- 1Hearing Sciences, Mental Health and Clinical Neurosciences, School of Medicine, University of Nottingham, Nottingham, United Kingdom
- 2National Institute for Health Research Nottingham Biomedical Research Centre, Nottingham, United Kingdom
- 3Department of Audiology, Mater Misericordiae University Hospital, Dublin, Ireland
- 4Audiology Department, University Hospitals Sussex NHS Foundation Trust, Brighton, United Kingdom
- 5Department of Otorhinolaryngology and Head and Neck Surgery, University Medical Center Utrecht, Utrecht, Netherlands
- 6UMC Utrecht Brain Center, University Medical Center Utrecht, Utrecht, Netherlands
- 7Wessex Institute, University of Southampton, Southampton, United Kingdom
- 8Nottingham University Hospitals NHS Trust, Queen's Medical Centre, Nottingham, United Kingdom
Single-sided deafness (SSD) is defined by severe-to-profound sensorineural hearing loss in one ear only. This article outlines the etiologies and associated functional, psychological, social, and other consequences of SSD in adulthood. The available hearing aids and auditory implants for SSD are described, alongside an overview of the methods adopted by clinicians and researchers to define and measure their benefits and harms. Current concepts and issues to consider in the field of rerouting and restoring device-based interventions are explored. A contemporary overview of the current challenges in outcome measurement of all available interventions in the field is also provided, and cost effectiveness of SSD interventions is discussed briefly. This article therefore proves a comprehensive summary of the current knowledge on interventions and outcome measurement for SSD for those interested or actively working in the field, and recommendations for future trials. These include recommendations on the timescale of measurements, long-term benefits (or harms), cost utility, and the use of the internationally agreed core outcome domain set for all future clinical trials of device-based interventions for SSD.
Introduction
Single-sided deafness (SSD) arises when there is normal or near-normal hearing in one ear and a severe-to-profound sensorineural hearing impairment in the other ear (Van de Heyning et al., 2017). SSD is defined by a specific audiological classification: the mean pure tone average at frequencies of 0.5, 1, 2, and 4 kHz should be ≥70 dB HL on the poorer ear, and ≤ 30 dB HL on the better ear, with an interaural threshold gap of ≥40 dB HL. This definition is in line with a previous definition published in a proceedings paper which aimed to differentiate between SSD and asymmetric hearing loss (AHL) (Vincent et al., 2015). With regards to terminology, SSD is sometimes used interchangeably with unilateral hearing loss which may incorporate conductive (middle ear related), or mixed (both middle and inner ear related) hearing losses (BSA, 2018). Developing a consensus around the definition allowed us to differentiate between SSD and AHL when comparing data.
This conceptual analysis aims to provide a narrative overview of SSD. The functional and psychological impact are discussed first, followed by the impact on wellbeing and quality of life (QoL). Finally, novel challenges in choice of device-based treatments faced by patients diagnosed with SSD, healthcare professionals, and clinical researchers working in the field are discussed.
Etiology
SSD can be (i) congenital or perinatal, (ii) of sudden acquired causes, or (iii) progressive. The most common causes of SSD in adulthood are sudden and idiopathic.
Congenital or perinatal causes
Cochlear nerve deficiency is one of the most common causes of pediatric SSD (Usami et al., 2017), followed by inner ear malformation (Acke et al., 2021). The causes can also be genetic, secondary to family history, syndromic (Widen et al., 2000; Fitzpatrick et al., 2017), or due to in-utero or post-natal infections such as cytomegalovirus or meningitis (Ghogomu et al., 2014; Huttunen et al., 2019; Dewyer et al., 2022). Perinatal causes include sudden idiopathic sensorineural hearing loss, or head trauma.
Sudden acquired causes
In adulthood, SSD can be of sudden causes secondary to conditions like Ménière's disease (Wu et al., 2019), follow viral infections such as labyrinthitis, be idiopathic (Chandrasekhar et al., 2019; Mirian and Ovesen, 2020; Simani et al., 2022), or due to autoimmune systemic diseases (McCabe, 1979; Rossini et al., 2017; Li et al., 2018). Sudden onset SSD can be caused by temporal bone fracture(s) following head trauma, or be iatrogenic following otological surgery (Bird and Bergin, 2018; Deep et al., 2021). More recently, case reports of sudden onset SSD following COrona VIrus Disease (COVID-19) (Koumpa et al., 2020; Asfour et al., 2021; Pokharel et al., 2021), or attributed to immunization for COVID-19 with SARS-CoV-2 mRNA vaccines (Ekobena et al., 2022) have been documented.
Progressive causes
SSD can be progressive, for example, in cases of cholesteatoma (Usami et al., 2017), cerebellopontine angle tumor(s), neurofibromatosis (Jia et al., 2020), or vestibular schwannoma (Daniels et al., 2000; Staecker et al., 2000; Douglas et al., 2007). Other progressive causes including ototoxicity, vascular conditions, demyelinating conditions, Lyme disease, otosyphilis, and human immunodeficiency virus have been proposed (Timon and Walsh, 1989; Peltomaa et al., 2000; Lee and Baloh, 2005; García-Berrocal et al., 2006; Chau et al., 2010; Schreiber et al., 2010).
Although the causal relationship between these causes and unilateral sensorineural hearing loss is difficult to verify, these causes should be outruled in cases of SSD in adulthood (Lawrence and Thevasagayam, 2015; Chandrasekhar et al., 2019; Twigg et al., 2020).
Prevalence and incidence of SSD
It has been suggested that SSD affects between 12 and 27 individuals in every 100,000 of the general population (Kitterick et al., 2014). More recently, it has been estimated that 10.4–25.4 individuals per 100,000 are at risk for SSD (Sprinzl and Wolf-Magele, 2016); 5–20 per 100,000 due to sudden sensorineural hearing loss (SSNHL) (Plaza et al., 2011). A more recent study containing information for more than 60 million unique patients estimated the prevalence of SSNHL in the USA to be 27 per 100,000 (Alexander and Harris, 2013). The NHANES epidemiologic study in the USA estimated the prevalence of SSD in adults to be 0.14% (Kay-Rivest et al., 2021); and to be higher in females (0.17%) vs. males (0.11%). The NHANES data also demonstrated that the prevalence of SSD was higher in individuals aged 60–79 years (prevalence of 0.25%) than in younger individuals (0.11% in ages 20–39 years, and 0.11% in ages 40–59 years).
The incidence of SSD in the UK has been estimated at 7,500 new adult cases per year (Baguley et al., 2006). Extrapolating to the 2023 population in the UK of ~67 million (www.ons.gov.uk), the incidence of SSD is ~9,000 new cases per year. Baguley et al. (2006) calculated that the highest incidence of SSD per 100,000 of the adult population per year was due to SSNHL, followed by Ménière's disease, with vestibular schwannoma being the lowest.
Associated otologic features
Tinnitus
SSD can be associated with tinnitus, defined as “the conscious awareness of a tonal or composite noise for which there is no identifiable corresponding external acoustic source” and/or tinnitus disorder, which arises when tinnitus is “associated with emotional distress, cognitive dysfunction, and/or autonomic arousal, leading to behavioral changes and functional disability” (De Ridder et al., 2021). It is estimated that ~80% of patients with sudden idiopathic sensorineural loss have tinnitus (Nosrati-Zarenoe et al., 2007; Schreiber et al., 2010; Levy et al., 2020). One hypothesis states that it can be due to reduced or absent auditory input that can lead to changes in neural activity (Eggermont and Roberts, 2012). Studies that used the Tinnitus Handicap Inventory (THI) (Newman et al., 1996) and the Hearing Handicap Inventory for Adults (HHIA) (Newman et al., 1991) to investigate the prevalence of tinnitus in those with idiopathic SSD showed that two thirds of patients reported intrusive tinnitus as per THI scores (Chiossoine-Kerdel et al., 2000). Visual Analog Scales (VAS) indicating the loudness of the tinnitus, and distress evoked by the tinnitus showed correlations between tinnitus loudness, distress, and hearing handicap (Chiossoine-Kerdel et al., 2000). Of those diagnosed with SSD secondary to Ménière's disease 78.6% report tinnitus (Young et al., 2022). In a retrospective study including 22 individuals with a vestibular schwannoma, tinnitus burden was measured using the THI (West et al., 2022). The authors highlighted that the methods adopted to date for evaluation of tinnitus may be a limitation due to participants being inadequately instructed to distinguish between the ears or situations (e.g., when wearing a hearing device or not). Tinnitus is also experienced by those who sustain SSD due to endolymphatic hydrops, labyrinthitis, trauma, iatrogenic causes, herpes zoster oticus, otosclerosis, cholesteatoma, or cerebrovascular accident (Van de Heyning et al., 2008; Buechner et al., 2010; Arndt et al., 2011a; Mertens et al., 2016; Ramos Macías et al., 2018).
Hyperacusis
Alongside the experience of tinnitus, some people with SSD also experience hyperacusis, which has been attributed to excessive gain increase in the central auditory pathway (Ramos Macías et al., 2018). Hyperacusis is a chronic disorder of loudness perception (Tyler et al., 2014) that involves reduced tolerance or increased sensitivity to regular noises (Baguley and Hoare, 2018; Fackrell et al., 2019; Adams et al., 2021). Hyperacusis is sometimes used interchangeably with loudness recruitment, which is a common symptom of peripheral hearing loss, defined as an abnormally fast growth of loudness perception of sound intensity (Shi et al., 2022). Hyperacusis in SSD has been linked to reduced median scores on the sub-scales of the Khalfa et al. (2002) hyperacusis questionnaire (HQ) (Mertens et al., 2016). The HQ however focuses on the psychological and social aspects of hearing, rather than on hyperacusis itself (Mertens et al., 2016). The Sound Hypersensitivity Questionnaire (SHQ) (Herráiz et al., 2006) was used in a Spanish multi-center study (Ramos Macías et al., 2018) to measure the impact of loud sounds and noise on the QoL in patients with SSD. It has 15 questions reported in four grades (Grade I: mild 1–10, Grade II: moderate 11–17, Grade III: severe 18–25, and Grade IV: very severe: 26–45) for three subscales of behaviors, cognitive reactions, and emotional reactions. Patients with SSSD score on average “severe” degree (Grade III) of incapacity (Ramos Macías et al., 2018).
Aural fullness
SSD due to sudden causes has been associated with aural fullness, which is described as “ear pressure,” “sense of fullness,” or “clogging sensation” (Westerlaken et al., 2003; Sakata and Kato, 2006; Park et al., 2012). Aural fullness in sudden onset SSD has no relationship to gender and age at the time of first assessment, however it is more common in low-frequency hearing loss audiograms (Sakata et al., 2008). In those diagnosed with Ménière's disease, 57.1% report unilateral aural fullness (Young et al., 2022), which is attributed to pressure imbalances between the round and oval windows in the inner ear (Sakata et al., 2008).
Vestibular dysfunction
The vestibular system can also be involved in 30–40% of cases with sudden unilateral loss (Nakashima and Yanagita, 1993; Schreiber et al., 2010; Shih et al., 2017). In Ménière's disease, individuals experience acute vestibular dysfunction (Thai-Van et al., 2001; Wu et al., 2019). In cases of SSD due to vestibulocochlear cranial nerve involvement individuals can experience instability while moving their head, imbalance, or vertigo (Nicoucar et al., 2006; Greene and Al-Dhahir, 2022).
Cortical changes in SSD
Studies have reported central auditory system re-organization in cases of unilateral deafness (Legris et al., 2018; Alzaher et al., 2021). These studies however, pool together data from individuals with various degrees of hearing loss that fall into the highly AHL category, as opposed to an SSD cohort explicitly. In adults, brain reorganization is detectable 5 weeks after onset of SSD (Suzuki et al., 2002), and functional magnetic resonance imaging (fMRI) studies have demonstrated that reorganization plateaus after 1 year (Bilecen et al., 2000). Magnetoencephalography studies of brain activation during performance of auditory syllable sequence reproduction tasks demonstrate that in adult-onset SSD there is both functional and structural alterations to the dorsal temporal and frontal-parietal areas of the brain (Shang et al., 2018). SSD also leads to physiological lateralization of auditory cortical activity, which has an impact on auditory spatial abilities (Karoui et al., 2022). An asymmetry of neuronal activity of the inferior colliculus and primary auditory cortex has been demonstrated using 18F-FDG PET imaging studies (Speck et al., 2020, 2022). AHL loss has a significant impact on glucose metabolism of the auditory pathway, which in turn can negatively influence audiological performance (e.g., speech recognition in noise) following cochlear implantation (Speck et al., 2022). Speck et al. (2022) enrolled nine participants with either AHL or SSD, with heterogeneous etiology, disease onset, and duration of deafness, so they suggest larger longitudinal studies to be able to confirm their hypothesis.
Impact of SSD
The disabling effects of SSD (speech, spatial, qualities domains), and impact of these effects on the degree of handicap experienced by the hearing impaired individual, vary considerably (Gatehouse and Noble, 2004; Noble and Gatehouse, 2004).
Why we need two ears
Good hearing in both ears (binaural hearing) helps us deal with everyday listening tasks (Dwyer et al., 2014; Snapp and Ausili, 2020). Auditory processing of speech in complex environments gives listeners with binaural hearing a benefit of 4–10 dB in processing speech (Hawley et al., 2004) in comparison to monaural (hearing with one ear only). This is known as binaural loudness summation, i.e., sounds presented to two healthy ears is perceived louder than the same level of sound presented to one healthy ear only (Avan et al., 2015). The squelch effect is another ability of a healthy auditory system, which allows listeners to combine signal(s) and competing noise information retrieved from both ears to improve speech perception in noise. Benefits of binaural hearing include understanding speech in noisy or reverberant environments and locating where sounds such as the telephone or car traffic are coming from (Levitt and Rabiner, 1967; Hawley et al., 2004; Snapp, 2019; Snapp and Ausili, 2020; Gallun, 2021).
Sound localization in the horizontal (azimuth) plane relies mainly on interaural time differences (ITDs) and interaural level differences (ILDs) (Agterberg et al., 2012; Rothpletz et al., 2012; Pedley and Kitterick, 2017). In other words, the auditory system helps us judge our positioning in space by dynamically calculating our interaction with signals that are constantly changing in terms of pitch (frequency spectrum), level (intensity), and time (latency) (Akeroyd, 2006; Arndt et al., 2011a; Snapp and Ausili, 2020). The integration of acoustic information from both ears is essential for spatial awareness (Güldner et al., 2013; Karoui et al., 2022), for example, determining where sounds are coming from (Douglas et al., 2007; Pedley and Kitterick, 2017; Snapp, 2019).
Although there can be a degree of adaptation in certain monaural listeners (Slattery and Middlebrooks, 1994; Rothpletz et al., 2012), and possible long-term compensation for loss of binaural cues (Liu et al., 2018; Alzaher et al., 2021), localization abilities can be severely impaired in those hearing monaurally (Wazen et al., 2005; Agterberg et al., 2012; Hoth et al., 2016; Pedley and Kitterick, 2017; Snapp, 2019). A further complication for monaural listeners is introduced by the head shadow effect, where the head acts as an acoustic barrier to signals that travel from one side of the head to the other, which can lead to significantly impaired speech understanding (Akeroyd, 2006; Pedley and Kitterick, 2017; Snapp, 2019). In cases of SSD, speech originating from the poor-hearing side of the head is reduced in intensity by 6.4 dB by the time it reaches the normal-hearing ear; it therefore arrives distorted due to loss of high-frequency information from the speech spectrum (McLeod et al., 2008).
Functional difficulties experienced by adults with SSD
Due to the challenges arising from monaural access to sound, individuals with SSD have difficulties dealing with everyday tasks such as speech recognition (Lieu et al., 2010; Dwyer et al., 2014) and impaired ability to understand speech in the presence of background noise (Welsh et al., 2004; Rothpletz et al., 2012; Firszt et al., 2017; Vannson et al., 2017; Peters et al., 2021; Kitoh et al., 2022). It is estimated that there is a reduction in speech understanding by approximately 3 dB in SNR in cases of SSD (McLeod et al., 2008). Speech understanding is reduced due to reduced signal loudness detected by the monaural listener.
Different features of the conversation context, for example the complexity of the acoustic environment, the type or loudness of the background noise, or the number of people in a group can influence speech perception and impact on individuals' need to modify their communication strategies (Hadley et al., 2021). Adults with unilateral hearing loss are more likely to report a higher level of communication difficulties than normal-hearing adults (Dwyer et al., 2014; Choi et al., 2021). SSD can also impact music appreciation. Music can sound unnatural, unpleasant and indistinct, lack perceptual qualities such as stereo sound, and be confounded by distortion effects and tinnitus (Meehan et al., 2017).
