- 1Department of Otolaryngology—Head and Neck Surgery, Columbia University Vagelos College of Physicians and Surgeons, NewYork-Presbyterian/Columbia University Irving Medical Center, New York, NY, United States
- 2Department of Otolaryngology-Head and Neck Surgery, Vanderbilt University Medical Center, Nashville, TN, United States
- 3Department of Mechanical Engineering, The Fu Foundation School of Engineering and Applied Science, Columbia University, New York, NY, United States
Objective: Hearing loss can cause speech and language delays, communication barriers, and learning problems. Such factors are associated with reduced academic achievement, social isolation, decreased quality of life, and poorer health outcomes. We use a national cohort of children to examine how subclinical hearing loss is associated with academic/educational performance. The goal of this study is to determine if different levels of subclinical hearing loss (pure tone average ≤ 25 dB HL) are associated with educational testing outcomes in children.
Design: Analysis of children 6–16 years old who participated in the National Health and Nutrition Examination Survey (NHANES-III, 1988–1994) was performed. Air-conduction thresholds were measured at 0.5, 1, 2, 4, 6, and 8 kHz. A four-frequency pure-tone average (PTA) was calculated from 0.5, 1, 2, and 4 kHz. Hearing thresholds were divided into categories (≤ 0, 1–10, and 11–25 dB) for analysis. The outcomes of interest were the Wide Range Achievement Test (WRAT-R) and Wechsler Intelligence Scale for Children (WISC-R). Analysis was conducted using ANOVA and logistic regression.
Results: We analyzed 3,965 participants. In univariable analysis, the average scores in scaled math, reading, digit span (short-term memory), and block design (visual-motor skills) were significantly lower with worsening hearing categories (p < 0.01). In multivariable regression PTAs of 1–10 dB HL (OR 1.72, 95% CI 1.29–2.29, p < 0.01) and 11-25 dB HL (OR: 2.99, 95% CI 1.3–6.65, p < 0.01), compared to PTA of ≤0 dB HL, were associated with poor reading test performance (<25th percentile).
Conclusion: Subclinical hearing loss is associated with worse performance on educational attainment (as measured by reading test performance) in children between the ages of 6–16.
Introduction
Hearing loss affects ~30 million individuals in the United States and ~10–20% of children (Rennels and Pickering, 2005; Su and Chan, 2017). Studies have shown that hearing loss in children can negatively impact speech and language development, learning ability, and communication (Lieu et al., 2020). In turn, these factors can manifest in reduced academic achievement, social isolation, decreased quality of life, and overall poorer health outcomes (Borg et al., 2002; Emmett and Francis, 2014; Wang et al., 2019a). The effects of hearing loss during childhood can persist and have a considerable longitudinal impact throughout adulthood (Lieu et al., 2012; Idstad et al., 2019). The impact of hearing loss on such outcomes has been seen even with a mild hearing loss (25–40 dB HL pure tone average) (Lewis et al., 2015; Wang et al., 2019b).
Although these categorizations have changed over several decades, hearing thresholds above 25 dB HL are often considered “clinically significant” hearing loss based on guidelines from associations such as the American Speech-Language-Hearing Association (Clark, 1981; Lieu et al., 2020). Many studies examining the impact of hearing loss on day-to-day functioning have used these categories in children (Wake et al., 2004; Ronner et al., 2020; Sindhar et al., 2021). Recent studies have established a relationship between lower cognition and subclinical hearing loss (SCHL) in adults, defined by a pure tone average ≤ 25 dB HL (averaged across 0.5, 1, 2, and 4 kHz) (Golub et al., 2020b). The designation of this as a hearing “loss” is based on preliminary data suggesting that hearing loss in this range (i.e., within the normal range of hearing) may be associated with decreased cognition. Although the current published data do not establish a clear cut-off for the detrimental effect of hearing loss on cognitive outcomes, studies by our group and others suggest that cognitive impairment in adults may begin at earlier stages of hearing loss than previously hypothesized, compared to their peers with better hearing (and still classified as normal under the current guidelines) (Golub et al., 2020a,d; Chern et al., 2021; Irace et al., 2022). Similarly, the effect of SCHL on children has been recognized over the past decade. It has already been well-established that children with hearing loss, specifically cochlear implant users and those with severe hearing loss, have impaired ability to perform academically. This is particularly true for reading and communication skills where decreased understanding of auditory communication can significantly impact these skills. Indeed, several studies have shown that even small amounts of hearing loss (e.g., a minimal or slight-to-mild sensorineural hearing loss, PTA 15–40 dB HL) in children are associated with decreased communication, social support, self-esteem, and cognitive performance (Lewis et al., 2015; Moore et al., 2019). However, the few existing studies examining minimal hearing loss and associated cognitive and educational performance in children are underpowered and demonstrate a lack of sample diversity (Bess et al., 1998; McFadden and Pittman, 2008; Lewis et al., 2015; Moore et al., 2020).
The primary objective of this study is to determine if different levels of SCHL (pure tone average ≤ 25 dB HL) are associated with educational testing outcomes [e.g., subsets of the Wechsler Intelligence Scale for Children-Revised (Grizzle, 2011) and the Wide Range Achievement Test-Revised (Caplan, 2011; National Center for Health Statistics, 2021)] in children from the NHANES-III (National Health and Nutrition Examination Survey), a national cross-sectional study of the United States population. We hypothesized that small differences in hearing will impact a child's ability to understand speech and instructions in unfavorable listening conditions relative to other children who have better hearing (Crandell, 1993; Bess et al., 1998). This could manifest as difficulty following conversation, understanding speech and instruction from a distance, and synthesizing new information in novel environments that require careful listening, perhaps in a challenging auditory environment. This has considerable, detrimental implications in classroom settings where much of a child's cognitive stamina is developed, and therefore can have implications for educational performance. In summary, if a child has small aberrations in hearing precluding them from developing skills needed to excel in the classroom, they will subsequently perform worse on educational outcome measures used in this manuscript. To the best of our knowledge, no prior study has examined minimal hearing loss and educational testing in United States children (Moore et al., 2019).
Methods
Participants
Participants included children 6–16 years of age who participated in the National Health and Nutrition Examination Survey (NHANES III, 1988–1994), a cyclic cross-sectional study that uses a stratified, multi-stage sampling design to achieve a nationally representative sample of the noninstitutionalized civilian US population in 50 states and District of Columbia. NHANES III is the only NHANES cycle that included both comprehensive educational/academic testing as well as audiometric data for children. The survey was conducted by the National Center for Health Statistics of the Centers for Disease Control and Prevention. The designs of the sample have been described in previous studies (Health USDo, 1998; Mohadjer et al., 2021; National Center for Health Statistics, 2021). The study encompasses both an in-home interview and physical examination conducted in a mobile examination center. The interview portion included questions addressing demographics (i.e., age, gender, and race), socioeconomic status (determined by poverty index), and health (i.e., medical comorbidities, smoking history, and medication use). Physical examinations included medical, physiological measurements, audiometric (hearing testing), and laboratory tests. The questionnaire included information regarding rheumatic heart disease, chronic bronchitis, epilepsy, cerebral palsy, and asthma.
