
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Astron. Space Sci. , 02 March 2023
Sec. Astrochemistry
Volume 10 - 2023 | https://doi.org/10.3389/fspas.2023.1135156
This article is part of the Research Topic Cosmic Dust – its Formation, Processing, and Destruction View all 5 articles
Reactions of SiO molecules have been postulated to initiate efficient formation of silicate dust particles in outflows around dying (AGB) stars. Both OH radicals and H2O molecules can be present in these environments and their reactions with SiO and the smallest SiO cluster, Si2O2, affect the efficiency of eventual dust formation. Rate coefficients of gas-phase oxidation and clustering reactions of SiO, Si2O2 and Si2O3 have been calculated using master equation calculations based on density functional theory calculations. The calculations show that the reactions involving OH are fast. Reactions involving H2O are not efficient routes to oxidation but may under the right conditions lead to hydroxylated species. The reaction of Si2O2 with H2O, which has been suggested as efficient producing Si2O3, is therefore not as efficient as previously thought. If H2O molecules dissociate to form OH radicals, oxidation of SiO and dust formation could be accelerated. Kinetics simulations of oxygen-rich circumstellar environments using our proposed reaction scheme suggest that under typical conditions only small amounts of SiO2 and Si2O2 are formed and that most of the silicon remains as molecular SiO.
The SiO molecule has been observed in the interstellar medium and in stellar outflows and is believed to be important for the formation of interstellar dust, which to a large extent consists of silicates (Hartquist et al., 1980; Clegg et al., 1983; Herbst et al., 1989; Langer and Glassgold, 1990; Sternberg and Dalgarno, 1995; Schilke et al., 1997; Gail and Sedlmayr, 1999; Smith et al., 2004; Gusdorf et al., 2008; Reber et al., 2008; Goumans and Bromley, 2012; Chakraborty et al., 2013; Plane, 2013; Krasnokutski et al., 2014; Bromley et al., 2016; Gobrecht et al., 2016). SiO is also found in terrestrial environments such as the upper atmosphere (from meteoric ablation; see Plane et al., 2016), in combustion of silicon compounds (Jachimowski and McLain, 1983; Britten et al., 1990; Tokuhashi et al., 1990; Chagger et al., 1996; Lindackers et al., 1997; Wooldridge, 1998; Moore et al., 2006) and in industrial silicon production processes (Johansen et al., 1998; Schei et al., 1998; Ravary and Johansen, 1999; Grådahl et al., 2007; Ravary et al., 2007; Kamfjord et al., 2012; Næss et al., 2014). SiO can react with oxygen-bearing species, to form SiO2 (Gómez Martín et al., 2009; Chakraborty et al., 2013). The reactions
and
have been suggested to be important for gas phase SiO2 formation in various environments. In contrast to its carbon analogue, CO2, the thermodynamically most stable state of SiO2 is a condensed phase, silica, at ambient conditions. Molecular SiO2 will readily form SiO2 clusters and eventually condense to silica particles at sufficiently high concentrations (in sufficiently dense media; see Lindackers et al., 1997; Wooldridge, 1998; Næss et al., 2014). SiO could also nucleate directly to form clusters, and through a series of reactions with different species form silica or silicate dust (Reber et al., 2008; Goumans and Bromley, 2012; Plane, 2013; Krasnokutski et al., 2014; Bromley et al., 2016). There is a lack of experimental data on the elementary reactions involved in SiO oxidation, with a few exceptions (Gómez Martín et al., 2009), and computational chemistry techniques are essential in providing mechanistic insight and estimates of the rates of reaction (Zachariah and Tsang, 1995; Becerra et al., 2005; Reber et al., 2008; Gómez Martín et al., 2009; Goumans and Bromley, 2012; Plane, 2013; Hao et al., 2014; Bromley et al., 2016; McCarthy and Gauss, 2016; Yang et al., 2018).
Experimentally, SiO2 formation has commonly been inferred from decay of SiO reactants in reactions with, e.g., H2O, OH, O2, and O3 (Gómez Martín et al., 2009). Molecular SiO2 products have proven harder to observe but have been detected directly in a few studies by time-of-flight mass spectrometry, for instance Yang et al. (2018) by electron-impact ionization of products of the SiH + O2 reaction and by Kostko et al. (2009) who generated gas phase SiO2 by laser ablation of silicon in a CO2 molecular beam followed by photoionization.
In space, SiO has been observed most abundantly in outflows in star forming regions where temperatures range from 10 K to 2000 K (Hartquist et al., 1980; Clegg et al., 1983; Schilke et al., 1997; Gusdorf et al., 2008). The SiO abundance increases rapidly with increasing temperatures. SiO is assumed to be formed mainly through the shock-induced evaporation of nano-to micrometer sized particles, “interstellar dust grains”, which to a large extent consist of silicates. SiO can be formed either directly from the grains or through sputtering of Si atoms that subsequently react with O2 and/or OH. The main gas-phase mechanism for removal of SiO is assumed to be the reaction with OH. The rate of this reaction is highly uncertain, especially at low temperatures.
A large portion of interstellar dust is assumed to be formed in the outflow from Asymptotic Giant Branch (AGB) stars, i.e., dying stars in a red giant phase (Gail and Sedlmayr, 1999; Gail et al 2013). Since the first observations of circumstellar silicate emission bands (Woolf and Ney, 1969; Hackwell et al., 1970) the formation of silicate dust grains represents a challenging problem for both theory and experiments. The theoretical study of Gail and Sedlmayr (1998) based on classical nucleation theory and thermodynamic equilibrium provoked a vibrant discussion in the community (Ali et al., 1998). This discussion points out the importance of non-equilibrium processes, the drawbacks of steady-state rates used in classical homogeneous nucleation theory, and the non-crystalline character of small cluster structures. This is in line with the chemical-kinetic bottom-up approach of the present study.
In oxygen-rich AGB stars the formed dust is mainly in the form of silicates, containing either magnesium or iron or both, and show an olivine-type [general formula (Mg,Fe)SiO4] or pyroxene-type [general formula (Mg,Fe)SiO3] stoichiometry. In AGB outflows, most circumstellar silicates are found to be magnesium-rich and iron-poor (Woitke, 2006; Höfner, 2008). The energies and structures of the most favourable Mg-rich olivine and pyroxene clusters were investigated in a recent study (Macià Escatllar et al., 2019). However, the formation of the corresponding monomer represents a major challenge in modeling the nucleation of astrophysical silicate dust.
