
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Astron. Space Sci. , 10 February 2022
Sec. Astrochemistry
Volume 8 - 2021 | https://doi.org/10.3389/fspas.2021.811342
This article is part of the Research Topic RNA World Hypothesis and the Origin of Life: Astrochemistry Perspective View all 13 articles
“Who” and how? In this simple question the complexity of the interstellar chemistry is encapsulated. “Who” refers to what molecules are present in the interstellar medium (ISM) and “how” to the mechanisms that led to their formation. While the large number of molecules discovered in the ISM (∼250) demonstrates the rich chemistry occurring there, a significant number of unknown species are waiting for an identification and the processes that led to the synthesis of the identified species are still hotly debated or even unknown. Gas-phase laboratory studies in the fields of rotational spectroscopy and quantum chemistry provide an important contribution to answering the question above. An overview on the role played by rotational spectroscopy and quantum chemistry in the unraveling of the gas-phase chemistry of the interstellar medium is presented.
The conditions of the interstellar medium (ISM) are extreme, with temperatures ranging between 10 and 102 K, very low number densities varying from 10–4–108 cm−3, and energetic or even ionizing radiation. Despite these extreme conditions, which severely hinder chemical reactivity, the chemistry of the ISM is a remarkably rich subject that is far from being disclosed. Nearly 50 years ago, the first polyatomic molecules, both organic and inorganic in nature, were discovered and led to the emergence of a new discipline: Astrochemistry. This is an interdisciplinary and multifaceted field, which is–in the words of A. Dalgarno (who is considered the “father of Astrochemistry”)– a “blending of astronomy and chemistry in which each area enriches the other in a mutually stimulating interaction” (Dalgarno, 2008).
Astrochemical modeling has been developing in parallel with the discovery of molecular species and the derivation of their formation routes. While the fist diatomic molecules were detected in the late 1930s, polyatomic molecules were first discovered in the late 1960s and the beginning of the 1970s owing to radioastronomy. Already in the seventies, the first so-called “complex organic molecules” (COMs; Herbst and van Dishoeck (2009)), namely C-bearing molecules with at least six atoms, were observed.
In the early seventies, molecular synthesis through gas-phase ion-molecule reactions was proposed to rationalize the molecular abundances observed in interstellar clouds. Later, the importance of gas-phase neutral-neutral reactions was also recognized. However, as observational capabilities advanced over the past decade, COMs began to be detected in regions where gas-phase reactions did not contribute significantly to chemical processing. It thus became clear that chemical reactions occurring on the surface of dust grains also play a crucial role in the interstellar chemistry (Caselli et al., 2004; Cuppen et al., 2017). However, the contribution of gas-phase chemistry cannot be overlooked and, indeed, a full explanation of observed molecular abundances is often obtained by accounting for both reactivity on grains and in the gas phase (Ruaud and Gorti, 2019; Laas et al., 2011; Codella et al., 2017; Baiano et al., 2020; Shingledecker et al., 2020). Despite the awareness of a rich interstellar chemistry occurring both on grains and in the gas phase, we are very far from sufficient knowledge to fully understand it: much information is still missing and only a small fraction of the elementary reactions have actually been adequately characterized. As a consequence, astrochemical networks and models still miss crucial information and species not predicted by any chemical model are detected in space.
Moving a step further, understanding how life originated on Earth is one of the greatest challenges in astrochemistry, which might be strongly related to the chemical reactivity taking place in the ISM. However, the characteristic features of the detected molecules are extremely different from the large and complex systems in the cell (see Figure 1). The link between these two extremes likely consists of a series of intermediate molecular species that are characterized by increasing complexity. In this sense we can identify the so-called “ingredients for life”: prebiotic molecules synthesized in abiotic processes. On this ground, we can envisage the first step to gaining insight into the origin-of-life issue as the investigation of how the abiotic synthesis occurs in the ISM. This gives further importance to the investigation of surface-grain and gas-phase chemistries, and to the elucidation of their mechanisms. In this respect, a key point is the derivation of chemical models able to quantitatively account for the observed abundances. Furthermore, the comparison of the results issuing from the astrochemical models and those from astronomical observations might also allow for obtaining insights into the chemistry at work in the environment under consideration, which means–for example–understanding whether gas-phase and/or grain-surface chemistry are taking place and to what extent. To give an example, in Codella et al. (2017), a Solar-like proto-stellar shock region (L1157-B1) was considered and astronomical observations coupled with a chemical modelling analysis demonstrated that formamide could only be formed by gas-phase reactions.
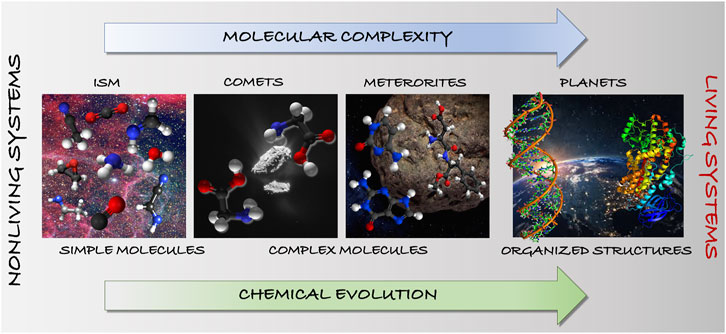
FIGURE 1. Chemical evolution in terms of molecular and astronomical complexity: from simple to complex molecules toward pre-biological and biological systems.
