
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Arachn. Sci. , 03 April 2024
Sec. Arachnid Ecology and Behavior
Volume 3 - 2024 | https://doi.org/10.3389/frchs.2024.1384128
This article is part of the Research Topic Function and Diversity of Arachnid Silk Structures View all 3 articles
Visual signal, mechanical reinforcement, protein storage, or non-functional stress response? Web decorations constructed by a number of orb web spider species puzzle behavioural ecologists. While some species use a variety of construction materials, it seems particularly difficult to solve the issue for silk decorations. The visual pattern of decoration structures has sparked the researchers’ imagination, and the conclusion that they act as signals is unsurprising. For over a century, however, we have not found a conclusive answer on a specific signal function of silk decorations. More recent studies even suggest that the construction mode of web decorations may render a specific signal function rather unlikely. In this review, I discuss reasons for the continuous struggle to find conclusive answers and what could be alternative routes for unravelling their adaptive significance. Based on my own experience in the field, I present a personal viewpoint, which I hope will be inspiring at a stage where research in this field seems to have reached a dead end. We are faced with a controversial debate, inconclusive and sometimes contradicting results; and an interest in new studies is fading. I draw the attention to three trouble areas, covering research gaps, logical inconsistencies and conceptual misunderstandings. More specifically: 1) Web decorations should be treated more as the dependent variable. 2) Experimental setups of several earlier studies appear flawed in retrospect, and their results thus overinterpreted. 3) We have not understood the evolutionary origin of web decoration. We may look at a signal that is still in an early phase of shaping, and inconclusive results may thus be inevitable. Finally, if web decorations do not act as signals, studies that look for exactly that cannot find conclusive results. In discussing these issues critically, I argue, we can open new routes for progress in finding a satisfying solution of the riddle of the silk decoration function.
When we ask a child to draw a spider web, the result is likely to be an orb web, as its rather simple, circular shape is the most prominent picture we have in mind. In fact, the orb web represents a highly evolved prey capture device (Blackledge et al., 2011), which can be regarded as part of a spider’s extended phenotype (Craig, 2003). The shape of the orb web has gone a long way through evolutionary times (Blackledge et al., 2009, 2011), yet its basic function has not changed much since the construction of primitive sheet webs built by the predecessors of orb weavers. Its function is to intercept prey animals with high retention efficiency (Sahni et al., 2014) and to signal the capture event to the host spider (Heiling and Herberstein, 2000). For their main prey, insects, thin spider silk threads are cryptic (Craig, 1986; Blackledge and Wenzel, 2000) and thus well-suited to be used in traps. In other words, the efficiency of an orb web to catch unsuspecting prey depends on its inconspicuousness. It is hence puzzling to any human observer to see some orb weaving spiders adding extra structures to their orb webs that increase its visibility. It is reasonable to conclude that web decorations must be associated with a selective advantage offsetting the apparent drawback of increased web visibility. Or, at least, the presence of web decorations cannot have any negative impact on the prey capture efficiency. So, are web decorations the result of adaptive evolution, or do they represent a non-adaptive trait that triggers human curiosity but is otherwise neutral to selection? Web decorations may also be regarded as an extended phenotype of the spider (Walter and Elgar, 2012), and the classical perspective is that the phenotype represents the current result of adaptive evolution (Ghalambor et al., 2007). However, evolutionary biologists debate lively about different ideas on how selection as well as non-adaptive processes affect the function or non-function of a certain trait (Putnins and Androulakis, 2021). It is known that the web decorating behaviour bears a genetic component (Craig et al., 2001), but the evolutionary origin and potential selective forces shaping the trait are yet unexplained. Consequently, it can be argued that it is unclear whether web decorations serve any function at all.
The human brain, however, has an aversion to accept randomness, but instead always tries to derive a meaning (Rominger et al., 2022), for example by assigning purpose to incidental patterns where there is actually none (Gladwell, 2005; Hanson et al., 2021). For the web decoration phenomenon this is interesting insofar as there are studies suggesting that these structures may well have no function and shaped into a reproducible pattern by non-intentional processes (Walter and Elgar, 2016; see also discussion below). Alternatively, we may be inclined to prematurely assume a function by simply deriving it from results of similar study systems. A familiar example is the lively discussion about the function of stripes in tigers and zebras. In tigers, the striped pattern has been demonstrated to disrupt their contours, which renders the predator cryptic in its surrounding habitat (Godfrey et al., 2008). A similar effect was at first also assumed for the stripes of zebras (see review in Ruxton, 2022). A number of studies tried to collect evidence, yet the support is not strong enough to conclude that the stripes render them cryptic too (Ruxton, 2022). Independent from its visual appearance, the stripes have been suggested to help thermoregulation (Cobb and Cobb, 2019), leading to the conclusion that their function is independent of its visual effect. Today, the most widely accepted function is the avoidance of tabanid flies. These insects would otherwise frequently bite, increasing the risk of pathogen transmissions, but the striped pattern helps to prevent the flies from landing on the zebras through optical nuisance (How et al., 2020). The web decoration research follows a similar zigzag route. The function of these curious structures may depend on its visual pattern, but perhaps a different one than initially assumed, or, it may be independent from the visual impression after all.
Web decoration patterns are quite distinct and often species specific (Bruce, 2006; Walter and Elgar, 2012). Consequently, the majority of hypotheses in previous studies presumed a pattern-specific function rather than considering the possibility of an independence from pattern. This is particularly the risk when web decorations are treated as the independent variable, yet it is what has happened numerous times in decoration research. Studies taking the alternative perspective, looking for factors that affect the decorating behaviour of the spiders, are in the minority, although they are likely to give very valuable functional insights.
Apart from the ambiguity of a visual signal effect, the number of other open research questions in the decoration field is surprisingly high, given that the debate has been lasting for more than a hundred years now. The most striking knowledge gap that may be key to the entire issue is that of the origin of the decorating behaviour. How has it evolved? Animal signals often derive from an elaboration of existing forms and from non-signalling predecessor traits (Endler, 1993). For web decorations, we have not identified any predecessors, and our current knowledge does not allow for a better conclusion than that they have come into rather sudden existence (Walter and Elgar, 2012). Unravelling the evolutionary origin is challenging, yet it may explain why we ended up with the dilemma of conflicting results on ultimate functions today, and it may give us a hint on how we could eventually unravel the adaptive significance of web decoration. This review aims to highlight both the potentially overrated explanatory power of previous studies as well as the numerous opportunities the field still offers to behavioural ecologists to make significant contributions.
A common habit in web decoration papers is to mention at some point how old the search for the function of these structures already is. Depending on the literature available, either Henry McCook (1889) or Eugène Simon (1892) are cited to have first described silken web decorations, specifically in Argiope spiders (family Araneidae), and have pondered on their alleged purpose. In a more recent publication, Alexander Kerr and co-authors (Kerr et al., 2021) dug a little deeper and found that none less than Charles Darwin already speculated about the web decoration function in an 1832 journal entry. Regardless of who has first discussed the issue, these records show that naturalists of the mid-late 19th century started the debate. However, as their work was more observational than experimental and as the methodological options were rather limited, their conclusions were merely based on intuition and logic. Darwin assumed, just from looking at the unusual web of this spider he never saw before, that the zigzag-shaped silk ribbons in the centre of its orb web (Figure 1) must strengthen it. Half a century later, Simon came to the same conclusion, arguing that the structure helps to secure the integrity of the capture web when the host spider struggles with larger prey. Accordingly, he called these zigzag-bands “stabilimenta”. To this date, this term is still synonymously used for web decorations and frequently found in the literature.
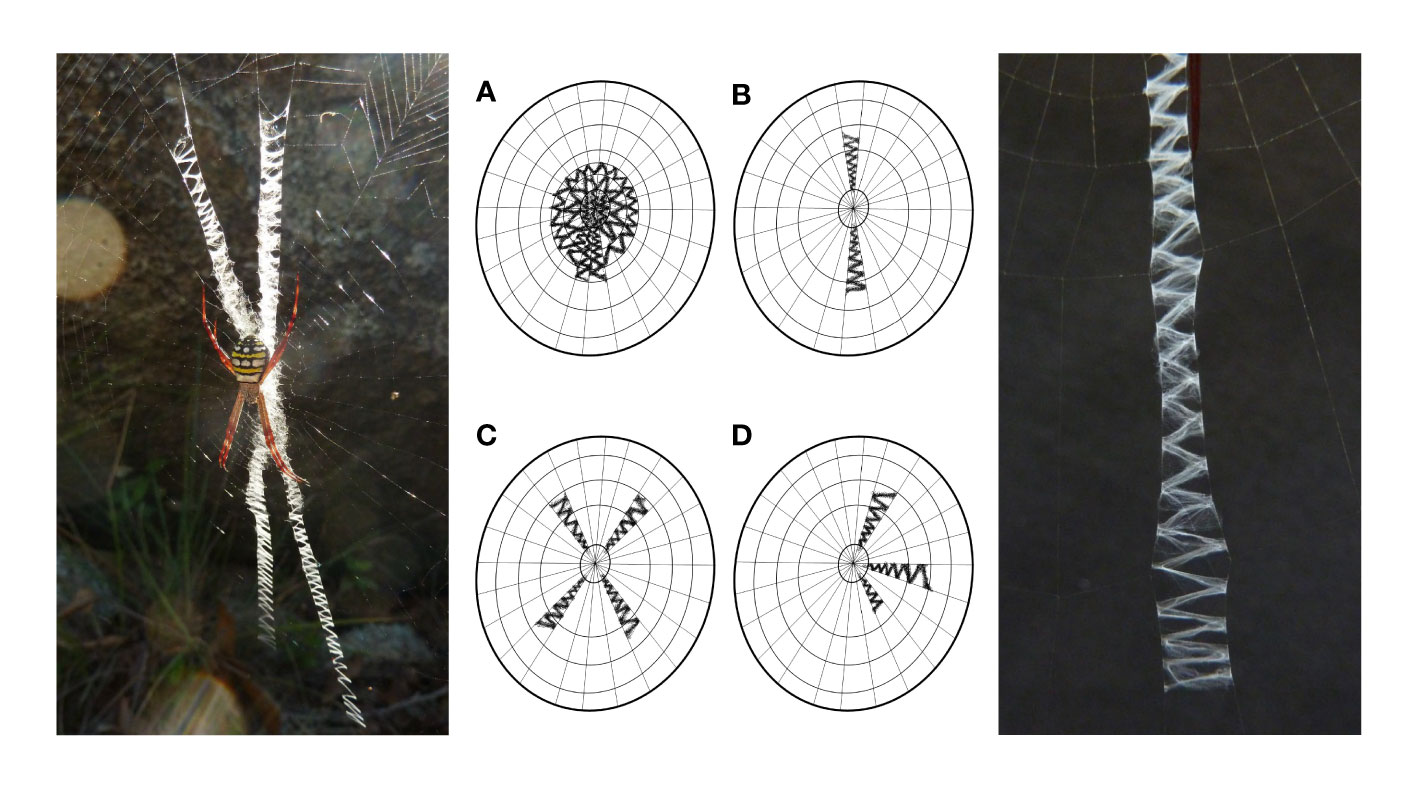
Figure 1 Typical zigzag structure of silk decoration bands in Argiope spiders. Left: Argiope picta female with cruciate silk decoration in its orb web; Centre: basic silk decoration arrangements in Argiope spiders: (A) circular or discoid, (B) linear, (C) cruciate and (D) irregular; Right: close-up of a decoration band of A. aetherea.
The idea of strengthening the web has never been extensively tested, but discarded, because the extra silk used to create decorations is not tightly woven into the web. It is conceivable that the typical zigzag-pattern may well convey the impression of a reinforcement, yet unlike the firmly strapped radii of an orb web, decoration silk is loosely dabbed on top of them (Blackledge et al., 2011; Foelix, 2011). Physical stress, like the impact of fast-moving prey, does not significantly change the overall architecture of the web, neither does a decoration help to keep it stable. Undecorated webs are as efficient in intercepting prey as decorated ones. Hence, no researcher found it interesting enough to even disprove the stability idea rigorously. Yet, a few studies tried to invoke a stabilising effect of silk decorations to explain their results. Li et al. (2003) suggested that cross shaped silk decorations help adult Argiope versicolor spiders to better bounce the web, a part of their anti-predator response (Robledo-Ospina et al., 2023). Watanabe (2000) argued that the silk decoration of Octonoba sybotides (family Uloboridae) gives the web more stiffness to better sense small prey insects hitting it. Apart from these excursions, the stability hypothesis has attracted only little attention though. This is a pity, because, as discussed later, there are intriguing hints that should make it worthwhile to revisit the stability function hypothesis.
It was the cleric naturalist Henry McCook (1889) who, at around the same time as Simon, studied spiders in the US and stumbled across the well-decorated webs of Argiope trifasciata and Argiope aurantia. His observations of the construction process were very accurate and never needed to be revised. Although also speculating about a web-strengthening function of that “winding stair”, he called these curious zigzag-bands “decorations”. Later, in the absence of clear results on their function, the term ‘decoration’ may have seemed neutral enough to be accepted by any party. Today, it is not a lack of empirical evidence, but the amount of inconsistent data that cause uncertainty. None of the numerous ultimate effects, as described below, has ever gained enough evidential power to be called conclusive. So, where will we go from here? While research on web decorations has been quite proliferate in the late 1990-s and early 2000s, with an average of five studies being published every year, there were only a very few papers added over the last ten years (Figure 2). Arachnologists and behavioural ecologists may be running out of ideas, or perhaps, experiments become increasingly elaborate and thus less and less attractive for smaller projects (Walter and Elgar, 2012).
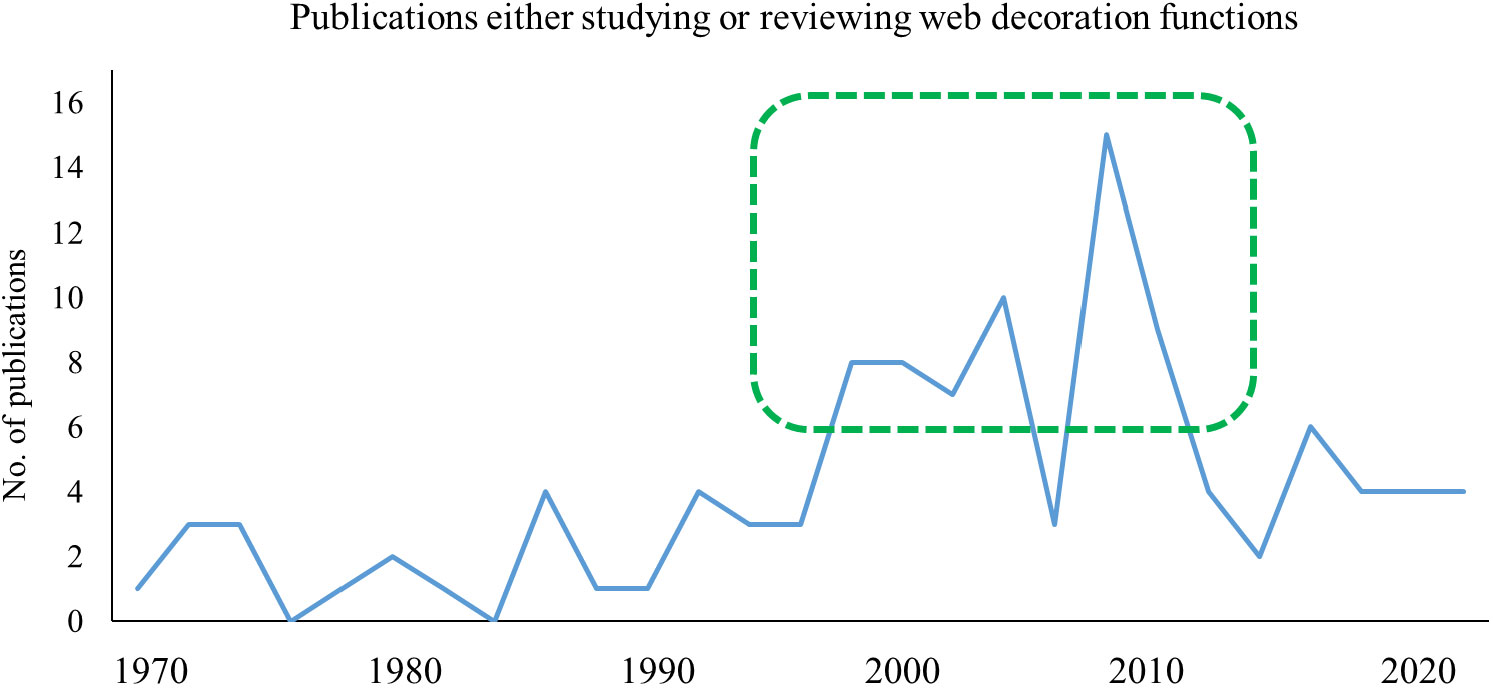
Figure 2 Number of publications that study and/or discuss the function of web decorations since 1970. The early 2000s have been the most productive phase in web decoration research. Despite many open questions, the frequency has later declined and remained low during the last decade.
