- 1College of Animal Science and Technology, Hunan Biological and Electromechanical Polytechnic, Changsha, China
- 2Changsha Luye Biotechnology Co., Ltd, Changsha, China
- 3College of Veterinary Medicine, Hunan Agricultural University, Changsha, China
Globally, the issue of antibiotic residues in agricultural products and their environments is increasingly critical, with the spread of microbial resistance becoming an urgent international challenge. Therefore, the development of ecological health feed additives is of paramount importance for advancing sustainable animal husbandry. Areca nut extract, derived from commonly available food sources, has garnered attention due to its exceptional bioactive properties. Its remarkable anti-inflammatory and antioxidant potential, along with its outstanding performance in antibacterial, antifungal, and antiviral activities, plays a crucial role in inhibiting various pathogens and protecting cells from oxidative damage. This review aims to comprehensively explore the biological activities of areca nut extract and delve into its practical application potential in enhancing animal production efficiency and promoting sustainable livestock development.
The pervasive presence of antibiotic residues—including tetracyclines, sulfonamides, and quinolones—in agricultural products such as meat, milk, and eggs has raised significant concerns due to their extensive use in animal husbandry. This issue is not only a formidable challenge for food safety but also exacerbates the global crisis of antimicrobial resistance (AMR). To address these challenges, there is an urgent need for safe and sustainable alternatives to antibiotics in animal production. Among these alternatives, plant extracts have garnered considerable attention for their natural bioactive properties. Notably, areca nut extract has emerged as a promising candidate due to its diverse biological activities and potential applications in livestock production.
Areca nut, derived from the dried ripe fruits, seeds, peels, and flowers of Areca catechu, is well-documented in traditional medicine sources such as the Pharmacopoeia of the People’s Republic of China (2010 Edition) for its medicinal properties, including antiparasitic effects, digestive support, and antimicrobial activity. This review focuses on the biological activities of areca nut extract, particularly its antioxidant, anti-inflammatory, antiparasitic, antibacterial, and microbiota-modulating effects, which collectively contribute to its potential role as a feed additive for enhancing animal health and performance.Key findings indicate that areca nut extract can promote livestock productivity by accelerating growth, enhancing immune responses, and reducing disease incidence. Additionally, its biological properties show potential for improving feed efficiency and mitigating the environmental footprint of livestock operations. By exploring these activities, we aim to provide theoretical insights and practical guidance for the application of areca nut extract in animal husbandry.
This review highlights the promise of areca nut extract as a natural, effective, and sustainable alternative to antibiotics, offering solutions to the pressing issues of antibiotic residues and AMR. Its potential contributions to sustainable livestock production underscore the importance of further scientific exploration and interdisciplinary collaboration in this field.
1 Introduction
The widespread presence of antibiotic residues in agricultural products and the environment poses a significant global challenge, primarily driven by the escalating threat of microbial resistance. This issue not only jeopardizes food safety and public health but also undermines environmental sustainability. In response, countries and regions such as China, the European Union, the United States, and the United Kingdom have implemented stringent regulations to restrict or ban the use of antibiotics in animal feed. These policy shifts have prompted the agricultural sector to seek safe, effective, and sustainable alternatives that ensure food safety, environmental protection, and animal health.
Among the numerous alternatives explored, plant-based feed additives have emerged as a promising solution. Notably, areca nut extract has garnered considerable attention due to its diverse bioactive properties, including antioxidant, anti-inflammatory, antimicrobial, and antiparasitic activities (Anonymous, 1953; Anonymous, 1977; Anonymous, 1999; Anonymous, 2010). These properties not only enhance gut health and improve disease resistance in animals but also align with global efforts to promote green and sustainable livestock production systems. By reducing the reliance on antibiotics, areca nut extract represents a natural and effective strategy to address pressing challenges in animal husbandry.
Recent studies have revealed that areca nut extract exhibits a wide range of pharmacological and biological effects, including regulatory impacts on blood glucose and lipid metabolism, as well as benefits to the digestive, nervous, and cardiovascular systems. For livestock, it has demonstrated the ability to improve growth performance, enhance immune responses, and reduce disease incidence (Chandra et al., 2008; Anonymous, 1953; Anonymous, 1977; Anonymous, 1999; Tian et al., 2002; Gilani et al., 2004; Anonymous, 2010; Li et al., 2010; Awais et al., 2011; Wang et al., 2011; Lee et al., 2013; Liu et al., 2013; Kheirabadi et al., 2014; Li et al., 2015; Li et al., 2016; Wei et al., 2016; Wang et al., 2018; Yao, 2023). These multifaceted effects underscore its potential as a natural feed additive that contributes to both animal health and production efficiency.
To comprehensively evaluate its applicability, this study systematically reviews the pharmacological activities and biological properties of areca nut extract, focusing on its potential applications in animal production. Data were collected from a wide range of sources, including government reports, national and local pharmacopeia, traditional literature, and authoritative scientific databases such as PubMed, SciFinder, Scopus, and the Web of Science. Through this analysis, we aim to elucidate the scientific basis for utilizing areca nut extract in animal feed, providing theoretical insights and practical recommendations for its further development.
In summary, this study seeks to systematically assess the biological activities of areca nut extract, its practical applications in animal feed, and its broader implications for sustainable and safe animal agriculture. By advancing research and application in this field, we aim to contribute to the ongoing global efforts to reduce antibiotic use and promote green agricultural practices.
2 Taxonomy of areca nut plant
The areca nut plant, scientifically known as Areca catechu L., is a species of the palm family (Arecaceae). It is an economically and culturally significant crop in many tropical and subtropical regions (Jahns, 1888; Anonymous, 1953; Anonymous, 1977; Anonymous, 1999; Gilani et al., 2004; Anonymous, 2010; Wang et al., 2011; Liu et al., 2013; Li et al., 2015; Wei et al., 2016). Below is the botanical classification of the areca nut plant:
Kingdom: Plantae
Clade: Angiosperms
Clade: Monocots
Order: Arecales
Family: Arecaceae
Genus: Areca
Species: Areca catechu
Areca catechu is commonly referred to as “betel nut” or “areca nut” in English and has various local names in different regions. The plant is primarily cultivated for its seeds (areca nuts), which are widely used in traditional medicines, food products, and cultural practices. This taxonomic information establishes a foundation for understanding the biological characteristics and the applications of the areca nut discussed in this review.
3 Methods for extracting bioactive compounds from areca nut after harvesting
Areca nut, as a significant tropical plant resource, contains various bioactive compounds in its fruit, such as polyphenols, alkaloids, and flavonoids. These active ingredients exhibit potential biological functions, including antibacterial, antioxidant, and immunomodulatory effects. Therefore, developing efficient and eco-friendly extraction techniques has become a research focus. To date, researchers have explored various methods for extracting bioactive compounds from areca nuts, including organic solvent extraction (Chunqin et al., 2013), reflux extraction (Qingqing et al., 2013), ultrasound-assisted extraction (UAE) (Sun et al., 2023), subcritical water extraction (Kang et al., 2016), and supercritical fluid extraction (Chunjiang et al., 2008). This section elaborates on the commonly used ultrasound-assisted extraction method as an example (Sun et al., 2023).
3.1 Raw material preparation
Proper preparation of areca nuts after harvesting is essential to ensure extraction efficiency and maintain the quality of the compounds:
Cleaning: Remove dirt, dust, and other impurities from the surface of the areca nuts.
Slicing or Grinding: Slice or pulverize the fruit to increase its surface area and facilitate the penetration and dissolution of solvents.
Drying: Use low-temperature drying methods, such as freeze-drying or hot air drying, to remove moisture and avoid degradation of thermolabile active compounds.
3.2 Selection of extraction solvent
The choice of extraction solvent significantly affects the yield and purity of the bioactive compounds. Commonly used solvents include ethanol, water, methanol, and their mixtures. Among these, the ethanol-water system is widely employed due to its low toxicity, high solubility, and sustainability. The solvent ratio (e.g., 70:30, v/v) and pH can be optimized depending on the properties of the target compounds.
3.3 Ultrasound-assisted extraction
Ultrasound-assisted extraction is a green and efficient extraction technique. Its principle lies in the cavitation effect of ultrasound, which enhances solvent penetration into the cell walls and releases intracellular compounds. This method significantly improves extraction efficiency, reduces extraction time, and minimizes solvent consumption. To better illustrate this process, we have prepared a visual diagram, as shown in Figure 1, to provide a clear understanding of the extraction procedure.
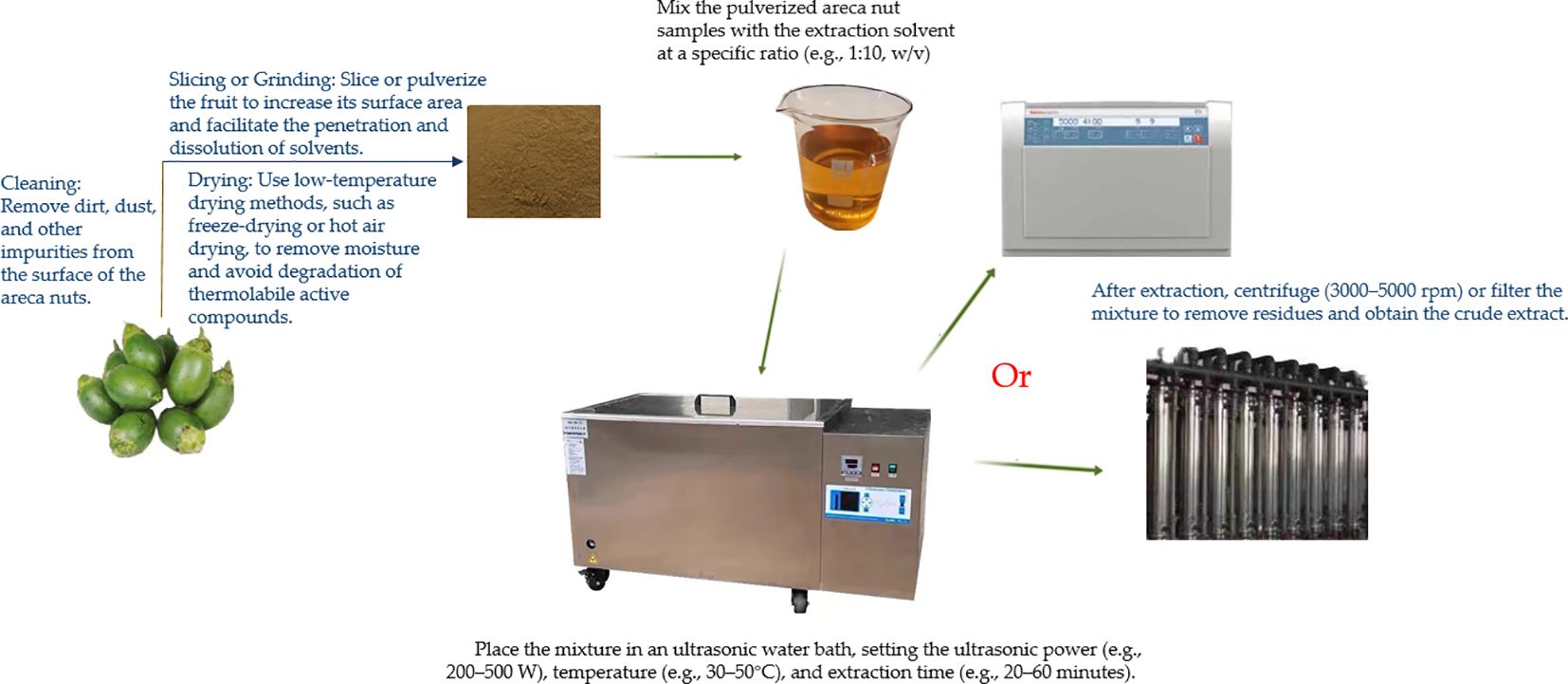
Figure 1. Ultrasound-assisted extraction technology for extracting bioactive compounds from areca nut.
