- 1Research Unit of Biodiversity and Resource Development in Mountain Areas of Tunisia (UR17AGR14), Higher School of Agriculture of Mateur, University of Carthage, Mateur, Tunisia
- 2Center Agriculture Food Environment (C3A), University of Trento, San Michele all’Adige, Trento, Italy
- 3Department of Animal Sciences, National Agronomic Institute of Tunisia, University of Carthage, Tunis, Tunisia
- 4NextProtein Tunisia, Ariana, Tunisia
- 5Animal and Food Resources Laboratory (LRAA), National Agronomic Institute of Tunisia, University of Carthage, Tunis, Tunisia
The current study was conducted to evaluate the effects of Black Soldier Fly (BSF) larvae meal in broiler chicken diets on growth performance, carcass characteristics, meat quality, and cecal microbiota. 300 unsexed Arbor Acres chicks were divided into two treatment groups, each with six replicates of 25 chicks. The experimental group was fed a diet in which soybean meal was partially substituted with 5% BSF larvae meal during the starter phase (1–14 days) and 10% during the grower phase (15–28 days). The finisher period is a BSF-free diet. Results showed no significant difference in growth performance between the experimental and control groups, except for a higher average weight in the second week (P=0.016). A significant difference was observed in the yield of hot eviscerated carcasses (P=0.001), while no significant differences were found in meat quality parameters, including pH, myoglobin, water-holding capacity, cooking loss, chemical composition (dry matter, ash, and ether extract), and bacterial counts such as total aerobic mesophilic flora, Enterobacteriaceae and E. coli. The inclusion of BSF larvae meal reduced harmful bacteria such as Enterobacteriaceae and increased beneficial bacteria such as Lactobacillus spp., in the chicken’s cecal microbiota. These findings suggest that BSF larvae provide health benefits in meal forms, supporting their potential as a viable and sustainable alternative protein source to partially replace soybean meal in broiler diets.
1 Introduction
Food security has become a major challenge due to rapid global population growth, escalating demand for animal protein, increased animal feed consumption, a reduction in arable land, and declining freshwater supplies (Alexandratos and Bruinsma, 2012). Furthermore, increasing meat production accounts for 20% of global greenhouse gas emissions (Rumpold and Schlüter, 2013), making it a significant contributor to climate change. Given their rapid growth rate, short generation interval, and efficient feed conversion, chickens are among the most economically viable sources of meat for human consumption. According to FAO (2021), poultry meat production has consistently increased worldwide, reaching over 135 million metric tons in 2020, making it the most widely consumed meat globally. However, the poultry industry faces major constraints, particularly in securing sustainable protein sources such as soybean meal (SBM) and fish meal (FM). While these conventional protein sources offer excellent amino acid profiles and high digestibility (Masagounder et al., 2016), their costs are continuously rising, threatening the economic sustainability of poultry farming, especially in developing countries (Boerema et al., 2016). This situation has led to a growing interest in alternative, sustainable protein sources that can maintain poultry dietary requirements while reducing feed costs (Eleroğlu et al., 2013a, 2013b; Gasco et al., 2019).
Insects are a natural component of wild birds’ diets and have been proposed as a suitable and more affordable alternative protein source for poultry (Onsongo et al., 2018; Affedzie-Obresi et al., 2020). Their high nutritional value, combined with their lower environmental footprint, makes them an attractive option (Gasco et al., 2019). Several wild bird species, including omnivorous and insectivorous birds, actively forage for insect larvae. For instance, the red junglefowl (Gallus gallus), the wild ancestor of domestic chickens, has been observed feeding on various insects, including fly larvae, in natural environments (Collias and Collias, 1967). Studies have also shown that birds commonly consume insects in agricultural settings, such as guava orchards, where various bird species fed on insect larvae, highlighting their natural inclination for insect consumption (Silva et al., 2021). Additionally, free-range chickens have been observed feeding on insect larvae, including black soldier fly (Hermetia illucens L., BSF) larvae, further supporting their potential as a natural feed ingredient (Makkar et al., 2014; Bellezza Oddon et al., 2024; Fiorilla et al., 2024).
Insects also have the unique ability to grow on by-product-based substrates and thus contribute to waste management (van Huis et al., 2013; Veldkamp and Bosch, 2015). Through bioconversion, they help reduce waste volume, recycle nutrients, and generate valuable products such as animal feed, biofertilizers, and biofuels (Pastor et al., 2015; Surendra et al., 2020; Sanchez Matos et al., 2021). BSF larvae, in particular, have been widely studied for their role in organic waste bioconversion, significantly reducing environmental pollution (Gold et al., 2020; Cattaneo et al., 2024). Additionally, insects play a role in entomoremediation, by breaking down plastic waste and removing heavy metals from contaminated soil (Shahanaz and Shankaraswamy, 2020). These attributes highlight the sustainability potential of insect farming in promoting a circular bioeconomy.
The growing interest in insect-based feeds is reflected in the recent approval of meals made from seven insect species under Regulation (European Union) 2021/1372, issued on August 17, which extends their use to poultry and pigs in addition to fish European Commission Regulation (2021).
Compared to SBM and FM, BSF larvae offer multiple advantages. Their protein content (32–53% dry matter, DM) is comparable to SBM (44% DM) and FM (65–72% DM) (Barragan-Fonseca et al., 2019; Abd El-Hack et al., 2020). Additionally, BSF larvae contain bioactive compounds such as antimicrobial peptides and lauric acid, which enhance gut health and improve pathogen resistance (Spranghers et al., 2017; Lee et al., 2018). Unlike SBM, which requires extensive land and water resources for cultivation, BSF larvae can be reared on organic by-products, making them more sustainable (van Huis et al., 2013; Veldkamp and Bosch, 2015). Furthermore, BSF production has a lower carbon footprint than FM, which depends on marine resources and is affected by overfishing concerns (Gasco et al., 2019). These attributes position BSF larvae as a promising alternative protein source for poultry feed.
Several studies have examined the use of BSF meal, live larvae, and dried larvae in broiler chickens (Dabbou et al., 2018; Schiavone et al., 2019; Ipema et al., 2020; Dörper et al., 2024), indicating that insect-based products can effectively replace conventional protein sources or serve as environmental enrichment, without compromising animal health or product quality. These products can also enhance feed efficiency and immune response (Dabbou et al., 2018; Bellezza Oddon et al., 2024). Given the above-mentioned background, this study aims to standardize inclusion levels of BSF larvae meal. This would optimize the performance and health of broiler chickens, promoting the use of BSF larvae meal as a novel protein source in poultry feeding. This study, in particular, proposes a feeding strategy program that includes an inclusion rate of 5% during the starter phase and 10% during the grower phase, followed by a diet without BSF during the finisher phase. The inclusion levels were determined based on digestibility constraints in young chicks and the progressive adaptation of the digestive system. A lower 5% inclusion rate in the starter phase minimizes potential adverse effects on nutrient absorption and gut health, as chicks have an immature enzymatic system. Increasing to 10% in the grower phase aligns with the birds’ improved digestive capacity, allowing for better utilization of insect meal without compromising performance.
BSF larvae meal were excluded from the finisher phase to achieve specified growth and feed efficiency goals. During this phase, dietary requirements vary to maximize final body weight and meat quality, with an emphasis on energy-dense feed and lower protein content. According to Popova et al. (2020), including 5% full-fat BSF meal was associated with a decrease in pH due to lauric acid, which is a key factor in muscle transformation and meat quality. Hence, including BSF during this phase may influence carcass composition and the optimum fat-to-lean ratio, as high inclusion levels have been associated with increased fat deposition, potentially altering meat texture and market quality. This effect may be linked to the chitin content in BSF larvae meal, which can influence lipid metabolism and nutrient digestibility. Additionally, the exclusion of BSF larvae meal in the finisher phase contributes to feed cost-effectiveness, as they can be more expensive or less practical for older animals. Furthermore, the exclusion of BSF larvae meal helps ensure consistency in feed ingredients and food safety, preventing potential issues with substrate quality, microbial contamination, or heavy metal accumulation, all of which could impact carcass uniformity and consumer safety.
