
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Anim. Sci. , 21 March 2025
Sec. Animal Nutrition
Volume 6 - 2025 | https://doi.org/10.3389/fanim.2025.1507440
 Wenzhong Li1
Wenzhong Li1 Mengli Zhao2,3
Mengli Zhao2,3 Lei Zhang2
Lei Zhang2 Guobo Sun2
Guobo Sun2 Hongchang Zhao2
Hongchang Zhao2 Gansheng Zhang2
Gansheng Zhang2 Rongchao Ji3
Rongchao Ji3 Jian Wang2,3
Jian Wang2,3 Xiaoming Li1,2,3*
Xiaoming Li1,2,3* Guoshun Chen1*
Guoshun Chen1*Herein, 364 one-day-old male Jiangnan white goslings were divided into four groups: PM (18.55% crude protein (CP) + main amino acids (AA)), PA (18.55% CP + all AA), LPM (15.55% CP + main AA), and LPA (15.55% CP + all AA). The main AAs were Lys, Met, Thr, Trp, Arg, and Cys, while all AAs included an additional six (Met, Lys, Arg, His, Ile, Leu, Cys, Phe, Tyr, Thr, Trp, and Val). At 28 days, all geese were switched to a 15.55% CP + main AA diet until 63 days. The results showed: (1) No significant impacts on average daily gain or feed-to-gain ratio at 29-63 days. Although numerical differences in average daily feed intake (ADFI) were observed between groups, statistical analysis indicated that the reduction in ADFI due to early low protein was marginal (P = 0.06). This suggests that the dietary protein level may influence feed intake patterns in goslings, but further studies with larger sample sizes are needed to confirm this effect. (2) Early low protein significantly affected abdominal fat percentage and leg muscle cooking loss at 63 days (P < 0.05). Pretreatment affected breast muscle rate at 63 days (P < 0.05). (3) Early low protein significantly increased Cys content in breast muscle at 63 days and increased Asp, Thr, Cys, and His in feces at 62-64 days (P < 0.05). (4) Early low protein led to a significant reduction in nitrogen excretion and an increase in nitrogen utilization (NU) in feces at 62-64 days (P < 0.05). Low-protein diets reduced nitrogen excretion by 12.3% (1.60 vs. 1.78 g/bird, P < 0.05) and improved nitrogen utilization from 56.03% to 59.48%. Leg muscle cooking loss decreased by 15.2% in the LPA group (15.01%) compared to PA (19.58%, P < 0.05). To conclude, different AA supplementation patterns with low protein (15.55%) in the early stage (1-28 days) had no significant effects on body weight, slaughter performance, and meat quality at 63 days. However, early low protein significantly reduced nitrogen excretion and improved NU, suggesting it is feasible for meat goslings to adopt a low-protein diet supplemented with main AAs during the early stage.
The extensive development of animal husbandry has led to a protein feed shortage, impeding further progress. Addressing this challenge, low-protein feed emerges as a crucial solution, conserving resources and mitigating environmental impact. This diet, tailored to diverse livestock needs, lowers protein levels by 2%–4% and is supplemented with crystal amino acids (AA) to meet nutritional requirements (Bezerra et al., 2016). The low-protein diet (LPD) use without additional AA supplementation can reduce body weight (BW) in broilers (De Cesare et al., 2019; Maynard et al., 2022), increase the feed-to-gain (F/G) (Hofmann et al., 2020), and cause intestinal damage in broilers to induce histological changes due to malnutrition (Buwjoom et al., 2010). However, positive effects have been observed in LPDs supplemented with one or more AA in chickens (Zamani et al., 2021), ducks (Xie et al., 2017), and geese (Liang et al., 2023).
The application of low-protein feed approach maintains the growth performance of poultry, reduces nitrogen excretion (Belloir et al., 2017; Chalova et al., 2016; Sigolo et al., 2017), and improves the nitrogen utilization (NU) rate (Hofmann et al., 2019; Rehman et al., 2017), which can play a good role in protecting the environment. Adding Lys, Met, and Thr to reduce the dietary crude protein (CP) content can reduce broilers’ average daily feed intake (ADFI), which is conducive to lowering dietary use and nitrogen pollutant emissions (De Cesare et al., 2019). Essential AA supplementations (Met, Lys, Thr, Val, Arg, Ile, Phe, Leu, His, and Trp) in three different LPDs increased BW, decreased Feed conversion ratio (FCR), and generally improved growth performance (Hilliar et al., 2020). Adding two of the most limiting AAs (methionine and lysine) to the LPD of broilers can simultaneously reduce FCR and maintain the growth rate of broilers, which is conducive to reducing dietary costs (Lee et al., 2020). In the control group, where Gly, Ser, or Thr was supplemented to the Low-Protein (LP) diet at 1.6%, both AA digestibility and plasma levels were reduced in the broilers. However, NU elevated by 9.6%, and the growth performance of the broilers was not negatively impacted. Simultaneously, CP reduction was expected to lower nitrogen excretion in broilers and enhance bird welfare. Reduce dependence on plant protein sources (van Harn et al., 2019). Essential AA supplementation (Val, Ile, Arg, Trp, Gly, and Ser) in LPDs can maintain product performance and reduce nitrogen discharge under specific environmental conditions (Hilliar et al., 2019). To prevent performance degradation, broilers must be supplemented with Val, Ile, Arg, and Gly at the growth stage. The recommended Thr content in the LPD is 110%, which improves average daily gain (ADG), ileal amino acid digestibility (IADF), amino acid digestibility efficiency index (ADEI), albeit to sub-optimal levels (ADPI), and visceral carcass weight, thus improving carcass yield (Ospina-Rojas et al., 2020) (Kriseldi et al., 2018). pointed out that AA supplementation can significantly reduce dietary CP levels, thus significantly reducing nitrogen excretion. In particular, when dietary protein level is insufficient and dietary AA supplement is excessive, production performance decreases, and nitrogen excretion increases (Applegate et al., 2008). Therefore, an LPD saves protein and protects the environment. The Jiangnan white goose (Anser cygnoides domesticus), a breed renowned for rapid growth and high feed efficiency under intensive farming systems in China, was selected for this study. Its adaptability to low-protein diets makes it a strategic model for sustainable poultry production (Chen et al., 2021).
