- 1Department of Clinical Pharmacy, School of Pharmacy, Section of Infectious Diseases, School of Medicine, West Virginia University, Morgantown, WV, United States
- 2Department of Pharmacy, West Virginia University (WVU) Medicine, Morgantown, WV, United States
- 3Department of Medicine, West Virginia University School of Medicine, Morgantown, WV, United States
We assessed the treatment of Serratia marcescens bacteremia and endocarditis in one of the largest single center studies. We could not identify an advantage with any particular antibiotic treatment regimen in this study. Induction of AmpC or selection of ESBL organisms was not displayed by any of the organisms.
Introduction
Serratia marcescens is an opportunist gram-negative bacilli that has been an occasional cause of healthcare-associated infection and as a cause of bacteremia and endocarditis in people who inject illicit drugs (PWID) (Phadke and Jacob, 2016). This bacteria remains somewhat of a mystery, and decisions about the treatment of S. marcescens infections are difficult given the limited clinical study data available and concerns about the potential for inducible multidrug resistance. Serratia marcescens has the ability to produce inducible AmpC β-lactamase and may even acquire extended-spectrum β-lactamase (ESBL) (Mahlen, 2011). Compared to other more virulent bacteria, S. marcescens is unlikely to overexpress AmpC β-lactamase (Tamma et al., 2021). Therefore, using broad-spectrum antibiotics that are typically stable to AmpC producing bacteria (carbapenems or cefepime) may not be required to treat S. marcescens infections successfully (Harris et al., 2016; Tamma et al., 2021). In terms of antimicrobial stewardship, use of carbapenem-sparing antibiotic regimens has become an attractive option as rates of carbapenem-resistant Enterobacteriacea (CRE) have increased worldwide (Tan et al., 2020).
Our university hospital has experienced a higher exposure to patients with S. marcescens bacteremia compared to many similar institutions in the United States. The higher rate may be fueled in particular by intravenous drug use practices and possibly environmental factors in the Appalachian region of the United States. It has been postulated that this particular organism occurs in the PWID population due to (A) recurrent exposure to medications directed at gram positive organisms, (B) exposure to hospitals, or (C) the practice of diluting the narcotic with water (Rosenblatt et al., 1973). Our clinicians have had to rely on their own clinical judgments for treating S. marcescens bacteremia and endocarditis, given the lack of good evidence-based guidelines. This brief study was designed to assess S. marcescens bacteremia/endocarditis treatment selection and outcomes in one of the largest collections of S. marcescens bacteremia/endocarditis from a single academic medical center.
Methods
This observational study included adult patients admitted to the University Hospital between 2017 and 2019 with S. marcescens bloodstream and/or endocarditis infections. Patients were excluded from the analysis, if they had a concomitant infection with another gram-negative organism, or had a previous S. marcescens infection within the previous 6 months. Our evaluation was designed to identify clinician-selected antibiotic therapy, compare clinical outcomes associated with different antibiotic regimens, evaluate how care differed in PWID patients vs. others, and identify factors associated with obtaining infectious diseases expert consultations. Phenotypic susceptibility patterns were also compared.
In addition to the initial positive bloodstream isolates, all subsequent isolates were assessed for clearance and/or recurrence. Clearance was defined as negative cultures after the start of antibiotic therapy (with no subsequent positive cultures during hospitalization). Recurrence was defined as a positive culture occurring after treatment initiation, negative cultures and hospital discharge. Susceptibility testing was performed primarily using standardized Vitek 2 automated susceptibility testing (bioMérieux, Inc. Durham, NC) with infrequent use of Kirby-Bauer disc diffusion testing for some agents. Patients' electronic medical records were followed for 90 days from the time of the first S. marcescens culture. Biostatistical analysis which included multi-logistic regression was performed using JMP Software, Version 14 (SAS Institute, Cary NC). This study was granted exempt status by the WVU Institutional Review Board due to its observational design.
