
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Audiol. Otol. , 22 June 2023
Sec. Clinical Research in Auditory Implants and Hearing Aids
Volume 1 - 2023 | https://doi.org/10.3389/fauot.2023.1207220
Introduction: To date, there is no quality-of-life questionnaire for people with hearing loss based on a multidisciplinary framework. Therefore, this study aimed to develop and validate a comprehensive assessment tool that addresses quality of life in people with hearing loss who use a cochlear implant based on the International Classification of Functioning, Disability, and Health (ICF).
Methods: In a first step, the Quality of Life in People with Hearing Loss Questionnaire (HL-QoL) was developed and tested for face validity. In a second step, the HL-QoL was evaluated and validated. In a third step, the HL-QoL was finalized based on the outcomes of the evaluation and validation.
Results: Eighty-four study participants fully completed the HL-QoL. The result of the test-retest reliability analysis was high and highly significant (n = 63; r = 0.914; p < 0.001). The mean total HL-QoL score (100.7 ± SD 24.58) suggests an overall high level of quality-of-life in this sample of people with hearing loss using a cochlear implant. The final version of the HL-QoL contains 21 items.
Conclusion: The HL-QoL has shown to be a valid and reliable tool to assess quality of life in people with hearing loss who use a cochlear implant. In addition to the total score, it is possible to calculate subscales based on the ICF components Body Functions and Activities and Participation.
The WHO defines health as “a state of complete physical, mental, and social wellbeing, not merely the absence of disease […]” (WHO). Consequently, the measurement of an individual's health and the effect of health care shall include two things: First, the changes in the frequency and severity of diseases, and second the improvement in health-related quality of life (QoL). WHO states that QoL is “an individual's perception of their position in life in the context of the culture and value systems in which they live and in relation to their goals, expectations, standards, and concerns” (WHO). QoL in the context of this research approach focuses on the health-related QoL, i.e., a multi-dimensional concept that includes domains related to physical, mental, emotional, and social functioning (Saikia, 2018). This can be obtained via general QoL measures which allow to compare treatment effectiveness and quality-adjusted life years across different diseases [e.g., the effect of a pacemaker compared to a cochlear implant (CI)], and disease-specific QoL measures that focus on a certain patient population [e.g., people with hearing loss (HL), CI recipients], or a certain indication [e.g., people with single-sided deafness (SSD)]. Patient-reported outcomes have gained in importance and attention in general (Mercieca-Bebber et al., 2018; Holch et al., 2020) and the acceptance of patient-reported QoL outcomes has increased in particular (Doward et al., 2010; Bansal et al., 2015; Squitieri et al., 2017; Rivera et al., 2019; Rudolph et al., 2019).
Hearing implants are a common treatment for HL of different degrees and with different indications. A widely-known and well-accepted treatment for people with severe to profound sensorineural HL is a CI (Lachowska et al., 2014; Ramos Macias et al., 2015).
Different disease-specific measures have been developed and are used to investigate the impact of HL and the benefits people get from hearing aid or hearing implant treatment across various indications (Newman et al., 1996; Hinderink et al., 2000; Gatehouse and Noble, 2004; Kompis et al., 2011; Umansky et al., 2011; Noble et al., 2013; Ambert-Dahan et al., 2018). However, disease-specific measures for people with HL like the Speech, Spatial and Qualities of Hearing Scale (SSQ, Gatehouse and Noble, 2004; Noble et al., 2013) strongly focus on hearing abilities (i.e., hearing performance) in everyday listening situations, hence they mainly focus on what an individual can hear or on the quality of hearing, or more specifically on sound quality, such as the Hearing Implant Sound Quality Index (HISQUI19, Amann and Anderson, 2014). Only few questionnaires focus on how HL impacts someone's QoL (e.g., the International Outcome Inventory for Hearing Aids (IOI-HA) and the Abbreviated Profile of Hearing Aid Benefit (APHAB); (Cox and Alexander, 1995; Aiello and Ferrari, 2011). However, these questionnaires were mainly developed for hearing aid users, not for CI or other hearing implant users (Cox and Alexander, 1995, 2002; Gatehouse, 1999). The APHAB questionnaire (Cox and Alexander, 1995) has been called the gold standard to address communication issues with and without the use of a hearing aid but has also been criticized as follows: the scoring system is rather complex; some items are formulated in a complex way; if the questionnaire has not been fully completed, the scores are not calculated according to the number of questions answered as the calculation does not consider missing answers and it has been claimed that it does not shed light on the impact of HL on the individual's QoL (Ambert-Dahan et al., 2018). The most widely used questionnaire in the CI field is the Nijmegen Cochlear Implant Questionnaire (NCIQ) (Hinderink et al., 2000). It was particularly developed for assessing QoL in CI users. However, it is not always easy to use in the clinical practice because it is rather long (60 items), i.e., it can be time-consuming and effortful to complete (particularly for the elderly). Moreover, all items focus on hearing abilities or problems in different listening situations rather than on QoL. Also, the authors did not perform a complete validation of all subdomains (Hinderink et al., 2000; Ambert-Dahan et al., 2018). Lastly, its items were formulated more than 20 years ago and may thus no longer comply with current expectations and outcomes in CI recipients. According to our literature review, there are two recently developed QoL questionnaires for people with HL. (1) The Évaluation du Retentissement de la Surdité chez l'Adulte, ERSA (Ambert-Dahan et al., 2018). The ERSA includes four sub-scores: QoL, personal life, occupational life, and social life. (2) The Cochlear Implant Quality of Life (CIQOL) (McRackan et al., 2019, 2021). Its 35 items are grouped to 6 domains (communication, emotional, entertainment, environment, listening effort, and social).
