- Department of Entomology, Plant Pathology and Nematology, University of Idaho, Moscow, ID, United States
Systematics provides the foundational knowledge about the units of biodiversity, i.e., species, and how we classify them. The results of this discipline extend across Biology and can have important impacts on conservation. Here we review the systematic and taxonomic practices within Theraphosidae over the last 260 years. We examine the rate of newly described species and investigate the contemporary practices being used in the description of new genera and species. There have been two large waves of theraphosid taxonomy, with an explosive growth of newly described species and author combinations in the last 60 years. We look back and find that during 2010–2024 contemporary practices in theraphosid systematics and taxonomy have remained largely static, being dominated by morphology-based approaches. Over this period, only 10% of newly described species incorporated DNA data or explicitly stated the species concept used. Similarly for genera, only five of the 37 newly described genera over that time were supported as distinct and monophyletic by DNA. We highlight the taxonomic movement of species among Theraphosidae, Barychelidae, and Paratropididae; however, given the limited molecular sampling for the two latter families, the boundaries of these families remain a significant area of needed research. To promote inclusivity, we provide a copy of this paper in Spanish as supplementary material.
1 Introduction
The fields of taxonomy and systematics form the foundation for all research in Biology. Because of this, the implications of accurately understanding classification and defining species boundaries extends beyond these two disciplines (Dayrat, 2005). These interwoven fields share objectives and methodologies, with taxonomy focused on the identification, delimitation, and description of new species and understanding how organisms are hierarchically ranked (i.e., alpha and beta taxonomy), while systematics endeavors to refine our comprehension of relationships between species, as well as higher taxonomic levels. Researchers from other scientific disciplines and policy makers rely on an accurate understanding of species because incorrect identifications can impact the interpretation of research (e.g., evolutionary, ecological, biochemical) (Bortolus, 2008; Bennett and Balick, 2014). Furthermore, the over-splitting or lumping of species can greatly impact conservation if species are overlooked, or conservation approaches are inappropriately applied to species or populations where it is not needed (Ely et al., 2017).
Being able to organize Earth’s biodiversity into unambiguous taxonomic ranks (e.g., species, genera, tribes, families, subfamilies) challenges biologists, even with modern data and techniques. Within the Araneae Clerck, 1757, there are over 52,000 described and valid spider species (World Spider Catalog WSC, Accessed 16 April 2024). The infraorder Mygalomorphae Pocock, 1892 comprises approximately 3000 valid species in 31 families (World Spider Catalog, 2024). These spiders (the tarantulas, trapdoor spiders, and funnel web spiders) pose several challenges to taxonomists because morphological homoplasy and morphological stasis are common (Wilson et al., 2023). These conserved morphologies have often stifled accurate classification and species delimitation, as well as obscuring evolutionary relationships (Opatova et al., 2020) – likely due to distantly related lineages having similar ecologies and niche preferences (Wilson et al., 2023). For example, the former Ctenizidae Thorell, 1887, Dipluridae Simon, 1889, and Nemesiidae Simon, 1889 families were found to be non-monophyletic and constituted multiple independent lineages that were raised to family status once genomic data was investigated (Opatova et al., 2020). Because of these morphological issues, Theraphosidae has been referred to as a “…nomenclature and taxonomic nightmare” (Raven, 1990a).
Of all the spiders, tarantulas (Theraphosidae Thorell, 1869) may be the most well-known to the general public (or at least most recognized) because of their large size, hairy appearance, and charismatic presence in popular culture (Figures 1A–K). This one family comprises over one-third of described mygalomorph diversity with over 1,100 valid species (as of 16 April 2024; World Spider Catalog, 2024). However, this does not include the many specific epithets that are now considered junior synonyms or have been classified as nomina dubia. Interestingly, because of their size and charisma there are likely significant numbers of undescribed species sitting in natural history collections waiting to be “discovered” (Paknia et al., 2015; Hilton et al., 2021). Compared to most mygalomorph families, theraphosid diversity is widespread with a near global, largely pan-tropical distribution. As such, tarantula systematics and taxonomy is a global endeavor with research being undertaken on species from all continents (except Antarctica): North America (Hendrixson et al., 2015; Ortiz and Francke, 2015, 2016, Hamilton et al., 2016; Turner et al., 2018; Graham et al., 2020; Mendoza and Francke, 2020); South America (Pérez-Miles and Locht, 2003; Bertani and Fukushima, 2009; Guadanucci, 2011, 2014; Perafán et al., 2015; Cifuentes et al., 2016; Ferretti et al., 2018; Hüsser, 2018; Nicoletta et al., 2020; Candia-Ramírez and Francke, 2021; Cifuentes and Bertani, 2022; Gabriel et al., 2023; Galleti-Lima et al., 2023; Kaderka et al., 2023; Sherwood et al., 2023; Ferretti et al., 2024; Peñaherrera-R et al., 2024); Europe (Korba et al., 2022); Africa (Gallon, 2003, 2005, Midgley and Engelbrecht, 2019); Asia (Schmidt and von Wirth, 1996; West et al., 2012; Prasanth and Jose, 2014; Sanap & Mizra, 2014; Nunn et al., 2016; Montemor et al., 2020; Sivayyapram et al., 2020; Yu et al., 2021; Songsangchote et al., 2022; Chomphuphuang et al., 2023); and Australia (Raven, 2005; Briggs et al., 2023).

Figure 1. Theraphosidae diversity. (A) Theraphosinae, Aphonopelma sp. (B) Aviculariinae, Typhochlaena seladonia. (C) Psalmopoeinae, Psalmopoeus irminia. (D) Eumenophorinae, Hysterocrates sp. (E) Harpactirinae, Augacephalus sp. (F) Stromatopelminae, Stromatopelma sp. (G) Selenocosmiinae, Selenocosmia crassipes. (H) Poecilotheriinae, Poecilotheria sp. (I) Ischnocolinae, Ischnocolus sp. (J) Ornithoctoninae, Cyriopagopus lividus. (K) Thrigmopoeinae, Thrigmopoeus sp. (L) Barychelidae, Rhianodes atratus. Photos used from iNaturalist were cropped to size. Photo credit in the same order: Chris Hamilton, Joao Mendes & Dimenor Santos (joaomendes/iNaturalist), Allan Hopkins (hoppy_1951/iNaturalist), Eric (Toganim/iNaturalist), Joubert Heymans (jouberth/iNaturalist), Nael Ajm (naelajm/iNaturalist), Michelle Woolley, Sanjaya Kanishka (Sanjaya_kanishka/iNaturalist), Vojtěch Víta (vojtechvita/iNaturalist), Wich’yanan L (plains-wanderer/iNaturalist), P. S. Sivaprasad (sivabirds/iNaturalist), Tan Kok Hui (kokhuitan/iNaturalist).
Our current understanding of the Theraphosidae Tree of Life is largely developed from a handful of molecular phylogenies (Hüsser, 2018; Lüddecke et al., 2018; Turner et al., 2018; Foley et al., 2019, 2021; Korba et al., 2022; Ortiz, 2023). These phylogenies challenged the results of the past (i.e., those that only used morphology), and began to provide a better understanding and stability of relationships through inter-subfamily sampling (Table 1; Figure 2). The subfamily rank has long been used to accommodate theraphosid diversity (Simon, 1892; Pocock 1895a), and over time many subfamilies have been modified to either expand their definition or new subfamilies have been erected to accommodate newly described or ‘hard-to-place’ species (Smith, 1990, 1995; Schmidt, 1993; Samm and Schmidt, 2008, 2010; Guadanucci, 2014) (Table 1). As of 2024, 13 extant subfamilies are largely accepted. These include the Aviculariinae Simon, 1892, Eumenophorinae Pocock, 1897, Harpactirinae Pocock, 1897, Ischnocolinae Simon, 1892, Ornithoctoninae Pocock, 1895a, Poecilotheriinae Simon, 1892, Psalmopoeinae Samm and Schmidt, 2010, Schismatothelinae Guadanucci, 2014, Selenocosmiinae Simon, 1889, Selenogyrinae Smith, 1990, Stromatopelminae Schmidt, 1993, Theraphosinae Thorell, 1870, and Thrigmopoeinae Pocock, 1900. Additionally, there is one extinct subfamily, Prototheraphosinae Wunderlich and Müller, 2020, known only from a single, mid Cretaceous fossil in Myanmar (Wunderlich and Müller, 2020) and is thought to be closely related to the Selenocosmiinae. Sanger sequencing phylogenies that used a handful of loci provided broad taxon sampling and were among the first to reject several morphological hypotheses; however, the limited phylogenetic signal in that data left many relationships unresolved (Hüsser, 2018; Lüddecke et al., 2018; Korba et al., 2022). The use of transcriptome-based phylogenies with hundreds of loci, albeit with less samples, lead to highly supported relationships within and between subfamilies for the first time, while also confirming the monophyly of most of the subfamilies found in Lüddecke et al. (2018). Several studies have since built on the Foley et al. (2019) phylogeny by adding the barychelid Rhianodes atratus (Thorell, 1890; Foley et al., 2021) and Bonnetina Vol, 2000 (Ortiz, 2023). These phylogenies all recover largely the same relationships, with all acknowledging the same basal node was unstable and sensitive to the data used. Importantly though, these phylogenies all suffer from the same issue, limited taxon sampling. With less than 5% of the known tarantula diversity sampled, there is significant room to explore and test hypotheses in Theraphosidae systematics.
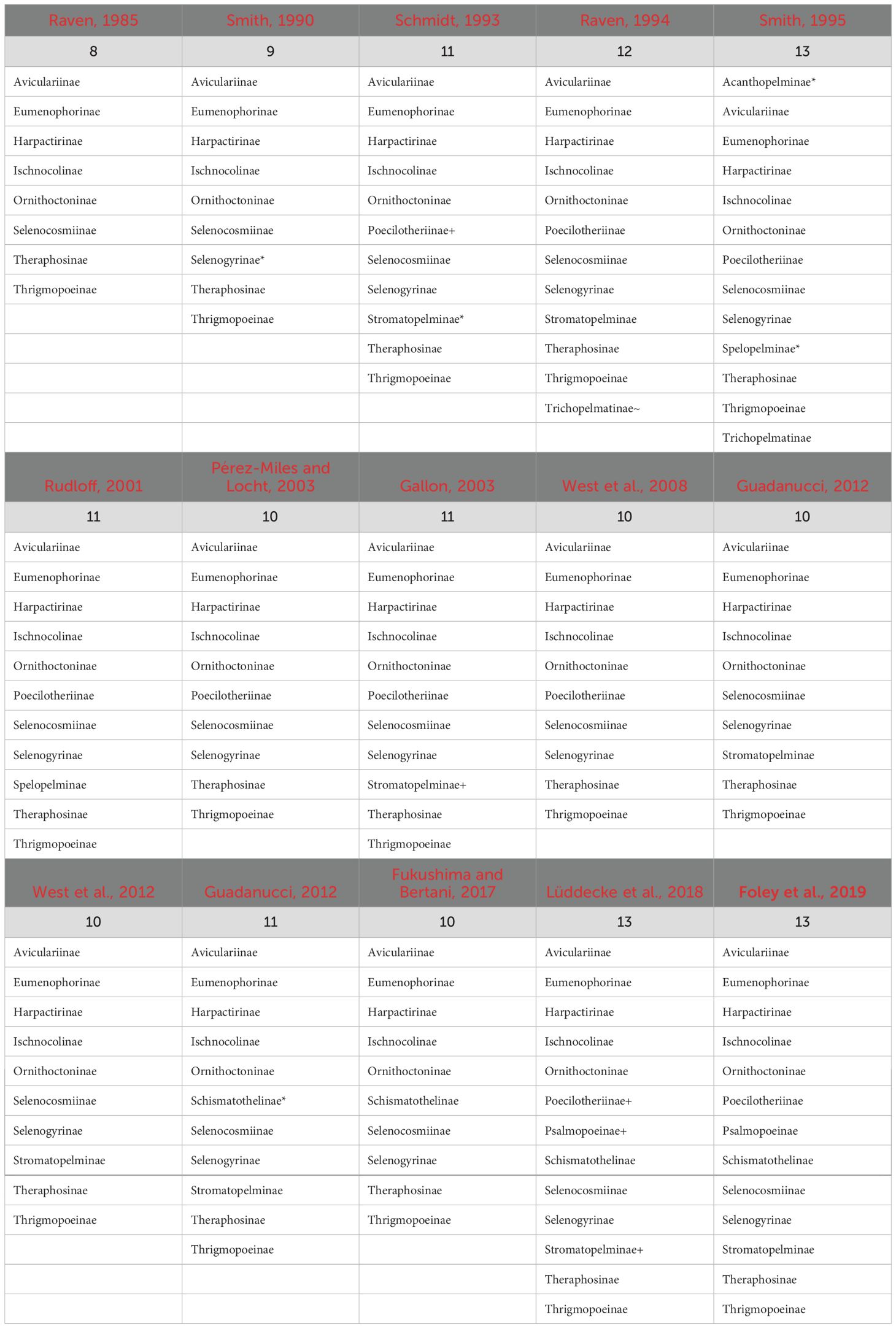
Table 1. Changes in the number of recognized Theraphosidae subfamilies from 1985–2019. *, Newly described subfamily; +, subfamily raised from synonymy; ~, subfamily moved to Theraphosidae from another family.
