
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Aquac., 16 December 2022
Sec. Production Biology
Volume 1 - 2022 | https://doi.org/10.3389/faquc.2022.1060335
 Matthew G. Hamilton1*
Matthew G. Hamilton1* Mohammed Yeasin1
Mohammed Yeasin1 Md. Badrul Alam1
Md. Badrul Alam1 Md. Rayhan Ali1
Md. Rayhan Ali1 Md. Fakhruddin1
Md. Fakhruddin1 Md. Mazharul Islam1
Md. Mazharul Islam1 Benoy K. Barman1
Benoy K. Barman1 Kelvin Mashisia Shikuku1
Kelvin Mashisia Shikuku1 Colin C. Shelley1
Colin C. Shelley1 Cristiano M. Rossignoli1
Cristiano M. Rossignoli1 John A. H. Benzie1,2
John A. H. Benzie1,2Introduction: Rohu (Labeo rohita Hamilton) is a globally significant aquaculture species for which genetically improved strains are increasingly available. In 2020, a multiplier population, comprised of highly–ranked generation-three (G3) families from the WorldFish Rohu Genetic Improvement Program, was released to hatcheries in Bangladesh for development into broodstock.
Methods: To estimate realised genetic gain for harvest weight in the G3-multiplier population, one pond from each of 19 Bangladeshi semi-commercial farms (ten in Jashore and nine in Natore–Rajshahi districts) were stocked with equal numbers of tagged fish from each of three strains. Strains included in the study were the ‘G3-multiplier’ released to hatcheries, a ‘control’ (putatively genetically equivalent to the unimproved base population which was spawned from fish of river origin) and fish sourced from a ‘commercial’ hatchery. Once stocked, fish were managed according to each farmer’s normal practices.
Results: The G3-multiplier was found to be the most rapidly growing strain. Back-transformed means for harvest weight across farms for the commercial, control and G3-multiplier strains were 594 g, 659 g and 914 g, respectively, in Jashore, and 545 g, 626 g and 845 g in Natore–Rajshahi.
Discussion: These results equate to realised genetic gains of 38.6% (Jashore) and 34.9% (Natore–Rajshahi) for the G3-multiplier over the control strain and provide evidence that a family-based (i.e. pedigree-based) approach to genetic improvement is able to generate substantial levels of genetic gain in rohu. Furthermore, the clear growth advantages exhibited by the G3-multiplier strain over the control and commercial strains in this study, should encourage more Bangladeshi hatcheries, nurseries and farmers to adopt genetically improved rohu.
Rohu (Labeo rohita Hamilton) is a cyprinid (i.e. carp) species and a member of the Indian major carps. The natural distribution of the species encompasses rivers in Bangladesh, India, Myanmar, Nepal and Pakistan (Jhingran and Pullin, 1985). It is a globally significant aquaculture species with over 2.0 Mt produced annually and, in Bangladesh, it is the most abundantly cultured carp species with 386 thousand tonnes produced over 12 months in 2019-2020 (DoF, 2020; FAO, 2020).
Rohu is almost exclusively farmed in polyculture. Polyculture systems efficiently utilise different spatial and trophic levels in ponds by stocking complimentary filter-feeding, herbivorous and bottom feeding species (Rahman et al., 1992; Wahab et al., 2011). Carp polyculture systems adopted in Bangladesh are diverse in terms of scale, intensity, species combinations, and targeted harvest age and weight (Wahab et al., 2011; Belton and Azad, 2012). Historically, low-input polyculture systems have been adopted in Bangladesh. These systems primarily rely on natural food production, generally enhanced through the application of organic or synthetic fertilizers. However, more intensive forms of production, involving supplementary feeding with locally-acquired and/or specialized formulated feeds, are increasingly adopted (Belton and Azad, 2012).
The Jashore District of Khulna Division (herein referred to as the Jashore region), and the Natore and Rajshahi Districts of the Rajshahi Division (herein referred to as the Natore–Rajshahi region) are important rohu growing areas in Bangladesh – they produce 7.3% (21,755 t) and 9.8% (29,335 t) of Bangladeshi pond-cultured rohu, respectively (DoF, 2020), while representing only 1.7% and 2.9% of the country by area. However, the two regions differ in climate (Rashid, 2019) and dominant carp polyculture practices. Natore–Rajshahi farms were located in the hottest and driest area of Bangladesh, the Western Dry climatic sub-region, which generally experiences mean summer maxima above 35°C, annual rainfall below 1524 mm and summer humidity less than 50%. The Jashore farms were located in the South-Western climatic sub-region, which experiences less extreme temperatures (mean summer maxima below 35°C), tempered by greater annual rainfall (1524 – 1778 mm) (Rashid, 2019). A specialized carp polyculture system is commonly practiced in the Natore-Rajshahi region, whereby farmers stock large fish (e.g. 500 g to 1 kg) at low density which are then grown out for extended periods to obtain greater weights (e.g. 3 to 5 kg) and premium prices. Carp aquaculture practices in Jashore region are similar to the practices followed in most other parts of the country, with fish generally harvested between 12 and 24 months of age at between 1 and 2 kg.
In Bangladesh and elsewhere, suboptimal genetic management of hatchery broodstock and a lack of genetically improved strains has historically resulted in the dissemination of rohu seed exhibiting poor performance (Reddy et al., 2002; Mahapatra et al., 2016; Khan et al., 2018; Sah et al., 2018; Vadhel et al., 2020). To address these issues, the WorldFish Rohu Genetic Improvement Program (WFRGIP) was initiated with the spawning of a base population in 2014. The WFRGIP has subsequently been managed as a family-based (i.e. pedigree-based) program with discrete generations – generation one (G1) was spawned in 2016, G2 in 2018 and G3 in 2020/21 – and selection based on harvest-age body weight (Keus et al., 2017; Hamilton et al., 2019b; Hamilton et al., 2022). To date three lines have been maintained within the WFRGIP – a positively selected line, a control line (putatively genetically equivalent to the base population which was spawned from fish of river origin) and a negatively selected line (not relevant to the current study) (Hamilton et al., 2022).
