- 1Huzhou Key Laboratory of Innovation and Application of Agricultural Germplasm Resources, Huzhou Academy of Agricultural Sciences, Huzhou, China
- 2College of Life Science, Huzhou Teachers College, Huzhou, China
- 3Central Plains Environmental Protection Co. Ltd, Treatment Plant Dispatch Center, Zhengzhou, China
In livestock production, ruminant feed resources are often scarce, and numerous challenges arise during production, such as immune disorders and oxidative stress. Mulberry leaves are rich in various nutrients and exhibit significant antioxidant and immune-regulating properties. Therefore, they can be used as an unconventional feed resource in livestock production. This study investigated the effects of mulberry leaves (ML) as a feed supplement on the blood biochemical parameters and hindgut microbial structure of Hu sheep. Sixteen Hu sheep were randomly divided into two groups and fed either 0 or 60 g/d of ML. Compared to the control group, sheep fed ML showed a significant increase in AKP (P = 0.027) and GPT (P = 0.002) levels in the blood, while TP (P = 0.001) levels decreased significantly. Additionally, there was an increasing trend in GSH-Px (P = 0.082) and CAT (P = 0.058) levels. After the addition of ML, the abundance of Campylobacterota, Campylobacter, and Mailhella in the hindgut significantly increased (P < 0.05), while the abundance of Alloprevotella, Roseburia, and Prevotellaceae UCG-003 significantly decreased (P < 0.05). Therefore, ML can serve as a natural feed supplement to regulate the immune status of animals, thereby promoting the healthy production of ruminants.
1 Introduction
Mulberry leaves are rich in protein, fiber, minerals, and other nutrients, making them an excellent source of feed with high biological yield, comprehensive nutritional value, good palatability, and high digestibility. They can serve as a new type of protein feed resource to address feed shortages (Geng et al., 2024). Mulberry leaves grow in regions with diverse climatic conditions worldwide, ranging from temperate to tropical areas. The annual yield of mulberry leaves can reach 25 to 30 tons per hectare, with a protein content of 18-25% (on a dry matter basis) (Guha et al., 2010; Iqbal et al., 2012; Wang et al., 2022). Moreover, the digestibility of protein in mulberry leaves for animals can reach 75-85% (on a dry matter basis) (Chan et al., 2016; Chen et al., 2018). Analysis reveals that mulberry leaves possess a relatively high crude fat content and low crude fiber content, positioning them as a valuable source of high-quality protein for livestock feed. Additionally, it is also rich in protein, containing 218.6 g/kg (Trabi et al., 2017). They are rich not only in amino acid content but also in diversity. Some essential amino acids, which may be lacking in other feeds, are present in sufficient quantities in mulberry leaves, meeting the amino acid requirements of livestock and poultry and helping to balance amino acid ratios (Leterme et al., 2005). Mulberry leaves are also high in minerals, with 100 g containing 2.699% calcium and 3.101% potassium (Batiha et al., 2023). The vitamin content in mulberry leaves is also abundant, especially in B-complex and C vitamins, which are beneficial for improving the immune systems of animals (Chen et al., 2021). Additionally, mulberry leaves contain various physiologically active substances such as polysaccharides, flavonoids, steroids, volatile oils, and alkaloids, which play important physiological roles (Liu et al., 2001). Multiple studies have demonstrated that incorporating mulberry leaves as a feed ingredient can significantly improve the health status, productivity, meat quality, and flavor of ruminant animals. Supplementing 600 g/d of mulberry leaf feed in a rice straw-based diet can significantly increase the dry matter intake of beef cattle (Tan et al., 2012). Moreover, adding 2 g of air-dried mulberry leaves per head in the daily ration does not adversely affect the feed intake of sheep (Chen et al., 2015). According to Liu et al. (2001), supplementing ammonia-treated rice straw with varying levels of mulberry leaf powder as a replacement for rapeseed meal can enhance feed intake in lambs and improve their growth performance. These findings suggest that mulberry leaf products can be added as a feed ingredient in the diets of ruminant animals.
In recent years, the use of unconventional plant feed ingredients has become increasingly popular, with beneficial plant-based feed ingredients being widely adopted in animal husbandry, especially in intensive farming systems (Hashem et al., 2020; Zhao et al., 2022a). Reports indicate that supplementing animal diets with plant feed can improve animal productivity and immune response while reducing oxidative stress and inflammation (Zhao et al., 2023a). These substances act on the gut microbiota by neutralizing free radicals and preventing their interaction with cellular DNA, while also enhancing nutrient digestion and absorption, thereby exerting immunomodulatory effects (Yu et al., 2023a). Therefore, adding feed ingredients containing natural plant nutrients such as polyphenols to animal diets is considered an optimal alternative for improving animal productivity (Yu et al., 2023b; Zhao et al., 2023b). In organic farms, a diverse grassland with high plant diversity ensures adequate intake of polyphenolic compounds. Achieving such results in intensive farming requires supplementing feed with polyphenol-rich food additives (Huang et al., 2018). This strategy has been supported by research conducted on some farms where the addition of natural plant extract antioxidants to animal feed has improved the quality of animal products (Ji et al., 2018). Additionally, these natural plant extracts play an important role in the hindgut of animals. Therefore, a comprehensive exploration of the effects of natural plant components on the blood and hindgut health of ruminant animals in intensive farming is a focal point of the modern livestock industry. Considering these factors, the aim of this study is to evaluate the effects of supplementing fattening Hu sheep with mulberry leaf powder and retrograde diets on blood parameters and hindgut microbiota.
2 Materials and methods
2.1 Source of dried tea residue
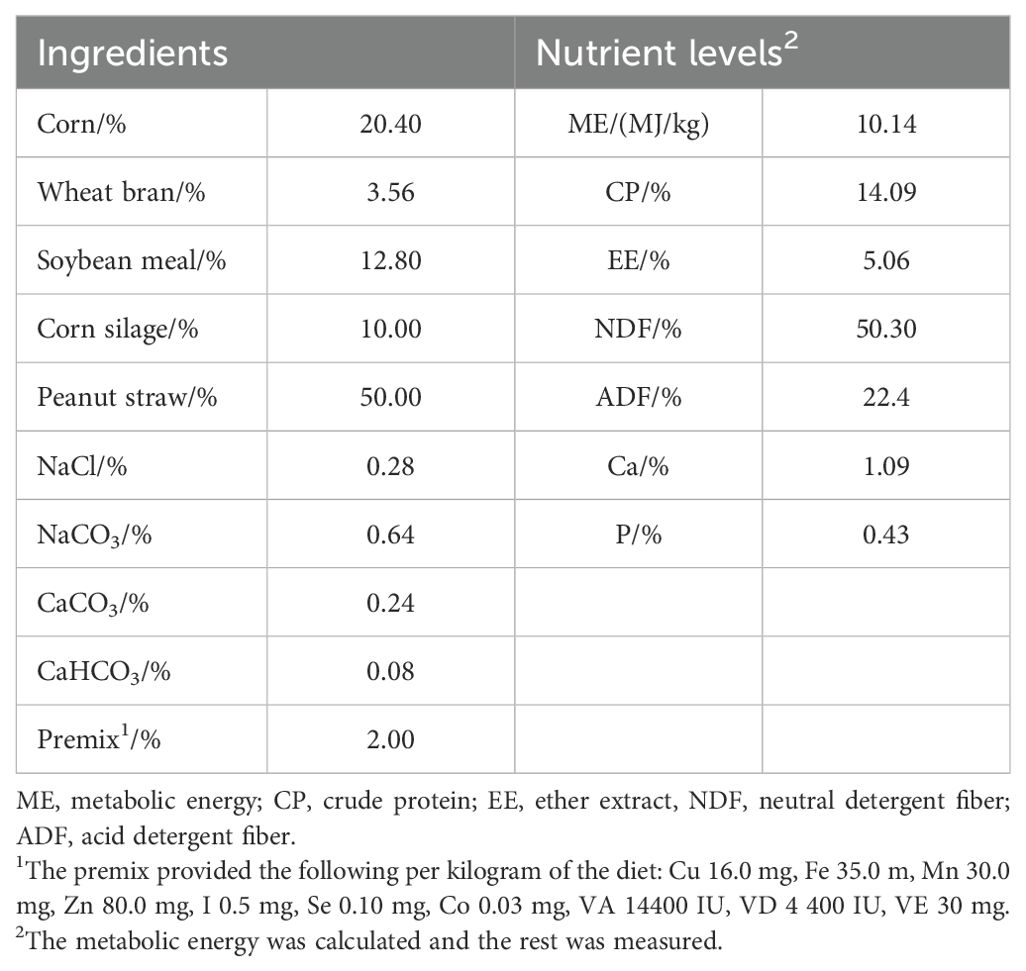
Mulberry leaves purchased from Huzhou, China, were tested for their nutritional levels, yielding the following results: moisture 11.46%, crude protein 19.30%, crude fat 8.25%, crude ash 7.46%, neutral detergent fiber 34.20%, acid detergent fiber 16.38%, calcium 1.54%, phosphorus 0.10%.
