
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Anim. Sci., 16 April 2025
Sec. Animal Nutrition
Volume 6 - 2025 | https://doi.org/10.3389/fanim.2025.1556967
 Mohamed S. El-Kholy1
Mohamed S. El-Kholy1 Samar S. Bassiony1
Samar S. Bassiony1 Adham A. Al-Sagheer2
Adham A. Al-Sagheer2 Mahmoud Alagawany1*
Mahmoud Alagawany1* Mohammed E. Ghonime3
Mohammed E. Ghonime3 Eman A. Elwakeel4
Eman A. Elwakeel4 Antonia Lestingi5*
Antonia Lestingi5* Ahmed A. Elolimy6
Ahmed A. Elolimy6 Mahmoud Madkour7
Mahmoud Madkour7 Mahmoud M. Azzam8
Mahmoud M. Azzam8 Shaaban S. Elnesr9
Shaaban S. Elnesr9Introduction: The current study was planned to evaluate the effect of using a combination of selenium (Se)-probiotic rabbit and colistin (COL) as a dietary supplement on growth, nutrient utilization, antioxidant and immune responses, blood metabolites, and cecal fermentation during summer conditions.
Methods: One hundred male New Zealand White rabbits aged 5 weeks with an initial body weight of (692.5 ± 5.19g) were randomly assigned to four groups (25 rabbits each) for 5–13 weeks of age experimental period. Four experimental groups in completely randomized design were used. (1) Control group: rabbits received a basal diet (BD) without any added supplements; (2) COL group: rabbits were given BD supplemented with 120 mg of COL/kg; (3) Se+EF group: rabbits consumed BD enriched with 0.3 mg of selenium plus 1 × 108 cfu of Enterococcus faecium/kg; and (4) Se+CB group: rabbits were fed BD fortified with 0.3 mg of selenium plus 2.5 × 106 cfu of Clostridium butyricum/kg.
Results: During overall period (5-13 weeks), body weight gain (BWG) and relative growth rate of rabbits consuming diets supplemented with COL, Se+EF, and Se+CB were greater to those of the control group. BWG (in groups Se+CB and Se+EF) and feed conversion ratio (FCR) (in groups COL and Se+EF) were improved compared with the control group. Malondialdehyde (MDA) values were declined in rabbits supplemented with Se plus probiotic (Se+CB or Se+EF) compared with the COL and control groups. Serum lipid profile in rabbits received selenium-probiotic combination (especially Se+EF) was improved. Furthermore, antioxidant levels were better in rabbits supplemented with Se plus probiotics compared to the control, and immune responses were significantly enhanced (P < 0.05).
Conclusion: Supplementing the diets of rabbits with a combination of selenium and probiotics (Se+CB or Se+EF) led to improvements in growth, antioxidant and immune responses, blood metrics, and cecal fermentation under heat stress conditions.
The production of rabbits has become essential in meeting the growing demand for flesh and guaranteeing global food security (Elsayed et al., 2024). The rabbit may also serve as a model species, since it has been utilized in studies investigating the impact of feeding on productive and physiological indicators (Alagawany et al., 2023). The heightened sensitivity of rabbits to heat stress (HS) is due to their thick fur and the scarcity of sweat glands, which hinder their ability to adequately remove excess heat (El-Ratel et al., 2025a). The world is currently experiencing extremely high temperatures, which have recently reached unprecedented levels. The biggest threat to the rabbit industry and main cause of stress source is HS (Liang et al., 2022). Thus, HS impairs rabbit meat quality, immune response, antioxidant properties; gut microbiome and performance, as well as gut morphology represented by duodenal lumen and villi which were negatively affected by HS (Bassiony et al., 2021; Dalle Zotte et al., 2025). Heat stress can affect production by disrupting the endocrine system, decreasing nutrient absorption, increasing energy demands for production maintenance, and increasing oxygen-derived free radical levels, which cause oxidative damage (Collier et al., 2005; Oladimeji et al., 2022; El-Ratel et al., 2025b). The adverse impacts of HS on rabbits can be mitigated through the implementation of feeding techniques (Alagawany et al., 2023). Various natural feed additives have been employed to alleviate the adverse effects of HS in rabbits in a manner that is practical, safe, and cost-effective (Hashem et al., 2013; Al-Sagheer et al., 2023). Finding effective substitutes for antibiotics in rabbit feed was an urgent necessity. The use of environmentally friendly functional feed additives in diets to improve health and growth is becoming more popular (Miranda et al., 2024; Placha et al., 2022). Elazab et al. (2022) stated that using natural feed additives (rosemary and ginger essential oils) as environmentally friendly supplementation improved physiological status, meat nutritive value, feed utilization, and growth performance of rabbits.
Probiotics, or living beneficial bacteria, have drawn a lot of attention lately as a secure substitute for antibiotics. Studies have indicated that supplementing feed with probiotics can enhance feed utilization, improve gut health, and promote growth rates (Bhatt et al., 2017; Phuoc and Jamikorn, 2017; Jameel and Kalef, 2023, 2024).
The realization of these benefits can be achieved through: (1) the enhancement of antioxidative capacity; (2) the improvement of nutrient digestion and absorption; (3) the strengthening of immune response; (4) the maintenance of gut health and functionality; and (5) the establishment of a favorable balance of gut microbes (Liu et al., 2019; Xia et al., 2024). Dietary probiotics inhibit the growth of harmful opportunistic infections and promote the establishment of beneficial bacteria, which are crucial for feed degradation, antimicrobial peptide production, vitamin synthesis, and volatile fatty acid (VFA) production (Mancini and Paci, 2021). Probiotic dietary supplements have been shown to mitigate the detrimental effects of HS in growing rabbits (Bassiony et al., 2021; Fathi et al., 2017). Dietary probiotic supplements may exhibit anti-inflammatory, immunostimulatory, and antioxidant effects, enhancing nutrients digestibility and performance (Ebeid et al., 2023; Saeed et al., 2023).
Enterococcus faecalis (EF) is a Gram-positive, facultative anaerobic bacterium and is classified among the bacterial species known to produce lactic acid (Domann et al., 2007). E. faecium has been shown to significantly enhance gut health, improve feed efficiency, and promote body weight gain (BWG) by inhibiting harmful gut pathogens and supporting beneficial bacterial growth (Pajarillo et al., 2015). Clostridium butyricum (CB) is a Gram-positive anaerobic bacterium known for its capacity to synthesize butyric acid in the digestive system of healthy animals (Wang et al., 2019). It also exhibits greater endurance to extreme temperatures, low pH, and bile salt. As a result, CB is now acknowledged as a safe and effective feed supplement (Kong et al., 2011; Bassiony et al., 2021). The ability of CB to synthesize butyric acid and other short-chain fatty acids (SCFAs), coupled with its spore-forming capability, establishes it as an effective dietary supplement for animals. This promotes the release of digestive enzymes and improves the efficiency of nutrient transporters (Duan et al., 2018; Obianwuna et al., 2023). The increases in immunological response, blood components, and growth performance were obviously caused by dietary supplementation with EF and/or CB (Bassiony et al., 2021).
Selenium (Se) is a naturally occurring trace mineral that may directly influence the functioning of animals owing to its antiviral, anti-inflammatory, and antioxidant characteristics (Al-Sagheer et al., 2023). Selenium is essential for growth, immunological response, and antioxidant status as it resides in the active site of glutathione peroxidase (Eid et al., 2019). Research has suggested that dietary Se supplementation may positively influence the performance and carcass traits of rabbits subjected to HS conditions (Marai et al., 2007). Ayyat et al. (2018) reported that supplementing rabbit diets with organic Se (0.03 ppm) enhanced growth rates and mitigated the adverse effects of HS on rabbit growth during the summer months. Moreover, it was noted that giving growing rabbits supplemental dietary Se enhanced growth and carcass weight, raised the amount of Se in meat, stabilized anti-oxidative status, and strengthened immune function (Verma et al., 2012; Ebeid et al., 2013). On the other hand, the positive impacts of selenium-enriched probiotics resulted from the modulation of antioxidant activities and cytokines release. The antioxidant effect of selenium-enriched probiotics was attributed to significantly enhanced activities of intracellular antioxidant enzymes, like glutathione peroxidase, thioredoxin reductase, and glutathione peroxidase reductase (Kieliszek et al., 2020).
In order to maintain animal productivity at peak levels, optimal combinations of various strategies must be employed alongside effective management and husbandry practices. Therefore, selenium-probiotic combination can be used as a feed supplement to improved growth of rabbits under summer conditions. It is hypothesized that the positive impacts of the prior feed additives could mitigate the detrimental effects of HS on rabbit performance. Thus, the objective of this research was to evaluate how dietary supplementation with colistin (COL) and a combination of probiotics and selenium would affect the growth rate, feed utilization, antioxidant and immunological indices, blood biochemistry, and cecal fermentation of rabbits subjected to thermal stress during the summer months.
The rabbits were housed in naturally ventilated rooms within galvanized wire cages measuring 40×35×60 cm. These cages were furnished with feeders and automated nipple drinkers, allowing for ad libitum access to water and feed. A one-week acclimatization period was provided before the commencement of the experiment, during which the rabbits were administered the basal diet (BD), serving as a control diet without additional supplements. The temperature-humidity index (THI) was calculated using data on relative humidity and air temperature gathered on the farm, in accordance with the equation outlined by Marai et al. (2001). The THI values were segmented into four categories: (1) <27.8 indicating an absence of HS; (2) 27.8 to < 28.9 representing a moderate level of HS; (3) 28.9 to 30.0 signifying a severe level of HS; and (4) >30.0 denoting an extremely severe level of HS. As shown in Table 1, rabbits were raised on BD that was formulated in accordance with Blas and Mateos (2010) recommendations. The AOAC (2006) methods were utilized to analyze the chemical composition of the BD. All groups’ rabbits were housed in identical environmental, managerial, and hygienic conditions for duration of eight weeks.
One hundred male New Zealand White rabbits, aged five weeks, were arbitrarily assigned to four distinct groups. Each group consisted of 25 replicate cages, housing one animal per cage. The experimental groups were: (1) Control group: animals received the BD without any additional supplement; (2) COL group: animals were fed BD fortified with 120 mg of colistin per kg feed according to Romero et al. (2012). (3) Se+EF group: rabbits were fed BD fortified with 0.3 mg of selenium plus 1 × 108 cfu of Enterococcus faecium per of feed; and (4) Se+CB group: Rabbits were fed BD fortified with 0.3 mg of selenium plus 2.5 × 106 cfu of Clostridium butyricum per kilogram of feed according to Bassiony et al. (2021) and Jiao et al. (2022).
Clostridium butyricum (Clostri-mixVR) was sourced from Cheil Bio Co., Ltd., located in Youngdungpo-Gu, Seoul, South Korea. Similarly, Enterococcus faecium, originally from Probiotics International Ltd., UK, was procured from the same company. The doses of both probiotics used in this study were determined based on previous research findings (Bassiony et al., 2021; Al-Sagheer et al., 2023).
Each rabbit was weighed separately at the start of the trial (5 weeks of age), and subsequently at 9 and 13 weeks of age, to determine BWG. The relative growth rate (RGR) was determined using the formula: . Where, W0 and W1 represent the initial and final body weights, respectively. The feed conversion ratio (FCR) was estimated by dividing total feed consumed (kg) by total weight gain (kg).
On the 91st day of the trial, 32 rabbits (eight from each group) were weighted and subsequently slaughtered for carcass analysis. Hot carcass weight and the weights of various organs, including lungs, heart, kidneys, spleen, and liver were documented. The dressing percentage was calculated as the ratio of hot carcass weight to the live rabbit weight before to slaughter, represented as a percentage. Organ weights were expressed relative to live body weight (g/kg).
