- 1Department of Animal Sciences, the Robert H. Smith Faculty of Agriculture, Food, and Environment, The Hebrew University of Jerusalem, Rehovot, Israel
- 2The Robert H. Smith Institute of Plant Sciences & Genetics in Agriculture, The Robert H. Smith Faculty of Agriculture, Food, and Environment, The Hebrew University of Jerusalem, Rehovot, Israel
This study evaluated the potential of low-glycoalkaloid potato haulm (LGPH) as a high-quality feed for ruminants. The low-glycoalkaloid potato plants were grown in a net house following standard agricultural practices. Dehaulming was performed a fortnight before the harvest of the tubers, as commercially practiced. Four healthy female lambs (aged 4 months) were employed in a 4×4 Latin square feeding experiment design. The lambs were fed with either a maintenance diet consisting of 73% roughage (control), or treatment diets with supplementation of 10% (P10), 15% (P15), and 20% (P20) LGPH on a dry matter (DM) basis for a period of 21 days, including 14 days of adaption and 7 days of sampling. Refusals and feces were collected for 6 days and pooled followed by a 24 h urine collection on the 7th day. On the 6th day, an hour after morning feeding, blood, and rumen liquid samples were collected. All the samples were analysed, and the data generated were analysed using ANOVA with diet and period as fixed effects and sheep as a random effect. Orthogonal contrasts were used to detect linear and quadratic effects of LGPH in the diet. Linear or polynomial equations were produced to extract the nutrient digestibility and metabolizable energy (ME) of LGPH. No toxicological clinical signs were observed, and the haematology profiles were normal throughout the trial. LGPH did not affect the daily intake of nutrients and energy. However, the apparent digestibility of DM, organic matter (OM), crude protein (CP), and gross energy increased (P= 0.01) linearly (P< 0.01) with supplementation of LGPH in the diet. The inclusion of LGPH had a quadratic effect on neutral detergent fiber (NDF) digestibility (P= 0.027), reaching a peak of 54.8 % at 12.3 % LGPH supplement on a DM basis. The N retention in lambs fed with the P20 diet was nearly twice that of the control diet (P= 0.032; 19.1 vs. 10.2 g/d). The computed digestibility values of DM, OM, CP, and NDF for LGPH were 76.1, 79.7, 84.2, and 72.1 %, respectively, and ME was 2.62 Mcal/kg DM. Conclusively, by significantly reducing the glycoalkaloids’ content in potato haulm, we can safely repurpose the currently wasted foliage as a new source of high-quality roughage feed for ruminants, equivalent to alfalfa hay, without imposing any additional environmental burden. However, further research is necessary to assess impact of LGPH on growth performance, and milk productivity before practical application on commercial farms.
1 Introduction
Driven by increase in global populations and economic growth in emerging markets, the demand for livestock products is growing swiftly (FAO, 2018; Makkar, 2018; OECD/FAO, 2021). This dietary change requires a significant increase in feed supply which exercebate the pressure on the world's water, land, and soil resources as well as the environment. More so, it heavily intensify the food-feed-fuel competition (Makkar, 2018; Food and Agriculture Organization Of The United Nation, 2021). One of the most promising strategies to alleviate this dilemma is identifying and developing novel, untapped, and unconventional high-quality feed sources (Makkar, 2014).
The Irish potato (Solanum tuberosum L.) has an outstanding history in both human and animal nutrition. Around 1.3 billion people worldwide consume potatoes as a staple food (more than 50kg per person annually), with Asia and Africa showing the strongest trends in both production and demand growth (CIP, 2017; Campos and Ortiz, 2019). Due to its great adaptability to a variety of climates and topographies, potatoes are farmed in more than 120 countries on about 19 million hectares worldwide. The annual yield of potatoes estimates to ~376 million tons, making it the fourth-most important food crop after wheat, corn, and rice (FAO, 2023).
In addition to its dietary importance, Irish potatoes serve as raw materials for many industries, the most important ones are alcohol production, nanotechnology, industrial starch production for medicine, cosmetics, papers and laundry, and feed resources (Scott and Suarez, 2012; Kaur and Singh, 2016).
Despite being highly nutritious, use of potato tubers for feeding ruminants is faced with several limitations such as high moisture content, short storability (Kilama et al., 2023) potential health risks such as digestive problems, acidosis, and choking (A. D. Pavlista and Rush, 2002), and toxicity to mammals (Morris and Lee, 1984).
On the other hand, potato haulm has received almost no attention in ruminant nutrition mainly because of its high levels of glycoalkaloids (GAs) mainly solanine and chaconine (Friedman and Levin, 2016; Baur et al., 2021). The Solanaceous potato produces GAs as bioactive secondary metabolites presumably for protection from herbivores and a variety of pests (Friedman and Levin, 2016). The GAs are toxic to both humans and animals due to their anticholinesterase activity on the neurological system and cell membrane disruption affecting digestive tracts and other organ systems (Friedman and McDonald, 1997). The GAs content of potato haulm varies with genotype, physiological age, environment (Nicholson et al., 1978; Maga, 1980; Jadhav et al., 1981; Kaplan et al., 2018) as well as insecticide application (Zarzecka et al., 2013).
Despite various reports highlighting the potential risks of GAs poisoning to both humans and animals, the research on potato GAs toxicity in ruminant feed is inadequate and insufficient (Chain et al., 2020). According to the scant information that is currently available for ruminants, the potentially toxic dosage of α-solanine is around 225 mg/kg body weight for sheep (Noordar et al., 2017). Nevertheless, ruminants are reported to resist higher levels of GAs than humans (Friedman and McDonald, 1997) mainly because rumen microbiomes are able to hydrolyse GAs (King and McQueen, 1981) and detoxify a number of secondary metabolites (Loh et al., 2020).
However, when consumed in large quantities, fresh potato vines carrying GAs levels ranging from 60 to 300 mg/100 g dry matter (DM) could pose a threat to ruminants. Consequently, the potential adverse effects of potato GAs on animal welfare and productivity such as morbidity, mortality, teratogenicity, pregnancy complications, and postnatal risks teratogenicity Wang et al. (2005) remain a major concern.
Potato dehaulming before tuber harvesting is practiced by growers worldwide to enhance tuber maturation, promote skin set, improve skin colour, decrease weight loss during storage, increase resistance to pathogens and bruising (Zotarelli et al., 2016; Karan, 2021) and control soil- or seed-borne pest (Kempenaar and Struik, 2007). Several studies suggested that biological and chemical methods can be employed to improve the quality of potato haulm for use in ruminant nutrition (Dijkstra and Reestman, 1943; Saleh, 2014; and El – Shinnawy and Eassawy, 2016). Nonetheless, dehaulming results in the loss of approximately 355-426kg/1,000 m2 of dry herbage yield of potato foliage (Kaplan et al., 2018) that could potentially be harnessed for feeding ruminants.
Currently, we are not aware of any practical commercial applications of potato haulm for feeding ruminants due to lingering uncertainties regarding the potential risk of potato GAs on animals.
Recently Rumafeed (a start-up company, Mishmar HaShivaa, Israel) made a breakthrough in breeding potato cultivars with low GAs levels (LGP) in both haulm and tubers by employing CRISPR/Cas9 technology, thus annulling the risk of haulm GAs to livestock. The novel genotypes offer a substantial solution to the global challenge of increased demand and rising costs of forage and feedstuffs for livestock.