Results
Two hundred-twenty unique patients had positive blood cultures for S. marcescens during the study assessment period. Of these, 43 patients met study inclusion/exclusion criteria and their characteristics are summarized in Table 1. Endocarditis was diagnosed in 13 (30.2%) of patients. Six patients had prosthetic heart valves in place prior to developing S. marcescens infections. Twenty-four (55.8%) of patients admitted to using intravenous drugs of abuse in their recent history, and 28 patients (65.1%) received infectious diseases specialist consults while hospitalized. When all characteristics were compared by multi-logistic regression, only PWID (p = 0.004), endocarditis (p = 0.0002), sepsis (p = 0.022), elevated hepatic enzymes (p = 0.030), and surgical intervention (p = 0.003), were independently associated with obtaining an Infectious Diseases Expert Consult.
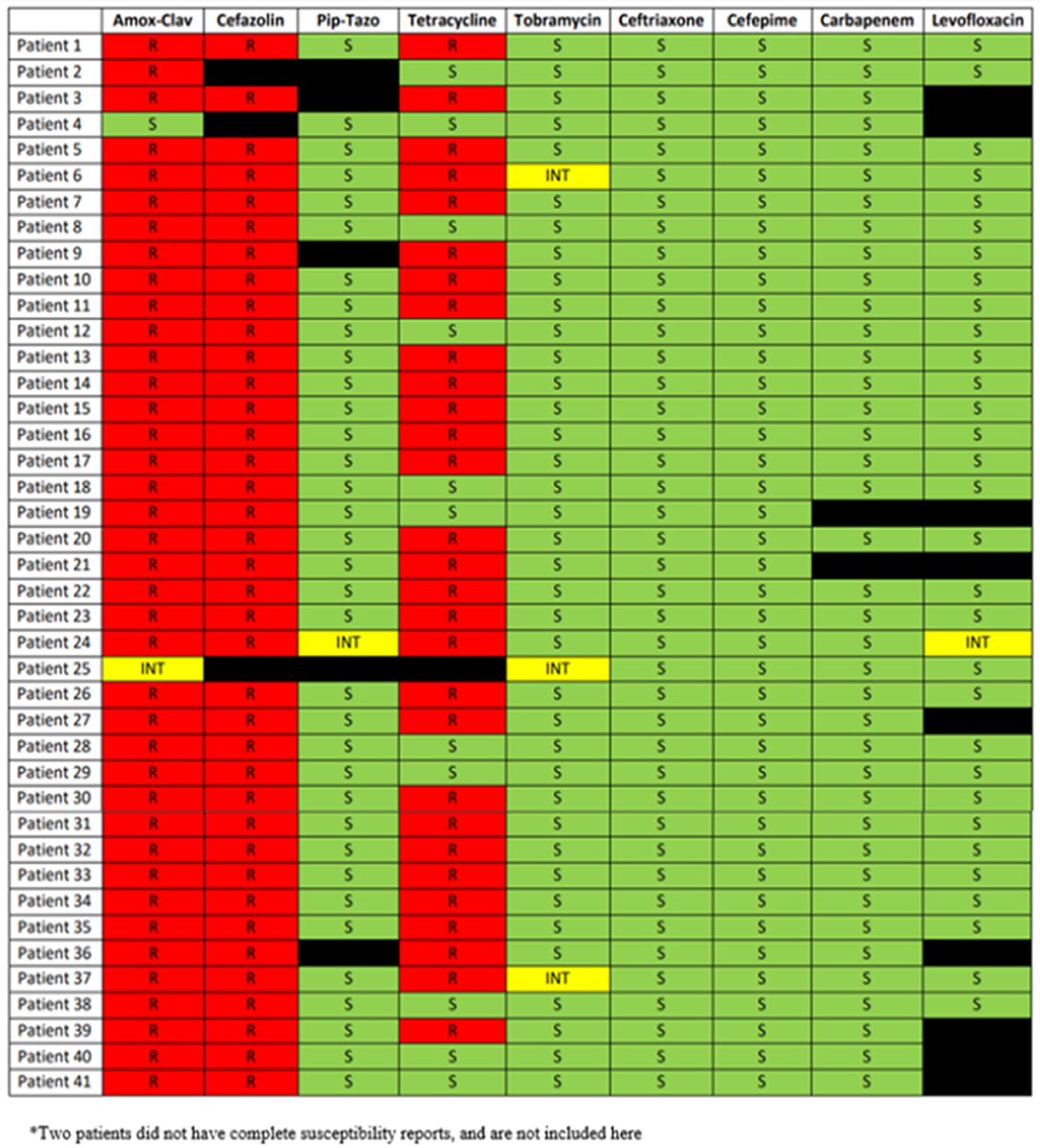
Full phenotypic antimicrobial susceptibility reports were available for 41 patients (see Figure 1). Automated phenotypic susceptibility testing did not identify either ESBL production or AmpC expression in any of the isolates. The few repeat positive cultures seen in this study did not display a difference in susceptibility from the baseline susceptibility report. The susceptibility patterns for the S. marcescens isolates were very consistent and displayed resistance to amoxicillin-clavulanate and cefazolin, while displaying susceptibility to tobramycin, ceftriaxone, cefepime, carbapenems, and levofloxacin. Tetracycline resistance was seen in 72.5% of the isolates.