However, to date, there (1) is no (international) consensus on which QoL questionnaire(s) are the most appropriate ones to use in both research and clinical practice for CI outcome evaluation (Andries et al., 2020, 2022a; Lassaletta et al., 2022). (2) Our intention was to develop a new viable tool based on an internationally accepted multidisciplinary framework – the International Classification of Functioning, Disability, and Health (ICF) model created by the WHO (https://www.who.int/standards/classifications/international-classification-of-functioning-disability-and-health). HL affects people's life in a multidimensional way and hence a multidisciplinary setting is needed to address their health care needs. To our knowledge, none of the questionnaires currently available is based on a multidisciplinary framework. A shared framework across interprofessional communication and collaboration would be beneficial to the improvement of healthcare. The ICF model may serve as such a framework. This classification model describes an individual's health status and functional capacity (body functions, activities, participation) and disability (impairment, activity limitations, participation limitations). To facilitate the use of the classification model in clinical practice, lists of ICF categories for HL, a “Brief ICF Core Set” and a “Comprehensive ICF Core Set” were developed (Danermark et al., 2013; Granberg et al., 2014a,b). The “Brief ICF Core Set” is a list of ICF categories that serves as a minimum data set that can be reported in any clinical study to provide a standardized description of individuals' experience of HL. The “Comprehensive ICF Core Set” is a list of ICF categories that includes as few categories as possible to make it easy to use, but as many as necessary to describe the typical spectrum of functional problems of individuals with HL in a comprehensive, multidisciplinary assessment. The ICF core sets should help clinicians better address both hearing loss and its consequences.
Based on the “Comprehensive ICF core set for hearing loss”, an international group of CI experts (Andries et al., 2022a,b) recently defined an ICF-based CI outcome assessment protocol for selected questionnaires, pure tone audiometry, speech audiometry, and localization to provide a comprehensive description and measurement of CI outcomes worldwide. The usefulness of the ICF for the categorization of results and framing of questionnaires in a larger concept within the field of HL and CI treatment is shown by two recent publications; (1) the ICF framework was used to categorize individuals according to their localization performance into one of five categories based on the severity of impairment to provide a clearer understanding of the degree of the sound localization impairment (Mertens et al., 2022) and (2) the Work Rehabilitation Questionnaire (WORQ), also based on the ICF, was revised and shortened to focus on items which are relevant to CI users (Andries et al., 2022a).
We aimed to develop and validate a new comprehensive assessment tool that addresses QoL in people with HL who use a CI. The tool should be able to assess the impact of HL on a person's life holistically and the questionnaire should be easy to administer (i.e., short, patient-reported). In a first step, the Quality of Life in People with Hearing Loss Questionnaire (HL-QoL) was developed and tested for face validity. In a second step, the HL-QoL was evaluated and validated. In a third step, the HL-QoL was finalized based on the outcomes of the evaluation and validation.
The following paragraphs describe the development of the initial version of the HL-QoL, the evaluation and validation of the HL-QoL through a longitudinal study, and the final version of the HL-QoL. The selected items were then linked to the biopsychosocial conceptual framework of the International Classification of Functioning, Disability and Health (WHO/ICF). This study focused on adult CI users.
First, existing scales were reviewed. A particular focus was placed on the NCIQ (Hinderink et al., 2000) because it is widely used in the field. The principal aim of a questionnaire in research is to obtain relevant information with the highest possible level of reliability and validity. Therefore, an expert panel (i.e., psychologists, audiologists, speech and language specialists) reviewed items especially from the NCIQ to determine whether they met current standards of CI outcome and latest concepts of QoL and discussed issues that arise during sessions with a rehabilitation specialist before and after CI treatment. Some items were formulated based on aspects and situations described in existing items. Other items describe situations and aspects of HL which have not yet been considered in existing QoL measures for people with HL. The items were then reviewed and finalized by a language professional with a degree in translation and interpretation and a career as senior medical writer to have clearly formulated items in lay language to ensure that all items are understandable to all study participants. This preliminary set of questions was tested for face validity in 11 CI users (Taherdoost, 2016). Based on their feedback, the questions were thoroughly reviewed and adapted, and the initial questionnaire version was created.
The initial questionnaire version consisted of 23 items on a 7-point Likert scale ranging from always (1) to never (7). Participants could tick the answer option “not applicable” (n/a) if the statement did not apply. If a question was not answered or the answer was “not applicable” (n/a) that question was treated as missing value. The maximum number of missing answers allowed per person is two items for the total score, and one item for the subscale analyses. The HL-QoL total score was obtained by adding the numerical values of all items. Likewise, the subscales scores are the sum of all items of each subscale. The higher the total score or the subscale score, the higher the perceived QoL benefit.
The newly developed HL-QOL questionnaire was compared to the NCIQ.
The NCIQ is a disease-specific QoL questionnaire which distinguishes three general domains: physical, psychological, and social functioning. These three domains are further split into six subdomains. The physical domain is divided into basic sound perception (1), advanced sound perception (2), and speech production (3). The psychological domain consists of only one subdomain: self-esteem (4). The social domain is divided into activity (5) and social interaction (6). Items can be answered on a five-point response scale ranging from “never” to “always” or “no” to “good”. If a statement does not apply to a participant, they can tick the answer “not applicable” (n/a). Total scores range from 0 (very poor) to 100 (optimal). As the NCIQ is an accepted and widely used questionnaire, the NCIQ total score and the HL-QoL total score were correlated to examine the criterion validity (see Section 2.5.3).
One hundred twenty-three CI users from the Hannover Medical School were enrolled in this study. The surveys were completed between June and October 2022. For the validation analyses, we only included participants who fully completed the questionnaires, see Section 3.1 for details on the participants included and their demographic data.
This study was conducted according to the principles of the Declaration of Helsinki. The study was reviewed and approved by the Ethics Committee at the Hannover Medical School (No. 10322_BO_K_2022). Participants participated voluntarily in the study.
