- 1Department of Animal and Food Sciences, Texas Tech University, Lubbock, TX, United States
- 2Division of Animal Sciences, University of Missouri, Columbia, MO, United States
- 3Adisseo USA, Inc., Alpharetta, GA, United States
The study aimed to evaluate the impact of carbohydrase supplementation, soluble fiber from sugar beet pulp (SBP; 20%), and insoluble fiber from corn dried distillers grains (DDGS; 20%) on water balance, serum electrolytes, gut motility, and fecal physicochemical properties in gestating sows. Thirty-six sows, with an initial body weight of 186 ± 4.6 kg, balanced by parity, were assigned randomly to a 2×2 factorial arrangement of treatments from day 28 to 109 of gestation. The factors were fiber type (insoluble (IF; 355 g/d) or soluble (SF; 98 g/d)) and enzyme supplementation (Rovabio Advance P10). The feeding level was 2.1 kg per day. Two 8-day metabolism periods occurred during mid- (days 50-59) and late-gestation (days 99-108). Fecal samples for physicochemical property analysis and serum samples were taken on day 1 of each period. Water balance was measured from days 4-7, with a water allowance set at 80 mL/kg of body weight per day. Data were analyzed using a linear mixed model, with parity as a random effect and fiber, enzyme, period, and their interactions as fixed effects. Daily water allowance was used as a covariate when necessary. Urine output increased by 22.3% in sows fed IF compared to SF, and by 30.5% from mid- to late-gestation (Fiber, Period P<0.05). Fecal moisture was 21.8% higher in sows fed SF and increased by 12.3% from mid- to late-gestation (Fiber, Period P<0.05). The SF treatment increased fecal water holding capacity (P<0.001) and fecal water binding capacity by 76.6% (P=0.044). Regardless of diet, fecal water binding capacity increased in late gestation (Period P=0.035). Urine output increased by 30.5% in late gestation (Period P=0.028) and fecal moisture output increased by 12.3% (Period P=0.015). Serum sodium and chloride concentrations were increased in late gestation (P<0.05). Plasma cholecystokinin tended to be 28% greater in sows fed SF (P=0.070), and motilin levels decreased among all groups from mid- to late-gestation (Fiber×Enzyme×Period P=0.006). Circulating 5-Hydroxytryptamine decreased in late gestating sows fed carbohydrases (Period×Enzyme P=0.002), as well as sows fed SF (Fiber P=0.004). These findings suggest a redistribution of water in the gastrointestinal tract of late gestational sows fed SF, altering fecal hydration and gut motility.
1 Introduction
Sows are commonly limit-fed in gestation to aid in weight management. Consequently, fiber sources are often included in gestating sow diets to help alleviate potential hunger, increase satiety, and enhance welfare. This use of fiber is attributed to the impact it can have on gut fill, satiety, and body condition management (Agyekum and Nyachoti, 2017). Feed ingredients with increased concentrations of soluble fiber exhibit greater physicochemical properties that alter digesta milieu transit and nutrient absorption (Slama et al., 2019). In human nutrition, the physicochemical properties of the non-digested polysaccharide matrix establish some of the therapeutic functions of dietary fiber such as water holding capacity, water binding capacity, fecal bulk, and buffering (Oakenfull, 2001). These properties are established through the interaction of the carbohydrate structure, ions, and water. Through this process, water acts as a carrier moving dietary fiber constituents within the gastrointestinal tract. Water plays a pivotal role in regulating homeostasis, cellular transport, absorption of nutrients, and motility of the gastrointestinal tract. Polysaccharides, such as dietary fiber contain multiple hydroxyl groups and thus have a strong affinity to water molecules (Guo et al., 2017). Thus, fiber solubility is a chemical property related to interaction with water, and feeding diets high in soluble fiber may alter water balance within an animal.
Fiber’s physicochemical properties may allow it to positively impact gut function, hydration, and overall sow welfare. In late gestation, these properties become essential as sows experience increased gastrointestinal distention, reduced rate of passage, and potentially clinical constipation. Soluble fibers enhance water retention and fecal consistency, while enzymatic supplementation may further modify these effects by altering fiber’s digestibility. Soluble fiber supplementation in sows has been shown to alter transit time and modulate gut motility hormone such as endothelin-1 (ET-1), serotonin (5-HT), cholecystokinin (CCK), peptide tyrosine tyrosine (PYY), and motilin (MTL; Tigchelaar et al., 2016; Ge et al., 2016). Additionally, carbohydrases can increase the solubility of dietary fiber within the gut by creating low molecular weight polysaccharides as a product of its hydrolyzation (Petry and Patience, 2020). A common source of soluble fiber is sugar beet pulp. It is a highly fermentable comprised predominately of pectins (Serena and Knudsen, 2007). The rapid fermentability of sugar beet pulp allows for it to be metabolized into short chain fatty acids in the hindgut, yielding anti-inflammatory effects on the body (Andoh et al., 2003). Due to the high solubility of sugar beet pulp, water holding capacity is also increased, allowing for greater water retention (Yan et al., 2017), and increased fecal water content in late gestation (Shang et al., 2021). However, there is a dearth of knowledge on the partitioning of water and the role that soluble fiber plays in gut motility of sows in late gestation. This study aims to address gaps in understanding how these interactions influence gut motility and water balance in gestating sows at two points in gestation. Therefore, the experimental objective was to investigate the effects of insoluble and soluble fiber sources with or without carbohydrase supplementation on water balance, fecal physicochemical properties, electrolyte balance, and markers of gut motility in gestating sows. It was hypothesized that both insoluble and soluble fiber, along with carbohydrase supplementation, will influence water balance, fecal physicochemical properties, electrolyte balance, and markers of gut motility in gestating sows.
2 Materials and methods
All experimental procedures followed ethical and humane use of animals for research as described by the Guide for the Care and Use of Agricultural Animals in Research and Teaching (FASS, 2010) and were approved by the Texas Tech University Institutional Animal Care and Use Committee (protocol# T-21065). Animal, experimental, diets, and feeding methods are previously reported in Crome et al., 2023. A brief explanation of experimental design is provided to orient readers, and all analytical methods of data reported herein are provided.