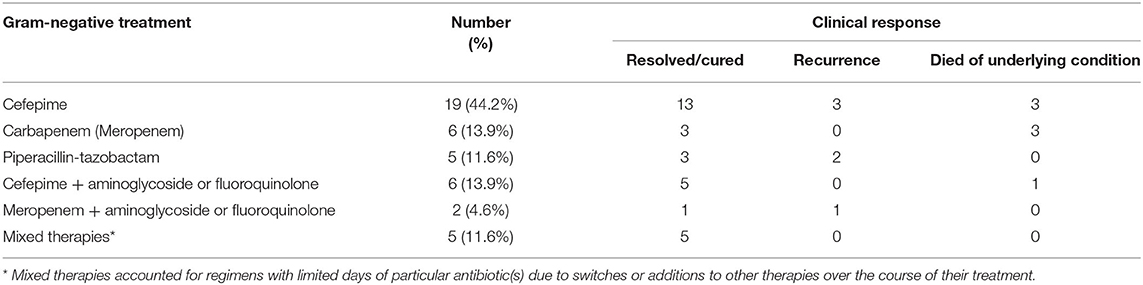
Table 2 highlights the treatment selection and clinical responses. The most common treatment regimen was cefepime (44.2%) followed by meropenem (13.9%) or cefepime + aminoglycoside or fluoroquinolone (13.9%). Piperacillin-tazobactam was used in 11.6% of patients. Some patients had “mixed” regimens that included limited days of particular antibiotic(s) with switches or additions to other therapies over the course of their treatment (11.6%).
Combination therapy was only recommended for endocarditis and with an Infectious Diseases Consult. When all characteristics were compared by multi-logistic regression, only PWID (p = 0.004), endocarditis (p = 0.0002), sepsis (p = 0.022), and surgical intervention (p = 0.003), were independently associated with obtaining an Infectious Diseases Consult. Most patients (90.7%) cleared their blood stream within 48 h of antibiotic start. Recurrence rates were generally low, with six cases seen overall. The patients with recurrences were treated with either a carbapenem or combination therapy. The patients with recurrences did receive therapies that were susceptible per microbiology lab report. Seven patients died within 60 days of their first positive S. marcescens culture, but the cause of death was attributed to underlying conditions in those cases.
Discussion
Our brief study provides some unique observations that can add to the thin patchwork of available information about S. marcescens bacteremia and endocarditis treatment. Our data comes from one of the largest single center S. marcescens bacteremia/endocarditis data sets. Evidence-based guidelines were not available at the time of this study to guide clinicians on the treatment of S. marcescens bacteremia or endocarditis. A brand new IDSA “guidance document” has just been released to provide clinicians with some guidance for the treatment of AmpC producing organisms (Tamma et al., 2021). That guidance document provides “suggested approaches” based on clinical experience, expert opinion, and a review of the available literature, rather than using evidence-based grading criteria like GRADE or the US Public Health criteria (Kavanagh, 2009). The document was only able to provide limited guidance on S. marcescens bacteremia and endocarditis treatment, due to the paucity of good clinical data in the medical literature.