On day 91 of the experiment, a total of 32 blood samples (eight rabbits per group) were obtained from the lateral ear vein. Two independent blood samples were obtained from each rabbit. The first sample, consisting of 2 ml, was collected into a tube containing an anticoagulant (EDTA). This sample was used to perform hematological analysis. The serum was separated from blood of the second sample after centrifugation for 20 min at 3000 rpm.
Hematological parameters were assessed using whole blood samples to measure white blood cell count (WBC), hemoglobin concentration (Hb), red blood cell count (RBC), mean corpuscular volume (MCV), mean corpuscular hemoglobin concentration (MCHC), mean corpuscular hemoglobin (MCH), and hematocrit,. The analyses were conducted in accordance with the methodology outlined by Jain (1986) using an automated cell counter (model XE-2100, Sysmex, Kobe, Japan). Serum samples were analyzed to assess lipid profiles, immune parameters, antioxidant status, and serum selenium levels. Lipid profiles included low-density lipoprotein (LDL), total cholesterol, high-density lipoprotein (HDL), total triglycerides, and very low-density lipoprotein (VLDL). Immunological indices such as immunoglobulin M (IgM) and immunoglobulin G (IgG), as well as antioxidant markers including catalase, malondialdehyde (MDA), and glutathione (GSH), were evaluated using Diamond Diagnostic Co. kits (Giza, Egypt) in accordance with the manufacturer’s instructions. Serum selenium was also quantified spectrophotometrically.
Following the slaughter of the rabbits, the cecal contents were promptly collected (n=8/group). The contents were extracted by applying gentle pressure and filtering through four layers of cheesecloth into a beaker. A 1 ml aliquot of the cecal content was mixed with 200 μL of 25% (w/v) meta-phosphoric acid in microcentrifuge tubes. The mixture was then centrifuged at a speed of 30,000×g (JA–17 rotor) for a duration of 20 minutes. The resulting supernatant was analyzed for VFA contents using gas chromatography (GC), following the methodology described by Palmquist and Conrad (1971).
Data (growth, nutrient utilization, antioxidant and immune responses, blood metabolites, and cecal fermentation) were subjected to statistical analysis using one-way analysis of variance (ANOVA), followed by Tukey’s post hoc test to evaluate mean differences, with significance established at P < 0.05. All statistical analyses were conducted using the statistical package SPSS (version 21, IBM Corporation, Armonk, NY, USA). Prior to ANOVA, the Shapiro-Wilk W test was used to confirm normality, and Levene’s test was employed to assess the homogeneity of variances. The statistical model was:
Yij = + Ti + eij, where Yij = observation, = general mean, Ti = Treatment effect, and eij = random error.
Throughout the trial, a mean ambient temperature of 32.87 ± 0.91°C and a humidity level of 58.64 ± 1.02% were documented inside the farm. The average computed THI of 30.50 ± 0.39 indicates that the rabbits were under high heat stress.
Table 2 illustrates the impact of selenium-probiotic supplementation and colistin on growth performance and feed efficiency in rabbits during summer conditions. The findings indicated that after 13 weeks of age, rabbits receiving diets enriched with Se plus Enterococcus faecium or Clostridium butyricum exhibited a considerably greater body weight (P = 0.001) compared to the control group. No statistically significant difference in body weight was observed at week 9 of age between the control group and the supplemented groups. Rabbits received Se plus EF showed the highest BWG during the period from 9 to 13 weeks of age, followed by groups received COL and Se plus CB, while the lowest were obtained in the control group. Throughout the overall period (5-13 weeks), BWG of rabbits fed diets enriched with COL, SE+EF, and Se+CB was greater than that of rabbits in the control group. The relative growth rate of rabbits remained unaltered by treatments during the 5-9 and 9-13 weeks of age; however, it was significantly greater (P = 0.003) in rabbits receiving COL, SE+EF, and Se+CB over the overall period (5-13 weeks of age) compared to control rabbits. None of the treatments affected feed intake of rabbits at all the studied ages. The values of FCR were comparable across groups during the periods of 5-9 weeks and 9-13 weeks of age. Rabbits receiving a diet supplemented with COL and Se+ EF exhibited elevated FCR values throughout the entire duration (5-13 weeks of age) when compared with the control group. The FCR values between the control group and the SE+CB group showed no significant difference.
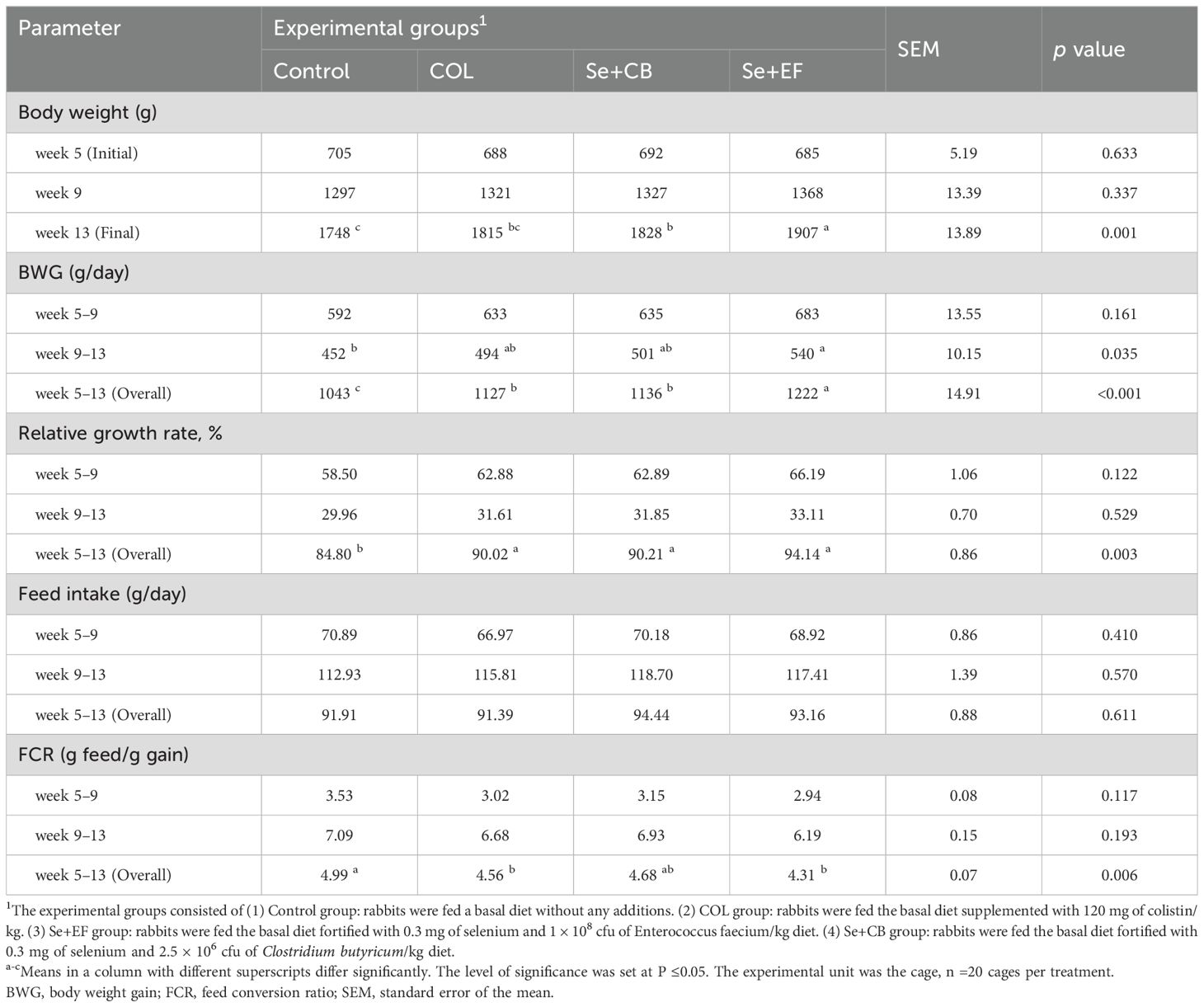
Table 2. Growth performance and feed utilization of growing rabbits as affected by dietary supplementation with selenium-probiotic combination and colistin under summer conditions.
Table 3 presents the carcass traits of NZW rabbits influenced by dietary supplementation with Se-probiotic (CB or EF) and COL during summer conditions. The results displayed that neither the relative weights of internal organs (liver, lungs, spleen, heart, and kidney) nor dressing percentages were significantly affected by supplemental COL, Se and probiotics used in the present study (P > 0.05).
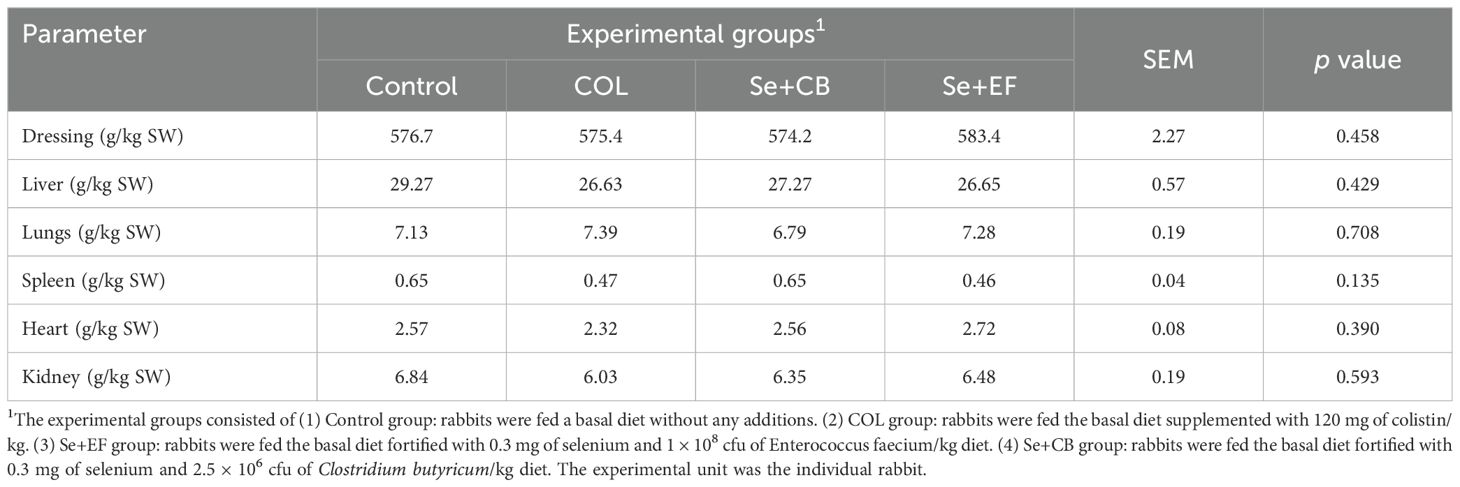
Table 3. Carcass traits of growing rabbits as affected by dietary supplementation with selenium-probiotic combination and colistin under summer conditions.
Table 4 presents the effects on blood hematology of growing rabbits under summer conditions of dietary supplementation with colistin and Se plus CB or EF. Non-significant increases were detected in hematological parameters including erythrogram (Hb, RBCs, MCV, MCH, MCHC, Hct and platelets) and leukogram (WBCs, lymphocyte, eosinophils, neutrophils and monocytes) pictures of blood in groups which received supplemental COL and Se probiotics (P > 0.05).