Procedure:
Mix the pulverized areca nut samples with the extraction solvent at a specific ratio (e.g., 1:10, w/v).
Place the mixture in an ultrasonic water bath, setting the ultrasonic power (e.g., 200–500 W), temperature (e.g., 30–50°C), and extraction time (e.g., 20–60 minutes).
After extraction, centrifuge (3000–5000 rpm) or filter the mixture to remove residues and obtain the crude extract.
Advantages:
Simple operation and high extraction efficiency;
Reduced energy consumption and environmental impact;
Better protection of the stability of bioactive compounds.
3.4 Concentration and purification of extracts
To enhance the concentration of bioactive compounds and improve the usability of the extracts, further concentration and purification steps are necessary:
Concentration: Use a rotary evaporator to remove excess solvent and obtain a concentrated solution of active ingredients.
Purification: The following methods can be employed to isolate and purify target compounds:
Resin Adsorption: Employ macroporous resin to adsorb and elute polyphenols while removing impurities.
Liquid-Liquid Extraction: Use solvents with different polarities to separate the desired compounds.
High-Performance Liquid Chromatography (HPLC): Perform accurate separation and quantitative analysis of the extracts.
3.5 Quality evaluation of extracts
The quality and content of the bioactive compounds in the extracts can be assessed using modern analytical techniques:
Chemical Composition Analysis: Identify and quantify the major active compounds using high-performance liquid chromatography (HPLC) or gas chromatography-mass spectrometry (GC-MS).
Biological Activity Testing: Evaluate the biological properties of the extracts through in vitro experiments, such as antioxidant capacity assays (e.g., DPPH radical scavenging activity) and antibacterial activity tests (e.g., minimum inhibitory concentration determination).
4 Bioactive effects of areca nut
4.1 Bioactive compound in areca nut
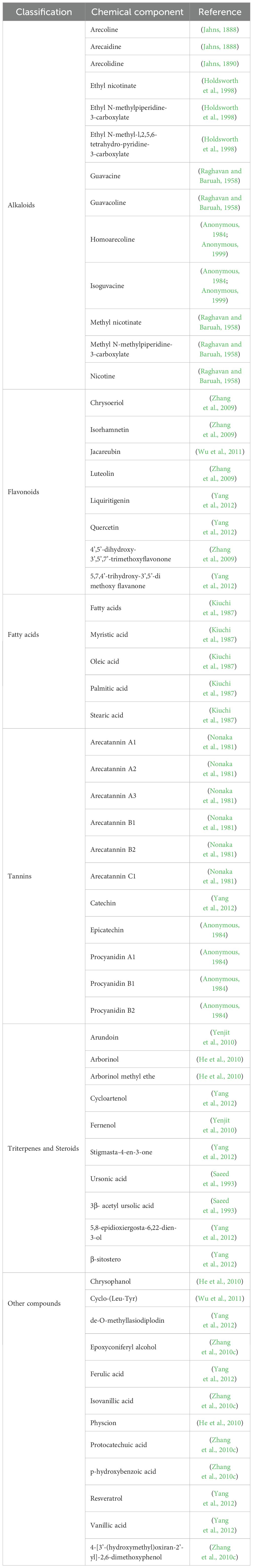
The chemical constituents of areca nut extract primarily comprise alkaloids, flavonoids, polysaccharides, among others. To date, over 59 compounds have been isolated and identified from this botanical source, with pyridine-type alkaloids and condensed tannins recognized as its characteristic components. These chemical constituents confer diverse biological activities upon the areca nut extract (Jahns, 1888; Jahns, 1890; Raghavan and Baruah, 1958; Nonaka et al., 1981; Anonymous, 1984; Kiuchi et al., 1987; Saeed et al., 1993; Holdsworth et al., 1998; Zhang et al., 2009; He et al., 2010; Yenjit et al., 2010; Zhang et al., 2010c; Wu et al., 2011; Yang et al., 2012). The major constituents of areca nut are presented in Table 1.
4.2 Revealing the pharmacological activity of areca nut extract
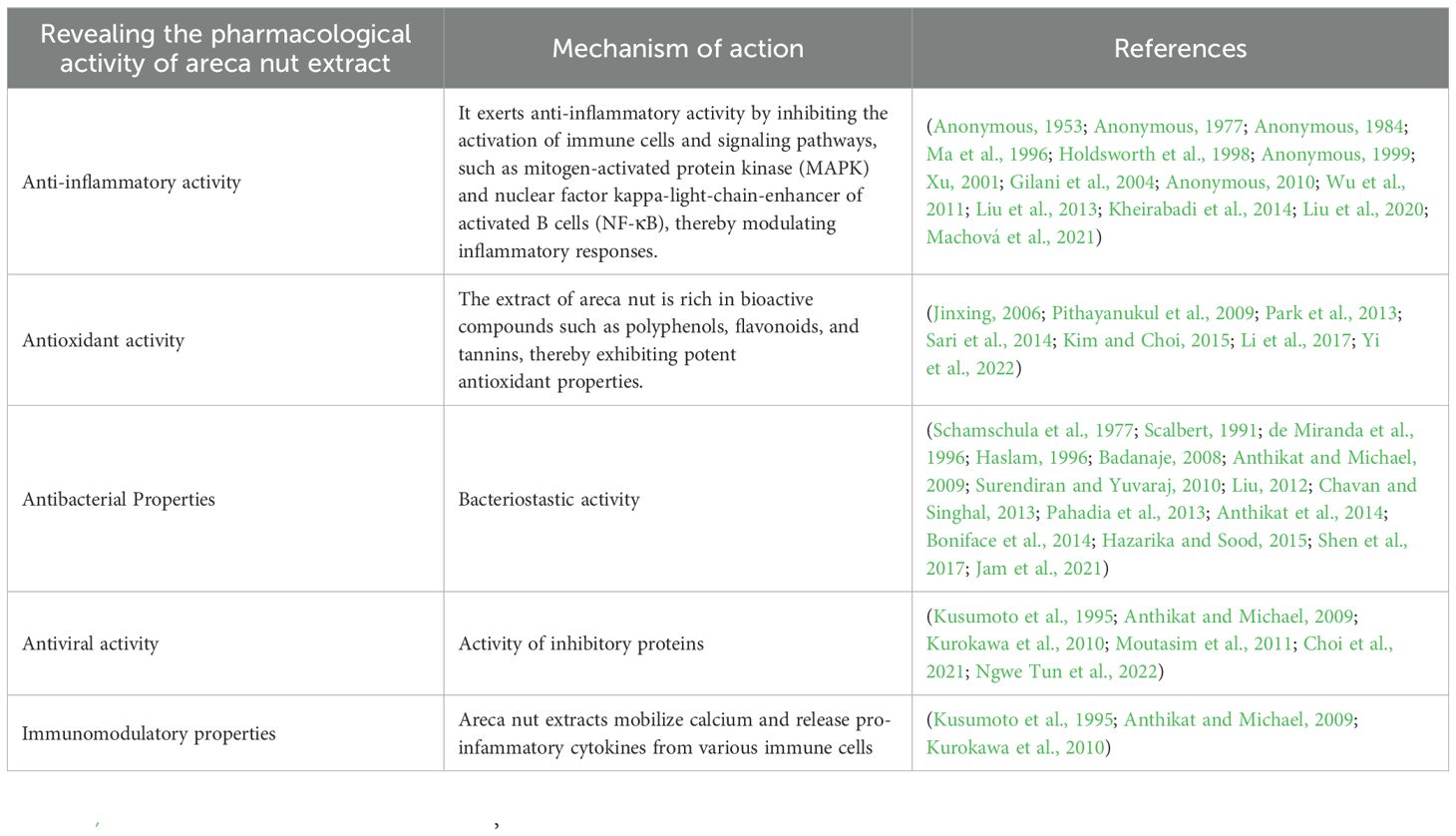
The plant extracts are rich in polysaccharides, phenols, flavonoids, and other bioactive compounds, exhibiting potent anti-inflammatory, antioxidant, immunomodulatory properties among others (Jahns, 1888; Jahns, 1890; Raghavan and Baruah, 1958; Schamschula et al., 1977; Nonaka et al., 1981; Anonymous, 1984; Kiuchi et al., 1987; Scalbert, 1991; Saeed et al., 1993; Kusumoto et al., 1995; de Miranda et al., 1996; Haslam, 1996; Ma et al., 1996; Chung et al., 1998; Holdsworth et al., 1998; Xu, 2001; Vermani and Garg, 2002; Lin et al., 2005; Jinxing, 2006; Taiping et al., 2007; Badanaje, 2008; Xiaoyan et al., 2008; Anthikat and Michael, 2009; Husvik et al., 2009; Pithayanukul et al., 2009; Zhang et al., 2009; Bhandare et al., 2010; He et al., 2010; Huang et al., 2010; Kurokawa et al., 2010; Lu et al., 2010; Surendiran and Yuvaraj, 2010; Yenjit et al., 2010; Zhang et al., 2010c; Barman et al., 2011; Hung et al., 2011; Wu et al., 2011; Liu, 2012; Yang et al., 2012; Chang et al., 2013a; Chavan and Singhal, 2013; Chin et al., 2013; Pahadia et al., 2013; Park et al., 2013; Sazwi et al., 2013; Amirkia and Heinrich, 2014; Anthikat et al., 2014; Boniface et al., 2014; Lee et al., 2014; Sari et al., 2014; Aizad et al., 2015; Arathi et al., 2015; Brousseau et al., 2015; Hazarika and Sood, 2015; Kim and Choi, 2015; Peng et al., 2015; Quiroz-Castañeda and Dantán-González, 2015; Li et al., 2017; Shen et al., 2017; Malika et al., 2018; Liu et al., 2020; Choi et al., 2021; Jam et al., 2021; Machová et al., 2021; Ngwe Tun et al., 2022; Yi et al., 2022). The main biological activities of areca nut are presented in Table 2.
