
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Artif. Intell., 16 April 2021
Sec. Medicine and Public Health
Volume 4 - 2021 | https://doi.org/10.3389/frai.2021.625573
Introduction: Prognostic scores are important tools in oncology to facilitate clinical decision-making based on patient characteristics. To date, classic survival analysis using Cox proportional hazards regression has been employed in the development of these prognostic scores. With the advance of analytical models, this study aimed to determine if more complex machine-learning algorithms could outperform classical survival analysis methods.
Methods: In this benchmarking study, two datasets were used to develop and compare different prognostic models for overall survival in pan-cancer populations: a nationwide EHR-derived de-identified database for training and in-sample testing and the OAK (phase III clinical trial) dataset for out-of-sample testing. A real-world database comprised 136K first-line treated cancer patients across multiple cancer types and was split into a 90% training and 10% testing dataset, respectively. The OAK dataset comprised 1,187 patients diagnosed with non-small cell lung cancer. To assess the effect of the covariate number on prognostic performance, we formed three feature sets with 27, 44 and 88 covariates. In terms of methods, we benchmarked ROPRO, a prognostic score based on the Cox model, against eight complex machine-learning models: regularized Cox, Random Survival Forests (RSF), Gradient Boosting (GB), DeepSurv (DS), Autoencoder (AE) and Super Learner (SL). The C-index was used as the performance metric to compare different models.
Results: For in-sample testing on the real-world database the resulting C-index [95% CI] values for RSF 0.720 [0.716, 0.725], GB 0.722 [0.718, 0.727], DS 0.721 [0.717, 0.726] and lastly, SL 0.723 [0.718, 0.728] showed significantly better performance as compared to ROPRO 0.701 [0.696, 0.706]. Similar results were derived across all feature sets. However, for the out-of-sample validation on OAK, the stronger performance of the more complex models was not apparent anymore. Consistently, the increase in the number of prognostic covariates did not lead to an increase in model performance.
Discussion: The stronger performance of the more complex models did not generalize when applied to an out-of-sample dataset. We hypothesize that future research may benefit by adding multimodal data to exploit advantages of more complex models.
With an estimated incidence of 18.1 million new cases and 9.6 million deaths worldwide in 2018, cancer is still one of the biggest healthcare challenges today (Ferlay et al., 2019). New paradigms such as cancer immunotherapy have led to an increase in survival for several hematological (Sant et al., 2014) and solid tumors (Pulte et al., 2019). Still, drug development in general, including in oncology, suffers from a high attrition rate. Most drugs (97%) fail during early development phases, a process that is both time-consuming (median duration of phase one clinical is 1.6 years) and costly (as much as $42,000 per patient) (Fogel, 2018; Wong et al., 2019). One of the reasons for such failures may be rooted in a suboptimal enrollment of patients in clinical trials. As a consequence, patients may dropout early due to adverse events, lack of tolerability and/or lack of efficacy which might lead to an early failure of potentially effective drugs (Fogel, 2018). In this context, an accurate characterization of the patients’ recovery (or response to medications) given their prognostic factors is key. Currently, the patients’ prognostic factors are used to determine 1) clinical trial eligibility, 2) toxicity monitoring and 3) treatment decisions. Furthermore, prognostic factors allow us to gain a deeper understanding of disease biology and thus may contribute to the development of more effective treatments (Bhimani et al., 2019).
To date, several prognostic scores in oncology have been published, such as the Royal Marsden Hospital Score (Arkenau et al., 2009), the international prognostic index (International Non-Hodgkin’s Lymphoma Prognostic Factors Project, 1993), the IMDC risk model (Ko et al., 2015) or the Glasgow prognostic score (Kinoshita et al., 2013). Due to prior lack of access to large-scale patient data, the previous prognostic scores were significantly limited on the modeling approaches. Additionally, previous databases also usually contained a small number of covariates, which typically were cast into a simple counting scheme (number of covariates above a threshold).
As a major enhancement, the ROPRO was introduced recently (Becker et al., 2020). The ROPRO is a new pan-cancer prognostic score developed from more than 125k patients in the EHR-derived de-identified database which consists of 27 highly prognostic covariates for overall survival. This prognostic score is based on the Cox proportional hazards model (in the following referred to as Cox model) (Becker et al., 2020), a widely used survival analysis model. In (Becker et al., 2020), ROPRO showed an increased prognostic power when compared to the aforementioned scores and was validated in independent clinical data. In general, the Cox model cannot model nonlinearities or interaction effects, unless all of these effects are explicitly specified (Harrell et al., 1996). While the ROPRO is a multivariate model it does not include covariate interactions and possibly could have missed nonlinearities in the covariates.
To overcome the Cox model’s limitations, recent models such as the regularized Cox model (Tibshirani 1997; Simon et al., 2011), random survival forests (Ishwaran et al., 2008), gradient boosting (Ridgeway 1999) and DeepSurv (Katzman et al., 2018) a deep neural network-modified version of the Cox model have been introduced.
Several studies (Chen et al., 2019; Christodoulou et al., 2019; Desai et al., 2020; Kim et al., 2019; Steele et al., 2018) have been published that compare the prognostic/predictive performance of some of these new survival models. Still, there remains the need for a more systematic and direct comparison. Hence, the objective of this study is to compare the prediction performance of a set of models with respect to model complexity and automated covariate selection. We aimed to address model complexity by implementing more complex survival models (regularized Cox (Tibshirani 1997; Simon et al., 2011), Random Survival Forests (Ishwaran et al., 2008), Gradient Boosting (Ridgeway 1999), DeepSurv (Katzman et al., 2018), a new autoencoder based model (Goodfellow et al., 2016) and Super Learner (van der Laan et al., 2007)) and compared them against the classical model (ROPRO (Becker et al., 2020)). To address the automated covariate selection, we investigated whether an increase in the covariate number, even though not present for all patients, led to an increase in model performance.
In this study we used two databases: 1) the nationwide Flatiron Health (FH) electronic health record (EHR)-derived de-identified database and 2) OAK clinical trial database. During the study period, the FH database included de-identified patient-level structured and unstructured data, curated via technology-enabled abstraction (Birnbaum et al., 2020; Ma et al., 2020) and includes data from over 280 cancer clinics (∼800 sites of care); Institutional Review Board approval of the FH study protocol was obtained prior to study conduct, and included a waiver of informed consent. The OAK dataset was derived from a phase III clinical trial (Rittmeyer et al., 2017) that evaluated the efficacy and safety of Atezolizumab monotherapy against a Docetaxel monotherapy in 1,187 patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) after the failure of platinum based chemotherapy.
From FH we derived a cohort with 136,719 patients across 18 different primary cancers (Table 1). The majority of patients were diagnosed with advanced non-small cell lung cancer (38,201–26.7%), followed by metastatic colorectal cancer (16,788–12.1%) and metastatic breast cancer (14,429–10.4%). We randomly split the samples in the FH dataset into train (90% - 121,644) and in-sample test (10% - 15,075) sets. In case the model required a validation dataset (e.g., neural network based models), the training set was further divided into subsets of 90% for training and 10% for validation. The OAK study (1,187 patients) was used exclusively for out-of-sample testing.
In terms of covariates used per sample, we created three feature sets with differing numbers of covariates that could be used for modeling by the respective method. The first feature set contained 27 covariates of FH inspired from (Becker et al., 2020) (Table 2). The second feature set consisted of 44 covariates that were present in at least 30% of patients in FH, and the third feature set comprised almost all covariates (88 covariates present for at least 1% of the FH patients). The 88 and 44 feature sets included all the covariates of the 44 and 27 feature sets, respectively (a complete list of the covariates in each set is available in Supplementary Table S1). The OAK dataset contained all the covariates used in the 27 covariates feature set except oxygen saturation in blood. In the 44 and 88 feature sets it was in addition lacking information on some covariates as compared to the FH dataset (for a complete list see Supplementary Table S2).
