- College of Animal Science and Veterinary Medicine, Shenyang Agricultural University, Shenyang, China
Introduction: The objective of this study was to evaluate the influences of dietary glucose oxidase (GOD) supplementation in the growth and slaughter performance, organ index, intestinal morphology and cecal microbiota in broilers.
Methods: A total of 480 one-day-old Arbor Acres male broiler chicks were randomly assigned to four groups, and the level of GOD in the diet was 0 (control), 200, 400, and 800 mg/kg. Each group had six replicates and each replicate had 20 chicks. The experimental period was 42 days.
Results: Compared to those in the control, the final body weight (BW) and average daily gain (ADG) were increased (p < 0.05) and the feed conversion ratio (FCR) was decreased (p < 0.05) in the 400 mg/kg and 800 mg/kg GOD groups. Dietary supplementation with 800 mg/kg GOD increased (p < 0.05) the dressing-out percentage; semieviscerated yield; relative weights of the thymus, spleen and bursa of Fabricius; and the lengths and weights of the duodenum, jejunum, ileum, and total small intestine compared to those in the control group. Additionally, dietary supplementation with GOD increased (p < 0.05) the villus height and villus height/crypt depth ratio and decreased (p < 0.05) the crypt depth of the duodenum and ileum compared to those of the control group. Illumina sequencing data indicated that the Simpson index of the cecal microbiota in the GOD group was decreased, indicative of increased microbial diversity. Compared to the control, GOD supplementation increased (p < 0.05) the abundances of the genera Ralstonia, Akkermansia and Parabacteroides.
Discussion: Therefore, the results from this study indicated that dietary GOD supplementation could improve the growth performance and carcass yields, promote immune organ and gut development, and enhance the intestinal morphology and cecal microbiota composition in broilers.
1 Introduction
The application of antibiotic growth promoters in livestock and poultry feeding has made a great contribution to meeting the demand for animal protein by humans in the last more than fifty years. However, the continuous overuse of antibiotics as growth promoters has led to severe problems in food safety, human health and environmental protection, such as the appearance of antibiotic-resistant bacteria and residues of antibiotics in animal products and the human body (Mehdi et al., 2018). The addition of antibiotics to animal feed has been prohibited in European since 2006 and in China since 2020, respectively. The dilemma between a comprehensive antibiotic prohibition policy and a higher demand for high-quality and safe meat products by consumers emphasizes the urgency and importance of developing cost-effective and green residue-free alternatives to antibiotics.
Glucose oxidase (GOD) is a dehydrogenase and can be produced through aerobic fermentation by Aspergillus niger, Penicillium notatum and other fungi. GOD utilizes molecular oxygen as an electron acceptor and catalyzes the oxidation of β-D-glucose to gluconic acid, along with hydrogen peroxide production (Wu et al., 2020). GOD has been reported to be used in many commercial applications including food preservation, biochemical detection, and textile bleaching (Bankar et al., 2009). In animal husbandry, the use of GOD is not vigorously implemented due to the low fermentation capacity by microbes and high application cost in feed. Even so, some previous studies have demonstrated that dietary supplementation with GOD has many positive effects on growth, immune function and gut health in pigs and broilers (Chen et al., 2023; Dang et al., 2021, 2022). It is unclear that the mechanism by which GOD affects animal growth performance in practice, but this may be associated with immune regulation and the intestinal microbiota. GOD has been previously demonstrated to play important roles in eliminating oxygen free radicals and alleviating the body’s oxidative damage, which contributes to maintaining health and promoting growth (Liu et al., 2020). When GOD catalyzes glucose oxidation, it consumes large amounts of oxygen, thus eliminating oxygen free radicals. In addition, GOD was also demonstrated to upregulate mRNA expression of superoxide dismutase (SOD) and catalase (CAT) in weaned piglets via activating the nuclear factor erythroid 2-related factor 2 (Nrf2) pathway (Sun et al., 2021), which contributes to improving the antioxidant capacity and alleviating the oxidative injury in the intestinal tract. It is generally accepted now that the intestinal structure and the microbiota play a crucial role in gut health, which is closely related to growth performance (Ducatelle et al., 2023). Gluconic acid formed by GOD catalytic reaction in the intestine has an ability of decreasing intestinal pH and improving the activity of digestive enzymes (Zhao et al., 2022). Besides, when gluconic acid reaches the hindgut, it would be fermented by specific bacteria to produce volatile fatty acids, mainly butyric acid, which has been widely proved to be able to provide energy for intestinal epithelial cells and maintain the morphology and structure of intestinal mucosa (Peng et al., 2009). In addition, hydrogen peroxide produced during the process of catalytic reaction of GOD naturally has a certain antimicrobial effect to inhibit pathogenic bacterial proliferation in the intestine (Tang et al., 2016). Therefore, GOD is considered to be a potential antibiotic alternative that could both maintain the intestinal health and improve the growth performance in farm animals (Chen et al., 2023; Wu et al., 2020).
To date, limited research has shown that GOD supplementation could improve intestinal barrier function and the microbial community profile in broilers (Wang et al., 2018; Wu et al., 2019), but the mechanism by which GOD improves animal growth performance should be studied further. Here, this study focused on evaluating the effects of GOD supplementation on the growth and slaughter performance, immune organ and intestine development and cecal microbiota in broilers.
2 Materials and methods
All experimental procedures were approved by the Animal Care and Use Committee of Shenyang Agricultural University (No. 2024030704). The GOD was fermented by Aspergillus niger and then purified and was provided by Heswof Biology Co., Ltd. (Yichun, Jiangxi, China). The purity was ≥ 98% and the activity of GOD was 2000 unit/g. One unit (U) of GOD activity is defined as the amount of enzyme which oxidizes 1 µmol β-D-glucose per minute to D-gluconic acid and hydrogen peroxide at 37°C and pH 5.5.