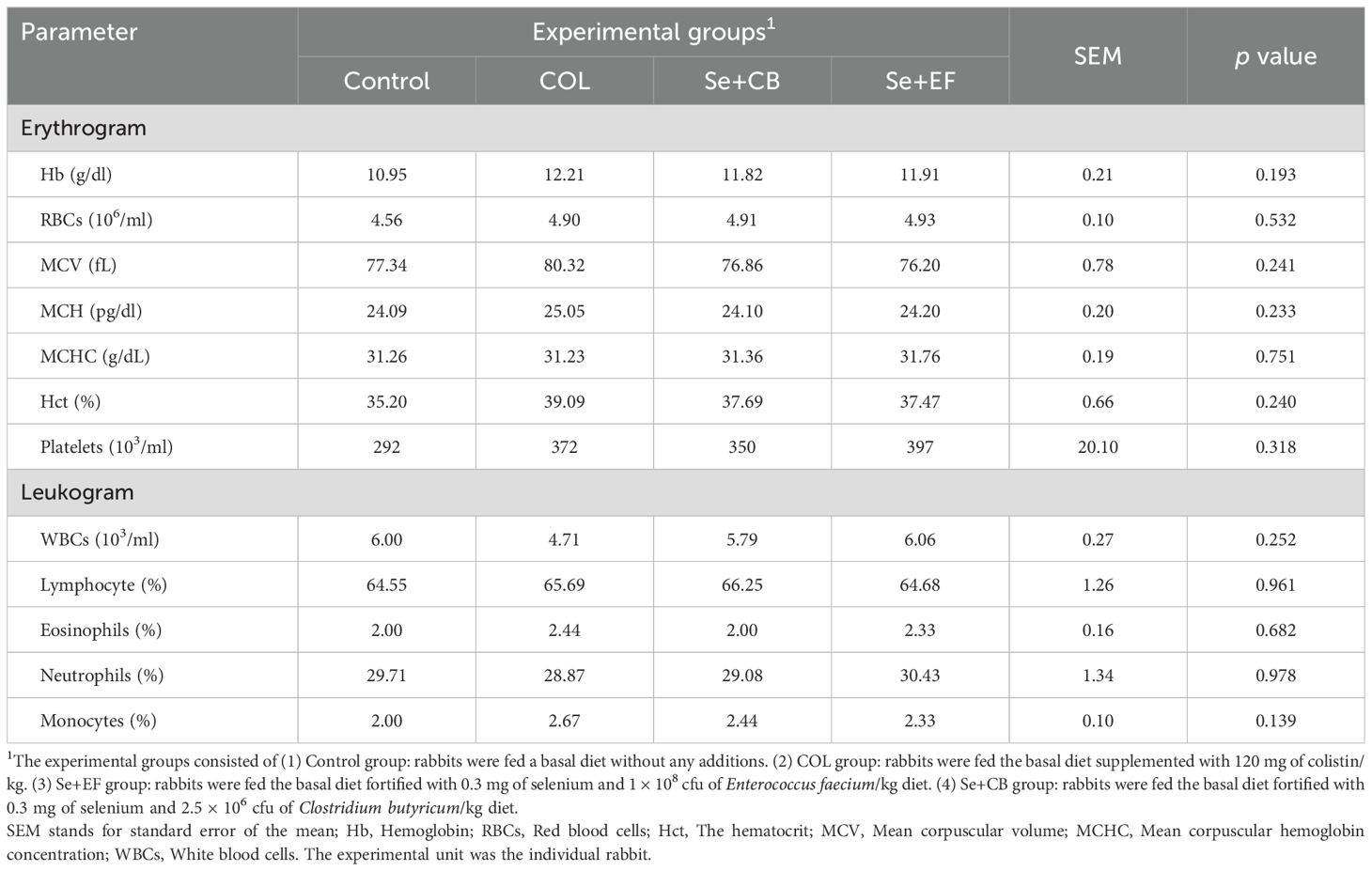
Table 4. Hematological parameters of growing rabbits as affected by dietary supplementation with selenium-probiotic combination and colistin under summer conditions.
Table 5 shows that dietary supplementation with Se-probiotic combination and colistin decreased the values (p= 0.030) of TC compared to the control group. Additionally, triglycerides and LDL values of the rabbit received diets containing Se plus EF significantly diminished compared to that of the control group (P=0.006 and P= 0.003, respectively). Moreover, values of VLDL were decreased with the treatment of COL and Se+CB (P=0.050). However, the findings exhibited that the influence of dietary treatments on HDL was not significant. Rabbit received the Se plus E. faecium and Se plus C. butyricum revealed the highest the blood Se content followed by the control and COL groups.
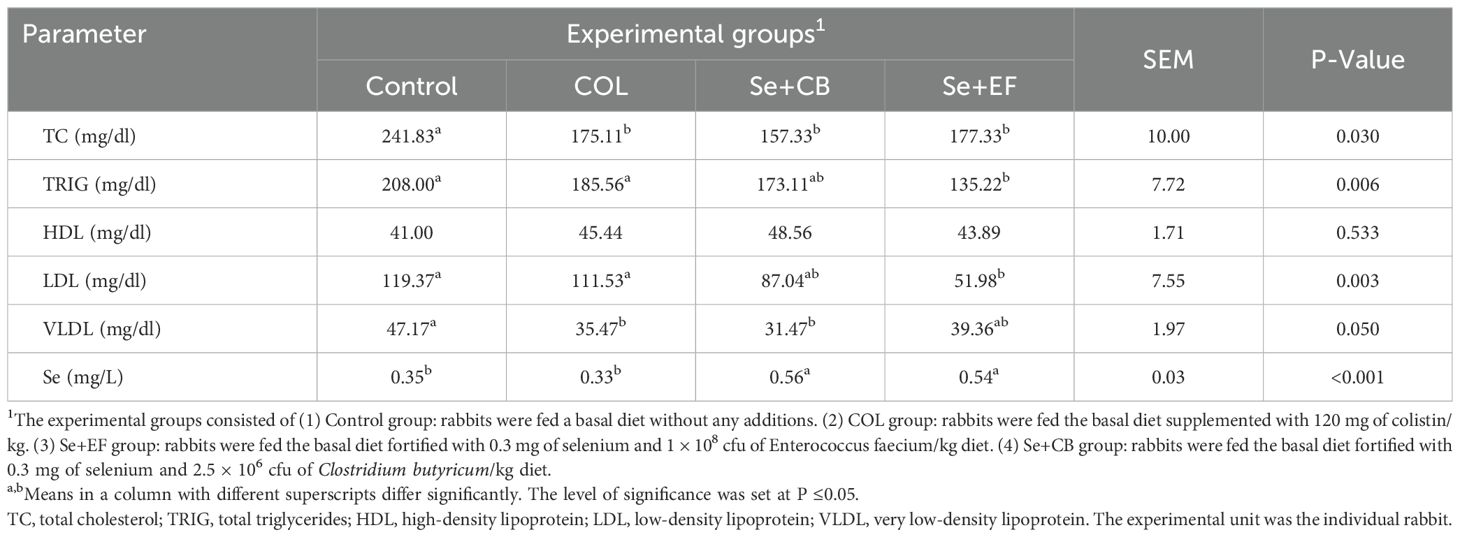
Table 5. Lipid profile and selenium content in growing rabbits serum as affected by dietary supplementation with selenium-probiotic combination and colistin under summer conditions.
Figure 1 displayed impacts of dietary supplementation on serum concentrations of catalase, GSH, and MDA. The results illuminated that rabbits fed on diets supplemented with Se plus probiotic (Se+CB or Se+EF) displayed significantly higher levels of catalase than the COL and control groups. Additionally, GSH activity was increased in rabbits received COL and Se-probiotics (Se+CB or Se+EF) compared to the control. However, MDA values were declined in rabbits supplemented with Se plus probiotic (Se+CB or Se+EF) compared with the COL and control groups.
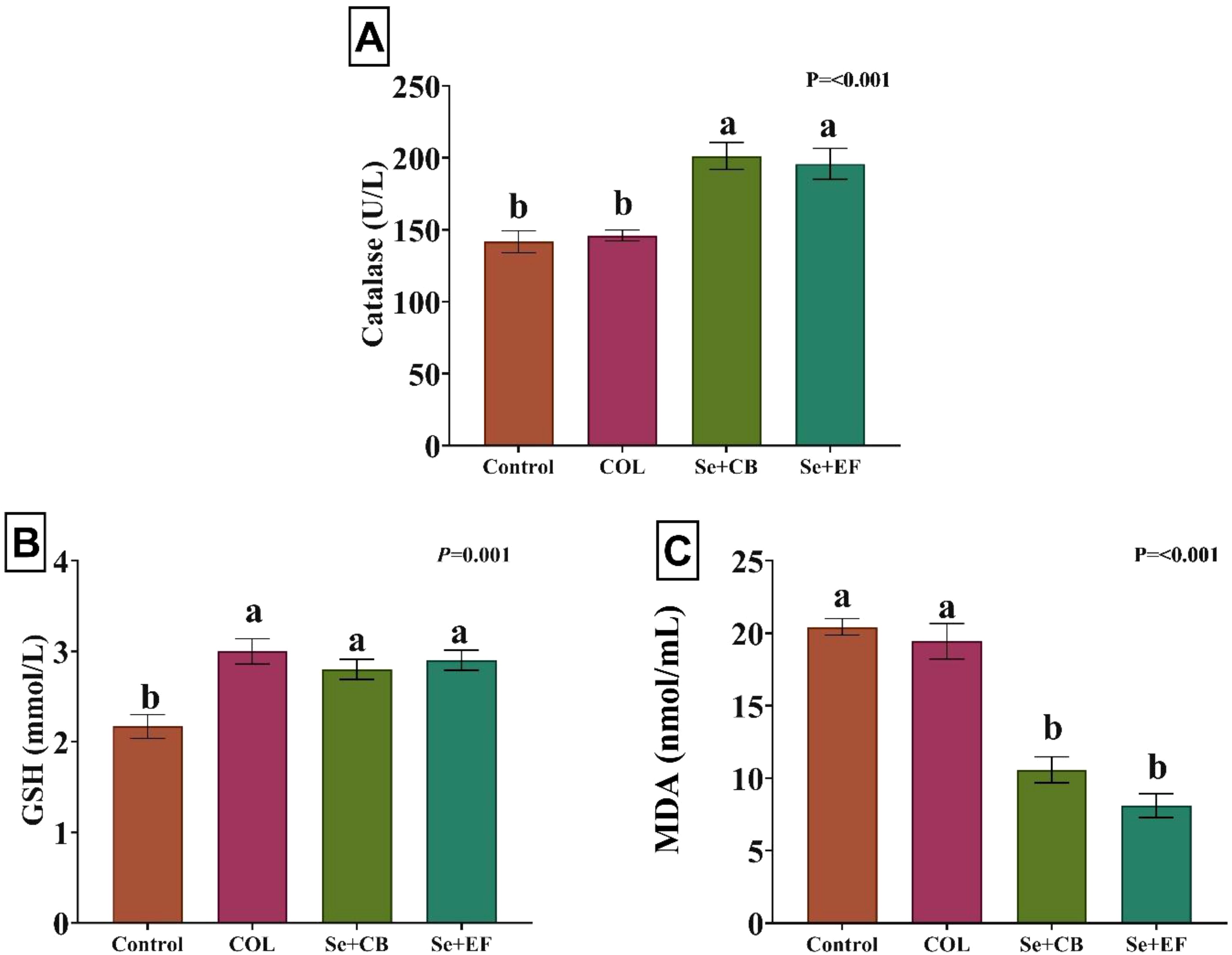
Figure 1. Effects of dietary supplementation on serum concentrations of catalase (A), reduced glutathione [GSH; (B)], and malondialdehyde [MDA; (C)]. Different letters above the error bar indicate significant differences at P < 0.05. Values are expressed as means ± standard error. The experimental groups consisted of (1) Control group: rabbits were fed a basal diet without any additions. (2) COL group: rabbits were fed the basal diet supplemented with 120 mg of colistin/kg. (3) Se+EF group: rabbits were fed the basal diet fortified with 0.3 mg of selenium and 1 × 108 cfu of Enterococcus faecium/kg diet. (4) Se+CB group: rabbits were fed the basal diet fortified with 0.3 mg of selenium and 2.5 × 106 cfu of Clostridium butyricum/kg diet.