To prepare the data for methods that require a full data matrix, all datasets were imputed with random forests by using the R package missForest (Stekhoven and Bühlmann, 2011). To prevent leakage of information between train and test sets, the imputation (random forest) was trained only on the train sets, and then applied to the FH test set and OAK test set.
One of our objectives in this paper was to determine if more complex survival models, that capture nonlinearities and feature dependence, are capable of predicting the patient’s risk better than the state of the art prognostic scores that are based on the classical Cox model. We selected the ROPRO (a Cox based model) as our baseline model and compared it against the regularized Cox model (Tibshirani 1997), random survival forest (Ishwaran et al., 2008), gradient boosting (Ridgeway, 1999), DeepSurv (Katzman et al., 2018) and a (to our knowledge) new autoencoder-based survival model (Goodfellow et al., 2016). In addition, we extended the super learner (van der Laan et al., 2007) framework to survival analysis problems and used it to aggregate the previous models into an ensemble that combined the predictions of all models, yielding a new weighted prediction.
Generally speaking, in survival analysis, the response variable is the time until an event occurs, such as death (Kalbfleisch and Prentice, 2002). If
The hazard function
The selected models in this paper all follow the underlying structure, but estimate the hazard function using different techniques.
The ROPRO, introduced in (Becker et al., 2020), is a prognostic score based on the Cox model. The Cox model (Cox, 1972) is a widely used model in survival analysis that estimates the hazard function based on a set of given covariates of the population. It assumes that the hazard function
where
where
The authors of ROPRO started with a Cox model with 44 covariates and applied backward selection, removing the least significant covariates, until a total of 27 covariates remained in the model. The 27 selected covariates are represented in Table 2. In this work, we used the ROPRO formula as published in (Becker et al., 2020).
The regularized Cox is a modification of the Cox proportional hazards algorithm where a regularization term is added to the cost function (Tibshirani 1997; Simon et al., 2011). The new regularized cost function has the form
The regularization term forces a penalization to the model weights
Gradient boosting (GB) is a machine learning algorithm used in classification and regression problems (Friedman 2001). It builds the predictive model in an iterative fashion, in each iteration adding a weak learner that reflects the current residuals. By doing so, in each iteration the model should fit better to the data and consequently, reduce the prediction error.
GB can be applied to survival analysis by using the Cox partial likelihood (Cox 1972) as the cost function to determine the residuals (Ridgeway 1999). The new GB partial likelihood has the form
Notice that the Cox model risk
where
Random survival forests (RSF) is a machine learning method that fits an ensemble of regression trees, a “forest”, that estimates the cumulative hazard function (Ishwaran et al., 2008). At each tree node, a covariate is used to separate the patients into groups. The RSF selects the split condition that maximizes the difference between the survival curves of the groups. Each tree is grown until it is not possible to create a new split that has more than a pre-specified number of unique events in each node.
DeepSurv uses a feed-forward neural network to predict the patient’s hazard
Autoencoders (AE) are unsupervized neural networks composed of two components: 1) an encoder function that transforms the input
Here we exploit the autoencoder to perform dimensionality reduction. By setting
The autoencoder does not model the hazard function directly. Therefore, we use a Cox model to estimate the hazard from the intermediate representation. The new hazard is given by
Above we introduced multiple models that are capable of predicting the hazard function. All these algorithms have distinct structures, leading to different strengths and weaknesses in their estimation capability. The Super Learner (SL) (van der Laan et al., 2007) offers a framework to combine these models into a single model with the aim of combining the strengths and mitigating the weaknesses.
The SL was originally proposed to handle classification and regression problems. In this work we extend it to address time-to-event data and to use all the models described above.
Consider the dataset
In this particular problem,
We learn a hazard function
The next step in SL is to combine the predicted hazards of all
where
As listed in Table 3, the more complex models require hyperparameters that adjust their complexity. Depending on the model, these hyperparameters were tuned by either cross-validation (for the regularized Cox models) or grid-search (for GB, RSF, DS and AE) on the FH training set.
All models were fit using the training set and tested using the two distinct testing sets: FH in-sample test and OAK out-of-sample test set. The ROPRO model was taken pre-trained from the formula published in (Becker et al., 2020) and was not trained again, however it was tested equally against our test sets.
To assess the discrimination performance of the models, we used Harrell’s C-index (C-index) (Harrell et al., 1982). The C-index is a generalization of the AUC. It is a goodness of fit measure for survival models and measures the concordance between the risk/hazard values given by the model and the time-to-event. More specifically, it measures if patients that died earlier in time have a higher risk score than patients that died later. The statistic is defined from 0 to 1. Where 1 means perfect concordance, 0.5 means that the model is equivalent to a random guess and 0 represents perfect discordance. The C-index 95% confidence intervals (CI) were determined by bootstrapping. We use the confidence intervals to determine if one model has a significantly higher C-index than the other. In essence, this process is a comparison of two means, where the null hypothesis is
To further evaluate the discrimination of the models we used Uno’s C-index (Uno et al., 2011). Uno’s C-index is an extension of Harrell’s C-index that incorporates the censoring distribution into the score. This modification should make the C-index independent on the study’s censoring distribution (Uno et al., 2011).
Given the differences between the FH test set and OAK, we performed additional analyses to validate our results. The additional analyses include: 1) PCA analysis (Hastie et al., 2009) between FH test and OAK to verify differences between the datasets; 2) create an additional FH test set without the covariates not available in OAK and impute them to check the effect of these covariates. Further, 3) stratify the FH test set by cancer entity to check if C-index varies with cancer entity, and 4) permute FH train and test sets.
All the analyses were done using R 3.5.1 (R Core Team 2018) and Python 3.6. The Cox model and C-index were used as implemented in the survival library (Therneau, 2015). The GB, RSF, and SL were used as implemented in the R libraries, gbm (Greenwell et al., 2019), RandomForestSRC (Ishwaran et al., 2008) and SuperLearner (Polley et al., 2019), respectively. DeepSurv was implemented in the Python DeepSurv package (Katzman et al., 2018). The random forest imputation was implemented in the missForest R library (Stekhoven and Bühlmann, 2011). The full analysis diagram is illustrated in Figure 1.
We modified the SuperLearner, DeepSurv and missForest packages to add new features used in this work. In the SuperLearner package, we added the functionality to process survival analysis problems. More specifically, we added new models (ROPRO, regularized Cox, RSF, GB, RF and AE) and a new fitting algorithm based on the C-index. In DeepSurv we added some functions to assess the quality of fit of the models. Finally, in the missForest package we added the functionality to save the fitted model and use it to impute new data, e.g. test sets that have to remain independent to the training. The modified packages and analysis files are available in the Supplementary materials.
A total of three datasets were used in this analysis, FH train, FH test and OAK (see Methods) including cancer cohorts with a median follow-up time of 19.33 months (95% confidence interval (CI) 19.10–19.57), 19.83 (95% CI 19.33–20.57) and 11.43 (95% CI 10.40–12.67), respectively (Table 2; Figure 2).

FIGURE 2. Kaplan Meier curves of the datasets. (A) - Kaplan Meier curves for the FH train and test datasets. (B) - Kaplan Meier curve for the OAK test set.
Table 2 illustrates the summary statistics for the covariates in the 27 covariate feature set. The summary statistics for the 44 and 88 covariate feature sets are available Supplementary Table S2.
We benchmarked the ROPRO against a set of eight more complex models - regularized cox with lasso, ridge regression and elastic net, GB, RSF, AE, DS and SL - across a total of three different feature sets, each with 27, 44 and 88 covariates yielding a total of 27 models.