Web decorations are found in the araneid genera Argiope, Gea, Neogea, Cyrtophora, Cyclosa, Allocyclosa, Witica, Micrathena, Gasteracantha, Austracantha, Thelacantha and Plebs, as well as in Nephila and Nephilengys of the family Nephilidae, Uloborus, Philoponella, Purumitra, Polenecia, Octonoba and Zosis of the Uloboridae. The boundaries of the term have been stretched from originally only referring to silk decorations (cf. above) to the inclusion of decorations build from non-silk materials too. Several Cyclosa species not only use silk, but also prey remains and plant material to construct web decorations (Marples, 1969; Tan et al., 2010), or even egg sacs (Craig, 1989; Allocyclosa: Eberhard, 2003, 2023), assembled to vertical lines in their orb webs. In the “group” of web decorations, the retreats of Phonognatha species (Araneidae) may take an interesting position. These spiders use silk to roll a leaf and attach it to the centre of their orb webs, thus abstractly resembling a web decoration. Many other araneid spiders build similar retreats, yet place them outside the actual capture web area. It seems that the sheer placement of this retreat let Herberstein et al. (2000) list Phonognatha graeffei as a decorating species in their review. This fact is intriguing insofar as there is an ongoing debate about whether web decorations may act as a replacement for retreats (Blackledge and Wenzel, 2001; Walter, 2018, see also discussion below). Finally, the term decoration has been extended even further to species without webs. Williams et al. (2006) describe turrets made from twigs, pebbles and debris around the burrow entrance of the wolf spider Lycosa tarantula (Lycosidae) as decoration.
The question as to which species to study in the quest for finding the web decoration function is simple and not. It is a simple question in a sense that there are species that are just easy to collect and to handle, like those from the genera Argiope or Nephila. It is a difficult one as well when we think about the variability of the behaviour. If different decoration patterns serve different functions, the researcher may have to make a critical decision before having even started the investigation. It is not only the material used, but also the arrangement of it that varies from species to species. Apart from the peculiar zigzag-shaped silk bands in Argiope-webs (Figure 1), McCook also described the web decorations of Gasteracantha (Araneidae) and Uloborus (Uloboridae), which, although made from the same material, turned out to look very different. The silk decorations of Uloborus are also attached to the web as a band (Eberhard and Opell, 2022), yet often less orderly than in Argiope and in some species not in a linear fashion but circular. The silk decorations of Gasteracantha again look quite different. These spiders add individual silk tufts to the radii and/or frame threads of their orbs, often arranged in rows. Considering a signal function of silk decorations, it becomes obvious that it takes some explanatory effort to argue for both Argiope- and Gasteracantha-decorations to have the same effect. Therefore, and depending on the experimental setup, choosing one over the other study system could alter the result.
The Argiope-Gasteracantha example illustrates a first key issue to address in any web decoration study: Is it reasonable to assume a similar function for all decoration types and patterns, or is there a strict dependency? There are good arguments for both. For example, a type-depending function appears plausible in the genus Cyclosa, as many species construct two decoration types of very different main components, silk or debris, which may relate to different purposes. Even their evolution may have followed different paths. We could easily see how a “lazy” spider may not have removed consumed prey items, leaving them hanging in the web, thereby concealing itself, equally dull coloured, sitting amongst those items. The occasionally built silk decorations may have a different evolutionary origin and may serve another function, like prey attraction (see below). Or, the different decorations serve the same function after all, with different materials and patterns representing replacements of each other depending on the availabilities in highly variable environments. Material or pattern variability alone is not inevitably an indication for variability of function (cf. Walter, 2019). In fact, different decoration patterns built by different species, but under the same circumstances, may be an indication for functional consistency.
Going back in evolutionary times can be a way to find a unifying functional explanation, as predecessors of a current, perhaps now species-specific decoration type may once have served the same purpose but were later selected differentially. However, the field of studying “the evolutionary origin of web decorations” is poorly tilled yet. When looking closer, we start to distinguish between an “original function”, which may have lost its significance over time, and an “ultimate function” or “ultimate functions” that may have evolved differently from species to species and environments to environments. The issue of not finding consensus then reaches another hurdle, once we realise that web decorations have evolved several times independently (Scharff and Coddington, 1997; Scharff et al., 2020). If we consider the evolutionary original function of web decorations being the same across species, we need to find an explanation for why this has happened repeatedly.
Debris decorations are absent in Argiope, yet the debate about functions has become most lively within this silk decorating genus. The “only” variability in the behaviour across the various species seems to be a variation of silk band arrangements. These spiders are categorised according to the mode they attach the typical zigzag-shaped silk bands in their webs: in a circular, linear, cruciate or irregular shape (cf. Bruce and Herberstein, 2005; Bruce, 2006; Walter and Elgar, 2012; cf. Figure 1). Also some Cyclosa species show pattern variation, either constructing circular or linear silk decorations (Gonzaga and Vasconcellos-Neto, 2005), but the genus Argiope has emerged as the more popular model. At a closer look, this is based on good reasons. First, Argiope has a worldwide distribution. There are species found on every continent (except Antarctica). Thus, researchers can easily compare their research results and adopt experimental protocols for their species of interest. Secondly, Argiope spiders are relatively large orb weavers, with adult females reaching 1-2.5cm in body length. They can be collected easily and build webs readily under lab conditions. They grow fast and are not demanding when it comes to the diet. The decorating behaviour has proven to have a genetic basis in Argiope (Craig et al., 2001) and shows an additionally interesting ontogenetic shift, with adults using either linear or cruciate arrangements of silk bands, while the juveniles of all species build circular web decorations, adding an intriguing aspect to experiments on the signal function. Based on these characteristics, the genus has become the favourite pet of web decoration researchers, and many of the results discussed in the following sections originate from Argiope-studies.
The body of evidence is weak for the evolutionary origin of the web decorating behaviour, and this is particularly true for silk decorations. We do not know anything about the reason why spiders started to deposit extra silk in their capture web. What proximate mechanism triggered them to attach an additional structure, when their webs would also function perfectly well without it? What condition allowed for an apparent excess of silk to accrue, which is not used for any other purpose but decorating? Orb web spiders can produce very different silk types, differing in amino acid composition, glycosylation patterns, hydration level etc. (Vollrath and Knight, 2001; Hayashi et al., 2004; Blamires et al., 2009; Blackledge et al., 2011, 2015, 2017). The different silk types have evolved as adaptations to specific uses, requiring differential properties from the material. There are silks used for covering the egg cases, for draglines, for frame threads of the web, the capture spiral; and there is a special silk type used for wrapping prey, aciniform silk.
It is known that in Argiope and Uloborus, the main silk used for web decorations also is aciniform silk (Peters, 1993). Based on this link, in an earlier review, Mark Elgar and I constructed a hypothetical scenario for an evolutionary origin of silk decorations (Walter and Elgar, 2012). Argiope spiders predominantly use wrap attacks and do not rely on the venomous bite to immobilise the struggling prey (Eberhard, 1967; Robinson et al., 1969; Robinson and Olazarri, 1971; Olive, 1980), and Uloborus spiders are left with this as their only option, since they lack venom glands altogether (Eberhard, 1967). Wrap attacking requires a fast provision of large amounts of aciniform silk, as the spiders throw dense silk mats over the struggling prey in order to immobilise it. The steady production of sufficient aciniform silk is therefore crucial to these spiders, and thus a high production level, or at least high plasticity of its synthesis can be expected. Walter et al. (2008a) found that an excessive use of aciniform silk leads to an increased production of it through positive feedback in Argiope. As a side effect, the decorating activity increases as well, thereby suggesting a direct link between prey capture and web decorating behaviours.
When having a closer look into the phylogeny of orb web spiders, a curious pattern emerges. Web decorating and wrap attack behaviours seem to be tightly linked in a large number of species (Scharff and Coddington, 1997). In a study on four Australian araneids, I showed that this link is also experimentally detectible (Walter, 2018). I compared the prey capture behaviour of two decorating and two non-decorating species occupying the same habitat and showed that the decorating species use wrap attacks significantly more frequently than non-decorators. How could this be functionally meaningful? In Walter et al. (2008a) we concluded that, originally, silk decorations may have represented the visible result of a regulation process that adjusts the production level of the aciniform silk glands. This regulation may be the proximate cause for a temporary silk deposition in the web. A highly fluctuating prey abundance and the varying demand for wrapping silk may have rendered this rather crude and straightforward strategy beneficial. Conversely, in times of high demand, the production of aciniform silk could, at least theoretically, be increased by increasing the web decoration activity. The latter is influenced by how much aciniform silk is used for wrapping, closing the feedback loop. Based on this idea, a hypothetical evolutionary progression from this starting point towards an ultimate signal function of web decorations has been proposed (Walter and Elgar, 2012; Walter, 2018).
Spiders recycle their webs before constructing a new one (Opell, 1998). If they do not abandon their webs then temporarily deposited silk is not lost, making the deposition of extra silk economically plausible. If the visual impression of the deposited extra silk does not significantly interfere with the function of an orb web, namely to catch prey, and if it does not reduce spider survival directly, e.g. by attracting predators, this behaviour may be maintained. However, the initial arrangement of the deposited silk in the web would be expected to vary considerably, not showing a recurring pattern. While the proposed regulatory origin of decoration silk may well be the same across various species, the ultimate function could differ, mirrored by a species-specific pattern shaped over evolutionary times.
The significance of ultimate decoration function may have increased during evolution, for example by a fine tuning of the signal. If we observe a detachment from its original meaning, it may explain why researchers find it so difficult to grasp the whole picture of the phenomenon. Moreover, if we consider web decorations to ultimately act as visual signals, it is even likely that the shaping is still ongoing. Various possible evolutionary paths and an incomplete fine tuning between sender and receiver may inevitably lead to inconclusive results. Attempts to reconcile contradicting data in the past often concluded with the notion that web decorations may represent multi-functional tools after all. Perhaps this “compromise” draws the best picture of the current situation. Or maybe, we just have not yet found the best suitable experimental approach to draw a better one.
Henry McCook (1889) was puzzled about what function silk decorations may serve, and he wrote “The purpose of this zigzag is an interesting problem; it evidently has no special purpose in the daily life habits of the spider; at least, close and continuous observation of many species colonized upon my premises have uncovered nothing.”. With the amount of data found in the literature today, it seems irrational to assume that web decorations have no functions. However, the interpretation of previous results often lacks some rigour, I argue. There are quite a few examples of narrowly focused experiments that tend to overestimate the functional significance of their results. On the other hand, it is impossible to prove the non-existence of a function. The crucial question is: When are we looking at a true function, a side effect, or just noise? For example, some animal receivers will always be there to pick up the visual impression of web decorations and exhibit some kind of response, but what does it mean? I try to highlight below that the literature of the last forty years is abundant of too narrowly designed experiments, actually hindering us to reach conclusive results on ultimate functions.
The visual impression of web decorations quickly leads to the conclusion that this structure may have a signal function. It seems counterintuitive that spiders reduce the prey capture efficiency by increasing the visibility of the web, and hence web decorations have been suspected not to deter prey but to actually improve prey capture. Craig and Bernard (1990) showed that web decorations reflect substantial amounts of UV-light and linked this with the fact that many prey insects use this part of the light spectrum for orientation and foraging (see also Kim et al., 2012). It should be noted here that decoration silk does not reflect more UV than other silk types (Zschokke, 2002), but that its local concentration may be suspected to generate a visual signal. In the mentioned study, Craig and Bernard (1990) found that the presence of silk decorations in the webs of Argiope argentata was indeed correlated with an increased prey interception rate. Later, Li et al. (2004); Bruce et al. (2005) and Blamires et al. (2008) studied the link between UV-reflectance of silk decorations and its prey attracting effect in more detail and supported the idea that these structures may exploit the sensory system of prey insects to lure them into the web. This prey attraction hypothesis was confirmed by a number of empirical studies (e.g. Tso, 1996, 1998; Herberstein, 2000; Bruce et al., 2001; Li et al., 2004; Li, 2005; Cheng and Tso, 2007; Rao et al., 2009; Gálvez, 2011; Kim et al., 2012; Gálvez, 2017a). Nevertheless, several studies also failed to detect the prey attracting effect (Blackledge, 1998a; Prokop and Grygláková, 2005; Rao, 2010) and some even revealed the opposite, that the presence of a decoration reduced the prey capture rate (Blackledge, 1998a; Blackledge and Wenzel, 1999; Kondo et al., 2012). Moreover, Argiope spiders often prey on insects that do not seem to be attracted to decorations, like cicadas and grasshoppers (Robinson and Robinson, 1970; Tso, 1996). So, what to conclude? Although the idea is compelling, the evidence for the prey attraction function is not unambiguous. Today, it is often recited that “web decorations are prey attractants”. However, while a prey attracting effect might be doubtlessly measurable, it remains questionable whether this generalisation is justified.
Web decorations could be built for a different purpose, yet once in the web also attract the attention of some insects. Nonetheless, even if we consider the benefit of attracting extra prey being rather small, it can of course still create a selective advantage, given that the costs are relatively low. However, the costs for building web decorations are not that low after all. Blackledge (1998a) showed that once silk decorations of Argiope spiders are treated as the dependent variable, the plausibility of the prey attraction function is shaken. He found that hungry spiders quickly stop decorating their webs, indicating that the spiders try to save resources instead of increasing their foraging effort by investing more in decorations. The other way around, well-fed spiders increase their web decoration activity, concluding that decorating is the consequence of higher food intake rather than its cause (Blackledge, 1998a). Apparently, only well-fed spiders can “afford” to build decorations, suggesting a different main function than prey attraction, or at least an additional one.
An alternative conclusion drawn from this observation may be that the spiders try to exploit a temporarily high prey abundance by building more of the attracting signal, in a frequency-dependent foraging effort (see Sherratt, 1993). However, this requires the assessment of the prey type, the prediction of its future relative abundance and the most suitable decoration tactic for the current conditions. There has been some discussion about the validity of the optimal foraging theory in web building spiders, and it remains questionable whether these sit-and-wait predators are capable of making an accurate assessment of their environment (Edwards et al., 2009; see also discussion in Pierce and Ollason, 1987).
The hunger level does not influence all foraging decisions directly. Web site tenacity, for example, may be less affected by hunger, as it is impossible for a spider to predict the quality of a new location of their trap (Scharf et al., 2010). In association with predator avoidance, however, hunger may well determine the degree of risk an individual is willing to take while using its foraging tactics. If a certain tactic increases the risk of attracting the attention of a predator, hungry individuals gain a higher benefit from taking this risk (Scharf et al., 2010). Several studies on the web decoration function describe a potential signalling conflict (Blackledge, 1998b; Blackledge and Wenzel, 1999; Bruce et al., 2001; Craig et al., 2001; Seah and Li, 2001; Cheng and Tso, 2007). Especially silk decorations are not only visible to the prey of the spiders, but also to their predators (Bruce and Herberstein, 2005; Cheng and Tso, 2007). If we look at the Hymenoptera, this is unsurprising. Bees represent a major prey guild of many Argiope spiders, while mud dauber wasps are often their main predators, both belonging to the same insect order, using the same visual system. In this regard, Craig et al. (2001) found that these wasps can learn to associate the presence of a decoration with a food source (here, the spider). Similarly, Bruce et al. (2001) showed that also mantids are attracted by silk decorations. Hence, the question is not whether to build decorations under high predation risks, but whether the construction of the decoration itself elevates that risk. When we investigate the decoration behaviour under different satiation levels, we should at least expect consistent tendencies. If the predation risk is low, well-fed spiders may build more decorations to exploit the prey abundance, but then hungry spiders can gain even higher benefits under these conditions and should thus also build more decorations. If the predation risk is high, then well-fed spiders as well as hungry ones should reduce their decorating activity. In fact, hungry spiders may be even more inclined to build decorations under high predation pressure. Instead, we find that well-fed spiders build more, and hungry spiders fewer decorations under the same conditions.