In 2020, a multiplier population comprised of highly-ranked WorldFish G3 rohu families was released to hatcheries in Bangladesh for development into broodstock. These G3-multiplier broodstock were spawned in commercial hatcheries for the first time in mid-2022.
The primary objective of this study was to quantify the magnitude of the realised genetic response to selection for harvest weight in the WFRGIP (i.e. realised genetic gain; Gjedrem and Baranski, 2009) in two regions of Bangladesh by comparing the performance of the G3-multiplier strain, grown under ‘real-world’ conditions on Bangladeshi farms, against fish from the WorldFish control line and a well-regarded commercial strain. Realised genetic gains represent gains from genetic improvement programs expressed in operational farms – in contrast with genetic gains estimated from trials in which the environment and management regimes are explicitly defined and controlled, but are not necessarily representative of targeted grow out systems (Hamilton et al., 2023).
Three strains of rohu were spawned within one day of each other in 2020. The generation-three multiplier (G3-multiplier) and control strains were spawned on the 9th of July at the WorldFish Carp Genetic Improvement Program (WFCGIP) facility, near Jashore, Bangladesh. Families contributing to the G3-multiplier and control strains were generated as part of routine genetic improvement activities (Hamilton et al., 2022). Families were made and maintained in separate upwelling hatching jars with mesh at the top to prevent the loss of eggs (i.e. one family per jar). Approximately 30 hours after hatching, fry from full-sib families were pooled to produce the G3-multiplier and control strains and transferred to hapas (i.e. nets) constructed of saree cloth (~300 μm mesh).
The ‘G3-multiplier strain’ was comprised of fish from 14 high-ranking full-sib families. The parents of these G3 families were selected on the basis of having a high estimated total additive genetic value – the sum of the estimated genetic group effect and estimated breeding value (EBV) (Wolak and Reid, 2017) – for harvest weight within the G2 positively-selected line. Furthermore, all parents were from different G2 families, to minimise relationships between individuals in the G3-multiplier and inbreeding in the progeny of the G3-multiplier (the expected level of inbreeding [Wright’s inbreeding coefficient; F] in the progeny of the G3-multiplier strain is 0.029).
The ‘control strain’ was comprised of fish from six full-sib families in the WFRGIP G3 control line and parents were selected on the basis of having an estimated total additive genetic value for harvest weight close to zero. Accordingly, the control strain is putatively genetically equivalent to the WFRGIP base population, which was spawned from river-sourced founders (Hamilton et al., 2019b; Hamilton et al., 2022).
The ‘commercial strain’ was spawned one day earlier than the G3-multiplier and control strains by a well-regarded commercial spawn producer. The commercial strain was derived from a mass spawning of five males and five females – the genetic relationships between these parents was unknown. The commercial strain was transported to the WorldFish Carp Genetic Improvement (WFCGIP) facility at five days of age. Upon arrival, the commercial strain was held in a separate pond to the G3-multiplier and control strains to allow observation and screening for key pathogens, before being transferred to nursing hapas at eleven days of age.
Fish were nursed in suspended hapas according to a randomized complete block design with 18 replicates (i.e. 54 hapas in total). Hapas were of the same dimensions as those used for routine family nursing in the WFRGIP (5.2 x 1.8 x 0.9 m) and each hapa was initially stocked with 1.5 g of hatchlings (Hamilton et al., 2022). Hapas were regularly cleaned and mesh size was progressively increased over the nursing phase – initially to mosquito netting (~1.4 mm; at 39-40 days of age) and ultimately to polyfilament netting (~5 mm; at 61-63 days of age). Once large enough (at 164-175 days of age), fingerlings were tagged with passive integrated transponder (PIT) tags and communally reared in a single pond.
Stocking density during nursing in hapas can affect growth rate (Hamilton et al., 2022). Accordingly, excess fish were removed from the hapas on three occasions during the nursing phase to ensure approximately equal numbers of fish were retained for each strain within a given replicate (Supplementary Material 1). Firstly, at the time of transfer from 300 μm to 1.4 mm hapas, the total weight of fry was determined in each hapa, the hapa with the lowest total weight in each replicate was identified and the weight of fry in the two other hapas was reduced to this weight. Secondly, soon after transfer to 5 mm hapas (at 90-92 days of age), the number of fingerlings in each hapa was counted, the hapa with the lowest number of fingerlings in each replicate was identified and the same number of fingerlings were retained, at random, in the two other hapas. Finally, at the conclusion of nursing in hapas fish were tagged with Passive Integrated Transponders (PIT) tags and weighed. Only healthy and non-deformed fish were tagged and, again, the hapa within each replicate with the minimum number of suitable fish was identified to determine the number of fish to be tagged from each hapa. The only deformity of note in fish at the time of tagging was a shortened or absent operculum, which was almost exclusively found in the commercial strain.
At between 304 and 316 days of age, tagged fish were transported to semi-commercial farms in two carp-growing regions of Bangladesh – Jashore district and Natore–Rajshahi districts. Ten private farms in Jashore (Farms A to J) and nine private farms in Natore–Rajshahi (Farms K to S) were identified and considered representative of ‘semi-commercial’ farms (Supplementary Material 2) in their respective regions (Table 1).
One pond at each farm was stocked with 82 randomly-selected rohu from each strain. No other rohu were stocked in the 19 ponds. Post-stocking mortalities were noted in Pond C in Jashore district, requiring the replacement of 142 fish ten days later, at the time of stocking ponds in Natore–Rajshahi. These mortalities were attributed to the unanticipated long transport time to this pond and associated stress placed on fish. No mortalities attributed to transport or stocking were recorded in the other 18 ponds.
Limited availability of tagged fish meant that stocking densities of rohu varied across farms, depending on pond size (Table 1). Once stocked, fish were managed according to each farmer’s normal practices. Water quality parameters, pond inputs (Table 1 and Supplementary Material 3) and fish weights were monitored over the period of the experiment.