Molecules can be defined as “cosmic chemical clocks” because they mark the stages of a star cycle as well as the parallelism between life development and evolution of the Universe (see Figure 1). To understand how life originated on Earth, a progressive hierarchy of “emergent” steps must be established, i.e. steps that add some chemical complexity to the previous system. This hierarchy is expected to lead from prebiotic molecules, to functionalized clusters of molecules (self-assembled or arrayed on a mineral surface), to self-replicating molecular systems (able to copy themselves), to encapsulation and eventually cellular life. In this hierarchical series of steps, the first one is the synthesis of the building blocks of biomolecules such as amino acids, sugars, and bases. However, these being already very complex systems for the ISM, the focus is usually shifted to small prebiotic molecules that can be precursors of the building blocks of biomolecules. Once these latter species are formed, subsequent steps then involve their assembly into macromolecules, possibly with specific functions. In this respect, it is very intriguing that nucleobases (fundamental elements of RNA and DNA) and amino acids are present in space, as demonstrated by the content of meteorites that have fallen to Earth (see, e.g., Callahan et al. (2011)) and comets (see, e.g., Hadraoui et al., 2019).
This paper is not meant to give an exhaustive contribution of the role played by laboratory astrochemistry in elucidating gas-phase chemistry. Instead, it aims at providing illustrative examples of the personal contribution of the author to the field. The manuscript is organized as follows. In the subsequent section, general considerations on the chemical reactivity in the ISM are briefly summarized. Then, we move to the specific topic of the gas-phase chemistry, therein underlining the contributions of quantum chemistry and rotational spectroscopy. Subsequently, two significant case studies are addressed: the reaction of methylamine with the cyano radical and the reaction of methanimine with small radical species. Finally, conclusions are summed up.
Figure 1 of Puzzarini (2020) summaries the steps required for the complete characterization of the chemistry occurring in an interstellar cloud. The first step is the derivation of its chemical composition, which results from a synergistic interplay of radioastronomical observations and laboratory spectroscopy (Zaleski et al., 2013; Melosso et al., 2018; McGuire, 2018; Puzzarini and Barone, 2020; Melosso et al., 2020). As explained in the following, astronomical observation of the spectroscopic features of a molecule provides unequivocal proof of its presence in the astronomical environment under consideration (Tennyson, 2005; Yamamoto, 2017; McGuire, 2018). The second step is the derivation of molecular abundances from the intensity of the observed/assigned lines (Nash, 1990; Goldsmith and Langer, 1999). In the third step, the reactivity needs to be completely characterized, which requires an investigation of all possible reactions leading to the molecules detected as well as those between the species identified. In this step, reactions in the gas phase, on grains and at their interface need to be considered. In the fourth step, the results from astronomical detections and reactivity are interpreted by suitable models (see, e.g., (Garrod et al., 2008; Holdship et al., 2017; Jiménez-Serra et al., 2018)). The simplest astrochemical models keep the physical conditions, such as the density and temperature, unchanged while the chemistry takes place. This latter is implemented in the model as a network of reactions that form and destroy all the species that have been identified in the specific environment under consideration. Therefore, astrochemical models typically contain hundreds of species and thousands of reactions. If the models are sufficiently reliable, they should be able to explain the detected molecules in the gas phase and their abundance.
The table in Figure 2 summarizes the types of reactions occurring in the ISM together with their typical rate coefficients. Because of the low densities, only binary ion-molecule or neutral-neutral reactions can occur, with the former taking place only in the gas phase. Because of the low temperature, reactions are expected to be exothermic and to have vanishing or nearly-vanishing activation energies. While in the gas phase the reaction energy is taken away as kinetic and vibrational energy of products or through emission of photons, on grains “third-body stabilization” occurs. Given all these constraints, neutral-neutral reactions must involve at least one highly reactive molecule, i.e. a radical species. Three-body reactions only become significant at number densities above ∼1013 cm−3 such as those encountered in the atmospheres of stars and exoplanets. While the order of magnitude of rate coefficients is approximately known, this is not at all sufficient. The reliability of the results of any chemical model depends on the accuracy of the rate coefficients of the hundreds or thousands of reactions involved.
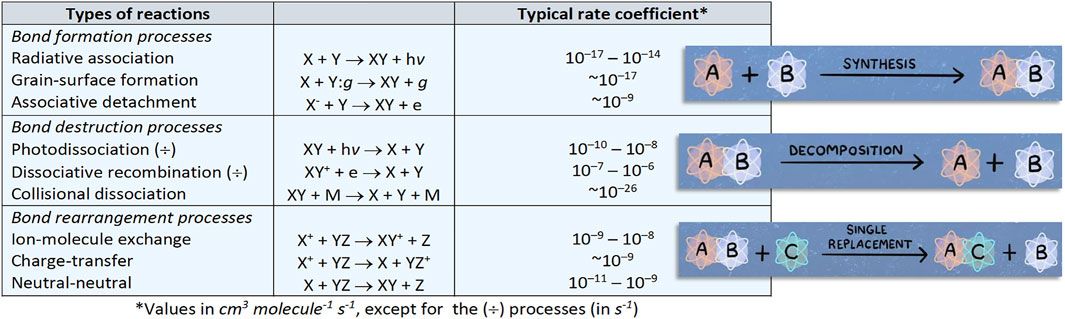
FIGURE 2. Schematic representation of the reaction types of relevance in astrochemistry together with their typical rate coefficients.
Even though–as mentioned in the Introduction–COMs were first detected decades ago, the processes that lead to their production are still a matter of debate. They can be synthesized either on grain surfaces or by means of gas-phase chemistry; however, the contribution of each type of chemistry is often unclear or even unknown. While grain chemistry is able to explain the molecules detected in hot cores surrounding protostars and young stars (Garrod et al., 2008), the formation of COMs in cold dense sources is not well understood. Analogously, the formation of COMs in diffuse or translucent material remains at least partially a mystery.
Detection of new molecular species in the ISM and the characterization of the interstellar chemistry are intimately related. From one side, the new identifications put constraints on the chemical network describing the astronomical object under consideration. From the other side, the investigation of possible chemical reactions might suggest new molecules to be searched for.