2.2 Animals and treatments
This study selected 16 male Hu sheep with a weight of 28.96 ± 1.04 kg at three months of age. These 16 sheep were randomly divided into a control group and a treatment group, with 8 sheep in each group. The control group was fed a basal diet (CON, n=8), while the treatment group was fed with 60 g of mulberry leaf powder (ML, n=8) daily. The dosage of mulberry leaf powder was determined based on the results of an unpublished in vitro experiment. The basal diet chosen for the experiment (Table 1) was a complete mixed ration, with a concentrate-to-roughage ratio of 7:3, meeting the requirements of Chinese sheep feeding standards (NY/T816-2004). The Hu sheep in the experiment were individually housed in pens and fed the basal diet twice daily (8:00 am and 5:00 pm). They had ad libitum access to feed and water throughout the experiment, which lasted for 56 days, including a 14-day adaptation period and a formal experimental period of 42 days. All experimental techniques followed the rules established by the Experimental Animal Management Committee of the Zhejiang Academy of Agricultural Sciences.
2.3 Sample collection
2.3.1 Blood sample collection
On the morning of the 56th day, 2 hours after feeding, blood samples were collected from the jugular vein of the Hu sheep using non-anticoagulant vacuum tubes. The samples were then centrifuged at 3,000 × g for 15 minutes at 4°C to collect serum. Commercial assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) and a microplate reader (Multiskan FC; Thermo Fisher Scientific, Waltham, MA, USA) were used to analyze the concentrations of catalase (CAT), total superoxide dismutase (T-SOD), glutathione peroxidase (GSH-Px), malondialdehyde (MDA), total antioxidant capacity (T-AOC), free fatty acids (FFA), albumin (ALB), total protein (TP), low-density lipoprotein (LDL), high-density lipoprotein (HDL), alkaline phosphatase (AKP), acid phosphatase (ACP), glutamate pyruvate transaminase (GPT), triglycerides (TG), total cholesterol (TCH), glutamate oxaloacetate transaminase (GOT), and lactate dehydrogenase (LD). All ELISA data were recorded using a microplate reader (Multiskan FC; Thermo Fisher Scientific, Waltham, MA, USA) and analyzed according to the instructions provided by the supplier using commercial ELISA assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
2.3.2 Fecal sample collection, 16S rRNA amplicon sequencing and analysis
On the morning of the 56th day, fecal samples were collected 2 hours after feeding and stored in liquid nitrogen until analysis. Genomic DNA was extracted from the collected fecal microbiota using the E.Z.N.A. Soil DNA Kit (Omega Bio-Tek, USA). The quantity and quality of the extracted DNA were evaluated using a ND-1000 spectrophotometer (NanoDrop, Wilmington, DE, USA), and validation was performed by running samples on a 1% agarose gel. Sequencing of the 16S rRNA gene was conducted using the Illumina MiSeq platform. For bacteria, the V3-V4 region of the 16S rRNA gene was amplified using primers 338F (5’-barcode-ACTCCTRCGGGAGGCAGCAG-3’) and 806R (5’-GGACTACCVGGGTATCTAAT-3’). PCR amplification was performed in a 25 μL reaction system, including 2 μL of DNA template, 12.5 μL of 2× Taq PCR MasterMix, 2.5 μL of each primer, and ddH2O to adjust the final volume. PCR products were evaluated by 2% agarose gel electrophoresis and purified using the QIAquick Gel Extraction Kit (Qiagen, Hilden, Germany). Amplified products were then mixed at equimolar ratios to generate the amplicon library. Paired-end sequencing (2×300 bp) was performed on