- 1Department of Animal Science, Faculty of Agriculture, Khon Kaen University, Khon Kaen, Thailand
- 2Department of Veterinary Public Health, Faculty of Veterinary Medicine, Khon Kaen University, Khon Kaen, Thailand
The aim of the present study was to evaluate the effects of replacing diet rice bran oil (RBO) with black soldier fly larva oil (BSFLO) on the growth performance, carcass traits, meat quality, and blood parameters of broiler chickens. At one day of age, a total of 180 male broiler chickens (Ross 308) were randomly allocated to 3 experimental groups (4 replicates and 15 birds/pen). To a basal control diet, either 50% or 75% of the RBO was replaced with BSFLO, respectively. The growth performance was monitored throughout the rearing period (divided into 3 periods: 1-10, 11-24, and 25-42 days). On days 24 and 42, Blood samples were taken from each treatment for hemato-biochemical index determination. At the termination period, 8 birds (two birds/pen) per group were slaughtered for carcass and meat quality measurement. Samples of the liver were submitted for fatty acid investigations. The results showed that the inclusion of 75% BSFLO in the broiler diet significantly increased FCR (Feed conversion ratio) in the finisher and overall periods. Interestingly, replacing 50% of RBO with BSFLO did not influence growth performance, carcass traits, and hematochemical parameters compared to 75% of BSFLO and control groups. The present study suggests that partially replacing RBO with 50% of BSFLO in broiler chicken diets has no adverse effects on growth performance, carcass-meat quality, or blood parameters.
1 Introduction
Insects are gaining traction as valuable additions to human diets in many countries (Borrelli et al., 2017). They also show potential substitutes for traditional sources of protein, such as fishmeal and soybean meal in livestock feed (Loponte et al., 2017; Bovera et al., 2018; Cutrignelli et al., 2018). The black soldier fly (Hermetia illucens) is a particularly attractive option. During its growth stage, this fly can efficiently convert large amounts of organic waste, which would otherwise be pollutants, into usable protein and fats for animal feed (Zheng et al., 2012; Li et al., 2016). Black soldier fly larvae (BSFL) boast a high nutrient content, containing up to 40% protein rich in essential amino acids, over 28% lipids, and essential minerals like calcium and phosphorus (Makkar et al., 2014; Wang and Shelomi, 2017).
Despite the nutritional advantages offered by insects as animal feed, concerns have emerged regarding potential hazards associated with their use. This is because insects raised on contaminated substrates may accumulate heavy metals and toxins, which could be passed on to the animals they are fed (Wang and Shelomi, 2017). However, insects have garnered attention as a promising alternative feed for monogastric animals due to their nutrient-rich composition and minimal environmental impact (Henry et al., 2015; Biasato et al., 2016; Overa et al., 2016). They boast low greenhouse gas and ammonia emissions, exhibit favorable feed conversion ratios as cold-blooded creatures, and require minimal water and soil for cultivation (van Huis, 2013; Makkar et al., 2014). Consumers appear increasingly receptive to products derived from these unconventional raw materials (Mancuso et al., 2016). Furthermore, insects can serve as bio-converters of food waste into animal feed, thus avoiding competition with humans for natural resources (Diener et al., 2011; Makkar et al., 2014).
Kierończyk et al. (2018) proposed that incorporating insect oils, such as those derived from Tenebrio molitor and Zophobas morio, into broiler chicken diets could effectively replace soybean oil (SBO) without compromising growth performance or nutrient digestibility. Additionally, Li et al. (2016) demonstrated that dietary black soldier fly larvae oil (BSFLO) led to increased deposition of omega-3 fatty acids in muscle tissues while reducing intraperitoneal fat accumulation in juvenile carp. BSFLO is particularly abundant in medium-chain fatty acids (MCFA) like lauric acid (C12:0), akin to coconut oil (CCO) (Li et al., 2016; Ushakova et al., 2016), which uniquely comprises approximately 50% lauric acid in its fatty acid composition (Dayrit, 2014). Wang et al. (2015) indicated that MCFA might contribute to abdominal fat reduction due to their preferential utilization for energy over long-chain saturated or unsaturated fatty acids. Furthermore, The MCFA, including lauric acid, exhibits antimicrobial properties against gut bacteria (Zeitz et al., 2015; Schiavone et al., 2017). Hence, it is possible to postulate that insect oils containing high levels of lauric acid may influence the growth performance and gut health of rapidly growing broiler chickens (Schiavone et al., 2018). The fatty acid composition of broiler diets has been found to influence that of meats and visceral organs, which are closely correlated with the storage and metabolism of fat (Taulescu et al., 2010; Khatun et al., 2018). Schiavone et al. (2017) observed that substituting SBO with BSFLO changed the fatty acid profile of broiler chickens. Nevertheless, a comprehensive understanding of the suitability of BSFLO as a poultry feed ingredient remains limited. Given the antimicrobial properties and capacity to modify body fatty acid composition associated with medium-chain fatty acids-rich insect oils, it is suggested that dietary BSFLO could enhance growth performance and gut health, influence the fatty acid composition of abdominal fat pads, and improve meat quality in broiler chickens. Previous studies have reported that dietary fat sources enhance broiler chickens' meat quality (Zeitz et al., 2015; Khatun et al., 2018).
In this study, we used two oil sources, including rice bran oil (RBO) and BSFLO, to see their effects on growth performance, carcass characteristics, meat quality, liver fatty acid composition, and serum parameters in broiler chickens.
2 Materials and methods
2.1 Ethics statement
This study was conducted following the ethics of animal experimentation recommendations of the National Research Council of Thailand. The research protocol was reviewed and approved by the Institutional Animal Care and Use Committee of Khon Kaen University, Thailand. (Record no. IACUC-KKU-60/66)
2.2 Source of BSFLO, experimental design, birds, and diets
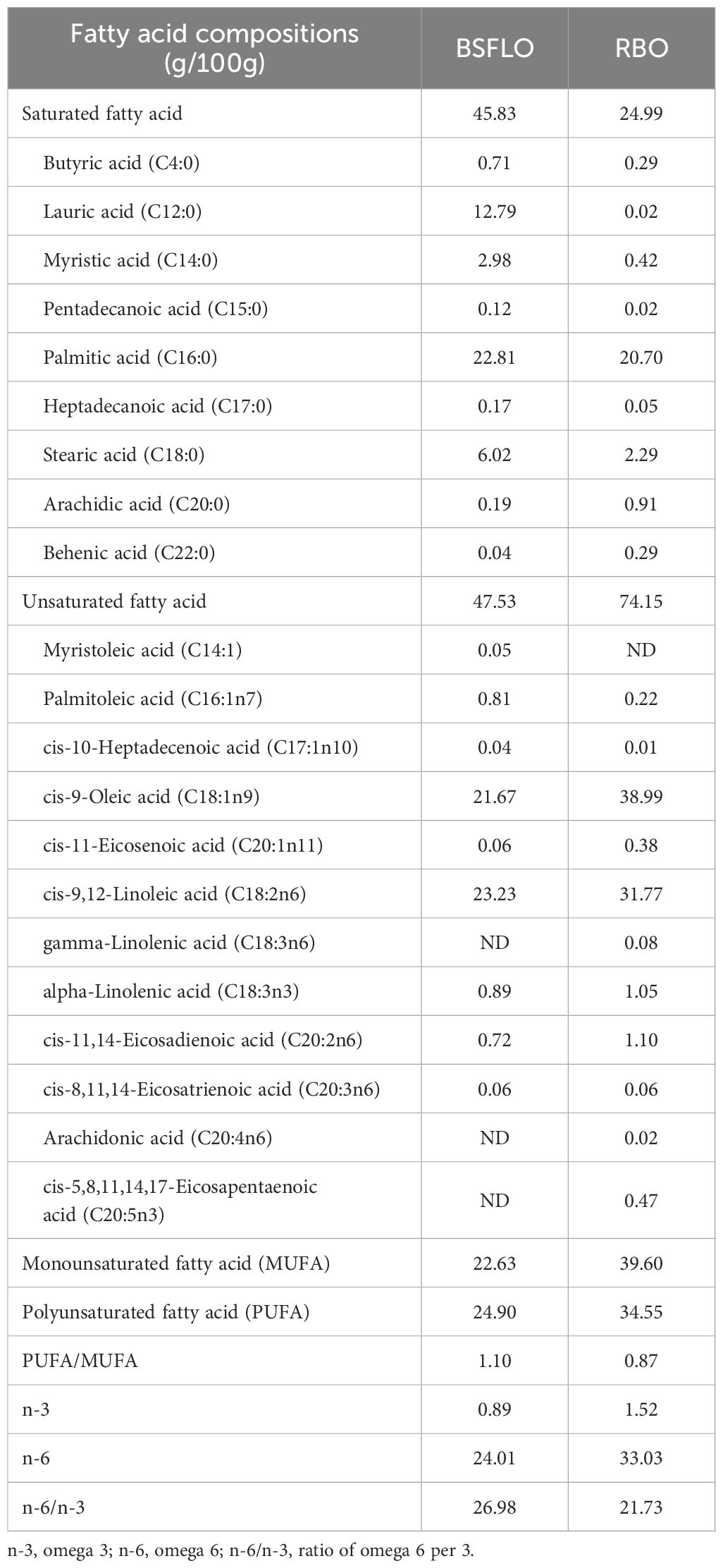
The BSFLO was purchased from BSFLY Co. Ltd. (Udonthani, Thailand), which is a reputable supplier that prioritizes quality, sustainability, and adheres to strict regulatory standards. The fatty acid content of BSFLO and rice bran oil was quantitatively determined using the gas chromatographic method 996.06 (Association of Official Analytical Chemists, 1990) and was expressed as g/100g of the total fatty acid identified (Table 1).