4.2.1 Anti-inflammatory activity
Areca nut extract has been reported to exhibit potent anti-inflammatory properties, suggesting its potential as a therapeutic agent for inflammatory diseases. Multiple in vitro and animal studies have highlighted the significant anti-inflammatory activity of areca nut extract (Anonymous, 1953; Anonymous, 1977; Anonymous, 1984; Ma et al., 1996; Anonymous, 1999; Xu, 2001; Gilani et al., 2004; Anonymous, 2010; Wu et al., 2011; Liu et al., 2013; Kheirabadi et al., 2014; Li et al., 2016; Liu et al., 2020; Machová et al., 2021). The immune system’s regulation of the inflammatory response, which can be triggered by cellular dysfunction or microbial infection (Quiroz-Castañeda and Dantán-González, 2015), is a complex process controlled by various mediators including transcription factors, pro-inflammatory cytokines, and adhesion enzymes. Areca nut extract is noted for its antibacterial, anti-inflammatory, and analgesic properties, which may enhance immune function and resistance against coccidia (Bhandare et al., 2010). This observation aligns with the report of Xiaoyan et al. (2008) on the immunomodulatory effects of areca nut extract in vitro against Coxsackievirus simplex virus type 1.
Inflammatory mediators such as Cyclooxygenase-2 (COX-2), Prostaglandin E2 (PGE2), and interleukin-1α (IL-1α) are often implicated in the development of tumors, including oral squamous cell carcinoma (OSCC) (Husvik et al., 2009). NF-κB, a transcription factor protein, plays a critical role in the pathogenesis of many diseases. Its role underscores the complexity of disease mechanisms (Taiping et al., 2007). COX-2 is regulated by NF-κB, with PGE2 as a metabolite of cyclooxygenase. Chang LY et al. observed that frequent areca nut chewing might enhance the expression of pro-inflammatory mediators by immune cells, creating a pro-inflammatory oral microenvironment conducive to cancer initiation (Chang et al., 2013a). Additionally, arecoline has been reported to induce reactive oxygen species (ROS) production in various cells (Hung et al., 2011), with NF-κB activation potentially serving as the mechanism for ROS generation (Lu et al., 2010). Thus, excessive consumption of areca nut may contribute to oxidative stress, upregulation of inflammatory factor expression, and prolonged inflammation.
Acetone extracts of the areca nut (AEAN), rich in procyanidins, have been shown to downregulate TPA-induced COX-2 expression at low concentrations (0.1-1 g/mL) by inhibiting ERK phosphorylation in SAS cells. In rats studies (1 and 10 mg/kg/d, p.o., for 5 days), administration of AEAN effectively reduced carrageenan-induced inflammatory edema and PGE2 levels (Huang et al., 2010). The anti-inflammatory efficacy of ursolic acid from areca nut leaves has been demonstrated in a mouse model of carrageenan-induced paw edema (Saeed et al., 1993). Lin et al. discussed the activation of the NF-κB signal transduction pathway and its role in COX-2 expression induced by areca nut extract, which is rich in polyphenols (Lin et al., 2005). Conversely, areca nut extract has also been reported to inhibit COX-2 expression. In the study conducted by Lee et al (Sari et al., 2014), it was discovered that treatment with 3 μg/mL of ethanol extract from betel nut leaves significantly inhibited the expression of proteins such as NF-κB, inducible nitric oxide synthase (iNOS), and cyclooxygenase-2 (COX-2), as well as the production of nitric oxide (NO) in LPS-stimulated Raw 264.7 cells. This finding suggests that the anti-inflammatory activity of the ethanol extract of betel nut leaves is correlated with the suppression of the NF-κB/iNOS/NO signaling pathway. Furthermore, the research indicated that the polyphenol extract of betel nuts could dose-dependently (40 to 320 μg/mL) inhibit the phosphorylation expression of proteins involved in the MAPK signaling pathway. These proteins include extracellular regulated protein kinase 1/2 (ERK1/2), p38 mitogen-activated protein kinase (p38 MAPK), and p-c-Jun N-terminal kinase (JNK), along with upstream signaling proteins like mitogen-activated protein kinase 1 (MAPKK1), MAPKK3, and MAPKK4. This indicates that the anti-inflammatory efficacy of betel nut extract is significantly dependent on its ability to inhibit the MAPK signaling pathway (Yi et al., 2022).
In summary, areca nut extract exhibits notable free radical scavenging capabilities and demonstrates antioxidant potential by enhancing intracellular antioxidant enzyme activity or activating the Nrf2 signaling pathway, thereby ameliorating oxidative damage in the body. The antioxidant activities of areca nut extract are likely associated with its rich content of polyphenols, flavonoids, and tannins. However, the precise mechanisms underlying these effects remain unclear and warrant further investigation.
4.2.2 Antioxidant activity
The extract of areca nut is abundant in bioactive constituents such as polyphenols, flavonoids, and tannins, thereby demonstrating potent antioxidant capabilities and the capacity to ameliorate oxidative stress. Oxidative stress refers to a cascade of stress reactions provoked by endogenous or exogenous stimuli, characterized by a cellular imbalance between oxidation and antioxidant systems, as well as the accumulation of free radicals within cells (Jinxing, 2006; Pithayanukul et al., 2009; Barman et al., 2011; Sazwi et al., 2013; Li et al., 2017).
The scavenging efficacy of areca water extract for oxygen free radicals has been found to be on par with that of vitamin C, as evidenced by an in vitro radical scavenging assay using 1,1-diphenyl-2-trinitrophenylhydrazine (DPPH). The ferric ion reduction capacity of areca nut extract is comparable to half the dose of vitamin C, while its lipid peroxidation inhibition capacity is fourfold greater than that of vitamin E. Furthermore, the administration of areca nut extract has been shown to augment the activity of antioxidant enzymes in animal models, including superoxide dismutase (SOD), myeloperoxidase (MPO), and catalase (CAT). Consequently, this intervention effectively mitigates organ damage induced by aging or exposure to environmental compounds (Jinxing, 2006; Pithayanukul et al., 2009; Li et al., 2017).
Research by Yi et al. (2022) reported that areca nut polyphenols (ANP) attenuated reactive oxygen species (ROS) levels in LPS-stimulated RAW264.7 cells and upregulated the expression of nuclear factor erythroid 2-related factor 2 (Nrf2) and heme oxygenase 1 (HO-1). RNA-seq analysis disclosed that ANP downregulated the transcription of genes associated with cancer pathways at a concentration of 160 μg/mL, as well as inflammatory and viral infection pathways at a concentration of 320 μg/mL. Moreover, cellular signaling analysis revealed that these gene expressions were regulated by the MAPK pathway, which was suppressed by ANP in response to LPS stimulation. Overall, ANP inhibits the MAPK pathway while activating Nrf2/HO-1 antioxidant pathways to mitigate ROS generation induced by LPS. Sustained LPS stimulation induces cellular inflammation, and the MAPK pathway is implicated in the generation of inflammatory responses; hence, ANP effectively suppresses both LPS-induced oxidative stress and inflammation (Park et al., 2013; Kim and Choi, 2015; Yi et al., 2022).
The acute oral toxicity test of areca extract was conducted in Sprague-Dawley rats, revealing no significant adverse effects, thus affirming its potential as a natural antioxidant suitable for incorporation into Chinese herbal medicine additives (Sari et al., 2014).
After thorough analysis, the extract of areca nut exhibits significant free radical scavenging ability and demonstrates potential to enhance the activity of antioxidant enzymes or activate the Nrf2 signaling pathway, thereby exerting an antioxidative effect and ameliorating oxidative damage in the body. The observed antioxidant activity of areca nut extract is likely attributed to its rich content of polyphenols, flavonoids, tannins, and other bioactive compounds. Nonetheless, further investigation is warranted to elucidate the precise underlying mechanisms.
4.2.3 Antibacterial properties
The areca nut exhibits, an element of profound interest in medicinal research, exhibits a spectrum of remarkable antibacterial and antifungal properties. Studies have consistently highlighted its efficacy against a myriad of bacterial strains. The aqueous extract of the areca nut, rich in natural polyphenols such as tannins, demonstrates therapeutic potential against both gram-negative and gram-positive bacteria, with minimum inhibitory concentrations reported between 3.3 to 7 µg/ml for the former and up to 16 µg/ml for the latter (Badanaje, 2008; Anthikat and Michael, 2009; Surendiran and Yuvaraj, 2010; Liu, 2012; Chavan and Singhal, 2013; Boniface et al., 2014; Hazarika and Sood, 2015; Jam et al., 2021). Moreover, its protein molecules, particularly peptides, have shown notable antibacterial properties, suggesting their potential as alternatives to synthetic antibiotics (Surendiran and Yuvaraj, 2010).
Research has further elucidated the superior efficacy of the butanol extract over methanol, ethyl acetate, and water extracts, particularly against strains such as Staphylococcus aureus and Mycobacterium smegmatis, with minimum inhibitory concentrations ranging from 62.5 to 250 µg/ml (Boniface et al., 2014). The areca nut fruit extract excels in its inhibitory effects on Escherichia coli, with a distinguished minimum inhibitory concentration of 1.56 mg/ml (Anthikat et al., 2014).
In addition to its antibacterial capabilities, the areca extract exhibits substantial antifungal activity. At a concentration of 50 µg/ml, it effectively inhibits Candida albicans, while a concentration of 16.67 µg/ml results in an inhibitory region of 18mm (de Miranda et al., 1996; Pahadia et al., 2013; Shen et al., 2017). Moreover, the aqueous extract has shown profound effects against fungi such as Mucor and Aspergillus niger, with concentrations as low as 16.67 µg/ml proving effective (Schamschula et al., 1977; Anthikat and Michael, 2009; Yenjit et al., 2010). Notably, ethyl acetate extracts, rich in tannins, exhibit the highest antifungal activity compared to other solvent extracts (Scalbert, 1991; Haslam, 1996; Chung et al., 1998).
Furthermore, the combination of areca extract with nano silver has been identified as a potent antibacterial agent, particularly against multi-drug-resistant strains, offering a promising avenue for developing novel antibacterial additives (Chin et al., 2013; Choi et al., 2021). This culmination of findings underscores the areca nut’s potential as a valuable resource in the development of new medicinal therapies.
4.2.4 Other biological activities
Incorporating betel nut extract into chicken feed at dosages of 100, 200, and 300 mg/kg over a period of 9 days demonstrated significant therapeutic effects against coccidial infections. This treatment notably ameliorated cecal damage caused by the infection. Furthermore, the betel nut extract enhanced the immune function of the chickens by increasing the concentrations of cytokines such as interleukin-2 (IL-2), interferon-gamma (IFN-γ), and macrophage migration inhibitory factor (MIF) in the bloodstream, thereby bolstering their disease resistance (Wei et al., 2016).
Following an extensive investigation, the antiviral efficacy of areca nut extract has been rigorously examined. It has unveiled that the extract exerts significant inhibitory effects on both Pediococcus acidilactici (Arathi et al., 2015) and Colletotrichum gloeosporioides (Aizad et al., 2015). Numerous scholars have meticulously reviewed the potential of various plant-based therapies in managing Sexually Transmitted Diseases (STDs) and Acquired Immune Deficiency Syndrome (AIDS) (Vermani and Garg, 2002). Their findings reveal that compounds derived from areca nut exert a potent inhibitory effect on the lethal pathways associated with Human Immunodeficiency Virus (HIV) and Herpes Simplex Virus (HSV-1). This antiviral property is attributed to its active constituents—plant tannins and alkaloids (Kusumoto et al., 1995; Kurokawa et al., 2010; Malika et al., 2018). Using high-performance liquid chromatography to assess enzyme activity, it was found that at a concentration of 0.2 mg/mL, the betel nut extract inhibited HIV-1 protease activity by more than 70% (Kusumoto et al., 1995).