We have investigated the nutritional value of low GAs potato haulm (LGPH) and its potential utilization as an alternative source of high-quality forage for livestock and reported their effect on dietary intake, nutrient digestibility, nitrogen and energy balance, rumen, and haematological parameters of sheep.
2 Materials and methods
2.1 Propagation of LGPH and experimental diets
A number of low-glycoalkaloids potato cultivars were developed by Rumafeed (Mishmar HaShivaa, Israel) using CRISPR/Cas9 gene editing technique (Johansen et al., 2019) for precise knock down of genes coding for GAs synthesis in the tetraploid plants (Krits et al., 2007; Sawai et al., 2014). Following tissue culturing, hardening (Mohapatra and Batra, 2017) and cultivation in a closed air-conditioned greenhouse, and MS-GC assays for GAs, post-storage tubers from selected mutants were planted and plants grown for more than 100 d in 7 L pots containing standard growing medium. Standard agricultural management was practiced throughout. Following dehaulming a fortnight prior to tuber harvesting, the fresh cut haulm was dried at 60°C for 48 h in an air-forced oven to make LGPH hay which is then stored in a polythene bag for experimental diet formulation.
2.2 Diet formulations
Using total mixed ration (TMR, Nahlal feed center, Nahlal, Israel) and pellets (25.5% CP #16100; Ambar feed mills, Hadera, Israel), combined with LGPH hay, the experimental diets were formulated to satisfy the nutritional requirements for small ruminants as outlined by the national research council (National Research Council, 2007). The constituents of TMR and the chemical composition of the ingredients used for dietary formulation are presented in Table 1.

Table 1 Ingredient proportions (%) and chemical compositions of TMR, pellets, and low glycoalkaloids potato haulm.
Control diet (CON) consisted of a maintenance diet made of TMR and pellets (ingredients are presented in Table 1) without addition of LGPH. Whereas treatment diets were formulated by addition of 10%, 15%, and 20% LGPH to make diets P10, P15, and P20, respectively on a DM basis (Table 2). All ingredients (TMR, pellets, and LGPH) diets were mixed manually by hand every morning to create the respected dietary treatments. The particle size distribution of the experimental diets was determined using a Penn State Particle Separator (PPS) with a 19-mm screen (Upper), an 8-mm screen (middle), a 4-mm screen (lower) and a pan (fine) as described by Lammers et al. (1996).
2.3 Ethical approval and experimental site
The experiment was approved by institutional ethics committee of the Hebrew University of Jerusalem (AG-16738) according to regulations of animal use for scientific purposes-directive 2010/63/EU and Israeli law.
The study was conducted at the metabolic experimental farm located at The Robert H. Smith Faculty of Agriculture, Food, and Environment, Rehovot campus (FAHU). The experimental environment was carefully controlled to maintain an ambient temperature between 21 and 25°C, relative humidity levels between 55 to 60% and a 12-hour light/dark cycle daily.
2.4 Animal management and experimental design
Four Asaf female lambs (4 months old) were purchased from a local trustworthy farmer in Israel and transported to the experimental facility at the FAHU. Upon arrival the facility, the sheep were dewormed and vaccinated according to the recommendations of Israeli Ministry of Agriculture and Rural Development and then allowed 1 month of acclimatization before the start of experiment.
The average sheep body weight at the start of the experiment was 44.1 ±1.7 kg. Each sheep was housed in an individual metabolic cage (1.5m x 0.8m) equipped with a drinker to provided ad libitum access to water, as well as an automatic feeder (Ankom Technology, Macedon, NY) set to deliver 12 equal meals per day. Each sheep was offered additional feed allowance to reach up to 10% refusal. A hard plastic net underneath each cage was used for collection of faecal samples.
The study utilized a 4 × 4 Latin square design with four experimental periods, each lasting a total of 21 days, subdivided into 14 days of adaptation followed by 7 days of sampling. During the adaptation period, sheep were put down in a free stall yard to socialize and stretch for a period of 3-4 hours every day. The sheep were randomly and concurrently assigned to one of the four experimental diets: CON, P10, P15, and P20 (Table 2). The dietary treatments were rotated after every 21 days while ensuring that each treatment was allocated once per row and column in the Latin square matrix. During the research, the animals were routinely monitored by a veterinarian for any potential clinical signs of GAs poisoning and other health problems. The lambs were weighed at both the start and end of each experimental period to monitor the average daily weight gain associated with each experimental diet. However, it is crucial to acknowledge that the Latin square design used in this study has its limitations because of the short duration. Therefore the experiments results do not comprehensively describe growth performance compared with continuous design (Hristov et al., 2019).
2.5 In vivo digestibility and sampling procedure
The digestibility experiment was performed according to Omar (2002); Al-zubiadi (2016) and Al-Baadani et al. (2022). During the 7 days of sample collection, the daily amount of feed offered and refused for each animal was recorded, and the daily dry matter intake (DMI) was calculated. Feed samples (both the diet offered and the refusals), and faeces for each sheep were collected daily, weighed, and stored in a labelled plastic bag at -25oC for chemical analysis.
On the 6th day, an hour after the first morning feeding, ≈50 mL of rumen liquid was collected using a vacuum pump (MRC, Holon, Israel) connected to an oro-ruminal tube (VetMarket, Modiin, Israel). To avoid contamination with saliva, the first 50 ml were discarded, and the ruminal tube was inserted to reach the ventral region of the rumen at a predetermined tube length. The pH of the rumen fluid was measured immediately using a pH meter (Satorius Ag-Gottingen, Germany). The concentration of NH3-N in rumen fluid was determined using a spectrophotometer after sample preparation by reacting ammonia with alkaline hypochlorite and phenol in the presence of sodium nitroprusside as a catalyst to create indophenol (Bower and Holm-Hansen, 1980).
Concomitantly, blood samples were collected after the first morning feeding through jugular puncture and quickly transferred into vacutainers (one with EDTA and another with clot activator). The blood samples were then immediately sent to Hebrew University Veterinary Hospital (Bet-Dagan, Rishon Letsiyon, Israel) for quantification of haematological and biochemical parameters using an autoanalyzer spectrophotometer (Roba et al., 2022).
On the last day of every experimental period, urine sample was collected using catheters and stored in urine bags (1.5 Litres; VetMarket, Modiin, Israel) filled with 20 mL of 10% sulphuric acid, (Foley catheter 16 Fr.; Degania Silicone Ltd., Hatzor HaGlili, Israel). The urine excreted by each animal over the duration of 24 hours was pooled, mixed thoroughly, recorded, and 150 mls sample/sheep were stored at -25˚C for determination of urinary nitrogen excretion and energy loss.
2.6 Sample analysis
At the end of each sampling period, the faeces, feeds, and refusals from each animal were thawed, pooled per animal, thoroughly mixed, and 10% (w/w) of the total samples were separately dried in an air-forced oven at 60°C for 48 h. The samples were weighed and ground to pass through a 2 mm screen using a knife mill (Thomas–Willey Laboratory Mill, model 4, Arthur H. Thomas Company, Philadelphia, PA, USA), and then stored in labelled paper bags awaiting analysis.