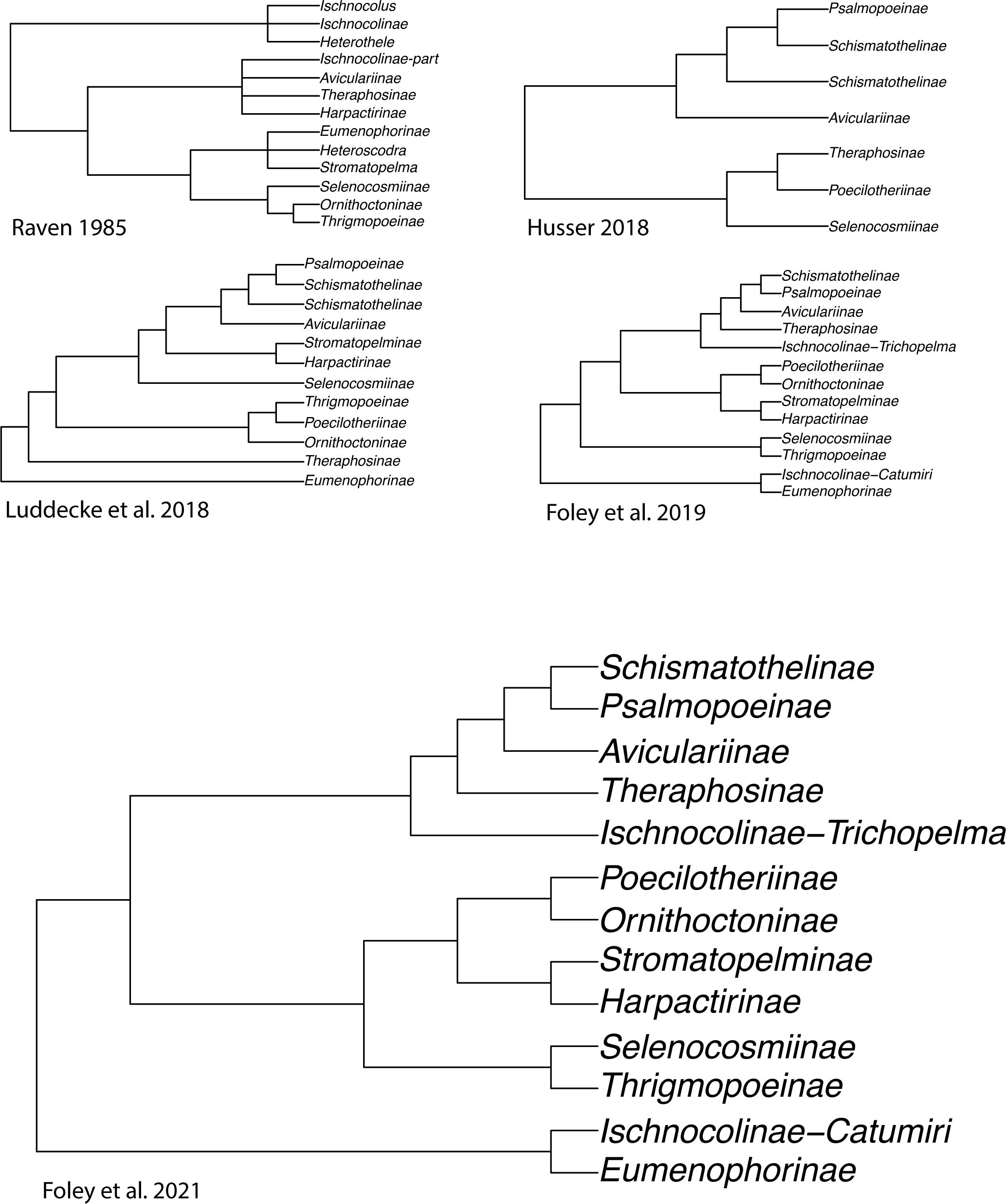
Figure 2. Simplified cladograms from key studies that have widespread taxon sampling in Theraphosidae systematics.
In this review, we highlight the surge in Theraphosidae systematic research over the past six decades, offer insights into the current diversity and classification landscape, and summarize the contemporary trends and methodologies employed up to this point. Extending beyond Theraphosidae, we explore the taxonomic boundaries between Theraphosidae and Barychelidae Simon, 1889 (Figure 1L), as well as the more recent inclusion of some Paratropididae Simon, 1889 species into Theraphosidae. Finally, we will show how this significant burst in recent theraphosid taxonomic work has provided the foundation for global researchers to collaborate and advance our understanding of the Theraphosidae Tree of Life.
2 Methods
2.1 Data acquisition & analysis
Theraphosidae systematics has been built cumulatively on the backs of many researchers, scholars, naturalists, and enthusiasts alike. While not every piece of work can be acknowledged here, this effort should not be considered overlooked or unrecognized. To investigate the general trends and statistics of Theraphosidae diversity through time, we downloaded species data from the World Spider Catalog (World Spider Catalog, 2024, Accessed 16 April 2024). The WSC is regarded as one of the best public, online taxonomic databases, with up-to-date decisions and access to all Araneae taxonomic papers. Only genera and species that are considered valid have data readily available for export, therefore readily downloadable statistics do not include data for nominal species considered as junior synonyms, nomina dubia, or nomina nuda. To accommodate the common use of subfamilies in Theraphosidae (Thorell, 1870; Raven, 1985; Lüddecke et al., 2018; Foley et al., 2021; Biswas et al., 2023), we added this data to our matrix (Supplementary Datasheet 1) by following Tarantupedia (Kambas, 2024) and the literature. Because there are some differences, we make several deviations from the classification provided by Kambas (2024). We place six genera as incertae sedis due to their uncertain placement (e.g., many have been placed in Barychelidae at some point in their taxonomic past); these include Acanthopelma F.O. Pickard-Cambridge, 1897, Cyrtogrammomma Pocock, 1895b, Melloina Brignoli, 1985, Psalistops Simon, 1889, Reichlingia Rudloff, 2001, and Thalerommata Ausserer, 1875. Most of these currently reside in Ischnocolinae, a subfamily that has been used as a historical ‘trash bin’ or ‘dumping ground’ to place species that do not fit into the other subfamilies (Guadanucci, 2014). Furthermore, we find that Neoheterophrictus Siliwal et al., 2012 and Heterophrictus Pocock, 1900 should be listed under Eumenophorinae rather than Ischnocolinae, as per Guadanucci (2011); Siliwal et al. (2012), and Mirza et al. (2014). Lastly, we find that Yanomamius Bertani and Almeida, 2021 should be listed under Schismatothelinae rather than Psalmopoeinae, as per the authorities of the genus (Bertani and Almeida, 2021).
To investigate contemporary trends in theraphosid taxonomy and systematics, we recorded the approach and data type used for newly described and currently valid genera and species, from 1 Jan 2010 to 16 April 2024. Newly described genera were categorized as one of the following: 1) Morphology – Descriptive; 2) Morphology – Cladistics; or 3) DNA+Morphology. Genera were categorized as Morphology – Descriptive if the study did not explicitly describe their testing framework to erect new genera. Genera were categorized as Morphology – Cladistics if a morphological cladistic analysis was included, supporting the monophyly of the new genera. Genera were categorized as DNA+Morphology if DNA and morphology were both used to confirm monophyly of the new genus. For species data, we followed Bond et al. (2022) and also recorded if a species concept was explicitly stated when testing species boundaries, and if so, which species concept. The approach used to delimit species were again categorized three ways: 1) Morphological – Descriptive; 2) Morphological – Cladistics; and 3) DNA+Morphology. Species were recorded as Morphological – Descriptive if no testing framework was stated. Species were recorded as Morphological – Cladistics if a morphological phylogeny was used to delimit species. Finally, species were recorded DNA+Morphology if DNA was used in any capacity that led to a new species being described, this included studies that produced a DNA barcode without phylogenetic context and studies using hundreds of loci. For DNA+Morphology, we also noted how morphology was used (e.g., cladistics, morphometrics, or descriptive), as well as if ecological information was used. Given the importance of preserving all aspects of holotypes, we also recorded if the holotype was sequenced and whether the sequence data was publicly available. To examine the proportion of valid names and synonyms for genera and species, we manually counted the number of generic and species synonyms for each valid genus, as well as the number of nomina dubia from WSC. Additionally, we examined which sex or sexes were described at the time of the species description (Supplementary Datasheets 2–4). Data was analyzed and plotted using Rstudio (R Core Team, 2024) and the following R packages: tidyverse (Wickham et al., 2019), RColorBrewer (Neuwirth, 2014), Patchwork (Pedersen, 2019), and ape (Paradis et al., 2004).
3 Results & discussion
3.1 The explosive waves of Theraphosidae taxonomy
Theraphosidae taxonomy is over 260 years old, with the majority of theraphosid species described during two large “waves” (Figure 3). Beginning in 1758, theraphosid taxonomic work slowly rose until the 1870s when the first, large burst of descriptions occurred, peaking in 1897 with 38 species. Following this peak, the number of species described each year decreased until the 1950s–1960s. In the 1980s a second, more explosive burst of activity began and continues to this day (Figure 3). During the first wave, a period of just over 200 years (1758–1960), 57 author combinations described 508 species which are still valid today; this makes up almost half of today’s formally recognized diversity with 46.1% (508/1110). This wave of theraphosid taxonomy was most prolific in Europe and North America, with 20 of the top 21 theraphosid taxonomists (measured in described and currently recognized species) coming from those two regions – all of which described at least six currently recognized species. However, it should be acknowledged that Brazilian arachnologist Cândido Firmino de Mello-Leitão described 36 tarantula species during this time. Only two prevalent theraphosid taxonomists described more, Reginald Pocock and Eugène Simon with 96 and 90 currently valid species, respectively.
