
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Audiol. Otol., 11 December 2023
Sec. Tinnitus
Volume 1 - 2023 | https://doi.org/10.3389/fauot.2023.1311186
This article is part of the Research TopicReviews in Tinnitus: Exploring the Scientific EvidenceView all articles
Identifying and implementing an effective tinnitus treatment has been a challenge. Despite efforts over many decades, there is no definitive cure for tinnitus yet. Implementation science may assist audiology practitioners and end-user patients in their pursuit of a cure by identifying ways to maximize the use of research findings. Within the context of therapeutic interventions, implementation science is the study of a successful treatment–system fit evidenced by use. Research evidence for tinnitus treatment efficacy is dominated by behavioral questionnaires as they are a pragmatic source of patient-driven data. Neurophysiological evidence of the underlying neural network change correlated with these behavioral findings enhances research conclusions and potential use. This implementation science review systematically sourced and analyzed neurophysiological evidence from 29 studies to find that targeting tinnitus core network neuroplasticity may be the most effective tinnitus treatment. Narrow-band sound treatment has the greatest body of correlated neurophysiological-behavioral evidence. This is the first tinnitus implementation science systematic review. It is hoped that new or improved treatments may emerge from pivoting the evidential lens toward the pragmatic use of neurophysiological evidence.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/, identifier: CRD42022335201.
The implementation of a universally effective tinnitus treatment is a challenge. Tinnitus, the “conscious awareness of a tonal or composite noise for which there is no identifiable corresponding external acoustic source” (De Ridder et al., 2021, p. 8), is prevalent in ~11.9%–30.3% of the general adult population (McCormack et al., 2016). As a comorbid or standalone pathology, it presents itself in many ways. Tinnitus may be characterized as unilateral, originating in one ear, or bilateral, found in both ears. It may be subjective, only perceptible by the individual, or objective, where it is generated by a source within the body and is perceptible by others. It can present with or without hearing loss. Most importantly, tinnitus may be acute, when it is temporarily present for a short duration, or chronic, when it persists for 3 months or more. The heterogeneity of its presentation is driven in part by the heterogeneity of its epidemiology, with research into risk factors such as hearing loss, aging, noise-related trauma, or head injury, illustrating that what drives tinnitus in one individual may not in another (Møller, 2011). Although no definitive cure has been found to date, multiple treatments have been developed including, but not limited to, cognitive behavioral therapy (CBT) and counseling, electrical stimulation, hearing aids, and sound-based therapy. Given these challenges, the most pragmatic treatment strategy has been trial and error. Inefficient and costly in time and resources, trial and error also runs the risk of producing a successive failure of treatments, only reinforcing the perception that tinnitus cannot be treated.
Implementation science may uncover solutions to this problem. Utilizing the scientific method, implementation science seeks to identify ways to increase the uptake of research findings into routine practice (Eccles et al., 2012). Applied to the systematic review of a health intervention, the implementation gap between efficacy under ideal research conditions and effectiveness under “normal” constraints (Villalobos Dintrans et al., 2019) can be overtly addressed. While this may improve health outcomes through increased adoption and early implementation of evidence-based practice, the greatest impact comes from long-term, consistent application (Tansella and Thornicroft, 2009) reliant on clear, rigorously tested research. Randomized control trials (RCTs) remain the gold standard of scientific research design, reducing the risk of bias through strict participant inclusion criteria, and (often blinded) randomization to highly controlled research protocols. Consistent RCT translation by clinical practitioners to ensure health equity (Yapa and Barnighausen, 2018; Woodward et al., 2021) is problematic without explicit implementation consideration (Wensing and Grol, 2019).
Evidential rigor competes with cost and accessibility in implementation translation. Subjective self-report questionnaires dominate evidence for tinnitus treatment efficacy such as the Tinnitus Handicap Inventory (THI), the Tinnitus Functional Index (TFI), and the Tinnitus Questionnaire (TQ). Pragmatic, low-cost, scientific behavioral metrics are captured firsthand, such as through the TFI eight subscales for intrusiveness, cognition, quality of life, and emotional distress. Advancements in non-invasive neuroimaging technology have enabled the growth of objective neurophysiological evidence. While magnetic resonance imaging captures the blood-oxygenation level-dependent response to treatment (McRobbie et al., 2017; Poldrack et al., 2011), electroencephalography (EEG) records cellular local field potential changes at the scalp (Kropotov, 2010; Schomer and Da Silva, 2018). Efficacy at a cellular level is captured at a regional and a global level by quantitatively comparing spatiotemporal neural activity in tinnitus participants with healthy control subjects, pre- and postintervention. Neuroimaging, however, is inaccessible to some researchers due to technology cost, location, and operational skill set. The implementation science dilemma is that the accessibility and equity necessary in practice must be weighed against research reliability and validity potentially achieved through expensive neurophysiological measures. For example, with tinnitus, questionnaire results alone underpin the claim that “only CBT treatment has been shown to have a definite improvement effect on tinnitus in a large randomized controlled trial” (Cima et al., 2012; Tang et al., 2019, p. 110). Using a low-cost, accessible, internet-based form, a positive effect on tinnitus distress using questionnaires has also been evidenced (Andersson et al., 2002; Beukes et al., 2018). Two Cochrane systematic reviews (Martinez Devesa et al., 2007; Fuller et al., 2020), however, reported contradicting results discussing the risk of bias when employing self-report questionnaires alone. Combined behavioral and neurophysiological data enhance the evidential rigor of research conclusions, increasing trust in treatment efficacy and potential use. Identifying ways to implement neurophysiological alongside behavioral evidence of tinnitus treatment efficacy, reconciling scientific rigor with equitable accessibility, is the goal of this systematic review.
This systematic review was conducted adhering to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Guidelines 2020 (Page et al., 2021) as shown in Figure 1. The review protocol was registered with the Prospective Register of Systematic Reviews, ID CRD42022335201, prior to data extraction. One deviation occurred post-extraction when the Assessing the Methodological Quality of Systematic Reviews (AMSTAR 2), with its treatment of non-randomized studies (Shea et al., 2017), was adapted to act as the individual study risk-of-bias (RoB) tool. Ethics committee approval was not required for this review.
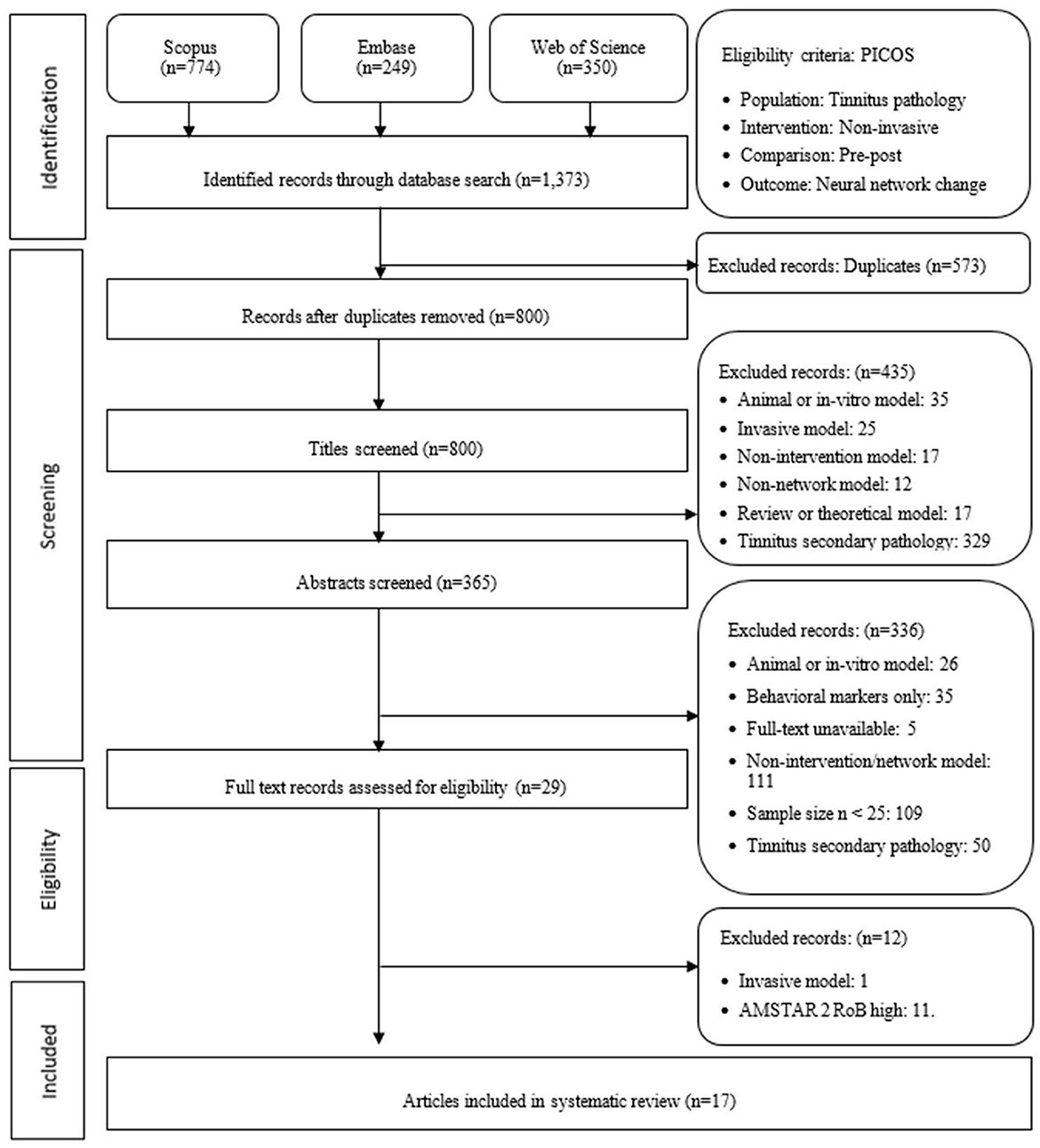
Figure 1. Preferred reporting items for systematic reviews and meta-analysis flow diagram detailing study selection process following 2020 guidelines. AMSTAR 2, assessing the methodological quality of systematic reviews; RoB, risk of bias.
