- 1Faculty of Life and Environmental Sciences, University of Iceland, Reykjavik, Iceland
- 2Department of Entomology, National Museum of Natural History, Smithsonian Institution, Washington, DC, United States
Kleptoparasitism, broadly defined, is the theft of extrinsic resources resulting in potential cost to the host. The stealing of resources, often food gathered by another, is perhaps best known in birds and mammals, but is even more common and widespread in arthropods like ants, bees, flies and spiders. Spiders are involved in myriad kleptoparasitic interactions, best studied as obligatory kleptoparasites of other spiders. However, less attention has been paid to the critical role of spiders as “superhosts” to commensal and kleptoparasitic organisms, and their variety of facultative kleptoparasitic strategies. To understand obligatory kleptoparasitism in spiders, it is first necessary to examine their role and characteristics as hosts and as facultative kleptoparasites. Most spider kleptoparasites utilize other spiders as hosts, a link that is not coincidental, and facultative resource stealing, in its many forms, is generally assumed to provide an evolutionary bridge to obligate kleptoparasitism. Here, I provide a brief review of these two roles through a summary of literature on all kleptoparasitic spiders and over 200 hosts. The phylogenetic distribution of spider hosts is distinctly non-random, involving about 200 species, in 86 genera, and 23 families. These then pertain to a few select lineages, out of total spider diversity: 23/136 families, 86/4,427 genera, and 200/52,765 known species. The vast majority of argyrodine hosts belong to four Araneoidea families (Araneidae, Nephilidae, Theridiidae, Linyphiidae), while the majority of hosts of mysmenid kleptoparasites are mygalomorphs, mostly Dipluridae and Ischnothelidae. Key spider hosts like Nephila, Trichonephila, Argiope, Cyrtophora, and Linothele, build large, often structurally complex, and persistent webs. Three-dimensionality, often in the form of auxiliary webbing, provides safe refuges for kleptoparasites, and the abundant prey and extended prey handling time of large spiders provide resources and opportunities for theft. Many of the favored hosts either interlink webs or are social. Key host traits to counter kleptoparasitism include web takedown and relocation, food concealment, and direct aggression. Facultative resource stealing in spiders includes web takeover, male kleptoparasitism of females in webs, and opportunistic prey theft. Among these, kleptotany, the facultative abandonment of own web and the invasion of a larger host web, to steal it and/or to prey on the host (araneophagy) are the most likely to link to obligatory kleptoparasitism.
1 Introduction
1.1 Kleptoparasitism and kleptoparasite hosts
Kleptoparasitism is typically characterized by resource theft that comes at a cost to the host (Vollrath, 1987; Elgar, 1993; Iyengar, 2008). Scrounging may refer to individuals benefiting from food remnants left or ignored by the host, without direct conflict or the overt act of stealing valuable resources. Such behavior can occur in a social context and be tolerated or even cooperative. Yet, the apparent distinction between scrounging and kleptoparasitism is blurred, e.g. by the dynamic nature of many such foraging systems where the potential cost to the host ranges from negative to none (Trager et al., 2010, Figure 1) to potentially positive (Peng et al., 2013). When resources are limited the value of food items increases so that any theft may come at some cost. The same species may interact in environments, or during seasons, where resources are abundant, and resource theft may occur less aggressively and have negligible impact on the host´s energy budget. I emphasize kleptoparasitic systems where theft of extrinsic resources results in potential cost to the host. As pointed out by Coyle et al. (1991) while direct cost can be small and difficult to measure, even a single interruption where an invader distracts a host momentarily from feeding represents a potential cost to the host. As such, kleptoparasitism is usefully delimited from other forms of species-interactions that center on exploitation of innate properties of individuals (such as parasitism and predation) or involve intimate association of species that may be aggressive (competition over non-secured resources), or where species cohabit with neither player incurring even potential cost from overlapping resource use (commensalism, mutualism). The primary goal here is to set the stage for understanding the origin and evolution of obligatory kleptoparasitism and the ecological context that may render this strategy successful in the long term as an evolutionarily stable strategy. Pivotal to this endeavor is to examine facultative kleptoparasitism as a strategy that is often presumed to precede—or be transitional to—the evolution of obligatory kleptoparasitism. It is also critical to understand the nature of hosts, whose relatively close relationships to kleptoparasites in numerous organisms (Wheeler, 1901; Iyengar, 2008) are not coincidental, but integral to the success of the strategy (a generalized form of Emery´s rule, Emery 1909).

Figure 1. The cost of resource theft to the ´host´ varies dramatically across systems and can be challenging to estimate. Top: when hosts and kleptoparasites are comparable in size, the cost is high. Hyenas form packs that can sometimes steal prey captured by lions. The cost to the host is obvious and significant: a lion will risk fighting over the prey with potentially harmful consequences and may lose prey that is challenging to capture. Bottom: When kleptoparasites are tiny compared to the host their impact may be marginal. Left: a tiny argyrodine spider with the towering Trichonephila host in the background that as adults are over 100x its mass. Lower right: kleptoparasitic milichiid flies soak up some digestive fluids from the prey of a crab spider. The individual consumption of these small kleptoparasites has negligible impact on the host but collectively, over time, and in circumstances of low food availability, they still have the potential to reduce the host’s fitness. Top, artwork modeled on a wirestock photo, lower left by I. Agnarsson, lower right by Jay Taylor.
For kleptoparasites, resource theft may conserve energy that would otherwise be spent in foraging while simultaneously avoiding exposure to potentially hazardous prey or predators (Vollrath, 1987; Elgar, 1993; Iyengar, 2008). From the perspectives of the host, the cost of kleptoparasitism ranges from sublethal/lethal to minimal, even putatively positive, temporarily, and under certain circumstances. The potential cost and the long-term interaction of kleptoparasite and host, regardless, is expected to result in an arms race and the evolution of adaptive traits in the host to respond to kleptoparasite selection pressures.
1.2 The scope of this review
I initially envisioned this invited review as an opportunity for a comparative synthesis of kleptobiosis across the tree of life—through the eyes of spiders. During months of effort, I gradually started to appreciate the gargantuan size and challenge of such a task. On the one hand, inordinately vast literature must be surveyed that offers a great wealth of information on different taxa and strategies. On the other hand, despite its volume, the literature is incredibly sparse on the most pertinent data needed for such meta-analyses, namely phylogenetic context. As for most lineages, this information is also largely lacking for spiders (but see Su and Smith, 2014). I therefore shifted my focus to a synthesis of kleptoparasitism involving spiders, but this too turned out to be elusive. Spiders are involved in a great variety of kleptoparasitic interactions both as hosts to a great variety of kleptoparasitic lineages, as well as containing many species involved both in facultative as well as obligatory kleptoparasitism (Table 1; Supplementary Table S1). The scope of this paper is therefore focused on providing a platform for understanding obligatory kleptoparasitism through a review of spiders as hosts to kleptoparasitic groups, including facultatively kleptoparasitic spiders. I highlight some of the ways in which spiders are unique and important hosts for multiple lineages of kleptoparasites, defensive host strategies, and the variety of opportunistic kleptoparasitism seen across the order. Facultative kleptoparasitism is probably extremely widespread in spiders, with scattered records that I attempt to summarize. Araneophagy through web invasion is seen in several lineages, perhaps best known in salticids like Portia. Such behavior has been reviewed before (e.g. Jackson, 1986) and does not seem to be explicitly linked to kleptoparasitism in general, with the possible exception of the argyrodine theridiids where both traits occur. Therefore, I offer a brief overview to summarize the variety of web invasion behaviors in spiders, with emphasis on those that may be linked to the origin and evolution of kleptoparasitism.

Table 1. Known hosts among spiders (see Supplementary Table S1 for detail).
Are there emerging shared patterns in the evolutionary ‘pathways’ to obligate kleptoparasitism across the countless independent evolutionary origins of kleptoparasitism? This question is challenging to answer and to do so properly, requires phylogenetic comparative meta-analyses that will not be attempted here and may be as yet unachievable given scattered phylogenetic knowledge of the various lineages. An obvious and often cited hypothesis is that obligatory kleptoparasitism has transitioned from facultative kleptoparasitism (Iyengar, 2008; Breed et al., 2012). Such a route where opportunistic resource theft as a part of a broader foraging strategy can act as an evolutionary bridge to obligate kleptoparasitism is intuitively appealing. Candidate examples may come from Koptothrips thrips initially utilizing damaged galls then moving to actively usurping intact galls (Crespi and Abbot, 1999), Lestrimellita stingless bees gradually losing pollen-gathering structures to become reliant solely on stolen food (Breed et al., 2012), sphaerocerid flies that range from facultatively stealing food from scavengers like dung beetles to specialized reliance on host (Sirvinski et al., 1999), and Ranzovius bugs that belong to a lineage containing many facultative kleptoparasites but where some species have become entirely reliant on their social spider hosts to capture prey (Henry, 1984; Wheeler and McCaffrey, 1984). In other cases, like spiders, such a transition between facultative and obligate kleptoparasitism is not obvious given the evidence at hand. Another hypothesis that may be specific to spiders suggests an evolutionary link between stealing from or predating on a host, though the direction of such transition is debated, and in fact, this link has not been clearly established (but see Su and Smith, 2014, and below).
Kleptoparasitism exemplifies the complex interplay between ecological opportunity, behavioral flexibility, and evolutionary adaptation. This versatile strategy has evolved independently across diverse taxa implying important ecological function and fitness consequences favored by natural selection. Below I provide the groundwork necessary to further our understanding of obligatory kleptoparasitism in spiders.
2 Spider superhosts: webs provide refuge and resources
2.1 Spider webs as habitats
Spider webs vary greatly in their size and web tenacity (Eberhard, 2020). Larger and more permanent webs may form habitats for a variety of arthropods, including other spiders (Figure 2). Webs provide a stable environment rich in resources such as shelter, prey remnants, and protection from environmental extremes (Eberhard, 2020), attracting a range of organisms that engage in complex interactions with their spider hosts. Proctor (1992), for example, discussed the co-inhabitants in colonial Cyrtophora moluccensis webs in Moorea, French Polynesia. In five Cyrtophora colonies she found five additional spider species whose combined abundance far outnumbered the host spiders. These included web builders using Cyrtophora webs as support, as well as the kleptoparasitic Argyrodes argentatus and the likely araneophagic Platnickina adamsoni. Social spider webs form an even more diverse habitat, for example the webs of Anelosimus studiosus can contain numerous other spiders, and a range of other arthropods (Deyrup et al., 2004; Perkins et al., 2007; Mock, 2008), and Jani et al. (2023) reported 21 spider species and many other arthropods and even vertebrates making the webs of social Stegodyphus sarasinorum their home. Further, in a survey of about 80 nests of subsocial Anelosimus in Madagascar Magnússon and Agnarsson (unpublished) found over 130 arthropod species associated with these nests.
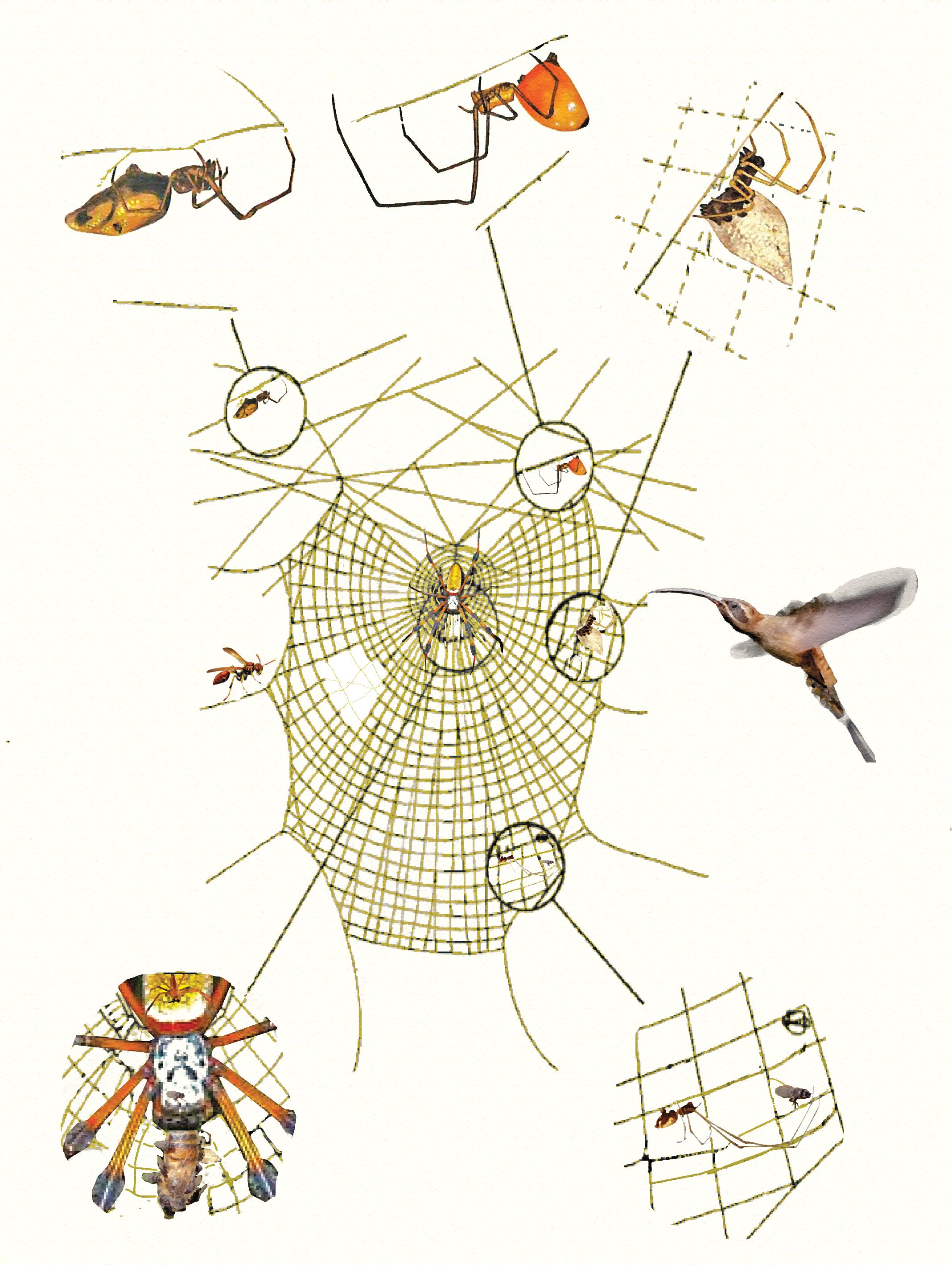
Figure 2. Spider webs are hosts for a variety of kleptoparasites. Here, a Trichonephila female sits at the hub of her web, feeding on prey. On her and the prey (inset lower left) kleptoparasitic flies share her prey. A wasp (left) and a hummingbird (right) may steal prey, and the latter also silk for its nest, from her web. Various obligatory kleptoparasite species (inset above and lower right) are constant guests in her web, gleaning insects, and prey packages, feeding with her, or on her juveniles. On the left side of the web, a hole has been cut out by a kleptoparasite, removing prey from the web. Artwork is a conceptual reproduction of figure 97 in Vollrath (1987).
The inhabitants of spider webs are varied. Some species, including certain beetles and lepidopteran larvae, act as scavengers, consuming waste materials and leftover prey within the web. In Phryganoporus candidus nests, scavengers like mealybugs thrive, benefitting from the sheltered environment and sometimes aiding in nest sanitation by breaking down waste (Downes, 1995). Some insects and spiders simply seek shelter in the nests of social spiders, while ants, wasps, assassin bugs, and other predatory insects have been documented as significant predators, sometimes killing spiders and stealing egg masses. Predatory wasps, for example—while not kleptoparasitic—invade host webs and have a major impact on Metepeira incrassata colonies (Uetz et al., 2002). As discussed below, in addition to opportunistic predators, many types of kleptoparasites are also attracted to this community thriving in a prey capturing abode.
2.2 Ecology and biogeography of spider webs as islands
Spider webs function as fascinating examples of “habitat islands”, uniquely structured and highly distinct and quantifiable ecosystems (Blackledge and Gillespie, 2002; Blackledge et al., 2011) that support various forms of life, notably among them, kleptoparasitic spiders (Elgar, 1989; Agnarsson, 2003). Spider webs resemble islands in the isolation of habitat, patchily dispersed within a matrix of strikingly contrasting habitats (Agnarsson, 2011). Therefore, general ecological models used for island biogeography and metapopulation biology (MacArthur and Wilson, 1967; Hanski, 1997) are applicable to spider webs as habitats. For kleptoparasites, host webs like those of golden orbweavers (Nephila, Trichonephila), are attractive due to their size, tenacity, and predictable provision of essential resources (Elgar, 1989; Grostal and Walter, 1999). Similarly, due to the size and ease of discovery and measurement, webs of golden orbweavers are a convenient system to study ecology, such as how kleptoparasites are distributed among ‘islands.’ Studies on nephilids (Robinson et al., 1973; Elgar, 1989; Grostal and Walter, 1997, 1999, Agnarsson, 2003, 2011), and other types of host webs (Cangialosi, 1990a, b, Rypstra and Binford, 1995) have clearly established that web size is the best predictor of kleptoparasite load (Su et al., 2021). Fernandez-Fournier and Avilés (2018) also showed that kleptoparasite diversity increases with web size across four different solitary and social host species, and McCrate and Uetz (2010) showed that the number of Argyrodinae kleptoparasite species increases with size of colonies made up by Metepeira incrassata and associated web builders. This is expected as large webs both contain more silk, and thus areas to occupy, and intercept more prey resulting in a relatively high-quality kleptoparasite habitat (Gregorič et al., 2021, 2024). What is intriguing is the scalability of the system—how abundance scales precisely with size (Agnarsson, 2011; Gregorič et al., 2024), down to some ´minimum´ size where kleptoparasites are generally absent (Agnarsson, 2002; Miyashita, 2002; Whitehouse et al., 2002). Thus, total host web area in a given region or habitat can approximately predict the expected number of spider kleptoparasites. Another interesting result from these studies is that a) while web clustering and interconnectedness—a common feature of many nephilid species—facilitate immigration and stabilization of kleptoparasite populations (Agnarsson, 2003, 2011), b) even highly isolated webs have kleptoparasite abundances proportional to their size (Gregorič et al., 2024). This suggests that habitat quality trumps connectivity when individuals freely distribute according to resources and implies that isolation is not limiting in the distribution of kleptoparasites. Spider kleptoparasites therefore seem both adept at locating host webs and highly mobile. Accordingly, it has been shown that the distribution of kleptoparasites among host webs closely follows the Ideal Free Distribution model, where the free movement of individuals leads to an equilibrium in habitat occupancy in relation to suitability (Elgar, 1989; Agnarsson, 2003, 2011, Gregorič et al., 2024). The ability of kleptoparasitic spiders to detect and evaluate habitat islands suggests the use of airborne cues, likely pheromones produced by the host (Agnarsson et al., 2025), to locate suitable patches. In conclusion, spider webs provide a compelling model for studying habitat islands and the ecological relationships they support. Through examining the relationship between orb weavers and their kleptoparasitic guests, we can gain a deeper understanding of how patch quality and connectivity shape community dynamics in isolated habitats (Agnarsson, 2011; Gregorič et al., 2024). The preference of kleptoparasitic spiders for larger webs, coupled with their impressive dispersal capabilities, demonstrates that for organisms in isolated ecosystems, the availability and quality of resources are paramount. Habitat islands, whether they are spider webs or isolated forest patches, illustrate the complex interactions between organisms and their environments, offering valuable insights into abundance distributions and resilience of ecological communities.
