- 1Neuroscience Institute, Georgia State University, Atlanta, GA, United States
- 2Department of Mathematics and Statistics, Georgia State University, Atlanta, GA, United States
We propose a minimalistic model called the
1. Introduction
Neural networks called central pattern generators (CPGs) [1–8] produce and control a great variety of rhythmic motor behaviors, including heartbeat, respiration, chewing, and locomotion. Many physiologically diverse CPGs involve 3-cell motifs, such as the spiny lobster pyloric network [6], the Tritonia swim circuit [4], and the Lymnaea respiratory CPGs [3]. Pairing experimental studies and modeling studies has been proven to be the key for disclosing basic operational and dynamical principles of CPGs [9–14]. Although various circuits and models of specific CPGs have been developed, the mystery of how CPGs gain the level of robustness and adaptation observed in nature remains unsolved. It is also not evident what mechanisms a single motor system can use to generate multiple rhythms, that is, whether CPGs need a specific circuitry for every function or whether it can be multifunctional to determine several behaviors [15–17]. Switching between multistable rhythms can be attributed to input-dependent switching between attractors of the CPG, where each attractor is associated with a specific rhythm. The goal of this article is to characterize how observed multistable states can emerge from the coupling using simple neural models on small networks.
This article, based on our original work, reemphasizes some basic principles well established in the characterization of 3-cell networks made of detailed Hodgkin-Huxley–type models of endogenous bursters [18–20] and the Fitzhugh-Nagumo–like neurons [21]. We use a bottom-up approach to show the universality of rhythm-generation principles in such 3-cell circuits regardless of the cell model selected, which can be the HH-type model of the leech heart interneuron [22, 23], the generalized Fitzhugh–Nagumo (gFN) model of neurons [24], or the minimalistic 2θ-burster suggested in this article, provided that all three meet some simple and generic criteria. We are convinced that one should first investigate the rules and mechanisms underlying the emergence of cooperative rhythms in basic neural motifs, as well as the role of coupling in generating a multiplicity of coexisting rhythmic outcomes in larger networks s[25].
The predecessor of a 2θ-burster proposed and examined below is the so-called spiking θ-neuron [26]. It is described by a phase differential equation with a specific term
The concept of the new model, called the
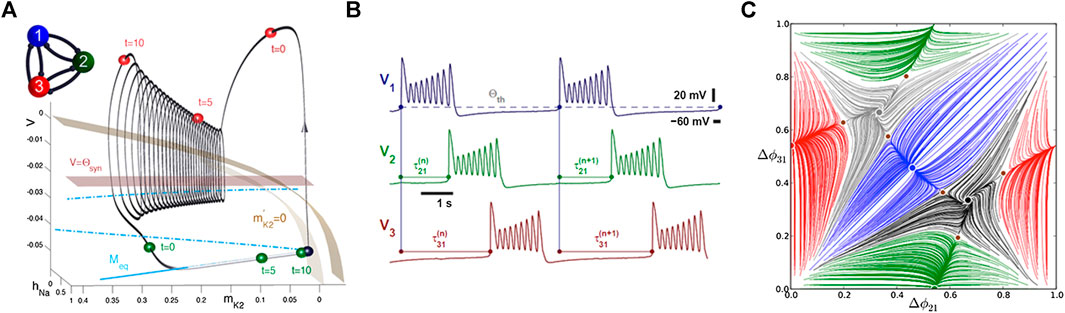
FIGURE 1. (A) Snapshots of the transient states (shown as the blue, green, and red spheres) of three inhibitory-coupled Hodgkin-Huxley–type cells at
2. Return Maps for Phase Lags
We developed a computational toolkit for oscillatory networks that reduces the problem of the occurrence of bursting and spiking rhythms generated by a CPG network to the bifurcation analysis of attractors in the corresponding Poincaré return maps for the phase lags between oscillatory neurons. The structure of the phase space of the map is a unique signature of the CPG as it discloses all characteristics of the functional range of the network. The recurrence of rhythms generated by the CPG (represented by a system of coupled Hodgkin-Huxley–type neurons [23]) allows us employ Poincaré return maps defined for phaselags between spike and burst initiations in the constituent neurons [27, 28], as illustrated in Figures 1, 2, and 6. With such return maps, we can predict and identify the set of robust outcomes in a CPG with mixed, inhibitory, excitatory, and electrical synapses, which are differentiated by phase-locked or periodically varying lags corresponding, respectively, to stable fixed points and invariant circles on the return map.