Audiometric hearing measures
Audiometric testing included a hearing questionnaire, tympanometry, and pure-tone air conduction audiometry. Testing was performed inside a sound booth using a standardized protocol from the National Center for Health Statistics. Air conduction thresholds were measured at 0.5, 1, 2, 4, 6, and 8 kHz in dB HL. Cochlear implant users and hearing aid users were instructed to remove their devices before testing. If a participant had two devices, they would need to remove both. If they were unable to remove them, they were excluded from the audiometry component of the study. A four-frequency pure tone average (PTA) was calculated from individual thresholds at 0.5, 1, 2, and 4 kHz. Hearing thresholds were arbitrarily grouped into 3 categories: PTA ≤ 0, 1–10, and 11–25 dB HL. This represented two levels of SCHL (1–10 dB HL and 11–25 dB HL) as well as a reference category of ≤ 0 dB HL, or the best possible performance on an audiometric exam. Participants with a PTA > 25 dB HL were excluded from analysis as the objective of the study was to explore the associations with subclinical hearing threshold levels. We defined this hearing loss as “subclinical” hearing loss (as used in previous studies) and is largely characterized as any hearing threshold under the criteria classically known as hearing loss (<25 dB HL) (Golub et al., 2020b; Chern et al., 2021; Irace et al., 2022). Participants with flat tympanogram results (peak ≤ 0.2 mL) were excluded since any related hearing loss may be from a middle ear effusion and thus transient. Similar to other epidemiologic hearing loss studies, analysis was limited to the hearing thresholds of the better ear (Deal et al., 2017; Golub et al., 2020a,d; Chern et al., 2021).
Intelligence/educational testing
NHANES III (1988–1994) includes multiple components that measure child intelligence, educational performance, and cognitive function. Subsets of the Wide Range Achievement Test–Revised (WRAT-R) and the Wechsler Intelligence Scale for Children—Revised (WISC-R) were included. These subsets included the arithmetic and reading components of the WRAT-R, as well as the verbal component (Forward Digit Span) and performance exam (Block Design) portions of the WISC-R. The WISC-R was initially designed to measure intelligence of children, and in a similar vein, the WRAT-R measures a child's ability read words, comprehend sentences, spell, and perform arithmetic all of which were designed to correlate with academic and educational achievement. Although not specifically designed to measure cognitive domains, the WRAT-R components indirectly extrapolate to various cognitive domains involved in reading and arithmetic. The reading components evaluate cognitive processes related to verbal learning, as well as decoding and printed word recognition. The math components evaluate cognitive processes involving inattention to detail (misread operational signs or misalignment of numbers), as well as skills involved in basic math operations. The Digit Span measures verbal short-term memory, and the block design addresses spatial visualization ability and motor skills. Raw scores, standardized scores, and age-corrected scaled scores for each subset are included in NHANES. For analysis, scaled scores were used.
The WISC-R and WRAT-R have been developed to evaluate intelligence and academic skills assessment using at several different tasks. Research has shown the utility of these tests in identifying children who are at risk of reading and other learning difficulties. Not only do they identify patterns of performance on academic achievement tests, but they also can characterize the relative cognitive deficits (i.e., compared to their peers) using measures of phonological awareness and rapid naming. They have also been shown to demonstrate an association with long-term neurocognitive outcomes (Andrews et al., 2001; D'Angiulli, 2003; Drotar et al., 2008; Sayegh et al., 2014; Chua et al., 2016). For all outcomes, higher numbers indicate better performance. For the main analysis, cognitive outcomes were binarized into high and low categories, where performing at the 25th percentile or lower was defined as low. This cut-off for poorer performance was arbitrarily determined to be a point at which a participant's educational performance would be meaningfully lower than his or her peers. Due to the arbitrary nature of these cut-offs, sensitivity analysis was completed using cutpoints at the 50th and 10th percentile as well to affirm if any association with hearing loss was maintained. Finally, multivariable linear regression was conducted to examine the effect of continuous hearing thresholds on continuous test scores to further test the sensitivity of any established relationships. This linear regression multivariable model also controlled for all factors included in Table 1. Educational assessments were performed as part of a standard protocol, described in NHANES III documentation (Aldea Martinez et al., 2019).
Covariates
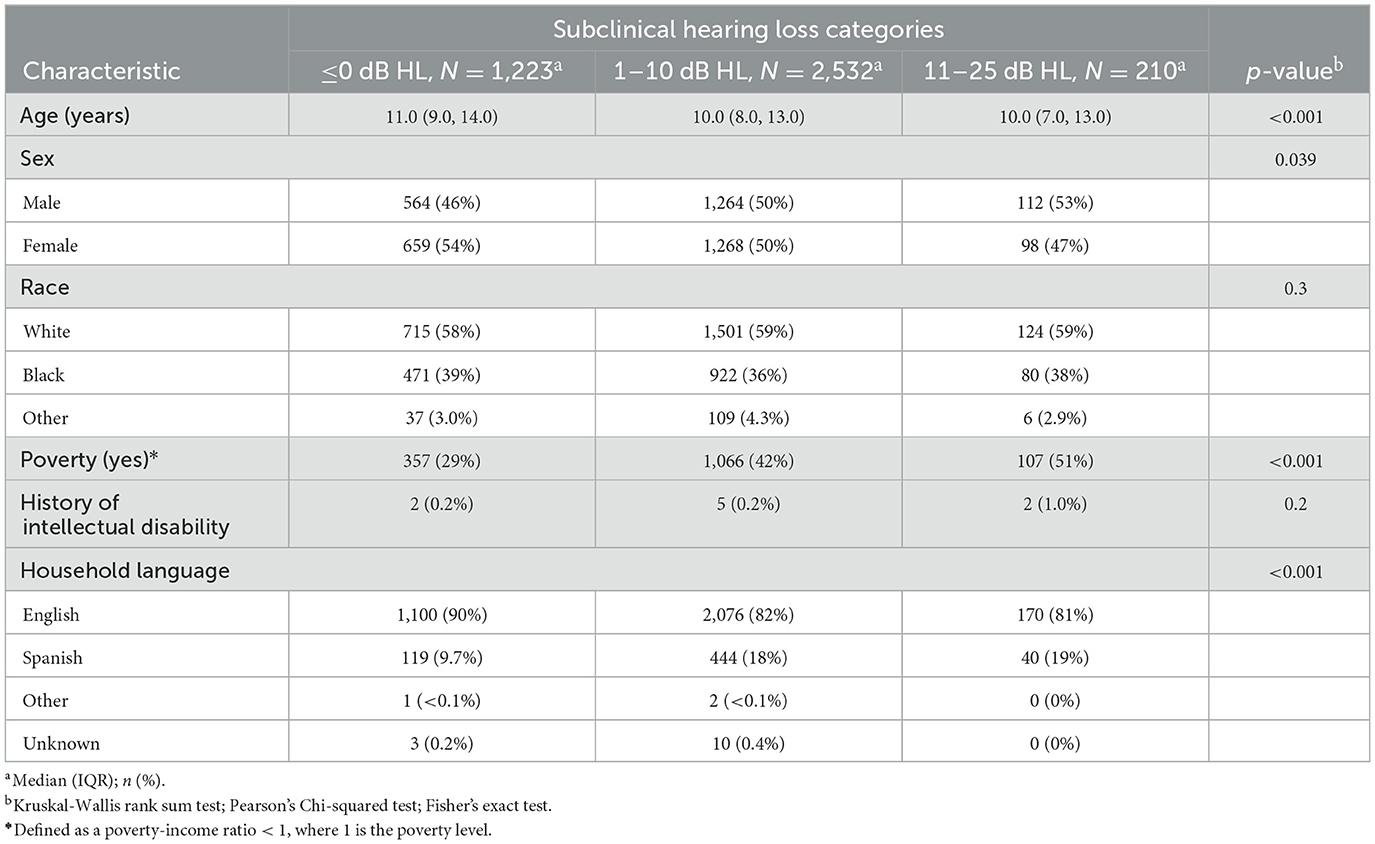
Covariates of interest included age, sex, race, poverty-income ratio, primary household language, history of intellectual disability, and the highest completed grade in school (e.g., 1st−12th grade or post-primary school) of the principal/head household member (as designated by the child's parent/guardian completing the survey). Age refers to the age (in years) at the time of the NHANES III Examination. Race was defined as White, Black, or other. The poverty income ratio is defined as the ratio of family income divided by the poverty threshold, as set by the Census bureau every year. Poverty ratios were categorized as <1 or ≥ 1 for analysis, where 1 is the poverty level. The highest completed grade (highest level of education attained) of the principal household member (head of the household defined as the child's parent or guardian) was defined as <12th grade or ≥12th grade (high school degree). Participants with a history of intellectual disability were recorded as yes/no by NHANES. Previous studies have established a relationship between the above socioeconomic factors (SES) and WISC-R and WRAT-R, which validates the inclusion of these variables (Lee et al., 2003; Wu et al., 2006). While NHANES contains many variables, these are the primary demographic/SES variables available.