2.1 Animals and experimental design
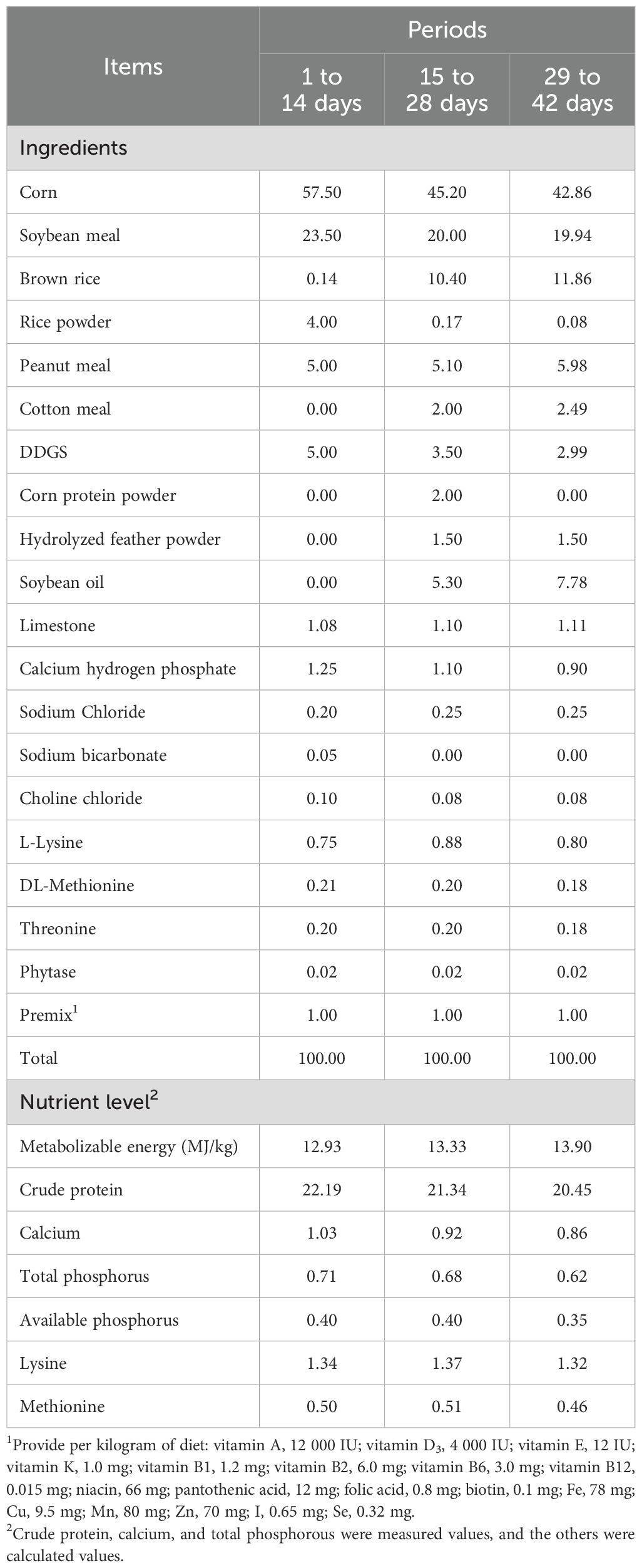
A single-factor experimental design method was adopted in this study. A total of 480 one-day-old Arbor Acres male broiler chicks were randomly assigned to four groups, in which each group had six replicates and each replicate had 20 chicks. The control group was fed a basal corn-soybean meal diet, and the experimental groups were fed a basal diet supplemented with 200, 400, and 800 mg/kg GOD. All diets were formulated to meet the nutritional requirements recommended by the National Research Council (1994), and the feedstuff composition and nutrient level of the control diet are shown in Table 1.
Five chicks were placed in each of the 1.4 m × 0.7 m cages, providing a floor area/bird of 0.19 m2, and all chicks were housed in 3-layer wire cages. The initial temperature was 35°C for the first 3 days of the experiment and then gradually decreased by 2°C weekly to reach a final temperature of 21°C, which was kept until the end of this study. The relative humidity was between 65% and 70%. The lighting was kept 20 h per day during the whole experiment. Feed and water were provided ad libitum for all chicks. The experiment lasted for 42 days.
2.2 Growth performance
On day 42, the chicks were weighed by replicate after a 12-h fast, and feed consumption was also recorded. Mortality was monitored when it occurred. The growth performance was evaluated by calculating the average daily gain (ADG), average daily feed intake (ADFI), and feed conversion ratio (FCR).
2.3 Slaughter performance
On day 42, based on the ADG and FCR results, 2 chicks with body weights similar to the mean body weight of the replicate from the control and 800 mg/kg GOD groups were selected and euthanized by cervical dislocation. The broilers were manually dissected, and the slaughter yields were determined as described by Zhu et al. (2022).
2.4 Immune organ and intestinal indices
The immune organs, including the thymus, spleen, and bursa of Fabricius, were collected and weighed individually. The index was presented as follows:
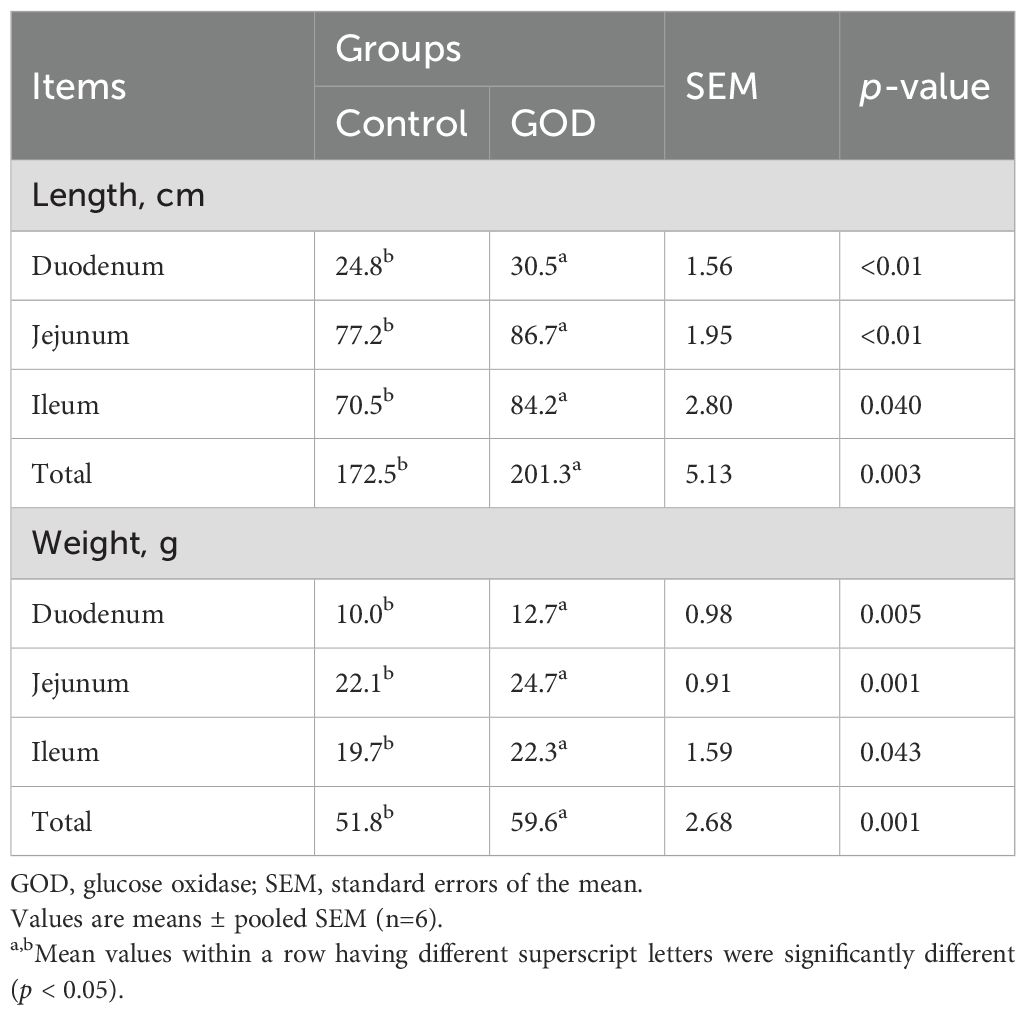
The parts of the small intestine, including the duodenum, jejunum, and ileum, were separated from the abdominal cavity, and their weights and lengths were determined after carefully removing the intestinal contents and the surrounding fat and mesentery.