The areca nut extract has also shown inhibitory effects on the replication of Newcastle Disease Virus (NDV) and Egg Drop Syndrome Virus (EDS) (Anthikat and Michael, 2009). Areca nut extract exhibits potent inhibitory activity against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in Vero E6 cells, with an IC50 of 1.2 μg/mL. This concentration is significantly lower than the dosage required to exert toxic effects, as indicated by the IC50 of 89.6 μg/mL for the viability of healthy Vero E6 cells (Ngwe Tun et al., 2022).
In summary, the extract derived from areca nut exhibits notable antimicrobial activity, including resistance against viruses and pathogenic microorganisms. This observed activity may be attributed to the presence of tannins within the extract (Aizad et al., 2015).
4.3 Toxicological properties of areca nut and their implications
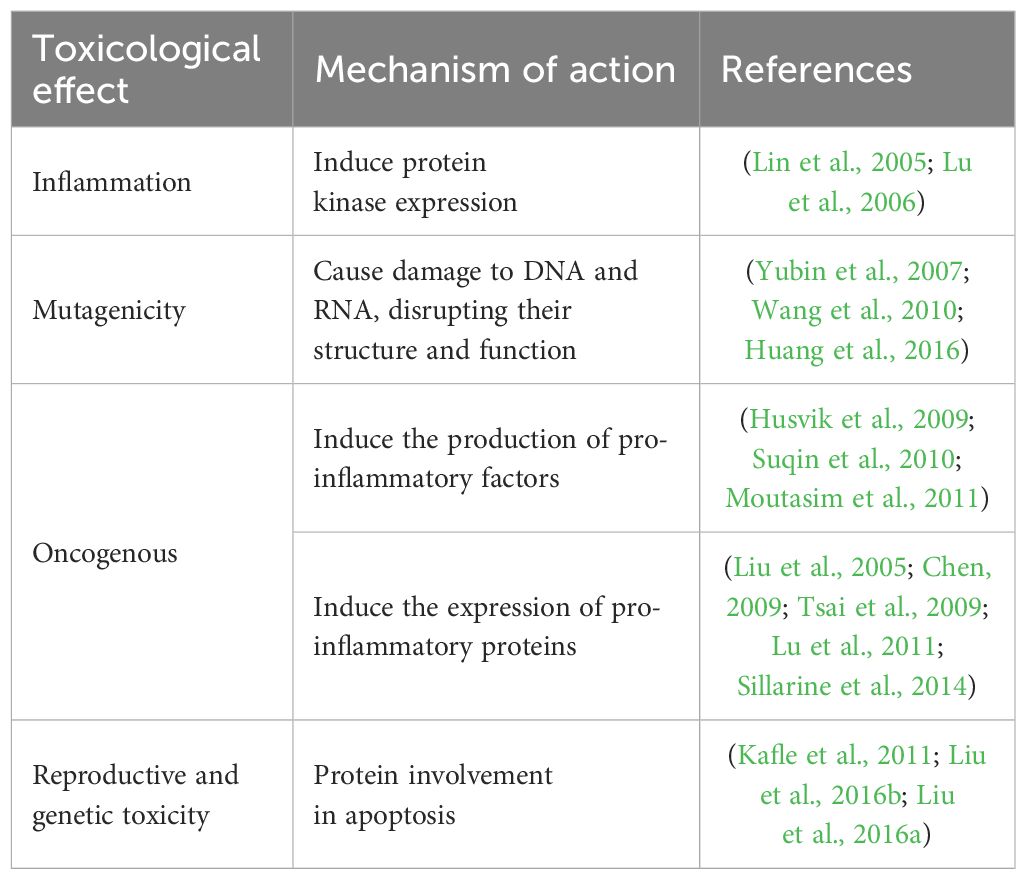
Areca nut extract, which has been extensively investigated for its diverse toxicological effects, holds significant implications for human health. Central to these concerns is its carcinogenic potential, primarily attributed to its capacity to induce the production of pro-inflammatory cytokines. These cytokines are instrumental in fostering an environment conducive to tumor growth (Husvik et al., 2009; Lu et al., 2010; Suqin et al., 2010; Hung et al., 2011; Moutasim et al., 2011; Khan et al., 2012; Chang et al., 2013b). Moreover, the extract amplifies the expression of pro-inflammatory proteins, thereby exacerbating inflammatory conditions that may lead to cancer development (Chang et al., 2013b). Table 3 provides a comprehensive overview of the primary toxicological effects of areca nut, highlighting its impact on health. Beyond its carcinogenic properties, areca nut extract also contributes to the onset of inflammatory diseases. This is facilitated through the induction of protein kinases, which are key enzymes in signaling pathways that regulate inflammation (Lin et al., 2005; Husvik et al., 2009; Hung et al., 2011; Moutasim et al., 2011; Khan et al., 2012). Dysregulation of these pathways can result in chronic inflammatory conditions, further complicating health outcomes.
The mutagenic effects of areca nut extract are particularly concerning, as it has been shown to cause damage to critical genetic materials, including DNA and RNA. Such genetic damage can lead to mutations, which are the underlying causes of various genetic disorders and cancers (Yubin et al., 2007; Cuadrado et al., 2009; Wang et al., 2010; Lee et al., 2011; Ji et al., 2012; Huang et al., 2016).
Moreover, areca nut extract poses significant reproductive and genetic toxicity risks. It has been implicated in inducing apoptosis, a process that can disrupt cellular homeostasis and adversely affect reproductive health by interfering with normal cellular functions (Kafle et al., 2011; Liu et al., 2016b; Liu et al., 2016a).
Research indicates that areca nut extract alters cellular signaling pathways and hinders DNA repair processes, which can have significant health implications. Specifically, it interferes with proteins that regulate cell growth and affects normal cell division, potentially leading to a range of biochemical and genetic issues (Wang et al., 2010).
Arecoline has been shown to induce apoptosis and exhibit significant cytotoxicity to a variety of normal cell types, including endothelial cells, lymphocytes, hepatocytes, myocytes, splenocytes, and epithelial cells. In various concentrations (0.1, 0.2, and 0.4 g/mL), areca nut and its extracts were used to treat cell lines L929, MOE1, and HSC-2 for 24, 48, and 72 hours, respectively. Compared to the control group, these extracts significantly reduced the viability of the cell lines (Al-Tayar et al., 2020). Stimulation of fibroblasts by arecoline significantly upregulated the expression of interleukin-2, interleukin-6, and interleukin-21, while downregulating transforming growth factor-beta (TGF-β). When the supernatants containing these cytokines were co-cultured with peripheral blood mononuclear cells, there was an observed increase in the number of T helper 17 (Th17) cells, while regulatory T cells (Treg) were significantly reduced. Additionally, the expression of RORγt was enhanced, while forkhead box P3 (FOXP3) expression decreased. These findings suggest that arecoline can influence the production of inflammatory cytokines by fibroblasts and is closely associated with its modulatory effects on immune cells Th17 and Treg (Wang et al., 2020). In studies on normal liver cells (Clone-9 cells), arecoline displayed significant cytotoxicity, capable of inducing apoptosis and causing cell cycle arrest at the G0/G1 phase. At high concentrations, arecoline significantly increased apoptosis in C2C12 myocytes and reduced their viability by inhibiting the activation of signal transducer and activator of transcription 3 (STAT3) (Juan et al., 2018).
We should not only be aware of the potential hazards of areca nut but also critically examine its beneficial aspects. As research and analysis of areca nut and its bioactive components expand, efforts should be made to explore its effective applications, thereby better harnessing and developing its potential value while minimizing risks.
5 Relevance of areca nut extract in animal husbandry applications
5.1 Feed supplement
Areca nut extract and its active components, such as areca nut, tannins, and polyphenols, possess remarkable antioxidant, antibacterial, antiviral, and anti-inflammatory properties. Additionally, they have the ability to regulate intestinal flora dynamics and promote gastrointestinal peristalsis while modulating the immune response. These multifaceted functions play a crucial role in maintaining the equilibrium of intestinal microbiota and REDOX homeostasis in animals, while alleviating stress responses within the realm of animal husbandry. Such capabilities are essential for enhancing animal performance (Haide et al., 2008; Hazarika and Sood, 2015; Wei et al., 2016; Salehi et al., 2020; Mei et al., 2021).
The utilization of areca nut extract as a feed additive demonstrates potential for improving animal performance. Reactive Oxygen Species (ROS) levels can serve as an indicative measure of oxidative stress and cytotoxicity in host cells following coccidia infection, with ROS accumulation showing a positive correlation with apoptosis in host cells (Sim et al., 2005). Throughout the course of coccidia infection, elevated ROS levels may facilitate the eradication of coccidia and influence signal transduction pathways associated with inflammatory response, cell proliferation, and immune response (Sareila et al., 2011).
Currently, research on the use of areca nut extract as a health feed additive is limited. However, previous studies have shown that areca extract can significantly improve feed intake and body weight in Wenchang chickens infected with coccidia, while reducing fecal oocyst levels compared to the negative control group. Moreover, its anti-coccidian efficacy is considered moderate (Awais et al., 2011; Kheirabadi et al., 2014; Li et al., 2016; Wei et al., 2016).
The supplementation of 1 g/L areca nut extract in the water of Litopenaeus vannamei over a period of 14 days significantly enhanced the immune function of the shrimp, thereby mitigating the weight loss and performance decline induced by hepatocenterocytosis infection. This beneficial effect may be attributed to the modulation of genes associated with growth, immunity, and drug metabolism by the areca extract, elucidating its underlying molecular mechanism (Li, 2022).
In a study conducted by Wei et al. (2016), the incorporation of areca nut extract into chicken diets at concentrations of 100, 200, and 300 mg/kg for nine consecutive days exhibited significant therapeutic efficacy against coccidia infection. Furthermore, it effectively alleviated the cecum damage induced by the infection (Ling and Kian, 2009; Wei et al., 2016; Salehi et al., 2020). Following administration of a 1.5% aqueous extract of areca nut for 90 consecutive days, Kunming mice showed significantly enhanced appetite, improved digestive and absorptive functions, as well as a notable increase in body weight (Shuhua et al., 2015). Compared to the control group, treatment with areca nut extract resulted in a significant reduction in fecal oocyst counts, ameliorated intestinal mucosal damage caused by coccidia infection, lowered circulating Nitric Oxide (NO) levels, and enhanced interleukin-2 concentration during post-treatment infection (Wang et al., 2018).
When assessing the impact of areca nut extract on serum antioxidant indicators in coccidia-infected chickens, it was observed that NO and nitric oxide synthase (NOS) concentrations were higher in the negative control group compared to the blank control group, whereas the treatment groups supplemented with 100, 200, and 300 mg/kg of areca nut extract, as well as the positive control group, exhibited lower concentrations of NO and NOS (Pinto et al., 2013). NO, a small, non-polar molecule, acts as a crucial messenger and effector in chickens. It is synthesized by NOS using L-arginine and molecular oxygen as substrates, resulting in the production of NO and L-guanidine. Its multifaceted role encompasses not only coccidia eradication but also interaction with superoxide ions to generate toxic derivatives, thereby effectively enhancing macrophage-mediated coccidia elimination (Shen et al., 2001; Tizard, 2009). Upon coccidium infection in chickens, the immune response transitions into the inflammatory phase, wherein IFN-γ binds to the membrane receptor of macrophages, thus activating them to upregulate NOS production and subsequently increase both NO concentration and NOS levels (Ma et al., 2013).