The chemical analyses were conducted according to AOAC methods (1980). Samples were dried overnight at 105°C in an air-forced oven (WTC Binder, Germany) to measure their dry matter (DM) content. Subsequently, the dried samples were burned in a muffle furnace at 600°C for 5 h to determine their organic matter (OM) content. The ether extract (EE) was quantified by extraction in a Soxhlet apparatus for 6 hours using petroleum ether (40-60˚C; Merck, Rehovot, Israel). The crude protein (CP) content was determined using an automatic Kjeldahl machine (KjelMaster K-375-BUTCHI, Switzerland) and starch was quantified by an enzymatic-colourimetric method (Hall et al., 2015). Neutral detergent fiber (NDF), acid detergent fiber (ADF), cellulose and lignin measurements were performed using the Ankom fiber analyzer (Ankom220 Technology, Macedon, NY) in accordance with Van Soest et al. (1991). A bomb calorimeter (6100, Parr Instrument Company, Moline, IL) was used to measure the gross energy (GE) of samples of experimental diets, refusals, feces, and urine. Benzoic acid was used as the reference standard. Urinary energy was performed on dried urine sample with the aid of paraffin mineral oil. Volatile fatty acids (VFAs) concentration in the rumen liquid samples were determined by gas chromatography (Erwin et al., 1961). Metabolic energy (ME) and combustible gas energy (GasE) were estimated in accordance with the National Research Council recommended protocol (NRC, 2001; National Research Council, 2007).
2.7 Statistical analysis and calculations
The daily dry matter intake (DMI) was calculated on a DM basis using formula: DMI = (amount of feed offered–refused feed). The apparent digestibility coefficient of nutrients was computed as the difference between the nutrients consumed in the diet and those excreted in faeces, expressed as a percentage of intake.
Data for daily dietary intake, nutrient digestibility, ruminal fermentation parameters and blood parameters were analysed using ANOVA on JMP Pro software (SAS Inc.), (2016) employing the Latin square design with diet and period as fixed effects and sheep as random effect. The statistical differences among the diets were determined using Tukey’s Honestly Significant Difference (HSD) for all pairwise experimental diet comparisons. The level of significance was established at P< 0.05. Furthermore, orthogonal contrasts were used to detect potential quadratic effects of LGPH inclusion in the experimental diets. Accordingly, respective equations (either linear or quadratic) were derived and used to estimate (extract) ME and nutrient digestibility of LGPH (Fonnesbeck et al., 1981).
After estimating the ME and GasE (NRC, 2001; National Research Council, 2007), metabolic energy concentration (Mcal/kg DM) was calculated as the ME intake (MEI) divided by its respective DM intake (DMI).
3 Results
3.1 Chemical composition and particle size distribution
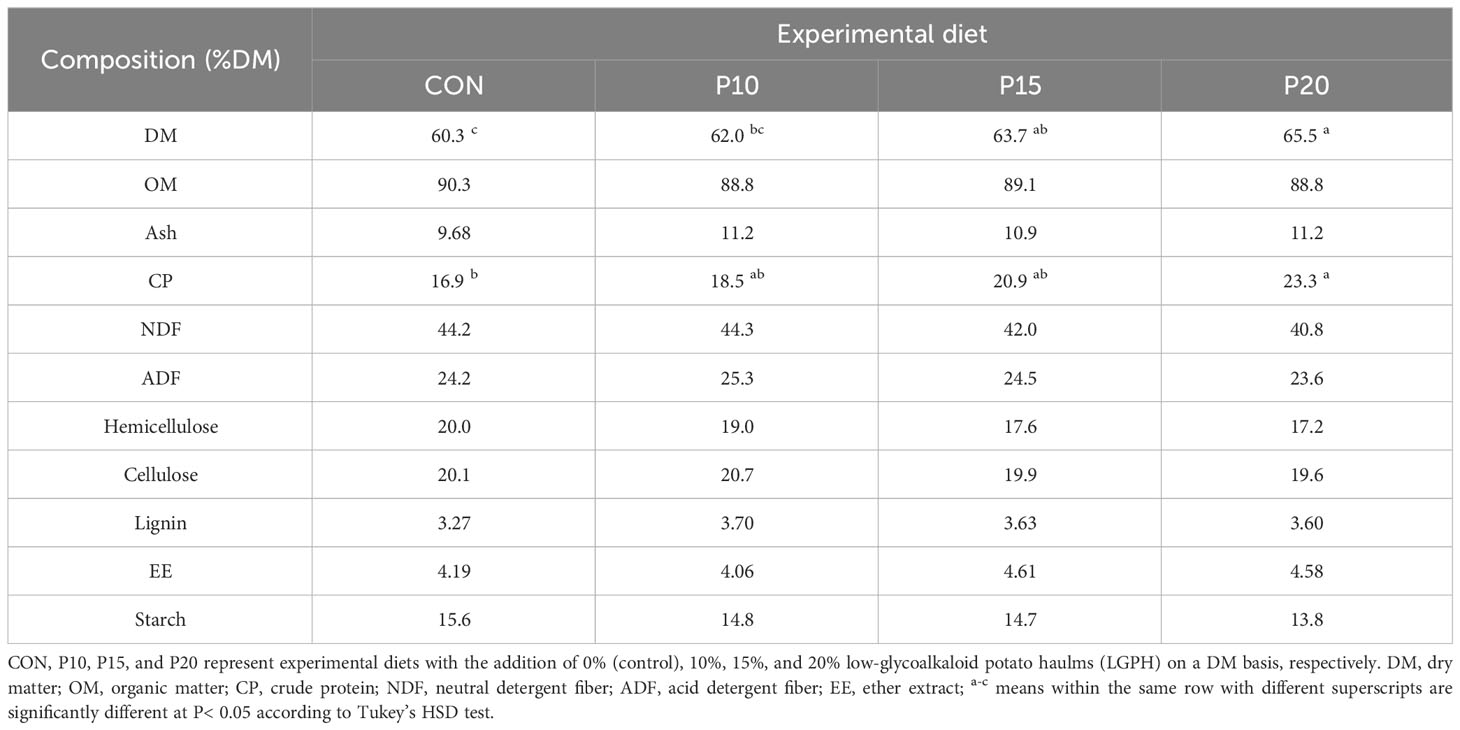
Freshly harvested LGPH has 9.27% DM content, and its chemical composition is shown in Table 1. All the experimental diets showed a similar composition of OM, ash, NDF, ADF, hemicellulose, cellulose, lignin, EE, and starch, whereas the DM and CP content increased linearly with the addition of LGPH (Table 2). Thus, demonstrating the high nutritious quality of the haulm.
The inclusion of LGPH had no effect on particle size distribution in the experimental diets (Table 3). Yet, there was a slight variation in the proportion of DM retained on the different sieves of PSPS. On average, the 4-8 mm particle size constituted the highest DM proportion (29.1 ± 2.32%) followed by 8-19 mm and > 19 mm particle sizes respectively, while the< 4mm particle size constituted the lowest DM proportion (21.7 ± 1.89%) on average.