2.5 Intestinal morphology
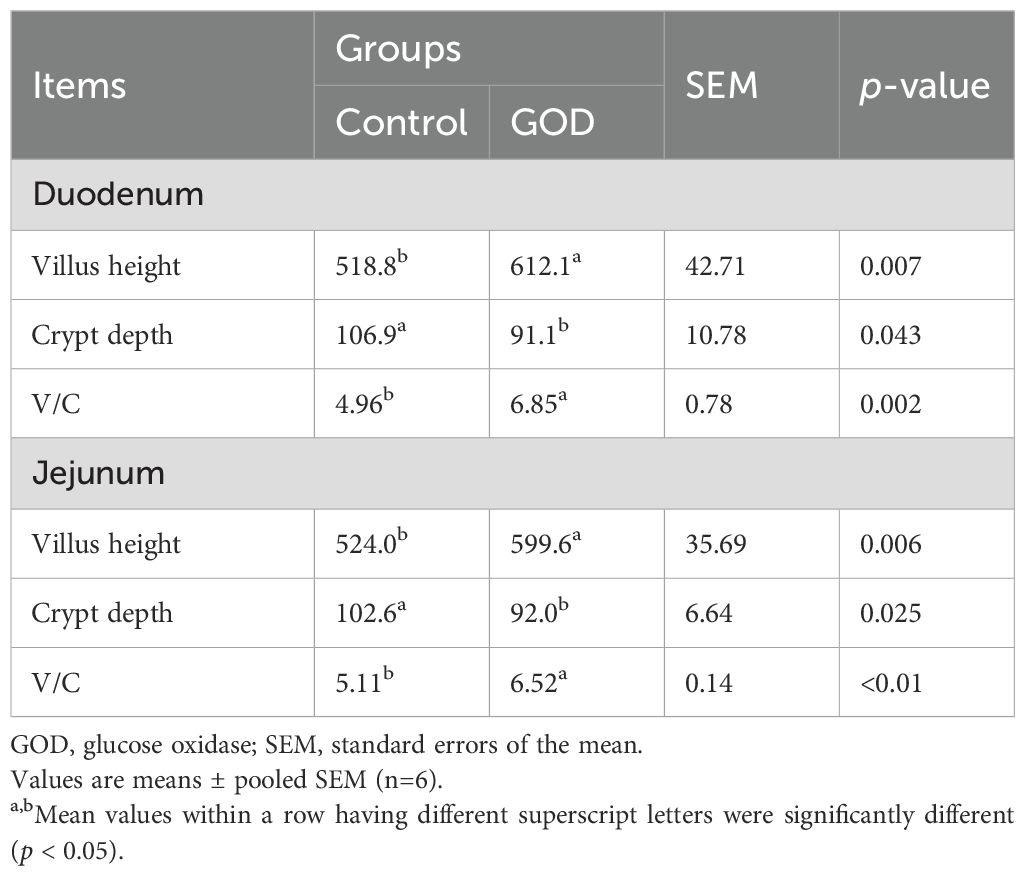
A section (1 cm) in the middle part of the duodenum and jejunum was collected. After being fixed in 4% paraformaldehyde solution for over 24 h, the samples were then embedded in paraffin. A microtome was used to transversely cut each sample into small slices (5-µm thickness), and the slices were placed on a glass slide. After being stained with hematoxylin and eosin, the slices were examined under an Olympus optical microscope with the Image-Pro plus software package. The villus height and crypt depth of the slice samples were measured, and the villus height/crypt depth ratio was calculated.
2.6 DNA extraction
Two chicks from each replicate in the control and 800 mg/kg GOD groups were randomly selected, and a total of twenty-four cecal content samples from broilers at 42 days of age were used for DNA extraction using a commercial DNA extraction kit (Noble Ryder, Beijing, China) by following the manufacturer’s instructions. After assessing the quantity and quality, the extracted DNA samples were diluted with sterile water to a final concentration of 1 ng/µl and then stored at -80°C for further analysis.
2.7 16S rRNA amplification and Illumina NovaSeq sequencing
The hypervariable region (V3 + V4) of the 16S rRNA gene was amplified from cecal content DNA samples using forward primer 341F (5’-CCTAYGGGRBGCASCAG-3’) and reverse primer 806F (5’-GGACTACNNGGGTATCTAAT-3’) with the barcode. A total volume of reaction system was 15 µl and a PCR amplification was performed as follows: 98°C for 1 min, 30 cycles at 98°C for 10 s, 50°C for 30 s and 72°C for 30 s with a final elongation at 72°C for 5 min in a T100 PCR amplifier (Bio-Red, USA). The quality of amplified PCR products was electrophoresed on a 2% agarose gel and then mixed in equidensity ratios. The mixed PCR products were purified with a commercial DNA kit (TianGen, China).
An NEB Next® Ultra DNA Library Prep Kit (Illumina, USA) was used to construct sequence libraries, accompanied by adding index codes. After the quality assessing, the constructed library was sequenced on an Illumina NovaSeq platform (Wekemo Tech Group Co., Ltd., Shenzhen, China), and 250-bp paired-end reads were generated.
2.8 Illumina sequencing data processing
The QIIME2 software package was used to process and analyze raw sequence data (https://docs.qiime2.org/2023.5/). The sequences from each sample were demultiplexed and quality filtered before being trimmed, denoised and merged. The QIIME2 DADA2 program was used to identify and exclude chimeras, and then the amplicon sequence variants (ASVs) were produced. The QIIME2 feature-classifier program was then used to align ASV sequences to a pretrained Greengenes 16S rRNA database (13-8 version) with 99% similarity to generate the taxonomy table. Feature-level alpha diversity indices (Chao1, Shannon and Simpson) of microbiota were also calculated.
2.9 Statistical analysis
One-way ANOVA with SPSS software (25.0 version, IBM, NY, USA) was used to analyze the data, and the treatment was included as a fixed effect. The replicate was the experiment unit for growth performance, while the selected chick from each replicate was used as an experimental unit for analysis of slaughter, immune organ, intestinal parameters and cecal microbiota. Duncan’s method was used to perform multiple range tests. Results were expressed as means ± pooled SEM. Differences were considered to be statistically significant at p < 0.05.
3 Results
3.1 Growth performance
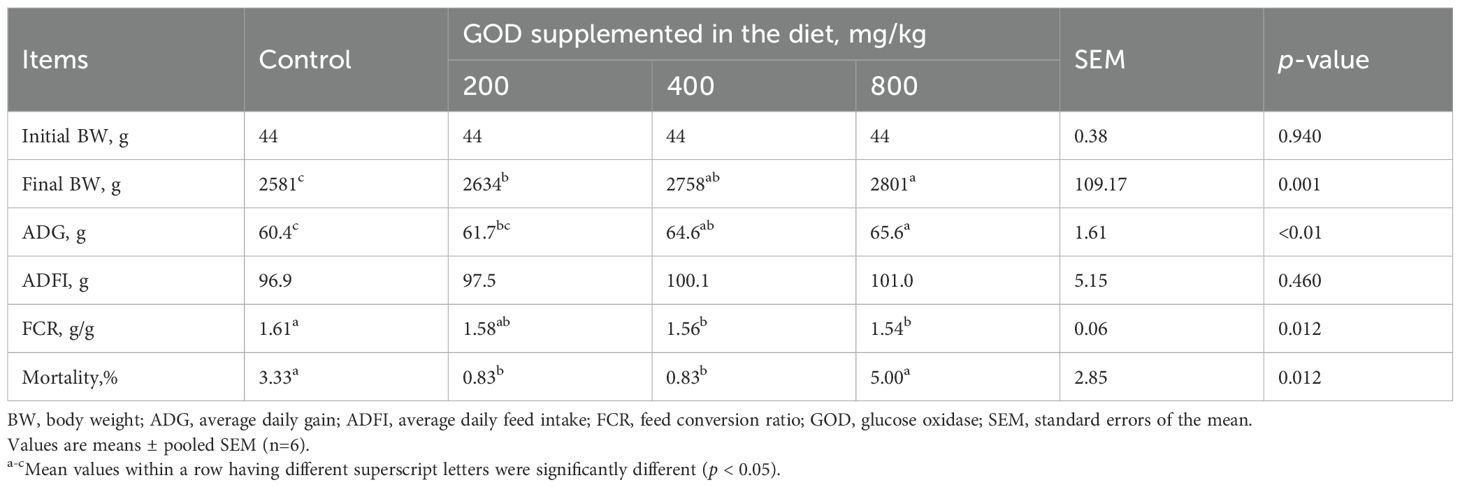
As shown in Table 2, dietary supplementation with GOD had significant (p < 0.05) effects on the final BW, ADG and FCR from 1 to 42 days. Compared to the control group, all GOD groups had a higher (p < 0.05) final BW. The ADGs in the 400 mg/kg and 800 mg/kg GOD groups were higher (p < 0.05) than that in the control group; moreover, the 400 mg/kg and 800 mg/kg GOD groups had lower (p < 0.05) FCRs than that in the control group. There was no significant difference (p > 0.05) in the ADFI among all groups. In addition, the 200 mg/kg and 400 mg/kg GOD groups had lower (p < 0.05) mortality than those in the control and 800 mg/kg GOD groups.