Rohu growth was monitored on farms by netting and weighing random fish. Fish were sampled on a monthly basis, although sampling was not possible every month in every farm due government-imposed constraints on the movement of people due to COVID-19 outbreaks. All sampling and measurement activities were overseen by WorldFish staff. A sample size of ten fish per strain per farm was targeted for each measurement event, although this target was not met in all cases. Ultimately, all rohu were harvested from farms between 623 and 703 days of age (Table 1). At the time of harvest, ponds were partially drained and repeatedly netted in an effort to recover all rohu. Farms O and P were harvested earlier than scheduled due to low pond water levels and the desire of farmers to harvest the trial fish given limited additional pond and water availability. At harvest, all netted and identifiable fish (i.e. with their tag retained) were assessed for body weight (i.e. whole weight; Holden and Raitt, 1974).
Data for fish from nursing replicates two and four (6 and 87 fish, respectively) were excluded from analyses, due to hapa failure resulting in the escape of large numbers of fish from one of the three hapas in each of these replicates (Supplementary Material 1). Data for 525 fish from nursing replicate one was also excluded due to the inadvertent retention of excess fish in one hapa post transfer to 5 mm hapas. Remaining data were log transformed to minimize heteroscedasticity in residuals (Figure 1). Log transformation was also in keeping with multiplicative differences in growth rate between strains (i.e. expressed as a ratio or percentage difference) – log transformation changes a multiplicative effect to an additive effect (Keene, 1995).
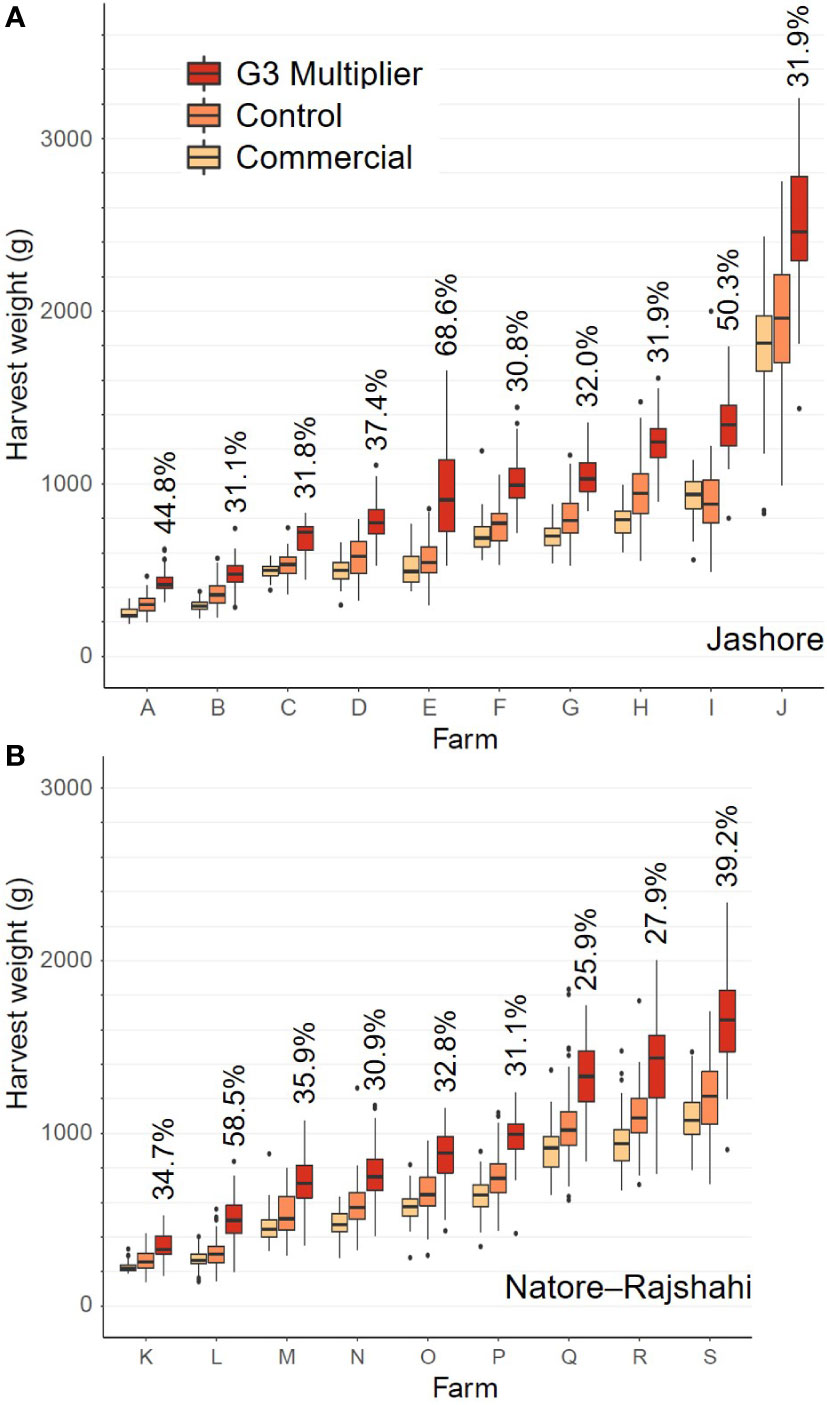
Figure 1 Boxplots of individual fish harvest weight data by strain and farm for the Jashore (A) and Natore-Rajshahi (B) regions. The G3-multipier mean expressed as a percentage of the control strain mean is indicated for each farm.