After hyperparameter tuning (see Supplementary Table S3 for a list of tested hyperparameters), the optimal shrinkage in the regularized cox resulted in the selection by lasso and elastic net of 23, 27 and 49 covariates in the 27, 44 and 88 covariate models, respectively. With grid search, we determined that the optimal
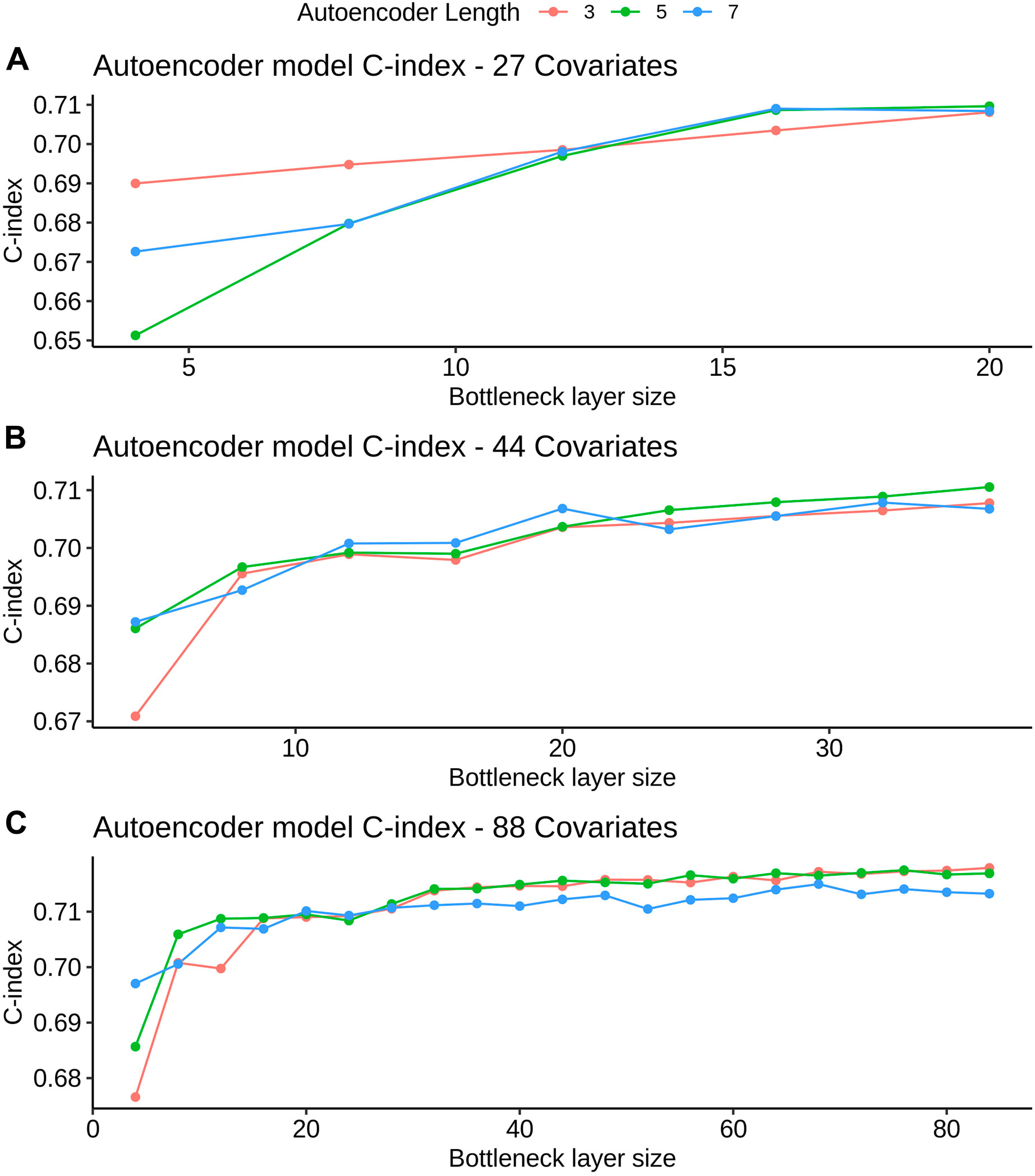
FIGURE 3. AE model C-index values for different bottleneck layer sizes and total layer sizes. All C-index values are referent to the validation set derived from FH train (see Datasets section for more details). Figures A, B and C refer to the 27, 44 and 88 covariate models, respectively.
K-fold cross-validation was used in the SL to calculate the contribution of each model (listed in Table 3) to the final score. Results show that independent from the feature set (27, 44 or 88 covariates) the only models that contributed to the SL score were the ROPRO, RSF and two versions of DS, one with tanh and another with SELU activation functions. Each model contributed to the SL distinctively (see Figure 4 for the models’ risk distributions). The models’ hazard value distributions varied for example, in center RSF (median 0.40–0.64) vs. ROPRO (median ‒0.161 to ‒0.0678), and in spread ROPRO (IQR 0.05–0.13) vs. DS with tanh (IQR 0.399–0.573). Additionally, the predicted risk values were stratified by the time-to-event of the patients (see Figure 5). In the 27 and 44 covariate models, RSF had the most sizable contribution for lower time-to-event (TTE). As the TTE increased, the contribution of RSF subsided while the contribution of both DS models increased. As a result, for later TTE, the model with the highest contribution changed from RSF to DS with tanh. Conversely, in the 88 covariate feature set, there was not a clear separation of the most contributive models.