2.3 Web building spiders as ideal hosts for kleptoparasites
Spider webs provide a diverse habitat for a variety of organisms (Figures 2, 3 and text above) that alone makes them attractive to other foragers. The efficacy of these prey catching devices further renders them a predictable source of insect cadavers and the sedentary nature of web-building spiders creates a stationary and accessible resource for kleptoparasites (Vollrath, 1987; Eberhard, 2020). Vollrath (1984) suggests that web spiders are vulnerable to kleptoparasitism because they cannot run away or hide food. Web building spiders, furthermore, may capture both large prey that require extended handling times and more prey than they can consume at a given time, requiring storage within the web (Champion de Crespigny et al., 2001). Additionally, spiders lack mandibles and generally do not chew on prey but rather inject venom and digestive enzymes into the prey. The prey is thus partially digested externally and spiders feed exclusively by sucking up the digestive juices (Foelix, 1982). Combined, the prolonged prey handling, availability of predigested stored prey in the web, and slow absorption of food from the prey by the host, all represent excellent opportunities for kleptoparasitic organisms to join in feeding with the host or steal its wrapped prey items (Table 1; Supplementary Table S1). In addition, while spider webs are not well characterized as sieves (Eberhard, 2020) their design, even if well-tuned to capture ´optimal´ prey, inevitably results in the by-catch of some very small insects that may not be important food source for the host. This presents an additional opportunity for kleptoparasites that are typically many times smaller than the host spider (regardless of developmental stage of either) or for whom such prey items represent valuable resources. It is no wonder then, that individual webs or colonies attract a great diversity of kleptoparasites, ranging from birds, large hymenopterans and scorpionflies, to tiny ants and ´freeloader´ flies, in addition to the specialized spider kleptoparasites like Argyrodes (Thornhill, 1975; Nyffler and Benz, 1980; Vollrath, 1987; Henschel, 1998; Sivinski et al., 1999).

Figure 3. Key spider hosts. (A–D) the most important hosts of argyrodine kleptoparasites. (A–C) Nephilidae. (A) Nephilingis livida female and B, her large orb web (c.a. 1m in total length). (C) The mother of all hosts, Trichonephila clavipes, sitting on her golden orb. (D) Cyrtophora citricola web, representing the second most affected host lineage. Note the abundant auxiliary webbing above and below the horizontal orb. (E) Linothele sp. female sitting in her retreat, leading to her brushed sheet web—the favorite home of mysmenid kleptoparasites. (D) is reproduced from Cyrtophora.citricola.net.7626.jpg in Wikipedia commons under Creative Commons Attribution-Share Alike 2.5 Generic. (E) is by Bastian Drolshagen, reproduced with permission from the author.
Spiders are potentially dangerous hosts, but large spiders may pay limited attention to smaller organisms in their webs, if they are not struggling or sending other similar vibratory signals to the web owner (Vollrath 1979b, 1987). While large spider webs thus attract a number of kleptoparasite species from diverse arthropod groups, the most numerous and speciose kleptoparasites in spider webs are other spiders. As pointed out by Vollrath (1987 p. 277) ´[t]he spider web is a very special structure in terms of material, configuration and information transmission. It is not surprising that web spiders are the most successful inquilines in spider webs (Brignoli, 1966), since they are superbly preadapted.’ In other words, spiders know how to operate spider webs and spider kleptoparasites can move about in webs stealthily and without becoming stuck on the sticky capture spiral of many of the hosts. Non-spider kleptoparasites that inhabit spider webs are, in fact, most common in webs that do not contain sticky silk, particularly in the webs of subsocial and social spiders that tend to show more tolerance toward other web inhabitants, many of which are, of course, their kin.
Kleptoparasites may impose significant costs on spider hosts. While some scavengers may only consume leftover prey, kleptoparasites often remove entire prey items, reducing the spider’s available resources (Henschel and Lubin, 1992; Vollrath, 1987). In some cases, kleptoparasites like ants or certain Argyrodes species also attack spider broods or egg sacs, increasing their impact on spider populations (Schneider and Lubin, 1997; Agnarsson, 2002). The persistent threat of kleptoparasites and predators has led to the evolution of several adaptive responses in spiders. Spiders like Stegodyphus lineatus exhibit defensive behaviors to reduce the risk of ant raids, such as altering web structure or abandoning heavily invaded areas (Henschel, 1998; Rypstra, 1981). Hosts may also relocate webs when kleptoparasitic pressure becomes unsustainable (Rypstra, 1981; Whitehouse, 1997a) offering short term respite from kleptoparasite activities (Robinson et al., 1973).
In sum, while spiders are potentially dangerous hosts as major arthropod predators that have evolved various means of countering kleptoparasitism, spider webs offer many advantages making them an ideal host to other organisms, among which kleptoparasites are typically most abundant.
2.4 Characteristics and phylogenetic distribution of spider hosts
Even a cursorial look at the distribution of kleptoparasitic hosts across the spider phylogeny reveals a starkly non-random pattern (Figures 4–6). The inset figure on the left side shows in broad strokes that hosts are not spread proportionately across all spider diversity. A total of about 200 hosts have been recorded, including approximately 200 named species, 88 genera, and 23 families, a tiny fraction of spider diversity (Figures 5, 6). But even among these, the distribution of the most critical hosts—those home to the majority of kleptoparasites—is non-random. Rather, as depicted in Figure 4, preferred kleptoparasite hosts, labeled in bold and green font, are concentrated in relatively few genera within a small subset of web-building spiders. These key hosts exhibit unique characteristics—large, often structurally complex, and persistent webs—that make them particularly suitable for kleptoparasitic exploitation (Exline, 1945b; Vollrath, 1984, 1987, Elgar, 1993; Whitehouse et al., 2002; Whitehouse, 2011). Kleptoparasitic hosts are disproportionately found among large sheet or funnel web spiders like web building mygalomorphs (Dipluridae, Ischnothelidae) and RTA clade spiders (Agelenidae, Pisauridae, Psechridae, Tengellidae), and among orbweavers (especially Nephilidae and Cyrtophora, but also in large Araneidae, Theridiidae, and Linyphiidae). Within these families, key genera like Diplura, Linothele, Ischnothele, Agelena, Psechrus, Nephila, Trichonephila, Argiope, Cyrtophora, Latrodectus, Linyphia and a few other araneoids emerge as primary hosts for kleptoparasites, through sharing some key traits. All build large webs, in absolute terms and certainly relative to confamiliars, that are long lasting, either in being in constant construction and repair (mygalomorphs, RTAs, Cyrtophora, Latrodectus, Linyphia), undergoing only partial reconstruction (Nephilidae), or webs that are reconstructed with high site fidelity (Argiope, Gasteracantha). Most of these webs are 3-dimensional in primary architecture, or through auxiliary webbing in the form of voluminous non-capture areas, either elaborate retreats where the host gets protected rest, or ´barrier´ webs that may serve to slow down prey or as protection against flying predators and parasitoids. The auxiliary webbing in these non-capture areas provides an excellent refuge for kleptoparasites where they can stay in contact with the prey capture web, but outside the prey monitoring zone of the host. In most cases, preferred hosts are also large spiders at least relative to the kleptoparasites. It is easier for a small kleptoparasite to go about unnoticed in a large host web, and some of the prey that gets stuck in large webs is so small as to be ignored by the host but represents an important meal for the kleptoparasite. Large prey items caught by large spiders may require long prey handling times, and excess capture results in prey storing, both of which provide additional opportunities for kleptoparasites (Champion de Crespigny et al., 2001). Finally, the most important host species tend to build clustered webs that may even be interconnected. For example, Trichonephila and Cyrtophora are the two genera that are hosts to the greatest diversity and highest abundances of kleptoparasitic spiders (Table 1; Supplementary Table S1). Both construct expansive orb webs that epitomize the key traits of ´good hosts´, though nevertheless, they are architecturally strikingly different as orb webs. Trichonephila builds a huge ´modified´ vertical orb (Kuntner et al. 2019) with extensive barrier webbing, especially as juveniles, while Cyrtophora constructs a horizontal modified ´sheet like´ orb with extensive vertical network of dense threads that intercept the flight of insects (Figure 3). Both tend to build webs in close proximity to others, frequently forming interconnected web clusters that may span significant areas. Aggregated webs provide large “arenas” where kleptoparasites may freely wander among multiple provisioning hosts, supplying rich prey capture opportunities and ample shelters. The large-scale composition of colonies inherently diffuses the host’s vigilance, creating a spatial limitation in policing and guarding resources effectively across the colony (Vollrath, 1984; Elgar, 1993). Clustered webs may also capture more prey, due for example to ´richochet effects´ (Uetz, 1989). The ultimate spider aggregations are found in social spiders constructing large communal webs housing hundreds or thousands of individuals (Avilés, 1997). It is no surprise then that social spiders complete the list of the most important host species for kleptoparasites (Vollrath, 1987; Cangialosi, 1990a, b, c; Straus and Avilés, 2018, 2023). Stegodyphus and Anelosimus, in particular, contain many social species all of which host kleptoparasites that in some cases are specialists, like Argyrodes ululans on Anelosimus eximius (Cangialosi, 1990a, b, –, 1991) and Archaeodictyna ulova in Stegadyphus webs (Griswold and Meikle, 1987). Faiditus subdolus also appears to prefer communal webs, hitherto found only in the nests of social Theridion nigroannulatum (Avilés et al., 2006) and the colonial Philoponella oweni (Smith Trail, 1980). Some particular traits of social spiders, in addition to web size, complexity, and permanence, are conducive to spider kleptoparasitism. Social spiders have generally reduced levels of aggression as—unlike typical solitary spiders—they must tolerate conspecifics sharing the web (Avilés, 1997; Agnarsson, 2002; 2004). Communal web-building and resource sharing also translates to reduced individual control over web sections and increased ´noise´ levels in the web, making it harder to detect invaders who are apt at navigating silk. Perhaps as a consequence of decreased host detection, kleptoparasites in social webs tend to be closer in size to the host spiders than kleptoparasites in solitary webs. As outlined above, social spiders are also favored hosts among non-spider kleptoparasites.

Figure 4. Phylogenetic distribution of major spider host species, obligate kleptoparasitic spiders, and theridiid araneophages. The inset figure highlights that both kleptoparasites and their hosts are clustered on certain branches unrelated to patterns of total species richness within the tree. This distribution indicates that while many spiders are web-builders, only a subset has evolved the necessary web traits to support kleptoparasitic lifestyles effectively (see also Figures 5, 6). This narrow host range likely reflects the stringent requirements that kleptoparasites have for host web characteristics—namely, webs that provide sustained resources, cover, and minimal interference from the host. It is notable, also, that kleptoparasitism is clustered within Theridiidae and symphytognathoids, even though multiple origins are implied. Hosts in bold appear to be the most important in terms of number and abundances of kleptoparasites in their webs. Species number (species#) indicates diversity of kleptoparasites (kl) and araneophages (ar). The phylogeny is an interpretive reconstruction of relationships within Theridiidae from Liu et al. (2016), while broader fundamental structure is from Wheeler et al. (2017) and Kulkarni et al. (2023). The putative placement of Nicodamidae and relationships within Araneoidea, especially the monophyly and interrelationships among ´symphytognthoids´ reflect the stronger character set of Kulkarni et al. (2023). Gushangzao is tentatively placed among hadrotarsine theridiids, as proposed by Lin et al. (2024).
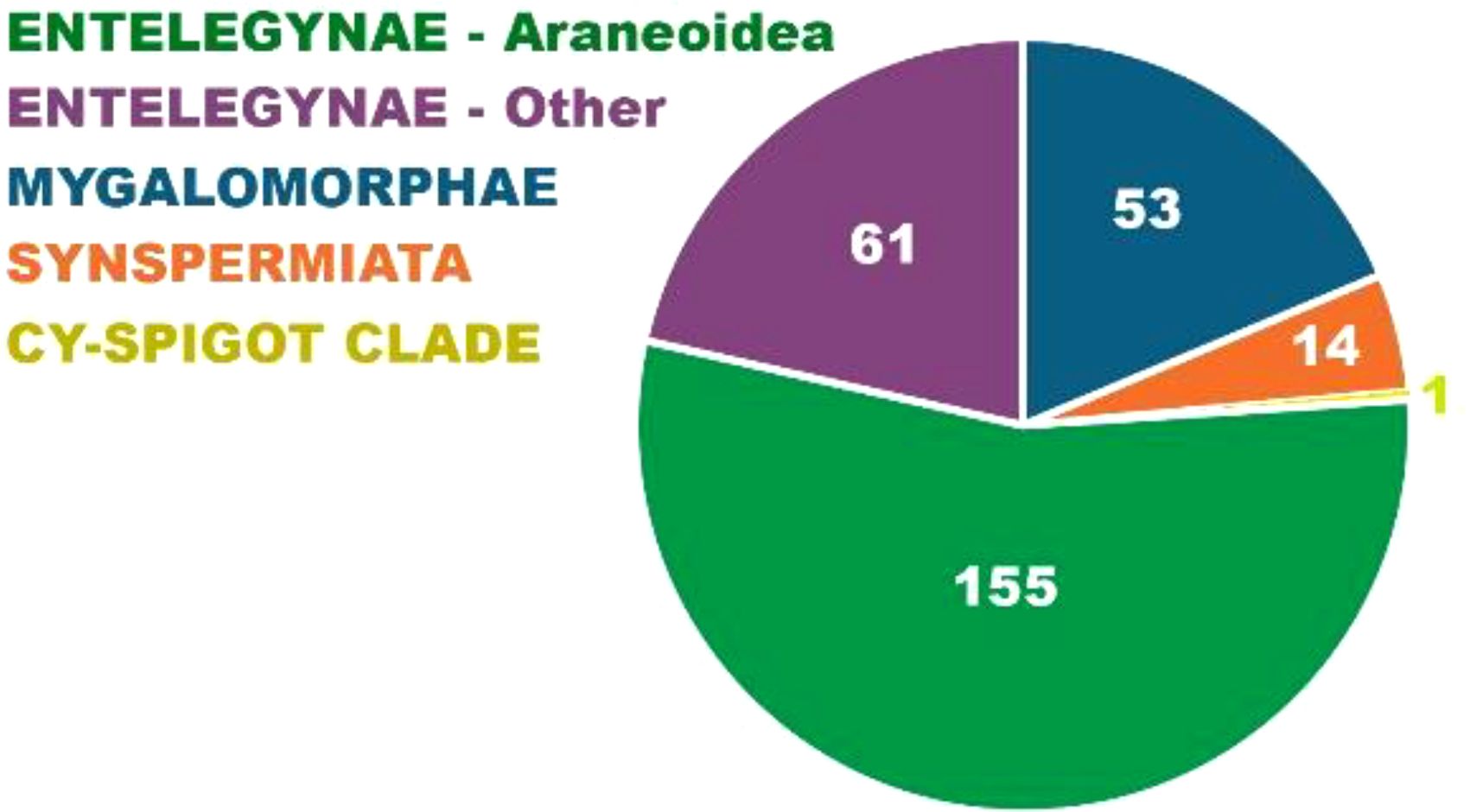
Figure 5. The number of kleptoparasite species associated with major spider clades. The majority of kleptoparasite species are associated with spiders building large and long lasting webs: primarily Entelegynae but also Mygalomorphae. In Entelegynae, araneoid spiders are hosts to the highest number of kleptoparasite species. The most prolific among those are the classical hosts in Nephilidae building large orbs with extensive barrier webs, along with the araneid Cyrtophora building complex horizontal sheets with extensive auxiliary webbing, and the social theridiids (Anelosimus) and eresids (Stegodyphus) (see Figure 6 for detail). Among mygalomorphs, the vast majority of kleptoparasites are found in two genera making large brushed sheets, Linothele and Ischnothele.
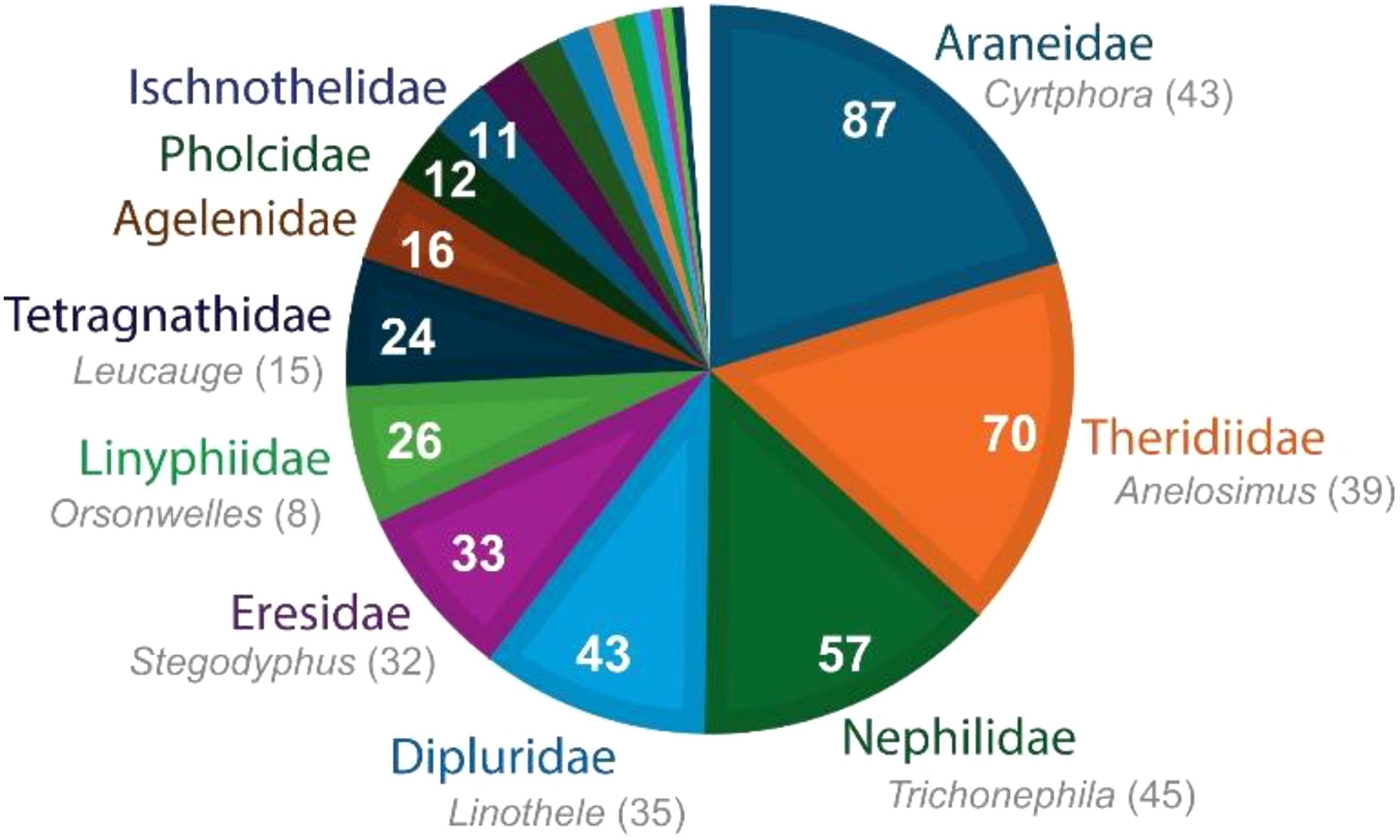
Figure 6. The total number of kleptoparasite species that have been documented associated with different spider families (only the most important host families labeled, see Table 1; Supplementary Table S1 for detail). The non-random distribution of hosts among spiders is evident. The ´superhosts´ belong to families representing a small portion of total spider diversity: Araneidae (6%), Theridiidae (5%), Nephilidae (0.1%), Dipluridae (0.3%), and Eresidae (0.2%). Thus, for example, about 25% of kleptoparasite species are associated with the tiny Nephilidae. Linyphiidae have about as many associated kleptoparasites as expected by their relative species diversity (9.4%), while most families, and the vast majority of spider genera and species have not been documented as hosts to kleptoparasites. Even within the key host families, a tiny fraction of the genera and species are important hosts, as highlighted by the number of kleptoparasites found documented from key genera in each of the major host families (gray subtext to family names). Indeed, the overwhelming majority of host records come from species making large, complex, and long lasting 3-dimensional webs—1) horizontal (Cyrtophora) or vertical (e.g. Trichonephila) orbs with extensive barrier webs, 2) expansive silk networks of social spiders (Anelosimus, Stegodyphus), 3) ´irregular´ sheets with swaths of very numerous silk lines (brushed sheets) containing a distinct retreat tunnel (Linothele), and 4) horizontal sheet webs with extensive auxiliary webbing above (Orsonwelles). Note that, commonly, kleptoparasite species are found in the webs of more than one family, though most of the kleptoparasite species that chose Dipluridae hosts (most mysmenid kleptoparasites) are exclusive to them.