However, there has been no research on the influences of distinct protein levels and AA supplementations in the early stages of meat geese in later goslings. Therefore, based on a corn-soybean meal diet, we investigated these effects in the early stages on goslings’ growth performance and nitrogen metabolism in the later stage (29–63 days). By evaluating growth and slaughter performances, meat quality, AA content of tissue and feces, nutrient availability, and nitrogen metabolism, the feasibility of early LPD application for goslings was explored.We hypothesized that a low-protein diet (15.55% CP) supplemented with key amino acids (Lys, Met, Thr, Trp, Arg, and Cys) during the early growth phase (1–28 days) would maintain growth performance and slaughter traits in goslings while significantly reducing nitrogen excretion, thereby improving environmental sustainability.
All experimental protocols were approved by the Administrative Committee for Jiangsu Agri-animal Husbandry Vocational College Animal Welfare and Ethics (Permission number: JAHV-2024-55). All methods were carried out in accordance with relevant guidelines and regulations. All methods are reported in accordance with ARRIVE guidelines (https://arriveguidelines.org).
Herein, 364 one-day-old male Jiangnan white goslings (National Gene Bank of Waterfowl Resources, Taizhou, China) were allocated at random into four groups of seven replicates and 13 geese each. The preliminary treatment (1–28 days) involved a two-factor, two-level factorial design with two protein levels (18.55% and 15.55%) and two AA supplementation patterns (main AA supplement and all AA supplements). A total of 364 goslings were randomly allocated into four dietary treatment groups (PM, PA, LPM, LPA), with each group containing 7 replicates. Each replicate consisted of 13 geese housed in a single pen, ensuring equal representation across all experimental conditions. The four groups included PM (CP 18.55% + main AA, MJ/kg 11.20), PA (CP 18.55% + all AA, MJ/kg 11.20), LPM (CP 15.55% + main AA, MJ/kg 11.20), and LPA (CP 15.55% + all AA, MJ/kg 11.20), encompassing six main AAs (Lys, Met, Thr, Trp, Arg, and Cys) and 12 all AAs (Met, Lys, Arg, His, Ile, Leu, Cys, Phe, Tyr, Thr, Trp, and Val). L-His was supplemented in the PA diet to ensure a complete amino acid profile, as His is often a limiting amino acid in plant-based diets (Hilliar et al., 2020). However, in this study, the “main AA” groups (PM and LPM) did not include L-His supplementation, whereas it was included in the “all AA” groups (PA and LPA). This differentiation was designed to evaluate the impact of different amino acid supplementation strategies on growth performance and nitrogen metabolism. During the post-test treatment (29–63 days), geese from the above groups were fed the same diet from day 29 (CP 15.55% + main AA, MJ/kg 10.91), with the main AAs continuing until day 63. The experiment lasted 35 days, with goslings housed on wire nets at a stocking density of 6/m2 and 18°C from days 29 to 63. Illumination was maintained daily for 14 h. Dietary nutritional levels followed the guidelines of the NRC (1994) and our laboratory’s previous reports (Zhiyue et al., 2010). Tables 1, 2 summarize details of the primary dietary composition and nutritional levels. The goslings were allowed to access food and water ad libitum during the experiment.
The weight of all goslings was determined at 28 and 63 days. The feed intake of each replicate was recorded weekly from 29 to 63 days, calculating ADG, ADFI, and F/G at the end of the experiment.
On 63 days, two geese with similar average BW were selected for each repetition, starved for 6 h, and slaughtered. Carcass weight was recorded after bleeding and plucking. For each goose, we weighed the eviscerated, semi-eviscerated, and eviscerated carcasses, as well as breast muscle (pectoralis main and minor), leg (whole leg), and abdominal fat (fat around the abdomen and gizzard) (Yu et al., 2022). This was followed by weighing the internal organs, including the heart, liver, gizzard, glandular stomach, spleen, and kidney, and calculating the relative organ index.
On day 63, a goose with a BW near the average was chosen for each replicate. The meat from the left breast and leg muscles was removed to assess meat color, pH level, cooking loss, and shear force. Using a chroma meter (Konica Minolta, CR-400, Osaka, Japan) and portable pH meter (pH-STAR, Matthaus, Berlin, Germany), meat colors: lightness (L*), redness (a*), and yellowness (b*) and pH value were measured at 45 min postmortem. The measurements of cooking loss and shear force were conducted via a thermostatic water bath (DK-S24, Shanghai Jinghong Experimental Equipment Co. Ltd, Shanghai, China) and a digital tenderness meter (C-LM3B, Tenovo, Beijing, China). Each sample index was measured three times, and the resulting average was utilized for statistical analysis.