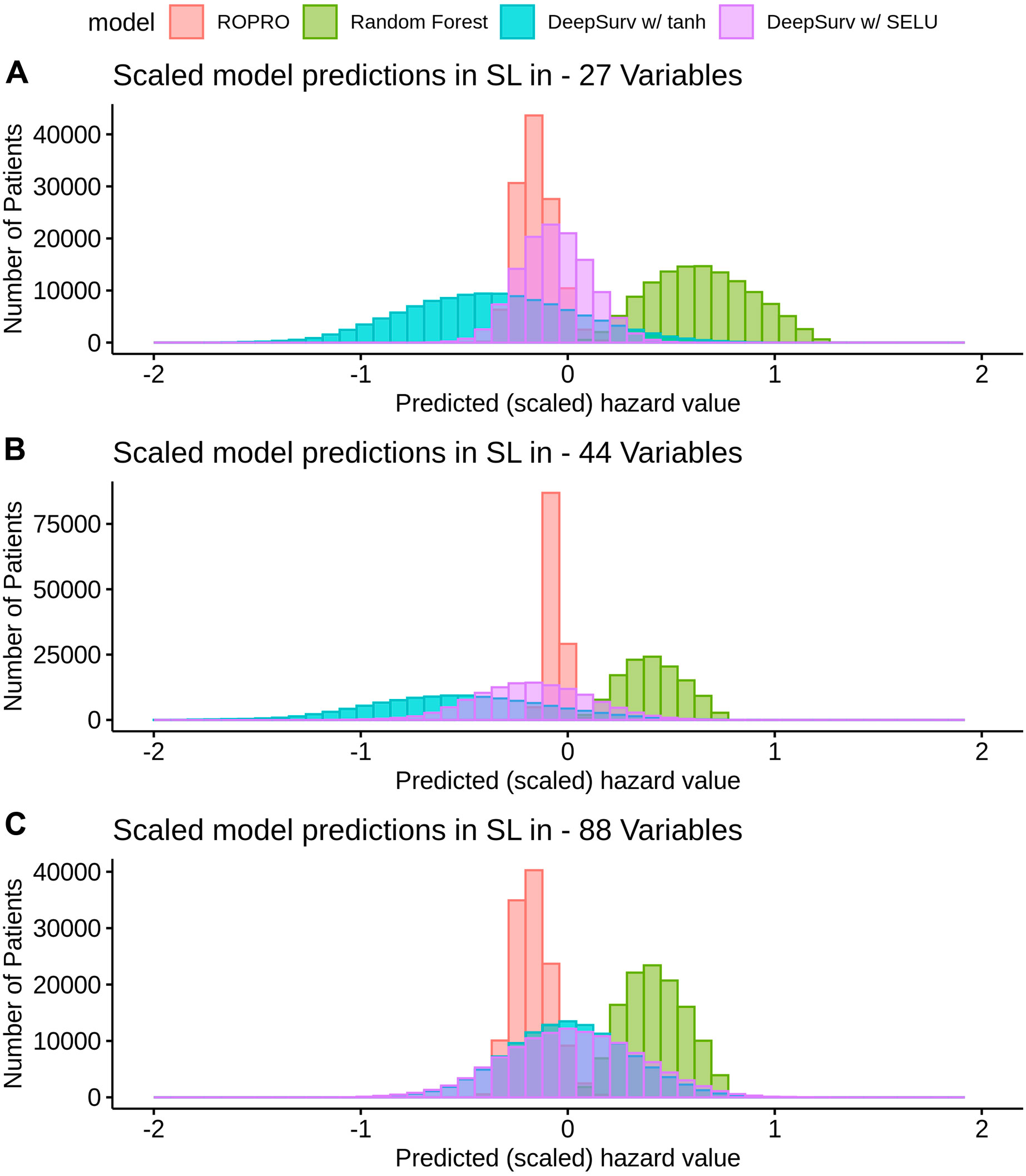
FIGURE 4. Histogram of the risk predictions for each model in the SL in the FH training dataset. The risk values correspond to the risk yielded by the original model, i.e., by ROPRO or DS. The risk was multiplied by the
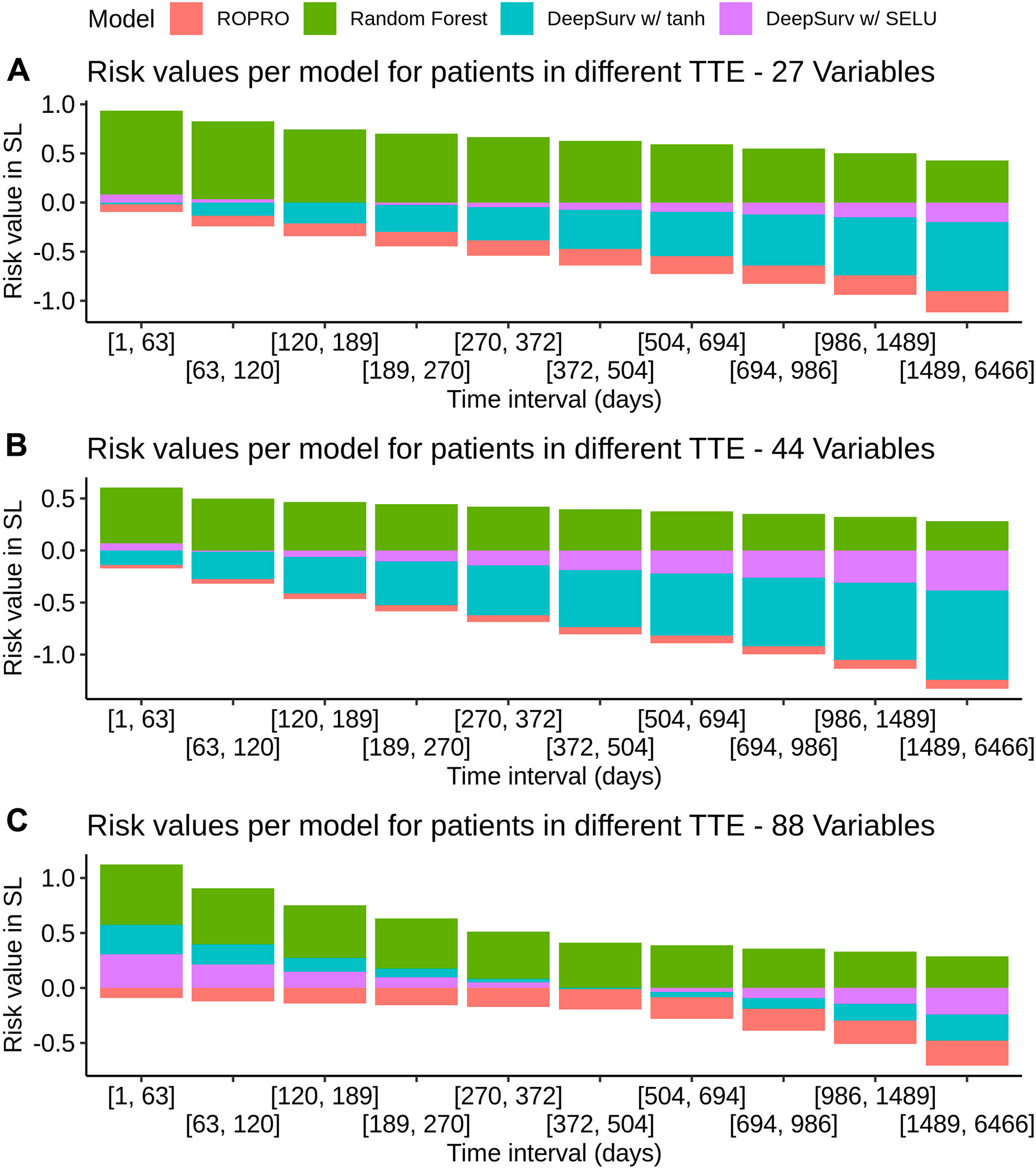
FIGURE 5. Individual model contribution to the SL risk by time-to-event in the FH training set. To create this visualization, the patients were split into groups based on their time to event (TTE). Each of these groups is represented in the x-axis. Then, for each group the median risk value per model was calculated and is displayed on the y-axis. The contribution changes over time because the models correctly assign higher risk for lower times-to-event and lower risk for later times-to-event.
The C-index and corresponding 95% confidence intervals (CI) for the FH test dataset and the OAK test dataset are displayed in Figure 6 and Table 4, and Table 5. Figure 6 contains the C-index distributions for all models, datasets (FH test and OAK test) and feature sets (27, 44 and 88 covariates). Table 4 offers a more granular view of the C-index distributions from Figure 6, with information on each models’ C-index and 95% CI. Furthermore, Table 5 includes the Uno C-index and corresponding 95% CI.

FIGURE 6. Violin plot of the C-index in the FH test dataset (top) and OAK III (bottom). The C-index results for the 27, 44 and 88 covariate sets are illustrated in Figure (A)–(C), respectively. The plot displays the distribution and a box-plot of the C-index. Bootstrap was used to determine the distribution of the C-index.
