- College of Computer Science and Engineering, University of Ha’il, Ha’il, Saudi Arabia
Introduction: In the intricate realm of enzymology, the precise quantification of enzyme efficiency, epitomized by the turnover number (kcat), is a paramount yet elusive objective. Existing methodologies, though sophisticated, often grapple with the inherent stochasticity and multifaceted nature of enzymatic reactions. Thus, there arises a necessity to explore avant-garde computational paradigms.
Methods: In this context, we introduce “enzyme catalytic efficiency prediction (ECEP),” leveraging advanced deep learning techniques to enhance the previous implementation, TurNuP, for predicting the enzyme catalase kcat. Our approach significantly outperforms prior methodologies, incorporating new features derived from enzyme sequences and chemical reaction dynamics. Through ECEP, we unravel the intricate enzyme-substrate interactions, capturing the nuanced interplay of molecular determinants.
Results: Preliminary assessments, compared against established models like TurNuP and DLKcat, underscore the superior predictive capabilities of ECEP, marking a pivotal shift in silico enzymatic turnover number estimation. This study enriches the computational toolkit available to enzymologists and lays the groundwork for future explorations in the burgeoning field of bioinformatics. This paper suggested a multi-feature ensemble deep learning-based approach to predict enzyme kinetic parameters using an ensemble convolution neural network and XGBoost by calculating weighted-average of each feature-based model’s output to outperform traditional machine learning methods. The proposed “ECEP” model significantly outperformed existing methodologies, achieving a mean squared error (MSE) reduction of 0.35 from 0.81 to 0.46 and R-squared score from 0.44 to 0.54, thereby demonstrating its superior accuracy and effectiveness in enzyme catalytic efficiency prediction.
Discussion: This improvement underscores the model’s potential to enhance the field of bioinformatics, setting a new benchmark for performance.
Introduction
The intricate tapestry of cellular metabolism is orchestrated by enzymes and bio-catalysts that expedite and modulate a plethora of biochemical reactions essential for life (Robinson, 2015). Central to understanding an enzyme’s catalytic prowess is the turnover number, kcat, a metric that quantifies the maximal number of substrate molecules an enzyme can convert to product per active site per unit time (Sánchez et al., 2017; Khodayari and Maranas, 2016). This parameter, emblematic of enzymatic efficiency, serves as a linchpin in the realm of enzymology, underpinning quantitative studies that span from cellular physiology to biotechnological applications (Ebrahim et al., 2016; Davidi et al., 2016). Accurate kcat values are indispensable for deciphering the kinetic intricacies of individual enzymes and are foundational in constructing and refining large-scale metabolic models that seek to emulate the holistic metabolic dynamics of organisms (Saa and Nielsen, 2017). Furthermore, with the burgeoning interest in synthetic biology and metabolic engineering, precise knowledge of kcat values becomes pivotal in designing enzymes with tailored functionalities (Strutz et al., 2019).
Despite the undeniable significance of kcat, the landscape of its experimental determination is fraught with challenges. High-throughput experimental assays for kcat remain conspicuously absent, rendering acquiring these values for most enzymatic reactions a labor-intensive and costly endeavor (Rives et al., 2021). Consequently, a substantial chasm exists between the number of biochemically characterized enzymes and those with empirically determined kcat values (Rao et al., 2019). This paucity of experimental data has spurred the development of computational methodologies aimed at predicting kcat values. However, extant prediction frameworks, while pioneering, often grapple with the multifarious and stochastic nature of enzymatic reactions, leading to predictions that, albeit insightful, are occasionally imprecise or bereft of quantified uncertainties (Detlefsen et al., 2022; Smallbone et al., 2013). Traditional methods, relying predominantly on deterministic algorithms, often fall short of capturing the nuanced interplay of molecular determinants governing enzymatic turnover (Goldman et al., 2022).
Artificial intelligence (AI) has significantly advanced in various fields, particularly machine learning (ML) and deep learning techniques. In medicine, deep learning, especially convolutional neural networks (CNN), has revolutionized medical detection systems, especially in analyzing medical images (Salas-Nuñez et al., 2024; Feehan et al., 2021). These developments hold great promise for early diagnosis, detection, and treatment of various diseases, demonstrating the potential of AI to tackle complex real-world problems, particularly in image analysis and computer vision (Du and Swamy, 2014; Pereira and Borysov, 2019). These developments hold great promise for the early diagnosis, detection, and treatment of various diseases, demonstrating the potential of AI to tackle complex real-world problems, particularly enzyme turnover number (kcat) prediction (Robinson et al., 2020). In this process, CNNs are used to predict the enzymatic turnover by process input data, such as protein and substrate molecular structures. By learning hierarchical representations from input data, CNNs can capture complex patterns and relationships within enzyme-substrate systems, improving prediction performance. Moreover, CNNs provide scalability and efficiency in managing extensive datasets, rendering them invaluable for expediting enzymatic turnover prediction and streamlining enzyme engineering endeavors across diverse biotechnological applications (Upadhyay et al., 2023; Mittal et al., 2021; Ge et al., 2023). The integration of AI into enzyme engineering has led to significant advancements, particularly in tailoring methods for optimizing lipase production and properties such as catalytic activity, stability, and substrate specificity. This progress involves using optimized network models and algorithms to predict and enhance lipase performance. Li et al. (2020) explored various AI-based approaches and their applications in lipase modification, discussing both their benefits and limitations. The author highlights the application of various neural networks and algorithms to optimize lipase production and predict molecular variations affecting its properties. Additionally, they emphasize the need to explore these research gaps and outline future perspectives for AI applications in enzyme engineering, particularly for lipases.
While existing models such as TurNuP and DLKcat have made significant progress in kcat prediction, there remains a critical need for methods that predict and quantify the uncertainty inherent in these predictions (Zhou et al., 2020). The biological realm is full of variability and stochasticity, and any model that ignores this inherent uncertainty risks oversimplification and possible misinterpretation (Davidi et al., 2016; Mittal et al., 2021). The enzyme catalytic efficiency prediction (ECEP) seeks to enhance the performance of existing models. It improves predictive accuracy and incorporates a CNNS deep learning algorithm, enabling it to surpass the capabilities of TurNuP and DLKcat. Additionally, we introduce new features derived from enzyme sequences and chemical reaction information, which further enhance performance. By refining the ensemble technique, we achieve significant improvements over current methodologies.
While previous implementations such as TurNuP employed simple mean averaging for ensemble predictions, assigning equal weight to each Kcat prediction, these approaches often failed to account for the varying predictive power of individual models. In this study, we introduce a novel methodology that improves upon this by utilizing a weighted mean approach for ensemble predictions. By optimizing the weights assigned to each model based on their performance, our method ensures that more accurate models have a greater influence on the final prediction. This refinement not only enhances predictive accuracy but also provides a more robust framework for enzyme catalytic efficiency prediction (see Figure 1).
Background
Enzyme performance prediction using deep learning models has become the cornerstone of contemporary bioinformatics. Kroll et al. (2023) presented a study highlighting TurNuP, a new computational method designed for accurately predicting turnover numbers (kcat) for enzymatic reactions, which are important for cellular biological activities. Contrary to current models that are limited to specific organisms or are restricted to enzymes closely resembling those in their training data, TurNuP is a generalized model capable of accurately predicting turnover rates for a wide range of enzymes in their natural reactions. TurNuP surpasses previous models and generalizes enzymes different from those in its training set. Incorporation of TurNuP-kcat values into metabolic models heightens predictions of proteome allocation. Li et al. (2022) highlighted DLKcat, a deep learning model that helps in the prediction of the turnover number (kcat) of enzymes. Unlike conventional methods, DLKcat demands less elaborate input characteristics, focusing mainly on information about the amino acid sequence of the enzyme and one of the reaction substrates. The model is intended to deliver accurate predictions of kcat especially when experimental data for closely related enzymes are lacking. DLKcat is imagined to have wide application in various enzymatic reactions. However, its efficiency drops significantly for enzymes that differ from those in the training dataset. This shortcoming highlights the significance of training deep learning models on dissimilar and representative datasets to guarantee vigorous performance across a broad range of enzyme types. Nevertheless, DLKcat is assumed as a tool to predict kcat values, especially in scenarios where experimental data for the respective enzymes are lacking.
Heckmann et al. (2020) demonstrated the potency of machine learning in the estimation of catalytic turnover numbers (kcat) of enzymes in Escherichia coli (E. coli). In particular, the study discovered novel protein structures corresponding to catalytic turnover, enlightening previously unexplored aspects of enzyme kinetics. The forecasting models developed in this research have broader implications. These models surpass previous methods, resulting in significantly better accuracy in predicting quantitative proteome data. They provide a powerful tool for investigating the complexities of cellular metabolism, helping to elucidate growth rates, proteome composition, and organismal physiology. Consequently, these developments offer a valuable tool for comprehensively understanding metabolism and the proteome at the genome-scale. Wendering et al. (2023) analyzed that turnover numbers are the main descriptors of enzyme activity. Integrating them into constraint-based metabolic modeling holds the promise of improving the prediction accuracy of various cellular properties. Despite efforts to integrate in vitro and in vivo turnover data, current methods provide inadequate predictions of condition-dependent growth rates in E. coli and Saccharomyces cerevisiae (S. cerevisiae), especially when considering protein abundance. The authors propose a new method that combines proteomic and physiological data to estimate turnover rates, leading to better predictions of growth rates under specific conditions. This approach not only increases accuracy but also offers a way to catalog the catalytic efficiency of other organisms, thereby advancing our understanding of cellular metabolism.
Tachibana et al. (2023) applied the Bayesian algorithm to enhance enzyme-catalyzed reactions, rooted in the probabilistic interpretation of knowledge, it has witnessed a renaissance in the field of bioinformatics and computational biology. Unlike frequentist approaches, which offer static point estimates, Bayesian methodologies provide a probabilistic framework, allowing for the incorporation of prior knowledge and the quantification of uncertainty in predictions (Shields et al., 2021; Braconi, 2023). This is particularly salient in enzymology, where data scarcity often impedes robust predictions. By leveraging prior distributions and updating them with new data through Bayes’ theorem, Bayesian methods offer a dynamic and adaptive approach to knowledge synthesis (Wang et al., 2023).
The ECEP methodology utilizes convolutional neural networks (CNNs), a sophisticated deep learning algorithm, to predict enzymatic turnover rates. CNNs are adept at autonomously extracting and learning complex feature patterns from intricate datasets (Memon et al., 2020). In this context, the input data consists of enzyme sequences and chemical reaction information. Through the analysis of these inputs, CNNs discern hidden features and patterns essential for comprehending enzymatic activities. These extracted features empower the model to precisely predict enzymatic turnover values, offering significant insights for biochemical research and applications (Gao et al., 2019). This approach harnesses the capabilities of CNNs in managing high-dimensional biological data, establishing it as a potent tool for enzymatic prediction tasks (Sikander et al., 2021).