A systematic search of the literature was undertaken using Scopus, EMBASE, and the Web of Science to ensure a biomedical focus was enhanced by a wider sciences scope while including one publisher independent of Elsevier. A comprehensive search strategy was employed to identify articles published using the English language without any limitation on the year of publication or the ages of participants. A combination of medical subject headings and keywords (tinnitus, network or system or region or area, and intervention or treatment or therapy) in the title, abstract, and/or keywords resulted in (n = 1,373) studies being retrieved for screening. Multiple searches were conducted commencing April 14, 2022, with a final search performed June 1, 2022.
Included in this systematic review were “articles” in which neural network change, using non-invasive experimental tinnitus interventions on human participants, was evidenced using a pre–post neurophysiological observation. Reviews, meta-analyses, book chapters, conference abstracts, non-English-language articles, and unpublished studies were excluded, along with in vitro and animal models, invasive interventions such as cochlear implantation, studies in which tinnitus was a secondary pathology or behavioral evidence alone was collected. A minimum treatment sample size of 25 was determined based on a two-sample power t-test (d = 0.8, sig. level = 0.05, power = 0.8) and analysis of variance power test (k = 2, f = 0.4, sig. level = 0.05, power = 0.8).
Summary data from (n = 1,373) studies, including title, source, authors, publication year, digital object identifier, URL, and abstract, were exported and de-duplicated by the primary reviewer (LJBH), resulting in 800 studies eligible for review. Two reviewers (LJBH and GS) independently reviewed eligibility. Any discrepancies between reviewers were documented in the protocol log, with final decisions undertaken by the tertiary reviewer (PS). Summary data screening resulted in 435 exclusions displayed in Figure 1. A total of 15 studies were entered into the protocol log and resolved by the tertiary reviewer (PS). Abstracts were then screened resulting in 336 exclusions. The full-text records of 29 studies were eligible for analysis. It is acknowledged that of the 29 studies, 12 were sourced from research conducted at the Department of Radiology, Beijing Friendship Hospital, Capital Medical University, including four connected to Trial ID: NCT02774122, three to NCT03764826, and two to NCT00927121. The independence of each study was reviewed by all three reviewers and confirmed to remain as an independent study.
To enhance consistency across reviewers, the primary reviewer extracted data for analysis in the Crowe Critical Appraisal Tool (CCAT; Crowe, 2013) format enabling a systematic eight-category quality review. A total score out of 40 was broken into the subcategories: preliminaries (title, abstract), introduction, research design, sampling (method, size, and protocol), data collection, ethics, results, and discussion. Exclusion for this systematic review was determined to be a score of <60% in total or for one individual section. No studies were excluded, with two studies reaching 60% but not below.
An additional risk of bias (RoB) assessment was conducted on 29 studies using an adapted AMSTAR 2 (Shea et al., 2017) 16-item protocol. Studies (n = 11) were excluded for lacking an explicit control group, sample data, and explicit statements regarding conflict of interest and ethics, as shown in Table 1. Only data presented within the published article were sourced for the RoB assessment on the basis that supplementary or additional data links contained data not imminently pertinent to the study findings. An additional study was excluded for an invasive intervention. The three-reviewer protocol resulted in no additional entries into the protocol log for either the CCAT or AMSTAR 2 assessments.
The analysis of each individual study produced a quantitative and a qualitative table of the remaining 17 studies. Statistical analysis of the quantitative table included central tendency and distribution data for sample size (control and pathology), tinnitus duration (standardized to months) and lateralization, and intervention duration (standardized to days), as well as demographic data (age, gender, education years, and handedness). Non-statistical analysis included treatment effect measure points (T0, T1, and T2), hearing loss, pretreatment (group only) baseline differences between healthy control and tinnitus pathology sample groups, posttreatment (Group × Time) intervention effects, and correlates between behavioral and neurophysiological results, as well as author-stated limitations. To ease the implementation of findings by end-user patients and clinicians, statistical and non-statistical results were then grouped by tinnitus duration (acute, <3 months; chronic, 3–60 months; and chronic extended, >60 months) and intervention type.
Figure 1 illustrates that of the 29 studies analyzed in Table 1, the results of 17 studies are presented in this systematic review. Primary affiliations across three nations, China, Germany, and the United States, are represented in Figure 2. Without any search restriction on publication year, most articles were published in 2020 or 2021. A significant majority of studies sourced included participant samples with a mean duration (months) classified as chronic tinnitus.
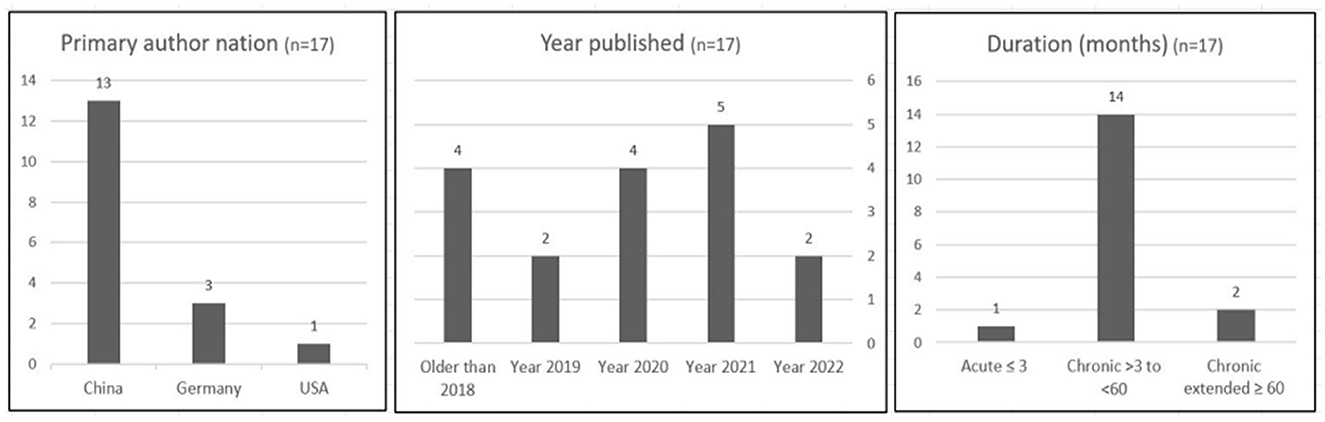
Figure 2. Heterogeneity of studies analyzed (n = 17) following the risk of bias assessment at Table 1.
The intervention with the greatest body of neurophysiological evidence for treatment-induced neural network change was narrow-band sound therapy (n = 12) displayed in Table 2. Demographical data for the large, consolidated tinnitus treatment sample (n = 464) include a mean age of 43.52 years (18–65), gender ratio 1.24 male:female, tinnitus duration 25.24 months (6–48, indicating all participants were chronic), and tinnitus lateralization ratio of 0.72 right:left, with 24.17 (mean) participants unilateral and 24.83 bilateral. A narrow-band noise set at the tinnitus frequency (Tf) ± 0.5 kHz for 20 min three times per day was applied with a mean intervention duration of 129 days (84–180). Following the review criteria of a minimum sample size of 25, from the large Table 2 control sample (n = 413), the mean control group size was 34.42 (25–63), while the mean treatment group size was 38.67 (25–78). All studies found behavioral evidence of improvement in tinnitus symptoms following sound treatment, measured by the THI.
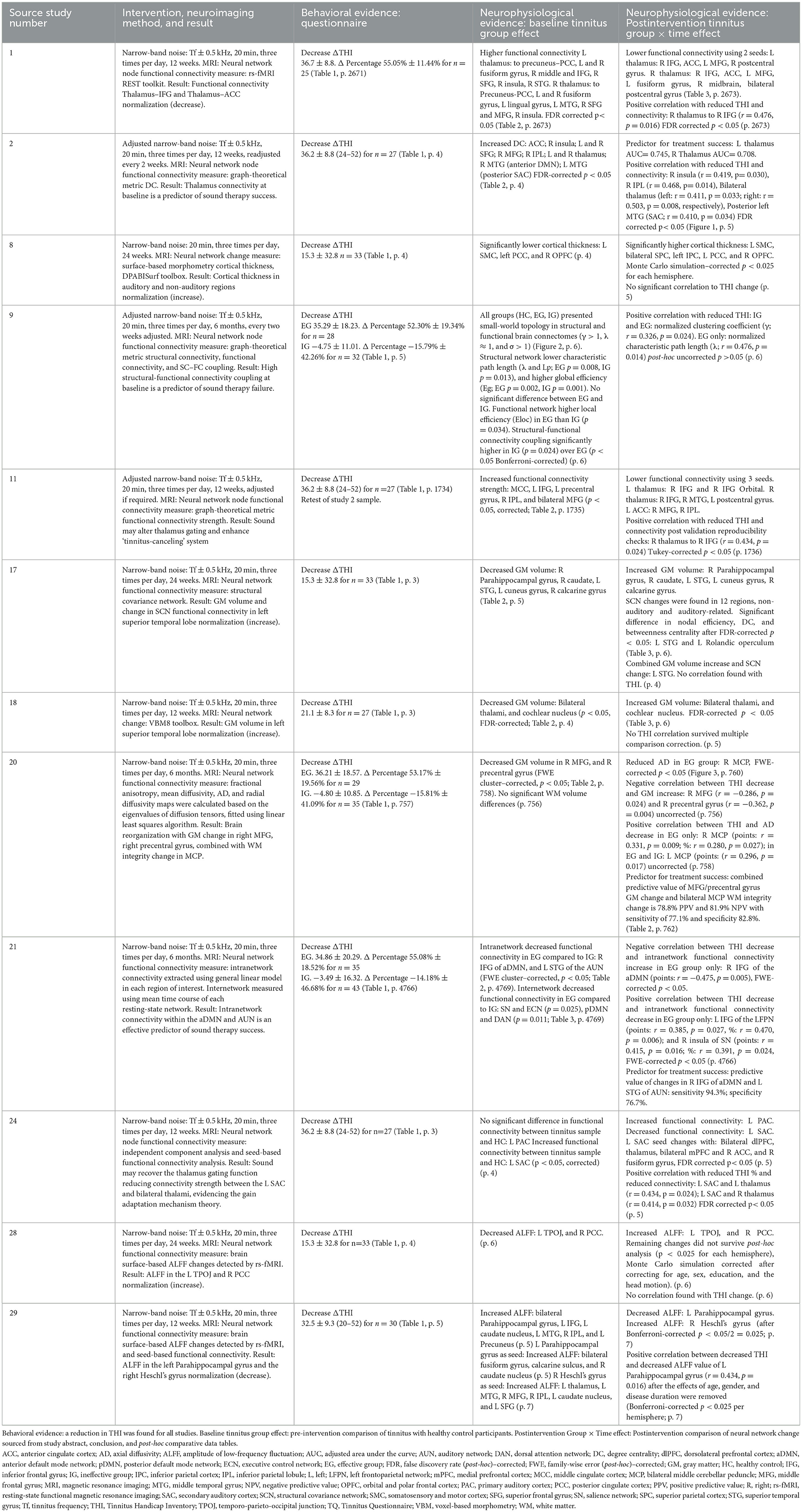
Table 2. Results from 12 studies applying a narrow-band noise sound treatment to chronic (3–60 months) tinnitus participants.