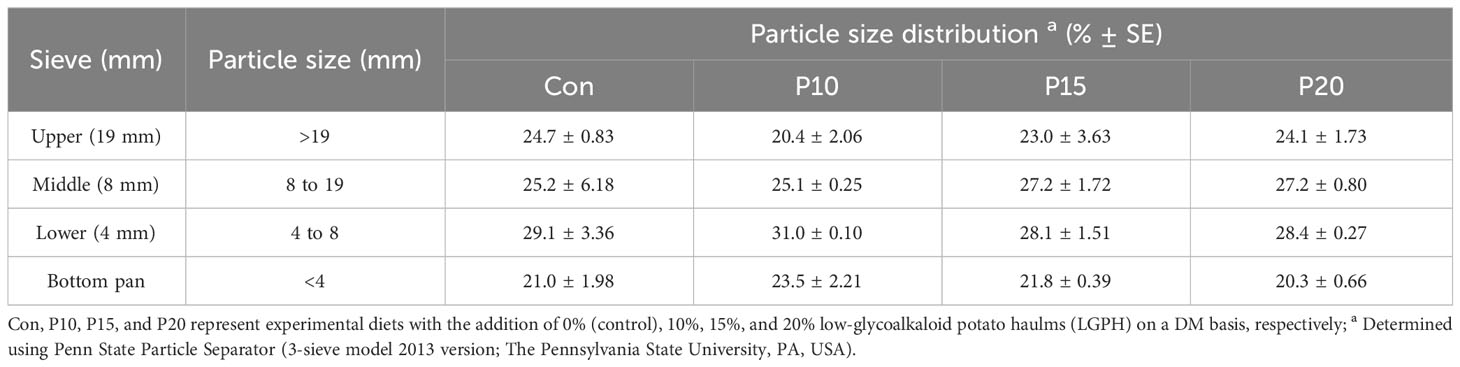
Table 3 Particle size distribution of experimental diets containing different levels of low glycoalkaloids potato haulm.
3.2 Intake and digestibility of experimental diets
The daily dietary intake of lambs fed on LGPH dietary treatments is shown in Table 4. The addition of LGPH had no effect on the daily intake of DM, OM, NDF, EE, starch, GE, and DE. Furthermore, LGPH inclusion in the experimental diets had a significant increase (P= 0.001) in daily CP intake, with diet P20 showing the highest CP intake at 281.7 g/day (Table 4). These findings provide additional evidence that potato haulm possesses a high level of nutritional quality.

Table 4 Nutrient intake, digestibility and metabolizable energy in sheep fed experimental diets containing different levels of low glycoalkaloids potato haulm.
The study further examined the apparent digestibility of nutrients in diets containing different levels of LGPH (Table 4). The apparent digestibility of ADF showed a linear tendency, while that of DM, OM, CP, and GE in lambs increased (P< 0.05) with the LGPH level in the diets. Hence the DM and CP levels of digestibility were 64.7% and 70.6%, respectively for P20, compared with 61.3% and 63.2% and in the control.
Additionally, LGPH had a quadratic effect on the NDF digestibility, with a peak digestibility (54.7%) occurring at 12.3% LGPH in the diet but had no significant effect on the EE, starch, and ADF digestibility. Furthermore, there was no significant differences in ME concentration among the experimental diets. Once again, our findings demonstrate the nutritional advantages of potato haulm.
3.3 Calculations of nutrient digestibility and metabolizable energy of LGPH
Orthogonal contrasts were employed to determine whether the effects of LGPH on the diet were linear or quadratic. Table 5 displays the computed digestibility of DM, OM, CP, NDF, and energy as well as metabolizable energy of LGPH using the linear or quadratic equations.
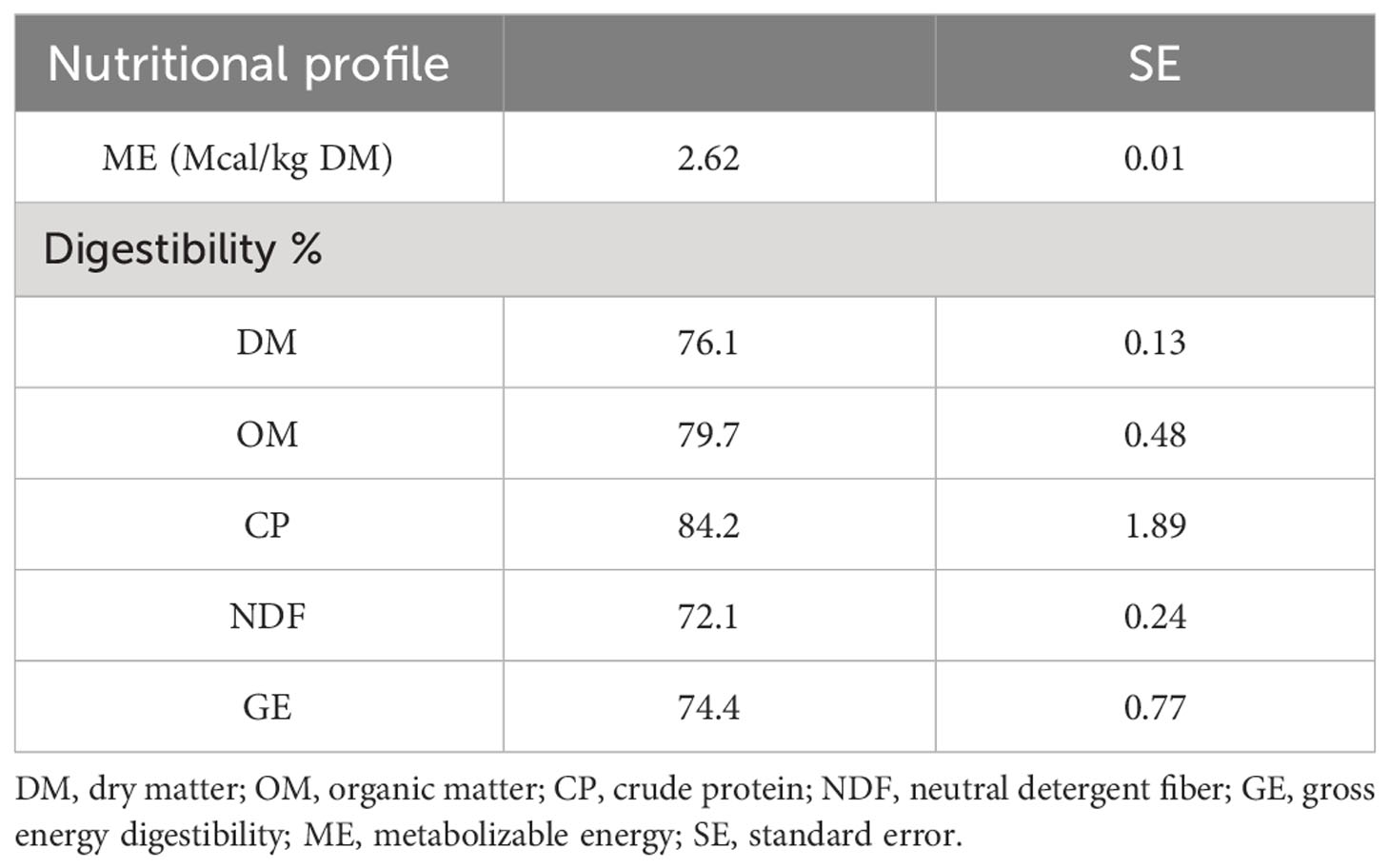
Table 5 Calculated metabolizable energy, and digestibility of nutrients and energy in low glycoalkaloids potato haulm.
The equations for dry matter digestibility (DMD), organic matter digestibility (OMD), crude protein digestibility (CPD), gross energy digestibility (GED), and metabolizable energy (ME) displayed a linear relationship, while the equation for NDF digestibility (NDFD) exhibited a quadratic pattern, as follows:
ME = metabolizable energy calculated based on gross energy of LGPH, and NRC equations (NRC, 2001; National Research Council, 2007). NDFD was extracted at proportion of LGPH (12.3%) in the diet which produced the maximum diet digestibility (54.8%) from the derivative equation. Therefore, supplementing diets with LGPH can potentially improve their nutritional value due to its excellent metabolizable energy and nutrient digestibility.