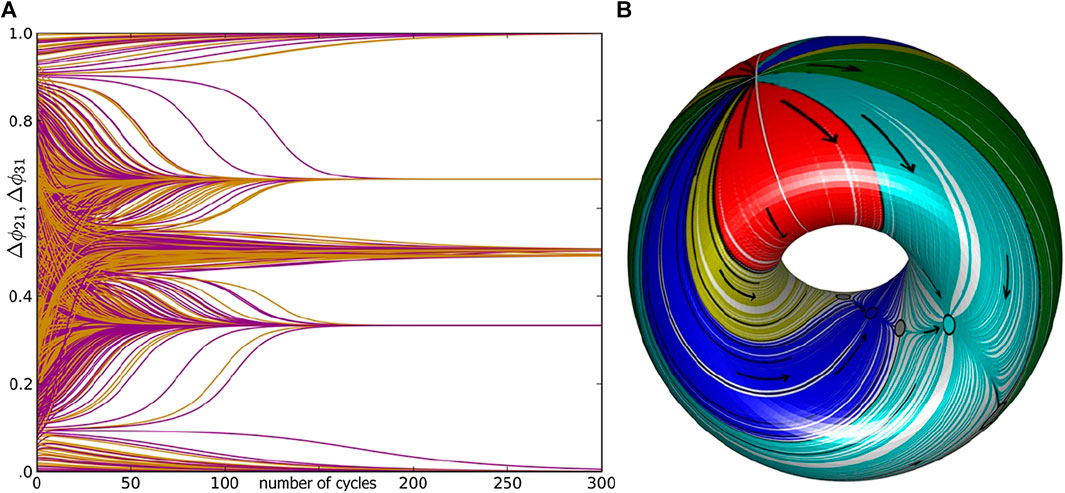
FIGURE 2. (A) The demonstration of the slow exponential convergence of initial states of
Let us introduce a 3-cell network (Figure 1A) made of weakly coupled HH-like bursters; see the equations in the Appendix. Here, “weakly” indicates that coupling cannot disturb the shape of the stable bursting orbit in the 3D phase space of the individual HH model (Figure 1A). Weak interactions, inhibitory (mainly repulsing) and excitatory/gap junction (mainly attracting), can only affect the phases of the periodically varying states of the neurons, represented by the color-coded spheres (blue/green/red, respectively, for cells 1/2/3), on the bursting orbit in the 3D phase space of the given interneuron model. As such, weak coupling can only gently alter the phase differences or phase lags between the networked neurons (Figure 2A). We also note that coupling remains weak as long as individual cell models stay away from bifurcations, such as a saddle-node bifurcation typical for 1D
Being inspired by neurophysiological recordings performed on various rhythmic CPGs, we employ only voltage traces generated by oscillatory networks to examine the time delays,
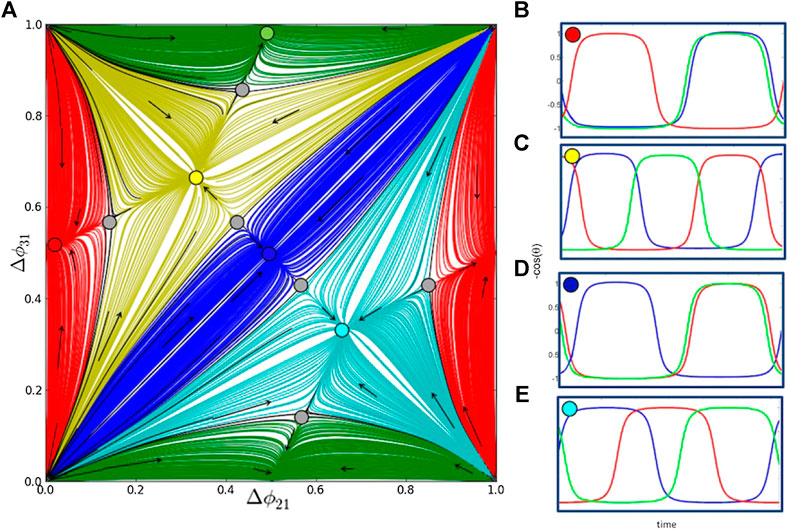
FIGURE 3. Multistable outputs of the 3-cell homogeneous network with six equal synaptic connections (all
To analyze the existence and the stability of various recurrent rhythms produced by such networks, we employ our previously developed approach using Poincaré return maps for phase lags between constituent neurons. We introduce phase lags at specific events in time when the voltage in cells reaches some threshold value, signaling the burst initiation (see Figure 1B). The phase lag
Sequences of phase lags
Other attractors in the return maps on a 2D torus can be a stable invariant curve (IC) corresponding to rhythmic patterns with periodically varying phase lags. Such a curve can enclose a focal fixed point on the torus or wrap around the 2D torus [27, 28] (see Figure 2B and Section 5.4 below). If the map has a single attractor, then the corresponding network is monostable; otherwise, it is a multifunctional or multistable network capable of producing several rhythmic outcomes robustly. Such as 2D return map, where