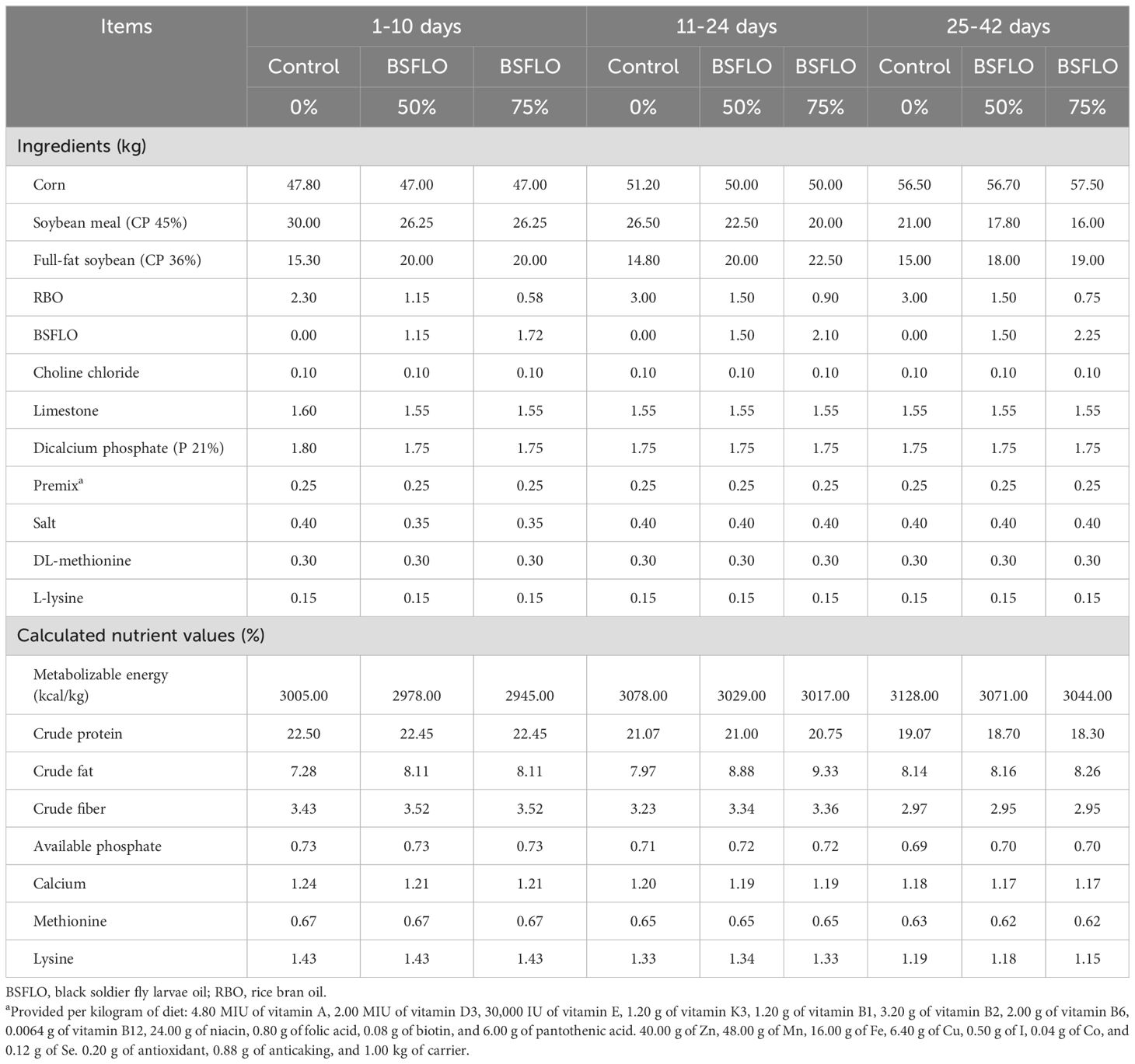
The present study used a completely randomized design with three treatments and four replications. A total of 180 one-day-old, male-sex, Ross 308 broiler chicks (initial body weight 47.49 ± 0.04 g) were obtained from a commercial hatchery (Charoen Pokphand Group Co. Ltd., Nakhon Ratchasima, Thailand). Chickens were randomly allocated into one of three dietary treatments (4 replication pens per treatment, with 15 chicks per pen) for a 42-day study period. Dietary treatments were as follows: the control group was basal diet based on corn-soybean meal and RBO; the BSFLO groups replaced RBO with BSFLO at 50% and 75%. The treatment diets and nutrition values were formulated to meet or exceed the nutrition specification (2022) of Ross broiler chickens in three phases – starter (1-10 days), grower (11-24 days), and finisher (25-42 days; Table 2). All broilers had free access to feed and water during the experiment. The temperature of the chicken coop was maintained at 32°C at the age of the first crucial 7 days and then reduced by 1 to 2°C each week in open housing under tropical conditions, to ensure a comfortable and healthy environment for growing chicks.
2.3 Sample collections and measurements
The broiler chickens were weighed, and their feed intake was recorded on days 1, 10, 24, and 42. Subsequently, the body weight gain (BWG), feed conversion ratio (FCR), survivor rate (SR), and productive index (PI; Equation 1) were calculated for each study period to comprehensively assess the growth performance.
At the end of days 24 and 42, four birds (one bird from each replicate pen) were randomly selected from each treatment, and 5 ml blood samples per bird were gently collected from the wing vein using a sterile syringe with a needle. The blood samples were then divided and transferred into the anticoagulant-free blood collecting tube 4 ml for analyzing blood serum biochemistry, i.e., alanine transaminase (ALT), aspartate transaminase (AST) alkaline phosphatase (ALP), glucose, albumin, globulin, total protein, triglyceride, cholesterol, low-density lipoprotein (LDL), and high-density lipoprotein (HDL). For serum biochemistry analysis, the blood samples were centrifuged at 1,500 g at 4°C for 15 min to separate the serum and stored at −20°C for analysis. The remaining 1 ml blood went into another tube that contained ethylene-diamine-tetra-acetic acid (EDTA) for plasma hematological analysis, i.e., complete blood count.
Upon completion of the 6-week experimental period, two birds per replication then proceeded to the euthanasia and slaughtering process. Prior to sample collection, the chicks were fasted for 12 h. Then, they were individually weighed, euthanized (by cervical dislocation), slaughtered and trimmed carcasses. The weight of each whole hot carcass was recorded. Following slaughter, the carcasses underwent immediate evisceration and subsequent sectioning. Visceral and edible meat organs were weighed and recorded to analyze carcass quality. The liver sample was immediately kept and stored at −20°C until the determination of fatty acid composition by gas chromatographic method 996.06 (25). The left side of the breast of each bird was pH-analyzed using a pH meter (CyberScan Ion 510, Eutech Instruments Pet. Ltd., Singapore), which was inserted directly into the breast muscle within 45 min and 24 h after disassembling the broiler. On the right side of each breast sample, meat color, drip loss, cooking loss, and texture profile were analyzed sequentially. About 45 min after the cut-apart, the meat was color-analyzed using the CIELAB system (Lightness: L* value, Redness: a* value, and Yellowness: b* value), along with the Colorimeter (Chroma Meter CR-410 Series, Konica Minolta Sensing Inc., Tokyo, Japan), then the samples were then individually bagged in polyethylene and stored at 4°C for further evaluation of meat quality.
Afterward, the meat was weighed, encased in multiple layers of gauze cloth, and suspended at 4°C for 24 h. Following this, the meat was carefully patted dry with a paper towel and weighed again. Drip loss (Equation 2) was calculated as a percentage based on the difference in weight before (W1) and after suspension (W2). The meat was then cooked in water at 85°C until the core temperature of the thickest section of the meat reached 80°C. Once cooked, the meat was cooled in a running tap water bath and allowed to rest at 4°C for 2 hours before being weighed once more. Cooking loss (Equation 2) was determined as the difference in weight before (W1) and after cooking (W2).