As already mentioned, the harsh conditions of the ISM puts severe constraints on the reactivity. Indeed, molecules do not have any additional thermal energy (kT, with k being the Boltzmann constant and T the absolute temperature) because of the low temperatures. The direct consequence is that only reactions that proceed with a barrierless attack and submerged barriers are allowed. This is exemplified in Figure 3. To fulfil this constraint, at least one of the reactants should be a reactive species such as a radical or an ion (the C species in Figure 3). In the gas phase, the very low densities add the additional constraint of bimolecular products: while a unimolecular species can be produced, often this is not the final product because it cannot get rid of the excess of energy through collisions, thus being unstable and either proceeding further along the reaction path or dissociatiing back to reactants. Exceptions are provided by radiative stabilization. The situation is different for grain-surface chemistry because the grain can efficiently remove the excess energy (see Figure 2: the “bond formation processes”).
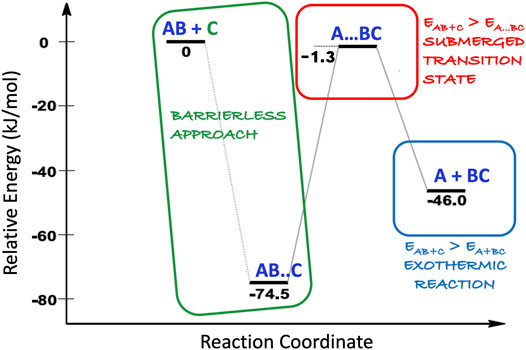
FIGURE 3. Sketch of a simple reaction mechanism between the AB molecule and the reactive C species: all the required conditions for a reaction occurring in the ISM are highlighted. Relative energies here considered (numbers in black) are ZPE-corrected electronic energies.
While a reaction is written emphasizing the reactants and the possible products, as evident in Figures 5, 6, the reaction mechanisms are often very complicated, and several reaction paths are possible. Therefore, investigation of gas-phase reactions requires, first of all, the full characterization of reactive potential energy surfaces (PESs), as shown in Figure 3, from an energetic point of view (thermochemistry). While the energetic characterization is a mandatory step to understand the possible routes open in the ISM, the definitive feasibility of the reaction paths under consideration is established by the rate at which they are expected to occur, i.e. by the corresponding kinetic study.
If the reaction is investigated for the purpose of explaining the formation route of a specific product, the study starts from purposely chosen precursors among molecules already detected in the astronomical source under consideration. Alternatively, again starting from small reactive species already identified, all possible reactive pathways are worked out, with those accessible in the conditions of the ISM further detailed to “discover” new molecules. An example in this respect is offered by Vazart et al. (2016), in which the gas-phase H2CO + NH2 and CH2NH + OH reactions have been investigated with an aim of understanding the formation of formamide (CHONH2). Among the possible products of the CH2NH + OH reaction, in addition to formamide, the E- and Z-methanimidic acids (CHOHNH) can be formed. These are entirely new species that deserve to be studied because a spectroscopic characterization is still missing.
Joint thermochemical-kinetic computational studies play a crucial role in astrochemistry because experiments able to reproduce the interstellar conditions are limited and, with regard to the gas phase, unable to reproduce at the same time the low temperature and the low pressure that are typical of the ISM. The preliminary investigation of the reactive PES for the identification of the reaction channels of interest can be effectively performed at a relatively low computational level, relying–for example–on models rooted in density functional theory (DFT). Among the different pathways, only those that are accessible in the typical conditions of the ISM are then further investigated at a higher level of theory. The re-investigation aims at improving the structural determination of the stationary points as well as their energetics. Since thermochemistry depends only marginally on the reference geometries, only the electronic energies are then further refined at the state of the art, usually resorting in composite schemes based on coupled-cluster theory (see, e.g., Tajti et al. (2004); Bomble et al. (2006); Harding et al. (2008); Alessandrini et al. (2020); Lupi et al. (2020b)). Indeed, the height of the energy barriers along the reaction path have a strong impact on the reaction rate. For this reason, computational methodologies able to provide energetics with a kJ/mol accuracy should be employed. The reader is referred, for example, to Lupi et al. (2020b); Tonolo et al. (2020); Puzzarini et al. (2020) for a thorough account of the methodology sketched above.
Finally, kinetic calculations are carried out in order to provide conclusive information on the feasibility of the suggested mechanisms as well as the branching ratios of the products. Noted is that the energies employed in the kinetic study should incorporate the corresponding zero-point energy (ZPE) corrections. The rate constants of elementary reactions involving a saddle point can be computed using conventional transition state theory, while the description of barrierless attack needs a specific approach. Then, the global rate constants are evaluated by employing a master equation approach.
While the first diatomic molecules were discovered in the late 1930s, it was with the advent of radioastronomy that the hunt for molecular species started at the end of 1960s. As mentioned in the Introduction, since then, about 250 molecules have been detected. Radioastronomy relies on the collection of microwave emission from the astronomical object under study. This emission contains the so-called “molecular fingerprints”, which are the rotational transitions. Indeed, the rotational features are extremely sensitive to the molecular species, and even allow for discriminating among the isotopic species of the same molecule. This is the reason why the overwhelming majority of gas-phase chemical species have been identified via their rotational signatures (McGuire, 2018). Radiotelescopes provide unbiased millimeter-/submillimeter-wave line surveys, which contain the rotational features of all molecules present in the interstellar cloud under consideration. As a consequence, their assignment requires accurate knowledge of the rotational transitions of all molecules that are expected to be present. This knowledge is obtained from rotational spectroscopy laboratory experiments. Figure 4 exemplifies the interplay of rotational spectroscopy and radioastronomy for the identification of molecules in the ISM: starting from a computational evaluation of the spectroscopic parameters, the experimental rotational spectrum is recorded and assigned, thus leading to a spectroscopic catalog to be employed in the assignment of astronomical spectra.