with small
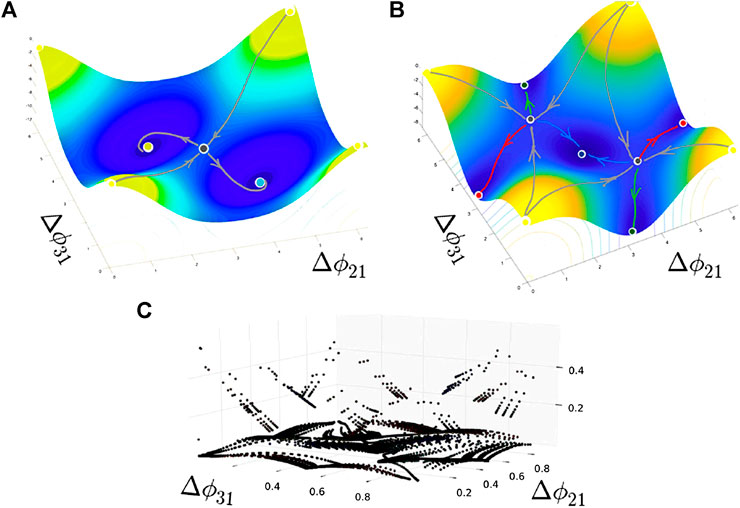
FIGURE 4. Critical points of the sketched “pseudopotentials” with periodic boundary conditions reveal the location of potential wells—attractors, as well as saddles (including with six separatrices in (B)) and repellers (a single local maximum at the origin) in the (
3. Minimalistic 2θ-Burster
The key feature of the
which is the occurrence of two saddle-node bifurcations giving rise to the two slow transient phases in its dynamics alternating with fast transitions in between. Likewise endogenous bursters with two such slow states (Figure 1), the duration of the active tonic spiking, and the quiescent phases can be controlled independently in the
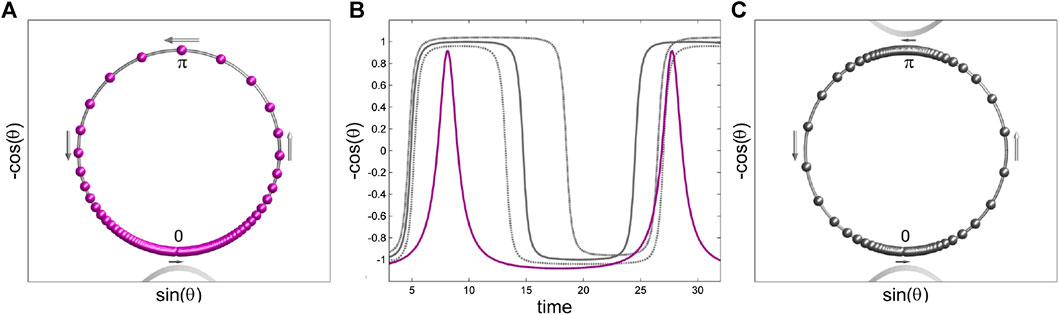
FIGURE 5. Comparison of the oscillatory dynamics generated by the spiking θ-neuron and the
First, let us observe from Eq. 3 that the dynamics of the individual
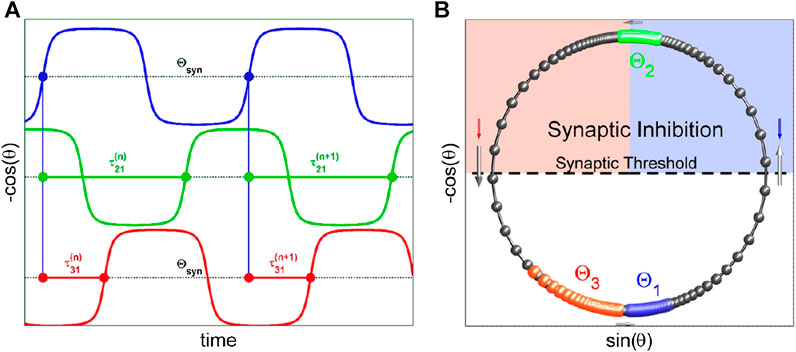
FIGURE 6. (A) Sampling the moments in phase traces,
4. Three Equations for 3-Cell Network
A 3-cell circuit of the
The
The last term
The electrical coupling or the gap junction between the neurons is handled by the other term
Let us note that unlike the bidirectional electrical synapse, a chemical one is unidirectional and hence asymmetric because it has a synaptic threshold: the chemical synapse becomes functional when the membrane voltage in the presynaptic cell rises above the only synaptic threshold in the active phase; otherwise the synapse is silent. This is the reason why a network composed of identical cell, and identical chemical synapses can only be called symmetric loosely, in some permutation sense. This is the reason why a permutation-symmetric 3-cell network always possesses a pair of traveling wave patterns (stable or not) where the cells burst sequentially and/or may generate three pacemaker patterns where one cell bursts in antiphase with the two others. Note that the last rhythms cannot be produced by a properly symmetric network by default.