The majority of antibiotic courses used at our hospital for S. marcescens bacteremia and endocarditis provided carbapenem-sparing treatment with a low rate of recurrence. Cefepime alone or in combination made up the majority of courses. Cefepime is often a preferred agent due to its stability against AmpC β-lactamase and low potential for AmpC induction (Tamma et al., 2021). Cefepime is generally well tolerated by most patients, but there are increasing concerns about the over use of this agent and its drug-associated neurotoxicity (Payne et al., 2017).
Piperacillin-tazobactam was the main therapy in five patients, despite the concern for any inducible AmpC expression. Piperacillin-tazobactam has been used with success in bacteremic infections with AmpC producing organisms (Harris et al., 2016; Tan et al., 2020). Use of piperacillin-tazobactam against S. marcescens is an option in the new IDSA guidance document, but the authors recommend caution in high inoculum infections like endocarditis or other serious infections (Tamma et al., 2021). Use of piperacillin-tazobactam as a carbapenem-avoidance strategy has been an area of active research in recent years. The MERINO-2 study group was not able to find a difference between piperacillin-tazobactam and meropenem among patients with bloodstream infections with AmpC producing organisms, unfortunately, there were only five Serratia infections in that study (Stewart et al., 2021). Similarly, a recent meta-analysis of bloodstream infections with AmpC-producing organism also failed to show a difference between piperacillin-tazobactam and carbapenems among studies with modest cases of S. marcescens infections (Cheng et al., 2017). In our study, we did see two recurrences in the five patients treated with piperacillin-tazobactam, but this subset is too small to denigrate piperacillin-tazobactam. Nonetheless, it does provide reason for additional study.
An interesting observation from our study was that combination therapy was only used for endocarditis treatment and with an Infectious Diseases Consult. This approach was deemed “reasonable” for non-HACEK gram-negative (not specific to S. marcescens) endocarditis in the most recent American Heart Association (AHA) endocarditis guidelines (Baddour et al., 2015). This may suggest that infectious diseases specialists are more familiar with the AHA guidelines than non-infectious diseases specialists. The level of evidence for that reasonable recommendation in the AHA guidelines was at the expert opinion level.
One of the encouraging observations from our study is that almost all patients experienced a quick bacteremia clearance rate (<48 h). This may reflect the oft-suggested low pathogenicity of S. marcescens, and could promote the use of shorter courses of intravenous antibiotics for this organism (Yu, 1979; Chotiprasitsakul et al., 2018; Yahav et al., 2019).
Conclusions
We could not identify an advantage of any particular antibiotic treatment regimen in this study. This may be due to the small sample size of infections with this relatively infrequent pathogen. Nonetheless, induction of AmpC β-lactamase or selection of ESBL organisms was not displayed by any of the organisms. Prospective randomized studies should be performed to better evaluate antimicrobial treatment options and duration for S. marcescens bacteremia and endocarditis.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The study involving human participants was reviewed and approved by West Virginia University Institutional Review Board. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
DS, CH, and CC contributed to the conception, study design, data analysis, and manuscript preparation and revision. DS and CH performed the data collection. DS worked with biostatistician to perform statistical analysis. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We wish to thank Dr. Gerald Hobbs, Ph.D. for his assistance with biostatistical analysis. An abstract of this study was presented at the 2020 ID Week Conference.