Moreover, SSD is associated to increased listening effort when compared to normally hearing individuals (Dwyer et al., 2014; Lopez et al., 2021). Listening effort is defined as the mental exertion required to attend to and understand an auditory message (McGarrigle et al., 2014). Hearing impaired listeners may experience increased listening effort in challenging listening situations than normally hearing individuals, even if they use hearing aids (Alhanbali et al., 2017). The constant effort applied by a listener with SSD to adjust to their listening environment is fatiguing, and can be unsustainable for many (Snapp, 2019). The real-world impact of increased fatigue is dependent on personal factors and lifestyle (Holman et al., 2019), and can influence social activity level (Holman et al., 2021a). Fatigue could arise due to decreased audibility of sounds, and in part, increased requirement for listening effort (McGarrigle et al., 2014). Other factors such as related challenges in auditory processing, and increased listening effort required in demanding listening environments have been proposed (Hornsby et al., 2016; Ohlenforst et al., 2017; Peelle, 2018). Associations to work, social, or physical activity levels, and wellbeing are also relevant and have implications on daily-life fatigue in people with hearing loss (Holman et al., 2021b). Objective measures of pupil dilation as an indicator of listening effort during listening tasks demonstrate that the individual's motivation is a factor that can influence objective measures of fatigue (Wang et al., 2018). Qualitative studies interviewing people with hearing loss identified factors such as lifestyle, personality, situational control, the relationship with those in conversation and the attribution of blame are key to individual emotional experiences (Holman et al., 2022).
Psychological impact of SSD
The psychological impact of SSD in adulthood has been well-documented in the literature, including worry about losing the hearing in the other ear, embarrassment related to the social stigma attached to hearing loss, and reduced confidence and belief in one's own abilities to participate (Sano et al., 2013b; Lucas et al., 2018; Choi et al., 2021). Individuals with unilateral hearing loss are at a disadvantage in social and emotional situations. They report being upset, anxious, frustrated and isolated due to their hearing handicap secondary to monaural listening (Araújo et al., 2010; Sano et al., 2013a; Lucas et al., 2018). It has also been reported that SSNHL is associated with anxiety and depression (Arslan et al., 2018). Furthermore, increased stress levels related to the need to find an optimal position in social settings, that will help with speech perception and participation, have been reported in interview studies (Lucas et al., 2018). Those who acquired SSD secondary to a vestibular disorder (e.g., labyrinthitis, Ménière's disease) could be at risk of chronic anxiety which could precede depressive states (Hilber, 2022). Analysis from the 2008 National Health Interview Survey, which included ~18 million people with vestibular vertigo in the USA, suggested that cognitive impairment (memory loss, difficulty concentrating, confusion) and psychiatric diagnoses (depression, anxiety, panic disorder) are comorbidities in those with vestibular deficiencies (Bigelow et al., 2016), which can also be linked to difficulties remembering in 32% of individuals. In addition, individuals diagnosed with SSD report decreased self-esteem when in places with background noise, which can leave them feeling frustrated and isolated (Lucas et al., 2018). They also report increased stress levels and exhaustion related to their constant attempts to maximize their abilities to hear and participate in complex social situations (Wie et al., 2010; Kuppler et al., 2013). Associated feelings of frustration, annoyance, helplessness, embarrassment, and depressive symptoms have been reported in multiple studies (Giolas and Wark, 1967; Gatehouse and Noble, 2004; Wie et al., 2010; Sano et al., 2013b; Lucas et al., 2018; Choi et al., 2021).
Impact of SSD on wellbeing
QoL is defined as “a patient's general wellbeing, including mental status, stress levels, sexual function, and self-perceived health status” (Farlex Partner Medical Dictionary, 2012). Population based studies evaluated the risks of adverse hearing and wellbeing outcomes (including self-reports on depression, health rating, satisfaction with health, happiness and loneliness), in 113,804 UK Biobank participants aged 40–69 years who self-reported unilateral hearing loss (Pierzycki et al., 2020). Participants with unilateral hearing impairment are significantly more likely to report poor health, dissatisfaction with health, and loneliness than those with normal hearing (Dawes et al., 2014; Pierzycki et al., 2020). The multi-dimensional burden of SSD on overall health is indicated by reductions in health-related QoL in individuals with a diagnosis of SSD, despite use of hearing-assistive devices for SSD (Arndt et al., 2011a; Kitterick et al., 2015; Vannson et al., 2015; Pierzycki et al., 2020). One study reports that the impact of SSD on QoL can exceed that reported by listeners with bilateral hearing loss (Sano et al., 2013a). This Japanese study included 167 adult participants with idiopathic SSNHL and 134 participants with bilateral hearing loss to act as controls. They measured health-related QoL with the Japanese version of the Short-Form Health Survey (SF-36) (Fukuhara et al., 1998). The term “health-related quality of life” (HRQoL) narrows QoL to aspects relevant to health (de Wit and Hajos, 2013). This study concluded that there was reduced mental functioning in those with idiopathic SSNHL, compared to averages in the Japanese population, which was similar to their participants with bilateral hearing loss. The psychosocial impact has also been documented, with annoying tinnitus and remaining vertigo after SSNHL to be the strongest predictors of negative effects on QoL (Baguley et al., 2006; Carlsson et al., 2011). QoL can be affected in those who have vestibular schwannomas surgically removed, as indicated by lower scores yielded on the SF-36 survey instrument (Ware and Sherbourne, 1992), in all categories, but more significantly in physical ability, social functioning, emotional status and vitality (Nicoucar et al., 2006).
Social consequences of SSD
Individuals with SSD report feeling excluded in conversations with multiple speakers, have reduced wellbeing in social settings, and a preference to avoid social gatherings in which they thought significant background noise would be present (Wie et al., 2010; Chang et al., 2020). Qualitative studies indicate that coping strategies of individuals with SSD include withdrawal from within a situation and in some cases, from the social situation completely (Lucas et al., 2018). The impact of SSD on communication can also affect intimate relationships (Hétu et al., 1993; Lucas et al., 2018). SSD can impact individual's vocational activities such as business negotiations, customer service, and meetings, contribute to absences or days away from work, and early retirement (McLeod et al., 2008; Härkönen et al., 2015; Marx et al., 2019; Snapp, 2019). These studies disagree with Colletti et al. (1988) who found no difference between monaurally and binaurally hearing individuals on educational, social, and employment achievement. Their participants however were aged 30–55 years, and had SSD since childhood (Colletti et al., 1988), rather than acquired in adulthood like the other studies. However, longitudinal studies in the USA with older adults with age-related hearing loss report that hearing loss may be associated with reduced engagement in physical and mental activities (Goman et al., 2021; Kuo et al., 2021). They also report higher odds of those with hearing loss reporting loneliness than those reporting excellent hearing, after adjusting for comorbidity index, functional and cognitive ability, self-reported health, and demographic characteristics (Huang et al., 2021). It is well-documented in recent literature that hearing impairment can impair social engagement, alter social roles, and impede the formation and maintenance of relationships (Barker et al., 2017; Vas et al., 2017; Heffernan et al., 2022).
Treatment options for SSD
The aim of SSD device-based treatments is to address the functional difficulties imposed and in turn improve everyday listening and communication. Devices are categorized as either rerouting or restoring.
Rerouting devices for SSD (“bilateral hearing”)
The most commonly used treatment options for SSD enable access to sounds on both sides of the head (bilateral hearing) by rerouting sounds from the impaired ear to the hearing ear.
Contralateral routing of signals hearing aid
Rerouting interventions include the CROS hearing aid (Leterme et al., 2015; Ryu et al., 2015; Snapp et al., 2017; Choi et al., 2021). The CROS system (Figure 1) is made of two parts: a wireless microphone which is mounted onto the poor-hearing ear and is paired wirelessly to a hearing aid that is worn on the better-hearing ear. The Snapp (2019) review lists several advantages of the CROS device including sound awareness on the poor-hearing side, improvement of the SNR for sounds directed to the poor-hearing ear in noisy environments, and ease of use. Limitations of this technology include that binaural input is still impaired, poor sound localization in the horizontal plane due to disruption of the available monaural level and spectral cues (Pedley and Kitterick, 2017), and impairments related to hearing in noise, especially if the interfering noise is amplified in the better-hearing ear (Snapp, 2019).
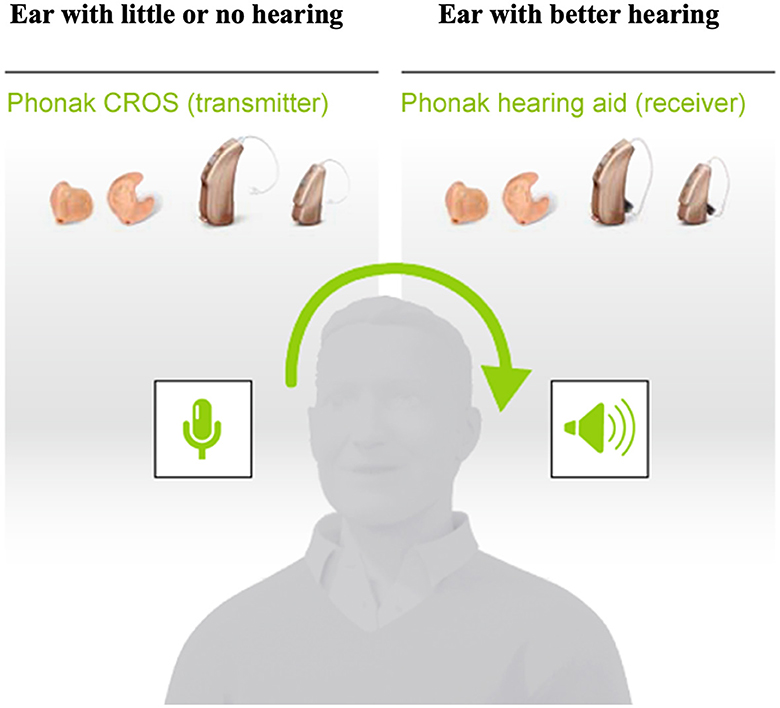
Figure 1. Schematic representation of the Phonak Contralateral Routing of Signals (CROS) hearing aid. Retrieved from www.phonakpro.com and used with permission, © 2023 Sonova AG.
Bone conduction devices
An alternative widely used rerouting intervention for SSD is the BCD which can be implanted on the poor-hearing side. BCDs were first implanted in the 1970s (Tjellström and Granström, 1994) and since then many variations have been developed (Håkansson et al., 2019; Iwasaki, 2022; Maier et al., 2022). A BCD fitting requires surgical implantation of a transcutaneous abutment (Figure 2), or a more recently used device with a subcutaneous magnet (Figure 3) into the skull bone behind the ear. It delivers sounds into the skull by means of sound vibrations, which transfer sound transcranially from the poor-hearing side to the contralateral side. Benefits of the BCD for SSD include overcoming the negative consequences of the head-shadow effect (Niparko et al., 2003; Snik et al., 2004), improvement in hearing speech in noise when noise is presented on the better hearing ear side (Hol et al., 2005), and improvement in QoL (Leterme et al., 2015).
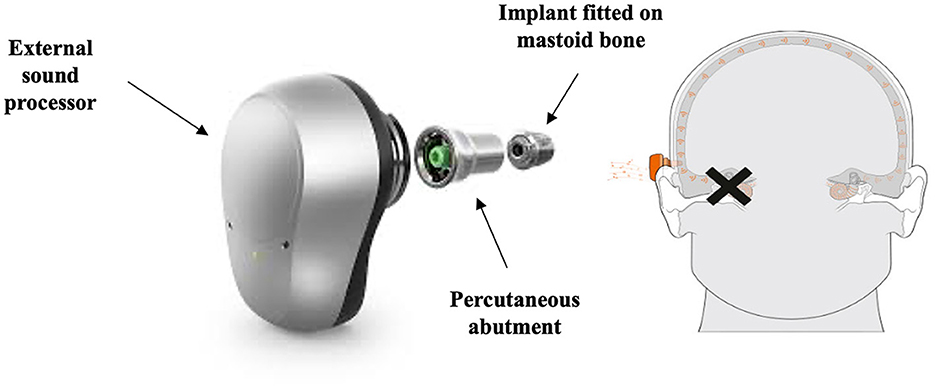
Figure 2. Schematic representation of the Oticon MedicalTM percutaneous bone conduction device (BCD). Retrieved from www.oticonmedical.com and used with permission from Oticon MedicalTM.
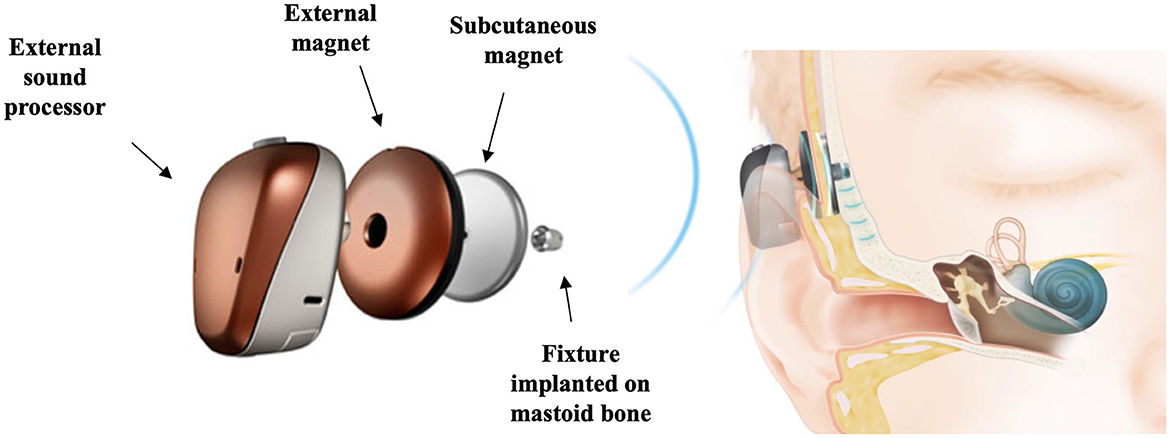
Figure 3. Schematic representation of the Cochlear™ Baha® 6 Max Attract system, a subcutaneous bone conduction device (BCD). Images courtesy of Cochlear Bone Anchored Solutions AB, © 2023.
A review of four controlled trials that attempted to determine the benefit of BCD vs. CROS vs. the unaided condition concluded that there is a paucity of evidence to support the efficacy of BCD in the treatment of acquired SSD (Baguley et al., 2006). However, they suggested that speech discrimination in noise and subjective questionnaire measures of auditory abilities showed an advantage for BCD over CROS and the unaided condition. A systematic review comparing the clinical outcomes of the CROS and BCD devices concluded that there is no difference between the two treatment options regarding speech perception in noise and localization, and a moderate improvement in subjective speech communication when using either a CROS or a BCD (Peters et al., 2015a). Other studies also concluded that the BCD does not improve nor deteriorate the localization abilities of individuals with SSD (Wazen et al., 2005; Lin et al., 2006; Agterberg et al., 2019). A study that included 44 individuals with SSD assessed the subjective benefits of BCD with four questionnaires, with a median of 50 months follow-up period (Desmet et al., 2014). Their findings suggest that the majority of individuals (86%) use their processors, and report an overall improvement; however, device use reduces at long-term follow-up, especially in noisy situations.
The subcutaneous magnet version of the BCD system (Figure 3) was compared to the percutaneous (Figure 2) on a prospective study evaluating the long-term audiological and clinical outcomes (Kruyt et al., 2020). The findings suggested that the percutaneous system provided statistically significant or near-significant improvement compared with the unaided condition in all audiometric tests throughout the 24-month follow-up, except for speech recognition in noise at the 24-month visit. However, the statistically significant clinical improvements recorded with questionnaires at 6 months were no longer present at 24 months. Another study that included five individuals with SSD and compared the percutaneous vs. subcutaneous devices during the first 6 months post implantation found an improvement in sound, speech understanding, and QoL in those implanted with the percutaneous device, but limited improvement in localization abilities, and there were no adverse effects noted (Kong et al., 2021).
Active bone conduction implant systems
Active bone conduction implant systems, like the MED-EL BoneBridgeTM (Figure 4) have been used to alleviate the impact of SSD in adults (Bianchin et al., 2015; Zernotti and Sarasty, 2015; Sprinzl and Wolf-Magele, 2016; Schmerber et al., 2017; Ratuszniak et al., 2022). The BoneBridgeTM consists of an external audio processor and an implantable bone conduction implant which lies completely under the skin on the poor-hearing side. The bone conduction implant is composed of an active electromagnetic bone conduction floating mass transducer, an electrical demodulator, and a receiver coil. Sound vibrations delivered through the skull are transmitted directly to the inner ear. The CochlearTM Carina, another active bone conduction implant, has not been reported as an intervention for SSD (Katiri et al., 2021).