While preferred spider hosts share many traits, the stark divide in host use between argyrodine and ´symphytognathoid´ (Mysmenopsis, Isela, Curimagua) kleptoparasites is intriguing and suggests we have a very partial understanding of host choice. The mygalomorphs Diplura and Linothele construct brushed sheet-like webs that span the ground or other surfaces and are key hosts for a clade of symphytognathoid kleptoparasites (excluding Sofanapsis) but are rarely used by argyrodines. In turn, symphytognthoids are relatively rarely found in orb webs suspended above the ground that are favored by argyrodines. Thus, it seems that the even ´good hosts´, as characterized above, building large, complex, and permanent webs, are not simply a free-for-all. Effective utilization of a given host type may require some adaptations. That two monophyletic lineages (argyrodines and mysmenids) have diverged to specialize on these different web types —sticky orbs vs mechanical (non-sticky) sheet and funnel webs, respectively--begs adaptive questions. It remains to be seen if we can identify traits that differ between argyrodine and symphytognathoid kleptoparasites and may help explain this division in host use. Certainly, certain kleptoparasite behaviors may be orb-web specific like silk consumption (spiders have not been documented consuming aciniform silks, used in the sheet of Diplurid webs) and stealing wrapped prey items. However, in both cases kleptoparasites feed with the host, possibly the primitive foraging mode of spider kleptoparasites. Curiously, the other symphytonathoid kleptoparasite, the anapid Sofanapis, is highly unusual in preferring host rarely used by the other two clades. It seems to prefer austrochilid hosts, while also found in the webs of Hahnidae, in addition to occurring in some classical orb webs. Regardless, Sofanapis displays the putatively primitive foraging mode of feeding with the host.
Of identified species, the host to the highest number of kleptoparasite species is probably also the best studied, the American Trichonephila clavipes where 21 species of mostly argyrodines have been documented (Table 1; Supplementary Table S1). While vastly less effort has been afforded to other nephilids as host, other species like T. inaurata, T. clavata, and Nephila pilipes are known to harbor multiple species of kleptoparasites (Table 1; Supplementary Table S1). Whitehouse et al. (2002) suggested a special importance of Nephilidae hosts globally, and field biologists certainly can confirm that large nephilid webs, as a rule, house multiple argyrodine individuals. In addition to Nephila and Trichonephila, larger Nephilengys and Nephilingis webs are rarely without kleptoparasites, that may also be found in Herennia. Cyrtophora citricola, and other Cyrtophora are similarly critical hosts harboring high abundances and numerouse species of kleptoparasites. These two groups seem to be the preferred hosts of the majority of argyrodine kleptoparasites, with some clear exceptions (Table 1; Supplementary Table S1), however, systematic research on kleptoparasite distributions and assembly into host webs is generally lacking. The tight link between Linothele mygalomorphs and Mysumenopsis kleptoparasites has become particularly clear recently with the effort of Dupérré and Tapia (2015, 2020), but again, almost no research on host choice and assembly into host webs exists on mysmenid kleptoparasites.
2.5 Deferred hosts
In contrast to the groups discussed above, the majority of web building spiders rarely host kleptoparasites and thus appear to be deferred hosts—that is their webs have much lower kleptoparasite loads than predicted based on web size. While many knowledge gaps exist and new research constantly adds to the list of kleptoparasitic species and their hosts, a ´list´ of deferred hosts among web building spiders would be very long, approximately the inverse of hosts listed in Table 1 (see also Supplementary Table S1). Even some found in Table 1, species that are utilized as hosts, are clearly deferred. For example spiders in the genus Leucauge are among the most common orbweavers in many habitats (own obs.). Yet they rarely contain kleptoparasitic spiders. In fact, many of the records of Leucauge as a host come from a study where McCrate and Uetz (2010) failed to find kleptoparasites in its webs, unless in multi-species colonies of web-builders associated with the colonial Metepeira incrassata. In some areas Leucauge webs are generally without kleptoparasites but may serve as interim hosts during seasons when preferred hosts are not available (Miyashita, 2002). Miyashita (2002) found Argyrodes bonadea and A. flavescens in Leucauge blanda webs in Japan but almost exclusively when preferred hosts (Nephila pilipes and Trichonephila clavata) were not in season. Regardless, Miyashita (2002 p. 34) found that L. blanda had “…significantly fewer [Argyrodes] … on their webs than expected from their web area”. Tetragnatha and other tetragnathids, most Araneidae genera, and nearly all small ‘‘orbicularians’’ (e.g. Cyclosa, Eustala, Mangora etc.), are further examples of extremely common, diverse, and abundant orbweavers that rarely host kleptoparasites. Similarly, amaurobiids, most pholcids, mysmenids, and most theridiids, are examples of diverse web builders underutilized by kleptoparasites (Supplementary Table S2). In fact, the vast majority of species of web-building spiders are deferred hosts, likely including many entire families of spiders like Theridiosomatidae, Symphytognathidae, and other groups of small spiders. The vast majority of species in the most diverse web-building groups Linyphiidae and Theridiidae are also deferred. In the latter two, nevertheless, certain species rank among clearly preferred hosts. It is logically obvious that what characterizes deferred hosts is lacking some or most of the traits of preferred hosts. There are vastly more species of small rather than large web builders, which may more readily detect similar-sized invaders and compete more directly with them for food. The majority of orbweavers make small webs with little auxiliary webbing, renew webs daily, and may frequently relocate. Leucauge are an exception in that their orbs are often associated with barrier webs, and indeed many kleptoparasites of many species have occasionally been found in their webs. However, these relatively small spiders are incredibly aggressive toward web intruders (Eberhard pers. comm.) which may explain why Leucauge webs seem mostly to be used as ´last resort´ hosts when preferred hosts are absent. Smaller spiders and webs capture fewer insects, are likely to have faster prey handling time, and less reason to store prey. Most web builders, furthermore, do not form interconnected clusters. In some cases, a single trait may separate preferred from avoided hosts. Large orbweavers have many attributes that are attractive to kleptoparasites, yet only a portion of them commonly host them, likely due to lack of certain critical traits. A point in case can be made regarding Gasteracantha and Micrathena. The two genera are diverse and highly similar as large, spiny, brightly colored spiders that build big and highly regular symmetric orb webs, in relatively open areas. Numerous species of argyrodines have been documented in a number of Gasteracantha species that, at least in certain habitats, appear to be among the most important hosts. In contrast, kleptoparasites are rarely encountered in Micrathena webs (except when associated with multi-species colonies McCrate and Uetz, 2010). While both genera remove webs at night and rebuild during the day, a key difference may be in what they do not remove. Micrathena removes the entire web leaving at most one or two lines while Gasteracantha leaves up a number of frame lines that that are reused as frames the following morning (Eberhard pers. comm.). Such minor differences may suffice to allow kleptoparasites to more easily find webs or web remains at night and stay associated with host webs that are taken down but rebuilt in the same spot.
2.6 Hosts from the perspective of kleptoparasites
It is clear that host choice is not random. First, only a small subset of web building spiders seem appropriate hosts. Second, even among spiders that build relatively large and permanent webs, kleptoparasite diversity and abundance is concentrated in the webs of a few host species. But how decisively do kleptoparasites chose among ´acceptable hosts´? There is a range of opinion but limited data on kleptoparasite host specialization. Vollrath (1984 p. 70), e.g. discussed argyrodines and claimed that ´[o]f the 50-odd neotropical Argyrodes two are presently known to be specialists, about ten to be generalists…´. Specialists are, he claims, in large araneids (Araneus, Cyrtophora, Argiope, Nephila), while generalists are found in multiple additional hosts (Vollrath, 1977). Since Vollrath (1984, 1987) kleptoparasites are commonly referred to as either specialists or generalists in literature (e.g. Elgar, 1993; Whitehouse, 2011; Su and Smith, 2014). Yet the data at hand suggest a more subtle picture. In an extensive study of argyrodines (treated together under the genus Conopistha at the time) of Peru and Ecuador, Exline (1945b p. 506) stated ´There is no correlation between the various species of Conopistha and species of their hosts. They inhabit indifferently webs of Gasteracantha, Argiope, Meta, and Aranea [= Araneus], in localities where these orb weavers occur.’ Similarly, as more data are gathered, species that once were thought to be specialists, like Argyrodes elevatus, have now been documented in a large sweep of hosts (Supplementary Table S2). On the other hand, Exline (1945b p. 506-507) discussed preferred and deferred hosts in general: ‘Some field observations on the relationships of Conopistha to host species may be important if correctly interpreted. In most areas where the genus is common, species of orb-weavers, including Meta, Gasteracantha, Aranea, Argiope, Tetragnatha, Cyclosa, and Leucauge, are common. Conopistha nearly always is present in the webs of Meta, Gasteracantha, Aranea, and Argiope, but almost never in webs of Tetragnatha, Cyclosa or Leucauge. Spiders of the first groups, hosts of Conopistha, feed upon large insects—beetles, large flies, bees, and Orthoptera—and disregard the small prey in their webs. Orb-weavers which do not harbor Conopistha feed on such minute insects as small flies, mosquitoes, and midges.’ Exline (1945a, b) believed host choice was essentially random and that kleptoparasites survived and thrived when they happened to be in webs where they did not compete with the host for prey or fall prey to it. There is little evidence for host choice to be random, especially with respect to preferred and deferred webs in general. However, establishing true host specialization requires large amounts of data that simply do not exist for most species. Studies that have been made over a short period of time, in a particular region or season, or particular habitat, may find evidence suggesting specialization, while the strength of any proposed association with a particular host tends to break down as more data are added. Vollrath (1976, 1977, 1979b, 1984), for example, suggested that Argyrodes elevatus is a specialist on large hosts, principally found in webs of Trichonephila clavipes and Argiope argentata, which is an expert in stealing food bundles from the hosts hub. Further studies on this species have found it utilizing a variety of host species, large and small. Thus, in stark contrast with Vollrath, Silveira and Japyassú (2012) instead consider A. elevatus to show extreme plasticity, employing diverse tactics to steal food from a great variety of hosts and web types. These include Nephilengys, various Argiope and Araneus, colonial Metepeira incrassata and Cyclosa huila, subsocial Anelosimus baeza, as well as in solitary webs of theridiid spiders such as Latrodectus, Tidarren, and Parasteatoda. What can we therefore claim about host specialization in argyrodine kleptoparasites? As proposed by Agnarsson et al. (2025) asking if one species is a specialist or a generalist is not a productive approach to understand the complex interplay between kleptoparasites and hosts. Rather “argyrodines may better be characterized as kleptoparasites generally capable of discriminating among host webs, but that choose hosts with varying degrees of astuteness or concern.” A more productive approach, therefore, will be to better assess kleptoparasite distribution and assembly into host webs at a community level, and the factors that impact host choice. In particular, what host traits do kleptoparasites cue in on and why do some species seem to choose hosts with greater astucity than others?
3 Diversity of organisms as kleptoparasites of spiders
3.1 Non-spider kleptoparasites on spider hosts
A number of animals regularly exploit spider webs, ranging from commensals to kleptoparasites, to predators and parasitoids, depending on whether symbionts compete for the same resource and the manner of their foraging or reproductive strategy (Supplementary Table S3, Robinson and Robinson, 1977). Some arthropods may be purely commensal, such as mealybugs found in Phryganoporus candidus nests, or scavenging beetles that rummage leftover prey and waste (Downes, 1995), or lepidopteran larvae feeding mostly on plant material incorporated into the webs of social spiders. Kleptoparasites (Supplementary Table S3), in addition to other spiders, include species of hemipterans (Davis and Russell, 1969; Henry, 1984) panorpoid scorpionflies (Thornhill, 1975), lepidopteran larvae (Robinson, 1977), wasps (Jeanne, 1972), damselflies (Vollrath, 1977), numerous dipteran species of at least 10 families (Sivinski et al., 1999), rove beetles and other scavenging beetles, Cheyletidae mites, and other arthropods (Supplementary Table S1, Knab, 1915; Bristowe, 1931, 1941, Richards, 1953; Robinson and Robinson, 1977), and even birds, such as a hummingbird that steals both insect prey and silk (Young, 1971). Many of these are attracted to prey captured by the host spider or entangled in its web, others may be more generally predators in the host webs where they may prey on host or commensal spiders, their eggs or juveniles, or other arthropods in the web (Schneider and Lubin, 1997; Henschel, 1998). Ants, for example, enter webs and may steal prey from the web and/or prey on the host and its offspring (Schneider and Lubin, 1997; Henschel, 1998). Parasitoid wasps target host spiders, their eggs, or even other symbionts (Hieber and Uetz, 1990).
Kleptoparasites that compete with spiders for prey, remove prey items secured by the spider, or feed with the host, clearly can negatively impact the hosts (Henschel and Lubin, 1992). Probably the majority of non-spider kleptoparasites in spider webs are facultative. Scorpionflies (Panorpidae) opportunistically enter webs to feed on captured prey (Thornhill, 1975).
Other facultative kleptoparasites include damselflies (Zygoptera), apocritan wasps, and lepidopteran larvae that may occasionally steal prey from spider webs or use the web or webbing as nesting material (Jeanne, 1972, Robinson, 1977; Vollrath, 1977), and mites and beetles that scavenge on debris left by the host spider or consume parts of prey remains (Faust et al., 2012). Hummingbirds may occasionally use silk taken from spider webs as nesting material and grab prey items as they pass by (Young, 1971). The impact of facultative kleptoparasites can be difficult to evaluate. Secondary consumption on decomposing prey bodies or web-bound detritus, can be of no consequence to the host, or even beneficial if it results in effectively reducing waste within the web. Yet, all these taxa may remove resources with potential fitness consequences for the host spider. On the other end of the spectrum, there is clear evidence that facultative kleptoparasites can be very harmful to the host. Ants provide a stark example of harmful occasional kleptoparasites. When they wander into spider webs, they have the ability to recruit large raids of foragers that opportunistically steal resources and predate upon hosts (Schneider and Lubin, 1997; Henschel, 1998; Hölldobler and Wilson, 1998). As ants may continuously patrol a network of routes around their nests, they can pose a persistent and ominous challenge to web building spiders (Henschel, 1998; Hölldobler and Wilson, 1998). Furthermore, ants like Veromessor pergandei, Oecophylla smaragdina, and Anoplolepis gracilipes can not only steal prey and forage on egg sacs but may also dismantle spider webs e.g. to salvage trapped nestmates.
Many groups then rely mostly on kleptoparasitism, where spiders may be among their hosts, or their exclusive hosts. Dipterans are adept kleptoparasites often feeding on prey along with the predators, and numerous species in families including Milichiidae, Phoridae, Chloropidae, and others (Supplementary Table S3) approach or invade spider webs (Sivinski and Stowe, 1980; Sivinskii et al., 1999). Milichiid flies are chemically attracted to prey caught in spider webs and often hover near the web until an opportunity arises to feed undetected. The majority of such flies apparently go unnoticed, perhaps adapted to hovering at a safe distance and landing on the spider carapace or on the prey only when the spider is occupied. Or the host opts to not detract them, however, some percentage of these flies may themselves end up as prey. Ranzovius bugs (Miridae) like R. morens and R. contubernalis are specialist kleptoparasites scavenging prey items in spider webs, many associated with the social Anelosimus spiders (Henry, 1984; Wheeler and McCaffrey, 1984; Guariso, 2000; Deyrup et al., 2004)
In conclusion, spider webs represent not only snares for prey capture but also complex habitats that sustain diverse communities where kleptoparasitic organisms are the most diverse. From the stealthy Argyrodes to opportunistic ants, each kleptoparasitic organism has developed strategies to exploit spider webs to its advantage. Clearly, spider webs play ecological roles beyond prey capture, providing important microhabitats within most terrestrial ecosystems.
4 Facultative kleptoparasitism in spiders and araneophages in kleptoparasitic lineages
4.1 Interactions among spider species in webs – webs as refuges and structural support, and web invasion
Spiders exhibit a fascinating array of web-invasive strategies exploiting the webs of other spiders in intra- and interspecific interactions including—in addition to kleptoparasitism—other resource usurpation, web takeover, predation, and mating.
Perhaps the least invasive usurpation of spider webs is using a stranger’s web as a refuge from predators, as seen in some wandering spiders in social spider webs or using the structural lines of the web to support one´s own web. Such connecting behavior characterizes colonial spiders that in addition to clustering around abundant resources may benefit from interconnectedness via ´ricochet effects´ (Uetz, 1989), the ability to sense struggling prey in multiple webs, predator defense, and others (Rypstra, 1989). Colonial species such as Metepeira incrassata (McCrate and Uetz, 2010) and Philoponella republicana (Binford and Rypstra, 1992), and those that more facultatively interlink webs like Cyrtophora spp. (Rypstra, 1979; Leborgne et al., 1998) and Trichonephila spp (Rypstra, 1981; Agnarsson, 2011), may also connect their nests to heterospecific webs. Many other species facultatively connect their webs to others, particularly to webs of colonial or clustering species. Leucauge, Uloborus, Micrathena, Holocnemus pulcei and some other pholcids, various small orbbweavers, linyphiids and theridiids are among those using ´host´ webs as structural support (Bristowe 1941a, b, Eberhard et al., 1993; Leborgne et al., 1998; McCrate and Uetz, 2010, Magnússon and Agnarsson unpublished). As webs of inter- and intraspecific individuals are thus frequently contiguous (Krafft, 1970; Burgess and Uetz, 1982; Jackson and Hallas, 1986; Hénaut et al., 2010), opportunities for web invasions abound. Indeed, spiders in interconnected complexes often enter neighboring webs to pursue insects (facultative kleptoparasitism, Supplementary Table S2), or opportunistically predate on the web owner (Kullmann, 1959a, b, 1960; Jackson and Hallas, 1986; Wise, 2006). Bristowe (1941a, b), for example, observed Pholcus sp. predate on the owners of various webs it connected to, including araneids, theridiids, amaurobiids, agelenids and other pholcids. Other spiders instead of linking their web will invade webs to aggressively steal prey from the host, kill the host, or attempt to take the web over. The best-known cases are wandering spiders like Olios (Sparassidae), Simaetha and Portia (Salticidae) that engage in aggressive facultative kleptoparasitism by removing the prey from their hosts (Jackson and Wilcox, 1990). Portia is unique in its versatility of foraging strategies, being among the very few jumping spiders that regularly build prey capture webs, in addition to hunting outside a web, and invading the webs of other species (Jackson and Hallas, 1986). It may or may not connect its own web to a host web and opportunistically forage kleptoparasitically or via araneophagy. Even though they will take insects, Portia species are specialized araneophages that will prey on eggs, juveniles, and capture host spiders using a variety of tactics including stealth and mimicry (Jackson, 1985, 1987, Jackson and Blest, 1982; Jackson and Hallas, 1986). Web building spiders may instead engage in web takeover. Kleptoteny refers to such theft were spiders abandon their own webs to invade and exploit the webs of others. While there are relatively few observations of web invasion by web building spiders (Enders, 1974; Buskirk, 1975; Eberhard et al., 1978; Wise, 1981, 2006, Wickler and Seibt, 1988; Eichenberger et al., 2009; Houser et al., 2014; Gan et al., 2015; Gould, 2021), web building spiders frequently abandon webs when insufficient prey is captured (Turnbull, 1964), providing an impetus to wander off web and possibly happen upon other webs inadvertently. It seems likely that web takeover is widespread as an opportunistic strategy (Bilsing, 1920, Bristowe 1941a, b). Web building spiders web takeover may take place intra- or interspecifically, can be passive or aggressive, and involve various life stages, including adult males. Enders (1974), in an experimental setup, observed passive (empty web) conspecific web takeover in Larinioides cornutus and Argiope trifasciata. Both species would also invade empty webs of some other orbweavers and Eustala sp. was observed taking over empty webs of Larinioides cornutus (Bilsing, 1920; Enders, 1974). Aggressive web takeover has been seen in a number of species. Colonial Cyrtophora citricola adults and juveniles will displace each other depending on the capture rate of individual webs (Blanke, 1972; Eberhard et al., 1978; Rypstra, 1979) and adult Trichonephila clavipes will displace smaller individuals from their webs (Farr, 1976; Christenson, 1984). Cyrtophora citricola often steal food from one another (Whitehouse and Lubin, 2005) and in some cases, rather than attempting to take over webs, some individuals may simply opt to be webless and operate as facultative kleptoparasites in conspecific webs. This has been observed, for example, in C. citricola and Agelenopsis aperta (Rypstra, 1979; Christenson, 1984). It is unclear if such ´intraspecific optional weblessness´ differs from other facultative kleptoparasite strategies. Gan et al. (2015) observed intra- and interspecific aggressive web takeover among various orbweaving species including Lariniaria argiopiformis, Neoscona inusta, N. punctigera, Parawixia dehaani, Zygiella x-notata, and Tetragnatha laboriosa. Webs built higher in the vegetation and those capturing more prey were more likely to be invaded, and typically, as in C. citricola and T. clavipes, the success rate of web takeover depends on the relative size of invader and host. Similarly, Eichenberger et al. (2009) observed interactions between the introduced linyphiid Mermessus trilobatus with native European sheetweb spiders (Erigone dentipalpis, E. atra, Gnathonarium dentatum, Dicymbium nigrum and Micrargus herbigradus). The impetus for the study was the indicated invasive takeover of webs by the introduced M. trilobatus, however, Eichenberger et al. (2009) found that all of these species may attempt web takeover. Like prior studies, they found that body size plays a critical role in the outcome of these interactions, whether intra- and interspecific web takeover. This kind of web takeover is a clear example of facultative kleptoparasitism (aggressive usurpation) where the stolen resource is not prey secured by the host, but its prey capturing device and, of course, the prey it subsequently entangles. Eberhard et al. (1978) observations of webs of Metazygia spp. in Colombia illustrate a form of web robbery in which male spiders seize webs from immature or conspecific individuals to capture prey. This is a particularly interesting case of size-based aggressive usurpation as the males themselves typically do not build webs. They observed a male Tetragnatha sp. invade the web of M. gregalis where it stole prey and eventually replaced the smaller owner in the hub of the web. Similarly, they saw males of Eustala sp. and Larinia directa take over webs of Metazygia and males of Metazygia taking over webs of conspecifics or other Metazygia species. The males would then capture prey in the stolen web. Male spiders may also feed on silk, male Leucauge marina may steal silk from the webs of larger orbweavers (Eberhard, 2020). Observations on male behavior after they molt to adulthood and abandon web building, but prior to courtship, is generally lacking. However, it is reasonable to assume that males wandering in search of females will opportunistically capture prey on and off webs and steal other resources. The males of many species that cohabit with females they (attempt to) mate with, will take prey from the webs (see below), and web building spiders are certainly capable of capturing prey outside their webs, Gould (2021), for example observed Tetragnatha sp. haul up a large dragonfly without the use of a web.