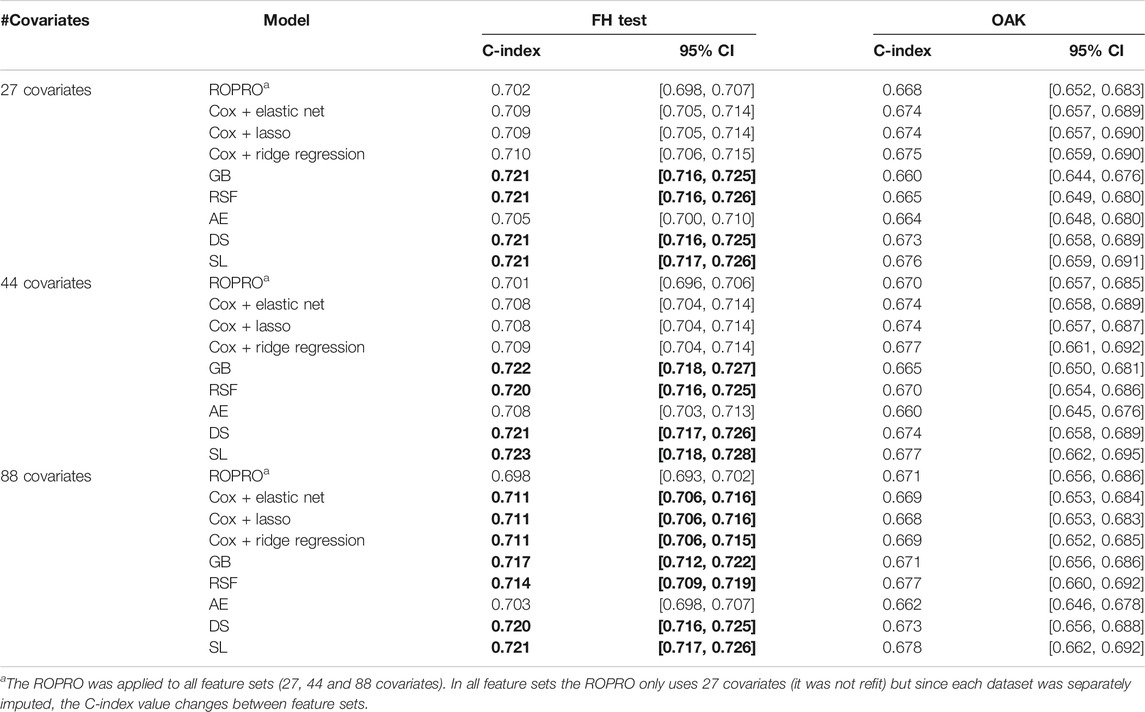
TABLE 4. C-index and corresponding 95% confidence interval (CI) for all the models (ROPRO, regularized Cox models, Gradient Boosting (GB), Random Survival Forests (RSF), autoencoder (AE), DeepSurv (DS) and Super Learner (SL)) and covariate sets. Significant increases in C-index are in bold. Please refer to Individual Model Development section for the complete model hyperparameters.
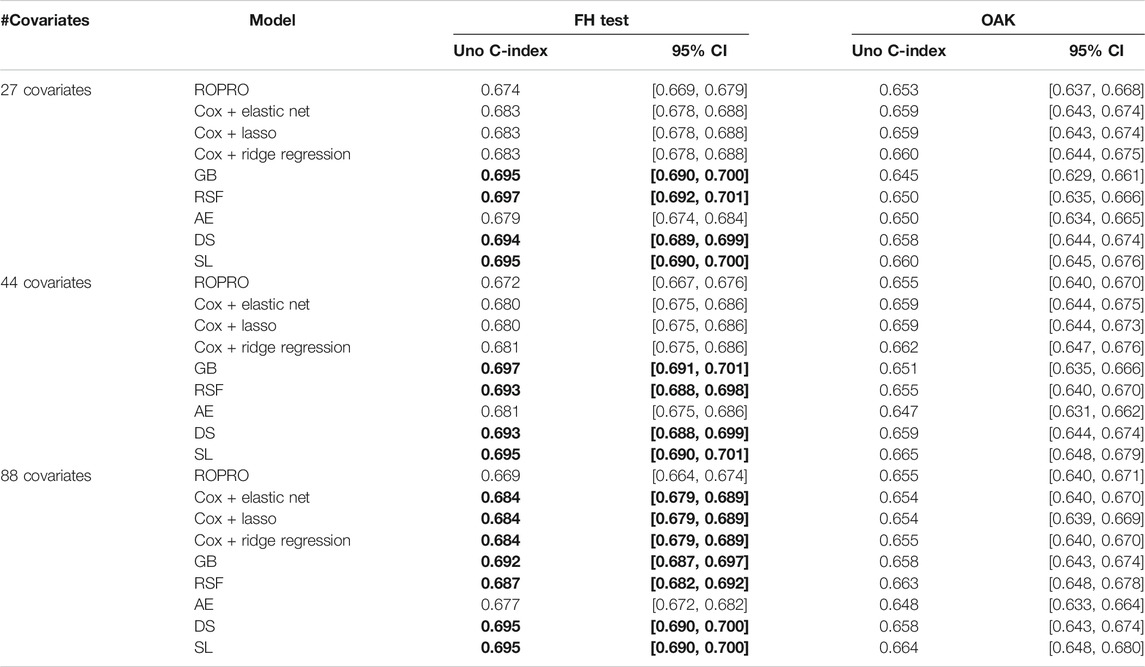
TABLE 5. Uno C-index and corresponding 95% confidence intervals. Significant increases in C-index over the ROPRO model are in bold.
As we observed similar patterns across all feature sets, we report here only results corresponding to the 44 covariate feature set. In the FH test dataset, the ROPRO achieved C-index values [95% CI] of 0.701 [0.696, 0.706]. In comparison, more complex models obtained slightly higher C-index values than ROPRO. Across all ML-derived models, the AE consistently yielded the lowest C-index values (0.708 [0.703, 0.713]), followed by lasso and elastic net (C-index 0.708 [0.704, 0.714]) and ridge regression (C-index 0.709 [0.704, 0.714]). The model performances improved using RSF (c-index 0.720 [0.716, 0.725]), GB (C-index 0.722 [0.718, 0.727]), DS (C-index 0.721 [0.717, 0.726]) and lastly, SL (C-index 0.723 [0.718, 0.728]). However, given their 95% CI only GB, RSF, DS and SL obtained significant increases in C-index for all feature sets when compared to ROPRO. As an exception, in the 88 covariates feature set, the regularized Cox models (C-index [95%CI] lasso and elastic net 0.711 [0.706, 0.716]; ridge regression 0.711 [0.706, 0.715]) also had significant increases in C-index when compared with ROPRO (C-index [95% CI] 0.698 [0.693, 0.702]). The increases in C-index for the remaining models were not significantly different.
All Uno C-index values were lower than the respective (Harrell) C-index. Regardless, the models that obtained significant (Harrell) C-index increases also had significant Uno C-index increases. For the 44 covariate feature set, the GB (Uno C-index [95% CI] 0.697 [0.691, 0.701]), RSF (Uno C-index [95% CI] 0.693 [0.688, 0.698]), DS (Uno C-index [95% CI] 0.693 [0.688, 0.699]) and SL (Uno C-index [95% CI] 0.695 [0.690, 0.701]) obtained significantly higher Uno C-index values than ROPRO (Uno C-index [95% CI] 0.672 [0.667, 0.676]). Additionally, in the 88 covariates feature set, the regularized Cox models also had significantly higher Uno C-index than ROPRO.
In OAK we observed similar patterns for the different feature sets. For easier reporting the following results similarly correspond to the 44 covariate feature set. The ROPRO resulted in a C-index value [95% CI] of 0.670 [0.657, 0.685]. In comparison, the model that yielded the highest C-index was SL 0.677 [0.662, 0.695]. Nevertheless, we observed that, contrary to the results in the FH test set, the confidence intervals between ROPRO and SL (and all the remaining models) overlapped, hence no statistically significant difference was found. Likewise, in the OAK dataset no model obtained a significantly higher Uno C-index than the ROPRO.
The PCA analysis with the first two principal components is shown in the Supplementary Figure S1. The C-index and 95% CI are displayed in Supplementary Tables S4–7 for FH test set without the covariates unavailable in OAK, FH test stratified by cancer entity, and FH train and test permutations, respectively.
The PCA analysis illustrated that the FH test distribution exhibited a higher variance than OAK, with the FH test set having a variance of (3.704, 2.137) while the OAK population exhibited a variance of (2.690, 1.421).