Data analysis
Analysis was performed in Stata IC v15 (StataCorp, College Station, TX). Differences in cognitive testing across hearing loss categories were determined by the unpaired ANOVA testing. Corrected Cohen's d (used to indicate the standardized differences between two means) was calculated to determine effect size between best and worst hearing threshold groups. Effect sizes are generally classified as small (d = 0.2), medium (d = 0.5), and large (d = 0.8) (Lakens, 2013). Multivariable logistic and linear regression with the above-mentioned covariates (also listed in Table 1) was conducted to explore the relationship between different levels of SCHL and cognitive performance.
For logistic regression, the binary outcome was a high or low cognitive test score, using the 25th percentile as the cutpoint to acquire an odds ratio (OR) for lower scores. As described previously, sensitivity analysis was completed using cutpoints at the 50th and 10th percentile as well to affirm if any association with hearing loss was maintained. This sensitivity analysis was only completed for reading scores as this was the only significant outcome from initial models. The odds ratio is the odds that an outcome will occur given the presence of a certain exposure, compared to the odds that an outcome will occur without the presence of the exposure (Szumilas, 2010). To summarize, a multivariable logistic model was created for each score (Reading, Math, Block Design, Digit Span). Sensitivity analysis involved three different logistic models using three different cutoffs (50th and 10th percentile) for only reading scores. Multivariable models included all relevant covariates collected in this study (Table 1).
Multivariable linear regression analysis was also performed and controlled for the same factors (Table 1) and examined the relationship between continuous hearing thresholds and continuous reading scores. Therefore, only one linear regression model was created. Statistical significance was defined at the α = 0.05 level.
Weighting
Raw audiometric and educational testing data are presented without using weighting protocols. NHANES weighting utilizes a complex survey design to reduce biases as a result of oversampling, undersampling, and survey non-response. Fundamentally, this allows the data to better match the actual US population makeup. For example, if a certain subset of the population is undersampled, the weighting will correct for this so these individuals will not be falsely underrepresented. ANOVA testing for hearing thresholds between PTA groups was conducted without weighting. Weighting was not used for this analysis to transparently show outcomes without any artificial weighting/modulation. However, weighting per NHANES protocols was used for univariable and multivariable logistic regression analysis, as well as sensitivity analysis using linear regression. Weighting protocols accounted for NHANES III's four-stage sample design, which accounted for (1) Primary Sampling Units (PSUs) comprising mostly single counties, (2) area segments within PSUs, (3) households within area segments, and (4) persons within households (Mohadjer et al., 2021).
Results
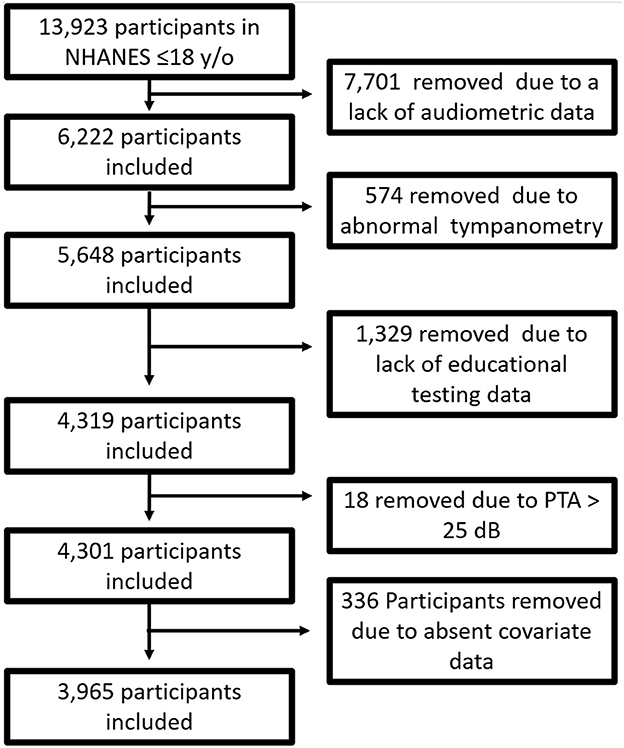
Of 13,923 participants under the age of 18 in NHANES III, 6,222 had complete audiogram data (only a random sample of children are assigned to audiometric testing). We removed 574 participants due to abnormal tympanometry. Only participants 16 years old and under had educational testing, which removed 1,329 participants due to missing data. Eighteen individuals were removed because they had a PTA > 25 dB HL; 336 individuals were removed due to incomplete covariates data. In total, 3,965 individuals were included in final analysis. Figure 1 diagrammatically shows the inclusion and exclusion of participants.
The average age of included participants was 10.8 years old (range 6–16); 51.1% were female, 59.0% were White, and 84.4% spoke English as the predominant language at home. Socioeconomic demographics are as follows: 38.6% of participants had a poverty income ratio of < 1 (indicating an income below the poverty limit) and 39.0% had a head of household who did not complete education past 11th grade. Of included participants, 0.2% had an intellectual disability. See Table 1 for participant demographics.