the Illumina MiSeq sequencing system. Paired-end reads were merged using FLASH (version 1.2.11). Sequence reads were processed and analyzed using the Quantitative Insights into Microbial Ecology (QIIME) pipeline software (version 1.9.1). Operational Taxonomic Units (OTUs) were assigned to reads based on 97% similarity using average-linkage clustering. OTU raw read counts were normalized against the total number of quality-filtered reads to calculate relative abundances. Taxonomic assignments were performed for each OTU using the RDP classifier (version 2.13) against the SILVA 16S rRNA database (version 138). Alpha diversity indices, including ACE, Chao1, Shannon, and Simpson, were calculated using Mothur (version 1.30.2). Beta diversity analysis was conducted using PCoA based on weighted and unweighted UniFrac distances in QIIME. ANOSIM statistical tests were performed to identify the statistical significance of between-group beta diversity.
2.4 Statistical analyses
The differences in blood biochemical parameters and microbial relative abundances observed in the experiment were analyzed using SPSS 22.0 software (SPSS Inc., Chicago, IL, USA). After conducting the Shapiro-Wilk test for normality, a two-tailed t-test was employed for analysis. A significance level of P < 0.05 was considered to indicate a significant difference, 0.10 < P ≤ 0.05 indicated a trend, and P ≥ 0.10 indicated no significant difference.
3 Results
3.1 Serum index
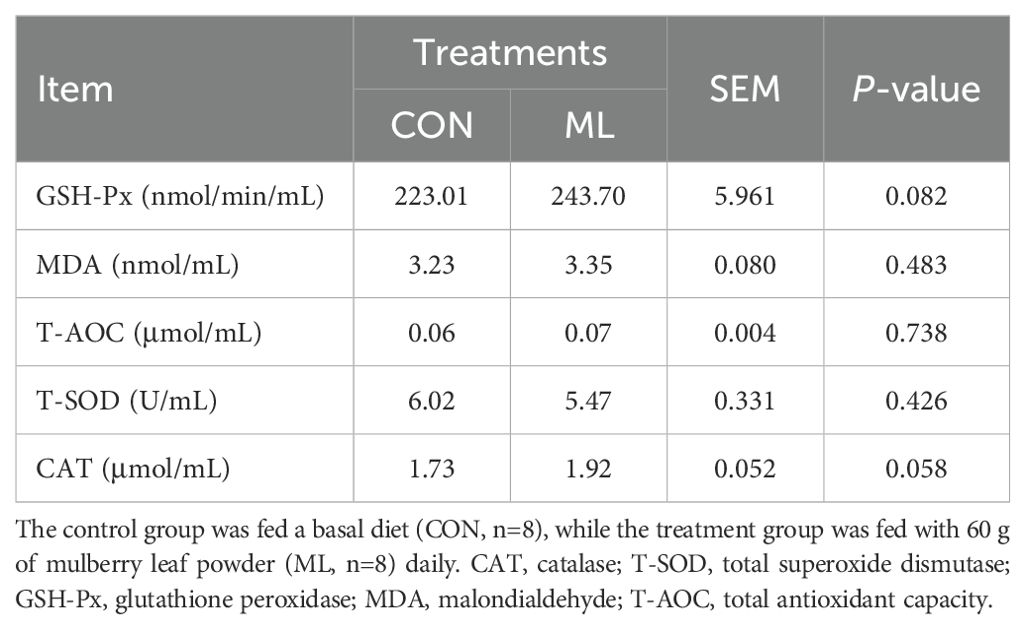
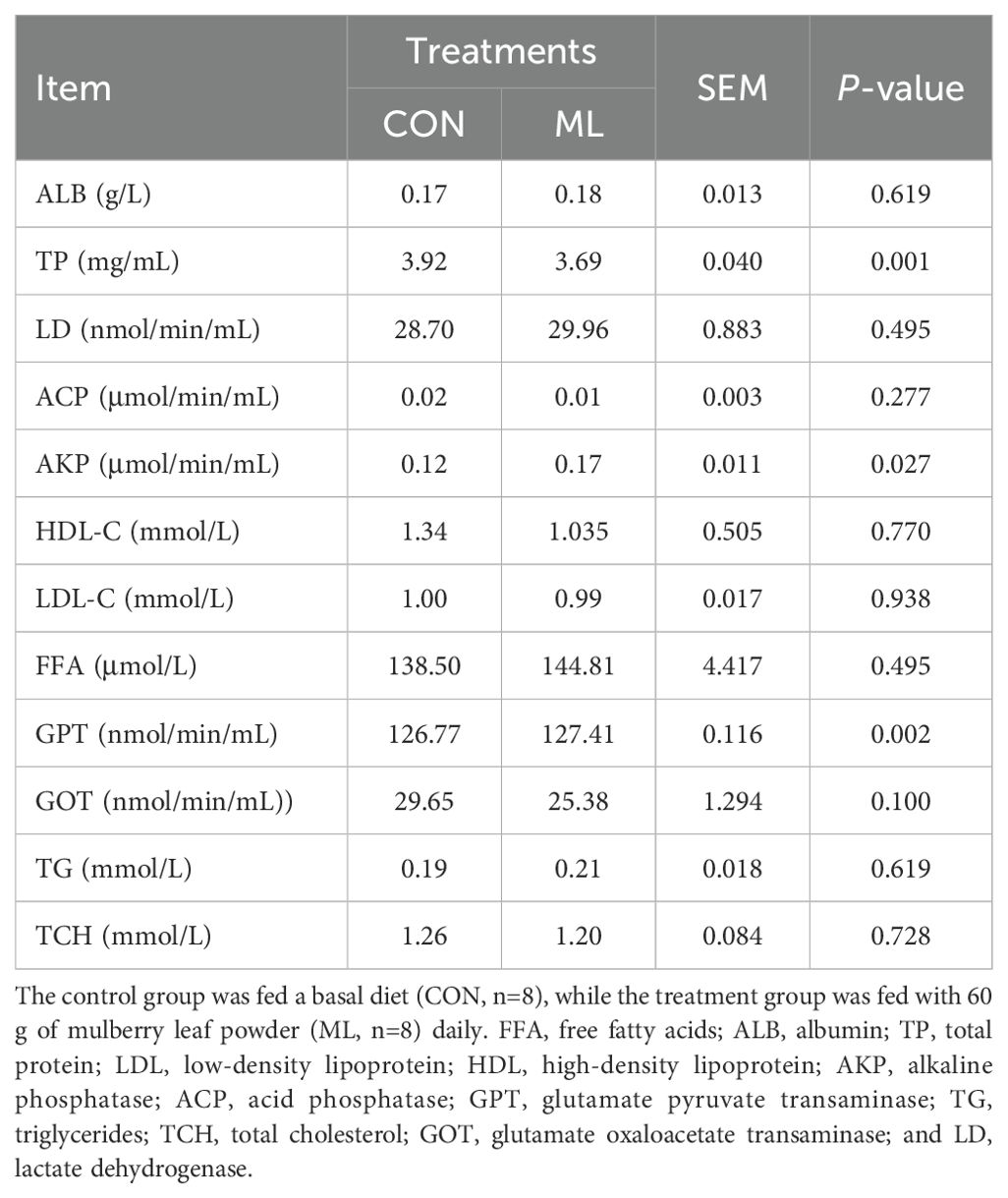
From Table 2, it can be observed that the addition of ML to the diet of Hu sheep does not significantly affect the concentrations of MDA, T-AOC, and T-SOD in the blood (P > 0.05). However, there is a trend of increased concentrations of GSH-Px (P = 0.082) and CAT (P = 0.058). This indicates that the addition of ML to the diet has the potential to enhance the antioxidant capacity of Hu sheep. From Table 3, it can be seen that the concentration of TP in the blood significantly decreases after adding ML to the diet (P = 0.001), while the concentrations of AKP (P = 0.027) and GPT (P = 0.002) significantly increase. However, there are no significant differences in the concentrations of ALB, LD, ACP, HDL-C, LDL-C, FFA, GOT, TG, and TCH (P > 0.05).
3.2 Structural changes in the gut microbiota
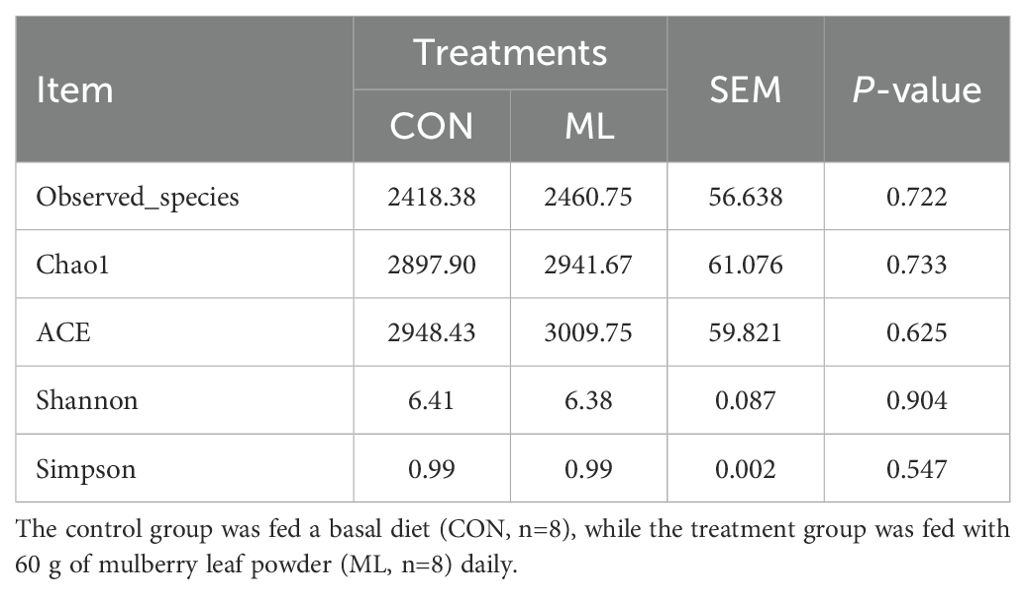
In Figure 1, the microbial composition of the CON and ML groups is presented. As seen in Figure 1A, the dilution curves of the sequencing samples reach a plateau, indicating that the sequencing depth meets the requirements. Figure 1B shows the PCA scores of the two groups, indicating that there is no significant separation between them. Further analysis of the microbial composition at the phylum level reveals that both the CON and ML groups have similar compositions, primarily composed of Firmicutes, Bacteroidota, Spirochaetota, Proteobacteria, Fibrobacterota, Verrucomicrobiota, Desulfobacterota, Cyanobacteria, Actinobacteriota, and Campylobacterota (Figure 1C). At the genus level, the microbial composition of both groups is also consistent, comprising Rikenellaceae RC9 gut group, UCG-005, Bacteroides, Treponema, Alistipes, Christensenellaceae R-7 group, Monoglobus, Lachnospiraceae NK4A136 group, Succinivibrio, and Prevotellaceae UCG-003 (Figure 1D). Additionally, according to Table 4, there are no significant differences in the α-diversity of the microbiota (P > 0.05). This suggests that the addition of ML to the diet does not have a negative impact on the composition of hindgut microbiota.
