- 1Directorate of Grant and Development, Mount Kenya University, Thika, Kenya
- 2Department of Pharmacy, Kiambu Level 5 Hospital, Kiambu, Kenya
- 3Department of Animal Sciences, Chuka University, Chuka, Kenya
Antibiotic resistance causes higher morbidity and mortality and higher healthcare costs. One of the factors influencing the emergence of antibiotic resistance is the inappropriate use of antibiotics. Clinical practitioners’ incorrect prescription patterns and a disregard for antibiotic usage recommendations are the leading causes of this resistance. This study examined the antibiotic prescription patterns among hospitalized patients at the Kiambu Level 5 hospital (KL5) to find potential for hospital quality improvement. This study was conducted in July 2021, and all patients hospitalized on the study day were included. The information was extracted from patient medical records using a World Health Organization Point Prevalence Survey (PPS) instrument. Anonymized data was gathered, entered, and then SPSS version 26 was used for analysis. Among the 308 surveyed patients, 191 (62%) received antibiotic medication, and 60.1% of the total were female. The pediatric ward, which had an antibiotic prescription rate of 94.1%, had the highest rate of antibiotic usage, followed by the medical ward (69.2%) and gynecological ward (65.6%). Over 40% of antibiotic prescriptions had a prophylactic medical indication. Penicillin G was the most prescribed antibiotic for community-acquired infections (32.2%), followed by 3rd generation cephalosporins (27.6%) and aminoglycosides (17.2%). Based on the AWaRe classification, 57% of the prescribed antibiotics were in the Access class while 42% were in the Watch class. Incomplete site of indication, lack of a method of administration, and length of administration are some of the conformities that were missing in the medical records. This study shows that antibiotic prescription rates are high, particularly for young patients, and there is a higher risk of antibiotic misuse. The data makes a compelling justification for using antibiotic stewardship practices in Kenyan hospitals.
Introduction
Antibiotics have been at the forefront of the fight against infection-related mortality and morbidity (Fourie et al., 2018). However, antibiotics must be used in a timely and appropriate manner to prevent antimicrobial resistance (Mulwa, 2014; Saleem et al., 2019; Afriyie et al., 2020; Saleem et al., 2020). Antibiotic misuse and abuse, particularly of broad-spectrum antibiotics, leads to the development of resistance (Saleem et al., 2019; Afriyie et al., 2020; Saleem et al., 2020). Antibiotic resistance has become a major concern, with a devastating trend associated with poor clinical outcomes and increased treatment costs (Mulwa, 2014; Ginting et al., 2019).
Antimicrobial stewardship (AST) has been incorporated into national action plans (NAPs) of low and middle-income countries (LMICs) to combat antimicrobial resistance (Seale et al., 2017; Saleem et al., 2019; Antonioli et al., 2020). Point prevalence surveys (PPS) serve as the backbone of the feedback loop for healthcare providers and policymakers in responding to antibiotic resistance policies (Elhajji et al., 2018; Kurdi et al., 2021). National standard treatment guidelines (NSTGs) are being developed in LMICs to raise awareness through the Drugs and Therapeutics Committees (DTCs). Antibiotic use in China (Xie et al., 2015) and Egypt (Umeokonkwo et al., 2019) has been reported to be up to 59% among in-patients. Most African countries report that most prescriptions are done empirically (Talaat et al., 2014; Nsofor et al., 2016; Labi et al., 2018; Umeokonkwo et al., 2019). There is a rising concern about high rates of antibiotic resistance across Africa, Asia, and Europe (Fourie et al., 2018), with accumulating reports indicating increased prescription of second and third-line antibiotics (Labi et al., 2018; Kurdi et al., 2021).
In Kenya, studies have found a high prevalence of inpatient antibiotic use, with some hospitals recording a prevalence close to 80% in inpatient departments (Mulwa, 2014). To date, only a handful of hospitals have had point prevalence studies on antibiotic use conducted here in Kenya (Okoth et al., 2018; Momanyi et al., 2019; Omulo et al., 2020; Maina et al., 2021). There is, therefore, a need to extend these studies to other hospitals to get a more representative figure on the prevalence of antibiotic use in Kenya. This study aimed to determine the variation in the quality and quantity of antibiotics prescribed to patients admitted to Kiambu Level 5 (KL5) hospital. The goal was to identify quality improvement targets and help the hospital design interventions for prudent antibiotic usage.
Methodology
Study site
The study was carried out at KL5 hospital, one of two major referral public hospitals in Kiambu County in Kenya. The hospital has an annual admission of up to 18,525 patients with a bed capacity of 426 beds.
Study design and target population
The study employed a point prevalence cross-sectional design to determine the variability in the use of antibiotics in all the wards in KL5 as well as to characterize the types of antibiotics used. The study targeted all the patients in the different wards, ranging from children to adult wards. Universal sampling was used, and this included all the patients admitted to the wards. Information about patient prescriptions and indications was collected at 8.00 am on the survey day since this timing was deemed appropriate. The timing did not interrupt the ward rounds and excluded patients admitted later in the day.
Data collection
Data collection took place between July 7th and July 16th 2021, and was collected using standardized forms from the Global PPS protocol with a few modifications (World Health Organization, 2018; Zumaya-Estrada et al., 2021). Each ward was allocated its day for data collection to ensure uniform sampling time was applied in all wards. A set of forms was used to collect the different data types. These forms were the hospital form, ward form, and patient form. The hospital form gathers information about the hospital, including the type of the hospital, the number of wards and the beds contained, and bed occupancy. The ward form collected data on the wards, including the dates the survey was conducted, the number of patients admitted to the ward, the type of the ward, and the capacity of the ward. The other form was the patient form which was used to gather data on each patient including their demographic characteristics, antibiotics administered if any, route, dose, duration, and indication among other variables. The indication form collected data on the indication for antibiotic use. The variables included in the form were the nature of the infection, the site of diagnosis, the date the treatment was started, and laboratory tests that were conducted. The antibiotic form collected data on the name of the antibiotics prescribed, dose, route, frequency, duration of use, diagnosis, and indications. Clinical pharmacists stationed in KL5 hospital were engaged to help with data collection. The data source was patient medical files which were collected in the morning and taken to a centralized place within the ward for data abstraction.