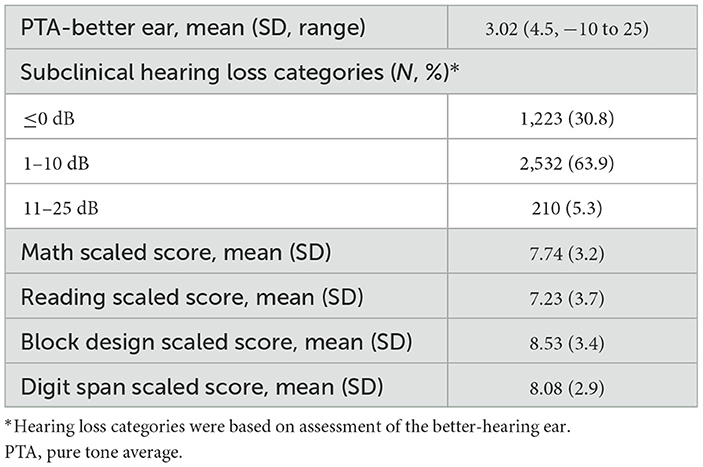
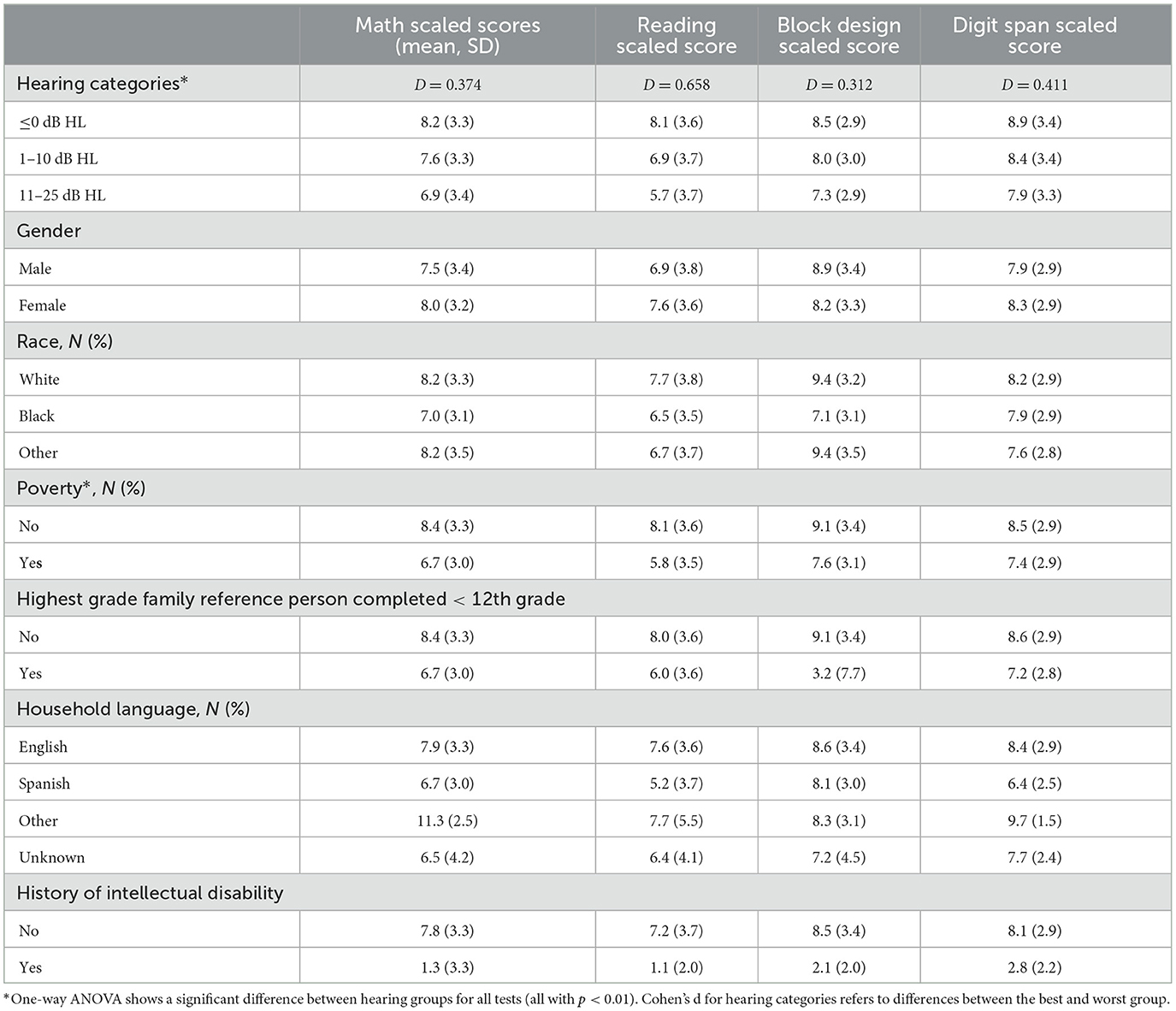
The average PTA was 3.0 dB HL (SD = 4.5) and ranged from−10 dB HL to 25 dB HL. Of the participants, 30.8% were classified with PTA ≤ 0 dB HL, 63.9% with PTA between 1 and 10 dB HL, and 5.3% with PTA between 11 and 25 dB HL. The average math scaled score was 7.74 (SD = 3.2), with a 25th percentile score being a score of 6. The average reading scaled score was 7.23 (SD = 3.7) and a 25th percentile score of 5. The average block scaled score was 8.53 (SD = 3.4) with a 25th percentile score of 6. The average digit span scaled score was 8.08 (SD = 2.9) with a 25th percentile score of 6. See Table 2 for hearing characteristics and average educational test scores. See Table 3 for average educational test scores stratified by hearing categories and other covariates.
ANOVA testing illustrates that test scores were significantly different between hearing categories (p < 0.01). Reading scores decreased most dramatically, specifically by ~2.5 points (Cohen's d = 0.658) from the PTA ≤ 0 dB HL category to the PTA between 11 and 25 dB HL category. In univariable analysis, the average scores in scaled math, reading, digit span (short-term memory), and block design (visual-motor skills) were significantly lower with worsening hearing categories (p < 0.01). Results of the univariable logistic regression analysis that did not control for confounders are included in Supplementary Table 1.
Multivariable logistic regression models using NHANES weighting protocols were created while controlling for all covariates in Table 1 (Table 4). The outcome of interest was a low educational test score (score < 25th percentile) in subsets of the WISC-R and WRAT-R (Reading, Math, Digit Span, Block Design). Worse hearing threshold categories were associated with lower reading scores; PTAs of 1–10 dB HL (OR 1.72, 95% CI 1.29–2.29, p < 0.01) and 11–25 dB HL (OR: 2.99, 95% CI 1.35–6.65, p < 0.01) were associated with scoring below the 25th percentile. On multivariable logistic regression analysis (Table 4), hearing thresholds were not significantly associated with math-scaled scores (p = 0.35–0.40), digit span scaled scores (p = 0.37–0.38), and block design scaled scores (p = 0.20–0.21).
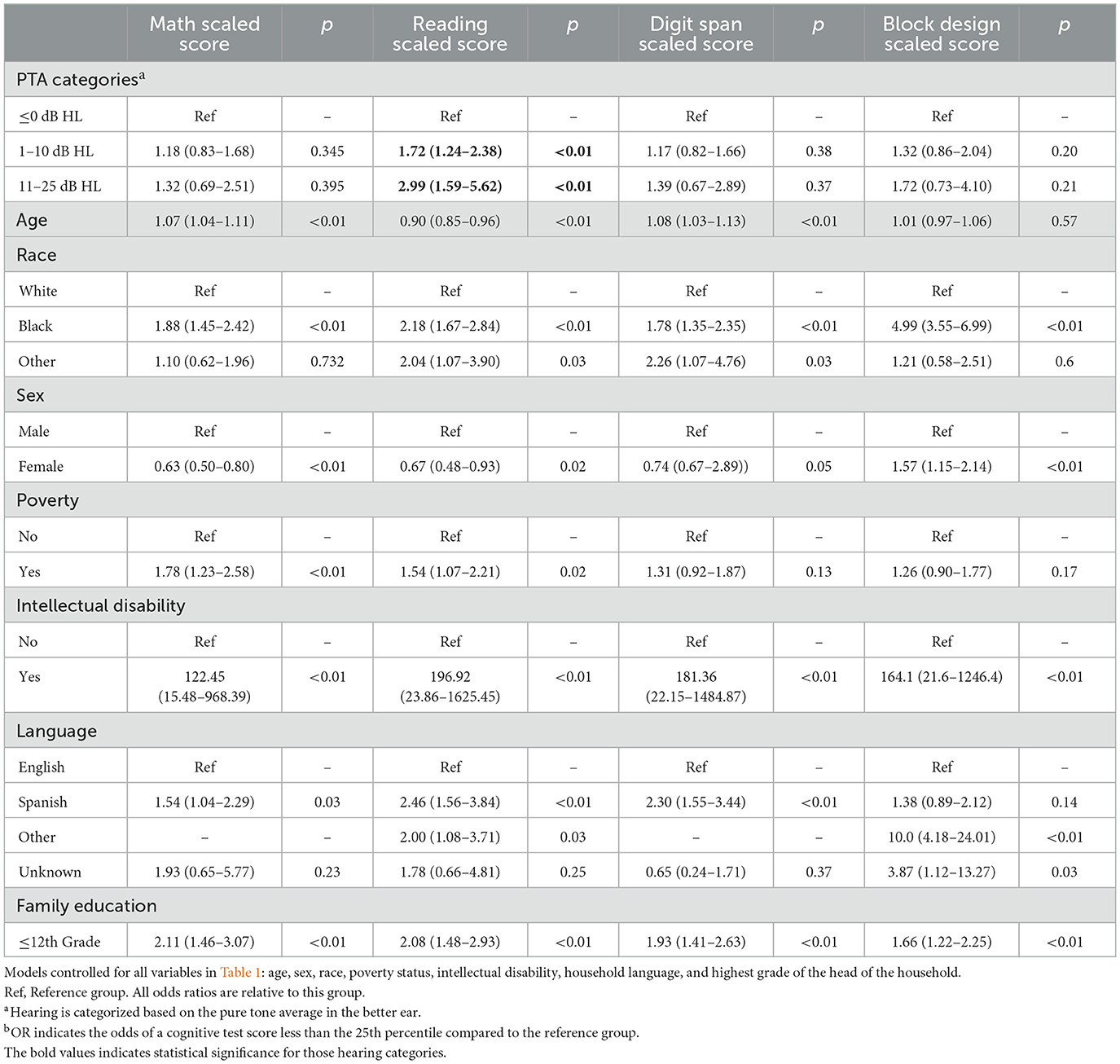
Table 4. Multivariable logistic regression between hearing and a cognitive test score less than the 25th percentile (weighted)b.