Materials and methods
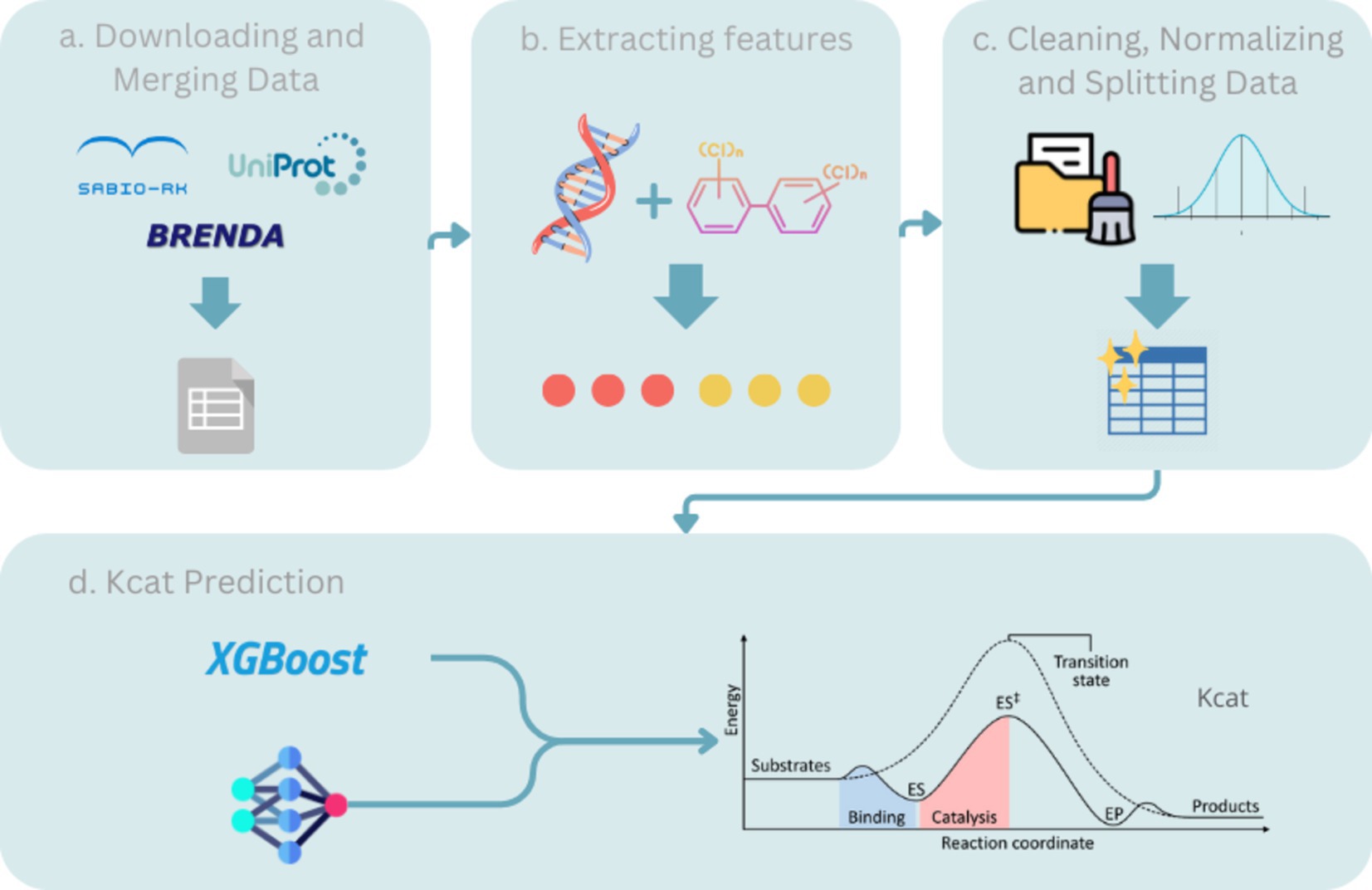
We incorporated TurNuP, which involves preprocessing data for model training, including the generation of datasets through the utilization of chemical reaction information.
Data sources
In our research, we employed the kcat dataset synthesized from the integration of multiple databases, thereby facilitating the development of advanced machine-learning models aimed at predicting enzyme turnover numbers. This dataset was deposited in Zenodo, ensuring public accessibility to uphold the principles of transparency and reproducibility inherent in our findings. The primary dataset amalgamated for this study underwent meticulous curation, drawing from three bioinformatic repositories.
• BRENDA: Renowned as a comprehensive enzyme information system, BRENDA furnishes intricate enzyme and metabolic data meticulously extracted from the primary literature. Its indispensability lies in the facilitation of biochemical pathway reconstruction and enzyme characterization (Schomburg et al., 2017).
• UniProt: A global repository of protein sequences and functional data. UniProt serves as a pivotal resource for associating protein sequences with functional insights, thus proving instrumental in elucidating the biochemical roles of the enzymes (The UniProt Consortium, 2018).
• Sabio-RK: Specialized repositories focusing on enzyme kinetics. Sabio-RK offers curated kinetic data about enzyme-catalyzed reactions, providing critical parameters, such as reaction rates and environmental conditions (Wittig et al., 2018).
The collaborative integration of these databases bolsters the robustness of our dataset, thereby empowering precise predictions of enzymatic activity through the application of sophisticated machine-learning methodologies. For comprehensive elucidation and access to the dataset, we refer to its deposition on Zenodo and its associated publication in Nature Communications.
This will give us a training set with 3,391 entries, and a testing set with 874 entries.
Preprocessing steps
We adopted an approach similar to that outlined in the TurNuP paper, with the addition of generating new features derived from the substrate and product components of chemical reactions.
• Data integration: Information sourced from the aforementioned repositories was amalgamated based on enzyme commission (EC) numbers and protein identifiers, thereby facilitating seamless fusion of enzyme sequences with their corresponding kcat values.
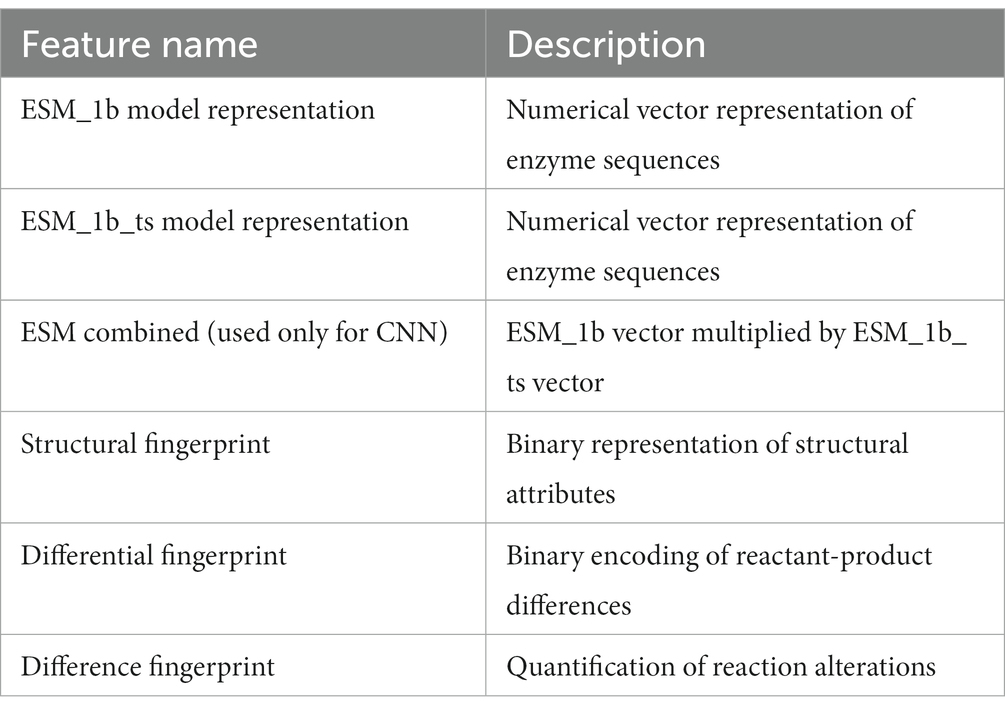
• Feature extraction: Pertinent attributes for each enzyme were abstracted, encompassing the conversion of amino acid sequences into numerical representation by utilizing the pre-trained ESM1b model and its fine-tuned version. Additionally, binary numerical representations of chemical reactions were computed using three methodologies: structured fingerprinting, difference fingerprinting, and the DRFP.
• Data cleaning: Instances with missing, duplicate, or outlier values were removed to ensure data integrity.
• Normalization: Numerical features were normalized to ensure uniform scaling by employing the Z-score normalization technique.
• Train/test splitting: The dataset was partitioned into a training set, utilized for model training, and a test set, employed for unbiased evaluation of the model performance post-training. This approach ensures the model’s ability to generalize effectively to new, unseen data, thereby upholding the reliability and robustness of the predictive models.
Final useful features
Upon the conclusion of preprocessing, we acquired the final train and test pickles, highlighting notable features embedded within this dataset.
A critical component of the ECEP model’s success is the detailed feature engineering process. Below, we outline the steps taken to extract and process features from raw data:
1. Data integration: We amalgamated enzyme sequence data with their corresponding kcat values from BRENDA, UniProt, and Sabio-RK databases.
2. Sequence representation: Enzyme sequences were converted into numerical vectors using the ESM-1b, and ESM_1b_ts models which provided 1,280-dimensional feature vectors for each sequence.
3. Reaction fingerprinting: Chemical reactions were represented using three fingerprinting methodologies:
o Structural fingerprinting: Captured physical and chemical properties (length: 4,096 binary values).
o Differential fingerprinting: Encoded differences between reactants and products (length: 2,048 binary values).
o Difference fingerprinting: Quantified alterations in chemical reactions (length: 2,048 binary values) (see Table 1).
Enzyme sequence representation
Enzyme sequences, initially presented as extensive strings of capitalized characters in fasta file format, necessitate conversion into a numerical format conducive to machine learning models. Leveraging the ESM-1b model, a transformer-based protein language model trained through unsupervised methods on vast protein sequences, these sequences are transformed into numeric vectors of length 1,280. The initial iteration of ESM-1b yields a standard numeric representation, while its fine-tuned counterpart, ESM-1b_ts, furnishes an adjusted numeric vector tailored to specific sequence intricacies. Both representations serve as instrumental components for the deep learning models deployed in the study, facilitating the effective prediction of enzyme characteristics.