Serum concentrations of immunoglobulins (IgG and IgM) are summarized in Figure 2. The inclusion of the Se plus EF in rabbit feed had the highest value (p=0.034), followed by group treated with Se plus CB compared with control group. A notable increase in IgM levels was detected in the rabbits of the Se-probiotic group relative to the COL and control groups (p=0.133).
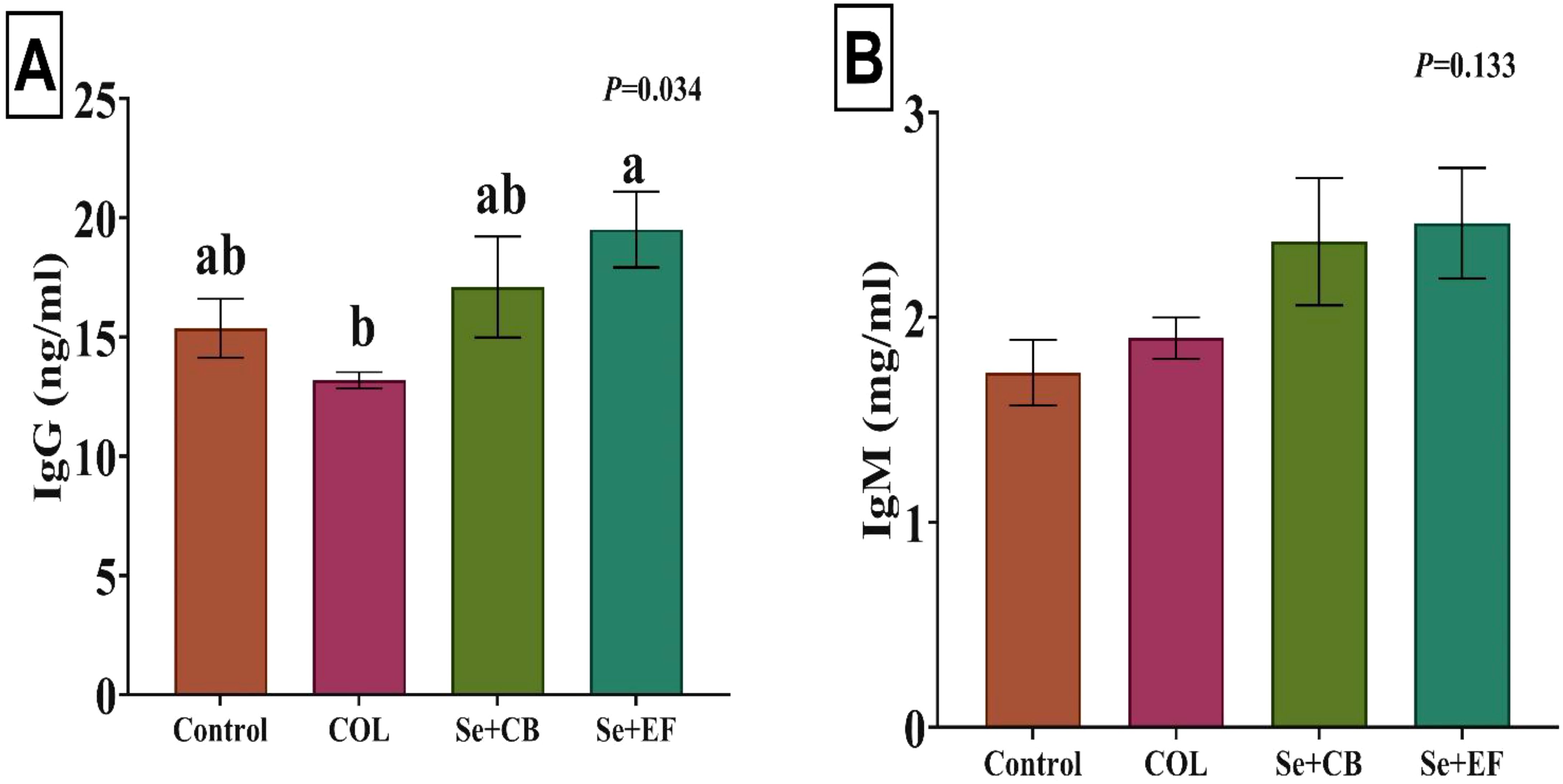
Figure 2. Effects of dietary supplementation on serum concentrations of immunoglobulin G [IgG; (A)] and immunoglobulin M [IgM; (B)]. Different letters above the error bar indicate significant differences at P < 0.05. Values are expressed as means ± standard error. The experimental groups consisted of (1) Control group: rabbits were fed a basal diet without any additions. (2) COL group: rabbits were fed the basal diet supplemented with 120 mg of colistin/kg. (3) Se+EF group: rabbits were fed the basal diet fortified with 0.3 mg of selenium and 1 × 108 cfu of Enterococcus faecium/kg diet. (4) Se+CB group: rabbits were fed the basal diet fortified with 0.3 mg of selenium and 2.5 × 106 cfu of Clostridium butyricum/kg diet.
The cecal fermentation parameters of growing rabbits, influenced by the dietary selenium-probiotic combination and colistin during summer conditions, are presented in Table 6. The results demonstrated that cecal VFA concentration (acetic, propionic, butyrate, valerate and total VFA; mmol/100 ml) and cecal VFA proportions (%) were statistically nonsignificant (P>0.05) for all groups. Influences of dietary supplementation on cecal concentrations of ammonia-N were presented in Figure 3. The finding showed that cecal ammonia concentration was significantly declined with the inclusion Se plus probiotic (Se+CB or Se+EF) and COL in rabbit diets compared with the control rabbits.
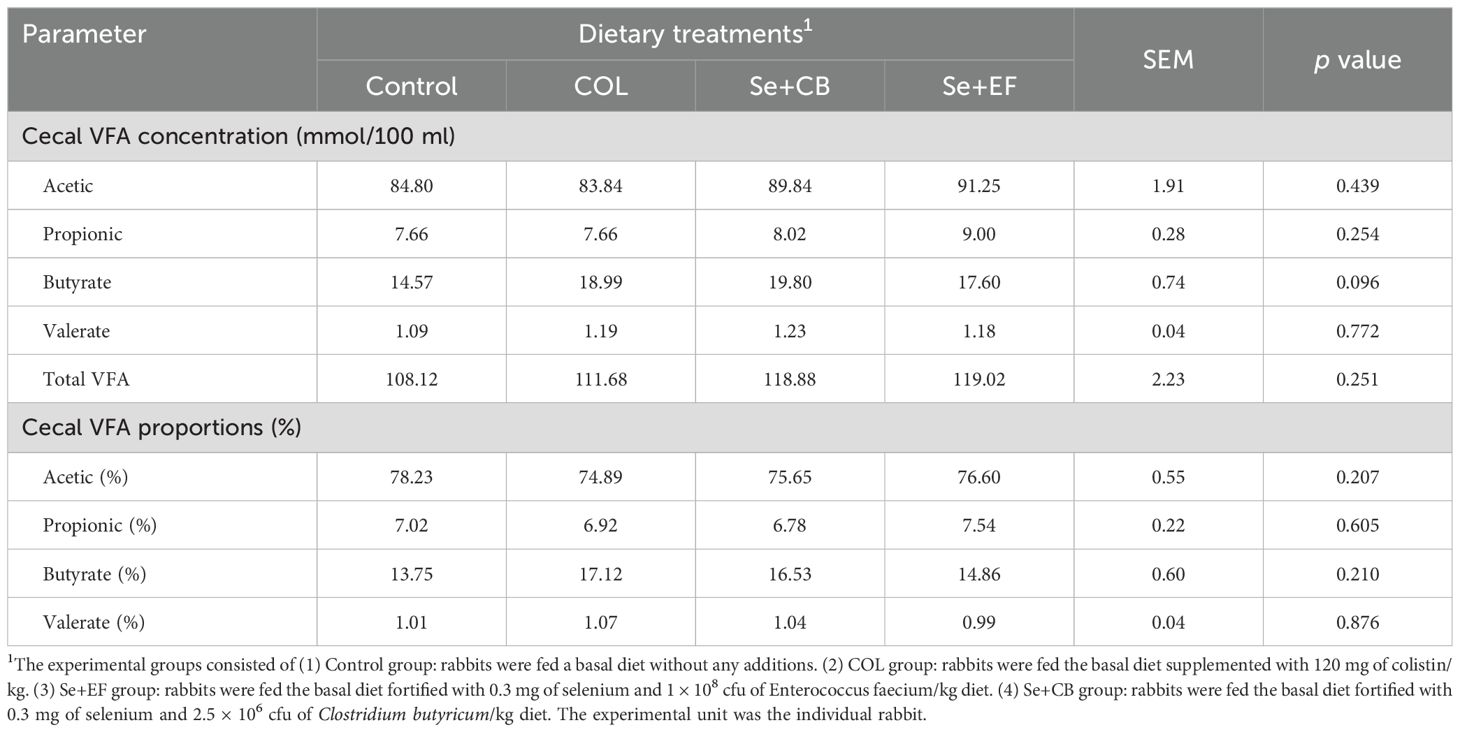
Table 6. Cecal fermentation parametersof growing rabbits as affected by dietary supplementation with selenium-probiotic combination and colistin under summer conditions.