Each strain was comprised of a large number of individuals from a relatively small number of families. However, families were pooled soon after spawning and no attempt to track or reconstruct pedigrees was made. Accordingly, to simply analyses and avoid the risk of underestimating error variances due to unknown genetic relationships between individuals within strains (Ponzoni et al., 2012), analyses were conducted on log10-transformed ‘adjusted mean harvest weights’ at each level of strain-by-farm (Figure 2). Adjusted mean harvest weights were computed by fitting the following model:
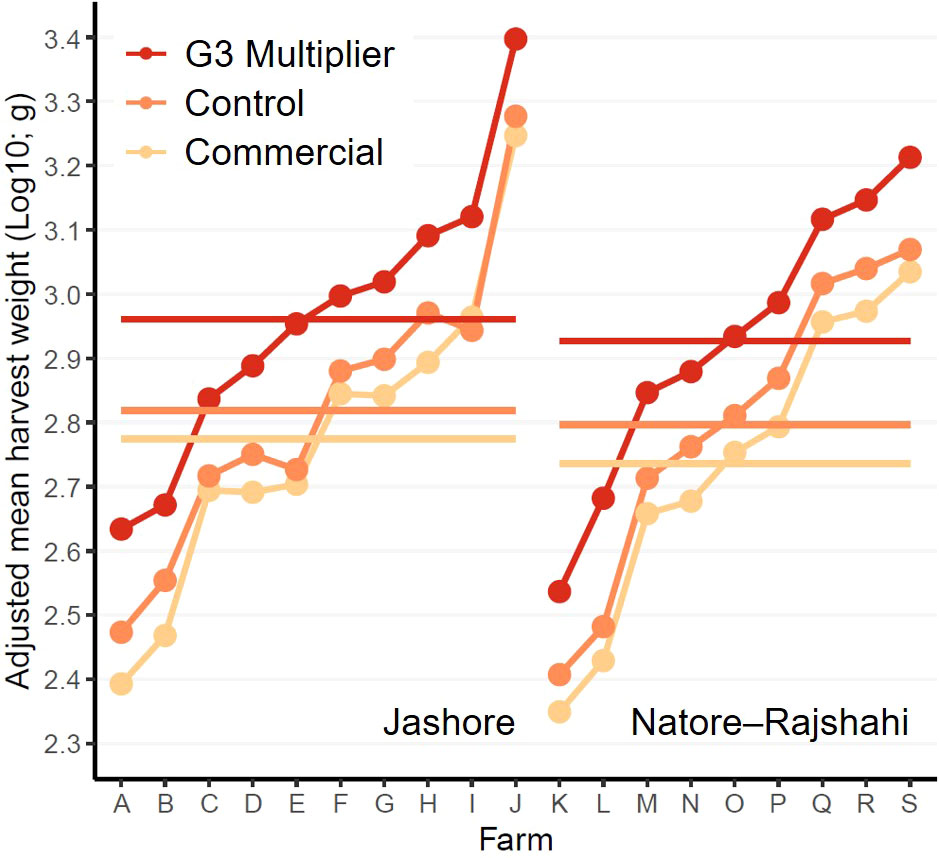
Figure 2 Interaction plot of log10 transformed adjusted mean harvest weights. Horizontal bars represent within-region means.
where μ is the grand mean, δ is a regression coefficient, is the log10-transformed nursing replicate mean tagging weight for individual l (Supplementary Material 4), is the log10-transformed arithmetic mean weight at tagging for all individuals, (αβ)ijk is the fixed effect of the ‘interaction’ between ‘strain’ i (i = ‘G3-multiplier’, ‘control’ or ‘commercial’) and ‘farm’ k (k = one of A to J, where ‘region’ j = ‘Jashore’, and k = one of K to S where ‘region’ j = ‘Natore–Rajshahi’), ϵijkl is the residual and Yijkl is the log10-transformed weight at harvest. The ‘covariate’, , was included in the model as a nuisance variable in an effort to adjust for enduring nursing replicate effects on harvest-age weight (Supplementary Material 5).
Analyses of adjusted mean harvest weights (Figure 2) were then conducted. Firstly, to test the significance of strain, region and strain-by-region interaction effects:
where μ is the grand mean, αi is the fixed effect of ‘strain’ i, γj is the fixed effect of ‘region’ j (j = ‘Jashore’ or ‘Natore–Rajshahi’), (αγ)ij is the fixed effect of the ‘interaction’ between strain and region, ϵijk is the residual, is the log10-transformed adjusted mean harvest weight.
Secondly, to test the significance of the strain effect separately within each ‘region’ j (j = ‘Jashore’ or ‘Natore–Rajshahi’):
where βjk is the fixed effect of ‘farm’ k (within ‘region’ j) and all other terms are as previously described. Model 3 analyses considered each farm to be an individual replicate of the strain treatments (Ponzoni et al., 2012), which considerably increased the statistical power of tests of the strain effect (Dixon, 2016).
In addition, growth trends between regions were compared by fitting Model 2 and Model 3 for weight at each sampling event over the grow-out period. In all cases, models were fitted using the lm function in R (R Core Team, 2020); ii) type III sums of squares were computed using the Anova function of the car package (Fox and Weisberg, 2019); iii) the significance of all effects were tested according to F-tests, with the mean sum of squares corresponding with the residual term taken as the error to compute the F value (Sahai and Ageel, 2012); iv) means (and standard errors) were computed using the emmeans function (Kassambara, 2021); and v) strain pairwise contrasts were conducted using the emmeans_test function with Bonferroni correction (Kassambara, 2021).
Across strains (i.e. using Model 2), the strain-by-region interaction effect (F2,51 = 0.0058, P = 0.994) and region effect (F1,51 = 0.2617, P = 0.611) were not significant at harvest. In contrast, the overall strain effect across regions was significant (F2,51 = 3.3329, P = 0.044), despite the limited number of farms studied and substantial inter-farm variation in growth (Table 2; Figures 1, 2).
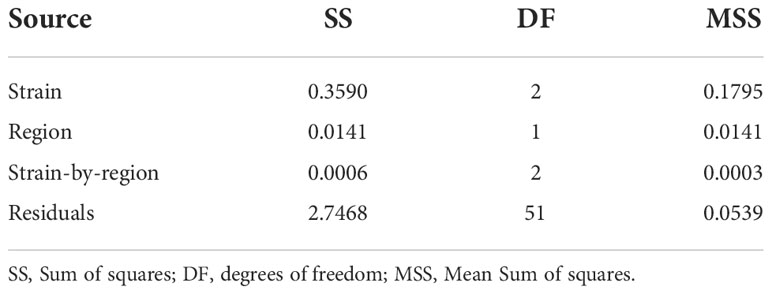
Table 2 Analysis of variance for Log10 transformed adjusted mean harvest weight across regions (i.e. Model 2).