4.2 Mating and males as facultative kleptoparasites
Webs typically serve as mating sites, with males abandoning their own webs and seeking to invade the web of a female to court her. It is common that males cohabit with females in their webs for some time, guarding a subadult female until she molts to maturity, defending her against other males, or awaiting an opportunity to sneak in and mate (Foelix, 2010). In the case of large spiders with pronounced sexual size dimorphism (female biased extreme sexual size dimorphism, eSSD sensu Kuntner and Coddington, 2020) males may even accumulate in significant numbers in female webs. In communal species, like Philoponella spp (Opell, 1979), it is also common to find males in the colonies and it seems likely they take prey opportunistically. Though observations are sparse for this aspect of male biology, the scattered records suggest that males may commonly act as facultative kleptoparasites in female webs. Linyphia litigosa males steal prey from females they cohabit with and will even fight her for prey (Rovner, 1968; Wise, 1975; Watson, 1990). Many male pholcids will, similarly, aggressively pursue food in female webs (Eberhard and Briceño, 1983, 1985, Blanchong et al., 1995). Males in some species are favored to win fights due to larger size (Eberhard and Briceño, 1983), or in species where the sexes are similarly sized, the larger individual tends to have the upper hand (Blanchong et al., 1995). Latrodectus revivensis males enter webs of females, even juveniles and subadults, and steal prey. The impact is particularly negative for younger females that may opt to relocate their webs to escape the male, at a high risk of mortality (Lubin et al., 1993). Male Stegodyphus lineatus cohabit with females and may steal and consume their eggs (if not their own) and steal prey from them, acting as costly kleptoparasites in female webs (Schneider and Lubin, 1996, 1997). Facultative male kleptoparasitism is typically less aggressive and costly to females. Male Metellina segmentata hang out at the edge of the female web and may enter the web when the female has captured prey and feed with her, both prior to and after mating (Blanke, 1974; Prenter et al., 1994; Elgar and Fahey, 1996). In the eSSD nephilids, where multiple males often cohabit with the giant female, their foraging behavior is quite similar to that of obligatory kleptoparasitic argyrodines. Trichonephila clavipes males, for example, await their opportunity in the barrier web and enter the web to glean insects ignored by the female, feed with the female, eat silk, or may even steal prey from argyrodines inside the female web (Christenson and Goist, 1979; Vollrath, 1977; 1980, 1987). In both Nephila and Trichonephila the largest male may reside near the hub and feed with the female while the smaller ´satellite´ males glean insects and rival argyrodine kleptoparasites (Robinson et al., 1973, 1976, Kuntner pers. comm.). It is likely that males of other nephilids and of other eSSD orb weavers where males accumulate in female webs display similar kleptoparasitic behaviors (Robinson et al., 1973; Christenson and Goist, 1979; Vollrath, 1980, Kuntner pers. comm.).
Male cohabitation may in some cases be more mutualistic than parasitic in nature. Males of the pholcids Blechrocelis sp. and Modisimus spp. cohabit with female and capture prey in her web (Eberhard and Briceño, 1983, 1985). While the generally larger males will contest with the female over prey, the males appear to ´concede´ the prey to females upon ´begging´ gestures she makes, vibrating her abdomen. In some instances, males will capture and wrap prey, signal the female by plugging the web, and then leave the prey to her, or even carry the prey to her. Eberhard and Briceño (1983, 1985) suggest that this kind of ´chivalry´ in Blechrocelis and Modisimus spp. may render the interaction between males and females mutualistic. On the other hand, where the sexes are similarly sized like in Holocnemus pluchei, the outcome of a struggle for prey depends on individual size, males not being favored in general. On such ´equal terms´ males that happened to secure a prey from a female, did not show any signs of ´chivalry (Blanchong et al., 1995). Perhaps the lack of a general size advantage means that males cannot exert control over females by stealing prey at will and then secondarily offering it to them.
4.3 Facultative kleptoparasites and araneophages
In addition to web takeover scattered across multiple web building groups, and male facultative kleptoparasitism that is also widespread but rather poorly known, a few cases of facultative kleptoparasitism that involve neither web takeover nor male spiders are relatively well documented. Information on a few groups of such facultatively kleptoparasitic taxa are summarized below. In addition, some taxa specializing in araneophagy are treated below, although they may be similar to taxa discussed under section 5.1. These are examples taken to highlight groups like Rhomphaea and Ariamnes that due to details of strategies and/or phylogenetic position, may display transitional traits related to the evolution of kleptoparasitism. Information on further facultative spider kleptoparasite and araneophages can be found above and in Supplementary Table S2, though it is far from exhaustive. It should be noted that not all spider kleptoparasites steal from other spiders. Henriksenia crab spiders take food from pitcher plants (Fage, 1928; Striffler and Rembold, 2009; Chua and Lim, 2012), and spiders may also take food from other predators and scavengers (Cushing, 2012). Jackson and Pollard (2008), for example, observed three Kenyan species of Menemerus jumping spiders snatch food from the mandibles of Crematogaster and Camponotus ants, a behavior first noted by Bhattacharya (1936) in the Indian M. bivittatus [as M. melanognathus]. Near the shores of a lake masses of Chaoboridae and Chironomidae midges that had emerged from a lake were found perishing on the walls of buildings scouted by ants. Jackson and Pollard (2008) describe how the spiders would track ants as they walked by and attack if they were carrying prey. The spiders would approach rapidly, grab the fly with their chelicerae and pull away as the ant let go of the prey. Only relatively small individuals would partake in prey theft, which complemented typical prey catching by these jumping spiders. Hence, the kleptoparasitic behavior is facultative and Jackson and Pollard (2008) suggest that it may secure fly prey more rapidly than stalking the fly directly. Further, as the flies are dead or dying, the spiders may use the ants as assessors picking out among the cadavers prey that is still palatable. This foraging tactic has been noted in multiple Menemerus species, but is not a common salticid behavior, suggesting this may be an adaptive, although facultative, strategy.
In most cases, spiders steal food from other spiders, and typically on the host´s web. Oonops is a fairly large genus of Oonopidae spiders containing one species known to be kleptoparasitic. Oonops pulcher is a small spider that has been observed in a variety of habitats, hunting on the ground as typical for oonopids, sometimes found in birds’ nests, but also in the webs of larger spiders (Bristowe, 1930, 1958). Bristowe (1930) observed O. pulcher move among the retreat strands of its hosts, ignored by the host agelenid or amaurobiid spider, feeding on remnants of the hosts meals. Oonops pulcher is clearly a facultative kleptoparasite, most commonly found outside a host webs (Le Peru, 2011).
Platnickina is a theridiid genus containing 12 species some of which have been observed invading other spider webs. Platnickina is an anomaly in this summary of genera as it likely rarely steals prey. It is discussed here to highlight the need for further studies of the genus, and to consider the potential links between opportunistic web invasion and obligatory kleptoparasitism in spiders. Platnickina species build their own web, a simple network of threads, and commonly capture prey there (Suzuki et al., 2022). The majority of prey consists of insects like nematoceran flies and paraneopterans, but some 40% of the prey were spiders. Platnickina will wander outside its web and into webs of hosts including other theridiids, Tetragnatha and Trichonephila (Bristowe 1941a, b; Namkung, 2003; Suzuki et al., 2022). There, it will attempt to attack and kill the host but has a much lower success rate in killing hosts in the host´s web than in capturing prey in its own web. The large size of potential spider host prey may make up for the challenge in capturing and killing it. Platnickina may be an opportunistic kleptoparasite as well, Suzuki (pers. comm.), for example, has observed P. sterninotana feeding on a large Brachycera fly in a theridiid web.
Rhomphaea contains 37 argyrodine species that are primarily thought to be araneophages and are often discussed in the context of the origin of web invasion and kleptoparasitism. Rhomphaea species are typically elongated, with long ´tails´ that they can move in a worm-like fashion (Archer, 1946)—their bodies appear well camouflaged, especially within host webs. While Rhomphaea species are often found in their own simple web consisting of several irregularly spaced threads, they are also frequent invaders in the webs of other spiders. ´Hosts´ of Rhomphaea include a variety of smaller sheet (Linyphiidae) and cobweb (Theridiidae) spiders—including social theridiids—as well as orbweavers (Archer, 1946; Exline and Levi, 1962; Smith Trail, 1980; Whitehouse, 1987b; Guarisco, 2000; Kim and Kim, 2007; McCrate and Uetz, 2010; Srinivasulu et al., 2013). In most cases, Rhomphaea are reported as host predators, but they have also been observed stealing food from host webs (Eberhard, 1979; Yaginuma, 1986, Yoshida, 2001a, b). Suzuki et al. (2022) found that the diet of adult Rhomphaea consists mostly of spiders, with some 15% of predation attempts directed at insects, while juvenile Rhomphaea capture mostly insects. Rhomphaea had a high predation success on its own simple thread webs (95%), but in host webs it often fails to capture the host spider (25% success rate), especially when the host is large. It is likely that Rhomphaea ventures into host webs in an attempt to catch larger prey than it gets on its own web, even if less successful.
Ariamnes is an extraordinary genus of argyrodine theridiids, containing 24 species with exceptionally elongated and worm-like abdomens (Vollrath, 1977). Ariamnes are relatively large spiders, and unlike most members of the subfamily, they appear to rarely wander into webs of other spiders (Clyne, 1979; Eberhard, 1979). Eberhard (1979) describes the web of Ariamnes as a sparse, irregular, three-dimensional network of long, non-sticky threads that can extend up to 1-2 meters. Rather than functioning as a snare, this web serves as a resting site for the spider and as a base for ambush attacks. Juveniles of A. attenuatus primarily capture nematocerous flies that tend to hang on these threads, while adults capture juvenile spiders, typically non-web builders larger than themselves. They use a specialized predatory technique shared with Rhomphaea, throwing sticky silk with their hind legs to immobilize prey. Suzuki et al. (2022) found that spiders constitute about 75% of the prey of Ariamnes, with a preference for cursorial spiders over web builders, and including other araneophagic spiders like Rhomphaea, Platnickina, and even other Ariamnes. Ariamnes foraging behavior appears to be quite homogenous across species. The spider’s resting posture, with legs outstretched, may be a camouflage resembling a pine needle caught in the web. Dönitz (1887) described the spider’s unique egg sac, a bell-shaped structure suspended by threads and resembling an elongated cocoon with a wool-like interior, which the adult females guard (Eberhard, 1979).
Spiders employ diverse web invasion tactics, utilizing webs for predation, resource competition, shelter, and mating. Diverse groups of both web builders and web-less hunters enter webs in search of resources or mating opportunities. Facultative kleptoparasitism is found in most such species, opportunistically stealing prey, silk, or even the web itself, regardless of the ultimate objective ranging from courtship to cannibalism. Facultative kleptoparasitism is also found in some spiders stealing prey not from a web spider. For reasons outlined in the Introduction, I have not attempted to exhaustively review facultative kleptoparasitism in spiders. It is a compelling but underexamined facet of spider biology, marked by dynamic and often intricate interactions: overt contests for webs, covert theft of prey and silk by males wandering in search of females, sexual conflict on display in facultative male kleptoparasitism, opportunistic resource theft by versatile taxa, and specialized araneophagy. Opportunities for innovative future research abound—given the phylogenetic spread of observed species, and the somewhat haphazard nature of such observations. I predict that facultative kleptoparasitism is far more widespread and common than currently appreciated. Spiders that engage in opportunistic web invasions demonstrate an evolutionary flexibility, using webs of others to reduce the energetic costs of web building and prey capture. If as pervasive as hypothesized above, the occurrence of web invasions and facultative kleptoparasitism across diverse spider lineages may imply that the evolutionary bridge to obligatory kleptoparasitism in spiders is rather short.
4.4 Obligate spider kleptoparasites
In total there are probably between 200-300 species of obligate kleptoparasites among the ~53,000 spider species currently known (WSC, 2025). Some behavioral or habitat data (host webs) are available for a little over 100 species (see Table 1; Supplementary Table S1 and References, summarized in Agnarsson in prep.), while the majority of species expected to be kleptoparasitic lack such data. Many new species also doubtless await discovery and description (Agnarsson et al., 2013) as evidenced by a proliferation in descriptions of species and genera in recent years (Dupérré and Tapia, 2020; Lin et al., 2024; Vanuytven et al., 2024). A synthesis on obligate kleptoparasitic spiders will be provided elsewhere (Agnarsson in prep.).
5 Host response to kleptoparasitism
The cost of kleptoparasitism is challenging to measure. In some cases, it is expected to be relatively small, when kleptoparasites only remove prey remains, prey ignored by the host, or silk. However, any removal of calories from the web has the potential to impact the host (Coyle et al., 1991; Whitehouse, 2011) and even calories contained in ignored prey and silk represent a cost as these could be ingested by the host when recycling the web. In many cases, kleptoparasitism inflicts a more readily measurable costs on host spiders, especially through prey depletion and its cascading effects on growth, reproductive output, and fitness (Vollrath, 1987; Elgar, 1993; Whitehouse, 2011). Studies on Anelosimus eximius demonstrate that Faiditus ululans may consume up to a quarter of the prey in a host’s web (Cangialosi, 1990b), and research on Trichonephila clavipes shows that the daily caloric requirements of argyrodine spiders can reach at least 20% of that of the host when kleptoparasite abundances are high—in this case when they reach about 40 per web (Vollrath, 1980; Elgar, 1993). While these may be extreme examples, even at a smaller cost, kleptoparasites divert significant energy reserves away from the host spider’s reproductive efforts and thus fitness. It is obvious that such resource diversion can be particularly damaging in energy-constrained environments, where each prey item represents a critical contribution to the host’s metabolic requirements. In social webs, the impact of kleptoparasites can amplify, as these webs represent a “public good” shared among colony members. The shared prey resources attract kleptoparasites who consume prey captured collectively, placing additional strain on the colony (Straus and Avilés, 2018, 2023). Thus, this resource-sharing model, though beneficial for maintaining the colony, becomes a point of vulnerability, making communal webs especially susceptible to kleptoparasitic exploitation and resource depletion (Leborgne et al., 2011). This highlights the ecological cost of maintaining large, accessible prey traps in communal settings (Vollrath, 1984), where kleptoparasitism can reach ´sublethal´ levels (Straus and Avilés, 2023).
The behavior and phylogenetic distribution of kleptoparasitic hosts suggests an evolutionary trade-off, perhaps even an arms race between web-building spiders and kleptoparasites. Hosts in clades like Nephilidae, Araneidae, and Dipluridae have evolved ´progressively´ (or repeatedly) larger and more intricate webs presumably under fecundity selection where larger females require ever more food to produce more offspring (see e.g. Kuntner et al., 2019; Kuntner and Coddington, 2020). However, large and elaborate webs with high prey capture success rate also attract kleptoparasites. This trade-off may have at least two consequences. First, one expects increased selective pressure on direct hosts countermeasures such as means of detecting and eliminating kleptoparasites while maintaining such webs. Second, the majority of orb weaving spiders take down their webs and rebuild daily. It is tempting to consider the possibility that daily web recycling, in addition to providing a fresh capture surface daily, is an example of a trade-off where the costs of kleptoparasitism outweigh the benefits of more elaborate permanent webs. However, testing this hypothesis may be challenging, and if orb recycling evolved once, as the evidence may suggest (e.g. Eberhard, 2020 and references therein), putative causative factors will ever remain speculative. One observation casts doubt on such co-evolutionary scenario; spider kleptoparasitism likely evolved later (and mostly within Araneoidea), than daily web recycling in orb-weavers. Thus, the origin of web recycling does not relate to (at least extant) spider kleptoparasites. The hypothesis is still tenable given the negative impact of heavy kleptoparasite load (Vollrath, 1987; Elgar, 1993), and facultative web takedown and relocation by spiders suffering such heavy loads. It is plausible that kleptoparasitism maintains natural selection for web recycling, and that it plays a trade-off role in the evolution of large and permanent webs. Nephila pilipes (Robinson et al., 1973) for example will take down and relocate its web when kleptoparasite load is high. A new web location may offer a respite from kleptoparasites for up to 48 hours. Web relocation in social Anelosimus and extreme ´boom and bust´ population growth in social Theridion nigroannulatum are both putatively linked to argyrodine kleptoparasites (Avilés et al., 2006; Straus and Avilés, 2018, 2023). Thus, kleptoparasite pressure can become a resource drain leading to colony fragmentation or collapse. These scenarios underscore the likely evolutionary arms race between resource conservation strategies and kleptoparasitic exploitation, with host spiders displaying adaptive flexibility in web structure, prey management, and interspecific interactions to mitigate the fitness impacts of kleptoparasitism (Whitehouse, 2011).