At 63 days, AA contents in breast muscle, leg muscle, serum, liver, and feces were analyzed using the following method: Weighed in a hydrolytic tube, around 100 mg of the sample was measured (recorded to 0.0001 g accuracy). Briefly, 9 mL of 6 mol/L hydrochloric acid (HCl) was added, and the tube was sealed, placed in a –20 °C refrigerator for 5 min, and nitrogen was blown onto the liquid surface for 1 min. The tube was sealed tightly and subjected to hydrolysis at 110°C for 23 h. After cooling, the liquid was transferred to a 100 mL volumetric bottle, adjusted to 100 mL, and thoroughly mixed. Vacuum drying was performed until no liquid remained. Subsequently, 1 mL of 0.02 mol/L HCl was introduced to the Ambrose bottle for further mixing. Using a 5 mL needle, all liquid was extracted and transferred through a 0.22 μ filter head to the upper bottle. Tryptophan was analyzed via alkaline hydrolysis (4.2 mol/L NaOH, 110°C, 20 h) to prevent degradation, followed by HPLC quantification (Waters Alliance System, Milford, MA) as per AOAC Method 988.15. A LA8080 automatic AA analyzer was utilized for AA determination.
At 56 days, one gosling per treatment was habituated in an individual wire-floor metabolic cage at 18 ± 2°C, with ad libitum access to water and food for a 5-day adaptation period and a 3-day excreta (feces) collection period. During fecal collection, precautions were taken to remove feathers and dander from the tray to prevent contamination. The fixation of excreta nitrogen was conducted by the addition of 10 mL of 10% HCl/100 g of feces. After a 65°C oven drying, the moisture was regained for 24 h, ground via a 40-mesh sieve, and general nutrient indexes (crude fat and acid and neutral detergent fiber, as well as calcium and phosphorus) were determined. Nitrogen metabolism-associated indicators (nitrogen intake, excretion, deposition, and utilization ratio) were assessed using the Kjeldahl method with the Kjeltec System 8400 (FOSS NIRSystems Inc., Hillerød, Denmark) with the following formulas:
Nitrogen intake (g) = feed intake × nitrogen content in feed
Nitrogen excretion (g) = fecal output × nitrogen content in feces
Nitrogen deposition (g) = nitrogen intake - nitrogen intake
Nitrogen utilization ratio (%) = (nitrogen deposition/nitrogen intake) ×100%
The diets were processed by grinding the ingredients to a uniform particle size, followed by mixing and pelleting at 65°C to ensure homogeneity and stability.
All data were preliminarily collated using WPS Excel 2023, and then a two-factor analysis of variance was performed through SPSS 26.0 (SPSS, Inc., Chicago, IL). Duncan’s method was utilized for the significance test, representing data by mean values and the standard error of the means (SEM), with P < 0.05 indicating a significant difference.A two-way ANOVA was performed to evaluate the main effects of protein level (18.55% vs. 15.55% CP), AA supplementation pattern (main AA vs. all AA), and their interaction. The statistical model was:
WhereYiik is the dependent variable, μ is the overall mean, Pi is the protein level effect, Aj is the AA supplementation effect, (P×A)ij is the interaction term, and ϵijk is the residual error. Significant interactions were further analyzed using Duncan’s multiple range test.
Table 3 illustrates the impact of early treatment on the growth performance of 29–63 day goslings. Through this period, early treatment did not significantly influence geese BW, ADG, and F/G ratio (P > 0.05), with no observed interaction (P > 0.05). However, varying protein levels in the early stage significantly influenced the ADFI of geese at 29–63 days, notably decreasing with low protein levels in the early stage (P < 0.05).
Table 4 presents the impact of early treatment on the slaughter performance of 63-day-old geese. Different protein levels in the early stage significantly influenced the abdominal fat percentage of geese at 63 days, notably decreasing with low protein levels in the early stage (P < 0.05). Herein, pretreatment significantly interacted with the breast muscle rate of geese at 63 days (P < 0.05). Nonetheless, early treatment had a nonsignificant impact on geese dressing, half-eviscerated, and wing percentages, as well as the leg ratio at 63 days, with no observed interaction (P > 0.05). Different protein levels in the early stage significantly affected the cooking loss of goose leg muscle at 63 days, showing a significant decrease with low protein levels in the early stage (P < 0.05, Table 5). The results showcased an interaction effect between pretreatment and cooking loss of the goose leg muscle at 63 days (P < 0.05). Pretreatment did not significantly impact the cooking loss of breast muscle as well as breast and leg muscle shear force, pH value, and flesh color (L*, a*, and b*) at 63 days, without interaction effect (P > 0.05).
The impact of early treatment on AA contents in breast muscle, leg muscle, serum, liver, and feces of 63-day-old geese is detailed in Tables 6–10. Different protein levels in the early stage significantly increased Cys content in goose breast muscle at 63 days, particularly at low protein levels (P < 0.05). In the geese’s breast muscle at 63 days, the AA supplementation mode in the early stage significantly influenced the Cys, Met, and His contents, with a notable increase under the primary AA supplementation mode (P < 0.05). Meanwhile, Ser content in geese liver at 63 days was significantly reduced by the AA supplementation mode in the early stage, particularly under the primary AA supplementation mode (P < 0.05). Asp, Thr, Cys, and His contents in goose feces at 62–64 days were significantly increased by different protein levels in the early stage, particularly under low protein levels (P < 0.05). Early treatment interacted with Arg in goose feces at 62–64 days (P < 0.05).
The impact of early treatment on nutrient utilization and nitrogen metabolism in 62-to 64-day-old geese is presented in Table 11. Early treatment did not significantly affect crude fat and neutral and acid detergent fibers, as well as calcium and phosphorus in goose feces at 62–64 days (P > 0.05). However, different protein levels in the early stage significantly influenced nitrogen excretion and NU in goose feces at 62–64 days. Low protein levels in the early stage significantly reduced nitrogen excretion and increased NU (P < 0.05).