Sensitivity analysis was conducted using 50th and 10th percentile cutpoints for reading scaled score thresholds on weighted multivariable logistic regression models: PTAs of 1–10 dB HL (OR 1.56, 95% CI 1.21–2.00, p = 0.001) and 11–25 dB HL (OR: 2.72, 95% CI 1.75–4.23) were associated with scoring below the 50th percentile on reading scores. PTAs of 1–10 dB HL (OR 1.83, 1.06–3.16, p = 0.031) and 11–25 dB HL (OR 3.96, 1.39–11.32, p = 0.011) were associated with scoring below the 10th percentile. This is included in Supplementary Table 2.
Finally, analysis was conducted using better hearing ear PTA and reading scores as continuous variables in a fully adjusted, multivariable, sample-weighted, linear regression model (see Table 5). PTA of the better hearing ear was negatively associated with reading scores (coef:−0.110, 95% CI −0.146, −0.0742, p < 0.001) when controlling for confounding factors (age, sex, race, poverty-income ratio, primary household language, history of intellectual disability, and the highest completed grade of the principal household member). This suggests that increased hearing loss even while still under 25 dB HL is significantly associated with worse reading test scores, adjusting for covariates.
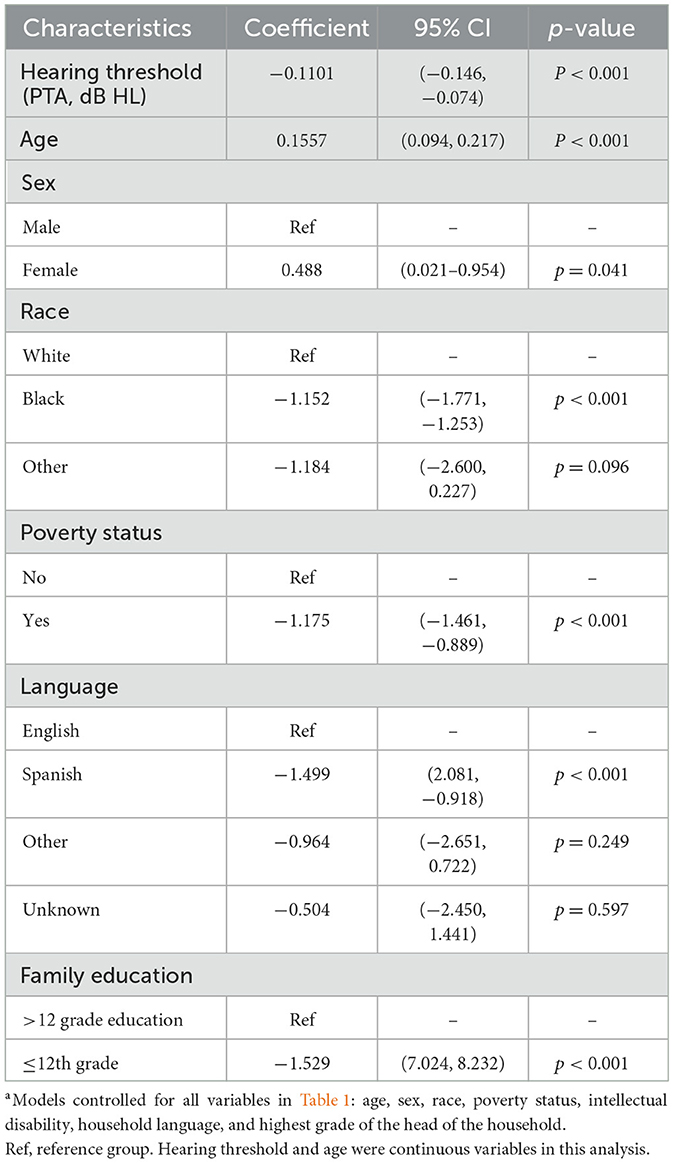
Table 5. Multivariable linear regression between hearing (continuous PTA threshold) and reading test scores (weighted)a.
Discussion
In this study of a national sample, we demonstrate that SCHL is associated with lower reading test scores in children aged 6–16 in the United States, adjusting for covariates. This reproduces an analogous finding previously reported in adults, which also includes measures of verbal learning and memory through the Spanish-English Verbal Learning Test (SEVLT) (Golub et al., 2020b). No prior United States population studies have explored the relationship between SCHL (1–25 dB HL) and reading test scores in children. The weighting protocol for NHANES is powerful, as it allows us to generalize our findings to the United States population. Although NHANES III was conducted several decades ago, it is the only national sample of US children containing rigorous audiometric and educational testing, as well as numerous comorbidities and socioeconomic factors captured through robust questionnaires given to both the participants and the family of the participants. Therefore, NHANES III represents a powerful tool for understanding the relationship between hearing and educational testing outcomes on a national scale in children. To the best of our knowledge, our study is the first and largest, national study of minimal hearing loss and educational testing in United States children (Moore et al., 2019).
The association between SCHL and reading/verbal reasoning educational test scores persists even when controlling for several potentially confounding covariates that may be associated with both hearing and cognition (e.g., age, sex, family educational level, household income, household language, and a history of intellectual disability). Using multivariable regression models, we have shown that increased hearing thresholds above 0 dB HL and under 25 dB HL, which are generally considered clinically “normal,” still exhibited increased odds of scoring below the 25th percentile on reading tests. For example, a PTA between 1-10 dB HL conferred a 72% increased odds of a score below the 25th percentile compared to those with a PTA ≤ 0 dB HL. Moreover, hearing thresholds within the range of SCHL (i.e., between 1–25 dB HL) exhibited a “dose-dependent” relationship with reading test scores: worse hearing categories, or a higher “dose” of hearing loss, were associated with larger odds of scoring below the 25th percentile.
A dose-dependent association between hearing loss and various domains of cognition, including reading/verbal functioning, executive function, and memory, has previously been shown in adults and children (Lin et al., 2011; Golub et al., 2019; Moore et al., 2019). It has generally been thought that the risk of cognitive impairment begins with mild hearing loss (e.g., 26–40 dB HL) and increases as the severity of HL increases. A recent cross-sectional study in adults in both NHANES and the Hispanic Community Health Study (HCHS) showed a robust and independent association between SCHL and cognitive impairment, specifically global cognitive function, verbal memory, and frontal-executive abilities (Golub et al., 2020c). The present study supports this phenomenon and suggests it may also be present in children. Our findings suggest that even a small hearing loss is associated with decreased educational attainment (i.e., as measured by reading testing performance). Of note, these educational attainment outcomes that assess math and verbal skills also require cognitive processes. Hearing primarily affects reading abilities through an impact on language development, as students struggle to grasp the nuances of oral communication, which subsequently impacts their ability to understand syntax and other factors involved in reading (Carney and Moeller, 1998). Hearing loss may also affect educational attainment through inability to hear and understanding of concepts being communicated by the teacher.