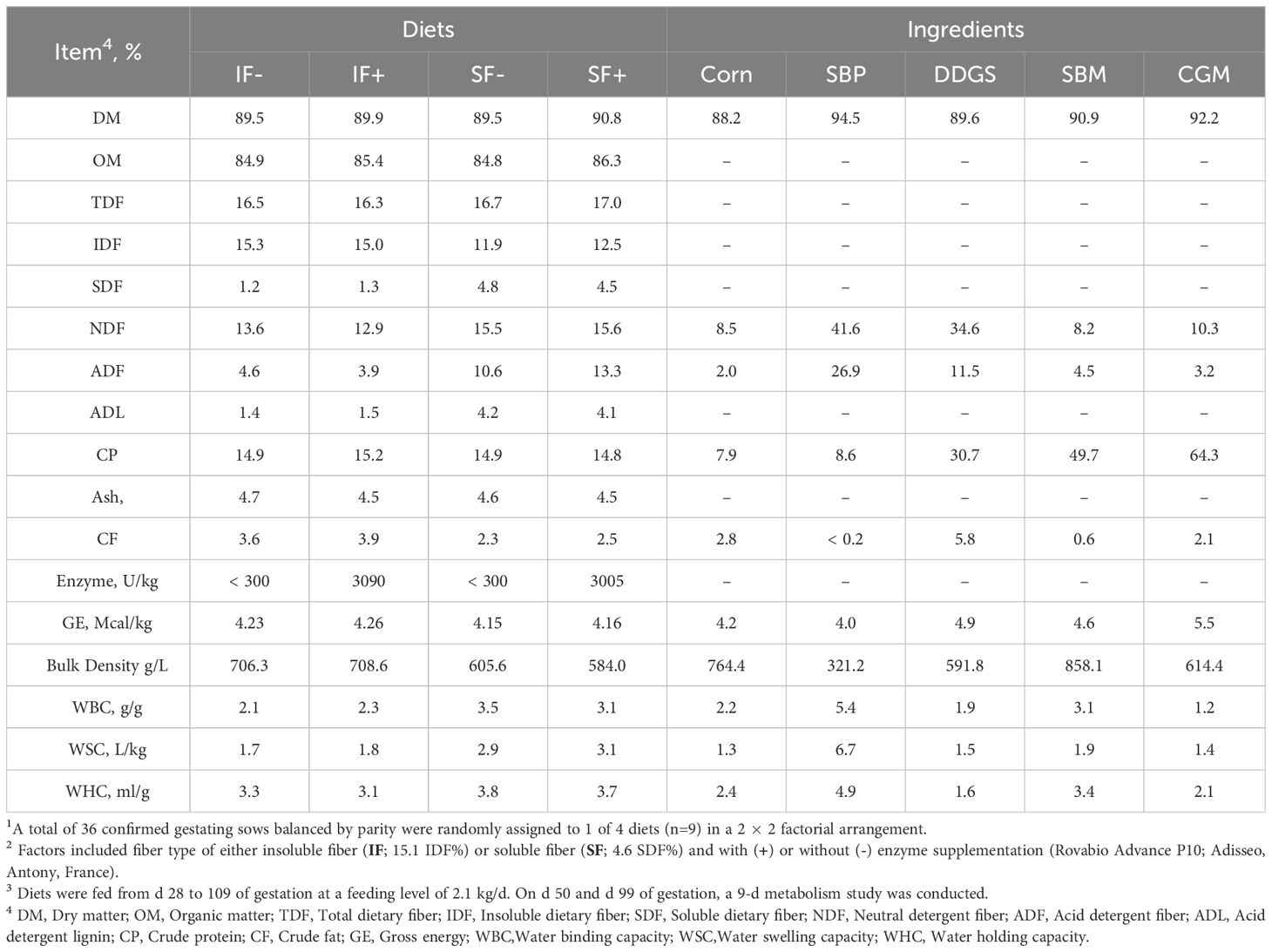
Briefly, 36 gestation stall-housed multiparous sows (Parity 3 0.73, Camborough; PIC Inc., Hendersonville, TN) weighing 186 ± 4.6 kg were used in three replicates of an 80-day (d) experiment. Sows were randomly assigned to 1 of 4 dietary treatments in a 2×2 factorial arrangement at d 28 of gestation. Factors included fiber type of insoluble (IF; 15.1 IDF%) or soluble fiber (SF; 4.6 SDF%) and with (+) or without (-) enzyme supplementation (0.05%, Rovabio Advance P10; Adisseo, Antony, France). Experimental diets were fed from d 28 to 109 of gestation at a feeding level of 2.1 kg/d as-fed to achieve 11 g of SID-Lys and 4.5 Mcal of NE per d, and thus were congruent with genetic line requirements and NRC (2012; Table 1). Diets were formulated with a fixed level of insoluble and soluble fiber allowing the energy level to float. On d 50 (mid-gestation) and 99 of gestation (late-gestation), sows underwent a 7-d metabolism study. For each metabolism period, d 1-3 served as an environmental adaptation period, d 4-7 total urine, feces, and water usage were collected (96-hours). Metabolism crates were equipped with a 9.4-liter individual nipple waterer above a sealed feeder and pan to account for water wastage and intake.
2.1 Sample and data collection
On d 1 of the adaptation period, sow weight was determined for calculating water allowance. Blood plasma was collected via jugular venipuncture with a 16 gauge 4-inch needle into a lithium heparinized syringe (Sarstedt, Nūmbrecht, Germany). Plasma was stored on ice and centrifuged at 1,500 g for 10 minutes at 4°C and aliquots stored at -80° C for subsequent analysis. Fresh fecal samples were collected for same day analysis of water binding capacity (WBC) and preparation of water holding capacity (WHC). Water intake was measured during both collection periods from d 4-8. An individual gravity fed waterer (Trojan Specialty Products; Dodge City, KS) with a nipple drinker was used for water intake measurements. The waterer was placed directly over a sealed feeder with a collection pan to account for and to measure waste. Water measurements were taken at the time of feeding (0600 h) and at the 12 h urine collection (1800 h). The volume of the waterer and the water volume added were recorded. Urine was collected every 12 h in an acid-washed container with 60 mL 6 N-HCl to minimize nitrogen volatilization. The urine containers were weighed, filtered through glass wool and cheesecloth, subsampled, and stored in acid-washed containers at -20°C for future analysis. Specific gravity of urine was determined by measuring 100 mL of urine in a graduated cylinder and recording the weight.
2.2 Fecal physicochemical property analysis
The WHC of feed, ingredients, and feces was measured using a modified procedure of Ruckman et al. (2020) and Giger-Reverdin (2000). A convection oven was used to dry 2 g of feces to a constant weight at 60°C in duplicate. Mortar and pestle were used to break apart dried fecal samples and 0.5 g of the subsampled fecal was soaked in 50 mL of deionized water for 24 h. After 24 h, the sample was filtered using a 60 ml fritted funnel with a porosity of 60 for 1 h, and the remaining wet sample was weighed. The WHC was calculated using the following equation (WHC = g of retained water/g of dry sample) and was expressed as mL of water per g of DM. The WBC of feces was measured in duplicate using a modified protocol from Serena and Knudsen (2007) as described by Ruckman et al. (2020). Fresh fecal (2 g) was centrifuged at 10,000 × g for 40 min at 4°C to separate the liquid and solid components. The liquid fraction was removed by suction immediately after centrifugation and again 12 h later. The solid fraction was weighed, and the WBC was calculated using the wet weight and dry weight of the fecal [WBC= (wet weight -dry weight) ÷ dry weight]. Fecal moisture excretion was estimated using total fecal dry matter output based on total tract dry matter digestibility and daily dry matter intake as reported in Crome et al., 2023. Fecal dry matter output was multiplied by the moisture: fecal dry matter ratio with the assumption that 1 g of moisture = 1 ml. Absolute fecal moisture concentration was determined by AOAC method 930.15.
2.3 Bulking and water swelling capacity analysis
The physicochemical characteristics of diets and ingredients including bulk density, swelling, WBC, and WHC were measured. Bulk density was determined by pouring samples into a 250 mL beaker and leveling off the top before weighing the sample as described by Cromwell et al. (2000). Swelling was measured using a procedure modified after Serena and Knudsen (2007). Briefly, 0.3 g of sample was weighed into a 15 mL conical centrifuge tube and dissolved in 10 mL of 0.9% NaCl with 0.02% NaN3 and placed in a shaking water bath at 39°C for 20 h. Samples were allowed to settle for 1 hour, and the swelling capacity was measured by determining volume the fiber occupied relative to water displacement.