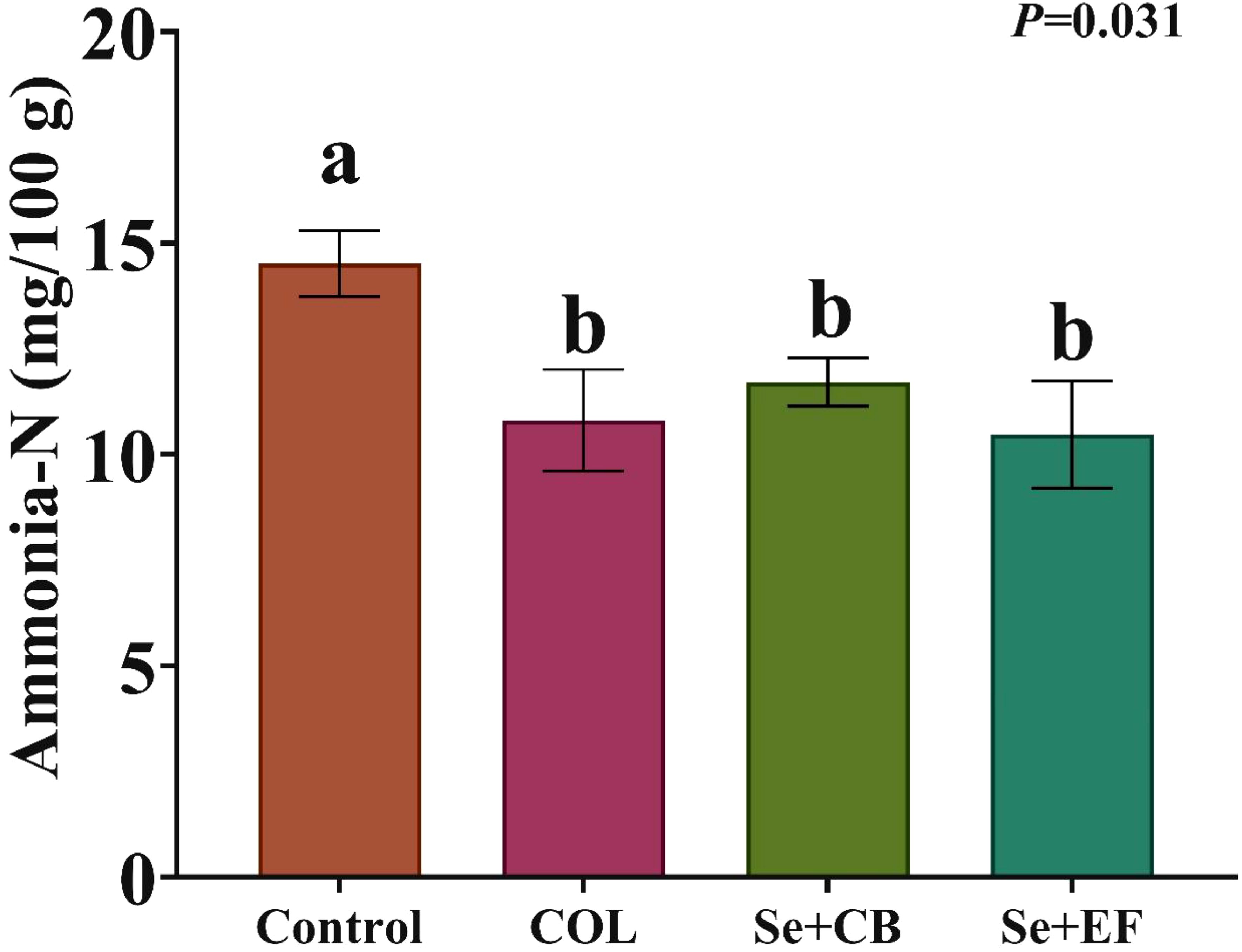
Figure 3. Effects of dietary supplementation on cecal concentrations of ammonia-N. Different letters above the error bar indicate significant differences at P < 0.05. Values are expressed as means ± standard error. The experimental groups consisted of (1) Control group: rabbits were fed a basal diet without any additions. (2) COL group: rabbits were fed the basal diet supplemented with 120 mg of colistin/kg. (3) Se+EF group: rabbits were fed the basal diet fortified with 0.3 mg of selenium and 1 × 108 cfu of Enterococcus faecium/kg diet. (4) Se+CB group: rabbits were fed the basal diet fortified with 0.3 mg of selenium and 2.5 × 106 cfu of Clostridium butyricum/kg diet.
Under hot summer temperatures, HS is a significant environmental stressor that results in substantial economic losses for the rabbit industry (El-Ratel et al., 2025a, b). Therefore, the addition of some feed additives in the diet is necessary as growth promoters and immune booster for improving rabbit tolerance against these stressful conditions (Alagawany et al., 2022; Reda et al., 2022; El-Ratel et al., 2025b). Good health and improved performance reflect the beneficial effect of feed additive (El-Wardany et al., 2016; Oladimeji et al., 2022; Ebeid et al., 2023; Mohamed et al., 2025). The findings of the current study demonstrated that the addition of a selenium-probiotic combination to rabbit diets during summer conditions significantly enhanced BW and BWG in comparison to the control group. The observed growth-promoting effect may be ascribed to the synergistic interaction between Se and probiotics when administered together. Research indicates that the inclusion of dietary Se can significantly reduce the negative impacts of HS on the growth performance of animals. It was noted that growing rabbits’ growth performance was enhanced by supplemental dietary Se (Ebeid et al., 2013). Some of the effects of Se were summarized as follows by Zheng et al. (2022): (I) Se can promote FCR by modulating the metabolism of carbohydrates, lipids, and proteins; (II) Se can augment the antioxidant defenses of animals, mitigating oxidative stress induced by heat and diminishing inflammatory responses, finally resulting in enhanced growth performance. Additionally, supplementation with probiotic Enterococcus faecium has been demonstrated to enhance broiler performance by improving FCR and increasing BWG, as reported by Samli et al. (2007). Hossain et al. (2015) found that broilers receiving diets supplemented with probiotic strains, including C. butyricum, exhibited a linear increase in BW and enhanced FCR compared to the control group. Ayyat et al. (2018) found that dietary Se and live yeast enhanced FCR in rabbits during summer conditions compared to the non-supplemented group. In the same context, values of FCR, net revenue and economic efficiency were improved with Nano-Se groups followed by organic Se (Se-probiotic) group compared to the control group (Moustafa et al., 2024).
Furthermore, Pogány Simonová et al. (2020) demonstrated that the inclusion of E. faecium in drinking water resulted in increased average daily body weight gain and final weight in rabbits compared to the control group. Bassiony et al. (2021) shown that the EF treatments enhanced FCR and BW in comparison to the COL treatment and control group. The supplementation of C. butyricum augmented BW of rabbits compared to control diet (Wang et al., 2023). Alagawany et al. (2023) indicated that providing the EF+CB complex to growing rabbits in hot summer conditions enhanced feed efficiency, as seen by FCR, and resulted in superior performance for BWG and final BW. Al-Sagheer et al. (2023) shown that the incorporation of selenium, inactivated yeast, and their combination in the diets of heat-stressed rabbits enhanced FCR over the period. The underlying mechanisms may involve several factors, including the production of intestinal enzymes, enhancement of the host immune system, increased resistance to colonization, reduction of toxin production, and lowering stress in rabbits (Abdel-Wareth et al., 2021; Elghandour et al., 2020). Overall, the productive performance improved due to this constructive action. The enhanced production of SCFAs in the caecum, especially butyric acid, which effectively improves feed utilization by stabilizing gut micro-ecological environments, may explain the observed performance improvements (Nakanishi et al., 2003). Furthermore, C. butyricum is purported to augment the activity of digestive enzymes, hence improving nutrient digestion and absorption (Fu et al., 2021). Finally, the enhancement in growth performance suggests a synergistic effect that facilitates a more efficient utilization of dietary Se and probiotics as feed additives.
Herein, the results indicate that the addition of a Se-probiotic combination did not affect carcass traits. Ayyat et al. (2018) found that rabbits kept in the summer days and supplemented with Se and live yeast did not affect carcass characteristics in comparison to the non-supplemented group. Bassiony et al. (2021) demonstrated that the relative organ weights and carcass yield percentages were not significantly influenced by all treatments. Al-Sagheer et al. (2023) reported that selenium supplementation did not influence carcass parameters. Consequently, the present findings confirm certain previous studies that indicated probiotics exerted no significant effect on rabbit organ and slaughter characteristics (Ayyat et al., 2018; Bhatt et al., 2017).
The current findings indicate that dietary supplements with colistin and Se, combined with either CB or EF, did not influence the blood variables in rabbits raised in summer environments. Al-Sagheer et al. (2023) shown that in heat-stressed growing rabbits, inactivated yeast and selenium did not significantly affect the levels of Hb, MCH, WBC, RBC, platelets, hematocrit%, and MCV. As well, Ayyat et al. (2018) found that under summertime conditions, Hb levels in growing rabbits were unaffected by dietary Se. This implies that the probiotics, at the supplemental doses administered, could maintain the regular hematopoietic function of rabbits. Conversely, Bassiony et al. (2021) reported that dietary addition with EF or EF+CB significantly increased the blood Hb content in growing rabbits raised in conditions of heat stress. The variances in results may stem from various factors, including the type of bioactive chemicals utilized, their origin, sample size, environmental stressors, and supplementation dosages.
In the current study, lipid profile in rabbits received selenium-probiotic combination (especially Se+EF) was improved. The improvement of lipid levels in rabbits fed diet containing Se-probiotic combination was due to that these supplements act as an effective antioxidant. The results presented here align with the findings of Amer et al. (2019), which shown that Se may reduce the blood concentrations of total cholesterol in growing rabbits. Guo et al. (2020) suggest that Se supplementation may exert an anti-cholesteremic effect by inhibiting adipose tissue degradation and reducing the diffusion of free fatty acids into the blood. Se-dependent antioxidant enzymes can aid in reducing hydrogen peroxide and lipid peroxide levels and may be beneficial in decreasing cholesterol levels (El-Deep et al., 2017). Wojcicki et al. (1991) found that decreasing the severity of atherosclerosis and lipid peroxidation was achieved by include Se in rabbits’ diet. According to Yazhini et al. (2018), giving broilers probiotic supplements positively changed their lipoprotein metabolism, resulting in a more marked decrease in LDL and TC and an increase in HDL. Bassiony et al. (2021) showed that adding EF and CB in rabbit diets raised in the summer significantly reduced serum triglycerides and extremely LDL while raising HDL. This finding suggests that these supplements may have a hypocholesterolemic effect. Probiotics can influence blood cholesterol levels by incorporating cholesterol into their cells, converting it into coprostanol in the gut for excretion, inhibiting the rate-limiting enzyme of cholesterogenesis, or hydrolyzing bile salts (Nour et al., 2021). The synergistic effect of probiotic and minerals including selenium mitigates the adverse effects of heat stress on the rabbits. The hypolipidemic effects of probiotic with Se in reducing TG and TC, and increasing HDL level in serum were reported in rabbits (El-Ratel et al., 2023a). Supplemental probiotic with Zinc and/or Selenium decreased total cholesterol and LDL levels in blood serum (Hassan et al., 2021). Such effect may be attributed to the fact that all supplements could decrease absorbed and synthesized cholesterol in the poultry gut (El-Ratel et al., 2023b).
For rabbits to be healthy, microbial fermentation must be stable (Pogány Simonová et al., 2020). Rabbits employ bacterial fermentation in their digestive process and rely on the total VFA obtained from the cecum as a consistent energy source (Abu Hafsa and Hassan, 2021). The present study investigates the potential for enhancing cecal fermentation and intestinal health in rabbits. Results indicate that the concentrations of cecal VFA including acetic, propionic, butyrate, valerate, and total VFA—as well as their proportions, exhibited non-significant improvements when a selenium-probiotic combination was incorporated into rabbit feed during heat stress conditions. Al-Sagheer et al. (2023) stated that cecal VFA was not influenced by the dietary Se. Besides, E. faecium CCM7420’s incorporation into rabbit feed resulted in an increase in fecal acetic acid levels in comparison to control rabbits. However, it did not affect the levels of other organic acids that were assessed, including butyric, succinic, and lactic acids (Simonová et al., 2008). Similarly to our findings, the molar proportion of VFA in the caecum was not influenced by the administration of additional probiotics (E. faecium CCM4231) to rabbits (Szabóová et al., 2011). Dietary C. butyricum supplementation augmented the levels of butyric acid, acetic acid, and total SCFAs in the intestinal digesta (Han et al., 2020). Furthermore, SCFAs benefit animals by providing intestinal mucosal epithelial cells with energy.