Craig and Bernard (1990) made the observation that web decoration silk reflects UV light; and especially for bees, the conclusion of an attracting effect was readily accepted. However, as it turned out, we are not entirely sure whether it really is the UV-part of the light spectrum alone that can be claimed to convey a visual effect. Blackledge and Wenzel (2000) showed that artificial decorations made of tarantula silk (Pterinochilus sp., Mygalomorphae: Theraphosidae) have a much stronger UV-peak than the aciniform decoration silk of Argiope. While one can train bees to find a “tarantula-decoration”, they fail to get trained on the Argiope’s decoration silk. Hence, it could be concluded that decoration silk has been evolving to become more cryptic rather than getting more visible. After all, Blackledge and Wenzel (2000) argue that when assessing the receivers’ perspective, all colours of the light spectrum have to be included, as it determines the overall visual conspicuousness. Aspects like background, the ambient environment and the resulting relative reflectance patterns play a role too. In an interplay of UV + blue + green light reflectance, web decorations gave a poor colour contrast against the natural grass background, suggesting that the decoration silk may be cryptic to many prey insects after all (Blackledge and Wenzel, 2001). Still, the decoration silk does not sufficiently match the colour spectrum of the spider body, to go so far to argue that decorations may provide visual protection through crypsis, e.g. against birds (Bruce et al., 2005).
Apart from the general question whether prey attraction is the main function of web decorations, when looking closer at the behaviour, it becomes obvious that any variation of decoration pattern and material may well influence the efficiency of the attracting signal. First of all, the deceptive signal luring prey to the capture web does not need to be a visual one. It has been demonstrated in Nephila that prey remains arranged as decoration bands in the web attract flies, and most likely so via the odour of the decaying material (Bjorkman-Chiswell et al., 2004). However, the same effect could not be confirmed for debris decorations of Cyclosa (Kondo et al., 2012). Pure silk decorations may also emit olfactory signals, like pheromones (see Henneken et al., 2015), but they have not been reported to attract prey in that way yet. Instead, the arrangement pattern of the decoration silk has been shown to play the major role for the efficiency via a visual attraction effect. There is no consensus on which of the basic decoration patterns observed in the various orb weavers may be the visually most effective prey attractant, linear, cruciate or spiral/circular.
Cheng et al. (2010) tried to address this question by proposing the idea that in adult Argiope spiders we may witness an evolutionary transition from a vertical, linear to a cruciate arrangement of their typical zigzag-shaped decoration bands (cf. Figure 1). The authors argue that the two patterns serve different functions, with the cruciate form being more effective in attracting prey insects, since insects have an inherent preference for inclined patterns (Bruce and Herberstein, 2005; Cheng et al., 2010). The less evolved, linear form has been suggested to be more efficient in deterring predators than attracting prey (Cheng et al., 2010). Along those lines, Starks (2002) pointed out that the circular decorations of juvenile Argiope spiders may also serve a different function than the cruciate arrangement of silk bands in adults, whereas Li et al. (2004) again presented results demonstrating a prey attracting function for both patterns in A. versicolor. However, when we refer to pattern variation, a consideration of the basic shapes may not even be sufficient. As mentioned above, the pattern may also vary regarding its completeness (see Nentwig and Heimer, 1987; Craig et al., 2001; Bruce, 2006; Walter and Elgar, 2016; Walter, 2019). The cross made of four zigzag-shaped decoration bands, which Cheng et al. (2010) described as being very effective, may well lack 1-3 arms. This becomes an important factor when we try to determine the minimum signal strength necessary to elicit a desired response by the receiver. Herberstein (2000) presents evidence for the prey attraction hypothesis, showing that an increasing number of decoration bands in the cruciate decorator Argiope keyserlingi increases the prey capture rate. However, the data also indicate that a single band seems to have no significant positive effect over undecorated webs. In accordance with this, Walter (2019) shows that Argiope bruennichi has a strong preference for two-banded (linear) decorations, constructing either the complete pattern or no decoration at all, with one-banded decorations being significantly rare in the species’ repertoire. The question arising from those observations is how has the decorating behaviour reached, and later passed, the threshold for the visual signal strong enough to elicit a receiver response? Because here, a gradual increase of the signal strength is unlikely to explain the early evolution of the signal (cf. discussion in Walter and Elgar, 2012).
There is undoubtable evidence that web decorations can attract prey insects. However, they do not attract all prey species equally well, and worse, they also attract unwelcome predators. Counterintuitively, well-fed spiders build more decorations than hungry ones. Together with the variability of decoration patterns and the unclear impact of their spectral properties, it appears almost questionable to straight-forwardly conclude that prey attraction is the main function of these structures. At this point, we may modestly assume that we have proven a beneficial effect that may facilitate the maintenance of the decorating behaviour, but we need better data on other potential decoration functions to discern the relative impacts on spider fitness and, consequently, the selective forces acting on the decorating behaviour.
In 1983, a paper by Eisner and Nowicki generated some considerable attention to the web decoration topic. It presented evidence that silk decorations may act as a warning signal, advertising the presence of the web to non-prey animals that might inadvertently damage it. The advantage for a hub-dwelling spider seems clear. It reduces the risk of injuries and the risk of a potential loss of the capture device and resting place. Eisner and Nowicki (1983) demonstrated that decorated webs persist longer intact than undecorated webs. Birds flying through the webs have been suggested to be a major cause of web damage (Lubin, 1975; Horton, 1980; Blackledge and Wenzel, 1999) and may thus be the intended receiver of such a warning signal. However, in 2017, Gálvez showed in a choice experiment with hummingbirds that these potential web damagers do not actively avoid silk decorated webs of Argiope submaronica (Gálvez, 2017b). Also, many decorating spider species build their webs in low vegetation strata, like in grasslands, rendering it rather unlikely for birds to damage them. Other vertebrates may need to be considered as frequent web damagers as well, but the literature provides no insights here.
Again, with this hypothesis, it remains unclear whether the avoidance of web damage is the main purpose of web decoration, or whether this may be considered a positive side effect, and the decorations have a different primary function. Can we say, “web decorations are protective devices”? Interestingly, unlike the situation with the prey attraction hypothesis, investigations that treat the decoration as the dependent variable seem to support a web protecting effect. Kerr (1993) presented a study conducted on Guam and neighbouring islands, showing that Argiope appensa reduces its web decorating activity in the absence of birds. This may indicate that with fewer web damage events, the spiders reduce their investment in the warning signal. However, birds may not only be considered potential web destroyers, but also major predators of these spiders (Horton, 1980; Blackledge and Wenzel, 1999), and competitors over insect prey (Kerr et al., 2021). Hence, it is alternatively possible that the release from predation pressure or competition over food can explain the observation. After birds had been eradicated on Guam by the brown tree snake, A. appensa may have no longer been in the need to protect itself against predators by building web decorations (see discussion under 4.2), or no longer needed to attract insects as the reduced inter-specific competition over prey has increased their abundance.
In an attempt to directly prove the impact of web damage on the spiders’ decorating behaviour, Walter and Elgar (2011) purposely damaged the webs of Argiope keyserlingi. The results show that after experiencing heavy web damage, these spiders increase the size of their silk decorations. Now the story seemed conclusive: Decorated webs persist longer undamaged, and more web damage causes an increase in decoration investment. However, what the study by Walter and Elgar (2011) also revealed was that spiders experiencing greater web damage also reduce the size of their capture web. After all, it could be concluded that this is the actual response to the damage, and as a compensation for the smaller capture area of the web the spiders increase the strength of the prey attracting signal, the decoration.
The last example nicely illustrates that with a growing number of ultimate functional explanations, the discussion of new results becomes more and more complicated, because alternative interpretations are readily available. Or, this may indicate that web decorations are indeed multifunctional tools. However, I think it is more likely that a more elaborate design of experimental setups can help to minimise the chances for conflicting interpretations. With the web protection/web advertisement hypothesis, a sharp distinction to the predator avoidance hypothesis is not always clear, as for the example of birds, which can be both predators and web damagers (cf. Horton, 1980; Blackledge, 1998b; Blackledge and Wenzel, 1999). For hub-dwelling spiders, the loss of the web is almost inevitably associated with high risk of injuries and an increased chance to be encountered by predators (Lubin et al., 1993). Apart from the prey attraction hypothesis, functional explanations claiming that web decorations protect the spider against predators are thus next most common (Bruce, 2006; Walter and Elgar, 2012; Robledo-Ospina and Rao, 2022).
Although there are many studies that have collected evidence for the predator avoidance function, its conclusiveness appears weaker than that of the prey attraction idea. Most papers conclude with indirect inferences based on observations, while direct experiments trying to go into detail are very rare. The reason may be that investigations of the foraging behaviour of the spiders’ predators quickly become rather elaborate. Lab studies are hardly feasible, because bringing predatory wasps, birds or other vertebrate predators into a controlled indoor setup comes with considerable limitations. The natural foraging behaviour can rarely be triggered under these conditions. A good example provides the comparison of two studies using a similar prey-predator species pair. Bruce et al. (2001) studied a mantis species preying upon Argiope keyserlingi in the lab, using a Y-maze choice experiment, with the result that the predator shows to be attracted to the silk decoration built by the spider. The maze was rather small in size (5x5cm tunnel), and a later experiment trying to reproduce the results using A. bruennichi and a different mantid species revealed that these predators do not show a natural prey search behaviour in the maze, but are rather busy attempting to escape the apparatus (Walter, 2008). While this represents a simple study system, the use of flying insects or birds as predators would complicate the situation even more. Experiments on the predator avoidance function are thus better advised to take place in the wild, but then they become technically elaborate and labour-intense.
Wayne Tolbert (1975) was one of the early supporters for a predator avoidance hypothesis. He suggested that the densely woven silk mats covering the web hub of Argiope aurantia may act as a physical shield to prevent a predator to reach the spiders’ body. Many orb web spiders shuttle around the web hub when being disturbed or threatened (Hingston, 1927; Tolbert, 1975; Jackson et al., 1993; Li et al., 2003; Eberhard, 2020; Wang et al., 2021). Decades later, Wang et al. (2021) confirmed Tolbert’s observations in one of the rare direct experiments on the predator avoidance hypothesis. The circular decorations around the web hubs of juvenile Argiope minuta spiders shield them from being spotted by chicks that would otherwise pick them from the web. However, shielding is just one possibility of how web decorations may protect the spiders from predation. Schoener and Spiller (1992) demonstrated that Argiope argentata spiders have a higher probability of survival if they decorate their webs (see also Blackledge and Wenzel, 2001 for A. trifasciata). The authors argue that the alignment of the spiders’ legs with the cruciate arrangement of their decoration bands makes them appear larger than they actually are, thus having the predator abandoning the idea of attacking an apparently too large prey item. In some earlier studies, it was further proposed that the silk bands may help to conceal the spiders’ outline, making it difficult for a predator to assess their true size (Lubin, 1975; Edmunds, 1986).
Blackledge and Wenzel (2001) suggested that in Argiope the zigzag-pattern of the decoration bands may play a crucial role. Argiope spiders respond to disturbances and attacks with ‘web bouncing’ or ‘web flexing’ (Tolbert, 1975; Jackson et al., 1993; Li et al., 2003; Blamires et al., 2007). By moving their body up and down against the web plane, they set the web into a low frequency vibration (Cedhagen and Björklund, 2007). This defensive behaviour is likely to confuse the attacker (Hingston, 1927; Soley, 2019), and visually, the result is a blurry picture of the spider and its decoration, which makes it difficult for the attacker to locate it in the web (Cloudsley-Thompson, 1995; Robledo-Ospina et al., 2023; Figure 3), to make it appear larger than it is (Li et al., 2003; Robledo-Ospina et al., 2023) and/or to give it enough time to escape, for example by eventually dropping from the web (Blackledge and Wenzel, 2001). Li et al. (2003) further showed that Argiope versicolor adjusts its predator escape behaviour depending on the presence and the shape of the web decoration. While juveniles shuttle around the circularly decorated web hub in response to a predator cue, adult spiders in cruciate-decorated webs bounce more frequently than those in undecorated webs, which then rather choose to drop from the web. However, a similar study on Argiope florida by Bateman and Fleming (2013) could not confirm the observation of that decoration-dependent fleeing behaviour.

Figure 3 Adult females of Argiope aetherea. Left: resting position; Right: bouncing in the web (another individual).
As discussed above, the spectral properties of decoration silk may not only attract the attention of prey, but also predators (Craig et al., 2001; Seah and Li, 2001; Bruce et al., 2005; Rao et al., 2007). This seems problematic also with respect to a proposed predator avoidance function. However, considering spectral properties of web decorations and the immediate environment, as well as the various distances of sender and receiver of the visual signal may at least partly conciliate the conflict. Taken separately, decoration or spider may be easy to spot, but in combination the decoration silk may actually help to reduce the visibility of the spider sitting on top of it. The contrast of the spider body against the decoration silk is strong enough for birds to spot the spider, yet they are undistinguishable from the background for hymenopteran predators at short distances (Bruce et al., 2005). In his review in 2006, Matthew Bruce pointed out that many animals use different receptors for short- and long-distance discrimination (see also Chittka, 1996), and consequently, it remains to be shown how silk decorations may be camouflaging the spiders over short but not over long distances.
For detritus decorations, a camouflaging effect has already been revealed by Tan and Li (2009); Cyclosa mulmeinensis individuals sitting on webs with decorations were invisible to both hymenopterans and birds over short distances, but visible to both predators and prey over long distances. Structural and spectral properties play a role in protecting spiders that build detritus decorations. Cyclosa morretes and C. fililineata build vertical decoration bands out of prey remains and sit in the centre of those “columns” (Gonzaga and Vasconcellos-Neto, 2005). With their legs tucked up, these spiders are visually indistinguishable from the linear arrangements of prey leftovers, disrupting the spiders’ outline and thus reducing the likelihood of being spotted by predators (Neet, 1990; McClintock and Dodson, 1999; Gonzaga and Vasconcellos-Neto, 2005). A similar protective effect of hiding the spiders’ location has been attributed to the egg sac decorations in Allocyclosa bifurca (Eberhard, 2003; Eberhard, 2019; 2023). Spectral properties play an additional role in concealing the spiders. Chou et al. (2005) demonstrated in Cyclosa confusa that hymenopteran predators cannot distinguish between the chromatic contrasts of the spiders and their detritus decorations. The same was observed for Cyclosa monticola (Ma et al., 2020). While the visual impression does still attract the attention of wasps and birds, interestingly, they more frequently attack the decoration rather than the spider (Chou et al., 2005; Tseng and Tso, 2009; Ma et al., 2020). Detritus decorations may protect Cyclosa spiders via spectral properties of the silk against insect predators and via structural characteristics against vertebrate predators (see also spider defence strategies reviewed in Robledo-Ospina and Rao, 2022). Not only detritus decorations but also silk decorations may function in a macroscopic masquerade fashion. Liu et al. (2014) described a protective function of circular silk decorations of Cyclosa ginnaga as an interplay of spider body colouration and the white silk. The authors argue that the spiders together with their decoration silk resemble bird droppings and that this may protect them against avian predation.
After all, the knowledge about the prey capture behaviour of many predators of web decorating spiders, like their approach paths and decision making is still scarce, which renders the design of meaningful experimental setups to test the predator avoidance function difficult. What becomes obvious is that if web decorations act as anti-predator devices, we will need to identify the intended receiver of the deterring signal first, because the visual systems of the predators vary considerably. As discussed above, predator attraction as well as predator avoidance can both be concurrently true. However, without a better resolution on the relative strengths of both effects, it poses the question of whether it would not be easier to omit web decorating in the first place. It seems reasonable to assume that the spiders need to be able to use their decorations tactically in response to fluctuating environmental conditions, in this case to changing predation pressures. However, previous studies have not yet convincingly demonstrated a sufficiently high degree of the spiders’ environmental awareness that could justify this assumption (with one notable exception of Li and Lee, 2004). More solid information on which environmental factors actually influence the decorating behaviour is required.
Wang et al. (2021) demonstrated that the function of web decorations may not only rely on their visual but also on their physical properties, shielding Argiope spiders against predatory attacks. The structure of the decoration silk attached to the web may also have another direct benefit. It can collect water originating from either rain or dew. Studies on Argiope bruennichi, A. aetherea and A. trifasciata showed that these spiders even use their silk decorations to drink the collected water (Walter et al., 2009, 2011, 2012). The dense meshwork of the decoration silk has the hygroscopic potential to retain substantial amounts of water (Walter et al., 2009), and under dry conditions the spiders use this cistern frequently to drink from (Walter et al., 2012). They systematically probe their decoration and ingest any adhering droplets. However, the difficulty with a potential “cistern function hypothesis” is that when treating the decoration as the dependent variable, ambient humidity does not seem to affect Argiope’s decorating behaviour (Nentwig and Rogg, 1988; Herberstein and Fleisch, 2003). If water collection is an important function, the spiders should be expected to increase their investment in web decorations in dry environments or under desiccating conditions, for example to collect more dew in the morning. A meta-analysis of the distribution of web decoration patterns of Argiope bruennichi across Europe using more than 3000 pictures taken by lay photographers (source: www.inaturalist.org) also did not reveal any correlation between web decorations patterns and sizes and the humidity/aridity of the region (Walter and Vanthournout, unpubl. Data).