Several stepwise mechanisms have been suggested as responsible for dust formation in these environments, where typical physical conditions are pressures of 0.001–0.1 Pa and temperatures of 1,000–1200 K (Goumans and Bromley, 2012; Plane, 2013; Bromley et al., 2016; Gobrecht et al., 2016). Based on density functional theory (DFT) calculations, Goumans and Bromley (2012) suggested a seven-step mechanism starting with the two reactions:
The latter reaction is exothermic and expected to be efficient even though the kinetics of the reaction are unknown. In this mechanism, Si2O3 is a key species, which, once formed, can lead to efficient growth of larger particles in reaction with other species, such as H2O and Mg atoms. Reaction with H2O would lead to formation of a hydroxylated species:
Cluster beam experiments were performed to investigate the agglomeration of SiO molecules (Reber et al., 2006). They revealed atomic segregation of silicon for (SiO)n clusters with sizes larger than n = 7. More recently, this size-dependent trend of silicon segregation in (SiO)n clusters was confirmed by Bromley et al. (2016). They investigated the kinetics of the nucleation of SiO into large (SiO)n clusters using DFT (B3LYP) to calculate structures and energetics. SiO nucleation had previously been suggested as a viable reaction route for initiating dust formation (Reber et al., 2006; Reber et al., 2008). However, this was found to not be an efficient process under the low pressures in the circumstellar environments studied, and only the smallest clusters, Si2O2 and Si3O3, were found to form in any significant amount during a reasonable time scale.
Small silicon oxide species like SiO2, Si2O3, and Si2O4 show strong absorption features between 7.0 μm and 8.0 μm (Gardner et al., 2020). These species, if present, could be detected with the observing facilities of the Atacama Large Millimeter/submillimeter Array (ALMA) and the James Webb Space Telescope (JWST).
Stellar dust formation is fundamental for the galactic chemical evolution as it is responsible for the vigorous mass loss of late-type stars enriching the interstellar medium. The formed silicate grains are later important as providing a catalytic surface for the initial formation of most molecules observed in space, including complex organic molecules, and as providing the solid material that makes up the dusty disks around newly-formed stars eventually creating planetary systems.
Based on the previous work, we decided to study the kinetics of the oxidations and clustering reactions leading to growth of silicon oxide molecules with up to three Si atoms. The reported work does to a large extent build upon parallel studies of the energetics of the reactions of SiO and Si2O2 with OH and H2O (Andersson, to be published).
This paper is structured as follows. First, we describe the computational methods used. Then we present results on master equation calculations of the rate coefficients of relevant reactions. This is followed by a description of the kinetic model. Subsequently, kinetic simulation results are presented on models of circumstellar regions of oxygen-rich AGB stars. Finally, we present some concluding remarks and a summary of the most important findings of this study.
For optimizing molecular structures and calculating vibrational frequencies of species involved in the reactions under study we have used the M06 density functional (Zhao and Truhlar, 2008a; Zhao and Truhlar, 2008b) with the maug-cc-pV (T+d)Z basis sets (Papajak and Truhlar, 2010). This approach was benchmarked against highly accurate CCSD(T) calculations for Si-O-H molecules and was shown to offer accurate energetics at a reasonable computational cost (Andersson, to be published). The mean deviations of M06 energies for stationary points on the potential energy surface for the SiO + OH and SiO + H2O reactions were 1.8 kJ/mol and 6.2 kJ/mol, respectively. In an earlier study by Ma et al. (2019), heats of formation calculated by M06/maug-cc-pV(T+d)Z were within the experimental error bars for SiO, SiO2, Si2O2, and SiO(OH)2 and deviated by only 10 kJ/mol from the experimental value for Si(OH)4. All DFT calculations were performed using the NWChem program package (Valiev et al., 2010).
For the calculation of rate coefficients, Rice–Ramsperger–Kassel–Markus (RRKM) calculations were employed using the Master Equation Solver for Multi-Energy well Reactions (MESMER) program (Glowacki et al., 2012). These calculations are run in a similar fashion as described in Gobrecht et al. (2022). The energetics, rotational constants and vibrational frequencies for molecules and intermediates were taken partly from a separate study (Andersson, to be published) but calculations on the reactions of Si2O3 with OH and H2O as well as clustering reactions of silicon oxide molecules were performed in the current study. Intermediate structures on the potential energy surfaces of the Si2O3 + H2O and Si2O3 + OH reactions are given in Supplementary Tables S1, S2, respectively. Since the potential energy surfaces (PESs) of some reactions are highly complex, especially the reactions with H2O, the reactions were simplified, ignoring high-energy pathways (likely to have insignificant rates) as well as lumping together intermediates with similar structures and energetics into single species (see Section 3.1). Intermediates are assumed to either dissociate back to reactants or to proceed to form products or be collisionally stabilized by background gas, in this case taken to be H2. The internal energies of each intermediate were divided into a contiguous set of grains with a typical width of 110 cm–1 containing a bundle of rovibrational states. Each grain was assigned a set of microcanonical rate coefficients for dissociation into reactants or products. The rate coefficients were determined using an inverse Laplace transform procedure to connect them to capture rate coefficients calculated using long-range transition state theory (Georgievskii and Klippenstein, 2005), in the cases where there is no potential energy barrier to reaction. The collisional transfer probability was estimated using the exponential down model, with the average energy for downward transitions designated <∆Edown> and the upward transition probability determined by detailed balance. <∆Edown> was assumed to be temperature independent and assigned a value of 200 cm–1, appropriate for H2 (Gilbert and Smith, 1990). The master equation, which describes the time evolution of grain populations, was expressed in matrix form and solved to yield rate coefficients of bimolecular and recombination reactions at given temperatures and pressures.
The set of small silicon oxide molecules (or clusters) that were considered in this study are shown in Figure 1 and hydroxylated species are shown in Figure 2. Two main types of reactions that are expected to be important in the initial stages of the growth of silica and silicate particles in AGB outflows starting from SiO, i.e., oxidation reactions where H2O and OH are the oxygen-bearing species as well as clustering reactions of the silicon oxide molecules. The included oxidation reactions are the following:
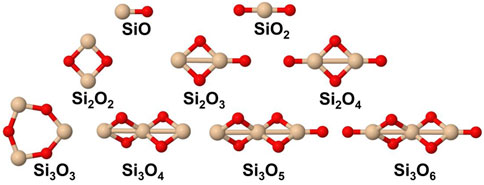
FIGURE 1. Silicon oxide species included in the present study. Silicon atoms are shown in beige and oxygen atoms in red.