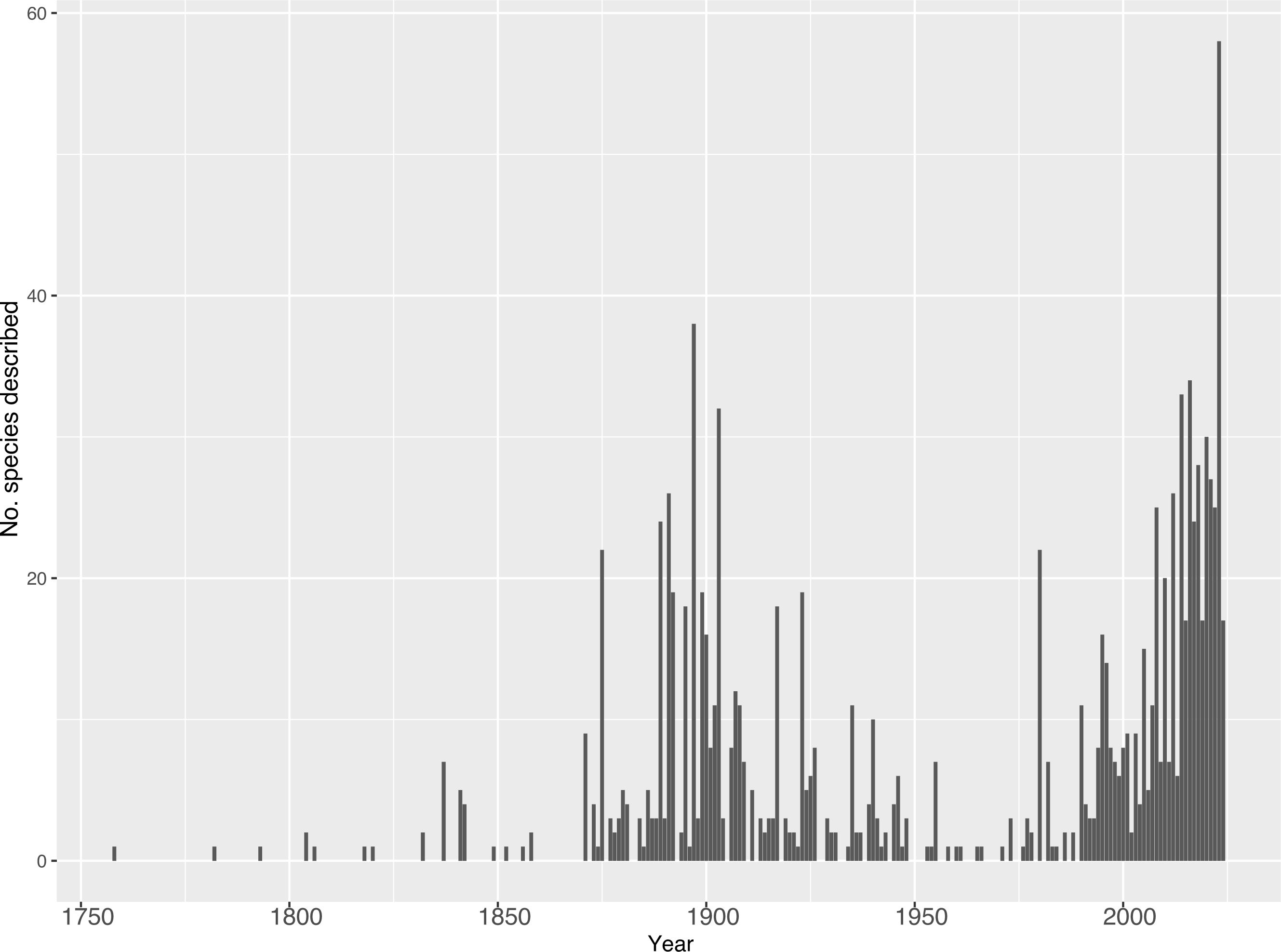
Figure 3. The number of valid species described per year. Data collected from the World Spider Catalog on 16 April 2024.
Currently, we are in the midst of a great resurgence or “second wave” in theraphosid taxonomy (Figure 3). From 1961 to 16 April 2024 (a 62-year span), arachnologists have described 592 currently valid species, more than 50% of the described tarantula diversity. In 2023 alone, 58 new theraphosid species were described. Interestingly, this second wave is occurring not only in species diversity, but also in the diversity of taxonomists describing these species – approximately half of the described species over the last 60 years were carried out by 204 author combinations, an almost 400% increase when compared to the 57 in the 200 years prior. This is important because these authors are living and working on the continents where the tarantulas they are describing live. Even though taxonomy is a field in crisis, where less and less taxonomists are employed due to underfunding (Bacher, 2012; Sluys, 2013; Bond et al., 2022), the number of theraphosid taxonomic papers and authors has been trending upwards.
The diversity of tarantulas, while nearly globally distributed, is far from uniform. On a continental scale, we see large disparities in the number of species. Regions such as Australia and Europe are relatively poor, with Australia having only six described species, while species from Europe/Eurasia are only found along the fringes of the Mediterranean. Africa and Asia have comparatively far more diversity, with ~150 and ~200 species respectively, yet all these regions are dwarfed by the Americas, where ~650 described species reside. When we compare diversity using geopolitical boundaries, we find several countries contain a relatively large proportion of diversity. The three countries with the most currently recognized species are Brazil, Mexico, and Peru, possessing ~210, ~100, and ~80 described species respectively (and a vast amount of undescribed diversity), making up ~35.4% (390/1100) of all described Theraphosidae diversity. There are likely a combination of factors contributing to the disparity in theraphosid diversity between subfamilies and regions, for example taxonomic bias, geology, and evolutionary innovation. One potential taxonomic bias is that the Americas have contributed much more taxonomic research in the last 60 years than other regions. Is this because more undescribed diversity has been examined here compared to other parts of the world? Are there more theraphosid taxonomists working on these continents? Or do the Americas truly harbor more diversity? These cannot be answered at this time, but we do know that Brazil, Mexico, and Peru are well known biodiversity hotspots, where factors such as long-term stability (Marin et al., 2018) and topographical complexity (Moeslund et al., 2013) can facilitate the accumulation and generation of new species. Additionally, evolutionary innovation often leads to differences in diversity, and the development of urticating hairs – an effective anti-predator mechanism found in two American subfamilies, has likely contributed to an increase in diversity, either through increased diversification or reduced extinction within the Theraphosinae subfamily (Biswas and Karanth, 2024).
We also investigate the breakdown of Theraphosidae classification across taxonomic levels, namely genus and subfamily. Here we acknowledge that measures of diversity above the species level may not be meaningful, as division between (and among) ranks above species (particularly genera and subfamily) can be arbitrary (Avise and Mitchell, 2007; Stork et al., 2015), but interesting nonetheless. As one taxonomist might determine a large clade to be one genus, another might recognize multiple smaller genera (see proposed changes to anole lizards: Nicholson et al., 2012, 2014, Poe, 2013; Poe et al., 2017). Currently, Theraphosidae includes 167 recognized genera. Of these, an astounding 25.1% (42/167) are monotypic! Most of these monotypic genera reside within the Theraphosinae subfamily – the dominant lineage throughout North, Central, and South America. Conversely, there are 11 genera with at least 20 species, comprising 28.9% (318/1100) of described tarantula diversity. When we look at subfamily classification, a notable pattern emerges (Figure 4). Within most subfamilies, one or two genera contain more diversity than the other genera in the same subfamily (Figure 4). This result begs the question: Is this a real, biological result or a taxonomic bias? In some cases, the more diverse genera could perhaps be a ‘dumping ground’ (like the Ischnocolinae), where new species with uncertain placement are put into these genera. Another answer is that there are more taxonomists working on these genera. Yet another potential answer lies in our desires to answer one of evolutionary biology’s most interesting questions – what mechanisms allowed some lineages on the Tree of Life to become more diverse than others? While the dumping of species into some genera helps reduce the inflation of monotypic genera, they may not always reflect natural monophyletic lineages. For example, the two most diverse genera Aphonopelma Pocock, 1901 (54 species) and Selenocosmia Ausserer, 1871 (40) (Figure 4), are likely not monophyletic (Schmidt, 1995; Raven, 2000; Hamilton et al., 2016; Turner et al., 2018), and will probably split into multiple genera after future revisions. Questions like these will only be answered by future collaborative research.
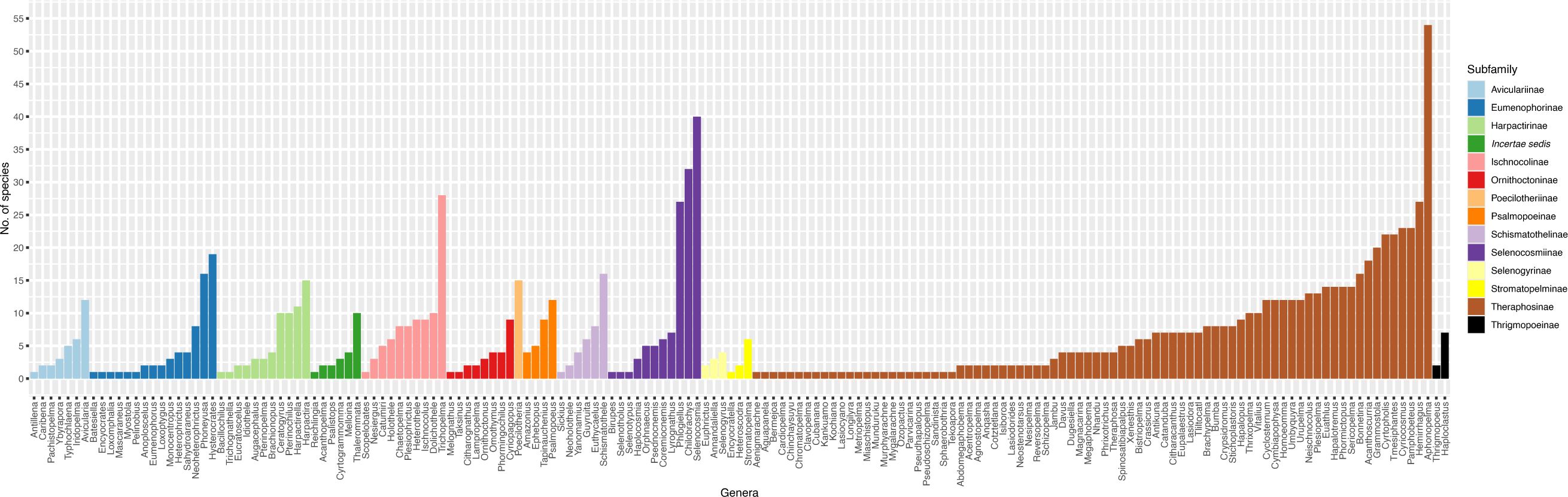
Figure 4. The number of valid species per genus. Genera are colored by subfamily classification. Data collected from the World Spider Catalog on 16 April 2024.
3.2 Contemporary practices in Theraphosidae systematics
It is well understood that the boundaries of different taxonomic ranks is hotly debated (Mahner, 1993; Mallet, 1995; Wheeler and Meier, 2000; De Queiroz, 2005, 2007; Kallal et al., 2020; Turk et al., 2020; Hormiga et al., 2023; Kuntner et al., 2023; Maddison and Whitton, 2023). Because of this, many researchers agree that taxonomy and systematics should be rigorous and employ “best practices” and current theory, as in any other scientific discipline (Dayrat, 2005; Cook et al., 2010; Kaiser et al., 2013; Wheeler, 2018, 2020; Bond et al., 2022; Valdecasas et al., 2022). In our field, “best practices” is synonymous with integrative taxonomy – i.e., using multiple data types (morphology, DNA, ecology, etc.) to test hypothesized taxonomic boundaries. Across Theraphosidae systematics, we have seen a variety of practices and methods, ranging from descriptive with no mention of a species hypothesis testing framework, to the “best practices” that utilizes morphology, DNA, and ecology.
Morphological data dominates theraphosid classification, particularly at the generic and species level. Since 2010, several years after the development of molecular tools that could be used for taxonomy (Hebert et al., 2003, 2004), there have been 38 theraphosid genera described (excluding Bumba Pérez-Miles et al., 2014 which is a replacement name for Maraca Pérez-Miles, 2006, which was also a replacement for Iracema Pérez-Miles, 2000). Surprisingly, only five genera (Lasiocyano, Parvicarina, Tekoapora Galleti-Lima et al., 2023, Tliltocatl Mendoza and Francke, 2020, and Urupelma Kaderka et al., 2023) used genetic data to support them as distinct monophyletic lineages – four of these were published in 2023 (Galleti-Lima et al., 2023; Kaderka et al., 2023). The other 33 genera can be considered untested hypotheses. Though nine of these 33 genera were supported as monophyletic by a morphological phylogeny, our understanding of these genera could change heavily following molecular investigations.
Molecular data can provide significant insight into theraphosid systematics by reconstructing evolutionary history and inferring extent of geneflow – a measure that cannot be tested using morphology. For example, molecular data revealed significant over-splitting in the North American Aphonopelma and aided in 33 synonymies, highlighting where morphology failed to accurately delimit species as distinct, independently evolving lineages. A recent review of taxonomic practices in Araneae between 2008–2018 revealed the use of molecular data was startlingly low at ~6% (see Bond et al., 2022). When we look at Theraphosidae, only 10.8% (40/369) of the newly described species during 2010–2024 were delimited with DNA (Figure 5) – interestingly this is biased by 2016 and 2017 where almost half of all newly described species included DNA. This trend of incorporating DNA quickly diminishes, with no new species described using DNA from 2020 to 2022. There are only a handful of studies that have incorporated molecular data into their species delimitation studies and generic revisions including: Aphonopelma (Hendrixson et al., 2013; Hamilton et al., 2011, 2014, 2016), the Australian species (Briggs et al., 2023), Bonnetina (Ortiz and Franke, 2015; 2016, 2017), Brachypelma Simon, 1891 (Mendoza and Francke, 2017), Davus O.Pickard-Cambridge, 1892 (Candia-Ramı́rez and Francke, 2021), Grammostola Simon, 1892 (Montes de Oca et al., 2016), Ischnocolus Ausserer, 1871 (Korba et al., 2022), Pamphobeteus Pocock, 1901 (Cifuentes et al., 2016), Plesiopelma Pocock, 1901 (Ferretti et al., 2024), Tliltocatl Mendoza and Francke, 2020 (Mendoza and Francke, 2020), Lasiocyano, Parvicarina, Tekoapora (Galleti-Lima et al., 2023), and Urupelma (Kaderka et al., 2023).
