- Division of Animal and Nutritional Sciences, West Virginia University, Morgantown, WV, United States
The development of resistance in parasites due to overuse of anthelmintics has resulted in a marked decrease in the efficacy of these drug classes. Recent research efforts have focused on exploring alternatives such as selection for parasite-resistant breeds with the implication that immunocompetence may align with parasite resistance. Two breeds that are often investigated are the St. Croix (STC), a resistant hair breed, and Suffolk (SUF), a susceptible wool breed sheep. The liver plays a vital role in metabolism in the body and metabolizes lipopolysaccharide (LPS), which triggers whole body response through the production of appropriate metabolites, cytokines and immune cells. The objective of this study was to investigate the breed differences in liver metabolome of sheep, with divergent resistance to parasites, in response to LPS. Both STC and SUF sheep (n = 9/breed) were challenged with LPS intravenously. Rectal temperatures and sheep grimace score (SGS) were recorded hourly, for each animal, and averaged across the study for both breeds. The average rectal temperature throughout the study was similar for STC and SUF sheep (40.4°C and 40.2°C respectively), but the pattern of response was different. STC had an average SGS of 0.8 while SUF had an average of 3.3. Liver biopsies were collected from 3 sheep that were not challenged with LPS (HR0; n = 3/breed), two hours post-challenge (HR2; n = 3/breed), and six hours post-challenge (HR6; n = 3/breed). Liver tissue samples were subjected to quantitative untargeted metabolome analysis using chemical isotope labeling/liquid chromatography-mass spectrometry. Pathway analysis of the HR0 metabolome data revealed that 8 pathways (and their associated metabolites) including beta-alanine metabolism, arginine and proline metabolism and glutathione metabolism were altered (false discovery rate-adjusted P-value (FDR) ≤ 0.05) between STC and SUF sheep. At HR2, 10 altered pathways such as folate biosynthesis, taurine and hypotaurine metabolism, and glutathione metabolism. At HR6, only 2 pathways (glycerophospholipid metabolism and purine metabolism) were altered (FDR ≤ 0.05) between STC and SUF sheep. Results highlight the differences in hepatic metabolome and physiological response to LPS challenge that exist between SUF and STC. These findings suggest breed-specific differences in metabolic response to immune challenge, potentially influencing the divergent resistance of the two breeds to parasitic infections.
Introduction
Parasite infections have consistently been one of the greatest challenges facing small ruminant production and were listed as one of the primary causes of non-predatory death loss in sheep in 2019 (Benford, 2022). Traditionally, the treatment of parasites depends on the administration of anthelmintics. This approach has, however, led to resistance in worms (Traoré et al., 2017). The development of resistance in these worms has resulted in a marked decrease in the efficacy of several anthelmintic drug classes (Fleming et al., 2006). Recent research efforts have focused on exploring alternatives including nutritional supplementation and selection for parasite-resistance within breeds (Sayers and Sweeney, 2005).
In sheep, St. Croix and Suffolk are two popular breeds with different resistance to parasites. St. Croix sheep respond quickly to Haemonchus contortus (Hc) larvae which prevents establishment of adult worms (Bowdridge et al., 2015). Conversely, Suffolk sheep’s delayed response favors greater fecal egg counts and adult worm establishment, making them more susceptible to infection (Weaver et al., 2021). In response to Hc larval antigen, St. Croix peripheral blood mononuclear cells (PBMC) upregulated significantly more genes associated with signal transduction, response to stress and immune system processes compared to Suffolk PBMC (Jacobs et al., 2020). Efficient and rapid response, in hair breeds, to parasites favors a Th2 response and may suggest STC are more immunocompetent than SUF (MacKinnon et al., 2015).
Lipopolysaccharide, a component of the outer membrane of gram-negative bacteria, stimulates immune cells to produce proinflammatory cytokines such as tumor necrosis factor alpha (TNFα) and interleukin 1-beta (IL-1β) (Lynn and Golenbock, 1992). Stimulation with LPS can reflect a bacterial infection and presents a model for research into immune response (Lynn and Golenbock, 1992). In a recent study, Bentley et al., 2023 evaluated the differences in immune response to LPS in St. Croix and Suffolk sheep. They observed an early decline in blood concentration of TNFα, a pro-inflammatory cytokine in the parasite-resistant group, compared to the susceptible group, which was associated with a reduced duration of sick behaviors in the former. The liver plays a vital role in the clearance of antigens such as LPS from the body, as Toll-like receptors (TLRs) recognize and help trigger the appropriate release of cytokines (Jirillo et al., 2002). However, the effect of LPS stimulation on liver metabolic profile of St. Croix and Suffolk sheep has not been fully described. The application of metabolomics has facilitated the characterization of metabolic phenotyping, providing insights into disease studies and metabolic mechanisms (Kenéz et al., 2016). Metabolomics enables the study of both exogenous and endogenous compounds as intermediate and final products of biological processes. Therefore, this study aimed to determine the effect of LPS on the liver metabolome profile in sheep breed with divergent parasite resistance (St. Croix and Suffolk breeds). We hypothesized that there would be discernible alterations in liver metabolome profiles in response to LPS between the two breeds.
Materials and methods
Animals
Animal use for this experiment was approved by the Institutional Animal Care and Use Committee of West Virginia University (protocol number # 2303064503). Nine (9) St. Croix wethers (BW= 66.8 ± 8.2 kg; 2.5 years of age) and 9 Suffolk (7 wethers and 2 ewes; BW = 83.0 ± 8.7 kg; 2.5 years of age) were housed and raised at West Virginia University Agronomy Farm, under parasite free conditions. A licensed veterinarian evaluated the health of all sheep 24 hours prior to start of experiment and only healthy animals were allowed to participate in the study. Live weights were recorded and used to calculate LPS dosage (E. coli O111:B4, Sigma-Aldrich, St Louis, MO). The LPS was administered via jugular venipuncture at 2.5 μg/kg then animals were euthanized via captive bolt gun at the following time points; HR0 (n = 3/breed; prior to LPS challenge), HR2 (n = 3/breed; 2 hours post- LPS challenge), and HR6 (n = 3/breed; 6 hours post- LPS challenge). This was immediately followed by exsanguination then liver and spleen were retrieved. Animal carcasses were disposed of via composting.
Sample collection and metabolome analysis
Rectal temperatures and sheep grimace score (SGS) were taken for each animal every hour from the start of the experiment using rectal thermometer. The orbital tightening, ear and head position, flehming of each animal were scored between 0 and 3 and the total of the three components were used to assess pain on a scale of 0 - 7 (Häger et al., 2017).