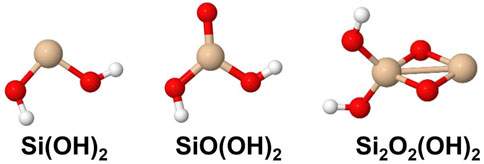
FIGURE 2. Hydroxylated silicon oxide species included in the present study. Silicon atoms are shown in beige, oxygen atoms in red and hydrogen in white.
The clustering reactions are the following:
In what follows the results of rate calculations on these sets of reactions will be presented and discussed. Parameters of modified Arrhenius type rate expressions fit to the calculated rate coefficients can be found in Supplementary Table S3.
The reactions of SiO, Si2O2 and Si2O3 with H2O are described schematically in the potential energy diagrams in Figures 3–5, respectively. Two things are apparent from these figures: (1) The reactions involve a multitude of pathways and reaction intermediates, and (2) there are high barriers to reaction to eventually lead to oxidized product plus H2 (i.e., reactions 1a, 2a, and 3a). As noted in Section 2, the reactions were simplified by ignoring several high-energy pathways and by combining several intermediates that are close in structure and energy. Such combinations are indicated by the dashed lines in the potential energy diagram where several lower barriers and intermediates are left out. In the case of the Si2O3 + H2O reaction, there are six initial reaction paths (see Figure 5) that go from initial intermolecular complexes to the relatively stable intermediate species H(Si2O3)OH (through TS1) or Si2O2(OH)2 (through TS2—TS6). The former reaction is the only pathway that can eventually lead to the formation of Si2O4 + H2 (reaction 4a) whereas the latter pathways either lead to formation of stabilized Si2O2(OH)2 (reaction 4c) or formation of SiO + SiO(OH)2 (reaction 4b). An analogous reaction of the latter reaction is also present for the Si2O2 + H2O reaction leading to the formation of SiO + Si(OH)2 (reaction 3b). In effect these reactions lead to destruction of the larger Si2O2 and Si2O3 back into smaller species. The destruction reactions are endothermic whereas the oxidation reactions forming H2 and Si2O3 and Si2O4 (reactions 3a and 4a), respectively, are somewhat exothermic. One would therefore expect the latter reactions to be more efficient. However, there are higher kinetic barriers leading to these exothermic reactions and as can be seen in Figure 6 the kinetics favors the destruction reactions that are several orders of magnitude faster than the oxidation reactions. Effectively, high abundances of H2O would therefore seem to counteract growth of larger silicon oxide species. The fate of the hydroxylated species, Si(OH)2 and SiO(OH)2, and whether they can initiate other reaction mechanisms is not taken into account here. Figure 7 shows the low-pressure rate constants for the recombination reactions forming the hydroxylated species Si(OH)2 and Si2O2(OH)2 (reactions 1b and 4c). The latter reaction has a large rate coefficient at low temperatures, but it decreases rapidly with increasing temperature. Goumans and Bromley (2012) identified this reaction (4c) as a key reaction in their suggested reaction scheme for the initial stages of silicate formation and even though our results do not directly contradict that assumption, the preceding reaction step would be the formation of Si2O3 from the reaction of Si2O2 and H2O (reaction 3a). Since this was shown to be inefficient above, some other mechanism for the formation of Si2O3 is needed for the reaction scheme to be valid.
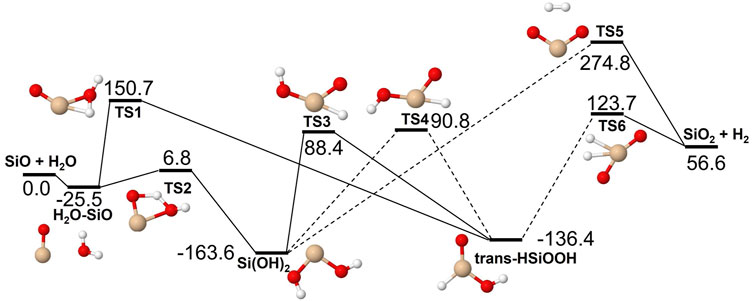
FIGURE 3. Potential energy surface based on M06 calculations for the SiO + H2O reaction as implemented in the RRKM calculations. The energies are given in kJ/mol and include vibrational zero-point energy corrections. Dashed lines indicate that the reaction step has been simplified by combining several similar intermediate isomers.
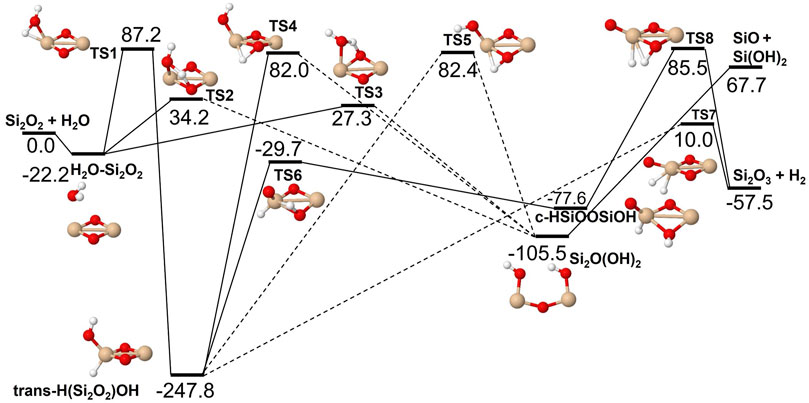
FIGURE 4. Potential energy surface based on M06 calculations for the Si2O2 + H2O reaction as implemented in the RRKM calculations. The energies are given in kJ/mol and include zero-point energy corrections. Dashed lines indicate that the reaction step has been simplified by combining several similar intermediate isomers.
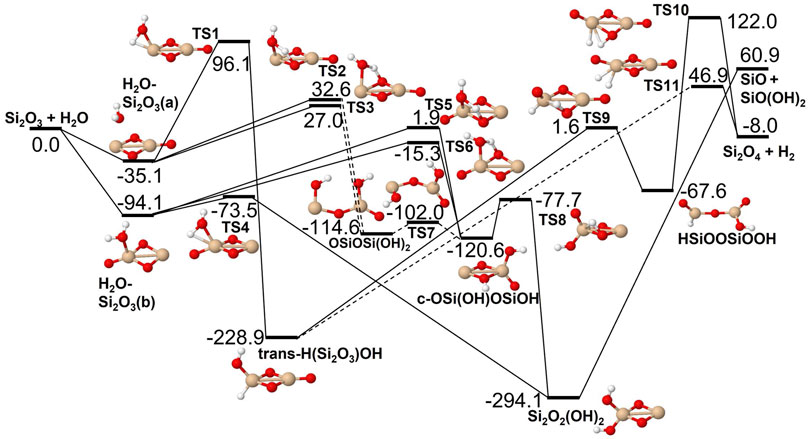
FIGURE 5. Potential energy surface based on M06 calculations for the Si2O3 + H2O reaction as implemented in the RRKM calculations. The energies are given in kJ/mol and include zero-point energy corrections. Dashed lines indicate that the reaction step has been simplified by combining several similar intermediate isomers.