There were only minor changes in the C-index values between the original FH test set and the FH test set without the covariates not present in OAK. Ultimately, the same models, GB, RSF, DS, and SL, obtained significantly higher C-index values that ROPRO for all feature sets. Furthermore, in the 88 covariate feature set, the regularized Cox models obtained significant increases in C-index.
The C-index values showed a considerable variation between cancer entities. Most cancer entities had lower C-index values than the complete FH test (that had C-index values between 0.698 and 0.723). Only diffuse large B-cell lymphoma (C-index between 0.715 and 0.741), and follicular cancer (C-index between 0.771 and 0.788) had C-index values higher than the whole FH test dataset. Acute myeloid leukemia, breast cancer, gastric cancer, head and neck cancer, and metastatic breast cancer had the lowest discriminative power with C-index values close to 0.650. Advanced non-small cell lung cancer, the largest cohort in the FH test set, had C-index values between 0.673 and 0.687. In all cohort/feature set combinations, none of the more complex models obtained a significant increase in C-index against the ROPRO.
We reshuffled the original FH dataset twice, generating two extra sets of FH train and test. There were only minor changes in C-index between the results of the primary analysis (in the section above) and the results from these two extra sets. More specifically, in the FH test, GB (C-index [95% CI] 0.724 [0.718, 0.729]; 0.723 [0.718, 0.728]), RSF (C-index [95% CI] 0.722 [0.717, 0.728]; 0.720 [0.715, 0.725]), DS (C-index [95% CI] 0.724 [0.718, 0.730]; 0.723 [0.718, 0.728]), and SL (C-index [95% CI] 0.725 [0.720, 0.731]; 0.724 [0.719, 0.729]) obtained significant C-index increases when compared with ROPRO (C-index [95% CI] 0.701 [0.695, 0.707]; 0.701 [0.695, 0.707]). As above, the C-index values refer to the 44 covariate feature set although we observed similar patterns for all feature sets. We presented for each model two C-index and 95% CI, each of which refers to one set of new FH train and test sets. In the 88 covariate feature set of the two new sets of FH train and test, the regularized Cox models also obtained significant increases in C-index. The only deviation in C-index significance observed compared to the primary analysis was that the AE model had a significant increase in C-index against ROPRO in the 88 covariate feature set of one of the sensitivity analyses.
We conducted an extensive benchmarking study to investigate: 1) whether the predictive power of prognostic scores in oncology could be improved by replacing the Cox model with more complex machine learning models and 2) whether increasing the number of covariates from 27 model-selected to 44 and 88 would increase the models’ performance. To that end, we performed a comprehensive head-to-head comparison between a classic Cox model-based approach (ROPRO) and more complex ML-based survival models including two novel methods employing autoencoder and super learner algorithms. Overall, our analysis suggests that neither increasing the number of covariates nor using complex machine learning models increases the performance of prognostic scores in oncology. In part, this might be explained by the absence in baseline clinical data (like blood work data and patient/disease characteristics) of complex covariate interactions that would have otherwise been learned by the more complex models. We hypothesize that the addition of rich patient/disease information in the form of imaging, genomics or longitudinal data could be the key to improving prognostic scores in cancer. These more complex data types, apart from adding prognostic factors to the models, should also contain information that are not easily extractable by classical methods (like the Cox model) which should lead to an increase in performance of the more complex models and therefore better prognostic performance.
To our knowledge, this is to date the largest benchmarking study of prognostic scores in oncology both in terms of number of models and patients. Previous analyses that compared the performance of simple and complex machine learning models have yielded rather inconsistent results. Some of these studies have demonstrated improvements in using complex models against the classic Cox model. For instance, a recent study challenged the Cox model against random survival forests (RSF) and DeepSurv (DS) to derive prognostic scores among patients with oral cancer (Kim et al., 2019). The DS was overall the model with the highest C-index. Yet, the study was limited by the low number of 255 patients and nine covariates. A separate study applied the Cox model, RSF and regularized cox to a larger dataset comprising a population of 80,000 patients with cardiovascular disease (Steele et al., 2018). The authors concluded that the elastic net model (C-index 0.801) using 600 covariates performed better than the Cox model (C-index 0.793) using 27 covariates, but the overall improvement was only moderate. In comparison, three other studies did not find any noticeable improvements by employing machine learning models (Chen et al., 2019; Christodoulou et al., 2019; Desai et al., 2020). However, these studies compared the use of logistic regression with a binary endpoint against machine learning methods instead of the Cox model with a time-to-event endpoint. Our results showed that some of the more complex models, that could model covariate interactions and non-linear effects, did obtain significantly higher C-index values when compared to ROPRO. Although the C-index improvement size was still only moderate. Hence, the main results of the here presented study may contextualize the findings from (Chen et al., 2019; Christodoulou et al., 2019; Desai et al., 2020) to a survival analysis framework concluding that more complex machine learning models may not lead to a significant increase in performance over the Cox model.
Additionally, some of these studies also analyzed the effect of the covariate number in the prognostic score performance. The study by Kim et al. (Kim et al., 2019) analyzed models employing a range of five–nine covariates and found that the model performance increased with an increase in the covariate number. The same increase in performance was also observed when the performance of established prognostic scores (Arkenau et al., 2009; International Non-Hodgkin’s Lymphoma Prognostic Factors Project 1993; Ko et al., 2015; Kinoshita et al., 2013), which used a maximum of six covariates, was compared to the more recently developed ROPRO (Becker et al., 2020) that reported a number of 27 highly prognostic and independent covariates. Therefore, there is evidence that increasing the covariate size from small (less than 10 covariates) to a larger, but still moderate, number (30 covariates) leads to an increase in the prognostic score performance. This finding is not unexpected as the addition of more covariates increases the chances that some of them contain prognostic information that could be used by the models to increase their performance. In our analysis, we built upon the progress made in (Becker et al., 2020) by increasing the feature set from 27 to 44 and 88 covariates. However, the addition of these extra covariates did not lead to an increase in performance of the models as in previous studies. This could be caused by multiple reasons: First, the higher missingness in the 44 and 88 feature sets could have led to an erroneous imputation of the covariates with high missingness. Second, the 27 covariates included had been previously selected as the most relevant in (Becker et al., 2020), hence any additional covariates could have lower prognostic value. Both reasons should contribute to the lack of performance improvement. Yet, since the regularized Cox models did incorporate additional covariates from the 44 and 88 feature sets it gives evidence that there was some prognostic value in them although they did not lead to an increase in performance.
Conversely, in (Steele et al., 2018) two datasets with differing covariate numbers were studied: an expert-selected covariate dataset with 27 covariates vs. a much larger dataset with 600 covariates. The results demonstrated that the best 600 covariate model (elastic net) obtained a slightly higher C-index value than the best 27 covariate model (Cox model). The elastic net model added covariates such as prescription of cardiovascular medication (that should indicate severe cardiovascular problems) and prescription of laxatives/home visits (that might indicate general frailty). All these covariates are possibly associated (proxies) with cardiovascular disease but were not identified by the experts as prognostic, which may explain the increase in performance of the elastic net model. This result illustrates the need to incorporate more diverse data into prognostic scores. As explained above, we followed a different approach in this analysis and instead focused on increasing the number of biomarkers (blood work/patient characteristics) from 27 model-selected to 44 and 88 feature sets. Our results showed that this addition did not result in an increase in performance. We hypothesized that, perhaps, we had exhausted the available information in the blood work/patient characteristics in the 27 covariate dataset and the covariates added in the 44 and 88 feature sets did not carry prognostic information. Therefore, these results might suggest that perhaps there is a hard limit on the predictive power of baseline blood work/patient characteristics. To further increase the performance it might be necessary to incorporate other types of covariates as suggested by Steele et al. (Steele et al., 2018) or data with increased richness, like images, genomics or longitudinal biomarkers. Although, we would suggest that further research in this area is still needed.