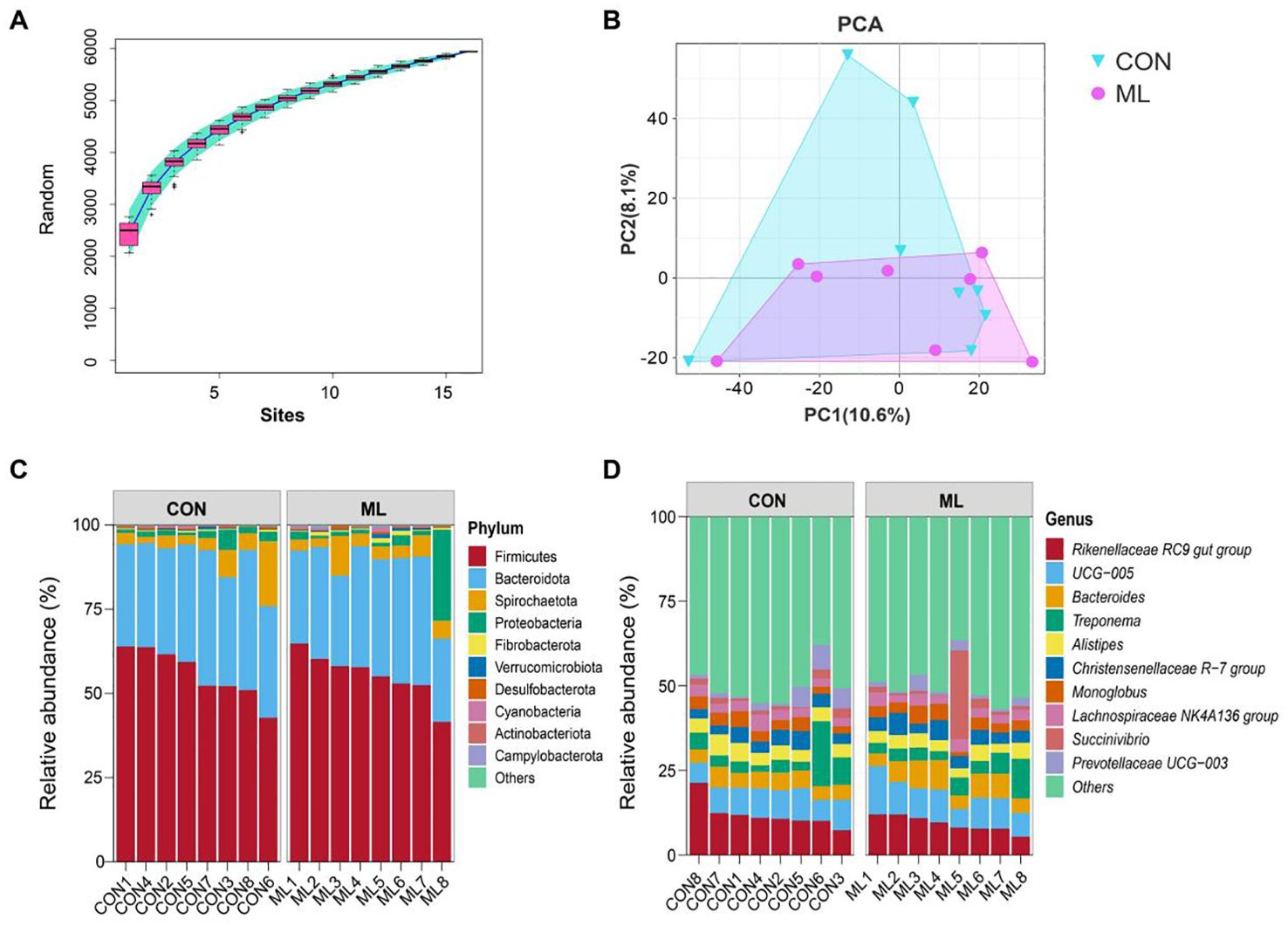
Figure 1. Overview of the structure and composition of gut microbiota. (A) Dilution curves of all samples. (B) PCA score plot based on unweighted analysis. (C) Composition of bacteria at the phylum level across all samples. (D) Composition of bacteria at the genus level across all samples. The control group was fed a basal diet (CON, n=8), while the treatment group was fed with 60 g of mulberry leaf powder (ML, n=8) daily.
We compared the specific microbial abundances and found that after adding ML to the diet, the abundance of Campylobacterota at the phylum level significantly increased (P < 0.05). At the genus level, the abundances of Campylobacter and Mailhella significantly increased (P < 0.05), while the abundances of Alloprevotella, Roseburia, and Prevotellaceae UCG-003 significantly decreased (P < 0.05). There was a decreasing trend in the abundances of Rikenellaceae RC9 gut group (P = 0.067) and UCG-002 (P = 0.081, Figure 2).
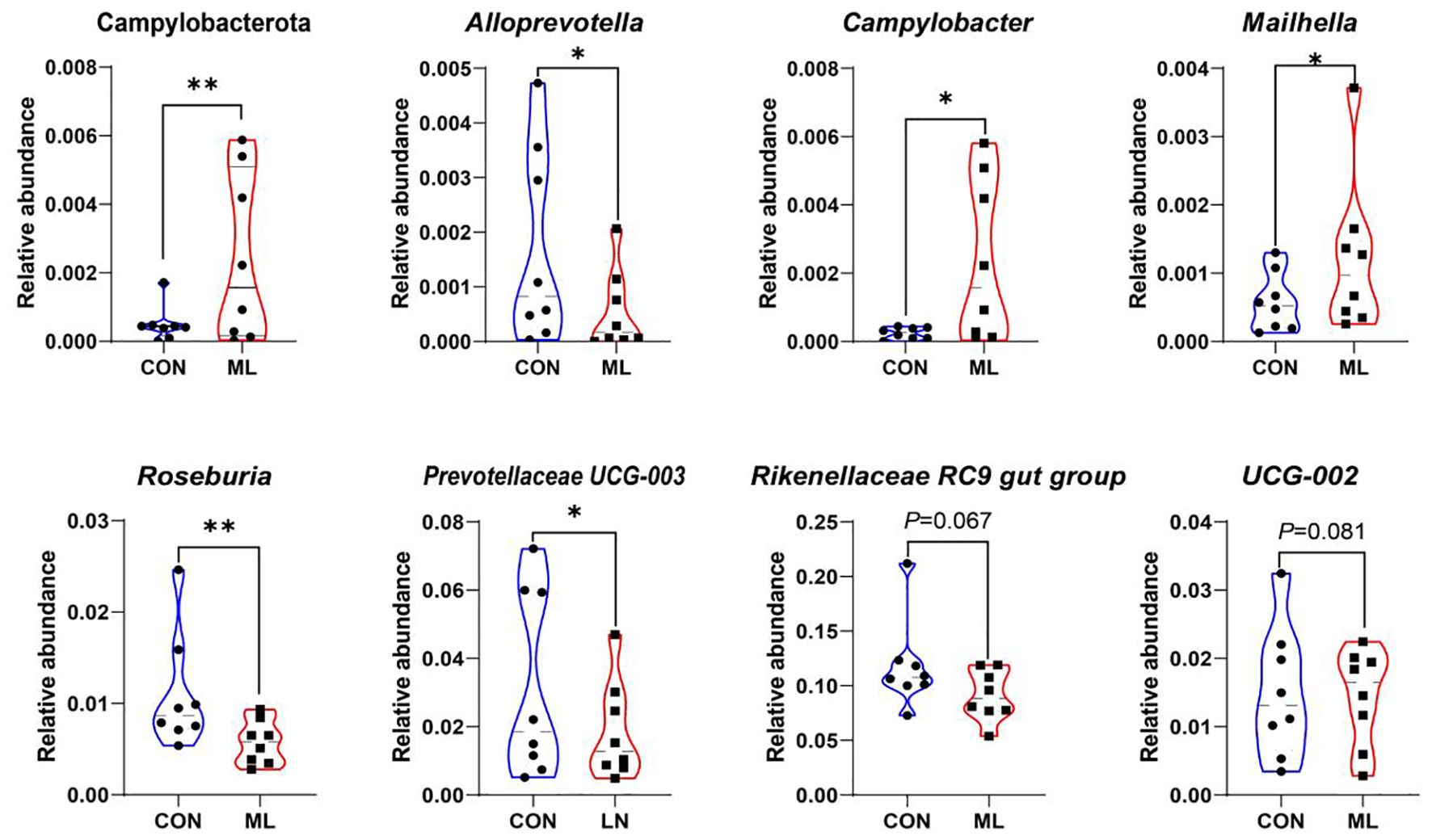
Figure 2. Comparative analysis of microbial relative abundance. * denotes P < 0.05, ** denotes P < 0.01. Data were presented as means ± SEM (n=8 per group). The control group was fed a basal diet (CON, n=8), while the treatment group was fed with 60 g of mulberry leaf powder (ML, n=8) daily.