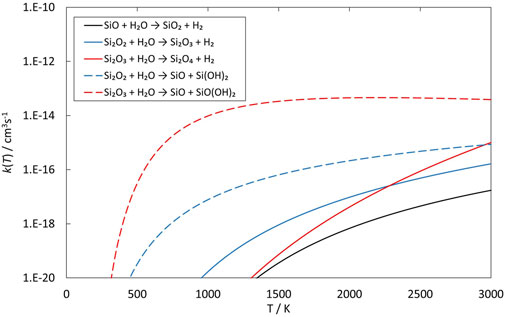
FIGURE 6. Rate coefficients (fit to RRKM calculations) of reactions involving SiO, Si2O2 and Si2O3 reacting with H2O as a function of temperature.
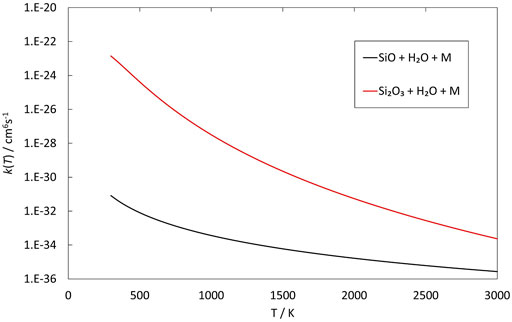
FIGURE 7. Rate coefficients (fit to RRKM calculations) of recombination reactions involving SiO and Si2O3 reacting with H2O as a function of temperature.
Reactions involving the reactive radical OH are expected to be significantly faster than the corresponding reactions with H2O. Figures 8–10 show potential energy surfaces for the reactions of SiO, Si2O2, and Si2O3 with OH (reactions 5, 6, and 7) to form oxidized silicon oxides and H atoms, respectively. These reactions are all exothermic, but the exothermicity of the SiO + OH reaction is so small that the reaction is effectively thermoneutral. Comparing to the reactions with H2O it is also clear that the intermediate reaction barriers are much lower and that there are reaction paths that are lower in energy than the reactant energies. This suggests that the reactions should be fast. It should be noted that in the case of the Si2O3 + OH reaction described in Figure 10, the initial point of attack of OH is important for the final outcome. Only when OH reacts with the Si atom that is bound to only two O atoms, the Si2O4 + H products can be formed. Reaction with the other Si atom, bound to three O atoms, does not lead to reaction or, more precisely, only leads to reaction through high-energy pathways with insignificant reaction rates (not shown in Figure 10). The calculated rate coefficients are shown in Figures 11, 12. In Figure 11, the forward reactions (involving OH) are presented. The reactions with OH are several orders of magnitude faster than the corresponding reactions with H2O (Figure 6), with the rate coefficients for Si2O2 and Si2O3 being basically temperature-independent and the one for SiO having a weakly positive temperature dependence. The reverse reaction of reaction 5, forming SiO + OH from H + SiO2, is faster than the forward reaction over the whole temperature range considered, as also noted by Gómez Martín et al. (2009). The other reverse reactions,
and
are slower than the forward reactions below about 2000 K and 3000 K, respectively, where the equilibrium is reversed, and they become faster instead. To efficiently form Si2O3 from Si2O2, as required in the scheme by Goumans and Bromley (2012), would be possible if a significant fraction of H2O has been dissociated into OH, but temperature should then preferably not be too high.
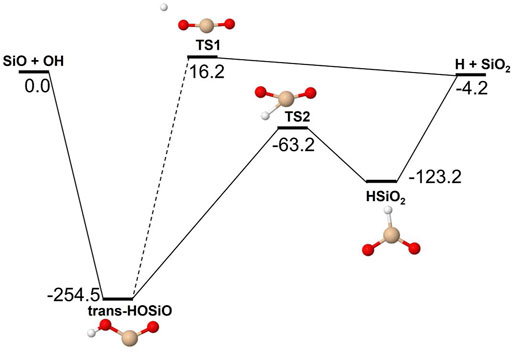
FIGURE 8. Potential energy surface based on M06 calculations for the SiO + OH reaction as implemented in the RRKM calculations. The energies are given in kJ/mol and include zero-point energy corrections. The dashed line indicates that the reaction step has been simplified by combining similar intermediate isomers.
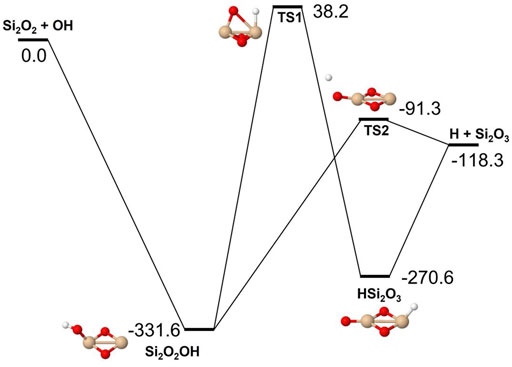
FIGURE 9. Potential energy surface based on M06 calculations for the Si2O2 + OH reaction as implemented in the RRKM calculations. The energies are given in kJ/mol and include zero-point energy corrections.
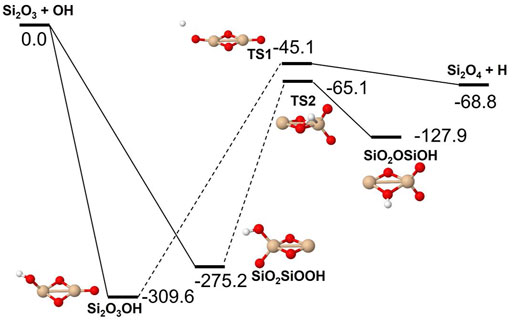
FIGURE 10. Potential energy surface based on M06 calculations for the Si2O3 + OH reaction as implemented in the RRKM calculations. The energies are given in kJ/mol and include zero-point energy corrections. Dashed lines indicate that the reaction step has been simplified by combining several similar intermediate isomers.