In most species, at least the decoration type (detritus, egg sac or silk) is consistent across individuals of a population. However, some Cyclosa species even vary the decoration type intra-individually, switching between silk and detritus as the building material. Silk decorations have thereby been suggested to function as a replacement form in the absence of detritus, which the spiders otherwise prefer to use (Gonzaga and Vasconcellos-Neto, 2005). McClintock and Dodson (1999) found that C. insulana builds more circular silk decorations in smaller webs and in windier areas. The linear detritus decorations seem to be the more permanent solution, and are only constructed under calm conditions when longer undisturbed periods allow for the accumulation of enough construction material. Accordingly, Gong et al. (2023) show that an elaborate detritus decoration is a substantial investment for Cyclosa monticola, negatively affecting their propensity to relocate their website after disturbance. The idea of silk decorations being a low-cost replacement implies that not only detritus decorations may be considered as protective devices (Neet, 1990), but that silk decorations also serve this purpose. In line with this idea, Eberhard (2003) describes how Allocyclosa bifurca switches between silk and egg sac decorations; and he argues that both decoration types serve the same function, to camouflage the spider, yet with egg sac decorations being more effective. However, since the material for detritus decorations, their pattern, as well as their spectral properties differ considerably from silk decorations, differential functions have also been proposed. While silk decorations may help to increase the prey capture rate by attracting insects (Tan et al., 2010), it is suggested that the detritus decoration protects the spiders against predators, once the capture success has been high enough to result in enough prey remains to be used for assembling the detritus band (Gonzaga and Vasconcellos-Neto, 2005). This is an interesting idea, yet the authors also note that further studies need to be conducted to confirm this hypothesis, which has not been done yet.
In pure silk decorations, the idea of a single main web decoration function is challenged by the dilemma of considerable decoration pattern variability. A question lingers in the room: Do different decoration patterns serve different functions or the same? Cheng and Kuntner (2014) show that species constructing either linear or cruciate decoration patterns are scattered all across the phylogeny of Argiope spiders. If any pattern-dependency of function exists, it is equally scattered then, which poses interesting follow-up questions as to the species’ common life history features. The variability of decoration patterns does not stop on species level. Within the genus, there are species that alternate between distinct patterns (linear, cruciate, circular) within the same population and even intra-individually, sometimes changing the decoration pattern on a daily basis (Walter and Elgar, 2016; Walter, 2019; Figure 4).
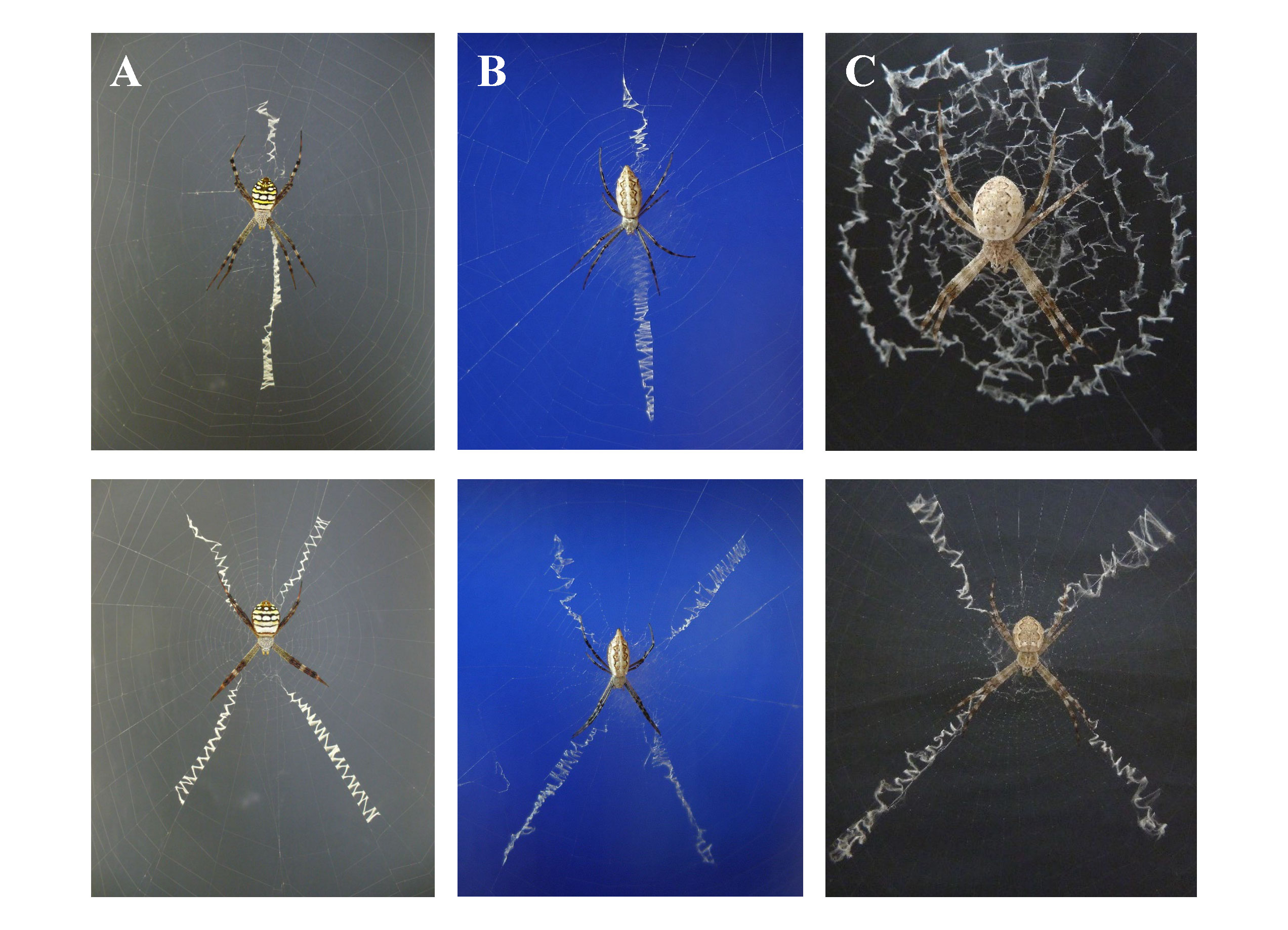
Figure 4 Argiope species known to construct more than only one of the basic decoration patterns. (A) linear and cruciate in A. picta, (B) linear and cruciate in juvenile A. sector, (C) circular and cruciate in A. mascordi.
Both the basic decoration shape as well as the completeness of a certain pattern have been suggested to significantly influence functionality. Cheng and Tso (2007) found that the absence of decorations in Argiope aemula webs reduces the predation risk, whereas Blackledge and Wenzel (2001) show that the absence of decorations increases the predation risk for A. trifasciata. Considering that the former species preferably constructs cruciate and the latter liner decorations, the linear pattern must be extraordinarily effective in protecting the spiders compared to the cruciate arrangement of the silk decoration bands. However, it is surprising then that compelling evidence for such strong pattern-effect is hard to find. Nevertheless, different functions have been proposed repeatedly for linear and cruciate patterns (Starks, 2002; Bruce and Herberstein, 2005; Cheng and Tso, 2007). Cheng et al. (2010) suggest that cruciate patterns represent the result of opposing selection pressures acting on the shape of decoration signal. Cruciate patterns may better exploit the sensory system of prey, increasing their attractiveness (see also Bruce and Herberstein, 2005), but linear arrangements may be favoured under conditions when other uses of the signal are more important, for example for predator avoidance (cf. discussion in 3.1).
The discussion about the possibility that linear and cruciate decorations serve different functions is ongoing, and we still lack direct comparative experiments. Beyond this dichotomy, we have even less insights into the functional significance of the ontogenetic shift from the juvenile decoration pattern to adult shape. It is a widespread phenomenon in Argiope that early instar spiders of almost all species build circular decorations and later shift to either linear or cruciate forms in late juvenile and subadult stages (Bruce, 2006; Walter and Elgar, 2012). Again, the question is, do the circular, juvenile patterns serve the same or a different function as the respective adult shape? Li et al. (2003) found evidence for circular silk decorations having a protective effect for juvenile Argiope versicolor (3.3). However, a little later, Li et al. (2004) also presented results that support a prey attracting function of circular decorations in the same species. It appears that studies on circular decorations do not resolve the dilemma of conflicting results either. I argue that this case is symptomatic for a widespread flaw in decoration research, that experimental setups try to seek agreement with narrow hypotheses, and that this hinders us to yield more conclusive results. If we predict a protective function, we may look for evidence only confirming this prediction. If we hypothesise that web decorations attract prey insects, we will find prey guilds that respond more than others, confirming the attraction. For example, direct comparisons of various species, decoration types and functional hypotheses within a single study do not yet exist.
Alternatively, we may assume that different decoration types and patterns do serve the same function despite pattern variation. This alternative is hard to prove though, because we need to explain the value of variation and to insist on more experimental consistency when comparing various studies. For example, if we consider web decorations to act as a visual signal, the minimum strength to elicit a response by the receiver becomes a crucial variable (cf. Walter and Elgar, 2012). If different studies measure the decoration signal in different environments, at different sizes and shapes, we may quickly end up with incomplete results that are prone to misinterpretation. As mentioned above, the study by Herberstein (2000) found that a four-armed, cruciate silk decoration has a prey attracting effect, whereas if a single-arm is measured, there is no difference to a web without decoration. With respect to the protective function, if web decorations make spiders appear larger than they are (Schoener and Spiller, 1992; Li et al., 2003), an increasing decoration size can be expected to increase its effectiveness to avoid predatory attacks. Yet, if we measure an incomplete decoration, it may turn out to be useless for protection.
Why do spiders then build incomplete decorations at all (cf. Walter, 2019)? And how has the complete decoration pattern evolved, if parts of it have no effect? An elaboration from smaller, previous versions is then implausible. Partly circumventing this discussion, the variation of silk decoration patterns and their completeness have been argued not to be symptom of imprecision, but to be an evolved feature. Serving the same function, the decoration pattern is suggested to be constantly altered by the spiders in order to avoid receiver learning by either prey or predators (Craig et al., 2001; Seah and Li, 2001), thereby acknowledging the possibility that the efficiency may be compromised. Ratz et al. (2023) discuss the issue of compromised signal efficiency as a result of a signalling conflict: If there is a trade-off between prey attraction and predator avoidance, it is expected that the prey attracting signal remains suboptimal despite the potential for greater attractiveness. The great diversity of prey types as well as predator species, whose sensory capabilities are overlapping, further makes it hard for the signal to evolve to become highly attractive to prey and concurrently cryptic to predators (Ratz et al., 2023). Thus, when assuming a signalling conflict being true for web decorations, pattern inconsistencies across species and even within species or populations may be expected. Apart from evolutionary reasons for the maintenance of pattern variability, Walter and Elgar (2016) note that the shape of a web decoration is additionally dictated by the architecture of the web and the amount of silk available at a given time. Pointing out that circular decorations are twice as large as cruciate decorations in Argiope mascordi, they argue that it might be a question of how much silk a spider has available to construct either the more expensive, circular pattern or the relatively cheaper cruciate form. This inevitably suggests that the circular pattern would be more effective than the cruciate form, irrespective of what the decoration function may be.
Eberhard (1973) found in Uloborus diversus, that these spiders change their decorating from linear to circular patterns when light intensity increases. This could be an indication that an increased visibility to predators triggers a shift to the more expensive yet more protective decoration form. Discerning the function of circular patterns may eventually shed light on the ontogenetic shape-shift recorded in so many Argiope species. Smaller, juvenile individuals may prefer the circular silk decoration pattern if it is more effective than linear and cruciate shapes. Adults, perhaps, had to come up with a cheaper solution. Spiders grow longitudinally more than laterally, but the circular shape of the juvenile silk decorations requires relatively greater amounts of extra silk per mm body length with each instar, as it grows equally in both directions. Hence, with an increasing body size, the construction of circular decorations becomes increasingly expensive for the spider, raising the selection pressure for developing cheaper solutions, which may have resulted in alternative, lined patterns, perhaps at the costs of a reduced efficiency. I suggest that future studies should test this idea further, thereby taking advantage of species that build circular decorations also in the adult stage. According species are rare, but they exist. Argiope mascordi (Figure 4C), A. ocyaloides and A. chloreis are examples. An interesting side question is, why would individuals of these species pay the relatively higher costs of circular decorations even as adults?
A meta-analysis of the literature presented above reveals that previous research appears to be biased in various ways. Figure 5A illustrates that web decoration studies in the past have been focussing on hypothesising and investigating single functions instead of approaching competing functional explanations or including the possibility of multifunctionality. They further have been treating web decorations predominantly as the independent variable instead of looking for factors that influence the decorating behaviour (Figure 5B). Moreover, while previous investigations tried to confirm a single ultimate function, they have missed the point of explaining the evolutionary origin of the behaviour almost completely (Figure 5C). Bringing genetics and genomic research into the system could shed some light into the evolution. Apart from its origin, we still do not know how plastic the decoration behaviour is, intra-individually as well as within and across populations. Perhaps, it is worth starting to investigate the response of certain ‘genetic lines’ to different environmental conditions, for example, by artificially selecting or sampling distinct decoration phenotypes (patterns, low- or high-frequency decorators, etc.). The focus on studying ultimate functions is understandable insofar as it is less elaborate and thus likely to yield publishable results reasonably faster. Once some basic data have been published, they can be easily supported or rejected by adopting the experimental setup but using a different species or environment. Results from those studies are valuable and shall not be belittled here, yet scientific progress in the field may have been slowed down by a trend of validating rather than exploring. Putting more effort in studies treating web decorations as the dependent variable and exploring the genetics and the proximate mechanisms of the behaviour in greater detail could help to resolve the issue, as those data will improve our understanding of the evolutionary progression of this intriguing behaviour.
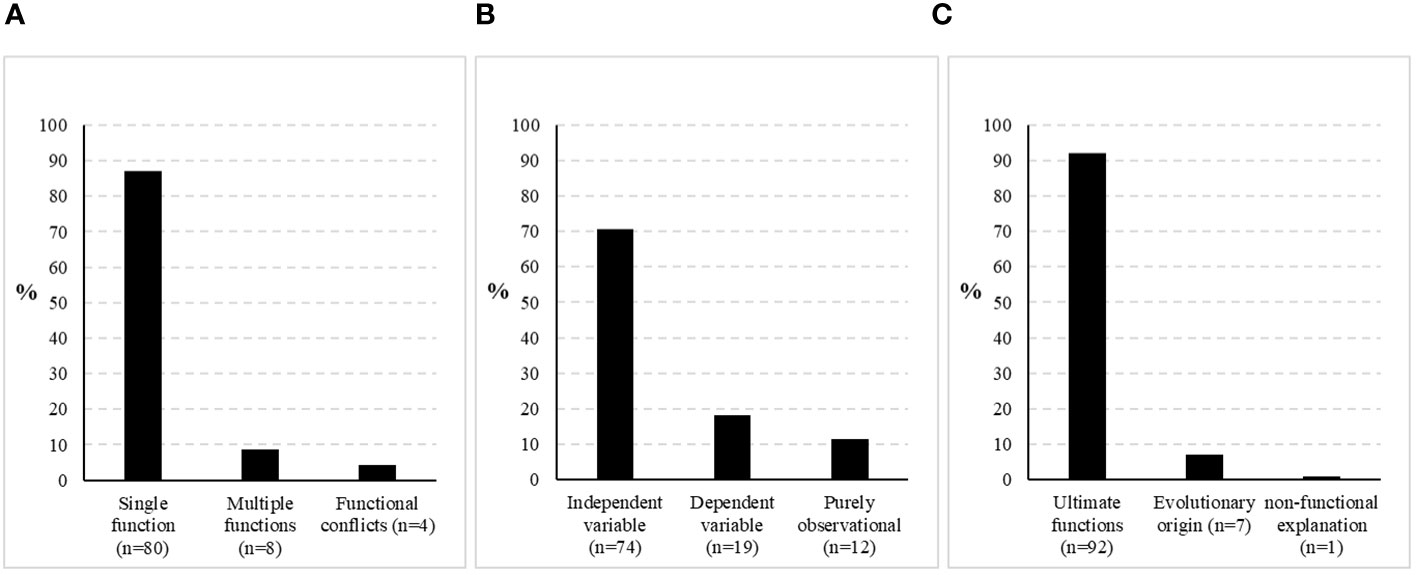
Figure 5 Spectrum of previous web decoration research according to various criteria. (A) Comparison of studies addressing a single function of web decorations, multiple functions or describing functional conflicts, (B) Comparison of studies treating web decorations as the independent or dependent variable, or which are purely observational, (C) Comparison of studies investigating ultimate functions of web decorations vs. those exploring their evolutionary origin or describing them as non-functional. *the body of literature for all three graphs is the same, but not all papers fulfil all criteria. The number of studies used for analysis is thus quite comprehensive, yet not meant to be exhaustive.