2.4 Electrolyte and gut motility marker analysis
Lithium heparin plasma was analyzed for electrolyte concentration by Clinical Pathology at the Veterinary Medical Diagnostic Laboratory at University of Missouri in Columbia, MO. Analysis included anion gap, bicarbonate, calcium (Ca), chloride (Cl), potassium (K), magnesium (Mg), and sodium (Na). Endothelin-1 (ET-1) and serotonin (5-HT) were analyzed in duplicate using commercially available ELISA kits (Enzo Life Sciences; Farmingdale, NY), and plasma samples were diluted 1:10 and 1:20 with provided assay buffer, respectively. Cholecystokinin (CCK), peptide tyrosine tyrosine (PYY), and motilin (MTL) were also analyzed by ELISA (MyBioSource; San Diego, CA) in duplicate, and plasma was diluted 1:20 with appropriate assay buffer. Both positive and negative controls were included with each plate for each assay, and a coefficient of variation threshold of 5% was used.
2.5 Statistical analysis
Data were analyzed according to the following linear mixed model using the MIXED procedure of SAS 9.4 (SAS Inst., Cary, NC):
Whereas, is the observed value for a given sow within the ith level of fiber, jth level of enzyme of the kth period; is the general mean; is the fixed effect of the ith fiber (i= 1 to 2); is the fixed effect of the jth enzyme (j= No or Yes); is the fixed effect of the kth period (k= 1 to 2); is the interaction term of Fiber Enzyme; is the interaction term of Fiber Period; is the interaction term of Enzyme Period; is the interaction term of Fiber Enzyme Period; is the random effect of parity; and is the associated variance as described by the model for (l=1 through 36); assuming ), where I is the identity matrix.
The normality and homogeneity of the studentized residuals were verified and outliers were removed if residuals varied more than 3 standard deviations away from the mean residual. Water allowance was used as a covariate where appropriate as deemed by Bayesian Information Criterion (BIC) fit statistics. Least square means were separated using Fisher’s Least Significant Difference test, and treatment differences were considered significant if P ≤ 0.05 and trends if 0.05 > P ≤ 0.10.
3 Results
All sows completed the experiment, and no pharmaceutical interventions were administered during the experimental period. There were no observed interactions among the main effects and replicates for the dependent variables. Thus, replicate was implemented in the model as a random effect.
3.1 Water balance
As anticipated, water allowance in late gestation increased by 11.8% (Period P<0.001; Table 2). This is by design, as BW increased from mid to late gestation from growth, and subsequent adjustments were made in water allowance. Urine output increased in sows fed IF compared to SF by 22.3% and increased from mid to late gestation by 30.5%, respectively (Fiber, Period P<0.05). In contrast, estimated fecal moisture excretion increased in sows fed SF, in comparison to IF, by 21.8% and increased from mid to late gestation by 12.3%, respectively (Fiber, Period P<0.05). Urine specific gravity increased from mid- to late gestation (P=0.047). The ratio of water intake to dry matter consumption did not differ among main effects (P>0.10).
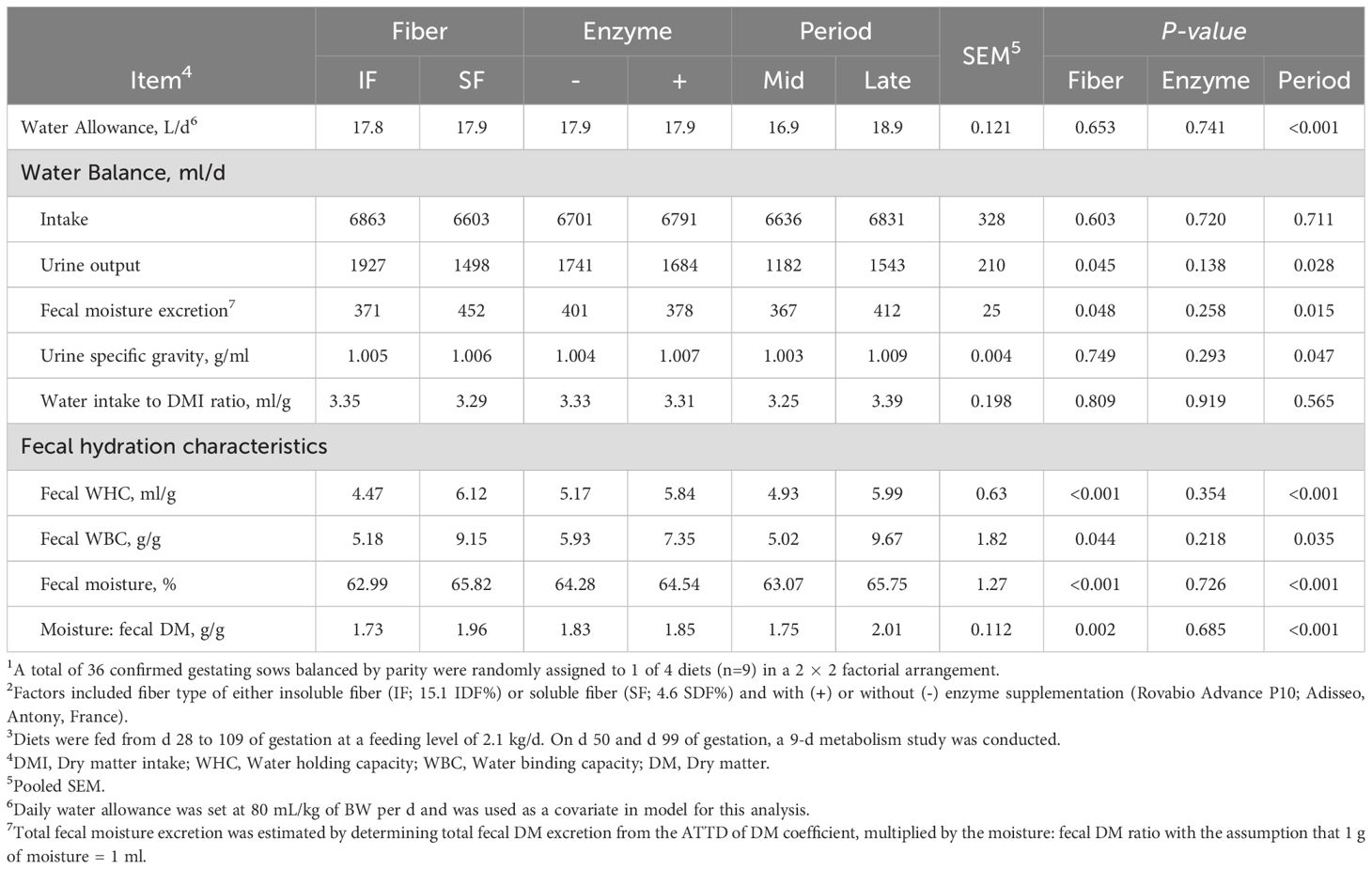
Table 2. The main effect of fiber type, enzyme supplementation, and collection period on water balance and fecal physicochemical properties of gestating sows1,2,3.
3.2 Fecal hydration characteristics
Fecal WBC increased in sows fed SF compared to sows fed IF by 76.6% and in late gestation compared to mid gestation by 92.6%, respectively (Fiber, Period P<0.05; Table 2). A similar effect of fiber was observed for fecal WHC (Fiber P<0.001). Fecal moisture in sows fed SF increased by 4.5% relative to sows fed IF and increased by 4.2% from mid to late gestation (Fiber, Period P<0.001). The ratio of fecal moisture to dry matter concentration was greater in sows fed SF and at late gestation (Fiber, Period P<0.05).
3.3 Serum electrolytes
Serum electrolytes were measured on d 50 and 99 of gestation. Sows fed SF had reduced serum P (Fiber P=0.023; Table 3). Carbohydrase supplementation increased serum Cl concentration (Enzyme P=0.041). Relative to d 50 of gestation, sows in late gestation have increased serum Cl (Period P=0.030) and tended to have increased serum Na (Period P=0.052). In contrast, at late gestation, serum Ca tended to decrease (Period P=0.063).