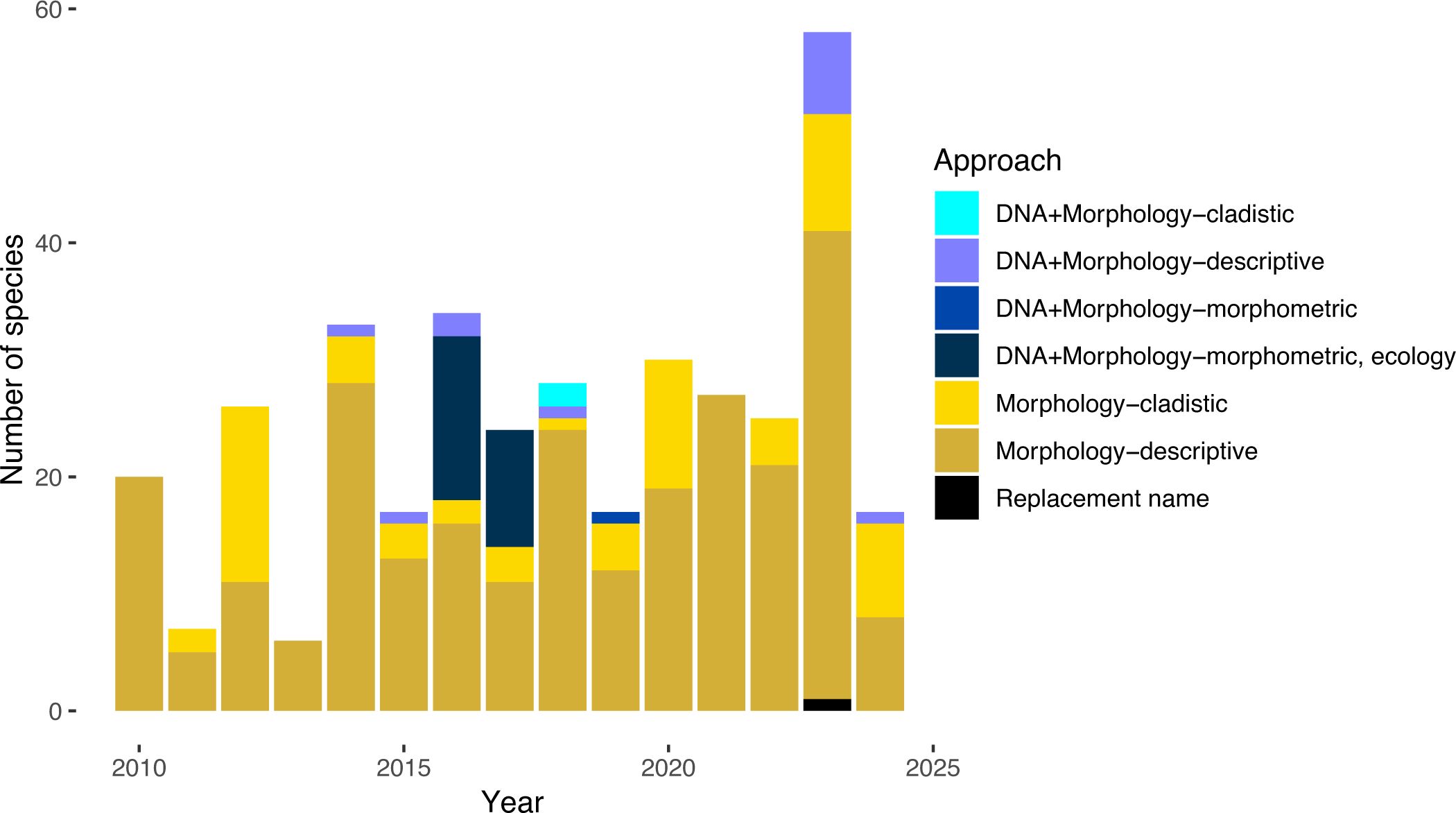
Figure 5. Approaches and data types used for newly described species from 2010–2014. Approaches were classified in the following ways: DNA+Morphology – cladistic if DNA and morphological cladistics were used for describing new species, DNA+Morphology – morphometrics if DNA and morphometrics were used, DNA+Morphology – morphometrics, ecology if DNA, morphometrics, and ecology was used, DNA+Morphology – descriptive if DNA and descriptive morphology was used, Morphology – cladistics if morphological cladistics was used, Morphology – descriptive if a descriptive taxonomic approach was used. Replacement name for cases where a species had to be renamed to avoid taxonomic confusion due to the species being a homonym.
Of the 40 species that have used DNA to aid in species delimitation, only 29 of them sequenced the holotype. However, in several studies it was unclear if the holotype was sequenced – i.e., the published phylogenies did not provide specimen identifiers or accession numbers, instead only supplying the species name, therefore it could not be confidently inferred if the holotype was used in the molecular phylogeny. In other studies, it was found that individual specimens had multiple codes, for example a holotype specimen was given one code in the species description (presumably a museum accession number) but another code in the molecular phylogeny and tables (presumably collection/collector identifiers). Additionally, some specimen codes were only able to be linked based on data in the Supplementary Material. For most holotypes, the sequence data was publicly available at the time of this publication; there were only two cases where a holotype’s data was not publicly available (Hüsser, 2018; Galleti-Lima et al., 2023).
Another important component of modern taxonomy and the “best practices” would be to explicitly state the species concept that was used. Species concepts provide a framework to test species hypotheses, however their use in theraphosid taxonomy has been very low, with only 10% (37/369) of new species (2010–2024) being described under a defined species concept. Of these 37 species, three species concepts have been utilized: the Phylogenetic Species Concept (inferred as Cracraft, 1983), the Unified Species Concept (sensu De Queiroz, 2007), or the Morphological Species Concept (MSC – generally using a typological viewpoint). Historically, theraphosid taxonomy has been based on the MSC, though not explicitly stated.
As of 1st April 2024, there have been 98 generic and 291 species synonyms within Theraphosidae taxonomic history, and a further two genera and 141 species considered nomina dubia (Figure 6). To put this into perspective, 36.9% of all available generic names (98/265) and 20.9% (291/1391) of all available species names are junior synonyms, comparable to previous examinations of similar statistics at the species level (see Platnick and Raven, 2013). We know that the “cryptic species problem” (i.e., morphologically indistinguishable using traditional approaches) is common throughout mygalomorphs. When using morphology alone for delimitation and classification, researchers must try to accurately find the boundary between intra- and inter-specific variation. Given the number of junior synonyms, both at the species and genus level in theraphosids, this is clearly difficult to do accurately. This should make taxonomists wary of inferring boundaries when only investigating morphology.

Figure 6. The proportion of species synonyms and nomina dubia for Theraphosidae genera and species, as of 16 April 2024.
When we look for potential biases in the sex of described species, we find that for most theraphosid species described since 2010, both sexes were described (Both: 226, Female only: 61, Male only: 82). In subsequent years, since their description, an additional six females were described for species that were only known from the male (Both: 232, Female: 55 and Male: 82). This is important to point out because Theraphosid taxonomists have described only one sex in 38.7% (143/369) of the examined species (from 2010–2024), comparable to the 35.6% of all spider species examined in Bond et al. (2022) from 2008–2018. While being able to describe males and females is of course best practice, we acknowledge the difficulty of being able to collect mature specimens of both sexes, based on our own experiences. In many cases, females can remain elusive with cryptic burrows and collecting mature males in the breeding season may not be possible based on location and climatic challenges. Furthermore, traits or character states can sometimes be hard to quantify objectively, particularly in the continuum of morphological variation. As such, different scoring of characters can lead to different inferred phylogenies (see Mori and Bertani, 2020; Goloboff-Szumik and Ríos-Tamayo, 2022), as well different opinions on what characters should define different genera and species (see Nunn et al., 2016; Sivayyapram et al., 2020). For example, theraphosids (and mygalomorphs, in general) have “simple” copulatory organs that do not provide many effective characters for comparison with other species. Because of this, small intraspecific variation in these simple structures might be perceived as interspecific ones by many taxonomists.
One of the best examples of convergent morphological evolution misleading theraphosid taxonomists is the arboreal African genera Stromatopelma Thorell, 1869 and Heteroscodra Pocock, 1900. They were placed into their own subfamily (Stromatopelminae) by Schmidt in 1993, though previously they had been placed in Eumenophorinae by Raven (1985) after being transferred from Aviculariinae. In 2003, Gallon included both genera in Stromatopelminae while also including Encyocratella olivacea Strand, 1907, one of only two tarantula species known to lack spermathecae (Bertani and da Silva Junior, 2002; Gallon, 2003), and proposing another African subfamily, the Harpactirinae, as the sister lineage. In later years, subsequent morphology-based phylogenies inferred the Stromatopelminae resided within the Aviculariinae once again (West et al., 2008; Fukushima and Bertani, 2017). Eventually, molecular data would refute the placement in either Aviculariinae or Eumenophorinae, affirming the positions of Schmidt (1993) and Gallon (2003, 2005) that the Stromatopelminae was an independent lineage, sister to Harpactirinae (Lüddecke et al., 2018; Foley et al., 2019). This is not a criticism of the use of morphology. The desire to use molecular data is prevalent but the funding is not. These research funding inequities are important because the vast majority of new diversity being described is coming from regions with the highest biodiversity, yet these researchers are not being supported.
There will be times when DNA is inaccessible and morphological data may be the only option. Many putatively undescribed species reside in biological collections around the globe, but because of their age or storage may not be suitable for DNA sequencing because of their preservation technique or a hesitancy to destructively sample small or very rare specimens. In these cases, researchers are left with few choices. They can either wait to resample fresh material, which of course requires additional time and resources, or proceed using a morphological-only approach. This is problematic due to significant anthropomorphic change where species are going extinct before being described (Bond, 2012). Ultimately, the more information researchers can use, the more robust our species and classification hypotheses will be. While species can be delimited and described solely from DNA sequence data following a hypothesis testing framework (Cook et al., 2010; Jörger and Schrödl, 2013; Renner, 2016; Briggs et al., 2023), systematics and taxonomy carried out only using DNA does not provide context about the organisms or their evolution, potentially leaving many interesting evolutionary stories behind (Wheeler, 2018). As said by Wheeler (2020): “But no single source of evidence can eclipse the others without sacrificing valuable knowledge”. Only an integrative approach using morphology, ecology, and molecular data will give a robust and informative classification.
3.3 Theraphosidae, Barychelidae, Paratropididae: Where to draw the line?
Phylogenomics has provided the much-needed stabilizing insight to the Mygalomorphae Tree of Life (Bond et al., 2014; Garrison et al., 2016; Starrett et al., 2017; Hedin et al., 2019; Kulkarni et al., 2020; Opatova et al., 2020; Kulkarni et al., 2023), however achieving widespread taxon sampling for phylogenomics is difficult given the sheer diversity of the group and the costs involved. Many taxonomically important genera and species have not yet been sampled in this context, leaving many untested hypotheses in mygalomorph systematics. In the case of the families Theraphosidae, Barychelidae, and Paratropididae, the frequent transfer of genera and species back and forth has blurred the taxonomic limits of these groups.
The Barychelidae (sometimes called brush-footed trapdoor spiders) are a widespread group of mygalomorphs, currently comprising 39 genera and 285 valid species. Barychelids are the sister lineage to Theraphosidae, with both families sharing many characteristics such as claw tufts, dense tarsal scopula, and hirsute appearance (Opatova et al., 2020), however recent phylogenetics suggest the family may not be monophyletic (Kulkarni et al., 2023). Barychelids have a broad distribution and can be found in Central and South America, Africa, Asia, and Australia. Interestingly, they are also found throughout Oceania in many of the pacific islands such as Hawaii, New Caledonia, and Fiji – areas where tarantulas have not been recorded. When compared to Theraphosidae, Barychelidae has received very limited taxonomic attention, with most studies focusing on Oceania and Asia (Raven, 1986, 1988, 1990b, 1994, 2008; Churchill and Raven, 1992; Yu et al., 2023), as well as a handful of studies from South America (Guadanucci, 2012; Mori and Bertani, 2016, Rios-Tamayo, 2023), Africa (Benoit, 1965, 1966, Gonzalez-Filho et al., 2023), and India (Jose and Sebastian, 2008; Siliwal and Molur, 2009; Siliwal et al., 2009). For example, where 38 new theraphosid genera have been erected since 2010, no new Barychelidae genera have been erected since 1995.