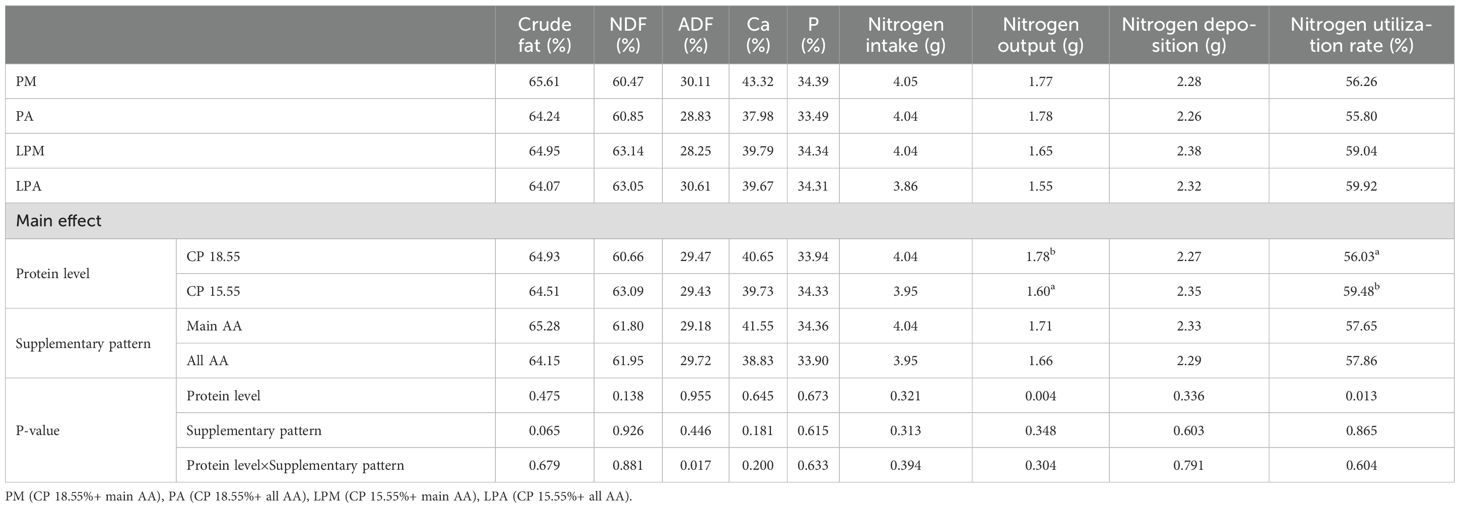
Table 11. Effects of early treatment on nutrient utilization and nitrogen metabolism of 62-64 d geese.
This study fed geese different protein-level diets alongside AA supplementation patterns from 1 to 28 days. Subsequently, the same diet was maintained for 29–63 days to observe regression changes and examine the effects of early treatment on various performances of late goslings (Ashour et al., 2020; Che et al., 2016). In a study on white-feathered broilers, six groups were established with a protein level gradient of 0.5% compared to the normal protein level control group (Yuan et al., 2022). The study included AA-supplemented diets (Lys, Met, Thr, Val, and Ile) and a normal protein level control group. The results showcased that ADG did not significantly differ between the low-protein and control groups on days 1–21. The findings of this study were consistent with those of the current investigation. The experiment revealed that varying protein levels and AA supplementation patterns had no significant impact on the ADG of geese during the early stage (1–28 days) and from 29–63 days, even after transitioning to the same diet.
In contrast to Lee’s discovery (Lee et al., 2020) that a low CP broiler diet supplemented with high lysine and standard methionine resulted in the lowest feed conversion rate, this study did not yield similar results, possibly due to the AA supplement not exceeding the normal protein level content in the control group (Wang et al., 2021). researched broilers, implementing a LPD supplemented with different Cys concentrations (0.05%, 0.1%, 0.15%, and 0.2%). Their results suggest that a 0.1% Cys-supplemented LPD might enhance broiler growth performance by regulating serum metabolic profiles. The study by (Jariyahatthakij et al., 2018) on broilers, LPDs supplemented with various AA combinations (Lys + Met, Lys + Thr, Met + Thr, and Lys + Met + Thr) elucidated that Met supplementation in a growing LPD, followed by feeding a control diet, enhanced dietary and protein conversion compared to the control diet. Our comprehensive analysis indicates that supplementing essential AAs in an LPD for goslings does not significantly affect BW, ADG, ADFI, and F/G. Thus, from an economical cost perspective, reducing dietary protein levels for goslings and employing a primary AA supplementation pattern in the LPD can meet normal growth performance requirements without adversely affecting production performance.
Slaughter performance is crucial for assessing animal production, reflecting carcass weight, and individual part performance across various physiological stages. There is a scarcity of research on the slaughtering performance of LPD-fed geese. Research on broiler fat deposition indicates that a Met-supplemented low CP diet throughput growth, followed by a control diet, improves feed and protein conversion rates, reduces fat accumulation, and lowers fat deposition costs (Jariyahatthakij et al., 2018). Lysine is pivotal for muscle protein production in broilers, and its deficiency can lead to a 40%–45% reduction in BW (Tesseraud et al., 1996). High Lys levels in brisket impact brisket yield, and reducing dietary protein while adding Lys improves broilers’ feed efficiency and brisket yield (Oliveira et al., 2022). This study demonstrates that reducing protein levels and AA supplementation in goslings’ diets can maintain carcass quality like the normal protein level control group without adversely affecting slaughter performance.