The liver was removed, weighed and photographed for each animal. Duplicate biopsy punches were taken from the same area of the right lobe and placed in cryogenic tubes for storage. Samples were stored in liquid nitrogen until time of analysis. A total of eighteen liver samples were subjected to untargeted metabolome analysis. To extract metabolites from the liver tissue, the samples were placed in plastic tubes with 6 ceramic beads, weighed, and homogenized at 6 m/s for 15 seconds. Exactly 500 μL LC-MS grade MeOH/water (4:1 v/v) was added before homogenizing again at 4 m/s for 10 seconds. Samples were then incubated at -20°C for 10 minutes and then centrifuged at 15,000 g for 10 minutes. All solvent was then transferred to a new vial and centrifuged for an additional 1 minute before the supernatants were removed and dried. Sample extracts were then re-suspended in 50 μL of LC-MS grade water. Metabolome analysis of the liver sample extracts were performed using chemical isotope labeling/liquid chromatography- mass spectrometry (Zhao et al., 2019). The technique uses 12C and 13C-isotope dansylation labeling to identify metabolites according to their chemical groups (amines/phenols, carboxylic acids, carbonyls, and hydroxyls (Zhao et al., 2019). Comprehensive details of the technique, encompassing sample preparation and analysis, have been previously documented (Zhao et al., 2019). A total of 18 raw LC-MS data files (n = 6/time point) were processed using IsoMS Pro 1.2.14 to remove redundant pairs (adduct ions and dimers) and singlet peaks. The peak pairs were identified as metabolites using CIL metabolite library (containing 1060 unique endogenous metabolites) at tier 1 and linked identity (LI) library (containing over 2000 metabolic-pathway-related metabolites extracted from the KEGG database at tier 2 (Li et al., 2013). All other metabolite features that were not identified at tiers 1 or 2 were removed from subsequent data analyses.
Statistical analysis