5. Poincaré Return Maps for the Phase Lags. Results
Figure 6A shows how phase lags between upstrokes are introduced (here, cell 1 (blue) is the reference) between the three-networked
Following the same approach used in the weakly coupled HH-type models above, we first create a uniform distribution of initial phases on
The fixed points and their attraction basins are coded with different colors in the map. For example, the Poincaré return map for the
Let us underline another handy feature of the
5.1.Homogeneous Motif With Identical Cells and Synapses
It will be shown below that
Let us first consider a symmetric network with two bifurcation parameters: the coupling strength
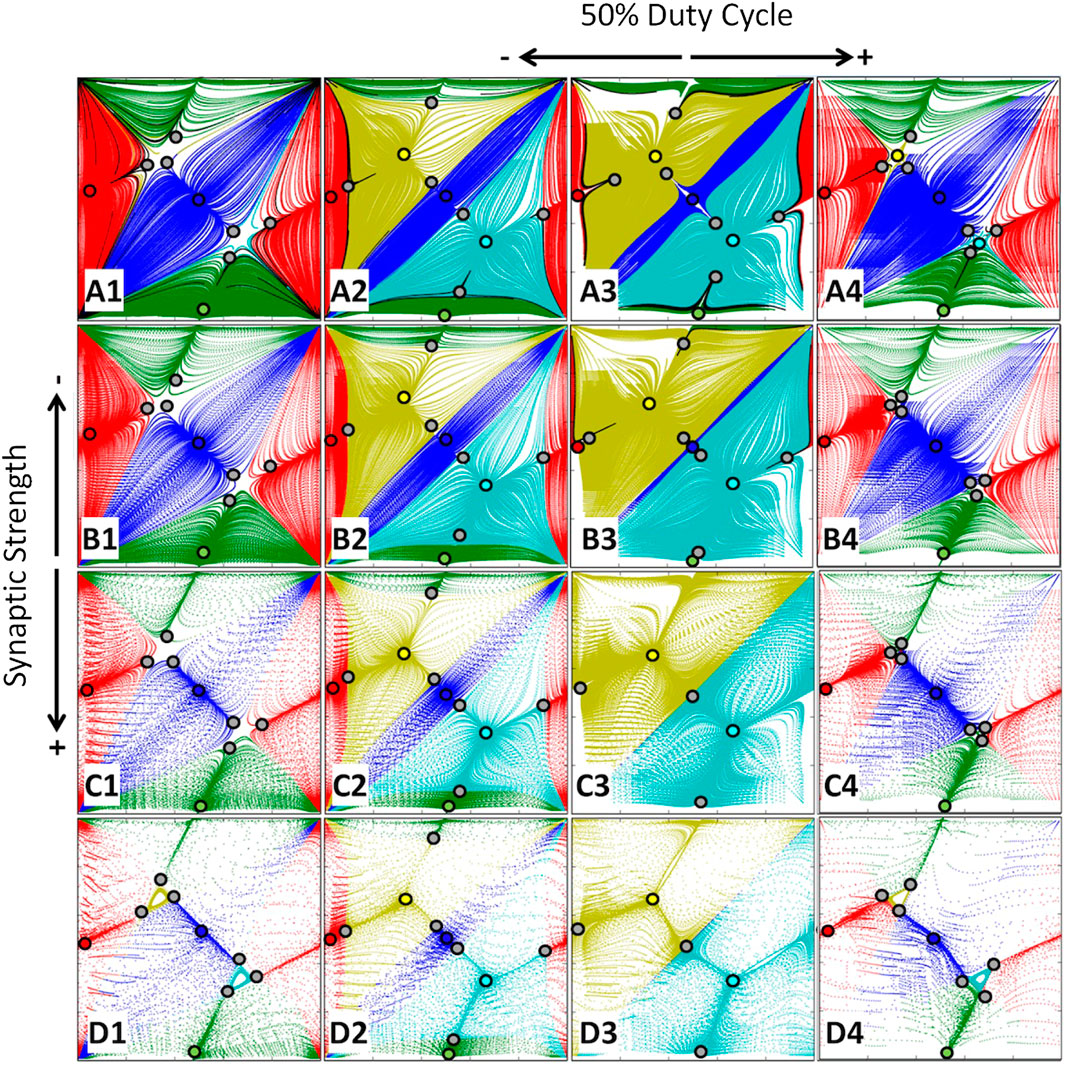
FIGURE 7. Bifurcations of FPs in the
The stable PMs promote or dominate the dynamics of the symmetric case at extreme α-values corresponding to the bursting rhythms with short or long burst durations. Once we compare panels, say A1 and D4 reveal this time, the separating saddles group around the stable TWs to minimize their attraction basins, and hence the likelihood of the occurrence of these rhythms in the network. These cases correspond to the “pseudopotential” depicted in Figure 4B.