Within regions (i.e. using Model 3), the strain effect was significant in both the Jashore (F2,18 = 147.9, P< 0.001) and Natore–Rajshahi regions (F2,16 = 292.1, P< 0.001) at harvest (Table 3). Furthermore, contrasts between strains revealed statistically significant differences between all three pairwise combinations of strain (P ≤ 0.003). The G3-multipier was the most rapidly growing strain at all farms (Figures 1, 2) with a mean harvest weight of 914 g in Jashore and 845 g in Natore–Rajshahi regions (Table 4) – equating to a realised genetic gain of 38.6% and 34.9%, respectively, for the G3-multipier over the control strain.
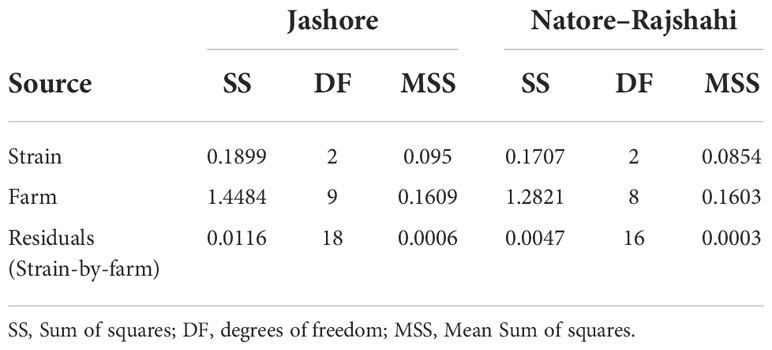
Table 3 Analysis of variance for Log10 transformed adjusted mean harvest weight within regions (i.e. Model 3).
In the Jashore region, fish grew consistently over the trial period (Figure 3A). In contrast, fish growth was initially rapid in the Natore–Rajshahi region but slowed from the onset of winter in late 2021 (Figure 3B). However, neither the strain-by-region interaction effect (P ≥ 0.459) nor the main region effect (P ≥ 0.191) was significant within any one measurement event.
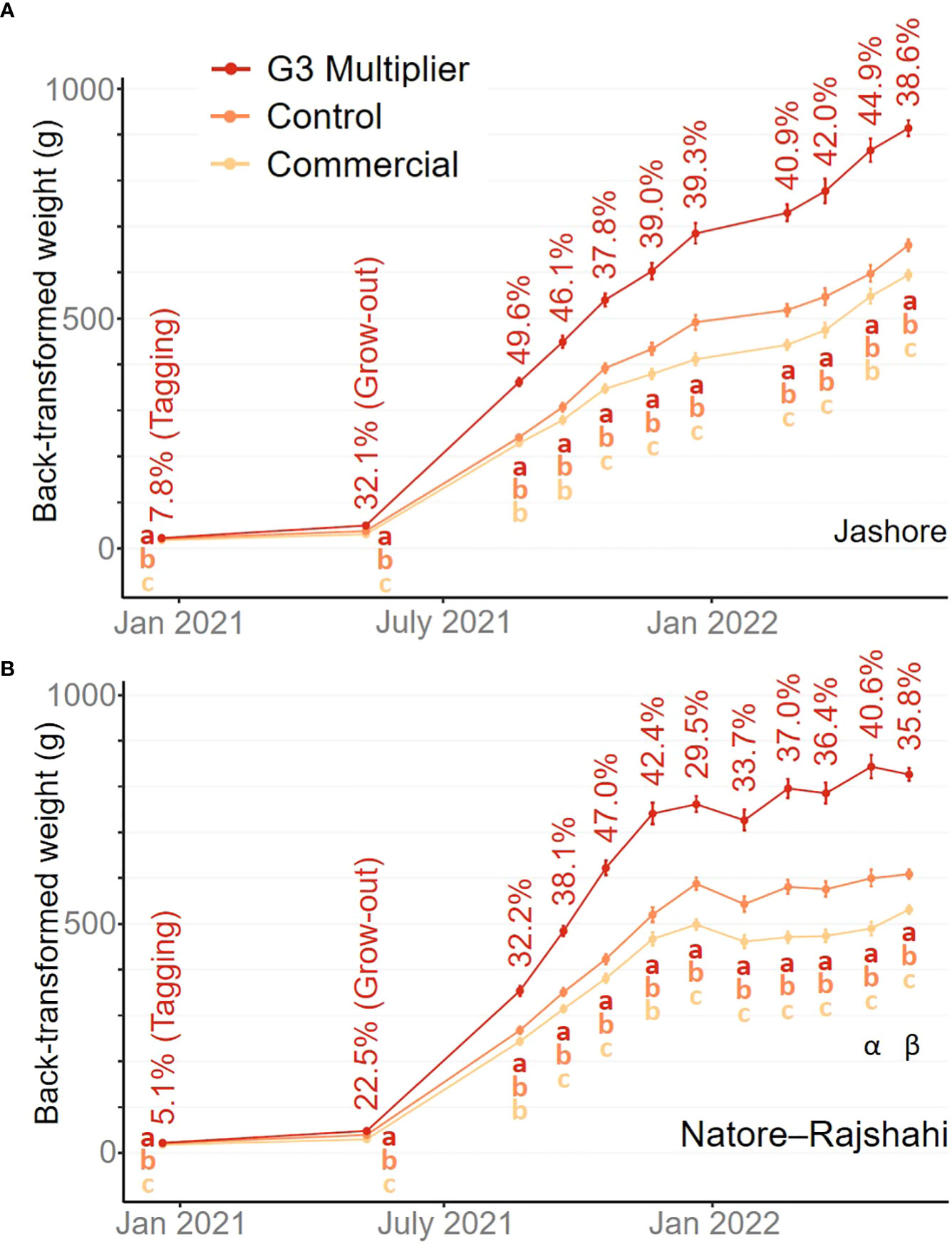
Figure 3 Back-transformed estimated marginal means for weight across farms by sampling event within the Jashore (A) and Natore–Rajshahi (B) regions. The G3-multipier mean expressed as a percentage of the control treatment mean is indicated for each sampling event. Error bars indicate 95% confidence intervals noting that the pond effect was fitted as fixed (Dixon, 2016). Letters indicate significance groups (P< 0.05) after Bonferroni adjustment. Note Pond P was not represented in the second last Natore–Rajshahi measurement (α) and pond O was not represented in the final Natore–Rajshahi measurement (β), as these ponds were harvested at an earlier date.