3.4 Nitrogen balance
The addition of LGPH to the experimental diets increased the daily N intake linearly (P = 0.001), with diet P20 showing the highest intake of 44.5 g/day compared to 33.6 g/day in control diet (Table 6). Furthermore, a corresponding) rise (P< 0.05 in N retention and the ratio of N retention to N intake were observed, with the diet P20 exhibiting the highest values of 19.1g/d and 0.42, respectively. However, dietary supplementation with LGPH had no significant effects on faecal and urinary nitrogen excretion. Therefore, the inclusion of LGPH in diets can lead to an enhanced efficiency of nitrogen utilization.
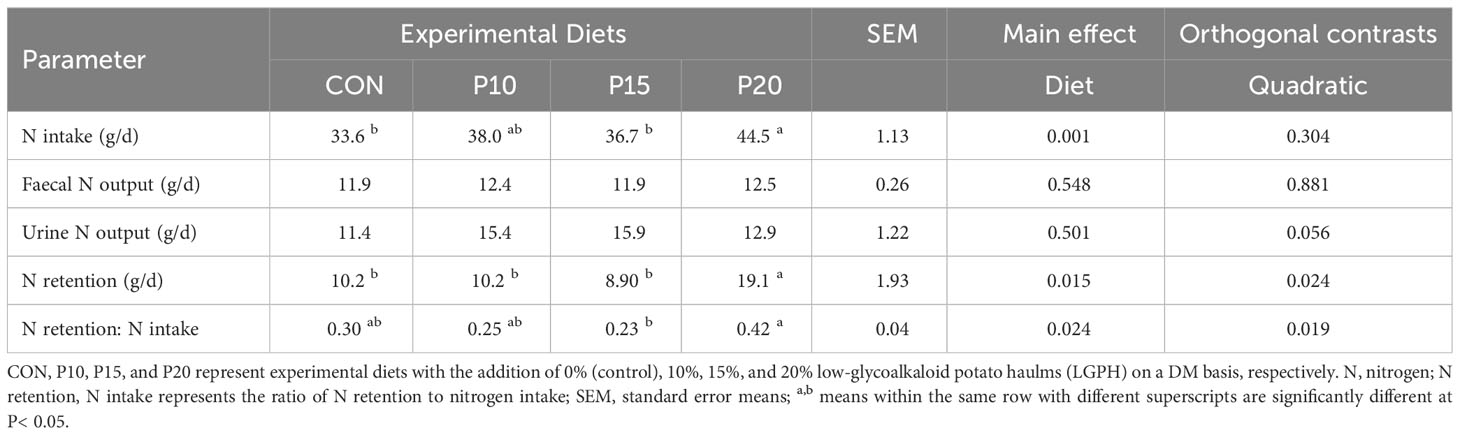
Table 6 Nitrogen balance in sheep fed experimental diets containing different levels of low glycoalkaloids potato haulm.
3.5 Rumen fermentation parameters
We analysed rumen liquids to determine whether addition LGPH to experimental diets affected the rumen fermentation parameters. It was noted that LGPH supplementation had no significant effect on pH, NH3-N, acetic (C2), propionic (C3), butyric (C4), caproic acid (C6) acids, C2:C3 ratio, and total VFA concentration (Table 7). Although the addition of LGPH had no significant effect on total VFA concentration, there was a tendency of a rise in total VFA concentration with increasing levels of LGPH in the treatment diets. However, it is important to highlight that addition LGPH to the treatment diets resulted in a significant (P< 0.05) decrease in levels of isovaleric (Ci5) and valeric (C5) acids in the rumen of the lambs. Therefore, LGPH supplementation maintains the integrity of rumen parameters appears to increase the concentration of total VFAs.
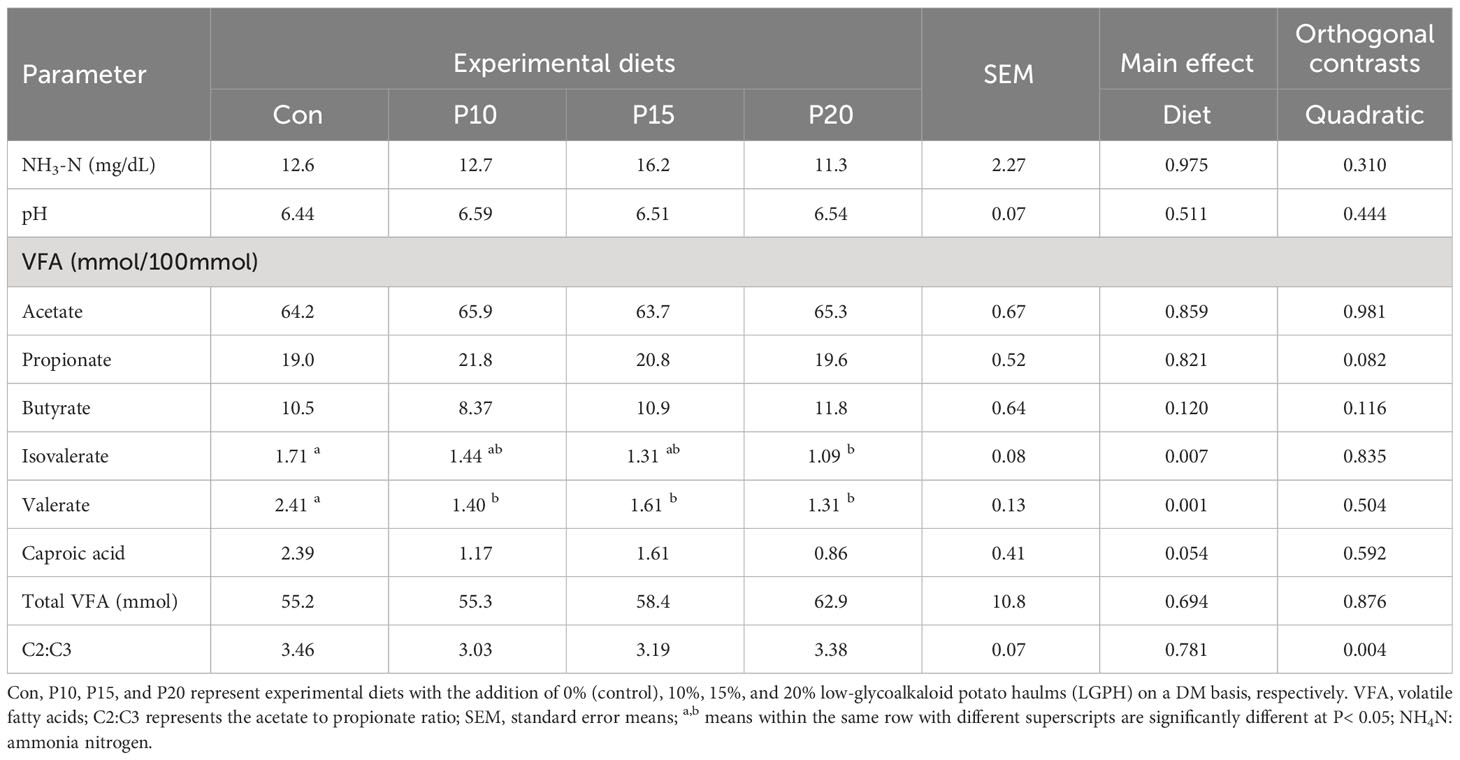
Table 7 Rumen fermentation parameters of sheep fed experimental diets containing different levels of low glycoalkaloids potato haulm.