In contrast (Jariyahatthakij et al., 2018), and (Ma et al., 2023) highlighted the complexity of dietary effects on fat accumulation. However (Jariyahatthakij et al., 2018), demonstrated that Met supplementation in a LPD reduced fat accumulation and production costs in broilers (Ma et al., 2023). found that LPDs for broilers could simultaneously alleviate lipid deposition. The higher abdominal fat percentage observed in geese fed conventional-CP diets (18.55%) compared to low-CP diets (15.55%) (Table 4) may be attributed to an imbalance in energy utilization. While high-protein diets theoretically enhance nitrogen retention, excess protein may be deaminated and converted to energy substrates, leading to increased lipogenesis. This hypothesis aligns with the lower nitrogen utilization rate (NU) in conventional-CP groups (Table 11), indicating inefficient protein metabolism and potential energy surplus. Consumer choices in meat selection often hinge on flesh color and overall appearance characteristics. Beyond yield, meat quality significantly influences consumer preferences in the meat industry. LPDs have been linked to improved meat quality in pigs by affecting fatty acid composition, intramuscular fat content, fiber characteristics, and free AA profiles (Li et al., 2018). The leg muscle cooking loss displayed significant differences with varying protein levels in the early stage (Table 5). Specifically, geese fed the LPA diet (15.55% CP + all AA) showed the lowest cooking loss (15.01%a), while the PA group (18.55% CP + all AA) exhibited the highest value (19.58%b). This interaction between protein level and AA supplementation pattern suggests that a low-protein diet combined with comprehensive AA supplementation may improve muscle water-holding capacity, potentially due to enhanced protein synthesis efficiency. This outcome may be attributed to the increased deposition efficiency in the leg muscles of geese fed an LPD in the early stage, resulting in denser muscle fibers and reduced cooking loss.Contrary to studies in broilers reporting increased fat-pad weights in low-CP diets (e.g., Belloir et al., 2017), our results showed no significant differences in abdominal fat percentage between geese fed low- and conventional-CP diets (Table 4). This discrepancy may stem from species-specific metabolic adaptations. Geese (Anser cygnoides), as herbivorous waterfowl, exhibit distinct energy partitioning strategies compared to broilers, favoring lean muscle deposition over adipose tissue under protein-restricted conditions (Liang et al., 2023).
Met is the primary limiting AA crucial for young geese’ growth and development, thereby being pivotal in their overall growth (Wu, 2013) (Hofmann et al., 2019). explored the impact of Gly concentration (0.12%, 0.15%, 0.18%, and 0.21%) and protein levels (CP 16.3%, 14.7%, and 13.2%) on nitrogen metabolism and plasma metabolome changes in broilers. Their findings revealed that reducing CP from 16.3% to 14.7% restricted broiler growth, with nutrients other than Gly becoming limiting factors. While AA studies have been conducted on chickens (Coon and Balling, 1984; Porteous, 1980), limited research has been conducted on geese. Accordingly, we ascertained the impact of different protein levels and AA supplementation patterns during the early stage on later-stage goslings, examining AA contents in breast muscle, leg muscle, serum, liver, and feces. The significant increase in Cys content in the breast muscle of geese fed low-CP diets (Table 6) may be explained by adaptive metabolic responses to protein restriction. Under low-protein conditions, sulfur-containing amino acids such as Cys and Met are prioritized for protein synthesis and antioxidant defense systems (Wu, 2013). The supplementation of Cys in the LCP diets (LPM and LPA groups) likely enhanced its retention in muscle tissue, compensating for potential deficiencies caused by reduced dietary protein. Additionally, the downregulation of Cys catabolism enzymes (e.g., cystathionase) under low-protein conditions may further contribute to its accumulation in breast muscle.
Interestingly, AA content did not significantly differ in the leg muscle and serum among the varying protein levels and AA supplementation patterns. This lack of difference may be because the meat deposition process in the leg muscle is nearly complete, and serum, as a major AA transport site in the body, maintains consistent AA levels within a certain range. Upon transporting AA to the liver via the serum, the Ser content in the liver was notably higher in the pre-supplementation pattern of total AA. This may be attributed to the comprehensive AA profile of the whole AA group, facilitating Ser metabolism in the liver and synthesizing and utilizing related products. In contrast, Asp, Thr, Cys, and His contents were significantly higher in the LPD group, possibly because the low-protein group exhibited AA lower digestion, absorption, and utilization rates in comparison to the standard protein level group, resulting in excreting excess unused AAs in the feces. Further studies are required to comprehensively understand the variations in AA contents in tissues and feces. The addition of L-Cys in the PM diet was based on previous findings that Cys plays a vital role in sulfur metabolism and antioxidant defense in poultry (Wu, 2013). While DL-Met can serve as a precursor for Cys synthesis, our goal was to isolate the effects of direct Cys supplementation under low-protein conditions. However, we recognize that optimizing sulfur-containing amino acid supplementation (e.g., prioritizing DL-Met) could enhance dietary efficiency. Future studies will explore balancing Cys and DL-Met to maximize nutrient utilization.
Poultry primarily excretes nitrogen in compounds like urea, creatinine, and AA, with around 70% to 80% being uric acid (Orosz and Echols, 2020). Microorganisms convert these excreta into ammonia (Ferguson et al., 1998) (De Cesare et al., 2019). manifested that lowering the protein level in broiler production by 7%, coupled with adding Lys, Met, and Thr, was beneficial for minimizing protein feed usage and nitrogen pollutant emissions (Cappelaere et al., 2021). demonstrated that a 2.2%–2.3% reduction in broiler protein levels was achieved through AA supplementation (Lys, Met, Thr, Arg, Ile, Gly, and Trp), reduced nitrogen emissions, enhanced animal welfare and reduced reliance on plant protein-based diets (Lemme et al., 2019). revealed that supplementing Lys to low-protein broiler diets did not effectively reduce nitrogen emissions.