The intensity values for the metabolites were analyzed using MetaboAnalyst 6.0 software (metaboanalyst.ca). Metabolome data for each time point were analyzed separately. The data at each time were normalized and pareto-scaled. Principal component analysis (PCA) score plots were used to visualize differences between the breeds of sheep at each time point. Volcano plot analysis was used to determine the metabolites that were different (false discovery rate-adjusted P-values (FDR) ≤ 0.05; Benjamini and Hochberg, 1995) between the two breeds at each time point. Pathway enrichment analyses of all the metabolites were conducted using the KEGG database, to determine pathways (and their associated metabolites) that were significantly altered (FDR ≤ 0.05) between STC and SUF at each time point.
Results
Physiological response to LPS
The average rectal temperature at HR0 was 39.5°C for STC and 39.0°C for SUF. STC sheep exhibited a more pronounced initial temperature response to LPS, with a 1.1°C change compared to a 0.6°C change in SUF, between hour 0 and hour 1. By HR2, STC showed minimal change in rectal temperature, which persisted for the remainder of the study. In contrast, SUF displayed a gradual increase in temperature, with the most significant change occurring between hours 5 and 6 (Supplementary Figure 1). Despite STC initially having a higher temperature than SUF, they maintained a consistent average rectal temperature for the duration of the study. Similarly, the average sheep grimace score (SGS) remained constant throughout the study for STC, with the highest score recorded as 2 on a 0 -7 scale. The average SGS for SUF peaked at HR2, averaging 3.3 compared to 0.8 for STC throughout the study (Supplementary Figure 2).
Liver metabolome prior to lipopolysaccharide challenge
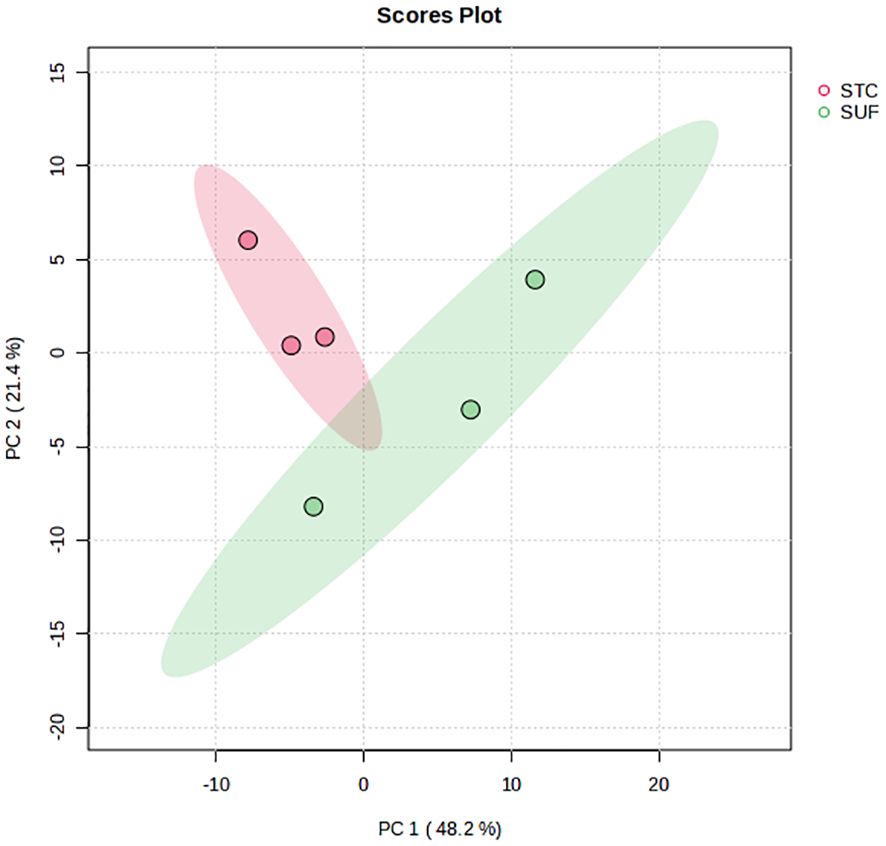
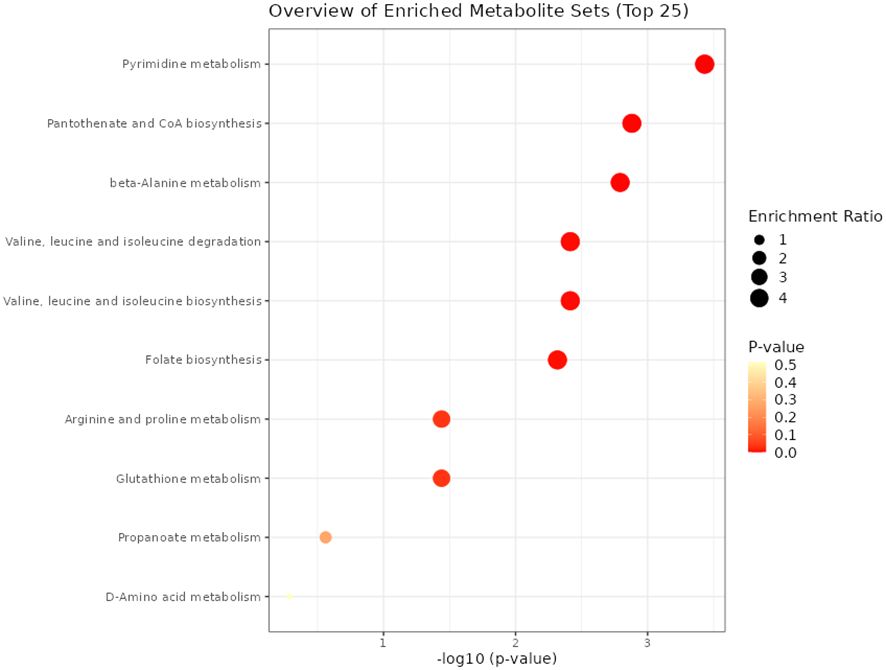
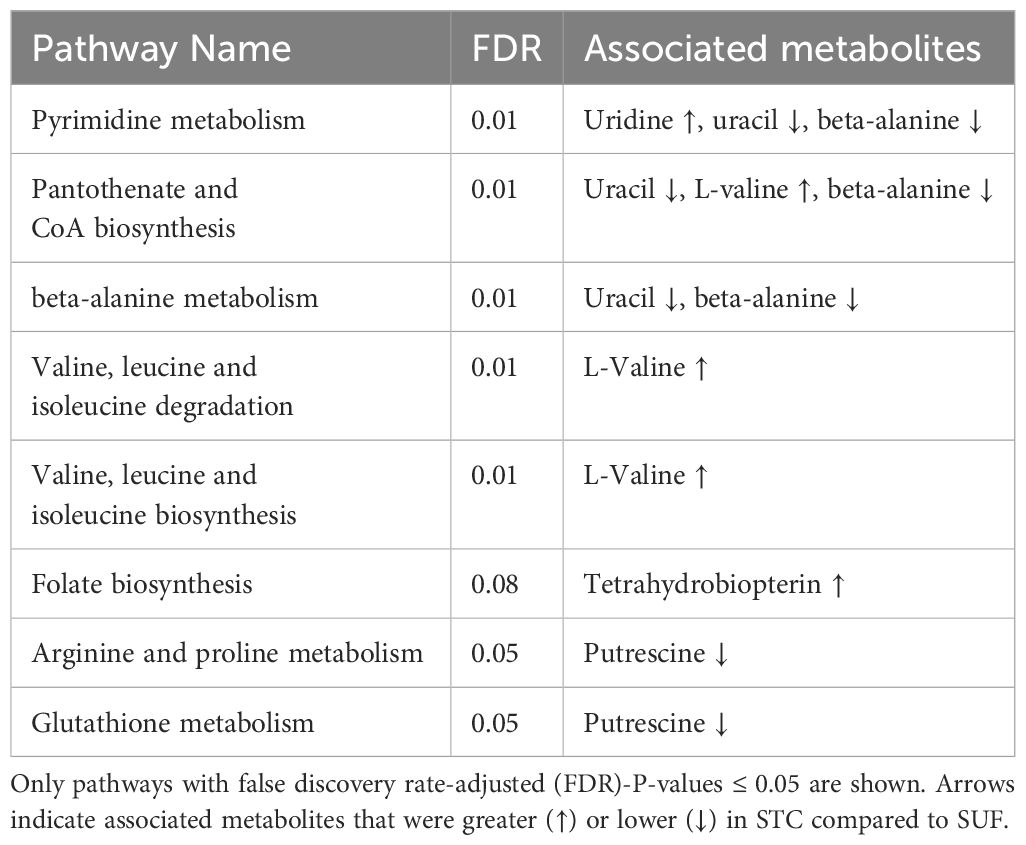
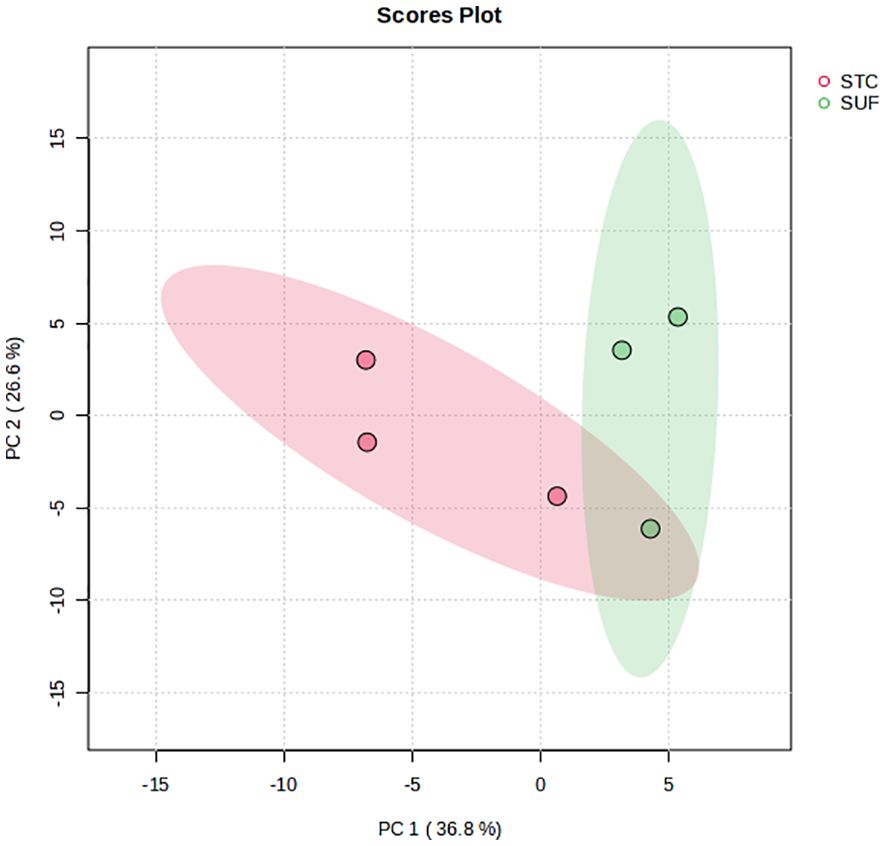
A total of 874 metabolites were detected and identified. The PCA score plot showed a clear separation between SUF and STC, indicating that there were clear breed differences (Figure 1) in the liver metabolome of the two breeds HR0. The volcano plot analysis revealed no metabolites with significant differential abundance (FDR > 0.05). However, the results of the pathway enrichment analysis showed that eight pathways (and their associated metabolites such as uridine, uracil, and valine), including pyrimidine metabolism, pantothenate and CoA biosynthesis, folate biosynthesis, arginine and proline metabolism, and glutathione metabolism, were altered (FDR ≤ 0.05) between STC and SUF sheep (Figure 2; Table 1).