In their review, Walter and Elgar (2012) described a potential evolutionary path of how silk decorations have emerged in Argiope spiders, which marks a first attempt to put the puzzle pieces together in this most prominent model system. It was trying to consolidate the findings on ultimate functions with the bit of knowledge we have on the origin of the behaviour. The authors suggest that the origin may be linked to the evolution of the wrap attack strategy based on the common use of aciniform silk (see 2.). Previously, Walter et al. (2008a) found that an increased use of wrapping silk in Argiope bruennichi, A. sector and A. keyserlingi resulted in an increase in the web decoration activity, suggesting a proximate connection. Yet, in a similar silk gland depletion experiment, Tso (2004) revealed in Argiope aetheroides that individuals that used less silk for wrapping built larger decorations than those having wrapped a lot of prey items. This shows that this “silk gland regulation hypothesis” for the origin of decorating is not yet well supported, and further studies need to look into this. Once better substantiated, this hypothesis may provide a proximate explanation for why it is aciniform silk that is used for web decorating.
After all, there may be another, and perhaps even simpler explanation for the occurrence of aciniform wrapping silk in Argiope webs, which has not yet been investigated. Once Argiope spiders have wrapped a prey item, they often leave it at the capture site in the web instead of immediately carrying it to the web hub (Robinson and Robinson, 1974). This is particularly true for large prey items, for which the spiders need a lot of wrapping material. When a spider returns to the web hub, it often drags more or less thick bands of remnant wrapping silk along (Walter, 2018), creating a white band in the web (Figure 6). This band may then be picked up as a visual signal (ME Herberstein, pers. comm.), and may even favour a selection for “messy” spiders. However, it remains to be explained how this initial “carelessness” de-coupled from the prey capture behaviour and became a part of the web building behaviour; but it is a hypothesis worth to be further pursued.

Figure 6 Argiope protensa female after having caught a house cricket. When the spider returned to the web hub, it dragged wrapping silk along, creating a white band that consists of the same silk as the actual web decoration.
“Silk-drag hypothesis” or “silk regulation hypothesis”, both scenarios may provide intriguing insights into how a signal may have evolved from meaningless concomitant circumstances of other behaviours. What followed could be more readily explained by a co-evolutionary fine-tuning between sender and receiver. Since animal signals are optimised for their use in a certain environment, another aspect needs to be included when tracing the shaping of the alleged decoration signal, physical or mechanical constraints. In their study on Argiope mascordi, Walter and Elgar (2016) discuss the potential reason for how the cruciate decoration pattern, as a cheaper alternative to the circular shape (see 3.5), may have resulted from architectural features of the orb web itself. While their observations could not identify any cause that may explain the intra-individual switching between the two decoration patterns, they revealed that cruciate decorations were significantly more frequent in old, worn webs. In A. mascordi, the circular pattern is otherwise more frequent (75% vs. 20%, with 5% mixed patterns). Yet regularly, spiders have been observed to add new decorations to webs that have not been renewed for a few days. In new webs, only 20% of the decorations are cruciate, whereas in those old webs this percentage is as high as 70%. Since worn webs often lack some fine structures, like the guide spiral, hub threads or even radii, Walter and Elgar (2016) suggested that the remaining structure of the web cannot accommodate a circular decoration, e.g. with the spider being unable to complete its revolving construction around the web hub due to a lack of suitable attachment points. The idea needs further support, yet is compelling, as it may also allow for inferences on how the ontogenetic pattern-switch in other Argiope species may have emerged (see 3.5). Following Ernst Haeckel’s biogenetic law (see reviews in Nelson, 1978 and Porges et al., 2019), ontogenesis reflects evolution/phylogeny, an idea that also holds for features of spider webs (cf. Nakata, 2010). Hence, combined with this idea, circular silk decoration patterns may well represent the evolutionary oldest decoration form, at least in Argiope spiders, while linear and cruciate arrangements may represent the cheaper, perhaps less effective alternative, with its initial evolutionary appearance being strongly dictated by the architecture of the web. Apart from additional studies on the proximate causes of the silk deposition in the web, more investigations on the pattern-shaping may not only provide more insights into the evolutionary progression of web decorating, but also into its ultimate function.
The scarce knowledge on the silk regulation hypothesis, the silk-dragging idea and the latest contemplations on an inevitable pattern-shaping highlight the need for more studies that treat the decoration as the dependent variable. We have been quick in concluding functionality from single experiments on ultimate signal effects, but we often do not understand how and under which conditions the spiders build their decorations or change their decorating behaviour. For example, web decorations protect against web damage by non-prey animals, but we do not know whether it is the evolved main purpose of the decoration to act as a warning signal. The same is true for the prey attraction hypothesis. Web decorations attract prey insects, but we do not know whether the spiders created this signal primarily to increase the prey capture rate. An interesting study by Li (2005) nicely illustrates how the dependency-issue can distort the interpretation of results. His study shows that an increased web decoration effort in Argiope versicolor leads to higher food intake via prey attraction, which eventually increases the fitness of the spiders, as they grow faster and bigger. The explanation seems coherent, but in fact has a flaw. For the effects presented and from the experimental setup described, it is not discernible what was cause and what consequence. An alternative conclusion from the same study could be that spiders with a random advantage, e.g. a profitable web location, catch more prey and grow faster accordingly. da Silva et al. (2020), for example, found that sun exposure had a strong positive effect on the prey capture rate of Argiope argentata. Accordingly, “lucky” spiders in the right location may have more silk to spare, which they can use to construct larger decorations. Simply by swapping the axes in the graphs in Li (2005), I could argue that the main function of A. versicolor’s web decoration is predator avoidance, without contradicting the data presented. Unfortunately, this is a recurring issue in the debate about silk decorations. When Schoener and Spiller (1992) suggested a protective function of web decorations in Argiope argentata, they argue that the higher survival of decorating spiders is a proof for it. However, one could debate this logic by saying that well-fed spiders have a survival benefit because they are in good condition, and spiders in good condition build more decorations. At the end, it is not entirely clear whether silk decorating and survival are linked in the way as it is proposed. Correlation does not necessarily allow to conclude causation, and we should be more careful when designing future studies to avoid spurious correlations.
The examples above indicate a dependency of the decorating behaviour on spider condition, but there are also examples of external factors that have a strikingly strong impact on the decorating behaviour, but are reprehensibly understudied. In fact, the literature already describes (but does not pursue) natural effects on the web decoration behaviour that vastly exceed anything ever measured after manipulations in the lab. The already mentioned investigations by Alexander Kerr on Argiope appensa on Guam provide an excellent example. In 1993 as well as more recently, in 2021, he could show that the absence of birds has a profound effect on the web decorating frequency in this species. While between 40-50% of A. appensa spiders in populations on neighbouring islands (still hosting birds in abundance) build web decorations, this number is significantly lower on Guam (only 16%), where the Brown Tree Snake, Boiga irregularis, has eradicated all birds. This remarkable difference has not changed over the 28 years in between both studies (Kerr et al., 2021). A similar observation was already made by Yael Lubin in 1975 on the Galapagos Islands, where the decoration frequency of Argiope argentata was negatively correlated with the abundance of birds. The link between bird abundance and web decorating is unclear, but in lab experiments intra-specific differences of such magnitude are never observed. If we could identify the key factor that let the spiders reduce their decoration effort, we might get a clearer picture of what functions these structures have. Yet, over the last 30 years, or with respect to the work of Lubin (1975) over the last 45 years, no-one has looked closer at this phenomenon.
Personally, I observed a similarly strong effect in Argiope mascordi (unpublished data), however, in a timely rather than a spatial manner. In 2012, I collected individuals for lab experiments at Lake Tinaroo in Queensland/Australia. It was February and I found them in large numbers building their webs on bare rocks all around the lake, which interestingly enough is quite unusual for Argiope. Since the body colouration of this species is very cryptic, I could only find them by spotting their silk decorations, which were shiny over relatively large distances. At this latitude of Australia, seasonal differences are weaker than further south, and A. mascordi has overlapping generations in this habitat, unlike species in the temperate regions of the world, where Argiope species follow a pronounced seasonal cycle. Hence, I was able to go on another field trip in June to stock up my laboratory population. However, this time, I was shocked as all spiders seemed to have disappeared. At a closer inspection of the rocks, I noticed that they were still there after all. I just could not spot them as they almost completely ceased to build any form of silk decoration (Figure 7). Unfortunately, I had no time to investigate this any further, but the effect was remarkable. What in the environment has caused this dramatic drop in decorating? Rao et al. (2007) also observed a seasonal change in the web decorating frequency of Argiope keyserlingi, with adults building fewer and fewer decorations as the season progressed. The authors argue that this might be the result of a selection process over the year, with non-decorating spiders being the survivors. This idea suggests that the construction of silk decorations is associated with a higher predation risk, perhaps through a signalling conflict between prey and predator attraction. We may be faced with two strategies, risky prey/predator attraction and fast growth when decorating the web vs save and slow growth without decorating. However, it may be noted here that Rao et al. (2007) found in the same study that more exposed spiders build larger decorations. This also allows for the alternative conclusion that it was actually the positioning of the spider alone that reduced their chance of survival, and that any link between decorating and survival would be indirect. Why spiders would invest more in web decorations when they are more exposed remains to be explored as well though.

Figure 7 Argiope mascordi. Upper row: easily spottable with silk decorations, Lower row: cryptic to the human eye without decorations.
For both, seasonal effects as well as the link between web decorating and the presence/absence of birds, we could invoke various causes, choosing from the repertoire of descriptions of ultimate decoration functions. The web protection hypothesis predicts that in the absence of web destroyers the decoration frequency is reduced, the predator avoidance hypothesis that it is reduced in the absence of predators, and the prey attraction hypothesis would predict that high prey abundance renders the construction of web decorations less important. All these hypotheses may explain the strikingly low decoration frequency in the two scenarios described above, yet we need to find out what the eliciting factor is in order to infer a function of the decoration.
After all, moulting events seem to have the greatest impact on silk decorating, by far. It is a consistent observation across species that a few days prior to moults, various spider species significantly increase the size of their web decorations (Edmunds, 1986; Nentwig and Heimer, 1987; Walter et al., 2008b). Robinson and Robinson (1973a, 1973b) reported the rare occurrence of silk decorations in Nephila pilipes (Figure 8) and Trichonephila clavipes only in relation to the moults of these species, with decorations not being constructed in inter-moult phases. If we do the exercise again and browse through the list of ultimate functional explanations, the prey attraction hypothesis fails to explain this phenomenon this time. In preparation of the moult, spiders stop foraging and feeding altogether (Higgins, 1990). Why would they try to increase the prey interception rate by investing more in an attracting signal? Often occurring in rudimentary moulting webs, called skeleton webs, Robinson and Robinson suggested that the decorations may reinforce the web in preparation of the moulting event, when the spiders need a stable abutment for the procedure.

Figure 8 Juvenile Nephila pilipes, Queensland/Australia. Silk decorating is rare in nephilids. It has only been found in juveniles and in relation to moulting events.
In Argiope, where web decorating is much more frequent, the effect of moults may be masked by the fact that decorations are also constructed in inter-moult phases. The observable size differences may be overlooked as “outliers”. However, the phenomenon has become obvious to investigators observing the web construction of these spiders over relatively long time spans. Edmunds (1986) noticed the much larger size of web decorations right before moults in Argiope flavipalpis, and Nentwig and Heimer (1987) observed the same in A. argentata. In 2008, Walter et al. collected empirical data on the phenomenon, measuring the web decoration size of juvenile and subadult Argiope keyserlingi. They found that, on average, the species’ silk decorations are three times larger within five days before moulting, compared to the “regular” decoration constructed during inter-moult phases (Figure 9). With the prey attraction hypothesis failing to explain the increased investment, web advertisement and predator avoidance hypotheses may hold here. If silk decorations protect web and/or spider, the pre-moulting state is the time where they would be most beneficial. Every moult is a critical life phase for these spiders, as they are particularly vulnerable to predation during that time (Robinson and Robinson, 1973a; Tanaka, 1984; Baba and Miyashita, 2006). Extra protection may thus increase their survival chances.
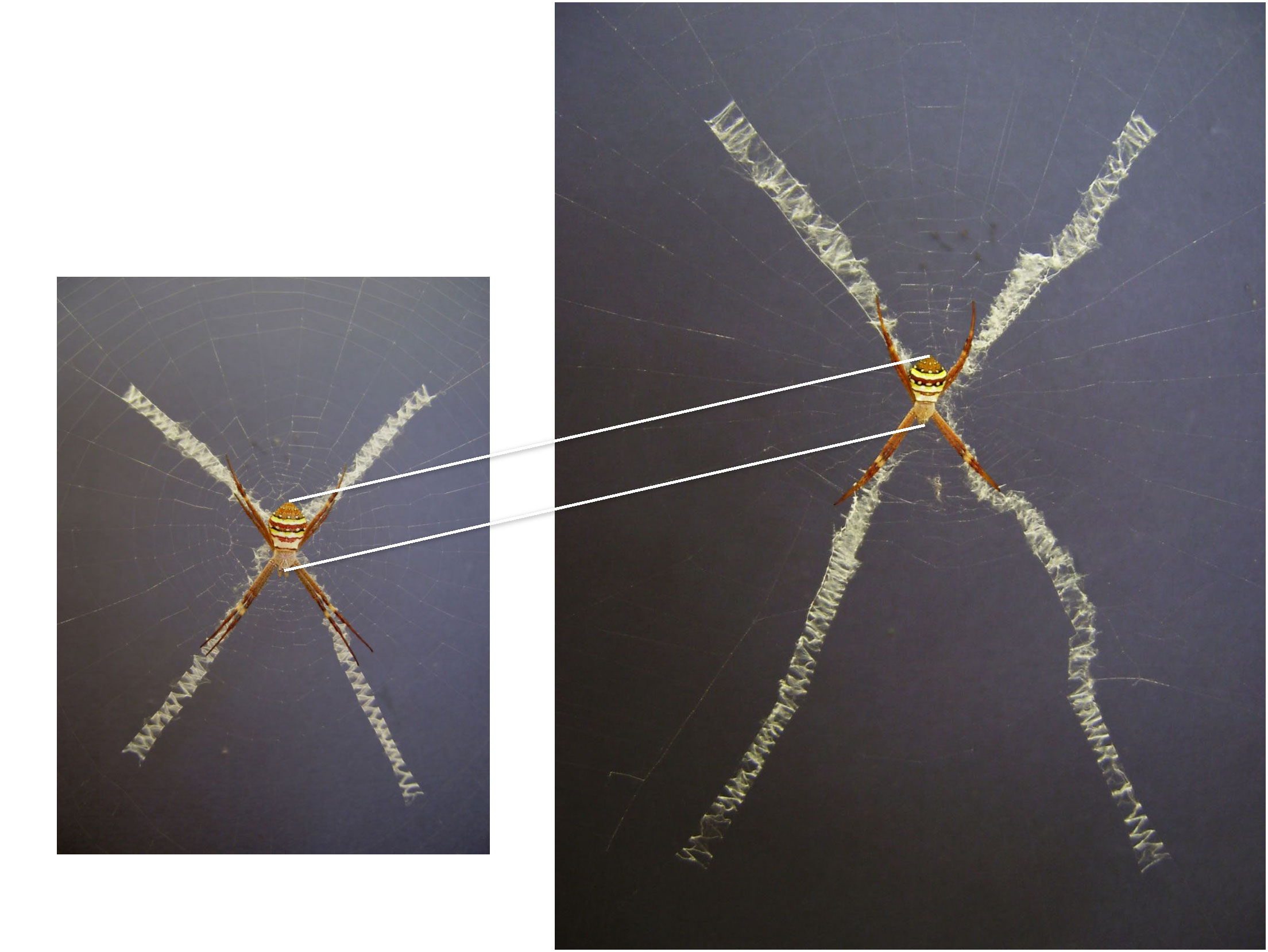
Figure 9 Subadult Argiope keyserlingi. Left: in inter-moult phase with species-typical cruciate decoration, Right: a few days before moulting with oversized cruciate decoration.