2 Materials and methods
2.1 Source of BSF larvae meal
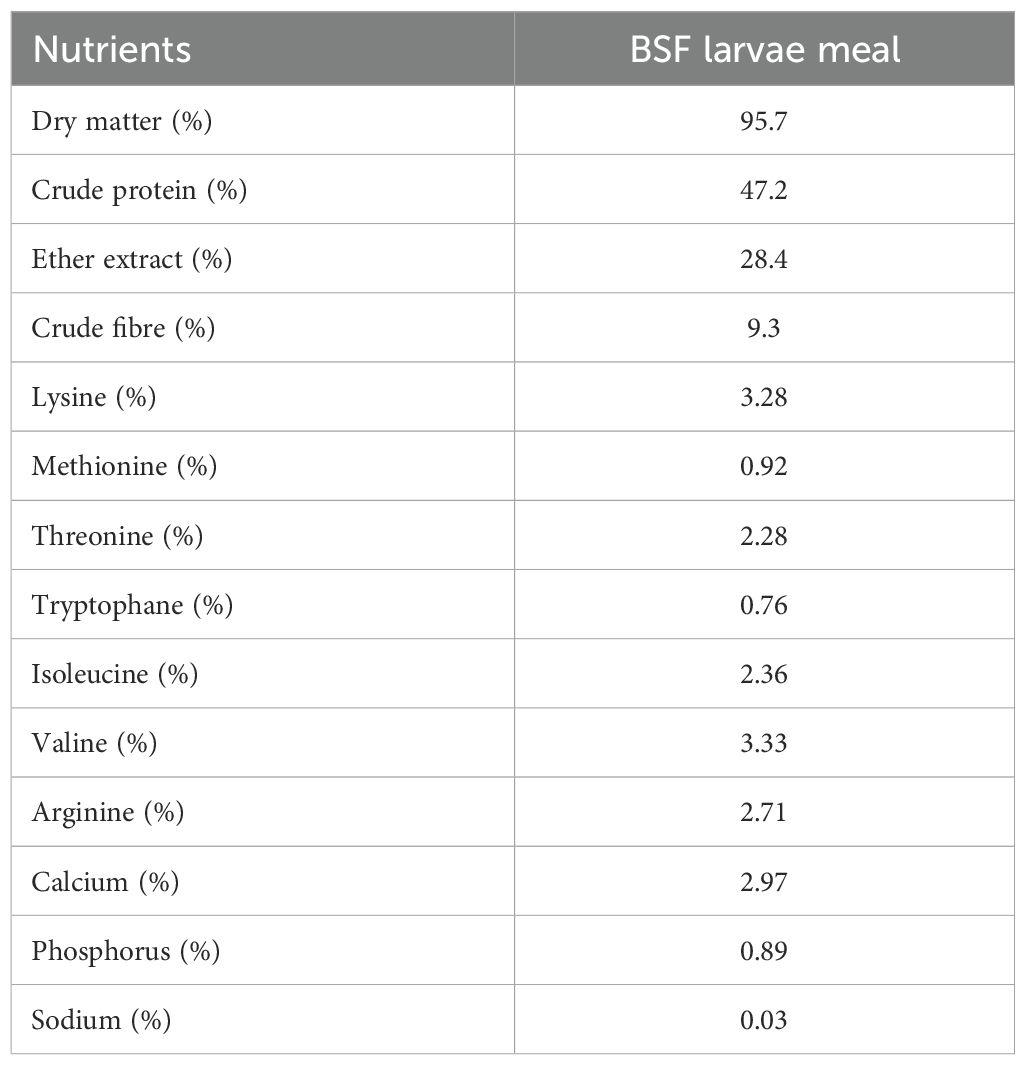
The BSF larva meal, obtained by processing larvae reared on inedible food substrates, consisting of biowaste, including fruit and vegetable peels, discarded crop residues, and food processing by-products, was provided by NextProtein Tunisia. The composition in terms of nutrients, essential amino acids, and minerals is presented in Table 1.
2.2 Birds, experimental design, housing, and feeding regimen
Three hundred unsexed one-day-old Arbor Acres broiler chicks having an average initial weight of 75 ± 2.5 g were acquired from a Tunisian commercial hatchery and delivered by an authorized truck to the pedagogical farm of the High School of Agriculture of Mateur, where the trial was held for 35 days, from 24 April to 28 May 2023. The chicks were transported in well-ventilated cardboard boxes, each with a capacity of 50 chicks. The transport duration was relatively short, approximately 2 hours, and the ambient temperature was around 23°C. The chicks were randomly split into two experimental treatment groups, the control group (n = 150) and the experimental group (n = 150), in a completely randomized block design. The two experimental treatments comprised six replicates, with twenty-five chicks per replicate, kept on a 5 cm-deep litter of wood shavings in an area of 2.5 × 1.5 m/ 25 chicks/pen. Each pen was equipped with one feeder and one drinker. The light/dark applied cycle was 23L/1D. Exhaust fans ensured air renewal and temperature control was managed by the pad cooling system. The chicks were raised according to the husbandry guidelines provided by Aviagen, Inc. A three-stage feeding regimen was implemented: starter: 1–14 days of age; grower: 15–28 days of age; and finisher: 29–35 days of age. Five diets were formulated for the starter (control and experimental), grower (control and experimental), and finisher (BSF-free) feeding regimen. The inclusion rates of full-fat BSF larvae meal were set at 5% for the starter diet and 10% for the grower diet, based on existing literature (Dabbou et al., 2018; Biasato et al., 2020) and a strategy to balance the chicks’ nutritional requirements with sustainability. The 5% inclusion in the starter phase minimizes potential disruptions to gut health, while positively influencing the cecal microbiota and gut mucin dynamics by selecting potentially beneficial bacteria and increasing villi mucins (Biasato et al., 2020). In contrast, the 10% inclusion in the grower phase takes advantage of their improved digestive capacity, allowing for better utilization of insect meal without compromising growth performance (Dabbou et al., 2018). These rates were chosen to optimize growth while reducing reliance on traditional protein sources like soybean, aligning with both nutritional requirements and sustainable feeding practices.
Experimental and control diets were formulated to be isocaloric/isonitrogenous. Table 2 presents the ingredients and the chemical composition of the control and experimental diets during the starter and grower phases, as well as the finisher’s BSF-free diet. Chemical analyses of the BSF larvae meal, control, and experimental diets were performed in triplicate according to the AOAC International (2004) procedures: Dry matter (method 934.01), ether extract (method 920.39), ash (method 942.05), crude protein (method 954.01), and crude fiber (method 945.18). Digestible lysine, digestible methionine, methionine + cysteine, and metabolizable energy were calculated according to Cerrate and Corzo (2019).
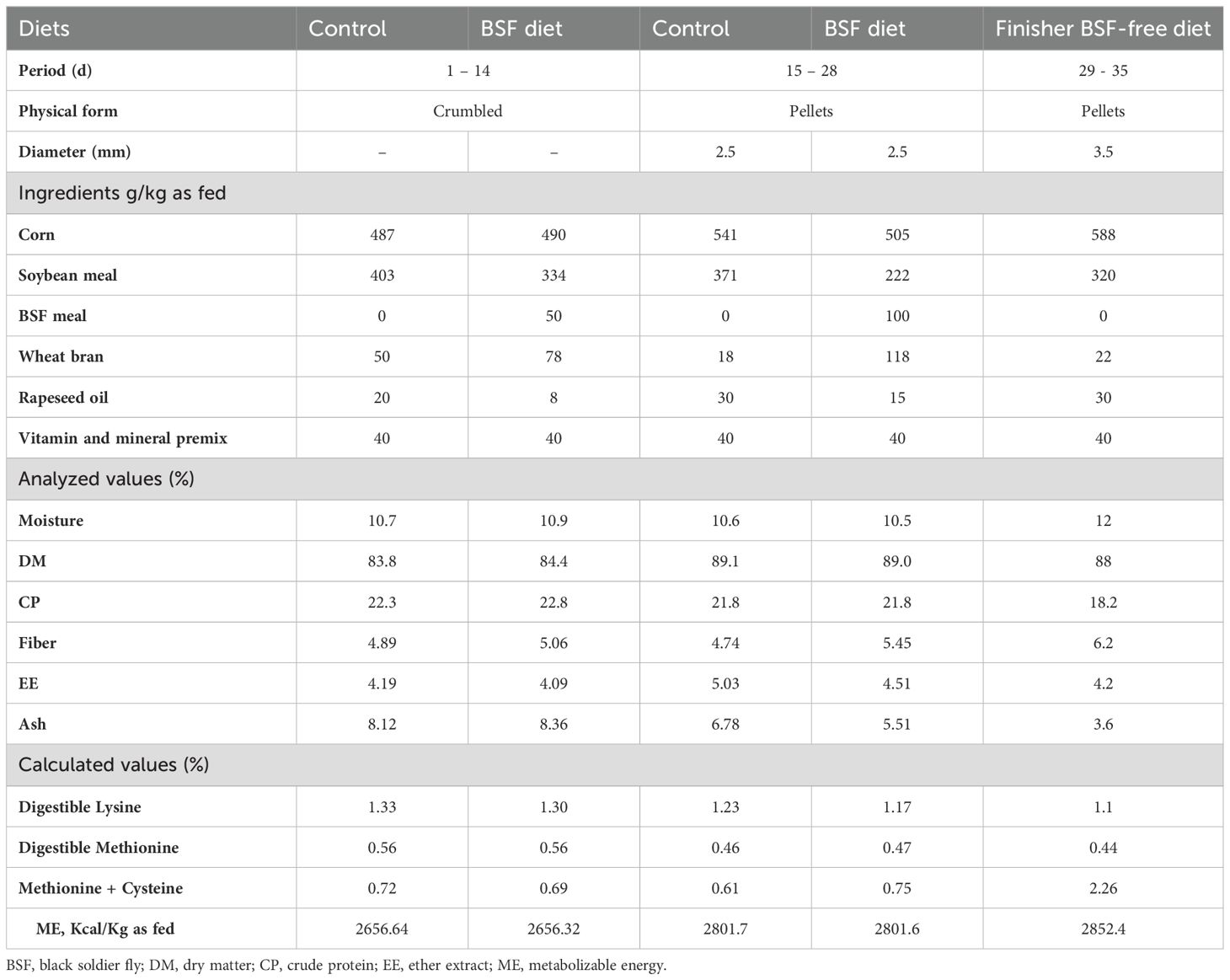
Table 2. Ingredients and chemical composition of control and experimental diets during the starter and grower phases and the finisher’s BSF-free diet.