Antibiotic usage definitions
Indications for antibiotics were divided into either therapeutic or prevention, while infections were classified as either community-acquired (symptoms present on admission or less than 48 hours after admission) or hospital-acquired infections (symptoms occurring more than 48 hours after admission). The preventive antibiotics were also classified as either medical or surgical. Antibiotic prescription data were obtained from the treatment sheets.
Statistical analysis
After cleaning the data in Microsoft Excel, it was exported to the Statistical Package for the Social Sciences (SPSS) version 26 for analysis. To summarize findings, descriptive statistics were used, while categorical data was summarized as counts and percentages. The results are presented in tables and figures. Antibiotics were distributed into the AWaRe classes as per the WHO AWaRe classification, 2021 (WHO, 2021).
Ethical consideration
Ethical approval to undertake the study was sought from Mount Kenya University ethical committee (Ref No. MKU/ERC/889) and a license to conduct the study was obtained from the National Commission of Science, Technology, and Innovation (NACOSTI) [NACOSTI/P/21/10948]. Authorization to conduct the study was also sought from Health Research Development in Kiambu County and the Kiambu Level 5 hospital administration.
Results
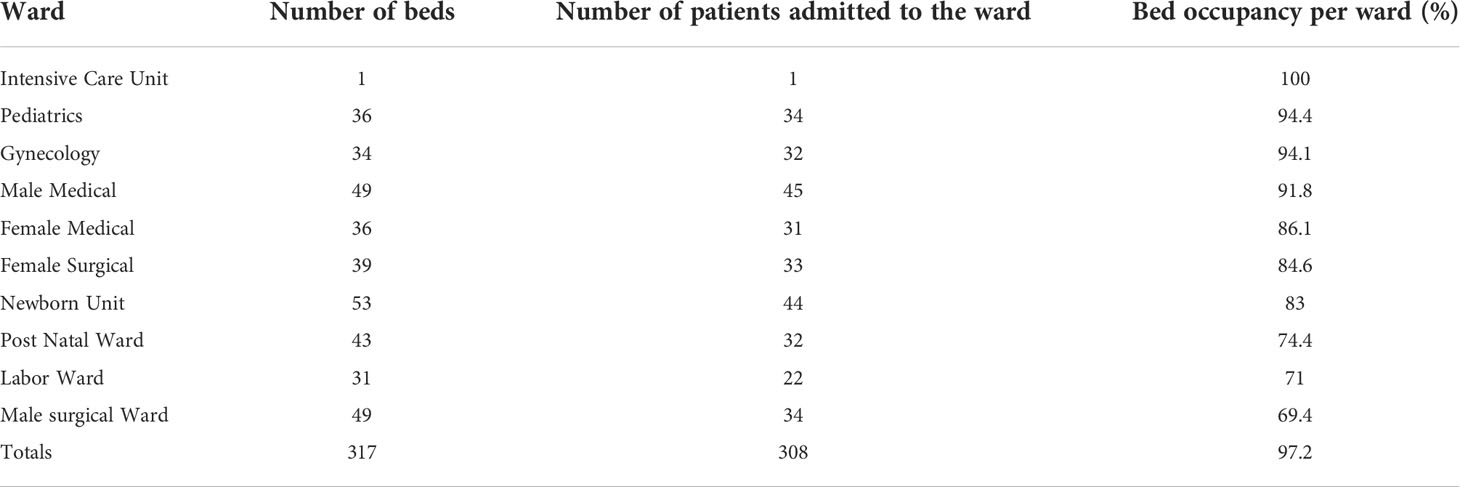
The wards had a total of 317 beds. Only one bed in the intensive care unit was occupied. The pediatric ward had the highest bed occupancy (94.4%, n = 34), followed by the gynecology ward (94.1%, n = 32). On the survey days, 308 patients were admitted to the hospital, resulting in a 97.2% occupancy rate (Table 1).
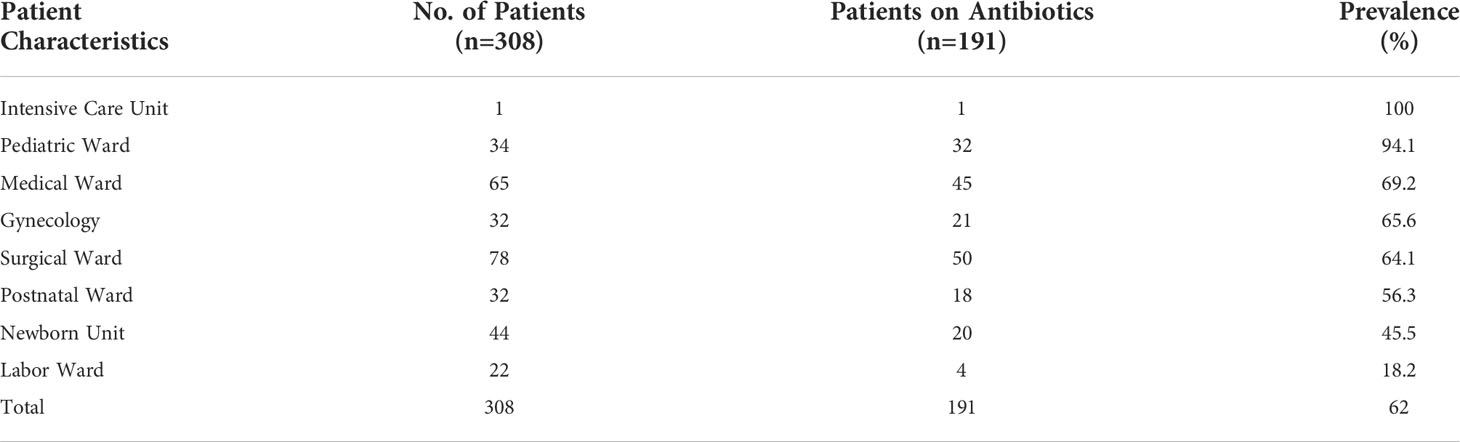
Most of the patients in all the wards were aged between 20 and 64 years old (182, 59.1%), with female being the majority (185, 60.1%). The top-3 wards that had the highest number of patients were surgical wards (78, 25.3%), medical wards (65, 21.1%), and newborn units, accounting for 14.3% (n = 44) of all the patients (Table 2).