Walter et al. (2008b) also suggest some functionality of these larger decorations, related to the physiology of the moulting spiders. One idea is that the spiders outsource specific compounds that would be otherwise metabolized during the moult or the days immediately prior to or after the moult. Another suggestion is, that the spiders may simply store proteins. That way, aciniform silk could be swiftly recycled and used again for capturing prey, since the spiders have to compensate for lost foraging opportunities of the days around the moulting event (Walter et al., 2008b). Several types of silk glands are remodelled during a moult, and they may not be fully operative right after (Tillinghast and Townley, 1986; Townley et al., 2006). This may well affect the aciniform glands too. Finally, the spiders may also need to reduce their body mass prior to the moulting. Higgins and Rankin (2001) demonstrated in Trichonephila clavipes that ‘‘well-fed’’ individuals more often suffer from moulting failures when exceeding a critical pre-moult mass.
Web decorations seem to have an important yet unknown function in connection with moulting events, and this does not necessarily need to be related to visual signalling. Similar to the silk gland regulation hypothesis, physiological reasons may explain the deposition of silk in the web. Perhaps it is moulting events that entail the explanation for the evolutionary origin of silk decorating. Whatever the reason for the increased silk deposition before moults, the easiest way for the spider to attach this silk to the web is to wipe it off between adjacent radii, which inevitably leads to the creation of the commonly observed zigzag-pattern of the decoration bands (cf. McCook, 1889; Walter and Elgar, 2016). Once picked up visually, these structures may then have evolved into a signal that proved useful in inter-moult phases as well. Selection may thus have favoured individuals that continued to construct decorations after having finished the moult, which for some reasons happened in Argiope but apparently not in Nephila. While we are spending a lot of time investigating the ultimate, inter-moult functions, the impact of moulting on the decorating behaviour has turned out to be many times stronger than any measurable difference between study groups in either field investigations or lab experiments.
No doubt, we need a better understanding of the proximate causes of web decorating. It is crucial for unravelling the evolution of this intriguing spider behaviour, but also to narrow down the number of possible, and plausible, ultimate functional explanations. A stronger knowledge base will help future researchers to better challenge existing functional hypotheses that often have become a tradition more than a fact. I argue that many interpretations of previous studies even lack some logic. Compared to straight-forward data on the prey attraction hypothesis, support for the predator avoidance function seems to be more ambiguous, because it is often based on circumstantial evidence and observations. However, I argue that the objective logic for the predator avoidance function is stronger. There is an undisputable link between web decorating and the hub-dwelling lifestyle of orb web spiders; no decorating species builds protective retreats, and no retreat builders build web decorations (Blackledge et al., 2011). Blackledge et al. (2011) have articulated the logical insufficiency of the prey attraction hypothesis in a thought experiment: “There is no obvious explanation for why diurnal, but retreat-dwelling spiders, such as Araneus, Metepeira, and Zygiella do not build stabilimenta, as they would certainly benefit from visual attraction of prey too”.
Given the mutual exclusiveness, and considering the primary function of retreats to be protecting the spiders against predation and environmental effects (Manicom et al., 2008), it would be logic to assume that web decorations represent some sort of replacement. For detritus decorations in Cyclosa, this view is likely to find wide acceptance (cf. Nakata and Ushimaru, 2004; Kondo et al., 2012; Ma et al., 2020; Gong et al., 2023). It may be less obvious for silk decorations, but several observations support the retreat-replacement perspective too. Spiders experiencing heavy web damage build more silk decorations (Walter and Elgar, 2011). In the absence of avian predators, spiders reduce their investment in silk decorations (Kerr, 1993; Kerr et al., 2021). And, spiders that build silk decorations have a higher chance of survival (Schoener and Spiller, 1992; Blackledge and Wenzel, 2001; Wang et al., 2021). Some kind of intermediate evolutionary state may be seen in Araneus expletus, a retreat-building species. Juveniles build web decoration-like structures, dense silk sheets, as an extension of their retreats, and Eberhard (2008) suggests a protective function against visually hunting predators. Another interesting study object to test the potential analogy of web decorations and retreats may be the araneid genus Cyrtophora, in which both behaviours can be found. C. hirta, for example, builds retreats (Manicom et al., 2008), while C. ikomosanensis and C. moluccensis construct silk decorations, albeit very rarely (Kerr, 2023, and pers. obs.).
The link between web decorating and a hub-dwelling lifestyle obviously allows for the inference of a protective function, a logical conclusion already drawn 50 years ago by Eberhard (1973). However, this link could also bring us back to the very early days of decoration research and revive the stability hypothesis. This hypothesis has never been rigorously examined, on the grounds that the decoration silk is only loosely attached to the radii of the orb web and does not provide any reinforcement of the web structure (Blackledge et al., 2011). This is the case in dry conditions, but the situation might change in rainy weather. Henry McCook (1889) described an according observation as follows: “After a heavy summer shower I once found two webs of this species temporarily marked by what is a quite fixed characteristic of the webs of Argiope. … Below the hub the notched zone was crossed by a disc of thick, sheeted silk which extended downward between two of the radii, uniting them. A similar band united two of the radii above the hub. I conjectured that these had been thrown out from the spinnerets to strengthen the web against the weight of the rain; or as a protection, a sort of umbrella, between the spider hanging on the side toward the bush and the shower driving from the opposite quarter.” What McCook observed is the typical picture of an Argiope web after heavy rain, if it had built a linear decoration. If wetted enough, the capture spiral and, eventually, the radii will rupture. The decorated radii, however, are less prone to rupture (pers. obs.). Each zigzag-band of the decoration will contract once wet, and perhaps then the decoration can indeed stabilise the two radii that were initially only dabbed. Similar, or perhaps in addition to the supercontraction of the orb web under high humidity (cf. Boutry and Blackledge, 2010), this mechanism may secure at least the centre of the web to stay in place after a shower. As mentioned above, losing the web is critical for the survival of hub-dwelling spiders that cannot hide in their retreats. Hence, reinforcing the web might be a facultative, but very beneficial behaviour in wet conditions. I strongly encourage to study this in more detail. Previous investigations have not yet found evidence that spiders adjust their decoration investment according to the humidity or the likelihood of rain though (Nentwig and Rogg, 1988; Herberstein and Fleisch, 2003). However, those were rather short-term studies, and it remains unclear how well spiders can predict weather conditions within a short time frame. The seasonality of the climate may play a more important role, hence long-term observations may provide more insights.
I here make a proposition of how the evolution of web decorations has progressed and what their main function is. I am happy to see myself proven wrong as well as confirmed. Given the strong indication that web decorations represent a replacement of protective retreats, I argue that they have directly evolved from them. Many orb web spiders build those retreats adjacent to their webs, by weaving together whatever plant material is available or by building silken shelters, like Zygiella (Levi, 1974; Gregorič et al., 2015). However, there are also species that construct them directly in the centre of their orbs. Phonognatha is known for rolling leaves into a retreat directly attached to the web hub (Kallal and Hormiga, 2018). A similar behaviour is found in Acusilas species (Kuntner et al., 2008) and in Araneus dimidiatus (Kallal and Hormiga, 2018). Retreats at the centre of the orb web are also seen in species using detritus for their construction, like Spilasma, Micrepeira and Nemoscolus (Kallal and Hormiga, 2018), and in Cyclosa, if we already count their detritus decorations as retreats (Gong et al., 2023). Blackledge et al. (2011) found evidence for a monophyletic evolution of orb webs, whereas more recent work by Kallal et al. (2021) indicates that orb webs may in fact have evolved repeatedly. However, there is agreement that retreats, like web decorations, have evolved several times within orb weavers (Gregorič et al., 2015). Sheet webs derived from silk lines dragged to and from retreats (Eberhard, 2020), and from those ancestral substrate-bound, rather irregular ground webs, orb webs have evolved by elevating the structure in the air (Blackledge et al., 2009). I suggest that the retreat followed this process, and in some cases evolved into web decorations by reducing the structural complexity. This may have happened in environments where the need for protection was less strong, while the benefit of resting on the web hub, like being quicker in responding to prey impacts, was a strong driver. Instead of 3D-structures, two-dimensional shields, like circular silk decorations, might have turned out to be sufficient to significantly reduce the predation pressure for those species (cf. Wang et al., 2021). This explains the widespread occurrence of circular versions of silk decorations, like in many uloborids and in Cyclosa and juvenile Argiope spiders (araneids). Architectural constraints as well as cost considerations later favoured the evolution of alternative silk arrangements in the web, perhaps by compromising functional efficiency (Walter and Elgar, 2016). During moults, when the need for protection temporarily grows, the only solution is to increase the investment in decorations, as the construction of a more protective 3D-retreat is no longer part of the behavioural repertoire. To me it seems most compelling to argue that web decorations primarily had, and may still have, a protective function. However, it cannot be denied that the visual impression of the decoration silk has also attracted the attention of prey insects, luring them to the web. This fortunate side effect for the spider has complicated the situation for the human investigator. The co-evolution of a single sender but a variety of receivers can be expected to result in high variation of decoration behaviours and measurable effects, respectively. I leave it open for debate whether the term “multifunctional” is a particularly helpful term to describe the situation, or whether it might be worthwhile to keep distinguishing between one main function and side effects otherwise.
The idea of the evolution of web decorations I proposed here is meant as an inspiration and cannot yet resolve all parts of the riddle. For example, the link between silk decorating and wrap attacking is still worthwhile to be pursued further (Walter et al., 2008a; Walter and Elgar, 2012; Walter, 2018). It remains to be shown, why spiders that reduce their efforts in building protective retreats would also shift their prey capture behaviour towards wrap attacking. The wrap attack strategy is considered to be the higher evolved prey capture strategy amongst orb weaving spiders compared to the more ancestral bite attack (Gregorič et al., 2015), a correlation that still has to be demonstrated for web decorating in comparison to retreat construction. We may be faced with a parallel evolution of two independent traits. Perhaps, the hub-welling lifestyles favours wrap attacks. Evolutionary, wrap attacking would then have followed web decorating and not vice versa, as suggested by the silk gland regulation hypothesis (Walter et al., 2008a; Walter and Elgar, 2012).
When screening the web decoration literature, it seems it can be divided into two “realms” of 1) less supported, but strong effects when decorations are treated as the dependent variable and 2) well supported, yet weaker effects when decorations are treated as the independent variable. Unfortunately, both realms have only little overlap (Figure 10).
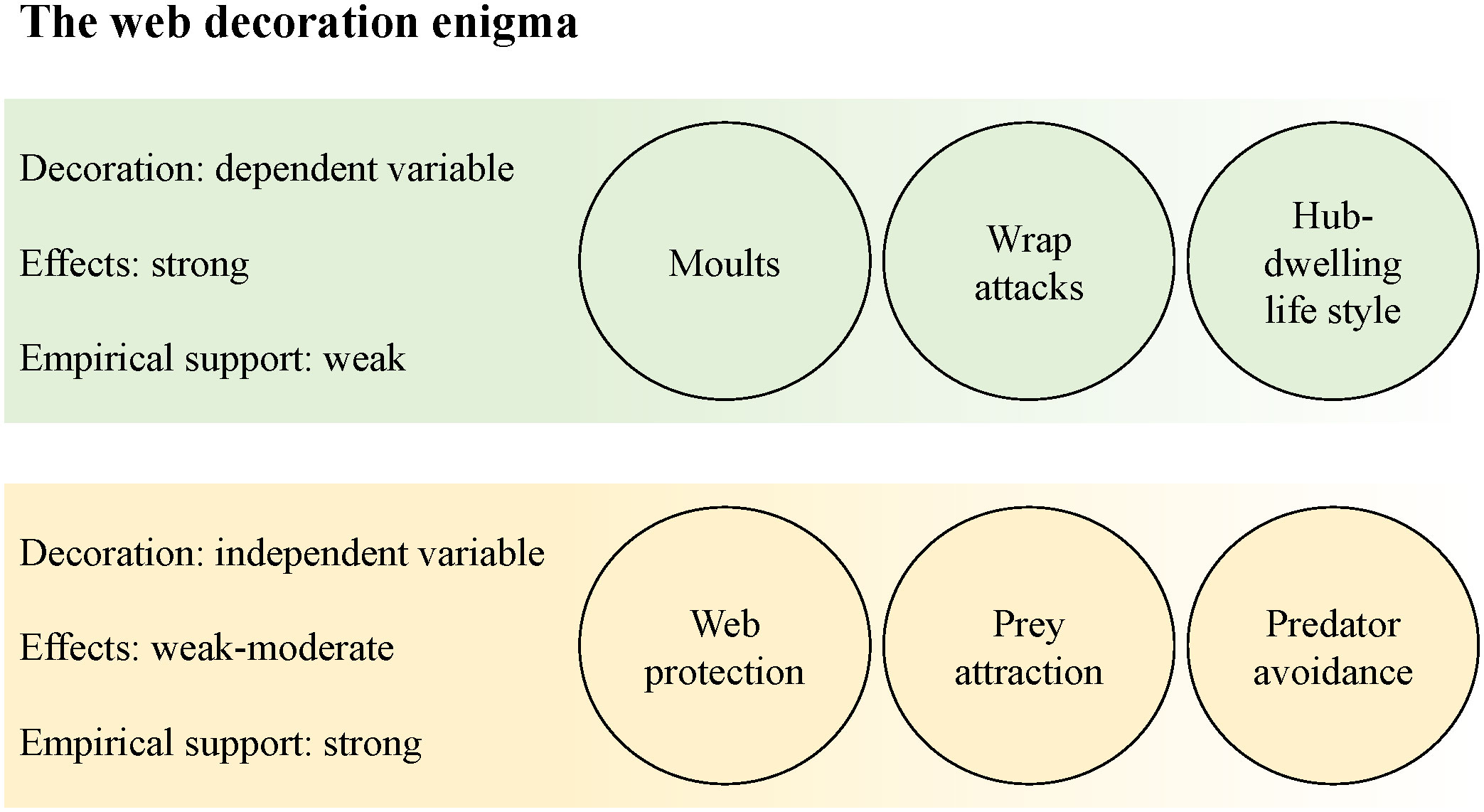
Figure 10 The issue with finding a conclusive functional explanation for web decorations is not least a lack of overlap of the two “realms” of previous research in this field. While we have observed strong effects when treating the decoration as the dependent variable, we have spent more time on investigating the weaker effects.
Henry McCook (1889) was an excellent observer, and most of his descriptions of spider behaviours are still not questioned to this day. However, he was a little too optimistic when he wrote about web decorating: “A future observer will doubtless find a simple explanation of the phenomenon …”. Finding a convincing explanation for the adaptive value of web decorations turned out to be very difficult. Studying the existing literature to find answers is like opening a long-forgotten fishing bag; everything is somehow there, but it seems impossible to disentangle the content. We need to tidy up, replace some stuff and re-evaluate others. Going one step back, we should first try to understand under which conditions spiders build and vary their decorations. Parallel investigations on species from different branches of the phylogenetic tree may facilitate the evolutionary interpretation of results. For example, why not conducting one and the same experiment on Uloborus, Cyclosa and Argiope more often, instead of comparing species of the same genus only? In doing so, we should refrain from presuming a pattern-dependency of web decoration functions, because there are good reasons that they may be independent from visual signalling or that different patterns serve the same function. Instead of being detail questions, it is fascinating to see that the unresolved riddles of the decoration research are the big ones. We have not yet understood why spiders build extraordinarily large decorations prior to moults, why they cease decorating in the absence of birds in their environment, and why there is a link to the hub-dwelling lifestyle and the wrap attack. Last not least, the stability hypothesis is not as unrewarding as many arachnologists continue to assume. I would not be at all surprised if McCook may have already described what we all have failed to prove in the 130 years after.