The cooked meat was subsequently sliced into cubes measuring 1 cm × 1 cm × 1 cm for texture profile analysis (TPA: Hardness), and into specimens measuring 1 cm × 2 cm × 1 cm for the shear force test. During the TPA and shear force analyses, the meat underwent either double compression or perpendicular cutting against the alignment of muscle fibers, utilizing a texture analyzer (model TA.HDplusC, Stable Micro Systems, Godalming, UK) equipped with either a 50 mm cylindrical aluminum probe or a V-slot WB Blade, respectively. All textural parameters were automatically computed and recorded by the Exponent software (Stable Micro Systems, Godalming, UK).
2.4 Statistical analysis
The data were checked for normal distribution using the Shapiro-Wilk test and consistency of variance using Levene's test. Any outliers were eliminated. Outliers were identified using the interquartile range (IQR) method and were excluded from the analysis if they significantly deviated from the overall dataset and were deemed unrelated to the experimental conditions. Statistical evaluations were carried out utilizing one-way analysis of variance (ANOVA) and conducted using SAS version 9.1 software. The significance of group differences was assessed using Tukey's studentized range test at a significance level of p < 0.05. The statistical model is as follows:
where Yijk = observation, µ = overall mean, αi = BSFLO levels (i = 0, 50, and 75%), and = the experimental error.
3 Results
The study investigated the effect of BSFLO in broiler chicken diets on growth performance across different stages, as presented in Table 3. Results indicated that BSFLO inclusion at 50% and 75% did not significantly affect initial body weight (IBW), final body weight (FBW), body weight gain (BWG), feed intake (FI), survival rate (SR), and production index (PI) compared to the control group during the starter, grower, and finisher periods (p > 0.05). However, a significant difference in FCR was observed in the finisher period (p < 0.05). Additionally, when considering the overall 1-42 days period, BSFLO inclusion did not significantly difference in BWG, FI, SR, and PI (p > 0.05), but differences were observed in FCR (p < 0.05), with a lower ratio in the BSFLO50% and control groups compared to 75% BSFLO.
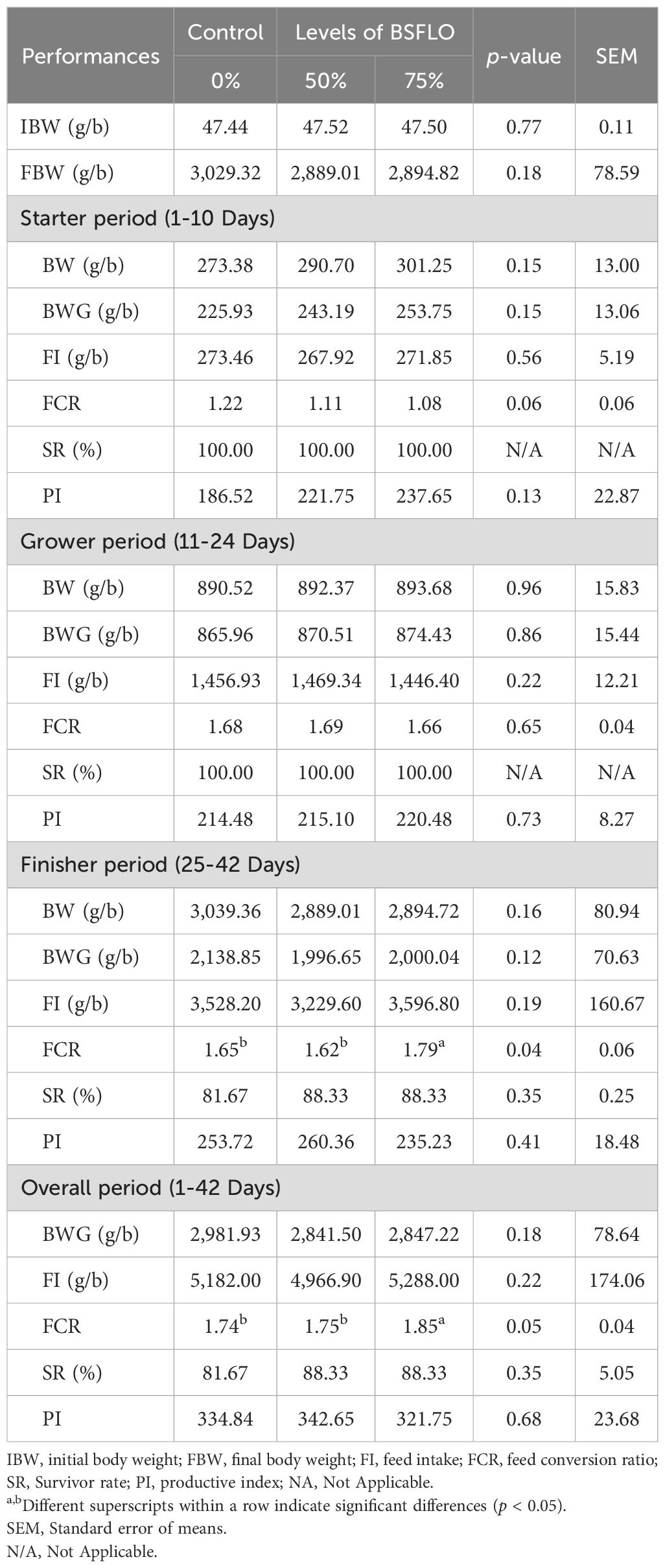
Table 3. The effect of inclusion of black soldier fly larvae oil (BSFLO) in broiler diets on performance.
The blood serum parameters of the broiler are shown in Table 4. The data found that replacing soybean oil with BSFLO in broiler chicken diets may affect blood cell counts. On day 21 (D21), the 50% BSFLO was significantly different in hemoglobin and white blood cells (WBCs) count (p < 0.05), higher than in other groups, and lower lymphocytes (p < 0.05), similar to those in the control group. By day 42, most blood cell parameters showed no significant differences between the groups. However, eosinophils were slightly lower with 50% BSFLO compared to the control and 75% BSFLO groups (p < 0.05).
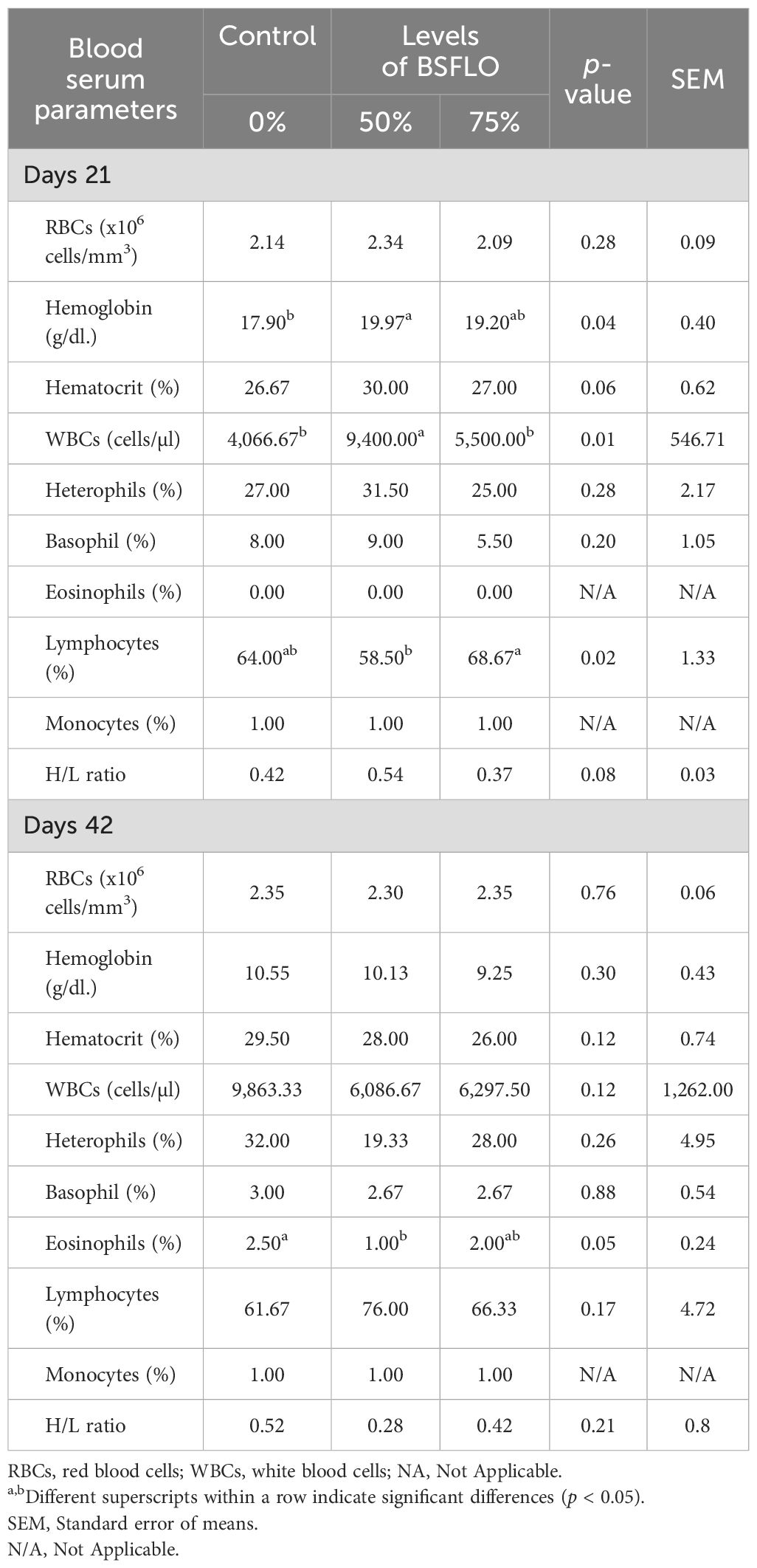
Table 4. The effect of inclusion of black soldier fly larvae oil (BSFLO) in broiler diets on hematology.