The superior growth performance of the G3-multipier strain was substantial, and similar in magnitude, across both regions under study (38.6% and 34.9% greater than the control strain at harvest, in the Jashore and Natore–Rajshahi regions respectively). These observations were in keeping with i) expectations at the commencement of the WFRGIP – a 10% improvement over the base population was anticipated with each generation of selection (Keus et al., 2017) – ii) results observed in other finfish genetic improvement programs (reviewed by Gjedrem and Rye, 2018); iii) genetic trends observed in common-garden trials of G1 and G2 rohu families conducted at the WFCGIP facility (Hamilton et al., 2022); and iv) a lack of substantive strain-by-region interaction – a form of genotype-by-environment interaction (Li et al., 2017). Indeed, there was no evidence of a statistically-significant strain-by-region interaction effect in harvest weight (P = 0.994; Table 1; Figure 2), suggesting that the substantial realised genetic gains observed in the current study are likely to be expressed in other regions with similar environments and management practices. However – given that only two regions and 19 farms, exhibiting highly variable growth rates, were studied – the possibility of Type II error in the current study, and the detection of statistically significant strain-by-region interaction effects in future studies, cannot be excluded.
The relatively slow growth rate of the commercial strain was unexpected. This slow growth was conceivably related to the prevalence of deformed opercula in these fish. Opercular deformities can affect the respiratory and feeding apparatus of rohu, potentially adversely impacting on growth (Blaker and Ellis, 2022). Although every effort was made to exclude fish exhibiting such deformities from our study at the time of tagging (Supplementary Material 1) it is possible that fish with minor opercular or related deformities were inadvertently retained. A wide range of non-genetic causal and risk factors have been proposed for operculum deformities (reviewed in Blaker and Ellis, 2022). Furthermore, the provider of the commercial strain indicated that opercular deformities are not routinely observed by their customers, suggesting that these deformities may have resulted from the unconventional transport and nursing environment experienced by these fish in the first 11 days of life, rather than underlying genetic factors. Fish in the commercial strain were spawned, transported (5 day of age), housed in a pond separate to other strains, and then placed in their final nursing hapa positions (11 days of age).
For the purpose of estimating realised genetic gains, the control strain included in the current study was considered genetically equivalent to the base population of the WFRGIP (i.e. unimproved – spawned from fish of river origin) (Hamilton et al., 2019b; Hamilton et al., 2022). However, maintaining control lines representative of an unimproved population, and sampling families from control lines for inclusion in performance trials, presents a number of difficulties (Gjedrem and Baranski, 2009). Indeed, the six families comprising the control strain in the current study had a mean total additive genetic worth for harvest-age weight marginally greater than zero (estimated using the method detailed in Hamilton et al., 2022), potentially resulting in corresponding underestimates of genetic gain in the current study. In contrast, the possibility of overestimation of genetic gain due to intraspecific and interspecific competition within ponds (i.e. repression of growth of small rohu by large rohu and other species) cannot be discounted (Ibrahim et al., 2013), particularly given the larger size of the G3-multipier animals at the time of grow-out-pond stocking (Figure 2). However, the larger size of G3-multipier fish at the time of stocking did not fully explain the superior performance of the G3-multipier strain. When analyses were repeated excluding the lightest 50% of control- and commercial-strain fish at tagging and the heaviest 50% of G3-multiplier fish at tagging, within each nursing replicate (i.e. hapa), the G3-multipier was 27.4% and 28.9% lighter than the control treatment the time of tagging in Jashore and Natore–Rajshahi, respectively, but 16.0% heavier at harvest in both regions (Supplementary Material 6).
Although the objective of the current study was to treat all fish as they would be in ‘real-world’ polyculture systems, a constraint was placed on the final harvest date – primarily to ensure the results of this study were available to hatcheries, nursery operators and farmers at the time G3-multipier broodstock were first spawned (i.e. mid 2022). If this constraint had not been imposed, some farmers would have grown their rohu for longer to achieve higher individual weights prior to sale, particularly in the Natore–Rajshahi region (Figure 2). However, given the consistently high estimates of realised genetic gain from the first monthly assessment (August 2021) to harvest (Mar-June 2022) in both regions (Figure 2), it is probable that comparable genetic gains would have been achieved if harvest had been delayed – the lowest estimate of genetic gain in Jashore was 37.8% in Oct 2021 and the lowest in Natore–Rajshahi was 29.5% in Dec 2021.
The clear growth advantages exhibited by the G3-multiplier strain over the control and commercial strains in this study should encourage more Bangladeshi hatcheries, nurseries and farmers to adopt genetically improved rohu. However, beyond enhancing productivity, the medium- to long-term implications of the widespread adoption of more rapidly-growing rohu by Bangladeshi farmers are difficult to predict. For example, it has the potential to alter optimal species ratios, harvest age and size, and management intensity adopted in polyculture systems, as well as the supply and price of rohu in the market – with corresponding economic implications for producers and consumers. The future availability of genetically improved stains of additional carp species (e.g. catla and silver carp; Hamilton et al., 2019a; Hamilton et al., 2021) will inevitably add further complexity to these management and economic considerations.