Overall, of the 308 patients admitted to all the wards, 62% (n = 191) of the patients were on one or more antibiotics. The ward that had the highest prevalence of antibiotic use was the pediatric ward at 94.1%, followed by the medical wards, gynecology, surgical, and postnatal wards at 69.2%, 65.6%, 64.1%, and 56.3%, respectively (Table 3).
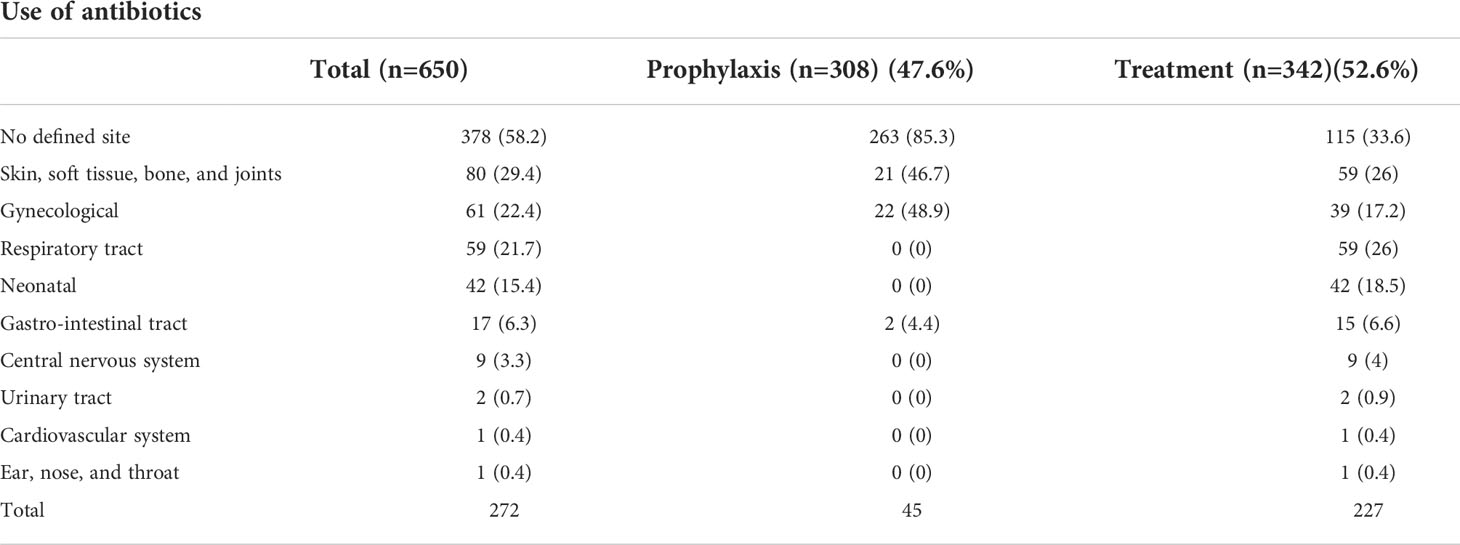
There were a total of 650 antibiotics that were prescribed to all 308 patients. Therapeutic use of antibiotics was more prevalent than prophylactic use (47.4% vs. 52.6%, respectively). Among those antibiotics that had a defined site, gynecological sites were the most common anatomical site for both medical and surgical prophylactic use at 48.9%, followed by skin, soft tissue, bone, and joints (46.7%), and the gastrointestinal tract (4.4%). Sites indicated for antibiotic treatment were skin, soft tissue, bone, and joints, accounting for 26%, followed by respiratory tract infections at 26% and gynecological sites at 17.2% (Table 4).