5.2.“Winner Takes all” Motif
The first asymmetric case considered is a motif termed the “winner takes it all.” In this modeling scenario, both outgoing inhibitory synapses from the given cell, here the reference blue burster 1, are evenly increased in the strength, see Figure 8A. It is observed that such a configuration breaks down the circular (and permutation) symmetries supporting traveling waves in the network. Let us start with Figure 8B: no surprise that with an initial increase in
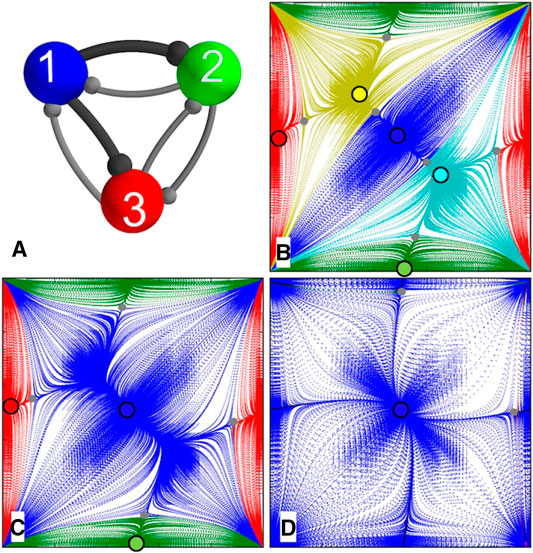
FIGURE 8. (A) “Winner takes all” motif with two synapse strengths,
5.3.Mono-Biased Motif
We refer as a mono-biased motif to another asymmetric network with a single different synapse. In this case, the strength
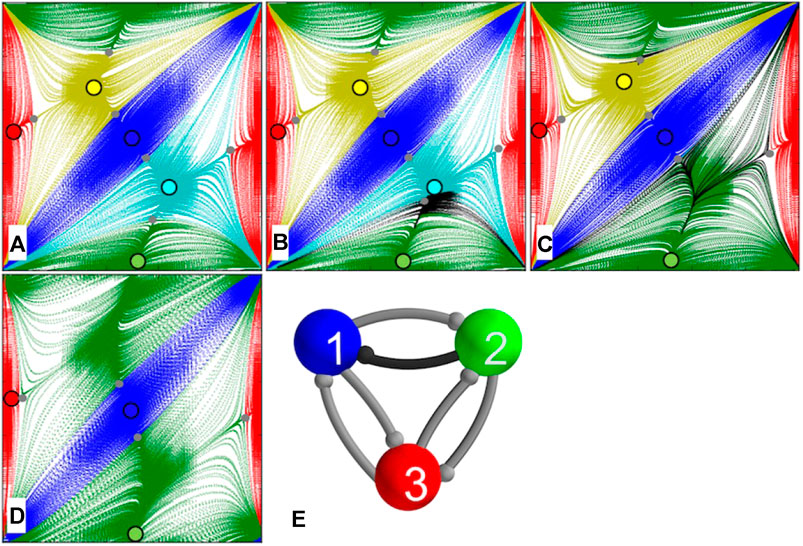
FIGURE 9. Mono-biased network motif (F) with one different synapse due to increasing
5.4.Dedicated HCO
The abbreviation HCO stands for a half-center oscillator, where a pair of neurons coupled reciprocally by inhibitory synapses to produce alternating bursting. Such a dedicated HCO is formed by cells 2 and 3 with stronger synapses due to
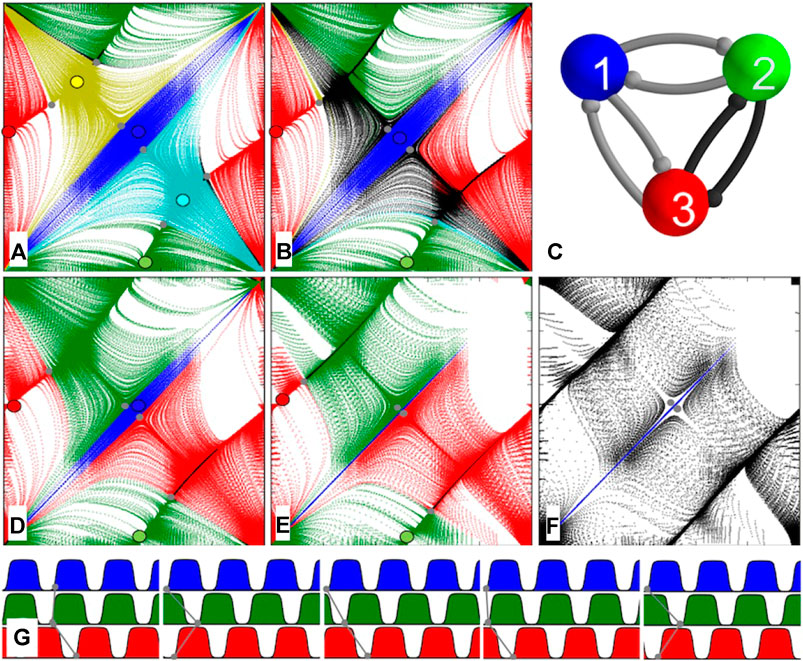
FIGURE 10. (C) “Pairwise-biased” network motif with two reciprocal synapse strengths
5.5.Clockwise-Biased Motif
The clockwise-biased motif in this case represents the 3-cell network with counterclockwise connections stronger than ones in the clockwise direction, see Figure 11E. This configuration does not break the circular symmetries of the network but implies that either TW should win over the opposite one, which should result in their attraction basins changing correspondingly. Figure 11 presents four transformation stages of the map as
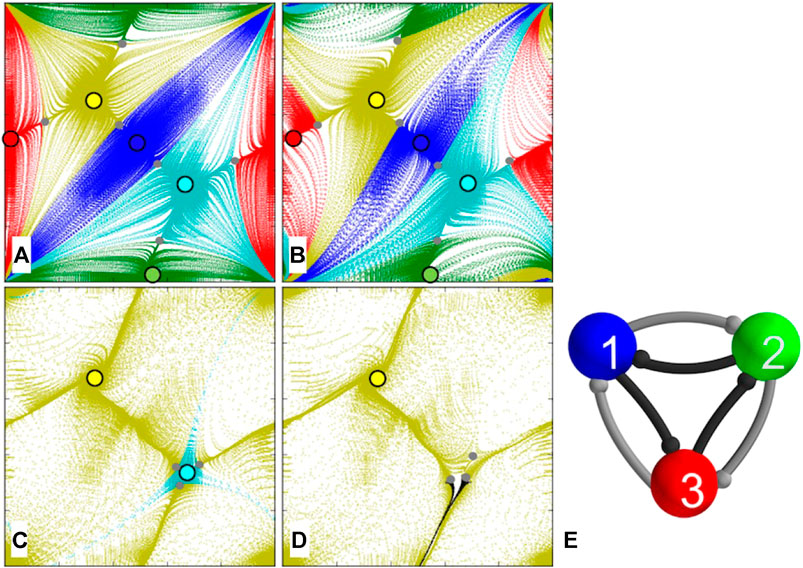
FIGURE 11. (E) Clockwise-biased motif with three synaptic strengths,
5.6.Gap Junction
In our last example, we consider the symmetric motif with a gap junction or electric synapse added between cells 1 and 2 as shown in Figure 12C. Recall that a gap junction is bidirectional unlike unidirectional chemical synapses with synaptic thresholds. Recall that it is modeled by this term
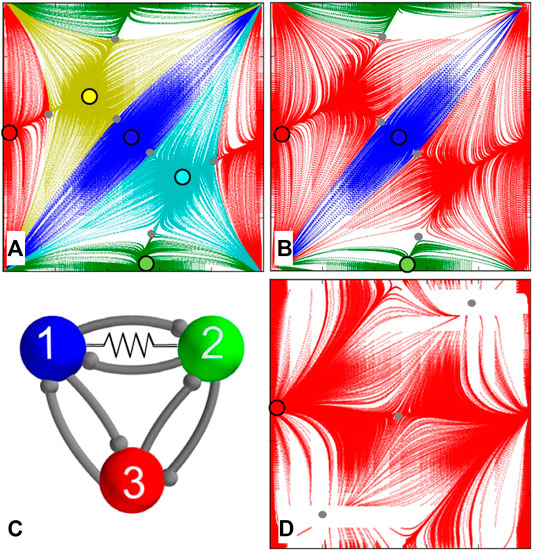