Liver metabolome two hours post lipopolysaccharide challenge
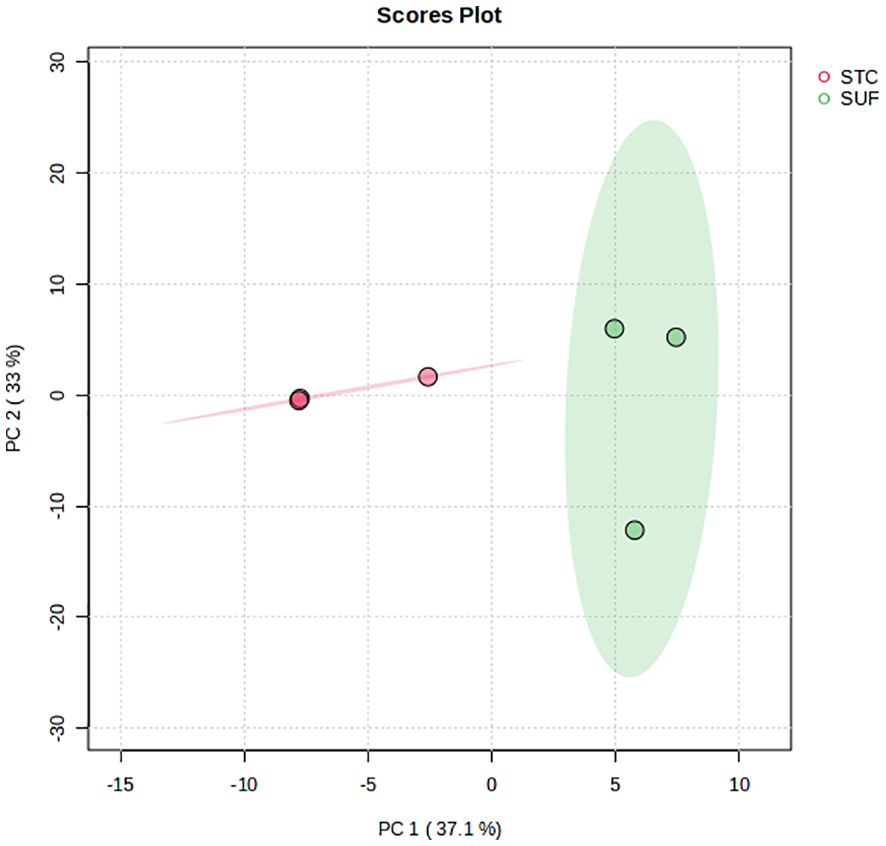
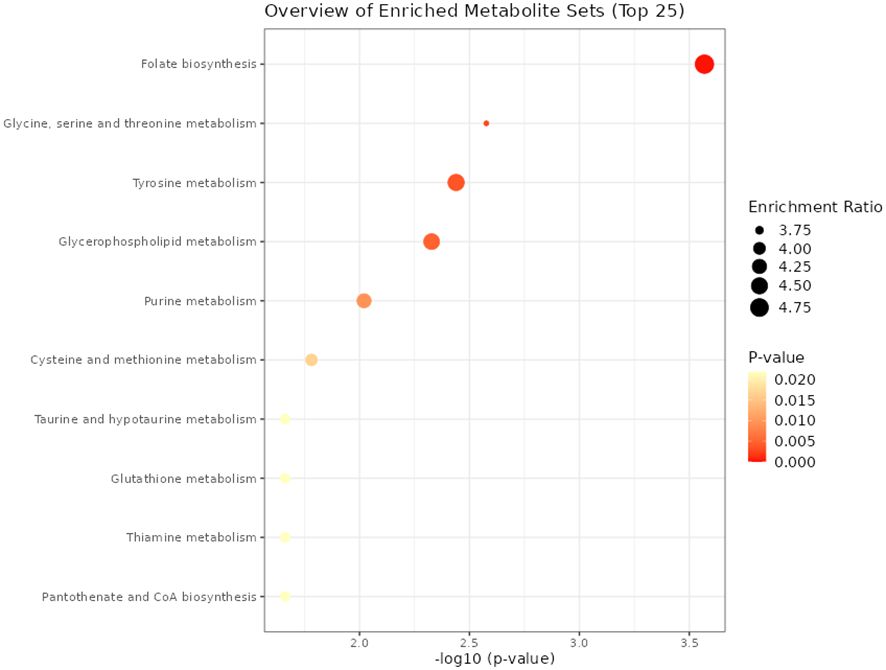
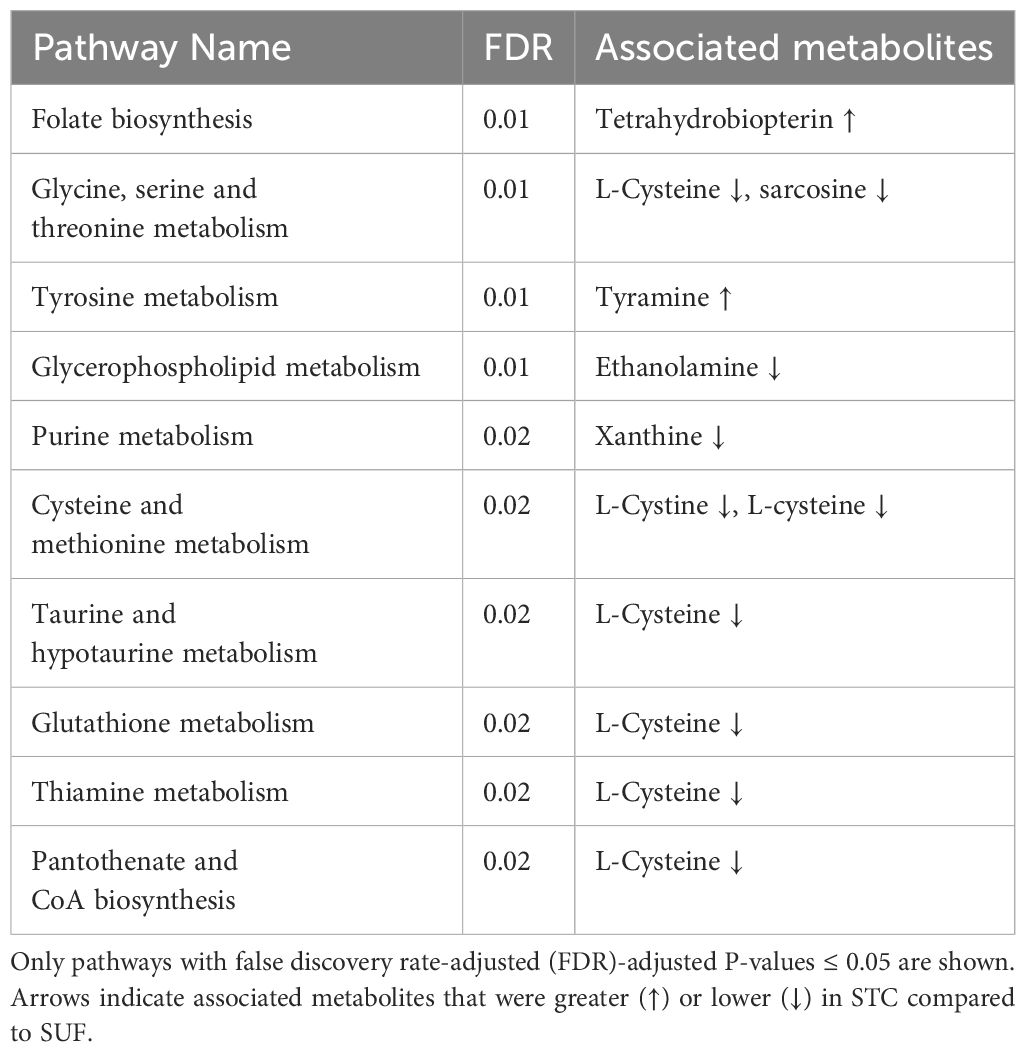
The PCA score plot showed a clear separation between SUF and STC at HR2 (Figure 3). The volcano plot analysis revealed no differentially abundant metabolites (FDR > 0.05). Results of the pathway enrichment showed that 10 pathways (and their associated metabolites such as tetrahydrobiopterin, L-cysteine and tyramine) including folate biosynthesis, glycine, serine and threonine metabolism were altered (FDR ≤ 0.05) between STC and SUF sheep (Figure 4; Table 2).