Participants were asked to complete the HL-QoL questionnaire and the NCIQ (Hinderink et al., 2000). Participants were also asked to complete the HL-QoL questionnaire a second time within 2 to 4 weeks after their first completion.
The psychometric characteristics of the HL-QoL items were examined based on the classical test theory model (Crocker and Algina, 1986; Rust and Golombok, 2000). Missing data was treated as “missing values”. The maximum number of incomplete answers allowed per participant was set to two items for the HL-QoL and three items per domain for the NCIQ. In case of more missing items, the participant's responses were excluded from the validation analyses.
IBM SPSS Statistics Version 25 (IBM, Armonk, New York, US) was used for all statistical analyses. The significance level was set to p ≤ 0.05. The Kolmogorov-Smirnov test and the Shapiro-Wilk test were used beforehand to check the data distribution. If both tests confirmed that the data were normally distributed, parametric statistical methods were applied. Otherwise, non-parametric statistical methods were applied.
The item difficulty index and the item discrimination were examined to confirm the selected items for the final version of the questionnaire. The difficulty index shows the proportion of respondents who agreed with the statement. An item difficulty from 0.5 to 0.7 reflects a balanced mix between respondents who agreed to the statement vs. those who disagreed; item difficulty from 0.7 to 0.9 reflects that more respondents agreed to the statement compared to those who disagreed; and an item difficulty from 0.9 to 1.0 reflects that the statement was agreed by most respondents and hence the item might not differentiate enough. A difficulty index between p = 0.3 and p = 0.9 is deemed to be satisfactory (Ebel and Frisbie, 1986).
Item discrimination reflects the extent to which the individual items correlated with the total score. The more single items correlated with the total score and the lower the variability of these correlations, the higher the item homogeneity (Adkins Wood, 1960). These relationships are described by the item discrimination index. In terms of discrimination index, a value of 0.5 or higher is considered satisfactory (Ebel and Frisbie, 1986).
The scale's internal consistency (reliability) was tested using Cronbach's alpha. Guttman‘s split-half-coefficient was calculated to estimate the full test reliability of the questionnaire. Typically, a score of 0.7 or above is considered an acceptable level for internal consistency. A value of α = 0.96 indicates very high internal consistency (Cronbach, 1951; Nunnally, 1978).
Test-Retest reliability shows how likely it is that a participant would obtain the same score if they took the test again. According to Kelley (1939), a coefficient between 0.80 and 0.90 is very good, and 0.90 or above is excellent, whereas a coefficient of 0.50 or below has questionable reliability.
Principal component analysis (PCA) was performed to model the inter-relationships among the items, and to check the underlying factor structure of the items (Bortz, 2005). A fixed number of three (3) factors was chosen, as the factor loadings clearly show which item contributes to which factor. Items loading moderately and highly on a factor (≥0.40) were retained. The oblique rotation method “Promax” was chosen because component values were ± >0.32 indicating that factors are correlated. To test the suitability of the items for factor analysis, the Kaiser-Meyer-Olkin (KMO) test (Kaiser and Rice, 1974), and the Bartlett test of sphericity were performed as measures of sampling adequacy.
As an additional approach, the relationship between items (intra-item correlation) was also examined using Pearson correlation.
For the criterion validity, the total score of the HL-QoL was correlated with the total score of the NCIQ. In the original validation paper of Hinderink et al. (2000), however, only (sub)domains were calculated for the NCIQ; a total score can also be calculated as described in several studies (Hirschfelder et al., 2008; Muigg et al., 2019; Vasil et al., 2020; Illg et al., 2022; Rasmussen et al., 2022) and questionnaire validations (Sanchez-Cuadrado et al., 2015; McRackan et al., 2021).
Eighty-four participants (48 female, 35 male, 0 diverse, 1 missing entry) with a mean age of 56.2 years (range: 22–80, SD 13.1 years) were included in the HL-QoL test validation (i.e., first HL-QoL questionnaire fully completed). 39 (32%) of the enrolled study participants did not fully complete the questionnaire.
Most participants stated that they used their audio processor 9 or more hours per day. Audio processors used included OPUS 2, RONDO, RONDO 2, RONDO 3, SONNET, SONNET 2. Further information is shown in Table 1.
Sixty-three participants (those who fully completed the first and second HL-QoL questionnaire) were included for the test-retest reliability. Seventy-four participants (those who fully completed the NCIQ and the first HL-QoL) were included in the criterion validity analysis.
The mean total HL-QOL score of the 84 fully completed questionnaires was 100.7 points (±SD 24.58, range 21–147). The total score was divided into five categories: very poor, poor, moderate, high, and very high self-perceived QoL benefit. Nobody reported a very poor QoL (0- < 30). Five participants (6.0%) reported a poor QoL benefit (≥30 to < 60 points), 22 participants (26.2%) reported a moderate QoL benefit (≥60 to < 90 points), 37 participants (44%) reported a high QoL benefit (≥90 to < 120 points), and 20 participants (23.8%) reported a very high QoL benefit (≥120 to 147 points).
Seventy-four participants completed the NCIQ (the NCIQ outcomes of 10 participants were excluded because of too many missing answers). The mean total score was 69.4 (SD ± 13.6). Table 2 shows the descriptive results of the NCIQ total score and the subdomains.
The distribution of the responses indicated that participants generally used the full range of answer options. Means ranged from 3.7 to 6.4 with an average deviation of ~1.6. No ceiling effects were detected, except for items 9 (41.7%), 14 (51.2%), and 19 (42.9%); no floor effects were identified.