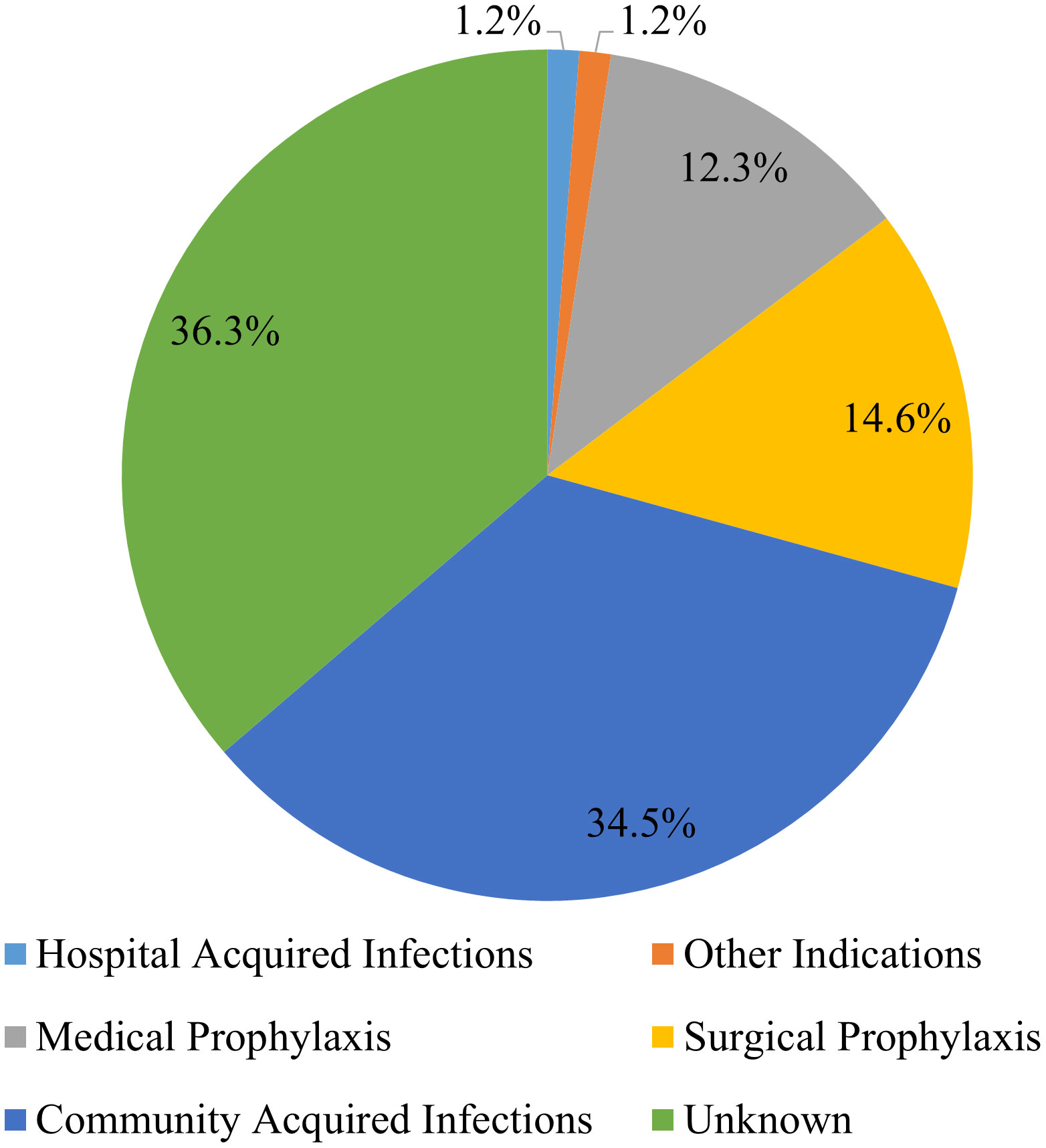
Among the different indications recorded, the most common indication for antibiotic use was community-acquired infections (35%), followed by surgical prophylaxis (15%). There were, however, 36% of the antibiotics whose indications were not reported in the patient files (Figure 1).
The proportional frequency of each antibiotic use indication also varied from one ward to another. For instance, whereas community-acquired infections (CAIs) accounted for most of the antibiotic usage in the medical ward (54.1%), most of the antibiotic usage in the postnatal ward (92.5%) was for surgical prophylaxis (SP). However, in the gynecological ward, medical prophylaxis (MP) accounted for 32.1% of all indications (Figure 2).
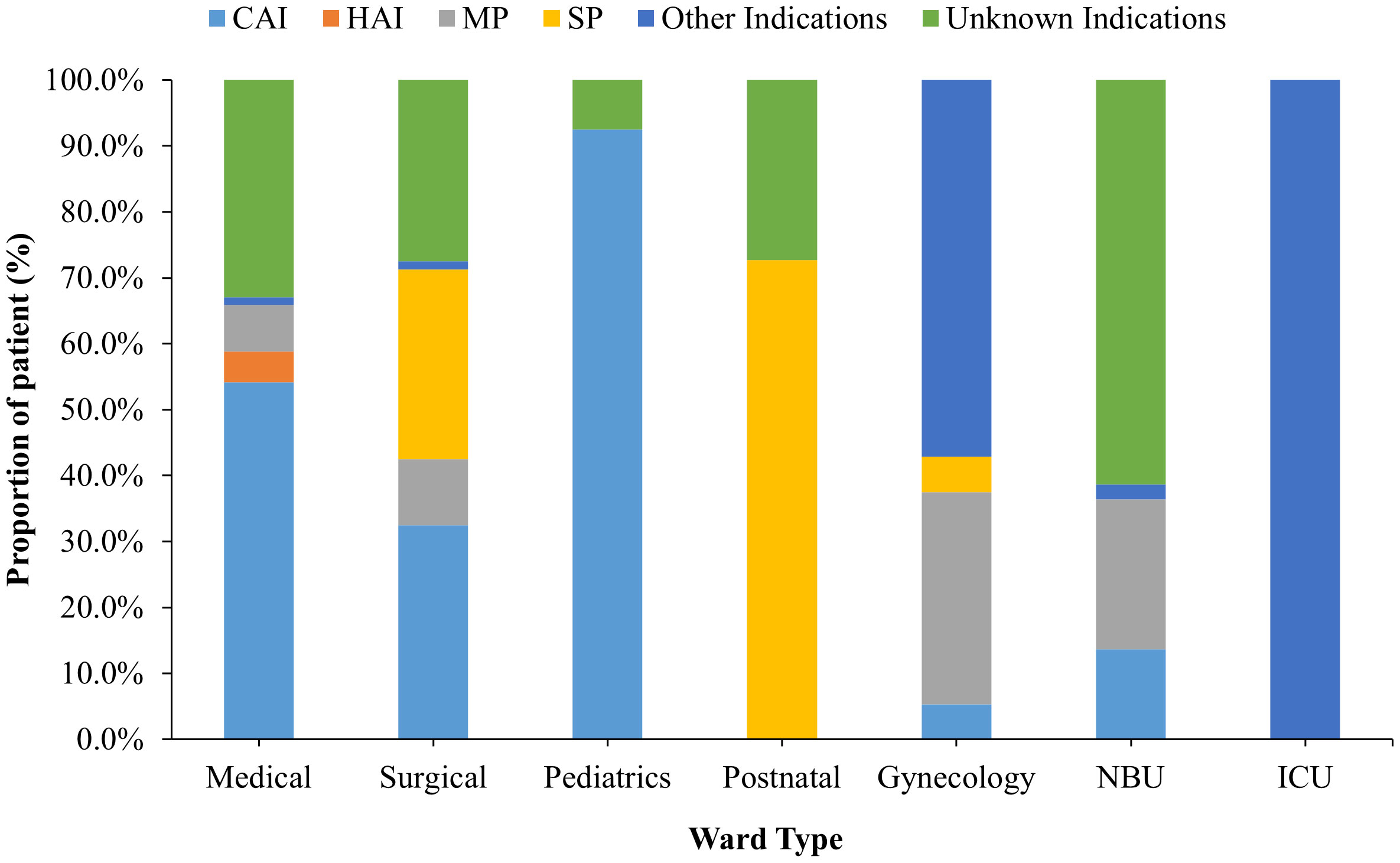
Figure 2 Patient distribution by indication and stratified by hospital wards for those receiving antibiotic treatment. NBU, Newborn Unit; ICU, – Intensive Care Unit.