Liver metabolome six hours post lipopolysaccharide challenge
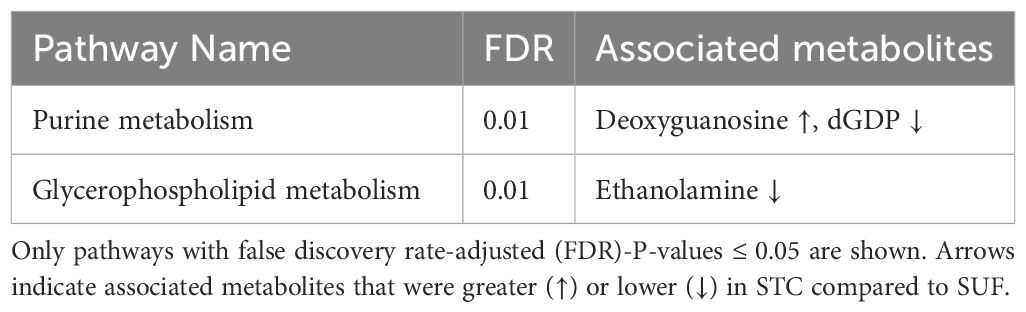
The PCA score plot showed a clear separation between SUF and STC, indicating that there were clear breed differences at HR6 (Figure 5). The volcano plot analysis revealed no differentially abundant metabolites (FDR > 0.05). However, pathway enrichment analysis revealed alterations (FDR ≤ 0.05) in two pathways, glycerophospholipid metabolism, and purine metabolism, along with their associated metabolites like deoxyguanosine, dGDP, and ethanolamine, between STC and SUF sheep (Figure 6; Table 3).
Discussion
Hair breeds like STC demonstrate greater adaptability to harsh climate conditions and increased resistance to internal parasites when compared to their wool counterparts (Wildeus, 1997). Though SUF sheep breeds are susceptible to parasites which poses a significant challenge (Leymaster, 1991; Bahirathan et al., 1996), they exhibit profitable growth rates and favorable carcass traits. They have emerged as a prominent meat breed in North America, leading to extensive efforts in generating crosses due to their impressive performance and maternal abilities (Shrestha et al., 2008). Fever response to LPS between breeds indicated a more rapid but less prolonged change in rectal temperature for STC sheep compared to SUF sheep, that presented longer lasting and slower response in temperature for this study. Assessment of pain, through SGS, revealed a more pronounced pain response to LPS in SUF sheep. Similar physiological responses were observed by Hadfield et al. (2018) in Dorset and Suffolk ewes’ response to LPS. They reported a greater and more frequent display of sick behavior in SUF ewes compared to Dorset ewes, and LPS treatment increased rectal temperature, peaking at hour 4 post-administration. A response akin to that of STC, as observed in this experiment, would be more desirable, where the immune system can promptly respond and resolve insults.
In this study, eight metabolic pathways, including those associated with some amino acid metabolism were found to be different between SUF and STC prior to LPS stimulation, suggesting increased altered metabolic activity. Amino acid metabolism in ruminants holds paramount importance, influencing various physiological functions crucial for their health and productivity (Titgemeyer and Löest, 2001). Serving as the fundamental building blocks of proteins, amino acids are indispensable for protein synthesis, and overall growth performance (Wu, 2009).
Wool production, a notable aspect of sheep husbandry, relies heavily on amino acids, particularly those containing sulfur, such as cysteine and methionine, which are pivotal for synthesizing keratin—the principal component of wool (Hynd and Masters, 2002). Furthermore, amino acids play a key role in supporting reproductive processes, from fertility to fetal development, highlighting their significance in sustaining the sheep population (Kwon et al., 2003; McCoard et al., 2016). The vast differences in metabolic profiles and pathways observed in this study may be attributed to the larger liver size and likely support the better growth performance, wool production, and maternal ability of SUF compared to STC.