For the HL-QoL, the item difficulty index ranged from 0.36 to 0.88 (see Table 3 for details) which reflects a balanced mix between agreement and disagreement to an item. For the HL-QoL items, the discrimination index was satisfying and ranged from 0.508 to 0.867 for 20 out of 23 items (see Table 3 for details). Items 14 and 19 had an index of 0.373 and 0.465 (see more information on excluding these items in the Section 4). We kept item 21 which had an index of 0.497 because it almost reached a good discrimination index of 0.5 and above.
The questionnaire has a high reliability with high internal consistency (Cronbach's α, 0.956; Guttman's split-half-coefficient, 0.909).
The result of the test-retest reliability analysis was high and highly significant (n = 63; r = 0.914; p < 0.001).
Results of the KMO test (KMO test result: 0.812), and the Bartlett test of sphericity (c2 = 895.572, df = 253, p < 0.001) confirmed that the items were appropriate for an exploratory factor analysis procedure (Kaiser and Rice, 1974). The 23 items loaded mainly on the first three factors which explained 66.7 % of the total variance. While 19 items loaded highly on factor 1 and on factor 2, items 9 and 21 loaded mainly on factor 2. Items 14 and 19 loaded mainly on factor 3 (see Table 4 for details).
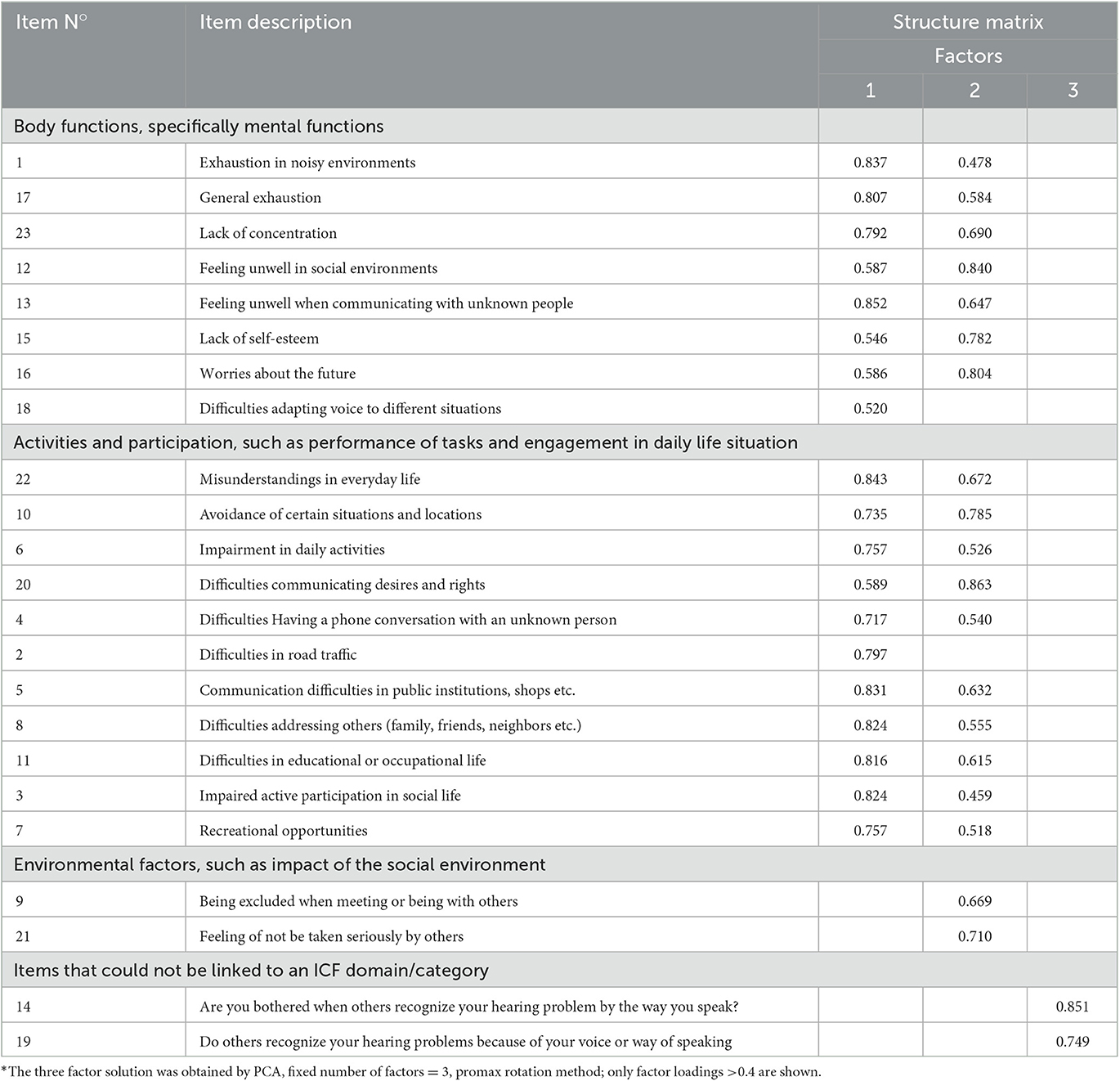
Table 4. Factor pattern matrix for the initial HL-QOL 23-item version* (items listed according to their ICF content).
Referring to the QoL concept of the International Classification of Functioning, Disability, and Health (ICF), those 19 items that loaded highly on factor 1 and factor 2 could be linked to the component Functioning and Disability: eight items could be linked to one or two ICF categories within the component Body Functions assessing possible impairments in “energy and drive functions, attention functions, emotional functions, and voice and speech functions” (see Table 5). 11 items could be linked to one or more ICF categories within the component Activities and Participation, such as “learning and applying knowledge, communication, mobility, relationships, and social life” (see Table 6).
Items 9 and 21 that loaded only on factor 2 could be linked to “e4 attitudes”, an ICF domain that belong to the component Environmental Factors part of the Contextual Factors of the classification (Table 7).