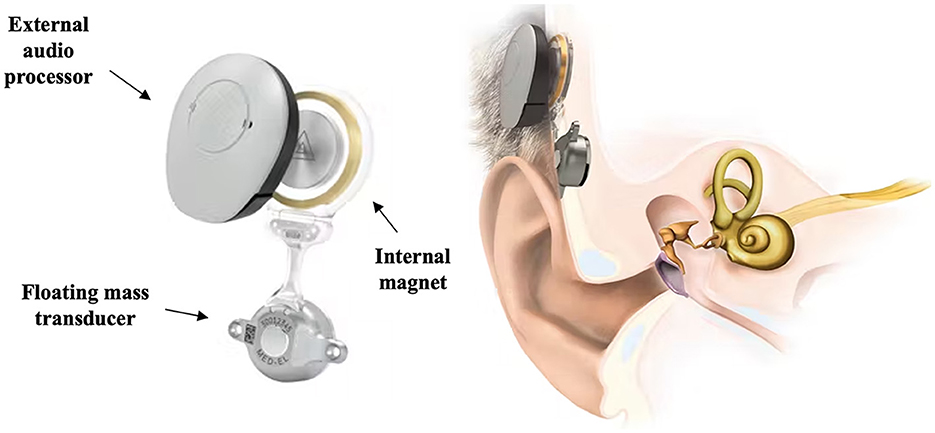
Figure 4. Schematic representation of the MED-EL BoneBridge™ bone conduction implant. Retrieved from www.medel.com and used with permission from MED-EL.
A longitudinal, 5-year follow-up economic analysis of the BoneBridgeTM compared to the percutaneous BCD (Figure 2) demonstrated that the BoneBridgeTM is a good alternative option with reduced skin complications reported due to the lack of a percutaneous abutment. However, a drawback of this device is the attenuation of high frequency auditory output by the skin (Amin et al., 2021). Another study that evaluated the post-operative pain following BoneBridgeTM implantation concluded that pain scores were similar to those experienced by individuals with other transcutaneous auditory implants (Lassaletta et al., 2016). Structured interviews conducted with 20 adult participants with SSD by Ratuszniak et al. (2022) demonstrated that the BoneBridgeTM device provided less subjective satisfaction in those with SSD vs. other types of hearing loss (conductive or mixed). Their interviews included questions on (i) satisfaction of the effect achieved, (ii) sound quality of the device, and (iii) change in hearing (improvement or deterioration).
Adhesive BCD
An adhesive BCD, the ADHEAR (Figure 5) by MED-EL, has also been used to alleviate the functional effects of SSD (Mertens et al., 2018; Moteki et al., 2020). The device comprises a removable, single use adhesive adapter and an audio processor that are worn behind the poor-hearing ear. The adhesive adapter secures the audio processor and provides sufficient contact force to provide good physical contact between the vibrating portion of the hearing aid and the user's skull (Mertens et al., 2018). A study aiming to obtain preliminary results regarding the use of ADHEAR in individuals with various types of hearing loss found no improvement in speech perception or sound localization, despite functional hearing gains in their three participants with SSD (Moteki et al., 2020). The speech perception findings mirror the Mertens et al. (2018) conclusions, although they did observe slight improvement in sound localization when wearing the ADHEAR with the omnidirectional microphone program enabled, when compared to the CROS device in 17 participants with SSD. However, due to the large variation in outcomes and limited statistical power no firm conclusions were made.
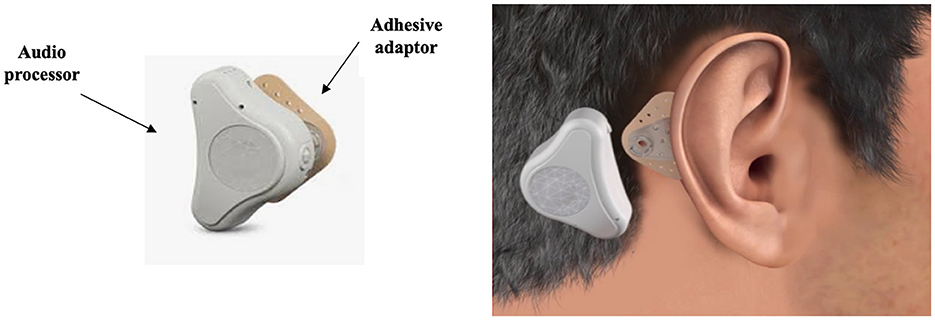
Figure 5. Schematic representation of the ADHEAR by MED-EL bone conduction device (BCD). Retrieved from www.medel.com and used with permission from MED-EL.
Dental implant
Another rerouting device, the SoundBiteTM dental implant (Figure 6) by Sonitus Medical, has been tested in the past (Popelka et al., 2010; Miller et al., 2011; Murray et al., 2011; Gurgel and Shelton, 2013; Moore and Popelka, 2013; Luo et al., 2020), but is currently only used in China (BusinessWire, 2022). The SoundBiteTM is a removable in-the-mouth device that is fixed onto the teeth, and directly coupled to the skull. A behind-the-ear hearing aid picks up signals and transmits them to the in-the-mouth device. The SoundBiteTM is directly fixed onto the dental bones, and it generates vibration that passes through the skull to the cochlea. A study that recruited nine Chinese individuals with SSD aged 24–61 years assessed speech recognition in quiet and noise, and QoL when using the SoundBiteTM compared to no intervention (Luo et al., 2020). The findings suggest an improvement in QoL and speech perception benefits in quiet and noise (when noise was presented on the better-hearing ear).
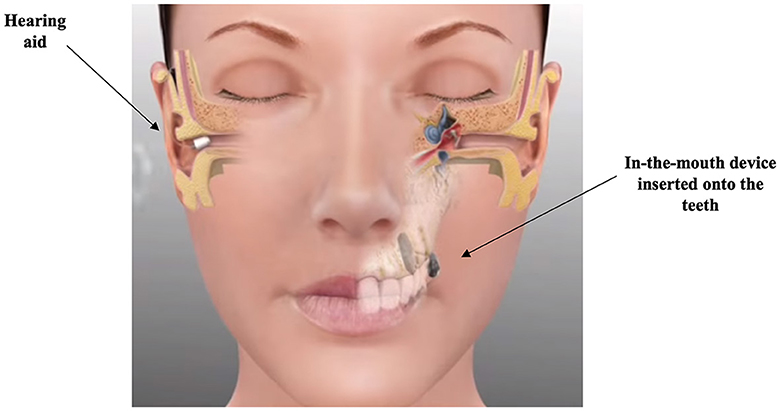
Figure 6. Schematic representation of the SoundBiteTM dental implant. Reproduced with permission from SoundBite hearing (SoundBite Hearing, 2013).
Restoring devices for SSD (“binaural hearing”)
Auditory input to the poor-hearing ear can be restored (binaural hearing) by delivering information about sounds directly to the auditory pathway on the side of the impaired ear.
Middle ear implants
Binaural hearing can be achieved using auditory prostheses like a MEI, such as the MED-EL Vibrant SoundBridgeTM (Figure 7). The device consists of an externally worn audio processor and an implant surgically positioned under the skin. The audio processor is held to the implant by magnetic attraction. The audio processor microphones pick up sound waves and the audio processor converts sounds into electrical signals, which are transmitted through the skin to the implant. A small part of the device, the floating mass transducer, converts the signals into mechanical vibrations which in turn stimulates the inner ear (Laske et al., 2015; Gerdes et al., 2016; Schmerber et al., 2017). Schmerber et al. (2017) included 12 individuals with SSD in their study aiming to validate the safety and efficacy of the Vibrant SoundBridgeTM to find an improvement in speech-in-noise performance when the speech was presented on the poor-hearing side with the device on. The findings are in agreement with Laske et al. (2015) who also found improvements when the speech signal was presented on the poor-hearing side with the device on.
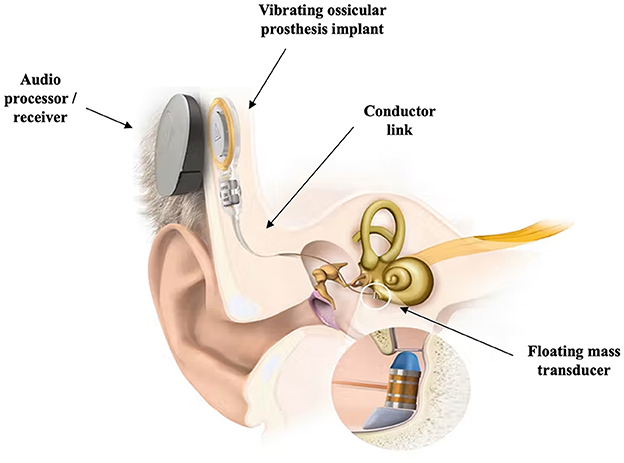
Figure 7. Schematic representation of the MED-EL Vibrant SoundBridgeTM middle ear implant. Retrieved from www.medel.com and used with permission from MED-EL.
More recently, the CochlearTM Osia® system (Figure 8) has been used for SSD (Rauch et al., 2021; Willenborg et al., 2022). The Osia® system is an active bone conduction hearing implant system that has a transcutaneous connection between an external processor and an implant. The vibrator (actuator) is piezoelectricity based and is connected directly to a titanium implant anchored and osseointegrated to the skull bone (Arndt et al., 2021; Hwa et al., 2022). Piezoelectricity is the ability of certain materials to generate an electric charge in response to applied mechanical stress (vibrations), or reversibly to generate vibrations in response to an external electric charge (Goycoolea et al., 2020). A multi-center study including five individuals with SSD investigated the clinical performance, safety, and patient-reported outcomes of the Osia® system (Briggs et al., 2022). They demonstrated a statistically significant and clinically relevant improvement in speech recognition in quiet and noisy situations compared to an unaided situation, and a subjective improvement in hearing benefit when compared to pre-operative scores.
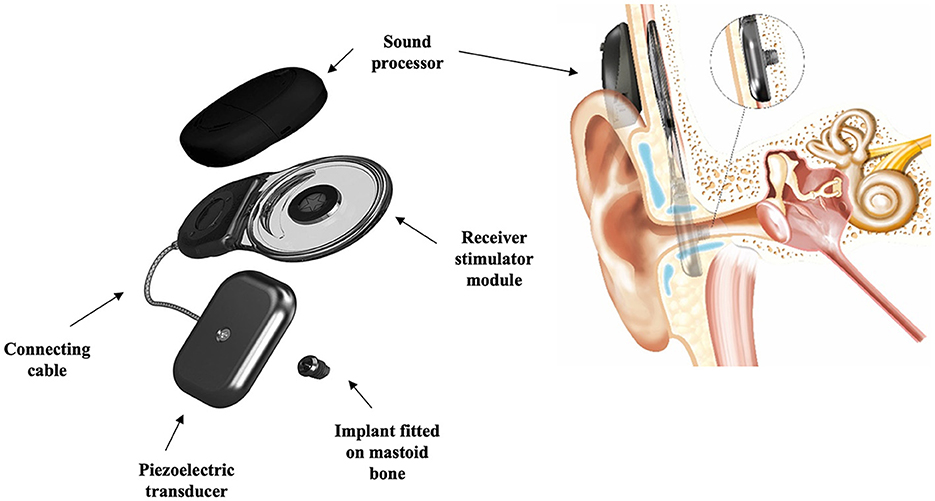
Figure 8. Schematic representation of the Cochlear™ Osia® system, an active bone conduction hearing implant. Images courtesy of Cochlear Bone Anchored Solutions AB, © 2023.
Cochlear implant
Cochlear implantation as an intervention for SSD was first piloted to assess the effect of electrical stimulation via a CI in individuals with SSD and incapacitating ipsilateral tinnitus (Van de Heyning et al., 2008). Since then has been utilized by several clinical teams, mainly in Europe, North America and Australia (Härkönen et al., 2015; Távora-Vieira et al., 2015; Arndt et al., 2017; Marx et al., 2019; Poncet-Wallet et al., 2020; Peters et al., 2021). A CI can deliver information about sounds directly to the auditory pathway, electrically stimulating the impaired ear (Figure 9), thus creating a sensation of binaural hearing (Arndt et al., 2011a). Auditory cortical plasticity studies have suggested that cochlear implantation in asymmetrical hearing loss enables reconstruction of the cortical mechanisms of spatial selectivity needed for sound localization (Karoui et al., 2022).
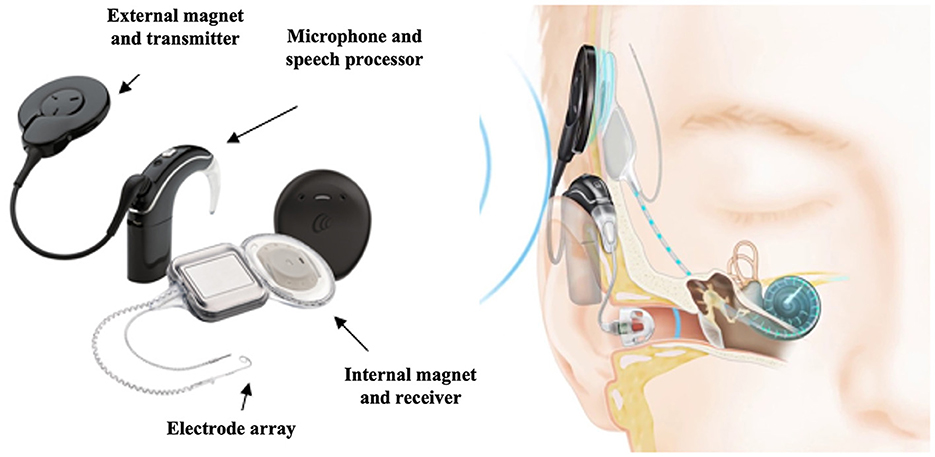
Figure 9. Schematic representation of a cochlear implant device. Images courtesy of Cochlear Limited, © 2023.
A systematic review of the literature up to 2015, analyzed the influence of cochlear implantation in a total of 137 individuals with SSD with regards to sound localization, speech perception, tinnitus, and QoL (Cabral Junior et al., 2016). Despite the variation in participant characteristics, onset and duration of SSD, and the diversity of outcomes reported, the authors conclude that cochlear implantation enhances sound localization, speech perception, and contributes to improvement in tinnitus. Another systematic review incorporating nine studies reporting on 112 participants assessed the clinical outcomes of cochlear implantation for SSD or AHL (van Zon et al., 2015). Due to large clinical heterogeneity in the reported measures, especially speech perception in noise, and high risk of bias, a meta-analysis was not conducted. A more recent systematic review included 50 studies totalling 674 adults with SSD aged 19–93 years, with an average duration of deafness ranging from 0.8 to 68 years (Oh et al., 2023). This review aimed to analyse the impact of CI on speech perception in quiet and noise, tinnitus control, sound localization, and QoL. Similar to Cabral Junior et al. (2016) the authors concluded that CI in individuals with SSD provides significant improvement in speech perception, tinnitus control, localization, and QoL. Oh et al. (2023) also highlighted a large variability in participant characteristics (e.g., etiology, onset, duration), numbers recruited in studies (e.g., ranged from 3 to 70 participants), choice and reporting of outcome measures (e.g., speech testing configurations, reporting parameters), follow-up time (e.g., ranged from 6 months to 3.5 years) across studies. Of note, a recently reported retrospective case series with 66 adults with SSD that were implanted with a CI report that duration of deafness was not associated with significant differences in speech recognition performance (Lindquist et al., 2022). They measured speech recognition with the Consonant-Nucleus-Consonant (CNC) words and the AzBio sentences in quiet. A systematic review including 31 studies, which aimed to provide a comprehensive overview of the short- and long-term effects of cochlear implantation on disabling tinnitus in adults with SSD, reported an improvement in tinnitus suppression scores despite variability in patient characteristics (Idriss et al., 2022).
Short-term outcomes of CI for SSD were compared to those for BCD, CROS aid, and no intervention in a Dutch randomized controlled trial (Cochlear Implantation for siNGLE-sided deafness, CINGLE-trial) involving 120 participants (Peters et al., 2015b, 2021). Peters et al. reported an improvement in speech perception in noise in various signal-to-noise configurations in their CI group. On the other hand speech perception in noise improved or deteriorated for the BCDs and CROS groups depending on the configuration. There was an improvement in sound localization, the tinnitus questionnaire (Goebel and Hiller, 1994; Meeus et al., 2007) scores, a reduction on tinnitus burden detected on the THI (Newman et al., 1996). In general, all treatment options improved disease-specific QoL on most subscales of the patient reported outcome measures that were used (Peters et al., 2021).