In these participants with SCHL, the effect sizes (i.e., differences in test scores per unit change in hearing) exhibit a moderate strength with the greatest difference between the mean average test scores being 2.5 points between the best and worst hearing threshold categories (Cohen's d = 0.658). Subsequently, a 2.5-point difference is equivalent to approximately three-quarters of a standard deviation. However, the true clinical impact of such a deficiency in scores for these tests in the real world is uncertain and warrants further research. Even small variations in hearing loss can impact absolute scores. On logistic regression analysis, children with hearing of 10-25 dB HL are 3 times as likely to perform within the bottom 25th percentile on intelligence tests compared to children with hearing within what is considered normal limits (i.e., ≤ 0 dB HL) after controlling for many factors associated with lower standardized test scores. This relationship was maintained for those within the bottom 50th and 10th percentile.
Several terms have referred to hearing ≤ 25 dB HL, including “minimal” HL, but fundamentally hearing under this dB HL threshold is ill-defined (Tharpe and Bess, 1991). Children in this category have also previously been assumed to have few, if any, special needs and require no accommodations in the academic setting (Tharpe and Bess, 1991). Our study supports these previous findings in a national sample. Although previous studies have investigated the effect of minimal hearing loss (varying definitions, but e.g., 10–25 dB HL) in children (McFadden and Pittman, 2008; Moore et al., 2020), few reports have looked at the potential effects of SCHL (1–25 dB HL), and none that look at specifically extremely minimal hearing thresholds (1–10 dB) in a population-based, national survey of the United States.
In our cohort, the association between SCHL and educational test performance is only maintained in the reading test, which is not surprising considering the impact of auditory deprivation on verbal skills (Furth, 1964; Wilson et al., 1975). This supports our overarching hypothesis that SCHL is linked to worsening educational attainment, as such a link should disproportionately affect skills that more heavily rely on auditory input (e.g., reading) over those that less heavily rely on auditory input (e.g., math). While worse hearing will likely impact all forms of intelligence (other than language-based skills), we are examining minimal hearing which likely affects only the skills that are most vulnerable or dependent on hearing. Therefore, our findings suggest that language and speech impairments may begin with very minimal hearing loss.
The acoustic environment of a classroom can greatly affect speech perception and learning in children, which ultimately affects academic achievement (Tharpe et al., 2009). Many studies have documented poor acoustic conditions in classrooms (McCroskey, 1975; Picard and Bradley, 2001; Knecht et al., 2002). Children have also been shown to require more favorable acoustic environments compared to adults to obtain equivalent speech recognition scores (Elliott, 1979; Johnson, 2000; Stuart, 2005). Research has suggested that children need approximately a +15 dB signal-to-noise ratio to learn effectively, a condition that most classrooms do not achieve (Goldberg and Richburg, 2004; American Speech-Language Hearing Association, 2005). As a result, children can lose voiceless consonant sounds (e.g., /f/,/th/,/s/) which can have a considerable effect on grammar development since these sounds are used to designate plurals, third-person verbs, and object possession. Children with SCHL will have an added difficulty, and therefore have slightly worse comprehension in learning environments. Children with SCHL may also experience more fatigue because the increased listening effort is required to perform at the same level (e.g., equivalent speech recognition) as their better-hearing peers; this has been shown in children with 15–40 dB HL of hearing loss (Hick and Tharpe, 2002). Thus, children with SCHL may overall have decreased opportunities to learn both at school and incidentally from their surrounding environment (Pagliaro and Kritzer, 2013).
Our study has several advantages. NHANES includes a national sample of children, which enables our study to have greater generalizability than prior studies with non-national cohorts. One noteworthy study of four centers in the UK established similar observations seen in our cohort (Moore et al., 2019). Similar to our study, this study identified a relationship between minimal hearing loss (defined as <20 dB HL but with one frequency ≥ 15 dB) with several different cognitive and learning outcomes. However, this sample did not examine the effect of 1–10 dB HL of hearing loss, and explored a less nationally acquired sample of the United Kingdom. The power and generalizability of our conclusions are amplified when applying the NHANES weighting protocol for our sample. Both the UK study and our own conclude that many children with classically undetected and untreated hearing could experience deficits in several different cognitive and educational functions.
This study has several limitations. Firstly, NHANES III is several decades old. However, it is the only national database that includes both audiograms and comprehensive cognitive testing for children. Additionally, since the NHANES audiometric protocol only included air conduction, we were not able to distinguish between conductive and sensorineural hearing loss. Even though we eliminated participants with flat tympanograms to attempt to eliminate those who may have conductive hearing loss from effusions or tympanic membrane perforations, this imperfect.mperfect. Additionally, this study lacks longitudinal data; as a result, we cannot establish a temporal relationship, i.e., that SCHL earlier on has a later association with cognitive performance. Finally, weighting protocols, while making the analysis more representative of the United States, carry their own biases and can skew conclusions. Additionally, while our educational testing is robust, there are limitations to these tests. The tests included are subscales from the WISC-R and WRAT-R to capture specific aspects of arithmetic, reading, and other performance tasks. While tests evaluating educational attainment may be related to cognition, they may not be directly indicative of cognition compared to tests that capture more abstract concepts of cognition. These cognitive tests should also be further investigated to fully characterize the effect of subclinical hearing loss on cognition in children. Some might argue that the included tests provide too broad of an estimate of academic achievement and contain little specific information within the various subsets. Finally, we chose to focus primarily on hearing as a risk factor for educational tests. Future studies should examine other risk factors, including the covariates we studied. Future studies should also look at effect modification, for example whether the effect of SCHL differs based on socioeconomic status.
The cross-sectional nature of the study prevents us from making definitive conclusions regarding the mechanism of association, as well as the ability to rule out reverse causation. It seems implausible that educational testing ability affects peripheral hearing levels. However, it is conceivable that young children with better cognition/educational performance can better pay attention and perform better on hearing testing. Studies have shown that audiometric testing has many sources of error including lack of the ability to participate, willingness to participate, instructions, learning, effects, and executive function which is associated with behavioral processes associated with attention (Pierson et al., 2007). This is especially true with manual audiometric techniques, which were used in the NHANES III survey, as opposed to automated (e.g., Bekesy) techniques. Given the mean age of 11 years old, participants should be fairly capable of relatively simple tasks such as audiometric testing. Moreover, because this is not a randomized controlled trial, there may be confounding variables that are associated with both hearing loss and cognition. Our study attempted to address this by including several model covariates that might be confounders. There is also debate regarding the effect of cultural biases and language differences on the cognitive tests included in NHANES (Neisser and Bouchard, 1996). Research suggests cultural and language differences may play a role in academic and educational testing performance, even within our study. Since NHANES does not capture these culture differences, future studies will require prospectively collected, matched cohorts to control for the effect of confounding.
These findings support the hypothesis that minor differences in hearing, even within the so-called “normal range” of hearing, is crucial for certain aspects of educational performance and learning in children. It is difficult to define the level of hearing loss at which treatment is indicated. Some specialists argue that the 25-dB HL threshold is too lenient, especially for children (Martin and Champlin, 2000). This may indeed be true if hearing loss is shown to have a clinically detectable impact on educational attainment. To mitigate this potential impact of SCHL on cognitive performance in children, it may be beneficial to optimize the acoustics of the learning environment to address variations in hearing among students. For example, the selective use of microphones and speakers can more effectively transfer sound to different parts of the classroom. Students with decreased hearing compared to their peers can be assigned preferential seating at the front of a classroom.
Conclusion
This study demonstrates the association and potential impact of SCHL on certain domains of educational/academic test performance in children. Further studies are necessary to fully understand the mechanism and implications of this phenomenon, in addition to how this should change hearing loss screening and management in schools.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: https://wwwn.cdc.gov/nchs/nhanes/nhanes3/default.aspx.
Author contributions
RS, AC, JG, and AL: writing, analysis, conception, data interpretation, and editing. All authors contributed to the article and approved the submitted version.