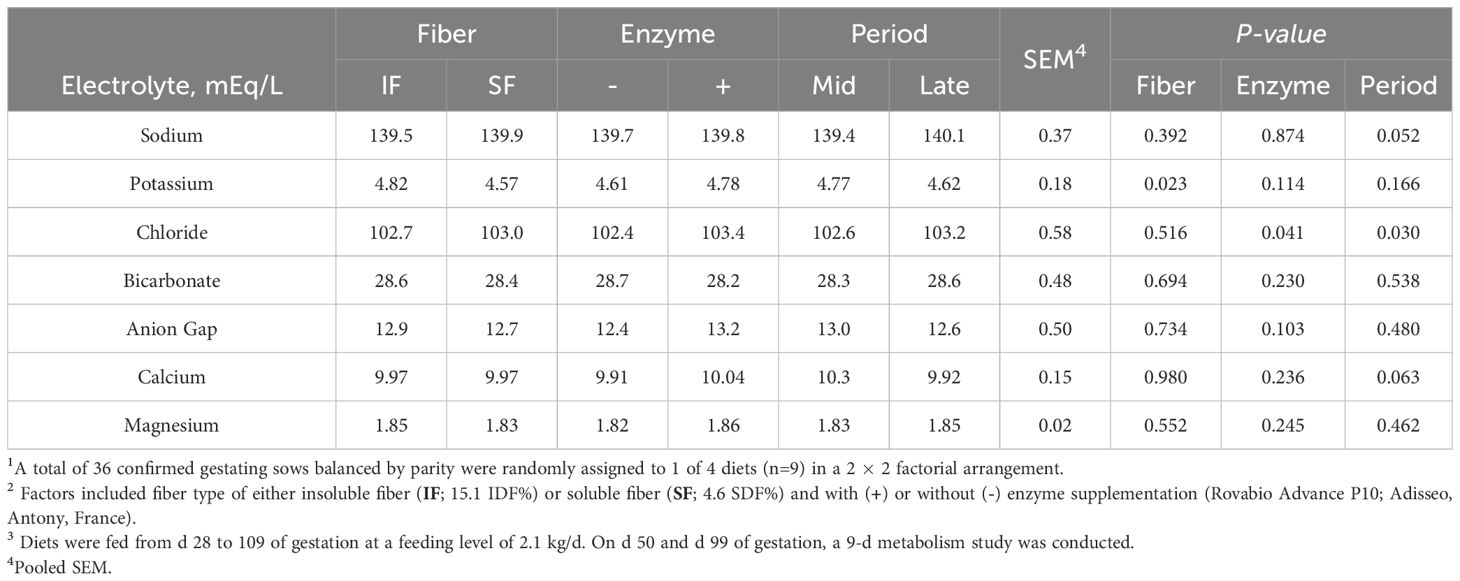
Table 3. The main effect of fiber type, enzyme supplementation, and collection period on serum electrolytes of gestating sows1,2,3.
3.4 Biomarkers of gut motility
Dietary fiber source and enzyme supplementation did not impact plasma PYY concentrations (P>0.10). However, on d 99 of gestation, PYY was 17.5% lower compared to mid-gestation (Period P=0.012; Figure 1A). There was a tendency for plasma CCK to be 28% greater in sows fed SF (P=0.070; Figure 1B). Endothelin-1 increased in sows fed IF in mid gestation compared to late gestation by 7.2%, respectively (Period × Fiber P=0.029; Figure 2). From mid to late gestation, MTL decreased by 11.1% in sows fed IF-, by 20.8% in sows fed IF+, and 17.7% in sows fed SF-, respectively (Fiber × Enzyme× Period P=0.006; Figure 3A). Sows in late gestation fed carbohydrases had decreased 5-HT (Period×Enzyme P=0.002; Figure 3B), and when fed SF (Fiber P=0.004).
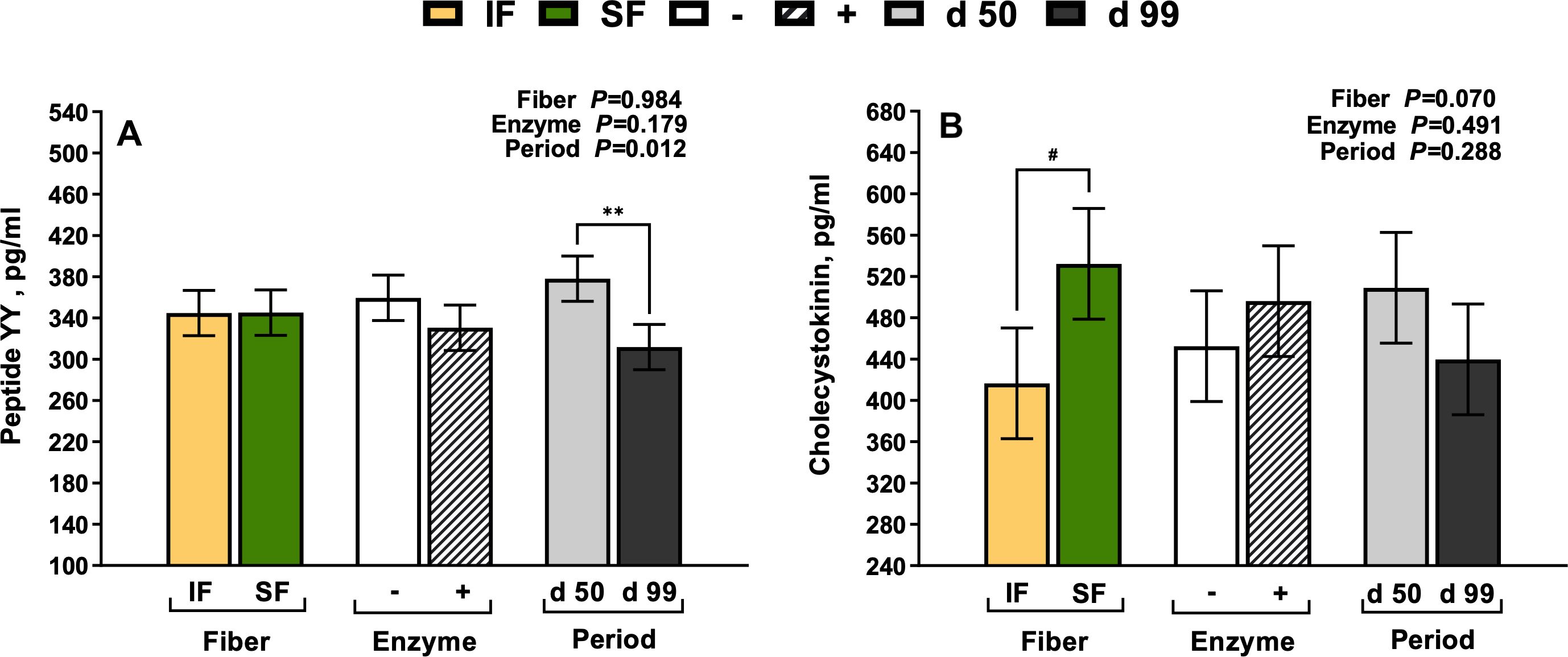
Figure 1. The main effects of fiber, enzyme, and period on peptide tyrosine tyrosine (A) and cholecystokinin (B) in gestating sows. **Main effects that differ as significant, P < 0.05; #Main effects that differ as a tendency, 0.05 > P < 0.100.