The G3-multipier strain exhibited a mean realised genetic gain of 38.6% and 34.9% for harvest weight over the control treatment in the Bangladeshi regions of Jashore and Natore–Rajshahi respectively, and exhibited the most rapid growth rate across all 19 studied farms. In contrast, the commercial strain exhibited the slowest growth rate in all but one farm. These findings should encourage more Bangladeshi hatcheries, nurseries and farmers to adopt genetically improved rohu and, more generally, provide confidence in the approach to genetic improvement adopted in the WFRGIP and comparable family-based programs.
The datasets analysed for this study can be found in the Harvard Dataverse (https://doi.org/10.7910/DVN/YWEBPK).
The animal study was reviewed and approved by National University of Malaysia (Universiti Kebangsaan Malaysia) Animal Ethics Committee. Written informed consent was obtained from the owners for the participation of their animals in this study.
Details of author contributions using CRediT (Contribution Roles Taxonomy) follow. MH: Conceptualization, data curation, formal analysis, funding acquisition, methodology, project administration, visualization, writing – original draft preparation. MY: Conceptualization, data curation, funding acquisition, investigation, methodology, project administration, writing – review & editing. MBA: Data curation, investigation, methodology, project administration. MRA: Data curation, investigation. MF: Data curation and investigation. MI: Conceptualization and methodology. BB: Conceptualization and methodology. KS: Conceptualization and methodology. CS: Conceptualization, funding acquisition and methodology. CR: Conceptualization, funding acquisition and methodology. JB: Conceptualization, funding acquisition, methodology, supervision, and writing – review & editing. All authors contributed to the article and approved the submitted version.
This work was undertaken as part of the CGIAR Research Initiative on Resilient Aquatic Food Systems for Healthy People and Planet, and funded by CGIAR Trust Fund donors. This work was also made possible by the support of the American People provided to the Feed the Future Bangladesh Aquaculture and Nutrition Activity [grant number 72038818IO00002] and Feed the Future Innovation Lab for Fish [Cooperative Agreement No. 7200AA18CA00030] through the United States Agency for International Development (USAID). Additional funding was provided by the Aquaculture: Increasing Income, Diversifying Diets and Empowering Women in Bangladesh and Nigeria project [INV009865] (funded by the Bill & Melinda Gates Foundation) and the CGIAR Research Program on Fish Agrifood Systems (FISH) (funded by contributors to the CGIAR Trust Fund).
The authors thank all the members of the WorldFish Carp Genetic Improvement Program technical team in Jashore for spawning, nursing and measuring fish up to the time of distribution to farms – Masud Akhter, Md. Mustafizur Rahman, Md. Sultan Mohmud, Uzzal Kumar Sarkar, Aashish Kumar Roy, Ram Prosad Kundu, Sirajum Monira Shanta, Md. Kamruzzaman, Jamal Hossain and Md. Tutul Hossain. The authors note the cooperation and assistance of the 19 farmers whose operations were disrupted by our study: Ahsanuzzaman (Sweet) (Jashore), Ali-Abdullah Dairy Farm and Matsha Khamar (Jashore), Ashroy Trainning Center (Rajshahi), Fahad Hatchery and Fish Farm (Jashore), Golden Fish and Nursery Complex (Jashore), Insar Ali (Jashore), Jalal Uddin (Jashore), Jui-Jerin Matsya Khamar (Natore), Madina Fish Nursery (Jashore), Md. Labu (Rajshahi), Md. Sofiuzzaman (Natore), Md. Abu Rayhan (Natore), Mehedi Enterprise (Natore), Molla Fish Nursery and Dairy Farm (Jashore), Muttakim Traders (Rajshahi), Osit Matsya Khamar (Natore), Razib Kumar Sarkar (Natore), Sagor Fish Hatchery (Jashore), Saifujjaman Pintu (Jashore). In particular, the authors offer condolences to the family, friends and colleagues of Zulfiker Ali Bhutto (Madina Fish Nursery), who passed away prior to the completion of this study. We also thank the commercial supplier of spawn used in the study and the reviewers of the manuscript for their insights and suggestions.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/faquc.2022.1060335/full#supplementary-material
Belton B., Azad A. (2012). The characteristics and status of pond aquaculture in Bangladesh. Aquaculture 358-359, 196–204. doi: 10.1016/j.aquaculture.2012.07.002
Blaker E., Ellis T. (2022). Assessment, causes and consequences of short opercula in laboratory reared Atlantic salmon (Salmo salar). Anim. Welf. 31, 79–89. doi: 10.7120/09627286.31.1.007
Dixon P. (2016). “Should blocks be fixed or random?,” in Conference on Applied Statistics in Agriculture (Kansas State University, Manhattan, Kansas: New Prairie Press). doi: 10.4148/2475-7772.1474
DoF (2020). “Yearbook of Fisheries Statistics of Bangladesh, 2019-20?,” in Fisheries Resources Survey System (FRSS), Department of Fisheries. Bangladesh: Ministry of Fisheries and Livestock, 2020 37, 141p.
FAO (2020). The state of world fisheries and aquaculture 2020. sustainability in action (Rome, Italy: FAO). doi: 10.4060/ca9229en
Fox J., Weisberg S. (2019). An r companion to applied regression. 3rd ed. (CA: Sage, Thousand Oaks).
Gjedrem T., Baranski M. (2009). Selective breeding in aquaculture: an introduction (New York: Springer).