FIGURE 12. Gap junction in the symmetric 3-cell network (C) is represented by a resistor symbol placed between cells 1 and 2. (A) At
It is observed that introducing an electrical synapse between only two of the cells of the motif breaks down both circular symmetries in the system. This is documented in Figures 12A,B depicting the maps for the networks with
6. Conclusion
Our ultimate goal is to use the top-down approach to single out the fundamental principles of the rhythm formation in small networks that can be systematically generalized and applied for understanding larger network architectures. Due to the rhythmic nature of the bursting patterns, we employed Poincaré return maps defined on phases and phase lags between burst initiations in the interneurons. These maps allow us to study quantitative and qualitative properties of the stable rhythms and their corresponding attractor basins. The specific goal of this study is to demonstrate the simplicity and usability of the 2θ-bursters to construct multistable, polyrhythmic neural networks that have the same dynamical and bifurcation properties as ones composed of biologically plausible models of Hodgkin-Huxley–type bursters and chemical synapses [34, 35].We argued that the maps derived from the HH-type bursters and ones from the
In this article, we did not discuss the rhythm-generating motifs composed of
Our computational approach based on the reduction to the evident Poincaré return maps for phase lags extracted from voltage traces were inspired by neurophysiological recordings from biological CPGs, such as the 3-cell pyloric one and swim CPGs of sea slugs. The predictive power of the map approach is that it allows constructing a desired neural circuit with some preset properties. With such maps, one also gains generalizable insights helpful for the better understanding of the fundamental and universal rules of the pattern formation in various models of central pattern generators. Our findings can be employed for identifying or implementing the conditions for normal and pathological functioning of basic CPGs of animals and artificially intelligent prosthetics that can regulate various movements.