2.3 Growth performance
Health status and mortality were monitored daily throughout the whole experimental period. The live weight (LW) was recorded individually at the beginning of the trial and weekly thereafter (days 7, 14, 21, 28, and 35). Feed intake was measured daily for each pen. The daily weight gain (DWG; Equation 1) in grams and the feed conversion ratio (FCR; Equation 2) were calculated weekly (weeks 1, 2, 3, 4, and 5) at individual and pen levels, respectively.
All the measurements were made on a pen basis using high-precision electronic scales (Accurex DSK, Gram Group, Barcelona, Spain).
2.4 Carcass traits
At 35 days of age, 24 animals (two birds per replicate pens) from each feeding group (chosen based on the average final LW in each pen) were individually identified with a shank ring and weighed. The chickens were slaughtered at a commercial abattoir. Plucked and eviscerated carcasses were obtained, and the head, neck, feet, liver, heart, spleen, bursa of Fabricius, and abdominal fat were removed to obtain the chilled carcass. Then, eviscerated carcass, thigh and breast weights were immediately recorded. The hot eviscerated carcass yield were expressed as percentages of the LW (Equation 3) and, the breast and thigh were expressed as percentage of eviscerated carcass weight (Equations 4, 5).
2.5 Meat quality analysis
For the measurements of pH, color, myoglobin concentration, cooking loss, and water-holding capacity (WHC), the meat was vacuum sealed and refrigerated (4 ± 1°C) to maintain its freshness. For the microbiological analyses, a sample of meat was placed in sterile containers and stored at -20°C to prevent bacterial proliferation during the storage period.
The pH of the breast was recorded immediately after slaughter and 24 hours postmortem (pHu) using a calibrated pH meter (Hanna HI-99163, Italy). The values of 2 replicates were considered, and their mean was subsequently utilized. The meat color was measured at room temperature (20°C) after slaughter and at 24 h postmortem on the inner surface of the Pectoralis major muscle on the breast and on the Biceps femoris muscle on the thigh and using a portable colorimeter Chroma Meter CR-410 Konica Minolta Sensing device (Minolta Sensing Inc., Osaka, Japan). The color measurements were reported in terms of lightness (L*), redness (a*), and yellowness (b*) in the CIELAB color space model (Commission Internationale de l’Éclairage, 1976). The values were recorded for the CIE standard illuminant D65 and the CIE 2° standard observer. The color values were obtained considering the average of three readings per sample.
Myoglobin concentration in the breast was determined using a spectrophotometric method as outlined by Viriyarattanasak et al. (2011). Water-holding capacity (WHC) was determined according to Bowker et al. (2014). Briefly, 10 g of minced muscle (initial weight) was placed in 15 mL of 0.6 M NaCl solution in a 50 mL centrifuge tube. The sample was mixed for 1 minute, stored at 4°C for 15 minutes, and then centrifuged at 7000 rpm for 15 minutes at 4°C. After centrifugation, the supernatant was removed, and the meat was collected and weighed. WHC was calculated as the percentage of retained water using the formula provided in Equation 6.
Cooking loss was measured following the procedure of Petracci and Baéza (2011). The process involved placing the breast meat into hermetically plastic food bags in a water bath operating at 80°C until reaching a final internal temperature at the thickest part of the breast of 75°C.
The temperature was monitored using a hand-held digital thermometer (Testo 104, Germany). Meat samples were then cooled under tap water, dried, weighed, and calculated according to Equation 7:
Meat chemical composition was performed by determining the dry matter (method 950.46), ash (method 920.153), and total fat contents (method 960.39) of the breast meat according to the AOAC’s official methods of analysis AOAC International (2000).
Meat microbiological analysis was based on the counts of total aerobic mesophilic flora, the Enterobacteriaceae family, and E. coli in 1 gram of minced meat. To determine colony-forming units (cfu/g), 1 ml of all decimal dilutions, ranging from 10-1 to 10-4 for each bacterial species, was inoculated in specialized bacterial growth media and incubated according to the times and temperatures specific for each bacterial class (Downes and Ito, 2001). To count total aerobic mesophilic flora, samples were inoculated on Plate count agar (PCA) and incubated at 30°C for 24 hours. Incubation of samples at 37°C for 48 hours was performed using HEKTOEN agar to isolate the Enterobacteriaceae family. Finally, for the determination of the total number of E. coli strains, samples were inoculated in Violet Red Bile Agar with Lactose (VRBL) agar and incubated at 44°C for 24 hours.
2.6 Cecal microbiota analysis
Cecal contents were carefully extracted during the dissection of broilers sampled from the control and experimental groups, using sterile gloves and a sterile spatula to collect the cecal material directly from the cecum, ensuring that no external surfaces came into contact with the sample. The samples were transferred into sterile containers and stored at -20°C until processing. Enterobacteriaceae, Echerichia coli, Clostridium spp., and Lactobacillus spp. were enumerated following the same protocol used for the microbiological quality of meat. For Clostridium spp. and Lactobacillus spp., samples were inoculated in Meat-Liver agar and De Man, Rogosa, and Sharpe (MRS) agar and incubated at 37°C for 24 to 48 hours and at 40°C for 24 to 48 hours, respectively. Two counts were conducted after 24 and 48 hours.
2.7 Data analysis
Data obtained from this study were analyzed using the SAS software (SAS Institute Inc. SAS® 9.4, 2014). Data were tested for normality and homogeneity of variance. All values were grouped, and the mean and standard error were calculated. A one-way analysis of variance (ANOVA) in a fully randomized design using the general linear model (GLM) was performed for all parameters to examine differences between groups. P <0.05 was considered a significant value.
3 Results
3.1 Growth performance
The overall mortality rate during the trial was identical for both groups (equal to 0.66%), and it was recorded during the two first weeks (starter period) as shown in Table 3. Statistical analysis revealed no significant differences in all measured parameters between the control and experimental groups (P> 0.05), except the average weight of broilers during the second week which was higher in the experimental group than the control group (545.7 ± 24.1 vs. 541.0 ± 43.0, P= 0.016).
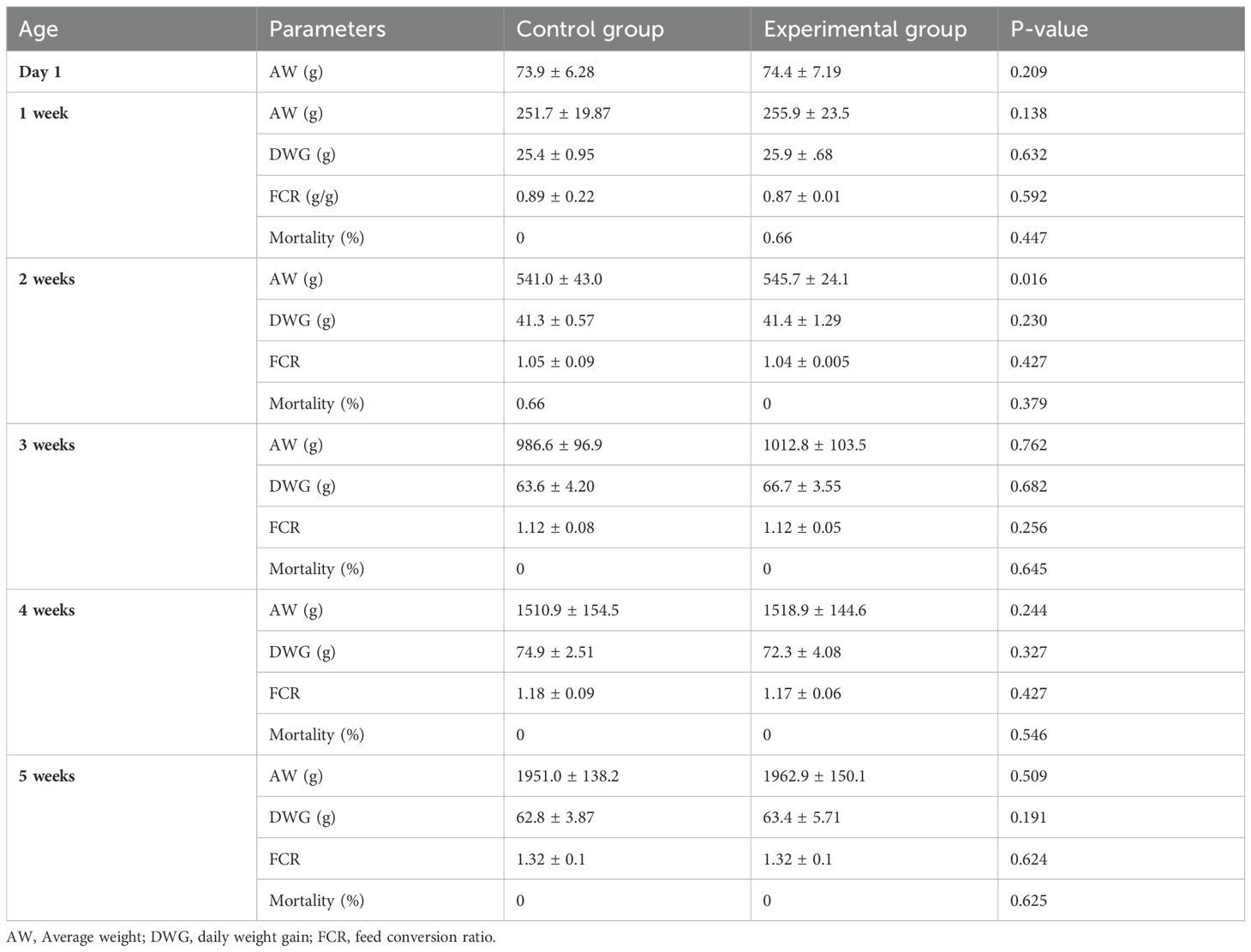
Table 3. Effect of the inclusion of BSF larvae meal on growth performances of broiler chickens during different phases of the trial (mean value ± standard deviation).