Certain solitary orb-weavers display a degree of tolerance toward minor kleptoparasitic activity, conserving energy by ignoring low-level incursions. Vollrath (1987) suggests that this tolerance may serve as an adaptive strategy, where active defense is reserved for more resource-intensive interactions. When implemented, hosts may engage in defensive behaviors aimed at excluding or confronting kleptoparasites. Trichonephila clavipes, for example, may respond to argyrodines with aggressive web shaking, or even pursuits. Many other host spiders have aggressive response to spider kleptoparasites that differs distinctly from their response to prey or predators (Vollrath, 1987). Further, the web itself may serve as a barrier modulating kleptoparasitic impact, e.g. through spatial separation of prey from potential kleptoparasites. For instance, layered retreats within Anelosimus colonies can limit direct access to prey-rich areas, creating a physical deterrent. It is likely that other traits, speed of prey capture and handling, retreats, and sensory biology are influenced by kleptoparasitism, but research is lacking on this front.
6 Conclusions
Certain spiders function as superhosts by providing structurally complex, resource-rich webs that support a wide range of kleptoparasites. Key superhost traits include large host and web size, high prey capture rates, web or website persistence, and three-dimensional auxiliary silk structures that offer refuges for kleptoparasites. These characteristics are most pronounced in genera such as the orbweaving Nephila, Trichonephila and Cyrtophora, and sheet weaving mygalomorphs like Diplura and Linothele. The webs of these hosts attract diverse kleptoparasites from multiple arthropod lineages, including spiders, flies, ants and bugs. Social spiders are also favored hosts likewise building large and complex webs. The most diverse communities, indeed, form in social spider webs, which have hosts that are more tolerant of co-inhabitants than solitary spiders and generally lack sticky silk. In such webs kleptoparasites bound, but also many arthropods scavenge leftovers and detritus, like beetles and lepidopteran larvae.
The spectrum of kleptoparasitism in spider webs is broad, encompassing obligate kleptoparasites like argyrodine theridiids, facultative prey thieves, web invaders, and even commensals that exploit spider webs without stealing resources useful to the host. Some kleptoparasites feed opportunistically on prey remnants, while others engage in stealthy theft or aggressive food robbery.
Additionally, spiders themselves exhibit secondary kleptoparasitism, with some species abandoning their own webs to invade and exploit the webs of other spiders (kleptotany). This facultative kleptoparasitism is seen in multiple lineages and can involve resource theft, web takeover, or even direct predation on the host (araneophagy). These interactions suggest a complex evolutionary pathway, where facultative kleptotany may serve as a transition to obligate kleptoparasitism.
This review highlights the importance of spiders in kleptoparasitic networks and suggests that further phylogenetic and ecological analyses are necessary to fully understand the evolutionary history of kleptoparasitism, and the assembly of kleptoparasitic species into host webs.
7 Future directions
Despite substantial progress in understanding spiders as superhosts and secondary kleptoparasites, this review reveals several key areas that urgently require future research.
7.1 Phylogenetic context of kleptoparasitism
While facultative kleptoparasitism and kleptotany (here) have been hypothesized as precursors to obligate kleptoparasitism, robust phylogenetic analyses are necessary to establish evolutionary transitions. Future work should focus on constructing comprehensive phylogenetic trees incorporating all kleptoparasitic and host lineages to discern evolutionary patterns and seek signatures of possible coevolutionary arms race between kleptoparasites and host.
7.2 Mechanisms of host selection and assembly into host webs
Further investigation is needed into how kleptoparasites locate, evaluate, and establish themselves in host webs. Ecological studies on species colonization, composition in host webs, and intra- and interspecific competition would clarify what rules may govern species assembly into these habitat islands. Studies employing behavioral assays and chemical ecology approaches should clarify the role of airborne cues, such as pheromones or web-borne signals, in host detection and selection.
7.3 Impact of kleptoparasitism on host fitness and web dynamics
Although some hosts appear resilient to kleptoparasitism, long-term studies tracking web persistence, prey capture rates, and reproductive success in the presence of kleptoparasites would provide a clearer picture of costs and benefits to hosts. Evaluating host responses to kleptoparasites in relation to species and kleptoparasite load could cast light on what strategies may be evolutionary responses to this type of resource theft. In particular, if both facultative and obligatory web takedown and relocation are influenced by kleptoparasitism, then web takedown may play a role in explaining the absence of kleptoparasites from the majority of spider webs.
7.4 Geographic and environmental influences
The distribution and abundance of kleptoparasites across different biogeographic regions remain underexplored. Comparative studies examining kleptoparasitism in varying climates, habitats, and ecological contexts, and the biogeography of kleptoparasites among geographical and habitat islands, would enhance understanding of environmental factors shaping these interactions.
7.5 Behavioral plasticity and evolutionary transitions
Some species exhibit facultative kleptoparasitism in specific contexts, suggesting behavioral plasticity that may facilitate evolutionary shifts. Experimental studies investigating the conditions under which facultative kleptoparasitism is expressed could illuminate pathways to obligate kleptoparasitism.
7.6 Community-level interactions in multi-species webs
Spider webs are often interlinked and can form multi-species assemblages. Given that existing studies suggest such web clusters are more than the combination of their parts, further research is needed to explore the dynamics of kleptoparasite assemblies in such transient communities. In particular, in what context do webs of species that are normally deferred become attractive to kleptoparasites? Understanding this context may offer key insight into the host traits that attract and detract kleptoparasites.
7.7 Application of (advanced) tracking technologies
An almost entirely unexplored field of study is that of tracking movement of kleptoparasites among host webs. Simple techniques like paint or dust tagging would allow the movement of individual kleptoparasites to be tracked and could provide novel insights into assembly into host webs, dispersal, and long-term site fidelity of kleptoparasitic spiders. The use of modern tracking tools, such as harmonic tags or miniature radio-frequency identification (micro RFID) tags would be ideal and is certainly feasible to track larger host species. The smallest harmonic tags may also be useful for tracking kleptoparasites and such data would lend themselves well to machine-learning-based behavioral analysis.
7.8 Molecular ‘tracking’ of kleptoparasites
A few studies have started to use modern sequencing methods to understand kinship and local adaptation in kleptoparasitic spiders. Population genomics offers tools that will advance our understanding on dispersal, kinship of individuals within host webs (potentially underlying some cooperative behavior), and host specialization. Such data may be pivotal in gaining complete understanding of species assembly into host webs.
Addressing these knowledge gaps will not only refine our understanding of kleptoparasitism in spiders but also contribute to broader ecological and evolutionary theories on community ecology, assembly rules, resource exploitation, parasitism, and host-parasite dynamics.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: Some unpublished sequence data from collaborative ongoing work was used to inform on the summary phylogeny. Requests to access these datasets should be directed to IA,aWFnbmFyc3NvbkBnbWFpbC5jb20=.
Author contributions
IA: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Funding for this work came, in part, from an Erasmus+ grant to the author and a University of Iceland grant RSJ2023-92897.
Acknowledgments
I thank the editors of Frontiers in Arachnid Science, in particular Matjaz Kuntner and Alejandro Cabezas-Cruz, for inviting me to contribute to the Horizons review collection and handling the manuscript, respectively. Many people contributed data and ideas to this paper and I am especially thankful to Robert Jackson, Mark Elgar, William Eberhard, Jonathan Coddington, Yuya Suzuki, Greg Anderson, Jillian Cowles, Matjaz Kuntner, and Matjaz Gregorič. Thanks to Danniella Sherwood, Franklyn Cala Riquelme, Jonathan Coddington, Matjaz Kuntner, Matjaz Gregorič., Elodie Ferreira, Brogan Pett and Snædís Björnsdóttir for comments that improved the manuscript.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. AI was used to extract data from literature, provide paper summaries, and in building bibliography. All text is original by the author and AI information was verified.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frchs.2025.1544428/full#supplementary-material
References
Abhijith A. P. C., Hill D. E., Pai M. J., Baliga V. (2021). Spiders that prey on jumping spiders (Araneae: Salticidae). Peckhamia 250, 1–7.
Agnarsson I. (2002). Sharing a web—On the relation of sociality and kleptoparasitism in theridiid spiders (Theridiidae, Araneae). J. Arachnology 30, 181–188. doi: 10.1636/0161-8202(2002)030[0181:SAWOTR]2.0.CO;2
Agnarsson I. (2003). Spider webs as habitat patches: The distribution of kleptoparasites (Argyrodes, Theridiidae) among host webs (Nephila, Tetragnathidae). J. Arachnology 31, 344–349. doi: 10.1636/s02-21
Agnarsson I. (2004). Morphological phylogeny of cobweb spiders and their relatives (Araneae, Araneoidea, Theridiidae). Zoological J. Linn. Soc. 141, 447–626. doi: 10.1111/j.1096-3642.2004.00120.x
Agnarsson I. (2006). A revision of the New World eximius lineage of Anelosimus (Araneae, Theridiidae) and a phylogenetic analysis using worldwide exemplars. Zoological J. Linn. Soc. 146, 453–593. doi: 10.1111/j.1096-3642.2006.00213.x
Agnarsson I. (2011). Habitat patch size and isolation as predictors of occupancy and number of argyrodine spider kleptoparasites in Nephila webs. Naturwissenschaften 98, 163–167. doi: 10.1007/s00114-010-0750-3
Agnarsson I., Coddington J. A., Kuntner M. (2013). “Systematics – progress in the study of spider diversity and evolution,” in Spider research in the 21st century. Ed. Penney D., 58–111.
Agnarsson I., Ramahefarison F. N., Matthíasdóttir. H. H., Kudari L., Dagsson M. M., Baldursdóttir N. G., et al. (2025). Which web to invade? argyrodine kleptoparasites differentiate among architecturally different host webs. Preprint at EcoEvoRxiv no 8729.
Agnarsson I., Gotelli N. J., Agostini D., Kuntner M. (2016). Limited role of character displacement in the coexistence of congeneric Anelosimus spiders in a Madagascan montane forest. Ecography 39, 743–753. doi: 10.1111/ecog.01930
Alayón García G. (1992). Descripción del macho de Ischnothele longicauda Franganillo (Araneae: Dipluridae). Poeyana 414, 1–7.
Andrade M. C. B. (1996). Sexual selection for male sacrifice in the Australian redback spider. Science 271, 70–72. doi: 10.1126/science.271.5245.70
Archer A. F. (1946). The Theridiidae or comb-footed spiders of Alabama. Museum Papers Alabama Museum Natural History 22, 1–67.
Archer A. F. (1948). A modified classification of spiders based in part on the evolution of the male palpus. J. Alabama Acad. Sci. 20, 3–9.
Avilés L., Maddison W. P., Agnarsson I. (2006). A new independently derived social spider with explosive colony proliferation and a female size dimorphism. Biotropica 38, 743–753. doi: 10.1111/j.1744-7429.2006.00202.x
Avilés L. (1997). “Causes and consequences of cooperation and permanent-sociality in spiders,” in The evolution of social insects and arachnids. Eds. Choe J. C., Crespi B. J. (Cambridge: Cambridge University Press), 476–498.
Baba Y. G., Miyashita T. (2005). Geographical host change in the kleptoparasitic spider Argyrodes kumadai associated with distribution of two host species. Acta Arachnologica 54, 75–76. doi: 10.2476/asjaa.54.75
Baba Y., Osada Y., Miyashita T. (2012). The effect of host web complexity on prey-stealing success in a kleptoparasitic spider mediated by locomotor ability. Anim. Behav. 84, 1261–1268. doi: 10.1016/j.anbehav.2012.02.019
Baba Y., Walters R. J., Miyashita T. (2007). Host-dependent differences in prey acquisition between populations of a kleptoparasitic spider Argyrodes kumadai (Araneae, Theridiidae). Ecol. Entomology 32, 38–44. doi: 10.1111/j.1365-2311.2006.00837.x
Baba Y., Walters R. J., Miyashita T. (2013). Complex latitudinal variation in the morphology of the kleptoparasitic spider Argyrodes kumadai associated with host use and climatic conditions. Population Ecol. 55, 43–51. doi: 10.1007/s10144-012-0334-5
Baert L. (1990). Mysmenidae (Araneae) from Peru. Bull. l'Institut R. Des. Sci. Naturelles Belgique 60, 5–18.
Baert L. L., Murphy J. A. (1987). Kilifia inquilina: a new mysmenid spider from Kenya (Araneae: Mysmenidae). Bull. Br. Arachnological Soc. 7, 194–196.
Baptista R. (1988). On mysmenopsis (Mysmenidae), a kleptoparasite of pholcidae. Am. Arachnology 38, 4.
Berland L. (1923). Le peuplement en Araignées de la Nouvelle Calédonie. Comptes Rendus l'Académie Des. Sci. 176, 1668–1670.
Berland L. (1924). Araignées de la Nouvelle Calédonie et des îles Loyalty Vol. 3. Eds. Sarazin F., Roux J. (Nova Caledonia: Zoologie), 159–255.
Berry J. W. (1987). Notes on the life history and behavior of the communal spider Cyrtophora moluccensis (Doleschall) (Araneae, Araneidae) in Yap, Caroline Islands. J. Arachnology 15, 309–319.
Bhattacharya G. C. (1936). Observations of some peculiar habits of the spider (Marpissa melanognathus). J. Bombay Natural History Soc. 39, 142–144.
Binford G. J., Rypstra A. L. (1992). Foraging behavior of the communal spider Philoponella republicana (Araneae: Uloboridae). J. Insect Behav. 5, 321–335. doi: 10.1007/bf01049841
Blackledge T. A., Gillespie R. G. (2002). Estimation of capture areas of spider orb webs in relation to asymmetry. J. Arachnology 30, 70–77. doi: 10.1636/0161-8202(2002)030[0070:eocaos]2.0.co;2
Blackledge T. A., Kuntner M., Agnarsson I. (2011). The form and function of spider orb webs: Evolution from silk to ecosystems. Adv. Insect Physiol. 41, 175–262. doi: 10.1016/B978-0-12-415919-8.00004-5
Blanchong J. A., Summerfield M. S., Popson M. A., Jakob E. M. (1995). Chivalry in pholcid spiders revisited. J. Arachnology 23, 165–170.
Blanke R. (1972). Untersuchungen zur Ökophysiologie und Ökethologie von Cyrtophora citricola Forsskål (Araneae, Araneidae) in Andalusien. Forma Functio (Braunschweig) 5, 125–206.
Blanke R. (1974). Der Zusammenhang zwischen Beuteangebot und Reproduktionsrate bei Cyrtophora citricola (Forsskål) (Araneae, Araneidae). Beiträge zur naturkundlichen Forschung Südwestdeutschland 33, 223–228.
Bosmans R., Colombo M. (2015). New species of spiders from Sardinia (Araneae), with ecological notes on Lipocrea epeiroides (O. Pickard-Cambridge 1872) (Araneae: Araneidae). Arachnology 16, 319–332. doi: 10.13156/arac.2015.16.9.319
Bradoo B. L. (1972). Some observations on the ecology of social spider Stegodyphus sarasinorum Karsch (Araneae: Eresidae) from India. Oriental Insects 6, 193–203. doi: 10.1080/00305316.1972.10434070
Bradoo B. L. (1979). Uloborus ferokus sp. nov. (Araneae: Uloboridae), a commensal of Stegodyphus sarasinorum Karsch. Bull. Br. Arachnological Soc. 4, 353–355.
Bradoo B. L. (1983). A new record of commensalism between Argyrodes progiles Tikader (Araneae: Theridiidae) and Stegodyphus sarasinorum Karsch. Curr. Sci. (Bangalore 52, 217–218.
Bradoo B. L. (1985). Some observations on the fecundity of Uloborus ferokus Bradoo (Araneae: Uloboridae). Indian Zoologist 9, 73–75.
Bradoo B. L. (1986). Feeding behavior of a non-poisonous spider Uloborus ferokus (Araneae, Uloboridae). Zoologischer Anzeiger 217, 75–88.
Breed M. D., Cook C., Krasnec M. O. (2012). Cleptobiosis in social insects. Psyche 2012, 484765. doi: 10.1155/2012/484765
Brignoli P. M. (1966). Le società eterotipiche degli Araneidi. Rendiconti dell'Accademia Nazionale dei Lincei XVI, 1–28.
Bristowe W. S. (1930). Notes on the biology of spiders. III. Miscellaneous. Ann. Magazine Natural History 6, 347–353.
Bristowe W. S. (1931). Notes on the biology of spiders. VII. Flies that triumph over spiders. Ann. Magazine Natural History 10, 471–474. doi: 10.1080/00222933108673425
Burgess J. W., Uetz C. W. (1982). “Social spacing strategies in spiders,” in Spider communication. mechanisms and ecological significance. Eds. Witt P. N., Rovner J. S. (Princeton: Princeton Univ. Press), 317–322.
Buskirk R. E. (1975). Aggressive display and orb defense in a colonial spider Metabus gravidus. Anim. Behav. 23, 560–567. doi: 10.1016/0003-3472(75)90133-5
Cangialosi K. R. (1990a). Kleptoparasitism in colonies of the social spider Anelosimus eximius (Araneae: Theridiidae). Acta Zoologica Fennici 190, 51–54.
Cangialosi K. R. (1990b). Social spider defense against kleptoparasitism. Behav. Ecol. Sociobiology 27, 49–54. doi: 10.1007/bf00183313
Cangialosi K. R. (1990c). Life cycle and behavior of the kleptoparasitic spider Argyrodes ululans (Araneae: Theridiidae). J. Arachnology 18, 347–358.
Cangialosi K. R. (1991). Attack strategies of a spider kleptoparasite: Effects of prey availability and host colony size. Anim. Behav. 41, 639–647. doi: 10.1016/S0003-3472(05)80902-9
Cangialosi K. R. (1997). Foraging versatility and the influence of host availability in Argyrodes trigonum (Araneae, Theridiidae). J. Arachnology 25, 182–193.
Cerveira A. M., Jackson R. R. (2005). Specialised predation by Palpimanus sp. (Araneae: Palpimanidae) on jumping spiders (Araneae: Salticidae). J. East Afr. Natural History 94, 303–317. doi: 10.2982/0012-8317(2005)94[303:SPBPSA]2.0.CO;2
Champion de Crespigny F. E., Herberstein M. E., Elgar M. A. (2001). Food caching in orb-web spiders (Araneae: Araneoidea). Naturwissenschaften 88, 42–45. doi: 10.1007/s001140000194
Chida T., Tanikawa A. (1999). A new species of the spider genus Argyrodes (Araneae: Theridiidae) from Japan previously misidentified with A. fissifrons. Acta Arachnologica 48, 31–36. doi: 10.2476/asjaa.48.31
Chinta S. P., Goller S., Uhl G., Schulz S. (2016). Identification and synthesis of branched wax-type esters, novel surface lipids from the spider argyrodes elevatus (Araneae: theridiidae). ChemBiodivers 13, 1202–1220. doi: 10.1002/cbdv.201600020
Christenson T. E. (1984). Behaviour of colonial and solitary spiders of the theridiid species Anelosimus eximius. Anim. Behav. 32, 725–734. doi: 10.1016/S0003-3472(84)80148-7
Christenson T. E., Goist K. C. (1979). Costs and benefits of male-male competition in the orb weaving spider, Nephila clavipes. Behav. Ecol. Sociobiology 5, 87–92. doi: 10.1007/BF00302697
Chua T. J. L., Lim M. L. M. (2012). Cross-habitat predation in Nepenthes gracilis: The red crab spider Misumenops nepenthicola influences abundance of pitcher dipteran larvae. J. Trop. Ecol. 28, 97–104. doi: 10.1017/S0266467411000629
Clark R. J., Jackson R. R. (1994). Self recognition in a jumping spider: Portia labiata females discriminate between their own draglines and those of conspecifics. Ethology Ecol. Evol. 6, 371–375. doi: 10.1080/08927014.1994.9522987
Cobbold S. M., Su Y.-C. (2010). The host becomes dinner: Possible use of Cyclosa as a nuptial gift by Argyrodes in a colonial web. J. Arachnology 38, 132–134. doi: 10.1636/HI09-42.1
Coyle F. A. (1995). A revision of the funnelweb mygalomorph spider subfamily Ischnothelinae (Araneae, Dipluridae). Bull. Am. Museum Natural History 226), 3–133.