A French multi-center prospective study aimed to assess the efficiency of CI in SSD, compared to that of CROS and BCD trials, using a cost-utility analysis (Marx et al., 2019). Initial findings indicate that approximately half of 104 participants opted for a CROS aid, but over one third of participants with SSD were dissatisfied with the CROS and BCD devices, and those that opted for CI experienced more severe handicap and had a poorer QoL than the other groups (Marx et al., 2021b). There was no significant difference between participants that opted for CROS, BCD, CI, or no intervention in terms of etiology of deafness, deafness duration, side of deafness, hearing thresholds in the better ear, or tinnitus severity. When the outcomes of the 51 participants that opted for a CI were considered with regards to generic and auditory-specific QoL, there was significant improvements noted, especially in participants with SSD and associated severe tinnitus (Marx et al., 2021a). The authors acknowledge the small participant number and the short-term follow-up, restricted to 6 months post implantation. A recent study including 20 participants with SSD implanted with CI demonstrated that localization abilities improve with long-term use, with more consistent responses in sound source localization performance at their 5-year visit (Thompson et al., 2022).
A systematic review by Kitterick et al. (2015) included 23 studies examining the impact of hearing-assistive devices on HRQoL in adults with SSD as measured using generic and disease-specific instruments. The average effect size of CROS aids was small and BCD devices was medium, whereas CI had a large effect with a caveat that it included within-subject comparisons of HRQoL before and after implantation.
Auditory brainstem implant
Finally, ABI has also been used as an intervention for SSD, but sporadically (Mueller et al., 2000). The ABI (Figure 10) was specifically designed to bypass both the cochlea and the cochlear nerve to directly stimulate the cochlear nucleus in the brainstem (van den Berge et al., 2019). Therefore, ABIs are suitable in cases of destruction of the cochlear nerve (Schwartz et al., 2008). When compared to no intervention, an ABI can provide a degree of improvement in sound recognition and speech perception to patients who are not CI candidates (Ontario Health, 2020a).
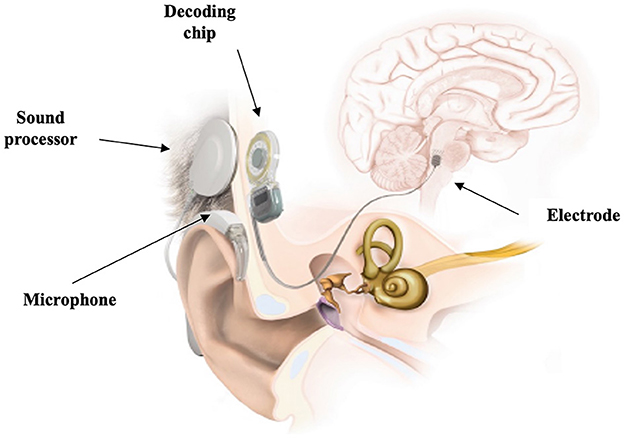
Figure 10. Schematic representation of an auditory brainstem implant. Retrieved from www.medel.com and used with permission from MED-EL.
Cost effectiveness of interventions
Hearing aid and auditory implant cost-effectiveness studies have become increasingly important (Theriou et al., 2019; Neve et al., 2021; Caspers et al., 2022). The device purchasing cost varies from a few hundred pounds for the rerouting hearing aid solutions to ~£20,000 for the restoring implants. A formal cost-effectiveness analysis for BCD devices in a prospective case-control study of 70 pathways found that there was limited data for cost effectiveness calculations for BCD devices (Monksfield et al., 2011). They presented total costs from initial evaluation, surgery, ongoing annual evaluation and maintenance, and processor upgrades after 5 years to the newest model for an estimated life expectancy of the individual patient (Monksfield et al., 2011). The Health Utilities Index (HUI) questionnaire (Horsman et al., 2003) was used in conjunction with life expectancy estimations to derive Quality-Adjusted Life Year (QALY) and Incremental Cost-Effectiveness Ratio (ICER) ratios. There is limited QoL data available for patients living with an osseointegrated implant. As a result, the cost-effectiveness of the osseointegrated implant, compared to conventional hearing aid devices remains unclear (Crowson and Tucci, 2016).
Amin et al. (2021) performed a retrospective case series analysis with a longitudinal economic analysis. They concluded that the mean total cost per patient of the MED-EL middle ear implant was significantly higher than percutaneous BCD at 1-year post-implantation. However, by 5-years post-implantation this difference was no longer statistically significant. Unfortunately, cost-effectiveness evaluations were limited by the lack of usable data on QoL and device usage (Caspers et al., 2022). Based on evidence of moderate quality, cochlear implantation and BCD improve functional and patient-important outcomes in adults and children with SSD (Ontario Health, 2020b). The Ontario health technology assessment report highlighted that among people with SSD, cochlear implantation may be more cost-effective than no intervention, whereas BCDs are unlikely to be although are acceptable to patients who cannot use CI.
Outcome measurement for SSD interventions
Existing literature has highlighted inconsistencies in what benefits and risks (side-effects) are measured when evaluating hearing aid(s) and auditory implant interventions for SSD (Kitterick et al., 2016). The challenge of synthesizing evidence for ENT and audiological interventions from trials, and the importance of utilizing valid measurement instruments that effectively measure the intended audiological outcomes has been highlighted in the case of SSD (Hall et al., 2019). Choosing the appropriate intervention for adults with SSD presents a clinical dilemma (Sin Wai and Chua Wei De, 2021; Underdown and Pryce, 2022).
Researchers investigating SSD intervention outcomes have measured a plethora of outcomes, such as speech understanding in quiet, or speech understanding in the presence of noise (Niparko et al., 2003; Firszt et al., 2012). When assessing speech outcomes in the presence of noise, various configurations are used. For example, Niparko et al. (2003) chose the conditions of (i) noise-front, (ii) noise-to-normal-ear, and (iii) noise-to-deaf-ear to compare BCD and CROS devices, using the Hearing in Noise Test (HINT) (Nilsson et al., 1994) that includes noise filtered to match the long-term average spectrum of sentences. In another example, Arndt et al. (2017) compared speech outcomes in noise with CROS, a BCD device, and CI in three conditions; (i) speech and noise from the front, (ii) speech from the hearing side/noise from the deaf side, and (iii) speech from the deaf side/noise from the hearing side, using the adaptive Oldenburger Sentence Test (OlSa) (Arndt et al., 2011a,b). Härkönen et al. (2015) compared CI outcomes in SSD using a speech-in-noise test that included phonetically balanced bisyllabic Finnish words at a level of 65 dB SPL from the loudspeaker at 0° of azimuth, and unmodulated artificial noise presented from four loudspeakers. Finally, a study assessing the masked speech recognition in 16 participants with SSD and a CI suggest a revised test battery to the Van de Heyning et al. (2017) recommendations to ensure binaural hearing abilities are captured (Anderson et al., 2022). Anderson et al. suggest presentation of the target from the front speaker and the masker co-located with the target, 90° toward the implanted-ear, and 90° toward the normally-hearing-ear, to incorporate real-world situations when the listener often faces the speaker of interest. This selection of studies demonstrates the diversity of test configurations chosen by researchers in the field to demonstrate the benefits and harms of SSD interventions.
The question of what outcome domains are important and relevant to individuals with SSD when deciding whether an intervention works has yet to be addressed. One attempt to harmonize evaluation of SSD interventions was made in 2017; it was based on two discussions among professional experts in CI (Van de Heyning et al., 2017) and was intended for adoption in clinical practice. Recommendations for a minimum set of outcome measures were made including daily device use, pure tone audiometry, free-field testing of speech perception in noise, and sound localization. Recommended instruments were the Speech, Spatial, and Qualities of hearing (SSQ) questionnaire (Noble et al., 2013), the HUI (Horsman et al., 2003) and, if applicable, the Tinnitus Functional Index (TFI) (Meikle et al., 2012). This consensus work by Van de Heyning et al. (2017) focussed on CI as a treatment for SSD and so the expert panels comprised professionals from CI centers. Furthermore, the recommendations included measurement instruments that were readily available in the hearing clinic (e.g., pure tone audiometry, standard audiometric and validated sentence test, binaural effect measures), and there was lack of patient involvement in decision-making. Therefore, it is unclear whether the recommended measures assess outcome domains that are most important or meaningful to patients (e.g., impact on individual's wellbeing, social identity) (Lucas et al., 2018; Underdown and Pryce, 2022). There has been no rigorous scrutiny of outcome reporting for rerouting or restoring interventions, no systematic patient involvement, and no specific consideration of what should be used in clinical trials. Consequently, investigators adopt markedly different methods when assessing the clinical benefit of rerouting and restoring interventions for SSD.
A systematic review and meta-analysis on the use of hearing instruments for SSD in adults has demonstrated ambiguity in the absolute benefit and efficacy of the SSD treatment options (Kitterick et al., 2016). For example, the meta-analysis showed that there was a statistically significant benefit (mean benefit: 2.5 dB) to speech perception in noise for devices that reroute speech signals from the poor-hearing ear to the better-hearing ear using either air or bone conduction. However, rerouting devices also significantly degrade speech understanding (mean deficit: 3.1 dB) when noise gets rerouted from the poor-hearing ear to the better-hearing ear. In relation to sound localization, there was inconsistency in the outcomes chosen by clinical researchers, precluding the synthesis of evidence across studies. Finally, HRQoL was measured in two studies (out of 27 included in the review), and the findings were inconclusive. In summary, Kitterick et al. (2016) concluded that inconsistent measurement of outcomes and observational biases amounted to a low quality of evidence. Kitterick et al. (2016) also concluded that outcome selection was biased toward assessing functional impairments for which measures are readily available and widely used, such as tests of speech perception in noise, with limited measurement of domains that are meaningful to patients such as HRQoL (Lucas et al., 2018). Another systematic review of outcomes of CI in patients with SSD focused on assessing (i) sound localization, (ii) speech perception, (iii) tinnitus, and (iv) QoL outcomes (Cabral Junior et al., 2016). The authors discovered a large variation in choice of outcomes in the included studies, and highlighted the need for high quality studies. Encouragingly, Mertens et al. (2022) have recently developed a consensus classification system for the reporting of sound localization testing results in the field of cochlear implantation. This builds on the Van de Heyning et al. (2017) recommendations, and application of this classification system will allow multi-center studies comparisons and improved meta-analysis in this field (Mertens et al., 2022).
Difficulties that SSD imposes can also affect the individual's psychological and social wellbeing (Carlsson et al., 2011; Sano et al., 2013a; Lucas et al., 2018), and therefore outcomes that assess the impact on an individual's overall health and wellbeing are also relevant and potentially as important (Kitterick et al., 2015). With respect to HRQoL, there is inconsistency in the choice of measurement instruments in trials assessing the benefits of SSD interventions (Kitterick et al., 2016). The authors highlight the need for consist use of patient-reported outcome measures that are sensitive to the impact of devices used by those with SSD, such as the HUI (Horsman et al., 2003). Often, it is unknown what plays a role in decision making and identifying better candidates for specific SSD interventions (Kosaner and Urban, 2014). Qualitative studies demonstrate that patients express uncertainty about choice of treatment options for SSD mainly due to a lack of clarity about their benefit (Underdown and Pryce, 2022), and they seek clinical advice when they need to make a decision. However, due to the varied evidence for different treatment options, clinicians may not know which option is ideal and for whom (Hall et al., 2019).
To address the inconsistency and diversity of outcome measure use in the field of SSD research, the Core Rehabilitation Outcome Set for Single Sided Deafness (CROSSSD) initiative set out to develop a core outcome set for SSD interventions (Katiri et al., 2020, 2022). The CROSSSD initiative developed an international consensus by including opinions of both healthcare users and professionals working in the field. Including a variety of stakeholders in core outcome set development, including the general public, can help demonstrate the impact of interventions on patients' lives (Dodd et al., 2023). The recommended CROSSSD study core outcome set included three outcome domains: spatial orientation, group conversations in noisy social situations, and impact on social situations. Adoption of the CROSSSD core outcome domain set will promote consistent assessment and reporting of outcomes that are meaningful and important to all relevant stakeholders. This consistency will in turn enable comparison of outcomes reported across clinical trials comparing SSD interventions in adults and reduce research waste (Chalmers and Glasziou, 2009; Tunis et al., 2016). The next step entails determining how these outcomes should be measured (Prinsen et al., 2014, 2018; Mokkink et al., 2016). It is important to choose measurement instruments that are comprehensive and sensitive to treatment-related change (Prinsen et al., 2016) as well as inclusive and equitable to ensure they incorporate the diversity of all patients being assessed with the condition of interest (Calvert et al., 2022). Strides are being made to identify available instruments to measure the three outcome domains in the core outcome domain set (Katiri et al., 2022).
Conclusion
SSD in adulthood is most commonly attributed to sudden and idiopathic causes. Although the prevalence and incidence is small, SSD can lead to significant functional, psychological, wellbeing, and social consequences for the individual. A variety of rerouting and restoring interventions have been utilized to date, aiming to alleviate the functional impact of SSD. Outcome measurement in the field of SSD has progressed significantly since the definition and clinical recommendations. Adopting the recently recommended algorithms for measuring sound localization will help with accurate comparison and evidence synthesis for the various SSD treatments. Finally, adoption of the internationally agreed core outcome domain set recommended by the CROSSSD initiative will ensure future clinical trials in the field report on outcomes consistently, and that outcomes that are relevant to both patients and healthcare professionals are reported. Future studies should consider the timescale of measurements, long-term benefits (or harms), and cost utility.
Author contributions
RK was the project administrator who conducted the analysis and drafted the manuscript. RK, KF, and DH were responsible for data curation, visualization, and resource management. KF and DH provided feedback and reviewed previous versions of the manuscript in their role as the PhD supervision team for RK. JP reviewed previous versions of the manuscript as an external examiner for RK's PhD thesis. KF, DH, and JP reviewed the manuscript and provided feedback prior to submission. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Institute for Health Research (NIHR) Nottingham Biomedical Research Centre (BRC), funding reference number BRC-1215-20003. KF was funded by the NIHR and Postdoctoral Fellowship PDF-2018-11-ST2-003. The funding bodies have no role in the study design and implementation, writing the report, or decision to submit the report for publication.
Acknowledgments
The authors would like to acknowledge the contributions of Professor Deborah A. Hall (Heriot-Watt University Malaysia, Putrajaya, Malaysia) and Professor Pádraig T. Kitterick (National Acoustic Laboratories, Sydney, Australia) for their input in designing and conducting a systematic review of outcome domains and instruments used in designs of clinical trials for interventions for single-sided deafness as part of the Core Rehabilitation Outcome Set for Single-Sided Deafness (CROSSSD) study group. Sections of this manuscript were taken from RK's PhD thesis submitted to the University of Nottingham, School of Medicine, in March 2023, which is under embargo until 31st of December 2024.
Conflict of interest
DH declares receiving a grant from Cochlear Europe Ltd outside of the submitted work.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ABI, Auditory Brainstem Implant; AHL, Asymmetric hearing loss; BCD, Bone conduction device; BSA, British Society of Audiology; CI, Cochlear implant; CINGLE, Cochlear Implantation for siNGLE-sided deafness; CNC, Consonant-Nucleus-Consonant; COVID-19, COrona VIrus Disease; CROS, Contralateral Routing of Signals; CROSSSD, Core Rehabilitation Outcome Set for Single-Sided Deafness; ENT, Ear, Nose, Throat; fMRI, functional Magnetic Resonance Imaging; HADS, Hyperacusis questionnaire; HHIA, Hearing Handicap Inventory for Adults; HINT, Hearing in Noise Test; HRQoL, Health-related quality of life; HUI, Health Utilities Index; ICER, Incremental Cost-Effectiveness Ratio; ILD, Interaural level difference; ITD, Interaural time difference; MEI, Middle Ear Implant; NHANES, National Health and Nutrition Examination Survey; NICE, National Institute for Health and Care Excellence; OlSa, Oldenburg Sentence Test; QALY, Quality-Adjusted Life Year; QoL, Quality of Life; SF-36, 36-Item Short Form Survey Instrument; SSD, Single-sided deafness; SSNHL, Sudden sensorineural hearing loss; SSQ, Speech, Spatial and Qualities hearing scale; TFI, Tinnitus Functional Index; THI, Tinnitus Handicap Inventory; US(A), United States (of America); VAS, Visual Analogue Scale.