3.2 Carcass traits, meat quality, and cecal microbiota analysis
The effect of BSF larvae meal on carcass characteristics, meat quality, and cecal analysis is shown in Table 4. The results indicated that BSF larvae meal had a positive impact (P=0.001) on hot eviscerated carcass yield. The experimental group exhibited a significant increase in carcass yield (80.2%) compared to the control group (76.8%; P=0.001). However, no significant differences were observed in breast and thigh yields (P>0.05). No significant differences were found in post-mortem or ultimate pH values, myoglobin content, water-holding capacity, cooking losses, or meat chemical composition between the control and experimental groups. Enterobacteriaceae counts in the cecal content showed a significant difference between the control and experimental groups (P=0.006), while no significant differences were observed in total aerobic mesophiles and E. coli counts between the groups (P>0.05).
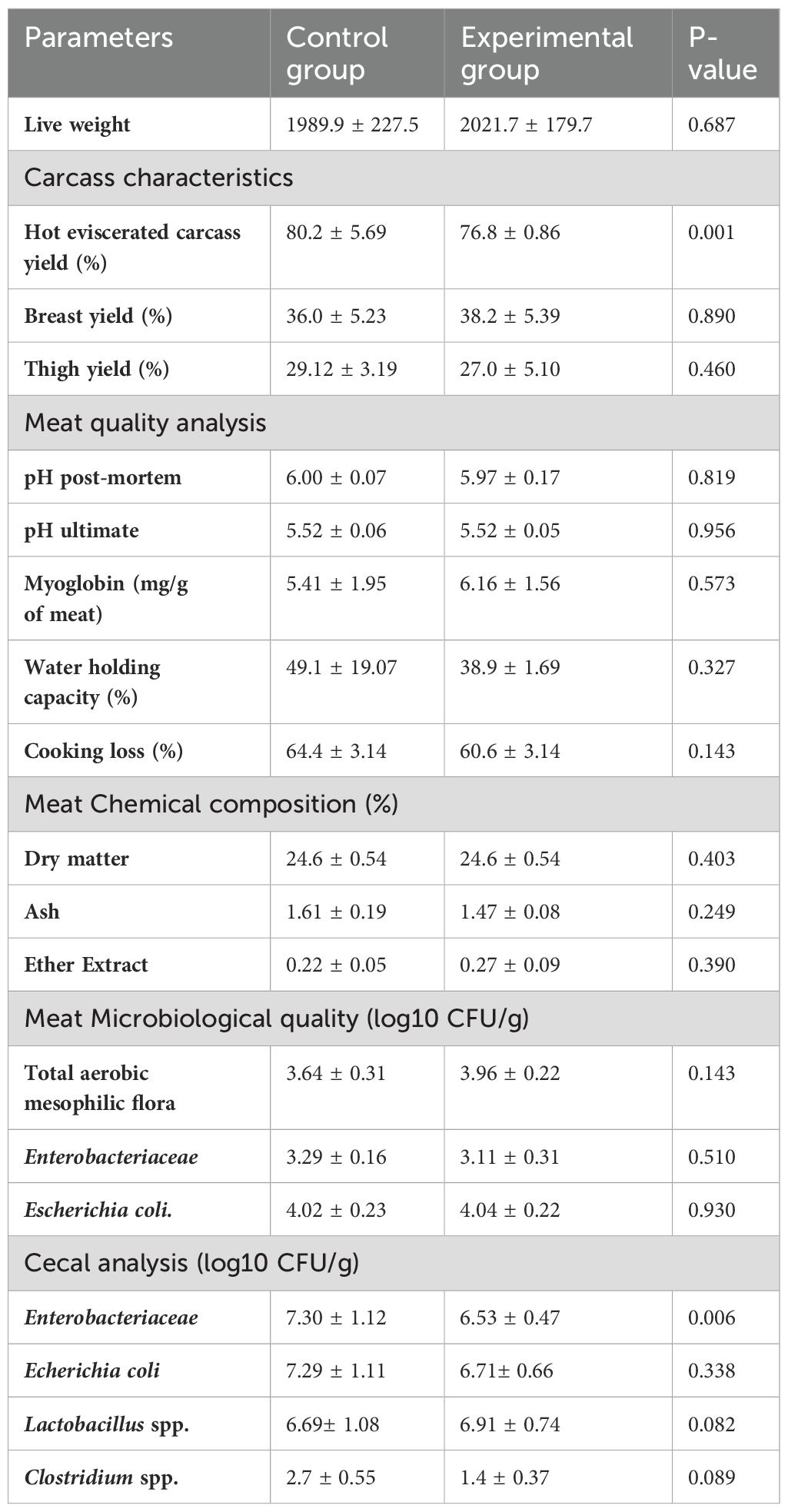
Table 4. Effect of BSF larvae meal supplementation on carcass characteristics, meat quality, and cecal analysis of broiler chickens (mean value ± standard deviation).
There were no significant differences in color parameters in broiler breast and thigh meat 24 hours after slaughter (Table 5). However, 1 hour post-mortem, the breast meat from the experimental group was redder (a*= 13.0 ± 2.68) than that from the control group (a*= 10.2 ± 1.70; P=0.026). For thigh meat, the experimental group had significantly lower yellowness (b*= 8.09 ± 1.68) compared to the control group (b*= 9.89 ± 1.06; P=0.023).
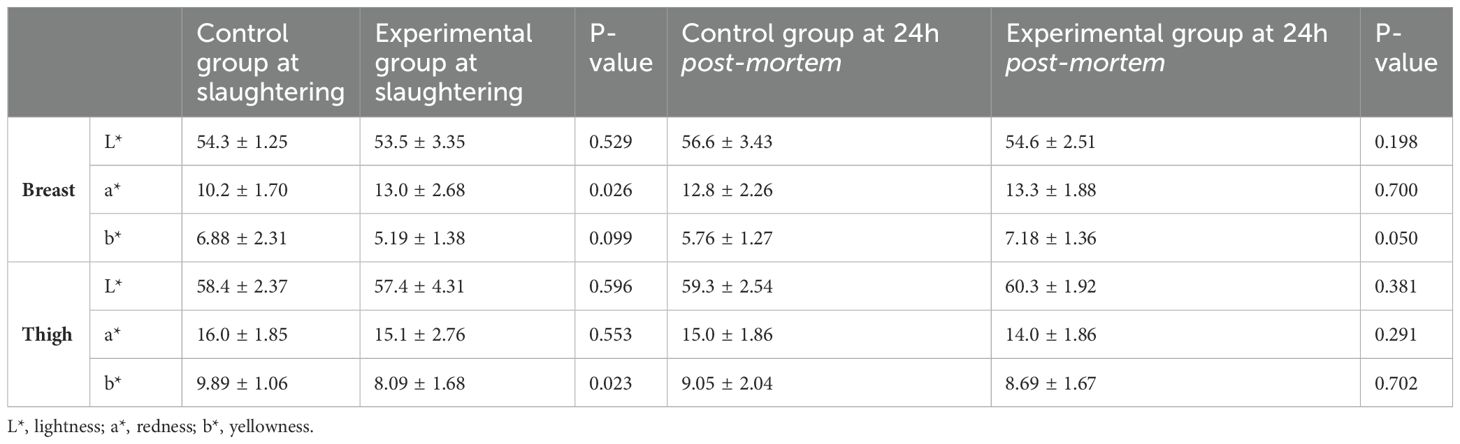
Table 5. Effect of the inclusion of BSF larvae meal in broiler diets on meat color (mean value ± standard deviation).