Furthermore, areca nut extract has been shown to effectively enhance gastrointestinal function and improve the overall health of animals to a certain extent. This effect may be attributed to the abundant presence of alkaloid active ingredients in areca nut.
Therapeutic potential of areca nut extract in parasitic disease management
The extract derived from the areca nut manifests distinct antiparasitic activity. Extensive research has corroborated that arecoline, a pivotal component of the areca nut, functions as a potent insect repellent by inducing paralysis in the insect’s nervous system, thereby impairing its locomotor abilities and manifesting antiparasitic properties (Awais et al., 2011; Kheirabadi et al., 2014; Li et al., 2016; Wei et al., 2016). Cell-mediated immunity is instrumental in the eradication of coccidia, with cytokines being indispensable elements (Zhao et al., 2014). Besides Tumor Necrosis Factor-alpha (TNF-α) and Tumor Necrosis Factor β (TNF-β), the influence of areca nut extract on serum cytokines in coccidia-infected chickens was observed by the 9th day post-infection. Furthermore, compared to the blank control group, the negative control group exhibited lower serum concentrations of IL-2, IFN-γ, TNF-α, TNF-β, and MIF from the 3rd to the 9th day post-infection (Park et al., 2007; Ma et al., 2013; Miska et al., 2013; Zhao et al., 2014; Amer et al., 2015). This phenomenon is attributed to the intricate life cycle of coccidia, which encompasses extracellular and intracellular stages as well as both asexual and sexual reproduction. Consequently, this complexity has engendered a multifaceted immune response against coccidia, engaging both antibody-mediated and cell-mediated immunity (Lillehoj, 1998; Dalloul and Lillehoj, 2006). Cytokines, as low molecular weight soluble polypeptides and glycopeptides secreted by diverse cells of the immune system, play a pivotal role in immune regulation and defense against intracellular parasites. As a class of signaling molecules, cytokines significantly contribute to the mediation and regulation of immunity, inflammation, and hematopoiesis, concurrently exerting influence on physiological processes through the modulation of cell proliferation, differentiation, activation, and migration (Rahman and Eo, 2012; Hoan et al., 2014).
As early as 1956, Mr. Feng Lanzhou (1956) conducted a systematic study on the pharmacological effects of areca nut and pumpkin seed (Cucurbitae semina) in the treatment of tapeworms. The results demonstrated that pumpkin seed induced paralysis in the middle and posterior segments of the tapeworm, whereas the areca nut exerted a paralytic effect on the head and immature segments of the tapeworm. This effect is primarily attributed to arecoline in the areca nut and cucurbitine in pumpkin seeds. Tian et al. (2002) discovered through ultrastructural observation that the combination of areca nut and pumpkin seed remains an effective mechanism for the eradication of Taenia tapeworm infection due to its numbing properties and non-injury to nerve tissue.
Areca nut exhibits a remarkable acaricidal effect against rabbit mites, leading to complete eradication of the mite population within 60 minutes under experimental conditions (Song et al., 2002). Jeyathilakan et al. (2010) evaluated the efficacy of areca hepatica extract in vitro for the eradication of Fasciola Linnaeus. The findings revealed that concentrations of 1%, 2.5%, and 5% of areca extract exhibited a remarkable inhibition rate of 100% against Fasciola hepatica, surpassing the effectiveness observed with oxyclozanide bpv.
The utilization of areca nut extract presents significant promise in the therapeutic management of coccidiosis in poultry and lagomorphs (Lu et al., 2007). The inclusion of areca extract at doses of 100, 200, and 300 mg/kg in the diet demonstrates a significant control effect on coccidial infection in chickens, thereby establishing it as an effective moderate anti-coccidial treatment. Specifically, administration of the extract at a dose of 200 mg/kg significantly reduces the number of Eimeria oocysts compared to other experimental groups at 8-11 days post-infection (Wei et al., 2016).
The anti-coccidia effect of areca nut extract may be attributed to the following mechanisms: Coccidia induces oxidative stress and lipid peroxidation damage in chicken cecal epithelial cells, while areca nut extract displays potent antioxidant activity (Lin et al., 2011), which can effectively scavenge oxygen free radicals and alleviate oxidative stress, thereby protecting the organism from harm. Moreover, areca extract contains alkaloids with cholinergic effects that could potentially induce paralysis in Eimeria tender and facilitate the expulsion of immature coccidium oocysts from cecal epithelial cells. Additionally, the extract demonstrates antibacterial, anti-inflammatory, and analgesic properties (Bhandare et al., 2010), which can enhance immune function and bolster resistance against coccidia. These findings align with a prior study by Xiaoyan et al. (2008), who observed inhibitory effects of areca extract on Coxsackie virus and herpes simplex virus type 1 infection in vitro, suggesting a potential role in modulating immune responses. Furthermore, Boniface et al. (2014) found that areca alcohol extract exhibited notable antimalarial and antimicrobial properties.
In the exploration of the anti-malarial efficacy of areca nut extract, it was observed that treatment with a daily dose of 150 mg/kg butanol extract from areca nut significantly enhanced the survival rate of infected mice by 60% after a four-day duration, as compared to the control group (Jiang et al., 2009; Keshavabhat et al., 2016).
The areca nut, a natural Chinese herb, possesses extensive repellent properties that effectively mitigate the risk of drug resistance due to its safety, non-toxicity, and lack of residual effects. Consequently, it can serve as an additive for the prevention and treatment of animal parasitic diseases, showcasing substantial potential across various applications.
6 Applications and future perspectives
Areca nut extract emerges as a compelling candidate for revolutionizing sustainable livestock production, offering the dual advantage of enhancing efficiency and mitigating antibiotic residues. However, its future integration necessitates a meticulous evaluation of potential limitations, including the assurance of consistent efficacy, comprehension of long-term health implications for animals, and consideration of any ecological ramifications.
7 Conclusions
Upon comprehensive analysis, areca nut extract demonstrates strong potential as an innovative feed additive with notable antioxidant, anti-inflammatory, antiparasitic, and antimicrobial properties. These characteristics contribute to improved gastrointestinal health in animals and enhanced disease resistance, offering a promising alternative to traditional antibiotics in addressing microbial resistance. However, the potential toxicity of areca nut extract at high doses remains a critical limitation that warrants further investigation. Future studies should focus on determining safe and effective dosage ranges to maximize its benefits while minimizing adverse effects. Additionally, long-term studies are necessary to evaluate its safety and efficacy, providing a solid foundation for its practical adoption in sustainable agriculture and livestock management.
Author contributions
ZL: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. XW: Conceptualization, Investigation, Software, Writing – original draft, Writing – review & editing. LW: Funding acquisition, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Scientific Research Project of the Education Department of Hunan Province (Grant No. 22C1048) and the Major University-Level Research Project (Grant No. 24YZD03).
Conflict of interest
Authors ZL and LW were employed by Changsha Luye Biotechnology Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahmad Rusdan A. I., Kadir J., Mahmud T. M. M., Ee G. C. L. (2015). Potential of the extract from the nut of Areca catechu to control mango anthracnose. Pertanika J. Trop. Agric. Sci. 38, 375–388.
Al-Tayar B. A., Ahmad A., Yusoff M. E., Abdullah S. F., Mohamad N. K., Hashim S. N. M., et al. (2020). Cytotoxic effects of betel quid and areca nut aqueous extracts on mouse fibroblast, human mouth-ordinary-epithelium 1 and human oral squamous cell carcinoma cell lines. Asian Pac J. Cancer Prev. 21, 1005–1009. doi: 10.31557/APJCP.2020.21.4.1005
Amer O. S. O., Dkhil M. A., Hikal W. M., Saleh A. Q. (2015). Antioxidant and Anti-Inflammatory Activities of Pomegranate (Punica granatum) on Eimeria papillata-Induced Infection in Mice. BioMed. Res. Int. 2015, 219670. doi: 10.1155/2015/219670
Amirkia V., Heinrich M. (2014). Alkaloids as drug leads – A predictive structural and biodiversity-based analysis. Phytochem. Letter. 10 (16). doi: 10.1016/j.phytol.2014.06.015
Anonymous (1953). Chinese pharmacopoeia (1953 ed.) Vol. 24 (Beijing: China Medical Science and Technology Press), 341.
Anonymous (1977). Dictionary of chinese materia medica (Shanghai: Science and Technology Press of Shanghai), 2525–2528.
Anonymous (1984). Study on the active constituents of Chinese herbal medicine Vol. 1 (Beijing: People’s Medical Publishing House), 445.
Anonymous (1999). Chinese material medica Vol. 8 (Shanghai: Science and Technology Press of Shanghai), 439–648.
Anonymous (2010). Chinese pharmacopoeia Vol. 1 (Beijing: China Medical Science and Technology Press), 342–343.
Anthikat R. R. N., Michael A. (2009). Study on the areca nut for its antimicrobial properties. J. Young Pharm. 1, 42–45. doi: 10.4103/0975-1483.51874
Anthikat R. R. N., Michael A., Kinsalin V. A., Ignacimuthu S. (2014). Antifungal activity of Areca cate-chu L. Int. J. Pharm. Clin. Sci. 4, 1–3.
Arathi G., Venkateshbabu N., Deepthi M., et al. (2015). In vitro antimicrobial efficacy of aqueous ex-tract of areca nut against Enterococcus fae-calis. Indian J. Res. Phar-macy Biotechnol. 3, 147–150.
Awais M. M., Akhtar M., Muhammad F., Haq A. U., Anwar M. I. (2011). Immunotherapeutic effects of some sugar cane (Saccharum officinarum L.) extracts against coccidiosis in industrial broiler chickens. Exp. Parasitol. 128, 104–110. doi: 10.1016/j.exppara.2011.02.024
Badanaje S. B. (2008). Areca nut - Medicinal and alternative uses. Mangalore India: Areca nut Res. Dev. Foundation®; p, 104.
Barman M. R., Uddin M. S., Akhter S., Ahmed M. N., Haque Z., Rahman S., et al. (2011). Antinociceptive activity of methanol extract of Areca catechu L.(Arecaceae) stems and leaves in mice. Adv. Nat. Appl. Sci. 5, 223–226.