References
Acke, F. R., Van Hoecke, H., and De Leenheer, E. M. (2021). Congenital unilateral hearing loss: characteristics and etiological analysis in 121 patients. Otol. Neurotol. 42, 1375–1381.
Adams, B., Sereda, M., Casey, A., Byrom, P., Stockdale, D., and Hoare, D. J. (2021). A Delphi survey to determine a definition and description of hyperacusis by clinician consensus. Int. J. Audiol. 60, 607–613. doi: 10.1080/14992027.2020.1855370
Agterberg, M. J. H., Snik, A. F. M., Hol, M. K. S., Van Wanrooij, M. M., and Van Opstal, A. J. (2012). Contribution of monaural and binaural cues to sound localization in listeners with acquired unilateral conductive hearing loss: Improved directional hearing with a bone-conduction device. Hear. Res. 286, 9–18. doi: 10.1016/j.heares.2012.02.012
Agterberg, M. J. H., Snik, A. F. M., Van de Goor, R. M. G., Hol, M. K. S., and Van Opstal, A. J. (2019). Sound-localization performance of patients with single-sided deafness is not improved when listening with a bone-conduction device. Hear. Res. 372, 62–68. doi: 10.1016/j.heares.2018.04.007
Akeroyd, M. A. (2006). The psychoacoustics of binaural hearing. Int. J. Audiol. 45, S25–33. doi: 10.1080/14992020600782626
Alexander, T. H., and Harris, J. P. (2013). Incidence of sudden sensorineural hearing loss. Otol. Neurotol. 34, 1586–1589. doi: 10.1097/MAO.0000000000000222
Alhanbali, S., Dawes, P., Lloyd, S., and Munro, K. J. (2017). Self-reported listening-related effort and fatigue in hearing-impaired adults. Ear Hear. 38, e39–e48. doi: 10.1097/AUD.0000000000000361
Alzaher, M., Vannson, N., Deguine, O., Marx, M., Barone, P., and Strelnikov, K. (2021). Brain plasticity and hearing disorders. Rev. Neurol. 177, 1121–1132. doi: 10.1016/j.neurol.2021.09.004
Amin, N., Soulby, A. J., Borsetto, D., and Pai, I. (2021). Longitudinal economic analysis of Bonebridge 601 versus percutaneous bone-anchored hearing devices over a 5-year follow-up period. Clin. Otolaryngol. 46, 263–272. doi: 10.1111/coa.13659
Anderson, K. M., Buss, E., Rooth, M. A., Richter, M. E., Overton, A. B., Brown, K. D., et al. (2022). Masked speech recognition as a function of masker location for cochlear implant users with single-sided deafness. Am. J. Audiol. 31, 757–763. doi: 10.1044/2022_AJA-21-00268
Araújo, P. G. V., de Mondelli, M. F. C. G., Lauris, J. R. P., Richiéri-Costa, A., and Feniman, M. R. (2010). Assessment of the auditory handicap in adults with unilateral hearing loss. Braz. J. Otorhinolaryngol. 76, 378–383. doi: 10.1590/S1808-86942010000300018
Arndt, S., Aschendorff, A., Laszig, R., Beck, R., Schild, C., Kroeger, S., et al. (2011a). Comparison of pseudobinaural hearing to real binaural hearing rehabilitation after cochlear implantation in patients with unilateral deafness and tinnitus. Otol. Neurotol. 32, 39–47. doi: 10.1097/MAO.0b013e3181fcf271
Arndt, S., Laszig, R., Aschendorff, A., Beck, R., Schild, C., Hassepass, F., et al. (2011b). Unilateral deafness and cochlear implantation: audiological diagnostic evaluation and outcomes. HNO 59, 437–446. doi: 10.1007/s00106-011-2318-8
Arndt, S., Laszig, R., Aschendorff, A., Hassepass, F., Beck, R., and Wesarg, T. (2017). Cochlear implant treatment of patients with single-sided deafness or asymmetric hearing loss. HNO 65, 586–598. doi: 10.1007/s00106-016-0294-8
Arndt, S., Rauch, A. K., and Speck, I. (2021). Active transcutaneous bone-anchored hearing implant: how I do it. Eur. Arch. Otorhinolaryngol. 278, 4119–4122. doi: 10.1007/s00405-021-06946-8
Arslan, F., Aydemir, E., Kaya, Y. S., Arslan, H., and Durmaz, A. (2018). Anxiety and depression in patients with sudden one-sided hearing loss. Ear Nose Throat J. 97, E7–E10. doi: 10.1177/0145561318097010-1101
Asfour, L., Kay-Rivest, E., and Roland, J. T. J. (2021). Cochlear implantation for single-sided deafness after COVID-19 hospitalization. Cochlear Implants Int. 22, 353–357. doi: 10.1080/14670100.2021.1936364
Avan, P., Giraudet, F., and Büki, B. (2015). Importance of binaural hearing. Audiol. Neurootol. 20(Suppl. 1), 3–6. doi: 10.1159/000380741
Baguley, D. M., Bird, J., Humphriss, R. L., and Prevost, A. T. (2006). The evidence base for the application of contralateral bone anchored hearing aids in acquired unilateral sensorineural hearing loss in adults. Clin. Otolaryngol. 31, 6–14. doi: 10.1111/j.1749-4486.2006.01137.x
Baguley, D. M., and Hoare, D. J. (2018). Hyperacusis: major research questions. HNO 66, 358–363. doi: 10.1007/s00106-017-0464-3
Barker, A. B., Leighton, P., and Ferguson, M. A. (2017). Coping together with hearing loss: a qualitative meta-synthesis of the psychosocial experiences of people with hearing loss and their communication partners. Int. J. Audiol. 56, 297–305. doi: 10.1080/14992027.2017.1286695
Bianchin, G., Bonali, M., Russo, M., and Tribi, L. (2015). Active bone conduction system: outcomes with the Bonebridge transcutaneous device. ORL 77, 17–26. doi: 10.1159/000371425
Bigelow, R. T., Semenov, Y. R., du Lac, S., Hoffman, H. J., and Agrawal, Y. (2016). Vestibular vertigo and comorbid cognitive and psychiatric impairment: the 2008 national health interview survey. J. Neurol. Neurosurg. Psychiatr. 87, 367–372. doi: 10.1136/jnnp-2015-310319
Bilecen, D., Seifritz, E., Radü, E. W., Schmid, N., Wetzel, S., Probst, R., et al. (2000). Cortical reorganization after acute unilateral hearing loss traced by fMRI. Neurology 54, 765–767. doi: 10.1212/WNL.54.3.765
Bird, P. A., and Bergin, M. J. (2018). Pharmacological issues in hearing rehabilitation. Adv. Otorhinolaryngol. 81, 114–122. doi: 10.1159/000485541
Briggs, R., Birman, C. S., Baulderstone, N., Lewis, A. T., Ng, I. H. Y., Östblom, A., et al. (2022). Clinical performance, safety, and patient-reported outcomes of an active osseointegrated steady-state implant system. Otol. Neurotol. 43, 827–834. doi: 10.1097/MAO.0000000000003590
BSA (2018). Recommended Procedure: Pure-Tone Air-Conduction and Bone-Conduction Threshold Audiometry With and Without Masking. Available online at: https://www.thebsa.org.uk/wp-content/uploads/2018/11/OD104-32-Recommended-Procedure-Pure-Tone-Audiometry-August-2018-FINAL.pdf (accessed December 3, 2022).
Buechner, A., Brendel, M., Lesinski-Schiedat, A., Wenzel, G., Frohne-Buechner, C., Jaeger, B., et al. (2010). Cochlear implantation in unilateral deaf subjects associated with ipsilateral tinnitus. Otol. Neurotol. 31, 1381–1385. doi: 10.1097/MAO.0b013e3181e3d353
BusinessWire (2022). Soundbite Medical Enters Into a License Agreement With VFLO Medical to Bring Its Products to Greater China. Available online at: https://www.businesswire.com/news/home/20221115005948/en/Soundbite-Medical-Enters-Into-A-License-Agreement-With-VFLO-Medical-To-Bring-Its-Products-To-Greater-China (accessed June 4, 2023).
Cabral Junior, F., Hausen Pinna, M., Dourado Alves, R., dos Santos Malerbi, A. F., and Ferreira Bento, R. (2016). Cochlear implantation and single-sided deafness: a systematic review of the literature. Int. Arch. Otorhinolaryngol. 20, 69–75. doi: 10.1055/s-0035-1559586
Calvert, M. J., Cruz Rivera, S., Retzer, A., Hughes, S. E., Campbell, L., Molony-Oates, B., et al. (2022). Patient reported outcome assessment must be inclusive and equitable. Nat. Med. 28, 1120–1124. doi: 10.1038/s41591-022-01781-8
Carlsson, P.-I., Hall, M., Lind, K.-J., and Danermark, B. (2011). Quality of life, psychosocial consequences, and audiological rehabilitation after sudden sensorineural hearing loss. Int. J. Audiol. 50, 139–144. doi: 10.3109/14992027.2010.533705
Caspers, C. J. I., Nelissen, R. C., Groenewoud, H. J. M. M., and Hol, M. K. S. (2022). Hearing-related quality of life in 75 patients with a percutaneous bone conduction device. Otol. Neurotol. 43, 345–351. doi: 10.1097/MAO.0000000000003442
Chalmers, I., and Glasziou, P. (2009). Avoidable waste in the production and reporting of research evidence. Lancet 374, 86–89. doi: 10.1016/S0140-6736(09)60329-9
Chandrasekhar, S. S., Tsai Do, B. S., Schwartz, S. R., Bontempo, L. J., Faucett, E. A., Finestone, S. A., et al. (2019). Clinical practice guideline: sudden hearing loss (update). Otolaryngol. Head Neck Surg. 161(1_suppl), S1–S45. doi: 10.1177/0194599819859885
Chang, P. F., Zhang, F., and Schaaf, A. J. (2020). Deaf in one ear: communication and social challenges of patients with single-sided deafness post-diagnosis. Patient Educ. Couns. 103, 530–536. doi: 10.1016/j.pec.2019.10.009
Chau, J. K., Lin, J. R. J., Atashband, S., Irvine, R. A., and Westerberg, B. D. (2010). Systematic review of the evidence for the etiology of adult sudden sensorineural hearing loss. Laryngoscope 120, 1011–1021. doi: 10.1002/lary.20873
Chiossoine-Kerdel, J. A., Baguley, D. M., Stoddart, R. L., and Moffat, D. A. (2000). An investigation of the audiologic handicap associated with unilateral sudden sensorineural hearing loss. Am. J. Otol. 21, 645–651.
Choi, J. S., Wu, F., Park, S., Friedman, R. A., Kari, E., and Volker, C. C. J. (2021). Factors associated with unilateral hearing loss and impact on communication in US adults. Otolaryngol. Head Neck Surg. 165, 868–875. doi: 10.1177/0194599821995485
Colletti, V., Fiorino, F. G., Carner, M., and Rizzi, R. (1988). Investigation of the long-term effects of unilateral hearing loss in adults. Br. J. Audiol. 22, 113–118. doi: 10.3109/03005368809077805
Crowson, M. G., and Tucci, D. L. (2016). Mini review of the cost-effectiveness of unilateral osseointegrated implants in adults: possibly cost-effective for the correct indication. Audiol. Neurootol. 21, 69–71. doi: 10.1159/000443629
Daniels, R. L., Swallow, C., Shelton, C., Davidson, H. C., Krejci, C. S., and Harnsberger, H. R. (2000). Causes of unilateral sensorineural hearing loss screened by high-resolution fast spin echo magnetic resonance imaging: review of 1,070 consecutive cases. Am. J. Otol. 21, 173–180. doi: 10.1016/S0196-0709(00)80005-8
Dawes, P., Fortnum, H., Moore, D. R., Emsley, R., Norman, P., Cruickshanks, K., et al. (2014). Hearing in middle age: a population snapshot of 40- to 69-year olds in the United Kingdom. Ear Hear. 35, e44–e51. doi: 10.1097/AUD.0000000000000010
De Ridder, D., Schlee, W., Vanneste, S., Londero, A., Weisz, N., Kleinjung, T., et al. (2021). Tinnitus and tinnitus disorder: theoretical and operational definitions (an international multidisciplinary proposal). Prog. Brain Res. 260, 1–25. doi: 10.1016/bs.pbr.2020.12.002
de Wit, M., and Hajos, T. (2013). “Health-related quality of life,” in Encyclopedia of Behavioral Medicine, eds M. D. Gellman, and J. R. Turner (New York, NY: Springer), 929–931.
Deep, N. L., Kay-Rivest, E., and Roland, J. T. J. (2021). Iatrogenic third window after retrosigmoid approach to a vestibular schwannoma managed with cochlear implantation. Otol. Neurotol. 42, 1355–1359. doi: 10.1097/MAO.0000000000003267
Desmet, J., Wouters, K., De Bodt, M., and Van de Heyning, P. (2014). Long-term subjective benefit with a bone conduction implant sound processor in 44 patients with single-sided deafness. Otol. Neurotol. 35, 1017–1025. doi: 10.1097/MAO.0000000000000297
Dewyer, N. A., Smith, S., Herrmann, B., Reinshagen, K. L., and Lee, D. J. (2022). Pediatric single-sided deafness: a review of prevalence, radiologic findings, and cochlear implant candidacy. Ann. Otol. Rhinol. Laryngol. 131, 233–238. doi: 10.1177/00034894211019519
Dodd, S., Gorst, S. L., Young, A., Lucas, S. W., and Williamson, P. R. (2023). Patient participation impacts outcome domain selection in core outcome sets for research: an updated systematic review. J. Clin. Epidemiol. 158, 127–133. doi: 10.1016/j.jclinepi.2023.03.022
Douglas, S. A., Yeung, P., Daudia, A., Gatehouse, S., and O'Donoghue, G. M. (2007). Spatial hearing disability after acoustic neuroma removal. Laryngoscope 117, 1648–1651. doi: 10.1097/MLG.0b013e3180caa162
Dwyer, N. Y., Firszt, J. B., and Reeder, R. M. (2014). Effects of unilateral input and mode of hearing in the better ear: self-reported performance using the speech, spatial and qualities of hearing scale. Ear Hear. 35, 126–136. doi: 10.1097/AUD.0b013e3182a3648b
Eggermont, J. J., and Roberts, L. E. (2012). The neuroscience of tinnitus: understanding abnormal and normal auditory perception. Front. Syst. Neurosci. 6, 53. doi: 10.3389/fnsys.2012.00053
Ekobena, P., Rothuizen, L. E., Bedussi, F., Guilcher, P., Meylan, S., Ceschi, A., et al. (2022). Four cases of audio-vestibular disorders related to immunisation with SARS-CoV-2 mRNA vaccines. Int. J. Audiol. 62, 587–591. doi: 10.1080/14992027.2022.2056721
Fackrell, K., Stratmann, L., Gronlund, T. A., and Hoare, D. J. (2019). Top ten hyperacusis research priorities in the UK. Lancet 393, 404–405. doi: 10.1016/S0140-6736(18)32616-3
Farlex Partner Medical Dictionary (2012). Quality of Life. Available online at: https://medical-dictionary.thefreedictionary.com/wellbeing (accessed October 22, 2022).
Firszt, J. B., Holden, L. K., Reeder, R. M., Waltzman, S. B., and Arndt, S. (2012). Auditory abilities after cochlear implantation in adults with unilateral deafness: a pilot study. Otol. Neurotol. 33, 1339–1346. doi: 10.1097/MAO.0b013e318268d52d
Firszt, J. B., Reeder, R. M., and Holden, L. K. (2017). Unilateral hearing loss: Understanding speech recognition and localization variability-implications for cochlear implant candidacy. Ear Hear. 38, 159–173. doi: 10.1097/AUD.0000000000000380
Fitzpatrick, E. M., Al-Essa, R. S., Whittingham, J. A., and Fitzpatrick, J. (2017). Characteristics of children with unilateral hearing loss. Int. J. Audiol. 56, 819–828. doi: 10.1080/14992027.2017.1337938
Fukuhara, S., Bito, S., Green, J., Hsiao, A., and Kurokawa, K. (1998). Translation, adaptation, and validation of the SF-36 Health Survey for use in Japan. J. Clin. Epidemiol. 51, 1037–1044. doi: 10.1016/S0895-4356(98)00095-X
Gallun, F. J. (2021). Impaired binaural hearing in adults: a selected review of the literature. Front. Neurosci. 15, 610957. doi: 10.3389/fnins.2021.610957
García-Berrocal, J. R., Górriz, C., Ramírez-Camacho, R., Trinidad, A., Ibáñez, A., Rodríguez Valiente, A., et al. (2006). Otosyphilis mimics immune disorders of the inner ear. Acta Otolaryngol. 126, 679–684. doi: 10.1080/00016480500491994
Gatehouse, S., and Noble, W. (2004). The speech, spatial and qualities of hearing scale (SSQ). Int. J. Audiol. 43, 85–99. doi: 10.1080/14992020400050014
Gerdes, T., Salcher, R. B., Schwab, B., Lenarz, T., and Maier, H. (2016). Comparison of audiological results between a transcutaneous and a percutaneous bone conduction instrument in conductive hearing loss. Otol. Neurotol. 37, 685–691. doi: 10.1097/MAO.0000000000001010
Ghogomu, N., Umansky, A., and Lieu, J. E. C. (2014). Epidemiology of unilateral sensorineural hearing loss with universal newborn hearing screening. Laryngoscope 124, 295–300. doi: 10.1002/lary.24059
Giolas, T. G., and Wark, D. J. (1967). Communication problems associated with unilateral hearing loss. J. Speech Hear. Disord. 32, 336–343. doi: 10.1044/jshd.3204.336
Goebel, G., and Hiller, W. (1994). The tinnitus questionnaire. A standard instrument for grading the degree of tinnitus. Results of a multicenter study with the tinnitus questionnaire. HNO 42, 166–172.