Narrow-band sound induced a reduction in network connectivity from the elevated baseline tinnitus brain to a healthy control level, positively correlated with symptom improvement. Correlated neurophysiological and behavioral evidence is displayed visually in Figure 3 and comparatively in Table 3. Using a seed-based analysis, Study 1 (Lv et al., 2020) found reductions in functional connectivity between the bilateral thalami and right inferior frontal gyrus, anterior cingulate cortex, left medial frontal gyrus, and right postcentral gyrus. Selecting the right thalamus in the seed-based analysis, functional connectivity also decreased posttreatment to the left fusiform gyrus, right midbrain, and left postcentral gyrus. Decreased connectivity between the right thalamus and right inferior frontal gyrus was positively correlated with the reduction in THI, (r = 0.476, p = 0.016) fake discovery rate (FDR)–corrected (p < 0.05). One year prior, applying a global analysis, Study 2 (Han et al., 2019a) evidenced a positive correlation between reduced THI and reduced functional connectivity in the right insula (r = 0.419, p = 0.030), bilateral thalamus (left: r = 0.411, p = 0.033; right: r = 0.503, p = 0.008), right inferior parietal lobule (r = 0.468, p = 0.014), and posterior left middle temporal gyrus (secondary auditory cortex A2; r = 0.410, p = 0.034) FDR-corrected (p < 0.05). Reduced connectivity between bilateral thalami and the left secondary auditory cortex was evidenced in Study 24 (left: r = 0.434, p = 0.024; right: r = 0.414, p = 0.032) FDR-corrected (p < 0.05; Lv et al., 2021). Study 11 (Han et al., 2019b) confirmed a correlate reduction in connectivity between the right thalamus and right inferior frontal gyrus (r = 0.434, p = 0.024) Tukey-corrected (p < 0.05), while Study 21 (Chen et al., 2021a) confirmed a reduction in the right insula of the salience network (THI points: r = 0.415, p = 0.016; %: r = 0.391, p = 0.024) along with the left inferior frontal gyrus of the left frontoparietal network (points: r = 0.385, p = 0.027, %: r = 0.470, p = 0.006) family-wise error (FWE)–corrected (p < 0.05). Similar normalization was found with cortical gray matter volume increasing to a healthy control level between limbic and auditory network structures, including the bilateral thalami (Study 18; Wei et al., 2020b), left posterior cingulate cortex (Study 8; Wei et al., 2021a), right parahippocampal gyrus, right caudate, left superior temporal gyrus (primary auditory cortex A1), left cuneus gyrus, and right calcarine gyrus (Study 17; Wei et al., 2020a). Negatively correlated with tinnitus symptom improvement, changes in gray matter are displayed in Figure 3 indicated by the dark circles.
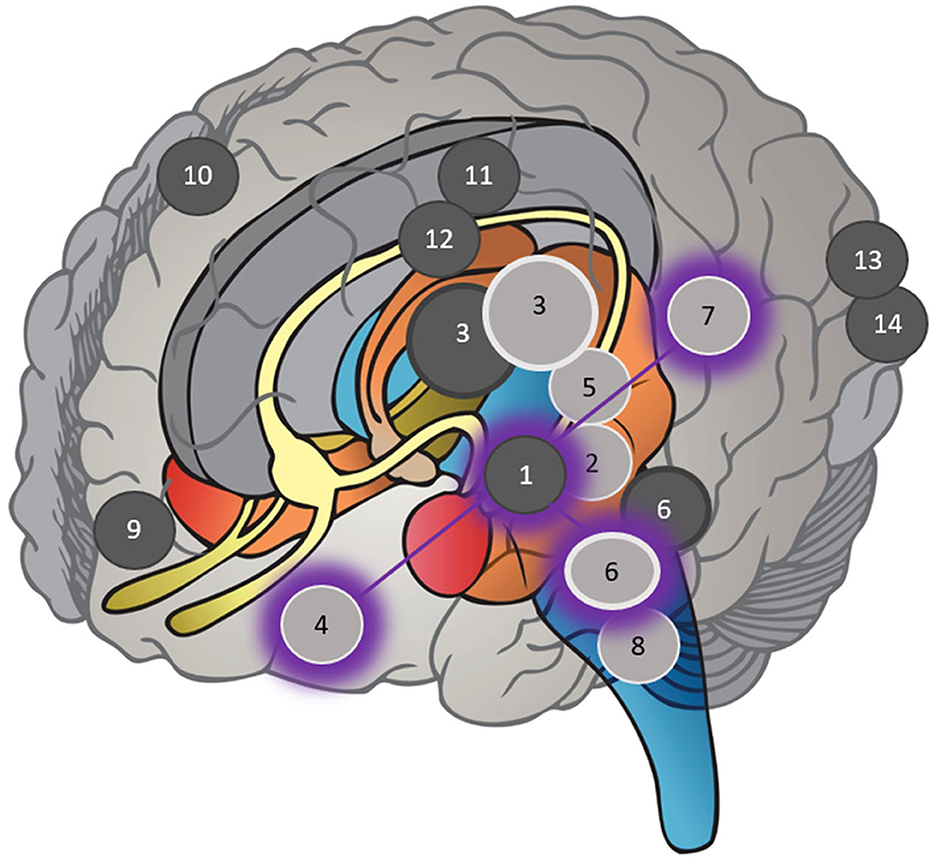
Figure 3. Specific locations named within each study in Table 2 in which neurophysiological evidence correlated with behavioral evidence following narrow-band sound stimulus, overlayed with the tinnitus core network. Dark circles represent increases in gray matter volume up to a healthy control level. Light circles represent decreases in functional connectivity to a healthy control level. Shadow circles represent the tinnitus core network adapted from De Ridder et al. (2014, p.27). Given the potential effect of hearing loss, tinnitus lateralization, and duration, specific hemisphere locations (left or right) have not been represented, only the region of interest. (1) Superior temporal gyrus (primary auditory cortex A1). (2) Middle temporal gyrus (secondary auditory cortex A2). (3) Thalamus. (4) Inferior frontal gyrus (overlaying ventrolateral prefrontal cortex in tinnitus core network). (5) Insula. (6) Parahippocampal gyrus. (7) Inferior parietal lobule. (8) Medial cerebellar peduncle. (9) Medial frontal gyrus. (10) Precentral gyrus. (11) Posterior cingulate cortex. (12) Caudate. (13) Cuneus. (14) Calcarine gyrus.
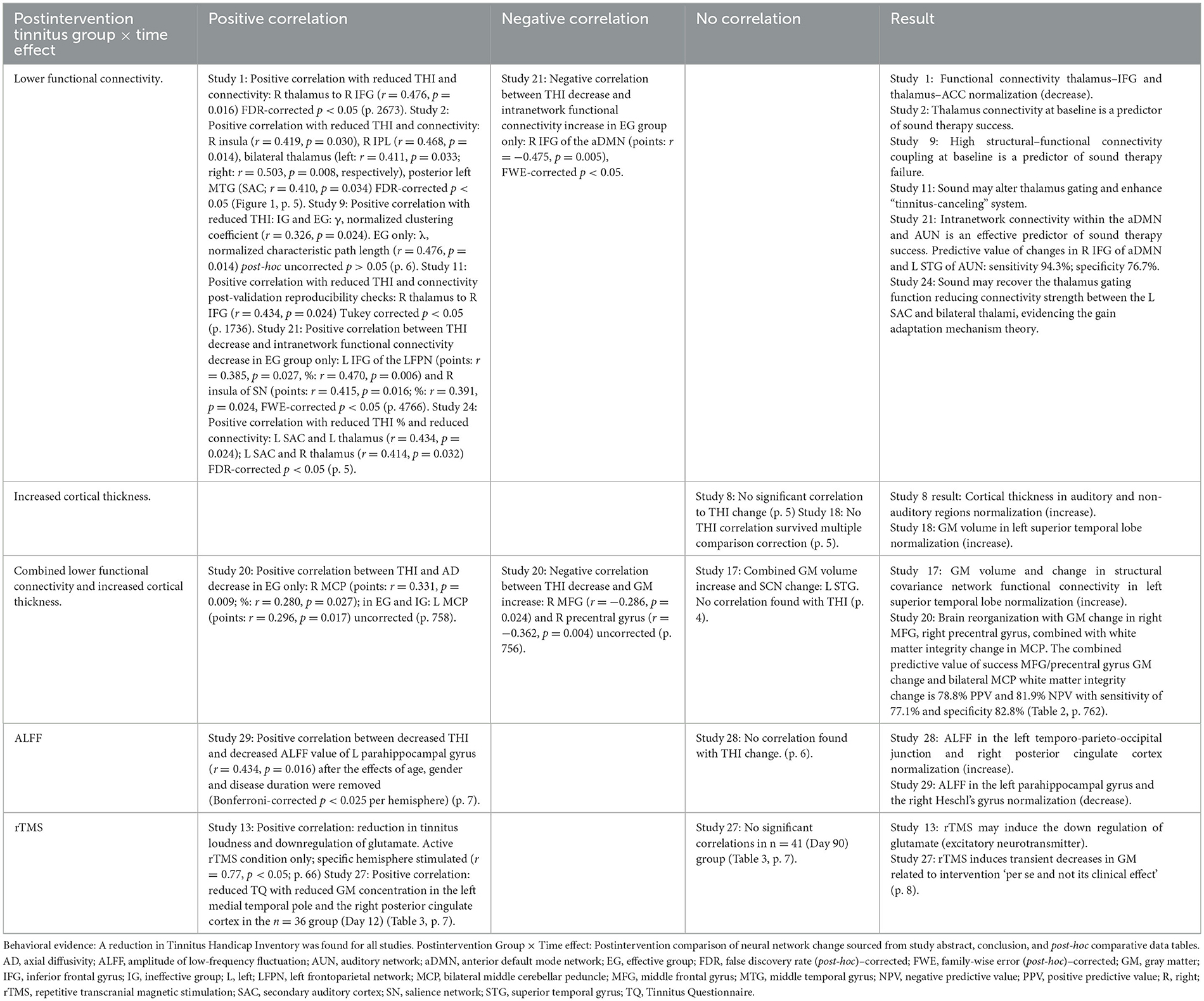
Table 3. Comparative evidence for correlation between neurophysiological and behavioral data in Table 2 (n = 12) and in Table 4 (n = 2).