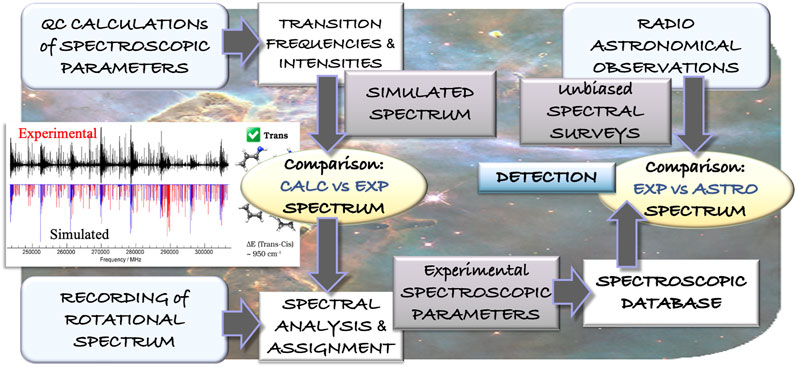
FIGURE 4. Schematic representation of the interplay of theory and experiment in the field of rotational spectroscopy leading to the guidance of astronomical observations.
As highlighted by the example mentioning the CH2NH + OH PES, the investigation of a specific reaction can lead to the suggestion of new species to be searched for in the ISM. In this respect, another example is offered by Puzzarini et al. (2020) reporting the reaction between methylamine (CH3NH2) and the CN radical. While this reaction will be addressed in the next section, we here anticipate that the two main products are two radical species: CH3NH and CH2NH2 (see Figure 5). These are new species not yet characterized even in the laboratory, and for this reason, in Puzzarini et al. (2020), an accurate computational study of their spectroscopic parameters was also carried out. For all cases analogous to the two examples above, to take a step forward, the first action item is the spectroscopic characterization of the new, potential interstellar species. This actually consists of two steps. First, a computational spectroscopy study is carried out in order to obtain a reliable and accurate prediction of the rotational parameters to guide the laboratory work (Puzzarini et al., 2010, 2019; Puzzarini and Barone, 2020; Barone et al., 2021). Because of the intrinsic high resolution of rotational spectroscopy, such an investigation should be performed at the state of the art (often employing composite schemes rooted in the coupled-cluster technique; see, e.g., Puzzarini et al. (2008); Alessandrini et al. (2018)). Second, the most promising rotational transitions are searched for and the assignment procedure of the rotational spectrum starts. This latter step is an iterative process in which the assigned transitions are fitted to an effective Hamiltonian, with the result of the fit allowing new, improved predictions and thus new assignments. The procedure is iterated until all the observed lines are properly assigned. The rotational parameters resulting from the fit can then be used to produce a line catalog to be subsequently employed in astronomical searches (Müller et al., 2005; McGuire, 2018).
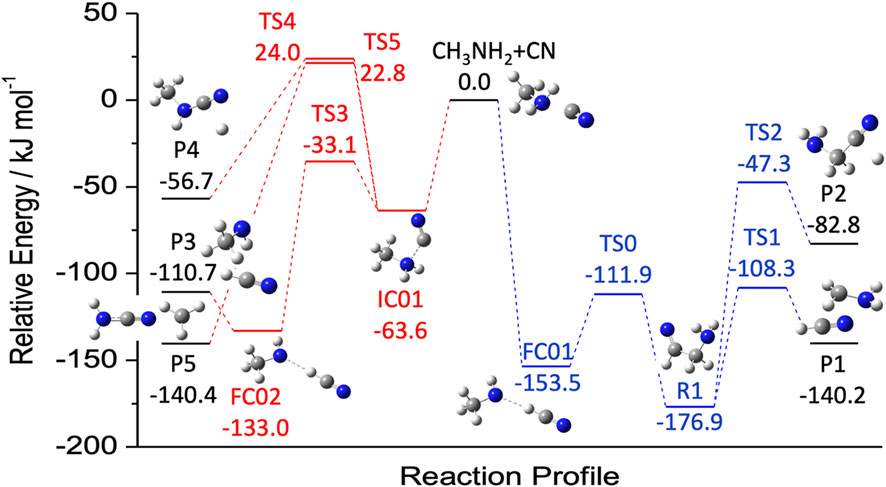
FIGURE 5. Reaction mechanism for the attack of CN to the N (red) and C (blue) moieties of methylamine. Energies, from Puzzarini et al. (2020), were evaluated using a coupled-cluster based composite scheme (the so-called ‘HEAT-like’) and augmented by anharmonic zero-point energy corrections.
The detection of propargylimine in the quiescent G+0.693−0.027 molecular cloud (Bizzocchi et al., 2020) is a significant example of the processes outlined above, with the interplay of computational and experimental spectroscopy with radioastronomy being graphically generalized in Figure 4. In Bizzocchi et al. (2020), the authors started from a high-level computational evaluation of all spectroscopic parameters required to predict the rotational spectrum (rotational constants vibrationally corrected, centrifugal-distortion constants, nuclear quadrupole-coupling constants, and electric dipole moment components), and then moved to the experimental recording of the rotational spectrum, with propargylimine being produced by pyrolizing (950°C) dipropargylamine ((HCCCH2)2NH) vapors. In such a case, the guidance of quantum chemistry in the assignment procedure of the rotational spectrum is crucial because pyrolysis leads to the formation of several byproducts. In the laboratory, the rotational spectra of both E and Z isomers of propargylimine were characterized. The spectroscopic catalogs (for E,Z species) obtained were then used to search for this imine in space, with only the most abundant (and most stable) Z isomer being detected in G+0.693−0.027.