3.2 Slaughter performance
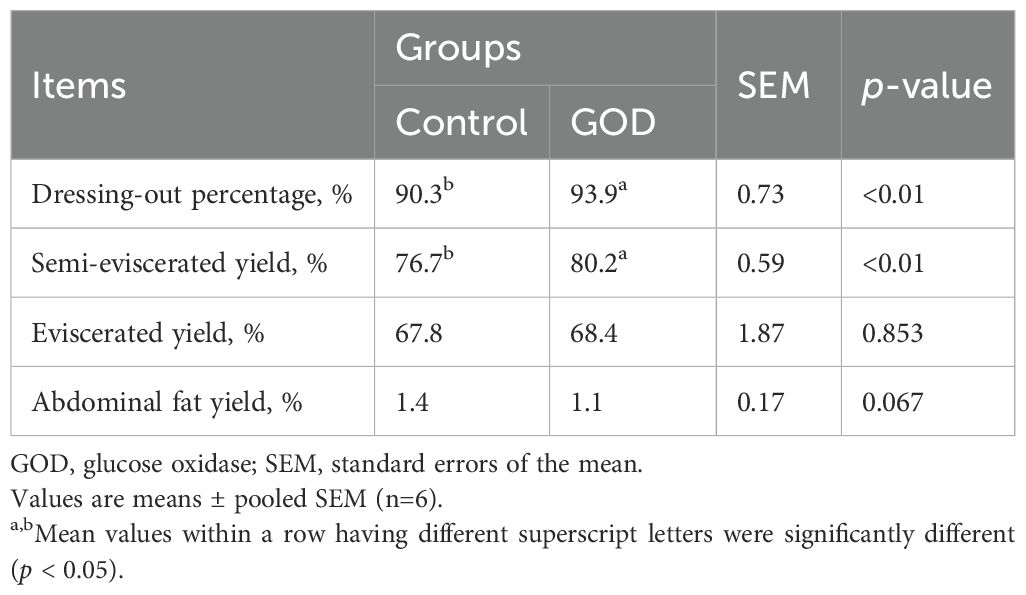
Based on the growth performance changes induced by dietary GOD supplementation, the 800 mg/kg GOD group was selected to evaluate the effects of GOD on broilers compared with the control group for further analysis. As shown in Table 3, compared to the control group, dietary supplementation with 800 mg/kg GOD significantly increased (p < 0.05) the dressing-out percentage and semieviscerated yield but did not affect (p > 0.05) the eviscerated yield. In addition, the 800 mg/kg GOD group had a decreased trend (p < 0.10) in abdominal fat yield compared to that in the control group.
3.3 Immune organ and intestinal indices
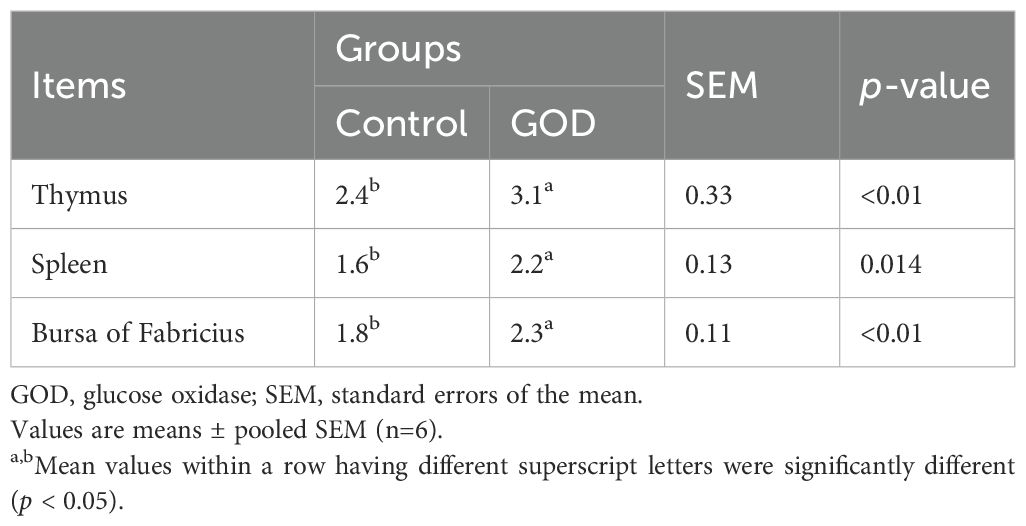
Compared to the control group, the 800 mg/kg GOD group showed increased (p < 0.05) indices of the thymus, spleen and bursa of Fabricius in broilers (Table 4). In terms of intestinal indices, dietary supplementation with GOD significantly increased (p < 0.05) the lengths and weights of the duodenum, jejunum, ileum, and total small intestine compared to values in the control group (Table 5).
3.4 Intestinal morphology
As shown in Table 6, in the duodenum, dietary supplementation with 800 mg/kg GOD significantly increased (p < 0.05) the villus height and ratio of villus height to crypt depth and decreased (p < 0.05) the crypt depth compared to those of the control group. Similarly, in the ileum, the villus height and ratio of villus height to crypt depth were significantly increased (p < 0.05) and the crypt depth was decreased (p < 0.05) in the 800 mg/kg GOD group compared with the control group.