Functional connectivity and gray matter neurophysiological evidence were also found to be predictors of sound therapy efficacy. Confirmed by both connectivity strength and degree centrality measures, bilateral thalami functional connectivity was found to be a predictor of sound therapy success (with area under the curve for left thalamus 0.745, and right thalamus 0.708; Study 2; Han et al., 2019a). Functional connectivity between the left superior temporal gyrus (A1) within the auditory network and the right inferior frontal gyrus of the anterior default mode network was found to be a predictor of sound treatment success with 94.3% sensitivity and 76.7% specificity (Study 21; Chen et al., 2021a). Gray matter volume in the right medial frontal gyrus and right precentral gyrus, alongside bilateral middle cerebellar peduncle white matter integrity, was found to predict sound therapy success in Study 20 (78.8% positive predictive value and 81.9% negative predictive value, with sensitivity of 77.1% and specificity 82.8%; Chen et al., 2021b). On a more global scale, higher structural-functional connectivity coupling was found to be predictive of sound therapy failure in Study 9 post-hoc uncorrected (Chen et al., 2022).
Sound-based therapy almost exclusively drives the systematic review results. Notably absent were any studies investigating CBT, tinnitus retraining therapy, or counseling. Minimal neurophysiological evidence was found for sound-based treatment that did not involve narrow-band noise. Table 4 illustrates evidence for the Heidelberg model of music in Study 7 (Krick et al., 2015), increasing gray matter density in the right Heschl's gyrus (p = 0.047), right Rolandic operculum (p = 0.022), and left superior frontal sulcus (p = 0.047), FWE-corrected (p < 0.05) in acute tinnitus patients (tinnitus duration of <3 months). As a form of sound therapy, the Heidelberg model of music was evidenced as potentially preventing the chronification of tinnitus by inducing long-lasting neuroplasticity. Acoustic coordinated reset neuromodulation was also found to normalize oscillatory power in temporal, parietal, and cingulate cortices to a healthy control baseline in participants with chronic extended tinnitus (more than 60 months in duration) in Study 23 (Adamchic et al., 2014). Only two studies applying non-sound brain stimulation met the review criteria for analysis. Study 13 (Cacace et al., 2018) found repetitive transcranial magnetic stimulation (rTMS) induced a downregulation of glutamate, an excitatory neurotransmitter, specific to the treatment condition and hemisphere positively correlates with a reduction in the THQ score (r = 0.77, p < 0.05). Study 25 (Lan et al., 2022) compared rTMS with tailor-made notched music training (TMNMT). Enhanced connectivity of the salience network to the right frontoparietal network indicated that rTMS may be successful, whereas reduced connectivity at baseline between the auditory network and salience or cerebellar networks indicated that TMNMT may be more successful.
Despite the synthesis of results by tinnitus duration, the small study number, collective sample size, and mixed efficacy results presented in Table 4 prevent any conclusion from being drawn. For example, the number of “not good” treatment respondents (TQ improvement ≤ 12 points) outweighed the “good” (TQ improvement > 12 points) in Study 23 (Adamchic et al., 2014). In Study 27 (Lehner et al., 2014), significant mean gray matter concentration changes in the bilateral insula, inferior frontal gyrus, and ventromedial prefrontal cortex measured on Day 12 posttreatment were absent by Day 90.
The consolidated evidential strengths and weaknesses presented by the study authors shape implementation discussion.
All studies presented in this review were published in scientifically rigorous, peer-reviewed, high–impact factor journals. The methods were meticulously described, enhancing reproducibility. Despite all studies in Table 2 originating from the same primary affiliation, a range of different research teams approached a homogeneous research question with a diverse set of measurement techniques. Results were obtained through seed-based, global, and mixed approaches. Functional connectivity within the tinnitus brain, for example, was examined using the resting-state functional magnetic resonance imaging (rs-fMRI) REST toolkit (Study 1), graph-theoretical metric degree centrality (Study 2), graph-theoretical metric functional connectivity strength (Study 11), and graph-theoretical metric structural connectivity–functional connectivity coupling (Study 9) to conclude local and global connectivity changes occurred following sound stimulus.
A coherent body of limitations was expressed by authors across all 17 studies. Generalizability was highly constrained by heterogeneity in sample demographics and tinnitus characteristics illustrated by large statistical ranges for each metric. A lack of data regarding hearing loss classification (mild or severe), tinnitus lateralization (unilateral, bilateral, left, and right), and distress quantification and qualification is notable potentially indicating heterogeneity is understated. A small sample size was cited by many authors as weakening the validity of individual study results. The absence of any study that did not find any behavioral improvement also raises the question as to whether collectively a publication bias is present, despite individually satisfying the RoB assessment. Despite these limitations, it is argued that sufficient validity is present for readers to confidently rely on the review findings for narrow-band sound treatment efficacy applying the protocol prescribed to the population characterized.
Given the evidential context and link between narrow-band sound treatment success and specific morphophysiological characteristics within the tinnitus brain, pivoting the evidential lens toward brain regions correlated with tinnitus symptom improvement may be of most use.
Narrow-band sound induced significant tinnitus core network neuroplasticity correlated with tinnitus symptom reduction as shown by the shadowed circles in Figure 3. A non-hierarchical tinnitus network was explored in 1990 when Jastreboff (1990) discussed distributed connections between the auditory network, prefrontal cortex, and limbic system when tinnitus was present. An ecological perspective on this auditory–non-auditory interplay considered tinnitus as a “failure of error correction” (Searchfield, 2014, p. 7) within overlapping bottom-up and top-down neural processes (Schilling et al., 2023). With continued research, a tinnitus core subnetwork specifically between the ventrolateral prefrontal cortex, auditory cortex, inferior parietal lobule, and parahippocampus was identified as the minimal subset of jointly active neural regions required for the conscious perception of tinnitus, excluding affective constituents (De Ridder et al., 2014). Interestingly, further research into the connectivity within an integrative model showed peripheral feeder nodes were ascertained as having a higher betweenness centrality value (reflecting influence on information transmission within a network) than other nodes as they transfer information from the periphery to the core in both tinnitus and control subjects using a “rich club” neural connectome (Mohan et al., 2017). The strikingly visual “T” first displayed in 2014 (De Ridder et al., 2014, p. 27), with the caveat that lines were not based on connectivity studies, is now displayed in 2023 with neurophysiological evidence directly sourced from connectivity and cortical gray matter studies.
Therapeutically targeting the multiplane tinnitus core network requires activation of an individual's underlying brain morphophysiology. Integrating review and tinnitus core network locations indicate tinnitus activates limbic association fibers linking structures within hemispheres, projection fibers descending from the cerebrum such as auditory cortices down to the thalamus and brainstem, as well as commissural fibers connecting homotopic areas across the midline between hemispheres. This is illustrated by the neurophysiological predictors of sound treatment success. Tinnitus participants who found narrow-band sound effective had reduced fractional anisotropy and increased radial diffusivity in the corpus callosum and cingulum (commissural), alongside decreased fractional anisotropy and axial diffusivity in the superior longitudinal fasciculus (association), altering white matter microstructure integrity combined with less gray matter atrophy in the medial frontal gyrus and pre-cingulate gyrus in Study 20 (Chen et al., 2020). A high structural connectivity–functional connectivity coupling was also found to predict sound treatment failure (Chen et al., 2022). This individual form–function neuroplasticity, a product of brain trauma, aging, experience, and genetic factors, may indicate a temporal window exists where sound treatment is most efficacious. The degree to which peripheral nodes dominate this core network also supports the importance of collecting tinnitus characteristic data, such as hearing loss, and tinnitus lateralization, as this may affect the window by altering the dominance of peripheral nodes following the effects of sensory deprivation–driven neuroplasticity on functional coupling (Noreña and Farley, 2013; Schilling et al., 2021).
Cellular repair is commonly sought when tinnitus is related to aging, blast and noise trauma, or a comorbid pathology. This review found decreased connectivity and increased gray matter volume around the thalamus and parahippocampal gyrus were correlated with improved tinnitus symptoms. Deep within the limbic system of the temporal lobe, thalamic functional nuclei in each hemisphere maintain an active reciprocal excitatory connection with highly specific cortical regions. For example, auditory peripheral–core communication is driven by thalamic medial geniculate nuclei receiving afferent fibers from the midbrain's inferior colliculus and projecting efferent fibers to the primary auditory cortex via the retrolenticular part of the internal capsule (Crossman and Neary, 2018). Thalamic connectivity dysfunction (Wu et al., 2022) and reduced gray matter volume (van de Mortel et al., 2021) have been found in the neurodegenerative Alzheimer's disease and Huntington disease (Ross et al., 2014). Despite ongoing debate (Boldrini et al., 2018; Sorrells et al., 2018), adult neurogenesis post-trauma or the proliferation of new neurons after birth is still considered a worthy pursuit (Kempermann et al., 2018). Located between the thalamus and parahippocampus, the hippocampus is one of two locations where adult neurogenesis has been found. Research links hippocampal neurogenesis, a complex unique form of neuroplasticity, to changes in learning and memory (Deng et al., 2010), memory resolution (Aimone et al., 2011), pattern separation (Sahay et al., 2011), and temporal encoding potentially relevant to tinnitus-related auditory memory formation, consolidation, and reconsolidation. Glial cells, such as microglia and astrocytes, are essential to the maintenance and repair of neural networks, as well as an integral aspect of adult neurogenesis. Stem cells originating in the hippocampus predominantly inhabit astroglia properties such that young astrocytes are produced alongside young neuronal cells (Götz and Huttner, 2005; Morrens et al., 2012). Neuroinflammatory signaling and resultant pro-inflammatory microglia and cytokines disrupt this neural repair system (Shulman et al., 2021). In the event of neuroinflammation, glial cells drive the initiation, progression, and resultant effect of neurogenesis (Yang and Zhou, 2019). Postmortem histopathological analysis of tinnitus brains, including the medial geniculate body and inferior colliculus, showed the presence of inflammatory and neurodegenerative processes (Almasabi et al., 2022). Glutamate release, also found following neuroinflammatory-induced microglial activation (Almeida et al., 2020), is consistent with the review in Study 13 (Cacace et al., 2018), where a positive correlation was found between reductions in tinnitus loudness and the downregulation of glutamate following five consecutive days of rTMS treatment, suggesting a potential mitigation of neuroinflammatory activity.