Most antibiotics were prescribed once daily (25.0%), with those prescribed twice daily (19.0%) coming in second. However, more than 26% of the antibiotics lacked an indication of their frequency. Parenteral administration (54.0%) was the most popular route of administration, followed by oral administration (20.8%). Use could last from one day to 14 days. Most of the antibiotics that were in use—roughly 223 (44.6%), had a duration of usage of between one and four days (Table 5).

Table 5 Frequency, route of administration, and duration of antibiotic use among the patients admitted to Kiambu Level 5 hospital.
Depending on the type of indication, different antibiotics were employed. For instance, penicillin G was the most frequently given antibiotic (32.2%), followed by 3rd generation cephalosporins (27.6%), and aminoglycosides (17.2%), for community-acquired illnesses (See Figure 3A). On the other hand, third-generation cephalosporins (40%) were the most used antibiotics for hospital-acquired infections (Figure 3B), while nitroimidazole analogs (30.8%) was the most used medication for medical prophylaxis (Figure 3C).

Figure 3 (A) Consumption of antibiotics prescribed for community-acquired infections (CAI); (B) consumption of antibiotics prescribed for hospital-acquired infections >(HAI) and (C) consumption of antibiotics prescribed for medical prophylaxis (MP).
Classified by the AWaRe classification, 57% of the antibiotics prescribed were in the Access class while 42% were in the Watch class. Only one percent of the antibiotics were not classified and comprised mainly of antitubercular drugs. Overall, Access class antibiotics were highly used in Newborn unit while Watch class antibiotics were highly used in medical wards (Figure 4).
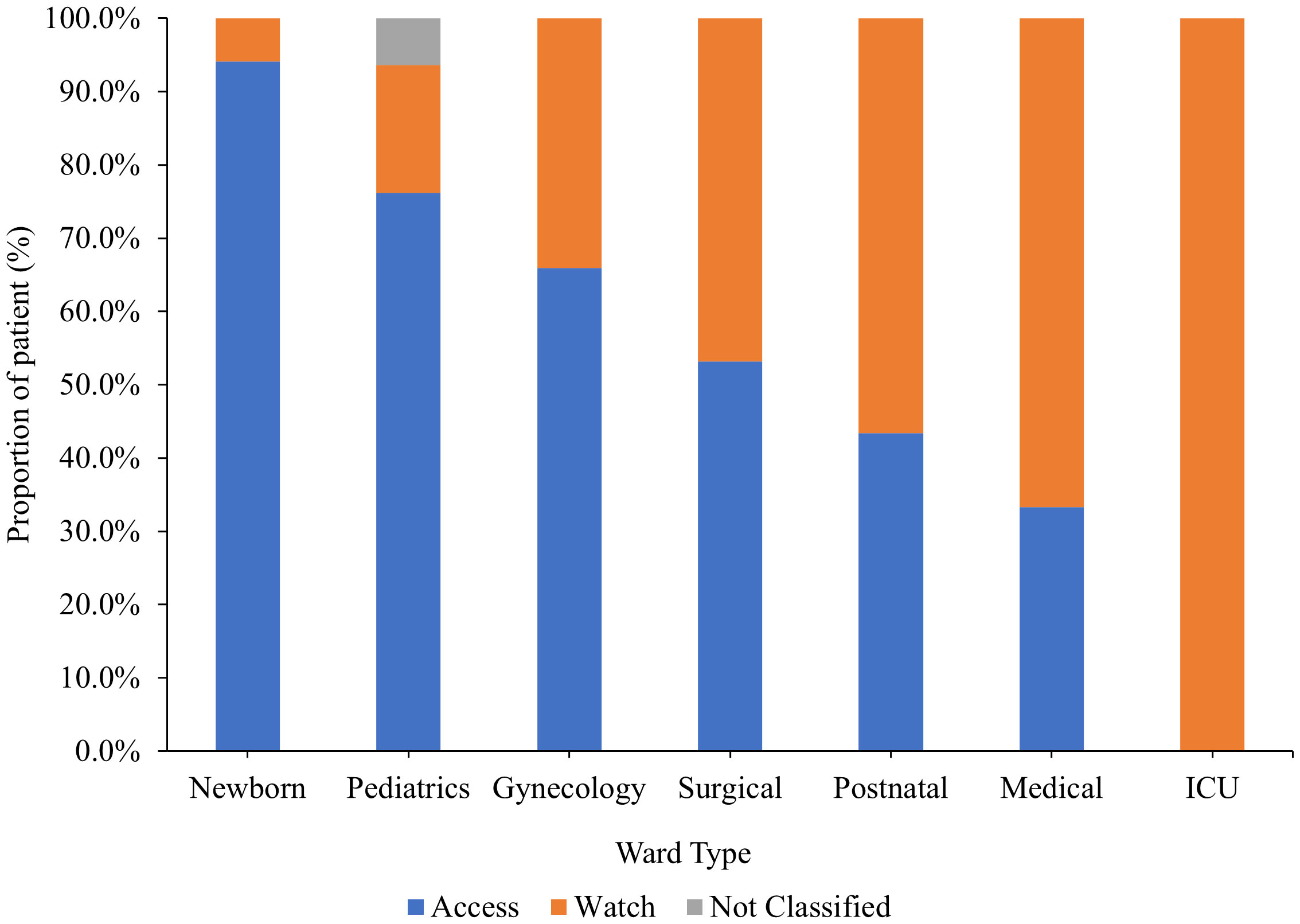
Figure 4 Antibiotics usage by ward type according to the WHO AWaRe classification. ICU, Intensive Care Unit.