3.5 Cecal microbiota diversity and composition
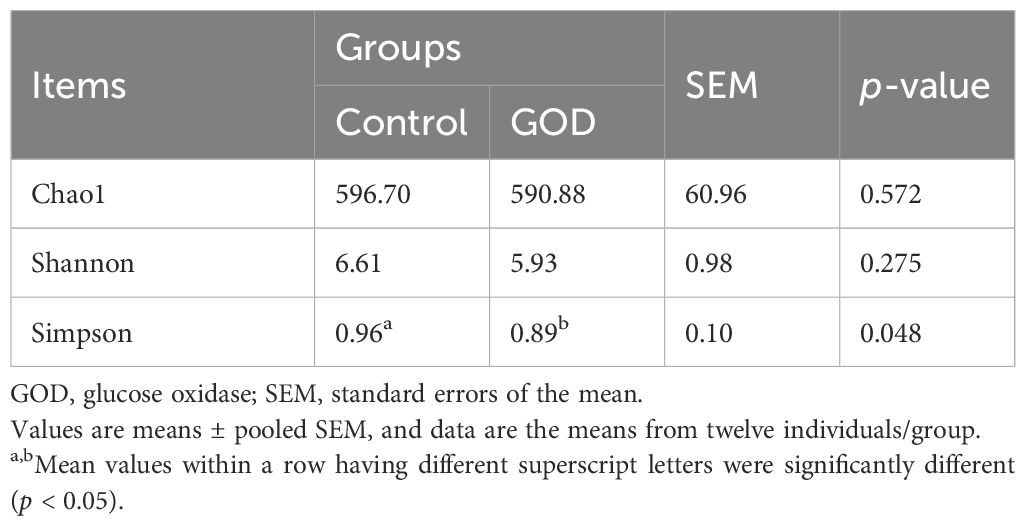
The results from Table 7 show that dietary supplementation with 800 mg/kg GOD did not affect (p > 0.05) the Chao1 and Shannon indices but decreased (p < 0.05) the Simpson index of the cecal microbiota compared to indices in the control group. At the phylum level, Firmicutes, Bacteroidetes, Proteobacteria and Verrucomicrobia were the most abundant, with abundances > 1.0% in all groups in this study (Figure 1). The phylum Verrucomicrobia was significantly more abundant (p < 0.05) in the GOD group, but there was no significant difference (p > 0.05) in the abundances of phyla Firmicutes, Bacteroidetes and Proteobacteria between the control and GOD groups. At the genus level, 20 genera with an abundance > 1.0% were present in all groups (Figure 2). Compared to abundances in the control group, the abundances of the genera Ralstonia, Akkermansia and Parabacteroides were increased (p < 0.05), and that of Ruminococcus was decreased (p < 0.05).
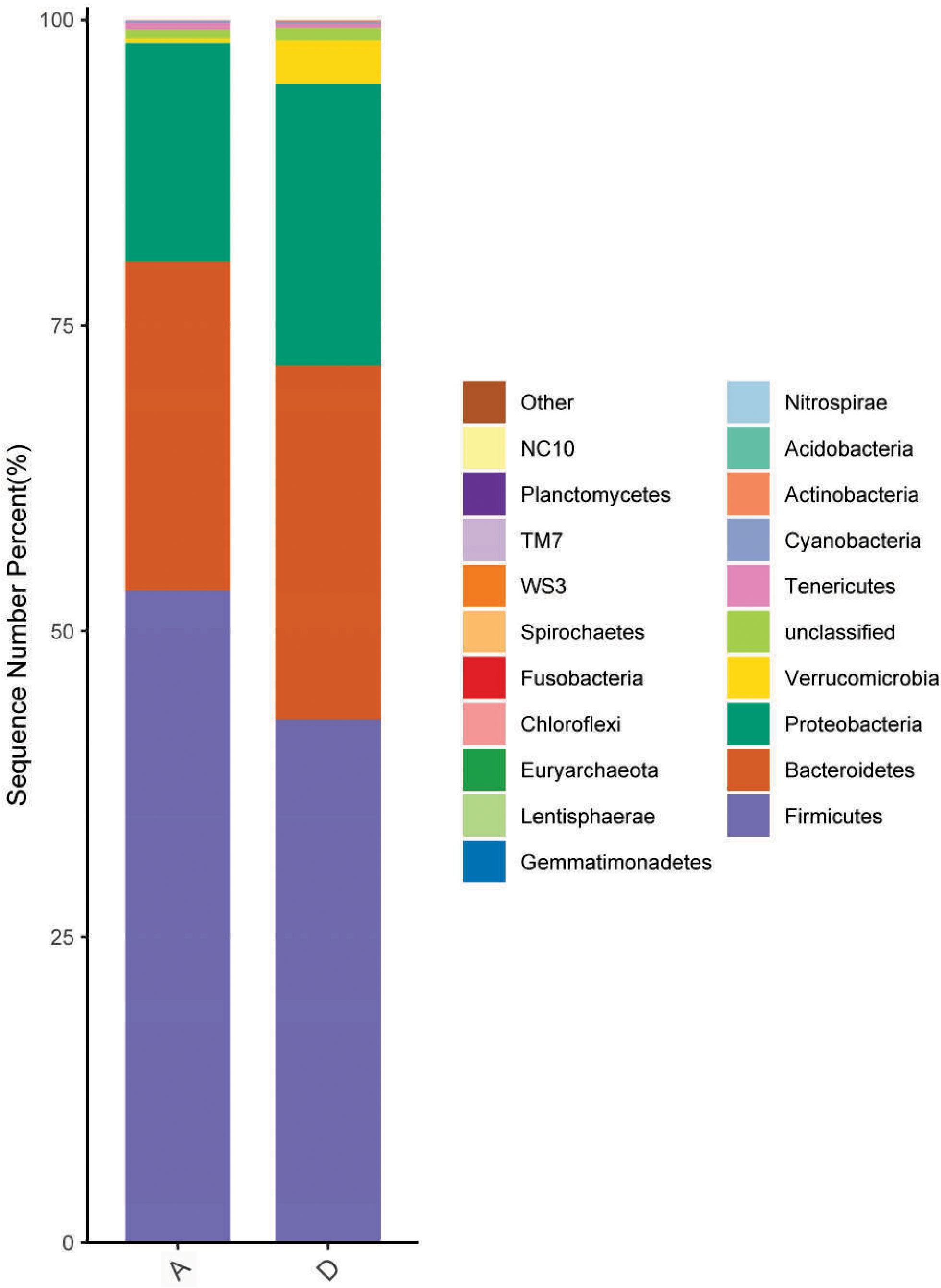
Figure 1. Effect of dietary supplementation with GOD on the cecal microbiota composition in broilers at the phylum level. A, the control group; D, the GOD group (data are the means from twelve individuals/group).
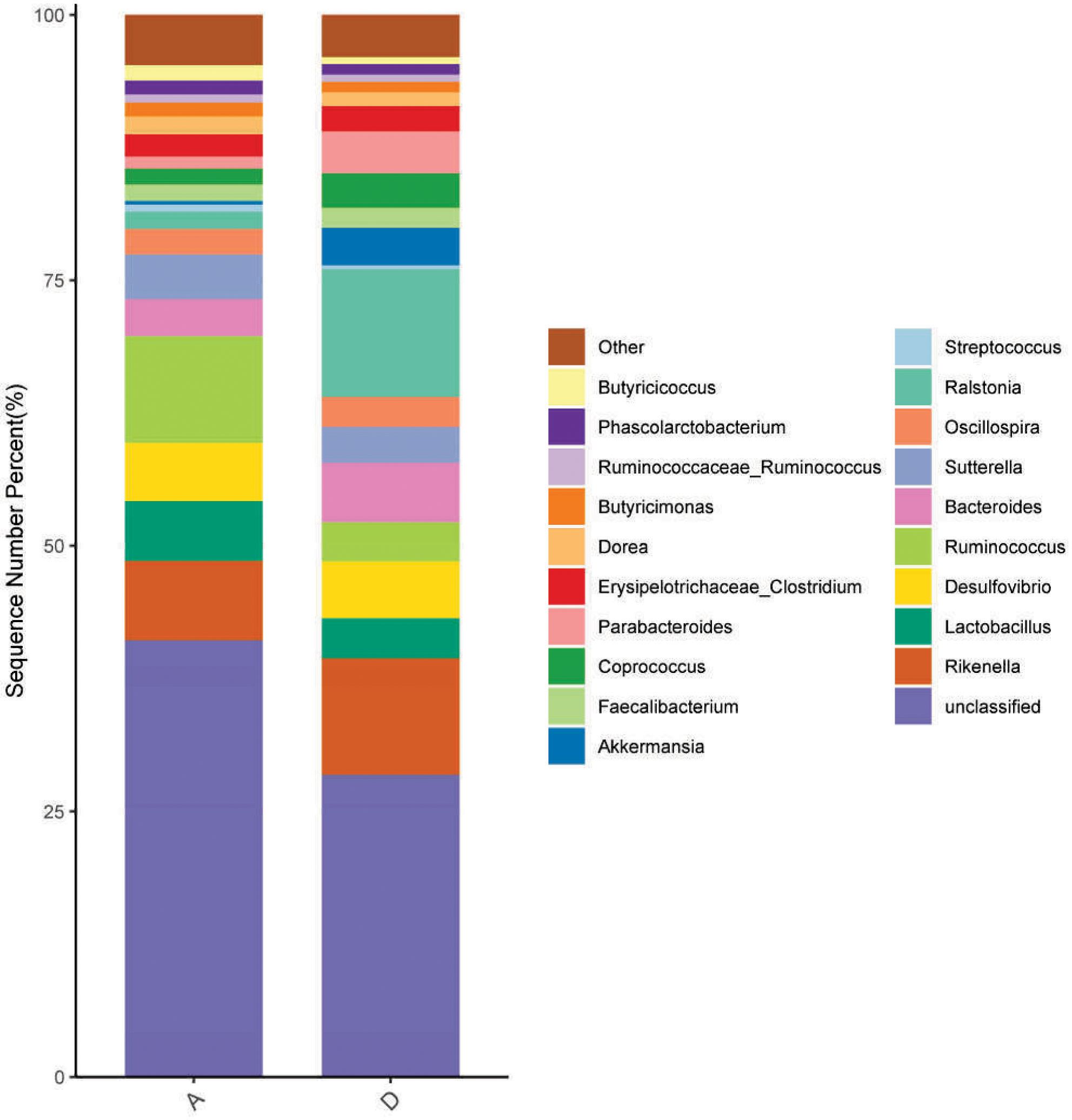
Figure 2. Effect of dietary supplementation with GOD on the cecal microbiota composition in broilers at the genus level. A, the control group; D, the GOD group (data are the means from twelve individuals/group).