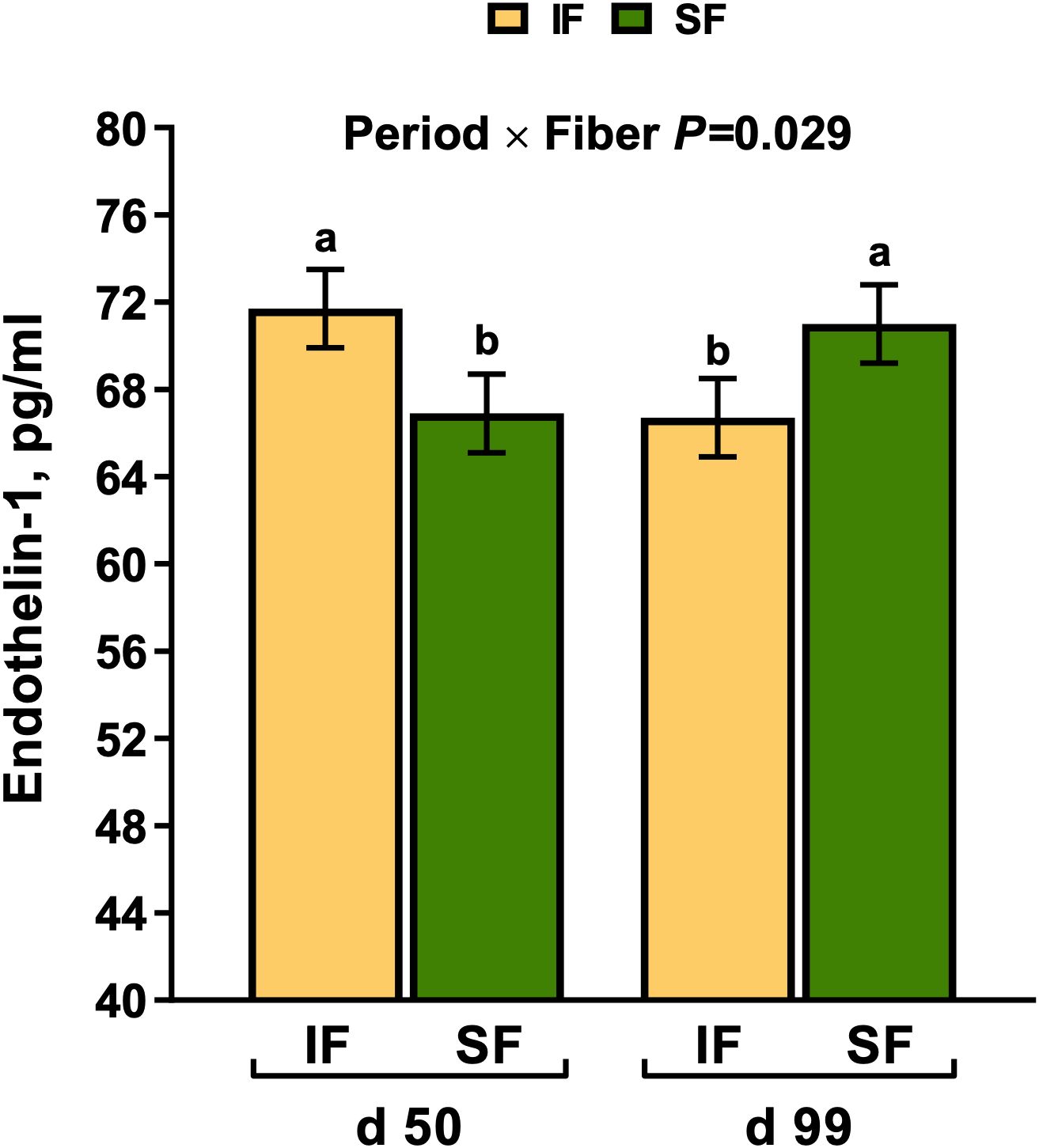
Figure 2. The influence of period fiber interaction on circulating plasma endothelin-1 in gestating sows. a-d Bars without a common superscript differ as significant and represent an interaction, P < 0.05.
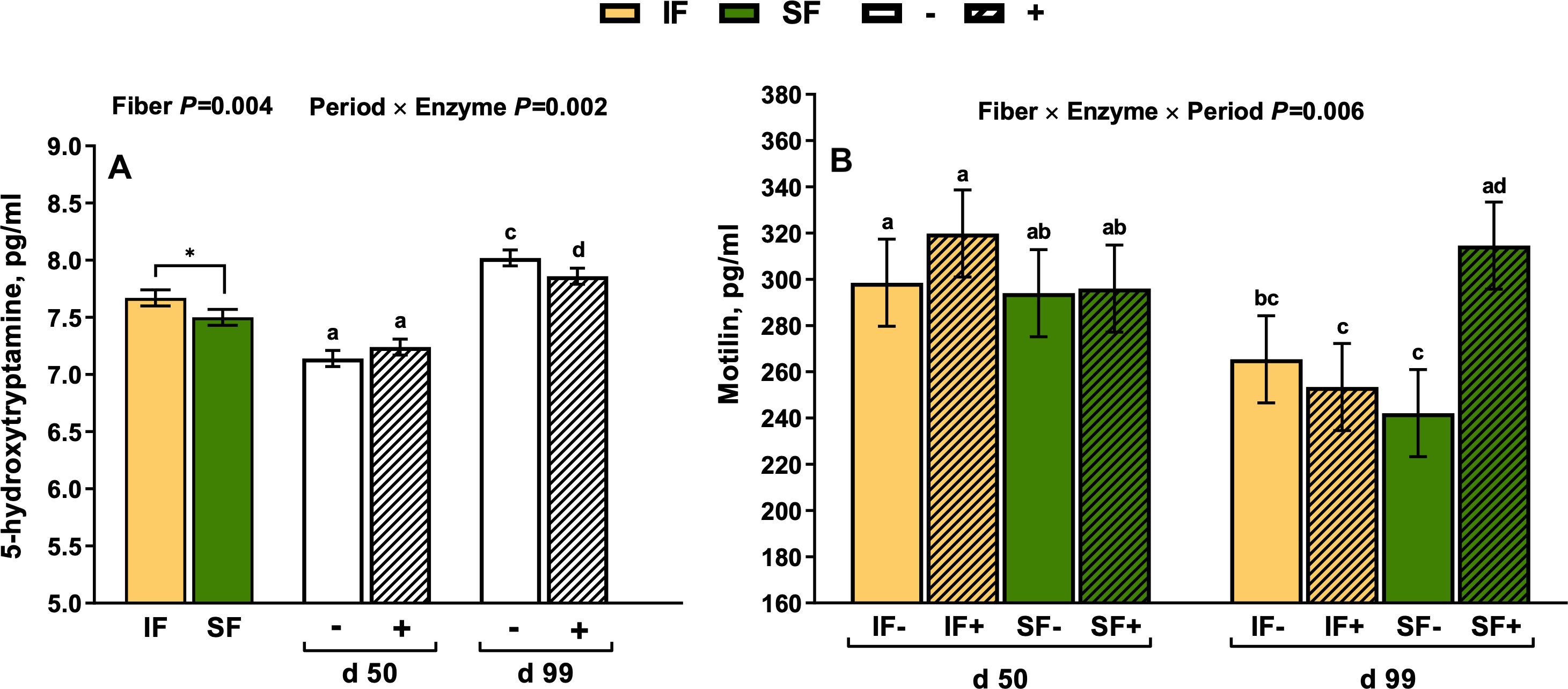
Figure 3. The influence of period × enzyme interaction on circulating plasma 5-hydroxytryptamine (A) and the fiber × enzyme × period interaction on circulating plasma motilin (B) in gestating sows. *Main effects that differ at a significance level of P < 0.01; a-d Bars without a common superscript differ as significant and represent an interaction, P < 0.05.