Reaction fingerprinting
Utilizing the same approach employed for reaction fingerprinting in chemical reaction representation, the process entails converting reactions into numerical representations. Three distinct fingerprinting methodologies are elucidated: structural fingerprinting, differential fingerprinting, and difference fingerprinting. Each methodology adopts a unique perspective on analyzing and representing chemical reactions in numeric or symbolic formats to facilitate diverse computational tasks.
• Structural fingerprinting: This methodology primarily concentrates on capturing the structural attributes of molecules engaged in reactions. It typically entails encoding the physical and chemical properties of each molecule, culminating in a detailed representation conducive to tasks such as similarity assessment and machine learning modeling. The structural fingerprint spans a length of 4,096, comprising binary values of 0 or 1.
• Differential fingerprinting: Differential fingerprinting extends beyond individual characteristics of reactants and products by explicitly encoding disparities between them. This approach proves particularly advantageous in predictive modeling scenarios, where the objective is to discern how alterations in reactant structures manifest in product configurations. The differential fingerprint spans a length of 2,048, featuring binary values of 0 or 1.
• Difference fingerprinting: Analogous to differential fingerprinting, this methodology focuses on delineating alterations occurring throughout a reaction. However, it often employs more complex algorithms to dissect and quantify the precise nature of these alterations, thereby providing deeper insights into reaction mechanics. This encompasses transformations of chemical groups, bond formation or cleavage, and other significant changes during the reaction process. The difference fingerprint spans a length of 2,048.
kcat predictions
Our predictive models for kcat values encompass both traditional machine learning and deep learning approaches.
XGBoost
XGBoost, a prominent algorithm in our research arsenal, is celebrated for its exceptional precision in handling regression tasks, including kcat value prediction. Operating within a gradient-boosting framework akin to its implementation in TurNuP, XGBoost constructs decision trees sequentially, iteratively refining predictions by addressing prior errors.
This algorithm incorporates regularization techniques to mitigate overfitting, employs tree pruning to enhance model simplicity, and leverages parallel processing for expedited computation. Moreover, its intrinsic support for cross-validation and adaptable loss functions enables tailored model optimization, rendering it highly adept at accommodating the intricacies of our expansive biochemical datasets (see Figure 2).
Convolution neural network
Convolutional neural networks (CNNs) represent a class of deep learning algorithms traditionally employed for image recognition and classification tasks. However, their adaptability extends to regression problems, including the prediction of enzyme kinetics such as kcat values. In our study, CNNs are harnessed to glean patterns and features from input data, comprising structural or sequence data about enzymes alongside numerical representations of chemical reactions (see Figure 3).
CNN architectures are characterized by multiple layers, including convolutional layers, pooling layers, and fully connected layers. Initially, the train and test datasets are loaded, essential features are filtered, and these features are vertically concatenated to form a multidimensional array, sequentially traversing each layer of the CNN. Convolutional layers apply filters to the input data, capturing local patterns and features, while pooling layers serve to reduce the spatial dimensions of the resultant feature maps, thereby mitigating computational complexity and curbing overfitting. Subsequently, fully connected layers amalgamate the features gleaned from preceding layers to facilitate predictions.
Training a CNN entails furnishing it with labeled data—comprising enzyme sequences, numerical representations of chemical reactions, and their corresponding kcat values—and optimizing its parameters, namely weights and biases, via methodologies such as backpropagation and gradient descent.
By leveraging CNNs for kcat value prediction, we capitalize on their innate capacity to autonomously extract pertinent features from input data, potentially encapsulating intricate relationships between enzyme structures or sequences and their catalytic efficacy. This affords the prospect of achieving more precise predictions vis-à-vis traditional regression techniques, particularly when confronted with high-dimensional or nonlinear datasets (see Figure 4).
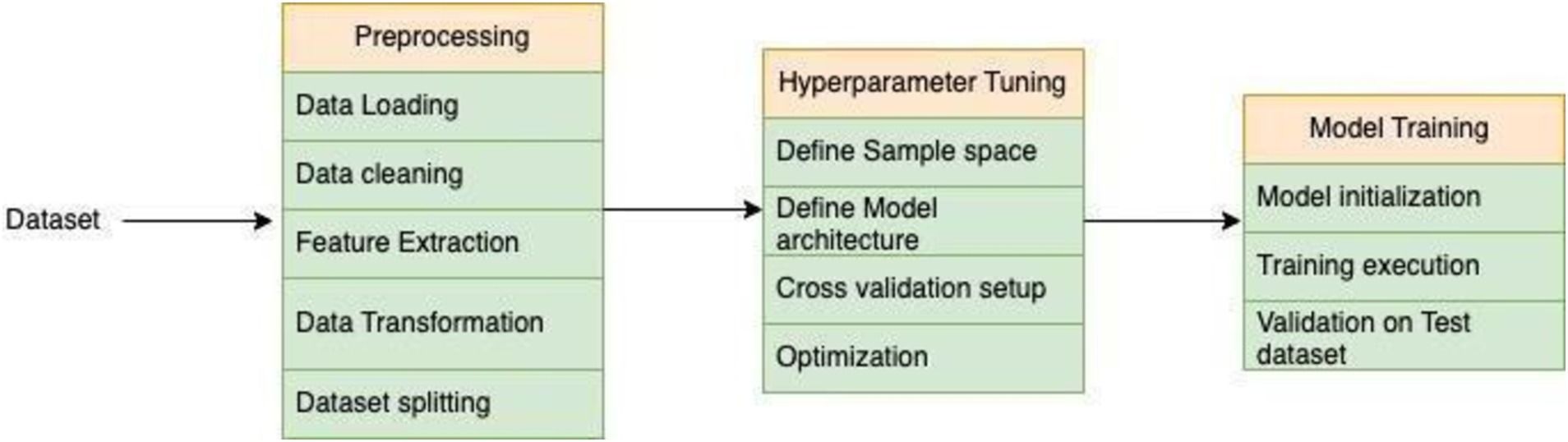
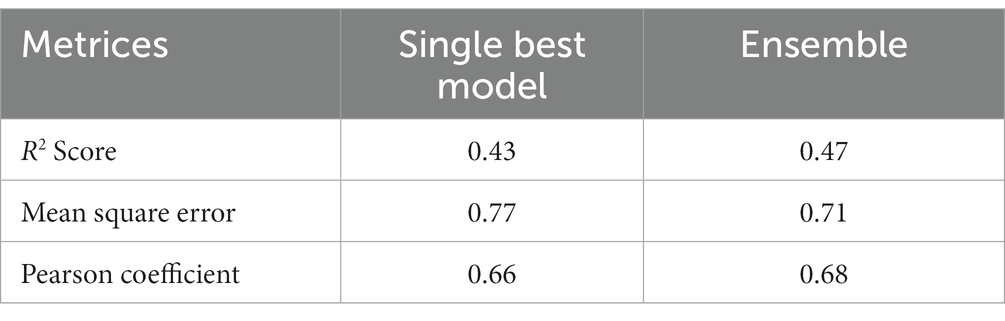
Figure 4. Loading the dataset for preprocessing, getting the optimized hyperparameter, used that hyperparameter to train the Model.
Model evaluation and validation
In order to gauge the efficacy and resilience of both the convolutional neural network (CNN) and XGBoost models, stringent evaluation protocols were implemented. The dataset underwent partitioning into distinct training, validation, and test sets, ensuring exposure of the model to a diverse array of enzymatic reactions throughout both the training and evaluation phases. For XGBoost, Cross-validation, a methodology involving further division of the training set into multiple folds, was employed to furnish a more intricate assessment of the model’s performance. This iterative procedure, wherein various folds are designated as validation sets in each iteration, provides an exhaustive perspective on the model’s capacity for generalization.
Hyperparameter tuning
The process of selecting optimal hyperparameters is paramount, as it intricately refines the configuration of the trained model, ensuring optimal performance across diverse datasets and tasks. Through systematic adjustment of these parameters, we augment the model’s capacity to discern intricate patterns and correlations within the data, thereby enhancing predictive accuracy and generalization capability. This iterative procedure entails experimenting with various hyperparameter combinations and assessing their impact on the model’s performance, with the aim of striking a delicate balance between model complexity and predictive prowess. The chosen hyperparameters wield significant influence over the model’s behavior and efficacy, underscoring the critical nature of their selection within the machine learning pipeline.
XGBoost hyperparameter tuning
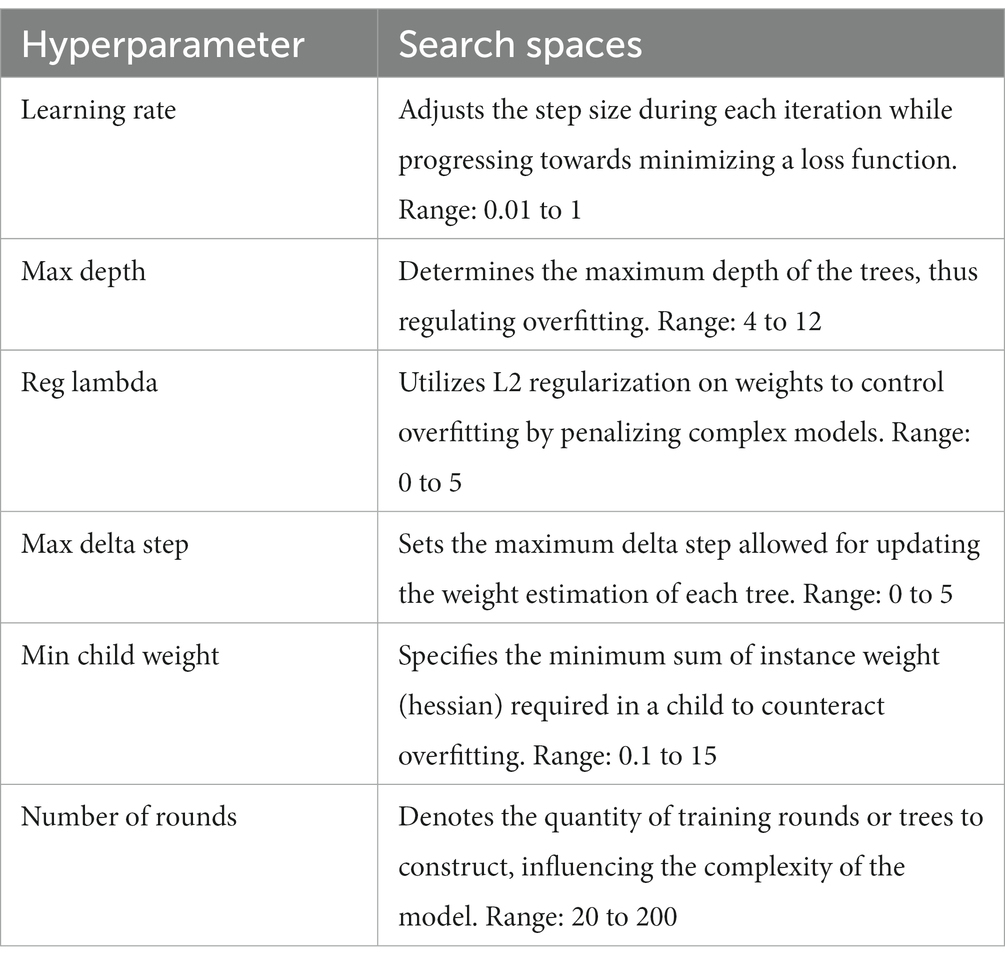
In our XGBoost implementation, we harnessed the capabilities of the hyperOpt package in Python to streamline the selection of optimal hyperparameters. This sophisticated tool automates the intricate process of hyperparameter tuning, allowing us to systematically explore the hyperparameter space and pinpoint configurations that yield superior model performance. Building upon the methodology detailed in the previous implementation elucidated in the TurNuP paper, we conducted a rigorous search for the most appropriate combination of hyperparameters.