3.6 Blood parameters
The results (Table 8) show that the LGPH incorporation in sheep’s diet led to an increase in the concentrations of total proteins (TP) and blood urea nitrogen (BUN) in sheep blood. Diet P15 showed a maximum TP level of 6.49 g/dL, whereas diet P20 exhibited the highest BUN concentration of 39.3 mg/dL compared with 5.22 g/dL and 26.6 mg/dL in the control, respectively.
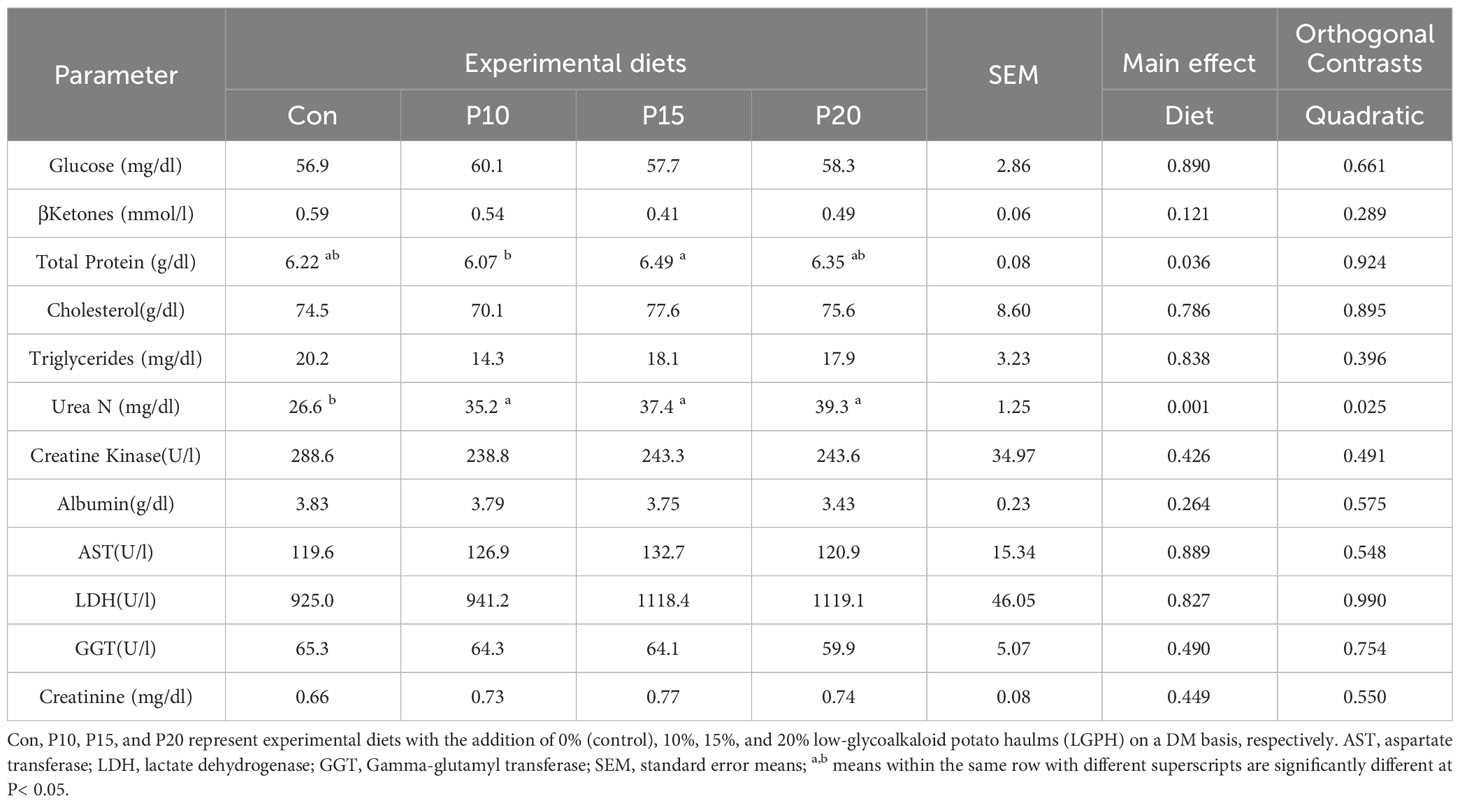
Table 8 Selected blood parameters of sheep fed experimental diets containing different levels of low glycoalkaloids potato haulm.
The dietary treatments had no effect on the levels of hepatic enzymes and the various blood parameters that were assessed in this study. The average body weight of the lambs increased from 44.1 ±1.7 kg at the beginning of the trial to 51.0± 2.4 kg at the end of the trial. Routine check by veterinarian didn’t observe any adverse clinical manifestation. Therefore, by maintaining blood biochemistry within the normal physiological range, it can be inferred that LGPH supplementation does not have any detrimental effects on the health of sheep.
4 Discussions
The current global shortage of feed and the rising demand for feed supply (Makkar, 2018) necessitate immediate attention and innovative approaches to address this pressing issue. Based on available knowledge, it is evident that crop productivity has not been successfully enhanced beyond the intrinsic genetic potential of crops. Consequently, efforts to increase productivity primarily concentrate on improving resilience to biological and climatic challenges, optimizing crop management techniques, and mitigating factors that restrict yield potential.
However, the current study examines the utilization of abundant yet wasted vegetative material, such as discarded potato haulm, as a potential solution to meet the growing demands for food and feed. Recent advancements in producing low-glycoalkaloids potato haulm opened profitable and sustainable possibilities. To ensure informed decision-making regarding the inclusion of LGPH in ruminant diets, it is crucial to assess their nutritional composition digestibility, as well as potential health risks (Assefa and Ledin, 2001). This study, therefore, conducted chemical analyses, in vivo digestibility studies, and evaluated blood and rumen parameters of sheep fed with LGPH for the first time.
4.1 Glycoalkaloid content and nutritional value of LGPH
In comparison to other potato varieties (Dao and Friedman, 1996; Zarzecka et al., 2013), the LGPH has very low levels of alpha-solanine (0.1-2.5 µg/g) and alpha-chaconine (0.4-5.5 µg/g) versus ~500 mg/100 g reported by (Friedman and Dao, 1992). The LGPH lines employed in our studies were developed using gene-editing techniques to target and knock down the genes which code for potato GAs (Krits et al., 2007).
Assessing the chemical composition of forage plants is critical for establishing their usefulness in animal nutrition (Assefa and Ledin, 2001). This study suggests that LGPH has high-potential application in ruminant diet formulation. Our results show that LGPH has higher CP and ash content but lower NDF and ADF content compared to other potato genotypes (Kaplan et al., 2018) studied in Turkey. This can possibly be attributable to differences in genotypes, physiological age, and growing environment (Nicholson et al., 1978; Maga, 1980; Jadhav et al., 1981; Assefa and Ledin, 2001; Kaplan et al., 2018). Nicholson et al. (1978) reported that the CP content in potato vines varies between 8-21% but decreases with prolonged harvest periods. The high CP content (26.3%) in the LGPH could have resulted from not only genetic and environmental factors but also maturity stage. This quality makes LGPH varieties exceptional since protein is one of the most expensive and limiting factor in ruminant nutrition (Parisi et al., 2020). The linear rise in the CP content noted in the treatment diet possibly resulted from the dietary supplementation with LGPH which has high CP content. Additionally, it’s worth noting that the addition of LGPH hay contributed to the observed linear increase in the DM content of the experimental diets which is in agreement with the earlier report by Koli et al. (2019).