The boundary between the Theraphosidae and Barychelidae has been vague for a very long time. This can be attributed to their similar morphology and lack of genetic taxon sampling. Barychelidae is thought to be distinct from Theraphosidae based on the number of cuspules and shape of the maxillary anterior lobe (Raven, 1985). However, this distinction does not represent a clear boundary as several barychelid genera (Brachionopus Pocock, 1897, Cyrtogrammomma, Dolichothele Mello-Leitão, 1923, Euthycaelus Simon, 1889, Harpactirella Purcell, 1902, Idiothele Hewitt, 1919, Psalistops, Reichlingia, Thalerommata, and Trichopelma Simon, 1888) have been transferred to Theraphosidae during the 1970s to 2023 (Bücherl et al., 1971; Raven, 1985; Mori and Bertani, 2020; Bertani and Raven, 2023) – and sometimes moving back and forth between the two families. Most of these decisions were based on morphology, though sometimes cladistics. Of these genera, only a small number of Brachionopus, Euthycaelus, Harpactirella, and Trichopelma species have been confirmed as theraphosids by molecular data (Bond et al., 2012; Wheeler et al., 2017; Opatova et al., 2020; Foley et al., 2021; Kulkarni et al., 2023; Yu et al., 2023). As such, barychelids are poorly represented in online repositories such as GenBank, SRA, or Dryad, and as argued in Mori and Bertani (2020) a wider sampling of Barychelidae should be a priority. A better understanding of the limits of Barychelidae would in turn dramatically impact our understanding of Theraphosidae classification, as well as providing further insight into their evolution and biogeography.
The Paratropididae are an enigmatic group of mygalomorph spiders due to them being rare, reclusive, and hard to find (Perafán et al., 2019), characteristics that have caused them to be difficult to place in the mygalomorph Tree of Life. Though Raven (1985) suggested they were sister to Theraphosidae, early molecular data had difficulty placing paratropidids in phylogenies, often with weak support (Hedin and Bond, 2006; Bond et al., 2012). Once phylogenomics were used, the family has been recovered either as an early branching and species poor lineage of Bipectina or sister to the Domiothelina (Opatova et al., 2020; Kulkarni et al., 2023) – though they have only ever been represented by Paratropis Simon, 1889. Recent work has questioned the placement of certain lineages, with Melloina being moved to Theraphosidae (Mori and Bertani, 2020; Goloboff-Szumik and Ríos-Tamayo, 2022) and morphological cladistic analyses placing the Melloina and Paratropis inside Theraphosidae (Mori and Bertani, 2020; Goloboff-Szumik and Ríos-Tamayo, 2022). Additionally, the Glabropelmatinae Raven, 1985 (and in particular Melloina) was placed in Theraphosidae (Echeverri et al., 2023). The cladistic analyses that placed Melloina in Theraphosidae (Mori and Bertani, 2020; Goloboff-Szumik and Ríos-Tamayo, 2022) conflicts with recent molecular phylogenies with regards to theraphosid subfamily relationships (Foley et al., 2019, 2021; Ortiz, 2023). The morphological phylogenies that proposed Eumenophorinae as sister to Selenocosmiinae, Theraphosinae sister to Ornithoctinae + Poecilotheriinae, and Psalmopoeinae sister to Stromatopelminae – all have been rejected in phylogenomic studies (Foley et al., 2019, 2021; Ortiz, 2023). Similar to the Barychelidae, increasing sampling for molecular work from paratropidid genera, such as Melloina, will be key to testing the correct placement and composition of this family.
3.4 The future of Theraphosidae systematics and taxonomy
From this review it is clear that the taxonomic practices throughout the history of Theraphosidae have remained largely static. Theraphosid systematists have been slow to adopt modern techniques and apply best practices, some of which we attribute to resource inequities – something global collaboration can help mitigate. If the fields of Theraphosidae taxonomy and systematics want to be more rigorous, then we must move toward a more integrative approach and explicit testing of species boundaries (i.e., hypothesis testing). Having a clear, reproducible framework for testing species or generic boundaries, even without DNA, will only help to increase the rigor and robustness of taxonomy. However, in cases where DNA can be used, sequencing of holotypes (either newly described species or historical specimens – more feasible now than ever before due to high-throughput sequencing methodologies) will be critical for future theraphosid and barychelid taxonomy by adding stability and clarity, and by providing the ability to confidently identify species regardless of stage of life or sex.
The systematics and taxonomy of Theraphosidae and their close relatives remains a fruitful area for discovery. While the number of newly described species continues to increase exponentially, there are likely still many more to be named. As mentioned earlier, widespread taxon sampling for molecular phylogenetics continues to be an issue, leaving many parts of the Theraphosidae (and Barychelidae) Tree of Life unknown and untested. With these spiders occurring across multiple continents, this is an opportunity for international collaboration in the face of disparity in resources. Global collaboration will help balance certain resource and acknowledgement inequities, collectively elevating the standard of Theraphosidae systematics and evolution. Theraphosid researchers, keep up the great work, collaborate, and continue this second wave of taxonomic research we are currently experiencing.
Author contributions
EB: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. CH: Conceptualization, Funding acquisition, Investigation, Methodology, Resources, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Funding for this work was partially provided by a National Science Foundation CAREER Award to CH (DEB-2144339); EB was funded by a University of Idaho P3R1 award to CAH.
Acknowledgments
We want to the thank the wonderful members of the Arthropod Molecular Systematics Lab at the University of Idaho who have helped by providing comments on earlier drafts of this work: Andrea Noble-Stuen, Arnau Calatayud-Mascarell, Erik Ciaccio, Karina Silvestre-Bringas, and Michelle Woolley. We would also like to thank the editors of the “Horizons in Arachnid Science” collection for the invitation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frchs.2024.1445731/full#supplementary-material
Supplementary Data Sheet 1 | THERAPHOSIDAE_species_export_20240416.csv, data downloaded from World Spider Catalog, 16 April 2014 with subfamily column added.
Supplementary Data Sheet 2 | Theraphosidae_2010_2024_FINAL.csv, reduced dataset for valid species described 2010-2024 with records of species concept, data type, approach, holotype sequences and availability.
Supplementary Data Sheet 3 | synonyms_nomina_dubia.xlsx, simple tally of the number of synonyms and for genera and species for each valid genus as well as records of nomina dubia.
Supplementary Data Sheet 4 | genera_described_post_2010.csv, dataset to record approach used for valid genera described from 2010-2024.
Supplementary Data Sheet 5 | español_MS_Briggs&Hamilton.pdf.
References
Ausserer A. (1871). Beiträge zur Kenntniss der Arachniden-Familie der Territelariae Thorell (Mygalidae Autor). Verhandlungen der Kaiserlich-Königlichen Zoologisch-Botanischen Gesellschaft Wien 21, 117–224, pl. 1.
Ausserer A. (1875). Zweiter Beitrag zur Kenntniss der Arachniden-Familie der Territelariae Thorell (Mygalidae Autor). Verhandlungen der Kaiserlich-Königlichen Zoologisch-Botanischen Gesellschaft Wien 25, 125–206, pl. 5-7.
Avise J. C., Mitchell D. (2007). Time to standardize taxonomies. Systematic Biol. 56, 130–133. doi: 10.1080/10635150601145365
Bacher S. (2012). Still not enough taxonomists: reply to Joppa et al. Trends Ecol. Evol. 27, 65–66. doi: 10.1016/j.tree.2011.11.003
Bennett B. C., Balick M. J. (2014). Does the name really matter? The importance of botanical nomenclature and plant taxonomy in biomedical research. J. Ethnopharmacol. 152, 387–392. doi: 10.1016/j.jep.2013.11.042
Benoit P. L. G. (1965). Les genres des Barychelidae-Leptopelmatinae africains (Araneae–Orthognatha). Rev. Zoologie Botanique africaines 72, 72–78.
Benoit P. L. G. (1966). Les Barychelidae-Barychelinae africains et malgaches (Aran.-Orthogn.). Revue de Zoologie et de Botanique Africaines 74 (3-4), 209–241.
Bertani R., Almeida M. Q. (2021). Yanomamius n. gen., a new genus of tarantula from Brazilian and Venezuelan Amazon (Araneae, Theraphosidae), with description of three new species. Zootaxa 4933, Zootaxa–4933. doi: 10.11646/zootaxa.4933.3.2
Bertani R., da Silva Junior P. I. (2002). The first mygalomorph spider without spermathecae: Sickius longibulbi, with a revalidation of Sickius (Araneae, Theraphosidae, Ischnocolinae). J. Arachnol. 30, 519–526. doi: 10.1636/0161-8202(2002)030[0519:TFMSWS]2.0.CO;2
Bertani R., Fukushima C. S. (2009). Description of two new species of Avicularia Lamarck 1818 and redescription of Avicularia diversipes (CL Koch 1842) (Araneae, Theraphosidae, Aviculariinae)—three possibly threatened Brazilian species. Zootaxa 2223, 25–47. doi: 10.11646/zootaxa.2223.1
Bertani R., Raven R. J. (2023). On the genus Thalerommata Ausserer 1875 (Araneae, Theraphosidae), with the description of six new species. Zootaxa 5271, 201–230. doi: 10.11646/zootaxa.5271.2
Biswas A., Chaitanya R., Karanth K. P. (2023). The tangled biogeographic history of tarantulas: An African centre of origin rules out the centrifugal model of speciation. J. Biogeogr. 50, 1341–1351. doi: 10.1111/jbi.14678
Biswas A., Karanth K. P. (2024). All about being old and shooting hairs: clade age and urticating hair explain the patterns of diversification in tarantulas. Evolution 78, 146–159. doi: 10.1093/evolut/qpad198
Bond J. E. (2012). Phylogenetic treatment and taxonomic revision of the trapdoor spider genus Aptostichus Simon (Araneae, Mygalomorphae, Euctenizidae). ZooKeys 252), 1. doi: 10.3897/zookeys.252.3588
Bond J. E., Garrison N. L., Hamilton C. A., Godwin R. L., Hedin M., Agnarsson I. (2014). Phylogenomics resolves a spider backbone phylogeny and rejects a prevailing paradigm for orb web evolution. Curr. Biol. 24, 1765–1771. doi: 10.1016/j.cub.2014.06.034
Bond J. E., Godwin R. L., Colby J. D., Newton L. G., Zahnle X. J., Agnarsson I., et al. (2022). Improving taxonomic practices and enhancing its extensibility—an example from araneology. Diversity 14, 5. doi: 10.3390/d14010005
Bond J. E., Hendrixson B. E., Hamilton C. A., Hedin M. (2012). A reconsideration of the classification of the spider infraorder Mygalomorphae (Arachnida: Araneae) based on three nuclear genes and morphology. PloS One 7, e38753. doi: 10.1371/journal.pone.0038753
Bortolus A. (2008). Error cascades in the biological sciences: the unwanted consequences of using bad taxonomy in ecology. AMBIO: A J. Hum. Environ. 37, 114–118. doi: 10.1579/0044-7447(2008)37[114:ECITBS]2.0.CO;2
Briggs E. J., Santana R. C., Raven R. J., Cook L. G. (2023). Assessing the diversity of Australian tarantulas (Araneae: Theraphosidae) using DNA barcoding and iterative species delimitation. Austral Entomol. 62, 464–478. doi: 10.1111/aen.12666
Brignoli P. M. (1985). On some generic homonymies in spiders (Araneae). Bull. Br. Arachnological Soc. 6, 380.
Bücherl W., Timotheo-da-Costa A., Lucas S. (1971). Revisão de alguns tipos de aranhas caranguejeiras (Orthognatha) estabelecidos por Candido de Mello-Leitão e depositados no Museu Nacional do Rio. Memórias do Instituto Butantan 35, 117–138.
Candia-Ramírez D. T., Francke O. F. (2021). Another stripe on the tiger makes no difference? Unexpected diversity in the widespread tiger tarantula Davus pentaloris (Araneae: Theraphosidae: Theraphosinae). Zoological J. Linn. Soc. 192, 75–104. doi: 10.1093/zoolinnean/zlaa107
Chomphuphuang N., Sippawat Z., Sriranan P., Piyatrakulchai P., Songsangchote C. (2023). A new electric-blue tarantula species of the genus Chilobrachys Karsh 1892 from Thailand (Araneae, Mygalomorphae, Theraphosidae). ZooKeys 1180, 105. doi: 10.3897/zookeys.1180.106278
Churchill T. B., Raven R. J. (1992). Systematics of the intertidal trapdoor spider genus Idioctis (Mygalomorphae: Barychelidae) in the western Pacific with a new genus from the northeast. Memoirs Queensland Museum 32, 9–30.