Figure 3 displays the core composition of gut microbiota in the CON and ML groups. In the CON group, the significant core microbiota include Ruminococcaceae uncultured, Hydrogenoanaerobacterium, Succinivibrio, Acetitomaculum, Clostridia norank, UCG-009, NK4A214 group, and Alloprevotella. In the ML group, the significant core microbiota consist of Lachnoclostridium, Rickettsiales norank, UCG-005, Marinbryantia, Oscillibacter, Ruminococcaceae norank, Papillibacter, Ruminiclostridium, Gastranaerophilales, Quinella, and Ruminococcus.
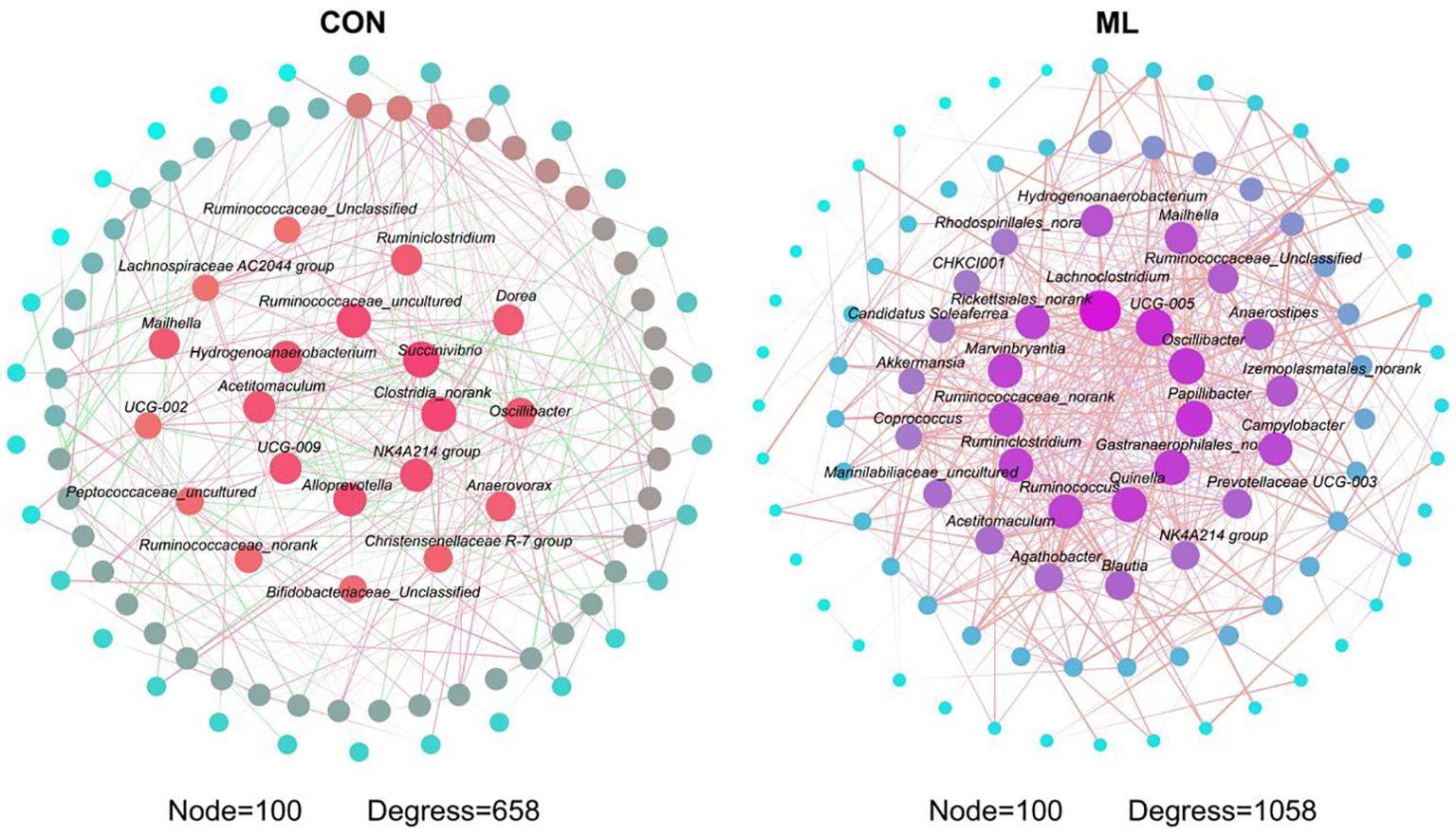
Figure 3. Overview of the composition of core microbiota network interactions. Data were presented as means ± SEM (n=8 per group). The control group was fed a basal diet (CON, n=8), while the treatment group was fed with 60 g of mulberry leaf powder (ML, n=8) daily.
3.3 Correlation between gut microbiota and blood indices
We utilized Spearman correlation analysis to examine the correlation between trending microbiota and all detected blood indices (Figure 4). We found a significant negative correlation (P < 0.05, R < -0.5) between Prevotellaceae UCG-003 and ABL, TG, LD, and FFA. Mailhella exhibited a significant positive correlation (P < 0.05, R > 0.5) with LD, FFA, and ACP. UCG-002 showed a significant positive correlation (P < 0.05, R > 0.5) with MDA and ACP, and a significant negative correlation (P < 0.05, R < -0.5) with T-AOC. ACP exhibited a significant positive correlation (P < 0.05, R > 0.5) with Campylobacter and a significant negative correlation (P < 0.05, R < -0.5) with Alloprevotella. Rikenellaceae RC9 gut group showed a significant positive correlation (P < 0.05, R > 0.5) with ALB.
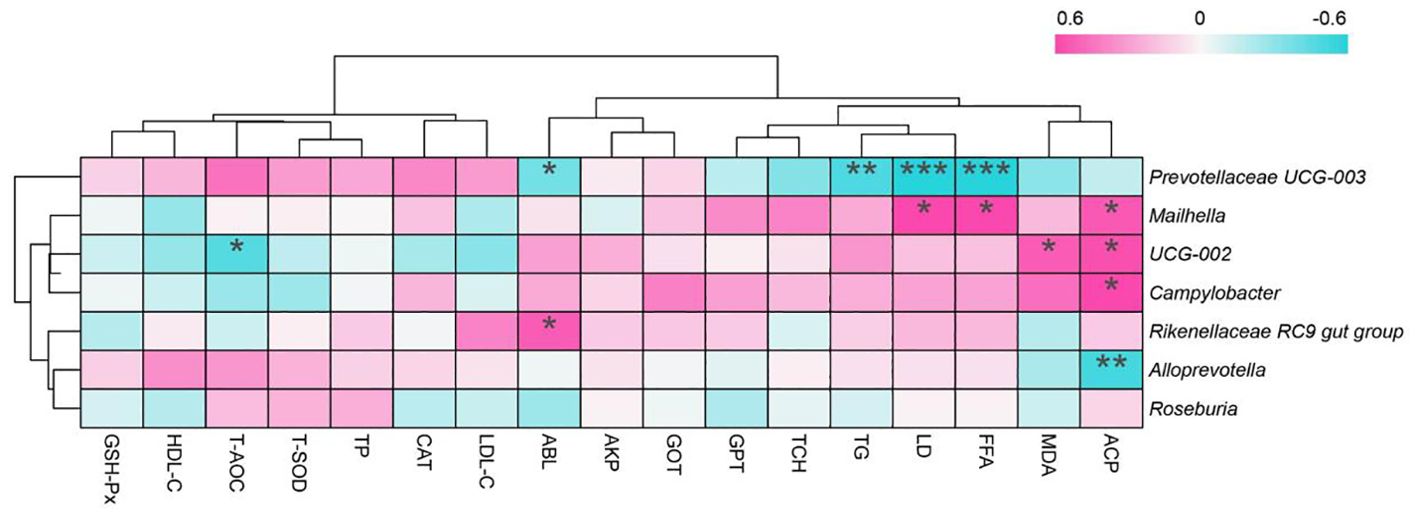
Figure 4. Correlation analysis between blood indices and microbiota showing trends of change. * denotes P < 0.05, ** denotes P < 0.01, *** denotes P < 0.001. The control group was fed a basal diet (CON, n=8), while the treatment group was fed with 60 g of mulberry leaf powder (ML, n=8) daily. CAT, catalase; T-SOD, total superoxide dismutase; GSH-Px, glutathione peroxidase; MDA, malondialdehyde; T-AOC, total antioxidant capacity. FFA, free fatty acids; ALB, albumin; TP, total protein; LDL, low-density lipoprotein; HDL, high-density lipoprotein; AKP, alkaline phosphatase; ACP, acid phosphatase; GPT, glutamate pyruvate transaminase; TG, triglycerides; TCH, total cholesterol; GOT, glutamate oxaloacetate transaminase, and LD, lactate dehydrogenase.