The Reader is welcome to download the open-source Motiftoolbox (supports GPUs) https://github.com/jusjusjus/Motiftoolbox to interactively explore various 3-cell, 4-cell, and large circuits composed of the HH-type, FN-like, and
Data Availability Statement
The computational toolkit supporting the findings of this study is openly available as Motiftoolbox in GitHub at https://github.com/jusjusjus/Motiftoolbox.
Author Contributions
AS supervised the findings of this work. All authors designed the model and the computational framework, analyzed the data, discussed the results, and contributed to the final manuscript.
Funding
We thank the Brains and Behavior initiative of the Georgia State University for the pilot grant support. This study was funded in part by the NSF grant IOS-1455527.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank the past and current lab-mates of the Shilnikov NeurDS lab (Neuro–Dynamical Systems), specifically, K. Wojcik, K. Pusuluri, J. Collens, and J. Bourahmah and J. Schwabedal (Motiftoolbox developer). The NeurDS lab is grateful to NVIDIA Corporation for donating the Tesla K40ring GPUs that were actively used in this study. This research was partially funded by the National Science Foundation, grant IOS-1455527.
Appendix
The time evolution of the membrane potential, V, of each neuron is modeled using the framework of the Hodgkin–Huxley formalism, based on a reduction in a leech heart interneuron model:
see ref. 23 and the references therein. Its dynamics involve a fast sodium current,
Fast, nondelayed synaptic currents in this study are modeled using the fast-threshold modulation (FTM) paradigm as follows [33]:
where
References
1. Marder, E, and Calabrese, RL. Principles of rhythmic motor pattern generation. Physiol Rev (1996). 76(3):687–717. doi:10.1152/physrev.1996.76.3.687.
2. Kristan, WB, Calabrese, RL, and Friesen, WO. Neuronal control of leech behavior. Prog Neurobiol (2005). 76:279–327. doi:10.1016/j.pneurobio.2005.09.004.
3. Marder, E. Invertebrate neurobiology: polymorphic neural networks. Curr Biol (1994). 4(8):752–4. doi:10.1016/s0960-9822(00)00169-x.
4. Calin-Jageman, RJ, Tunstall, MJ, Mensh, BD, Katz, PS, and Frost, WN. Parameter space analysis suggests multi-site plasticity contributes to motor pattern initiation in tritonia. J Neurophysiol (2007). 98:2382–98. doi:10.1152/jn.00572.2007.
5. Newcomb, JM, Sakurai, A, Lillvis, JL, Gunaratne, CA, and Katz, PS. Homology and homoplasy of swimming behaviors and neural circuits in the nudipleura (mollusca, gastropoda, opistho-branchia). Proc Natl Acad Sci USA (2012). 109(1):10669–76. doi:10.1073/pnas.1201877109.
7. Bal, T, Nagy, F, and Moulins, M. The pyloric central pattern generator in Crustacea: a set of conditional neuronal oscillators. J Comp Physiol (1988). 163(6):715–27. doi:10.1007/bf00604049.
8. Katz, PS, and Hooper, SL. Invertebrate central pattern generators. In: G North RR Greenspan, editors Invertebrate neurobiology. New York, NY: Cold Spring Harbor Laboratory Press (2007).
9. Kopell, N, and Ermentrout, B. Chemical and electrical synapses perform complementary roles in the synchronization of interneuronal networks. Proc Natl Acad Sci USA (2004). 101(43):15482–7. doi:10.1073/pnas.0406343101.
10. Matsuoka, K. Mechanisms of frequency and pattern control in the neural rhythms generators. Biol Cybern (1987). 56:345–53.
11. Kopell, N. Toward a theory of modeling central pattern generators. In: AH Cohen, S Rossingol, S Grillner, editors Neural control of rhythmic movements in vertebrates. New York, NY: Wiley (1988).
12. Canavier, CC, Baxter, DA, Clark, JW, and Byrne, JH. Multiple modes of activity in a model neuron suggest a novel mechanism for the effects of neuromodulators. J Neurophysiol (1994). 72(2):872–82. doi:10.1152/jn.1994.72.2.872.
13. Skinner, FK, Kopell, N, and Marder, E. Mechanisms for oscillation and frequency control in reciprocally inhibitory model neural networks. J Comput Neurosci (1994). 1:69–87. doi:10.1007/bf00962719.
14. Dror, RO, Canavier, CC, Butera, RJ, Clark, JW, and Byrne, JH. A mathematical criterion based on phase response curves for stability in a ring of coupled oscillators. Biol Cybern (1999). 80(1):11–23. doi:10.1007/s004220050501.
15. Shilnikov, A, Gordon, R, and Belykh, I. Polyrhythmic synchronization in bursting networking motifs. Chaos (2008). 18(3):037120. doi:10.1063/1.2959850.