This study shows that the LGPH genotypes employed have higher nutrient digestibility compared with potato haulm from other cultivars (Parfitt et al., 1982; Kaplan et al., 2018). The high digestibility noted in this study could be explained by low NDF, ADF and lignin in the LGPH compared other cultivars. High levels of NDF and ADF in the diet is known to slow down digestion but merely give animals sensation of physical fullness and satiety (Riaz et al., 2014). This occurs when high forage diets accumulate in the reticulo-rumen, triggering the stretch receptors on its muscular wall to signal satiety regions of the brain and cause a feeling of fullness (Allen, 2000). Furthermore, it is also possible that the nutrient digestibility in the present study was not affected by the GAs since LGPH had negligible GAs concentration. Previous studies have indicated that GAs can disrupt the integrity of the digestive system (EFSA, 2020). Therefore, LGPH exhibits not only exceptional nutritional composition but also high nutrient digestibility, making it a desirable quality for forage.
4.2 Dry matter intake and nutrient digestibility
Our results show that LGPH was acceptable by the sheep and didn’t affect intake of DM, OM, NDF, EE, starch, and energy in the experimental diets. These findings supports Parfitt et al. (1982) who studied the nutritional value of pressed potato vine silage and reported uniform voluntary intake in goats. This observation in the current study could be because the experimental diets were well-blended with a uniform particle size distribution, which resulted in a consistent palatability of the diets and reduced feed selection by the lambs. It is worth considering that the blocking of GAs biosynthesis also resulted in the absence of GAs-associated bitter sensations in LGPH thus contributing to the normalcy in the voluntary feed intake in the treatment diets (Baumont, 1996; EFSA, 2020).
The increase in CP intake is attributed to the corresponding linear rise in CP content in the experimental diets. This consequently improved nutrient (DM, OM, CP) and energy digestibility, owing to the stimulatory effect of CP on dietary intake and nutrient utilization (Silanikove et al., 1997). Moreover, supplementing protein from foliage has been shown to improve forage digestion (Bodine et al., 2000; Aregheore and Perera, 2004). It is also possible that the higher CP intake resulting from LGPH inclusion synchronized better with nutrient availability, ultimately leading to optimal rumen fermentation and microbial growth (Tripathi et al., 2007). Therefore, the increased digestibility observed in the treatment diets compared to control could have resulted from contribution of LGPH as a supplemented component (Patra, 2010).
The incorporation of LGPH in the treatment diets resulted in a quadratic increase in NDF digestibility, peaking at 54.8% when LGPH was included at a proportion of 12.3% in the diet. The increase in NDF digestibility could be attributed to the optimal degradability of NDF by the rumen microbiomes (Lavrenčič et al., 1997). However, when LGPH was added above 12.3%, NDF digestibility decreased probably due to a reduction in ruminal mat formation and an increased ruminal passage rate (Patra, 2010). Waldo et al. (1972) proposed a compartmental model to calculate digestibility of potential digestible nutrients (PD) as follows: Digestibility = PD × [kd/(kd + kp)]where; kd = the rate of digestion, and kp = rate of passage.
It is generally suggested that an increased kp limits the extent of digestion of potential digestible components of feeds (Waldo et al., 1972). This could explain the decrease in NDF digestibility observed when LGPH was added above 12.3%, as the high LGPH levels may have led to an increased ruminal passage rate, limiting the extent of NDF digestion. To maximize the digestibility of NDF, the inclusion of LGPH in the diet should be optimized in conjunction with coarse roughages and carefully regulated within appropriate limits.
4.3 Nitrogen balance and retention
A synchronized balance between nitrogen and energy availability in the rumen is crucial for attaining maximum microbial protein production, efficient ruminal fermentation, and improved feed utilization in livestock (Cabrita, 2006). Evidently, excess N in the rumen is not utilized for microbial protein production but is added to the ammonia pool, which is then transported to the liver, converted into urea, and largely excreted in as urine (Patra, 2010). This was not the case in the present study where the levels of ruminal NH3-N and urinary N excretion were not affected by dietary treatment. Hence, the inclusion of LGPH in the diets resulted in an increase (P< 0.01) in N retention (g/day), primarily due to higher CP intake and increased CP digestibility, implying optimal level of amino acids are supplied to match physiological requirements of sheep.
The increase in N retention (NR) in animals could have resulted from optimizing the protein-energy balance (P/E) in the treatment diets (Setyono et al., 2022). Consequently, efficiency of N utilisation, expressed as the ratio of N intake to N retention correspondingly exhibited a linear increase. Similar linear increase in N retention upon inclusion of high CP soya hulls in the forage diets response was reported by De Araujo et al. (2008).
Our results show a positive P/E balance that could potentially enhance productivity, particularly weight gain, as reported by Setyono et al. (2022). The metabolizable energy values recorded in the current study are higher than those reported by Kaplan et al. (2018). This dissimilarity could be attributed to the difference in methodologies applied. The current study was conducted in vivo, whereas the former used 24hr gas production and chemical composition to calculate metabolizable energy. Furthermore, the high energy values reported in our study could be due to the physiological age of the sheep, which were young and fast growing (Costa et al., 2013). As sheep grow at high rates, their basal metabolic rates increase, stimulating and enhancing the activities of both the cardiovascular and digestive systems to digest, absorb, and supply the nutrients necessary for their rapid growth (Yang et al., 2020). Our results demonstrate the beneficial effects of adding LGPH to experimental diets, as it improves both N retention and energy balance in lambs.
4.4 Rumen fermentation parameters
Rumen pH, NH3-N, and VFA concentrations are key parameters that reflect the microbial growth and fermentation processes occurring in the rumen. These parameters can be influenced by various factors, such as dietary intake, composition, degradability, fermentation and absorption rate as well as synchrony of available N and energy in the diets (Chumpawadee et al., 2006). To maximise the efficiency of microbial protein synthesis the diets should provide optimum rumen conditions (Cabrita, 2006). For instance, rumen pH as the main variable of fermentation has to remain within the critical range of 6.0-7.0 for optimal nutrient utilisation and microbial growth (Soto et al., 1994). In the current study, rumen pH was not affected by addition of LGPH in the diets and varied between 6.44 and 6.59 probably due to the fact that the diets were mainly made of forage with low proportion of quickly fermentable non-structural carbohydrates that might cause a rapid decline in the rumen pH (Ryle and Ørskov, 1990).
Additionally, VFAs as weak acids that dissociate and release protons in the rumen, potentially contribute to lowering and stabilizing ruminal pH by either eliminating protons via passive diffusion or by secreting bicarbonate via anion exchange pathways (Penner, 2014). Primarily VFAs are synthesised through the fermentation of carbohydrates, as well as by the partial breakdown of proteins and cleavage of lipids (Owens and Basalan, 2016). It is also possible that production of VFAs didn’t exceed the buffering capacity of the saliva and bicarbonate ions (e.g., rumination activity). The lower concentration of total VFAs in our study compared with Ramos et al. (2009) report could be attributed to the difference in the experimental design and timing of rumen liquid collection, as our collection was done in a semi steady-state metabolic conditions, whereas their collection was done two hours after feeding.
Bergman (1990) reported that 75% of metabolizable energy originates from VFAs. Therefore, diets that promote fermentation and VFAs production, also promote productivity. We thus conclude that dietary supplementation with LGPH not only enhanced CP digestibility but also promoted sufficient production of VFAs which potentially improve weight gain and milk production.