Coyle F. A., Meigs T. E. (1989). Two new species of kleptoparasitic Mysmenopsis (Araneae, Mysmenidae) from Jamaica. J. Arachnology 17, 59–70.
Coyle F. A., O'Shields T. C., Perlmutter D. G. (1991). Observations on the behavior of the kleptoparasitic spider Mysmenopsis furtiva. J. Arachnology 19, 62–66.
Crespi B., Abbot P. (1999). The behavioral ecology and evolution of kleptoparasitism in Australian gall thrips. Florida Entomologist 82, 147–164. doi: 10.2307/3496568
Crespo L. C., Cardoso P., Henriques S., Gaspar C. (2009). Spiders (Araneae) from Porto Santo (Madeira, Portugal): additions to the current knowledge. Boletín la Sociedad Entomologica Aragonesa 45, 471–475.
Cushing P. E. (2012). Spider-ant associations: An updated review of myrmecomorphy, myrmecophily, and myrmecophagy in spiders. Psyche: A J. Entomology 2012, 1–23. doi: 10.1155/2012/151989
Davis R. M., Russell M. P. (1969). Commensalism between Ranzovius moerens (Reuter) (Hemiptera: Miridae) and Hololena curta (McCook) (Araneida: Agelenidae). Psyche 76, 262–269.
Deyrup M., Kraus J. M., Eisner T. (2004). A Florida caterpillar and other arthropods inhabiting the webs of a subsocial spider (Lepidoptera: Pyralidae; Araneida: Theridiidae). Florida Entomologist 87, 554–558. doi: 10.1653/0015-4040(2004)087[0554:afcaoa]2.0.co;2
Dippenaar-Schoeman A. S., Haddad C. R., Foord S. H., Lotz L. N. (2021a). South African National Survey of Arachnida Photo Identification Guide: The Mysmenidae of South Africa (Version 1: 1–6). doi: 10.5281/zenodo.6802505 (Zenodo: online)
Dippenaar-Schoeman A. S., Haddad C. R., Foord S. H., Lotz L. N. (2021b). South African National Survey of Arachnida Photo Identification Guide: The Theridiidae of South Africa. Part 1 (A-P): 1–69 Part 2 (R–Z) version 1: 1–23. doi: 10.5281/zenodo.7515997 (Zenodo: online)
Dönitz (1887). Über die Copulation von Spinnen. Sitzungsberichte der Gesellschaft naturforschender Freunde zu Berlin 1887, 49–51.
Downes M. F. (1994). Arthropod nest associates of the social spider Phryganoporus candidus (Araneae: Desidae). Bull. Br. arachnol. Soc. 9, 249–255.
Downes M. F. (1995). Australian social spiders: what is meant by ‘social’ records of the western australian museum supplement, Vol. 52. 25–32.
Drisya-Mohan O. M., Kavyamol P., Sudhikumar A. V. (2019). Effect of kleptoparasitic ants on the foraging behavior of a social spider (Stegodyphus sarasinorum Karsch 1891). Zoological Stud. 58, 3. doi: 10.6620/ZS.2019.58-03
Dupérré N., Tapia E. (2015). Descriptions of four kleptoparasitic spiders of the genus Mysmenopsis (Araneae, Mysmenidae) and their potential host spider species in the genus Linothele (Araneae, Dipluridae) from Ecuador. Zootaxa 3972, 343–368. doi: 10.11646/zootaxa.3972.3.3
Dupérré N., Tapia E. (2020). Megadiverse Ecuador: A review of Mysmenopsis (Araneae, Mysmenidae) of Ecuador, with the description of twenty-one new kleptoparasitic spider species. Zootaxa 4761, 1. doi: 10.11646/zootaxa.4761.1.1
Eberhard W. G. (1979). Argyrodes attenuatus (Theridiidae): a web that is not a snare. Psyche: A Journal of Entomology 86, 407–413. doi: 10.1155/1979/31273
Eberhard W. G. (2020). Spider webs: behavior, function, and evolution (Chicago: University of Chicago Press). doi: 10.7208/chicago/9780226534749.001.0001
Eberhard W. G., Barreto M., Pfizenmaier W. (1978). Web robbery by mature male orb-weaving spiders. Bull. Br. arachnol. Soc. 4, 228–230. doi: 10.1007/BF00345159
Eberhard W. G., Briceño R. D. (1983). Chivalry in pholcid spiders. Behav. Ecol. Sociobiology 13, 189–195. doi: 10.1007/BF00299922
Eberhard W. G., Briceño R. D. (1985). Behavior and ecology of four species of Modisimus and Blechroscelis (Araneae, Pholcidae). Rev. Arachnologique 6, 29–36.
Eberhard W. G., Platnick N. I., Schuh R. T. (1993). Natural history and systematics of arthropod symbionts (Araneae, Hemiptera, Diptera) inhabiting webs of the spider Tengella radiata (Araneae: Tengellidae). Am. Museum Novitates No. 3065, 1–17.
Eichenberger B., Siegenthaler E., Schmidt-Entling M. H. (2009). Body size determines the outcome of competition for webs among alien and native sheetweb spiders (Araneae: Linyphiidae). Ecol. Entomology 34, 363–368. doi: 10.1111/j.1365-2311.2008.01085.x
Elgar M. A. (1989). Kleptoparasitism: a cost of aggregating for an orb-weaving spider. Anim. Behav. 37, 1052–1055. doi: 10.1016/0003-3472(89)90152-8
Elgar M. A. (1993). Inter-specific associations involving spiders: kleptoparasitism, mimicry and mutualism. Memoirs Queensland Museum 33, 411–430.
Elgar M. A. (1994). Experimental evidence of a mutualistic association between two web-building spiders. J. Anim. Ecol. 63, 880–886. doi: 10.2307/5265
Elgar M. A., Fahey B. F. (1996). Sexual cannibalism, competition, and size dimorphism in the orb-weaving spider Nephila plumipes Latreille (Araneae: Araneoidea). Behav. Ecol. 7, 195–198. doi: 10.1093/beheco/7.2.195
Elgar M. A., Pope B., Williamson I. (1983). Observations on the spatial distribution and natural history of Cyrtophora hirta (L. Koch) (Araneae: Araneidae) in Queensland, Australia. Bull. Br. Arachnological Soc. 6, 83–87.
Elias N. U., Responte M. A., Wu C.-Y., Chiu Y.-F., Peng P., Liao H., et al. (2024). A shift in the host web occupancy of dew-drop spiders associated with genetic divergence in the Southwest Pacific. J. Biogeography 51, 1049–1063. doi: 10.1111/jbi.14803
Emerton J. H. (1882). New England Spiders of the Family Theridiidae. Trans. Connecticut Acad. Arts Sci. 6, 1–86. doi: 10.5962/bhl.part.7410
Emerton J. H. (1909). Supplement to the New England spiders. Trans. Connecticut Acad. Arts Sci. 14, 171–236.
Emery C. (1909). Über den ursprung der dulotischen, parasitischen und myrmekophilen ameisen. Biologisches Centralblatt 29, 352–362.
Enders F. (1974). Vertical stratification in orb-web spiders (Araneidae-Araneae) and a consideration of other methods of coexistence. Ecology 55, 317–328. doi: 10.2307/1935219
Exline H. (1945a). A new group of the genus Conopistha Karsch 1881 and three new species from Ecuador and Peru. Trans. Connecticut Acad. Arts Sci. 36, 177–189.
Exline H. (1945b). Spiders of the genus Conopistha (Theridiidae, Conopisthinae) from northwestern Peru and Ecuador. Ann. Entomological Soc. America 38, 505–528. doi: 10.1093/aesa/38.4.505
Exline H., Levi H. W. (1962). American spiders of the genus Argyrodes (Araneae: Theridiidae). Bull. Museum Comp. Zoology at Harvard Coll. 127, 75–204.
Fage L. (1928). Part IV. Araneae. In notes on the fauna of pitcher plants. J. Malay Branch Asiatic Soc. 6, 13–19.
Farr J. A. (1976). Social behavior of the golden silk spider, Nephila clavipes (Linnaeus) (Araneae: Araneidae). J. Arachnology 4, 137–144.
Faust L. F., De Cock R., Lewis S. M. (2012). Thieves in the night: Kleptoparasitism by fireflies in the genus Photuris Dejean (Coleoptera: Lampyridae). Coleopterists Bull. 66, 1–6. doi: 10.1649/072.066.0101
Fernandez-Fournier P., Avilés L. (2018). Environmental filtering and dispersal as drivers of metacommunity composition: complex spider webs as habitat patches. Ecosphere 9, e02101. doi: 10.1002/ecs2.2101
Forster R. R., Platnick N. I. (1977). A review of the spider family Symphytognathidae (Arachnida: Araneae). Am. Museum Novitates 2619, 1–29.
Fowler H. G., Venticinque E. M. (1996). Interference competition and scavenging by Crematogaster ants associated with the webs of the social spider Anelosimus eximius. J. Kansas Entomological Soc. 69, 267–269.
Gan W., Liu S., Yang X., Li D., Lei C. (2015). Prey interception drives web invasion and spider size determines successful web takeover in nocturnal orb-web spiders. Biol. Open 4, 1326–1329. doi: 10.1242/bio.012799
Gillespie R. G., Rivera M. A. J. (2007). Free-living spiders of the genus Ariamnes (Araneae: Theridiidae) in Hawaii. J. Arachnology 35, 11–37. doi: 10.1636/H04-05.1
González A., Castro C. D. (1996). Neotropical spiders of the genus Argyrodes Simon (Araneae, Theridiidae). Bull. Br. Arachnol. Soc. 10, 127–137.
González M., Toscano-Gadea C. A. (2021). Can’t even trust the family? The web of the unusual web-building wolf spider Aglaoctenus lagotis (Araneae: Lycosidae) invaded by typical wandering wolf spiders. Arachnology 18, 710–714. doi: 10.13156/arac.2020.18.7.710
Gould J. (2021). Hauling up a hefty meal: Long-jawed spider (Araneae, Tetragnathidae) uses silk lines to transport large prey vertically through the air in the absence of a web. Ethology 127, 438–442. doi: 10.1111/eth.13137
Gray M. R., Anderson G. J. (1989). A new Australian species of Argyrodes Simon (Araneoidea: Theridiidae) which preys on its host. Proc. Linn. Soc. New South Wales 111, 25–30.
Gregorič M., Agnarsson I., Blackledge T. A., Kuntner M. (2011). Darwin's bark spider: Giant prey in giant orb webs (Caerostris darwini, Araneae: Araneidae)? J. Arachnology 39, 287–295. doi: 10.1636/CB10-95.1
Gregorič M., Quiñones-Lebrón S. G., Kuntner M., Agnarsson I. (2024). Exploring resource patch occupancy: Patch size, but not connectivity, explains the abundance of spider kleptoparasites in golden orb webs. J. Zoology 324, 244–252. doi: 10.1111/jzo.13212
Griswold C. E. (1985). Isela okuncana: A new genus and species of kleptoparasitic spider from southern Africa. Ann. Natal Mus. 27, 207–217.
Griswold C. E., Meikle T. (1987). Archaeodictyna ulova, new species: A remarkable kleptoparasite of group-living eresid spiders (Stegodyphus spp.). Am. Museum Novitates 2897, 1–11.
Grostal P. (1999). Five species of kleptobiotic Argyrodes Simon (Theridiidae: Araneae) from eastern Australia: descriptions and ecology with special reference to southeastern Queensland. Memoirs of the Queensland Museum 43, 621–638.
Grostal P., Walter D. E. (1997). Kleptoparasites or commensals? Effects of Argyrodes antipodianus (Araneae: Theridiidae) on Nephila plumipes (Araneae: Tetragnathidae). Oecologia 111, 570–574. doi: 10.1007/s004420050273
Grostal P., Walter D. E. (1999). Host specificity and distribution of the kleptobiotic spider Argyrodes antipodianus (Araneae: Theridiidae) on orb webs in Queensland, Australia. J. Arachnology 27, 522–530.
Guarisco H. (1999). Distributional status and natural history observations of the genus Argyrodes (Araneae: Theridiidae) in Kansas. Trans. Kansas Acad. Sci. 102, 138–141. doi: 10.2307/3627875
Guarisco H. (2000). Three cobweb spider genera (Anelosimus, Tidarren, and Thymoites) and Argyrodes fictilium recently discovered in Kansas. J. Kansas Ent. Soc. 73, 155–163.
Hall D. W. (2019). Orchard Orbweaver, Orchard Spider Leucauge argyrobapta (White), Leucauge venusta (Walckenaer) (Arachnida: Araneae: Tetragnathidae): EENY728/IN1243, 3/2019. Featured Creatures Collection. doi: 10.32473/edis-in1243-2019
Hanski I. A. (1997). Metapopulation biology: Ecology, genetics, and evolution (San Diego: Academic Press), 512 pages.
Hénaut Y. (2000). Host selection by a kleptoparasitic spider. J. Natural History 34, 747–753. doi: 10.1080/002229300299390
Hénaut Y., Delme J., Williams T. (2005). Host selection by a kleptobiotic spider. Naturwissenschaften 92, 95–99. doi: 10.1007/s00114-004-0597-6
Hénaut Y., Machkour-M’Rabet S. (2010). Interspecific aggregation around the web of the orb spider Nephila clavipes: consequences for the web architecture of Leucauge venusta. Ethology Ecol. Evol. 22, 203–209. doi: 10.1080/03949371003708016
Hénaut Y., Machkour-M’Rabet S., Winterton P., Calmé S. (2010). Insect attraction by webs of Nephila clavipes (Araneae: Nephilidae). J. Arachnology 38, 135–138. doi: 10.1636/t08-72.1
Henry T. J. (1984). Revision of the spider-commensal plant bug genus Ranzovius Distant (Heteroptera: Miridae). Proc. Entomological Soc. Washington 86, 53–67.
Henschel J. R. (1998). Predation on social and solitary individuals of the spider Stegodyphus dumicola (Araneae, Eresidae). J. Arachnology 26, 61–69.
Henschel J. R., Lubin Y. D. (1992). Environmentalfactors affecting the web and activity of a psam-mophilous spider in the namib desert. J. Arid Environments 22, 173–189. doi: 10.1016/s0140-1963(18)30590-1
Hentz N. M. (1850). Descriptions and figures of the araneides of the United States. Boston J. Natural History 6, 18–35.
Hieber C. S., Uetz G. W. (1990). Colony size and parasitoid load in two species of colonial Metepeira spiders from Mexico (Araneae: Araneidae). Oecologia 82, 145–150. doi: 10.1007/bf00323527
Higgins L. E., Buskirk R. E. (1998). Spider-web kleptoparasites as a model for studying producer-consumer interactions. Behav. Ecol. 9, 384–387. doi: 10.1093/beheco/9.4.384
Hiramatsu T. (2012). Kleptoparasitic behavior of Rhomphaea sp. in a web of Nephila clavata. Kishidaia 101, 48–49.
Hiramatsu T. (2019). Predation of Argyrodes kumadai by Chorizopes nipponicus in the web of Nephila clavata. 114, 56–57.
Hölldobler B., Wilson E. O. (1998). Journey to the ants a story of scientific exploration (Harvard University Press).
Hormiga G. (2002). Orsonwelles: A new genus of giant linyphiid spiders (Araneae) from the Hawaiian Islands. Invertebrate Systematics 16, 369–448. doi: 10.1071/it01026
Houser J. D., Ginsberg H. S., Jakob E. M. (2014). Competition between introduced and native spiders (Araneae: Linyphiidae). Biol. Invasions 16, 2479–2488. doi: 10.1007/s10530-014-0679-0
Houser J. D., Jennings D. T., Jakob E. M. (2005). Predation by Argyrodes trigonum on Linyphia triangularis, an invasive sheet-web weaver in coastal Maine. J. Arachnology 33, 193–195. doi: 10.1636/s03-15
Iyengar E. V. (2008). Kleptoparasitic interactions throughout the animal kingdom and a re-evaluation, based on participant mobility, of the conditions promoting the evolution of kleptoparasitism. Biol. J. Linn. Soc. 93, 745–762. doi: 10.1111/j.1095-8312.2008.00954.x
Jackson R. R. (1985). The biology of Simaetha paetula and Simaetha thoracica web-building jumping spiders (Araneae: Salticidae) from Queensland, Australia: cohabitation with social spiders, utilization of silk, predatory behavior, and intraspecific interactions. J. Zoological Ser. B 1, 175–210. doi: 10.1111/j.1469-7998.1985.tb00071.x
Jackson R. R. (1986). Communal jumping spiders (Araneae: Salticidae) from Kenya: Interspecific nest complexes, cohabitation with web-building spiders, and intraspecific interactions. New Z. J. Zoology 13, 13–26. doi: 10.1080/03014223.1986.10422643
Jackson R. R. (1987). The biology of Olios spp., huntsman spiders (Araneae, Sparassidae) from Queensland and Sri Lanka: predatory behaviour and cohabitation with social spiders. Bull. Br. Arachnological Soc. 7, 133–136.
Jackson R. R., Blest A. D. (1982). The biology of Portia fimbriata, a web-building jumping spider (Araneae, Salticidae) from Queensland: Utilization of webs and predatory versatility. J. Zoology 196, 255–293. doi: 10.1111/j.1469-7998.1982.tb03504.x
Jackson R. R., Hallas S. E. A. (1986). Comparative biology of Portia africana, P. albimana, P. fimbriata, P. labiata, and P. shultzi: Utilisation of webs, predatory versatility, and intraspecific interactions. New Z. J. Zoology 13, 423–489. doi: 10.1080/03014223.1986.10422978
Jackson R. R., Pollard S. D. (2008). Observations of Portia africana, an araneophagic jumping spider, living together and sharing prey. New Z. J. Zoology 35, 237–242. doi: 10.1080/03014220809510119
Jackson R. R., Whitehouse M. E. A. (1986). The biology of New Zealand and Queensland pirate spiders (Araneae, Mimetidae): aggressive mimicry, araneophagy and prey specialization. J. Zoology 210, 279–303. doi: 10.1111/j.1469-7998.1986.tb03635.x
Jackson R. R., Wilcox R. S. (1990). Aggressive mimicry, prey-specific predatory behavior, and predator recognition in the predator-prey interactions of Portia fimbriata and Euryattus sp., jumping spiders from Queensland. Behav. Ecol. Sociobiology 26, 111–119. doi: 10.1007/bf00171580
Jäger P., Praxaysombath B. (2011). Spiders from Laos with forty-three new records and first results from the provinces Bolikhamsay and Champasak (Arachnida: Araneae). Acta Arachnologica 60, 9–31. doi: 10.2476/asjaa.60.9
Jani M. B., Caleb J. T. D., Kapoor V., Kulkarni S., Uma D. (2023). Aliens in the society: Foreign arthropods and small vertebrates associated with the social spider Stegodyphus sarasinorum Karsch 1892 (Araneae: Eresidae). J. Arachnology 51, 57–62. doi: 10.1636/joa-s-22-004
Javed S. M. M., Srinivasulu C., Tampal F. (2010). Addition to araneofauna of Andhra Pradesh, India: occurrence of three species of Argyrodes Simon 1864 (Araneae: Theridiidae). J. Threatened Taxa 2, 980–985. doi: 10.11609/JoTT.o2194.980-5
Jeanne R. L. (1972). Social biology of the neotropical wasp Mischocyttarus drewseni. Bull. Museum Comp. Zoology 144, 63–150.
Justice M. J., Justice T. C., Vesci R. L. (2005). Web orientation, stabilimentum structure, and predatory behavior of Argiope florida Chamberlin & Ivie 1944 (Araneae, Araneidae, Argiopinae). J. Arachnology 33, 82–92. doi: 10.1636/S03-53
Kaston B. J. (1948). Spiders of connecticut. Bull. Connecticut Geological Natural History Survey 70, 1–874.