In response to LPS challenge, the traditional reaction involves the activation of proinflammatory cytokines and mediators like TLR4, IL-1β, and TNF-α, accompanied by the generation of reactive oxygen species (ROS) and the initiation of NF-kB activation (Page et al., 2022). NADPH oxidase is implicated in driving proinflammatory responses to LPS, such as NF-kB activation (Koay et al., 2001; Qin et al., 2004). NADH oxidase, particularly in phagocytic cells like neutrophils and macrophages, is crucial for ROS production, contributing to inflammatory responses against invaders like LPS (Forman and Torres, 2002; Qin et al., 2004). Franchini et al. (2013) identified NADPH oxidase, specifically the NADPH oxidase complex (NOX2), as a crucial element in the complex essential for ROS production in macrophages, leading to subsequent IL-6 production in response to insults such as bacterial invasion. In this study, L-Cysteine drives most of the pathways (such as cysteine and methionine metabolism, taurine and hypotaurine metabolism, and glutathione metabolism) altered at HR2, and its relative concentration was lower in STC compared to SUF. Cysteine is directly derived from homocysteine and is required for glutathione and taurine synthesis, which both play significant roles in combating oxidative stress (Malmezat et al., 2000). Cysteine is also one of the products of methionine degradation in the liver (Yin et al., 2016; Coleman et al., 2020). It has been demonstrated that dietary methionine supplementation improved oxidative status in ruminants, through increases in glutathione transferase activity and glutathione concentrations in plasma and liver (Osorio et al., 2014; Tsiplakou et al., 2017). Batistel et al. (2018) further underscored the importance of methionine in combating oxidative stress by facilitating ROS scavenging, antioxidant activity, increased neutrophil phagocytic activity, and oxidative burst activity, through taurine and glutathione metabolism. Glutathione is an important component of living cells and more specifically plays a protective role in red blood cells against oxidative stress (Tucker et al., 1981). Given the central role cysteine plays in combating oxidative stress, it can be concluded that by HR2, STC sheep are actively combating LPS-induced oxidative stress. Reduced cysteine concentration in STC suggests a shift in metabolic functions to defend against LPS through oxidative stress interventions as a result of inflammation.
The relative concentration of tetrahydrobiopterin (BH4) was greater in STC, relative to SUF at 2 hours post LPS stimulation, and this metabolite is associated with folate biosynthesis. Tetrahydrobiopterin is a cofactor in nitric oxide (NO) production and helps mediate excessive oxidative stress, through reduction in superoxide radical anions (Gamal et al., 2018). Coupling of endothelium nitric oxide synthases is facilitated by BH4, which ameliorates oxidative stress (He et al., 2012). Folate is essential in many metabolic processes such as nucleic acid synthesis, methionine regeneration and purine and pyrimidine synthesis (Bailey and Gregory, 1999). Xu et al., 2014 showed that folate metabolism is affected by biopterins, such as BH4, through increases in oxidative stress abatement pathways. Also, at HR2, purine metabolism was driven by xanthine, which was lower in STC. Xanthine is oxidized into uric acid, catalyzed by xanthine oxidoreductase (XOR), as the end product of purine metabolism (Al-Shehri et al., 2020). This enzyme, XOR, is most abundant in the liver (hepatocytes) and is rate-limiting in the degradation of nucleic acid (Harrison, 2002; Battelli et al., 2016). Xanthine oxidase, a form of XOR, generates ROS. The elevated level of BH4 and reduced relative concentration of xanthine in STC probably suggest a mitigation of LPS-induced oxidative stress.
Tyramine was the only other metabolite that was enriched in STC at HR2. Tyramine is a biogenic amine derived from the decarboxylation of tyrosine (Scherer et al., 2015). (Glymenaki et al., 2023) reported the negative impacts of tyramine on colon cells include cytotoxicity, DNA damage, necrosis and upregulation of oxidative stress-related genes. Despite its detrimental effects, tyramine exerts an indirect influence on the nervous system, promoting pupil dilation, respiration elevation, and blood sugar increase in humans (Shalaby, 1996). Tyramine has been reported to have a dose-dependent effect on immune regulation, energy uptake, and feed uptake modulation in response to starvation in marine shrimp (Kuo et al., 2024). The result of this study suggests potential immunological implications of tyramine concerning its response to LPS in sheep.
Purine metabolism and glycerophospholipid metabolism were the only two pathways altered at HR 6 post-LPS challenge. These two pathways were driven by deoxyguanosine and ethanolamine, respectively. The relative concentration of ethanolamine was lower in STC, compared to SUF sheep, and its associated glycerophospholipid metabolism was found to be a strong indicator of copper toxicity in pig kidney (Qiao et al., 2021; Bi et al., 2022; Mukhopadhyay and Trauner, 2023). Reduced relative concentration of ethanolamine in STC may suggest non-toxic liver tissue. The relative concentration of deoxyguanosine was greater in STC. Deoxyguanosine is derived from guanine, a very sensitive base nucleotide, which is known to be very susceptible to oxidative damage (Nikolova et al., 2022). Increased relative concentration of deoxyguanosine suggests that STC was able to resolve the LPS insult with no oxidative damage to the liver tissue which aligns with the results of sick behavior and rectal temperature pattern of STC compared to SUF sheep breed.
Conclusion
This study highlights hepatic metabolic distinctions between STC and SUF sheep breeds before and after an LPS challenge. The enriched amino acid metabolism before the LPS challenge supports the better growth performance, wool production, and reproduction of the SUF, compared to STC breed. The observed variations in the relative concentrations of several metabolites associated with important metabolic pathways further contribute to understanding the responses of SUF and STC to the LPS challenge. Notably, the lower concentration of ethanolamine in STC indicates potentially non-toxic liver tissue, while the increased relative concentration of deoxyguanosine suggests the effective resolution of the LPS insult without oxidative damage. These findings enhance our understanding of the hepatic metabolome changes of SUF and STC sheep breeds to inflammatory challenges.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The animal studies were approved by Institutional Animal Care and Use Committee of West Virginia University (protocol number # 2303064503). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
SJ: Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. KB: Data curation, Investigation, Methodology, Project administration, Writing – review & editing. SB: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – review & editing. IO: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The research study was funded by the U.S. Department of Agriculture hatch multi-state regional project W-3010 and West Virginia University Animal Health hatch project #923.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fanim.2024.1407533/full#supplementary-material
References
Al-Shehri S. S., Duley J. A., Bansal N. (2020). Xanthine oxidase-lactoperoxidase system and innate immunity: Biochemical actions and physiological roles. Redox Biol. 34, 101524. doi: 10.1016/j.redox.2020.101524
Bahirathan M., Miller J. E., Barras S. R., Kearney M. T. (1996). Susceptibility of Suffolk and Gulf Coast Native suckling lambs to naturally acquired strongylate nematode infection. Vet. Parasitol. 65, 259–268. doi: 10.1016/S0304–4017(96)00969–7
Bailey L. B., Gregory J. F. (1999). Folate metabolism and requirements12. J. Nutr. 129, 779–782. doi: 10.1093/jn/129.4.779
Batistel F., Arroyo J. M., Garces C. I. M., Trevisi E., Parys C., Ballou M. A., et al. (2018). Ethyl-cellulose rumen-protected methionine alleviates inflammation and oxidative stress and improves neutrophil function during the periparturient period and early lactation in holstein dairy cows. J. Dairy Sci. 101, 480–490. doi: 10.3168/jds.2017-13185
Battelli M. G., Polito L., Bortolotti M., Bolognesi A. (2016). Xanthine oxidoreductase in drug metabolism: Beyond a role as a Detoxifying enzyme. Curr. Med. Chem. 23, 4027–4036. doi: 10.2174/0929867323666160725091915
Benford R.-A. (2022). Death loss trends in the U.S. Sheep industry: 1994–2019. USDA-NASS. Available at: https://www.aphis.usda.gov/sites/default/files/sheep-death-loss-trends-us-2020.pdf.