The effects of BSFLO on blood biochemistry in broiler chicken diets are shown in Table 5. At day 21, the 50% BSFLO showed the lowest aspartate transaminase (AST) and LDL (low-density lipoprotein) cholesterol levels significantly compared to the control group (p < 0.05). By day 42, The alanine transaminase (ALP) was significantly lower in both BSFLO groups (p < 0.05). Meanwhile, triglyceride was highly significant in 50% BSFLO than the other groups (p < 0.05). On the contrary, the dietary BSFLO inclusion did not significantly (p > 0.05) influence the liver enzyme (alanine transaminase; ALT) and serum protein (albumin, globulin, and total protein).
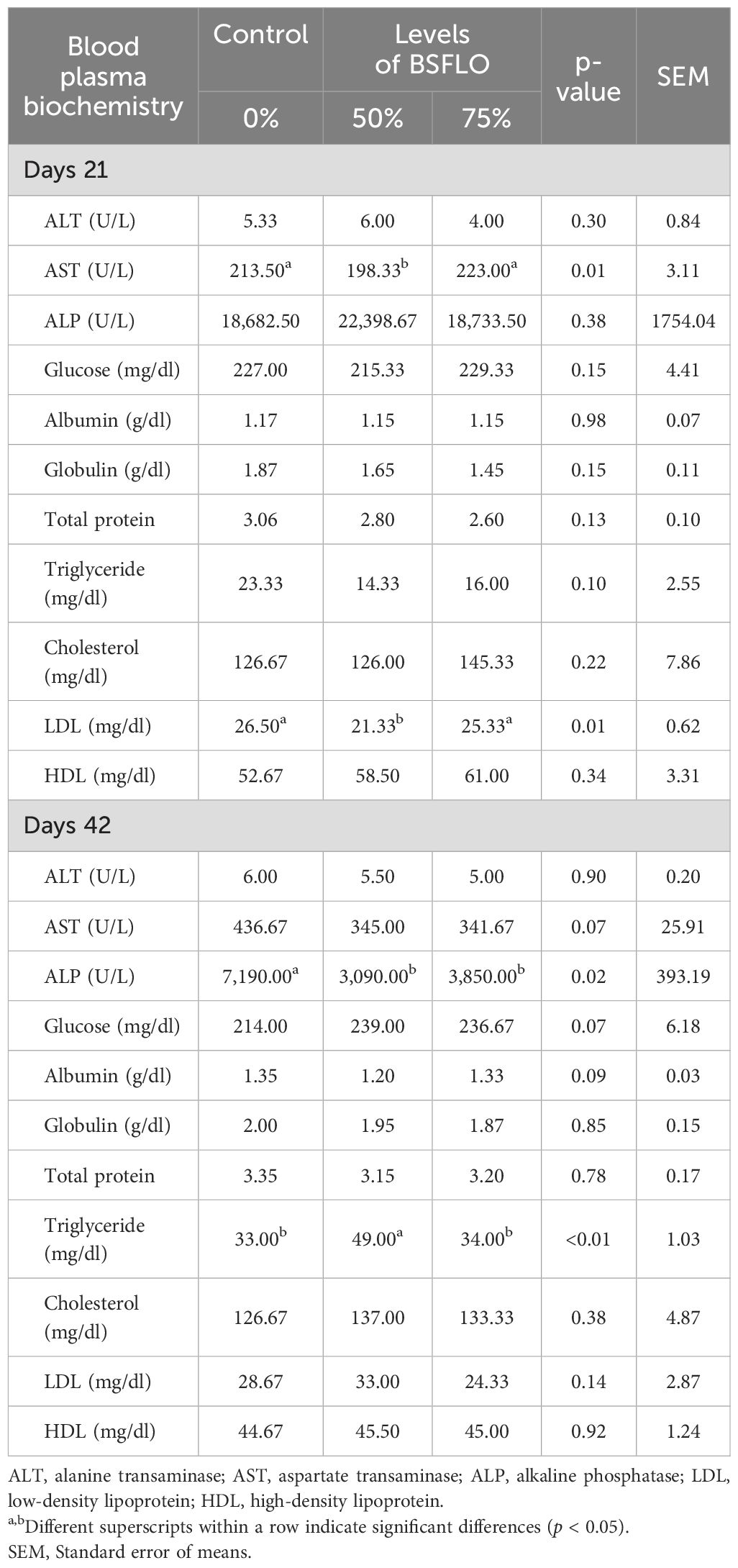
Table 5. The effect of inclusion of black soldier fly larvae oil (BSFLO) in broiler diets on blood plasma biochemistry.
The carcass quality parameters are shown in Table 6. The data found that replacing rice bran oil with BSFLO in broiler chicken diets may have some effects on carcass yield. The dressing percentage was slightly lower with higher BSFLO inclusion (p < 0.05). There were no significant differences (p > 0.05) in the part of giblets, including the percentage of liver, heart, pancreas, spleen, and gizzard. However, broilers in BSFLO groups had higher abdominal fat percentage than those in control group (p < 0.05). Part of external organs, the percentage of the wing tended to increase when supplementing high levels of BSFLO (p < 0.05). Meanwhile, the thigh, breast, and drumstick percentages did not differ significantly among the treatments.
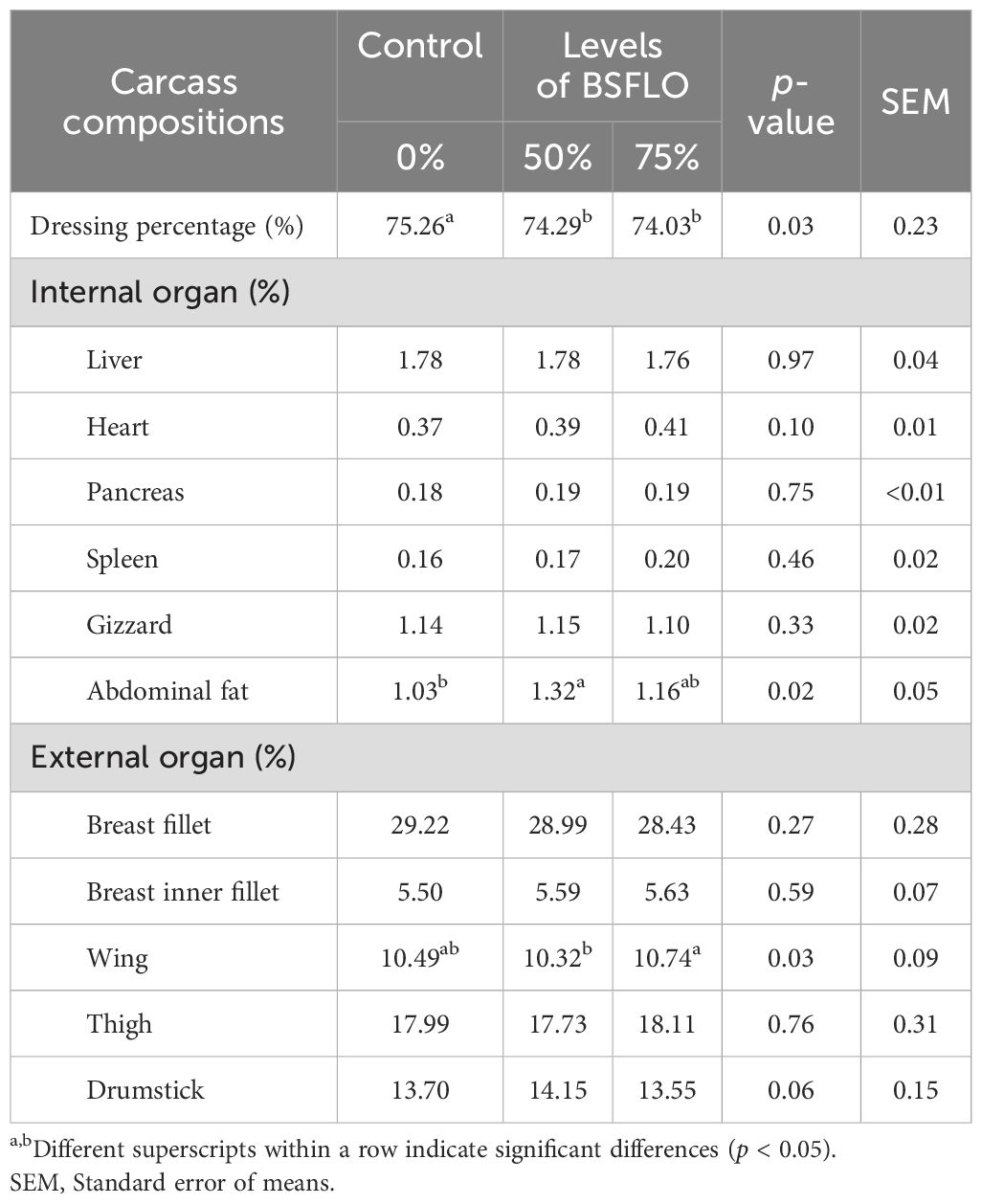
Table 6. The effect of inclusion of black soldier fly larvae oil (BSFLO) in broiler diets on carcass yield.