Items 14 and 19 loaded on factor 3, but could not be assigned to any other item or to any ICF domain, and in addition did not obtain good item analysis results. Items 14 and 19 were therefore excluded from the final questionnaire version (see Section 4). This resulted in a reduction of the 3-factor solution, to a 2-factor solution and a 21 item version of the questionnaire. The 2-factor solution adequately captured the underlying structure and the items loaded clearly on the 2 factors based on their content (see Tables 4, 8).
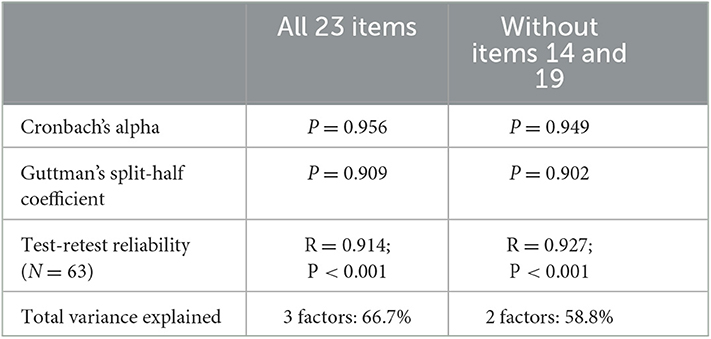
Table 8. Reliability results and total variance explained compared when all 23 items are included to when items 14 and 19 are excluded.
Three HL-QoL items that could be linked to ICF categories from the ICF Core Set could also be linked to another ICF category that is not explicitly included in the ICF Core Set but belongs to the same ICF domain: Item 10 was linked to d175 “Solving Problems” which is included in the ICF Core Set, but this item could also be linked to d177 “Making decisions” which is not included in the ICF Core Set, but is present in the same ICF domain d1 “Learning and applying knowledge”, or could even be seen as a coping strategy, which is a Personal Factor (PF) but has not yet been developed by the WHO. Item 6 was linked to d240 “Handling stress and other demands” but could also be linked to d230 “Carrying out daily routine” within the same ICF domain, d2 “General tasks and demands”. Item 2 was linked to d470 “Using transportation” which is included in the ICF Core Set but could also be linked to d460 “Moving around in different locations” which is not included in the ICF Core Set but is present in the same ICF domain d4 “Mobility”.
Overall, the items were positively and significantly inter-correlated (Pearson correlation: p < 0.001 to p = 0.036). However, item 21 did not significantly correlate with item 2 (p = 0.053) and item 18 (p = 0.067). Items 14 and 19 did not significantly correlate with 16 items (items 1, 2, 3, 5, 6, 7, 9, 10, 11, 12, 13, 14, 16, 17, 18, and 21; p = 0.086–0.883).
A significant correlation between the total HL-QoL score and the total NCIQ score was observed (n = 74; r = 0.866; p < 0.001), which shows high criterion validity of the newly developed QoL questionnaire. Significant correlations (Pearson correlation) were also found between the ICF components Body Functions (BF) and Activities and Participation (AandP), and appropriate NCIQ subdomains.
We investigated age, gender, and wearing time of the audio-processor as potential variables influencing HL-QoL. No significant correlation was found between the total HL-QoL score and age (Pearson correlation: r = 0.059; p = 0.596). When stratified for age (< 60 yrs. vs. ≥60 yrs.), the relationship between the total HL-QoL score and age remained non-significant (independent samples t-test: t = −644; df = 82; p = 0.522). Gender did not influence self-perceived QoL (independent samples t-test: t = 1.537; df = 81; p = 0.128). Wearing time of the audio processor did not have a significant effect on QoL (left ear: ANOVA: F = 1.933; df = 4; p = 0.118; right ear: ANOVA: F = 2.328; df = 2; p = 0.107).
The aim of the present study was to develop and validate a short and user-friendly QoL questionnaire for individuals with a HL who use a CI. Results from 84 CI users showed that the HL-QoL is a valid and reliable tool to assess QoL. With only 21 items in its final version, the questionnaire is not only quick and easy to complete but can also be easily integrated in clinical routine aftercare. Scoring is straightforward (i.e., 7-point Likert scaling, no reversed items, one total score). The items were linked to the ICF model taking the multidimensional and multidisciplinary setting of HL and its treatment into account. Unlike many published questionnaires, the items are not limited to the ability to hear or to the quality of hearing. The questionnaire provides a holistic perspective on how HL can impact an individual's QoL. Based on the ICF model, the questionnaire may facilitate interdisciplinary exchange and communication within the team of professionals involved in an individual's treatment. Together with other standards and questionnaires using the ICF model (Granberg et al., 2014a; van Leeuwen et al., 2020; Andries et al., 2022b; Mertens et al., 2022), we want to contribute toward standardized measurement and reporting in the field of HL and CI treatment.
Two items which were neither related to any other items, nor to any ICF domain, did not obtain good item analyses results, and loaded separately on the third factor were excluded. For the 21 items of the final questionnaire version, item analyses results were satisfying. Internal consistency and repeatability across time was acceptable (see Table 8). The remaining items explained 58.8% of the total variance (see Tables 4, 8). Validation results and the strong spread of the total score results show that the HL-QoL is sensitive enough to depict self-perceived QoL benefit in participants with a HL who use a CI.
We linked the selected items to the biopsychosocial conceptual framework of the WHO, the ICF classification model, as this classification describes an individual's health status and functional capacity (body functions, activities, participation) and disability (impairment, activity limitations, participation limitations). The selected items particularly covered the components Body Functions and Activities and Participation, and additionally the component Environmental Factors. Selected ICF categories of the component Body Functions can be used to describe potential impairments of people with HL. The component Activities and Participation shows limitations or difficulties that an individual with HL may have when performing tasks in different daily life situations. The component Environmental Factors reflects the general or specific opinions and beliefs of others about an individual with HL that may reflect as a barrier either through presence (for example, negative attitudes toward people with disabilities) or absence (for example, the unavailability of a needed service).