Cifuentes Y., Bertani R. (2022). Taxonomic revision and cladistic analysis of the tarantula genera Tapinauchenius Ausserer 1871, Psalmopoeus Pocock 1985, and Amazonius n. gen.(Theraphosidae, Psalmopoeinae). Zootaxa 5101, 1–123. doi: 10.11646/zootaxa.5101.1
Cifuentes Y., Estrada-Gomez S., Vargas-Muñoz L. J., Perafán C. (2016). Description and molecular characterization of a new species of tarantula, Pamphobeteus verdolaga, from Colombia (Araneae: Mygalomorphae: Theraphosidae). Zoologia (Curitiba) 33, e20160113. doi: 10.1590/s1984-4689zool-20160113
Clerck C. (1757). Svenska Spindlar: uti sina hufvud-slågter indelte samt under några och sextio särskildte arter; beskrefne och med illuminerade figurer uplyste (Stockholm: Salvius). doi: 10.5962/bhl.title.119890
Cook L. G., Edwards R. D., Crisp M. D., Hardy N. B. (2010). Need morphology always be required for new species descriptions? Invertebrate Systematics 24, 322–326. doi: 10.1071/IS10011
Dayrat B. (2005). Towards integrative taxonomy. Biol. J. Linn. Soc. 85, 407–417. doi: 10.1111/bij.2005.85.issue-3
De Queiroz K. (2005). A unified concept of species and its consequences for the future of taxonomy. Proc. California Acad. Sci. 56, 196–215.
De Queiroz K. (2007). Species concepts and species delimitation. Syst. Biol. 56, 879–886. doi: 10.1080/10635150701701083
Echeverri M., Torres S. G., Pinel N., Perafán C. (2023). Four new species of mygalomorph spiders (Araneae, Halonoproctidae and Theraphosidae) from the Colombian Pacific region (Bahía Solano, Chocó). ZooKeys 1166, 49. doi: 10.3897/zookeys.1166.101069
Ely C. V., de Loreto Bordignon S. A., Trevisan R., Boldrini I. I. (2017). Implications of poor taxonomy in conservation. J. Nat. Conserv. 36, 10–13. doi: 10.1016/j.jnc.2017.01.003
Ferretti N., Cavallo P., Chaparro J. C., Ríos-Tamayo D., Seimon T. A., West R. (2018). The neotropical genus Hapalotremus Simon 1903 (Araneae: Theraphosidae), with the description of seven new species and the highest altitude record for the family. J. Natural History 52, 1927–1984. doi: 10.1080/00222933.2018.1506521
Ferretti N. E., Nicoletta M. M., Soresi D. S. (2024). An integrative taxonomy approach evaluates the limits of the widespread tarantula Plesiopelma longisternale (Araneae: Mygalomorphae: Theraphosidae) and reveals a new species from Argentina. Zoologischer Anzeiger 308, 131–143. doi: 10.1016/j.jcz.2023.12.003
Foley S., Krehenwinkel H., Cheng D. Q., Piel W. H. (2021). Phylogenomic analyses reveal a Gondwanan origin and repeated out of India colonizations into Asia by tarantulas (Araneae: Theraphosidae). PeerJ 9, e11162. doi: 10.7717/peerj.11162
Foley S., Lüddecke T., Cheng D. Q., Krehenwinkel H., Künzel S., Longhorn S. J., et al. (2019). Tarantula phylogenomics: a robust phylogeny of deep theraphosid clades inferred from transcriptome data sheds light on the prickly issue of urticating setae evolution. Mol. Phylogenet. Evol. 140, 106573. doi: 10.1016/j.ympev.2019.106573
Fukushima C. S., Bertani R. (2017). Taxonomic revision and cladistic analysis of Avicularia Lamarck 1818 (Araneae, Theraphosidae, Aviculariinae) with description of three new aviculariine genera. ZooKeys 659), 1. doi: 10.3897/zookeys.659.10717
Gabriel R., Sherwood D., Pérez-Miles F. (2023). Four new species and two new genera of theraphosid spider from Bolivia (Araneae: Theraphosidae). Arachnology 19, pp.944–pp.951. doi: 10.13156/arac.2023.19.6.944
Galleti-Lima A., Hamilton C. A., Borges L. M., Guadanucci J. P. L. (2023). Phylogenomics of Lasiodoriforms: reclassification of the South American genus Vitalius Lucas, Silva and Bertani and allied genera (Araneae: Theraphosidae). Front. Ecol. Evol. 11, 1177627. doi: 10.3389/fevo.2023.1177627
Gallon R. C. (2003). A new African arboreal genus and species of theraphosid spider (Araneae, Theraphosidae, Stromatopelminae) which lacks spermathecae. Bulletin-British Arachnological Soc. 12, 405–411.
Gallon R. C. (2005). Encyocratella olivacea Strand 1907, a senior synonym of Xenodendrophila gabrieli Gallon 2003 (Araneae: Theraphosidae: Stromatopelminae) with a description of the male. Zootaxa 1003, 45–56. doi: 10.11646/zootaxa.1003.1
Garrison N. L., Rodriguez J., Agnarsson I., Coddington J. A., Griswold C. E., Hamilton C. A., et al. (2016). Spider phylogenomics: untangling the Spider Tree of Life. PeerJ 4, e1719. doi: 10.7717/peerj.1719
Goloboff-Szumik V. E., Ríos-Tamayo D. (2022). Description of the female of Melloina gracilis (Schenkel 1953) (Mygalomorphae: Theraphosidae) with comments on the familial placement of Melloina. Rev. del Museo Argentino Cienc. Naturales 24, 249–255. doi: 10.22179/REVMACN.24.781
Gonzalez-Filho H. M., Guadanucci J. P. L., Brescovit A. D. (2023). On the genus Ammonius Thorell 1899 (Mygalomorphae, Barychelidae): description of the female of A. pupulus, a new species and new distribution records. Eur. J. Taxonomy 861, 113–131. doi: 10.5852/ejt.2023.861.2071
Graham M. R., Santibáñez-López C. E., Derkarabetian S., Hendrixson B. E. (2020). Pleistocene persistence and expansion in tarantulas on the Colorado Plateau and the effects of missing data on phylogeographical inferences from RADseq. Mol. Ecol. 29, 3684–3701. doi: 10.1111/mec.15588
Guadanucci J. P. L. (2011). Cladistic analysis and biogeography of the genus Oligoxystre Vellard 1924 (Araneae: Mygalomorphae: Theraphosidae). J. Arachnol. 39, 320–326. doi: 10.1636/CA10-81.1
Guadanucci J. P. L. (2012). Trichobothrial morphology of Theraphosidae and Barychelidae spiders (Araneae, Mygalomorphae). Zootaxa 3439, 1–42. doi: 10.11646/zootaxa.3439.1
Guadanucci J. P. L. (2014). Theraphosidae phylogeny: relationships of the ‘Ischnocolinae’ genera (Araneae, Mygalomorphae). Zoologica Scripta 43, 508–518. doi: 10.1111/zsc.12065
Hamilton C. A., Formanowicz D. R., Bond J. E. (2011). Species delimitation and phylogeography of Aphonopelma hentzi (Araneae, Mygalomorphae, Theraphosidae): cryptic diversity in North American tarantulas. PloS One 6, e26207. doi: 10.1371/journal.pone.0026207
Hamilton C. A., Hendrixson B. E., Bond J. E. (2016). Taxonomic revision of the tarantula genus Aphonopelma Pocock 1901(Araneae, mygalomorphae, theraphosidae) within the United States. ZooKeys 560), 1. doi: 10.3897/zookeys.560.6264
Hamilton C. A., Hendrixson B. E., Brewer M. S., Bond J. E. (2014). An evaluation of sampling effects on multiple DNA barcoding methods leads to an integrative approach for delimiting species: a case study of the North American tarantula genus Aphonopelma (Araneae, Mygalomorphae, Theraphosidae). Mol. Phylogenet. Evol. 71, 79–93. doi: 10.1016/j.ympev.2013.11.007
Hebert P. D., Cywinska A., Ball S. L., DeWaard J. R. (2003). Biological identifications through DNA barcodes. Proc. R. Soc. London Ser. B: Biol. Sci. 270, 313–321. doi: 10.1098/rspb.2002.2218
Hebert P. D., Penton E. H., Burns J. M., Janzen D. H., Hallwachs W. (2004). Ten species in one: DNA barcoding reveals cryptic species in the neotropical skipper butterfly Astraptes fulgerator. Proc. Natl. Acad. Sci. 101, 14812–14817. doi: 10.1073/pnas.0406166101
Hedin M., Bond J. E. (2006). Molecular phylogenetics of the spider infraorder Mygalomorphae using nuclear rRNA genes (18S and 28S): conflict and agreement with the current system of classification. Mol. Phylogenet. Evol. 41, 454–471. doi: 10.1016/j.ympev.2006.05.017
Hedin M., Derkarabetian S., Alfaro A., Ramírez M. J., Bond J. E. (2019). Phylogenomic analysis and revised classification of atypoid mygalomorph spiders (Araneae, Mygalomorphae), with notes on arachnid ultraconserved element loci. PeerJ 7, e6864. doi: 10.7717/peerj.6864
Hendrixson B. E., DeRussy B. M., Hamilton C. A., Bond J. E. (2013). An exploration of species boundaries in turret-building tarantulas of the Mojave Desert (Araneae, Mygalomorphae, Theraphosidae, Aphonopelma). Mol. Phylogenet. Evol. 66 (1), 327–340. doi: 10.1016/j.ympev.2012.10.004
Hendrixson B. E., Guice A. V., Bond J. E. (2015). Integrative species delimitation and conservation of tarantulas (Araneae, Mygalomorphae, Theraphosidae) from a North American biodiversity hotspot. Insect Conserv. Diversity 8, 120–131. doi: 10.1111/icad.12089
Hewitt J. (1919). Descriptions of new South African araneae and solifugae. Ann. Transvaal Museum 6, 63–111.
Hilton E. J., Watkins-Colwell G. J., Huber S. K. (2021). The expanding role of natural history collections. Ichthyol. Herpetol. 109, 379–391. doi: 10.1643/t2020018
Hormiga G., Kulkarni S., Arnedo M., Dimitrov D., Giribet G., Kallal R. J., et al. (2023). Genitalic morphology and phylogenomic placement of the Australian spider Paraplectanoides crassipes Keyserling 1886 (Araneae, Araneidae) with a discussion on the classification of the family Araneidae. Invertebrate Systematics 37, 797–818. doi: 10.1071/IS23050
Hüsser M. (2018). A first phylogenetic analysis reveals a new arboreal tarantula genus from South America with description of a new species and two new species of Tapinauchenius Ausserer 1871 (Araneae, Mygalomorphae and Theraphosidae). ZooKeys 784), 59. doi: 10.3897/zookeys.784.26521
Jörger K. M., Schrödl M. (2013). How to describe a cryptic species? Practical challenges of molecular taxonomy. Front. zool. 10, 1–27. doi: 10.1186/1742-9994-10-59
Jose K. S., Sebastian P. A. (2008). Description of two mygalomorph spiders from south India (Araneae: Barychelidae, Theraphosidae). Rev. ibérica aracnología 15, 29–34.
Kaderka R., Lüddecke T., Řezáč M., Řezáčová V., Hüsser M. (2023). Revision of the Peruvian tarantula Homoeomma Peruvianum (Chamberlin 1916): description of a new genus with eleven new species and insights to the evolution of montane tarantulas (Araneae: Theraphosidae: Theraphosinae). J. Natural History 57, 1710–1824. doi: 10.1080/00222933.2023.2265621
Kaiser H., Crother B. I., Kelly C. M., Luiselli L., O’Shea M., Ota H., et al. (2013). Best practices: in the 21st century, taxonomic decisions in herpetology are acceptable only when supported by a body of evidence and published via peer-review. Herpetological Rev. 44, 8–23.
Kallal R. J., Dimitrov D., Arnedo M. A., Giribet G., Hormiga G. (2020). Monophyly, taxon sampling, and the nature of ranks in the classification of orb-weaving spiders (Araneae: Araneoidea). Systematic Biol. 69, 401–411. doi: 10.1093/sysbio/syz043
Kambas D. (2024). Tarantupedia: an online taxonomic database for the world’s largest spiders. www.tarantupedia.com. Accessed on 2024-4-16.