This endeavor entailed defining a comprehensive search space encompassing a multitude of hyperparameters, including but not limited to learning rate, tree depth, and regularization terms. Through iterative evaluation of diverse parameter configurations and meticulous assessment of their impact on model performance, our objective was to identify settings that optimize predictive accuracy and generalization capability. This meticulous approach ensures that our XGBoost model is finely attuned to the nuances of our dataset, thereby augmenting its efficacy in predicting enzyme kcat values. Table 2 indicates the detail of the hyperparameter tuning search space.
Convolutional neural network hyperparameter tuning
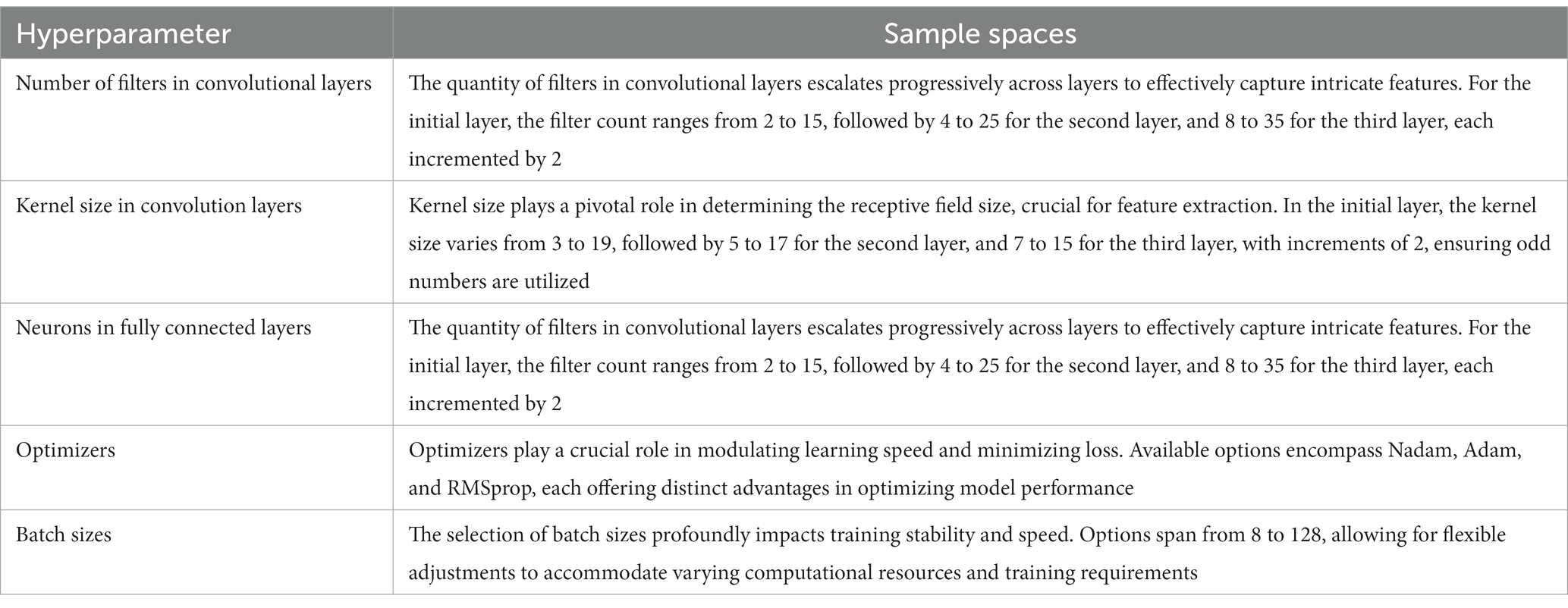
In our pursuit of optimal CNN performance, we embraced a randomized approach within the defined search space for hyperparameter selection. This expansive search space encapsulated a spectrum of parameters, including the number of filters, kernel dimensions, neurons in the fully connected layer, choice of optimizer, batch size, and dropout rate. Furthermore, we incorporated callback functions to dynamically adjust the learning rate and establish early stopping criteria, thus mitigating concerns related to overfitting. Acknowledging the intrinsic variability in deep learning outcomes stemming from random weight initialization and data shuffling, we implemented an ensemble learning strategy to bolster model robustness.
Specifically, we amalgamated the predictions from three distinct models utilizing a weighted averaging technique. This methodology allocates greater weights to the predictions of well-performing models, thereby magnifying their impact within the final prediction ensemble. Through the utilization of this ensemble approach, our aim was to alleviate the inherent variability in deep learning models and bolster the reliability of our predictions. Table 3 indicates the detail of the hyperparameter tuning search space.
Results
The fruition of any research undertaking, particularly within the realm of computational biology and machine learning, culminates in the presentation of results. Within the scope of the “ECEP” model, crafted to forecast enzymatic turnover numbers, the results serve as more than mere evidence of the model’s effectiveness; they symbolize its potential to catalyze advancements within the broader scientific community.
The advent of the “ECEP” model ushers in a new era of enzymatic turnover prediction. Our implementation techniques, encompassing both XGBoost and the newly introduced CNN deep learning approach, have surpassed the performance benchmarks set by previous methodologies.
XGBoost
Through enhancements to the previous XGBoost results, we have attained the following updated outcomes. Employing an ensemble technique, we amalgamated the results from all trained models using a weighted average mean. This comprehensive approach resulted in refined predictions, further elevating the model’s performance and predictive accuracy.
Table 4 indicates XGBoost results were notably enhanced with only previous features within a single model, as well as by ensembling the predictions from the best-performing models. This dual strategy yielded a significant improvement in predictive performance, underscoring the efficacy of feature augmentation and ensemble methodologies in refining model outcomes (see Figure 5).

Figure 5. The graph illustrates the relationship between predicted kcat values (obtained by training XGBoost using previous features) and ground truth kcat values. The red line represents the ground truth predictions, while the blue dots signify predictions generated by the individual XGBoost-trained models. Notably, the predictions from the XGBoost Ensemble model appear to cluster closely around the ground truth predictions, indicating a higher level of precision and agreement between the predicted and actual values compared to the predictions from the single XGBoost model.
Convolution neural network
Convolutional neural networks (CNNs) have markedly enhanced the predictive performance of our model. Not only we have optimized the performance of existing features from the TurNuP methodology, but we have also elevated model efficacy by incorporating new features derived from enzyme sequences and chemical reactions. The ensuing tables delineate the outcomes achieved with and without the integration of these new features (see Figure 6 and Table 5).
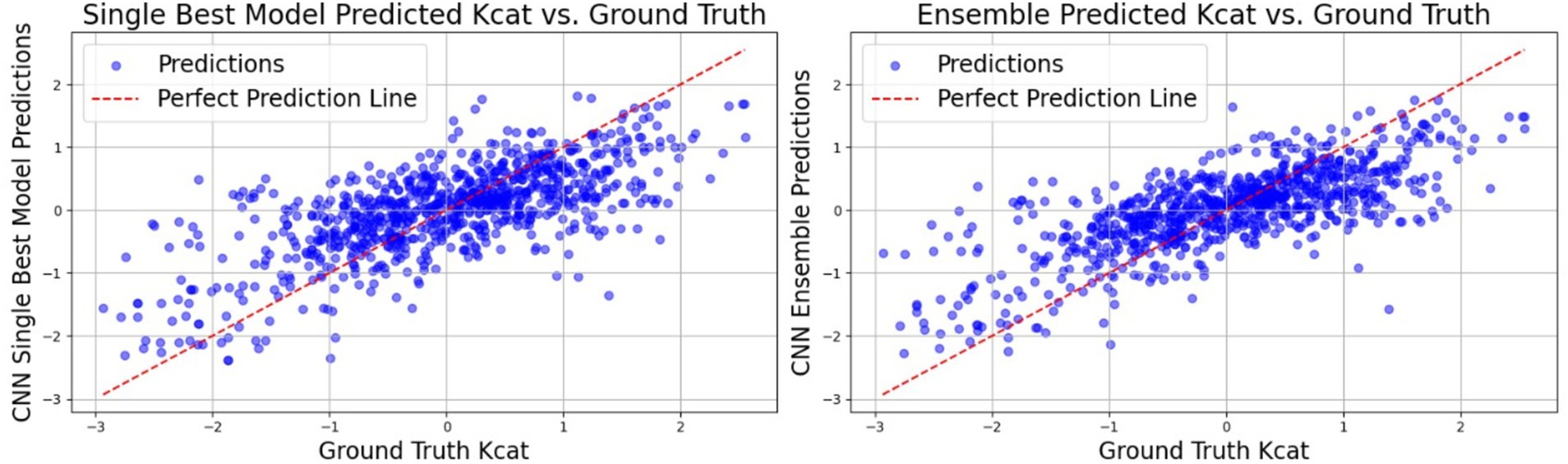
Figure 6. In the graph depicting the relationship between predicted kcat values (obtained through CNN models trained on previous feature only) and ground truth kcat values, the red line represents the ground truth predictions, while the blue dots signify predictions generated by the individual CNN-trained models. Notably, the predictions from the CNN Ensemble model appear to cluster closely around the ground truth predictions, indicating a higher level of precision and agreement between the predicted and actual values compared to the predictions from the single CNN model.