However (Donato et al., 2016), found that supplementing methionine + cysteine and threonine caused a significant decrease in environmental nitrogen excretion in broilers. Feeding geese diets with different protein levels and AA supplementation patterns in the early stage showed no significant impact on nutrient utilization-related metrics in the later stage but did affect nitrogen metabolism. Specifically, feeding varied protein levels in the early stage and significantly reduced nitrogen output in geese fed the same diet later, contributing positively to environmental protection. Despite no significant differences in nitrogen intake and deposition, different protein levels and AA supplementation patterns significantly improved the NU rate of goslings in the early stage, aiding in reducing environmental pollution during farming. The potential metabolic mechanism lies in the varied protein levels during the early stage, allowing for more effective regulation of nitrogen digestion, absorption, and utilization in the gosling intestines. This enhanced efficiency in NU persists even when geese are fed the same diet in the later period. In conclusion, adjusting protein levels and AA supplementation patterns during the early feeding period can enhance NU efficiency in goslings, reducing environmental pollution during breeding.
Although amino acid supplementation in geese diets is less common than in broilers, recent studies have demonstrated its feasibility. Liang et al. (2023) reported that amino acid supplementation in low-protein diets effectively maintained growth performance and reduced nitrogen excretion in goslings. Similarly, Xie et al. (2017) found positive effects in ducks. Our findings align with these results, showing that amino acid supplementation in low-protein diets reduced nitrogen excretion by 12% while maintaining growth performance (Table 11). Economically, reducing dietary protein from 18.55% to 15.55% with main AA supplementation could lower feed costs by 8–10% (based on soybean meal pricing trends in 2023). Concurrently, the 12.3% reduction in nitrogen excretion may reduce environmental management costs by mitigating ammonia emissions and eutrophication risks, enhancing the sustainability of goose production systems.
In summary, varying AA supplementation modes in the LPD during the early stage (1–28 days) had no notable impact on 63-day geese BW, slaughter performance, and meat quality. However, adopting an LPD in the early stage caused a significant reduction in nitrogen excretion and enhancement in NU. The observed growth potential of late-stage goslings may be owing to improved NU. Therefore, in the early stage, utilizing an LPD (15.55%) supplemented with critical AAs (Lys, Met, Thr, Trp, Arg, and Cys) is a viable approach for meat goslings. Significantly, this dietary strategy did not compromise the BW and meat quality of late-stage goslings.
Future studies should focus on supplementing only essential amino acids (e.g., Lys, Met, Thr) to improve cost-effectiveness while maintaining performance. This strategy aligns with practical feeding approaches in commercial poultry production and could enhance the economic feasibility of amino acid-supplemented low-protein diets.
In summary, varying AA supplementation modes in the LPD during the early stage (1-28 days) had no notable impact on the 63-day geese BW, slaughter performance, and meat quality. However, adopting an LPD in the early stage significantly reduced nitrogen excretion besides enhancing NU. This growth potential of late-stage goslings can be ascribed to enhanced NU. Therefore, in the early stage, utilizing an LPD (15.55%) supplemented with critical AAs (Lys, Met, Thr, Trp, Arg, and Cys) is a viable approach for meat goslings. Significantly, this dietary strategy did not compromise the late-stage goslings’ BW and meat quality.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
All animal care and experimental procedures in the study followed the Regulations for the Administration of Affairs Concerning Experimental Animals of the People’s Republic of China and were authorized by the Yangzhou University Animal Care and Use Committee (Yangzhou, China). SYXK (Su) IACUC 2021-0036. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
WL: Writing – review & editing, Data curation, Validation, Writing – original draft. MZ: Data curation, Writing – review & editing. LZ: Investigation, Writing – review & editing. GS: Methodology, Writing – review & editing. HZ: Formal Analysis, Project administration, Writing – review & editing. GZ: Validation, Writing – review & editing. RJ: Data curation, Project administration, Writing – review & editing. JW: Supervision, Writing – review & editing. XL: Funding acquisition, Resources, Writing – review & editing. GC: Funding acquisition, Resources, Writing – review & editing.
The author(s) declare that financial support was received for the research and/or publication of this article. Funded by the Academy-level Scientific Research Project (NSF2022CB19), The JBGS Project of Seed Industry Revitalization in Jiangsu Province (JBGS[2021]030), Lanzhou Talent Innovation and Entrepreneurship Project (23-3-110), Key Research and Development Program of Gansu Province (23YFNA002), and Protection of livestock and poultry genetic resources (2023-SJ-019).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Applegate T., Powers W., Angel R., Hoehler D. (2008). Effect of amino acid formulation and amino acid supplementation on performance and nitrogen excretion in Turkey toms. Poult. Sci. 87, 514–520. doi: 10.3382/ps.2007-00375