Korba J., Opatova V., Calatayud-Mascarell A., Enguídanos A., Bellvert A., Adrián S., et al. (2022). Systematics and phylogeography of western Mediterranean tarantulas (Araneae: Theraphosidae). Zoological J. Linn. Soc. 196, 845–884. doi: 10.1093/zoolinnean/zlac042
Kulkarni S., Wood H. M., Hormiga G. (2023). Advances in the reconstruction of the Spider Tree of Life: a roadmap for spider systematics and comparative studies. Cladistics 39, 479–532. doi: 10.1111/cla.12557
Kulkarni S., Wood H., Lloyd M., Hormiga G. (2020). Spider-specific probe set for ultraconserved elements offers new perspectives on the evolutionary history of spiders (Arachnida, Araneae). Mol. Ecol. Resour. 20, 185–203. doi: 10.1111/1755-0998.13099
Kuntner M., Čandek K., Gregorič M., Turk E., Hamilton C. A., Chamberland L., et al. (2023). Increasing information content and diagnosability in family-level classifications. Systematic Biol. 72, 964–971. doi: 10.1093/sysbio/syad021
Lüddecke T., Krehenwinkel H., Canning G., Glaw F., Longhorn S. J., Tänzler R., et al. (2018). Discovering the silk road: Nuclear and mitochondrial sequence data resolve the phylogenetic relationships among theraphosid spider subfamilies. Mol. Phylogenet. Evol. 119, 63–70. doi: 10.1016/j.ympev.2017.10.015
Maddison W. P., Whitton J. (2023). The species as a reproductive community emerging from the past. Bull. Soc. Systematic Biologists 2, 1–35. doi: 10.18061/bssb.v2i1
Mahner M. (1993). What is a species? A contribution to the never ending species debate in biology. J. Gen. philosophy Sci. 24, 103–126. doi: 10.1007/BF00769517
Mallet J. (1995). A species definition for the modern synthesis. Trends Ecol. Evol. 10, 294–299. doi: 10.1016/0169-5347(95)90031-4
Marin J., Rapacciuolo G., Costa G. C., Graham C. H., Brooks T. M., Young B. E., et al. (2018). Evolutionary time drives global tetrapod diversity. Proc. R. Soc. B: Biol. Sci. 285, 20172378. doi: 10.1098/rspb.2017.2378
Mendoza J., Francke O. (2017). Systematic revision of Brachypelma red-kneed tarantulas (Araneae: Theraphosidae), and the use of DNA barcodes to assist in the identification and conservation of CITES-listed species. Invertebrate Systematics 31 (2), 157–179. doi: 10.1071/IS16023
Mendoza J., Francke O. (2020). Systematic revision of Mexican threatened tarantulas Brachypelma (Araneae: Theraphosidae: Theraphosinae), with a description of a new genus, and implications on the conservation. Zoological J. Linn. Soc. 188, 82–147. doi: 10.1093/zoolinnean/zlz046
Midgley J. M., Engelbrecht I. (2019). New collection records for Theraphosidae (Araneae, Mygalomorphae) in Angola, with the description of a remarkable new species of Ceratogyrus. Afr. Invertebrates 60, 1–13. doi: 10.3897/afrinvertebr.60.32141
Mirza Z. A., Sanap R. V., Bhosale H. (2014). Preliminary review of Indian Eumenophorinae (Araneae: Theraphosidae) with description of a new genus and five new species from the Western Ghats. PloS One 9, e87928. doi: 10.1371/journal.pone.0087928
Moeslund J. E., Arge L., Bøcher P. K., Dalgaard T., Svenning J. C. (2013). Topography as a driver of local terrestrial vascular plant diversity patterns. Nordic J. Bot. 31, 129–144. doi: 10.1111/j.1756-1051.2013.00082.x
Montemor V. M., West R. C., Zamani A., Moradmand M., Wirth V. V., Wendt I., et al. (2020). Taxonomy of the genus Ischnocolus in the Middle East, with description of a new species from Oman and Iran (Araneae: Theraphosidae). Zool. Middle East 66, 76–90. doi: 10.1080/09397140.2020.1675994
Montes de Oca L., D’Elía G., Pérez-Miles F. (2016). An integrative approach for species delimitation in the spider genus Grammostola (Theraphosidae, Mygalomorphae). Zoologica Scripta 45, 322–333. doi: 10.1111/zsc.12152
Mori A., Bertani R. (2016). On the genus Cosmopelma Simon 1889 (Araneae, barychelidae). Zootaxa 4137, pp.520–pp.534. doi: 10.11646/zootaxa.4137.4
Mori A., Bertani R. (2020). Revision and cladistic analysis of Psalistops Simon 1889, Trichopelma Simon 1888 and Cyrtogrammomma Pocock 1895 (Araneae: Theraphosidae) based on a cladistic analysis of relationships of Theraphosidae, Barychelidae and Paratropididae. Zootaxa 4873, 1–132. doi: 10.11646/zootaxa.4873.1
Nicholson K. E., Crother B. I., Guyer C., Savage J. M. (2014). Anole classification: a response to Poe. Zootaxa 3814, 109–120. doi: 10.11646/zootaxa.3814.1
Nicholson K. E., Crother B. I., Guyer C., Savage J. M. (2012). It is time for a new classification of anoles (Squamata: Dactyloidae). Zootaxa 3477, 1–108. doi: 10.11646/zootaxa.3477.1
Nicoletta M., Chaparro J. C., Mamani L., Ochoa J. A., West R. C., Ferretti N. E. (2020). Two new endemic species of Bistriopelma (Araneae: Theraphosidae) from Peru, including a new remarkable horned tarantula. Eur. J. Taxonomy 644, 1–20. doi: 10.5852/ejt.2020.644
Nunn S. C., West R. C., Von Wirth V. (2016). A revision of the selenocosmiine tarantula genus Phlogiellus Pocock 1897 (Araneae: Theraphosidae), with description of 4 new species. Int. J. Zool. 2016, 1–54. doi: 10.1155/2016/9895234
Opatova V., Hamilton C. A., Hedin M., de Oca L. M., Král J., Bond J. E. (2020). Phylogenetic systematics and evolution of the spider infraorder Mygalomorphae using genomic scale data. Systematic Biol. 69, 671–707. doi: 10.1093/sysbio/syz064
Ortiz D. (2023). High utility of Ultraconserved Elements (UCE) for disentangling the elusive relationships of tarantulas. Zoologica Scripta 52, 645–653. doi: 10.1111/zsc.12619
Ortiz D., Francke O. F. (2015). Two new species of Bonnetina tarantulas (Theraphosidae: Theraphosinae) from Mexico: contributions to morphological nomenclature and molecular characterization of types. J. Natural History 49, 685–707. doi: 10.1080/00222933.2014.924770
Ortiz D., Francke O. F. (2016). Two DNA barcodes and morphology for multi-method species delimitation in Bonnetina tarantulas (Araneae: Theraphosidae). Mol. Phylogenet. Evol. 101, 176–193. doi: 10.1016/j.ympev.2016.05.003
Ortiz D., Francke O. F. (2017). Reconciling morphological and molecular systematics in tarantulas (Araneae: Theraphosidae): revision of the Mexican endemic genus Bonnetina. Zoological J. Linn. Soc. 180, 819–886. doi: 10.1093/zoolinnean/zlw013
Paknia O., Rajaei Sh H., Koch A. (2015). Lack of well-maintained natural history collections and taxonomists in megadiverse developing countries hampers global biodiversity exploration. Organisms Diversity Evol. 15, 619–629. doi: 10.1007/s13127-015-0202-1
Paradis E., Claude J., Strimmer K. (2004). APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20, 289–290. doi: 10.1093/bioinformatics/btg412
Peñaherrera-R P., Ghia T., Sherwood D., Gabriel R. (2024). New insights on male palpal bulb morphology in Cymbiapophysa Gabriel & Sherwood 2020, with four new species from Ecuador (Araneae: Theraphosidae). Arachnology 19, 1003–1017. doi: 10.13156/arac.2024.19.7.1003
Perafán C., Cifuentes Y., Estrada-Gomez S. (2015). Aguapanela, a new tarantula genus from the Colombian Andes (Araneae, Theraphosidae). Zootaxa 4033, 529–542. doi: 10.11646/zootaxa.4033.4
Perafán C., Galvis W., Pérez-Miles F. (2019). The first Paratropididae (Araneae, Mygalomorphae) from Colombia: new genus, species and records. ZooKeys 830, 1. doi: 10.3897/zookeys.830.31433
Pérez-Miles F., Bonaldo A. B., Miglio L. T. (2014). Bumba, a replacement name for Maraca Pérez-Miles 2005 and Bumba lennoni, a new tarantula species from western Amazonia (Araneae, Theraphosidae, Theraphosinae). ZooKeys 448, 1–8. doi: 10.3897/zookeys.448.7920
Pérez-Miles F. (2006). A replacement name for Iracema Pérez-Miles 2000 (Araneae, Theraphosidae). J. Arachnol. 34, 247. doi: 10.1636/H04-15.1
Pérez-Miles F., Locht A. (2003). Revision and cladistic analysis of the genus Hemirrhagus Simon 1903 (Araneae, theraphosidae, theraphosinae). Bulletin-British Arachnological Soc. 12, 365–375.
Pérez-Miles F. (2000). Iracema cabocla new genus and species of a theraphosid spider from Amazonic Brazil (Araneae, theraphosinae). J. Arachnology 28, 141–148. doi: 10.1636/H04-15.1
Pickard-Cambridge F. O. (1897). “Arachnida - Araneida and Opiliones,” in Porter] R. H. editor. Biologia Centrali-Americana, Zoology, vol. 2. (London), 1–40, pl. 1-3.
Platnick N. I., Raven R. J. (2013). Spider systematics: past and future. Zootaxa 3683, 595–600. doi: 10.11646/zootaxa.3683.5
Pocock R. I. (1892). XXXVIII.—Liphistius and its bearing upon the classification of spiders. J. Natural History 10, 306–314.
Pocock R. I. (1895a). XIX.—On a new and natural grouping of some of the Oriental genera of Mygalomorphae, with descriptions of new genera and species. J. Natural History 15, 165–184.
Pocock R. I. (1895b). Description of two new spiders obtained by Messrs. J. J. Quelch and F. McConnell on the summit of Mount Roraima, in Demerara; with a note upon the systematic position of the genus Desis. Ann. Magazine Natural History 16, 139–143. doi: 10.1080/00222939508680241
Pocock R. I. (1897). On the spiders of the suborder Mygalomorphae from the Ethiopian Region, contained in the collection of the British Museum. Proc. Zoological Soc. London 65, 724–774, pl. 46-48. doi: 10.1111/j.1096-3642.1897.tb03116.x
Pocock R. I. (1900). The fauna of British India, including Ceylon and Burma. Arachnida (London: Taylor and Francis), 279.
Pocock R. I. (1901). Some new and old genera of S.-American Avicularidae. Ann. Magazine Natural History 8, 540–555.
Poe S. (2013). 1986 Redux: New genera of anoles (Squamata: Dactyloidae) are unwarranted. Zootaxa 3626, 295–299. doi: 10.11646/zootaxa.3626.2
Poe S., Nieto-Montes de Oca A., Torres-Carvajal O., De Queiroz K., Velasco J. A., Truett B., et al. (2017). A phylogenetic, biogeographic, and taxonomic study of all extant species of Anolis (Squamata; Iguanidae). Systematic Biol. 66, 663–697. doi: 10.1093/sysbio/syx029
Prasanth M. T., Jose K. S. (2014). A new species of the genus Haploclastus from Western Ghats, India (Araneae: Theraphosidae). Munis Entomol. Zool. 9, 494–500.
Purcell W. F. (1902). On the South African Theraphosidae or “Baviaan” spiders, in the collection of the South African Museum. Trans. South Afr. Philos. Soc. 11, 319–347.
Raven R. J. (1985). The spider infraorder Mygalomorphae (Araneae): cladistics and systematics. Bulletin of the AMNH. American Museum of Natural History Museum USA, Vol. 182. article 1.
Raven R. J. (1986). A revision of the spider genus Sason Simon (Sasoninae, Barychelidae, Mygalomorphae) and its historical biogeography. J. Arachnol. 14, 47–70.
Raven R. J. (1988). A revision of the mygalomorph spider genus Idioctis (Araneae, Barychelidae). Am. Museum Novitates 2929, 14.