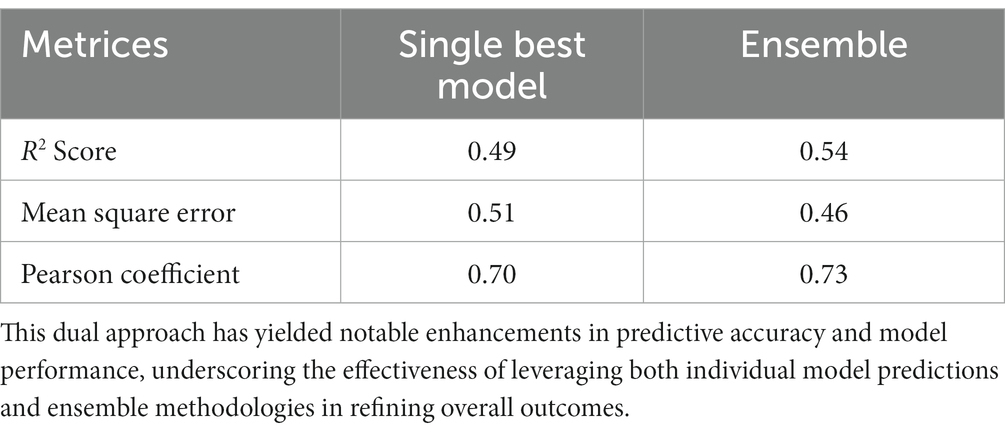
Table 5. This table illustrates the CNN results attained through the utilization of legacy features within a single model, as well as via the aggregation of predictions from the most proficient models in an ensemble.
Ensembling
In contrast to the previous TurNuP implementation, where ensemble averaging was conducted by simply taking the mean of all model predictions which gives equal weightage to each predicted value of kcat, we have enhanced our ensemble methodology by employing a weighted mean approach. This refined method optimizes weights for each model’s predictions by minimizing a loss function against true values. It ensures that weights sum to one and are constrained between zero and one. By dynamically adjusting weights based on predictive performance, more accurate models exert a greater influence on the final prediction, thereby enhancing the ensemble’s robustness and accuracy compared to simpler averaging methods.
While it is possible to ensemble predictions from all trained models, doing so would escalate computational costs. Thus, by ensembling only the best-performing model and leveraging diverse learning features from this model, we can mitigate computational expenses while still achieving superior ensemble performance.
Comparative analysis
To truly appreciate the prowess of the “ECEP” model, it’s essential to juxtapose its performance against that of its contemporaries. When benchmarked against models like DLKCat and TurNuP, “ECEP” consistently outperformed in terms of both R2 and mean squared error (MSE). Such comparative superiority is not just a testament to “ECEP”’s advanced architecture but also its adaptability to the nuances of enzymatic data. A deeper dive into the results reveals some intriguing insights.
The landscape of enzymatic turnover prediction has been punctuated by several innovative models over the years. However, the introduction of “ECEP” has stirred the waters, prompting a re-evaluation of established methodologies. To truly gauge the prowess of “ECEP,” it’s imperative to benchmark it against its contemporaries, notably TurNuP and DLKcat. Before embarking on the comparative analysis, it’s imperative to delve into the historical context. Both TurNuP and DLKcat have established themselves as stalwarts in the field, each offering a distinctive approach:
• TurNuP: Rooted in deep learning architectures, TurNuP harnessed the formidable capabilities of neural networks to unravel the intricate relationships within enzymatic data. Its multi-layered design was crafted to capture both linear and non-linear patterns, rendering it a preferred choice among researchers for numerous years.
• DLKcat: In contrast, DLKcat employed a hybrid approach, amalgamating traditional regression techniques with machine learning algorithms. Its hallmark was its adaptability, with the model being meticulously tailored to accommodate various enzyme families and experimental conditions.
Performance metrics a side-by-side view
R-squared (coefficient of determination)
• ECEP: With an R2 value of 0.54, “ECEP” showcased its superior predictive capabilities, capturing 55% of the variance in the observed kcat values.
• TurNuP: TurNuP, with its deep learning foundation, achieved an R2 value of 0.44. While commendable, it fell short of the benchmark set by “ECEP.”
• DLKcat: DLKcat’s hybrid approach yielded an R2 of 0.44, reflecting its balanced methodology but also highlighting areas of potential improvement (see Figures 7, 8).
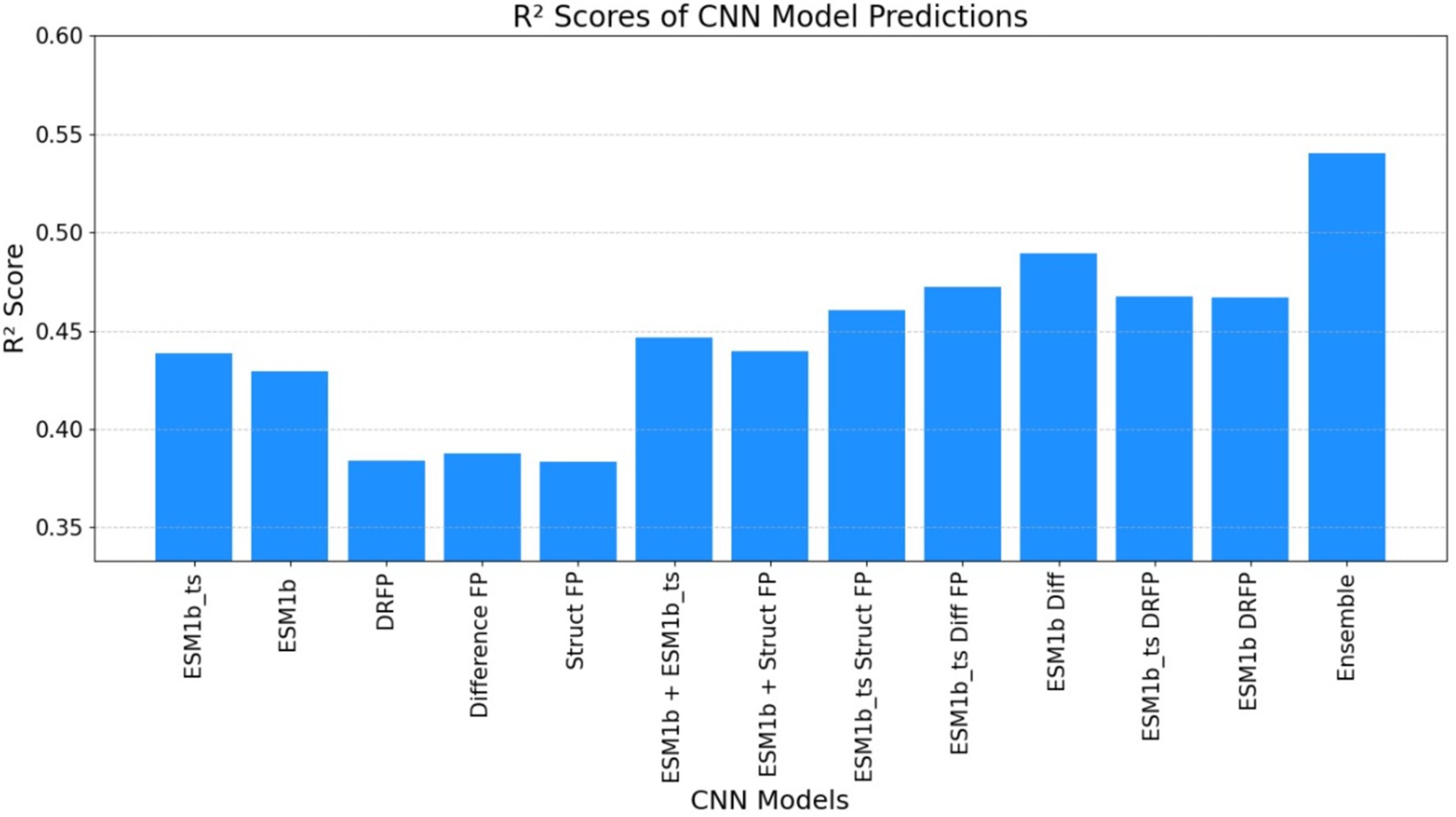
Figure 7. The graph below illustrates the R-squared (R2) score obtained by the ECEP model, which was trained using the CNN approach. This depiction showcases the model’s performance in capturing the variance in the observed kcat values, thereby providing insights into its predictive capabilities.
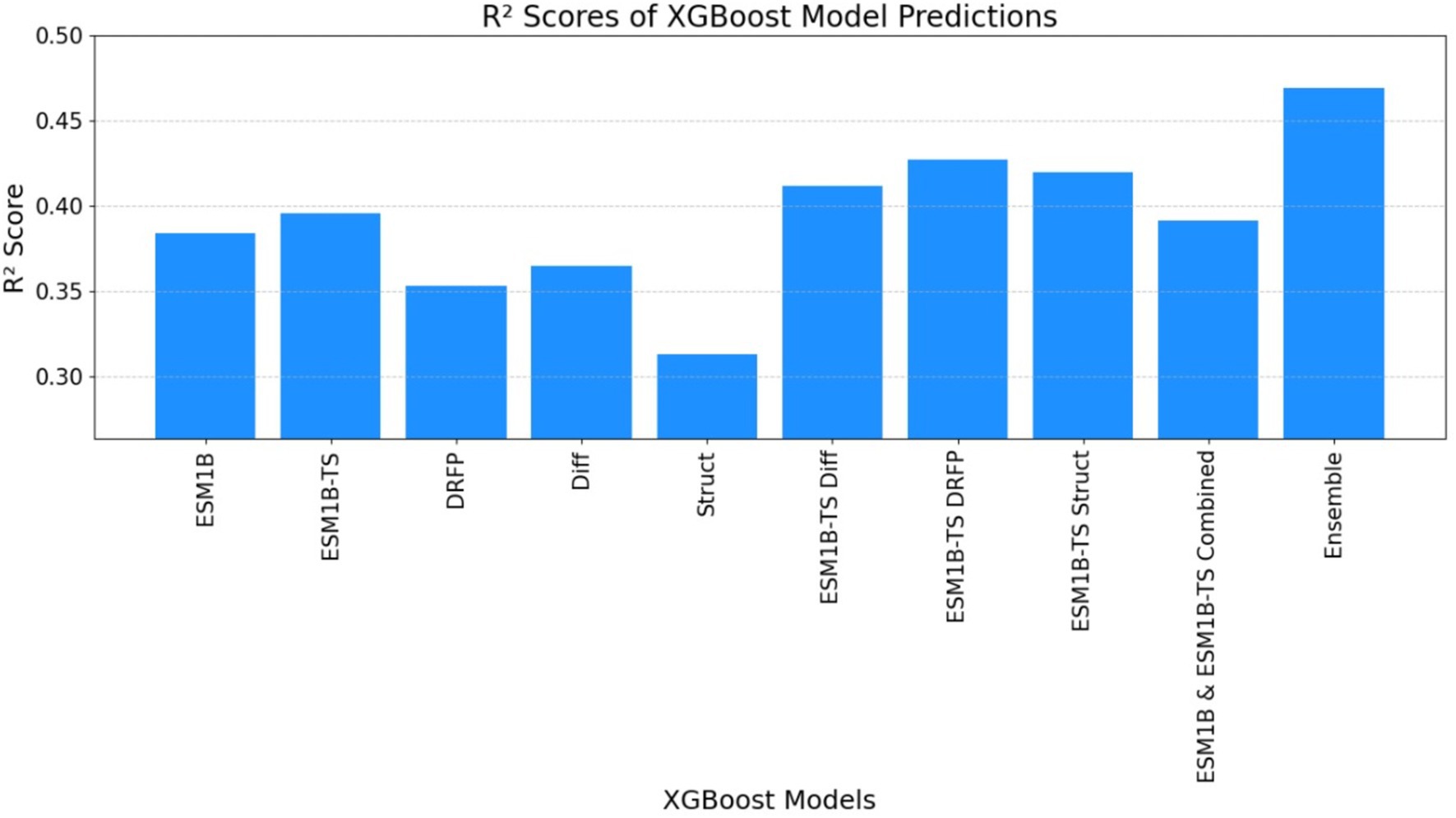
Figure 8. The graph illustrates the R-squared (R2) score obtained by the ECEP model, trained using the XGBoost.