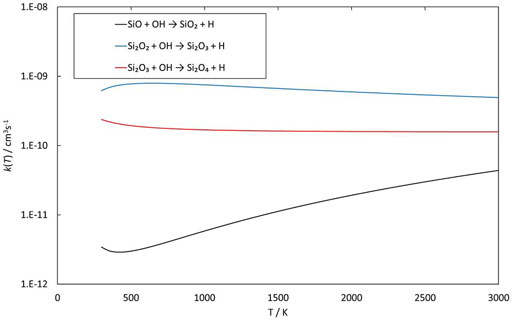
FIGURE 11. Rate coefficients (fit to calculations) as a function of temperature of reactions involving SiO, Si2O2 and Si2O3 reacting with OH.
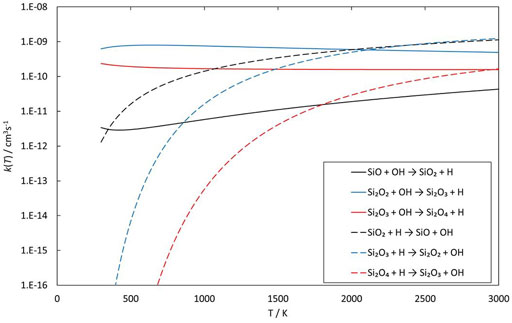
FIGURE 12. Rate coefficients (fit to calculations) as a function of temperature of reactions involving SiO, Si2O2 and Si2O3 reacting with OH and their reverse reactions.
The clustering reactions (reactions 8—16) are all treated as a simple direct recombination of species in a single reaction step, i.e., without intermediates or reaction barriers. Some studies have suggested that there are intermediates and/or small barriers to formation of for instance Si3O3 (reaction 11, Avramov et al., 2005; Pimentel et al., 2006), but we follow Bromley et al. (2016) who concluded that any reaction barriers would be below the reactants and would not appreciably affect the kinetics of SiO clustering reactions. The energetics of the reactions are summarized in Table 1 and calculated rate coefficients (in the low-pressure limit) are shown in Figure 13. All rate coefficients decrease with temperature above 500 K but a few of the reactions are most efficient around 400—500 K. Most of the rate coefficients are quite similar in magnitude and it is only reaction 8, the formation of Si2O2 from two SiO molecules, that is clearly slower than the other reactions. As mentioned earlier, this is one of the key reactions in the silicate formation scheme by Goumans and Bromley (2012), and it does clearly seem to be a bottleneck for further growth of silicon oxides.
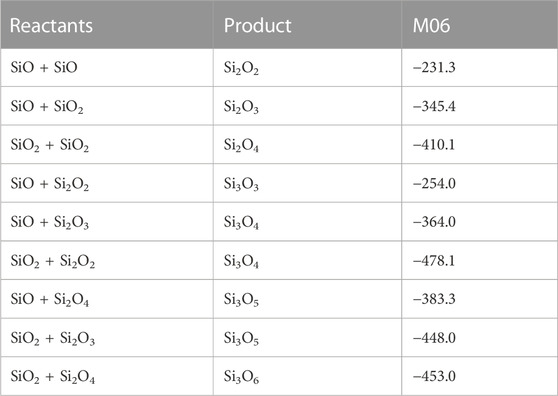
TABLE 1. Reaction energies of clustering reactions calculated using M06/maug-cc-pV(T+d)Z calculations. The energies (at T = 0 K) are given in kJ/mol relative to the respective reactants and include zero-point energy corrections.
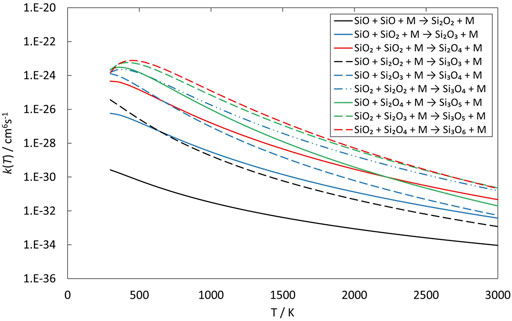
FIGURE 13. Rate coefficients (fit to RRKM calculations) of clustering reactions of small silicon oxide molecules as a function of temperature.
For the hydrodynamic trajectories pertaining to the circumstellar environment of an oxygen-rich AGB star we use the pulsating MIRA model reported in Gobrecht et al. (2022). This model is characterized by a C/O ratio of 0.75, an effective temperature of T = 2000 K and a photospheric density at 1R* of n = 4 × 1014 cm-3. The computational domain extends from 1 to 3 stellar radii and is subject to pulsation-induced shocks that periodically cross the atmosphere with a period of p = 470 days. The resulting post-shock density and temperature profiles are shown in the top and middle panel of Figure 14 as a function of pulsation phase and radial distance.
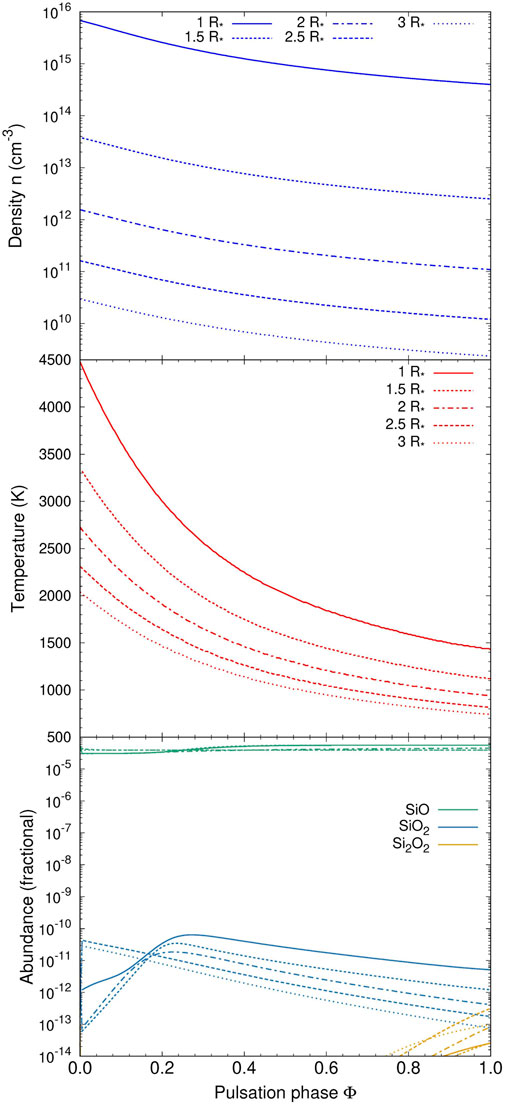
FIGURE 14. Density (top), temperature (middle), and fractional abundances in the kinetic model of an oxygen-rich AGB star as a function of pulsation phase (see text for details).