As mentioned above, the temperature of the ISM requires that reactions do not have energy barriers toward products. Therefore, the first condition to be met is their exothermicity. This condition allows for discriminating among possible products. To address this issue, we can consider a specific example: the reaction between methaylamine (CH3NH2) and the cyano (CN) radical. Figure 4 of Sleiman et al. (2018) shows that starting from these two reactants, several products can be formed, some lying above the reactants and most of them lying below. Since endothermic reactions require an external energy input, they do not proceed. In Puzzarini et al. (2020), the exothermic paths of the CH3NH2 + CN reaction have been further and more thoroughly investigated. This latter study is a nice example to point out that exothermicity is a necessary but not sufficient condition. Indeed, exothermic reactions can have one or more energy barriers toward the formation of products, which require an external energy input to be overcome, thus rendering them closed in the ISM.
Inspection of Figure 5 (based on the results of Puzzarini et al. (2020)) points out that the extent of exothermicity is not a guarantee that reaction can actually occur at low temperature. Indeed, it is noted that the P5 product (NH2CN + CH3), which is the most exothermic, cannot be formed because the transition state TS5 is emerged, i.e., it lies above the reactants. Analogously, TS4 prevents the formation of CH3NHCN + H (denoted as P4).
The investigation carried out in Puzzarini et al. (2020) also allowed us to stress the importance of the level of theory employed in the thermochemical characterization of a reactive PES. Indeed, because of the extreme conditions, the rate at which a reaction can occur strongly depends on the barrier heights to be overcome. It is therefore mandatory to employ computational methodologies at the state of the art, possibly providing a kJ/mol accuracy, such as the approach used in Puzzarini et al. (2020) (see, e.g., Tajti et al. (2004); Bomble et al. (2006); Harding et al. (2008); Alessandrini et al. (2020, 2021)). Actually, a low level of theory might underestimate the barrier, thus leading to a wrong conclusion. In fact, in Sleiman et al. (2018), the authors relied on DFT (the hybrid B3LYP functional in conjunction with the aug-cc-pVTZ level (Becke, 1993; Dunning Jr, 1989; Kendall et al., 1992)) and discarded instead CCSD(T)/aug-cc-pVTZ calculations (Raghavachari et al., 1989), thus erroneously concluding that the main product of the CH3NH2 + CN reaction is cyanamide (NH2CN). In passing we note that, even if the CCSD(T)/aug-cc-pVTZ level of theory predicts an emerged transition state, this is only 8.3 kJ/mol above the reactants, to be compared with the value of 22.8 kJ/mol provided by the so-called ‘HEAT-like’ approach (Tajti et al., 2004; Lupi et al., 2020b) employed in Puzzarini et al. (2020). This suggests that even the CCSD(T) method (which is often denoted as the ‘gold standard’ for quantum-chemical calculations) in conjunction with a medium-sized basis set cannot be sufficiently accurate when it is used for characterizing borderline transition states.
Astrochemical models are designed to simulate the interstellar chemistry, with the most complete models accounting for both gas-phase and dust-grain surface chemical processes. As already mentioned, these models use large reaction networks to link all the chemical species that are present in the interstellar object under consideration. One of the major challenges is to have a complete picture of the composition and reactivity of the interstellar object under consideration. In this respect, the derivation of general mechanisms can provide an important step forward.
In Lupi et al. (2020a), it was pointed out that the reaction of methanimine (CH2NH) with a small radical can provide a general mechanism leading to the formation of complex imines. This general mechanism is shown in Figure 6. The attack on the C-end of CH2NH leads to the formation of a C-substituted methanimine having two forms, Z and E, according to the position of the attached fragment with respect to the H (N) atom. Instead, the attack on the N-side of CH2NH leads to a methanimine substituted at the N atom. Finally, the attack to the C-N double bond leads to an intermediate that links the C- and N-paths. This general mechanism has proven to be effective for the OH, CN, CP, and CCH radicals (Vazart et al., 2016; Lupi et al., 2020a; Alessandrini et al., 2021); however, it should be taken with caution because species like the CH3 radical leads to a barrier in the entrance channel, likely due to its structural re-arrangement (from planar to pyramidal geometry).
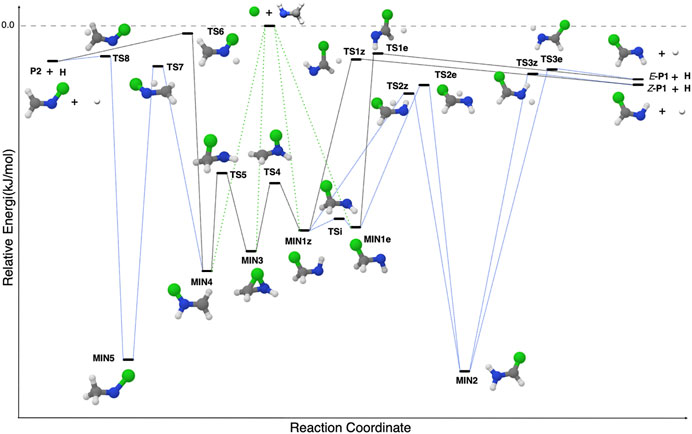
FIGURE 6. The general mechanism for the X + CH2NH reaction, where X (the ‘green ball’ in the figure) is a generic small radical.