Conflict of interest
JG: consulting fees (Alcon). AL: advisory board (Advanced Bionics, Med El, Spiral Therapeutics).
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author AL declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fauot.2023.1214188/full#supplementary-material
References
Aldea Martinez, J., Aldea Viana, L., Lopez Martinez, J. L., and Ruiz Perez, E. (2019). Radiofrequency ablation of thyroid nodules: a long-term prospective study of 24 patients. J. Vasc. Interv. Radiol. 30, 1567–1573. doi: 10.1016/j.jvir.2019.04.022
American Speech-Language Hearing Association (2005). Guidelines for Addressing Acoustics in Educational Settings. ASHA Working Group on Classroom Acoustics. American Speech-Language-Hearing Association.
Andrews, J. J. W. S., Donald, H. J., and Henry, L. (2001). Handbook of Psychoeducational Assessment - Ability, Achievement, and Behavior in Children. Elsevier (2001).
Bess, F. H., Dodd-Murphy, J., and Parker, R. A. (1998). Children with minimal sensorineural hearing loss: prevalence, educational performance, and functional status. Ear Hear. 19, 339–354. doi: 10.1097/00003446-199810000-00001
Borg, E., Risberg, A., McAllister, B., Undemar, M., Edquist, G., Reinholdson, A.-C., et al. (2002). Language development in hearing-impaired children. Establishment of a reference material for a 'Language test for hearing-impaired children', LATHIC. Int. J. Pediatr. Otorhinolaryngol. 65, 15–26. doi: 10.1016/S0165-5876(02)00120-9
Caplan, B. (2011). “Wide range achievement test – 4,” in Encyclopedia of Clinical Neuropsychology, eds J. S. Kreutzer, J. DeLuca, and B. Caplan (New York, NY: Springer), 2710–2711.
Carney, A. E., and Moeller, M. P. (1998). Treatment efficacy: hearing loss in children. J. Speech Lang. Hear. Res. 41, S61–84. doi: 10.1044/jslhr.4101.s61
Chern, A., Irace, A. L., Sharma, R. K., Zhang, Y., Chen, Q., Golub, J. S., et al. (2021). The longitudinal association of subclinical hearing loss with cognition in the health, aging and body composition study. Front. Aging Neurosci. 13, 789515. doi: 10.3389/fnagi.2021.789515
Chua, S. M., Rickard Liow, S. J., and Yeong, S. H. (2016). Using spelling to screen bilingual kindergarteners at risk for reading difficulties. J. Learn. Disabil. 49, 227–239. doi: 10.1177/0022219414538519
Crandell, C. C. (1993). Speech recognition in noise by children with minimal degrees of sensorineural hearing loss. Ear Hear. 14, 210–216. doi: 10.1097/00003446-199306000-00008
D'Angiulli, A. S. L. (2003). cognitive functioning as measured by the WISC-R: do children with learning disabilities have distinctive patterns of performance? J. Learn. Disabil. 36, 48–58. doi: 10.1177/00222194030360010601
Deal, J. A., Betz, J., Yaffe, K., Harris, T., Purchase-Helzner, E., Satterfield, S., et al. (2017). Hearing impairment and incident dementia and cognitive decline in older adults: The health ABC study. J. Gerontol. A Biol. Sci. Med. Sci. 72, 703–709. doi: 10.1093/gerona/glw069
Drotar, D. D., Wolraich, M., and Dworkin, P. H. (2008). Developmental-Behavioral Pediatrics - Evidence and Practice. Elsevier (2008).
Elliott, L. L. (1979). Performance of children aged 9 to 17 years on a test of speech intelligibility in noise using sentence material with controlled word predictability. J. Acoust. Soc. Am. 66, 651–653. doi: 10.1121/1.383691
Emmett, S. D., and Francis, H. W. (2014). Bilateral hearing loss is associated with decreased nonverbal intelligence in US children aged 6 to 16 years. Laryngoscope 124, 2176–2181. doi: 10.1002/lary.24746
Furth, H. G. (1964). Research with the deaf: implications for language and cognition. Psychol. Bull. 62, 145–164. doi: 10.1037/h0046080
Goldberg, L. R., and Richburg, C. M. (2004). Minimal hearing impairment: major myths with more than minimal implications. Commun. Disord. Q. 25, 152+. doi: 10.1177/15257401040250030601
Golub, J. S., Brewster, K. K., Brickman, A. M., Ciarleglio, A. J., Kim, A. H., Luchsinger, J. A., et al. (2019). Association of audiometric age-related hearing loss with depressive symptoms among hispanic individuals. JAMA Otolaryngol. Head Neck Surg. 145, 132–139. doi: 10.1001/jamaoto.2018.3270
Golub, J. S., Brewster, K. K., Brickman, A. M., Ciarleglio, A. J., Kim, A. H., Luchsinger, J. A., et al. (2020a). Subclinical hearing loss is associated with depressive symptoms. Am. J. Geriatr. Psychiatry 28, 545–556. doi: 10.1016/j.jagp.2019.12.008
Golub, J. S., Brickman, A. M., Ciarleglio, A. J., Schupf, N., and Luchsinger, J. A. (2020b). Association of subclinical hearing loss with cognitive performance. JAMA Otolaryngol. Head Neck Surg. 146, 57–67. doi: 10.1001/jamaoto.2019.3375
Golub, J. S., Brickman, A. M., Ciarleglio, A. J., Schupf, N., and Luchsinger, J. A. (2020c). Audiometric age-related hearing loss and cognition in the hispanic community health study. J. Gerontol. A Biol. Sci. Med. Sci. 75, 552–560. doi: 10.1093./gerona/glz119
Golub, J. S., Brickman, A. M., Ciarleglio, A. J., Schupf, N., and Luchsinger, J. A. (2020d). Association of subclinical hearing loss with cognitive performance. JAMA Otolaryngol. Head Neck Surg. 146, 57–67.
Grizzle, R. (2011). “Wechsler intelligence scale for children, 4th Edn,” in Encyclopedia of Child Behavior and Development, eds S. Goldstein, and J. A. Naglieri (New York, NY: Springer US), 1553–1555.
Health USDo Control HSCfD Statistics PNCfH.. (1998). Data From: National Health and Nutrition Examination Survey III, 1988–1994. National Center for Health Statistics. Available online at: https://wwwn.cdc.gov/nchs/nhanes/nhanes3/default.aspx
Hick, C. B., and Tharpe, A. M. (2002). Listening effort and fatigue in school-age children with and without hearing loss. J. Speech Lang. Hear. Res. 45, 573–584. doi: 10.1044/1092-4388(2002/046)
Idstad, M., Tambs, K., Aarhus, L., and Engdahl, B. L. (2019). Childhood sensorineural hearing loss and adult mental health up to 43 years later: results from the HUNT study. BMC Public Health 19, 168. doi: 10.1186/s12889-019-6449-2
Irace, A. L., Armstrong, N. M., Deal, J. A., Chern, A., Ferrucci, L., Lin, F. R., et al. (2022). Longitudinal associations of subclinical hearing loss with cognitive decline. J. Gerontol. A Biol. Sci. Med. Sci. 77, 623–631. doi: 10.1093/gerona/glab263
Johnson, C. E. (2000). Children's phoneme identification in reverberation and noise. J. Speech Lang. Hear. Res. 43, 144–157. doi: 10.1044/jslhr.4301.144
Knecht, H. A., Nelson, P. B., Whitelaw, G. M., and Feth, L. L. (2002). Background noise levels and reverberation times in unoccupied classrooms: predictions and measurements. Am. J. Audiol. 11, 65–71. doi: 10.1044/1059-0889(2002/009)
Lakens, D. (2013). Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front. Psychol. 4, 863. doi: 10.3389/fpsyg.2013.00863
Lee, S., Kawachi, I., Berkman, L. F., and Grodstein, F. (2003). Education, other socioeconomic indicators, and cognitive function. Am. J. Epidemiol. 157, 712–720. doi: 10.1093/aje/kwg042
Lewis, D. E., Valente, D. L., and Spalding, J. L. (2015). Effect of minimal/mild hearing loss on children's speech understanding in a simulated classroom. Ear Hear. 36, 136–144. doi: 10.1097/AUD.0000000000000092
Lieu, J. E., Tye-Murray, N., and Fu, Q. (2012). Longitudinal study of children with unilateral hearing loss. Laryngoscope 122, 2088–2095. doi: 10.1002/lary.23454
Lieu, J. E. C., Kenna, M., Anne, S., and Davidson, L. (2020). hearing loss in children: a review. JAMA 324, 2195–2205. doi: 10.1001/jama.2020.17647
Lin, F. R., Metter, E. J., O'Brien, R. J., Resnick, S. M., Zonderman, A. B., Ferrucci, L., et al. (2011). Hearing loss and incident dementia. Arch. Neurol. 68, 214–220. doi: 10.1001/archneurol.2010.362
Martin, F. N., and Champlin, C. A. (2000). Reconsidering the limits of normal hearing. J. Am. Acad. Audiol. 11, 64–66. doi: 10.1055/s-0042-1748011
McCroskey, F. D. J. (1975). “Acoustic characteristics of public school classrooms constructed between 1890 and 1960,” in NOISEXPO Proceedings, 101–103.