Bhandare A. M., Kshirsagar A. D., Vyawahare N. S., Hadambar A. A., Thorve V. S. (2010). Potential analgesic, anti-inflammatory and antioxidant activities of hydroalcoholic extract of Areca catechu L. nut. Food Chem. Toxicol. 48, 3412–3417. doi: 10.1016/j.fct.2010.09.013
Boniface P., Verma S. K., CheeCheema H. S., Daroka M. P., Pal A. (2014). Evaluation of antimalarial and antimicrobial activities of extract and fractions from Areca catechu. Int. J. Infect. Dis-eases 21, 228–229. doi: 10.1016/j.ijid.2014.03.897
Brousseau J.-P., Talbot G., Beaudoin F., Lauzon K., Roy D., Lessard M. (2015). Effects of probiotics Pediococcus acidilactici strain MA18/5M and Saccharomyces cerevisiae ssp. boulardii strain SB-CNCM I-1079 on fecal and intestinal microbiota of nursing and weanling piglets. J. Anim. Sci. 93, 5313–5326. doi: 10.2527/jas.2015-9190
Narendra Sharath Chandra J. N., Malviya M., Sadashiva C. T., Subhash M. N., Rangappa K. S. (2008). Effect of novel arecoline thiazolidinones as muscarinic receptor 1 agonist in Alzheimer’s dementia models. Neurochem. Int. 52 (3), 376. doi: 10.1016/j.neuint.2007.07.006
Chang L. Y., Wan H. C., Lai Y. L., Chou I. C., Chen Y. T., Huang S. L. (2013a). Areca nut extracts increased the expression of cyclooxygenase-2, prostaglandin E2 and interleukin1a in human immune cells via oxidative stress. Arch. Oral. Biol. 58, 1523–1531. doi: 10.1016/j.archoralbio.2013.05.006
Chang L. Y., Wan H. C., Lai Y. L., Chou C., Chen Y. T., Hung S. L. (2013b). Areca nut extracts increased the expression of cyclooxygenase-2, prostaglandin E2 and interleukinla in human immune cells via oxidative stress. Arch. Oral. Biol. 58, 1523–1531. doi: 10.1016/j.archoralbio.2013.05.006
Chavan Y. V., Singhal R. S. (2013). Separation of polyphenols and arecoline from areca nut (Areca catechu L.) by solvent extraction, its antioxidant activity and identification of pol-yphenols. J. Sci. Food Agric. 93, 2580–2589. doi: 10.1002/jsfa.2013.93.issue-10
Chen H. J. (2009). Effects of arecoline on CyclinD1 and P16 expression in HaCaT cell line (Changsha: Central South University), 1–42.
Chin A. A., Clariza D. F., Renalyn B. S., Marie S. S. B., Regine F. T., Frederick R. M. (2013). Antimicrobial performance of ethanolic extract of Areca catechu L seeds against mixed-oral flora from tooth scum and gram negative laboratory isolates. Int. J. Res. Ayurveda Pharm. 4, 876–880. doi: 10.7897/2277-4343.04620
Choi J. S., Jung H. C., Baek Y. J., Kim B. Y., Lee M. W., Kim H. D., et al. (2021). Antibacterial activity of green-synthesized silver nanoparticles using Areca catechu extract against antibiotic-resistant bacteria. Nanomaterials (Basel) 11, 205. doi: 10.3390/nano11010205
Chung K. T., Wong T. Y., Wei C. L., Huang Y. V. V., Lin Y. (1998). Tannins and human health: a review. Crit. Rev. Food Sci. Nutr. 38, 421–464. doi: 10.1080/10408699891274273
Chunjiang Z., Haiteng T., Feijie Lv, jianxiang T., zhunian W. (2008). Supercritical CO2 fluid extraction of arecoline from Areca nut. Trans. Chin. Soc. Agric. Eng. (06), 250–253. doi: 10.3969/j.issn.1002-6819.2008.6.038
Chunqin M., Tuling Lu, De Ji, Yuan Z., Junyang Hu, Ying W., et al. (2013). HPLC determination of arecoline in arecae pericarpium from different habitats. Chin. Pharm. J. 48 (11), 909–911. doi: 10.11669/cpj.2013.11.017
Cuadrado M., Gutierrez-Martinez P., Swat A., Nebreda A. R., Fernandez-Capetillo O. (2009). p27Kip1 stabilization is essential for the maintenance of cell cycle arrest in responseto DNA damage. Cancer Res. 69, 8726–8732. doi: 10.1158/0008-5472.CAN-09-0729
Dalloul R. A., Lillehoj H. S. (2006). Poultry coccidiosis: recent advancements in control measures andvaccine development. Expert Rev. Vaccines 5, 143–163. doi: 10.1586/14760584.5.1.143
de Miranda C. M., van Wyk C. W., van der Biji P., Basson N. J. (1996). The effect of areca nut on sali-vary and selected oral microorganisms. Int. Dental J. 46 (4), 350–356. doi: 10.1006/eesa.1996.0071
Gilani A. H., Ghayur M. N., Saify Z. S., Ahmed S. P., Choudhary M. I., Khalid A. (2004). Presence of cholinomimetic and acetylcholinesterase inhibitory constituents in betel nut. Life Sci. 75, 2377–2389. doi: 10.1016/j.lfs.2004.03.035
Haide Z., Yulin H., Yanzhong. F. (2008). Study on DPPH radical scavenging ability of raw betel nut extract. Food Sci. 8), 74–77.
Haslam E. (1996). Natural polyphenols (vegetable tannins) as drugs: possible modes of ac-tion. J. Nat. Prod 59, 205–215. doi: 10.1021/np960040+
Hazarika D. J., Sood K. (2015). In vitro antibacterial activity of peptides isolated from Areca catechu Linn. Der Pharmacia Lettre 7, 1–7.
He X. X., Li Y. J., Hu X. P., Zhang C. X., Li Q. G., Wang J. L. (2010). Isolation and structural identification of triterpenoids and anthraquinones from pericarpium Arecae. Traditional Chin. Drug Res. Clin. Pharmacol. 21, 634–636. doi: 10.19378/j.issn.1003-9783.2010.06.022
Hoan T. D., Thao D. T., Gadahi J. A., Song X., Xu L., Yan R., et al. (2014). Analysis of humoral immune response and cytokines in chickens vaccinated with Eimeria brunetti apical membrane antigen-1 (EbAMA1) DNA vaccine. Exp. Parasitol. 144, 65–72. doi: 10.1016/j.exppara.2014.04.015
Holdsworth D. K., Jones R. A., Self R. (1998). Volatile alkaloids from Areca catechu. Phytochemistry 48, 581–582. doi: 10.1016/S0031-9422(98)00016-8
Huang P. L., Chi C. W., Liu T. Y. (2010). Effects of Areca catechu L. containing procyanidins on cyclooxygenase-2 expression in vitro and in vivo. Food Chem. Toxicol. 48, 306–313. doi: 10.1016/j.fct.2009.10.014
Huang J. L., Lu H. H., Lu Y. N., Hung P. S., Lin Y. J., Lin C. C., et al. (2016). Enhancement of the genotoxicity of benzo[a]pyrene by arecoline through suppression of DNA repair in HEp-2 cells. Toxicol. Vitro 33, 80–87. doi: 10.1016/j.tiv.2016.02.007
Hung T. C., Huang L. W., Su S. J., Hsieh B. S., Cheng H. L., Hu Y. C., et al. (2011). Hemeoxygenase-1 expression in response to arecoline-induced oxidative stress in human umbili-cal vein endothelial cells. Int. J. Cardiol. 151, 187–194. doi: 10.1016/j.ijcard.2010.05.015
Husvik C., Khuu C., Bryne M., Halstensen T. S. (2009). PGE2 production in oral cancer cell lines is COX-2-dependent. Dent. Res. 88, 164–169. doi: 10.1177/0022034508329519
Jahns E. (1888). Ueber die Alkalo de der Arecanuss. Berichte der Deutschen Chemischen Gesellschaft 21, 3404–3409. doi: 10.1002/cber.188802102233
Jahns E. (1890). Ueber die Alkalo de der Arecanuss. Berichte der Deutschen Chemischen Gesellschaft 23, 2972–2978. doi: 10.1002/cber.189002302219
Jam N., Hajimohammadi R., Gharbani P., Mehrizad A. (2021). Evaluation of antibacterial activ-ity of aqueous, ethanolic and methanolic extracts of areca nut fruit on selected bacteria. BioMed. Res. Int. 2021, 6663399. doi: 10.1155/2021/6663399
Jeyathilakan N., Murali K., Anandaraj A., Basith S. A. (2010). In vitro evaluation of anthelmintic property of herbal plants against Fasciola gigantica. Indian J. Anim. Sci. 80 (11), 1070–1074. doi: 10.1186/1297-9686-42-40
Ji W. T., Yang S. R., Chen J. Y. (2012). Arecoline downregulates levels of p21 and p27 through the reactive oxygen species/mTORcomplex 1 pathway and may contribute to oral squamous cell carcinoma. Cancer Sci. 103, 1221–1229. doi: 10.1111/j.1349-7006.2012.02294.x
Jiang J. H., Jung S. Y., Kim Y. C., Shin S. R., Yu S. T., Park H. (2009). Antimalarial effects of Areca catechu L. Korean J. Oriental Physiol. Pathol. 23, 494–498.
Jinxing Z. (2006). Study on the health care function of areca catechu extracts (Changsha: Central South University of Forestry and Technology).
Juan S., Lixing C., Zhiqiang C., Qicheng C., Zhi J. (2018). Review of pharmacological and toxicological studies on semen arecae and its main component. J. Guangzhou Univ. Traditional Chin. Med. 35 (6), 1143–1146. doi: 10.13359/j.cnki.gzxbtcm.2018.06.040
Kafle S., Shanbhag T., Shenoy S., Amuthan A., Shrestha J. (2011). Antinfertility effect of Areca catechu in male albino rats. Int. J. Pharm. Sci. Rev. Res. 10, 79–82.
Kang L. R., Fu S. F., Zeng G. L., Ji Y. Y., Zhang H. D., Zhang W. M. (2016). Optimization of subcritical water extraction process of arecoline from areca seeds by response surface methodology. J. Food Saf. Qual. Inspection 7 (09), 3773–3780. doi: 10.19812/j.cnki.jfsq11-5956/ts.2016.09.059
Keshavabhat S., Ashwin D., Mythri S. (2016). Areca Palm (Areca catechu L.) leaf as a potent biopestiside against mosquitoes. Pestology 40, 52–57.
Khan I., Kumar N., Pant I., Narra S., Kondaiah P. (2012). Activation of TGF -b pathway by areca nut constituents: A possible cause of oral submucous fibrosis. PloS One 7, e51806. doi: 10.1371/journal.pone.0051806
Kheirabadi K. P., Katadj J. K., Bahadoran S., Silva J. A. T. D., Bashi M. C. (2014). Comparison of the anticoccidial effect of granulated extract of Artemisia sieberi with monensin in experimenta coccidiosis in broiler chickens. Exp. Parasitol. 141, 129–133. doi: 10.1016/j.exppara.2014.03.022
Kim E. K., Choi E.-J. (2015). Compromised MAPK signaling in human diseases: An update. Arch. Toxicol. 89, 867–882. doi: 10.1007/s00204-015-1472-2
Kiuchi F., Miyashita N., Tsuda Y., Konda K., Yoshimura H. (1987). Studies on crude drugs effective on Visceral Larva Migrans. I. identification of Larvicidal principles in Betel nuts. Chem. Pharm. Bull. 35, 2880–2886. doi: 10.1248/cpb.35.2880
Kurokawa M., Shimizu T., Watanabe W., Shiraki K. (2010). Development of new antiviral agents from natural products. Open Antimicrobial Agents J. 2, 49–57. doi: 10.2174/1876518101002020049
Kusumoto I. T., Nakabayashi T., Kida H., Miyashiro M., Hattori M., Namba T., et al. (1995). Screening of various plant extracts used in ayurvedic medicine for inhibitory effects on human immunodeficiency virus type 1 (HIV-1) protease. Phytotherapy Res. 9, 180–184. doi: 10.1002/ptr.2650090305
Lanzhou F. (1956). Study on the treatment of insect with pumpkin seeds and combina-tion. Chin. J. Med. 3, 138–147.