Kaston B. J. (1965). Some little-known aspects of spider behavior. Am. Midland Nat. 73, 336–356. doi: 10.2307/2423458
Kerr A. M. (2005). Behavior of web-invading spiders Argyrodes argentatus (Theridiidae) in Argiope appensa (Araneidae) host webs in Guam. J. Arachnology 33, 1–6. doi: 10.1636/s02-29
Kerr A. M., Quenga A. S. (2004). Population variation of web-invading spiders (Theridiidae: Argyrodes spp.) on host webs in Guam, Mariana Islands, Micronesia. J. Natural History 38, 671–680. doi: 10.1080/0022293031000064413
Kim S.-T. (2021). Invertebrate Fauna of Korea, Volume 21, Number 47: Spiders V (Arthropoda: Arachnida: Araneae: Theridiidae, Amaurobiidae, Eresidae, Zoropsidae, Anyphaenidae) (Incheon, Republic of Korea: Published by the National Institute of Biological Resources).
Kim B.-W., Kim J.-P. (2007). Taxonomic study of the spider subfamily Argyrodinae (Arachnida: Araneae: Theridiidae) in Korea. Korean J. Sys. Zool. 23, 213–222. doi: 10.5635/kjsz.2007.23.2.213
Kim J. P., Ye S. H., Kim T. W., Kim D. H. (2015). One new record species of the genus Neospintharus Exline 1950 (Araneae: Theridiidae) from Korea. Korean Arachnology 31, 25–28.
Koh T. H., Li D. (2003). State-dependent prey type preferences of a kleptoparasitic spider Argyrodes flavescens (Araneae: Theridiidae). J. Zoology 260, 227–233. doi: 10.1017/S0952836903003674
Krafft B. (1970). Contribution a la biologie et a l'ethologie d'Agelena consociata denis (araignee sociale du gabon) . i. Biologica Gabonica 4, 197–301.
Kulkarni S., Wood H. M., Hormiga G. (2023). Advances in the reconstruction of the spider tree of life: A roadmap for spider systematics and comparative studies. Cladistics 39, 479–532. doi: 10.1111/cla.12557
Kullmann E. (1959a). Beobachtungen und Betrachtungen zum Verhalten der Theridiide Conopistha argyrodes Walckenaer (Araneae). Mitt. aus dem Zoologischen Museum Berlin 35, 275–292. doi: 10.1002/mmnz.19590350204
Kullmann E. (1959b). Über parasitäres Verhalten der Spinne Theridium tepidariorum C. L. Koch. Verhandlungen der Deutschen Zoologischen Gesellschaft Münster 1959, 332–342.
Kullmann E. (1960). Beobachtungen an Theridium tepidariorum C. L. Koch als Mitbewohner von Cyrtophora-Netzen. Deutsche Entomologische Z. Berlin 7, 146–163. doi: 10.1002/mmnd.19600070105
Kumada K. (1986). Theridion adamsoni Berland 1934, a newly recorded species from Japan. Atypus 88, 1–6.
Kumada K. (1990). A new species of the genus Argyrodes from Japan (Araneae, Theridiidae). Acta Arachnologica 39, 1–5. doi: 10.2476/asjaa.39.1
Kuntner M. (2005). Phylogenetic systematics of the Gondwanan nephilid spider lineage Clitaetrinae (Araneae, Nephilidae). Zoologica Scripta 35, 19–62. doi: 10.1111/j.1463-6409.2006.00220.x
Kuntner M. (2007). A monograph of Nephilengys, the pantropical ´hermit spiders´ (Araneae, Nephilidae, Nephilinae). Systematic Entomology 32, 95–135. doi: 10.1111/j.1365-3113.2006.00348.x
Kuntner M., Agnarsson I. (2010). Web gigantism in Darwin's bark spider, a new species from Madagascar (Araneidae: Caerostris). J. Arachnology 38, 346–356. doi: 10.1636/B09-113.1
Kuntner M., Agnarsson I. (2011). Biogeography and diversification of hermit spiders on Indian Ocean islands (Nephilidae: Nephilengys). Mol. Phylogenet. Evol. 59, 477–488. doi: 10.1016/j.ympev.2011.02.002
Kuntner M., Coddington J. A. (2020). Sexual size dimorphism: evolution and perils of extreme phenotypes in spiders. Annu. Rev. Entomology 65, 57–80. doi: 10.1146/annurev-ento-011019-025032
Kuntner M., Hamilton C., Cheng R.-C., Gregorič M., Lupše N., Moriarty Lemmon E., et al. (2019). Golden orbweavers ignore biological rules: Phylogenomic and comparative analyses unravel a complex evolution of sexual size dimorphism. Systematic Biol. 68, 555–572. doi: 10.1093/sysbio/syy082
Lamore D. H. (1957). The spider Conopistha trigona as a commensal of Allepeira lemniscata in Maryland. Proc. Entomological Soc. Washington 59, 79.
Lamore D. H. (1958). The jumping spider Phidippus audax Hentz and the spider Conopistha trigona Hentz as predators of the basilica spider, Allepeira lemniscata Walckenaer, in Maryland. Proc. Entomological Soc. Washington 60, 286.
Larcher S. F., Wise D. H. (1985). Experimental studies of the interactions between a web-invading spider and two host species. J. Arachnology 13, 43–59.
Le Peru B. (2011). The spiders of europe, a synthesis of data: Volume 1 atypidae to theridiidae. Mémoires la Société Linnéenne Lyon 2, 1–522.
Leborgne R., Cantarella T., Pasquet A. (1998). Colonial life versus solitary life in Cyrtophora citricola (Araneae: Araneidae). Insectes Sociaux 45, 125–134. doi: 10.1007/s000400050074
Leborgne R., Lubin Y., Pasquet A. (2011). Kleptoparasites influence foraging behaviour of the spider Stegodyphus lineatus (Araneae: Eresidae). Insectes Sociaux 58, 255–261. doi: 10.1007/s00040-010-0144-8
Legendre R. (1960). Quelques remarques sur le comportement des Argyrodes malgaches. Annales Des. Sci. Naturelles (Zoologie Biologie Animale) 2, 507–512.
Legendre R. (1961). Études sur les Archaea (Aranéides). II. La capture des proies et la prise de nourriture. Bull. la Société Zoologique France 86, 316–319.
Levi H. W. (1967). Cosmopolitan and pantropical species of theridiid spiders (Araneae: Theridiidae). Pacific Insects 9, 175–186.
Levy G. (1985). Spiders of the genera Episinus, Argyrodes, and Coscinida from Israel with additional notes on Theridion (Araneae: Theridiidae). J. Zoology Ser. A 207, 87–123.
Lin Y.-J., Hu C.-H., Pham D.-S., Li S. (2024). Taxonomic notes of theridiid spiders (Araneae: Theridiidae) from China and Vietnam. Zoological Res.: Diversity and Conservation 1, 141–168. doi: 10.24272/j.issn.2097-3772.2024.604
Liu J., May-Collado L. J., Pekár S., Agnarsson I. (2016). A revised and dated phylogeny of cobweb spiders (Araneae, Araneoidea, Theridiidae): A predatory Cretaceous lineage diversifying in the era of the ants (Hymenoptera, Formicidae). Mol. Phylogenet. Evol. 94, 658–675. doi: 10.1016/j.ympev.2015.09.023
Lopez A. (1986). Observations on some spiders of Tenerife (Canary Islands). Br. Arachnological Soc. Newslett. 45, 6–7.
Lopez A. (1988). Les appareils stridulatoires d’Argyrodes dracus Chamb. & Iv. (Theridiidae), d’Holocnemus pluchei (Scop.) (Pholcidae) et autres araignées. Bull. la Société d’Étude Des. Sci. Naturelles Béziers (Nouvelle Série) 12, 21–31.
Lopez A. (1990). Contribution to the study of spiders from Reunion: Introductory note. Bull. la Société Sci. Naturelles 67, 13–22.
Lubin Y. D. (1974). Adaptive advantages and the evolution of colony formation in Cyrtophora (Araneae: Araneidae). Zoological J. Linn. Soc. 54, 321–339. doi: 10.1111/j.1096-3642.1974.tb00806.x
Lubin Y., Ellner S., Kotzman M. (1993). Web relocation and habitat selection in a desert widow spider. Ecology 74, 1915–1928. doi: 10.2307/1940835
Lubin Y. D., Robinson M. H. (1982). Dispersal by swarming in a social spider. Science 216, 319–321. doi: 10.1126/science.216.4543.319
Luczak J., Dabrowska-Prot E. (1970). Preliminary observations on the food of the spider Theridion pictum (Walck.) and its predators. Bull. Br. Arachnological Soc. 1, 109.
MacArthur R. H., Wilson E. O. (1967). The theory of island biogeography (Princeton University Press).
Marples B. J. (1955). Spiders from Western Samoa. J. Linn. Soc. London 42, 453–504. doi: 10.1111/j.1096-3642.1955.tb02217.x
McCrate A., Uetz G. W. (2010). Kleptoparasites: a twofold cost of group living for the colonial spider Metepeira incrassata (Araneae: Araneidae). Behav. Ecol. Sociobiology 64, 389–399. doi: 10.1007/s00265-009-0855-x
Meira F. A., Gonzaga M. O. (2021). Araneophagy as an alternative foraging tactic to kleptoparasitism in two Argyrodinae (Araneae: Theridiidae) species. Behav. Processes 189, 104445. doi: 10.1016/j.beproc.2021.104445
Michálek O., Petráková L., Pekár S. (2017). Capture efficiency and trophic adaptations of a specialist and generalist predator: A comparison. Ecol. Evol. 7, 2756–2766. doi: 10.1002/ece3.2812
Miyashita T. (2001). Competition for a limited space in kleptoparasitic Argyrodes spiders revealed by field experiments. Population Ecology 43, 97–103. doi: 10.1007/PL00012020
Miyashita T. (2002). Population dynamics of two species of kleptoparasitic spiders under different host availabilities. J. Arachnology 30, 31–38. doi: 10.1636/0161-8202(2002)030[0031:PDOTSO]2.0.CO;2
Miyashita T., Maezono Y., Shimazaki A. (2004). Silk feeding as an alternative foraging tactic in a kleptoparasitic spider under seasonally changing environments. J. Zoology 262, 225–229. doi: 10.1017/S0952836903004540
Mock S. N. (2008). Arthropod community associated with the webs of the subsocial spider Anelosimus studiosus. Electronic Theses and Dissertations. 702. Available online at: https://digitalcommons.georgiasouthern.edu/etd/702.
Moran J. A. (1993). Misumenops nepenthicola: The top aquatic predator of the Nepenthes food web? Brunei Museum J. 8, 83–84.
Moura R. R., Oliveira I. D., Vasconcellos-Neto J., Gonzaga M. O. (2021). Where ignorance is bliss,’ tis folly to be wise”: Indiscriminate male care in a neotropical spider. Ethology 127, 223–230. doi: 10.1111/eth.13112
Moura R. R., Vasconcellos-Neto J., Gonzaga M. O. (2017). Extended male care in Manogea porracea (Araneae: Araneidae): The exceptional case of a spider with amphisexual care. Anim. Behav. 123, 1–9. doi: 10.1016/j.anbehav.2016.09.018
Moura R. R., Vasconcellos-Neto J., Gonzaga M. O. (2020). Female sexual maturity as a determining factor of size-assortative pairing in the protandrous spider Manogea porracea (Araneae, Araneidae). Zoologischer Anzeiger 284, 1–6. doi: 10.1016/j.jcz.2019.11.003
Nentwig W., Christenson T. E. (1986). Natural history of the non-solitary sheet-weaving spider Anelosimus jucundus (Araneae: Theridiidae). Zoological J. Linn. Soc. 87, 27–35. doi: 10.1111/j.1096-3642.1986.tb01328.x
Nyffeler M., Benz G. (1980). Kleptoparasitismus von juvenilen Kreuzspinnen und Skorpionsfliegen in den Netzen adulter Spinnen. Rev. Suisse de Zoologie 87, 907–918. doi: 10.3929/ETHZ-A-005779248
Opell B. D. (1979). Revision of the genera and tropical American species of the spider family Uloboridae. Bull. Museum Comp. Zoology 148, 443–549.
Osses F., Martins E. G., Romero G. Q. (2007). Association of the stilt bug Jalysus ossesae Henry (Hemiptera: Heteroptera: Berytidae) with myrmecophytic plants of the genus Maieta (Melastomataceae) in an upland forest area in central Amazon, Brazil. Proc. Entomological Soc. Washington 109, 331–337.
Ovatt Mohanan D. M., Neisseril A. K., Ambalaparambil V. S. (2019). Is cooperation in prey capture flexible in the Indian social spider Stegodyphus sarasinorum. Arachnologische Mitt. / Arachnology Lett. 58, 97–102. doi: 10.30963/aramit5813
Pasquet A., Leborgne R., Cantarella T. (1997). Opportunistic egg feeding in the kleptoparasitic spider Argyrodes gibbosus. Ethology 103, 160–170. doi: 10.1111/j.1439-0310.1997.tb00015.x
Patel B. H., Bradoo B. L. (1981). The cocoon spinning behaviour and maternal care in Uloborus ferokus Bradoo (Araneae: Uloboridae). Zool. Anz. Leipzig 207, 78–87.
Pekár S., Sobotník J., Lubin Y. (2011). Armoured spiderman: morphological and behavioural adaptations of a specialised araneophagous predator (Araneae: Palpimanidae). Naturwissenschaften 98, 593–603. doi: 10.1007/s00114-011-0804-1
Peng P., Blamires S. J., Agnarsson I., Lin H.-C., Tso I.-M. (2013). A color-mediated mutualism between two arthropod predators. Curr. Biol. 23, 172–176. doi: 10.1016/j.cub.2012.11.057
Peng P., Stuart-Fox D., Su Y.-C., Baba Y. G., Elgar M. A. (2024). Fitness effects of symbiotic relationships between arthropod predators: Synergy in a three-way spider symbiosis. Funct. Ecol. 38, 1590–1599. doi: 10.1111/1365-2435.14575
Perkins T. A., Riechert S. E., Jones T. S., Jones T. C. (2007). Interactions between the social spider Anelosimus studiosus (Araneae, Theridiidae) and foreign spiders that frequent its nests. J. Arachnology 35, 143–152. doi: 10.1636/t06-43.1
Petrunkevitch A. (1930). The spiders of Porto Rico. Part two. Trans. Conn. Acad. Arts Sci. 30, 159–356.
Pickard-Cambridge O. (1872). General list of the spiders of Palestine and Syria, with descriptions of numerous new species, and characters of two new genera. Proc. Zoological Soc. London 1872, 212–354.
Platnick N. I., Forster R. R. (1989). A revision of the temperate South American and Australasian spiders of the family Anapidae (Araneae, Araneoidea). Bull. Am. Mus. Nat. Hist. 190, 1–139.
Platnick N. I., Shadab M. U. (1978). A review of the spider genus Mysmenopsis (Araneae: Mysmenidae). Am. Museum Novitates No. 2661, 1–29.
Platnick N. I., Shadab M. U. (1979). A review of the spider genera Anapisona and Pseudanapis (Araneae, Anapidae). Am. Mus. Novit. 2672, 1–20.
Pocock R. I. (1898). The Arachnida from the Province of Natal, South Africa, contained in the collection of the British Museum. Ann. Mag. Nat. Hist. 7) 2, 197–226. doi: 10.1080/00222939808678036
Prenter J., Elwood R., Montgomery I. (1994). Male exploitation of female predatory behaviour reduces sexual cannibalism in male autumn spiders, Metellina segmentata. Anim. Behav. 47, 235–236. doi: 10.1006/anbe.1994.1031
Proctor H. C. (1992). Cohabitation of six species of spiders in webs of Cyrtophora moluccensis (Araneae: Araneidae) in Moorea, French Polynesia. J. Arachnology 20, 144–145.
Quero A., Gonzaga M. O., Vasconcellos-Neto J., Moura R. R. (2023). Offspring mortality factors and parental care efficiency of the spider Manogea porracea (Araneidae) in the Brazilian savanna. Ethology Ecol. Evol. 35, 551–567. doi: 10.1080/03949370.2022.2152197
Ramírez M. J., Platnick N. I. (1999). On Sofanapis antillanca (Araneae, Anapidae) as a kleptoparasite of austrochiline spiders (Araneae, Austrochilidae). J. Arachnol. 27, 547–549.
Reimoser E. (1931). Echte Spinnen der deutschen limnologischen Sunda-Expedition. Arch. Hydrobiol. 8, 759–770.
Responte M., Chiu Y.-F., Peng P., Brown R. M., Yu M.-L., Dai C.-Y., et al. (2021). Northward geographic diversification of a kleptoparasitic spider Argyrodes lanyuensis (Araneae: Theridiidae) from the Philippine Archipelago to Orchid Island. Ecol. Evol. 11, 11241–11266. doi: 10.1002/ece3.7910
Richards O. W. (1953). A communication on commensalism of Desmometopa (Diptera: Milichiidae) with predacious insects and spiders. Proc. R. Entomological Soc. London C 18, 55–56.
Riechert S. E., Gillespie R. G. (1986). “Habitat choice and utilization in web building spiders,” in Spiders: Webs, Behavior, and Evolution. Ed. Shear W. A. (Stanford University Press, Stanford, CA), 23–48.
Rivera M. A. J., Gillespie R. G. (2010). New species of endemic kleptoparasitic spiders of the genus Argyrodes (Araneae: Theridiidae) in the Hawaiian Islands. Pacific Sci. 64, 221–231. doi: 10.2984/64.2.221
Roberts N. L. (1952). “A contrast in snares,” in Australian Spiders., vol. 138–141. Ed. McKeown K. C. (Angus & Robertson, London), 287 pp.
Roberts M. J. (1978). Contributions à l'étude de la faune terrestre des îles granitiques de l'archipel des Séchelles. (Mission P. L. G. Benoit - J. J. van Mol 1972). Theridiidae, Mysmenidae and gen. Theridiosoma (Araneidae) (Araneae). Rev. Zool. Afr. Belgium 92, 663–674.
Robinson M. H. (1977). Symbioses between insects and spiders: An association between lepidopteran larvae and the social spider Anelosimus eximius (Araneae: Theridiidae). Psyche: A Journal of Entomology 84, 225–232. doi: 10.1155/1977/87867
Robinson M. H., Olazarri J. (1971). Units of behavior and complex sequences in the predatory behavior of Argiope argentata (Fabricius): (Araneae: Araneidae). In Smithsonian Contributions to Zoology. Washington: Smithsonian Institution Press. 65. doi: 10.5479/si.00810282.65
Robinson M. H., Robinson B. C. (1976). Ecology and behavior of Nephila maculata: a supplement. Smithson. Contrib. Zool. 218, 1–22. doi: 10.5479/si.00810282.218
Robinson M. H., Robinson M. E., Robinson B. (1973). Ecology and behavior of the giant wood spider Nephila maculata (Fabricius) in New Guinea. Smithsonian Contributions to Zoology 149, 1–73. doi: 10.5479/si.00810282.149
Rovner J. S. (1968). Territoriality in the sheet web spider Linyphia triangularis (Clerck) (Araneae, Linyphiidae). Z. Tierpsychol. 25, 232–242. doi: 10.1111/j.1439-0310.1968.tb00015.x
Rypstra A. (1979). Foraging flocks of spiders: A study of aggregate behavior in Cyrtophora citricola (Forskal) (Araneae: Araneidae) in West Africa. Behavioral Ecology and Sociobiology. 5, 291–300. doi: 10.1007/BF00293677
Rypstra A. L. (1981). The effect of kleptoparasitism on prey consumption and web relocation in a Peruvian population of the spider Nephila clavipes. Oikos 37, 179–182. doi: 10.2307/3544463
Rypstra A. L. (1989). Foraging success of solitary and aggregated spiders: insights into flock formation. Anim. Behav. 37, 274–281. doi: 10.1016/0003-3472(89)90116-4
Rypstra A. L., Binford G. J. (1995). Philoponella republicana (Araneae: Uloboridae) as a commensal in the webs of other spiders. J. Arachnology 23, 1–8.