Furthermore, all models had a comparable internal performance (C-index 0.70–0.72 within the FH test and C-index 0.66–0.68 within OAK) while the performance between datasets, which may be an indicator for model generalizability, was less strong. Particularly when the same models were compared between datasets, the C-index differences were more apparent. Some models had a considerable loss in performance with a decrease in C-index between FH test and OAK as high as 0.060 for GB or 0.056 for RSF. The ROPRO showed the most stable performance between datasets with a C-index difference as low as 0.027. These results suggest that the slight gains in performance achieved by the more complex models in the FH test dataset are not generalizable to other datasets. We hypothesize that this could happen due to multiple reasons: First, differences in the cohort number between FH test and OAK could cause differences in the C-index as the model performance could depend on the type of cancer. Second, the lack of some of the covariates in OAK, e.g., blood oxygen or granulocytes, could lead to a decrease in performance in the OAK dataset. Third, since OAK is a clinical trial, it is likely that the patient population is more homogeneous than in FH. Therefore, more extreme values in highly prognostic covariates (e.g. ECOG > 1) should be inexistent or rare, making it harder for prognostic prediction. Additionally, the study start date was defined differently between FH (first day of first line of treatment) and OAK (first day of second or third lines of treatment). We investigated some of these hypotheses in the sensitivity analyses above. For the first hypothesis, we tested whether the models had different performance for different cohorts. The FH test set C-index for advanced non-small cell lung cancer (the only cohort in OAK) ranged between 0.68–0.69, which is closer to the C-index in OAK. For the second hypothesis, we removed the covariates inexistent in OAK from the FH test and imputed them. This had little effect on the C-index of the FH test, therefore, we discard the effect of the absent OAK covariates. For the third hypothesis, we performed a PCA analysis where we compared both datasets, which supported the hypothesis that OAK has less extreme values. Given the sensitivity analyses, we argue that a combination of the first and third hypotheses is more likely. The C-index for the advanced non-small cell cancer in FH test was closer to the OAK value. Additionally, the other differences introduced in the third hypothesis might further decrease the C-index in the OAK dataset. Furthermore, there could have also been some overfitting to the FH test set that caused the decrease in OAK. Unfortunately, the compared prognostic scores in literature utilized test sets from the same data-source as the training set which makes a valid comparison not feasible. We suggest that further studies should be performed to investigate the true cause of this effect.
In general, we argue that in order to develop better prognostic scores in oncology, rather than focusing on more complex models on the same dataset, we should focus on getting access to larger and optimally multimodal data describing the patients in more detail. In particular, adding data about tumor biology via rich data types, e.g., via imaging, genomics or longitudinal data might be more beneficial and could lead to improved clinical decision-making when using prognostic scores. Consequently, these rich data types should contain complex information that the classical models cannot interpret, in that case, the more complex models tested in this work should demonstrate increased performance. Another area for improvement is related to the response of patients to treatments. By combining the patients’ treatments with longitudinal data, e.g. biomarkers, it might be possible to model the disease progression, leading to models that could offer real-time decision-making support. Overall, there remains an unmet clinical need for precise survival prediction to enable improved toxicity monitoring, treatment selection and assessment of clinical trial eligibility and hence further work is required to improve prognostic scores in oncology.
Prognostic scores are important clinical decision-making tools for treatment decisions, monitoring adverse events, and clinical trial eligibility. Our results show that complex machine learning-derived models did not improve prognostic scores in oncology compared to a classical Cox-based framework. We argue that further research should focus on the impact of adding other data types (e.g. imaging, genomics or longitudinal biomarkers) describing complementary features of disease biology. In these scenarios, complex machine learning architectures might still prove beneficial.
The data analyzed in this study is subject to the following licenses/restrictions: The data presented is owned by either Flatiron Health Inc. (train and in-sample test datasets) or F. Hoffmann-la Roche LTD (test dataset). Access to Flatiron Health may be made available upon request, and are subject to a license agreement with Flatiron Health. Requests to access these datasets should be directed to DataAccess@flatiron.com.
Institutional Review Board approval of the Flatiron Health study protocol was obtained prior to study conduct, and included a waiver of informed consent. For the OAK dataset, an independent data monitoring committee reviewed safety. Protocol approval was obtained from independent ethics committees for each site.
HL wrote the codes, performed the analysis and wrote the draft of the publication. TB contributed to the conceptualization of this study, supported the data preparation and contributed significantly to the manuscript. AB-M contributed to the conceptualization of this study, supervised the study project with respect to clinical application and contributed to the manuscript. NA supervised ML aspects of the project, edited the manuscript significantly. JW contributed to the conceptualization of this study, supervised the study project and contributed to the manuscript.
This study was funded by F. Hoffmann-la Roche LTD. No grant number is applicable.
HL, AB-M and JW are employed by F. Hoffmann-La Roche. JW and AB-M hold shares of F. Hoffmann-La Roche. TB is a contractor paid by F. Hoffmann-La Roche.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors thank Fabian Schmich, Roche Innovation Center Munich; Sarah McGough, Svetlana Lyalina and Devin Incerti from PHC RWDS Analytics, Genentech Inc., San Francisco, United States and Stefka Tyanova from Data Science, Roche Innovation Center Basel for their valuable input regarding methodology and results. HL is supported by the Helmholtz Association under the joint research school “Munich School for Data Science - MUDS”.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frai.2021.625573/full#supplementary-material.
Arkenau, H. T., Barriuso, J., Olmos, D., Ang, J. E., de Bono, J., Judson, I., et al. (2009). Prospective validation of a prognostic score to improve patient selection for oncology phase I trials. J. Clin. Oncol. 27 (16), 2692–2696. doi:10.1200/JCO.2008.19.5081
Becker, T., Weberpals, J., Jegg, A. M., So, W. V., Fischer, A., Weisser, M., et al. (2020). An enhanced prognostic score for overall survival of patients with cancer derived from a large real-world cohort. Ann. Oncol. 31 (11), 1561–1568. doi:10.1016/j.annonc.2020.07.013
Bhimani, J., Philipps, L., Simpson, L., Lythgoe, M., Soultati, A., Webb, A., et al. (2020). The impact of new cancer drug therapies on site specialized cancer treatment activity in a UK cancer network 2014-2018. J. Oncol. Pharm. Pract. 26 (1), 93–98. doi:10.1177/1078155219839445
Birnbaum, B., Nathan, N., Seidl-Rathkopf, K., Agrawal, M., Estevez, M., Estola, E., et al. (2020). Model-assisted cohort selection with bias analysis for generating large-scale cohorts from the EHR for oncology research. Available at: http://arxiv.org/abs/2001.09765.