4 Discussion
4.1 Growth performance
Throughout the trial, broilers exhibited normal vitality, with no pathological symptoms observed. The mortality rate was extremely low, at 0.66% in both the control and experimental groups. One chick in the experimental group was stunted from the beginning of the trial, while another in the control group developed joint inflammation in its leg, which prevented it from eating and drinking.
Our results are consistent with those of Dabbou et al. (2018), who reported a zero-mortality rate in all groups of broiler chickens fed diets containing 5%, 10%, and 15% BSF larvae meal. Similarly, Cullere et al. (2016) observed mortality rates ranging from 0% to 0.2% in broiler quails fed diets incorporating 15% and 10% BSF larvae meal, respectively. However, Hartinger et al. (2022) reported a higher mortality rate of 16.66% in chickens fed a diet containing 30% BSF larvae meal, whereas the group fed a 15% BSF larvae meal diet had a mortality rate of 1.38%. In our study, no significant differences were observed in average weight, daily weight gain, or feed conversion ratio between the control and experimental groups, except for the average weight during the second week. It should be noted that throughout the 5 weeks of broiler chicken rearing, the experimental group numerically showed higher weights during the second, third, fourth, and fifth weeks. However, the difference was only statistically significant during the second week. This suggests that while the experimental group exhibited higher weights across the later weeks, the effect was most pronounced in the second week. The lack of statistical significance in the subsequent weeks may indicate that the initial advantage diminished over time, possibly due to adaptation to the diet. Overall, these findings suggest that the inclusion of BSF larvae meal as a protein source in broiler chicken diets did not negatively affect growth performance. Similar conclusions were drawn by Hartinger et al. (2022), who reported no adverse effects with a 15% inclusion level of BSF larvae meal in broiler diets, and by Al-Qazzaz et al. (2016) in laying hens supplemented with 1% or 5% BSF larvae meal. Other studies have reported a positive impact of insect meal inclusion on growth performance. For instance, De Souza Vilela et al. (2021) found that a diet containing 20% BSF larvae meal reduced the FCR by 10% in broilers. Similarly, Hwangbo et al. (2009) demonstrated that diets supplemented with 10% to 15% maggots improved the growth performance of broilers. Dabbou et al. (2018) found that BSF larvae meal positively influenced broiler growth performance at inclusion levels up to 10%, although performance declined with a 15% inclusion level. Meanwhile, Cullere et al. (2016) observed no significant differences in body weight gain, feed intake, FCR, or mortality rate between control and experimental groups of broiler quails fed 10% or 15% BSF larvae meal.
The variability in findings among these studies may be attributed to several factors, including differences in the nutrient profiles of the insect meals used, which are influenced by the substrates used for larvae production and directly affect their chemical composition (Tschirner and Simon, 2015). Additionally, factors such as insect processing methods, inclusion rates, experimental protocols, and the specific bird species studied play a critical role. These results highlight the potential of BSF meal as a sustainable protein source for the broiler feed industry. Moreover, this study provides valuable insights into how BSF meal influences broiler performance, and overall feed efficiency, contributing to efforts aimed at reducing variability in its composition. However, further research is needed to establish consistent and universal standards that ensure the reliability of insect-based feed ingredients.
4.2 Carcass traits
The inclusion of BSF larvae meal in poultry diets did not affect carcass traits in this study. Research on BSF larvae meal as a protein source for poultry has shown mixed effects on carcass characteristics. Some studies align with our findings, reporting no significant impact on carcass traits in Cobb 500 broilers fed diets containing 5%, 10%, and 15% BSF larvae meal (Pieterse et al., 2019), in broiler quails (Coturnix coturnix japonica) fed diets with 10% and 15% BSF larvae meal (Cullere et al., 2016), and in Gramasree hens receiving BSF larvae as a protein replacer at varying levels (25%, 50%, 75%, and 100%) (Avinash et al., 2022). In contrast, other studies have reported improvements in carcass traits following BSF larvae meal inclusion (Hoffman, 2019; Chia et al., 2021). Popova et al. (2020) found that while BSF larvae meal enhanced carcass weight and breast yield, it reduced thigh yield. These discrepancies may be attributed to differences in bird species, dietary formulations, or environmental factors influencing growth.
4.3 Meat quality analysis
Colorimetric analysis showed that chicken thighs from birds fed BSF larvae meal exhibited significantly lower yellowness one hour post-slaughter and slightly reduced yellowness after 24 hours. Meat color is influenced by multiple factors, including heme pigments, genetics, and diet composition (Fletcher, 1999). The lower yellowness observed in this study could be due to the reduced carotenoid content in BSF larvae, which is highly dependent on the rearing substrate (Borel et al., 2021; Leni et al., 2022). Similarly, Schiavone et al. (2019) reported a decrease in breast meat yellowness with increasing levels of BSF larvae meal. In contrast, redness (a*) values increased in the breast meat of broilers fed BSF larvae meal. This finding aligns with previous studies showing that BSF larvae meal or oil increases breast meat redness (Schiavone et al., 2019; Aprianto et al., 2023; Kierończyk et al., 2023). The increase in a* values may be attributed to xanthophyll pigments accumulated in BSF larvae, which originate from vegetables and fruits in their rearing substrate (Aprianto et al., 2023).
In this study, dietary treatments did not significantly affect (P>0.05) the physico-chemical, nutritional, technological, or microbiological quality of breast and thigh meat. This consistency in meat quality can be attributed to the formulation of diets as isocaloric and isonitrogenous, ensuring similar levels of essential nutrients across all groups. Additionally, our dietary strategy, starting with a lower inclusion rate during the starter phase to avoid digestibility issues and excluding BSF larvae meal during the finisher phase, may have limited the duration of exposure, preventing significant differences from manifesting. Similar findings have been reported by Schiavone et al. (2019), who observed no changes in meat quality parameters, such as cooking loss, with BSF larvae meal inclusion up to 10%. Pieterse et al. (2019) also found no significant effect of diets containing up to 15% BSF larvae meal on broiler meat pH, color, and cooking loss. However, Popova et al. (2020) reported that BSF larvae meal reduced pH and myoglobin concentration in broiler meat, attributing this effect to the lauric acid content of the larvae. Differences in inclusion levels, basal diet composition, bird breed, or BSF larvae processing and storage methods may explain these conflicting results, as such factors could influence the bioavailability of lauric acid and its impact on meat quality.
To our knowledge, no prior studies have assessed the effect of BSF larvae meal on the microbiological quality of broiler meat. In this study, its inclusion did not affect total aerobic mesophilic flora, Enterobacteriaceae, or Escherichia coli counts. This outcome is likely due to identical handling conditions during slaughter, storage, and analysis for all groups. However, further research is needed to explore the impact of different BSF larvae meal inclusion levels on meat microbiological quality.
4.4 Cecal microbiota analysis
BSF larvae are recognized as a source of antimicrobial compounds that can modulate fecal microbiota, potentially reducing harmful bacteria (Borrelli et al., 2017). This effect is attributed to their bioactive molecules, including lauric acid (Borrelli et al., 2021), chitin (Lagat et al., 2021; Kemboi et al., 2022), and various antimicrobial peptides (Moretta et al., 2020; Xu et al., 2020). Lauric acid, which makes up to 60% of BSF larvae fat (Spranghers et al., 2017), exhibits strong antimicrobial activity against various bacterial species. It achieves this through a membrane-lytic mechanism, destabilizing bacterial cell membranes, increasing permeability, and ultimately leading to cell lysis (Yoon et al., 2018). Additionally, lauric acid inhibits the MurA enzyme, which is essential for bacterial cell wall biosynthesis. Chitin, the primary component of the insect exoskeleton and crustacean shells (Joseph et al., 2021), is a natural polysaccharide and the second most abundant polymer after cellulose. It holds significant value in the pharmaceutical, food, and agricultural industries due to its diverse biological activities, including antimicrobial properties (Kumar et al., 2020; Lagat et al., 2021). Insects, including BSF larvae, are also rich sources of antimicrobial peptides that can target both Gram-positive and Gram-negative bacteria. These include Staphylococcus aureus, Micrococcus gluteus, Bacillus subtilis, Bacillus thuringiensis, Aerococcus viridans, and Bacillus megaterium, as well as Escherichia coli (Moretta et al., 2020). These peptides exert antimicrobial effects either through membranolytic mechanisms or by interacting with intracellular targets such as DNA, RNA, and proteins. This is consistent with our findings, which showed a significant decrease (P=0.006) in Enterobacteriacae counts, a group of gram-negative bacteria.