fMRI evidence links tinnitus with impaired thalamic gating. Gating dysfunction results in disinhibited signals increasing from the thalamus to cortical regions evidenced by increased activation of the medial geniculate body (Jimoh et al., 2023). Multiple review studies found tinnitus improvement was correlated with reduced functional connectivity between the thalamus and inferior frontal gyrus (IFG) as well as the right insula. Located within the frontal lobe, the inferior frontal gyrus lies above the Sylvian fissure or lateral sulcus, constituting the inferior-lateral cortical surface (Rasuli, 2020). Broca's area, known for speech production, is located within the dominant, commonly left (Crossman and Neary, 2018), hemisphere of the IFG. The IFG plays an active role in response inhibition or externally driven inhibition as demonstrated through research using the go/no-go task stimulus (Chikazoe et al., 2007, Schel et al., 2014). The insula as part of the ventral attention network acts to reorient attention to salient task-based activities. Directly participating in the pain pathway and language, the anterior insula has also been found to mediate bottom-up-driven affective distress and top-down avoidance during distress phases in a positive-vs.-negative auditory feedback task (Addicott et al., 2018). Receiving signals from these and other regions connected to a distributed inhibitory network, subthalamic nuclei suppress or inhibit thalamocortical output. Specific metabotropic glutamate receptor subtype activation induces the up- or downregulation of thalamic relay neuron inhibitory activity levels, differentially altering thalamocortical gating of sensory information transfer (Govindaiah and Cox, 2006) and dendritic output of thalamic interneurons (Govindaiah and Cox, 2004). Astrocytic activation also plays a role in thalamocortical gating, modulating sensory inhibition through metabotropic glutamate receptor receptor regulation (Copeland et al., 2017). Aging has been found to alter auditory gating and medial geniculate body temporal processing by reducing the density of gamma-aminobutyric type A receptors, driving fast (phasic) inhibition and long-lasting (tonic) inhibition (Richardson et al., 2013, 2021). Neurophysiological evidence of the tinnitus brain illustrates that neuromodulation, such as impaired inhibition, is part of a complex system interacting and responding to changes in neuroprotective mechanisms and neuroplasticity change (Abraham et al., 2013, Shahsavarani et al., 2019) such that pain and distress emerge for some individuals (De Ridder et al., 2021). Within the tinnitus core network, where gray matter volume of the ventromedial prefrontal cortex was not found to be correlated with levels of distress, anterior insula cortical thickness was positively correlated with distress levels, while subcallosal anterior cingulate cortex was negatively correlated with depression and anxiety (Leaver et al., 2012) reinforcing the link between underlying cortico-limbic morphophysiology, tinnitus symptoms, and potential treatment efficacy.
Implementing the scientifically rigorous neurophysiological evidence found within this review that narrow-band sound induces tinnitus core neural network change correlated with tinnitus improvement is difficult when the underlying morphophysiological tinnitus brain is unknown. Seeking ways to implement research regarding the neuroprotective and rehabilitative properties of tinnitus core network activation, to make possible the necessary cortico-thalamic neuronal cell density and connectivity strength required for therapy success, invites both old and new treatment opportunities.
Experience-dependent neuroplasticity is an old but effective treatment driven by the end-user patient. End-user patients can target their tinnitus core network through exercise and environmental enrichment that in consultation with their primary health care team, clinician, or practitioner meets their unique rehabilitative needs. Animal models enabling the direct observation of cellular change show the restorative properties of environmental enrichment and physical exercise. Exercise drives neurovascular adaptation with an increase in the density of dentate gyrus blood vessels (Clark et al., 2009) as well as neurogenesis in the form of hippocampal dentate gyrus stem cell proliferation (van Praag et al., 1999). Enrichment and exercise also increase the expression of brain-derived neurotrophic factor (BDNF), a neurotrophin that supports neurite growth and survival in existing neurons, and vascular endothelial growth factor that promotes vasculogenesis and angiogenesis driving new blood vessel formation (Clemenson et al., 2015). A three-phase enriched environment protocol matching a multistage rehabilitation program applied post–head trauma or stroke, was found to improve cerebral blood flow, reducing cortical cellular loss (Zhan et al., 2020). Specific examples of implementation include encouraging end-user patients to identify new ways to deepen their experience of naturally occurring sound (Welch et al., 2022), incorporating mindfulness (Tran et al., 2020, 2023) that may engage tinnitus core inhibitory systems and reduce stress. Multisensory novel stimuli, such as visual–auditory (experiencing unexpected or new sights and sounds) or motion–auditory stimuli (such as singing and dancing), may activate similar limbic structures to narrow-band noise. Tinnitus individuals appear hypersensitive to cross-modal interference (Araneda et al., 2015), indicating impaired inhibitory systems are active during multimodal experiences, with decreased sensitivity to novel visual stimuli as we age (Czigler et al., 2006), potentially altering this dynamic in tinnitus individuals. Novel or new stimulus also propels inhibitory activity at a cellular level through Parvalbumin-expressing (PV+) inhibitory neurons driving gamma oscillations (Hayden et al., 2021). Long-term benefits of novel experiences driving gamma oscillations within the brain include delaying the onset of dementia and Alzheimer's disease (Martorell et al., 2019, Iaccarino et al., 2016). Mobile digital applications that implement the latest tinnitus research are encouraged (Searchfield and Sanders, 2022), especially where user input shapes treatment design. Given that this review highlights the importance of trying to match the unique form and functional health of the individual tinnitus brain to the treatment, end-user patients are encouraged to record their own research into the role exercise, enrichment, and specific treatments play for them, sharing this valuable data with clinicians and practitioners over the time course of their tinnitus journey.
The clinician-practitioner arguably plays the most critical role in research implementation. As the trusted source of tinnitus education and support to patients, as well as the gatekeeper to the largest potential cohort of tinnitus research participants, clinicians and practitioners must be continually empowered to be the health change makers they are and continually strive to be. To address the generalizability limitations of this review finding and implement the knowledge that treatment success requires a specific morphophysiological tinnitus brain, clinicians and practitioners are encouraged to collaborate with researchers to improve the secure standardized capture of individual patient histories. As well as capturing hearing loss classification, tinnitus lateralization, duration, and distress, neurophysiological data, such as cognitive, neurovascular, and neuronal health, evident through cognitive testing and exercise and enrichment data are important. Supporting end-user patients to be their own researchers of themselves may reduce the burden of this data capture and facilitate the recording of between-visit changes. Compensating the commercially situated clinician-practitioner may improve access for researchers to their frontline pragmatic view when seeking new ways to improve the standardization of tinnitus behavioral questionnaires or capture behavioral data, such as using ecological momentary evaluation (Goldberg et al., 2017, Schleicher et al., 2020). A standardized tinnitus health literacy metric to improve the monitoring of equitable access to treatments may also occur through a commercially mediated collaboration between clinicians, practitioners, and academic researchers. Future implementation of neurophysiological evidence is currently being supported by innovation across three streams: commercially available low-cost mobile neuroimaging, artificial intelligence, and investment in secure data-sharing infrastructure. For example, sound-based tinnitus treatment efficacy prediction (Sanders et al., 2021; Doborjeh et al., 2023) and mindfulness efficacy were able to be predicted with 0.87 accuracy (F-score, 89%) using EEG data fed into a brain-inspired spiking neural network model (Doborjeh et al., 2019).
Closing the evidential gaps identified in this review requires resources. Quality research design within an agile scientific, legal, financial, and ethical research environment requires the careful allocation of finite, competing resources. The dominance of reviewed articles published in 2020 and 2021 suggests the capture of neurophysiological-based evidence has improved within tinnitus research in recent times, in line perhaps with advancements in methodologies and tools for data capture. To continue this improvement, a range of research opportunities are presented with varying levels of resource intensity. Given the importance of the underlying morphophysiological characteristics within the tinnitus brain determining treatment success, observed through neurophysiological data, explicitly designing neurophysiological subgroups at the protocol phase may facilitate greater sample homogeneity balanced against impact on effect sample size. Collaborating with clinicians and practitioners to share participant history over their life span may also inform subgrouping and strengthen the generalizability of research findings. Incorporating active, passive, and sham controls, with pre–postintervention data captured for both treatment and control, is encouraged, especially when normalization from a pathological state to a healthy state is the primary research question. Given the chronification of tinnitus (potentially 0–3 months) and wide tinnitus duration deemed chronic when narrow-band sound was effective (6–48 months), increasing the number of data capture points between 0 and 48 months may narrow or inform the temporal window for treatment efficacy. On a wider scale, while restorative enriched environment research paradigms are not new, the control of their implementation by end-user patients is perhaps more recent. Participatory research and codesign are rapidly evolving, which may increase the adoption and use of findings through intellectual and creative multistakeholder investment in outcomes (Tu'akoi et al., 2022). Collaborating with other researchers to spread the resource burden may enable more multiphase tinnitus studies where RCT and participatory research designs are given the opportunity to collectively maximize the adoption and pragmatic use of evidentially rigorous findings to improve health outcomes.
Applying the same rigorous standards of AMSTAR 2 to this systematic review highlights three key limitations. First, the exclusion of non-English-language articles may adversely constrain the findings presented. Second, it must be acknowledged that the minimum sample size may have introduced a bias toward research from nations with a large population, evidenced by the nations displayed in Figure 2 (China, the United States, and Germany). Third, the sample selection may also have been affected by the COVID-19 pandemic, with most studies published between 2020 and 2022. Participation by those situated long distances from research hubs or confined by lockdown protocols may have been impeded. The lack of studies from smaller nations, as well as rural and remote sample participants, further constrains the generalizability and thereby implementation of results.