As mentioned above, C-substituted imines such as cyanomethanimine, ethanimine and propargylimine exist in two forms: Z and E (Melosso et al., 2018; Baiano et al., 2020; Lupi et al., 2020a). As pointed out in several works (see, e.g., Zhang et al. (2020); Shingledecker et al. (2020)), known reaction mechanisms are not able to explain the isomer abundance ratio obtained from astronomical observations. In Lupi et al. (2020a), assuming similar destruction rates for both E and Z forms of propalgylimine and cyanomethanimine, the branching ratios for the CH2NH + CCH and, in particular, CH2NH + CN reactions were found to semi-quantitatively predict observations. However, to obtain quantitative agreement, de la Concepción et al. (2021) pointed out the need to account for quantum tunneling effects in the E-Z isomerization reaction to allow the system to reach the thermodynamic equilibrium. Such an outcome leads to the consequence that the origin of the E/Z ratio of imines depends exclusively on their relative stabilities. Indeed, in Puzzarini and Barone (2020), it was shown that accurate energetics (also accounting for zero-point energy corrections) are required to reproduce the kinetic temperature of cyanomethanimine assuming the observational Z/E ratio.
In this contribution, we have provided a flavor of the research focusing on the role of gas-phase chemistry in solving two crucial challenges: having a complete census of interstellar molecules and a clear understanding of their formation/destruction pathways. Despite all efforts, we are still far from a complete picture, but important steps have been taken and road forward is marked. This perspective was not meant to be an exhaustive account, but instead to provide an overview of the general problem. One aim was also to underline the role played by rotational spectroscopy (which can potentially even be extended to kinetic investigations (Kidwell et al., 2014; Oldham et al., 2014; Abeysekera et al., 2015; Porterfield et al., 2018)) and the importance of carrying out computational studies at the state of the art.
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
The author confirms being the sole contributor of this work and has approved it for publication.
The works discussed in this manuscript received the support by the Italian Space Agency (ASI; ‘Life in Space’ project, N. 2019-3-U.0) and University of Bologna (RFO).
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
I would like to acknowledge all my group (https://site.unibo.it/rotational-computational-spectroscopy/en) for the continuous support and fruitful discussions.
Abeysekera, C., Joalland, B., Ariyasingha, N., Zack, L. N., Sims, I. R., Field, R. W., et al. (2015). Product Branching in the Low Temperature Reaction of Cn with Propyne by Chirped-Pulse Microwave Spectroscopy in a Uniform Supersonic Flow. J. Phys. Chem. Lett. 6, 1599–1604. doi:10.1021/acs.jpclett.5b00519
Alessandrini, S., Barone, V., and Puzzarini, C. (2020). Extension of the “Cheap” Composite Approach to Noncovalent Interactions: The Jun-ChS Scheme. J. Chem. Theor. Comput. 16, 988–1006. doi:10.1021/acs.jctc.9b01037
Alessandrini, S., Gauss, J., and Puzzarini, C. (2018). Accuracy of Rotational Parameters Predicted by High-Level Quantum-Chemical Calculations: Case Study of Sulfur-Containing Molecules of Astrochemical Interest. J. Chem. Theor. Comput. 14, 5360–5371. doi:10.1021/acs.jctc.8b00695
Alessandrini, S., Tonolo, F., and Puzzarini, C. (2021). In Search of Phosphorus in Astronomical Environments: The Reaction between the CP Radical (X 2Σ+) and Methanimine. J. Chem. Phys. 154, 054306. doi:10.1063/5.0038072
Baiano, C., Lupi, J., Tasinato, N., Puzzarini, C., and Barone, V. (2020). The Role of State-Of-The-Art Quantum-Chemical Calculations in Astrochemistry: Formation Route and Spectroscopy of Ethanimine as a Paradigmatic Case. Molecules 25, 2873. doi:10.3390/molecules25122873
Barone, V., Alessandrini, S., Biczysko, M., Cheeseman, J. R., Clary, D. C., McCoy, A. B., et al. (2021). Computational Molecular Spectroscopy. Nat. Rev. Methods Primers 1, 38. doi:10.1038/s43586-021-00034-1
Becke, A. D. (1993). Density-functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 98, 5648–5652. doi:10.1063/1.464913
Bizzocchi, L., Prudenzano, D., Rivilla, V. M., Pietropolli-Charmet, A., Giuliano, B. M., Caselli, P., et al. (2020). Propargylimine in the Laboratory and in Space: Millimetre-Wave Spectroscopy and its First Detection in the ISM. Astron. Astrophys. 640, A98. doi:10.1051/0004-6361/202038083
Bomble, Y. J., Vázquez, J., Kállay, M., Michauk, C., Szalay, P. G., Császár, A. G., et al. (2006). High-accuracy Extrapolated Ab Initio Thermochemistry. II. Minor Improvements to the Protocol and a Vital Simplification. J. Chem. Phys. 125, 064108. doi:10.1063/1.2206789
Callahan, M. P., Smith, K. E., Cleaves, H. J., Ruzicka, J., Stern, J. C., Glavin, D. P., et al. (2011). Carbonaceous Meteorites Contain a Wide Range of Extraterrestrial Nucleobases. PNAS 108, 13995–13998. doi:10.1073/pnas.1106493108
Caselli, P., Stantcheva, T., and Herbst, E. (2004). “Grain Surface Chemistry,” in The Dense Interstellar Medium in Galaxies. Editors S. Pfalzner, C. Kramer, C. Straubmeier, and A. Heithausen (Berlin, Heidelberg: Springer Berlin Heidelberg), 479–486.