The study also confirmed that the obtained ICF categories were all included in the “Comprehensive ICF Core Set for Hearing Loss”, a list of ICF categories to facilitate the use of the classification model in clinical practice in people with HL within the framework of a comprehensive and multidisciplinary assessment (Danermark et al., 2013; Granberg et al., 2014a,b).
The criterion validity of the HL-QoL was confirmed by correlating its total score with the NCIQ, one of the most frequently used QoL questionnaires in the field of CIs. The mean NCIQ total score obtained from our sample resembles those reported in the literature, suggesting our sample to be representative (Hirschfelder et al., 2008; Vasil et al., 2020; Rasmussen et al., 2022).
Improved speech perception in noise can have an impact on QoL. However, there is conflicting evidence in the literature as to whether and how strongly QoL measures and speech perception outcomes correlate (or not) with each other (McRackan et al., 2018). It is difficult to correlate disability which depends strongly on environmental and personal factors with a quantitative assessment of deficit (Ambert-Dahan et al., 2018), especially as speech perception tests are performed in a standardized way and an audiologic booth hardly resembles hearing in everyday situations (Lassaletta et al., 2022). Therefore, we did not evaluate speech perception outcomes in this study.
Further research will evaluate the questionnaire's sensitivity to CI and hearing aid treatment and investigate its validity in different languages and settings.
The new HL-QoL questionnaire provides a holistic perspective on how HL can impact an individual's QoL. The questionnaire is based on the ICF model. Hence, it enables interdisciplinary exchange and communication within the team of professionals involved in an individual's treatment.
According to our validation analyses, the total HL-QoL score clearly and reliably represents the concept of QoL. The HL-QoL has shown to be a valid and reliable tool to assess QoL in people with hearing loss who use a CI. In addition to the total score, it is possible to calculate subscales based on the ICF components Body Functions. This may be beneficial to patient counseling, (re)habilitation, or specific research.
The final version of the HL-QoL contains 21 items. The mean total HL-QoL score (100.7 ± SD 24.58) and the mean NCIQ score (69.4, SD ± 13.6) suggest an overall high QoL benefit in our sample of people with HL using a CI.
The data presented in this study are available on reasonable request from the corresponding author.
The studies involving human participants were reviewed and approved by the Ethics Committee at the Hannover Medical School (No. 10322_BO_K_2022). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Conceptualization and writing—review and editing: AI, EA, KK, IA, TL, and MB-F. Methodology: AI, EA, KK, IA, and MB-F. Formal analysis: EA and KK. Data curation: AI. Writing—original draft preparation: MB-F. Visualization: EA. All authors have read and agreed to the published version of the manuscript.
MED-EL covers the processing costs of the publication.
The authors would like to thank the study participants for their time and effort. Dovile Jankunaite and Laura Sturm provided data management support and Ursula Lehner-Mayrhofer provided writing assistance on a version of this manuscript (all MED-EL).
TL declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision. EA, KK, IA, and MB-F were employed by MED-EL Elektromedizinische Geräte GmbH. The authors declare that this study received funding from MED-EL, Elektromedizinische Geräte GmbH. The funder had the following involvement in the study: Conceptualization and writing—review and editing: EA, KK, IA, MB-F. Methodology: EA, KK, IA, and MB-F. Formal analysis: EA and KK. Writing—original draft preparation: MB-F. Visualization: EA.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Adkins Wood, D. (1960). Test. construction:. Development. and. Interpretation. of. Achievement. Tests. Columbus OH: Charles E. Merrill Books, Inc.
Aiello, C. P., and Ferrari, D. V. (2011). Validity and reliability of the hearing handicap inventory for adults. Braz. J. Otorhinolaryngol. 77, 432–438. doi: 10.1590/S1808-86942011000400005
Amann, E., and Anderson, I. (2014). Development and validation of a questionnaire for hearing implant users to self-assess their auditory abilities in everyday communication situations: the Hearing Implant Sound Quality Index (HISQUI19). Acta. Otolaryngol. 134, 915–923. doi: 10.3109/00016489.2014.909604
Ambert-Dahan, E., Laouénan, C., Lebredonchel, M., Borel, S., Carillo, C., Bouccara, D., et al. (2018). Evaluation of the impact of hearing loss in adults: Validation of a quality of life questionnaire. Eur. Ann. Otorhinolaryngol. Head. Neck. Dis. 135, 25–31. doi: 10.1016/j.anorl.2017.09.003
Andries, E., Gilles, A., Topsakal, V., Vanderveken, O., Van de Heyning, P., Van Rompaey, V., et al. (2022a). The impact of cochlear implantation on health-related quality of life in older adults, measured with the Health Utilities Index Mark 2 and Mark 3. Eu. Archives. Oto-Rhino-Laryngol. 279, 739–750. doi: 10.1007/s00405-021-06727-3
Andries, E., Gilles, A., Topsakal, V., Vanderveken, O. M., Van de Heyning, P., Van Rompaey, V., et al. (2020). Systematic review of quality of life assessments after cochlear implantation in older adults. Audiol. Neurootol. 4, 1–15. doi: 10.1159/000508433
Andries, E., Lorens, A., Skarżyński, P. H., SkarZynski, H., Calvino, M., Gavilan, J., et al. (2022b). Evaluating the revised work rehabilitation questionnaire in cochlear implant users cochlear implant outcome assessment based on the international classification of functioning, disability, and health (ICF). Otol. Neurotol. 43, e571–7. doi: 10.1097/MAO.0000000000003524
Bansal, D., Bhagat, A., Schifano, F., and Gudala, K. (2015). Role of patient-reported outcomes and other efficacy endpoints in the drug approval process in Europe (2008-2012). J. Epidemiol. Glob. Health 5, 385–395. doi: 10.1016/j.jegh.2015.04.006
Cox, R. M., and Alexander, G. C. (1995). The abbreviated profile of hearing aid benefit. Ear. Hear. 16, 176–186. doi: 10.1097/00003446-199504000-00005
Cox, R. M., and Alexander, G. C. (2002). The International Outcome Inventory for Hearing Aids (IOI-HA): psychometric properties of the English version. Int. J. Audiol. 41, 30–35. doi: 10.3109/14992020209101309
Crocker, L., and Algina, J. (1986). Introduction to Classical and Modern Test Theory. Hoboken, NJ: ERIC.