Implementing an effective tinnitus treatment through trial and error is a challenge. This review has found that the greatest body of neurophysiological evidence for treatment success correlated to symptom improvement is narrow-band sound when applied to chronic tinnitus individuals. Underlying treatment efficacy, however, is a specific morphophysiological tinnitus core network that both facilitates and predicts success. In the absence of neuroimaging equipment in audiology practice or in the home of tinnitus individuals to assess the health of this tinnitus core network prior to treatment, implementation science reveals both old and new opportunities for implementing this important body of research today. From end-user patient-driven experience and clinician-practitioner empowerment to researcher collaboration, it is hoped that new or improved treatments will continue to emerge as the evidential lens pivots toward the pragmatic use of neurophysiological evidence. Alongside the rapid development of mobile neuroimaging tools and artificial intelligence, implementation science has a vital role to play in ensuring the link between tinnitus research and practice continues to narrow to make visible when knowledge boundaries dissipate and the co-construction of research-informed practice between the end-user patient, the clinician-practitioner, and the academic researcher emerges.
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author/s.
LB-H: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. PS: Formal analysis, Supervision, Validation, Writing – review & editing. GS: Formal analysis, Resources, Supervision, Validation, Visualization, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The primary researcher wishes to acknowledge the contribution of Professor Steven B. Heymsfield, MD, as the original source that inspired the exploration of implementation science in public health research.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abraham, S., Barbara, G., and Arnold, S. (2013). Electrical stimulation and tinnitus: neuroplasticity, neuromodulation, neuroprotection. Int. Tinnitus J. 18, 75–95. doi: 10.5935/0946-5448.20130010
Adamchic, I., Hauptmann, C., and Tass, P. A. (2012). Changes of oscillatory activity in pitch processing network and related tinnitus relief induced by acoustic CR neuromodulation. Front. Syst. Neurosci. 6, 18. doi: 10.3389/fnsys.2012.00018
Adamchic, I., Toth, T., Hauptmann, C., and Tass, P. A. (2014). Reversing pathologically increased EEG power by acoustic coordinated reset neuromodulation. Hum. Brain Mapp. 35, 2099–2118. doi: 10.1002/hbm.22314
Addicott, M. A., Daughters, S. B., Strauman, T. J., and Appelbaum, L. G. (2018). Distress tolerance to auditory feedback and functional connectivity with the auditory cortex. Psychiat. Res.: Neuroimag. 282, 1–10. doi: 10.1016/j.pscychresns.2018.10.003
Aimone, J. B., Deng, W., and Gage, F. H. (2011). Resolving new memories: a critical look at the dentate gyrus, adult neurogenesis, and pattern separation. Neuron 70, 589–596. doi: 10.1016/j.neuron.2011.05.010
Almasabi, F., Alosaimi, F., Corrales-Terron, M., Wolters, A., Strikwerda, D., Smit, J. V., et al. (2022). Post-mortem analysis of neuropathological changes in human tinnitus. Brain Sci. 12, 1024. doi: 10.3390/brainsci12081024
Almeida, P. G. C., Nani, J. V., Oses, J. P., Brietzke, E., and Hayashi, M. A. F. (2020). Neuroinflammation and glial cell activation in mental disorders. Brain Behav. Immun. Health 2. doi: 10.1016/j.bbih.2019.100034
Andersson, G., Stromgren, T., Strom, L., and Lyttkens, L. (2002). Randomized controlled trial of internet-based cognitive behavior therapy for distress associated with tinnitus. Psychosom. Med. 64, 810–816. doi: 10.1097/00006842-200209000-00014
Araneda, R., De Volder, A. G., Deggouj, N., and Renier, L. (2015). Altered inhibitory control and increased sensitivity to cross-modal interference in tinnitus during auditory and visual tasks. PLoS ONE 10, e0120387. doi: 10.1371/journal.pone.0120387
Beukes, E. W., Baguley, D. M., Allen, P. M., Manchaiah, V., and Andersson, G. (2018). Audiologist-guided Internet-based cognitive behavior therapy for adults with tinnitus in the United Kingdom: a randomized controlled trial. Ear Hear. 39, 423–433. doi: 10.1097/AUD.0000000000000505
Boldrini, M., Fulmore, C. A., Tartt, A. N., Simeon, L. R., Pavlova, I., Poposka, V., et al. (2018). Human hippocampal neurogenesis persists throughout aging. Cell Stem Cell 22, 589–599 e5. doi: 10.1016/j.stem.2018.03.015
Cacace, A. T., Hu, J., Romero, S., Xuan, Y., Burkard, R. F., and Tyler, R. S. (2018). Glutamate is down-regulated and tinnitus loudness-levels decreased following rTMS over auditory cortex of the left hemisphere: a prospective randomized single-blinded sham-controlled cross-over study. Hear. Res. 358, 59–73. doi: 10.1016/j.heares.2017.10.017
Carpenter-Thompson, J. R., Schmidt, S., McAuley, E., and Husain, F. T. (2015). Increased frontal response may underlie decreased tinnitus severity. PLoS ONE 10, e0144419. doi: 10.1371/journal.pone.0144419
Chen, Q., Lv, H., Wang, Z., Wei, X., Liu, J., Liu, F., et al. (2022). Distinct brain structural-functional network topological coupling explains different outcomes in tinnitus patients treated with sound therapy. Hum. Brain Mapp. 43, 3245–3256. doi: 10.1002/hbm.25848
Chen, Q., Lv, H., Wang, Z., Wei, X., Liu, J., Zhao, P., et al. (2021a). Pretreatment intranetwork connectivity can predict the outcomes in idiopathic tinnitus patients treated with sound therapy. Hum. Brain Mapp. 42, 4762–4776. doi: 10.1002/hbm.25584
Chen, Q., Lv, H., Wang, Z., Wei, X., Zhao, P., Yang, Z., et al. (2021b). Outcomes at 6 months are related to brain structural and white matter microstructural reorganization in idiopathic tinnitus patients treated with sound therapy. Hum. Brain Mapp. 42, 753–765. doi: 10.1002/hbm.25260
Chen, Q., Wang, Z., Lv, H., Zhao, P., Yang, Z., Gong, S., et al. (2020). Reorganization of brain white matter in persistent idiopathic tinnitus patients without hearing loss: evidence from baseline data. Front. Neurosci. 14, 591. doi: 10.3389/fnins.2020.00591
Chikazoe, J., Konishi, S., Asari, T., Jimura, K., and Miyashita, Y. (2007). Activation of right inferior frontal gyrus during response inhibition across response modalities. J. Cogn. Neurosci. 19, 69–80. doi: 10.1162/jocn.2007.19.1.69
Cima, R. F., Maes, I. H., Joore, M. A., Scheyen, D. J., El Refaie, A., Baguley, D. M., et al. (2012). Specialised treatment based on cognitive behaviour therapy versus usual care for tinnitus: a randomised controlled trial. Lancet 379, 1951–1959. doi: 10.1016/S0140-6736(12)60469-3
Clark, P. J., Brzezinska, W. J., Puchalski, E. K., Krone, D. A., and Rhodes, J. S. (2009). Functional analysis of neurovascular adaptations to exercise in the dentate gyrus of young adult mice associated with cognitive gain. Hippocampus 19, 937–950. doi: 10.1002/hipo.20543
Clemenson, G. D., Deng, W., and Gage, F. H. (2015). Environmental enrichment and neurogenesis: from mice to humans. Curr. Opin. Behav. Sci. 4, 56–62. doi: 10.1016/j.cobeha.2015.02.005
Copeland, C. S., Wall, T. M., Sims, R. E., Neale, S. A., Nisenbaum, E., Parri, H. R., et al. (2017). Astrocytes modulate thalamic sensory processing via mGlu2 receptor activation. Neuropharmacology 121, 100–110. doi: 10.1016/j.neuropharm.2017.04.019
Crossman, A. R., and Neary, D. (2018). Neuroanatomy: an Illustrated Colour Text. London: Elsevier Health Sciences.
Crowe, M. (2013). Crowe Critical Appraisal Tool (CCAT) User Guide. Version 1.4. Kyle: Conchra House, 11.