Building on the well-documented antimicrobial properties of chitin and insect-derived peptides, the inclusion of BSF larvae meal in animal diets may enhance gut microbiota composition by leveraging these bioactive compounds. The insect-based diet led to an increase in Lactobacillus spp. and a decrease in Clostridium spp. and Enterobacteriaceae, while Escherichia coli strains abundance was not significantly affected. Lagat et al. (2021) reported that chitin and chitosan extracted from BSF pupal exuviae exhibit broad-spectrum antimicrobial activity, inhibiting the growth of both Gram-negative (Escherichia coli and Pseudomonas aeruginosa) and Gram-positive (Bacillus subtilis and Staphylococcus aureus) bacteria. Similarly, Borrelli et al. (2021) demonstrated that lauric acid inhibits Clostridium difficile by disrupting cell membranes. Borrelli et al. (2017) suggested that BSF larvae may act as a prebiotic, with chitin playing a key role in this effect. The inclusion of BSF larvae in poultry diets has been associated with an increase in Oscillospira, a genus considered one of the next-generation probiotics, known to prevent Clostridium difficile infections (Yang et al., 2021). Bonomini et al. (2025) further confirmed that BSF larvae and their lipid fractions exhibit both antibacterial and prebiotic effects, supporting their potential role in gut health optimization. Lauric acid, a major component of BSF larvae, exhibits strong antimicrobial activity against pathogenic Clostridium species, such as C. perfringens, a causative agent of necrotic enteritis in poultry (Matsue et al., 2019). Importantly, lauric acid selectively targets pathogens while sparing beneficial bacteria like Lactobacillus spp., potentially modulating intestinal health. This could explain the increase in Lactobacillus spp. and decrease in Clostridium spp. observed in our study. Furthermore, chitin, a major structural component of BSF larvae, has been reported to act as a functional fiber, promoting the growth of beneficial bacteria and enhancing gut barrier function (Edo et al., 2024). The combined effects of chitin and lauric acid may synergistically inhibit pathogens while fostering a microbiota composition favorable for gut health (Borrelli et al., 2021). Future research should explore ways to enhance the functional benefits of BSF meal, including adjusting inclusion levels, processing methods (e.g., defatting, enzymatic hydrolysis), or combining BSF-derived bioactives with other functional feed additives.
Additionally, Lactobacillus spp. exhibit antibacterial activity, making them valuable for poultry feed formulation. Their probiotic properties help inhibit pathogen growth through competitive exclusion in the gastrointestinal tract (Ahmed et al., 2019) and contribute to improved feed efficiency (Yan et al., 2017). Based on these findings, we hypothesize that an insect-based diet could positively influence cecal microbiota, creating a gut environment that supports poultry health and performance. Given the antimicrobial properties of BSF-derived compounds, future research should investigate BSF meal as a microbial modulator, particularly its impact on gut microbiota composition and its potential role in improving meat safety and quality.
5 Conclusion
The study assessed the effects of black soldier fly larvae meal on various performance indicators in broiler chickens, focusing on growth metrics, carcass characteristics, meat quality and cecal microbiota composition. The experimental group demonstrated a statistically significant increase in average weight at two weeks, suggesting a potential short-term advantage of BSF larvae meal in promoting early growth. Significant enhancements were observed in the yield of hot eviscerated carcasses in the group receiving BSF larvae, indicating improved productivity and efficiency in meat production attributable to the inclusion of BSF in the diet. The study also revealed that the incorporation of BSF larvae meal significantly modified the composition of cecal microbiota. There was a notable decrease in pathogenic bacteria, particularly Enterobacteriaceae and Clostridium spp., alongside an increase in beneficial Lactobacillus species, which indicates potential health advantages related to gut integrity and function. These microbiota changes may contribute to improved overall growth performance and meat quality. The findings of this study support the hypothesis that BSF larvae meal can function as a viable and sustainable protein source for broiler nutrition, potentially substituting conventional protein sources such as soybean meal. The inclusion of BSF larvae meal not only promotes gut health through a more balanced microbiota but also provides economic advantages by increasing carcass yield without compromising meat quality. Thus, BSF larvae meal emerge as a promising alternative in poultry nutrition, contributing to sustainable agricultural practices by reducing dependence on traditional feed ingredients.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal studies were approved by Official Animal Care and Use Committee of the National Institute of Agronomy of Tunisia (Protocol No. 05/15). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
MS: Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. SD: Validation, Visualization, Supervision, Writing – review & editing. MB: Conceptualization, Methodology, Supervision, Writing – review & editing. IB: Validation, Writing – review & editing. WF: Conceptualization, Writing – review & editing. TA: Methodology, Writing – review & editing. MA: Data curation, Writing – review & editing. NM: Data curation, Software, Supervision, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Acknowledgments
The authors thank SAVINORD company for providing animals, and NextProtein, Tunisia for providing the BSF larvae meal.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abd El-Hack M. E., Shafi M. E., Alghamdi W. Y., Abdelnour S. A., Shehata A. M., Noreldin A. E., et al. (2020). Black soldier fly (Hermetia illucens) meal as a promising feed ingredient for poultry: A comprehensive review. Agriculture 10, 339. doi: 10.3390/agriculture10080339
Affedzie-Obresi S., Adu-Aboagye G., Nkegbe E. K., Asuming-Bediako N., Ansah K., Mensah-Bonsu A., et al. (2020). Black soldier fly (Hermitia illucens) larvae meal as alternative protein in broiler production in Ghana. Ghana J. Agric. Sci. 55, 1–13. doi: 10.4314/gjas.v55i1.1
Ahmed Z., Vohra M. S., Khan M. N., Ahmed A., Khan T. A. (2019). Antimicrobial role of Lactobacillus species as potential probiotics against enteropathogenic bacteria in chickens. J. Infect. Dev. Ctries 13, 130–136. doi: 10.3855/jidc.10542
Alexandratos N., Bruinsma J. (2012). World Agriculture Towards 2030/2050: The 2012 Revision. FAO Agricultural Development Economics Division. Viale delle Terme di Caracalla 00153 Rome, Italy. 1–147.
Al-Qazzaz M., Ismail D., Akit H., Lokman I. H. (2016). Effect of using insect larvae meal as a complete protein source on quality and productivity characteristics of laying hens. Rev. Bras. Zootecnia 45, 518–523. doi: 10.1590/s1806-92902016000900003
AOAC (2000). Official methods of analysis of AOAC international. 16th ed. Gaithersburg: Association of Official Analytical Chemists.
AOAC (2004). Official methods of analysis of AOAC international. 16th ed. Gaithersburg: Association of Official Analytical Chemists.
Aprianto M. A., Muhlisin , Kurniawati A., Hanim C., Ariyadi B., Anas M. A. (2023). Effect supplementation of black soldier fly larvae oil (Hermetia illucens L.) calcium salt on performance, blood biochemical profile, carcass characteristic, meat quality, and gene expression in fat metabolism broilers. Poultry Sci. 102, 102984. doi: 10.1016/j.psj.2023.102984
Avinash N., Sankaralingam S., Anitha P., Deepak M. D. K., Aswathi P. B. (2022). Influence of Black Soldier Fly (Hermetia illucens) larvae feeding on carcass characteristics of Gramasree hens. J. Vet. Anim. Sci. 53 (3), 429–434. doi: 10.51966/jvas.2022.53.3.429-434
Barragan-Fonseca K. B., Gort G., Dicke M., Van Loon J. J. A. (2019). Effects of dietary protein and carbohydrate on life-history traits and body protein and fat contents of the black soldier fly. Physiol. Entomology 44, 148–159.
Bellezza Oddon S., Biasato I., Ferrocino I., Imarisio A., Renna M., Caimi C., et al (2024). Live black soldier fly larvae as environmental enrichment for native chickens: implications for bird performance, welfare, and excreta microbiota. Animal 18, 101341. doi: 10.1016/j.animal.2024.101341
Biasato I., Ferrocino I., Dabbou S., Evangelista R., Gai F., Gasco L., et al. (2020). Black soldier fly and gut health in broiler chickens: insights into the relationship between cecal microbiota and intestinal mucin composition. J. Anim. Sci. Biotechnol. 11, 11. doi: 10.1186/s40104-019-0413-y
Boerema A., Peeters A., Swolfs S., Vandevenne F., Jacobs S., Staes J., et al. (2016). Soybean trade: balancing environmental and socio-economic impacts of an intercontinental market. PloS One 11, e0155222. doi: 10.1371/journal.pone.0155222
Bonomini M. G., Verstringe S., Bruggeman G., Vandercruyssen R., Carmans H., Caligiani A. (2025). Characterisation, antibacterial activity, and prebiotic potential of dried Hermetia illucens L. larvae and of their fractions. Journal of Insects as Food and Feed 11, 273–288.