The capacity of natural feed additives to induce an overproduction of enzymes in the antioxidant defense system, which can neutralize reactive oxygen species (ROS) and reduce lipid peroxidation, may account for the improved antioxidant function (Mohammed et al., 2019). Wang et al. (2017b) elucidated that probiotics enhanced the activity of antioxidant enzymes such as SOD and GSH-Px, hence bolstering the system that protects against free radicals. Antioxidant enzymes help to degrade hydrogen peroxide and superoxide anions as well as inactivate ROS (Shen et al., 2014). Diets supplemented with C. butyricum augmented the concentration of GSH-Px, CAT, and SOD in gut of Rex rabbits and broiler chickens (Liao et al., 2015; Liu et al., 2019). Obianwuna et al. (2023) indicated that dietary probiotics supplementation significantly augmented GSH-Px, T-SOD, and CAT diminished MDA. Palkovicsné Pézsa (2023) summarized that probiotics have the potential to modify the redox status of the host in a number of ways, including: (1) decomposing ROS with their own antioxidant enzymes, (2) chelating metal ions, (3) controlling the host’s enzymes producing ROS, (6) controlling the host’s intestinal microbiota, (4) generating metabolites with antioxidant capacity, and (5) controlling cell signalling pathways. As for the role of selenium, it lowers lipid peroxidation and activates antioxidant enzymes, both of which are essential to the body’s antioxidant defense system (Ebeid et al., 2013). The improvement of antioxidant state was positively correlated with the Se role in GSH-Px activation. According to Al-Sagheer et al. (2023), Se and inactivated yeast together have the ability to lower MDA growth levels and raise blood CAT and GPx levels. Supplementation with a combination of probiotic and SeNPs for five weeks pre-mating reduced MDA of heat-stressed rabbit does (El-Ratel et al., 2023a). Selenoproteins (TRxR and GSH-Px), which are crucial for the scavenging of ROS and the cellular redox system, have been linked to the primary antioxidant activity of Se (Brigelius-Flohé et al., 2001; Pilarczyk et al., 2012).
Immunomodulatory feed additives are primarily intended to reduce inflammation and prevent immune function impairment. The enhanced immune status in the current study is a sign of the immunomodulatory effects of the Se-probiotic combination under summer conditions and the potential for supporting the rabbit immune system component. It has been documented that probiotic microbes alter the host immunological response to infection by stimulating the creation of immunoglobulin (Fukushima et al., 1998). Wang et al. (2017a) observed that dietary supplementation with probiotics elevated serum levels of IgG and IgM. Some findings presented higher IgG and IgM levels and boosted cell-mediated immunity after probiotic administration to rabbits (Fathi et al., 2017; Wang et al., 2017a). Liu et al. (2019) showed that giving weaned rabbits the Clostridium butyricum probiotic resulted in improved immunoglobulin production and preserved intestinal barrier function. According to Wang et al. (2019), EF and CB regulate the structure of the intestinal flora to promote the production of VAFs, which in turn enhances immune function, augmenting the serum IgA, IgM and IgG levels. Han et al. (2020) discovered that C. butyricum can increase immunological function, allowing animals to fight off harmful microorganisms. They also showed that C. butyricum supplementation led to higher serum levels of IgM, IgG, and IgA than the control group. Improved immune function may partially contribute to the enhancement of antioxidant status and the suppression of ROS formation (Aviello and Knaus, 2018). Bassiony et al. (2021) shown that the inclusion of EF and CB enhanced immunological indicators in rabbits subjected to HS environments. The group of rabbits that received probiotics exhibited higher levels of serum lysozyme activity and complement component 3 compared to the control group (Bassiony et al., 2021). The synthesis of selenoprotein, which facilitates the proliferation and differentiation of immune cells, is generally associated with the biological effects of selenium on livestock (Pisoschi and Pop, 2015). Increased immunoglobulin levels can be attributed to the essential biological role of selenium in augmenting T helper cell activity and stimulating cytokine release. Diet Selenium-enriched probiotics enhanced antioxidant function, increased immune responses (Yanez-Lemus et al., 2022), and alleviated inflammation-induced intestinal injury (Liu et al., 2022). Furthermore, selenium-enriched probiotics significantly regulated intestinal flora, and significantly reduced the number of pathogenic bacteria (Kheradmand et al., 2014). Liu et al. (2023) stated that nano selenium-enriched probiotics play an important role in the treatment of immune modulation and diseases.
The sustainability and health of animals depend on the enhancement of environmental conditions and the management of excreted metabolic products (Emam et al., 2023). Reduced ammonia production by rabbits improves their growth and general health and lowers the mortality rate. According to Alagawany et al. (2023), rabbits that received (EF + CB) had the lowest concentrations of ammonia. Additionally, probiotic supplementation was linked to a lower cecum ammonia concentration, which may indicate that the liver is using more ammonia to produce protein (Alagawany et al., 2023). Ammonia impairs the structural integrity of cecal tissues and reduces the levels of butyrate, acetate, and propionate in rabbit cecums (Cui et al., 2021). Huang et al. (2021) reported that feeding rabbits with C. butyricum improves their intestinal morphology, gut microbiota, and growth performance.
The dietary supplementation of selenium has synergetic influence with probiotic (Enterococcus faecium and Clostridium butyricum) and enhanced growth performance antioxidant and immunological indices, blood parameters and cecal fermentation in growing rabbits reared under summer conditions. These results suggest that selenium-probiotic combination is a novel additive that can be included in the diet of rabbits to improve health parameters. Therefore, a better understanding of the relationship between probiotics and selenium in feed could enable the targeted improvement of nutritional strategies that mitigate heat stress, which is detrimental to the rabbit industry.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
The experimental protocols applied in this study were approved by Ethics Committee of the Local Experimental Animals Care of Zagazig University with the assigned approval number ZU-IACU/2/F/1/2024. The authors confirm that they have followed EU standards for the protection of animals used for scientific purposes. The study was conducted in accordance with the local legislation and institutional requirements.
MSE: Writing – original draft, Writing – review & editing. SSB: Writing – original draft, Writing – review & editing. AAA: Writing – original draft, Writing – review & editing. MA: Conceptualization, Investigation, Project administration, Visualization, Writing – original draft, Writing – review & editing. MEG: Writing – original draft, Writing – review & editing. EAE: Writing – original draft, Writing – review & editing. AL: Writing – original draft, Writing – review & editing. AAE: Writing – original draft, Writing – review & editing. MM: Writing – original draft, Writing – review & editing. MMA: Writing – original draft, Writing – review & editing. SSE: Writing – original draft, Writing – review & editing.
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Researchers Supporting Project (RSPD2025R731), King Saud University (Riyadh, Saudi Arabia).
This work was supported by the Researchers Supporting Project(RSPD2025R731), King Saud University (Riyadh, Saudi Arabia).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abdel-Wareth A. A., Elkhateeb F. S., Ismail Z. S., Ghazalah A. A., Lohakare J. (2021). Combined effects of fenugreek seeds and probiotics on growth performance, nutrient digestibility, carcass criteria, and serum hormones in growing rabbits. Livest. Sci. 251, 104616. doi: 10.1016/j.livsci.2021.104616
Abu Hafsa S. H. A., Hassan A. A. (2021). Partial berseem hay replacement by Panicum maximum grass on caecal fermentation activity, performance, and carcass characteristics of growing rabbits. Livest. Sci. 250, 104593. doi: 10.1016/j.livsci.2021.104593
Alagawany M., Bassiony S. S., El-Kholy M. S., El-Naggar K., El-Metwally A. E., Al-Sagheer A. A. (2023). Comparison of the effects of probiotic-based formulations on growth, feed utilization, blood constituents, cecal fermentation, and duodenal morphology of rabbits reared under hot environmental conditions. Ann. Anim. Sci. 23, 777–787. doi: 10.2478/aoas-2023-0004
Alagawany M., El-Saadony M. T., El-Rayes T. K., Madkour M., Loschi A. R., Di Cerbo A., et al. (2022). Evaluation of dried tomato pomace as a non-conventional feed: Its effect on growth, nutrients digestibility, digestive enzyme, blood chemistry and intestinal microbiota of growing quails. Food Energ. Sec. 11, e373. doi: 10.1002/fes3.373
Al-Sagheer A. A., Alagawany M., Bassiony S. S., Shehata A. M., El-Metwally A. E., El-Kholy M. S. (2023). Inactivated Saccharomyces cerevisiae and selenium as alternatives to antibiotic in rabbits reared under summer conditions: Effects on growth, nutrient utilization, cecal fermentation, blood components, and intestinal architecture. Anim. Feed Sci. Technol. 302, 115688. doi: 10.1016/j.anifeedsci.2023.115688
Amer S. A., Omar A. E., El-Hack A., Mohamed E. (2019). Effects of selenium-and chromium-enriched diets on growth performance, lipid profile, and mineral concentration in different tissues of growing rabbits. Biol. Trace Elem. Res. 187, 92–99. doi: 10.1007/s12011-018-1356-4
AOAC (2006). Official Methods of Analysis. 18th ed. (Washington, DC, USA: Association of Official Analytical Chemists International).
Aviello G., Knaus U. G. (2018). NADPH oxidases and ROS signaling in the gastrointestinal tract. Mucosal Immunol. 11, 1011–1023. doi: 10.1038/s41385-018-0021-8
Ayyat M. S., Al-Sagheer A. A., El-Latif A., Khaled M., Khalil B. A. (2018). Organic selenium, probiotics, and prebiotics effects on growth, blood biochemistry, and carcass traits of growing rabbits during summer and winter seasons. Biol. Trace Elem. Res. 186, 162–173. doi: 10.1007/s12011-018-1293-2
Bassiony S. S., Al-Sagheer A. A., El-Kholy M. S., Elwakeel E. A., Helal A. A., Alagawany M. (2021). Evaluation of Enterococcus faecium NCIMB 11181 and Clostridium butyricum probiotic supplements in post-weaning rabbits reared under thermal stress conditions. Ital. J. Anim. Sci. 20, 1232–1243. doi: 10.1080/1828051X.2021.1941334
Bhatt R., Agrawal A., Sahoo A. (2017). Effect of probiotic supplementation on growth performance, nutrient utilization and carcass characteristics of growing Chinchilla rabbits. J. Appl. Anim. Res. 45, 304–309. doi: 10.1080/09712119.2016.1174126
Blas C. D., Mateos G. G. (2010). Feed formulation. In Nutrition of the rabbit. (Wallingford UK: CABI), pp. 222–232. doi: 10.1079/9781845936693.0222
Brigelius-Flohé R., Maiorino M., Ursini F., Flohé L. (2001). Selenium: an antioxidant?. In Handbook of antioxidants. (CRC Press), pp. 652–683. doi: 10.1201/9780203904046
Collier R. J., Baumgard L. H., Lock A. L., Bauman D. E. (2005). “Physiological limitations, nutrient partitioning,” in Yield of farmed species Constraints and opportunities in the 21st Century. Eds. Sylvester-Bradley R., Wiseman J., 351–377. Nottingham University Press.