Lee K. P., Sudjarwo G. W., Kim J. S., Dirgantara S., Hong H. (2014). The anti-inflammatory effect of Indonesian Areca catechu leaf extract in vitro and in vivo. Nutr. Res. Pract. 8, 267–271. doi: 10.4162/nrp.2014.8.3.267
Lee M., Theodoropoulou M., Graw J., Roncaroli F., Zatelli M. C., Pellegata N. S. (2011). Levels of p27 sensitize to dual PI3K/mTOR inhibition. Mol. Cancer Ther. 10, 1450–1459. doi: 10.1158/1535-7163.MCT-11-0188
Lee S. C., Tsai C. C., Yao C. H., Hsu Y. M., Chen Y. S., Wu M. C. (2013). Effect of arecoline on regeneration of injured peripheral nerves. Am. J. Chin. Med. 41, 865. doi: 10.1142/S0192415X13500584
Li N. A. (2022). Study on the control effect and transcriptional regulation mechanism of areca ex-tract against Hepatocenterocytosis of Litopenaeus vannamei (Haikou: Hainan Univer-sity).
Li S., Chen R., Luo K., Guo Y., Xiao M., Du G. (2017). Areca nut extract protects against ovariecto-my-induced osteoporosis in mice. Exp. Ther. Med. 13, 2893–2899. doi: 10.3892/etm.2017.4362
Li W., Wang D. F., Zhou L. L., Zhou H. L., Hu L., Li Q. (2015). Research progress on the anthelmintic effects of areca nut. Breed. Feed 2, 6–9. doi: 10.3969/j.issn.1671-427X.2015.02.002
Li C. B., Yang X., Tang W. B., Liu C. Y., Xie D. P. (2010). Arecoline excites the contraction of distal colonic smooth muscle strips in rats via the M3 receptor- extracellular Ca2 + influx- Ca2 + store release pathway. J. Physiol. Pharmacol. 88, 439. doi: 10.1139/Y10-024
Li W., Zhou L. L., Wang D. F., Zhou H., Hu L., Zhang Y., et al. (2016). Effects of areca nut extract on the cecal tissue structure of chickens infected with coccidia. Heilongjiang Anim. Sci. Veterinary Med. 17), 187–189+298-300. doi: 10.13881/j.cnki.hljxmsy.2016.1718
Lillehoj H. S. (1998). Role of T lymphocytes and cytokines in coccidiosis. Int. J. Parasitol. 28, 1071–1081. doi: 10.1016/S0020-7519(98)00075-7
Lin H., Hai De Z., Shi Shu L. (2011). Optimization of ultrasound-assisted extraction of total phenol from betel. Afr. J. Biotechnol. 10 (46). doi: 10.5897/AJB11.703
Lin S. C., Lu S. Y., Lee S. Y., Lin C. Y., Chen C. H., Chang K. W. (2005). Areca (betel) nut extract activates mitogen-activated protein kinases and NF-kappaB in oral keratinocytes. Int. J. Cancer. 116, 526–535. doi: 10.1002/ijc.v116:4
Ling K. H., Kian C. T. (2009). A guide to medicinal plants (Singapore: World Sci-entific Publishing Co).
Liu W. J. (2012). Study on extraction and purification of alkaloids from areca nut and their antibacterial properties (Beijing: Beijing Forestry University), 1–54.
Liu F. L., Chen C. L., Lai C. C., Lee C. C., Chen D. M. (2020). Arecoline suppresses RANKL induced osteo-clast differentiation in vitro and attenuates LPS induced bone loss in vivo. Phyto-medicine 69, 153195. doi: 10.1016/j.phymed.2020.153195
Liu H. W., Hu B. Q., Cao C. F., Wei M. J., Chen Y., Gao Y. (2005). Comparative study on the expression of p16 and p53 proteins in oral leukoplakia and squamous cell carcinoma. J. Modern Stomatology 19, 607–611.
Liu S. W., Wang Y., Dou E. X., Jinzhao H. (2016a). Influence of Lime, Piper betle Processing Betelnut on Mice'Genital Toxicity and Body Temperature [J]. Henan Agric. Sci. 45 (10), 151–154. doi: 10.15933/j.cnki.1004-3268.2016.10.034
Liu S. W., Wang Y., Hu J. Z. (2016b). Effects of water extracts from different parts of areca nut on physiological indicators of mice. China Anim. Husbandry Veterinary Med. 43 (10), 2648–2654. doi: 10.16431/j.cnki.1671-7236.2016.10.020
Liu D. L., Wang X. Y., Yang B., Zhang H. (2013). Review of pharmacological effects and toxicological information of Arecae Semen. China J. Chin. Materia Med. 38, 2273–2275.
Lu S. Y., Chang K. W., Liu C. J., Tseng Y. H., Lu H. H., Lee S. Y., et al. (2006). Ripe areca nut extract induces G1phase arrests and senescence-associated phenotypes in normal human oral keratinocyte. Carcinogenesis 27, 1273–1284. doi: 10.1093/carcin/bgi357
Lu H. H., Kao S. Y., Liu T. Y., Liu S. T., Huang W. P., Chang K. W., et al. (2010). Areca nut extract induced oxidative stress and upregulated hypoxia inducing factor leading to autophagy in oral cancer cells. Autophagy 6, 725–737. doi: 10.4161/auto.6.6.12423
Lu Z. Z., Yao X. W., Chen S. S., Yang L. H., Huang Y. H., Lin M. X., et al. (2011). Expression and significance of p53 and proliferating cell nuclear antigen in oral leukoplakia and oral squamous cell carcinoma. Chin. J. Oral. Med. Res. 5, 477–482.
Lu F. Z., Zhang X. J., Fu Y., Zhu W. Y., Ji J. Y. (2007). A survey on preventing and curing chicken and rabbit coccidiosis using chinese medicinal herbs. Acta Agriculturae Zhejiangensis, 253–257.
Ma D., Gao M., Li J., Ma C., Li G. (2013). Construction of novel cytokine by fusion of chicken IL-2 signal peptide to mature chicken IL-15 and comparison of the adjuvant effects by DNA immunization against Eimeria challenge. Veterinary Immunol. Immunopathology 156, 114–120. doi: 10.1016/j.vetimm.2013.09.005
Ma Y. T., Hsu F. L., Lan S. J., Chen C. F. (1996). Tannins from betel nuts. J. Chin. Chem. Soc. (Taipei) 4, 77–81. doi: 10.1002/jccs.199600013
Machová M., Bajer T., Šilha D., Ventura K., Bajerová P. (2021). Volatiles composition and antimicrobial activities of areca nut extracts obtained by simultane ous distillation-extraction and headspace solid-phase microextraction. Molecules 26 (24), 7422. doi: 10.3390/molecules26247422
Malika F., Neupane Ram P., Jian Y., Philip W., Reinhold P. (2018). Areca nut extracts mobilize calcium and release pro-inflammatory cytokines from various immune cells. Sci. Rep. 8, 1075. doi: 10.1038/s41598-017-18996-2
Mei F., Meng K., Gu Z., Yun Y., Zhang W., Zhang C., et al. (2021). Arecanut (Areca catechu L) seed polyphe-nol-ameliorated osteoporosis by altering gut microbiome via LYZ and the immune system in estrogen-deficient rats. J. Agric. Food Chem. 69, 246–258. doi: 10.1021/acs.jafc.0c06671
Miska K. B., Kim S., Fetterer R. H., Dalloul R., Jenkins M. (2013). Macrophage migration inhibitory factor (MIF) of the protozoan parasite Eimeria influences the components of the immune system of its host, the chicken. Parasitol. Res. 112, 1935–1944. doi: 10.1007/s00436-013-3345-z
Moutasim K. A., Jenei V., Sapienza K., Marsh D., Weinreb P. H., Violette S. M., et al. (2011). Betelderived alkaloid upregulates keratinocyte alphavbeta6 integrin expression and promotes oral submucous fibrosis. Pathol 223, 366–377. doi: 10.1002/path.v223.3
Ngwe Tun M. M., Toume K., Luvai E., Nwe K. M., Mizukami S., Hirayama K., et al. (2022). The discovery of herbal drugs and natural compounds as inhibitors of SARS-CoV-2 infection in vitro. J. Natural Medicines 76, 402–409. doi: 10.1007/s11418-021-01596-w
Nonaka G. I., Hsu F. L., Nishioka I. (1981). Structures of dimeric, trimeric, and tetrameric procyanidins from Areca catechu L. J. Chem. Soc. Chem. Communication 9, 781–783. doi: 10.1039/c39810000781
Pahadia A., Gawde R., Agrawal S. (2013). Antimicrobial activity of hydro alcoholic extract of Areca catechu. Int. J. Pharma Erudition 3, 18–25.
Park E. J., Kim Y. M., Park S. W., Kim H. J., Lee J. H., Lee D.-U., et al. (2013). Induction of HO-1 through p38 MAPK/Nrf2 signaling pathway by ethanol extract of Inula helenium L. reduces inflammation in LPS-activated RAW 264.7 cells and CLP-induced septic mice. Food Chem. Toxicol. 55, 386–395. doi: 10.1016/j.fct.2012.12.027
Park S. S., Lillehoj H. S., Hong Y. H., Lee S. H. (2007). Functional characterization of tumor ne-crosis factor superfamily 15 (TNFSF15) induced by lipopolysaccharides and Eimeria in-fection. Dev. Comp. Immunol. 31, 934–944. doi: 10.1016/j.dci.2006.12.010
Peng W., Liu Y. J., Wu N., Sun T., He X. Y., Gao Y. X., et al. (2015). Areca catechu L. (Arecaceae): A review of its traditional uses, botany, phytochemistry, pharmacology and toxicology. J. Ethnopharmacology. 164, 340–356. doi: 10.1016/j.jep.2015.02.010
Pinto A., Cordeiro R. D., Sidrim J. J. C., Melo Leite A., Girão V., Brilhante R., et al. (2013). Anti-inflammatory and immunomodulatory effect of an extract of Coccidioides posadasii in experimental arthritis. Mycopathologia 175, 193–206. doi: 10.1007/s11046-013-9621-8
Pithayanukul P., Nithitanakool S., Bavovada R. (2009). Hepatoprotective potential of ex-tracts from seeds of Areca catechu and nutgalls of Quercus infectoria. Molecules 14, 4987–5000. doi: 10.3390/molecules14124987
Qingqing Z., Yaqi Z., Lilian Lu, yangyang L., bingchun G. (2013). Optimization of heat reflux extraction of arecoline from areca inflorescence. Chin. J. Exp. Prescription 19, 77–79.