Gjedrem T., Rye M. (2018). Selection response in fish and shellfish: a review. Rev. Aquac. 10, 168–179. doi: 10.1111/raq.12154
Hamilton M. G., Mekkawy W., Alam M. B., Barman B. K., Karim M., Benzie J. A. H. (2023). Genotype-by-culture-system interaction in catla and silver carp: Monoculture and biculture. Aquaculture 562, 738846. doi: 10.1016/j.aquaculture.2022.738846
Hamilton M. G., Mekkawy W., Alam M. B., Benzie J. A. H. (2022). Early selection to enhance genetic gain in a rohu (Labeo rohita) genetic improvement program. Aquaculture 553, 738058. doi: 10.1016/j.aquaculture.2022.738058
Hamilton M. G., Mekkawy W., Barman B. K., Alam M. B., Karim M., Benzie J. A. H. (2021). Genetic relationships among founders of a silver carp (Hypophthalmichthys molitrix) genetic improvement program in Bangladesh. Aquaculture 540, 736715. doi: 10.1016/j.aquaculture.2021.736715
Hamilton M. G., Mekkawy W., Benzie J. A. H. (2019a). Sibship assignment to the founders of a Bangladeshi Catla catla breeding population. Genet. Sel. Evol. 51, 17. doi: 10.1186/s12711-019-0454-x
Hamilton M. G., Mekkawy W., Kilian A., Benzie J. A. H. (2019b). Single nucleotide polymorphisms (SNPs) reveal sibship among founders of a Bangladeshi rohu (Labeo rohita) breeding population. Front. Genet. 10. doi: 10.3389/fgene.2019.00597
Holden M. J., Raitt D. F. S. (1974). Manual of fisheries science. part 2-methods of resource investigation and their application (Rome: Food and Agriculture Organisation of the United Nations (FAO).
Ibrahim N. A., Zaid M. Y. A., Khaw H. L., El-Naggar G. O., Ponzoni R. W. (2013). Relative performance of two Nile tilapia (Oreochromis niloticus Linnaeus) strains in Egypt: The abbassa selection line and the kafr El sheikh commercial strain. Aquac. Res. 44, 508–517. doi: 10.1111/j.1365-2109.2012.03240.x
Jhingran V. G., Pullin R. S. V. (1985). “A hatchery manual for the common Chinese and Indian major carps,” in ICLARM studies and reviews, 2nd rev ed (Manila, Philippines: ICLARM).
Kassambara A. (2021). rstatix: Pipe-Friendly Framework for Basic Statistical Tests (R package version 0.7.0). Available at: https://CRAN.R-project.org/package=rstatix.
Keene O. N. (1995). The log transformation is special. Stat Med. 14, 811–819. doi: 10.1002/sim.4780140810
Keus E., Subasinghe R., Aleem N., Sarwer R., Islam M., Hossain M., et al. (2017). Aquaculture for income and nutrition: final report (Penang, Malaysia: WorldFish).
Khan M. R. I., Parvez M. T., Talukder M. G. S., Hossain M. A., Karim M. S. (2018). Production and economics of carp polyculture in ponds stocked with wild and hatchery produced seeds. J. Fish. 6, 541–548. doi: 10.17017/jfish.v6i1.2018.306
Li Y., Suontama M., Burdon R. D., Dungey H. S. (2017). Genotype by environment interactions in forest tree breeding: review of methodology and perspectives on research and application. Tree Genet. Genomes 13, 60. doi: 10.1007/s11295-017-1144-x
Mahapatra K. D., Saha J. N., Murmu K., Sahoo P. K. (2016). “Genetic improvement and dissemination of rohu (Labeo rohita) in India: impact and lessons learned,” in Sustainable intensification of aquaculture in the Asia-pacific region. documentation of successful practices. Eds. Miao W., Lal K. K. (Bangkok, Thailand: FAO), 28–39.
Ponzoni R. W., James J. W., Nguyen N. H., Mekkawy W., Khaw H. L. (2012). Strain comparisons in aquaculture species: a manual (Penang, Malaysia: WorldFish).
Rahman M., Varga I., Chowdhury S. (1992). Manual on polyculture and integrated fish farming in Bangladesh (Rome: Food and Agriculture Organisation of the United Nations (FAO).
R Core Team (2020). R: A language and environment for statistical computing (Vienna, Austria: R Foundation for Statistical Computing).
Reddy P. V. G. K., Gjerde B., Tripathi S. D., Jana R. K., Mahapatra K. D., Gupta S. D., et al. (2002). Growth and survival of six stocks of rohu (Labeo rohita, Hamilton) in mono and polyculture production systems. Aquaculture 203, 239–250. doi: 10.1016/S0044-8486(01)00540-3
Sah U., Mukhiya Y., Wagle S. K. (2018). Comparative evaluation of genetically improved and farmed rohu (Labeo rohita) on growth and yield at initial stage of rearing. Int. J. Fish. Aquat. Stud. 6, 47–50.
Sahai H., Ageel M.I. (2012). The analysis of variance: fixed, random and mixed models. Boston: Birkhäuser.
Vadhel N., Pathan J., Shrivastava V., Akolkar N., Kumar S., Misra C. K., et al. (2020). Comparative study on the performance of genetically improved rohu “Jayanti” and native rohu, Labeo rohita fingerlings reared in biofloc system. Aquaculture 523, 735201. doi: 10.1016/j.aquaculture.2020.735201
Wahab M. A., Kadir A., Milstein A., Kunda M. (2011). Manipulation of species combination for enhancing fish production in polyculture systems involving major carps and small indigenous fish species. Aquaculture 321, 289–297. doi: 10.1016/j.aquaculture.2011.09.020
Keywords: rohu, Labeo rohita, cyprinidae, Indian major carp, genetic improvement program, growth rate, harvest weight, realised genetic gain
Citation: Hamilton MG, Yeasin M, Alam MB, Ali MR, Fakhruddin M, Islam MM, Barman BK, Shikuku KM, Shelley CC, Rossignoli CM and Benzie JAH (2022) On-farm performance of genetically-improved rohu (Labeo rohita) in Bangladesh. Front. Aquac. 1:1060335. doi: 10.3389/faquc.2022.1060335
Received: 03 October 2022; Accepted: 23 November 2022;
Published: 16 December 2022.
Edited by:
Guozhi Luo, Shanghai Ocean University, ChinaReviewed by:
Edison D. Macusi, Davao Oriental State University (DOrSU), PhilippinesCopyright © 2022 Hamilton, Yeasin, Alam, Ali, Fakhruddin, Islam, Barman, Shikuku, Shelley, Rossignoli and Benzie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Matthew G. Hamilton, TS5IYW1pbHRvbkBjZ2lhci5vcmc=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.