Saaristo M. I. (1978). Spiders (Arachnida, Araneae) from Seychelles Islands, with notes on taxonomy. Ann. Zool. Fennici 15, 99–126.
Saaristo M. I. (2000). Kleptoparasitic theridiid spiders of the granitic Seychelles (Araneae, Theridiidae). Phelsuma 8, 49–89.
Saaristo M. I. (2010). “Araneae,” in Arachnida and Myriapoda of the Seychelles Islands. Eds. Gerlach J., Marusik Y. M. (Siri Scientific Press, Manchester), 8–306.
Schmidt G. E. W. (1980). “Zur Spinnenfauna von Lanzarote und Graciosa,” in Verhandlungen 8. Internationaler Arachnologen-Kongress abgehalten an der Universität für Bodenkultur Wien, 7–12 Jul. Ed. Gruber J. (H. Egermann, Vienna), 421–423.
Schmidt G. (1999). Was führt bei Nephila inaurata Madagascariensis (Vinson 1863) (Araneae: Araneidae: Nephilinae) zur Koloniebildung? Arachnol. Mag. 7, 38720.
Schmidt G. (2005). Das Weibchen von Grammostola aureostriata Schmidt & Bullmer 2001 (Araneae: Theraphosidae: Theraphosinae). Tarantulas World 106, 3–6.
Schmidt G., Geisthardt M., Piepho F. (1994). Zur Kenntnis der Spinnenfauna der Kapverdischen Inseln (Arachnida: Araneida). Mitt. Des. Internationalen Entomologischen Vereins 19, 3–4.
Schneider J. M. (1997). Timing of maturation and the mating system of the spider, Stegodyphus lineatus (Eresidae): how important is body size? Biol. J. Linn. Soc. 60, 517–525.
Schneider J. M., Lubin Y. (1996). Infanticidal male eresid spiders. Nature 381, 655–656. doi: 10.1038/381655a0
Schneider J. M., Lubin Y. (1997). Infanticide by males in a spider with suicidal maternal care, Stegodyphus lineatus (Eresidae). Anim. Behav. 54, 305–312. doi: 10.1006/anbe.1996.0454
Scott C. E., McCann S. (2023). They mostly come at night: Predation on sleeping insects by introduced candy-striped spiders in North America. Ecology 104, e4025. doi: 10.1002/ecy.4025
Sekhar R., Jose S. K. (2018). First record of Rhomphaea labiata (Zhu & Song 1991) from India (Araneae: Theridiidae). J. Entomology Zoology Stud. 6, 2774–2776.
Shinkai A. (1988). A note on the web silk theft by Argyrodes cylindratus Thorell (Araneae: Theridiidae). Acta Arachnologica 36, 115–119. doi: 10.2476/asjaa.36.115
Shinkai A. (2000). Argyrodes flavescens caught by Rhomphaea sp. in a web of Nephila maculata. Kishidaia 78, 37.
Sibi K. K., Gigi P., Sudhikumar A. V. (2022). First report of the kleptoparasitic spider Argyrodes miniaceus (Doleschall 1857) (Araneae: Theridiidae) from India. Serket 19, 90–94.
Sierwald P., Fenzl T. (1999). Argyrodes in webs of the Floridian red widow spider (Araneae: Theridiidae). Florida Entomologist 82, 359–361. doi: 10.2307/3496591
Silveira M. C., Japyassú H. F. (2012). Notes on the behavior of the kleptoparasitic spider Argyrodes elevatus. Revista de Etologia 11, 56–67.
Sivinski J., Marshall S. D., Petersson E. (1999). Kleptoparasitism and phoresy in the diptera. Florida Entomologist 82, 179–197. doi: 10.2307/3496570
Sivinski J., Stowe M. K. (1980). A kleptoparasitic cecidomyiid and other flies associated with spiders. Psyche 87, 337–348. doi: 10.1155/1980/27685
Smith Trail D. R. (1980). Predation by Argyrodes (Theridiidae) on solitary and communal spiders. Psyche (Cambridge) 87, 349–355. doi: 10.1155/1980/74071
Spear D. M., Foster W. A., Advento A. D., Naim M., Caliman J. P., Luke S. H., et al. (2018). Simplifying understory complexity in oil palm plantations is associated with a reduction in the density of a kleptoparasitic spider, Argyrodes miniaceus (Araneae: Theridiidae), in host (Araneae: Nephilinae) webs. Ecol. Evol. 8, 1595–1603. doi: 10.1002/ece3.3772
Srinivasulu C., Srinivasulu B., Javed S. M. M., Tampal F., Subramaniyam M., Nagulu V., et al. (2013). Additions to the araneofauna of Andhra Pradesh, India - Part II. Records of interesting species of the comb-footed genera Latrodectus, Rhomphaea, and Coleosoma (Araneae: Theridiidae). J. Threatened Taxa 5, 4483–4491. doi: 10.11609/JoTT.o2660.4483-91
Straus S., Avilés L. (2018). Effects of host colony size and hygiene behaviours on social spider kleptoparasite loads along an elevation gradient. Funct. Ecol. 32, 2707–2716. doi: 10.1111/1365-2435.13225
Straus S., Avilés L. (2023). Sublethal effects of kleptoparasitism on experimental social spider colonies. Ethology 129, 649–654. doi: 10.1111/eth.13401
Striffler B. F., Rembold K. (2009). “Henriksenia labuanica nom. nov., a replacement name for Misumenops nepenthicola Bristowe, (1930) and the clarification of the current taxonomic status of Misumenops nepenthicola Fage, (1928) (Arachnida, Araneae, Thomisidae),” in Pitcher Plants of the Old World. Ed. McPherson S. (Redfern Natural History Publications, Dorset), 1334–1338.
Struhsaker T. T. (1969). Notes on the spiders Uloborus mundior (Chamberlin and Ivie) and Nephila clavipes (Linnaeus) in Panama. Am. Midland Nat. 82, 611–613. doi: 10.2307/2423802
Su Y.-C. (2012). A gang of thieves: Evolution of cooperative kleptoparasitism in the subfamily Argyrodinae (Araneae: Theridiidae). University of Kansas, Lawrence.
Su Y.-C., Peng P., Elgar M. A., Smith D. R. (2018). Dual pathways in social evolution: Population genetic structure of group-living and solitary species of kleptoparasitic spiders (Argyrodinae: Theridiidae). PLoS One 13, e0208123. doi: 10.1371/journal.pone.0208123
Su Y.-C., Smith D. R. (2014). Evolution of host use, group-living, and foraging behaviours in kleptoparasitic spiders: Molecular phylogeny of the Argyrodinae (Araneae: Theridiidae). Invertebrate Systematics 28, 415–431. doi: 10.1071/is14010
Su Y.-C., Wu C.-Y., Hung J.-Y., Yang C.-H., Li B., Moi S.-H., et al. (2021). Machine learning data imputation and prediction of foraging group size in a kleptoparasitic spider. Mathematics 9, 415. doi: 10.3390/math9040415
Suzuki Y., Ikemoto M., Yokoi T. (2022). The ontogenetic dietary shift from non-dangerous to dangerous prey in predator-eating predators under capture risk. Ecol. Evol. 12, ece3–9609. doi: 10.1002/ece3.9609
Taczanowski L. (1873). Les Aranéides de la Guyane française. Horae Societatis Entomologicae Rossicae 113-150, 261–286.
Tanaka K. (1984). Rate of predation by a kleptoparasitic spider, Argyrodes fissifrons, upon a large host spider, Agelena limbata. J. Arachnology 12, 363–367.
Tanikawa A. (2017). Uloborus plumipes and Dipoena pelorosa (Araneae: Uloboridae, Theridiidae): two newly recorded spiders in Japan. Acta Arachnologica 66, 5–8. doi: 10.2476/asjaa.66.5
Thorell T. (1887). Viaggio di L. Fea in Birmania e regioni vicine. II. Primo saggio sui Ragni birmani. Annali del Museo Civico di Storia Naturale di Genova, 5–417.
Thornhill R. (1975). Scorpionflies as kleptoparasites of web-building spiders. Nature 258, 709–711. doi: 10.1038/258709b0
Tikader B. K. (1970). Spider fauna of sikkim. Records Zoological Survey India 64, 1–83. doi: 10.26515/rzsi/v64/i1-4/1966/161523
Tikader B. K. (1977). Studies on spider fauna of Andaman and Nicobar Islands, Indian Ocean. Records Zoological Survey India 72, 153–212. doi: 10.26515/rzsi/v72/i1-4/1977/161929
Trager M. D., Bhotika S., Hostetler J. A., Andrade G. V., Rodriguez-Cabal M. A., McKeon C. S., et al. (2010). Benefits for plants in ant-plant protective mutualisms: A meta-analysis. PLoS One 5, e14308. doi: 10.1371/journal.pone.0014308
Tso I.-M., Severinghaus L. L. (1998). Silk stealing by Argyrodes lanyuensis (Araneae: Theridiidae): a unique form of kleptoparasitism. Anim. Behav. 56, 219–225. doi: 10.1006/anbe.1998.0770
Tso I. M., Severinghaus L. L. (2000). Argyrodes fissifrons inhabiting webs of Cyrtophora hosts: Prey size distribution and population characteristics. Zoological Stud. 39, 236–242.
Uetz G. W. (1989). The "ricochet effect" and prey capture in colonial spiders. Oecologia 81, 154–159. doi: 10.1007/bf00379799
Uetz G. W., Boyle J., Hieber C. S., Wilcox R. S. (2002). Anti-predatorbenefits of group living in colonial web-building spiders – the“Early Warning“ effect. Anim. Behav. 63, 445–452. doi: 10.1006/anbe.2001.1918
Vanuytven H., Jocqué R., Deeleman-Reinhold C. (2024). Two new theridiid genera from Southeast Asia (Araneae: Theridiidae, Argyrodinae): males with a nose for courtship. J. Belgian Arachnological Soc. 39, 1–96.
Vinson A. (1863). Aranéides des Iles de la Réunion, Maurice et Madagascar (Paris: Librairie Encyclopédique de Roret). doi: 10.5962/bhl.title.125517
Vollrath F. (1976). Konkurrenzvermeidung bei tropischen kleptoparasitischen Haubennetzspinnen der Gattung Argyrodes (Arachnida, Araneae, Theridiidae). Entomologia Generalis 3, 104–108.
Vollrath F. (1977). Zur Ökologie und Biologie von kleptoparasitischen Argyrodes elevatus und synöken Argyrodes-Arten (Araneae, Theridiidae) (Doctoral Thesis). University of Freiburg.
Vollrath F. (1978). A close relationship between two spiders (Arachnida, Araneidae): Curimagua bayano synecious on a Diplura species. Psyche 85, 347–353. doi: 10.1155/1978/27439
Vollrath F. (1979a). Behaviour of the kleptoparasitic spider Argyrodes elevatus (Araneae: Theridiidae). Anim. Behav. 27, 519–521. doi: 10.1016/0003-3472(79)90186-6
Vollrath F. (1979b). Vibrations: Their signal function for a spider kleptoparasite. Science 205, 1149–1151. doi: 10.1126/science.205.4411.1149
Vollrath F. (1980). Male body size and fitness in the web-building spider Nephila clavipes. Z. für Tierpsychologie - J. Comp. Ethology 53, 61–78. doi: 10.1111/j.1439-0310.1980.tb01052.x
Vollrath F. (1982). Colony foundation in a social spider. Ethology 60, 313–325. doi: 10.1111/j.1439-0310.1982.tb01089.x
Vollrath F. (1984). Kleptobiotic interactions in invertebrates. In: Barnard C. J., ed. Producers and scroungers: strategies of exploitation and parasitism. London: Croom-Helm, 61–66.
Vollrath F. (1987). “Kleptobiosis in spiders,” in Ecophysiology of spiders. Ed. Nentwig W. (Springer-Verlag, Berlin), 274–286. doi: 10.1007/978-3-642-71552-5_20
Vollrath F. (1977). Zur (kologie und biologie yon kleptoparasitischen Argyrodes elevatus und synoken Argyrodes arten. Dissertation Univ. Freiburg..
Watson P. J. (1990). Female-enhanced male competition determines the first mate and principal sire in the spider Linyphia litigiosa (Linyphiidae). Behav. Ecol. Sociobiology 26, 77–90. doi: 10.1007/BF00171577
Wheeler W. M. (1901). The compound and mixed nests of American ants. Am. Nat. 35, 431–448. doi: 10.1086/277964
Wheeler C. C., Coddington J. A., Crowley L. M., Dimitrov D., Goloboff P. A., Griswold C. E., et al. (2017). The spider tree of life: phylogeny of Araneae based on target-gene analyses from an extensive taxon sampling. Cladistics 33, 574–616. doi: 10.1111/cla.12182
Wheeler A. G., McCaffrey J. P. (1984). Ranzovius contubernalis: Seasonal history, habits, and description of fifth instar, with speculation on the origin of spider commensalism in the genus Ranzovius (Hemiptera: Miridae). Proc. Entomol. Soc. Wash. 86, 68–81.
Whitehouse M. E. A. (1986). The foraging behaviours of Argyrodes antipodiana (Theridiidae), a kleptoparasitic spider from New Zealand. New Z. J. Zoology 13, 151–159. doi: 10.1080/03014223.1986.10422658
Whitehouse M. E. A. (1987b). Spider eat spider": the predatory behavior of Rhomphaea sp. from New Zealand. J. Arachnology 15, 355–366.
Whitehouse M. E. A. (1988). Factors influencing specificity and choice of host in Argyrodes antipodiana (Theridiidae: Araneae). J. Arachnology 16, 349–355.
Whitehouse M. E. A. (1991). To mate or fight? Male-male competition and alternative mating strategies in Argyrodes antipodiana (Theridiidae: Araneae). Behav. Processes 23, 163–172. doi: 10.1016/0376-6357(91)90046-3
Whitehouse M. E. A. (1997a). The benefits of stealing from a predator: foraging rates, predation risk, and intraspecific aggression in the kleptoparasitic spider Argyrodes antipodiana. Behav. Ecol. 8, 665–667. doi: 10.1093/beheco/8.6.665
Whitehouse M. E. A. (1997b). Experience influences male–male contests in the spider Argyrodes antipodiana (Theridiidae: Araneae). Anim. Behav. 53, 913–923. doi: 10.1006/anbe.1996.0313
Whitehouse M. (2011). “Kleptoparasitic spiders of the subfamily Argyrodinae: A special case of behavioural plasticity,” in Spider Behaviour: Flexibility and Versatility. Ed. Herberstein M. E. (Cambridge: Cambridge University Press), 348–386. doi: 10.1017/CBO9780511974496.011
Whitehouse M. E. A. (2016). Sex-linked differences in learning to improve foraging techniques in the group-living kleptoparasitic spider Argyrodes antipodianus (Theridiidae). New Z. J. Zoology 43, 96–111. doi: 10.1080/03014223.2015.1127264
Whitehouse M. E. A., Agnarsson I., Miyashita T., Smith D. R., Cangialosi K. R., Masumoto T., et al. (2002). Argyrodes: Phylogeny, sociality, and interspecific interactions: A report on the Argyrodes Symposium, Badplaas 2001. J. Arachnology 30, 238–245. doi: 10.1636/0161-8202(2002)030[0238:APSAII]2.0.CO;2
Whitehouse M. E. A., Jackson R. R. (1993). Group structure and time budgets of Argyrodes antipodiana (Araneae: Theridiidae), a kleptoparasitic spider from New Zealand. New Z. J. Zoology 20, 201–206. doi: 10.1080/03014223.1993.10422860
Whitehouse M. E. A., Jackson R. R. (1994). Intraspecific interactions of Argyrodes antipodiana, a kleptoparasitic spider from New Zealand. New Z. J. Zoology 21, 253–268. doi: 10.1080/03014223.1994.9517993
Whitehouse M. E. A., Jackson R. R. (1998). Predatory behaviour and parental care in Argyrodes flavipes, a social spider from Queensland. J. Zoology 244, 95–105. doi: 10.1111/j.1469-7998.1998.tb00011.x
Whitehouse M.-E. A., Lubin Y. (2005). The functions of societies and the evolution of group living: Spider societies as a test case. Biol. Rev. 80, 347–361. doi: 10.1017/S1464793104006694
Wickler W., Seibt U. (1988). Two species of Stegodyphus spiders as solitary parasites in social S. dumicola colonies (Araneida, Eresidae). Verhandlungen Des. Naturwissenschaftlichen Vereins Hamburg 30, 311–317.
Wiehle H. (1928). Beiträge zur Biologie der Araneen, insbesondere zur Kenntis der Radnetzbaues. Z. für Morphologie und Ökologie der Tiere 11, 115–151. doi: 10.1007/BF02425772
Wise D. H. (1975). Food limitation of the spider Linyphia marginata: Experimental field studies. Ecology 56, 637–646. doi: 10.2307/1935497
Wise D. H. (1981). Inters- and intraspecific effects of density manipulations upon females of two orb-weaving spiders (Araneae, Araneidae). Oecologia 48, 252–256. doi: 10.1007/BF00347972
Wise D. H. (1982). Predation by a commensal spider, Argyrodes trigonum, upon its host: an experimental study. J. Arachnology 10, 111–116.
Wise D. H. (2006). Cannibalism, food limitation, intraspecific competition, and the regulation of spider populations. Annu. Rev. Entomology 51, 441–465. doi: 10.1146/annurev.ento.51.110104.150947
Wood H. M., Griswold C. E., Gillespie R. G. (2012). Phylogenetic placement of pelican spiders (Archaeidae, Araneae), with insight into evolution of the "neck" and predatory behaviours of the superfamily Palpimanoidea. Cladistics 28(6), 598–626. doi: 10.1111/j.1096-0031.2012.00411.x
Yoshida M. (2001a). Kleptoparasitic behaviors of two orb-weaving spiders in webs abandoned by Metleucauge kompirensis (Araneae: Tetragnathidae). Acta Arachnologica 50, 5–10. doi: 10.2476/asjaa.50.5
Yoshida M. (2001b). Seasonal changes in the frequency of intrusion by web spiders into webs abandoned by Metleucauge kompirensis (Araneae: Tetragnathidae). Acta Arachnologica 50, 117–125. doi: 10.2476/asjaa.50.117
Yoshida H., Tso I. M., Severinghaus L.-L. (1998). Description of a new species of the genus Argyrodes (Araneae: Theridiidae) from Orchid Island, Taiwan, with notes on its ecology and behavior. Acta Arachnologica 47, 1–5. doi: 10.2476/asjaa.47.1
Young A. M. (1971). Foraging for insects by a tropical hummingbird. Condor 73, 36–45. doi: 10.2307/1366122
Zhang X., Lan T., Nie L., Li S. (2020). Eight new species of the spider genus Pimoa (Araneae, Pimoidae) from Tibet, China. ZooKeys 940, 79–104. doi: 10.3897/zookeys.940.49793
Zhang H., Zhong R., Yu L., Chen J., Agnarsson I., Liu J. (2022). Safety is increasingly important in cobweb spiders based on life history. Integr. Zoology 18, 736–745. doi: 10.1111/1749-4877.12682
Keywords: Kleptoparasitism, commensal, Theridiidae, spider webs, kleptotany
Citation: Agnarsson I (2025) Spiders as superhosts and secondary kleptoparasites. Front. Arachn. Sci. 4:1544428. doi: 10.3389/frchs.2025.1544428
Received: 12 December 2024; Accepted: 19 February 2025;
Published: 15 April 2025.
Edited by:
Alejandro Cabezas-Cruz, Institut national de recherche pour l’agriculture, l’alimentation et l’environnement (INRAE), FranceReviewed by:
Danniella Sherwood, Arachnology Research Association, United KingdomFranklyn Cala Riquelme, California Academy of Sciences, United States
Copyright © 2025 Agnarsson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ingi Agnarsson, aWFnbmFyc3NvbkBnbWFpbC5jb20=
 Ingi Agnarsson
Ingi Agnarsson