Cronbach, L. J. (1951). Coefficient alpha and the internal structure of tests. Psychometrika. 16, 297–334. doi: 10.1007/BF02310555
Danermark, B., Granberg, S., Kramer, S. E., Selb, M., and Möller, C. (2013). The creation of a comprehensive and a brief core set for hearing loss using the international classification of functioning, disability and health. Am. J. Audiol. 22, 323–328. doi: 10.1044/1059-0889(2013/12-0052)
Doward, L. C., Gnanasakthy, A., and Baker, M. G. (2010). Patient reported outcomes: looking beyond the label claim. Health Q. Life. Outcomes 8, 89. doi: 10.1186/1477-7525-8-89
Ebel, R., and Frisbie, D. (1986). Essentials of Educational Measurement. Englewood Cliffs, NJ: Prentice-Hall.
Gatehouse, S. (1999). Glasgow Hearing Aid Benefit Profile: Derivation and validation of a client-centered outcome measure for hearing aid services. J. Am Acad. Audiol. 10, 80–103. doi: 10.1055/s-0042-1748460
Gatehouse, S., and Noble, W. (2004). The speech, spatial and qualities of hearing scale (SSQ). Int. J. Audiol. 43, 85–99. doi: 10.1080/14992020400050014
Granberg, S., Dahlström, J., Möller, C., Kähäri, K., and Danermark, B. (2014a). The ICF Core Sets for hearing loss–researcher perspective. Part I: systematic review of outcome measures identified in audiological research. Int. J. Audiol. 53, 65–76. doi: 10.3109/14992027.2013.851799
Granberg, S., Möller, K., Skagerstrand, Å., Möller, C., and Danermark, B. (2014b). The ICF Core Sets for hearing loss: researcher perspective, Part II: linking outcome measures to the international classification of functioning, disability and health (ICF). Int. J. Audiol. 53, 77–87. doi: 10.3109/14992027.2013.858279
Hinderink, J. B., Krabbe, P. F., and Van Den Broek, P. (2000). Development and application of a health-related quality-of-life instrument for adults with cochlear implants: the Nijmegen cochlear implant questionnaire. Otolaryngol. Head Neck Surg. 123, 756–765. doi: 10.1067/mhn.2000.108203
Hirschfelder, A., Grabel, S., and Olze, H. (2008). The impact of cochlear implantation on quality of life: the role of audiologic performance and variables. Otolaryngol. Head Neck Surg. 138, 357–362. doi: 10.1016/j.otohns.2007.10.019
Holch, P., Absolom, K., Brooke, C., and Wang, X. (2020). Advances in patient reported outcomes: integration and innovation. J. Pat.-Rep. Outcomes 4, 28. doi: 10.1186/s41687-020-00193-x
Illg, A., Lukaschyk, J., Kludt, E., Lesinski-Schiedat, A., and Billinger-Finke, M. (2022). Do not go gentle into that deaf night: a holistic perspective on cochlear implant use as part of healthy aging. J. Pers. Med. 12, 1658. doi: 10.3390/jpm12101658
Kaiser, H. F., and Rice, J. (1974). Educational and psychological Measurement 34, 111–117. doi: 10.1177/001316447403400115
Kelley, T. (1939). The selection of upper and lower groups for the validation of test items. J. Educ. Psychol 30, 17–24. doi: 10.1037/h0057123
Kompis, M., Pfiffner, F., Krebs, M., and Caversaccio, M. D. (2011). Factors influencing the decision for Baha in unilateral deafness: the Bern benefit in single-sided deafness questionnaire. Adv. Otorhinolaryngol 71, 103–111. doi: 10.1159/000323591
Lachowska, M., Pastuszka, A., Glinka, P., and Niemczyk, K. (2014). Benefits of cochlear implantation in deafened adults. Audiol. Neurootol 19, 40–44. doi: 10.1159/000371609
Lassaletta, L., Calvino, M., Sanchez-Cuadrado, I., Skarzynski, P. H., Cywka, K. B., Czajka, N., et al. (2022). Using generic and disease-specific measures to assess quality of life before and after 12 months of hearing implant use: a prospective, longitudinal, multicenter, observational clinical study. Int. J. Environ. Res. Public. Health 19, 503. doi: 10.3390/ijerph19052503
McRackan, T. R., Bauschard, M., Hatch, J. L., Franko-Tobin, E., Droghini, H. R., Nguyen, S. A., et al. (2018). Meta-analysis of quality-of-life improvement after cochlear implantation and associations with speech recognition abilities. The Laryngoscope 128, 982–990. doi: 10.1002/lary.26738
McRackan, T. R., Hand, B. N., Velozo, C. A., and Dubno, J. R. (2019). Development of the cochlear implant quality of life item bank. Ear. Hear. 40, 1016–1024. doi: 10.1097/AUD.0000000000000684
McRackan, T. R., Hand, B. N., Velozo, C. A., and Dubno, J. R. (2021). Validity and reliability of the cochlear implant quality of life (CIQOL)-35 profile and CIQOL-10 Global instruments in comparison to legacy instruments. Ear. Hear. 42, 896–908. doi: 10.1097/AUD.0000000000001022
Mercieca-Bebber, R., King, M. T., Calvert, M. J., Stockler, M. R., and Friedlander, M. (2018). The importance of patient-reported outcomes in clinical trials and strategies for future optimization. Patient. Relat. Outcome. Meas. 9, 353–367. doi: 10.2147/PROM.S156279