The results of the breast meat quality values when replacing with BSFLO to broiler chicken diets until termination at 42 days are shown in Table 7. There were no significant changes in breast meat pH at processing (Hour-1; p > 0.05) but a slight decrease at 24 hours (Hour-24; p < 0.05) with higher BSFLO levels. Alternatively, the meat color (L*, a*, and b*), drip loss, cooking loss, shear force, and hardness were not significantly affected (p > 0.05).
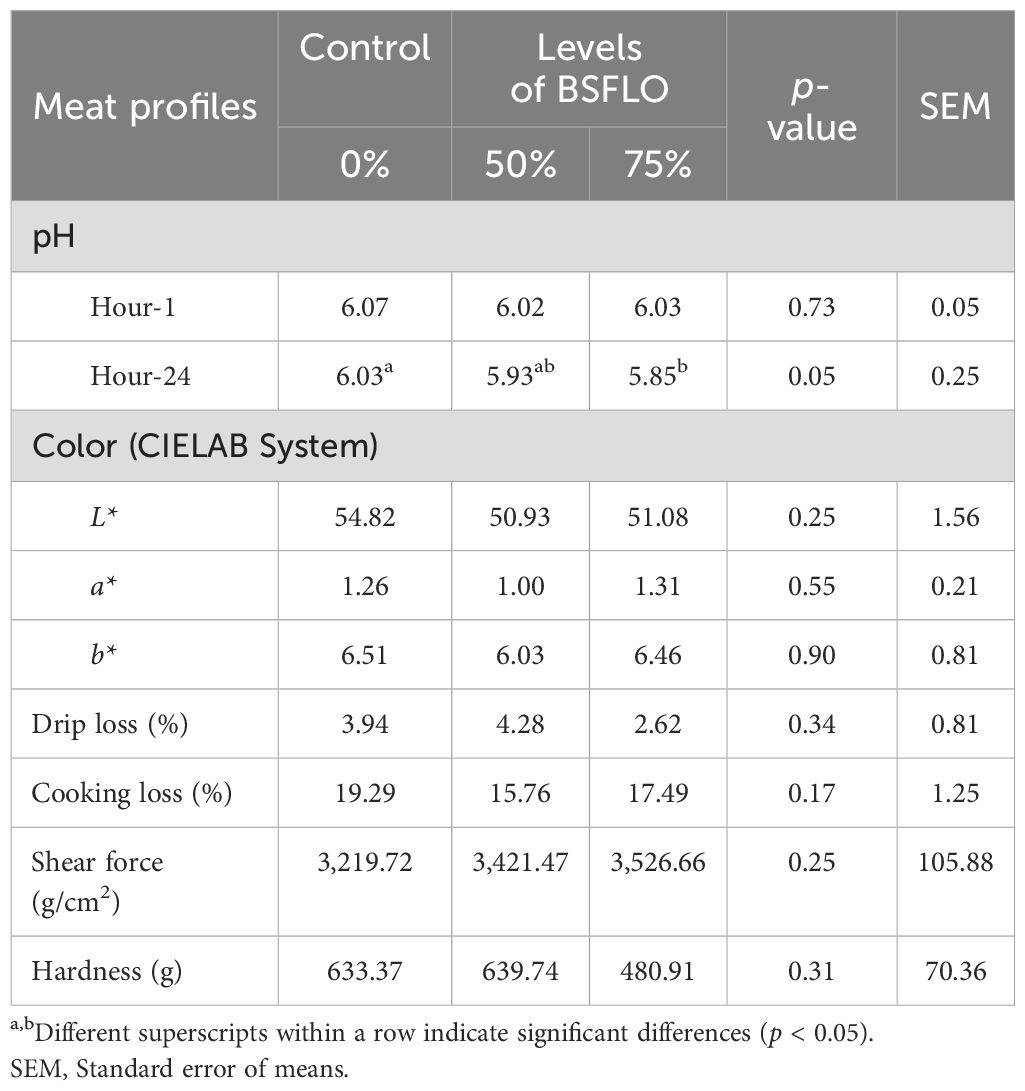
Table 7. The effect of inclusion of black soldier fly larvae oil (BSFLO) in broiler diets on meat quality.
The fatty acid profiles of the liver are presented in Table 8. Broilers fed diets with high levels of BSFLO (75%) had significantly higher (p < 0.05) levels of saturated fatty acids compared to the control or 50% BSFLO groups, particularly butyric acid (C4:0), palmitic acid (C16:0), and stearic acid (C18:0). Conversely, 50% BSFLO inclusion significantly decreased (p < 0.05) unsaturated fatty acids compared to the control and 75% BSFLO groups, leading to a notable decrease in palmitoleic acid (C16:1n7) and cis-9-oleic acid (C18:1n9c) levels. Moreover, polyunsaturated fatty acid (PUFA) levels were also lowered (p < 0.05) in both BSFLO groups, reducing linoleic acid (C18:2n6) and alpha-linolenic acid (C18:3n3) content. The total levels of PUFA, n-3, n-6, and n-9 were significantly higher (p < 0.05) in the control group compared to the BSFLO treatments. In contrast, broilers receiving the 75% BSFLO diet had a higher concentration of monounsaturated fatty acid (MUFA) and a lower PUFA/MUFA ratio (p < 0.05). Additionally, the n-6/n-3 ratio was significantly lower (p < 0.05) in both the 50% BSFLO and control groups.
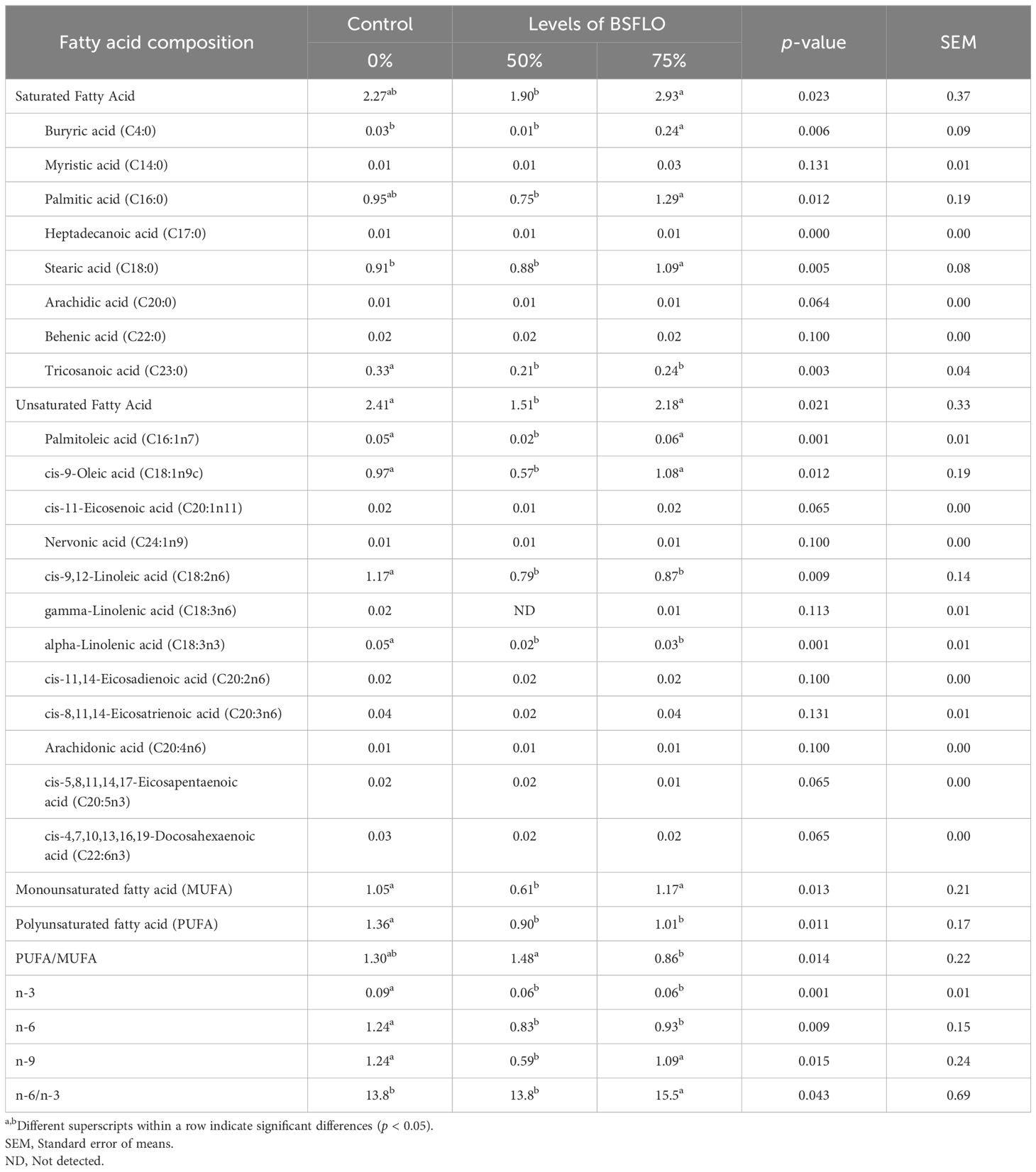
Table 8. The effect of inclusion of black soldier fly larvae oil (BSFLO) in broiler diets on fatty acid profile of liver.