Ashour E. A., Abou-Kassem D. E., Abd-El-Hack M. E., Alagawany M. (2020). Effect of dietary protein and tsaa levels on performance, carcass traits, meat composition and some blood components of Egyptian geese during the rearing period. Animals 10, 549. doi: 10.3390/ani10040549
Belloir P., Meda B., Lambert W., Corrent E., Juin H., Lessire M., et al. (2017). Reducing the CP content in broiler feeds: impact on animal performance, meat quality and nitrogen utilization. Animal 11, 1881–1889. doi: 10.1017/S1751731117000660
Bezerra R. M., Costa F. G., Givisiez P. E., Freitas E. R., Goulart C. C., Santos R. A., et al. (2016). Effect of l-glutamic acid supplementation on performance and nitrogen balance of broilers fed low protein diets. J. Anim. Physiol. Anim. Nutr. (Berl) 100, 590–600. doi: 10.1111/jpn.12405
Buwjoom T., Yamauchi K., Erikawa T., Goto H. (2010). Histological intestinal alterations in chickens fed low protein diet. J. Anim. Physiol. Anim. Nutr. (Berl) 94, 354–361. doi: 10.1111/j.1439-0396.2008.00915.x
Cappelaere L., Le Cour Grandmaison J., Martin N., Lambert W. (2021). Amino acid supplementation to reduce environmental impacts of broiler and pig production: A review. Front. Vet. Sci. 8, 689259. doi: 10.3389/fvets.2021.689259
Chalova V. I., Kim J., Patterson P. H., Ricke S. C., Kim W. K. (2016). Reduction of nitrogen excretion and emission in poultry: A review for organic poultry. J. Environ. Sci. Health B 51, 230–235. doi: 10.1080/03601234.2015.1120616
Che L. Q., Chen D. W., Fang Z. F., Hu L., Li J., Lin Y., et al. (2016). Effects of low-protein diets supplemented with indispensable amino acids on growth performance, intestinal morphology and immunological parameters in 13 to 35 kg pigs. Animal 10, 1812–1820. doi: 10.1017/S1751731116000999
Chen F., Zhang H., Du E., Fan Q., Zhao N., Jin F., et al. (2021). Supplemental magnolol or honokiol attenuates adverse effects in broilers infected with Salmonella pullorum by modulating mucosal gene expression and the gut microbiota. J. Anim. Sci. Biotechnol. 12, 87. doi: 10.1186/s40104-021-00611-0
Coon C., Balling R. (1984). Asparagine and glutamine metabolism in chicks. Poult. Sci. 63, 717–729. doi: 10.3382/ps.0630717
De Cesare A., Faria do Valle I., Sala C., Sirri F., Astolfi A., Castellani G., et al. (2019). Effect of a low protein diet on chicken ceca microbiome and productive performances. Poult. Sci. 98, 3963–3976. doi: 10.3382/ps/pez132
Donato D. C., Sakomura N. K., Silva E. P., Troni A. R., Vargas L., Guagnoni M. A., et al. (2016). Manipulation of dietary methionine+cysteine and threonine in broilers significantly decreases environmental nitrogen excretion. Animal 10, 903–910. doi: 10.1017/s175173111500289x
Ferguson N. S., Gates R. S., Taraba J. L., Cantor A. H., Pescatore A. J., Straw M. L., et al. (1998). The effect of dietary protein and phosphorus on ammonia concentration and litter composition in broilers. Poult. Sci. 77, 1085–1093. doi: 10.1093/ps/77.8.1085
Hilliar M., Hargreave G., Girish C. K., Barekatain R., Wu S. B., Swick R. A. (2020). Using crystalline amino acids to supplement broiler chicken requirements in reduced protein diets. Poult. Sci. 99, 1551–1563. doi: 10.1016/j.psj.2019.12.005
Hilliar M., Huyen N., Girish C. K., Barekatain R., Wu S., Swick R. A. (2019). Supplementing glycine, serine, and threonine in low protein diets for meat type chickens. Poult. Sci. 98, 6857–6865. doi: 10.3382/ps/pez435
Hofmann P., Siegert W., Kenez A., Naranjo V. D., Rodehutscord M. (2019). Very low crude protein and varying glycine concentrations in the diet affect growth performance, characteristics of nitrogen excretion, and the blood metabolome of broiler chickens. J. Nutr. 149, 1122–1132. doi: 10.1093/jn/nxz022
Hofmann P., Siegert W., Naranjo V. D., Rodehutscord M. (2020). Effects of supplemented nonessential amino acids and nonprotein nitrogen on growth and nitrogen excretion characteristics of broiler chickens fed diets with very low crude protein concentrations. Poult. Sci. 99, 6848–6858. doi: 10.1016/j.psj.2020.09.003
Jariyahatthakij P., Chomtee B., Poeikhampha T., Loongyai W., Bunchasak C. (2018). Effects of adding methionine in low-protein diet and subsequently fed low-energy diet on productive performance, blood chemical profile, and lipid metabolism-related gene expression of broiler chickens. Poult. Sci. 97, 2021–2033. doi: 10.3382/ps/pey034
Kriseldi R., Tillman P. B., Jiang Z., Dozier W. A. 3rd (2018). Effects of feeding reduced crude protein diets on growth performance, nitrogen excretion, and plasma uric acid concentration of broiler chicks during the starter period. Poult. Sci. 97, 1614–1626. doi: 10.3382/ps/pex395
Lee C. Y., Song A. A., Loh T. C., Abdul Rahim R. (2020). Effects of lysine and methionine in a low crude protein diet on the growth performance and gene expression of immunity genes in broilers. Poult. Sci. 99, 2916–2925. doi: 10.1016/j.psj.2020.03.013
Lemme A., Hiller P., Klahsen M., Taube V., Stegemann J., Simon I. (2019). Reduction of dietary protein in broiler diets not only reduces n-emissions but is also accompanied by several further benefits. J. Appl. Poultry Res. 28, 867–880. doi: 10.3382/japr/pfz045