4 Discussion
GOD was previously demonstrated to have the ability to catalyze β-D-glucose to produce hydrogen peroxide (H2O2) and β-D-gluconolactone by molecular oxygen as an electron acceptor, and the latter was spontaneously hydrolyzed to gluconic acid (Hztzinikolaou and Macris, 1995). The presence of gluconic acid and H2O2 is supposed to reduce the pH of the digestive tract and resist pathogen proliferation, thus contributing to improving growth performance (Hoque et al., 2022). However, the results from many previous studies on the effects of GOD supplementation on growth performance in broilers were inconsistent. No effects on the ADG, ADFI and FCR were observed in Chinese male Lingnan yellow-feathered broilers by supplementation with 75 U/kg GOD throughout a 52-day feeding period (Wang et al., 2018) and in male Arbor Acres broilers by supplementation with 0.01% GOD in a 42-day feeding experiment (Qu and Liu, 2021). Zhao et al. (2022) found that dietary supplementation with 200 U/kg GOD decreased the FCR in Chinese male You chickens from 42 to 77 days of age. The results from Hoque et al. (2022) suggested that feeding Ross 308 male broilers with 0.02% to 0.03% GOD increased the ADG and decreased the FCR from 1 to 35 days of age. Similarly, in this study, we found that supplementation with 400 to 800 mg/kg GOD in the diet could increase the ADG and decrease the FCR but did not affect the ADFI from 1 to 42 days of age. This improved growth performance in the present study may be due to increased nutrient digestion and absorption. Dang et al. (2021) reported that dietary supplementation with 0.03% GOD increased the apparent total tract digestibility of energy and nitrogen in growing pigs. In addition, Wu et al. (2019) observed that the supplement with 60 U/kg GOD not only improved apparent digestibility of crude protein, calcium, total phosphorus, and ether extract, but also increased the small intestinal activities of amylase, chymotrypsin, and lipase in 42-day-old broilers. The improvement in growth performance by dietary supplementation with GOD, as observed in this study, may be related to the gluconic acid production. Gluconic acid can be produced by catalytic reaction of GOD and could be further fermented by specific bacteria to produce short-chain fatty acids, such as acetic acid, butyric acid and so on, in the hindgut (Zhao et al., 2022). These acids could be conducive to lowering intestinal pH and improving the activity of digestive enzymes, thus increasing digestion and absorption so that could promote the growth performance.
Broilers fed an 800 mg/kg GOD diet had a higher dressing-out percentage and semieviscerated yield compared to those of the control group, indicating that GOD supplementation had the ability to increase slaughter performance. An explanation may be that dietary GOD supplementation could lower gut pH and increase digestive enzyme activity by producing gluconic acid and improving nutrient digestion, absorption and deposition, thus increasing slaughter performance. In addition, we also found a decreased trend in abdominal fat yield in the GOD group, while no significant difference in eviscerated yield was observed between the control and GOD groups, further suggesting that dietary GOD supplementation may affect nutrient metabolism, decreasing fat anabolism and contributing to an increased carcass yield.
Generally, the immune organ index can be used to estimate the growth and development of immune organs. In broilers, a higher immune organ index usually means an increase in immune function and disease resistance (Xing et al., 2020). Liu et al. (2020) reported that dietary supplementation with 200 mg/kg GOD did not affect spleen and bursa of Fabricius indices of 28-day-old ducks. Similarly, Wang et al. (2018) found that adding 15 mg/kg GOD into the diet had no significant effect on the relative weights of the thymus, spleen and bursa of Fabricius in Chinese yellow-feathered broilers from 1 to 52 days of age. In this study, dietary GOD supplementation had significant effects on the immune organ indices compared to the control group, and GOD-fed broilers had a higher relative weight of the thymus, spleen, and bursa of Fabricius, indicating that GOD had a protective role in promoting immune organ development. This inconsistency might be mainly due to the difference in supplemental levels of GOD, feeding environment and animal breed, etc.
The small intestine, which is composed of three segments, namely, the duodenum, jejunum, and ileum, is a very important organ of the digestive system where ingested feed is digested and nutrients are absorbed (Bazira, 2023). In terms of the intestinal index, in this study, we found that GOD supplementation in the diet significantly increased the lengths and weights of the duodenum, jejunum and ileum, indicative of a promoting activity of GOD in digestive organ development. It is reasonable to infer that a longer small intestine tract would expand the time of transit through the tract of intestinal contents, thus contributing to the digestion and absorption of nutrients and growth performance. The explanation for this result whereby the dietary GOD supplementation promoted intestinal development might have been the production of the gluconic acid metabolites in the gut. According to other reports, the end products of gluconic acid formed through bacterial fermentation, such as butyric acid, were demonstrated to accelerate the proliferation and differentiation of intestinal epithelial stem cells and enhance the relative weight and length of the small intestine (Lan et al., 2020; Zabek et al., 2020), thus contributing to the intestinal development.
The morphology of the small intestine is often closely related to the ability to absorb of nutrients. The villi have finger-like projections and dictate the surface area of nutrition absorption, while the crypts have crevice-like invaginations and dictate the maturation rate of the cells (Cloft et al., 2023). Usually, a larger ratio of villus height to crypt depth reflects a better intestinal absorption function (Zhao et al., 2022). In this study, dietary GOD supplementation significantly increased the villus height and the ratio of villus height to crypt depth and decreased the crypt depth, indicating that GOD could improve the morphology and structure of the intestine, which is consistent with the results of previous studies (Qu and Liu, 2021; Zhao et al., 2022).