Borel P., Hammaz F., Morand-Laffargue L., Creton B., Halimi C., Sabatier D., et al. (2021). Using black soldier fly larvae reared on fruits and vegetables waste as a sustainable dietary source of provitamin a carotenoids. Food Chem. 359, 129911. doi: 10.1016/j.foodchem.2021.129911
Borrelli L., Coretti L., Dipineto L., Bovera F., Menna F., Chiariotti L., et al. (2017). Insect-based diet, a promising nutritional source, modulates gut microbiota composition and SCFAs production in laying hens. Sci. Rep. 7, 16269. doi: 10.1038/s41598-017-16560-6
Borrelli L., Varriale L., Dipineto L., Pace A., Menna L. F., Fioretti A. (2021). Insect derived lauric acid as promising alternative strategy to antibiotics in the antimicrobial resistance scenario. Front. Microbiol. 12. doi: 10.3389/fmicb.2021.620798
Bowker B., Hawkins S., Zhuang H. (2014). Measurement of water-holding capacity in raw and freeze-dried broiler breast meat with visible and near-infrared spectroscopy1 1The use of trade, firm, or corporation names in this publication is for the information and convenience of the reader. Such use does not constitute an official endorsement or approval by the USDA or the Agricultural Research Service of any product or service to the exclusion of others that may be suitable. Poultry Sci. 93, 1834–1841. doi: 10.3382/ps.2013-03651
Cattaneo N., Zarantoniello M., Conti F., Tavano A., Frontini A., Sener I., et al. (2024). Natural-based solutions to mitigate dietary microplastics side effects in fish. Chemosphere 367, 143587. doi: 10.1016/j.chemosphere.2024.143587
Cerrate S., Corzo A. (2019). Lysine and energy trends in feeding modern commercial broilers. Int. J. Poultry Sci. 18, 28–38. doi: 10.3923/ijps.2019.28.38
Chia S. Y., Tanga C. M., Osuga I. M., Alaru A. O., Mwangi D. M., Githinji M., et al. (2021). Black soldier fly larval meal in feed enhances growth performance, carcass yield and meat quality of finishing pigs. J. Insects as Food Feed 7, 433–448. doi: 10.3920/JIFF2020.0072
CIE (CommissionInternationale de l’Éclairage) (1976). Recommendations on uniform colour spaces-colour difference equations, psychometric colour terms (supplement no. 2 to CIE publication no. 15) (Paris, France: Commission Internationale de l’Éclairage).
Collias N. E., Collias E. C. (1967). A field study of the red jungle fowl in north-central India. Condor 69, 360–386. doi: 10.2307/1366199
Cullere M., Tasoniero G., Giaccone V., Miotti-Scapin R., Claeys E., De Smet S., et al. (2016). Black soldier fly as dietary protein source for broiler quails: apparent digestibility, excreta microbial load, feed choice, performance, carcass and meat traits. Animal 10, 1923–1930. doi: 10.1017/S1751731116001270
Dabbou S., Gai F., Biasato I., Capucchio M. T., Biasibetti E., Dezzutto D., et al. (2018). Black soldier fly defatted meal as a dietary protein source for broiler chickens: Effects on growth performance, blood traits, gut morphology and histological features. J. Anim. Sci. Biotechnol. 9, 49. doi: 10.1186/s40104-018-0266-9
De Souza Vilela J., Andronicos N. M., Kolakshyapati M., Hilliar M., Sibanda T. Z., Andrew N. R., et al. (2021). Black soldier fly larvae in broiler diets improve broiler performance and modulate the immune system. Anim. Nutr. 7, 695–706. doi: 10.1016/j.aninu.2020.08.014
Dörper A., Berman H. M., Gort G., Van Harn J., Dicke M., Veldkamp T. (2024). Effects of different black soldier fly larvae products on slow-growing broiler performance and carcass characteristics. Poultry Sci. 103, 103481. doi: 10.1016/j.psj.2024.103481
Downes F. P., Ito H. (2001). Compendium of methods for the microbiological examination of foods (Washington: American Public. Health Association (APHA).
Edo G. I., Yousif E., Al-Mashhadani M. H. (2024). Chitosan: An overview of biological activities, derivatives, properties, and current advancements in biomedical applications. Carbohydr. Res. 542, 109199. doi: 10.1016/j.carres.2024.109199
Eleroğlu H., Yıldırım A., Işıklı N., Şekeroğlu A., Duman M. (2013a). Comparison of meat quality and fatty acid profile in slow-growing chicken genotypes fed diets supplemented with origanum vulgare or melissa officinalis leaves under the organic system. Ital. J. Anim. Sci. 12, 395–403. doi: 10.4081/ijas.2013.e64
Eleroğlu H., Yıldırım A., Şekeroğlu A., Duman M. (2013b). Comparison of the Growth Performance and Carcass Characteristics of Two Slow-Growing Broiler Genotypes Fed Diets Supplemented with Dry Oregano (Origanum vulgare L.) or Lemon Balm (Melissa officinalis L.) Leaves under the Organic System. Kafkas Üniversitesi Veteriner Fakültesi Dergisi 20, 49–58.
European Commission Regulation. (2021). Commission Regulation. 2021/1372 of 17 August 2021 amending Annex IV to Regulation (EC) No 999/2001 of the European Parliament and of the Council as regards the prohibition to feed non-ruminant farmed animals, other than fur animals, with protein derived from animals. Off. J. Eur. Union.
Fiorilla E., Gariglio M., Gai F., Zambotto V., Bongiorno V., Cappone E. E., et al. (2024). Dehydrated and live black soldier fly larvae as environmental enrichment in indigenous slow-growing chickens: performance, gut health, and chitinolytic enzyme activity. animal 18, 101239. doi: 10.1016/j.animal.2024.101239
Fletcher D. L. (1999). Broiler breast meat color variation, pH, and texture. Poult Sci. 78, 1323–1327. doi: 10.1093/ps/78.9.1323
Food and Agriculture Organization of the United Nations (FAO) (2021). OECD-FAO agricultural outlook 2021-2030. (Rome: FAO). Available at: https://www.fao.org.
Gasco L., Biasato I., Dabbou S., Schiavone A., Gai F. (2019). Animals fed insect-based diets: state-of-the-art on digestibility, performance and product quality. Animals 9, 170. doi: 10.3390/ani9040170
Gold M., Cassar C. M., Zurbrügg C., Kreuzer M., Boulos S., Diener S., et al. (2020). Biowaste treatment with black soldier fly larvae: Increasing performance through the formulation of biowastes based on protein and carbohydrates. Waste Manage. 102, 319–329. doi: 10.1016/j.wasman.2019.10.036
Hartinger K., Fröschl K., Ebbing M. A., Bruschek-Pfleger B., Schedle K., Schwarz C., et al. (2022). Suitability of Hermetia illucens larvae meal and fat in broiler diets: effects on animal performance, apparent ileal digestibility, gut histology, and microbial metabolites. J. Anim. Sci. Biotechnol. 13, 50. doi: 10.1186/s40104-022-00701-7
Hartinger K., Greinix J., Thaler N., Ebbing M. A., Yacoubi N., Schedle K., et al. (2021). Effect of graded substitution of soybean meal by hermetia illucens larvae meal on animal performance, apparent ileal digestibility, gut histology and microbial metabolites of broilers. Anim. (Basel) 11. doi: 10.3390/ani11061628
Hoffman L. C. (2019). Impact of insect larvae on meat quality. Proceedings 36, 186. doi: 10.3390/proceedings2019036186
Hwangbo J., Hong E. C., Jang A., Kang H. K., Oh J. S., Kim B. W., et al. (2009). Utilization of house fly-maggots, a feed supplement in the production of broiler chickens. J. Environ. Biol. 30(4), 609–641.
Ipema A. F., Gerrits W. J. J., Bokkers E., Kemp B., Bolhuis J. E. (2020). Provisioning of live black soldier fly larvae (Hermetia illucens) benefits broiler activity and leg health in a frequency- and dose-dependent manner. Appl. Anim. Behav. Sci. 230, 105082. doi: 10.1016/j.applanim.2020.105082
Joseph S. M., Krishnamoorthy S., Paranthaman R., Moses J. A., Anandharamakrishnan C. (2021). A review on source-specific chemistry, functionality, and applications of chitin and chitosan. Carbohydr. Polym. Technol. Appl. 2, 100036. doi: 10.1016/j.carpta.2021.100036
Kemboi V. J., Kipkoech C., Njire M., Were S., Lagat M. K., Ndwiga F., et al. (2022). Biocontrol potential of chitin and chitosan extracted from black soldier fly pupal exuviae against bacterial wilt of tomato. Microorganisms 10. doi: 10.3390/microorganisms10010165
Kierończyk B., Rawski M., Mikołajczak Z., Szymkowiak P., Stuper-Szablewska K., Józefiak D. (2023). Black soldier fly larva fat in broiler chicken diets affects breast meat quality. Animals 13, 1137. doi: 10.3390/ani13071137
Kumar M., Rajput M., Soni T., Vivekanand V., Pareek N. (2020). Chemoenzymatic production and engineering of chitooligosaccharides and N-acetyl glucosamine for refining biological activities. Front. Chem. 8, 469. doi: 10.3389/fchem.2020.00469
Lagat M. K., Were S., Ndwigah F., Kemboi V. J., Kipkoech C., Tanga C. M. (2021). Antimicrobial activity of chemically and biologically treated chitosan prepared from black soldier fly (Hermetia illucens) pupal shell waste. Microorganisms 9, 2417. doi: 10.3390/microorganisms9122417
Lee J., Kim Y.-M., Park Y.-K., Yang Y.-C., Jung B.-G., Lee B.-J. (2018). Black soldier fly (Hermetia illucens) larvae enhances immune activities and increases survivability of broiler chicks against experimental infection of Salmonella Gallinarum. J. Veterinary Med. Sci. 80, 736–740. doi: 10.1292/jvms.17-0236
Leni G., Maistrello L., Pinotti G., Sforza S., Caligiani A. (2022). Production of carotenoid-rich Hermetia illucens larvae using specific agri-food by-products. J. Insects as Food Feed 9, 1–12.