Cui J., Yang X., Wang F., Liu S., Han S., Chen B. (2021). Effects of ammonia on growth performance, lipid metabolism and cecal microbial community of rabbits. PloS One 16, e0252065. doi: 10.1371/journal.pone.0252065
Dalle Zotte A., Pontalti E., Cullere M., Gerencsér Z. S., Matics Z. S., Szendrő Z. S. (2025). Effect of heat stress on meat quality of growing rabbits divergently selected for body fat content. Ital. J. Anim. Sci. 24, 13–24. doi: 10.1080/1828051X.2024.2438840
Domann E., Hain T., Ghai R., Billion A., Kuenne C., Zimmermann K., et al. (2007). Comparative genomic analysis for the presence of potential enterococcal virulence factors in the probiotic Enterococcus faecalis strain Symbioflor 1. Int. J. Med. Microbiol. 297, 533–539. doi: 10.1016/j.ijmm.2007.02.008
Duan Y., Wang Y., Dong H., Ding X., Liu Q., Li H., et al. (2018). Changes in the intestine microbial, digestive, and immune-related genes of Litopenaeus vannamei in response to dietary probiotic Clostridium butyricum supplementation. Front. Microbiol. 9, 2191. doi: 10.3389/fmicb.2018.02191
Ebeid T. A., Aljabeili H. S., Al-Homidan I. H., Volek Z., Barakat H. (2023). Ramifications of heat stress on rabbit production and role of nutraceuticals in alleviating its negative impacts: an updated review. Antioxidants 12, 1407. doi: 10.3390/antiox12071407
Ebeid T., Zeweil H., Basyony M., Dosoky W., Badry H. (2013). Fortification of rabbit diets with vitamin E or selenium affects growth performance, lipid peroxidation, oxidative status and immune response in growing rabbits. Livest. Sci. 155, 323–331. doi: 10.1016/j.livsci.2013.05.011
Eid S. Y., El-Zaher H. M., Emara S. S., Farid O. A.-H., Michael M. I. (2019). Nano selenium treatment effects on thyroid hormones, immunity and antioxidant status in rabbits. World Rabbit Sci. 27, 93–100. doi: 10.4995/wrs.2019.11251
Elazab M. A., Khalifah A. M., Elokil A. A., Elkomy A. E., Rabie M. M., Mansour A. T., et al. (2022). Effect of dietary rosemary and ginger essential oils on the growth performance, feed utilization, meat nutritive value, blood biochemicals, and redox status of growing NZW rabbits. Animals 12, 375. doi: 10.3390/ani12030375
El-Deep M., Shabaan M., Assar M., Attia K. M., Sayed M. (2017). Comparative effects of different dietary selenium sources on productive performance, antioxidative properties and immunity in local laying hens exposed to high ambient temperature. J. Anim. Poult. Prod. 8, 335–343. doi: 10.21608/jappmu.2017.45998
Elghandour M., Tan Z., Abu Hafsa S., Adegbeye M., Greiner R., Ugbogu E., et al. (2020). Saccharomyces cerevisiae as a probiotic feed additive to non and pseudo-ruminant feeding: a review. J. Appl. Microbiol. 128, 658–674. doi: 10.1111/jam.14416
El-Ratel I. T., Elbasuny M. E., El-Nagar H. A., Abdel-Khalek A. E., El-Raghi A. A., El Basuini M. F., et al. (2023b). The synergistic impact of Spirulina and selenium nanoparticles mitigates the adverse effects of heat stress on the physiology of rabbits bucks. PloS One 18, e0287644. doi: 10.1371/journal.pone.0287644
El-Ratel I. T., El-Kholy K. H., Elgmmal S. M., Fouda S. F., Abdel-Khalek A. E., Hassan M. A., et al. (2025a). The synergetic effect of selenium or zinc oxide nanoparticles with chromium on mitigating thermal stress for sustainable production and improving antioxidant capacity and inflammatory cytokines of growing rabbits. Arch. Anim. Breed. 68, 43–55. doi: 10.5194/aab-68-43-2025
El-Ratel I. T., El-Kholy K. H., Mousa N. A., El-Said E. A. (2023a). Impacts of selenium nanoparticles and spirulina alga to alleviate the deleterious effects of heat stress on reproductive efficiency, oxidative capacity and immunity of doe rabbits. Anim. Biotechnol. 34, 3519–3532. doi: 10.1080/10495398.2023.2168198
El-Ratel I. T., Mekawy A., Hassab S. H., Abdelnour S. (2025b). Enhancing growing rabbit heat stress resilience through dietary supplementation with natural antioxidants. BMC Vet. Res. 21, 28. doi: 10.1186/s12917-024-04466-1
Elsayed N., Mandour A. E. E., Amin M. K. A., Reda F. M., Taha H. S. A., Di Cerbo A., et al. (2024). Evaluation of genetic diversity within different rabbit (Oryctolagus cuniculus) genotypes utilizing start codon targeted (SCoT) and inter-simple sequence repeat (ISSR) molecular markers. Arch. Anim. Breed. 67, 285–295. doi: 10.5194/aab-67-285-2024
El-Wardany I., Shourrap M. I., Madkour M., Abd El-Azeem N. A. (2016). Effect of Age at mating and silver nanoparticles administration on progeny productive performance and some blood constituents in Japanese quail. Int. J. Chem.Tech. Res. 9, 21–34.
Emam A. M., Elnesr S. S., El-Full E. A., Mahmoud B. Y., Elwan H. (2023). Influence of improved microclimate conditions on growth and physiological performance of two Japanese quail lines. Animals 13 (6), 1118. doi: 10.3390/ani13061118
Fathi M., Abdelsalam M., Al-Homidan I., Ebeid T., El-Zarei M., Abou-Emera O. (2017). Effect of probiotic supplementation and genotype on growth performance, carcass traits, hematological parameters and immunity of growing rabbits under hot environmental conditions. Anim. Sci. J. 88, 1644–1650. doi: 10.1111/asj.2017.88.issue-10
Fu J., Wang T., Xiao X., Cheng Y., Wang F., Jin M., et al. (2021). Clostridium butyricum ZJU-F1 benefits the intestinal barrier function and immune response associated with its modulation of gut microbiota in weaned piglets. Cells 10, 527. doi: 10.3390/cells10030527
Fukushima Y., Kawata Y., Hara H., Terada A., Mitsuoka T. (1998). Effect of a probiotic formula on intestinal immunoglobulin A production in healthy children. Int. J. Food Microbiol. 42, 39–44. doi: 10.1016/S0168-1605(98)00056-7
Guo L., Xiao J., Liu H., Liu H. (2020). Selenium nanoparticles alleviate hyperlipidemia and vascular injury in ApoE-deficient mice by regulating cholesterol metabolism and reducing oxidative stress. Metallomics 12, 204–217. doi: 10.1039/c9mt00215d
Han Y., Tang C., Li Y., Yu Y., Zhan T., Zhao Q., et al. (2020). Effects of dietary supplementation with Clostridium butyricum on growth performance, serum immunity, intestinal morphology, and microbiota as an antibiotic alternative in weaned piglets. Animals 10, 2287. doi: 10.3390/ani10122287
Hashem N., Abd El-Hady A., Hassan O. (2013). Effect of vitamin E or propolis supplementation on semen quality, oxidative status and hemato-biochemical changes of rabbit bucks during hot season. Livest. Sci. 157, 520–526. doi: 10.1016/j.livsci.2013.09.003
Hassan F., Mobarez S., Mohamed M., Attia Y., Mekawy A., Mahrose K. (2021). Zinc and/or selenium enriched spirulina as antioxidants in growing rabbit diets to alleviate the deleterious impacts of heat stress during summer season. Animals 11, 756. doi: 10.3390/ani11030756
Hossain M., Begum M., Kim I. (2015). Effect of Bacillus subtilis, Clostridium butyricum and Lactobacillus acidophilus endospores on growth performance, nutrient digestibility, meat quality, relative organ weight, microbial shedding and excreta noxious gas emission in broilers. Vet. Med.- Czech 60, 77–86. doi: 10.17221/7981-VETMED
Huang P., Cui X., Wang Z., Xiao C., Ji Q., Wei Q., et al. (2021). Effects of clostridium butyricum and a bacteriophage cocktail on growth performance, serum biochemistry, digestive enzyme activities, intestinal morphology, immune responses, and the intestinal microbiota in rabbits. Antibiotics 10, 1347. doi: 10.3390/antibiotics10111347
Jameel M. S., Kalef D. A. (2023). Investigations on the role of commercial probiotics on New Zealand white rabbits experimentally infected with eimeria stiedae. Comp. Parasitol. 90, 27–33. doi: 10.1654/COPA-D-22-00019
Jameel M. S., Kalef D. A. (2024). Probiotics lessens pathological changes in rabbits infected with hepatic coccidiosis. One Health Bull. 4, 124–132. doi: 10.4103/ohbl.ohbl_16_24
Jiao P., Wang Z., Wang X., Zuo Y., Yang Y., Hu G., et al. (2022). Effect of Clostridium butyricum supplementation on in vitro rumen fermentation and microbiota with high grain substrate varying with media pH levels. Front. Microbiol. 13, 912042. doi: 10.3389/fmicb.2022.912042
Kheradmand E., Rafii F., Yazdi M. H., Sepahi A. A., Shahverdi A. R., Oveisi M. R. (2014). The antimicrobial effects of selenium nanoparticle-enriched probiotics and their fermented broth against candida albicans. Daru 22, 48. doi: 10.1186/2008-2231-22-48
Kieliszek M., Bierla K., Jimenez-Lamana J., Kot A. M., Alcantara-Duran J., Piwowarek K., et al. (2020). Metabolic response of the yeastcandida utilisduring enrichment in selenium. Int. J. Mol. Sci. 21, 5287. doi: 10.3390/ijms21155287
Kong Q., He G.-Q., Jia J.-L., Zhu Q.-L., Ruan H. (2011). Oral administration of Clostridium butyricum for modulating gastrointestinal microflora in mice. Curr. Microbiol. 62, 512–517. doi: 10.1007/s00284-010-9737-8
Liang Z.-L., Chen F., Park S., Balasubramanian B., Liu W.-C. (2022). Impacts of heat stress on rabbit immune function, endocrine, blood biochemical changes, antioxidant capacity and production performance, and the potential mitigation strategies of nutritional intervention. Front. Vet. Sci. 9, 906084. doi: 10.3389/fvets.2022.906084
Liao X. D., Ma G., Cai J., Fu Y., Yan X. Y., Wei X. B., et al (2015). Effects ofClostridium butyricum on growth performance, antioxidation, and immune function of broilers. Poult. Sci. 94 (4), 662–667. doi: 10.3382/ps/pev038
Liu J., Shi L., Tuo X., Ma X., Hou X., Jiang S., et al. (2022). Preparation, characteristic and anti-inflammatory effect of selenium nanoparticle-enriched probiotic strain enterococcus durans A8-1. J. Trace Elem. Med. Biol. 74, 127056. doi: 10.1016/j.jtemb.2022.127056
Liu R., Sun W., Sun T., Zhang W., Nan Y., Zhang Z., et al. (2023). Nano selenium-enriched probiotic Lactobacillus enhances alum adjuvanticity and promotes antigen-specific systemic and mucosal immunity. Front. Immunol. 14, 1116223. doi: 10.3389/fimmu.2023.1116223
Liu L., Zeng D., Yang M., Wen B., Lai J., Zhou Y., et al. (2019). Probiotic Clostridium butyricum improves the growth performance, immune function, and gut microbiota of weaning rex rabbits. Probiotics Antimicrob Proteins 11, 1278–1292. doi: 10.1007/s12602-018-9476-x
Maertens L., Perez J. M., Villamide M., Cervera C., Gidenne T., Xiccato G. (2002). Nutritive value of raw materials for rabbits: Egran tables 2002. World Rabbit Sci. 10 (4), 157–166. doi: 10.4995/wrs.2002.488
Mancini S., Paci G. (2021). Probiotics in rabbit farming: Growth performance, health status, and meat quality. Animals 11, 3388. doi: 10.3390/ani11123388
Marai I., Ayyat M., El-Monem A. (2001). Growth performance and reproductive traits at first parity of New Zealand White female rabbits as affected by heat stress and its alleviation under Egyptian conditions. Trop. Anim. Health Prod. 33, 451–462. doi: 10.1023/A:1012772311177
Marai I., Haeeb A., Gad A. (2007). Biological functions in young pregnant rabbit does as affected by heat stress and lighting regime under subtropical conditions of Egypt. Trop. Subtrop. Agroecosyst 7, 165–176.