The microbial community within the intestinal tract plays a significant role in overall health and digestion, and the cecal microbiota has the highest bacterial richness and diversity in broiler chickens. In the ceca, more than 90% of the bacteria are gram-positive, and the most dominant phylum was Firmicutes, followed by Bacteroidetes and Actinobacteria (Feye et al., 2020). In this study, we found that dietary supplementation with GOD significantly decreased the Simpson index of the cecal microbiota, indicating that GOD supplementation could increase the diversity of the cecal microbial community in broilers. The main phyla of the cecum were composed of Firmicutes, Bacteroidetes, Proteobacteria and Verrucomicrobia in this study, and dietary GOD supplementation increased the abundance of Verrucomicrobia in the cecum. Verrucomicrobia is known to improve glucose metabolism in animals, and the increased abundance of Verrucomicrobia could contribute to the depletion of pathogenic microorganisms, such as Escherichia and Shigella (Li et al., 2022). At the genus level, we found that dietary supplementation with GOD increased the abundances of Ralstonia, Akkermansia and Parabacteroides and decreased that of Ruminococcus in the cecum. Ralstonia was previously demonstrated to have the ability to oxidize trace levels of H2 using O2 as the terminal electron acceptor to obtain metabolic energy, indicative of energy conversion (Ludwig et al., 2009). Although the role of this genus in poultry is unknown, Ralstonia was proved to present antimicrobial activity and biosynthesis of bioactive compounds and can produce beneficial secondary metabolites for the host, contributing to the intestinal development and health (Cerezo-Ortega et al., 2021). Akkermansia has been identified as a dominant gut bacterium that abundantly colonizes in a nutrient-rich environment and is usually considered to be a “lean microbe” (Cui et al., 2022). Dietary supplementation with raffinose and oligofructose can stimulate the proliferation of Akkermansia (Everard et al., 2011; Zhu et al., 2020), and the abundance of Akkermansia in the gut was previously demonstrated to decrease high-fat diet-induced fat-mass gain and oxidative stress in mice (Everard et al., 2013), indicative of glucose homeostasis regulation and antioxidant activity. In broilers, Akkermansia in the intestinal mucosa can synthesize short-chain fatty acids to promote their colonization and provide energy to the host and thus enhances gut integrity (Rassmidatta et al., 2024). The genus Parabacteroides is also an important member of the human and mammalian normal intestinal microbiota and was reported to be involved in the degradation of polysaccharides, the production of short-chain fatty acids and the modulation of host immune response (Qiao et al., 2022). In addition, Wang et al. (2019) reported that oral gavage of live Parabacteroides to mice could alleviate obesity and metabolic dysfunctions via the production of succinate and secondary bile acids, which were involved in the central control of energy and glucose homeostasis. Overall, these results indicated that the GOD supplement may consume oxygen by the catalytic reaction to produce gluconic acid and hydrogen peroxide (Hztzinikolaou and Macris, 1995), which provide an antipathogen environment and stimulate the growth of beneficial bacteria, such as Ralstonia, Akkermansia and Parabacteroides. Thus, a positive regulation in the structure of intestinal microbiota by GOD treatment may improve broiler gut health and growth performance.
In conclusion, our findings demonstrated that dietary supplementation with GOD increased the growth performance and slaughter yield of broilers. The dietary GOD could promote immune organ and small intestine development and improve the intestinal morphology. GOD also regulated cecal microbiota with increasing the abundance of beneficial bacteria (Ralstonia, Akkermansia and Parabacteroides), which might be related to the improvement of the growth performance and gut health in broilers.
Data availability statement
The original contributions presented in the study are publicly available. This data can be found here: NCBI BioProject, accession PRJNA1185495.
Ethics statement
The animal study was approved by Animal Care and Use Committee of Shenyang Agricultural University. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
XZ: Conceptualization, Data curation, Writing – original draft. XJZ: Formal Analysis, Investigation, Writing – review & editing. YZ: Methodology, Resources, Writing – review & editing. YJZ: Software, Validation, Writing – review & editing. FL: Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bankar S. B., Bule M. V., Singhal R. S., Ananthanarayan L. (2009). Glucose oxidase-an overview. Biotechnol. Adv. 27, 489–501. doi: 10.1016/j.bioteChadv.2009.04.003
Bazira P. J. (2023). Anatomy of the small intestine. Surg. (Oxford). 41, 389–396. doi: 10.1016/j.mpsur.2023.02.031
Cerezo-Ortega I. M., Di Zeo-Sanchez D. E., Garcia-Marquez J., Ruiz-Jarabo I., Saez-Casado M. I., Balebona M. C., et al. (2021). Microbiota composition and intestinal integrity remain unaltered after the inclusion of hydrolysed Nannochloropsis gaditana in Sparus autata diet. Sci. Rep. 11, 18779. doi: 10.1038/s41598-021-98087-5
Chen J., Wang P., Liu C., Yin Q., Chang J., Wang L., et al. (2023). Effects of compound feed additive on growth performance and intestinal microbiota of broilers. Poultry. Sci. 102, 102302. doi: 10.1016/j.psj.2022.102302
Cloft S. E., Uni Z., Wong E. A. (2023). Profiling intestinal stem and proliferative cells in the small intestine of broiler chickens via in situ hybridization during the peri-hatch period. Poultry. Sci. 102, 102495. doi: 10.1016/j.psj.2023.102495
Cui X., Gou Z., Jiang Z., Li L., Lin X., Fan Q., et al. (2022). Dietary fiber modulates abdominal fat deposition associated with cecal microbiota and metabolites in yellow chickens. Poultry. Sci. 101, 101721. doi: 10.1016/j.psj.2022.101721
Dang D. X., Hoque M. R., Liu Y., Chen N., Kim I. H. (2021). Dietary glucose oxidase supplementation improves growth performance, apparent nutrient digestibility, and serum antioxidant enzyme parameters in growing pigs. Ital. J. Anim. Sci. 20, 1568–1574. doi: 10.1080/1828051X.2021.1984853
Dang D. X., Liu Y., Chen N., Kim I. H. (2022). Dietary supplementation of Aspergillus Niger-expressed glucose oxidase ameliorates weaning stress and improves growth performance in weaning pigs. J. Anim. Physiol. An. N. 106, 258–265. doi: 10.1111/jpn.13576
Ducatelle R., Goossens E., Eeckhaut V., Immerseel F. V. (2023). Poultry gut health and beyond. Anim. Nutr. 13, 240–248. doi: 10.1016/j.aninu.2023.03.005
Everard A., Belzer C., Geurts L., Ouwerkerk J. P., Druart C., Bindels L. B., et al. (2013). Cross-talk between Akkermansia munciniphila and intestinal epithelium controls diet-induced obesity. PNAS 110, 9066–9071. doi: 10.1073/pnas.1219451110
Everard A., Lazarevic V., Derrien M., Girard M., Muccioli G. G., Neyrinck A. M., et al. (2011). Response of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice. Diabetes 60, 2775–2786. doi: 10.2337/db11-0227
Feye K. M., Baxter M. F. A., Tellez-Isaias G., Kogut M. H., Ricke S. C. (2020). Influential factors on the composition of the conventionally raised broiler gastrointestinal microbiomes. Poultry. Sci. 99, 653–659. doi: 10.1016/j.psj.2019.12.013
Hoque M. R., Chen N., Liu Y., Kim I. H. (2022). Possibility of using glucose oxidase in the diet to improve selected indicators of blood antioxidant defense, digestibility and growth performance of broiler chickens. Ital. J. Anim. Sci. 21, 455–462. doi: 10.1080/1828051X.2021.2024457
Hztzinikolaou D. G., Macris B. J. (1995). Factors regulating production of glucose oxidase by Aspergillus Niger. Enzyme Microb. Tech. 17, 530–534. doi: 10.1016/0141-0229(95)91708-7
Lan R., Li S., Zhao Z., An L. (2020). Sodium butyrate as an effective feed additive to improve growth performance and gastrointestinal development in broilers. Vet. Med. Sci. 6, 491–499. doi: 10.1002/vms3.250
Li C., Cai H., Li S., Liu G., Deng X., Bryden W. L., et al. (2022). Comparing the potential of Bacillus amyloliquefaciens CGMCC18230 with antimicrobial growth promoters for growth performance, bone development, expression of phosphorus transporters, and excreta microbiome in broiler chickens. Poultry. Sci. 101, 102126. doi: 10.1016/j.psj.2022.102126
Liu J., Liu G., Chen Z., Zheng A., Cai H., Chang W., et al. (2020). Effects of glucose oxidase on growth performance, immune function, and intestinal barrier of ducks infected with Escherichia coli O88. Poultry. Sci. 99, 6549–6558. doi: 10.1016/j.psj.2020.09.038
Ludwig M., Cracknell J. A., Vincent K. A., Armstrong F. A., Lenz O. (2009). Oxygen-tolerant H2 oxidation by membrane-bound [NiFe] hydrogenases of Ralstonia species. J. Biol. Chem. 284, 465–747. doi: 10.1074/jbc.M803676200
Mehdi Y., Letourneau-Montminy M. P., Gaucher M. L., Chorfi Y., Suresh G., Rouissi T., et al. (2018). Use of antibiotics in broiler production: global impacts and alternatives. Anim. Nutr. 4, 170–178. doi: 10.1016/j.aninu.2018.03.002
National Research Council. (1994). Nutrient requirements of poultry. 9th ed. Washington, USA: National Academy Press.