AW: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Baba Y., Miyashita T. (2006). Does individual internal state affect the presence of a barrier web in Argiope bruennichii (Araneae: Araneidae)? J. Ethol. 24, 75–78. doi: 10.1007/s10164-005-0164-4
Bateman P. W., Fleming P. A. (2013). The influence of web silk decorations on fleeing behaviour of Florida orb weaver spiders, Argiope florida (Aranaeidae). Can. J. Zool. 91, 468–472. doi: 10.1139/cjz-2012-0312
Bjorkman-Chiswell B. T., Kulinski M. M., Muscat R. L., Nguyen K. A., Norton B. A., Symonds M. R. E., et al. (2004). Web building spiders attract prey by storing decaying matter. Sci. Nat. 91, 245–248. doi: 10.1007/s00114-004-0524-x
Blackledge T. A. (1998a). Stabilimentum variation and foraging success in Argiope aurantia and Argiope trifasciata (Araneae: Araneidae). J. Zool. 246, 21–27. doi: 10.1017/S0952836998009030
Blackledge T. A. (1998b). Signal conflict in spider webs driven by predators and prey. Proc. Royal. Soc B. 265, 1991–1996. doi: 10.1098/rspb.1998.0530
Blackledge T. A., Kuntner M., Agnarsson I. (2011). The form and function of spider orb webs: evolution from silk to ecosystems. Adv. In Insect. Phys. 41, 175–262. doi: 10.1016/B978-0-12-415919-8.00004-5
Blackledge T. A., Scharff N., Coddington J. A., Szüts T., Wenzel J. W., Hayashi C. Y., et al. (2009). Reconstructing web evolution and spider diversification in the molecular era. Proc. Natl. Acad. Sci. U.S.A. 106, 5229–5234. doi: 10.1073/pnas.0901377106
Blackledge T. A., Wenzel J. W. (1999). Do stabilimenta in orb webs attract prey or defend spiders? Behav. Ecol. 10, 372–376. doi: 10.1093/beheco/10.4.372
Blackledge T. A., Wenzel J. W. (2000). The evolution of cryptic spider silk: a behavioral test. Behav. Ecol. 11, 142–145. doi: 10.1093/beheco/11.2.142
Blackledge T. A., Wenzel J. W. (2001). Silk mediated defense by an orb web spider against predatory mud-dauber wasps. Behaviour 138, 155–171. doi: 10.1163/15685390151074357
Blamires S. J., Blackledge T. A., Tso I.-M. (2017). Physicochemical property variation in spider silk: ecology, evolution, and synthetic production. Annu. Rev. Entomol. 62, 443–460. doi: 10.1146/annurev-ento-031616-035615
Blamires S. J., Hochuli D. F., Thompson M. B. (2007). Does decoration building influence antipredator responses in an orb-web spider (Argiope keyserlingi) in its natural habitat? Austral. J. Zool. 55, 1–7. doi: 10.1071/ZO06098
Blamires S. J., Hochuli D. F., Thompson M. B. (2008). Why cross the web: decoration spectral properties and prey capture in an orb spider (Argiope keyserlingi) web. Biol. J. Linn. Soc 94, 221–229. doi: 10.1111/j.1095-8312.2008.00999.x
Blamires S. J., Hochuli D. F., Thompson M. B. (2009). Prey protein influences growth and decoration building in the orb web spider Argiope keyserlingi. Ecol. Entomol. 34, 545–550. doi: 10.1111/j.1365-2311.2009.01095.x
Blamires S. J., Liao C.-P., Chang C.-K., Chuang Y.-C., Wu C.-L., Blackledge T. A., et al. (2015). Mechanical performance of spider silk is robust to nutrient-mediated changes in protein composition. Biomacromolecules 16, 1218–1225. doi: 10.1021/acs.biomac.5b00006
Boutry C., Blackledge T. A. (2010). Evolution of supercontraction in spider silk: structure-function relationship from tarantulas to orb-weavers. J. Exp. Biol. 213, 3505–3514. doi: 10.1242/jeb.046110
Bruce M. J. (2006). Silk decorations: controversy and consensus. J. Zool. 269, 89–97. doi: 10.1111/j.1469-7998.2006.00047.x
Bruce M. J., Heiling A. M., Herberstein M. E. (2005). Spider signals: are web decorations visible to birds and bees? Biol. Lett. 1 299, 302. doi: 10.1098/rsbl.2005.0307
Bruce M. J., Herberstein M. E. (2005). Web decoration polymorphism in Argiope Audouin 1826(Araneidae) spiders: ontogenetic and interspecific variation. J. Nat. Hist. 39, 3833–3844. doi: 10.1080/00222930500432182
Bruce M. J., Herberstein M. E., Elgar M. A. (2001). Signalling conflict between prey and predator attraction. J. Evol. Biol. 14, 786–794. doi: 10.1046/j.1420-9101.2001.00326.x
Cedhagen T., Björklund S. (2007). Is web oscillation in the orb-web spider Argiope lobata (Pallas 1772) (Araneidae) an anti-predatory behaviour? Newsl. Br. Arachnol. Soc 109, 10–11.
Cheng R.-C., Kuntner M. (2014). Phylogeny suggests nondirectional and isometric evolution of sexual size dimorphism in argiopine spiders. Evolution 68, 2861–2872. doi: 10.1111/evo.12504
Cheng R.-C., Tso I.-M. (2007). Signaling by decorating webs: luring prey or deterring predators? Behav. Ecol. 18, 1085–1091. doi: 10.1093/beheco/arm081
Cheng R.-C., Yang E.-C., Lin C.-P., Herberstein M. E., Tso I.-M. (2010). Insect form vision as one potential shaping force of spider web decoration design. J. Exp. Biol. 213, 759–768. doi: 10.1242/jeb.037291
Chittka L. (1996). Optimal sets of color receptors and color opponent systems for coding of natural objects in insect vision. J. Theor. Biol. 181, 179–196. doi: 10.1006/jtbi.1996.0124
Chou I.-C., Wang P.-H., Shen P. S., Tso I.-M. (2005). A test of prey-attracting and predator defence functions of prey carcass decorations built by Cyclosa spiders. Anim. Behav. 69, 1055–1061. doi: 10.1016/j.anbehav.2004.09.010
Cloudsley-Thompson J. L. (1995). A review of the antipredator devices of spiders. Bull. Br. Arachnol. Soc 10, 81–96.
Cobb A., Cobb S. (2019). Do zebra stripes influence thermoregulation? J. Nat. Hist. 53, 863–879. doi: 10.1080/00222933.2019.1607600
Craig C. L. (1986). Orb-web visibility: the influence of insect flight behaviour and visual physiology on the evolution of web designs within the Araneoidea. Anim. Behav. 34, 54–68. doi: 10.1016/0003-3472(86)90006-0
Craig C. L. (1989). Alternative foraging modes of orb web weaving spiders. Biotropica 21, 257–264. doi: 10.2307/2388653
Craig C. L. (2003). Spiderwebs and Silks: Tracing Evolution from Molecules to Genes to Phenotypes (Oxford: Oxford University Press). doi: 10.1093/oso/9780195129168.001.0001
Craig C. L., Bernard G. D. (1990). Insect attraction to ultraviolet reflecting spider webs and web decorations. Ecology 71, 616–623. doi: 10.2307/1940315
Craig C. L., Wolf S. G., Davis L. D., Hauber M. E., Maas J. L. (2001). Signal polymorphism in the web-decorating spider Argiope argentata is correlated with reduced survivorship and the presence of stingless bees, its primary prey. Evolution 55, 986–993. doi: 10.1554/0014-3820(2001)055[0986:SPITWD]2.0.CO;2
da Silva F. C., Moleta M., Dos Anjos C. A., Marra SChade G., Staichak G., Tozetto L., et al. (2020). Testing traditional hypotheses about prey capture efficiency in orb−web spiders. J. Ethol. 39, 3–8. doi: 10.1007/s10164-020-00663-1
Eberhard W. G. (1967). Attack behavior of diguetid spiders and the origin of prey wrapping in spiders. Psyche 74, 173–181. doi: 10.1155/1967/867474
Eberhard W. G. (1973). Stabilimenta on the webs of Uloborus diversus (Araneae: Uloboridae) and other spiders. J. Zool. Lond. 171, 367–384. doi: 10.1111/j.1469-7998.1973.tb05345.x
Eberhard W. G. (2003). Substitution of silk stabilimenta for egg sacs by Allocyclosa bifurca (Araneae: Araneidae) suggests that silk stabilimenta function as camouflage devices. Behaviour 140, 847–868. doi: 10.1163/156853903770238346
Eberhard W. G. (2019). Hunting behavior of the wasp Polysphincta gutfreundi and related Polysphinctine wasps (Hymenoptera, Ichneumonidae). J. Kansas Entomol. Soc. 91, 177–191.
Eberhard W. G. (2008). Araneus expletus (Araneae, Araneidae): Another stabilimentum that does not function to attract prey. J. Arachnol. 36, 191–194. doi: 10.1636/St07-35SC.1
Eberhard W. G. (2020). Spider webs: behavior, function, and evolution (Chicago: University of Chicago Press). doi: 10.7208/chicago/9780226534749.001.0001
Eberhard W. G. (2023). A suite of behavioural and morphological traits camouflage Allocyclosa bifurca (McCook 1887)(Araneae: Araneidae). Arachnology 19, 809–815. doi: 10.13156/arac.2023.19.5.809
Eberhard W. G., Opell B. D. (2022). Orb web traits typical of Uloboridae (Araneae). J. Arachnol. 50, 351–384. doi: 10.1636/JoA-S-21-050
Edmunds J. (1986). “The stabilimenta of Argiope flavipalpis and Argiope trifasciata in West Africa, with a discussion of the function of stabilimenta,” in Proceedings of the 9th International Congress of Arachnology, PANAMA, Panama 1983. Eds. Eberhard W. G., Lubin Y. D., Robinson B. C. (Smithsonian Institution Press, Washington), 61–72.
Edwards W., Whytlaw P. A., Congdon B. C., Gaskett C. (2009). Is optimal foraging a realistic expectation in orb-web spiders? Ecol. Entomol. 34, 527–534. doi: 10.1111/j.1365-2311.2009.01099.x
Eisner T., Nowicki S. (1983). Spiderweb protection through visual advertisement: role of the stabilimentum. Science 219, 185–187. doi: 10.1126/science.219.4581.185
Endler J. A. (1993). Some general comments on the evolution and design of animal communication systems. Phil. Trans. R. Soc Lond. B. 340, 215–225. doi: 10.1098/rstb.1993.0060
Gálvez D. (2011). Web decoration of Micrathena sexpinosa (Araneae: Araneidae): a frame-web-choice experiment with stingless bees. J. Arachnol. 39, 128–132. doi: 10.1636/Hi09-76.1
Gálvez D. (2017a). Luring prey to the web: the case of Argiope and Nephila. Anim. Biol. 67, 149–156. doi: 10.1163/15707563-00002528
Gálvez D. (2017b). Argiope submaronica (Araneidae) silk decoration does not reduce web damage by birds. J. Entomol. Sci. 52, 301–303. doi: 10.18474/JES17-38.1
Ghalambor C. K., McKay J. K., Carroll S. P., Reznick D. N. (2007). Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct. Ecol. 21, 394–407. doi: 10.1111/j.1365-2435.2007.01283.x
Gladwell M. (2005). Blink: The power of thinking without thinking (New York: Back Bay Books, Little, Brown And Co).
Godfrey D., Lythgoe J. N., Rumball D. A. (2008). Zebra stripes and tiger stripes: the spatial frequency distribution of the pattern compared to that of the background is significant in display and crypsis. Biol. J. Linn. Soc Lond. 32, 427–433. doi: 10.1111/bij.1987.32.issue-4
Gong D., Hua Z., Mao A., Chen L., Peng Y., Zhang S. (2023). Web decoration is important in mediating foraging site fidelity as well as prey supplement and predation risk in an orb-web spider. Oikos 2023, e09961. doi: 10.1111/oik.09961
Gonzaga M. O., Vasconcellos-Neto J. (2005). Testing the functions of detritus stabilimenta in webs of Cyclosa fililineata and Cyclosa morretes (Araneae: Araneidae): Do they attract prey or reduce the risk of predation? Ethology 111, 479–491. doi: 10.1111/j.1439-0310.2005.01074.x
Gregorič M., Agnarsson I., Blackledge T. A., Kuntner M. (2015). Phylogenetic position and composition of Zygiellinae and Caerostris, with new insight into orb-web evolution and gigantism. Zool. J. Linn. Soc 175, 225–243. doi: 10.1111/zoj.12281
Hanson N. A., Lavallee M. B., Thiele R. H. (2021). Apophenia and anesthesia: how we sometimes change our practice prematurely. Can. J. Anesth. 68, 1185–1196. doi: 10.1007/s12630-021-02005-2
Hayashi C. Y., Blackledge T. A., Lewis R. V. (2004). Molecular and mechanical characterization of aciniform silk: uniformity of iterated sequence modules in a novel member of the spider silk fibroin gene family. Mol. Biol. Evol. 21, 1950–1959. doi: 10.1093/molbev/msh204
Heiling A. M., Herberstein M. E. (2000). “Interpretations of orb-web variability: a review of past and current ideas,” in Proceedings of the 18th European Colloquium of Arachnology, Stará Lesná, 1999, vol. 19 . Eds. Gajdoš P., Pekár S. (Bratislava: Ekológia), 97–106.
Henneken J., Jones T. M., Goodger J. Q. D., Dias D. A., Walter A., Elgar M. A. (2015). Diet influences female signal reliability for male mate choice. Anim. Behav. 108, 215–221. doi: 10.1016/j.anbehav.2015.07.023
Herberstein M. E. (2000). Foraging behaviour in orb-web spiders (Araneidae): do web decorations increase prey capture success in Argiope keyserlingi Karsch 1878? Aust. J. Zool. 48, 217–223. doi: 10.1071/ZO00007
Herberstein M. E., Craig C. L., Coddington J. A., Elgar M. A. (2000). The functional significance of silk decorations of orb-web spiders: a critical review of the empirical evidence. Biol. Rev. 75, 649–669. doi: 10.1111/j.1469-185X.2000.tb00056.x
Herberstein M. E., Fleisch A. F. (2003). Effect of abiotic factors on the foraging strategy of the orb-web spider Argiope keyserlingi (Araneae: Araneidae). Aust. Ecol. 28, 622–628. doi: 10.1046/j.1442-9993.2003.t01-1-01319.x
Higgins L. E. (1990). Variation in foraging investment during the intermolt interval and before egg-laying in the spider Nephila clavipes (Araneae: Araneidae). J. Insect Behav. 3, 773–783. doi: 10.1007/BF01065965
Higgins L. E., Rankin M. A. (2001). Mortality risk of high rate of weight gain in the spider Nephila clavipes. Funct. Ecol. 15, 24–28. doi: 10.1046/j.1365-2435.2001.00491.x
Hingston R. W. G. (1927). Protective devices in spider’s snares, with a description of seven new species of orb-weaving spiders. Proc. Zool. Soc Lond. 28, 259–293. doi: 10.1111/j.1096-3642.1927.tb02261.x
Horton C. C. (1980). A defensive function for the stabilimentum of two orb-weaving spiders (Araneae, Araneidae). Psyche 87, 13–20. doi: 10.1155/1980/57246
How M. J., Gonzales D., Irwin A., Caro T. (2020). Zebra stripes, tabanid biting flies and the aperture effect. Proc. Biol. Sci. 287, 20201521. doi: 10.1098/rspb.2020.1521
Jackson R. R., Rowe R. J., Wilcox R. S. (1993). Anti-predator defences of Argiope appensa (Araneae, Araneidae), a tropical orb-weaving spider. J. Zool. 229, 121–132. doi: 10.1111/j.1469-7998.1993.tb02625.x
Kallal R. J., Hormiga G. (2018). Systematics, phylogeny and biogeography of the Australasian leaf-curling orb-weaving spiders (Araneae: Araneidae: Zygiellinae), with a comparative analysis of retreat evolution. Zool. J. Linn. Soc 184, 1055–1141. doi: 10.1093/zoolinnean/zly014
Kallal R. J., Kulkarni S. S., Dimitrov D., Benavides L. R., Arnedo M. A., Giribet G., et al. (2021). Converging on the orb: denser taxon sampling elucidates spider phylogeny and new analytical methods support repeated evolution of the orb web. Cladistics 37, 298–316. doi: 10.1111/cla.12439
Kerr A. M. (1993). Low frequency of stabilimenta in orb webs of Argiope appensa (Araneae: Araneidae) from Guam: an indirect effect of an introduced avian predator? Pac. Sci. 47, 328–337.
Kerr A. M. (2023). Some Cyrtophora spp. (Araneae: Araneidae) build silk stabilimenta. Acta Arachnologica 72, 39–48. doi: 10.2476/asjaa.72.39
Kerr A., Sablan J. D., Williams M. K., Galsim F., Guerrero P. C., Townsend A. L., et al. (2021). Long-term, low incidence of web-decorating by spiders in the Mariana Islands, Micronesia. Ecology 102, e03433. doi: 10.1002/ecy.3433
Kim K. W., Kim K., Choe J. C. (2012). Functional values of stabilimenta in a wasp spider, Argiope bruennichi: support for the prey-attraction hypothesis. Behav. Ecol. Sociobiol. 66, 1569–1576. doi: 10.1007/s00265-012-1410-8
Kondo S., Tatsuta H., Tsuji K. (2012). Carcass decoration changes web structure and prey capture rate in an orb-web spider, Cyclosa mulmeinensis (Araneae, Araneidae). J. Insect. Behav. 25, 518–528. doi: 10.1007/s10905-012-9319-7
Kuntner M., Coddington J. A., Hormiga G. (2008). Phylogeny of extant nephilid orb-weaving spiders (Araneae, Nephilidae): testing morphological and ethological homologies. Cladistics 24, 147–217. doi: 10.1111/j.1096-0031.2007.00176.x
Levi H. W. (1974). The orb-weaver genus Zygiella (Araneae: Araneidae). Bull. Mus. Comp. Zool. 146, 267–290.