Raven R. J. (1990a). Comments on the proposed precedence of Aphonopelma Pocock 1901 (Arachnida, araneae) over Rhechostica Simon 1892. Bull. Zoological Nomenclature 47, 126–127. doi: 10.5962/bhl.part.2678
Raven R. J. (1990b). A revision of the Australian spider genus Trittame Koch (Mygalomorphae: Barychelidae) and a new related genus. Invertebrate Systematics 4, 21–54. doi: 10.1071/IT9900021
Raven R. J. (1994). Mygalomorph spiders of the Barychelidae in Australia and the western Pacific. Memoirs Queensland Museum 35, 291–706.
Raven R. J. (2000). Taxonomica araneae I: barychelidae, theraphosidae, nemesiidae and dipluridae (Araneae). Memoirs Queensland Museum 45, 569–575.
Raven R. J. (2005). A new tarantula species from northern Australia (Araneae, Theraphosidae). Zootaxa 1004, 15–28. doi: 10.11646/zootaxa.1004.1
Raven R. J. (2008). A revision of the mygalomorph spider genus Monodontium Kulczynski (Barychelidae: Araneae). Raffles B Zool 56, 29–44.
Renner S. S. (2016). A return to Linnaeus’s focus on diagnosis, not description: the use of DNA characters in the formal naming of species. Systematic Biol. 65, 1085–1095. doi: 10.1093/sysbio/syw032
Ríos-Tamayo D. (2023). A new species of the mygalomorph genus Strophaeus Ausserer 1875 (Araneae: barychelidae) from Colombia. Arachnology 19, 881–884. doi: 10.13156/arac.2023.19.6.881
Rudloff J.-P. (2001). Anmerkungen zur systematischen Stellung von Acanthopelma rufescens F.O.P.-Cambridge 1897 und Acanthopelma annae Reichling 1997 (Ischnocolinae: Theraphosidae: Mygalomorphae), sowie die Einrichtung einer neuen Gattung Reichlingia gen. nov. (Mygalomorphae: Barychelidae: Trichopelmatinae). Arthropoda 9, 14–20.
Samm R., Schmidt G. (2008). Sinurticantinae subfamilia nov.—eine neue Unterfamilie der Theraphosidae (Araneae). Tarantulas World 141, 3–14.
Samm R., Schmidt G. (2010). Psalmopoeinae subfamilia nov.–eine neue Unterfamilie der Theraphosidae (Araneae). Tarantulas World 142, 35–41.
Sanap R. V., Mirza Z. A. (2014). A new iridescent tarantula of the genus Thrigmopoeus Pocock, 1899 from Western Ghats, India. Comptes Rendus Biologies 337 (7-8), 480–486. doi: 10.1016/j.crvi.2014.06.003
Schmidt G. (1993). Vogelspinnen: Vorkommen, Lebensweise, Haltung und Zucht. Hanover, Germany: Landbuch.
Schmidt G. E. W. (1995). Gehören, ”Selenocosmia” crassipes (L. Koch 1873) und” Selenocosmia” stirlingi Hogg 1901 (Araneida: Theraphosidae: Selenocosmiinae) wirklich zu Selenocosmia Ausserer 1871. Arachnologisches Magazin 3, 1–12.
Schmidt G., von Wirth V. (1996). Haplocosmia Nepalensis gen. et sp. n., die erste Vogelspinne aus Nepal (Araneida: Theraphosidae: Selenocosmiinae). Arthropoda 4, 12–15.
Sherwood D., Gabriel R., Peñaherrera-R P., Brescovit A. D., Lucas S. M. (2023). On the tarantula genus Xenesthis Simon 1891, with description of a new species from Venezuela (Araneae: Theraphosidae). Taxonomy 3, 509–527. doi: 10.3390/taxonomy3040029
Siliwal M., Gupta N., Raven R. (2012). A new genus of the family Theraphosidae (Araneae: Mygalomorphae) with description of three new species from the Western Ghats of Karnataka, India. J. Threatened Taxa 4, 3233–3254. doi: 10.11609/JoTT
Siliwal M., Molur S. (2009). A new species of the genus Sason (Araneae: Barychelidae) from Rameshwaram Island, Tamil Nadu, India. Zootaxa 2283, 60–68. doi: 10.11646/zootaxa.2283.1
Siliwal M., Molur S., Raven R. (2009). Two new species of the genus Diplothele (Araneae, Barychelidae) from Orissa, India with notes on D. walshi. J. Arachnol. 37, 178–187. doi: 10.1636/A08-64.1
Simon E. (1889). “Voyage de ME Simon au Venezuela (Décembre 1887–Avril 1888). 4e Mémoire. Arachnides,” in Annales de la Société entomologique de France. Paris, France: Annals of the Entomological Society of France, vol. 6. , 169–220.
Simon E. (1892). Histoire naturelle des araignées Vol. 1 (Paris: Libraire Encyclopedique de Roret), 1–256. doi: 10.5962/bhl.title.51973
Simon E. (1888). Etudes arachnologiques. 21e mémoire. XXIX. descriptions d'espèces et de genres nouveaux de l'Amérique centrale et des antilles. Annales la Société Entomologique France 8 (6), 203–216.
Simon E. (1891). Liste des espéces de la famille des Aviculariidae qui habitent le Mexique et l'Amérique du Nord. Actes de la Société Linnéenne de Bordeaux 44 (1890), 307–339.
Sivayyapram V., Kunsete C., Songsangchote C., Thanoosing C., Traiyasut P., Warrit N. (2020). Two new species of the Southeast Asian dwarf tarantula genus Phlogiellus Pocock 1887(Theraphosidae, Selenocosmiinae) and a discussion on the taxonomic problem of the genus. Zootaxa 4859, 487–506. doi: 10.11646/zootaxa.4859.4
Sluys R. (2013). The unappreciated, fundamentally analytical nature of taxonomy and the implications for the inventory of biodiversity. Biodiversity Conserv. 22, 1095–1105. doi: 10.1007/s10531-013-0472-x
Smith A. M. (1990). Baboon spiders: tarantulas of Africa and the Middle East Vol. 1 (London: Fitzgerald Pub).
Smith A. M. (1995). Tarantula Spiders: Tarantulas of the U.S.A. and Mexico (London: Fitzgerald Publishing), 196.
Songsangchote C., Sippawat Z., Khaikaew W., Chomphuphuang N. (2022). A new genus of bamboo culm tarantula from Thailand (Araneae, Mygalomorphae, Theraphosidae). ZooKeys 1080, 1. doi: 10.3897/zookeys.1080.76876
Starrett J., Derkarabetian S., Hedin M., Bryson R. W. Jr., McCormack J. E., Faircloth B. C. (2017). High phylogenetic utility of an ultraconserved element probe set designed for Arachnida. Mol. Ecol. Resour. 17, 812–823. doi: 10.1111/1755-0998.12621
Stork N. E., McBroom J., Gely C., Hamilton A. J. (2015). New approaches narrow global species estimates for beetles, insects, and terrestrial arthropods. Proc. Natl. Acad. Sci. 112, 7519–7523. doi: 10.1073/pnas.1502408112
Strand E. (1907). Vorläufige Diagnosen afrikanischer und südamerikanischer Spinnen. Zoologischer Anzeiger 31, 525–558.
Thorell T. (1869). On European spiders. Review of the European genera of spiders, preceded by some observations on zoological nomenclature [first part]. Nova Acta Regiae Societatis Scientiarum Upsaliensis 7, 1–108.
Thorell T. (1887). Viaggio di L. Fea in Birmania e regioni vicine. II. Primo saggio sui ragni birmani. Annali del Museo Civico di Storia Naturale di Genova 25, 5–417.
Thorell T. (1890). Aracnidi di Pinang raccolti nel 1889 dai Signori L. Loria e L. Fea. Genoa, Italy: Annals of the Civic Museum of Natural History.
Thorell T. (1870). On european spiders. review of the european genera of spiders, preceded by some observations on zoological nomenclature [second part]. Nova Acta Regiae Societatis Scientiarum Upsaliensis 7 (3), 109–242.
Turk E., Čandek K., Kralj-Fišer S., Kuntner M. (2020). Biogeographical history of golden orbweavers: Chronology of a global conquest. J. Biogeogr. 47, 1333–1344. doi: 10.1111/jbi.13838
Turner S. P., Longhorn S. J., Hamilton C. A., Gabriel R., Pérez-Miles F., Vogler A. P. (2018). Re-evaluating conservation priorities of New World tarantulas (Araneae: Theraphosidae) in a molecular framework indicates non-monophyly of the genera, Aphonopelma and Brachypelma. Systematics Biodiversity 16, 89–107. doi: 10.1080/14772000.2017.1346719
Valdecasas A. G., Pelaéz M. L., Wheeler Q. D., de Carvalho M. R. (2022). Evidence-based taxonomy: labels as illocutionary acts. Taxonomy 2, 339–346. doi: 10.3390/taxonomy2030026
Vol F. (2000). Description de Bonnetina cyaneifemur, gen. n. & sp. n. Araneae, Theraphosidae, Theraphosinae) du Mexique. Arachnides. 44, 2–9.
West R. C., Marshall S. D., Fukushima C. S., Bertani R. (2008). Review and cladistic analysis of the Neotropical tarantula genus Ephebopus Simon 1892 (Araneae: Theraphosidae) with notes on the Aviculariinae. Zootaxa 1849), 35–58. doi: 10.11646/zootaxa.1849.1
West R. C., Nunn S. C., Hogg S. (2012). A new tarantula genus, Psednocnemis, from West Malaysia (Araneae: Theraphosidae), with cladistic analyses and biogeography of Selenocosmiinae Simon 1889. Zootaxa 3299, 1–43. doi: 10.11646/zootaxa.3299.1
Wheeler Q. (2018). Blank canvas: The case for descriptive taxonomy. Integr. Comp. Biol. 58, 1118–1121. doi: 10.1093/icb/icy067
Wheeler Q. (2020). A taxonomic renaissance in three acts. Megataxa 1, 4–8. doi: 10.11646/megataxa.1.1.2
Wheeler W. C., Coddington J. A., Crowley L. M., Dimitrov D., Goloboff P. A., Griswold C. E., et al. (2017). The spider tree of life: phylogeny of Araneae based on target-gene analyses from an extensive taxon sampling. Cladistics 33, 574–616. doi: 10.1111/cla.12182
Wheeler Q. D., Meier R. (Eds.) (2000). Species concepts and phylogenetic theory: a debate (USA: Columbia University Press).
Wickham H., Averick M., Bryan J., Chang W., McGowan L. D. A., François R., et al. (2019). Welcome to the tidyverse. J. Open Source software 4, 1686. doi: 10.21105/joss.01686
Wilson J. D., Bond J. E., Harvey M. S., Ramírez M. J., Rix M. G. (2023). Correlation with a limited set of behavioral niches explains the convergence of somatic morphology in mygalomorph spiders. Ecol. Evol. 13, e9706. doi: 10.1002/ece3.9706
World Spider Catalog (2024). World spider catalog. Version 25.0 (Switzerland: Natural History Museum Bern). Available at: http://wsc.nmbe.ch.
Wunderlich J., Müller P. (2020). New and already described fossil spiders (Araneae) of 20 families in mid cretaceous Burmese amber with notes on spider phylogeny, evolution and classification. Beiträge zur Araneologie 13, 22164.
Yu K. P., Lo Y. Y., Cheng R. C., Raven R. J., Kuntner M. (2023). Discovery of a new intertidal trapdoor spider of the genus Idioctis (Araneae: Barychelidae), with a generic range extension to Taiwan. J. Arachnol. 51, 238–248. doi: 10.1636/JoA-S-22-020
Keywords: Barychelidae, Paratropididae, spider, review, Mygalomorphae, species concept
Citation: Briggs EJ and Hamilton CA (2024) What does the history of Theraphosidae systematics tell us about the future of tarantula taxonomy? Front. Arachn. Sci. 3:1445731. doi: 10.3389/frchs.2024.1445731
Received: 08 June 2024; Accepted: 30 July 2024;
Published: 30 August 2024.
Edited by:
Jason E. Bond, University of California, Davis, United StatesReviewed by:
Alireza Zamani, University of Turku, FinlandNelson Ferretti, CONICET Centro de Recursos Naturales Renovables de la Zona Semiárida (CERZOS), Argentina
Copyright © 2024 Briggs and Hamilton. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ethan J. Briggs, ZWJyaWdnc0B1aWRhaG8uZWR1; Chris A. Hamilton, aGFtaWx0b25sYWJAdWlkYWhvLmVkdQ==
 Ethan J. Briggs
Ethan J. Briggs Chris A. Hamilton
Chris A. Hamilton