The chemistry is modeled by a kinetic rate network that includes the prevalent gas phase species H2, CO, CO2, H2O, OH, and SiO, whose initial pre-shock abundances at R = 1R* are given by thermodynamic equilibrium. The corresponding reaction rates are listed as reactions 1–25 in Gobrecht et al. (2022). In addition, rates linking the SixOyHz, x = 1—3, y = 2—6, z = 0—2, clusters are an integral part of the present study, and these were included in our chemical-kinetic network (see Supplementary Table S3). Kinetic parameters were taken from fits to the RRKM calculations described in Section 3.1, except for those for reaction 2 (Plane et al., 2016). The integration of differential rate equations was performed using the Linear Solving of Ordinary Differential Equations (LSODE) solver (Hindmarsh, 1980).
In the bottom panel of Figure 14, the fractional abundances of silicon-, and oxygen-bearing molecules and clusters are shown. The chemistry is dominated by the SiO molecule for all considered radial distances and pulsation phases. SiO2 is formed in modest quantities peaking at phases 0.25—0.3 for radial distances ≤2 R*. In these early post-shock regions, some OH is available and small concentrations of SiO2 are synthesized by its predominant formation channel SiO + OH. The Si2O2 cluster forms only in tiny amounts at late phases (>0.8). All other considered SixOyHz species show negligible abundances with values below 10−14 in the entire computational domain.
We did not include alternative reaction mechanism that could be important, most prominently radiative association. Plane and Robertson (2022) showed that for the SiO2 + H2O recombination reaction, radiative association becomes the dominant mechanism for densities lower than 109 cm-3, which is five orders of magnitude lower than the unshocked photospheric density used in our study. At this and higher densities termolecular recombinations dominate over radiative association.
DFT and RRKM calculations were performed for a number of reactions involving SiO, Si2O2, and Si2O3. Rate coefficients were evaluated in the temperature range 298–3000 K. Reactions involving H2O do not efficiently form the oxidized forms of the silicon oxides, i.e., SiO2, Si2O3, and Si2O4, respectively, but might lead to formation of hydroxylated species instead. The reaction of Si2O3 with H2O to form Si2O2(OH)2 seems to be particularly efficient under the right conditions, i.e., low temperatures and sufficiently high pressure. In contrast, reactions with OH radicals are fast at all temperatures and lead to formation of SiO2, Si2O3, and Si2O4. Especially at high temperatures the reverse reactions become faster than the forward reactions, potentially decreasing the efficiency of formation of the oxidized silicon oxide species. The clustering reactions leading to build-up of larger silicon oxide species are all most efficient at low temperature. A reaction scheme for the initial stages of circumstellar silicate formation proposed by Goumans and Bromley (2012) based on reaction thermodynamics, and starting from SiO, H2O and Mg, depends on the efficient formation of Si2O2 from clustering of two SiO molecules, formation of Si2O3 from the reaction of Si2O2 with H2O, and subsequently formation of Si2O2(OH)2 from the reaction of Si2O3 and H2O. Out of these three reaction steps, the efficiency of Si2O3 formation is not supported by our results. However, if there would be sufficiently high abundances of OH, for instance through thermal or photo-induced dissociation of H2O, the reaction might still be sufficiently efficient for the reaction scheme to be valid. Kinetic modeling of the outflows from AGB stars based on our results lead to only minor amounts of SiO2 and Si2O2 being formed. This seems to indicate that silicon oxide molecules alone will not initiate growth of larger oxide particles in stellar outflows.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
SA performed the DFT and RRKM calculations and wrote most of the manuscript. DG performed the kinetic modeling of AGB stars, wrote the corresponding part and edited parts of the introduction. RV provided valuable input to the study of the SiO + OH reaction.
The Funding from Knut and Alice Wallenberg foundation (research grant KAW 2020.0081) is gratefully acknowledged.
RV was employed by Zhejiang Huayou Cobalt Co., Ltd.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fspas.2023.1135156/full#supplementary-material
Ali, A., Sedlmayr, E., Lobel, A., Rowe, B. R., Schutte, W. A., Richards, A. M. S., et al. (1998). General discussion. Faraday Discuss. 109, 361–401. doi:10.1039/FD109361
Avramov, P. V., Adamovic, I., Ho, K.-M., Wang, C. Z., Lu, W. C., and Gordon, M. S. (2005). Potential energy surfaces of SimOn cluster formation and isomerization. J. Phys. Chem. A 109, 6294–6302. doi:10.1021/jp058078v
Becerra, R., Bowes, S. J., Ogden, J. S., Cannady, J. P., Adamovic, I., Gordon, M. S., et al. (2005). Time-resolved gas-phase kinetic and quantum chemical studies of the reaction of silylene with oxygen. Phys. Chem. Chem. Phys. 7, 2900–2908. doi:10.1039/B504760A
Britten, J. A., Tong, J., and Westbrook, C. K. (1990). “A numerical study of silane combustion,” in Twenty-third symposium (international) on combustion (The Combustion Institute), 195–202.
Bromley, S. T., Gómez Martín, J. C., and Plane, J. M. C. (2016). Under what conditions does (SiO)N nucleation occur? A bottom-up kinetic modelling evaluation. Phys. Chem. Chem. Phys. 18, 26913–26922. doi:10.1039/C6CP03629E
Chagger, H. K., Hainsworth, D., Patterson, P. M., Pourkashanian, M., and Williams, A. (1996). “The formation of SiO2 from hexamethyldisiloxane combustion in counterflow methane-air flames,” in Twenty-sixth symposium (international) on combustion (The Combustion Institute), 1859–1865.
Chakraborty, S., Yanchulova, P., and Thiemens, M. H. (2013). Mass-independent oxygen isotopic partitioning during gas-phase SiO2 formation. Science 342, 463–466. doi:10.1126/science.1242237
Clegg, R. E. S., van Ijzendoorn, L. J., and Allamandola, L. J. (1983). Circumstellar silicon chemistry and the SiO maser. Mon. Not. R. Astron. Soc. 203, 125–146. doi:10.1093/mnras/203.1.125
Gail, H.-P., and Sedlmayr, E. (1998). Inorganic dust formation in astrophysical environments. Faraday Discuss. 109, 303–319. doi:10.1039/A709290C
Gail, H.-P., and Sedlmayr, E. (1999). Mineral formation in stellar winds I. Condensation sequence of silicate and iron grains in stationary oxygen rich outflows. Astron. Astrophys. 347, 594–616.