4 Discussion
The main constituents of BSFL are proteins and lipids, with their composition varying depending on the substrate used for rearing. Typically, the BSFL contains around 40% protein and 30% lipid on a dry matter basis. While BSFL are primarily valued as a feed source due to their high protein content, their lipid content is often overlooked and considered a by-product in animal feed production. As a result, lipids or fatty acids derived from BSFL are frequently underutilized despite research suggesting their potential for alternative fat sources. The present experiment tests the BSFLO as a partial replacement in half and three-quarters of the RBO in the diet for growing chickens.
In the present study, the replacement of RBO with BSFLO for the broilers' diet did not significantly affect their growth performance, except FCR, which was identified as increasing in the finisher and overall phase when levels of BSFLO inclusion increased (75% BSFLO). The current study was not consistent with previous research, which also reported that the partial (50%) or total (100%) inclusion of BSFLO in a broiler diet had no significant impact on performance in all periods, as well as nutrient digestibility, intestinal morphological features, and even carcass and meat quality were not adversely affected by supplementation or addition of BSFLO to the diet (Schiavone et al., 2017; Kim et al., 2020; Dabbou et al., 2021). The disparity among all the studies could be attributed to variations in factors such as age, breed, trial conditions, management practices, dietary composition, and the wide variation in fatty acid compositions of BSFLO (Danieli et al., 2019; Gao et al., 2019; Kawasaki et al., 2019). Comparing the effect of three oil sources, i.e., BSFLO, coconut oil (CCO), and corn oil (CO) in broiler chickens, Kim et al. (2020) did not observe any impact on BW, BWG, or FI in chicks at 15 and 30 days old, they did report a noteworthy decrease in FCR for chicks fed CCO and BSFLO compared to the CO group. The authors suggest that this improvement in FCR is likely due to the MCFA abundant in insect oils, which can promote better nutrient digestion and absorption. According to our results, the inclusion of 75% BSFLO likely did not negatively affect the growth performance, especially the BW and FCR of starter and grower periods, compared to a middle level of BSFLO or control groups (Table 3).
The blood serum parameters of broiler chickens play a crucial role in assessing their health and physiological status. The findings presented in Table 4 indicate that replacing RBO with BSFLO in broiler diets exerts effects on some blood cell counts. Specifically, chickens fed with a diet containing 50% BSFLO exhibited higher levels of hemoglobin and WBCs counts on day 21, although increasing WBCs were identified for 50% BSFLO. This finding appears difficult to explain since none of the broilers showed any signs of physical distress. Meanwhile, at day 42, most blood cell parameters showed no significant differences among the groups, indicating potential adaptation or normalization of blood cell counts over time. Notably, eosinophil levels were slightly lower in chickens fed with 50% BSFLO compared to the control and 75% BSFLO groups. Nevertheless, all the hemato-chemical parameters analyzed for the broilers in the present study fell within the physiological reference intervals reported for chicken (Mat et al., 2022), thus suggesting that the BSFLO utilization did not negatively influence the health status or immune system of the broiler chickens. Moreover, The H/L ratio usually has been used for the measurement of distress conditions in chickens in which an increased H/L ratio may indicate that the animals suffered from infections, inflammation, or stress (Salamano et al., 2010; DeMarco et al., 2013; Pozzo et al., 2013). In the present study, the H/L ratio of chickens fed the experimental diets did not present any significant differences compared with the control group.
The activities of plasma ALT, AST, and ALP are generally related to liver damage, acting as biomarkers of liver health when they are damaged (Hyder et al., 2013). The blood biochemistry traits (Table 5) were significantly affected on AST and ALP by the dietary treatments, which showed lower value than the control group, but the value present trial fell and is still within the physiological normal ranges (Schiavone et al., 2018), suggesting that BSFLO may not cause negative effects on the hepatopancreas or liver health. The results of the present research agree with those of Schiavone et al. (2017) and Li et al. (2016), who showed that the use of BSFLO in substitution for soybean oil in broilers and juvenile Jian carp diets, respectively, had no negative effects on the blood traits of the animals and confirmed the nutritional adequacy of these diets.
The replacement of RBO with BSFLO in broiler chickens did not negatively affect liver function indicators, as measured by AST and ALP activity, between birds fed diets containing different fat sources, consistent with a previous report (Taulescu et al., 2010). This suggests that BSFLO supplementation did not induce liver toxicity or impair liver function. However, the effects on plasma cholesterol were inconclusive. Meanwhile, medium-chain fatty acids are generally known to decrease triglycerides, total cholesterol, and LDL cholesterol, as well as increase HDL cholesterol in chickens (Wang et al., 2015; Khatibjoo et al., 2018). Our results did not show a consistent pattern; we found that triglyceride was higher by, on average, 18.8% in 75% BSFLO-fed chickens than in 50% BSFLO-fed ones (Table 5). However, the BSFLO did not significantly impact total and HDL cholesterol levels compared to chickens fed RBO. It is not clear how BSFLO affected cholesterol metabolism in chickens, necessitating further investigation. In line with our study, dietary BSFLO did not affect blood parameters in chickens, which aligns with previous studies where dietary BSFLO supplementation did not affect blood parameters in chickens (Schiavone et al., 2017), juvenile carp (Li et al., 2016), and rabbits (Gasco et al., 2019). Thus, BSFLO's influence on cholesterol metabolism appears to differ from RBO, although BSFLO is rich in MCFA. It should be kept in mind that RBO has a higher amount of linolenic acid than BSFLO, which is typically known to have a hypocholesterolemic effect (Lorenzo et al., 2014). It is likely that BSFLO would disturb or curb the MCFA–induced increase in plasma cholesterol. Thus, it may be likely that MCFA of BSFLO would disturb or curb–the induced increase in plasma cholesterol, similar to observations with CCO (Wang et al., 2015). Lekshmi Sheela et al. (2016) report that the coconut phyto-compounds, especially lauric acid, were found effective against HMG-CoA reductase enzyme which plays a central role in the synthesis of cholesterol.
None of the viscera was significantly impacted by dietary fat sources. The results of the present study agree with the findings of Schiavone et al. (2017), which reported that the partial or total replacement SBO with BSFLO in growing broiler diets did not affect the growth performance, the feed choice or the carcass traits. In the same context, Cullere et al. (2019) showed that defatted BSFL meal could be introduced into the diet for growing broiler quails at 10% and 15% inclusion levels, partially replacing conventional soybean meal and SBO, with no negative effects on productive performance, mortality or carcass traits. However, RBO partial replacement with both BSFLO groups increased abdominal fat percentages by 32% and 16%, respectively (Table 6). BSF oil is rich in saturated fatty acids (SFA) 1.8 times compared to SBO (Table 1), particularly lauric and myristic acids. In order that, the high SFA intake may promote lipogenesis (fat synthesis) and adipocyte hypertrophy (fat cell enlargement) in abdominal adipose tissue. As research has shown, the fatty acid profile of a broiler's diet directly influences the fatty acid composition of its meat and internal organs. This influence is closely linked to fat storage and metabolism within the animal (Taulescu et al., 2010; Khatun et al., 2018).