4 Discussion
It is well-established that dietary fiber possesses various physicochemical properties that play a crucial role in influencing gastrointestinal physiology and are associated with metabolic implications in humans (Guillon and Champ, 2000). Sow nutrition capitalizes on the bulk density, viscosity, hydration, and satiety properties of fiber to mitigate vices associated with feed restriction in gestation (Agyekum and Nyachoti, 2017). The type, concentration, and source of fiber will dictate which physicochemical properties are present in the gastrointestinal tract (Jha and Berrocoso, 2015). Classically, IDF and SDF have distinct differences in their physical and rheological properties. Sources of SDF, such as SBP utilized herein, are hallmarked by their hydration properties such as WBC, WHC, viscosity, and water swelling capacity (Oakenfull, 2001). In contrast, corn DDGS is predominately composed of IDF and has stool bulking and porosity-driven water retention physical characteristics (Guillon and Champ, 2000). This is distinguishable for the diets and ingredients utilized herein, as SBP and SF diets have increased SDF and WBC, WHC, and water swelling capacity as depicted in Table 4. These distinct physicochemical differences are largely driven by their interaction with water and can influence their efficacy in stimulating peristalsis and intestinal function (Cummings, 1984). In the context of sow production, the utilization of fiber proves relevant, as it can assist in mitigating constipation, facilitating a smoother passage of piglets during farrowing (Krogh et al., 2015; Oliviero et al., 2010). However, the influence of dietary fiber from DDGS and SBP on water balance, gut motility, and fecal physicochemical properties in sows is largely unknown.
Due to the interplay of fiber with water within the gastrointestinal tract, it was hypothesized that feeding fiber and supplementing carbohydrate degrading enzymes will alter water balance within the sow and influence fecal physicochemical properties. Indeed, urine output and was greater in sows fed IF, compared to SF. In contrast, fecal moisture excretion increased in sows fed SF and in late gestation. These observations demonstrate a diet influenced repartitioning of water in sows fed SF likely due to the hydration and cation-binding properties of SBP within the large intestine (Guillon and Champ, 2000). Navarro et al. (2018) presented that under in vitro conditions, corn DDGS and SBP have unique water hydration and binding properties driven by forming hydrogen bonds with water. In this study, SBP had 115% greater WBC and 132% greater WSC compared to DDGS. Thus, due to this increased hydrogen bonding and water sequestering, it could be less accessible for reabsorption in the large intestine. Likewise, it is plausible more water was excreted in feces due to physicochemical properties of fiber such as viscous gel formation and cation-binding by the pectin polysaccharides in SBP (Wenk, 2001). It is important to note, that alterations in water balance are often attributed to changes in water intake or incidences of polydipsia under experimental conditions. Falk (1961) introduced the observation that under intermittent feeding schedules, rats would drink large amounts of water at the conclusion of the meal. This schedule-induced drinking phenomenon was confirmed in the pig by Stephens et al. (1983). Since water intake did not differ in this study, and water allowance was controlled to limit water wastage and polydipsia behaviors, greater confidence can be placed that observed responses are driven by dietary treatment or the hormonal influence on renal function during the last third of gestation (Cheung and Lafayette, 2013).
The impact of the physicochemical properties of dietary fiber on fecal hydration characteristics aligns with the water balance data observed here in, as well as findings from Zhao et al. (2015) and Oh et al. (2022). Fiber physicochemical properties can be defined as the combined physical and chemical characteristics that determine their behavior and functionality in a media driven environment (Capon and Overend, 1961). These properties influence how carbohydrates interact with water, enzymes, and other molecules in a food matrix or biological system. As studied in Slama et al. (2019), fiber physicochemical properties are measured with in vitro analyses such as water buffering capacity (the ability to stabilize pH), water holding capacity (the ability to retain water), water swelling capacity (the increase in volume after absorbing water), and binding capacity indexes (the ability to bind with various solutes in a given media). In this study, those principles were applied to collected fecal material, as undigested fiber residues predominately compose feces. In sows fed SF, fecal WBC and WHC were increased, irrespective of gestation stage or enzyme supplementation. The high pectin content of SBP could partially explain this increase in water retention within feces. Pectic polysaccharides contain charged ions that prevent increased electrostatic repulsion between the polymers and other organic matter (Oakenfull, 2001). Furthermore, the unmethylated galacturonic acids comprising the pectin can form hydrogen bonds with water, decreasing water available for absorption within the gastrointestinal tract (Bertin et al., 1988). This observation aligns with other studies indicating that pectin-based DF has increased WBC (Johansen et al., 1996; Priester et al., 2020).
In contrast to the effect of diet, the difference in fecal moisture characteristics observed across gestation periods could potentially be elucidated by alterations in transit time resulting from the enlarged size of reproductive organs exerting pressure on the posterior gastrointestinal tract, as proposed by Bradley et al. (2007). Alternatively, shifts in whole-body osmotic regulation due to progesterone may also contribute to these effects. Likewise, the increase in urinary excretion in late gestation can be explained by alterations in the renin-angiotensin-aldosterone system resulting in increased blood volume, hyperglomerular filtration, and increased plasma osmolarity (Cheung and Lafayette, 2013). This also supports the increased serum Na, Cl, and Ca observed in late gestation which is congruent with what has been observed in late gestating rats (Atherton et al., 1982).
Increases in uterine mass throughout gestation and the physicochemical properties of DF can both influence gastrointestinal motility, digesta rate of passage through the intestine, and defecation frequency (Bradley et al., 2007; Gill et al., 2021). This is important as alterations in gastrointestinal motility can increase constipation incidences and colonic impactions in late gestation can act as a barrier to the birth canal, resulting in dystocia (Moturi et al., 2022). There was a tendency for plasma CCK to be 28% greater in sows fed SF. Cholecystokinin is a hormone that modulates postprandial gut motility and increases satiety (Wu et al., 2013). An increase in plasma CCK by feeding SBP is in alignment with human nutrition studies (Meier et al., 1993). It is thought the rheological properties of viscous gels formed by soluble dietary fiber in the duodenum delay substrate release and increase CCK release by enteroendocrine cells (Burton-Freeman et al., 2002). There was no effect of fiber or carbohydrases on PYY, another marker associated with satiety, but in late gestation, it decreased by 17.5%. The hormone PYY functions as an ‘ileal break’ inhibiting gastrointestinal motility of the colon by delaying gastric emptying (Van Citters and Lin, 1999), and this response is alignment with other sow studies (Huang et al., 2020). A plausible explanation for PYY reduction in late gestation could be the aforementioned influence of increased uterine mass and subsequently altered transit time. Interestingly, there was a period by fiber interaction for ET-1. Whereas sows fed IF in mid gestation had greater ET-1 than SF, and the inverse was observed in late gestation. While difficult to interpret, Sugiura et al. (1989) reported ET-1 is associated with mediation of endotoxin pressures from lipopolysaccharides being translocated from the gastrointestinal tract. In the same study, we observed a similar interactive response for serum lipopolysaccharide binding protein (Crome et al., 2023). Plausibly, the influence of fiber and gestation period on gastrointestinal integrity may partially explain this response, but further research is warranted.