McFadden, B., and Pittman, A. (2008). Effect of minimal hearing loss on children's ability to multitask in quiet and in noise. Lang. Speech Hear. Serv. Sch. 39, 342–351. doi: 10.1044/0161-1461(2008/032)
Mohadjer, L. M., Waksberg, J., Bell, B., James, P., Flores-Cervantes, M., Montes, I., et al (2021). National 11 Health And Nutrition Examination Survey III Weighting, and Estimation Methodology - Executive Summary National Center for Health Sciences. Available online at: https://wwwn.cdc.gov/nchs/data/nhanes3/manuals/wgt_exec.pdf (accessed December 2022).
Moore, D. R., Zobay, O., and Ferguson, M. A. (2019). Minimal and mild hearing loss in children: association with auditory perception, cognition, and communication problems. Ear Hear. doi: 10.1101/723635
Moore, D. R., Zobay, O., and Ferguson, M. A. (2020). Minimal and mild hearing loss in children: association with auditory perception, cognition, and communication problems. Ear Hear. 41, 720–732. doi: 10.1097/AUD.0000000000000802
National Center for Health Statistics (2021). NHANES III (1988-1994). Available online at: https://wwwn.cdc.gov/nchs/nhanes/nhanes3/default.aspx (accessed December 2022).
Neisser, U. B. G., and Bouchard, T. J. Jr. (1996). Intelligence: knowns and unknowns. Am. Psychol. 55, 77–101. doi: 10.1037/0003-066X.51.2.77
Pagliaro, C. M., and Kritzer, K. L. (2013). The Math Gap: a description of the mathematics performance of preschool-aged deaf/hard-of-hearing children. J. Deaf Stud. Deaf Educ. 18, 139–160. doi: 10.1093/deafed/ens070
Picard, M., and Bradley, J. S. (2001). Revisiting speech interference in classrooms. Audiology 40, 221–244. doi: 10.3109/00206090109073117
Pierson, S. K., Caudle, S. E., Krull, K. R., Haymond, J., Tonini, R., Oghalai, J. S., et al. (2007). Cognition in children with sensorineural hearing loss: etiologic considerations. Laryngoscope 117, 1661–1665. doi: 10.1097/MLG.0b013e3180ca7834
Rennels, M., and Pickering, L. K. (2005). Sensorineural hearing loss in children. Lancet 365, 2085–2086. doi: 10.1016/S0140-6736(05)66724-4
Ronner, E. A., Benchetrit, L., Levesque, P., Basonbul, R. A., and Cohen, M. S. (2020). Quality of life in children with sensorineural hearing loss. Otolaryngol. Head Neck Surg. 162, 129–136. doi: 10.1177/0194599819886122
Sayegh, P., Arentoft, A., Thaler, N. S., Dean, A. C., and Thames, A. D. (2014). Quality of education predicts performance on the Wide Range Achievement Test-4th Edition Word Reading subtest. Arch. Clin. Neuropsychol. 29, 731–736. doi: 10.1093/arclin/acu059
Sindhar, S., Friesen, T. L., Carpenter, D., Kesser, B., and Lieu, J. E. C. (2021). Fatigue in children with unilateral and bilateral hearing loss. Otol. Neurotol. 42, e1301–e1307. doi: 10.1097/MAO.0000000000003225
Stuart, A. (2005). Development of auditory temporal resolution in school-age children revealed by word recognition in continuous and interrupted noise. Ear Hear. 26, 78–88. doi: 10.1097/00003446-200502000-00007
Su, B. M., and Chan, D. K. (2017). Prevalence of hearing loss in US children and adolescents: findings from NHANES 1988-2010. JAMA Otolaryngol. Head Neck Surg. 143, 920–927. doi: 10.1001/jamaoto.2017.0953
Tharpe, A., Sladen, D., Murphy, J. D., and Boney, S. (2009). Minimal hearing loss in children: minimal but not inconsequential. Semin. Hear. 30, 080–093. doi: 10.1055/s-0029-1215437
Tharpe, A. M., and Bess, F. H. (1991). Identification and management of children with minimal hearing loss. Int. J. Pediatr. Otorhinolaryngol. 21, 41–50. doi: 10.1016/0165-5876(91)90058-J
Wake, M., Hughes, E. K., Poulakis, Z., Collins, C., and Rickards, F. W. (2004). Outcomes of children with mild-profound congenital hearing loss at 7 to 8 years: a population study. Ear Hear. 25, 1–8. doi: 10.1097/01.AUD.0000111262.12219.2F
Wang, J., Quach, J., Sung, V., Carew, P., Edwards, B., Grobler, A., et al. (2019b). Academic, behavioural and quality of life outcomes of slight to mild hearing loss in late childhood: a population-based study. Arch. Dis. Child. 104, 1056–1063. doi: 10.1136/archdischild-2019-316917
Wang, J., Sung, V., Carew, P., Burt, R. A., Liu, M., Wang, Y., et al. (2019a). Prevalence of childhood hearing loss and secular trends: a systematic review and meta-analysis. Acad. Pediatr. 19, 504–514. doi: 10.1016/j.acap.2019.01.010
Wilson, J. J., Rapin, I., Wilson, B. C., and Van Denburg, F. V. (1975). Neuropsychologic function of children with severe hearing impairment. J. Speech Hear. Res. 18, 634–652. doi: 10.1044/jshr.1804.634
Keywords: subclinical hearing loss, pediatric hearing loss, educational performance, cognition, NHANES
Citation: Sharma RK, Chern A, Golub JS and Lalwani AK (2023) Subclinical hearing loss and educational performance in children: a national study. Front. Audiol. Otol. 1:1214188. doi: 10.3389/fauot.2023.1214188
Received: 29 April 2023; Accepted: 17 July 2023;
Published: 03 August 2023.
Edited by:
Z. Jason Qian, Stanford University, United StatesReviewed by:
Adrian Fuente, Montreal University, CanadaPatrizia Mancini, Sapienza University of Rome, Italy
Copyright © 2023 Sharma, Chern, Golub and Lalwani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anil K. Lalwani, YWtsMjE0NEBjdW1jLmNvbHVtYmlhLmVkdQ==
 Rahul K. Sharma
Rahul K. Sharma Alexander Chern
Alexander Chern Justin S. Golub1
Justin S. Golub1 Anil K. Lalwani
Anil K. Lalwani