Different fats did not affect thigh and breast meat but affected wing yield traits. Meanwhile, the pH of the breast meat was decreased in chickens fed a diet containing BSFLO compared with those fed on SBO. Nonetheless, the negative correlation between the lightness and pH of fresh meat (Qiao et al., 2001) was not observed in this study (Table 7). Of interest is the BSFLO trend in medium-chain fatty acids vs. SBO, which has increased b* values. Increased breast meat yellowness has been postulated with an increase in carotenoid contents (da Silva et al., 2017) or lipid contents (Zhao et al., 2018) in broiler chickens. Nonetheless, all observed values regarding pH, meat colors, and cooking loss of breast meat still fall within the acceptable range according to meat quality characteristics (Cullere et al., 2019). Further analysis of the nutrient profile, fatty acid composition, and antioxidant content of breast and leg meat could provide insights into the differences in meat quality observed between the various fat sources used in the diets.
The liver plays a central role in maintaining healthy fat balance (lipid homeostasis) through a complex network of precisely regulated biochemical pathways, signaling mechanisms, and cellular processes. Hepatocytes, the main functional cells of the liver, are responsible for these biochemical and metabolic functions, including lipid metabolism. In a healthy state, the liver processes large amounts of fatty acids daily while storing only a small portion as triglycerides. Omega-3 fatty acids, particularly docosahexaenoic acid (DHA) and its precursor docosapentaenoic acid (DPA), are critical factors reflecting changes in liver health (Schiavone et al., 2017). Notably, omega-3 fatty acids and their derivatives possess potent anti-inflammatory properties that help mitigate the metabolic effects of oxidative stress in the liver and other tissues (Dabbou et al., 2021; Schiavone et al., 2017). Additionally, maintaining a proper balance between different fatty acid types is crucial. This includes the n-6/n-3 and PUFA/MUFA ratios, which should not be excessively high. Our findings revealed that partially replacing refined RBO with BSFLO at 50% and 75% inclusion levels resulted in favorable n-6/n-3 (0.86) and PUFA/MUFA (1.38) ratios, respectively (Table 8).
In conclusion, the important differences in the dietary contents of lauric and myristic FAs with increasing BSFLO inclusion had no beneficial effect on amount of fat accumulated in the abdominal fat and also FCR in the final period. Despite this, the findings of this study suggest that BSFLO could partially replace RBO in chickens' diet at 50% without any adverse effects on growth performance, hematological parameters, serum biochemical indices, or carcass and meat quality. These findings suggest that BSFLO can be a suitable ingredient for poultry diets. Further research efforts are necessary to investigate the impact of BSFLO on the meat quality traits and fatty acid profile of broiler chickens in depth.
Data availability statement
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by the Institutional Animal Care and Use Committee of Khon Kaen University, Thailand. (Record no. IACUC-KKU-60/66). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
NS: Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing. PP: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. WP: Conceptualization, Investigation, Methodology, Validation, Writing – original draft. NP: Investigation, Methodology, Validation, Writing – original draft. TS: Investigation, Validation, Writing – original draft. BT: Funding acquisition, Writing – review & editing. AC: Writing – review & editing. SW: Conceptualization, Data curation, Formal analysis, Funding acquisition, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was funded by the Fundamental Fund of Khon Kaen University (FF66). The FF66 is funded by the National Science, Research, and Innovation Fund (NSRF). Additionally, support was provided by the Toxic Substances, Microorganisms, and Feed Additives in Livestock and Aquatic Animals for Food Safety Research Program, Khon Kaen University, Thailand, under Grant No. KKU-RP64-10-005.
Acknowledgments
The authors gratefully acknowledge the Department of Animal Science, Faculty of Agriculture, Khon Kaen University, for providing access to the research facilities. The authors acknowledge the use of ChatGPT 3.5 (Open AI, https://chat.openai.com) to assist with grammar checks during the manuscript preparation process.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Association of Official Analytical Chemists (1990). Official Methods of Analysis. 15th ed (Arlington, VA, USA: Association of Official Analytical Chemists (AOAC).
Biasato I., De Marco M., Rotolo L., Renna M., Dabbou S., Capucchio M. T., et al. (2016). Effects of dietary Tenebrio molitor meal inclusion in free-range chickens. J. Anim. Physiol. Anim. Nutr. 100, 1104–1112. doi: 10.1111/jpn.12487
Borrelli L., Coretti L., Dipineto L., Bovera F., Menna F., Chiariotti L., et al. (2017). Insect-based diet, a promising nutritional source, modulates gut microbiota composition and SCFAs production in laying hens. Sci. Rep. 7, 16269. doi: 10.1038/s41598-017-16560-6
Bovera F., Loponte R., Pero M. E., Cutrignelli M. I., Calabrò S., Musco N., et al. (2018). Laying performance, blood profiles, nutrient digestibility and inner organs traits of hens fed an insect meal from Hermetia illucens larvae. Res. Vet. Sci. 120, 86–93. doi: 10.1016/j.rvsc.2018.09.006
Cullere M., Schiavone A., Dabbou S., Gasco L., Dalle Zotte A. (2019). Meat quality and sensory traits of finisher broiler chickens fed with black soldier fly (Hermetia illucens L.) larvae fat as alternative fat source. Animals 9, 140. doi: 10.3390/ani9040140
Cutrignelli M. I., Messina M., Tulli F., Randazzi B., Olivotto I., Gasco L., et al. (2018). Evaluation of an insect meal of the black soldier fly (Hermetia illucens) as soybean substitute: intestinal morphometry, enzymatic and microbial activity in laying hens. Res. Vet. Sci. 117, 209–215. doi: 10.1016/j.rvsc.2017.12.020
Dabbou S., Lauwaerts A., Ferrocino I., Biasato I., Sirri F., Zampiga M., et al. (2021). Modified black soldier fly larva fat in broiler diet: effects on performance, carcass traits, blood parameters, histomorphological features and gut microbiota. Animals 11, 1837. doi: 10.3390/ani11061837
Danieli P. P., Lussiana C., Gasco L., Amici A., Ronchi B. (2019). The effects of diet formulation on the yield, proximate composition, and fatty acid profile of the black soldier fly (Hermetia illucens L.) prepupae intended for animal feed. Animals 9, 178. doi: 10.3390/ani9040178
da Silva D. C. F., de Arruda A. M. V., Gonçalves A. A. (2017). Quality characteristics of broiler chicken meat from free-range and industrial poultry system for the consumers. J. Food Sci. Technol. 54, 1818–1826. doi: 10.1007/s13197-017-2612-x
Dayrit F. M. (2014). Lauric acid is a medium-chain fatty acid, coconut oil is a medium-chain triglyceride. Philipp. J. Sci. 143, 157–166.
DeMarco M., Martinez S., Tarantola M., Bergagna S., Mellia E., Gennero M. S., et al. (2013). Effect of genotype and transport on tonic immobility and heterophil/lymphocyte ratio in two local Italian breeds and Isa Brown hens kept under free-range conditions. Ital. J. Anim. Sci. 12, 481–485. doi: 10.4081/ijas.2013.e78
Diener S., Studt Solano N. M., Roa Gutierrez F., Zurbrugg C., Tockner K. (2011). Biological treatment of municipal organic waste using black soldier fly larvae. Waste Biomass Valour 2, 357–363. doi: 10.1007/s12649-011-9079-1
Gao Z., Wang W., Lu X., Zhu F., Liu W., Wang X. P., et al. (2019). Bioconversion performance and life table of black soldier fly (Hermetia illucens) on fermented maize straw. J. Clean. Prod 230, 974–980. doi: 10.1016/j.jclepro.2019.05.074
Gasco L., Dabbou S., Trocino A., Xiccato G., Capucchio M. T., Biasato I., et al. (2019). Effect of dietary supplementation with insect fats on growth performance, digestive efficiency and health of rabbits. J. Anim. Sci. Biotechnol. 10, 4. doi: 10.1186/s40104-018-0309-2
Henry M., Gasco L., Piccolo G., Fountoulaki E. (2015). Review on the use of insects in the diet of farmed fish: past and future. Anim. Feed Sci. Technol. 203, 1–22. doi: 10.1016/j.anifeedsci.2015.03.001
Hyder M. A., Hasan M., Mohieldein A. H. (2013). Comparative levels of ALT, AST, ALP and GGT in liver associated diseases. Eur. J. Exp. Biol. 3, 280–284.