Codella, C., Ceccarelli, C., Caselli, P., Balucani, N., Barone, V., Fontani, F., et al. (2017). Seeds of Life in Space (SOLIS): II. Formamide in Protostellar Shocks: Evidence for Gas-phase Formation. Astron. Astrophys 605, L3. doi:10.1051/0004-6361/201731249
Cuppen, H., Walsh, C., Lamberts, T., Semenov, D., Garrod, R., Penteado, E., et al. (2017). Grain Surface Models and Data for Astrochemistry. Space Sci. Rev. 212, 1–58. doi:10.1007/s11214-016-0319-3
Dalgarno, A. (2008). A Serendipitous Journey. Ann. Rev. Astron. Astrophys. 46, 1–20. doi:10.1146/annurev.astro.46.060407.145216
de la Concepción, J. G., Jiménez-Serra, I., Corchado, J. C., Rivilla, V. M., and Martín-Pintado, J. (2021). The Origin of the E/Z Isomer Ratio of Imines in the Interstellar Medium. Astrophys. J. Lett. 912, L6. doi:10.3847/2041-8213/abf650
Dunning, T. H. (1989). Gaussian Basis Sets for Use in Correlated Molecular Calculations. I. The Atoms boron through Neon and Hydrogen. J. Chem. Phys. 90, 1007–1023. doi:10.1063/1.456153
Garrod, R. T., Weaver, S. L. W., and Herbst, E. (2008). Complex Chemistry in star-forming Regions: An Expanded Gas-Grain Warm-Up Chemical Model. Astrophys. J. 682, 283–302. doi:10.1086/588035
Goldsmith, P. F., and Langer, W. D. (1999). Population Diagram Analysis of Molecular Line Emission. Astrophys. J. 517, 209–225. doi:10.1086/307195
Hadraoui, K., Cottin, H., Ivanovski, S. L., Zapf, P., Altwegg, K., Benilan, Y., et al. (2019). Distributed glycine in Comet 67P/Churyumov-Gerasimenko. Astron. Astrophys. 630, A32. doi:10.1051/0004-6361/201935018
Harding, M. E., Vázquez, J., Ruscic, B., Wilson, A. K., Gauss, J., and Stanton, J. F. (2008). High-accuracy Extrapolated Ab Initio Thermochemistry. III. Additional Improvements and Overview. J. Chem. Phys. 128, 114111. doi:10.1063/1.2835612
Herbst, E., and van Dishoeck, E. F. (2009). Complex Organic Interstellar Molecules. Ann. Rev. Astron. Astrophys. 47, 427–480. doi:10.1146/annurev-astro-082708-101654
Holdship, J., Viti, S., Jiménez-Serra, I., Makrymallis, A., and Priestley, F. (2017). UCLCHEM: A Gas-Grain Chemical Code for Clouds, Cores, and C-Shocks. Astron. J. 154, 38. doi:10.3847/1538-3881/aa773f
J. Tennyson (Editor) (2005). Astronomical Spectroscopy (London, United Kingdom: Imperial College Press).
Jiménez-Serra, I., Viti, S., Quénard, D., and Holdship, J. (2018). The Chemistry of Phosphorus-Bearing Molecules under Energetic Phenomena. Astrophys. J. 862, 16. doi:10.3847/1538-4357/aacdf2
Kendall, A., Dunning, T. H., and Harrison, R. J. (1992). Electron Affinities of the First-Row Atoms Revisited. Systematic Basis Sets and Wave Functions. J. Chem. Phys. 96, 6796. doi:10.1063/1.462569
Kidwell, N. M., Vaquero-Vara, V., Ormond, T. K., Buckingham, G. T., Zhang, D., Mehta-Hurt, D. N., et al. (2014). Chirped-pulse Fourier Transform Microwave Spectroscopy Coupled with a Flash Pyrolysis Microreactor: Structural Determination of the Reactive Intermediate Cyclopentadienone. J. Phys. Chem. Lett. 5, 2201–2207. doi:10.1021/jz5010895
Laas, J. C., Garrod, R. T., Herbst, E., and Weaver, S. L. W. (2011). Contributions from Grain Surface and Gas Phase Chemistry to the Formation of Methyl Formate and its Structural Isomers. Astrophys. J. 728, 71. doi:10.1088/0004-637x/728/1/71
Lupi, J., Puzzarini, C., and Barone, V. (2020a). Methanimine as a Key Precursor of Imines in the Interstellar Medium: The Case of Propargylimine. Astrophys. J. Lett. 903, L35. doi:10.3847/2041-8213/abc25c
Lupi, J., Puzzarini, C., Cavallotti, C., and Barone, V. (2020b). State-of-the-art Quantum Chemistry Meets Variable Reaction Coordinate Transition State Theory to Solve the Puzzling Case of the H2S + Cl System. J. Chem. Theor. Comput. 16, 5090–5104. doi:10.1021/acs.jctc.0c00354
McGuire, B. A. (2018). 2018 Census of Interstellar, Circumstellar, Extragalactic, Protoplanetary Disk, and Exoplanetary Molecules. Astrophys. J. Suppl. Ser. 239, 17. doi:10.3847/1538-4365/aae5d2
Melosso, M., Belloche, A., Martin-Drumel, M.-A., Pirali, O., Tamassia, F., Bizzocchi, L., et al. (2020). Far-infrared Laboratory Spectroscopy of Aminoacetonitrile and First Interstellar Detection of its Vibrationally Excited Transitions. Astron. Astrophys. 641, A160. doi:10.1051/0004-6361/202038466
Melosso, M., Melli, A., Puzzarini, C., Codella, C., Spada, L., Dore, L., et al. (2018). Laboratory Measurements and Astronomical Search for Cyanomethanimine. Astron. Astrophys. 609, A121. doi:10.1051/0004-6361/201731972
Müller, H. S., Schlöder, F., Stutzki, J., and Winnewisser, G. (2005). The cologne Database for Molecular Spectroscopy, CDMS: a Useful Tool for Astronomers and Spectroscopists. J. Mol. Struct. 742, 215–227. doi:10.1016/j.molstruc.2005.01.027