4 Discussion
Adding food, industrial by-products, or unconventional feed products to animal diets is becoming an important approach in the development of animal husbandry today. For example, adding apple pomace (Maslovarić et al., 2017), citrus peel (Zhao et al., 2022b), and other vegetable and fruit waste to feed has become increasingly common (Sahoo et al., 2021). Previous studies have demonstrated that mulberry leaves are rich in various bioactive compounds, including flavonoids and phenolic acids, which contribute to their efficacy as a high-quality immune modulator (Cui X. et al., 2023). These bioactive components, such as flavonoids, can effectively improve the health status of the host through pathways like the PI3K-Akt signaling pathway and the AGE-RAGE pathway (Lv et al., 2022). Additionally, many studies have reported that mulberry leaves also have a certain growth-promoting effect, which will be an important focus of our future research (Ding et al., 2021; Mengistu et al., 2020).
In the animal biological system, the redox system plays a crucial role in the host’s immune and health status. GSH-Px, MDA, T-AOC, T-SOD, and CAT are the main indicators used to evaluate the antioxidant capacity of animals. Among these, GSH-Px reduces toxic hydrogen peroxide and organic peroxides to relatively harmless water and corresponding alcohols, playing a role in clearing peroxides within cells (Czyżowska et al., 2023; Sharma et al., 2021). CAT, on the other hand, is another antioxidant enzyme responsible for breaking down hydrogen peroxide (a type of oxygen free radical) into water and oxygen, thus protecting cells from oxidative stress damage, especially in tissues with active aerobic metabolism such as the liver and lungs (Kang et al., 2013). In this study, there was an increasing trend in GSH-Px and CAT after adding mulberry leaves to the diet, indicating that mulberry leaves have a positive effect on improving the immune status of sheep. This finding is consistent with previous research results (Jin et al., 2022; Li et al., 2022; Shi et al., 2022). This effect may be attributed to the various bioactive components abundant in mulberry leaves, similar to the significant antioxidant capacity reported previously for polysaccharides and flavonoids in mulberry leaves (Kim et al., 2020; Liao et al., 2017). Our current research does not offer a comprehensive analysis of antioxidant indicators. While some indicators show an improving trend, this does not fully substantiate the antioxidant effects of ML. It merely suggests that ML does not negatively impact the antioxidant system of Hu sheep. The specific effects will be explored in greater detail in future studies.
During the growth and development of animals, the concentrations of AKP and GPT reflect the growth and remodeling of bone and muscle tissues (Eriksson et al., 2016; Sobhani et al., 2021; Wang et al., 2017). In bone tissue, AKP is primarily secreted by osteoblasts and plays a critical role in promoting the deposition of calcium and phosphate, thereby facilitating bone mineralization and formation. AKP catalyzes the hydrolysis of phosphate esters, converting organic phosphate esters into inorganic phosphate and alcohols or phenols, a process essential for bone mineralization (Ansari et al., 2022). GPT catalyzes the transamination reaction between glutamate and pyruvate, converting glutamate to α-ketoglutarate and pyruvate to alanine. This process is essential for amino acid metabolism and nitrogen transport (Wei et al., 2022). In this study, the addition of mulberry leaves to the animal diet resulted in increased concentrations of AKP and GPT, suggesting that mulberry leaves may influence certain blood hormones to regulate the host’s development. However, the specific mechanisms underlying this effect warrant further investigation. The concentration of TP also reflects the metabolism within the animal’s body. A slight decrease in TP levels may indicate metabolic adjustments within the animal’s body (Mapfumo and Muchenje, 2015; Rogaly et al., 1982). In this study, the observed decrease in TP levels may be associated with the liver and kidney functions of Hu sheep. However, it remains unclear whether ML have a beneficial or detrimental effect on TP regulation. Some studies suggest that a moderate reduction in TP may be linked to immune system modulation, potentially lowering hyperactive immune responses and reducing inflammation-related damage (Azzini et al., 2022; Fuellen et al., 2023). Although there are indirect reports indicating that supplementing Hu sheep diets with ML may enhance protein utilization efficiency in intensive farming, the specific mechanisms underlying this effect remain unexplored and require further investigation (Ariyarathne et al., 2021; Laroche et al., 2022). This suggests that the addition of mulberry leaves to the diet has the potential to influence the host’s physiological processes. However, further investigation is needed to determine whether all these effects are indeed beneficial (Cui W. et al., 2023).
In this study, we focused on the influence of mulberry leaves on the composition of the intestinal microbiota. At the phylum level, there was a significant increase in the abundance of Campylobacterota, which may be due to the relative decrease in the abundance of other phyla. At the genus level, Campylobacterin the intestine may play a role in food degradation and digestion, potentially secreting enzymes that break down complex food components and facilitate nutrient absorption in the host (Burnham and Hendrixson, 2018). However, most studies identify Campylobacter as a harmful pathogen responsible for animal diseases. The observed increase in abundance in this study might be linked to the host’s nutrient metabolism. Campylobacter possesses unique metabolic pathways, primarily utilizing amino acids and organic acids as carbon and energy sources, instead of relying on carbohydrate fermentation. This adaptation enables their survival in low-oxygen environments, such as the gut and foodborne microbial niches (Stahl et al., 2012). Moreover, there are few reports regarding Mailhella, but it may contribute to maintaining the diversity and stability of the intestinal microbiota, interacting with other microbial communities to collectively maintain normal intestinal function. The significant increase in the abundance of Campylobacterota, Campylobacter, and Mailhella in this study may be related to changes in certain indicators in the blood, as demonstrated in the correlation analysis. Meanwhile, the observed decrease in the abundance of Alloprevotella, Roseburia, and Prevotellaceae UCG-003 may be attributed to changes in the microbial network. Additionally, their changes are strongly correlated with immune metabolic capacity (Shen et al., 2022; Ye et al., 2022; Yu et al, 2023c). Finally, we observed a significant increase in the complexity of the microbial network in the hindgut following the addition of mulberry leaves to the diet. More complex microbial networks are known to possess greater resistance to adverse external factors (Yu et al., 2023b; Yu et al., 2024), further supporting the notion that mulberry leaves can modulate intestinal microbiota structure and enhance host health.
5 Conclusions
This study offers new insights into the use of ML in ruminant production. The inclusion of ML can elevate the levels of AKP and GPT in the blood, and showing potential to enhance the host’s antioxidant capacity and improve the hindgut microbiota structure of Hu sheep. These findings are significant for the development of alternative feed resources. ML is a promising feed ingredient that benefits both animal health and environmental sustainability. Further investigation into ML’s impact on rumen fermentation, metabolism, and microbial communities in ruminants will support its wider adoption in ruminant production.
Data availability statement
The original contributions presented in the study are publicly available. This data can be found here: NCBI BioProject, accession PRJNA1191173.
Ethics statement
The animal study was approved by Animal Care and Use Committee at Zhejiang Academy of Agricultural Sciences. The study was conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
LG: Conceptualization, Data curation, Formal analysis, Writing – original draft, Writing – review & editing. XS: Software, Supervision, Validation, Visualization, Writing – original draft. FC: Data curation, Methodology, Project administration, Resources, Software, Writing – original draft. SH: Investigation, Software, Supervision, Validation, Visualization, Writing – original draft. WQ: Conceptualization, Formal analysis, Funding acquisition, Investigation, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the China Agriculture Research System of MOF and MARA (CARS-18-SYZ05) and Zhejiang Province Industrial Technology Team Project (2022CSSFD08).