Benjamini Y., Hochberg Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: Series B (Methodological) 57, 289300. doi: 10.1111/j.2517–6161.1995.tb02031.x
Bentley K., Wright D. L., Greiner S. P., Bowdridge S. A. (2023). 158 evaluating lipopolysaccharide-induced behavioral and immune response differences in sheep divergently bred for parasite resistance. J. Anim. Sci. 101, 105–106. doi: 10.1093/jas/skad068.127
Bi S., Shao J., Qu Y., Hu W., Ma Y., Cao L. (2022). Hepatic transcriptomics and metabolomics indicated pathways associated with immune stress of broilers induced by lipopolysaccharide. Poult. Sci. 101, 102199. doi: 10.1016/j.psj.2022.102199
Bowdridge S. A., Zajac A. M., Notter D. R. (2015). St. Croix sheep produce a rapid and greater cellular immune response contributing to reduced establishment of Haemonchus contortus. Vet. Parasitol. 208, 204–210. doi: 10.1016/j.vetpar.2015.01.019
Coleman D. N., Lopreiato V., Alharthi A., Loor J. J. (2020). Amino acids and the regulation of oxidative stress and immune function in dairy cattle. J. Anim. Sci. 98, S175–S193. doi: 10.1093/jas/skaa138
Fleming S. A., Craig T., Kaplan R. M., Miller J. E., Navarre C., Rings M. (2006). Anthelmintic resistance of gastrointestinal parasites in small ruminants. J. Vet. Intern. Med. 20, 435–444. doi: 10.1111/j.1939-1676.2006.tb02881.x
Forman H. J., Torres M. (2002). Reactive oxygen species and cell signaling. Am. J. Respir. Crit. Care Med. 166, S4–S8. doi: 10.1164/rccm.2206007
Franchini A. M., Hunt D., Melendez J. A., Drake J. R. (2013). FcγR-driven release of IL-6 by macrophages requires NOX2-dependent production of reactive oxygen species. J. Biol. Chem. 288, 25098–25108. doi: 10.1074/jbc.M113.474106
Gamal M., Moawad J., Rashed L., Morcos M. A., Sharawy N. (2018). Possible involvement of tetrahydrobiopterin in the disturbance of redox homeostasis in sepsis – Induced brain dysfunction. Brain Res. 1685, 19–28. doi: 10.1016/j.brainres.2018.02.008
Glymenaki M., Curio S., Shrestha S., El-Bahrawy M., Wang Y., Gooderham N. J., et al. (2023). Tyramine promotes colon cancer risk and development by inducing DNA damage and inflammation. biorxiv. doi: 10.1101/2023.05.25.542254
Hadfield J. M., Bowdridge E. C., Holásková I., Elsasser T. H., Dailey R. A. (2018). Breed-specific differences in the immune response to lipopolysaccharide in ewes. J. Anim. Sci. 96, 4220–4228. doi: 10.1093/jas/sky288
Harrison R. (2002). Structure and function of xanthine oxidoreductase: where are we now? Free Radic. Biol. Med. 33, 774–797. doi: 10.1016/S0891-5849(02)00956-5
Häger C., Biernot S., Buettner M., Glage S., Keubler L. M., Held N., et al. (2017). The Sheep Grimace Scale as an indicator of post-operative distress and pain in laboratory sheep. PloS One 12, e0175839. doi: 10.1371/journal.pone.0175839
He X., Su F., Velissaris D., Salgado D. R., Barros D., de S., et al. (2012). Administration of tetrahydrobiopterin improves the microcirculation and outcome in an ovine model of septic shock*. Crit. Care Med. 40, 2833. doi: 10.1097/CCM.0b013e31825b88ba
Hynd P. I., Masters D. G. (2002)Nutrition and wool growth. | Sheep Nutrition. In: CABI Books (CABI Publishing) (Accessed February 15, 2024).
Jacobs J. R., Middleton D., Greiner S. P., Bowdridge S. A. (2020). RNA-Sequencing of ovine PBMC after exposure to Haemonchus contortus larval antigen. Parasite. Immunol. 42, e12697. doi: 10.1111/pim.12697
Jirillo E., Caccavo D., Magrone T., Piccigallo E., Amati L., Lembo A., et al. (2002). Review: The role of the liver in the response to LPS: experimental and clinical findings. J. Endotoxin. Res. 8, 319–327. doi: 10.1177/09680519020080050501
Kenéz Á., Dänicke S., Rolle-Kampczyk U., von Bergen M., Huber K. (2016). A metabolomics approach to characterize phenotypes of metabolic transition from late pregnancy to early lactation in dairy cows. Metabolomics 12, 165. doi: 10.1007/s11306-016-1112-8
Koay M. A., Christman J. W., Segal B. H., Venkatakrishnan A., Blackwell T. R., Holland S. M., et al. (2001). Impaired pulmonary NF-κB activation in response to lipopolysaccharide in NADPH oxidase-deficient mice. Infect. Immun. 69, 5991–5996. doi: 10.1128/iai.69.10.5991-5996.2001
Kuo H.-W., Hsu L.-Y., Su W.-Y., Cheng W. (2024). Tyramine mediates growth performance, immune response, and physiological regulation of Litopenaeus vannamei through dietary administration. Aquaculture 578, 740098. doi: 10.1016/j.aquaculture.2023.740098
Kwon H., Spencer T. E., Bazer F. W., Wu G. (2003). Developmental changes of amino acids in ovine fetal fluids1. Biol. Reprod. 68, 1813–1820. doi: 10.1095/biolreprod.102.012971
Leymaster K. A. (1991). Straightbred comparison of a composite population and the Suffolk breed for performance traits of sheep. J. Anim. Sci. 69, 993–999. doi: 10.2527/1991.693993x
Li L., Li R., Zhou J., Zuniga A., Stanislaus A. E., Wu Y., et al. (2013). MyCompoundID: using an evidence-based metabolome library for metabolite identification. Anal. Chem. 85, 3401–3408. doi: 10.1021/ac400099b
Lynn W. A., Golenbock D. T. (1992). Lipopolysaccharide antagonists. Immunol. Today 13, 271–276. doi: 10.1016/0167–5699(92)90009-V
MacKinnon K. M., Bowdridge S. A., Kanevsky-Mullarky I., Zajac A. M., Notter D. R. (2015). Gene expression profiles of hair and wool sheep reveal importance of Th2 immune mechanisms for increased resistance to Haemonchus contortus. J. Anim. Sci. 93, 2074–2082. doi: 10.2527/jas.2014–8652
Malmezat T., Breuillé D., Pouyet C., Buffière C., Denis P., Mirand P. P., et al. (2000). Methionine transsulfuration is increased during sepsis in rats. Am. J. Physiol.-Endocrinol. Metab. 279, E1391–E1397. doi: 10.1152/ajpendo.2000.279.6.E1391
McCoard S. A., Sales F. A., Sciascia Q. L. (2016). Amino acids in sheep production. Front. Biosci. Elite. Ed. 8, 264–288. doi: 10.2741/E766
Mukhopadhyay T. K., Trauner D. (2023). Concise synthesis of glycerophospholipids. J. Org. Chem. 88, 11253–11257. doi: 10.1021/acs.joc.2c02096
Nikolova G., Karamalakova Y., Oblakova M., Gadjeva V., Vasilev N. (2022). Role of oxidative stress biomarkers in the assessment of metabolic status in the ruminant. J. Hygenic Eng. Des. 41, 464–471.