Makkar H. P. S., Tran G., Heuzé V., Ankers P. (2014). State-of-the-art on use of insects as animal feed. Anim. Feed Sci. Technol. 197, 1–33. doi: 10.1016/j.anifeedsci.2014.07.008
Masagounder K., Ramos S., Reimann I., Channarayapatna G. (2016). Optimizing nutritional quality of aquafeeds. In: Aquafeed formulation. Academic Press, pp 239–264. https://doi.org/10.1016/B978-0-12-800873-7.00006-3
Matsue M., Mori Y., Nagase S., Sugiyama Y., Hirano R., Ogai K., et al. (2019). Measuring the antimicrobial activity of lauric acid against various bacteria in human gut microbiota using a new method. Cell Transplant. 28, 1528–1541. doi: 10.1177/0963689719881366
Moretta A., Salvia R., Scieuzo C., Di Somma A., Vogel H., Pucci P., et al. (2020). A bioinformatic study of antimicrobial peptides identified in the Black Soldier Fly (BSF) Hermetia illucens (Diptera: Stratiomyidae). Sci. Rep. 10, 16875. doi: 10.1038/s41598-020-74017-9
Onsongo V. O., Osuga I. M., Gachuiri C. K., Wachira A. M., Miano D. M., Tanga C. M., et al. (2018). Insects for income generation through animal feed: effect of dietary replacement of soybean and fish meal with black soldier fly meal on broiler growth and economic performance. J. Econ Entomol 111, 1966–1973. doi: 10.1093/jee/toy118
Pastor B., Velasquez Y., Gobbi P., Rojo S. (2015). Conversion of organic wastes into fly larval biomass: bottlenecks and challenges. J. Insects as Food Feed 1, 179–194. doi: 10.3920/JIFF2014.0024
Petracci M., Baéza E. (2011). Harmonization of methodologies for the assessment of poultry meat quality features. World's Poult. Sci. J. 68, 137–153. doi: 10.1017/S0043933911000122
Pieterse E., Erasmus S. W., Uushona T., Hoffman L. C. (2019). Black soldier fly (Hermetia illucens) pre-pupae meal as a dietary protein source for broiler production ensures a tasty chicken with standard meat quality for every pot. J. Sci. Food Agric. 99, 893–903. doi: 10.1002/jsfa.2019.99.issue-2
Popova T. L., Petkov E., Ignatova M. (2020). Effect of Black Soldier Fly (Hermetia illucens) meals on the meat quality in broilers. Agric. Food Sci. 29, 177–188. doi: 10.23986/afsci.88098
Rumpold B. A., Schlüter O. K. (2013). Nutritional composition and safety aspects of edible insects. Mol. Nutr. Food Res. 57, 802–823. doi: 10.1002/mnfr.201200735
Sanchez Matos J., Barberino A. T. M. S., De Araujo L. P., Lôbo I. P., De Almeida Neto J. A. (2021). Potentials and limitations of the bioconversion of animal manure using fly larvae. Waste Biomass Valorization 12, 3497–3520. doi: 10.1007/s12649-020-01141-y
Schiavone A., Dabbou S., Petracci M., Zampiga M., Sirri F., Biasato I., et al. (2019). Black soldier fly defatted meal as a dietary protein source for broiler chickens: effects on carcass traits, breast meat quality and safety. Animal 13, 2397–2405. doi: 10.1017/S1751731119000685
Shahanaz Shankaraswamy J. (2020). Entomoremediation: An ecofriendly approach for waste management: A review. J. Entomology Zoology Stud. 8, 2022–2025. doi: 10.22271/j.ento.2020.v8.i6aa.9171
Silva C. D., Ruiz-Esparza J., Silva F. O. D., Santos J. C., Ribeiro A. D. S. (2021). Consumption of insects by birds in guava orchards (Psidium guajava L.). J. Environ. Anal. Prog. 6, 113–118. doi: 10.24221/jeap.6.2.2021.4116.113-118
Spranghers T., Ottoboni M., Klootwijk C., Ovyn A., Deboosere S., De Meulenaer B., et al. (2017). Nutritional composition of black soldier fly (Hermetia illucens) prepupae reared on different organic waste substrates. J. Sci. Food Agric. 97, 2594–2600. doi: 10.1002/jsfa.2017.97.issue-8
Surendra K. C., Tomberlin J. K., Van Huis A., Cammack J. A., Heckmann L.-H. L., Khanal S. K. (2020). Rethinking organic wastes bioconversion: Evaluating the potential of the black soldier fly (Hermetia illucens (L.)) (Diptera: Stratiomyidae) (BSF). Waste Manage. 117, 58–80. doi: 10.1016/j.wasman.2020.07.050
Tschirner M., Simon A. (2015). Influence of different growing substrates and processing on the nutrient composition of black soldier fly larvae destined for animal feed. J. Insects as Food Feed 1(4), 249–259. doi: 10.3920/JIFF2014.0008
van Huis A., Van Itterbeeck J., Klunder H., Mertens E., Halloran A., Muir G., et al. (2013). Edible insects: future prospects for food and feed security. (FAO forestry paper; No. 171). FAO. https://edepot.wur.nl/258042.
Veldkamp T., Bosch G. (2015). Insects: a protein-rich feed ingredient in pig and poultry diets. Anim. Front. 5(2), 45–50. doi: 10.2527/af.2015-0019
Viriyarattanasak C., Hamada-Sato N., Watanabe M., Kajiwara K., Suzuki T. (2011). Equations for spectrophotometric determination of relative concentrations of myoglobin derivatives in aqueous tuna meat extracts. Food Chem. 127, 656–661. doi: 10.1016/j.foodchem.2011.01.001
Xu J., Luo X., Fang G., Zhan S., Wu J., Wang D., et al. (2020). Transgenic expression of antimicrobial peptides from black soldier fly enhance resistance against entomopathogenic bacteria in the silkworm, Bombyx mori. Insect Biochem. Mol. Biol. 127, 103487. doi: 10.1016/j.ibmb.2020.103487
Yan W., Sun C., Yuan J., Yang N. (2017). Gut metagenomic analysis reveals prominent roles of Lactobacillus and cecal microbiota in chicken feed efficiency. Sci. Rep. 7, 45308. doi: 10.1038/srep45308
Yang J., Li Y., Wen Z., Liu W., Meng L., Huang H. (2021). Oscillospira - a candidate for the next-generation probiotics. Gut Microbes 13, 1987783. doi: 10.1080/19490976.2021.1987783
Keywords: black soldier fly, larvae meal, broiler chickens, growth performance, meat quality, cecal microbiota
Citation: Saidani M, Dabbou S, Ben Larbi M, Belhadj Slimen I, Fraihi W, Arbi T, Amraoui M and M’Hamdi N (2025) Effect of black soldier fly (Hermetia illucens L.) larvae meal on growth performance, carcass characteristics, meat quality, and cecal microbiota in broiler chickens. Front. Anim. Sci. 6:1531773. doi: 10.3389/fanim.2025.1531773
Received: 20 November 2024; Accepted: 18 February 2025;
Published: 20 March 2025.
Edited by:
Rayudika Aprilia Patindra Purba, Veterinary Paramedic Program, Airlangga University, IndonesiaReviewed by:
Arda Yıldırım, Gaziosmanpaşa University, TürkiyeAnusorn Cherdthong, Khon Kaen University, Thailand
Copyright © 2025 Saidani, Dabbou, Ben Larbi, Belhadj Slimen, Fraihi, Arbi, Amraoui and M’Hamdi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sihem Dabbou, c2loZW0uZGFiYm91QHVuaXRuLml0
 Mariem Saidani
Mariem Saidani Sihem Dabbou
Sihem Dabbou Manel Ben Larbi
Manel Ben Larbi Imen Belhadj Slimen
Imen Belhadj Slimen Wael Fraihi4
Wael Fraihi4 Naceur M’Hamdi
Naceur M’Hamdi