Discussion
This study discovered a high prevalence of antibiotic prescribing, with 62% of all hospitalized patients receiving antibiotics during the study period. This finding exceeds the 54.7% prevalence reported in a referral hospital in western Kenya (Momanyi et al., 2019) but it is lower than the reported 74% in a study conducted in Makueni, Kenya (Mulwa, 2014). Our study’s prevalence of 62% compares favorably with other similar studies conducted in other parts of the country, ranging from 58.2% to 67.7% (Okoth et al., 2018; Momanyi et al., 2019) as well as other studies in African countries (Talaat et al., 2014; Nsofor et al., 2016; Labi et al., 2019). The prevalence of antibiotic use varies from one hospital to another in Kenya and seems to depend on the capacity of a particular facility and not necessary on their hierarchy level. For instance, while both Kiambu hospital (this study) and Rift Valley Provincial General hospital (Momanyi et al., 2019) are both level 5 hospitals, Rift Valley Provincial General hospital is more equipped than Kiambu Level 5 hospital. This can explain why the prevalence of antibiotic use is lower in Rift Valley Provincial General Hospital than Kiambu Level 5 hospital. Similarly national teaching and referral hospitals have been reported to have much lower antibiotic prevalence (Omulo et al., 2020) than lower level facilities. These results indicate that efforts to ensure judicious use of antibiotics is more of a facility effort than general health practice across health facilities. In comparison to research conducted in developed nations where antibiotic use and standards are strictly adhered to through well-established antibiotic stewardship programs (Versporten et al., 2018), the finding is significantly higher. Due to insufficient laboratory capacity to assist in the detection of specific diseases, broad-spectrum antibiotics are typically prescribed empirically, which accounts for the high prescription rate in this hospital.
When compared to other wards that primarily house adult patients, the pediatric ward’s rate of antibiotic prescriptions was extremely high, at 94.1%, according to our study. This occurrence could be linked to local hospital policies recommending antibiotic treatment for postpartum women and newborns with low birth weight and hypoxia. This finding is in line with research from other African countries, where several studies (Amaha et al., 2018; Apostolakos et al., 2019) reported proportions of patients under the age of five that were higher than 90%. The main causes of antibiotic usage were community-acquired illnesses and surgical prophylaxis (35% and 15%, respectively), which is consistent with findings from other studies (Seni et al., 2020). Overall, the majority (52.6%) of prescriptions were for therapeutic purposes. This result is consistent with research from other African nations (Maina et al., 2017; Anand Paramadhas et al., 2019; Afriyie et al., 2020). Without the aid of a laboratory, the bulk of antibiotic prescriptions is made empirically. Empirical prescribing is mainly caused by difficulties such as a shortage of laboratory technicians, insufficient laboratory supplies, patient cost apprehensions, and patients’ delayed presentation to the hospital after self-medication with antibiotics fails (Dat et al., 2022).
Penicillin G, third-generation cephalosporins, and aminoglycosides were the most frequently prescribed antibiotics among community-acquired infections, accounting for 32.2%, 27.6%, and 17.2% of all prescriptions, respectively. Penicillin G and cephalosporin use have significantly increased globally over the past ten years because of higher health care spending (Amdany and Mcmillan, 2014) and easier access to medications (Maina et al., 2020). Similar studies in poor nations have also found that the use of cephalosporin is preferred over other antibiotics with numerous daily doses in situations where hospital staff is shorthanded because of the frequency of administration and the broad-spectrum nature of the drug (Labi et al., 2018). Metronidazole (30.8%) and penicillin G (32.2%) were the two drugs most frequently used for medical prophylaxis, respectively. The two antibiotics are frequently used for surgical prophylaxis and the treatment of invasive infections (Amdany and Mcmillan, 2014) because they are readily available and reasonably priced in Kenya. WHO has provided a list of antibiotics grouped into three major classes to support antibiotic stewardship. These classes are Access, Watch and Reserve classes. In this study, only 57% of the antibiotics prescribed were in the Access class while 42% were in the Watch class. The use of 42% of the antibiotics in the Watch class is higher than rates reported in Ghana (Amponsah et al., 2021) and Finland (Hsia et al., 2019). However, the use of the Watch class antibiotics was relatively lower than reported in a study conducted in Bangladesh where 64% of the prescribed antibiotics were in the Watch class. Similar to a previous study (Rashid et al., 2022), medical wards had the highest use of antibiotics in the Watch class.
The parenteral route was the most used (54.0%), followed by the oral route (20.8%). Other studies have found that the parenteral route is the most prescribed route of drug administration (Thu et al., 2012). This observation may be explained by the late presentation of critically ill patients to the hospital, the patient’s advanced age, and a contraindication to oral intake. This situation is consistent with the most prescribed antibiotics, penicillin G, and cephalosporins, which are primarily available as parenteral injections. A reduced parenteral route of administration is considered a better antimicrobial stewardship practice and, thus, a good metric for stewardship in a hospital (Amdany and Mcmillan, 2014).