Miranda C., Igrejas G., Poeta P. (2024). “Administration of Antimicrobials and resistance in food-producing rabbits: dietary supplements as potential alternatives,” in Veterinary Care of Farm Rabbits: A Complete Practice Guide to Rabbit Medicine and Production (Cham: Springer), 409–430.
Mohamed S. M., Alagawany M., El-Kholy M. S., El-Mekkawy M. M., Salah A. S., Attia Y. A., et al. (2025). Effect of dietary microalgae on growth performance and health in meat-type quails. Poult. Sci. 104, 104709. doi: 10.1016/j.psj.2024.104709
Mohammed A., Jiang S., Jacobs J., Cheng H.-W. (2019). Effect of a synbiotic supplement on cecal microbial ecology, antioxidant status, and immune response of broiler chickens reared under heat stress. Poult. Sci. 98, 4408–4415. doi: 10.3382/ps/pez246
Moustafa K. E. M. E., El-Hosseiny H. M., Shaheen G. F., El-Kotamy E. M., Ghoniem A. E., Younan G. E., et al. (2024). Impact of different forms of selenium supplementation on growth and physiological performance of New Zealand white rabbits. Trop. Anim. Health Prod. 56, 131. doi: 10.1007/s11250-024-03970-8
Nakanishi S., Kataoka K., Kuwahara T., Ohnishi Y. (2003). Effects of high amylose maize starch and Clostridium butyricum on metabolism in colonic microbiota and formation of azoxymethane-induced aberrant crypt foci in the rat colon. Microbiol. Immunol. 47, 951–958. doi: 10.1111/j.1348-0421.2003.tb03469.x
Nour M. A., El-Hindawy M. M., Abou-Kassem D. E., Ashour E. A., Abd El-Hack M. E., Mahgoub S., et al. (2021). Productive performance, fertility and hatchability, blood indices and gut microbial load in laying quails as affected by two types of probiotic bacteria. Saudi J. Biol. Sci. 28, 6544–6555. doi: 10.1016/j.sjbs.2021.07.030
Obianwuna U. E., Qiu K., Wang J., Zhang H.-J., Qi G.-H., Huang L.-L., et al. (2023). Effects of dietary Clostridium butyricum and fructooligosaccharides, alone or in combination, on performance, egg quality, amino acid digestibility, jejunal morphology, immune function, and antioxidant capacity of laying hens. Front. Microbiol. 14, 1125897. doi: 10.3389/fmicb.2023.1125897
Oladimeji A. M., Johnson T. G., Metwally K., Farghly M., Mahrose K. M. (2022). Environmental heat stress in rabbits: implications and ameliorations. Int. J. Biometeorol. 66, 1–11. doi: 10.1007/s00484-021-02191-0
Pajarillo E. A. B., Chae J. P., Balolong M. P., Kim H. B., Park C.-S., Kang D.-K. (2015). Effects of probiotic Enterococcus faecium NCIMB 11181 administration on swine fecal microbiota diversity and composition using barcoded pyrosequencing. Anim. Feed Sci. Technol. 201, 80–88. doi: 10.1016/j.anifeedsci.2015.01.011
Palkovicsné Pézsa N. (2023). Evaluation of probiotics on porcine intestinal epithelial cells (Budapest, Hungary: University of Veterinary Medicine Budapest).
Palmquist D., Conrad H. (1971). Origin of plasma fatty acids in lactating cows fed high grain or high fat diets. J. Dairy Sci. 54, 1025–1033. doi: 10.3168/jds.S0022-0302(71)85966-0
Phuoc T. L., Jamikorn U. (2017). Effects of probiotic supplement (Bacillus subtilis and Lactobacillus acidophilus) on feed efficiency, growth performance, and microbial population of weaning rabbits. Asian-Austral. J. Anim. Sci. 30, 198. doi: 10.5713/ajas.15.0823
Pilarczyk B., Jankowiak D., Tomza-Marciniak A., Pilarczyk R., Sablik P., Drozd R., et al. (2012). Selenium concentration and glutathione peroxidase (GSH-Px) activity in serum of cows at different stages of lactation. Biol. Trace Elem. Res. 147, 91–96. doi: 10.1007/s12011-011-9271-y
Pisoschi A. M., Pop A. (2015). The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 97, 55–74. doi: 10.1016/j.ejmech.2015.04.040
Placha I., Gai F., Pogány Simonová M. (2022). Natural feed additives in animal nutrition–Their potential as functional feed. Front Vet Sci. 9, 1062724. doi: 10.3389/fvets.2022.1062724
Pogány Simonová M., Lauková A., Chrastinová Ľ., Plachá I., Szabóová R., Kandričáková A., et al. (2020). Beneficial effects of Enterococcus faecium EF9a administration in rabbit diet. World Rabbit Sci. 28, 169–179. doi: 10.4995/wrs.2020.11189
Reda F. M., Madkour M., Abd El-Azeem N., Aboelazab O., Ahmed S. Y., Alagawany M. (2022). Tomato pomace as a nontraditional feedstuff: Productive and reproductive performance, digestive enzymes, blood metabolites, and the deposition of carotenoids into egg yolk in quail breeders. Poult. Sci. 101, 101730. doi: 10.1016/j.psj.2022.101730
Romero C., Rebollar P. G., Moscati L., Dal Bosco A., Castellini C., Cardinali R. (2012). Effect of substitution of medium-chain organic acids for zinc bacitracin in a diet containing colistin on performance and development of intestinal lymphoid tissues in growing rabbits experimentally infected with Escherichia coli O103 and Clostridium perfringens toxinotype A. Anim. Feed Sci. Technol. 174, 174–181. doi: 10.1016/j.anifeedsci.2012.03.012
Saeed M., Afzal Z., Afzal F., Khan R. U., Elnesr S. S., Alagawany M., et al. (2023). Use of postbiotic as growth promoter in poultry industry: A review of current knowledge and future prospects. Food Sci. Anim. Res. 43, 1111. doi: 10.5851/kosfa.2023.e52
Samli H. E., Senkoylu N., Koc F., Kanter M., Agma A. (2007). Effects of Enterococcus faecium and dried whey on broiler performance, gut histomorphology and intestinal microbiota. Arch. Anim. Nutr. 61, 42–49. doi: 10.1080/17450390601106655
Shen X., Yi D., Ni X., Zeng D., Jing B., Lei M., et al. (2014). Effects of Lactobacillus plantarum on production performance, immune characteristics, antioxidant status, and intestinal microflora of bursin-immunized broilers. Can. J. Microbiol. 60, 193–202. doi: 10.1139/cjm-2013-0680
Simonová M., Marcináková M., Strompfová V., Cobanová K., Gancarcíková S., Vasilková Z., et al. (2008). Effect of probiotics Lactobacillus rhamnosus GG and new isolate Enterococcus faecium EF2019 (CCM 7420) on growth, blood parameters, microbiota and coccidia oocysts excretion in rabbits. Int. J. Probiot. Prebiot. 3, 7.
Szabóová R., Lauková A., Pogány-Simonová M., Strompfová V., Plachá I., Čobanová K., et al. (2011). Enterocin 4231 produced by Enterococcus faecium CCM 4231 and its use in rabbits. Acta Vet. 61, 523–529. doi: 10.2298/AVB1106523S
Verma A. K., Kumar A., Rahal A., Kumar V., Roy D. (2012). Inorganic versus organic selenium supplementation: a review. Pak. J. Biol. Sci.: PJBS 15, 418–425. doi: 10.3923/pjbs.2012.418.425
Wang K., Cao G., Zhang H., Li Q., Yang C. (2019). Effects of Clostridium butyricum and Enterococcus faecalis on growth performance, immune function, intestinal morphology, volatile fatty acids, and intestinal flora in a piglet model. Food Funct. 10, 7844–7854. doi: 10.1039/C9FO01650C
Wang Y., Wu Y., Wang Y., Xu H., Mei X., Yu. D., et al. (2017b). Antioxidant properties of probiotic bacteria. Nutrients 9, 521. doi: 10.3390/nu9050521
Wang Y., Zhang Y., Ren H., Fan Z., Yang X., Zhang C., et al. (2023). Dietary yucca extract and Clostridium butyricum promote growth performance of weaned rabbits by improving nutrient digestibility, intestinal development, and microbial composition. Front. Vet. Sci. 10, 1088219. doi: 10.3389/fvets.2023.1088219
Wang C., Zhu Y., Li F., Huang L. (2017a). The Effect of Lactobacillus isolates on growth performance, immune response, intestinal bacterial community composition of growing Rex Rabbits. J. Anim. Physiol. Anim. Nutr. 101, e1–e13. doi: 10.1111/jpn.2017.101.issue-5
Wojcicki J., Barcew-Wiszniewska B., Samochowiec L., Juiwiak S., Kadłubowska D., Tustanowski S., et al. (1991). Effect of selenium and vitamin E on the development of experimental atherosclerosis in rabbits. Atherosclerosis 87, 9–16. doi: 10.1016/0021-9150(91)90227-T
Xia M., Li C., Wu D., Wu F., Kong L., Jia Z., et al. (2024). Benefits of heat-killed Lactobacillus acidophilus on growth performance, nutrient digestibility, antioxidant status, immunity, and cecal microbiota of rabbits. Front.Vet. Sci. 11, 1361908. doi: 10.3389/fvets.2024.1361908
Yanez-Lemus F., Moraga R., Smith C. T., Aguayo P., Sánchez-Alonzo K., García-Cancino A., et al. (2022). Selenium nanoparticle-enriched and potential probiotic, S14 strain, a diet supplement beneficial for rainbow trout. Biol. (Basel) 11, 1523. doi: 10.3390/biology11101523
Yazhini P., Visha P., Selvaraj P., Vasanthakumar P., Chandran V. (2018). Dietary encapsulated probiotic effect on broiler serum biochemical parameters. Vet. World 11, 1344. doi: 10.14202/vetworld.2018.1344-1348
Keywords: selenium, probiotic, hot climate, antioxidant, immunity, cecal fermentation
Citation: El-Kholy MS, Bassiony SS, Al-Sagheer AA, Alagawany M, Ghonime ME, Elwakeel EA, Lestingi A, Elolimy AA, Madkour M, Azzam MM and Elnesr SS (2025) Enterococcus faecium and Clostridium butyricum combined with selenium as alternatives to the antibiotic colistin: impacts on growth, cecal fermentation, and immune function in rabbits raised under hot environmental conditions. Front. Anim. Sci. 6:1556967. doi: 10.3389/fanim.2025.1556967
Received: 29 January 2025; Accepted: 13 March 2025;
Published: 16 April 2025.
Edited by:
Bianca Castiglioni, National Research Council (CNR), ItalyReviewed by:
Baojiang Chen, Agricultural University of Hebei, ChinaCopyright © 2025 El-Kholy, Bassiony, Al-Sagheer, Alagawany, Ghonime, Elwakeel, Lestingi, Elolimy, Madkour, Azzam and Elnesr. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Antonia Lestingi, YW50b25pYS5sZXN0aW5naUB1bmliYS5pdA==; Mahmoud Alagawany, ZHIubWFobW91ZC5hbGFnd2FueUBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.