Gail, H.-P., Wetzel, S., Pucci, A., and Tamanai, A. (2013). Seed particle formation for silicate dust condensation by SiO nucleation. Astron. Astrophys. 555, A119. doi:10.1051/0004-6361/201321807
Gardner, M. B., Westbrook, B. R., and Fortenberry, R. C. (2020). Spectral characterization for small clusters of silicon and oxygen: SiO2, SiO3, Si2O3, & Si2O4. Planet. Space Sci. 193, 105076. doi:10.1016/j.pss.2020.105076
Georgievskii, Y., and Klippenstein, S. J. (2005). Long-range transition state theory. J. Chem. Phys. 122, 194103. doi:10.1063/1.1899603
Gilbert, R. G., and Smith, S. C. (1990). Theory of unimolecular and recombination reactions. Oxford: Blackwell.
Glowacki, D. R., Liang, C.-H., Morley, C., Pilling, M. J., and Robertson, S. H. (2012). Mesmer: An open-source master equation solver for multi-energy well reactions. J. Phys. Chem. A 112, 9545–9560. doi:10.1021/jp3051033
Gobrecht, D., Cherchneff, I., Sarangi, A., Plane, J. M. C., and Bromley, S. T. (2016). Dust formation in the oxygen-rich AGB star Ik Tauri. Astron. Astrophys. 585, A6. doi:10.1051/0004-6361/201425363
Gobrecht, D., Plane, J. M. C., Bromley, S. T., Decin, L., Cristallo, S., and Sekaran, S. (2022). Bottom-up dust nucleation theory in oxygen-rich evolved stars I. Aluminium oxide clusters. Astron. Astrophys. 658, A167. doi:10.1051/0004-6361/202141976
Gómez Martín, J. C., Blitz, M. A., and Plane, J. M. C. (2009). Kinetic studies of atmospherically relevant silicon chemistry. Part II: Silicon monoxide reactions. Phys. Chem. Chem. Phys. 11, 10945–10954. doi:10.1039/B911745H
Goumans, T. P. M., and Bromley, S. T. (2012). Efficient nucleation of stardust silicates via heteromolecular homogeneous condensation. Mon. Not. R. Astron. Soc. 420, 3344–3349. doi:10.1111/j.1365-2966.2011.20255.x
Grådahl, S., Johansen, S. T., Ravary, B., Andresen, B., and Tveit, H. (2007). “Reduction of emissions from ferroalloy furnaces,” in Innovations in ferro alloy industry (INFACON XI), 479–488. Available at: http://www.pyrometallurgy.co.za/InfaconXI/046.pdf.
Gusdorf, A., Cabrit, S., Flower, D. R., and Pineau des Forêts, G. (2008). SiO line emission from C-type shock waves: interstellar jets and outflows. Astron. Astrophys. 482, 809–829. doi:10.1051/0004-6361:20078900
Hackwell, J. A., Gehrz, R. D., and Woolf, N. J. (1970). Interstellar silicate absorption bands. Nature 227, 822–823. doi:10.1038/227822a0
Hao, Y., Xie, Y., and Schaefer, H. F. (2014). Features of the potential energy surface for the SiO + OH → SiO2 + H reaction: Relationship to oxygen isotopic partitioning during gas phase SiO2 formation. RSC Adv. 4, 47163–47168. doi:10.1039/C4RA09829C
Hartquist, T. W., Oppenheimer, M., and Dalgarno, A. (1980). Molecular diagnostics of interstellar shocks. Astrophys. J. 236, 182–188. doi:10.1086/157731
Herbst, E., Millar, T. J., Wlodek, S., and Bohme, D. K. (1989). The chemistry of silicon in dense interstellar clouds. Astron. Astrophys. 222, 205–210.
Hindmarsh, A. C. (1980). LSODE and LSODI, two new initial value ordinary differential equation solvers. Signum Newsl. 15, 10–11. doi:10.1145/1218052.1218054
Höfner, S. (2008). Winds of M-type AGB stars driven by micron-sized grains. Astron. Astrophys. 491, L1–L4. doi:10.1051/0004-6361:200810641
Jachimowski, C. J., and McLain, A. G. (1983). A chemical kinetic mechanism for the ignition of silane/hydrogen mixtures. Nasa Technical Paper 2129.
Johansen, S. T., Tveit, H., Grådahl, S., Valderhaug, A., and Byberg, J. Å. (1998). “Environmental aspects of ferro-silicon furnace operations – an investigation of waste gas dynamics,” in 8th international ferroalloys congress proceedings (China Science & Technology Press), 59–63.
Kamfjord, N. E., Tveit, H., Næss, M. K., and Myrhaug, E. H. (2012). “Mechanisms of NO formation during SiO combustion,” in 3rd international symposium on high temperature metallurgical processing. Editors T. Jiang, J. Y. Hwang, P. Masset, O. Yucel, R. Padilla, and G. Zhou (Hoboken: Wiley), 401–409.