Conclusion
This study demonstrates the widespread use of antibiotics, particularly in pediatric patients. Most prescriptions are empirical, without the guidance of laboratory microbiological testing. The primary indications for antibiotic use are community-acquired infections and surgical prophylaxis. A significant portion of antibiotic prescriptions was for penicillin G, third-generation cephalosporins, and aminoglycosides. Most medications were in the form of parenteral drug formulations. As demonstrated by this study, there is a risk of inappropriate antibiotic use due to incomplete prescriptions. The study makes a compelling case for implementing antimicrobial stewardship policies that can help reduce empirical treatment and increase the use of more specific and targeted prescriptions of oral antimicrobial formulations. There is a need for a more detailed study to assess the reason behind these prescription practices by the clinicians.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics and Review Committee, Mount Kenya University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
JG and DM conceptualized the study. JG, MK and RM designed the study. MK, MM, RK, RM, and CN collected data. MK and MM analyzed the data. MK, MM and DM prepared the first draft manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by The Kenya National Research Fund (grant number NRF/MKU/2017/007 to JG).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Afriyie D. K., Sefah I. A., Sneddon J., Malcolm W., McKinney R., Cooper L., et al. (2020). Antimicrobial point prevalence surveys in two ghanaian hospitals: Opportunities for antimicrobial stewardship. JAC-Antimicrobial Resistance 2, 1–9. doi: 10.1093/jacamr/dlaa001
Amaha N. D., Berhe Y. H., Kaushik A. (2018). Assessment of inpatient antibiotic use in halibet national referral hospital using WHO indicators: A retrospective study. BMC Res. Notes. 11 (1), 1–5. doi: 10.1186/s13104-018-4000-7
Amdany H. K., Mcmillan M. (2014). Metronidazole intravenous formulation use in in-patients in kapkatet district hospital, Kenya: a best practice implementation project. JBI Database Syst. Rev. Implement Rep. 12 (3), 419–432. doi: 10.11124/jbisrir-2014-1381
Amponsah O. K. O., Buabeng K. O., Owusu-Ofori A., Ayisi-Boateng N. K., Hämeen-Anttila K., Enlund H. (2021). Point prevalence survey of antibiotic consumption across three hospitals in Ghana. JAC-Antimicrobial Resist. 3 (1), 1–7. doi: 10.1093/jacamr/dlab008
Anand Paramadhas B. D., Tiroyakgosi C., Mpinda-Joseph P., Morokotso M., Matome M., Sinkala F., et al. (2019). Point prevalence study of antimicrobial use among hospitals across botswana; findings and implications. Expert Rev. Anti Infect. Ther. 17 (7), 535–546. doi: 10.1080/14787210.2019.1629288
Antonioli P., Bolognesi N., Valpiani G., Morotti C., Bernardini D., Bravi F., et al. (2020). A 2-year point-prevalence surveillance of healthcare-associated infections and antimicrobial use in ferrara university hospital, Italy. BMC Infect. Dis. 20 (1), 1–8. doi: 10.1186/s12879-020-4791-8
Apostolakos I., Mughini-Gras L., Fasolato L., Piccirillo A. (2019). Assessing the occurrence and transfer dynamics of ESBL/pAmpC-producing escherichia coli across the broiler production pyramid. PloS One 14 (5), e0217174. doi: 10.1371/journal.pone.0217174
Dat V. Q., Dat T. T., Hieu V. Q., Giang K. B., Otsu S. (2022). Antibiotic use for empirical therapy in the critical care units in primary and secondary hospitals in Vietnam: a multicenter cross-sectional study. Lancet Reg. Heal - West Pacific. 18, 1–10. doi: 10.1016/j.lanwpc.2021.100306
Elhajji F. D., Al-Taani G. M., Anani L., Al-Masri S., Abdalaziz H., Qabba’H S. H., et al. (2018). Comparative point prevalence survey of antimicrobial consumption between a hospital in northern Ireland and a hospital in Jordan 11 medical and health sciences 1103 clinical sciences. BMC Health Serv. Res. 18 (1), 1–8. doi: 10.1186/s12913-018-3656-y
Fourie T., Schellack N., Bronkhorst E., Coetzee J., Godman B. (2018). Antibiotic prescribing practices in the presence of extended-spectrum β-lactamase (ESBL) positive organisms in an adult intensive care unit in south Africa – a pilot study. Alexandria J. Med. 54 (4), 541–547. doi: 10.1016/j.ajme.2018.09.001
Ginting F., Sugianli A. K., Bijl G., Saragih R. H., Kusumawati R. L., Parwati I., et al. (2019). Rethinking antimicrobial resistance surveillance: A role for lot quality assurance sampling. Am. J. Epidemiol. 188 (4), 734–742. doi: 10.1093/aje/kwy276
Hsia Y., Lee B. R., Versporten A., Yang Y., Bielicki J., Jackson C., et al. (2019). Use of the WHO access, watch, and reserve classification to define patterns of hospital antibiotic use (AWaRe): an analysis of paediatric survey data from 56 countries. Lancet Glob Heal. 7 (7), e861–e871. doi: 10.1016/S2214-109X(19)30071-3
Kurdi A., Hasan A. J., Baker K. I., Seaton R. A., Ramzi Z. S., Sneddon J., et al. (2021). A multicentre point prevalence survey of hospital antibiotic prescribing and quality indices in the Kurdistan regional government of northern Iraq: the need for urgent action. Expert Rev. Anti Infect. Ther. 19 (6), 805–814. doi: 10.1080/14787210.2021.1834852
Labi A. K., Obeng-Nkrumah N., Owusu E., Bjerrum S., Bediako-Bowan A., Sunkwa-Mills G., et al. (2019). Multi-centre point-prevalence survey of hospital-acquired infections in Ghana. J. Hosp Infect. 101 (1), 60–68. doi: 10.1016/j.jhin.2018.04.019
Labi A. K., Obeng-Nkrumah N., Sunkwa-Mills G., Bediako-Bowan A., Akufo C., Bjerrum S., et al. (2018). Antibiotic prescribing in paediatric inpatients in Ghana: A multi-centre point prevalence survey. BMC Pediatr. 18, 1–9. doi: 10.1186/s12887-018-1367-5
Maina M., Akech S., Mwaniki P., Gachau S., Ogero M., Julius T., et al. (2017). Inappropriate prescription of cough remedies among children hospitalised with respiratory illness over the period 2002–2015 in Kenya. Trop. Med. Int. Heal. 22 (3), 363–369. doi: 10.1111/tmi.12831
Maina M., McKnight J., Tosas-Auguet O., Schultsz C., English M. (2021). Using treatment guidelines to improve antibiotic use: Insights from an antibiotic point prevalence survey in Kenya. BMJ Global Health 6, e003836. doi: 10.1136/bmjgh-2020-003836
Maina M., Mwaniki P., Odira E., Kiko N., McKnight J., Schultsz C., et al. (2020). Antibiotic use in Kenyan public hospitals: Prevalence, appropriateness and link to guideline availability. Int. J. Infect. Dis. 99, 10–18. doi: 10.1016/j.ijid.2020.07.084
Momanyi L., Opanga S., Nyamu D., Oluka M., Kurdi A., Godman B. (2019). Antibiotic prescribing patterns at a leading referral hospital in Kenya: A point prevalence survey. J. Res. Pharm. Pract. 8 (3), 149. doi: 10.4103/jrpp.JRPP_18_68
Mulwa C. (2014). Patterns of prescribing practices in makueni county referral hospital, (dissertation) Kenya 2014. Afr. J. Pharmacol. Ther. 4 (4), 161–168, 63. Available at: http://journals.uonbi.ac.ke/ajpt/article/view/1401.