There was a 3-way interaction across main effects for motilin. From mid to late gestation, motilin decreased by 11.1% in sows fed IF-, by 20.8% in sows fed IF+, and 17.7% in sows fed SF-, respectively. Motilin alleviates constipation and improves intestinal peristalsis by binding to G protein-coupled receptors on gastrointestinal enteric neurons and in the central nervous system (Kitazawa and Kaiya, 2021). Sows in late gestation fed carbohydrases also had decreased 5-HT, and when fed SF. 5-hydroxytryptamine, otherwise known as serotonin, is a marker of motility associated with alleviating constipation, and over 90% of 5-hydroxytryptamine secreted in the whole body is secreted in the gut (Kim and Camilleri, 2000). The exact cause of these interactions between gestation length and enzyme supplementation on motilin and 5-HT remains unclear. One plausible relationship could be modulation of the gut-brain axis by microbial metabolites due to the prebiotic effect of dietary fiber and oligosaccharides released by carbohydrases. Lu et al. (2021) observed that motilin and 5-HT release from enterochromaffin cells was modulated by Lactobacillus and Bifidobacterium in a constipated mouse model. These microbial communities are upregulated with carbohydrase supplementation and SBP in growing swine (Petry and Patience, 2020; Diao et al., 2020), but further research is warranted among sows to better delineate this potential mechanism between microbial fiber metabolism and gut motility.
5 Conclusions
In conclusion, this study demonstrates that the physicochemical properties of dietary fiber considerably impact water balance and fecal hydration characteristics in sows. The distinct hydration properties of soluble fiber from SBP increase water binding and swelling capacity in feces, leading to higher moisture excretion, particularly in late gestation. Additionally, the interplay between gestation periods and dietary treatments affects gastrointestinal motility and hormone regulation, influencing key markers like CCK, PYY, ET-1, motilin, and 5-HT. In late gestation, alterations in transit time and increased pressure from the reproductive organs may affect gastrointestinal physiology and regulate plasma osmolarity and water balance. However, the controlled conditions and specific fiber sources used in this study may limit its direct application to commercial production systems with greater variability or limitations in feed ingredients. Furthermore, the role of microbial interactions with fiber and carbohydrase supplementation, particularly in the gut-brain axis, remains unexplored and warrants further investigation. Despite these limitations, the findings offer strategies for optimizing sow diets to improve comfort, productivity, and sustainability in swine production. Validating these results in diverse commercial settings will further enhance their applicability.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by Texas Tech University Institutional Animal Care and Use Committee. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
TC: Data curation, Formal analysis, Methodology, Project administration, Validation, Writing – original draft, Writing – review & editing. KV: Data curation, Investigation, Methodology, Writing – review & editing. RS: Data curation, Investigation, Methodology, Writing – review & editing. MG: Conceptualization, Funding acquisition, Methodology, Writing – review & editing. AP: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The authors declare that this study received funding from Adisseo USA, Inc.
Acknowledgments
We extend our sincere gratitude to Edward Carrasco for his assistance with the sow trial.
Conflict of interest
The authors declare that this study received funding from Adisseo. They were involved in the conceptualization of the project and study design, and they provided the multicarbohydrase used in this trial. MG was employed by Adisseo and contributed to the study design.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Agyekum A. K., Nyachoti C. M. (2017). Nutritional and metabolic consequences of feeding high-fiber diets to swine: a review. Engineering 3, 716–725. doi: 10.1016/J.ENG.2017.03.010
Andoh A., Tsujikawa T., Fujiyama Y. (2003). Role of dietary fiber and short-chain fatty acids in the colon. Curr. Pharm. design 9, 347–358. doi: 10.2174/1381612033391973
Atherton J. C., Dark J. M., Garland H. O., Morgan M. R., Pidgeon J., Soni S. (1982). Changes in water and electrolyte balance, plasma volume and composition during pregnancy in the rat. J. Physiol. 330, 81–93. doi: 10.1113/jphysiol.1982.sp014330
Bertin C., Rouau X., Thibault J. F. (1988). Structure and properties of sugar beet fibres. J. Sci. Food Agric. 44, 15–29. doi: 10.1002/jsfa.2740440104
Bradley C. S., Kennedy C. M., Turcea A. M., Rao S. S., Nygaard I. E. (2007). Constipation in pregnancy: prevalence, symptoms, and risk factors. Obstetrics Gynecology 110, 1351–1357. doi: 10.1097/01.AOG.0000295723.94624.b1
Burton-Freeman B., Davis P. A., Schneeman B. O. (2002). Plasma cholecystokinin is associated with subjective measures of satiety in women. Am. J. Clin. Nutr. 76, 659–667. doi: 10.1093/ajcn/76.3.659
Capon B., Overend W. G. (1961). “Constitution and physicochemical properties of carbohydrates,” in Advances in Carbohydrate Chemistry, vol. 15. (Academic Press), 11–51. doi: 10.1016/s0096-5332(08)60184-8
Cheung K. L., Lafayette R. A. (2013). Renal physiology of pregnancy. Adv. chronic Kidney Dis. 20, 209–214. doi: 10.1053/j.ackd.2013.01.012
Crome T. A., Giesemann M. A., Miller H. E., Petry A. L. (2023). Influence of fiber type and carbohydrase supplementation on nutrient digestibility, energy and nitrogen balance, and physiology of sows at mid and late gestation. J. Anim. Sci. 101, skad390. doi: 10.1093/jas/skad390
Cromwell G. L., Cline T. R., Crenshaw J. D., Crenshaw T. D., Easter R. A., Ewan R. C., et al. (2000). Variability among sources and laboratories in analyses of wheat middlings. J. Anim. Sci. 78, 2652–2658. doi: 10.2527/2000.78102652x
Cummings J. H. (1984). Constipation, dietary fibre and the control of large bowel function. Postgraduate Med. J. 60, 811. doi: 10.1136/pgmj.60.709.811
Diao H., Jiao A., Yu B., He J., Zheng P., Yu J., et al. (2020). Beet pulp: an alternative to improve the gut health of growing pigs. Animals 10, 186. doi: 10.3390/ani10101860
Falk J. L. (1961). Production of polydipsia in normal rats by an intermittent food schedule. Science 133, 195–196. doi: 10.1126/science.133.3447.195
FASS (2010). Guide for the Care and Use of Agricultural Animals in Research and Teaching. 3rd ed. (Champaign, IL: Federation of Animal Science Societies), 169.
Ge X., Ding C., Gong J., Li N., Li J. (2016). Fecal microbiota transplantation in combination with soluble dietary fiber for the chronic constipation: 2450. Off. J. Am. Coll. Gastroenterology| ACG 111, 1251. doi: 10.14309/00000434-201610001-02450
Giger-Reverdin S. (2000). Characterization of feedstuffs for ruminants using some physical parameters. Anim. Feed Sci. Technol. 86, 53–69. doi: 10.1016/S0377-8401(00)00159-0
Gill S. K., Rossi M., Bajka B., Whelan K. (2021). Dietary fibre in gastrointestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 18, 101–116. doi: 10.1038/s41575-020-00375-4
Guillon F., Champ M. (2000). Structural and physical properties of dietary fibres, and consequences of processing on human physiology. Food Res. Int. 33, 233–245. doi: 10.1016/S0963-9969(00)00038-7
Guo M. Q., Hu X., Wang C., Ai L. (2017). Polysaccharides: structure and solubility. Solubility polysaccharides, 8–21.