Mean squared error
• ECEP: “ECEP” demonstrated remarkable precision and reliability with an MSE of 0.46, indicative of its superior performance.
• TurNuP: TurNuP achieved an MSE of 0.81, showcasing impressive performance but also highlighting areas for potential refinement to enhance accuracy.
• DLKcat: DLKcat recorded an MSE of 0.87, reflecting the inherent challenges associated with its hybrid approach and the need for careful balancing of traditional and machine learning techniques (see Figures 9–11 and Table 6).
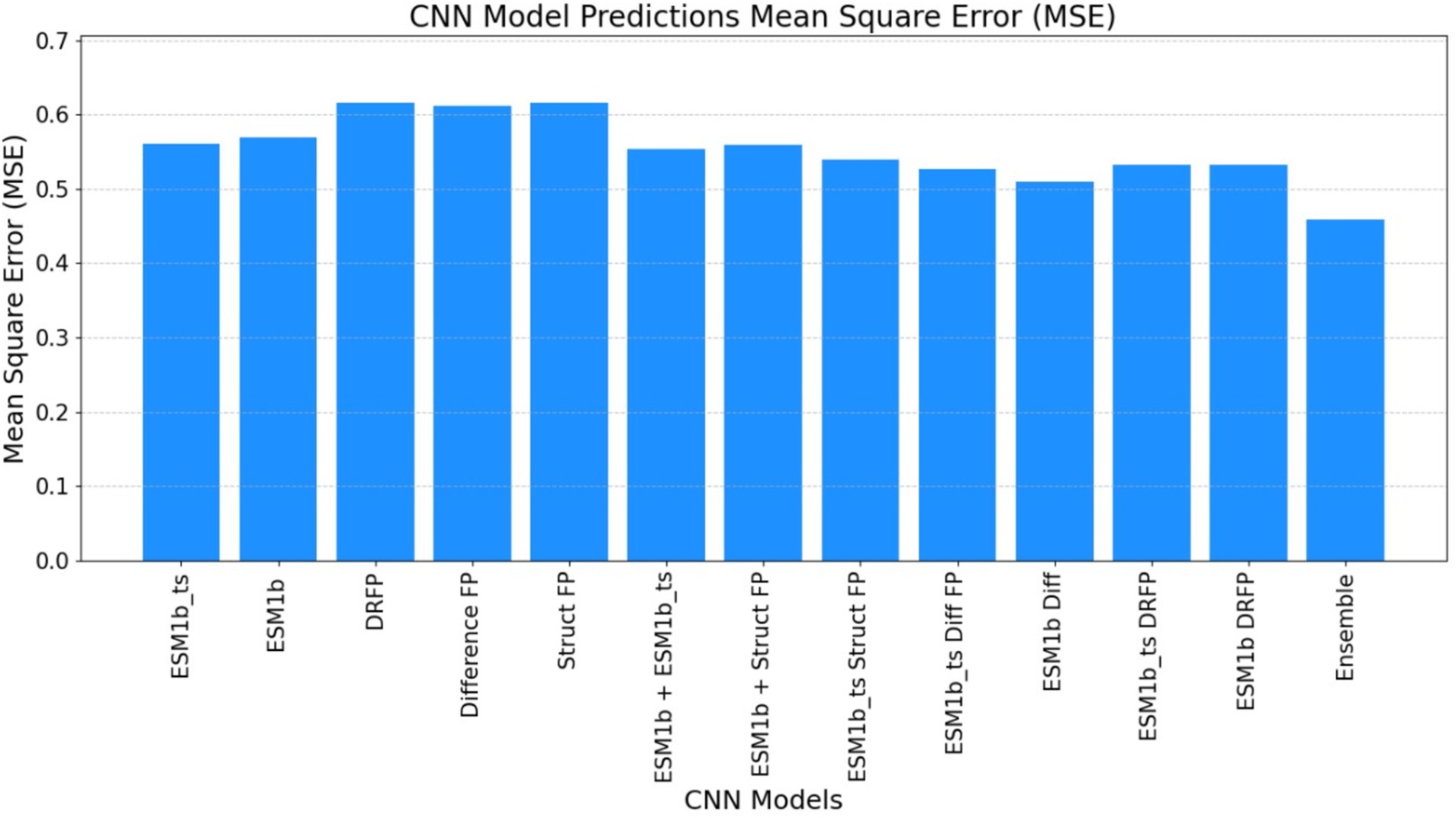
Figure 9. The mean square error (MSE) obtained by the ECEP model, trained using the convolutional neural network (CNN), was 0.46, underscoring the model’s precision and reliability.
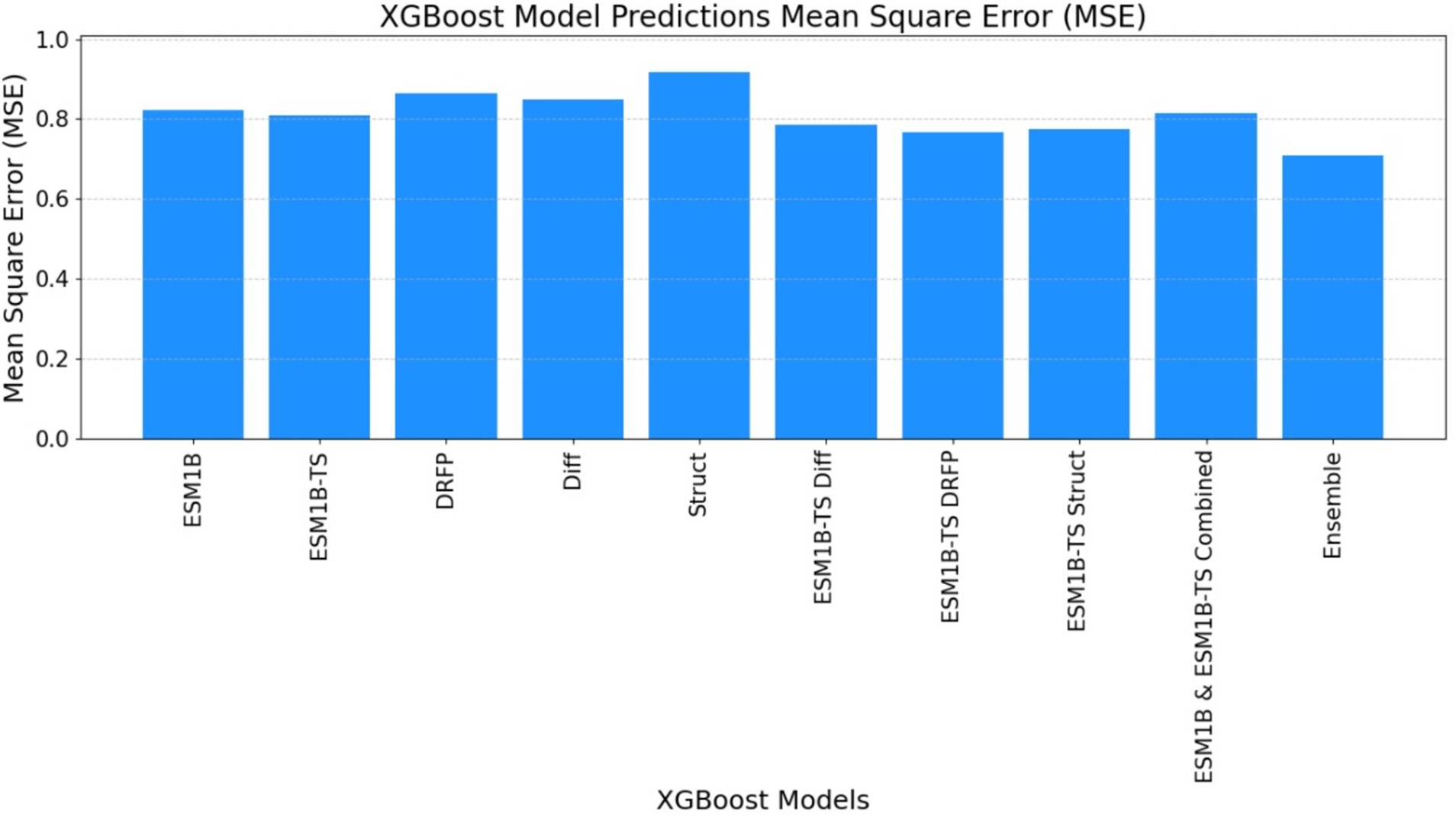
Figure 10. The mean square error (MSE) obtained by the ECEP model, trained using the XGBoost algorithm, was 0.69, affirming the model’s precision and reliability.