Nsofor C. A., Amadi E. S., Ukwandu N., Obijuru C. E., Ohalete C. V. (2016). Prevalence of antimicrobial use in major hospitals in owerri, Nigeria. EC Microbiol. 35, 522–527. Available at: https://www.ecronicon.com/ecmi/pdf/ECMI-03-000075.pdf.
Okoth C., Opanga S., Okalebo F., Oluka M., Baker Kurdi A., Godman B. (2018). Point prevalence survey of antibiotic use and resistance at a referral hospital in Kenya: findings and implications. Hosp Pract. (1995). 46 (3), 128–136. doi: 10.1080/21548331.2018.1464872
Omulo S., Oluka M., Ombajo L., Osoro E., Kinuthia R., Guantai A., et al. (2020). Point-prevalence surveys of antibiotic use at three Large public hospitals in Kenya. Infect. Control Hosp Epidemiol. 41 (S1), s353–s354. doi: 10.1017/ice.2020.971
Rashid M. M., Akhtar Z., Chowdhury S., Islam M. A., Parveen S., Ghosh P. K., et al. (2022). Pattern of antibiotic use among hospitalized patients according to WHO access, watch, reserve (AWaRe) classification: Findings from a point prevalence survey in Bangladesh. Antibiotics. 11 (6), 1–14. doi: 10.3390/antibiotics11060810
Saleem Z., Hassali M. A., Godman B., Versporten A., Hashmi F. K., Saeed H., et al. (2020). Point prevalence surveys of antimicrobial use: a systematic review and the implications. Expert Rev. Anti-Infective Ther. 18, 897–910. doi: 10.1080/14787210.2020.1767593
Saleem Z., Hassali M. A., Versporten A., Godman B., Hashmi F. K., Goossens H., et al. (2019). A multicenter point prevalence survey of antibiotic use in punjab, Pakistan: findings and implications. Expert Rev. Anti Infect. Ther. 17 (4), 285–293. doi: 10.1080/14787210.2019.1581063
Seale A. C., Gordon N. C., Islam J., Peacock S. J., Scott J. A. G. (2017). AMR surveillance in low and middle-income settings - a roadmap for participation in the global antimicrobial surveillance system (GLASS). Wellcome Open Res. 2, 1–17. doi: 10.12688/wellcomeopenres.12527.1
Seni J., Mapunjo S. G., Wittenauer R., Valimba R., Stergachis A., Werth B. J., et al. (2020). Antimicrobial use across six referral hospitals in Tanzania: A point prevalence survey. BMJ Open 10, e042819. doi: 10.1136/bmjopen-2020-042819
Talaat M., Saied T., Kandeel A., Abo El-Ata G. A., El-Kholy A., Hafez S., et al. (2014). A point prevalence survey of antibiotic use in 18 hospitals in Egypt. Antibiotics. 3 (3), 450–460. doi: 10.3390/antibiotics3030450
Thu T. A., Rahman M., Coffin S., Harun-Or-Rashid M., Sakamoto J., Hung N. V. (2012). Antibiotic use in Vietnamese hospitals: A multicenter point-prevalence study. Am. J. Infect. Control. 40 (9), 840–844. doi: 10.1016/j.ajic.2011.10.020
Umeokonkwo C. D., Madubueze U. C., Onah C. K., Okedo-Alex I. N., Adeke A. S., Versporten A., et al. (2019). Point prevalence survey of antimicrobial prescription in a tertiary hospital in south East Nigeria: A call for improved antibiotic stewardship. J. Glob Antimicrob. Resist. 17, 291–295. doi: 10.1016/j.jgar.2019.01.013
Versporten A., Zarb P., Caniaux I., Gros M. F., Drapier N., Miller M., et al. (2018). Antimicrobial consumption and resistance in adult hospital inpatients in 53 countries: results of an internet-based global point prevalence survey. Lancet Glob Heal. 6 (6), e619–e629. doi: 10.1016/S2214-109X(18)30186-4
WHO (2021) 2021 AWaRe classification. Available at: https://www.who.int/publications/i/item/2021-aware-classification.
World Health Organization (2018). WHO methodology for point prevalence survey on antibiotic use in hospitals. World Heal Organ, 1–102. Available at: https://www.who.int/publications/i/item/WHO-EMP-IAU-2018.01.
Xie D.s., Xiang L., Li R., Hu Q., Luo Q.q., Xiong W. (2015). A multicenter point-prevalence survey of antibiotic use in 13 Chinese hospitals. J. Infect. Public Health 8 (1), 55–61. doi: 10.1016/j.jiph.2014.07.001
Keywords: point prevalence, antibiotic use, Kenya, antibiotic stewardship (ABS), referral hospital
Citation: Kamita M, Maina M, Kimani R, Mwangi R, Mureithi D, Nduta C and Gitaka J (2022) Point prevalence survey to assess antibiotic prescribing pattern among hospitalized patients in a county referral hospital in Kenya. Front. Antibiot. 1:993271. doi: 10.3389/frabi.2022.993271
Received: 13 July 2022; Accepted: 13 October 2022;
Published: 26 October 2022.
Edited by:
Stephen Henry Gillespie, University of St Andrews, United KingdomReviewed by:
Douglas Slain, West Virginia University, United StatesMd Latiful Bari, University of Dhaka, Bangladesh
Copyright © 2022 Kamita, Maina, Kimani, Mwangi, Mureithi, Nduta and Gitaka. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jesse Gitaka, amdpdGFrYUBta3UuYWMua2U=
 Moses Kamita
Moses Kamita Michael Maina
Michael Maina Racheal Kimani
Racheal Kimani Robert Mwangi
Robert Mwangi Dominic Mureithi
Dominic Mureithi Cynthia Nduta
Cynthia Nduta Jesse Gitaka
Jesse Gitaka