Goman, A. M., Gao, T., Betz, J., Reed, N. S., Deal, J. A., and Lin, F. R. (2021). Association of hearing loss with physical, social, and mental activity engagement. Semin. Hear. 42, 59–65. doi: 10.1055/s-0041-1726001
Goycoolea, M., Ribalta, G., Tocornal, F., Levy, R., Alarcón, P., Bryman, M., et al. (2020). Clinical performance of the OsiaTM system, a new active osseointegrated implant system. Results from a prospective clinical investigation. Acta Otolaryngol. 140, 212–219. doi: 10.1080/00016489.2019.1691744
Greene, J., and Al-Dhahir, M. A. (2022). Acoustic Neuroma. Available online at: https://www.ncbi.nlm.nih.gov/books/NBK470177/ (accessed December 3, 2022).
Güldner, C., Heinrichs, J., Weiß, R., Zimmermann, A. P., Dassinger, B., Bien, S., et al. (2013). Visualisation of the Bonebridge by means of CT and CBCT. Eur. J. Med. Res. 18, 30. doi: 10.1186/2047-783X-18-30
Gurgel, R. K., and Shelton, C. (2013). The SoundBite hearing system: patient-assessed safety and benefit study. Laryngoscope 123, 2807–2812. doi: 10.1002/lary.24091
Håkansson, B., Reinfeldt, S., Persson, A.-C., Jansson, K.-J. F., Rigato, C., Hultcrantz, M., et al. (2019). The bone conduction implant - a review and 1-year follow-up. Int. J. Audiol. 58, 945–955. doi: 10.1080/14992027.2019.1657243
Hadley, L. V., Whitmer, W. M., Brimijoin, W. O., and Naylor, G. (2021). Conversation in small groups: speaking and listening strategies depend on the complexities of the environment and group. Psychon. Bull. Rev. 28, 632–640. doi: 10.3758/s13423-020-01821-9
Hall, D. A., Kitterick, P. T., Heffernan, E., Fackrell, K., Lucas, L., and Ferguson, M. (2019). How do we know that our patients have benefitted from our ENT/Audiological interventions? Presented at the annual meeting of ADANO 2016 in Berlin. Otol. Neurotol. 40, e474–e481. doi: 10.1097/MAO.0000000000001937
Härkönen, K., Kivekas, I., Rautiainen, M., Kotti, V., Sivonen, V., and Vasama, J.-P. (2015). Single-sided deafness: the effect of cochlear implantation on quality of life, quality of hearing, and working performance. ORL 77, 339–345. doi: 10.1159/000439176
Hawley, M. L., Litovsky, R. Y., and Culling, J. F. (2004). The benefit of binaural hearing in a cocktail party: effect of location and type of interferer. J. Acoust. Soc. Am. 115, 833–843. doi: 10.1121/1.1639908
Heffernan, E., Withanachchi, C. M., and Ferguson, M. A. (2022). ‘The worse my hearing got, the less sociable I got': a qualitative study of patient and professional views of the management of social isolation and hearing loss. Age Ageing 51, afac019. doi: 10.1093/ageing/afac019
Herráiz, C., de los Santos, G., Diges, I., Díez, R., and Aparicio, J. M. (2006). Assessment of hyperacusis: the self-rating questionnaire on hypersensitivity to sound. Acta Otorrinolaringol. Esp. 57, 303–306. doi: 10.1016/S0001-6519(06)78716-7
Hétu, R., Jones, L., and Getty, L. (1993). The impact of acquired hearing impairment on intimate relationships: implications for rehabilitation. Audiology 32, 363–381. doi: 10.3109/00206099309071867
Hilber, P. (2022). The role of the cerebellar and vestibular networks in anxiety disorders and depression: the internal model hypothesis. Cerebellum 21, 791–800. doi: 10.1007/s12311-022-01400-9
Hol, M. K. S., Bosman, A. J., Snik, A. F. M., Mylanus, E. A. M., and Cremers, C. W. R. J. (2005). Bone-anchored hearing aids in unilateral inner ear deafness: an evaluation of audiometric and patient outcome measurements. Otol. Neurotol. 26, 999–1006. doi: 10.1097/01.mao.0000185065.04834.95
Holman, J. A., Ali, Y. H. K., and Naylor, G. (2022). A qualitative investigation of the hearing and hearing-aid related emotional states experienced by adults with hearing loss. Int. J. Audiol. 1–10. doi: 10.1080/14992027.2022.2111373
Holman, J. A., Drummond, A., Hughes, S. E., and Naylor, G. (2019). Hearing impairment and daily-life fatigue: a qualitative study. Int. J. Audiol. 58, 408–416. doi: 10.1080/14992027.2019.1597284
Holman, J. A., Drummond, A., and Naylor, G. (2021a). Hearing aids reduce daily-life fatigue and increase social activity: a longitudinal study. Trends Hear. 25, 23312165211052784. doi: 10.1177/23312165211052786
Holman, J. A., Hornsby, B. W. Y., Bess, F. H., and Naylor, G. (2021b). Can listening-related fatigue influence well-being? Examining associations between hearing loss, fatigue, activity levels and well-being. Int. J. Audiol. 60, 47–59. doi: 10.1080/14992027.2020.1853261
Hornsby, B. W. Y., Naylor, G., and Bess, F. H. (2016). A taxonomy of fatigue concepts and their relation to hearing loss. Ear Hear. 37, 136S−44S. doi: 10.1097/AUD.0000000000000289
Horsman, J., Furlong, W., Feeny, D., and Torrance, G. (2003). The Health Utilities Index (HUI): concepts, measurement properties and applications. Health Qual. Life Outcomes 1, 54. doi: 10.1186/1477-7525-1-54
Hoth, S., Rösli-Khabas, M., Herisanu, I., Plinkert, P. K., and Praetorius, M. (2016). Cochlear implantation in recipients with single-sided deafness: audiological performance. Coch. Implants Int. 17, 190–199. doi: 10.1080/14670100.2016.1176778
Huang, A. R., Deal, J. A., Rebok, G. W., Pinto, J. M., Waite, L., and Lin, F. R. (2021). Hearing impairment and loneliness in older adults in the United States. J. Appl. Gerontol. 40, 1366–1371. doi: 10.1177/0733464820944082
Huttunen, K., Erixon, E., Löfkvist, U., and Mäki-Torkko, E. (2019). The impact of permanent early-onset unilateral hearing impairment in children - a systematic review. Int. J. Pediatr. Otorhinolaryngol. 120, 173–183. doi: 10.1016/j.ijporl.2019.02.029
Hwa, T. P., Locketz, G., and Ruckenstein, M. J. (2022). Novel surgical technique in active bone conduction: Minimally invasive approach to fully implantable osseointegrated implant. Otolaryngol. Head Neck Surg. 167, 206–208. doi: 10.1177/01945998211044408
Idriss, S. A., Reynard, P., Marx, M., Mainguy, A., Joly, C.-A., Ionescu, E. C., et al. (2022). Short- and long-term effect of cochlear implantation on disabling tinnitus in single-sided deafness patients: a systematic review. J. Clin. Med. 11, 5664. doi: 10.3390/jcm11195664
Iwasaki, S. (2022). Advances in auditory implants. Auris Nasus Larynx 50, 321–326. doi: 10.1016/j.anl.2022.09.003
Jia, H., Nguyen, Y., Hochet, B., Smail, M., Mosnier, I., Wu, H., et al. (2020). NF2-related intravestibular schwannomas: long-term outcomes of cochlear implantation. Otol. Neurotol. 41, 94–99. doi: 10.1097/MAO.0000000000002431
Karoui, C., Strelnikov, K., Payoux, P., Salabert, A.-S., James, C. J., Deguine, O., et al. (2022). Auditory cortical plasticity after cochlear implantation in asymmetric hearing loss is related to spatial hearing: a PET H215O study. Cereb. Cortex. 33, 2229–2244. doi: 10.1093/cercor/bhac204
Katiri, R., Hall, D. A., Buggy, N., Hogan, N., Horobin, A., Van de Heyning, P., et al. (2020). Core Rehabilitation Outcome Set for Single Sided Deafness (CROSSSD) study: protocol for an international consensus on outcome measures for single sided deafness interventions using a modified Delphi survey. Trials 21, 238. doi: 10.1186/s13063-020-4094-9
Katiri, R., Hall, D. A., Hoare, D. J., Fackrell, K., Horobin, A., Hogan, N., et al. (2022). The Core Rehabilitation Outcome Set for Single-Sided Deafness (CROSSSD) study: international consensus on outcome measures for trials of interventions for adults with single-sided deafness. Trials 23, 764. doi: 10.1186/s13063-022-06702-1
Katiri, R., Hall, D. A., Killan, C. F., Smith, S., Prayuenyong, P., and Kitterick, P. T. (2021). Systematic review of outcome domains and instruments used in designs of clinical trials for interventions that seek to restore bilateral and binaural hearing in adults with unilateral severe to profound sensorineural hearing loss ('single-sided deafness'). Trials 22, 220. doi: 10.1186/s13063-021-05160-5
Kay-Rivest, E., Irace, A. L., Golub, J. S., and Svirsky, M. A. (2021). Prevalence of single-sided deafness in the United States. Laryngoscope 132, 1652–1656. doi: 10.1002/lary.29941
Khalfa, S., Dubal, S., Veuillet, E., Perez-Diaz, F., Jouvent, R., and Collet, L. (2002). Psychometric normalization of a hyperacusis questionnaire. Orl. 64, 436–442. doi: 10.1159/00067570
Kitoh, R., Nishio, S.-Y., and Usami, S.-I. (2022). Speech perception in noise in patients with idiopathic sudden hearing loss. Acta Otolaryngol. 142, 302–307. doi: 10.1080/00016489.2022.2059565
Kitterick, P. T., Lucas, L., and Smith, S. N. (2015). Improving health-related quality of life in single-sided deafness: a systematic review and meta-analysis. Audiol. Neurootol. 20, 79–86. doi: 10.1159/000380753
Kitterick, P. T., O'Donoghue, G. M., Edmondson-Jones, M., Marshall, A., Jeffs, E., Craddock, L., et al. (2014). Comparison of the benefits of cochlear implantation versus contra-lateral routing of signal hearing aids in adult patients with single-sided deafness: study protocol for a prospective within-subject longitudinal trial. BMC Ear Nose Throat Disord. 14, 7. doi: 10.1186/1472-6815-14-7
Kitterick, P. T., Smith, S. N., and Lucas, L. (2016). Hearing instruments for unilateral severe-to-profound sensorineural hearing loss in adults: a systematic review and meta-analysis. Ear Hear. 37, 495–507. doi: 10.1097/AUD.0000000000000313
Kong, T. H., Lee, J., Kwak, C., Han, W., Gwon, O.-H., and Seo, Y. J. (2021). Audiological benefits and performance improvements of Baha® attract implantation in patients with unilateral hearing loss. Coch. Implants Int. 22, 270–282. doi: 10.1080/14670100.2021.1903713
Kosaner, M., and Urban, M. (2014). The decision making process in receiving Bone Conduction Implants (BCI) for single sided deafness. Value Health 17, A611. doi: 10.1016/j.jval.2014.08.2143
Koumpa, F. S., Forde, C. T., and Manjaly, J. G. (2020). Sudden irreversible hearing loss post COVID-19. BMJ Case Rep. 13. doi: 10.1136/bcr-2020-238419
Kruyt, I. J., Monksfield, P., Skarzynski, P. H., Green, K., Runge, C., Bosman, A., et al. (2020). Results of a 2-year prospective multicenter study evaluating long-term audiological and clinical outcomes of a transcutaneous implant for bone conduction hearing. Otol. Neurotol. 41, 901–911. doi: 10.1097/MAO.0000000000002689
Kuo, P.-L., Di, J., Ferrucci, L., and Lin, F. R. (2021). Analysis of hearing loss and physical activity among US adults aged 60-69 years. JAMA Netw. Open 4, e215484. doi: 10.1001/jamanetworkopen.2021.5484
Kuppler, K., Lewis, M., and Evans, A. K. (2013). A review of unilateral hearing loss and academic performance: is it time to reassess traditional dogmata? Int. J. Pediatr. Otorhinolaryngol. 77, 617–622. doi: 10.1016/j.ijporl.2013.01.014
Laske, R. D., Röösli, C., Pfiffner, F., Veraguth, D., and Huber, A. M. (2015). Functional results and subjective benefit of a transcutaneous bone conduction device in patients with single-sided deafness. Otol. Neurotol. 36, 1151–1156. doi: 10.1097/MAO.0000000000000791
Lassaletta, L., Calvino, M., Zernotti, M., and Gavilan, J. (2016). Postoperative pain in patients undergoing a transcutaneous active bone conduction implant (Bonebridge). Eur. Arch. Otorhinolaryngol. 273, 4103–4110. doi: 10.1007/s00405-016-3972-y
Lawrence, R., and Thevasagayam, R. (2015). Controversies in the management of sudden sensorineural hearing loss: an evidence-based review. Clin. Otolaryngol. 40, 176–182. doi: 10.1111/coa.12363
Lee, H., and Baloh, R. W. (2005). Sudden deafness in vertebrobasilar ischemia: clinical features, vascular topographical patterns and long-term outcome. J. Neurol. Sci. 228, 99–104. doi: 10.1016/j.jns.2004.10.016
Legris, E., Galvin, J., Roux, S., Gomot, M., Aoustin, J. M., Marx, M., et al. (2018). Cortical reorganization after cochlear implantation for adults with single-sided deafness. PLoS ONE 13, e0204402. doi: 10.1371/journal.pone.0204402
Leterme, G., Bernardeschi, D., Bensemman, A., Coudert, C., Portal, J. J., Ferrary, E., et al. (2015). Contralateral routing of signal hearing aid versus transcutaneous bone conduction in single-sided deafness. Audiol. Neurootol. 20, 251–260. doi: 10.1159/000381329
Levitt, H., and Rabiner, L. R. (1967). Predicting binaural gain in intelligibility and release from masking for speech. J. Acoust. Soc. Am. 42, 820–829. doi: 10.1121/1.1910654
Levy, D. A., Lee, J. A., Nguyen, S. A., McRackan, T. R., Meyer, T. A., and Lambert, P. R. (2020). Cochlear implantation for treatment of tinnitus in single-sided deafness: a systematic review and meta-analysis. Otol. Neurotol. 41, e1004–e1012. doi: 10.1097/MAO.0000000000002711
Li, G., You, D., Ma, J., Li, W., Li, H., and Sun, S. (2018). The role of autoimmunity in the pathogenesis of sudden sensorineural hearing loss. Neural Plast. 2018, 7691473. doi: 10.1155/2018/7691473
Lieu, J. E. C., Tye-Murray, N., Karzon, R. K., and Piccirillo, J. F. (2010). Unilateral hearing loss is associated with worse speech-language scores in children. Pediatrics 125, e1348–e1355. doi: 10.1542/peds.2009-2448
Lin, L.-M., Bowditch, S., Anderson, M. J., May, B., Cox, K. M., and Niparko, J. K. (2006). Amplification in the rehabilitation of unilateral deafness: speech in noise and directional hearing effects with bone-anchored hearing and contralateral routing of signal amplification. Otol. Neurotol. 27, 172–182. doi: 10.1097/01.mao.0000196421.30275.73
Lindquist, N. R., Holder, J. T., Patro, A., Cass, N. D., Tawfik, K. O., O'Malley, M. R., et al. (2022). Cochlear implants for single-sided deafness: quality of life, daily usage, and duration of deafness. Laryngoscope. doi: 10.1002/lary.30452
Liu, Y. W., Cheng, X., Chen, B., Peng, K., Ishiyama, A., and Fu, Q. J. (2018). Effect of tinnitus and duration of deafness on sound localization and speech recognition in noise in patients With single-sided deafness. Trends Hear. 22, 2331216518813802. doi: 10.1177/2331216518813802
Lopez, E. M., Dillon, M. T., Park, L. R., Rooth, M. A., Richter, M. E., Thompson, N. J., et al. (2021). Influence of cochlear implant use on perceived listening effort in adult and pediatric cases of unilateral and asymmetric hearing loss. Otol. Neurotol. 42, e1234–e1241. doi: 10.1097/MAO.0000000000003261
Lucas, L., Katiri, R., and Kitterick, P. T. (2018). The psychological and social consequences of single-sided deafness in adulthood. Int. J. Audiol. 57, 21–30. doi: 10.1080/14992027.2017.1398420
Luo, Q., Shen, Y., Chen, T., Zheng, Z., Shi, H., Feng, Y., et al. (2020). Effects of SoundBite bone conduction hearing aids on speech recognition and quality of life in patients with single-sided deafness. Neural Plast. 2020, 4106949. doi: 10.1155/2020/4106949
Maier, H., Lenarz, T., Agha-Mir-Salim, P., Agterberg, M. J. H., Anagiotos, A., Arndt, S., et al. (2022). Consensus statement on bone conduction devices and active middle ear implants in conductive and mixed hearing loss. Otol. Neurotol. 43, 513–529. doi: 10.1097/MAO.0000000000003491
Marx, M., Costa, N. N., Lepage, B., Taoui, S., Molinier, L., Deguine, O., et al. (2019). Cochlear implantation as a treatment for single-sided deafness and asymmetric hearing loss: a randomized controlled evaluation of cost-utility. BMC Ear Nose Throat Disord. 19, 1. doi: 10.1186/s12901-019-0066-7
Marx, M., Mosnier, I., Venail, F., Mondain, M., Uziel, A., Bakhos, D., et al. (2021a). Cochlear implantation and other treatments in single-sided deafness and asymmetric hearing loss: results of a national multicenter study including a randomized controlled trial. Audiol. Neurootol. 26, 414–424. doi: 10.1159/000514085
Marx, M., Mosnier, I., Vincent, C., Bonne, N., Bakhos, D., Lescanne, E., et al. (2021b). Treatment choice in single-sided deafness and asymmetric hearing loss. a prospective, multicentre cohort study on 155 patients. Clin. Otolaryngol. 46, 736–743. doi: 10.1111/coa.13672
McCabe, B. F. (1979). Autoimmune sensorineural hearing loss. Ann. Otol. Rhinol. Laryngol. 88 (5 Pt 1), 585–589. doi: 10.1177/000348947908800501
McGarrigle, R., Munro, K. J., Dawes, P., Stewart, A. J., Moore, D. R., Barry, J. G., et al. (2014). Listening effort and fatigue: what exactly are we measuring? A British Society of Audiology Cognition in Hearing Special Interest Group “white paper”. Int. J. Audiol. 53, 433–440. doi: 10.3109/14992027.2014.890296
McLeod, B., Upfold, L., and Taylor, A. (2008). Self reported hearing difficulties following excision of vestibular schwannoma. Int. J. Audiol. 47, 420–430. doi: 10.1080/14992020802033083
Meehan, S., Hough, E. A., Crundwell, G., Knappett, R., Smith, M., and Baguley, D. M. (2017). The impact of single-sided deafness upon music appreciation. J. Am. Acad. Audiol. 28, 444–462. doi: 10.3766/jaaa.16063
Meeus, O., Blaivie, C., and Van de Heyning, P. (2007). Validation of the Dutch and the French version of the Tinnitus Questionnaire. B-ENT 3(Suppl. 7), 11–17.