Czigler, I., Pato, L., Poszet, E., and Balazs, L. (2006). Age and novelty: event-related potentials to visual stimuli within an auditory oddball–visual detection task. Int. J. Psychophysiol. 62, 290–299. doi: 10.1016/j.ijpsycho.2006.05.008
De Ridder, D., Schlee, W., Vanneste, S., Londero, A., Weisz, N., Kleinjung, T., et al. (2021). Tinnitus and tinnitus disorder: Theoretical and operational definitions (an international multidisciplinary proposal). Prog. Brain Res. 260, 1–25. doi: 10.1016/bs.pbr.2020.12.002
De Ridder, D., Vanneste, S., Weisz, N., Londero, A., Schlee, W., Elgoyhen, A. B., et al. (2014). An integrative model of auditory phantom perception: tinnitus as a unified percept of interacting separable subnetworks. Neurosci. Biobehav. Rev. 44, 16–32. doi: 10.1016/j.neubiorev.2013.03.021
Deng, W., Aimone, J. B., and Gage, F. H. (2010). New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat. Rev. Neurosci. 11, 339–350. doi: 10.1038/nrn2822
Doborjeh, M., Liu, X., Doborjeh, Z., Shen, Y., Searchfield, G., Sanders, P., et al. (2023). Prediction of tinnitus treatment outcomes based on eeg sensors and tfi score using deep learning. Sensors (Basel) 23, 902. doi: 10.3390/s23020902
Doborjeh, Z., Doborjeh, M., Taylor, T., Kasabov, N., Wang, G. Y., Siegert, R., et al. (2019). Spiking neural network modelling approach reveals how mindfulness training rewires the brain. Sci. Rep. 9, 6367. doi: 10.1038/s41598-019-42863-x
Eccles, M. P., Foy, R., Sales, A., Wensing, M., and Mittman, B. (2012). Implementation Science six years on our evolving scope and common reasons for rejection without review. Implement. Sci. 7, 1–6. doi: 10.1186/1748-5908-7-71
Feng, T., Wang, M., Xiong, H., Zheng, Y., and Yang, H. (2020). Efficacy of an Integrative treatment for tinnitus combining music and cognitive-behavioral therapy-assessed with behavioral and EEG data. Front. Integr. Neurosci. 14, 12. doi: 10.3389/fnint.2020.00012
Fuller, T., Cima, R., Langguth, B., Mazurek, B., Vlaeyen, J. W., and Hoare, D. J. (2020). Cognitive behavioural therapy for tinnitus. Cochrane Database Syst Rev, 1, CD012614. doi: 10.1002/14651858.CD012614.pub2
Goldberg, R. L., Piccirillo, M. L., Nicklaus, J., Skillington, A., Lenze, E., Rodebaugh, T. L., et al. (2017). Evaluation of ecological momentary assessment for tinnitus severity. JAMA Otolaryngology–Head and Neck Surg, 143, 700–706. doi: 10.1001/jamaoto.2017.0020
Götz, M., and Huttner, W. B. (2005). The cell biology of neurogenesis. Nat. Rev. Mol. Cell Biol. 6, 777–788. doi: 10.1038/nrm1739
Govindaiah, G., and Cox, C. L. (2004). Synaptic activation of metabotropic glutamate receptors regulates dendritic outputs of thalamic interneurons. Neuron 41, 611–623. doi: 10.1016/S0896-6273(04)00013-3
Govindaiah, G., and Cox, C. L. (2006). Metabotropic glutamate receptors differentially regulate GABAergic inhibition in thalamus. J. Neurosci. 26, 13443–13453. doi: 10.1523/JNEUROSCI.3578-06.2006
Han, J. J., De Ridder, D., Vanneste, S., Chen, Y. C., Koo, J. W., and Song, J. J. (2020a). Pre-treatment ongoing cortical oscillatory activity predicts improvement of tinnitus after partial peripheral reafferentation with hearing aids. Front. Neurosci. 14, 410. doi: 10.3389/fnins.2020.00410
Han, L., Na, Z., Chunli, L., Yuchen, C., Pengfei, Z., Hao, W., et al. (2019a). Baseline functional connectivity features of neural network nodes can predict improvement after sound therapy through adjusted narrow band noise in tinnitus patients. Front. Neurosci. 13, 614. doi: 10.3389/fnins.2019.00614
Han, L., Pengfei, Z., Chunli, L., Zhaodi, W., Xindi, W., Qian, C., et al. (2020b). The effects of sound therapy in tinnitus are characterized by altered limbic and auditory networks. Brain Commun. 2, fcaa131. doi: 10.1093/braincomms/fcaa131
Han, L., Yawen, L., Hao, W., Chunli, L., Pengfei, Z., Zhengyu, Z., et al. (2019b). Effects of sound therapy on resting-state functional brain networks in patients with tinnitus: a graph-theoretical-based study. J. Magn. Reson. Imaging 50, 1731–1741. doi: 10.1002/jmri.26796
Hayden, D. J., Montgomery, D. P., Cooke, S. F., and Bear, M. F. (2021). Visual recognition is heralded by shifts in local field potential oscillations and inhibitory networks in primary visual cortex. J. Neurosci. 41, 6257–6272. doi: 10.1523/JNEUROSCI.0391-21.2021
Huang, B., Wang, X., Wei, F., Sun, Q., Sun, J., Liang, Y., et al. (2021). Notched sound alleviates tinnitus by reorganization emotional center. Front. Hum. Neurosci. 15, 762492. doi: 10.3389/fnhum.2021.762492
Iaccarino, H. F., Singer, A. C., Martorell, A. J., Rudenko, A., Gao, F., Gillingham, T. Z., et al. (2016). Gamma frequency entrainment attenuates amyloid load and modifies microglia. Nature 540, 230–235. doi: 10.1038/nature20587
Jastreboff, P. J. (1990). Phantom auditory perception (tinnitus): mechanisms of generation and perception. Neurosci. Res. 8, 221–254. doi: 10.1016/0168-0102(90)90031-9
Jimoh, Z., Marouf, A., Zenke, J., Leung, A. W. S., and Gomaa, N. A. (2023). Functional brain regions linked to tinnitus pathology and compensation during task performance: a systematic review. Otolaryngol Head Neck Surg. 169, 1409–1423. doi: 10.1002/ohn.459
Kallogjeri, D., Piccirillo, J. F., Spitznagel, E. Jr., Hale, S., Nicklaus, J. E., Hardin, F. M., et al. (2017). Cognitive training for adults with bothersome tinnitus: a randomized clinical trial. JAMA Otolaryngol. Head Neck Surg. 143, 443–451. doi: 10.1001/jamaoto.2016.3779
Kempermann, G., Gage, F. H., Aigner, L., Song, H., Curtis, M. A., Thuret, S., et al. (2018). Human adult neurogenesis: evidence and remaining questions. Cell Stem Cell 23, 25–30. doi: 10.1016/j.stem.2018.04.004
Kim, S. H., Jang, J. H., Lee, S. Y., Han, J. J., Koo, J. W., Vanneste, S., et al. (2016). Neural substrates predicting short-term improvement of tinnitus loudness and distress after modified tinnitus retraining therapy. Sci. Rep. 6, 29140. doi: 10.1038/srep29140
Krick, C. M., Grapp, M., Daneshvar-Talebi, J., Reith, W., Plinkert, P. K., and Bolay, H. V. (2015). Cortical reorganization in recent-onset tinnitus patients by the Heidelberg Model of Music Therapy. Front. Neurosci. 9, 49. doi: 10.3389/fnins.2015.00049
Kropotov, J. (2010). Quantitative EEG, Event-Related Potentials and Neurotherapy. Cambridge, MA: Academic Press.
Lan, L., Liu, Y., Wu, Y., Xu, Z. G., Xu, J. J., Song, J. J., et al. (2022). Specific brain network predictors of interventions with different mechanisms for tinnitus patients. EBioMedicine 76, 103862. doi: 10.1016/j.ebiom.2022.103862
Leaver, A. M., Seydell-Greenwald, A., Turesky, T. K., Morgan, S., Kim, H. J., and Rauschecker, J. P. (2012). Cortico-limbic morphology separates tinnitus from tinnitus distress. Front. Syst. Neurosci. 6, 21. doi: 10.3389/fnsys.2012.00021
Lee, S. Y., Rhee, J., Shim, Y. J., Kim, Y., Koo, J. W., De Ridder, D., et al. (2019). Changes in the resting-state cortical oscillatory activity 6 months after modified tinnitus retraining therapy. Front. Neurosci. 13, 1123. doi: 10.3389/fnins.2019.01123
Lehner, A., Langguth, B., Poeppl, T. B., Rupprecht, R., Hajak, G., Landgrebe, M., et al. (2014). Structural brain changes following left temporal low-frequency rTMS in patients with subjective tinnitus. Neural Plast. 2014, 132058. doi: 10.1155/2014/132058
Lv, H., Chen, Q., Wei, X., Liu, C., Zhao, P., Wang, Z., et al. (2021). Sound therapy can modulate the functional connectivity of the auditory network. Prog. Neuropsychopharmacol. Biol. Psychiatry 110, 110323. doi: 10.1016/j.pnpbp.2021.110323
Lv, H., Liu, C., Wang, Z., Zhao, P., Cheng, X., Yang, Z., et al. (2020). Altered functional connectivity of the thalamus in tinnitus patients is correlated with symptom alleviation after sound therapy. Brain Imaging Behav. 14, 2668–2678. doi: 10.1007/s11682-019-00218-0
Martinez Devesa, P., Waddell, A., Perera, R., and Theodoulou, M. (2007). Cognitive behavioural therapy for tinnitus. Cochrane Database Syst Rev, CD005233. doi: 10.1002/14651858.CD005233.pub2
Martorell, A. J., Paulson, A. L., Suk, H. J., Abdurrob, F., Drummond, G. T., Guan, W., et al. (2019). Multi-sensory gamma stimulation ameliorates Alzheimer's-associated pathology and improves cognition. Cell 177, 256–271. doi: 10.1016/j.cell.2019.02.014
McCormack, A., Edmondson-Jones, M., Somerset, S., and Hall, D. (2016). A systematic review of the reporting of tinnitus prevalence and severity. Hear. Res. 337, 70–79. doi: 10.1016/j.heares.2016.05.009
McRobbie, D. W., Moore, E. A., Graves, M. J., and Prince, M. R. (2017). MRI from Picture to Proton. Cambridge: Cambridge University Press.
Mohan, A., De Ridder, D., and Vanneste, S. (2017). Robustness and dynamicity of functional networks in phantom sound. Neuroimage 146, 171–187. doi: 10.1016/j.neuroimage.2016.04.033
Møller, A. R. (2011). “Epidemiology of tinnitus in adults,” in Textbook of Tinnitus, eds. M. R. Møller, B. Langguth, D. DeRidder, T. Kleinjung, T. Springer Science and Business Media, 29–37.
Morrens, J., Van Den Broeck, W., and Kempermann, G. (2012). Glial cells in adult neurogenesis. Glia 60, 159–174. doi: 10.1002/glia.21247
Noreña, A. J., and Farley, B. J. (2013). Tinnitus-related neural activity: theories of generation, propagation, and centralization. Hear. Res. 295, 161–171. doi: 10.1016/j.heares.2012.09.010
Page, M. J., Mckenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int. J. Surg. 88, 105906. doi: 10.1016/j.ijsu.2021.105906
Poldrack, R. A., Mumford, J. A., and Nichols, T. E. (2011). Handbook of Functional MRI and Data Analysis. Cambridge: Cambridge University Press.
Rasuli, B. (2020). Radiopaedia. Available online at: https://radiopaedia.org/articles/inferior-frontal-gyrus (accessed 14 August, 2023).