Mertens, G., Andries, E., Kurz, A., Tȧvora-Vieira, D., Calvino, M., Amann, E., et al. (2022). Towards a consensus on an icf-based classification system for horizontal sound-source localization. J. Pers. Med. 12, 1971. doi: 10.3390/jpm12121971
Muigg, F., Bliem, H. R., Kuhn, H., Seebacher, J., Holzner, B., Weichbold, V. W., et al. (2019). Cochlear implantation in adults with single-sided deafness: generic and disease-specific long-term quality of life. Eur. Arch. Otorhinolaryngol. 277, 695–704. doi: 10.1007/s00405-019-05737-6
Newman, C. W., Jacobson, G. P., and Spitzer, J. B. (1996). Development of the Tinnitus Handicap Inventory. Arch Otolaryngol Head Neck Surg. 122, 143–148. doi: 10.1001/archotol.1996.01890140029007
Noble, W., Jensen, N. S., Naylor, G., Bhullar, N., and Akeroyd, M. A. (2013). A short form of the speech, spatial and qualities of hearing scale suitable for clinical use: the SSQ12. Int. J. Audiol. 52, 409–412. doi: 10.3109/14992027.2013.781278
Ramos Macias, A., Falcon Gonzalez, J. C., Manrique, M., Morera, C., Garcia-Ibanez, L., Cenjor, C., et al. (2015). Cochlear implants as a treatment option for unilateral hearing loss, severe tinnitus and hyperacusis. Audiol. Neurootol. 20, 60–66. doi: 10.1159/000380750
Rasmussen, K. M. B., West, N. C., Bille, M., Sandvej, M. G., and Cayé-Thomasen, P. (2022). Cochlear implantation improves both speech perception and patient-reported outcomes: a prospective follow-up study of treatment benefits among adult cochlear implant recipients. J. Clin. Med. 11, 2257. doi: 10.3390/jcm11082257
Rivera, S. C., Kyte, D. G., Aiyegbusi, O. L., Slade, A. L., McMullan, C., Calvert, M. J., et al. (2019). The impact of patient-reported outcome (PRO) data from clinical trials: a systematic review and critical analysis. Health. Q. Life Outcomes 17, 156. doi: 10.1186/s12955-019-1220-z
Rudolph, C., Petersen, G. S., Pritzkuleit, R., Storm, H., and Katalinic, A. (2019). The acceptance and applicability of a patient-reported experience measurement tool in oncological care: a descriptive feasibility study in northern Germany. BMC. Health Serv. Res. 19, 786. doi: 10.1186/s12913-019-4646-4
Rust, J., and Golombok, S. (2000). Modern Psychometrics: The Science of Psychological Assessment. London: Routledge.
Saikia, L. (2018). Review on health related quality of life (HRQOL) of patients after stroke. Int. J. Adv. Res. 6, 57–61. doi: 10.21474/IJAR01/6825
Sanchez-Cuadrado, I., Gavilan, J., Perez-Mora, R., Muñoz, E., and Lassaletta, L. (2015). Reliability and validity of the Nijmegen Cochlear Implant Questionnaire in Spanish. Eur. Arch. Otorhinolaryngol 272, 1621–1625. doi: 10.1007/s00405-014-2983-9
Squitieri, L., Bozic, K. J., and Pusic, A. L. (2017). The role of patient-reported outcome measures in value-based payment reform. Value. Health 20, 834–836. doi: 10.1016/j.jval.2017.02.003
Taherdoost, H. (2016). Validity and reliability of the research instrument; how to test the validation of a questionnaire/survey in a research. Questionnaire/Survey. Res. 10, 1–7. doi: 10.2139/ssrn.3205040
Umansky, A. M., Jeffe, D. B., and Lieu, J. E. (2011). The HEAR-QL: quality of life questionnaire for children with hearing loss. J. Am. Acad. Audiol. 22, 644–653. doi: 10.3766/jaaa.22.10.3
van Leeuwen, L. M., Pronk, M., Merkus, P., Goverts, S. T., Terwee, C. B., Kramer, S. E., et al. (2020). Operationalization of the brief ICF core set for hearing loss: an ICF-based e-intake tool in clinical otology and audiology practice. Ear. Hear. 41, 1533–1544. doi: 10.1097/AUD.0000000000000867
Keywords: hearing loss, quality of life, cochlear implant, patient-report outcomes, HL-QoL, NCIQ
Citation: Illg A, Amann E, Koinig KA, Anderson I, Lenarz T and Billinger-Finke M (2023) A holistic perspective on hearing loss: first quality-of-life questionnaire (HL-QOL) for people with hearing loss based on the international classification of functioning, disability, and health. Front. Audiol. Otol. 1:1207220. doi: 10.3389/fauot.2023.1207220
Received: 17 April 2023; Accepted: 05 June 2023;
Published: 22 June 2023.
Edited by:
Z. Jason Qian, Stanford University, United StatesReviewed by:
Richard Charles Dowell, The University of Melbourne, AustraliaCopyright © 2023 Illg, Amann, Koinig, Anderson, Lenarz and Billinger-Finke. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mareike Billinger-Finke, bWFyZWlrZS5maW5rZUBtZWRlbC5jb20=
†ORCID: Ilona Anderson orcid.org/0000-0001-7518-6661
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.