Meikle, M. B., Henry, J. A., Griest, S. E., Stewart, B. J., Abrams, H. B., McArdle, R., et al. (2012). The Tinnitus Functional Index: development of a new clinical measure for chronic, intrusive tinnitus. Ear Hear. 33, 153–176. doi: 10.1097/AUD.0b013e31822f67c0
Mertens, G., Andries, E., Kurz, A., Tȧvora-Vieira, D., Calvino, M., Amann, E., et al. (2022). Towards a consensus on an ICF-based classification system for horizontal sound-source localization. J. Pers. Med. 12, 1971. doi: 10.3390/jpm12121971
Mertens, G., De Bodt, M., and Van de Heyning, P. (2016). Cochlear implantation as a long-term treatment for ipsilateral incapacitating tinnitus in subjects with unilateral hearing loss up to 10 years. Hear. Res. 331, 1–6. doi: 10.1016/j.heares.2015.09.016
Mertens, G., Gilles, A., Bouzegta, R., and Van de Heyning, P. (2018). A prospective randomized crossover study in single sided deafness on the new non-invasive adhesive bone conduction hearing system. Otol. Neurotol. 39, 940–949. doi: 10.1097/MAO.0000000000001892
Miller, R., Hujoel, P., Murray, M., and Popelka, G. R. (2011). Safety of an intra-oral hearing device utilizing a split-mouth research design. J. Clin. Dent. 22, 159–162.
Mirian, C., and Ovesen, T. (2020). Intratympanic vs systemic corticosteroids in first-line treatment of idiopathic sudden sensorineural hearing loss: a systematic review and meta-analysis. JAMA Otolaryngol. Head Neck Surg. 146, 421–428. doi: 10.1001/jamaoto.2020.0047
Mokkink, L. B., Prinsen, C. A. C., Bouter, L. M., Vet, H. C. W., and de, Terwee, C. B. (2016). The COnsensus-based Standards for the selection of health Measurement INstruments (COSMIN) and how to select an outcome measurement instrument. Braz. J. Phys. Ther. 20, 105–113. doi: 10.1590/bjpt-rbf.2014.0143
Monksfield, P., Jowett, S., Reid, A., and Proops, D. (2011). Cost-effectiveness analysis of the bone-anchored hearing device. Otol. Neurotol. 32, 1192–1197. doi: 10.1097/MAO.0b013e31822e5ae6
Moore, B. C. J., and Popelka, G. R. (2013). Preliminary comparison of bone-anchored hearing instruments and a dental device as treatments for unilateral hearing loss. Int. J. Audiol. 52, 678–686. doi: 10.3109/14992027.2013.809483
Moteki, H., Kitoh, R., and Usami, S. (2020). The availability of an adhesive bone conduction hearing device: a preliminary report of a single-center experience. Acta Otolaryngol. 140, 319–326. doi: 10.1080/00016489.2019.1708969
Mueller, J., Behr, R., Knaus, C., Milewski, C., Schoen, F., and Helms, J. (2000). Electrical stimulation of the auditory pathway in deaf patients following acoustic neurinoma surgery and initial results with a new auditory brainstem implant system. Adv. Otorhinolaryngol. 57, 229–235. doi: 10.1159/000059169
Murray, M., Miller, R., Hujoel, P., and Popelka, G. R. (2011). Long-term safety and benefit of a new intraoral device for single-sided deafness. Otol. Neurotol. 32, 1262–1269. doi: 10.1097/MAO.0b013e31822a1cac
Nakashima, T., and Yanagita, N. (1993). Outcome of sudden deafness with and without vertigo. Laryngoscope. 103, 1145–1149. doi: 10.1288/00005537-199310000-00012
Neve, O. M., Boerman, J. A., van den Hout, W. B., Briaire, J. J., van Benthem, P. P. G., and Frijns, J. H. M. (2021). Cost-benefit analysis of cochlear implants: a societal perspective. Ear Hear. 42, 1338–1350. doi: 10.1097/AUD.0000000000001021
Newman, C. W., Jacobson, G. P., and Spitzer, J. B. (1996). Development of the Tinnitus Handicap Inventory. Arch. Otolaryngol. Head Neck Surg. 122, 143–148. doi: 10.1001/archotol.1996.01890140029007
Newman, C. W., Weinstein, B. E., Jacobson, G. P., and Hug, G. A. (1991). Test-retest reliability of the hearing handicap inventory for adults. Ear Hear. 12, 355–357. doi: 10.1097/00003446-199110000-00009
Nicoucar, K., Momjian, S., Vader, J.-P., and De Tribolet, N. (2006). Surgery for large vestibular schwannomas: How patients and surgeons perceive quality of life. J. Neurosurg. 105, 205–212. doi: 10.3171/jns.2006.105.2.205
Nilsson, M., Soli, S. D., and Sullivan, J. A. (1994). Development of the Hearing in Noise Test for the measurement of speech reception thresholds in quiet and in noise. J. Acoust. Soc. Am. 95, 1085–1099. doi: 10.1121/1.408469
Niparko, J. K., Cox, K. M., and Lustig, L. R. (2003). Comparison of the bone anchored hearing aid implantable hearing device with contralateral routing of offside signal amplification in the rehabilitation of unilateral deafness. Otol. Neurotol. 24, 73–78. doi: 10.1097/00129492-200301000-00015
Noble, W., and Gatehouse, S. (2004). Interaural asymmetry of hearing loss, Speech, Spatial and Qualities of Hearing Scale (SSQ) disabilities, and handicap. Int. J. Audiol. 43, 100–114. doi: 10.1080/14992020400050015
Noble, W., Jensen, N. S., Naylor, G., Bhullar, N., and Akeroyd, M. A. (2013). A short form of the Speech, Spatial and Qualities of Hearing scale suitable for clinical use: the SSQ12. Int. J. Audiol. 52, 409–412. doi: 10.3109/14992027.2013.781278
Nosrati-Zarenoe, R., Arlinger, S., and Hultcrantz, E. (2007). Idiopathic sudden sensorineural hearing loss: results drawn from the Swedish national database. Acta Otolaryngol. 127, 1168–1175. doi: 10.1080/00016480701242477
Oh, S. J., Mavrommatis, M. A., Fan, C. J., DiRisio, A. C., Villavisanis, D. F., Berson, E. R., et al. (2023). Cochlear implantation in adults with single-sided deafness: a systematic review and meta-analysis. Otolaryngol. Head Neck Surg. 168, 131–142. doi: 10.1177/01945998221083283
Ohlenforst, B., Zekveld, A. A., Jansma, E. P., Wang, Y., Naylor, G., Lorens, A., et al. (2017). Effects of hearing impairment and hearing aid amplification on listening effort: a systematic review. Ear Hear. 38, 267–281. doi: 10.1097/AUD.0000000000000396
Ontario Health (2020a). Auditory brainstem implantation for adults with neurofibromatosis 2 or severe inner ear abnormalities: a health technology assessment. Ont. Health Technol. Assess. Ser. 20, 1–85.
Ontario Health (2020b). Implantable devices for single-sided deafness and conductive or mixed hearing loss: A health technology assessment. Ont. Health Technol. Assess. Ser. 20, 1–165.
Park, M. S., Lee, H. Y., Kang, H. M., Ryu, E. W., Lee, S. K., and Yeo, S. G. (2012). Clinical manifestations of aural fullness. Yonsei Med. J. 53, 985–991. doi: 10.3349/ymj.2012.53.5.985
Pedley, A. J., and Kitterick, P. T. (2017). Contralateral routing of signals disrupts monaural level and spectral cues to sound localisation on the horizontal plane. Hear. Res. 353, 104–111. doi: 10.1016/j.heares.2017.06.007
Peelle, J. E. (2018). Listening effort: how the cognitive consequences of acoustic challenge are reflected in brain and behavior. Ear Hear. 39, 204–214. doi: 10.1097/AUD.0000000000000494
Peltomaa, M., Pyykkö, I., Sappälä, I., Viitanen, L., and Viljanen, M. (2000). Lyme borreliosis, an etiological factor in sensorineural hearing loss? Eur. Arch. Otorhinolaryngol. 257, 317–322. doi: 10.1007/s004059900206
Peters, J. P., van Heteren, J. A. A., Wendrich, A. W., van Zanten, G. A., Grolman, W., Stokroos, R. J., et al. (2021). Short-term outcomes of cochlear implantation for single-sided deafness compared to bone conduction devices and contralateral routing of sound hearing aids-Results of a Randomised controlled trial (CINGLE-trial). PLoS ONE 16, e0257447. doi: 10.1371/journal.pone.0257447
Peters, J. P., van Zon, A., Smit, A. L., van Zanten, G. A., de Wit, G. A., Stegeman, I., et al. (2015b). CINGLE-trial: cochlear implantation for siNGLE-sided deafness, a randomised controlled trial and economic evaluation. BMC Ear Nose Throat Disord. 15, 3. doi: 10.1186/s12901-015-0016-y
Peters, J. P. M., Smit, A. L., Stegeman, I., and Grolman, W. (2015a). Review: bone conduction devices and contralateral routing of sound systems in single-sided deafness. Laryngoscope 125, 218–226. doi: 10.1002/lary.24865
Pierzycki, R. H., Edmondson-Jones, M., Dawes, P., Munro, K. J., Moore, D. R., and Kitterick, P. T. (2020). Associations between hearing health and well-being in unilateral hearing impairment. Ear Hear. 42, 520–530. doi: 10.1097/AUD.0000000000000969
Plaza, G., Durio, E., Herráiz, C., Rivera, T., and García-Berrocal, J. R. (2011). [Consensus on diagnosis and treatment of sudden hearing loss. Acta Otorrinolaringol. Esp. 62, 144–157. doi: 10.1016/j.otorri.2010.09.001
Pokharel, S., Tamang, S., Pokharel, S., and Mahaseth, R. K. (2021). Sudden sensorineural hearing loss in a post-COVID-19 patient. Clin. Case Rep. 9, e04956. doi: 10.1002/ccr3.4956
Poncet-Wallet, C., Mamelle, E., Godey, B., Truy, E., Guevara, N., Ardoint, M., et al. (2020). Prospective multicentric follow-up study of cochlear implantation in adults with single-sided deafness: tinnitus and audiological outcomes. Otol. Neurotol. 41, 458–466. doi: 10.1097/MAO.0000000000002564
Popelka, G. R., Derebery, J., Blevins, N. H., Murray, M., Moore, B. C. J., Sweetow, R. W., et al. (2010). Preliminary evaluation of a novel bone-conduction device for single-sided deafness. Otol. Neurotol. 31, 492–497. doi: 10.1097/MAO.0b013e3181be6741
Prinsen, C. A. C., Mokkink, L. B., Bouter, L. M., Alonso, J., Patrick, D. L., de Vet, H. C. W., et al. (2018). COSMIN guideline for systematic reviews of patient-reported outcome measures. Qual. Life Res. 27, 1147–1157. doi: 10.1007/s11136-018-1798-3
Prinsen, C. A. C., Vohra, S., Rose, M. R., Boers, M., Tugwell, P., Clarke, M., et al. (2016). How to select outcome measurement instruments for outcomes included in a “Core Outcome Set” - a practical guideline. Trials 17, 449. doi: 10.1186/s13063-016-1555-2
Prinsen, C. A. C., Vohra, S., Rose, M. R., King-Jones, S., Ishaque, S., Bhaloo, Z., et al. (2014). Core Outcome Measures in Effectiveness Trials (COMET) initiative: protocol for an international Delphi study to achieve consensus on how to select outcome measurement instruments for outcomes included in a “core outcome set”. Trials 15, 247. doi: 10.1186/1745-6215-15-247
Ramos Macías, A., Falcón-González, J. C., Manrique Rodríguez, M., Morera Pérez, C., García-Ibáñez, L., Cenjor Español, C., et al. (2018). One-year results for patients with unilateral hearing loss and accompanying severe tinnitus and hyperacusis treated with a cochlear implant. Audiol. Neurootol. 23, 8–19. doi: 10.1159/000488755
Ratuszniak, A., Skarzynski, P. H., Gos, E., and Skarzynski, H. (2022). Self-rated benefits of auditory performance after Bonebridge implantation in patients with conductive or mixed hearing loss, or single-sided deafness. Life 12, 137. doi: 10.3390/life12020137
Rauch, A.-K., Wesarg, T., Aschendorff, A., Speck, I., and Arndt, S. (2021). Long-term data of the new transcutaneous partially implantable bone conduction hearing system Osia®. Eur. Arch. Otorhinolaryngol. 279, 4279–4288. doi: 10.1007/s00405-021-07167-9
Rossini, B. A. A., Penido, N., de, O., Munhoz, M. S. L., Bogaz, E. A., and Curi, R. S. (2017). Sudden sensorioneural hearing loss and autoimmune systemic diseases. Int. Arch. Otorhinolaryngol. 21, 213–223. doi: 10.1055/s-0036-1586162
Rothpletz, A. M., Wightman, F. L., and Kistler, D. J. (2012). Informational masking and spatial hearing in listeners with and without unilateral hearing loss. J. Speech Lang. Hear. Res. 55, 511–531. doi: 10.1044/1092-4388(2011/10-0205)
Ryu, N. G., Moon, I. J., Byun, H., Jin, S. H., Park, H., Jang, K. S., et al. (2015). Clinical effectiveness of wireless CROS (contralateral routing of offside signals) hearing aids. European Arch. Oto-Rhino-Laryngol. 272, 2213–2219. doi: 10.1007/s00405-014-3133-0
Sakata, T., Esaki, Y., Yamano, T., Sueta, N., and Nakagawa, T. (2008). A comparison between the feeling of ear fullness and tinnitus in acute sensorineural hearing loss. Int. J. Audiol. 47, 134–140. doi: 10.1080/14992020701760547
Sakata, T., and Kato, T. (2006). Feeling of ear fullness in acute sensorineural hearing loss. Acta Otol. 126, 828–833. doi: 10.1080/00016480500527268
Sano, H., Okamoto, M., Ohhashi, K., Ino, T., Iwasaki, S., and Ogawa, K. (2013a). Self-reported symptoms in patients with idiopathic sudden sensorineural hearing loss. Otol. Neurotol. 34, 1405–1410. doi: 10.1097/MAO.0b013e3182a03705
Sano, H., Okamoto, M., Ohhashi, K., Iwasaki, S., and Ogawa, K. (2013b). Quality of life reported by patients with idiopathic sudden sensorineural hearing loss. Otol. Neurotol. 34, 36–40. doi: 10.1097/MAO.0b013e318278540e