Kawasaki K., Hashimoto Y., Hori A., Kawasaki T., Hirayasu H., Iwase S., et al. (2019). Evaluation of black soldier fly (Hermetia illucens) larvae and pre-pupae raised on household organic waste, as potential ingredients for poultry feed. Animals 9, 98. doi: 10.3390/ani9030098
Khatibjoo A., Mahmoodi M., Fattahnia F., Akbari-Gharaei M., Shokri A. N., Soltani S. (2018). Effects of dietary short- and medium-chain fatty acids on performance, carcass traits, jejunum morphology, and serum parameters of broiler chickens. J. Appl. Anim. Res. 46, 492–498. doi: 10.1080/09712119.2017.1345741
Khatun J., Loh T. C., Akit H., Foo H. L., Mohamad R. (2018). Influence of different sources of oil on performance, meat quality, gut morphology, ileal digestibility and serum lipid profile in broilers. J. Appl. Anim. Res. 46, 479–485. doi: 10.1080/09712119.2017.1337580
Kierończyk B., Rawski M., Józefiak A., Mazurkiewicz J., Świątkiewicz S., Siwek M., et al. (2018). Effects of replacing soybean oil with selected insect fats on broilers. Anim. Feed Sci. Technol. 240, 170–183. doi: 10.1016/j.anifeedsci.2018.04.002
Kim Y. B., Kim D. H., Jeong S. B., Lee J. W., Kim T. H., Lee H. G., et al. (2020). Black soldier fly larvae oil as an alternative fat source in broiler nutrition. Poultry Sci. 99, 3133–3143. doi: 10.1016/j.psj.2020.01.018
Lekshmi Sheela D., Nazeem P. A., Narayanankutty A. (2016). In silico and wet lab studies reveal the cholesterol lowering efficacy of lauric acid, a medium chain fat of coconut oil. Plant Foods Hum. Nutr. 71, 410–415. doi: 10.1007/s11130-016-0577-y
Li S., Ji H., Zhang B., Tian J., Zhou J., Yu H. (2016). Influence of black soldier fly (Hermetia illucens) larvae oil on growth performance, body composition, tissue fatty acid composition and lipid deposition in juvenile Jian carp (Cyprinus carpio var. Jian). Aquaculture 465, 43–52. doi: 10.1016/j.aquaculture.2016.08.020
Loponte R., Nizza S., Bovera F., DeRiu N., Fliegerova K., Lombardi P., et al. (2017). Growth performance, blood profiles and carcass traits of Barbary partridge (Alectoris barbara) fed two different insect larvae meals (Tenebrio molitor and Hermetia illucens). Res. Vet. Sci. 115, 183–188. doi: 10.1016/j.rvsc.2017.04.017
Lorenzo J. M., Crecente S., Franco D., Sarriés M. V., Gómez M. (2014). The effect of livestock production system and concentrate level on carcass traits and meat quality of foals slaughtered at 18 months of age. Animal 8, 494–503. doi: 10.1017/S175173111300236X
Makkar H. P. S., Tran G., Heuzé V., Ankers P. (2014). State-of-the-art on use of insects as animal feed. Anim. Feed Sci. Technol. 197, 1–33. doi: 10.1016/j.anifeedsci.2014.07.008
Mancuso T., Baldi L., Gasco L. (2016). An empirical study on consumer acceptance of farmed fish fed on insect meals: the Italian case. Aquacult. Int. 24, 1498–1507. doi: 10.1007/s10499-01600007-z
Mat K., Kari Z. A., Rusli N. D., Rahman M. M., Harun H. C., Al-Amsyar S. M., et al. (2022). Effects of the inclusion of black soldier fly larvae (Hermetia illucens) meal on growth performance and blood plasma constituents in broiler chicken (Gallus domesticus) production. Saudi J. Biol. Sci. 29, 809–815. doi: 10.1016/j.sjbs.2021.10.027
Overa F., Loponte R., Marono S., Piccolo G., Parisi G., Iaconisi V., et al. (2016). Use of Tenebrio molitor larvae meal as protein source in broiler diet: effect on growth performance, nutrient digestibility, and carcass and meat traits. J. Anim. Sci. 94, 639–647. doi: 10.2527/jas.2015-9201
Pozzo L., Salamano G., Mellia E., Gennero M. S., Doglione L., Cavallarin L., et al. (2013). Feeding a diet contaminated with ochratoxin A for broiler chickens at the maximum level recommended by the EU for poultry feeds (0.1 mg/kg). 1. Effects on growth and slaughter performance, haematological and serum traits. J. Anim. Physiol. Anim. Nutr. 97, 13–22. doi: 10.1111/jpn.12050
Qiao M., Fletcher D. L., Smith D. P., Northcutt J. K. (2001). The effect of broiler breast meat color on pH, moisture, water-holding capacity, and emulsification capacity. Poult. Sci. 80, 676–680. doi: 10.1093/ps/80.5.676
Salamano G., Mellia E., Tarantola M., Gennero M. S., Doglione L., Schiavone A. (2010). Acute phase proteins and heterophil ratio in laying hens in different housing systems. Veterinary Rec. 167, 749–751. doi: 10.1136/vr.c5349
Schiavone A., Cullere M., DeMarco M., Meneguz M., Biasato I., Bergagna S., et al. (2017). Partial or total replacement of soybean oil by black soldier fly larvae (Hermetia illucens L.) fat in broiler diets: effect on growth performances, feed-choice, blood traits, carcass characteristics and meat quality. Ital. J. Anim. Sci. 16, 93–100. doi: 10.1080/1828051X.2016.1249968
Schiavone A., Dabbou S., DeMarco M., Cullere M., Biasato I., Biasibetti E., et al. (2018). Black soldier fly larva fat inclusion in finisher broiler chicken diet as an alternative fat source. Animal 12, 2032–2039. doi: 10.1017/S1751731117003743
Taulescu C., Mihaiu M., Bele C., Matea C., Dan S. D., Mihaiu R., et al. (2010). Manipulating the fatty acid composition of poultry meat for improving consumer’s health. Bull. UASVM Vet. Med. 67, 220–225. doi: 10.15835/buasvmcn-vm:67:2:6034
Ushakova N. A., Brodskii E. S., Kovalenko A. A., Bastrakov A. I., Kozlova A. A., Pavlov D. S. (2016). Characteristics of lipid fractions of larvae of the black soldier fly Hermetia illucens. Dokl. Biochem. Biophys. 468, 209–212. doi: 10.1134/S1607672916030145
van Huis A. (2013). Potential of insects as food and feed in assuring food security. Annu. Rev. Entomol 58, 563–583. doi: 10.1146/annurev-ento-120811-153704
Wang Y. S., Shelomi M. (2017). Review of black soldier fly (Hermetia illucens) as animal feed and human food. Foods 6, 91. doi: 10.3390/foods6100091
Wang J., Wang X., Li J., Chen Y., Yang W., Zhang L. (2015). Effects of dietary coconut oil as a medium-chain fatty acid source on performance, carcass composition and serum lipids in male broilers. Asian-Australasian J. Anim. Sci. 28, 223–230. doi: 10.5713/ajas.14.0328
Zeitz J. O., Fennhoff J., Kluge H., Stangl G. I., Eder K. (2015). Effects of dietary fats rich in lauric and myristic acid on performance, intestinal morphology, gut microbes, and meat quality in broilers. Poult. Sci. 94, 2404–2413. doi: 10.3382/ps/pev191
Zhao X., Ren W., Siegel P. B., Li J., Wang Y., Yin H., et al. (2018). Meat quality characteristics of chickens as influenced by housing system, sex, and genetic line interactions. Ital. J. Anim. Sci. 17, 462–468. doi: 10.1080/1828051X.2017.1363639
Keywords: insect oil, Hermetia illucens L., broiler chicken, productivity, blood profiles
Citation: Somparn N, Pootthachaya P, Puangsap W, Pintaphrom N, Srikha T, Tengjaroenkul B, Cherdthong A and Wongtangtintharn S (2024) Evaluation of black soldier fly larvae oil as a feed ingredient for broiler chickens: effects on performance, carcass traits, meat characteristics, and blood parameters. Front. Anim. Sci. 5:1496763. doi: 10.3389/fanim.2024.1496763
Received: 15 September 2024; Accepted: 31 October 2024;
Published: 19 November 2024.
Edited by:
Geoffrey E. Dahl, University of Florida, United StatesReviewed by:
Mürsel Özdoğan, Aydin Adnan Menderes University, TürkiyeMargaret Bryer, University of Wisconsin-Madison, United States
Copyright © 2024 Somparn, Pootthachaya, Puangsap, Pintaphrom, Srikha, Tengjaroenkul, Cherdthong and Wongtangtintharn. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sawitree Wongtangtintharn, c2F3aXdvQGtrdS5hYy50aA==
 Nantanant Somparn
Nantanant Somparn Padsakorn Pootthachaya
Padsakorn Pootthachaya Warin Puangsap1,2
Warin Puangsap1,2 Anusorn Cherdthong
Anusorn Cherdthong Sawitree Wongtangtintharn
Sawitree Wongtangtintharn