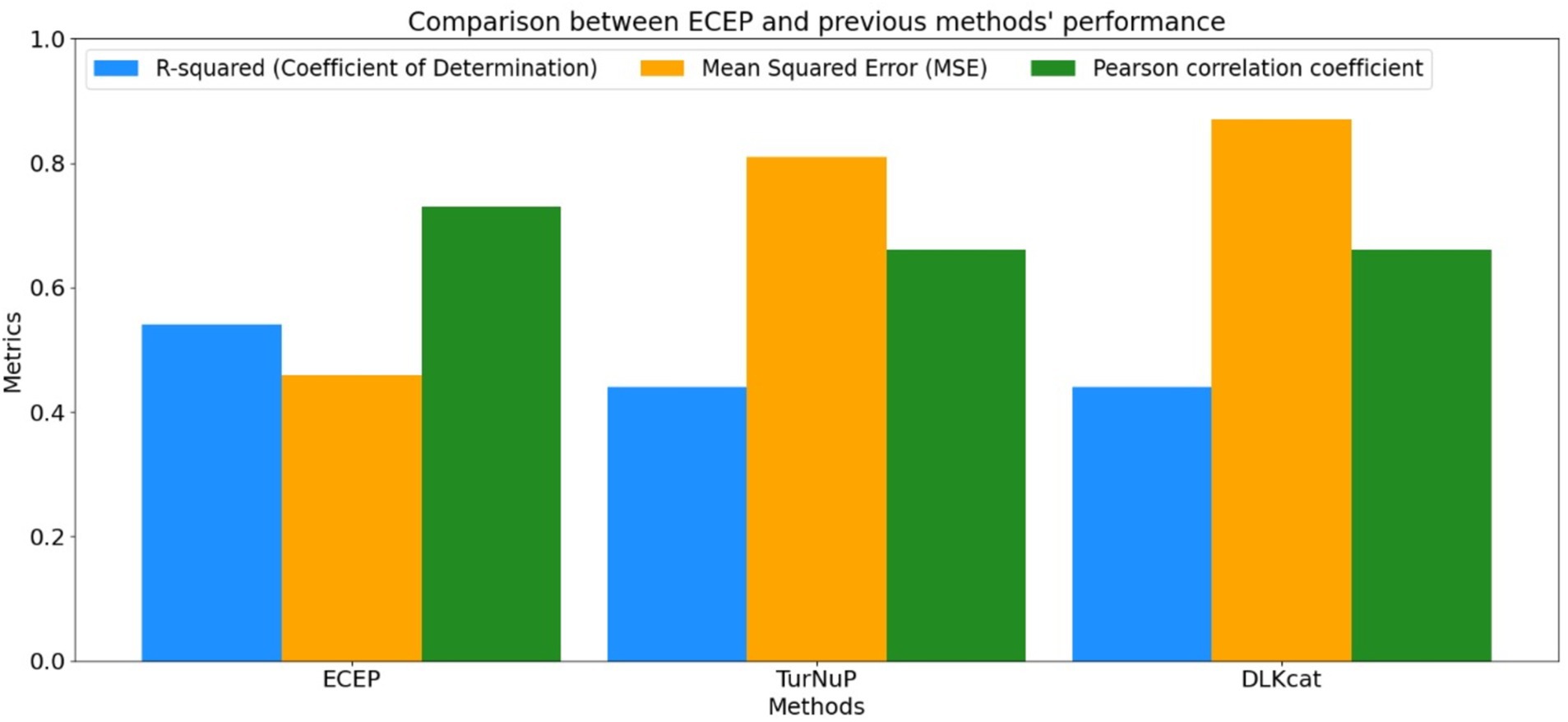
Figure 11. Comparison between R-squared, MSE, and Pearson correlation coefficient obtained by ECEP and previous methods; TurNuP, and DLKcat.
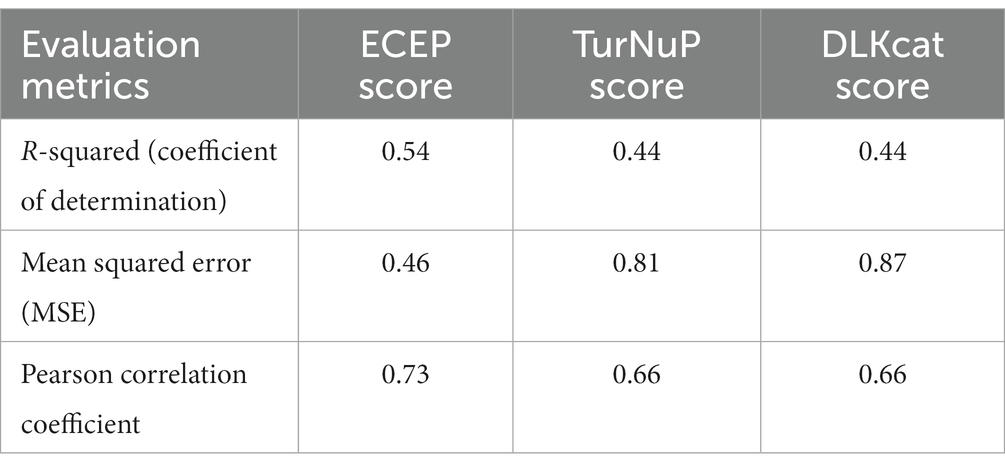
Table 6. This table illustrates a comparison of evaluation metrics scores across various techniques.
Underlying architectures: strengths and limitations
• ECEP: ECEP represents a significant advancement over previous implementations such as TurNuP, leveraging the convolutional neural network algorithm to elevate the R2 score from 44% to an impressive 55%. Its innovative approach not only enhances predictive accuracy but also provides a transparent and interpretable framework.
• TurNuP: TurNuP’s deep learning architecture empowers it to effectively model complex, non-linear relationships within enzymatic data. Its multi-layered neural network excels at capturing intricate patterns, making it highly adaptable to diverse datasets. However, TurNuP’s reliance on extensive data for training and its inherent black-box nature can pose challenges in terms of interpretability, limiting its utility in certain applications.
• DLKcat: DLKcat’s hybrid approach combines traditional regression techniques with machine learning algorithms, endowing it with the flexibility to adapt to various data types and enzyme families. While commendable, this versatility sometimes leads to challenges in finding the optimal balance between regression and machine learning methodologies. DLKcat’s performance highlights its potential for refinement and optimization to achieve even greater predictive accuracy.
The introduction of ECEP has set new standards in enzymatic turnover prediction. While TurNuP and DLKcat have contributed significantly to the field, ECEP emerges as a frontrunner due to its blend of accuracy, flexibility, and interpretability. Nonetheless, scientific progress thrives on collaboration and the integration of insights from diverse methodologies. By amalgamating insights from ECEP, TurNuP, and DLKcat, future methodologies can advance even further, paving the way for more sophisticated approaches in enzymatic turnover prediction.
One of the challenges posed by “ECEP” is its computational intensity, especially with large datasets. The complexity of processing and analyzing vast amounts of data can lead to significant computational demands, which may limit the practical application of the model in real-world scenarios. Future research can focus on optimizing the model for scalability, ensuring that it remains efficient even as the data volume grows. This could involve developing more advanced algorithms that reduce computational load, enhancing parallel processing capabilities, or leveraging high-performance computing resources to manage larger datasets more effectively.
As the field of enzymology evolves, new types of data, from molecular dynamics simulations to quantum mechanical calculations, emerge. These advancements offer detailed insights into enzyme behavior and interactions at unprecedented levels of precision. Adapting “ECEP” to incorporate and leverage these data types can enhance its predictive capabilities, allowing it to provide more accurate and comprehensive analyses. This adaptation could involve integrating new computational techniques and data sources into the model, as well as refining its algorithms to handle the increased complexity and volume of information.
Furthermore, collaboration with other scientific disciplines, such as bioinformatics and structural biology, may provide additional insights and methodologies that can be incorporated into “ECEP.” Such interdisciplinary approaches could lead to the development of hybrid models that combine the strengths of various computational and experimental techniques, thereby improving the overall performance and applicability of “ECEP.”
In summary, addressing the computational challenges and adapting to emerging data types are crucial for the advancement of “ECEP.” By focusing on these areas, future research can enhance the model’s efficiency and predictive power, ultimately contributing to more precise and reliable enzymological studies.
Discussion
Predicting enzyme turnover numbers is challenging due to small and noisy datasets. Bar-Even et al. (2011) discovered discrepancies of up to 20% between BRENDA entries and reference papers, possibly due to copying errors and unit replacements. Additionally, differences in kcat measurements for identical enzyme reaction pairs in different studies can be significant, highlighting the challenge of ensuring consistency and accuracy in enzyme data. Our “ECEP” has performed remarkably well outperforming previous implementations, but it comes with its own set of limitations. The utilization of deep learning models demands significant computing power for optimizing hyperparameters and processing inferences, thereby impacting computational resources and time.
However, the presented results for “ECEP” signify more than just numerical values; they underscore the model’s capabilities. An R2 value of 0.55 indicates high predictive accuracy, suggesting adept capture of underlying enzyme-feature relationships, while a low MSE reinforces precision and alignment with observed values. These metrics, along with case studies, portray a robust, reliable, and nuanced model tailored to enzymology intricacies. In the broader enzymatic turnover prediction landscape, “ECEP” stands out not only due to superior metrics but also its unique approach. Compared to TurNuP and DLKcat, “ECEP” consistently outperforms, especially in complex enzyme families, sparse data, or non-linear dynamics scenarios. However, acknowledging the contributions of TurNuP and DLKcat is essential; they have laid the foundation for advancements in the field.
To further enhance “ECEP,” leveraging convolutional neural networks (CNNs) with powerful computing resources for hyperparameter optimization, acquiring more datasets, and refining feature engineering and extraction could be beneficial. Deep learning models thrive on ample data and sophisticated feature representation techniques, which can bolster predictive accuracy significantly.
In conclusion, the fusion of XGBoost and CNN Regression in the “ECEP” model presents a promising avenue for enzymatic turnover prediction. Despite challenges, its advantages and innovative approach position it as a transformative force in enzymology research. Its true impact will be realized through real-world applications and its contribution to advancing our comprehension of enzymes and their intricate dynamics.
Conclusion
The journey of “ECEP” from conception to validation has been both enlightening and transformative. As we reflect on its performance, implications, and the road ahead, it’s evident that this model represents a significant leap in the realm of enzymatic turnover prediction.
• The introduction of “ECEP” has the potential to redefine the benchmarks in enzymatic turnover prediction. Its ability to provide nuanced, probabilistic outputs can guide experimental designs, risk assessments, and decision-making processes in enzyme kinetics research.
• The insights derived from the model, especially regarding feature importance, can pave the way for novel hypotheses, experimental designs, and a deeper understanding of enzyme dynamics.
• The model’s adaptability ensures that it remains relevant even as new data emerges, making it a valuable tool for ongoing and future research in the field.
Building on the foundations laid by models like TurNuP and DLKcat, future iterations of “ECEP” can explore hybrid approaches, amalgamating the strengths of different models to achieve even higher predictive accuracy.
In essence, “ECEP” represents a beacon of progress in the field of enzymatic turnover prediction. Its introduction promises not just enhanced predictive capabilities but also a deeper understanding of the intricate dance of enzymes. As with any scientific endeavor, the journey is ongoing, and “ECEP” is poised to lead the way, illuminating the path for future research and discoveries in enzyme kinetics.