Richardson, B. D., Ling, L. L., Uteshev, V. V., and Caspary, D. M. (2013). Reduced GABA(A) receptor-mediated tonic inhibition in aged rat auditory thalamus. J. Neurosci. 33, 1218–27a. doi: 10.1523/JNEUROSCI.3277-12.2013
Richardson, B. D., Sottile, S. Y., and Caspary, D. M. (2021). Mechanisms of GABAergic and cholinergic neurotransmission in auditory thalamus: Impact of aging. Hear. Res. 402, 108003. doi: 10.1016/j.heares.2020.108003
Ross, C. A., Aylward, E. H., Wild, E. J., Langbehn, D. R., Long, J. D., Warner, J. H., et al. (2014). Huntington disease: natural history, biomarkers and prospects for therapeutics. Nature Reviews Neurology 10, 204–216. doi: 10.1038/nrneurol.2014.24
Sahay, A., Wilson, D. A., and Hen, R. (2011). Pattern separation: a common function for new neurons in hippocampus and olfactory bulb. Neuron 70, 582–588. doi: 10.1016/j.neuron.2011.05.012
Sanders, P. J., Doborjeh, Z. G., Doborjeh, M. G., Kasabov, N. K., and Searchfield, G. D. (2021). Prediction of acoustic residual inhibition of tinnitus using a brain-inspired spiking neural network model. Brain Sci. 11, 52. doi: 10.3390/brainsci11010052
Schel, M. A., Kuhn, S., Brass, M., Haggard, P., Ridderinkhof, K. R., and Crone, E. A. (2014). Neural correlates of intentional and stimulus-driven inhibition: a comparison. Front. Hum. Neurosci. 8, 27. doi: 10.3389/fnhum.2014.00027
Schilling, A., Sedley, W., Gerum, R., Metzner, C., Tziridis, K., Maier, A., et al. (2023). Predictive coding and stochastic resonance as fundamental principles of auditory phantom perception. Brain 2023, awad255. doi: 10.1093/brain/awad255
Schilling, A., Tziridis, K., Schulze, H., and Krauss, P. (2021). The Stochastic Resonance model of auditory perception: a unified explanation of tinnitus development, Zwicker tone illusion, and residual inhibition. Prog. Brain Res. 262, pp.139–157. doi: 10.1016/bs.pbr.2021.01.025
Schleicher, M., Unnikrishnan, V., Neff, P., Simoes, J., Probst, T., Pryss, R., et al. (2020). Understanding adherence to the recording of ecological momentary assessments in the example of tinnitus monitoring. Sci. Rep. 10, 22459. doi: 10.1038/s41598-020-79527-0
Schomer, D. L., and Da Silva, F. L. (2018). Niedermeyer's Electroencephalography: Basic Principles, Clinical Applications, and Related Fields. Philadelphia, PA: Lippincott Williams and Wilkins.
Searchfield, G. D. (2014). Tinnitus what and where: an ecological framework. Front. Neurol. 5, 271. doi: 10.3389/fneur.2014.00271
Searchfield, G. D., and Sanders, P. J. (2022). A randomized single-blind controlled trial of a prototype digital polytherapeutic for tinnitus. Front. Neurol. 13, 958730. doi: 10.3389/fneur.2022.958730
Shahsavarani, S., Khan, R. A., and Husain, F. T. (2019). Tinnitus and the brain: a review of functional and anatomical magnetic resonance imaging studies. Persp. ASHA Spec. Int. Groups 4, 896–909. doi: 10.1044/2019_PERS-SIG6-2019-0001
Shea, B. J., Reeves, B. C., Wells, G., Thuku, M., Hamel, C., Moran, J., et al. (2017). AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 358, j4008. doi: 10.1136/bmj.j4008
Shulman, A., Wang, W., Luo, H., Bao, S., Searchfield, G., and Zhang, J. (2021). “Neuroinflammation and tinnitus,” in The Behavioral Neuroscience of Tinnitus, eds. G. D. Searchfield, J. Zhang, J. Cham: Springer International Publishing, 161–174.
Silchenko, A. N., Adamchic, I., Hauptmann, C., and Tass, P. A. (2013). Impact of acoustic coordinated reset neuromodulation on effective connectivity in a neural network of phantom sound. Neuroimage 77, 133–147. doi: 10.1016/j.neuroimage.2013.03.013
Sorrells, S. F., Paredes, M. F., Cebrian-Silla, A., Sandoval, K., Qi, D., Kelley, K. W., et al. (2018). Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature 555, 377–381. doi: 10.1038/nature25975
Tang, D., Li, H., and Chen, L. (2019). “Advances in understanding, diagnosis, and treatment of tinnitus,” in Hearing Loss: Mechanisms, Prevention and Cure, eds. H. Li, and R. Chai. Cham: Springer (2019). p. 109–128. doi: 10.1007/978-981-13-6123-4_7
Tansella, M., and Thornicroft, G. (2009). Implementation science: understanding the translation of evidence into practice. Br. J. Psychiatry 195, 283–285. doi: 10.1192/bjp.bp.109.065565
Tran, T., Donnelly, C., Nalder, E., Trothen, T., and Finlayson, M. (2023). Mindfulness-based stress reduction for community-dwelling older adults with subjective cognitive decline (SCD) and mild cognitive impairment (MCI) in primary care: a mixed-methods feasibility randomized control trial. BMC Primary Care 24, 2. doi: 10.1186/s12875-023-02002-y
Tran, T., Donnelly, C., Nalder, E. J., Trothen, T., and Finlayson, M. (2020). Occupational therapist-led mindfulness-based stress reduction for older adults living with subjective cognitive decline or mild cognitive impairment in primary care: a feasibility randomised control trial protocol. BMJ Open 10, e035299. doi: 10.1136/bmjopen-2019-035299
Tu'akoi, S., Ofanoa, M., Ofanoa, S., Lutui, H., Heather, M., Jansen, R. M., et al. (2022). Co-designing an intervention to prevent rheumatic fever in Pacific People in South Auckland: a study protocol. Int. J. Equity Health 21, 9. doi: 10.1186/s12939-022-01701-9
van de Mortel, L. A., Thomas, R. M., and van Wingen, G. A. (2021). Grey Matter Loss at Different Stages of Cognitive Decline: A Role for the Thalamus in Developing Alzheimer's Disease. J. Alzheimers. Dis. 83, 705–720. doi: 10.3233/JAD-210173
van Praag, H., Kempermann, G., and Gage, F. H. (1999). Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat. Neurosci. 2, 266–270. doi: 10.1038/6368
Vanneste, S., van Dongen, M., De Vree, B., Hiseni, S., van der Velden, E., Strydis, C., et al. (2013). Does enriched acoustic environment in humans abolish chronic tinnitus clinically and electrophysiologically? A double blind placebo controlled study. Hear. Res. 296, 141–148. doi: 10.1016/j.heares.2012.10.003
Villalobos Dintrans, P., Bossert, T. J., Sherry, J., and Kruk, M. E. (2019). A synthesis of implementation science frameworks and application to global health gaps. Glob Health Res Policy 4, 25. doi: 10.1186/s41256-019-0115-1
Wei, X., Lv, H., Chen, Q., Wang, Z., Liu, C., Zhao, P., et al. (2020a). Neuroanatomical alterations in patients with tinnitus before and after sound therapy: a combined VBM and SCN study. Front. Hum. Neurosci. 14, 607452. doi: 10.3389/fnhum.2020.607452
Wei, X., Lv, H., Chen, Q., Wang, Z., Liu, C., Zhao, P., et al. (2021a). Cortical thickness alterations in patients with tinnitus before and after sound therapy: a surface-based morphometry study. Front. Neurosci. 15, 633364. doi: 10.3389/fnins.2021.633364
Wei, X., Lv, H., Chen, Q., Wang, Z., Zhao, P., Liu, C., et al. (2021b). Surface-based amplitude of low-frequency fluctuation alterations in patients with tinnitus before and after sound therapy: a resting-state functional magnetic resonance imaging study. Front. Neurosci. 15, 709482. doi: 10.3389/fnins.2021.709482
Wei, X., Lv, H., Wang, Z., Liu, C., Ren, P., Zhang, P., et al. (2020b). Neuroanatomical alterations in patients with tinnitus before and after sound therapy: a voxel-based morphometry study. Front. Neurosci. 14, 911. doi: 10.3389/fnins.2020.00911
Welch, D., Reybrouck, M., and Podlipniak, P. (2022). Meaning in music is intentional, but in soundscape it is not-a naturalistic approach to the qualia of sounds. Int. J. Environ. Res. Public Health 20, 269. doi: 10.3390/ijerph20010269
Wensing, M., and Grol, R. (2019). Knowledge translation in health: how implementation science could contribute more. BMC Med. 17, 88. doi: 10.1186/s12916-019-1322-9
Woodward, E. N., Singh, R. S., Ndebele-Ngwenya, P., Melgar Castillo, A., Dickson, K. S., and Kirchner, J. E. (2021). A more practical guide to incorporating health equity domains in implementation determinant frameworks. Implement. Sci. Commun 2, 61. doi: 10.1186/s43058-021-00146-5
Wu, Y., Wu, X., Gao, L., Yan, Y., Geng, Z., Zhou, S., et al. (2022). Abnormal functional connectivity of thalamic subdivisions in Alzheimer's disease: a functional magnetic resonance imaging study. Neuroscience 496, 73–82. doi: 10.1016/j.neuroscience.2022.06.006
Yakunina, N., Kim, S. S., and Nam, E. C. (2018). BOLD fMRI effects of transcutaneous vagus nerve stimulation in patients with chronic tinnitus. PLoS ONE 13, e0207281. doi: 10.1371/journal.pone.0207281
Yang, Q. Q., and Zhou, J. W. (2019). Neuroinflammation in the central nervous system: Symphony of glial cells. Glia 67, 1017–1035. doi: 10.1002/glia.23571
Yapa, H. M., and Barnighausen, T. (2018). Implementation science in resource-poor countries and communities. Implement. Sci. 13, 154. doi: 10.1186/s13012-018-0847-1
Keywords: tinnitus, implementation science, systematic review, tinnitus core network, neurophysiological evidence, cognitive neuroscience, limbic system, audiology
Citation: Burton-Harris LJ, Sanders PJ and Searchfield GD (2023) An implementation science systematic review of neurophysiological evidence indicates the tinnitus core network as a therapeutic target. Front. Audiol. Otol. 1:1311186. doi: 10.3389/fauot.2023.1311186
Received: 09 October 2023; Accepted: 22 November 2023;
Published: 11 December 2023.
Edited by:
Nilesh Washnik, Ohio University, United StatesReviewed by:
Konstantin Tziridis, University Hospital Erlangen, GermanyCopyright © 2023 Burton-Harris, Sanders and Searchfield. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Grant D. Searchfield, Zy5zZWFyY2hmaWVsZEBhdWNrbGFuZC5hYy5ueg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.