16. Kristan, WB. Neuronal decision-making circuits. Curr Biol (2008). 18(19):R928–32. doi:10.1016/j.cub.2008.07.081.
17. Briggman, KL, and Kristan, WB. Multifunctional pattern-generating circuits. Annu Rev Neurosci (2008). 31:271–94. doi:10.1146/annurev.neuro.31.060407.125552.
18. Belykh, IV, and Shilnikov, AL. When weak inhibition synchronizes strongly desynchronizing networks of bursting neurons. Phys Rev Lett (2008). 101(7):078102. doi:10.1103/physrevlett.101.078102.
19. Jalil, S, Belykh, I, and Shilnikov, AL. Spikes matter in phase-locking of inhibitory bursting networks. Phys Rev E (2012). 85:36214. doi:10.1103/physreve.85.036214.
20. Jalil, S, Allen, D, Youker, J, and Shilnikov, A. Toward robust phase-locking inMelibeswim central pattern generator models. Chaos (2013). 23(4), 046105. doi:10.1063/1.4825389.
21. Knapper, D, Schwabedal, D, and Shilnikov, AL. Qualitative and quantitative stability analysis of penta-rhythmic circuits. Nonlinearity (2016). 29(12):3647–76. doi:10.1088/0951-7715/29/12/3647.
22. Shilnikov, A, and Cymbalyuk, G. Homoclinic bifurcations of periodic orbits en a route from tonic spiking to bursting in neuron models. Reg Chaot Dyn (2004). 9, 281–97. doi:10.1070/rd2004v009n03abeh000281.
23. Shilnikov, A. Complete dynamical analysis of a neuron model. Nonlinear Dyn (2012). 68(3):305–28. doi:10.1007/s11071-011-0046-y.
24. Collens, J, Pusuluri, K, Kelley, A, Knapper, D, Xing, T, Basodi, S, et al. Dynamics and bifurcations in multistable 3-cell neural networks. Chaos (2020). 30:072101. doi:https://doi.org/10.1063/5.00113742020.
25. Pusuluri, K, Basodi, S, and Shilnikov, A. Computational exposition of multistable rhythms in 4-cell neural circuits. Commun Nonlinear Sci Numer Simulat (2020). 83:105139. doi:10.1016/j.cnsns.2019.105139.
26. Ermentrout, B, and Kopell, N. Parabolic bursting in an excitable system coupled with a slow oscillation. SIAM J Appl Math (1986). 46:0146017. doi:10.1137/0146017.
27. Wojcik, J, Clewley, R, and Shilnikov, AL. Order parameter for bursting polyrhythms in multifunctional central pattern generators. Phys Rev E (2011). 83:056209. doi:10.1103/physreve.83.056209.
28. Wojcik, J, Clewley, R, Schwabedal, J, and Shilnikov, AL. Key bifurcations of bursting polyrhythms in 3-cell central pattern generators. PLoS One (2014). 9(4):e92918. doi:10.1371/journal.pone.0092918.
29. Shilnikov, AL, and Cymbalyuk, G. Transition between tonic spiking and bursting in a neuron model via the blue-sky catastrophe. Phys Rev Lett (2005). 94(4):048101. doi:10.1103/physrevlett.94.048101.
30. Shilnikov, A, and Turaev, D. Blue-sky catastrophe. Scholarpedia (2007). 2(8):1889. doi:10.4249/scholarpedia.1889.
31. Shilnikov, LP, Shilnikov, AL, and Turaev, DV. Showcase of blue sky catastrophes. Int. J. Bifurcation Chaos (2014). 24, 1440003. doi:https://doi.org/10.1142/S0218127414400033.
32. Turaev, AL, Shilnikov, LP, and Turaev, DV. Blue-sky catastrophe in singularly perturbed systems. Moscow Math J (2005). 5(1):269–282. doi:10.17323/1609-4514-2005-5-1-269-282.
33. Kopell, N, and Somers, D. Rapid synchronization through fast threshold modulation. Biol Cybern (1993). 68(1):393–407. doi:10.1007/BF00198772.
34. Channell, P, Cymbalyuk, G, and Shilnikov, AL. Origin of bursting through homoclinic spike adding in a neuron model. Phys Rev Lett (2007). 98(13):134101. doi:10.1103/physrevlett.98.134101.
Keywords: central pattern generator, r multistability, phase-lag, neuron, model
Citation: Kelley A and Shilnikov A (2020) 2θ-Burster for Rhythm-Generating Circuits. Front. Appl. Math. Stat. 6:588904. doi: 10.3389/fams.2020.588904
Received: 29 July 2020; Accepted: 14 September 2020;
Published: 27 November 2020.
Edited by:
Vijay Kumar Yadav, Nirma University, IndiaReviewed by:
Valeri Makarov, Complutense University of Madrid, SpainJames Rankin, University of Exeter, United Kingdom
Copyright © 2020 Shilnikov and Kelley. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrey Shilnikov, YXNoaWxuaWtvdkBnc3UuZWR1
 Aaron Kelley1
Aaron Kelley1 Andrey Shilnikov
Andrey Shilnikov