Byrd, R. H., Lu, P., Nocedal, J., and Zhu, C. (1995). A limited memory algorithm for bound constrained optimization. SIAM J. Sci. Comput. 16 (5), 1190–1208. doi:10.1137/0916069
Chen, D., Liu, S., Kingsbury, P., Sohn, S., Storlie, C. B., Habermann, E. B., et al. (2019). Deep learning and alternative learning strategies for retrospective real-world clinical data. NPJ Digit Med. 2 (1), 43. doi:10.1038/s41746-019-0122-0
Christodoulou, E., Ma, J., Collins, G. S., Steyerberg, E. W., Verbakel, J. Y., and Van Calster, B. (2019). A systematic Review shows No performance benefit of machine learning over logistic regression for clinical prediction models. J. Clin. Epidemiol. 110, 12–22. doi:10.1016/j.jclinepi.2019.02.004
Cox, D. R. (1972). Regression models and life-tables. J. R. Stat. Soc. Ser. B 34 (2), 187–202. doi:10.1111/j.2517-6161.1972.tb00899.x
Desai, R. J., Wang, S. V., Vaduganathan, M., Evers, T., and Schneeweiss, S. (2020). Comparison of machine learning methods with traditional models for use of administrative claims with electronic medical records to predict heart failure outcomes. JAMA Netw. Open 3, e1918962. doi:10.1001/jamanetworkopen.2019.18962
Ferlay, J., Colombet, M., Soerjomataram, I., Mathers, C., Parkin, D. M., Piñeros, M., et al. (2019). Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 144 (8), 1941–1953. doi:10.1002/ijc.31937
Fogel, D. B. (2018). Factors associated with clinical trials that fail and opportunities for improving the likelihood of success: a Review. Contemp. Clin. Trials Commun. 11, 156–164. doi:10.1016/j.conctc.2018.08.001
Greenwell, B., Bradley, B., Cunningham, J., and Developers, G. B. M. (2019). Gbm: generalized boosted regression models. Available at: https://CRAN.R-project.org/package=gbm.
Harrell, F. E., Califf, R. M., Pryor, D. B., Lee, K. L., and Rosati, R. A. (1982). Evaluating the yield of medical tests. JAMA 247 (18), 2543–2546. doi:10.1001/jama.1982.03320430047030
Harrell, F. E., Lee, K. L., and Mark, D. B. (1996). Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat. Med. 15 (4), 361–387. doi:10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4
Hastie, T., Tibshirani, R., and Friedman, J. (2009). The elements of statistical learning: data mining, inference, and prediction. 2nd Edn. New York, NY: Springer Science & Business Media.
International Non-Hodgkin’s Lymphoma Prognostic Factors Project (1993). A predictive model for aggressive non-hodgkin’s lymphoma. New Engl. J. Med. 329 (14), 987–994. doi:10.1056/NEJM199309303291402
Ishwaran, H., Kogalur, U. B., Blackstone, E. H., and Lauer, M. S. (2008). Random survival forests, Ann. Appl. Stat. 2, 841–860. doi:10.1214/08-aoas169
Kalbfleisch, J. D., and Prentice, R. L. (2002). The statistical analysis of failure time data. New Jersey: John Wiley and Sons.
Katzman, J. L., Shaham, U., Cloninger, A., Bates, J., Jiang, T., and Kluger, Y. (2018). DeepSurv: personalized treatment recommender system using a cox proportional hazards deep neural network. BMC Med. Res. Methodol. 18 (1), 24. doi:10.1186/s12874-018-0482-1
Kim, D. W., Lee, S., Kwon, S., Nam, W., Cha, I. H., and Kim, H. J. (2019). Deep learning-based survival prediction of oral cancer patients. Sci. Rep. 9 (1), 6994. doi:10.1038/s41598-019-43372-7
Kinoshita, A., Onoda, H., Imai, N., Iwaku, A., Oishi, M., Tanaka, K., et al. (2013). The Glasgow prognostic score, an inflammation based prognostic score, predicts survival in patients with hepatocellular carcinoma. BMC Cancer 13 (1), 52. doi:10.1186/1471-2407-13-52
Ko, J. J., Xie, W., Kroeger, N., Lee, J. L., Rini, B. I., Knox, J. J., et al. (2015). The international metastatic renal cell carcinoma database consortium model as a prognostic tool in patients with metastatic renal cell carcinoma previously treated with first-line targeted therapy: a population-based study. Lancet Oncol. 16 (3), 293–300. doi:10.1016/S1470-2045(14)71222-7
LeDell, E., van der Laan, M. J., and Petersen, M. (2016). AUC-maximizing ensembles through metalearning. Int. J. Biostat 12 (1), 203–218. doi:10.1515/ijb-2015-0035
Ma, X., Long, L., Moon, S., Blythe, J., Adamson, S., and Baxi, S. S. (2020). Comparison of population characteristics in real-world clinical oncology databases in the US: flatiron health, SEER, and NPCR. Available at: https://www.medrxiv.org/content/10.1101/2020.03.16.20037143v2.
Polley, E., LeDell, E., Kennedy, C., and van der Laan, M. (2019). SuperLearner: super learner prediction. Available at: https://github.com/ecpolley/SuperLearner.
Pulte, D., Weberpals, J., Jansen, L., and Brenner, H. (2019). Changes in population-level survival for advanced solid malignancies with new treatment options in the second decade of the 21st century. Cancer 125 (15), 2656–2665. doi:10.1002/cncr.32160
R Core Team (2018). R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing.
Rittmeyer, A., Barlesi, F., Waterkamp, D., Park, K., Ciardiello, F., von Pawel, J., et al. (2017). Atezolizumab versus Docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 389 (10066), 255–265. doi:10.1016/S0140-6736(16)32517-X
Sant, M., Minicozzi, P., Mounier, M., Anderson, L. A., Brenner, H., Holleczek, B., et al. (2014). Survival for hematological malignancies in europe between 1997 and 2008 by region and age: results of EUROCARE-5, a population-based study. Lancet Oncol. 15 (9), 931–942. doi:10.1016/S1470-2045(14)70282-7
Simon, N., Friedman, J., Hastie, T., and Tibshirani, R. (2011). Regularization paths for cox's proportional hazards model via coordinate descent. J. Stat. Softw. 39 (5), 1–13. doi:10.18637/jss.v039.i05
Steele, A. J., Denaxas, S. C., Shah, A. D., Hemingway, H., and Luscombe, N. M. (2018). Machine learning models in electronic health records can outperform conventional survival models for predicting patient mortality in coronary artery disease. PLOS ONE 13 (8), e0202344. doi:10.1371/journal.pone.0202344
Stekhoven, D. J., and Bühlmann, P. (2011). MissForest--non-parametric missing value imputation for mixed-type data. Bioinformatics 28 (1), 112–118. doi:10.1093/bioinformatics/btr597
Therneau, T. M. (2015). A package for survival analysis in S. Available at: https://CRAN.R-project.org/package=survival.
Tibshirani, R. (1997). The lasso method for variable selection in the cox model. Stat. Med. 16 (4), 385–395. doi:10.1002/(SICI)1097-0258(19970228)16:4<385::AID-SIM380>3.0.CO;2-3
Uno, H., Cai, T., Pencina, M. J., D’Agostino, R. B., and Wei, L. J. (2011). On the C-statistics for evaluating overall adequacy of risk prediction procedures with censored survival data. Stat. Med. 30 (10), 1105–1117. doi:10.1002/sim.4154
van der Lann, M. J., Polley, E. C., and Hubbard, A. E. (2007). Super learner. Stat. Appl. Genet. Mol. Biol. 6 (1), 25. doi:10.2202/1544-6115.1309
Keywords: electronic health records, machine learning, prognostic scores, real world data, survival analyisis
Citation: Loureiro H, Becker T, Bauer-Mehren A, Ahmidi N and Weberpals J (2021) Artificial Intelligence for Prognostic Scores in Oncology: a Benchmarking Study. Front. Artif. Intell. 4:625573. doi: 10.3389/frai.2021.625573
Received: 03 November 2020; Accepted: 19 January 2021;
Published: 16 April 2021.
Edited by:
Enkelejda Miho, University of Applied Sciences and Arts Northwestern Switzerland, SwitzerlandReviewed by:
Gregory R Hart, Yale University, United StatesCopyright © 2021 Loureiro, Becker, Bauer-Mehren, Ahmidi and Weberpals. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anna Bauer-Mehren, YW5uYS5iYXVlci1tZWhyZW5Acm9jaGUuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.