Li D. (2005). Spiders that decorate their webs at higher frequency intercept more prey and grow faster. Proc. R. Soc B. 272, 1753–1757. doi: 10.1098/rspb.2005.3160
Li D., Lee W. S. (2004). Predator-induced plasticity in web-building behaviour. Anim. Behav. 67, 309–318.
Li D., Kok L. M., Seah W. K., Lim M. L. M. (2003). Age-dependent stabilimentum-associated predator avoidance behaviours in orb-weaving spiders. Behaviour 140, 1135–1152. doi: 10.1163/156853903322589678
Li D., Lim M. L. M., Seah W. K., Tay S. L. (2004). Prey attraction as a possible function of discoid stabilimenta of juvenile orb-spinning spiders. Anim. Behav. 68, 629–635. doi: 10.1016/j.anbehav.2003.12.018
Liu M.-H., Blamires S. J., Liao C.-P., Tso I.-M. (2014). Evidence of bird dropping masquerading by a spider to avoid predators. Sci. Rep. 4, 5058. doi: 10.1038/srep05058
Lubin Y. D. (1975). Stabilimenta and barrier webs in the orb webs of Argiope argentata (Araneae, Araneidae) on Daphne and Santa Cruz Islands, Galapagos. J. Arachnol. 2, 119–126.
Lubin Y. D., Ellner S., Kotzman M. (1993). Web relocation and habitat selection in desert widow spider. Ecology 74, 1915–1928. doi: 10.2307/1940835
Ma N., Yu L., Gong D., Hua Z., Zeng H., Chen L., et al. (2020). Detritus decorations as the extended phenotype deflect avian predator attack in an orb-web spider. Funct. Ecol. 34, 2110–2119. doi: 10.1111/1365-2435.13636
Manicom C., Schwarzkopf L., Alford R. A., Schoener T. W. (2008). Self-made shelters protect spiders from predation. Proc. Natl. Acad. Sci. U.S.A. 105, 14903–14907. doi: 10.1073/pnas.0807107105
McClintock W. J., Dodson G. N. (1999). Notes on cyclosa insulana (Araneae, araneidae) of Papua New Guinea. J. Arachnol. 27, 685–688.
McCook H. C. (1889). American Spiders and their Spinning Work Vol. 1 (Philadelphia: Academy of Natural Sciences).
Nakata K. (2010). Does ontogenetic change in orb web asymmetry reflect biogenetic law? Sci. Nat. 97, 1029–1032. doi: 10.1007/s00114-010-0719-2
Nakata K., Ushimaru A. (2004). Difference in web construction behavior at newly occupied web sites between two Cyclosa species. Ethology 110, 397–411. doi: 10.1111/j.1439-0310.2004.00983.x
Neet C. R. (1990). Function and structural variability of the stabilimenta of Cyclosa insulana (Costa) (Araneae, Araneidae). Bull. Br. Arachnol. Soc 8, 161–164.
Nelson G. (1978). Ontogeny, phylogeny, paleontology, and the biogenetic law. Syst. Zool. 27, 324–345. doi: 10.2307/2412883
Nentwig W., Heimer S. (1987). “Ecological aspects of spider webs,” in Ecophysiology of Spiders. Ed. Nentwig W. (Springer, Berlin, Heidelberg, New York), 211–225. doi: 10.1007/978-3-642-71552-5
Nentwig W., Rogg H. (1988). The cross stabilimentum of Argiope argentata - nonfunctional or a nonspecific stress reaction? Zool. Anz. 221, 246–266.
Olive C. W. (1980). Foraging specializations in orb-weaving spiders. Ecology 61, 1133–1144. doi: 10.2307/1936833
Opell B. D. (1998). Economics of spider orb-webs: the benefits of producing adhesive capture thread and of recycling silk. Funct. Ecol. 12, 613–624. doi: 10.1046/j.1365-2435.1998.00222.x
Peters H. M. (1993). Über das Problem der Stabilimente in Spinnennetzen. Zoologische Jahrbücher (Physiologie) 97, 245–264.
Pierce G. J., Ollason J. G. (1987). Eight reasons why optimal foraging theory is a complete waste of time. Oikos 49, 111–118. doi: 10.2307/3565560
Porges K., Stewart E. G., Hoßfeld U., Levit G. S. (2019). From idea to law: theory, concept and terminological formation in Ernst Haeckel’s works. Russ. J. Dev. Biol. 6, 290–302. doi: 10.1134/S1062360419060079
Prokop P., Grygláková D. (2005). Factors affecting the foraging success of the wasp-like spider Argiope bruennichi (Araneae): role of web design. Biologia 60, 165–169.
Putnins M., Androulakis I. P. (2021). Self-selection of evolutionary strategies: adaptive versus non-adaptive forces. Heliyon. 7, e06997.
Rao D. (2010). Stingless bee interception is not affected by variations in spider silk decoration. J. Arachnol. 38, 157–161. doi: 10.1636/St08-41.1
Rao D., Cheng K., Herberstein M. E. (2007). A natural history of web decorations in the St Andrew’s Cross spider (Argiope keyserlingi). Aust. J. Zool. 55, 9–14. doi: 10.1071/ZO06010
Rao D., Webster M., Heiling A. M., Bruce M. J., Herberstein M. E. (2009). The aggregating behaviour of Argiope radon, with special reference to web decorations. J. Ethol. 27, 35–42. doi: 10.1007/s10164-007-0080-x
Ratz T., Bourdiol J., Moreau S., Vadnais C., Montiglio P.-O. (2023). The evolution of prey−attraction strategies in spiders: the interplay between foraging and predator avoidance. Oecologia 202, 669–684. doi: 10.1007/s00442-023-05427-5
Robinson M. H., Mirick H., Turner O. (1969). The predatory behaviour of some araneid spiders and the origin of immobilization wrapping. Psyche 76, 487–501. doi: 10.1155/1969/68029
Robinson M. H., Olazarri J. (1971). Units of behavior and complex sequences in the predatory behavior of Argiope argentata (Fabricius): (Araneae: Araneidae). Contrib. Zool. 65, 1–36. doi: 10.5479/si.00810282.65
Robinson M. H., Robinson B. C. (1970). Prey caught by a sample population of the spider Argiope argentata (Araneae: Araneidae) in Panama: a year's census data. Zool. J. Linn. Soc 49, 345–358. doi: 10.1111/zoj.1970.49.issue-4
Robinson M. H., Robinson B. C. (1973a). The stabilimenta of Nephila clavipes and the origins of stabilimentum-building in araneids. Psyche 80, 277–288. doi: 10.1155/1973/86980
Robinson M. H., Robinson B. C. (1973b). Ecology and behavior of the giant wood spider Nephila maculata (Fabricius) in New Guinea. Contrib. Zool. 149, 1–76. doi: 10.5479/si.00810282.149
Robinson B., Robinson M. H. (1974). The biology of some Argiope species from New Guinea: predatory behaviour and stabilimentum construction (Araneae: Araneidae). Zool. J. Linn. Soc 54, 145–159. doi: 10.1111/j.1096-3642.1974.tb00796.x
Robledo-Ospina L. E., Morehouse N., Escobar F., Tapia−McClung H., Narendra A., Rao D. (2023). Visual antipredator effects of web flexing in an orb web spider, with special reference to web decorations. Sci. Nat. 110, 23. doi: 10.1007/s00114-023-01849-6
Robledo-Ospina L. E., Rao D. (2022). Dangerous visions: a review of visual antipredator strategies in spiders. Evol. Ecol. 2, 163–180. doi: 10.1007/s10682-022-10156-x
Rominger C., Fink A., Perchtold-Stefan C. M., Schulter G., Weiss E. M., Papousek I. (2022). Creative, yet not unique? Paranormal belief, but not self-rated creative ideation behavior is associated with a higher propensity to perceive unique meanings in randomness. Heliyon 8, e09269. doi: 10.1016/j.heliyon.2022.e09269
Ruxton G. D. (2022). The possible fitness benefits of striped coat coloration for zebra. Mammal Rev. 32, 237–244. doi: 10.1046/j.1365-2907.2002.00108.x
Sahni V., Dhinojvala A., Opell B. D., Blackledge T. A. (2014). “Prey capture adhesives produced by orb-weaving spiders,” in Biotechnology of Silk. Eds. Asakura T., Miller T. (Springer, Berlin, Heidelberg, New York), 272.
Scharf I., Lubin Y., Ovadia O. (2010). Foraging decisions and behavioural flexibility in trap-building predators: a review. Biol. Rev. 86, 626–639. doi: 10.1111/j.1469-185X.2010.00163.x
Scharff N., Coddington J. A. (1997). A phylogenetic analysis of the orb-weaving spider family Araneidae (Arachnida, Araneae). Zool. J. Linn. Soc 120, 355–424. doi: 10.1111/zoj.1997.120.issue-4
Scharff N., Coddington J. A., Blackledge T. A., Agnarsson I., Framenau V. W., Szüts T., et al. (2020). Phylogeny of the orb-weaving spider family Araneidae (Araneae: Araneoidea). Cladistics 36, 1–21. doi: 10.1111/cla.12382
Schoener T. W., Spiller D. A. (1992). Stabilimenta characteristics of the spider Argiope argentata on small islands: Support of the predator-defense hypothesis. Behav. Ecol. Sociobiol. 31, 309–318. doi: 10.1007/BF00177771
Seah W. K., Li D. (2001). Stabilimenta attract unwelcome predators to orb-webs. Proc. R. Soc B. 268, 1553–1558. doi: 10.1098/rspb.2001.1709
Sherratt T. N. (1993). Frequency-dependent food selection by arthropods: a review. Zool. J. Linn. Soc 48, 167–186. doi: 10.1111/bij.1993.48.issue-2
Simon E. (1892). Histoire Naturelle des Araignées. Tome Premiere. Deuxième Édition (Paris: Libraire Encyclopédique de Roret). doi: 10.5962/bhl.title.51973
Soley F. G. (2019). A possible role of decorations in spiderwebs as protection devices that distract predators. Rev. Biol. Trop. 67, 2. doi: 10.15517/rbt.v67i2SUPL
Starks P. T. (2002). The adaptive significance of stabilimenta in orb-webs: a hierarchical approach. Ann. Zool. Fenn. 39, 307–315.
Tan E. J., Li D. (2009). Detritus decorations of an orb-weaving spider, Cyclosa mulmeinensis (Thorell): for food or camouflage? J. Exp. Biol. 212, 1832–1839. doi: 10.1242/jeb.030502
Tan E. J., Seah S. W. H., Yap L-M. Y. L., Goh P. M., Gan W., Liu F., et al. (2010). Why do orb-weaving spiders (Cyclosa ginnaga) decorate their webs with silk spirals and plant detritus? Anim. Behav. 79, 179–186. doi: 10.1016/j.anbehav.2009.10.025
Tanaka K. (1984). Rate of predation by a kleptoparasitic spider, Argyrodes fissifrons, upon a large host spider, Agelena limbata. J. Arachnol. 12, 363–367.
Tillinghast E. K., Townley M. A. (1986). The independent regulation of protein synthesis in the major ampullate glands of Araneus cavaticus Keyserling. J. Insect Physiol. 32, 117–123. doi: 10.1016/0022-1910(86)90130-7
Tolbert W. W. (1975). Predator avoidance behaviors and web defensive structures in the orb weavers Argiope aurantia and Argiope trifasciata (Araneae, Araneidae). Psyche 82, 29–52. doi: 10.1155/1975/39173
Townley M. A., Tillinghast E. K., Neefus C. D. (2006). Changes in composition of spider orb web sticky droplets with starvation and web removal, and synthesis of sticky droplet compounds. J. Exp. Biol. 209, 1463–1486. doi: 10.1242/jeb.02147
Tseng L., Tso I.-M. (2009). A risky defence by a spider using conspicuous decoys resembling itself in appearance. Anim. Behav. 78, 425–431. doi: 10.1016/j.anbehav.2009.05.017
Tso I.-M. (1996). Stabilimentum of the garden spider Argiope trifasciata: a possible prey attractant. Anim. Behav. 52, 183–191. doi: 10.1006/anbe.1996.0163
Tso I.-M. (1998). Isolated spider web stabilimentum attracts insects. Behaviour 135, 311–319. doi: 10.1163/156853998793066276
Tso I.-M. (2004). The effect of food and silk reserve manipulation on decoration building of Argiope aetheroides. Behaviour 141, 603–616. doi: 10.1163/1568539041166690
Vollrath F., Knight D. P. (2001). Liquid crystalline spinning of spider silk. Nature 410, 541–548. doi: 10.1038/35069000
Walter A. (2008). Are web stabilimenta attractive to praying mantids? Rev. Ibérica Aracnologia 15, 55–61.
Walter A. (2018). Tracing the evolutionary origin of a visual signal: the coincidence of wrap attack and web decorating behaviours in orb web spiders (Araneidae). Evol. Ecol. 32, 159–170. doi: 10.1007/s10682-018-9930-y
Walter A. (2019). Silk decorations in Argiope spiders: Consolidation of pattern variation and specific signal function. J. Arachnol. 47, 271–275. doi: 10.1636/JoA-S-18-013
Walter A., Bliss P., Elgar M. A., Moritz R. F. A. (2009). Argiope bruennichi shows a drinking like behaviour in web hub decorations (Araneae, Araneidae). J. Ethol. 27, 25–29. doi: 10.1007/s10164-007-0077-5
Walter A., Cadenhead N., Lee Sze Weii V., Dove C., Milley E., Elgar M. A. (2012). Water as an essential resource: Orb web spiders cannot balance their water budget by prey alone. Ethology 118, 534–542. doi: 10.1111/j.1439-0310.2012.02041.x
Walter A., Elgar M. A. (2011). Signals for damage control: web decorations in Argiope keyserlingi (Araneae, Araneidae). Behav. Ecol. Sociobiol. 65, 1909–1915. doi: 10.1007/s00265-011-1200-8
Walter A., Elgar M. A. (2012). The evolution of novel animal signals: silk decorations as a model system. Biol. Rev. 87, 686–700. doi: 10.1111/j.1469-185X.2012.00219.x
Walter A., Elgar M. A. (2016). Signal polymorphism under a constant environment: the odd cross in a web decorating spider. Sci. Nat. 103, 93. doi: 10.1007/s00114-016-1415-7
Walter A., Elgar M. A., Bliss P., Moritz R. F. A. (2008a). ‘Wrap attack’ activates web decorating behavior in Argiope spiders. Behav. Ecol. 19, 799–804. doi: 10.1093/beheco/arn030
Walter A., Elgar M. A., Bliss P., Moritz R. F. A. (2008b). Moulting interferes with web decorating behaviour in Argiope keyserlingi (Araneae: Araneidae). J. Arachnol. 36, 538–544. doi: 10.1636/ST07-58.1
Walter A., Westphal C., Bliss P., Moritz R. F. A. (2011). Drinking behaviour of the orb web spider Argiope bruennichi (Araneae; Araneidae). Behaviour 148, 1297–1311. doi: 10.1163/000579511X603292
Wang B., Yu L., Ma N., Zhang Z., Liu Q., Fan W., et al. (2021). Discoid decorations function to shield juvenile Argiope spiders from avian predator attacks. Behav. Ecol. 32, 1230–1239. doi: 10.1093/beheco/arab089
Watanabe T. (2000). Web tuning of an orb-web spider, Octonoba sybotides, regulates prey-catching behaviour. Proc. R. Soc B. 267, 565–569. doi: 10.1098/rspb.2000.1038
Williams J. L., Moya-Laraño J., Wise D. H. (2006). Burrow decorations as antipredatory devices. Behav. Ecol. 17, 586–590. doi: 10.1093/beheco/ark003
Keywords: web decorations, stabilimenta, visual signals, signalling conflict, orb web spiders, orb weavers, Argiope
Citation: Walter A (2024) The function of web decorations in orb web spiders. Front. Arachn. Sci. 3:1384128. doi: 10.3389/frchs.2024.1384128
Received: 08 February 2024; Accepted: 19 March 2024;
Published: 03 April 2024.
Edited by:
Stanislav Korenko, Czech University of Life Sciences Prague, CzechiaReviewed by:
Dumas Galvez, University of Panama, PanamaCopyright © 2024 Walter. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: André Walter, YW5kcmUud2FsdGVyQGJpb21lZC5hdS5kaw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.