Huang S., Wei J., Yu H., Hao X., Zuo J., Tan C., et al. (2020). Effects of dietary fiber sources during gestation on stress status, abnormal behaviors and reproductive performance of sows. Animals 10, 141. doi: 10.3390/ani10010141
Jha R., Berrocoso J. D. (2015). Dietary fiber utilization and its effects on physiological functions and gut health of swine. Animal 9, 1441–1452. doi: 10.1017/S1751731115000919
Johansen H. N., Knudsen K. B., Sandström B., Skjøth F. (1996). Effects of varying content of soluble dietary fibre from wheat flour and oat milling fractions on gastric emptying in pigs. Br. J. Nutr. 75, 339–351. doi: 10.1079/BJN19960138
Kim D. Y., Camilleri M. (2000). Serotonin: a mediator of the brain–gut connection. Off. J. Am. Coll. Gastroenterology| ACG 95, 2698–2709. doi: 10.1111/j.1572-0241.2000.03177.x
Kitazawa T., Kaiya H. (2021). Motilin comparative study: structure, distribution, receptors, and gastrointestinal motility. Front. Endocrinol. 12, 700884. doi: 10.3389/fendo.2021.700884
Krogh U., Bruun T. S., Amdi C., Flummer C., Poulsen J., Theil P. K. (2015). Colostrum production in sows fed different sources of fiber and fat during late gestation. Can. J. Anim. Sci. 95, 211–223. doi: 10.4141/cjas-2014-060
Lu Y., Zhang Z., Tong L., Zhou X., Liang X., Yi H., et al. (2021). Mechanisms underlying the promotion of 5-hydroxytryptamine secretion in enterochromaffin cells of constipation mice by Bifidobacterium and Lactobacillus. Neurogastroenterol. Motil. 33, 14082. doi: 10.1111/nmo.14082
Meier R., Beglinger C., Schneider H., Rowedder A., Gyr K. (1993). Effect of a liquid diet with and without soluble fiber supplementation on intestinal transit and cholecystokinin release in volunteers. J. Parenteral Enteral Nutr. 17, 231–235. doi: 10.1177/0148607193017003231
Moturi J., Hosseindoust A., Tajudeen H., Mun J. Y., Ha S. H., Kim J. S. (2022). Influence of dietary fiber intake and soluble to insoluble fiber ratio on reproductive performance of sows during late gestation under hot climatic conditions. Sci. Rep. 12, 19749. doi: 10.1038/s41598-022-23811-8
National Research Council, Life Studies, & Committee on Nutrient Requirements of Swine (2012). Nutrient requirements of swine.
Navarro D. M. D. L., Bruininx E. M. A. M., De Jong L., Stein H. H. (2018). The contribution of digestible and metabolizable energy from high-fiber dietary ingredients is not affected by inclusion rate in mixed diets fed to growing pigs. J. Anim. Sci. 96, 1860–1868. doi: 10.1093/jas/sky090
Oakenfull D. (2001). Physicochemical properties of dietary fiber: Overview. Handb. dietary fiber, 195–206.
Oh S., Hosseindoust A., Ha S., Moturi J., Mun J., Tajudeen H., et al. (2022). Metabolic responses of dietary fiber during heat stress: Effects on reproductive performance and stress level of gestating sows. Metabolites 12, 280. doi: 10.3390/metabo12040280
Oliviero C., Heinonen M., Valros A., Peltoniemi O. (2010). Environmental and sow-related factors affecting the duration of farrowing. Anim. Reprod. Sci. 119, 85–91. doi: 10.1016/j.anireprosci.2009.12.009
Petry A. L., Patience J. F. (2020). Xylanase supplementation in corn-based swine diets: a review with emphasis on potential mechanisms of action. J. Anim. Sci. 98, 318. doi: 10.1093/jas/skaa318
Priester M., Visscher C., Fels M., Rohn K., Dusel G. (2020). Fibre supply for breeding sows and its effects on social behaviour in group-housed sows and performance during lactation. Porcine Health Manage. 6, 1–16. doi: 10.1186/s40813-020-00153-3
Ruckman L. A., Petry A. L., Gould S. A., Kerr B. J., Patience J. F. (2020). The effects of enzymatically treated soybean meal on growth performance and intestinal structure, barrier integrity, inflammation, oxidative status, and volatile fatty acid production of nursery pigs. Trans. Anim. Sci. 4, 170. doi: 10.1093/tas/txaa170
Serena A., Knudsen K. B. (2007). Chemical and physicochemical characterization of co-products from the vegetable food and agro industries. Anim. feed Sci. Technol. 139, 109–124. doi: 10.1016/j.anifeedsci.2006.12.003
Shang Q., Liu S., Liu H., Mahfuz S., Piao X. (2021). Impact of sugar beet pulp and wheat bran on serum biochemical profile, inflammatory responses and gut microbiota in sows during late gestation and lactation. J. Anim. Sci. Biotechnol. 12, 1–14. doi: 10.1186/s40104-021-00573-3
Slama J., Schedle K., Wurzer G. K., Gierus M. (2019). Physicochemical properties to support fibre characterization in monogastric animal nutrition. J. Sci. Food Agric. 99, 3895–3902. doi: 10.1002/jsfa.2019.99.issue-8
Stephens D. B., Ingram D. L., Sharman D. F. (1983). An investigation into some cerebral mechanisms involved in schedule-induced drinking in the pig. Quarterly J. Exp. Physiology: Translation Integration 68, 653–660.
Sugiura M., Inagami T., Kon V. (1989). Endotoxin stimulates endothelin-release in vivo and in vitro as determined by radioimmunoassay. Biochem. Biophys. Res. Commun. 161, 1220–1227. doi: 10.1016/0006-291X(89)91372-7
Tigchelaar E. F., Bonder M. J., Jankipersadsing S. A., Fu J., Wijmenga C., Zhernakova A. (2016). Gut microbiota composition associated with stool consistency. Gut 65, 540–542. doi: 10.1136/gutjnl-2015-310328
Van Citters G. W., Lin H. C. (1999). The ileal brake: a fifteen-year progress report. Curr. Gastroenterol. Rep. 1, 404–409. doi: 10.1007/s11894-999-0022-6
Wenk C. (2001). The role of dietary fibre in the digestive physiology of the pig. Anim. Feed Sci. Technol. 90, 21–33. doi: 10.1016/S0377-8401(01)00194-8
Wu T., Rayner C. K., Young R. L., Horowitz M. (2013). Gut motility and enteroendocrine secretion. Curr. Opin. Pharmacol. 13, 928–934. doi: 10.1016/j.coph.2013.09.002
Yan C. L., Kim H. S., Hong J. S., Lee J. H., Han Y. G., Jin Y. H., et al. (2017). Effect of dietary sugar beet pulp supplementation on growth performance, nutrient digestibility, fecal microflora, blood profiles and diarrhea incidence in weaning pigs. J. Anim. Sci. Technol. 59, 1–8. doi: 10.1186/s40781-017-0142-8
Keywords: carbohydrases, dietary fiber, sows, water balance, gut motility
Citation: Crome TA, Vahlenkamp KD, Self RM, Giesemann MA and Petry AL (2025) Dietary fiber source and stage of gestation impact water balance, fecal physicochemical properties, serum electrolytes, and markers of gut motility in sows. Front. Anim. Sci. 5:1433187. doi: 10.3389/fanim.2024.1433187
Received: 15 May 2024; Accepted: 16 December 2024;
Published: 16 January 2025.
Edited by:
Luciano Pinotti, University of Milan, ItalyReviewed by:
Ravikanth Reddy Poonooru, University of Missouri, United StatesGiovanni Buonaiuto, University of Bologna, Italy
Copyright © 2025 Crome, Vahlenkamp, Self, Giesemann and Petry. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amy L. Petry, YW15cGV0cnlAbWlzc291cmkuZWR1
 Thomas A. Crome
Thomas A. Crome Kyle D. Vahlenkamp1
Kyle D. Vahlenkamp1 Amy L. Petry
Amy L. Petry