Conflict of interest
Author SH was employed by the company Central Plains Environmental Protection Co. Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ansari S., Ito K., Hofmann S. (2022). Alkaline phosphatase activity of serum affects osteogenic differentiation cultures. ACS Omega 7, 12724–12733. doi: 10.1021/acsomega.1c07225
Ariyarathne H., Correa-Luna M., Blair H. T., Garrick D. J., Lopez-Villalobos N. (2021). Identification of genomic regions associated with concentrations of milk fat, protein, urea and efficiency of crude protein utilization in grazing dairy cows. Genes 12, 456. doi: 10.3390/genes12030456
Azzini E., Peluso I., Intorre F., Barnaba L., Venneria E., Foddai M. S., et al. (2022). Total and plant protein consumption: the role of inflammation and risk of non-communicable disease. Int. J. Mol. Sci. 23, 8008. doi: 10.3390/ijms23148008
Batiha G. E., Al-Snafi A. E., Thuwaini M. M., Teibo J. O., Shaheen H. M., Akomolafe A. P., et al. (2023). Morus alba: a comprehensive phytochemical and pharmacological review. Naunyn. Schmiedebergs. Arch. Pharmacol. 396, 1399–1413. doi: 10.1007/s00210-023-02434-4
Burnham P. M., Hendrixson D. R. (2018). Campylobacter jejuni: collective components promoting a successful enteric lifestyle. Nat. Rev. Microbiol. 16, 551–565. doi: 10.1038/s41579-018-0037-9
Chan E. W., Lye P., Wong S. (2016). Phytochemistry, pharmacology, and clinical trials of morus alba. Chin. J. Nat. Med. 14, 17–30. doi: 10.3724/SP.J.1009.2016.00017
Chen C., Mohamad Razali U. H., Saikim F. H., Mahyudin A., Mohd Noor N. Q. I. (2021). Morus alba l. Plant: bioactive compounds and potential as a functional food ingredient. Foods 10, 689. doi: 10.3390/foods10030689
Chen D., Chen X., Tu Y., Wang B., Lou C., Ma T., et al. (2015). Effects of mulberry leaf flavonoid and resveratrol on methane emission and nutrient digestion in sheep. Anim. Nutr. 1, 362–367. doi: 10.1016/j.aninu.2015.12.008
Chen X., Zhang T., Wang X., Hamann M. T., Kang J., Yu D., et al. (2018). A chemical investigation of the leaves of morus alba l. Molecules 23, 1018. doi: 10.3390/molecules23051018
Cui W., Luo K., Xiao Q., Sun Z., Wang Y., Cui C., et al. (2023). Effect of mulberry leaf or mulberry leaf extract on glycemic traits: a systematic review and meta-analysis. Food Funct. 14, 1277–1289. doi: 10.1039/d2fo02645g
Cui X., Yang Y., Zhang M., Liu S., Wang H., Jiao F., et al. (2023). Transcriptomics and metabolomics analysis reveal the anti-oxidation and immune boosting effects of mulberry leaves in growing mutton sheep. Front. Immunol. 13. doi: 10.3389/fimmu.2022.1088850
Czyżowska A., Brown J., Xu H., Sataranatarajan K., Kinter M., Tyrell V. J., et al. (2023). Elevated phospholipid hydroperoxide glutathione peroxidase (gpx4) expression modulates oxylipin formation and inhibits age-related skeletal muscle atrophy and weakness. Redox Biol. 64, 102761. doi: 10.1016/j.redox.2023.102761
Ding Y., Jiang X., Yao X., Zhang H., Song Z., He X., et al. (2021). Effects of feeding fermented mulberry leaf powder on growth performance, slaughter performance, and meat quality in chicken broilers. Animals 11, 3294. doi: 10.3390/ani11113294
Eriksson M., Strandberg G., Lipcsey M., Larsson A. (2016). Evaluation of intraosseous sampling for measurements of alanine aminotransferase, alkaline phosphatase, aspartate aminotransferase, creatinine kinase, gamma-glutamyl transferase and lactate dehydrogenase. Scand. J. Clin. Lab. Invest. 76, 597–600. doi: 10.1080/00365513.2016.1230774
Fuellen G., Walter U., Henze L., Bohmert J., Palmer D., Lee S., et al. (2023). Protein biomarkers in blood reflect the interrelationships between stroke outcome, inflammation, coagulation, adhesion, senescence and cancer. Cell. Mol. Neurobiol. 43, 1413–1424. doi: 10.1007/s10571-022-01260-1
Geng B., Gao J., Cheng H., Guo G., Wang Z. (2024). Effects of dietary mulberry leaves on growth, production performance, gut microbiota, and immunological parameters in poultry and livestock: a systematic review and meta-analysis. Anim. Biosci. 37, 1065–1076. doi: 10.5713/ab.23.0449
Guha A., Sengupta D., Reddy A. R. (2010). Physiological optimality, allocation trade-offs and antioxidant protection linked to better leaf yield performance in drought exposed mulberry. J. Sci. Food. Agric. 90, 2649–2659. doi: 10.1002/jsfa.4135
Hashem N. M., Gonzalez-Bulnes A., Simal-Gandara J. (2020). Polyphenols in farm animals: source of reproductive gain or waste? Antioxidants 9, 1023. doi: 10.3390/antiox9101023
Huang Q., Liu X., Zhao G., Hu T., Wang Y. (2018). Potential and challenges of tannins as an alternative to in-feed antibiotics for farm animal production. Anim. Nutr. 4, 137–150. doi: 10.1016/j.aninu.2017.09.004
Iqbal S., Younas U., Sirajuddin, Chan K. W., Sarfraz R. A., Uddin M. K. (2012). Proximate composition and antioxidant potential of leaves from three varieties of mulberry (morus sp.): A comparative study. Int. J. Mol. Sci. 13, 6651–6664. doi: 10.3390/ijms13066651
Ji Z. K., Zhao Y., Yu S. S., Zhao H. (2018). The application progress of 3d printing technology in ophthalmology. Chung-Hua Yen K’o Tsa Chih. 54, 72–76. doi: 10.3760/cma.j.issn.0412-4081.2018.01.014
Jin Y., Tu J., Han X., Zhuo J., Liu G., Han Y., et al. (2022). Characteristics of mulberry leaf powder enriched with gamma-aminobutyric acid and its antioxidant capacity as a potential functional food ingredient. Front. Nutr. 9. doi: 10.3389/fnut.2022.900718
Kang M. Y., Kim H., Piao C., Lee K. H., Hyun J. W., Chang I., et al. (2013). The critical role of catalase in prooxidant and antioxidant function of p53. Cell Death Differ. 20, 117–129. doi: 10.1038/cdd.2012.102
Kim M., Nam D., Ju W., Choe J., Choi A. (2020). Response surface methodology for optimization of process parameters and antioxidant properties of mulberry (morus alba l.) Leaves by extrusion. Molecules 25, 5231. doi: 10.3390/molecules25225231
Laroche J. P., Gervais R., Lapierre H., Ouellet D. R., Tremblay G. F., Halde C., et al. (2022). Milk production and efficiency of utilization of nitrogen, metabolizable protein, and amino acids are affected by protein and energy supplies in dairy cows fed alfalfa-based diets. J. Dairy Sci. 105, 329–346. doi: 10.3168/jds.2021-20923
Leterme P., Londo O A. M., Estrada F., Souffrant W. B., Buldgen A. (2005). Chemical composition, nutritive value and voluntary intake of tropical tree foliage and cocoyam in pigs. J. Sci. Food Agric. 85, 1725–1732. doi: 10.1002/jsfa.2177
Li R., Zhu Q., Wang X., Wang H. (2022). Mulberry leaf polyphenols alleviated high-fat diet-induced obesity in mice. Front. Nutr. 9. doi: 10.3389/fnut.2022.979058
Liao B., Zhu D., Thakur K., Li L., Zhang J., Wei Z. (2017). Thermal and antioxidant properties of polysaccharides sequentially extracted from mulberry leaves (morus alba l.). Mol. (Basel Switzerland) 22, 2271. doi: 10.3390/molecules22122271
Liu J. X., Yao J., Yan B., Yu J. Q., Shi Z. Q. (2001). Effects of mulberry leaves to replace rapeseed meal on performance of sheep feeding on ammoniated rice straw diet. Small Ruminant Res. 39, 131–136. doi: 10.1016/S0921-4488(00)00180-2