References
Baddour, L. M., Wilson, W. R., Bayer, A. S., Fowler, V. G. Jr, Tleyjeh, I. M., Rybak, M. J., et al. (2015). Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications a scientific statement for healthcare professionals from the American Heart Association. Circulation 132, 1435–1486. doi: 10.1161/CIR.0000000000000296
Cheng, L., Nelson, B. C., Mehta, M., Seval, N., Park, S., Giddins, M. J., et al. (2017). Piperacillin-tazobactam versus other antibacterial agents for the treatment of bloodstream infections due to AmpC β-lactamase-producing Enterobacteriaceae. Antimicrob. Agents Chemother. 61, e00276–e00217. doi: 10.1128/AAC.00276-17
Chotiprasitsakul, D., Han, J. H., Cosgrove, S. E., Harris, A. D., Lautenbach, E., Conley, A. T., et al. (2018). Comparing the outcomes of adults with Enterobacteriaceae bacteremia receiving short-course versus prolonged-course antibiotic therapy in a multicenter, propensity score-matched cohort. Clin. Infect. Dis. 66, 172–177. doi: 10.1093/cid/cix767
Harris, P. N., Wei, J. Y., Shen, A. W., Abdile, A. A., Paynter, S., Huxley, R. R., et al. (2016). Carbapenems versus alternative antibiotics for the treatment of bloodstream infections caused by Enterobacter, Citrobacter or Serratia species: a systematic review with a meta-analysis. J. Antimicrob. Chemother. 71, 296–306. doi: 10.1093/jac/dkv346
Kavanagh, B. P. (2009). The GRADE system for rating clinical guidelines. PLoS Med 6, e1000094. doi: 10.1371/journal.pmed.1000094
Mahlen, S. D. (2011). Serratia infections: from military experiments to current practice. Clin. Microbiol. Rev. 24, 755–791. doi: 10.1128/CMR.00017-11
Payne, L. E., Gagnon, D. J., Riker, R. R., Seder, D. B., Glisic, E. K., Morris, J. G., et al. (2017). Cefepime-induced neurotoxicity: a systematic review. Crit. Care 21, 276. doi: 10.1186/s13054-017-1856-1
Phadke, V. K., Jacob, J. T. (2016). Marvelous but morbid: infective endocarditis due to Serratia marcescens. Infect. Dis. Clin. Pract. 24, 143–150. doi: 10.1097/IPC.0000000000000360
Rosenblatt, J. E., Dahlgren, J. G., Fishbach, R. S., Tally, F. P. (1973). Gram-negative bacterial endocarditis in narcotic addicts. Calif. Med. 118, 1–4.
Stewart, A. G., Paterson, D. L., Young, B., Lye, D. C., Davis, J. S., Schneider, K., et al. (2021). Meropenem versus piperacillin-tazobactam for definitive treatment of bloodstream infections caused by AmpC β-lactamase-producing Enterobacter spp, Citrobacter freundii, Morganella morganii, Providencia spp, or Serratia marcescens: a pilot multicenter randomized controlled trial (MERINO-2). Open Forum Infect. Dis. 8, ofab387. doi: 10.1093/ofid/ofab387
Tamma, P. D., Aitken, S. L., Bonomo, R. A., Mathers, A. J., van Duin, D., Clancy, C. J. (2021). Infectious diseases society of America guidance on the treatment of AmpC β-lactamase-producing enterobacterales, carbapenem-resistant Acinetobacter baumannii, and Stenotrophomonas maltophilia infections. Clin. Infect. Dis. ciab1013. doi: 10.1093/cid/ciab1013
Tan, S. H., Ng, T. M., Chew, K. L., Yong, J., Wu, J. E., Yap, M. Y., et al. (2020). Outcomes of treating AmpC-producing Enterobacterales bacteraemia with carbapenems vs. non-carbapenems. Int. J. Antimicrob. Agents 55, 105860. doi: 10.1016/j.ijantimicag.2019.105860
Yahav, D., Franceschini, E., Koppel, F., Turjeman, A., Babich, T., Bitterman, R., et al. (2019). Seven versus 14 days of antibiotic therapy for uncomplicated gram-negative bacteremia: a non-inferiority randomized controlled trial. Clin. Infect. Dis. 69, 1091–1098. doi: 10.1093/cid/ciy1054
Keywords: antibacterials, Serratia marcescens, AmpC β-lactamase, bacteremia, endocarditis
Citation: Slain D, Howard C and Cooper CG (2022) An Antimicrobial Treatment Assessment of Serratia marcescens Bacteremia and Endocarditis. Front. Antibiot. 1:942721. doi: 10.3389/frabi.2022.942721
Received: 12 May 2022; Accepted: 08 June 2022;
Published: 29 June 2022.
Edited by:
Wilson Mandala, Malawi University of Science and Technology, MalawiReviewed by:
Chisomo Msefula, Kamuzu University of Health Sciences, MalawiKurt Neumann, Independent Researcher, Kerékteleki, Hungary
Copyright © 2022 Slain, Howard and Cooper. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Douglas Slain, ZHNsYWluQGhzYy53dnUuZWR1; orcid.org/0000-0002-4318-7165
 Douglas Slain
Douglas Slain Catessa Howard2
Catessa Howard2