In conclusion, our proposed methodology represents a significant advancement in the field of enzyme catalytic efficiency prediction. By shifting from simple mean averaging to a weighted mean approach, we address a critical limitation of previous models like TurNuP. Our weighted ensemble technique optimizes the contribution of each model based on its accuracy, resulting in more reliable and precise predictions. This innovative approach not only demonstrates superior performance but also offers a scalable and adaptable framework for future research in enzymology. Our findings underscore the importance of methodological improvements in predictive modeling, paving the way for more accurate and effective tools in bioinformatics.
Data availability statement
We used the BRENDA, UniProt, and Sabio-RK databases to create the kcat dataset. All data and the code used to generate all results is publicly available only at https://github.com/misharisaud/ECEP. All figures are available in high quality at https://figshare.com/articles/figure/ecep-figures/27165615.
Author contributions
MA: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The author thanks Alexander Kroll for making the data and code available online.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bar-Even, A., Noor, E., Savir, Y., Liebermeister, W., Davidi, D., Tawfik, D., et al. (2011). The moderately efficient enzyme: evolutionary and physicochemical trends shaping enzyme parameters. Biochemistry 50, 4402–4410. doi: 10.1021/bi2002289
Braconi, E. (2023). Bayesian optimization as a valuable tool for sustainable chemical reaction development. Nat. Rev. Methods Primers 3:74. doi: 10.1038/s43586-023-00266-3
Davidi, D., Noor, E., Liebermeister, W., Bar-Even, A., Flamholz, A., Tummler, K., et al. (2016). Global characterization of in vivo enzyme catalytic rates and their correspondence to in vitro kcat measurements. Proc. Natl. Acad. Sci. U.S.A. 113, 3401–3406. doi: 10.1073/pnas.1514240113
Detlefsen, N. S., Hauberg, S., and Boomsma, W. (2022). Learning meaningful representations of protein sequences. Nat. Commun. 13:1914. doi: 10.1038/s41467-022-29443-w
Du, K. L., and Swamy, M. S. (2014). “Fundamentals of machine learning” in Neural networks and statistical learning (London: Springer).
Ebrahim, A., Brunk, E., Tan, J., O’Brien, E. J., Kim, D., Szubin, R., et al. (2016). Multi-omic data integration enables discovery of hidden biological regularities. Nat. Commun. 7:13091. doi: 10.1038/ncomms13091
Feehan, R., Montezano, D., and Slusky, J. S. (2021). Machine learning for enzyme engineering, selection and design. Protein Eng. Des. Sel. 34:gzab019. doi: 10.1093/protein/gzab019
Gao, R., Wang, M., Zhou, J., Fu, Y., Liang, M., Guo, D., et al. (2019). Prediction of enzyme function based on three parallel deep CNN and amino acid mutation. Int. J. Mol. Sci. 20:2845. doi: 10.3390/ijms20112845
Ge, F., Chen, G., Qian, M., Xu, C., Liu, J., Cao, J., et al. (2023). Artificial intelligence aided lipase production and engineering for enzymatic performance improvement. J. Agric. Food Chem. 71, 14911–14930. doi: 10.1021/acs.jafc.3c05029
Goldman, S., Das, R., Yang, K. K., and Coley, C. W. (2022). Machine learning modeling of family wide enzyme-substrate specificity screens. PLoS Comput. Biol. 18:e1009853. doi: 10.1371/journal.pcbi.1009853
Heckmann, D., Campeau, A., Lloyd, C. J., Phaneuf, P. V., Hefner, Y., Carrillo-Terrazas, M., et al. (2020). Kinetic profiling of metabolic specialists demonstrates stability and consistency of in vivo enzyme turnover numbers. Proc. Natl. Acad. Sci. U.S.A. 117, 23182–23190. doi: 10.1073/pnas.2001562117
Khodayari, A., and Maranas, C. D. (2016). A genome-scale Escherichia coli kinetic metabolic model k-ecoli457 satisfying flux data for multiple mutant strains. Nat. Commun. 7:13806. doi: 10.1038/ncomms13806
Kroll, A., Rousset, Y., Hu, X. P., Liebrand, N. A., and Lercher, M. J. (2023). Turnover number predictions for kinetically uncharacterized enzymes using machine and deep learning. Nat. Commun. 14:4139. doi: 10.1038/s41467-023-39840-4
Li, F., Yuan, L., Lu, H., Li, G., Chen, Y., Engqvist, M. K., et al. (2022). Deep learning-based kcat prediction enables improved enzyme-constrained model reconstruction. Nat. Catal. 5, 662–672. doi: 10.1038/s41929-022-00798-z
Li, S., Wan, F., Shu, H., Jiang, T., Zhao, D., and Zeng, J. (2020). MONN: a multi-objective neural network for predicting compound-protein interactions and affinities. Cell Syst. 10, 308–322.e11. doi: 10.1016/j.cels.2020.03.002
Memon, S. A., Khan, K. A., and Naveed, H. (2020). Enzyme function prediction using deep learning. Biophys. J. 118:533a. doi: 10.1016/j.bpj.2019.11.2926
Mittal, R., Arora, S., Bansal, V., and Bhatia, M. S. (2021). An extensive study on deep learning: techniques, applications. Arch. Computat. Methods Eng. 28, 4471–4485. doi: 10.1007/s11831-021-09542-5
Pereira, F. C., and Borysov, S. S. (2019). “Machine learning fundamentals” in Mobility patterns, big data and transport analytics (Amsterdam: Elsevier).
Rao, R., Bhattacharya, N., Thomas, N., Duan, Y., Chen, P., Canny, J., et al. (2019). Evaluating protein transfer learning with TAPE. Adv. Neural Inf. Proces. Syst. 32, 9689–9701. doi: 10.1101/676825
Rives, A., Meier, J., Sercu, T., Goyal, S., Lin, Z., Liu, J., et al. (2021). Biological structure and function emerge from scaling unsupervised learning to 250 million protein sequences. Proc. Natl. Acad. Sci. U.S.A. 118:e2016239118. doi: 10.1073/pnas.2016239118
Robinson, P. K. (2015). Enzymes: principles and biotechnological applications. Essays Biochem. 59, 1–41. doi: 10.1042/bse0590001
Robinson, S. L., Smith, M. D., Richman, J. E., Aukema, K. G., and Wackett, L. P. (2020). Machine learning-based prediction of activity and substrate specificity for OleA enzymes in the thiolase superfamily. Synth. Biol. 5:ysaa004. doi: 10.1093/synbio/ysaa004
Saa, P. A., and Nielsen, L. K. (2017). Formulation, construction and analysis of kinetic models of metabolism: a review of modelling frameworks. Biotechnol. Adv. 35, 981–1003. doi: 10.1016/j.biotechadv.2017.09.005
Salas-Nuñez, L. F., Barrera-Ocampo, A., Caicedo, P. A., Cortes, N., Osorio, E. H., Villegas-Torres, M. F., et al. (2024). Machine learning to predict enzyme-substrate interactions in elucidation of synthesis pathways: a review. Metabolites 14:154. doi: 10.3390/metabo14030154
Sánchez, B. J., Zhang, C., Nilsson, A., Lahtvee, P. J., Kerkhoven, E. J., and Nielsen, J. (2017). Improving the phenotype predictions of a yeast genome-scale metabolic model by incorporating enzymatic constraints. Mol. Syst. Biol. 13:935. doi: 10.15252/msb.20167411
Schomburg, I., Jeske, L., Ulbrich, M., Placzek, S., Chang, A., and Schomburg, D. (2017). The BRENDA enzyme information system–from a database to an expert system. J. Biotechnol. 261, 194–206. doi: 10.1016/j.jbiotec.2017.04.020
Shields, B. J., Stevens, J., Li, J., Parasram, M., Damani, F., Alvarado, J. M., et al. (2021). Bayesian reaction optimization as a tool for chemical synthesis. Nature 590, 89–96. doi: 10.1038/s41586-021-03213-y
Sikander, R., Wang, Y., Ghulam, A., and Xianjuan, W. (2021). Identification of enzymes-specific protein domain based on DDE, and convolutional neural network. Front. Genet. 12:759384. doi: 10.3389/fgene.2021.759384
Smallbone, K., Messiha, H. L., Carroll, K. M., Winder, C. L., Malys, N., Dunn, W. B., et al. (2013). A model of yeast glycolysis based on a consistent kinetic characterisation of all its enzymes. FEBS Lett. 587, 2832–2841. doi: 10.1016/j.febslet.2013.06.043
Strutz, J., Martin, J., Greene, J., Broadbelt, L., and Tyo, K. (2019). Metabolic kinetic modeling provides insight into complex biological questions, but hurdles remain. Curr. Opin. Biotechnol. 59, 24–30. doi: 10.1016/j.copbio.2019.02.005
Tachibana, R., Zhang, K., Zou, Z., Burgener, S., and Ward, T. R. (2023). A customized Bayesian algorithm to optimize enzyme-catalyzed reactions. ACS Sustain. Chem. Eng. 11, 12336–12344. doi: 10.1021/acssuschemeng.3c02402
The UniProt Consortium (2018). UniProt: the universal protein knowledgebase. Nucleic Acids Res. 46:2699. doi: 10.1093/nar/gky092
Upadhyay, V., Boorla, V. S., and Maranas, C. D. (2023). Rank-ordering of known enzymes as starting points for re-engineering novel substrate activity using a convolutional neural network. Metab. Eng. 78, 171–182. doi: 10.1016/j.ymben.2023.06.001
Wang, T., Xiang, G., He, S., Su, L., Yan, X., and Lu, H. (2023). DeepEnzyme: a robust deep learning model for improved enzyme turnover number prediction by utilizing features of protein 3D structures. Brief. Bioinform. 25:bbae409. doi: 10.1093/bib/bbae409
Wendering, P., Arend, M., Razaghi-Moghadam, Z., and Nikoloski, Z. (2023). Data integration across conditions improves turnover number estimates and metabolic predictions. Nat. Commun. 14:1485. doi: 10.1038/s41467-023-37151-2
Wittig, U., Rey, M., Weidemann, A., Kania, R., and Müller, W. (2018). SABIO-RK: an updated resource for manually curated biochemical reaction kinetics. Nucleic Acids Res. 46, D656–D660. doi: 10.1093/nar/gkx1065
Keywords: deep learning, CNN, turnover number, kcat , enzyme efficiency
Citation: Alazmi M (2024) Enzyme catalytic efficiency prediction: employing convolutional neural networks and XGBoost. Front. Artif. Intell. 7:1446063. doi: 10.3389/frai.2024.1446063
Edited by:
Anderson Rodrigues dos Santos, Federal University of Uberlandia, BrazilReviewed by:
Balu Bhasuran, Florida State University, United StatesSaleena YounusKunju, Lund University, Sweden
Copyright © 2024 Alazmi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Meshari Alazmi, bXMuYWxhem1pQHVvaC5lZHUuc2E=
 Meshari Alazmi
Meshari Alazmi