Li Y. H., Li F. N., Duan Y. H., Guo Q. P., Wen C. Y., Wang W. L., et al. (2018). Low-protein diet improves meat quality of growing and finishing pigs through changing lipid metabolism, fiber characteristics, and free amino acid profile of the muscle. J. Anim. Sci. 96, 3221–3232. doi: 10.1093/jas/sky116
Liang Y. Q., Zheng X. C., Wang J., Yang H. M., Wang Z. Y. (2023). Different amino acid supplementation patterns in low-protein diets on growth performance and nitrogen metabolism of goslings from 1 to 28 days of age. Poult. Sci. 102, 102395. doi: 10.1016/j.psj.2022.102395
Ma S., Zhang K., Shi S., Li X., Che C., Chen P., et al. (2023). Low-protein diets supplemented with isoleucine alleviate lipid deposition in broilers through activating 5’ adenosine monophosphate-activated protein kinase and janus kinase 2/signal transducer and activator of transcription 3 signaling pathways. Poult. Sci. 102, 102441. doi: 10.1016/j.psj.2022.102441
Maynard C. W., Kidd M. T., Chrystal P. V., McQuade L. R., McInerney B. V., Selle P. H., et al. (2022). Assessment of limiting dietary amino acids in broiler chickens offered reduced crude protein diets. Anim. Nutr. 10, 1–11. doi: 10.1016/j.aninu.2021.11.010
Oliveira C. H., Dias K. M. M., Bernardes R. D., Diana T. F., Rodrigueiro R. J. B., Calderano A. A., et al. (2022). The effects of arginine supplementation through different ratios of arginine:lysine on performance, skin quality and creatine levels of broiler chickens fed diets reduced in protein content. Poult. Sci. 101, 102148. doi: 10.1016/j.psj.2022.102148
Orosz S. E., Echols M. S. (2020). The urinary and osmoregulatory systems of birds. Vet. Clin. North Am. Exot Anim. Pract. 23, 1–19. doi: 10.1016/j.cvex.2019.09.001
Ospina-Rojas I. C., Pozza P. C., Rodrigueiro R. J. B., Gasparino E., Khatlab A. S., Murakami A. E. (2020). High leucine levels affecting valine and isoleucine recommendations in low-protein diets for broiler chickens. Poult. Sci. 99, 5946–5959. doi: 10.1016/j.psj.2020.08.053
Porteous J. W. (1980). Glutamate, glutamine, aspartate, asparagine, glucose and ketone-body metabolism in chick intestinal brush-border cells. Biochem. J. 188, 619–632. doi: 10.1042/bj1880619
Rehman Z., Kamran J., El-Hack M., Alagawany M., Bhatti S., Ahmed G., et al. (2017). Influence of low-protein and low-Amino acid diets with different sources of protease on performance, carcasses and nitrogen retention of broiler chickens. Anim. Prod. Sci. 58, 1625–1631. doi: 10.1071/AN16687
Sigolo S., Zohrabi Z., Gallo A., Seidavi A., Prandini A. (2017). Effect of a low crude protein diet supplemented with different levels of threonine on growth performance, carcass traits, blood parameters, and immune responses of growing broilers. Poult. Sci. 96, 2751–2760. doi: 10.3382/ps/pex086
Tesseraud S., Maaa N., Peresson R., Chagneau A. M. (1996). Relative responses of protein turnover in three different skeletal muscles to dietary lysine deficiency in chicks. Br. Poult. Sci. 37, 641–650. doi: 10.1080/00071669608417893
van Harn J., Dijkslag M. A., van Krimpen M. M. (2019). Effect of low protein diets supplemented with free amino acids on growth performance, slaughter yield, litter quality, and footpad lesions of male broilers. Poult. Sci. 98, 4868–4877. doi: 10.3382/ps/pez229
Wang W. W., Feng Q. Q., Wang J., Wu S. G., Qi G. H., Zhang H. J. (2021). Cyst(e)ine fortification in low crude protein diet improves growth performance of broilers by modulating serum metabolite profile. J. Proteomics 238, 104–154. doi: 10.1016/j.jprot.2021.104154
Wu G. (2013). Functional amino acids in nutrition and health. Amino Acids 45, 407–411. doi: 10.1007/s00726-013-1500-6
Xie M., Jiang Y., Tang J., Wen Z. G., Zhang Q., Huang W., et al. (2017). Effects of low-protein diets on growth performance and carcass yield of growing White Pekin ducks. Poult. Sci. 96, 1370–1375. doi: 10.3382/ps/pew349
Yu J., Zhang H., Yang H. M., Wang Z. Y. (2022). Effects of dietary paddy rice on growth performance, carcass traits, bare skin color, and nutrient digestibility in geese. Poult. Sci. 101, 101865. doi: 10.1016/j.psj.2022.101865
Yuan C., Jiang Y., Wang Z., Chen G., Bai H., Chang G. (2022). Indigenous, yellow-feathered chickens body measurements, carcass traits, and meat quality depending on marketable age. Animals 12, 2422. doi: 10.3390/ani12182422
Zamani M., Zaghari M., Ghaziani F. (2021). Comparison of absorption kinetics and utilisation of DL-methionine (DL-Met), Met-Met product (AQUAVI(R) Met-Met), and protein-bound methionine (PB-Met) by female broiler chickens. Br. Poult. Sci. 62, 539–551. doi: 10.1080/00071668.2021.1884653
Keywords: low-protein diet, amino acid supplementation pattern, growth performance, nitrogen metabolism, goose
Citation: Li W, Zhao M, Zhang L, Sun G, Zhao H, Zhang G, Ji R, Wang J, Li X and Chen G (2025) Impact of low-protein diet on geese growth: early low-protein diets with amino acid supplementation improve nitrogen utilization and maintain growth performance in meat geese. Front. Anim. Sci. 6:1507440. doi: 10.3389/fanim.2025.1507440
Received: 08 October 2024; Accepted: 26 February 2025;
Published: 21 March 2025.
Edited by:
Anusorn Cherdthong, Khon Kaen University, ThailandReviewed by:
Shemil Priyan Macelline, The University of Sydney, AustraliaCopyright © 2025 Li, Zhao, Zhang, Sun, Zhao, Zhang, Ji, Wang, Li and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoming Li, MTUyOTUyMDE2MDdAMTYzLmNvbQ==; Guoshun Chen, Y2hlbmdzODA1OUAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.