Nash, A. G. (1990). The Abundance Ratio of Formaldehyde to Ammonia in Molecular Clouds Observed toward Radio Continuum Sources. Astrophys. J. Suppl. Ser. 72, 303. doi:10.1086/191418
Oldham, J. M., Abeysekera, C., Joalland, B., Zack, L. N., Prozument, K., Sims, I. R., et al. (2014). A Chirped-Pulse Fourier-Transform Microwave/pulsed Uniform Flow Spectrometer. I. The Low-Temperature Flow System. J. Chem. Phys. 141, 154202. doi:10.1063/1.4897979
Porterfield, J. P., Eibenberger, S., Patterson, D., and McCarthy, M. C. (2018). The Ozonolysis of Isoprene in a Cryogenic Buffer Gas Cell by High Resolution Microwave Spectroscopy. Phys. Chem. Chem. Phys. 20, 16828–16834. doi:10.1039/c8cp02055h
Puzzarini, C., and Barone, V. (2020). The Challenging Playground of Astrochemistry: an Integrated Rotational Spectroscopy – Quantum Chemistry Strategy. Phys. Chem. Chem. Phys. 22, 6507–6523. doi:10.1039/d0cp00561d
Puzzarini, C., Bloino, J., Tasinato, N., and Barone, V. (2019). Accuracy and Interpretability: The Devil and the Holy Grail. New Routes across Old Boundaries in Computational Spectroscopy. Chem. Rev. 119, 8131–8191. doi:10.1021/acs.chemrev.9b00007
Puzzarini, C. (2020). Grand Challenges in Astrochemistry. Front. Astron. Space Sci. 7, 19. doi:10.3389/fspas.2020.00019
Puzzarini, C., Heckert, M., and Gauss, J. (2008). The Accuracy of Rotational Constants Predicted by High-Level Quantum-Chemical Calculations. I. Molecules Containing First-Row Atoms. J. Chem. Phys. 128, 194108. doi:10.1063/1.2912941
Puzzarini, C., Salta, Z., Tasinato, N., Lupi, J., Cavallotti, C., and Barone, V. (2020). A Twist on the Reaction of the CN Radical with Methylamine in the Interstellar Medium: New Hints from a State-Of-The-Art Quantum-Chemical Study. MNRAS 496, 4298–4310. doi:10.1093/mnras/staa1652
Puzzarini, C., Stanton, J. F., and Gauss, J. (2010). Quantum-chemical Calculation of Spectroscopic Parameters for Rotational Spectroscopy. Int. Rev. Phys. Chem. 29, 273–367. doi:10.1080/01442351003643401
Raghavachari, K., Trucks, G. W., Pople, J. A., and Head-Gordon, M. (1989). A Fifth-Order Perturbation Comparison of Electron Correlation Theories. Chem. Phys. Lett. 157, 479–483. doi:10.1016/s0009-2614(89)87395-6
Ruaud, M., and Gorti, U. (2019). A Three-phase Approach to Grain Surface Chemistry in Protoplanetary Disks: Gas, Ice Surfaces, and Ice Mantles of Dust Grains. Astrophys. J. 885, 146. doi:10.3847/1538-4357/ab4996
S. Yamamoto (Editor) (2017). Introduction to Astrochemistry (Chemical Evolution from Interstellar Clouds to Star and Planet Formation (Berlin/Heidelberg, Germany: Springer).
Shingledecker, C. N., Molpeceres, G., Rivilla, V. M., Majumdar, L., and Kästner, J. (2020). Isomers in Interstellar Environments. I. The Case of Z- and E-Cyanomethanimine. Astrophys. J. 897, 158. doi:10.3847/1538-4357/ab94b5
Sleiman, C., El Dib, G., Rosi, M., Skouteris, D., Balucani, N., and Canosa, A. (2018). Low Temperature Kinetics and Theoretical Studies of the Reaction CN + CH3NH2: a Potential Source of Cyanamide and Methyl Cyanamide in the Interstellar Medium. Phys. Chem. Chem. Phys. 20, 5478–5489. doi:10.1039/c7cp05746f
Tajti, A., Szalay, P. G., Császár, A. G., Kállay, M., Gauss, J., Valeev, E. F., et al. (2004). HEAT: High Accuracy Extrapolated Ab Initio Thermochemistry. J. Chem. Phys. 121, 11599–11613. doi:10.1063/1.1811608
Tonolo, F., Lupi, J., Puzzarini, C., and Barone, V. (2020). The Quest for a Plausible Formation Route of Formyl Cyanide in the Interstellar Medium: A State-Of-The-Art Quantum-Chemical and Kinetic Approach. Astrophys. J. 900, 85. doi:10.3847/1538-4357/aba628
Vazart, F., Calderini, D., Puzzarini, C., Skouteris, D., and Barone, V. (2016). State-of-the-Art Thermochemical and Kinetic Computations for Astrochemical Complex Organic Molecules: Formamide Formation in Cold Interstellar Clouds as a Case Study. J. Chem. Theor. Comput. 12, 5385–5397. doi:10.1021/acs.jctc.6b00379
Zaleski, D. P., Seifert, N. A., Steber, A. L., Muckle, M. T., Loomis, R. A., Corby, J. F., et al. (2013). Detection of E-Cyanomethanimine toward Sagittarius B2(N) in the Green Bank Telescope PRIMOS Survey. ApJ 765, L10. doi:10.1088/2041-8205/765/1/l10
Keywords: astrochemistry, gas-phase reactivity, rotational spectroscopy, prebiotic molecules, ISM
Citation: Puzzarini C (2022) Gas-phase Chemistry in the Interstellar Medium: The Role of Laboratory Astrochemistry. Front. Astron. Space Sci. 8:811342. doi: 10.3389/fspas.2021.811342
Received: 08 November 2021; Accepted: 20 December 2021;
Published: 10 February 2022.
Edited by:
Ashraf—Ali, University of Maryland, United StatesReviewed by:
Jonathan Tennyson, University College London, United KingdomCopyright © 2022 Puzzarini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cristina Puzzarini, Y3Jpc3RpbmEucHV6emFyaW5pQHVuaWJvLml0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.