Kostko, O., Ahmed, M., and Metz, R. B. (2009). Vacuum-ultraviolet photoionization measurement and ab initio calculation of the ionization energy of gas-phase SiO2. J. Phys. Chem. A 113, 1225–1230. doi:10.1021/jp8091495
Krasnokutski, S. A., Rouillé, G., Jäger, C., Zhukovska, S., and Henning, Th. (2014). Formation of silicon oxide grains at low temperature. Astrophys. J. 782, 15. doi:10.1088/0004-637X/782/1/15
Langer, W. D., and Glassgold, A. E. (1990). Silicon chemistry in interstellar clouds. Astrophys. J. 352, 123–131. doi:10.1086/168519
Lindackers, D., Strecker, M. G. D., Roth, P., Janzen, C., and Pratsinis, S. E. (1997). formation and growth of SiO2 particles in low pressure H2/O2/Ar flames doped with SiH4. Combust. Sci. Tech. 123, 287–315. doi:10.1080/00102209708935632
Ma, Y., Jiang, B., Moosavi-Khoonsari, E., Andersson, S., Opila, E. J., and Tranell, G. M. (2019). Oxidation of liquid silicon in air atmospheres containing water vapor. Ind. Eng. Chem. Res. 58, 6785–6795. doi:10.1021/acs.iecr.9b00313
Macià Escatllar, A., Lazaukas, T., Woodley, S. M., and Bromley, S. (2019). Structure and properties of nanosilicates with olivine (Mg2SiO4)N and pyroxene (mgSiO3)N compositions. ACS Earth Space Chem. 3, 2390–2403. doi:10.1021/acsearthspacechem.9b00139
McCarthy, M. C., and Gauss, J. (2016). Exotic SiO2H2 isomers: Theory and experiment working in harmony. J. Phys. Chem. Lett. 7, 1895–1900. doi:10.1021/acs.jpclett.6b00632
Moore, T., Brady, B., and Martin, L. R. (2006). Measurements and modeling of SiCl4 combustion in a low-pressure H2/O2 flame. Combust. Flame 146, 407–418. doi:10.1016/j.combustflame.2006.06.003
Næss, M. K., Olsen, J. E., Andersson, S., and Tranell, G. (2014). Parameters affecting the rate and product of liquid silicon oxidation. Oxid. Met. 82, 395–413. doi:10.1007/s11085-014-9498-z
Papajak, E., and Truhlar, D. G. (2010). Efficient diffuse basis sets for density functional theory. J. Chem. Theory Comp. 6, 597–601. doi:10.1021/ct900566x
Pimentel, A. S., Lima, F. d. C. A., and da Silva, A. B. F. (2006). Rate coefficient for the reaction SiO + Si2O2 at T = 10-1000 K. J. Phys. Chem. A 110, 13221–13226. doi:10.1021/jp065462z
Plane, J. M. C., and Robertson, S. H. (2022). Master equation modelling of nonequilibrium chemistry in stellar outflows. Faraday Discuss. 238, 461–474. doi:10.1039/d2fd00025c
Plane, J. M. C., Gómez Martín, J. C., Feng, W., and Janches, D. (2016). Silicon chemistry in the mesosphere and lower thermosphere. J. Geophys. Res. Atmos. 121, 3718–3728. doi:10.1002/2015JD024691
Plane, J. M. C. (2013). On the nucleation of dust in oxygen-rich stellar outflows. Phil. Trans. R. Soc. A 371, 20120335. doi:10.1098/rsta.2012.0335
Ravary, B., and Johansen, S. T. (1999). “2D modeling of the combustion and NOx formation in furnaces producing FeSi,” in Second International Conference on CFD in the Minerals and Process Industries, 305–310. Available at: http://www.cfd.com.au/cfd_conf99/papers/020RAVA.PDF.
Ravary, B., Colomb, C., and Johansen, S. T. (2007). “Modeling combustion and thermal NOx formation in electric arc furnaces for the production of ferro-silicon and silicon metal,” in Innovations in ferro alloy industry (INFACON XI), 499–506. Available at: http://www.pyrometallurgy.co.za/InfaconXI/048.pdf.
Reber, A. C., Clayborne, P. A., Reveles, J. U., Khanna, S. N., Castleman, A. W., and Ali, A. (2006). Silicon oxide nanoparticles reveal the origin of silicate grains in circumstellar environments. Nano Lett. 6, 1190–1195. doi:10.1021/nl0605521
Reber, A. C., Paranthaman, S., Clayborne, P. A., Khanna, S. N., and Castleman, A. W. (2008). From SiO molecules to silicates in circumstellar space: Atomic structures, growth patterns, and optical signatures of SinOm clusters. ACS Nano 8, 1729–1737. doi:10.1021/nn7003958
Schilke, P., Walmsley, C. M., Pineau des Forêts, G., and Flower, D. R. (1997). SiO production in interstellar shocks. Astron. Astrophys. 321, 293–304.
Smith, I. W. M., Herbst, E., and Chang, Q. (2004). Rapid neutral–neutral reactions at low temperatures: a new network and first results for TMC-1. Mon. Not. R. Astron. Soc. 350, 323–330. doi:10.1111/j.1365-2966.2004.07656.x
Sternberg, A., and Dalgarno, A. (1995). Chemistry in dense photon-dominated regions. Astrophys. J. Suppl. Ser. 99, 565–607. doi:10.1086/192198
Tokuhashi, K., Horiguchi, S., Urano, Y., Iwasaki, M., Ohtani, H., and Kondo, S. (1990). Premixed silane-oxygen-nitrogen flames. Combust. Flame 82, 40–50. doi:10.1016/0010-2180(90)90076-4
Valiev, M., Bylaska, E. J., Govind, N., Kowalski, K., Straatsma, T. P., van Dam, H. J. J., et al. (2010). NWChem: a comprehensive and scalable open-source solution for large scale molecular simulations. Comput. Phys. Commun. 181, 1477–1489. doi:10.1016/j.cpc.2010.04.018
Woitke, P. (2006). Too little radiation pressure on dust in the winds of oxygen-rich AGB stars. Astron. Astrophys. 460, L9–L12. doi:10.1051/0004-6361:20066322
Wooldridge, M. S. (1998). Gas-phase combustion synthesis of particles. Prog. Energy. Combust. Sci. 24, 63–87. doi:10.1016/S0360-1285(97)00024-5
Woolf, N. J., and Ney, E. P. (1969). Circumstellar infrared emission from cool stars. Astrophys. J. 155, L181–L184. doi:10.1086/180331
Yang, T., Thomas, A. M., Dangi, B. B., Kaiser, R. I., Mebel, A. M., and Millar, T. J. (2018). Directed gas phase formation of silicon dioxide and implications for the formation of interstellar silicates. Nat. Comm. 9, 774. doi:10.1038/s41467-018-03172-5
Zachariah, M. R., and Tsang, W. (1995). Theoretical calculation of thermochemistry, energetics, and kinetics of high-temperature SixHyOz reactions. J. Phys. Chem. 99, 5308–5318. doi:10.1021/j100015a012
Zhao, Y., and Truhlar, D. G. (2008a). The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 120, 215–241. doi:10.1007/s00214-007-0310-x
Keywords: SiO, circumstellar, dust, DFT, rate coefficients, kinetics
Citation: Andersson S, Gobrecht D and Valero R (2023) Mechanisms of SiO oxidation: Implications for dust formation. Front. Astron. Space Sci. 10:1135156. doi: 10.3389/fspas.2023.1135156
Received: 31 December 2022; Accepted: 15 February 2023;
Published: 02 March 2023.
Edited by:
Ashraf Ali, College Park, United StatesReviewed by:
Marzio Rosi, University of Perugia, ItalyCopyright © 2023 Andersson, Gobrecht and Valero. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stefan Andersson, c3RhQGNoZW0uZ3Uuc2U=, c3RlZmFuLmFuZGVyc3NvbkBzaW50ZWYubm8=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.