Osorio J. S., Trevisi E., Ji P., Drackley J. K., Luchini D., Bertoni G., et al. (2014). Biomarkers of inflammation, metabolism, and oxidative stress in blood, liver, and milk reveal a better immunometabolic status in peripartal cows supplemented with smartamine m or MetaSmart. J. Dairy Sci. 97, 7437–7450. doi: 10.3168/jds.2013-7679
Page M. J., Kell D. B., Pretorius E. (2022). The role of lipopolysaccharide-induced cell signalling in chronic inflammation. Chronic Stress 6, 24705470221076390. doi: 10.1177/24705470221076390
Qiao N., Yang Y., Liao J., Zhang H., Yang F., Ma F., et al. (2021). Metabolomics and transcriptomics indicated the molecular targets of copper to the pig kidney. Ecotoxicol. Environ. Saf. 218, 112284. doi: 10.1016/j.ecoenv.2021.112284
Qin L., Liu Y., Wang T., Wei S.-J., Block M. L., Wilson B., et al. (2004). NADPH oxidase mediates lipopolysaccharide-induced neurotoxicity and proinflammatory gene expression in activated microglia*. J. Biol. Chem. 279, 1415–1421. doi: 10.1074/jbc.M307657200
Sayers G., Sweeney T. (2005). Gastrointestinal nematode infection in sheep – a review of the alternatives to anthelmintics in parasite control. Anim. Health Res. Rev. 6, 159–171. doi: 10.1079/AHR2005108
Scherer R., Gerlach K., Südekum K.-H. (2015). Biogenic amines and gamma-amino butyric acid in silages: Formation, occurrence and influence on dry matter intake and ruminant production. Anim. Feed. Sci. Technol. 210, 1–16. doi: 10.1016/j.anifeedsci.2015.10.001
Shalaby A. R. (1996). Significance of biogenic amines to food safety and human health. Food Res. Int. 29, 675–690. doi: 10.1016/S0963–9969(96)00066-X
Shrestha J. N. B., Boylan W. J., Rempel W. E. (2008). Evaluation of sheep genetic resources in north america: Lamb productivity of purebred, crossbred and synthetic populations. Can. J. Anim. Sci. 88, 391–398. doi: 10.4141/CJAS07049
Titgemeyer E. C., Löest C. A. (2001). Amino acid nutrition: Demand and supply in forage-fed ruminants. J. Anim. Sci. 79, E180. doi: 10.2527/jas2001.79E-SupplE180x
Traoré A., Notter D. R., Soudre A., Kaboré A., Álvarez I., Fernández I., et al. (2017). Resistance to gastrointestinal parasite infection in Djallonké sheep. animal 11, 1354–1362. doi: 10.1017/S1751731116002640
Tsiplakou E., Mavrommatis A., Kalogeropoulos T., Chatzikonstantinou M., Koutsouli P., Sotirakoglou K., et al. (2017). The effect of dietary supplementation with rumen-protected methionine alone or in combination with rumen-protected choline and betaine on sheep milk and antioxidant capacity. J. Anim. Physiol. Anim. Nutr. 101, 1004–1013. doi: 10.1111/jpn.12537
Tucker E. M., Young J. D., Crowley C. (1981). Red cell glutathione deficiency: Clinical and biochemical investigations using sheep as an experimental model system. Br. J. Haematol. 48, 403–415. doi: 10.1111/j.1365-2141.1981.tb02732.x
Weaver A. R., Garza J. J., Greiner S. P., Bowdridge S. A. (2021). Immune mechanisms of Texel sheep to adult and egg stages of Haemonchus contortus. Parasite. Immunol. 43, e12876. doi: 10.1111/pim.12876
Wildeus S. (1997). Hair sheep genetic resources and their contribution to diversified small ruminant production in the United States. J. Anim. Sci. 75, 630. doi: 10.2527/1997.753630x
Wu G. (2009). Amino acids: metabolism, functions, and nutrition. Amino Acids 37, 1–17. doi: 10.1007/s00726–009-0269–0
Xu F., Sudo Y., Sanechika S., Yamashita J., Shimaguchi S., Honda S., et al. (2014). Disturbed biopterin and folate metabolism in the Qdpr-deficient mouse. FEBS Lett. 588, 3924–3931. doi: 10.1016/j.febslet.2014.09.004
Yin J., Ren W., Yang G., Duan J., Huang X., Fang R., et al. (2016). L-cysteine metabolism and its nutritional implications. Mol. Nutr. Food Res. 60, 134–146. doi: 10.1002/mnfr.201500031
Keywords: metabolism, parasite, LPS, liver, oxidative stress
Citation: Johnson SR, Bentley K, Bowdridge S and Ogunade IM (2024) Lipopolysaccharide-induced alterations in the liver metabolome of St. Croix and Suffolk sheep. Front. Anim. Sci. 5:1407533. doi: 10.3389/fanim.2024.1407533
Received: 26 March 2024; Accepted: 09 May 2024;
Published: 24 May 2024.
Edited by:
Vishwajit S. Chowdhury, Kyushu University, JapanReviewed by:
Masatoshi Matsuzaki, Hirosaki University, JapanÁkos Kenéz, City University of Hong Kong, Hong Kong SAR, China
Copyright © 2024 Johnson, Bentley, Bowdridge and Ogunade. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ibukun M. Ogunade, SWJ1a3VuLm9ndW5hZGVAbWFpbC53dnUuZWR1; Scott Bowdridge, c2NvdHQuYm93ZHJpZGdlQG1haWwud3Z1LmVkdQ==
 Samanthia R. Johnson
Samanthia R. Johnson Kelsey Bentley
Kelsey Bentley Scott Bowdridge*
Scott Bowdridge* Ibukun M. Ogunade
Ibukun M. Ogunade