Lv Q., Lin J., Wu X., Pu H., Guan Y., Xiao P., et al. (2022). Novel active compounds and the anti-diabetic mechanism of mulberry leaves. Front. Pharmacol. 13. doi: 10.3389/fphar.2022.986931
Mapfumo L., Muchenje V. (2015). Comparative changes in monthly blood urea nitrogen, total protein concentrations, and body condition scores of nguni cows and heifers raised on sweetveld. S. Afr. J. Anim. Sci. 45, 96–103. doi: 10.4314/SAJAS.V45I1.12
Maslovarić M. D., Vukmirović Đ., Pezo L., Čolović R., Jovanović R., Spasevski N., et al. (2017). Influence of apple pomace inclusion on the process of animal feed pelleting. Food Additives Contaminants: Part A. 34, 1353–1363. doi: 10.1080/19440049.2017.1303851
Mengistu G., Assefa G., Tilahun S. (2020). Noug seed (guizotia abyssinica) cake substituted with dried mulberry (morus indica) andvernonia amygdalina mixed leaves’ meal on growth performances of bonga sheep at teppi, Ethiopia. J. Nutr. Metab. 2020, 1–10. doi: 10.1155/2020/9308761
Rogaly E., Clague M. B., Carmichael M. J., Wright P. D., Johnston I. D. A. (1982). Comparison of body protein metabolism during total parenteral nutrition using glucose or glucose and fat as the energy source. Clin. Nutr. 1, 81–90. doi: 10.1016/0261-5614(82)90008-5
Sahoo A., Sarkar S., Lal B., Kumawat P., Sharma S., De K. (2021). Utilization of fruit and vegetable waste as an alternative feed resource for sustainable and eco-friendly sheep farming. Waste Manage. 128, 232–242. doi: 10.1016/j.wasman.2021.04.050
Sharma G., Shin E., Sharma N., Nah S., Mai H. N., Nguyen B. T., et al. (2021). Glutathione peroxidase-1 and neuromodulation: novel potentials of an old enzyme. Food. Chem. Toxicol. 148, 111945. doi: 10.1016/j.fct.2020.111945
Shen Z., Luo W., Tan B., Nie K., Deng M., Wu S., et al. (2022). Roseburia intestinalis stimulates tlr5-dependent intestinal immunity against crohn’s disease. Ebiomedicine 85, 104285. doi: 10.1016/j.ebiom.2022.104285
Shi Y., Zhong L., Fan Y., Zhang J., Zhong H., Liu X., et al. (2022). The protective effect of mulberry leaf flavonoids on high-carbohydrate-induced liver oxidative stress, inflammatory response and intestinal microbiota disturbance in monopterus albus. Antioxidants 11, 976. doi: 10.3390/antiox11050976
Sobhani S., Aryan R., AkbariRad M., Ebrahimi M. E., Alinezhad-Namaghi M., Sobhani S. R., et al. (2021). The association between anthropometry indices and serum concentrations of gamma-glutamyl transferase, alkaline phosphatase, alanine aminotransferase, and aspartate aminotransferase. BioMed. Res. Int. 2021, 2365399. doi: 10.1155/2021/2365399
Stahl M., Butcher J., Stintzi A. (2012). Nutrient acquisition and metabolism by campylobacter jejuni. Front. Cell. Infect. Microbiol. 2. doi: 10.3389/fcimb.2012.00005
Tan N. D., Wanapat M., Uriyapongson S., Cherdthong A., Pilajun R. (2012). Enhancing mulberry leaf meal with urea by pelleting to improve rumen fermentation in cattle. Asian Australas. J. Anim. Sci. 25, 452–461. doi: 10.5713/ajas.2011.11270
Trabi E. B., Yuan X., Li J., Dong Z., Shah A. A., Shao T. (2017). Effect of glucose and lactic acid bacteria on the fermentation quality, chemical compositions effect of glucose and lactic acid bacteria on the fermentation quality, chemical compositions and in vitro digestibility of mulberry (morus alba) leaf silage. Pak. J. Zool. 49, 2271–2277. doi: 10.17582/journal.pjz/2017.49.6.2271.2277
Wang F., Wang X., Wang K., Han L., Wang J., Xue C. (2017). Phosphorylated peptides from antarctic krill (euphausia superba) improve fracture healing in mice with ovariectomy induced osteoporosis. J. Food Biochem. 41, e12408. doi: 10.1111/jfbc.12408
Wang L., Gao H., Sun C., Huang L. (2022). Protective application of morus and its extracts in animal production. Animals 12, 3541. doi: 10.3390/ani12243541
Wei P., Bott A. J., Cluntun A. A., Morgan J. T., Cunningham C. N., Schell J. C., et al. (2022). Mitochondrial pyruvate supports lymphoma proliferation by fueling a glutamate pyruvate transaminase 2-dependent glutaminolysis pathway. Sci. Adv. 8, eabq117. doi: 10.1126/sciadv.abq0117
Ye M., Hou M., Peng Q., Jia S., Peng B., Yin F., et al. (2022). The microbiota and cytokines correlation between the jejunum and colon in altay sheep. Animals 12, 1564. doi: 10.3390/ani12121564
Yu S., Li L., Zhao H., Liu M., Jiang L., Zhao Y. (2023a). Citrus flavonoid extracts alter the profiling of rumen antibiotic resistance genes and virulence factors of dairy cows. Front. Microbiol. 14. doi: 10.3389/fmicb.2023.1201262
Yu S., Li L., Zhao H., Tu Y., Liu M., Jiang L., et al. (2023b). Characterization of the dynamic changes of ruminal microbiota colonizing citrus pomace waste during rumen incubation for volatile fatty acid production. Microbiol. Spectr. 11, e351722. doi: 10.1128/spectrum.03517-22
Yu S., Li L., Zhao H., Zhang S., Tu Y., Liu M., et al. (2023c). Dietary citrus flavonoid extract improves lactational performance through modulating rumen microbiome and metabolites in dairy cows. Food Funct. 14, 94–111. doi: 10.1039/d2fo02751h
Yu S., Zhao Y., Li L., Zhao H., Liu M., Jiang L. (2024). Flavonoids from citrus peel display potential synergistic effects on inhibiting rumen methanogenesis and ammoniagenesis: a microbiome perspective. Environ. Sci. pollut. Res. 31, 21208–21223. doi: 10.1007/s11356-024-32509-5
Zhao Y., Yu S., Li L., Zhao H., Li Y., Jiang L., et al. (2023a). Feeding citrus flavonoid extracts decreases bacterial endotoxin and systemic inflammation and improves immunometabolic status by modulating hindgut microbiome and metabolome in lactating dairy cows. Anim. Nutr. 13, 386–400. doi: 10.1016/j.aninu.2023.03.007
Zhao Y., Yu S., Zhang S., Li Y., Tu Y., Liu M., et al. (2022a). Microbiome-metabolomics insights into the milk of lactating dairy cows to reveal the health-promoting effects of dietary citrus peel extracts on the mammary metabolism. Foods 11, 4119. doi: 10.3390/foods11244119
Zhao Y., Yu S., Zhao H., Li L., Li Y., Liu M., et al. (2023b). Integrated multi-omics analysis reveals the positive leverage of citrus flavonoids on hindgut microbiota and host homeostasis by modulating sphingolipid metabolism in mid-lactation dairy cows consuming a high-starch diet. Microbiome 11, 236. doi: 10.1186/s40168-023-01661-4
Keywords: Hu sheep, mulberry leaf powder, blood metabolism, fecal microbiota, healthy
Citation: Guo L, Shi X, Cao F, Hu S and Qian W (2025) Effects of dietary addition of mulberry leaf powder on blood metabolites and fecal microbiota composition in Hu sheep. Front. Anim. Sci. 5:1469850. doi: 10.3389/fanim.2024.1469850
Received: 24 July 2024; Accepted: 18 November 2024;
Published: 03 January 2025.
Edited by:
David L. Harmon, University of Kentucky, United StatesReviewed by:
Assar Ali Shah, Jiangsu University, ChinaRavikanth Reddy Poonooru, University of Missouri, United States
Copyright © 2025 Guo, Shi, Cao, Hu and Qian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenchun Qian, Y2JiMjA2ODAwM0BzaW5hLmNvbQ==
†These authors have contributed equally to this work
 Liangyong Guo
Liangyong Guo Xingyun Shi1†
Xingyun Shi1†