Schmerber, S., Deguine, O., Marx, M., Van de Heyning, P., Sterkers, O., Mosnier, I., et al. (2017). Safety and effectiveness of the Bonebridge transcutaneous active direct-drive bone-conduction hearing implant at 1-year device use. Eur. Arch. Otorhinolaryngol. 274, 1835–1851. doi: 10.1007/s00405-016-4228-6
Schreiber, B. E., Agrup, C., Haskard, D. O., and Luxon, L. M. (2010). Sudden sensorineural hearing loss. Lancet 375, 1203–1211. doi: 10.1016/S0140-6736(09)62071-7
Schwartz, M. S., Otto, S. R., Shannon, R. V., Hitselberger, W. E., and Brackmann, D. E. (2008). Auditory brainstem implants. Neurotherapeutics 5, 128–136. doi: 10.1016/j.nurt.2007.10.068
Shang, Y., Hinkley, L. B., Cai, C., Subramaniam, K., Chang, Y. S., Owen, J. P., et al. (2018). Functional and structural brain plasticity in adult onset single-sided deafness. Front. Hum. Neurosci. 12, 474. doi: 10.3389/fnhum.2018.00474
Shi, L., Zhao, R., Li, X., Sun, W., and Liu, X. (2022). A review of the neurobiological mechanisms that distinguish between loudness recruitment and hyperacusis. Med. Sci. Monit. 28, e936373. doi: 10.12659/MSM.936373
Shih, C. P., Chou, Y. C., Chen, H. C., Lee, J. C., Chu, Y. H., and Wang, C. H. (2017). Analysis of caloric test responses in sudden hearing loss. Ear Nose Throat J. 96, 59–64. doi: 10.1177/014556131709600207
Simani, L., Oron, Y., Shapira, U., Handzel, O., Abu Eta, R., Warshavsky, A., et al. (2022). Is idiopathic sudden sensorineural hearing loss seasonal? Otol. Neurotol. 43, 1016–1021. doi: 10.1097/MAO.0000000000003661
Sin Wai, L., and Chua Wei De, K. (2021). Single-sided deafness, cochlear or bone conduction implants: a clinical dilemma. Hear. J. 74, 14–15. doi: 10.1097/01.HJ.0000792644.84692.00
Slattery W. H. III. and Middlebrooks J. C. (1994). Monaural sound localization: acute versus chronic unilateral impairment. Hear. Res. 75, 38–46. doi: 10.1016/0378-5955(94)90053-1
Snapp, H. A. (2019). Nonsurgical management of single-sided deafness: contralateral routing of signal. J. Neurol. Surg. B Skull Base 80, 132–138. doi: 10.1055/s-0039-1677687
Snapp, H. A., and Ausili, S. A. (2020). Hearing with one ear: consequences and treatments for profound unilateral hearing loss. J. Clin. Med. 9, 1010. doi: 10.3390/jcm9041010
Snapp, H. A., Hoffer, M. E., Liu, X., and Rajguru, S. M. (2017). Effectiveness in rehabilitation of current wireless CROS technology in experienced bone-anchored implant users. Otol. Neurotol. 38, 1397–1404. doi: 10.1097/MAO.0000000000001614
Snik, A. F. M., Bosman, A. J., Mylanus, E. A. M., and Cremers, C. W. R. J. (2004). Candidacy for the bone-anchored hearing aid. Audiol. Neurootol. 9, 190–196. doi: 10.1159/000078388
SoundBite Hearing (2013). What is SoundBiteTM?. Available online at: https://www.youtube.com/watch?v=ExTSW5Ogat4 (accessed December 3, 2022).
Speck, I., Arndt, S., Thurow, J., Blazhenets, G., Aschendorff, A., Meyer, P. T., et al. (2020). 18F-FDG PET Imaging of the inferior colliculus in asymmetric hearing loss. J. Nucl. Med. 61, 418–422. doi: 10.2967/jnumed.119.231407
Speck, I., Arndt, S., Thurow, J., Rau, A., Aschendorff, A., Meyer, P. T., et al. (2022). Neural activity of the auditory cortex predicts speech recognition of patients with asymmetric hearing loss after cochlear implantation. Sci. Rep. 12, 8068. doi: 10.1038/s41598-022-12139-y
Sprinzl, G. M., and Wolf-Magele, A. (2016). The Bonebridge bone conduction hearing implant: indication criteria, surgery and a systematic review of the literature. Clin. Otolaryngol. 41, 131–143. doi: 10.1111/coa.12484
Staecker, H., Nadol, J. B. J., Ojeman, R., Ronner, S., and McKenna, M. J. (2000). Hearing preservation in acoustic neuroma surgery: middle fossa versus retrosigmoid approach. Am. J. Otol. 21, 399–404. doi: 10.1016/S0196-0709(00)80051-4
Suzuki, M., Kouzaki, H., Nishida, Y., Shiino, A., Ito, R., and Kitano, H. (2002). Cortical representation of hearing restoration in patients with sudden deafness. Neuroreport 13, 1829–1832. doi: 10.1097/00001756-200210070-00029
Távora-Vieira, D., De Ceulaer, G., Govaerts, P. J., and Rajan, G. P. (2015). Cochlear implantation improves localization ability in patients with unilateral deafness. Ear Hear. 36, e93–98. doi: 10.1097/AUD.0000000000000130
Thai-Van, H., Bounaix, M. J., and Fraysse, B. (2001). Menière's disease: pathophysiology and treatment. Drugs 61, 1089–1102. doi: 10.2165/00003495-200161080-00005
Theriou, C., Fielden, C. A., and Kitterick, P. T. (2019). The cost-effectiveness of bimodal stimulation compared to unilateral and bilateral cochlear implant use in adults with bilateral severe to profound deafness. Ear Hear. 40, 1425–1436. doi: 10.1097/AUD.0000000000000727
Thompson, N. J., Dillon, M. T., Buss, E., Rooth, M. A., Richter, M. E., Pillsbury, H. C., et al. (2022). Long-term improvement in localization for cochlear implant users with single-sided deafness. Laryngoscope 132, 2453–2458. doi: 10.1002/lary.30065
Timon, C. I., and Walsh, M. A. (1989). Sudden sensorineural hearing loss as a presentation of HIV infection. J. Laryngol. Otol. 103, 1071–1072. doi: 10.1017/S0022215100111028
Tjellström, A., and Granström, G. (1994). Long-term follow-up with the bone-anchored hearing aid: a review of the first 100 patients between 1977 and 1985. Ear Nose Throat J. 73, 112–114. doi: 10.1177/014556139407300210
Tunis, S. R., Clarke, M., Gorst, S. L., Gargon, E., Blazeby, J. M., Altman, D. G., et al. (2016). Improving the relevance and consistency of outcomes in comparative effectiveness research. J. Comp. Eff. Res. 5, 193–205. doi: 10.2217/cer-2015-0007
Twigg, V., Lawrence, R., Thevasagayam, R., Fergie, N., and Daniel, M. (2020). Management of Suspected Unilateral Idiopathic Sudden Sensorineural Hearing Loss in Adults. ENT UK. Available online at: https://www.entuk.org/resources/98/management_of_suspected_unilateral_idiopathic_sudden_sensorineural_hearing_loss_in_adults/ (accessed April 15, 2023).
Tyler, R. S., Pienkowski, M., Roncancio, E. R., Jun, H. J., Brozoski, T., Dauman, N., et al. (2014). A review of hyperacusis and future directions: Part I. Definitions and manifestations. Am. J. Audiol. 23, 402–419. doi: 10.1044/2014_AJA-14-0010
Underdown, T., and Pryce, H. (2022). How do patients decide on interventions for single sided deafness? A qualitative investigation of patient views. Int. J. Audiol. 61, 551–560. doi: 10.1080/14992027.2021.1951853
Usami, S.-I., Kitoh, R., Moteki, H., Nishio, S.-Y., Kitano, T., Kobayashi, M., et al. (2017). Etiology of single-sided deafness and asymmetrical hearing loss. Acta Otolaryngol. 137, 2–7. doi: 10.1080/00016489.2017.1300321
Van de Heyning, P., Távora-Vieira, D., Mertens, G., Van Rompaey, V., Rajan, G. P., Müller, J., et al. (2017). Towards a unified testing framework for single-sided deafness studies: a consensus paper. Audiol. Neurootol. 21, 391–398. doi: 10.1159/000455058
Van de Heyning, P., Vermeire, K., Diebl, M., Nopp, P., Anderson, I., and De Ridder, D. (2008). Incapacitating unilateral tinnitus in single-sided deafness treated by cochlear implantation. Ann. Otol. Rhinol. Laryngol. 117, 645–652. doi: 10.1177/000348940811700903
van den Berge, M. J. C., van Dijk, J. M. C., Metzemaekers, J. D. M., Maat, B., Free, R. H., and van Dijk, P. (2019). An auditory brainstem implant for treatment of unilateral tinnitus: protocol for an interventional pilot study. BMJ Open 9, e026185. doi: 10.1136/bmjopen-2018-026185
van Zon, A., Peters, J. P. M., Stegeman, I., Smit, A. L., and Grolman, W. (2015). Cochlear implantation for patients with single-sided deafness or asymmetrical hearing loss: a systematic review of the evidence. Otol. Neurotol. 36, 209–219. doi: 10.1097/MAO.0000000000000681
Vannson, N., James, C., Fraysse, B., Strelnikov, K., Barone, P., Deguine, O., et al. (2015). Quality of life and auditory performance in adults with asymmetric hearing loss. Audiol. Neurootol. 20, 38–43. doi: 10.1159/000380746
Vannson, N., James, C. J., Fraysse, B., Lescure, B., Strelnikov, K., Deguine, O., et al. (2017). Speech-in-noise perception in unilateral hearing loss: relation to pure-tone thresholds and brainstem plasticity. Neuropsychologia 102, 135–143. doi: 10.1016/j.neuropsychologia.2017.06.013
Vas, V., Akeroyd, M. A., and Hall, D. A. (2017). A data-driven synthesis of research evidence for domains of hearing loss, as reported by adults with hearing loss and their communication partners. Trends Hear. 21, 2331216517734088. doi: 10.1177/2331216517734088
Vincent, C., Arndt, S., Firszt, J. B., Fraysse, B., Kitterick, P. T., Papsin, B. C., et al. (2015). Identification and evaluation of cochlear implant candidates with asymmetrical hearing loss. Audiol. Neurootol. 20, 87–89. doi: 10.1159/000380754
Wang, Y., Naylor, G., Kramer, S. E., Zekveld, A. A., Wendt, D., Ohlenforst, B., et al. (2018). Relations between self-reported daily-life fatigue, hearing status, and pupil dilation during a speech perception in noise task. Ear Hear. 39, 573–582. doi: 10.1097/AUD.0000000000000512
Ware, J. E. J., and Sherbourne, C. D. (1992). The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care 30, 473–483. doi: 10.1097/00005650-199206000-00002
Wazen, J. J., Ghossaini, S. N., Spitzer, J. B., and Kuller, M. (2005). Localization by unilateral BAHA users. Otolaryngol. Head Neck Surg. 132, 928–932. doi: 10.1016/j.otohns.2005.03.014
Welsh, L. W., Welsh, J. J., Rosen, L. F., and Dragonette, J. E. (2004). Functional impairments due to unilateral deafness. Ann. Otol. Rhinol. Laryngol. 113, 987–993. doi: 10.1177/000348940411301209
West, N., Bunne, M., Sass, H., and Cayé-Thomasen, P. (2022). Cochlear implantation for patients with a vestibular schwannoma: Effect on tinnitus handicap. J. Int. Adv. Otol. 18, 382–387. doi: 10.5152/iao.2022.21541
Westerlaken, B. O., Stokroos, R. J., Dhooge, I. J. M., Wit, H. P., and Albers, F. W. J. (2003). Treatment of idiopathic sudden sensorineural hearing loss with antiviral therapy: a prospective, randomized, double-blind clinical trial. Ann. Otol. Rhinol. Laryngol. 112, 993–1000. doi: 10.1177/000348940311201113
Widen, J. E., Folsom, R. C., Cone-Wesson, B., Carty, L., Dunnell, J. J., Koebsell, K., et al. (2000). Identification of neonatal hearing impairment: hearing status at 8 to 12 months corrected age using a visual reinforcement audiometry protocol. Ear Hear. 21, 471–487. doi: 10.1097/00003446-200010000-00011
Wie, O. B., Pripp, A. H., Tvete, O., Wie, O. B., Pripp, A. H., and Tvete, O. (2010). Unilateral deafness in adults: effects on communication and social interaction. Ann. Otol. Rhinol. Laryngol. 119, 772–781.
Willenborg, K., Avallone, E., Maier, H., Lenarz, T., and Busch, S. (2022). A new active osseointegrated implant system in patients with single-sided deafness. Audiol. Neurootol. 27, 83–92. doi: 10.1159/000515489
Wu, Q., Li, X., Sha, Y., and Dai, C. (2019). Clinical features and management of Ménière's disease patients with drop attacks. Eur. Arch. Otorhinolaryngol. 276, 665–672. doi: 10.1007/s00405-018-5260-5
Young, A. S., Nham, B., Bradshaw, A. P., Calic, Z., Pogson, J. M., Gibson, W. P., et al. (2022). Clinical, oculographic and vestibular test characteristics of Ménière's disease. J. Neurol. 269, 1927–1944. doi: 10.1007/s00415-021-10699-z
Keywords: CROS hearing aid, bone conduction device, cochlear implant, single-sided deafness (SSD), unilateral hearing loss, hearing interventions, auditory implant
Citation: Katiri R, Peters JPM, Fackrell K and Hoare DJ (2023) Device-based interventions that seek to restore bilateral and binaural hearing in adults with single-sided deafness: a conceptual analysis. Front. Audiol. Otol. 1:1242196. doi: 10.3389/fauot.2023.1242196
Received: 18 June 2023; Accepted: 07 August 2023;
Published: 29 August 2023.
Edited by:
Jorge Humberto Ferreira Martins, Polytechnic Institute of Porto, PortugalReviewed by:
Cristina Caroça, CUF Infante Santo Hospital, PortugalAntonio Vasco Oliveira, Polytechnic Institute of Porto, Portugal
Copyright © 2023 Katiri, Peters, Fackrell and Hoare. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Roulla Katiri, cm91bGxhLmthdGlyaUBuaHMubmV0
†ORCID: Roulla Katiri orcid.org/0000-0001-7098-8024
Jeroen P. M. Peters orcid.org/0000-0003-3189-9103
Kathryn Fackrell orcid.org/0000-0001-6529-8643
Derek J. Hoare orcid.org/0000-0002-8768-1392
 Roulla Katiri
Roulla Katiri Jeroen P. M. Peters
Jeroen P. M. Peters Kathryn Fackrell
Kathryn Fackrell Derek J. Hoare
Derek J. Hoare