Peng L., Li Z., Green R., Holzman I., Lin J. (2009). Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in caco-2 cell monolayers. J. Nutr. 139, 1619–1625. doi: 10.3945/jn.109.104638
Qiao Y., Liu C., Guo Y., Zhang W., Guo W., Oleksandr K., et al. (2022). Polysaccharides derived from Astragalus membranaceus and Glycyrrhiza uralensis improve growth performance of broilers by enhancing intestinal health and modulating gut microbiota. Poultry. Sci. 101, 101905. doi: 10.1016/j.psj.2022.101905
Qu W., Liu J. (2021). Effects of glucose oxidase supplementation on the growth performance, antioxidative and inflammatory status, gut function, and microbiota composition of broilers fed moldy corn. Front. Physiol. 12. doi: 10.3389/fphys.2021.646393
Rassmidatta K., Theapparat Y., Chanaksorn N., Carcano P., Adeyemi K., Ruangpanit Y. (2024). Dietary Kluyveromyces marxianus hydrolysate alters humoral immunity, jejunal morphology, cecal microbiota and metabolic pathways in broiler chickens raised under a high stocking density. Poultry. Sci. 103, 103970. doi: 10.1016/j.psj.2024.103970
Sun X., Piao L., Jin H., Nogoy K., Zhang J., Sun B., et al. (2021). Dietary glucose oxidase and/or catalase supplementation alleviates intestinal oxidative stress induced by diquat in weaned piglets. Anim. Sci. J. 92, e13634. doi: 10.1111/asj.13634
Tang H., Yao B., Gao X., Yang P., Wang Z., Zhang G. (2016). Effects of glucose oxidase on the growth performance, serum parameters and faecal microflora of piglets. South Afr. J. Anim. Sci. 16, 14–20. doi: 10.4314/sajas.v46i1.2
Wang K., Liao M., Zhou N., Bao L., Ma K., Zheng Z., et al. (2019). Parabacteroides distasonis alleviates obesity and metabolic dysfunctions via production of succinate and secondary bile acids. Cell Rep. 26, 222–235. doi: 10.1016/j.celrep.2018.12.028
Wang Y., Wang Y., Xu H., Mei X., Gong L., Wang B., et al. (2018). Direct-fed glucose oxidase and its combination with B.amyloliquefaciens SC06 on growth performance, meat quality, intestinal barrier, antioxidative status, and immunity of yellow-feathered broilers. Poultry. Sci. 97, 3540–3549. doi: 10.3382/ps/pey216
Wu S., Chen X., Li T., Ren H., Zheng L., Yang X. (2020). Changes in the gut microbiota mediate the differential regulatory effects of two glucose oxidase produced by Aspergillus Niger and Penicillium amagasakiense on the meat quality and growth performance of broilers. J. Anim. Sci. Biotechno. 11, 73. doi: 10.1186/s40104-020-00480-z
Wu S., Li T., Niu H., Zhu Y., Liu Y., Duan Y., et al. (2019). Effects of glucose oxidase on growth performance, gut function, and cecal microbiota of broiler chickens. Poultry. Sci. 98, 828–841. doi: 10.3382/ps/pey393
Xing R., Yang H., Wang X., Yu H., Liu S., Li P. (2020). Effects of calcium source and calcium level on growth performance, immune organ indexes, serum components, intestinal microbiota, and intestinal morphology of broiler chickens. J. Appl. Poultry. Res. 29, 106–120. doi: 10.3382/japr/pfz033
Zabek K., Szkopek D., Michalczuk M., Konieczka P. (2020). Dietary phytogenic combination hops and a mixture of a free butyrate acidifier and gluconic acid maintaining the health status of the gut and performance in chickens. Aniamls 10, 1335. doi: 10.3390/ani10081335
Zhao Y., Fu J., Li P., Chen N., Liu Y., Liu D., et al. (2022). Effects of dietary glucose oxidase on growth performance and intestinal health of AA broilers challenged by Clostridium perfringens. Poultry. Sci. 101, 101553. doi: 10.1016/j.psj.2021.101553
Zhu X., Liu J., Liu H., Yang G. (2020). Soybean oligosaccharide, stachyose, and raffinose in broilers diets: effects on odor compound concentration and microbiota in cecal digesta. Poultry. Sci. 99, 3532–3539. doi: 10.1016/j.psj.2020.03.034
Zhu X., Zhang Y., Zhao Y., Tao L., Liu H., Dong W., et al. (2022). Effects of dietary supplementation with itaconic acid on the growth performance, nutrient digestibility, slaughter variables, blood biochemical parameters, and intestinal morphology of broiler chickens. Poultry. Sci. 101, 101732. doi: 10.1016/j.psj.2022.101732
Keywords: glucose oxidase, broiler, growth performance, gut health, microbiota
Citation: Zhu X, Zhang X, Zhang Y, Zhu Y and Li F (2025) Dietary glucose oxidase supplementation improves growth performance, intestinal morphology, and microbial community of broilers. Front. Anim. Sci. 5:1491519. doi: 10.3389/fanim.2024.1491519
Received: 05 September 2024; Accepted: 20 November 2024;
Published: 17 February 2025.
Edited by:
David L. Harmon, University of Kentucky, United StatesReviewed by:
Arda Yıldırım, Gaziosmanpaşa University, TürkiyeDaniel Marco Paredes Lopez, Universidad Nacional Agraria de la Selva, Peru
Copyright © 2025 Zhu, Zhang, Zhang, Zhu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fangfang Li, bGZmc3lhdUBzeWF1LmVkdS5jbg==
 Xin Zhu
Xin Zhu Xinjie Zhang
Xinjie Zhang