instruments for medical product evaluation : ISPOR PRO good research practices task force report : part 1 — eliciting concepts for a new PRO inst. Value Health 14, 967–977. doi: 10.1016/j.jval.2011.06.014
Patrick, D. L., Burke, L. B., Gwaltney, C. J., Leidy, N. K., Martin, M. L., Molsen, E., et al. (2011b). Content Validity — establishing and reporting the evidence in newly developed patient-reported outcomes (PRO) instruments for medical product evaluation : ISPOR PRO good research practices task force report : part 2 — assessing respondent understanding. Value Health 14, 978–988. doi: 10.1016/j.jval.2011.06.013
Raj-Koziak, D., Gos, E., Swierniak, W., Skarzynski, H., and Skarzynski, P. H. (2020). Prevalence of tinnitus in a sample of 43,064 children in Warsaw, Poland. Int. J. Audiol. 60, 614−620. doi: 10.1080/14992027.2020.1849829
ReadabilityFormulas.com. (2023). Automatic Readability Checker. Available online at: https://readabilityformulas.com/free-readability-formula-tests.php (accessed February 9, 2021).
Rosing, S. N., Schmidt, J. H. S., Wedderkopp, N., and Baguley, D. (2016). Prevalence of tinnitus and hyperacusis in children and adolescents: a systematic review. BMJ Open 6, e010596. doi: 10.1136/bmjopen-2015-010596
Rothrock, N. E., Kaiser, K. A., and Cella, D. (2011). Developing a valid patient-reported outcome measure. Clin. Pharmacol. Ther. 23, 1–7. doi: 10.1038/clpt.2011.195
Savastano, M. (2007). Characteristics of tinnitus in childhood. Eur. J. Pediatr. 166, 797–801. doi: 10.1007/s00431-006-0320-z
Skinner, T. C., Howells, L., Greene, S., Edgart, K., McEvilly, A., Johansson, A., et al. (2003). Development, reliability and validity of the diabetes illness representations questionnaire: four studies with adolescents. Diabet. Med. 20, 283–289. doi: 10.1046/j.1464-5491.2003.00923.x
Smith, H. (2018). “How should tinnitus be measured in children (Oral Presentation),” in British Tinnitus Association Annual Conference (Sheffield).
Smith, H., Fackrell, K., Kennedy, V., Barry, J., Partridge, L., Hoare, D. J., et al. (2019). A scoping review to catalogue tinnitus problems in children. Int. J. Pediatr. Otorhinolaryngol. 122, 141–151. doi: 10.1016/j.ijporl.2019.04.006
Smith, H., Fackrell, K., Kennedy, V., Barry, J. G., Broomhead, E., Hoare, D. J., et al. (2020). An evaluation of paediatric tinnitus services in UK National Health Service audiology departments. BMC Health Serv. Res. 20, 214. doi: 10.1186/s12913-020-5040-y
Streiner, D. L., Norman, G. R., and Cairney, J. (2015). Health Measurement Scales: A Practical Guide to their Development and Use, 5th ed. Oxford: Oxford University Press. doi: 10.1093/med/9780199685219.001.0001
Tegg-Quinn, S., Eikelboom, R. H., Baguley, D. M., Brennan-Jones, C. G., Mulders, W. H. A. M., Bennett, R. J., et al. (2023). The use of patient-report measures and intervention strategies for children and adolescents with chronic tinnitus: a scoping review. Int. J. Audiol. 62, 1109–1117. doi: 10.1080/14992027.2022.2111371
Tegg-Quinn, S., Eikelboom, R. H., Brennan-Jones, C. G., Barabash, S., Mulders, W. H. A. M., Bennett, R. J., et al. (2021). Reflections on how tinnitus impacts the lives of children and adolescents. Am. J. Audiol. 30, 544–556. doi: 10.1044/2021_AJA-20-00178
Terwee, C. B., Prinsen, C. A. C., Chiarotto, A., De Vet, H. C. W., Westerman, M. J., Patrick, D. L., et al (2018). COSMIN Methodology for Assessing the Content Validity of PROMs. Available online at: https://www.cosmin.nl/wp-content/uploads/COSMIN-methodology-for-content-validity-user-manual-v1.pdf
Varni, J. W., Seid, M., and Rode, C. A. (1999). The PedsQL TM : measurement model for the pediatric quality of life inventory. Med. Care 37, 126–139. doi: 10.1097/00005650-199902000-00003
Vetter, T. R., Bridgewater, C. L., and McGwin, G. (2012). An observational study of patient versus parental perceptions of health-related quality of life in children and adolescents with a chronic pain condition: who should the clinician believe? Health Qual. Life Outcomes 10, 85. doi: 10.1186/1477-7525-10-85
Williams, K. (2018). Patient-reported Outcome Measures: Stakeholder Interviews | Australian Commission on Safety and Quality in Health Care. Available online at: https://www.safetyandquality.gov.au/publications-and-resources/resource-library/patient-reported-outcome-measures-stakeholder-interviews (accessed December 27, 2023).
Willis, G. (2005). Cognitive Interviewing: A Tool for Improving Questionnaire Design. London: SAGE Publications. doi: 10.1037/e538062007-001
Keywords: tinnitus, child, measurement, questionnaire, PROM, content validity
Citation: Smith H, Fackrell K, Kennedy V, Barry JG, Benton C, Partridge L and Hoare DJ (2024) Development of the impact of tinnitus in children questionnaire (iTICQ). Front. Audiol. Otol. 1:1323864. doi: 10.3389/fauot.2023.1323864
Received: 18 October 2023; Accepted: 15 December 2023;
Published: 11 January 2024.
Edited by:
Vinaya Manchaiah, University of Colorado Anschutz Medical Campus, United StatesReviewed by:
Maren Stropahl, Sonova, SwitzerlandCandice M. Quinn, United States Department of Veterans Affairs, United States
Emma Stapleton, The University of Manchester, United Kingdom
Copyright © 2024 Smith, Fackrell, Kennedy, Barry, Benton, Partridge and Hoare. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Harriet Smith, aGFycmlldC5zbWl0aEBzaGVmZmllbGQuYWMudWs=
 Harriet Smith
Harriet Smith Kathryn Fackrell
Kathryn Fackrell Veronica Kennedy
Veronica Kennedy Johanna G. Barry1,5
Johanna G. Barry1,5 Derek J. Hoare
Derek J. Hoare