Quiroz-Castañeda R. E., Dantán-González E. (2015). Control of avian coccidiosis: future and pre-sent natural alternatives. BioMed. Res. Int. 2015, 430610. doi: 10.1155/2015/430610
Raghavan V., Baruah H. (1958). Areca nut: India's popular masticatory history, chemistry and utilization. Economic Bot. 12, 315–345. doi: 10.1007/BF02860022
Rahman M. M., Eo S. K. (2012). Prospects and challenges of using chicken cytokines in disease prevention. Vaccine 30, 7165–7173. doi: 10.1016/j.vaccine.2012.10.011
Saeed S. A., Farnaz S., Simjee R. U., Malik A. (1993). Triterpenes and sitosterol from piper betle: isolation, antiplatelet and anti-inflammatory effects. Biochem. Soc. Trans. 21, 462s. doi: 10.1042/bst021462s
Salehi B., Konovalov D. A., Fru P., Kapewangolo P., Peron G., Ksenija M. S., et al. (2020). Areca catechu-from farm to food and bio-medical applications. Phytotherapy Res. 34, 2140–2158. doi: 10.1002/ptr.v34.9
Sareila O., Kelkka T., Pizzolla A., Hultqvist M., Holmdahl R. (2011). NOX2 complex-derived ROS as immune regulators. Antioxid Redox Signal 15, 2197–2208. doi: 10.1089/ars.2010.3635
Sari L. M., Suyatna D., Utami S., Chairul C., Subita G. P., Whulandhary Y. S., et al. (2014). Acute oral toxicity study of Areca catechu linn. aqueous extract in sprague-dawley rats. Asian J. Pharm. Clin. Res. 7, 20–22. doi: 10.22159/ajpcr.2016.v9s3.14462
Sazwi N. N., Nalina T., Abdul Rahim Z. H. (2013). Antioxidant and cytoprotective activities of Piper betle, Areca catechu, Uncaria gambir and betel quid with and without calcium hydroxide. BMC Complementary Med. Therapies 13, 351. doi: 10.1186/1472-6882-13-351
Scalbert A. (1991). Antimicrobial properties of tannins. Phytochemistry 30, 3875–3 83. doi: 10.1016/0031-9422(91)83426-L
Schamschula R. G., Adkins B. L., Barmes D. E., Charlton G. (1977). Betal chewing and caries experi-ence in New Guinea. Community Dent. Oral. Epidemiol. 5, 284–286. doi: 10.1111/j.1600-0528.1977.tb01015.x
Shen X., Chen W., Zheng Y., Lei X., Tang M., Wang H., et al. (2017). Chemical composition, antibacterial and antioxidant activities of hydrosols from different parts of Areca catechu L. and Cocos nucifera L. Ind. Crops Products 96, 110–119. doi: 10.1016/j.indcrop.2016.11.053
Shen Z. J., Zhu B. L., Jiang J. S. (2001). Nitric oxide production during Eimeria tenella infection in chickens. Acta Veterinaria et Zootechnica Sinica. (03), 270–276.
Shuhua T., Qiong L., Zhi X., Qin M., Jia Y., Fu H. (2015). Effect of Areca catechu extracts on immunity functions and antioxidant activities in mice [J]. Food Industry Sci. Technol. 36 (17), 343–346. doi: 10.13386/j.issn1002-0306.2015.17.062
Sillarine K., Banerjee A., Dkhar H., Nongrum H. B., Ganguly B., Islam M., et al. (2014). Precocious anaphase and expression of Securin and p53 genes as candidate biomarkers for the early detection in areca nut-induced carcinogenesis. Mutagenesis 30 (3), 381–389. doi: 10.1093/mutage/geu083
Sim S., Yong T. S., Parks J., Im K., Kong Y., Ryu J., et al. (2005). NADPH oxidase-derived reactive oxygen species-mediated activation of ERK1/2 is required for apoptosis of human neutrophils induced by Entamoeba histolytica. J. Immunol. (Baltimore Md.: 1950) 174, 4279–4288. doi: 10.4049/jimmunol.174.7.4279
Song P., Yu S. K., Zhang W. M., Zhang D., Qiu J. (2002). Selection of acarid-killing plants and their effective parts in vitro [J]. J. Northwest A F Univ. (Natural Sci. Edition). 30 (6), 69–72. doi: 10.13207/j.cnki.jnwafu.2002.06.017
Sun Y., Lu J., Li J., Li P., Zhao M., Xia G. (2023). Optimization of ultrasonic-assisted extraction of polyphenol from Areca nut(Areca catechu L,)seeds using response surface methodology and its effects on osteogenic activity. Ultrason Sonochem 98, 106511–106519. doi: 10.1016/j.ultsonch.2023.106511
Suqin C., Jun Li, Jinying X. (2010). The role of nuclear transcription factor κB in inflammation-related cancers. Clin. Compendium 25, 1471–1473.
Surendiran N. S., Yuvaraj T. V. (2010). Antibacterial, antioxidant, in vitro and in vivo immunomod-ulatory studies of Areca catechu in mice. J. Pharm. Res. 3, 2678–2681.
Taiping Yu, Yong L. I., Tao W. (2007). The role of nuclear transcription factor κB in inflammation-related cancers. Int. J. Stomatology 34, 418–420.
Tian X. F., Dai J. J., Dong L., He B. L., Yang Z. Y., Zhao L. N. (2002). Ultrastructure observation on Taenia solium expelled by decoction of areca and pumpkin seeds. China Acad. J. Electronic Publishing House, 15 (6), 47–48.
Tizard I. (2009). How inflammation is triggered [M], Veterinary immunology an introduction. (St. Louis, Missouri, USA: Saunders Elsevier MO). 1, 1–27.
Tsai C. H., Yang S. F., Lee S. S., Chang Y. C. (2009). Augmented heme oxygenase-1 expression in areca quid chewing associated oral submucous fibrosis. Oral. Dis. 15, 281–286. doi: 10.1111/j.1601-0825.2009.01523.x
Vermani K., Garg S. (2002). Herbal medicines for sexually transmitted diseases and AIDS. J. Eth-nopharmacol 80, 49–66. doi: 10.1016/S0378-8741(02)00009-0
Wang L., Gu L., Tang Z. (2020). Cytokines secreted by arecoline activate fibroblasts that affect the balance of TH17 and Treg. J. Oral. Pathol. Med. 49, 156–163. doi: 10.1111/jop.12965
Wang C. C., Lin H. L., Wey S. P., Jan T. R. (2011). Areca-nut extract modulates antigen- specific immunity and augments inflammation in ovalbumin-sensitized mice. Immunopharmacol Immunotoxicol 32 (2), 315. doi: 10.3109/08923973.2010.507208
Wang Y. C., Tsai Y. S., Huang J. L., Lee K. W., Kuo C. C., Wang C. S., et al. (2010). Arecoline arrests cells at prometaphase by deregulating mitotic spindle assembly and spindle assembly checkpoint: implication for carcinogenesis. Oral. Oncol. 46, 255–262. doi: 10.1016/j.oraloncology.2010.01.003
Wang D., Zhou L., Li W., Zhou H., Hou G. (2018). Anticoccidial effects of areca nut (Areca catechu L.) extract on broiler chicks experimentally infected with Eimeria tenella. Exp. Parasitol. 184, 16–21. doi: 10.1016/j.exppara.2017.11.002
Wei L., Luli Z., Dingfa W., Hanlin Z., Lin H., Yage Z., et al. (2016). Effect of areca nut extract on caecal histological structure in Wenchang chickens infected by Eimeria tenella. Heilongjiang Anim. Husbandry Veterinary Sci. 187 189 + 298–300. doi: 10.13881/j.cnki.hljxmsy.2016.1718
Wu J., Wang H., Li X. N., Mei W. L., Dai H. F. (2011). Study on cytotoxic chemical constituents in Areca catechu. J. Henan Univ. (Nat Sci) 41, 511–514.
Xiaoyan W., Meiying Z., Yafeng W., Hao J., Liu Q., Wang Y., et al. (2008). Investigation on antiviral effect of areca catechu L. Extract vitro. Shi Zhen Chin. Med. 19, 2954–2955.
Xu J. (2001). Determination of total alkaloid in Semen Arecae by acid dye colorimetry. Strait Pharm. J. 13, 30–31.
Yang W. Q., Wang H. C., Wang W. J., Wang Y., Zhang X. Q., Ye W. C. (2012). Chemical constituents from the fruits of Areca catechu. J. Chin. Med. Materials 35, 400–402.
Yao N. (2023). Effects and mechanisms of arecoline on production performance, digestion and absorption capacity, and intestinal structural integrity in late-stage grass carp [D] (Ya’an, Sichuan, China: Sichuan Agricultural University). doi: 10.27345/d.cnki.gsnyu.2023.000280
Yenjit P., Issarakraisila M., Intana W., Chantrapromma K. (2010). Fungicidal activity of compounds extracted from the pericarp of Areca catechu against Colletotrichum gloeosporioides in vitro and in mango fruit. Postharvest Biol. Technol. 55, 129–132. doi: 10.1016/j.postharvbio.2009.09.003
Yi S., Zou L., Li Z., Sakao K., Wang Y., Hou D.-X. (2022). In vitro antioxidant activity of areca nut polyphenol extracts on RAW264.7 cells. Foods 11, 3607. doi: 10.3390/foods11223607
Yubin Ji, Lianchuang Li, Lei Yu (2007). Effects of arecoline on DNA in bone marrow cells. Chin. Herbal Medicines 38, 573–575.
Zhang X., Mei W. L., Zeng Y. B., Liu J., Dai W. J., Dai H. F. (2009). Phenolic constituents from the fruits of Areca catechu and their Anti-bacterial activities. J. Trop. 41 Subtropical Bot. 17, 74–76.
Zhang X., Wu J., Han Z., Mei W. L., Dai H. F. (2010c). Antioxidant and cytotoxic phenolic compounds of areca nut (Areca catechu). Chem. Res. Chin. Univ. 26, 161–164.
Keywords: areca nut extract, antibiotic alternatives, natural bioactive compounds, antiinflammatory, sustainable animal agriculture
Citation: Liu Z, Wang X and Wen L (2025) Exploring the potential benefits of areca nut extract in animal production: a review. Front. Anim. Sci. 6:1495886. doi: 10.3389/fanim.2025.1495886
Received: 13 September 2024; Accepted: 21 January 2025;
Published: 06 February 2025.
Edited by:
Massimo Bionaz, Oregon State University, United StatesReviewed by:
Patrick Valere Tsouh Fokou, The University of Bamenda, CameroonIsaac Oluseun Adejumo, University of Ibadan, Nigeria
Katia Scortecci, Federal University of Rio Grande do Norte, Brazil
Ashfaq Ahmad Khan, Govt. Postgraduate College, Pakistan
Copyright © 2025 Liu, Wang and Wen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lixin Wen, c2Z3bHg4MDE1QHNpbmEuY29t
 Zhuying Liu
Zhuying Liu Xiaolong Wang
Xiaolong Wang Lixin Wen2,3*
Lixin Wen2,3*