The observed decrease (P< 0.05) in isovalerate and valerate levels in the treatment diets compared to the control diets could be attributed to a lower extent of protein breakdown, since the production of these branched-chain VFAs is related to the fermentation of branched-chain amino acids (Ramos et al., 2009).
NH3-N in rumen fluids is mainly produced from the proteolytic breakdown of dietary CP and Non-Protein Nitrogen (NPN). It is then being utilized by rumen microbes for the synthesis of amino acid, proteins, and nucleic acids (Hidanah et al., 2016). Its concentration in the rumen fluids was not affected by diet supplementation with LGPH and remained within the normal ranges of 8.5 to 30mg//100ml (McDonald et al., 2002). It is concluded that supplementation with LGPH supplied sufficient fermentable energy for optimal capture of the N produced.
4.5 Blood parameters
Our findings show that all the values of biochemical parameters fall within the normal physiological range of healthy sheep (Radostits et al., 2007; Kaneko and John Harvey, 2008; Roba et al., 2022). The linear increase in blood TP and BUN in the sheep fed with higher LGPH diets (P15, and P20) could be attributed to corresponding linearly increased dietary CP intake and nutrient digestibility (Yang et al., 1999). Kaneko and John Harvey (2008) showed that serum TP and BUN of healthy sheep range between 6-7.9 g/dL and 10-35mg/dL, respectively. Blood TP levels reflect amino acid synthesis and absorption, while BUN levels accurately indicate short-term dietary impacts on the nitrogen cycle in the rumen, urea synthesis in the liver, recycling, or catabolism (Faryabi et al., 2023). The level of TP in our study were within the normal range, however, BUN was relatively higher in P15, and P20 fed sheep, being 37.4 and 39.3 mg/dL, respectively, probably as a reflection of a high dietary N supply (Luo et al., 2022). The normal levels of serum metabolites enzymes such as creatinine, alanine transferase, aspartate transferase, lactate dehydrogenase, and creatine kinase imply that trace levels of GAs in diets with LGPH didn’t cause adverse toxicological effects on the liver, muscles, and kidney functions in sheep. It is important to note that high GAs in potato vines can be deadly to livestock (Pavlista, 2013). In humans, when the concentration of glycoalkaloids in potato tubers exceeds 200 mg/kg fresh weight, it can cause harmful health effects (Rymuza et al., 2020). As a result, the recommended safe limit for new potato varieties is 100 mg/kg fresh weight (Knuthsen et al., 2009).
In the present study, the low levels of GAs detected in LGPH ranged between 0.4-5.5 µg/g dry weight were probably hydrolysed to solanidine by the rumen microbes (King and McQueen, 1981; El – Shinnawy and Eassawy, 2016). This is consistent with the fact that no observable clinical signs were noticed throughout the trial, suggesting that LGPH is safe and has no toxicological effects on sheep. While our experiment did not primarily focus on growth performance, it is worth noting that we found no significant difference in the daily average weight gain (ADG) of sheep between the LGPH and control diets.
5 Conclusion
The present study demonstrates compelling evidence that LGPH has an excellent nutritional composition comparable to traditional fodder crops like alfalfa with no adverse health effects on sheep. Dietary supplementation with LGPH increases N retention, and DM, OM, CP, NDF, and energy digestibility offering enormous potential to improve growth performance of sheep. Additionally, LGPH has no effect on ruminal pH, NH3-N and total VFAs concentration. In conclusion, LGPH could serve as an excellent valuable source of high-quality protein-rich roughage feedstuff for ruminants with zero environmental burden and beneficial effect on production thus reducing feed-food competition. However, further research is necessary to assess impact of LGPH on growth performance, and milk productivity before practical application on commercial farms.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The animal studies were approved by The Hebrew University of Jerusalem (AG-16738) according to regulations of animal use for scientific purposes-directive 2010/63/EU and Israeli law. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
JK conducted this work and did the analysis and wrote the first draft of the MS. BI helped in conducting the actual experiment with sheep and helped with analysis. PW, and GN contributed to analysis. CS is the lab manager. HR contributed to writing and finalizing the MS. SJM contributed to designing the trail, guidelines, analysis, calculations, funding, and writing. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Al-Baadani H. H., Alowaimer A. N., Al-Badwi M. A., Abdelrahman M. M., Soufan W. H., Alhidary I. A. (2022). Evaluation of the nutritive value and digestibility of sprouted barley as feed for growing lambs: in vivo and in vitro studies. Animals 12 (9), 1–12. doi: 10.3390/ani12091206
Allen M. S. (2000). Effects of diet on short-term regulation of feed intake by lactating dairy cattle. J. Dairy Sci. 83 (7), 1598–1624. doi: 10.3168/jds.S0022-0302(00)75030-2
Al-zubiadi I. A. H. (2016). Effects of substitution barley by 10%, 30% of sprouted barley on rumen characters, digestibility and feed efficiency in diet of Awassi male lambs. Int. J. Sci. Res. (IJSR) 5 (4), 2228–2233. doi: 10.21275/v5i4.nov163174
Aregheore E. M., Perera D. (2004). Effects of Erythrina variegata, Gliricidia sepium and Leucaena leucocephala on dry matter intake and nutrient digestibility of maize stover, before and after spraying with molasses. Anim. Feed Sci. Technol. 111 (1–4), 191–201. doi: 10.1016/j.anifeedsci.2003.06.001
Assefa G., Ledin I. (2001). Effect of variety, soil type and fertiliser on the establishment, growth, forage yield, quality and voluntary intake by cattle of oats and vetches cultivated in pure stands and mixtures. Anim. Feed Sci. Technol. 92 (1–2), 95–111. doi: 10.1016/S0377-8401(01)00242-5
Baumont R. (1996). Palatability and feeding behaviour in ruminants. A review. Annales Zootechnie 45, 385–400. doi: 10.1051/animres:19960501
Baur S., Frank O., Hausladen H., Hückelhoven R., Hofmann T., Eisenreich W., et al. (2021). Biosynthesis of α-solanine and α-chaconine in potato leaves (Solanum tuberosum L.) – A 13CO2 study. Food Chem. 365, 130461. doi: 10.1016/j.foodchem.2021.130461
Bergman E. N. (1990). Energy conttributions of ùvolatile fatty acidds. Int. J. Coaching Sci. 3 (2), 67–78. doi: 10.1152/physrev.1990.70.2.567
Bodine T. N., Purvis H. T., Ackerman C. J., Goad C. L. (2000). Effects of supplementing prairie hay with corn and soybean meal on intake, digestion, and ruminal measurements by beef steers. J. Anim. Sci. 78 (12), 3144–3154. doi: 10.2527/2000.78123144x
Bower C. E., Holm-Hansen T. (1980). A salicylate–hypochlorite method for determining ammonia in seawater. Can. J. Fisheries Aquat. Sci. 37 (5), 413–420. doi: 10.1139/f80-106
Cabrita A. R. J. (2006). Evaluation of the effects of synchronising the availability of N and energy on rumen function and production responses of dairy cows – a review. Anim. Res. 55 (February), 1–24. doi: 10.1051/animres:2005045
Campos H., Ortiz O. (2019). “The potato crop: Its agricultural, nutritional and social contribution to humankind,” in The Potato Crop: Its Agricultural, Nutritional and Social Contribution to Humankind. (Switzerland AG, Cham, Switzerland: Springer Nature). doi: 10.1007/978-3-030-28683-5
Chain E. P., Schrenk D., Bignami M., Bodin L., Chipman J. K., del Mazo J., et al (2020). Risk assessment of glycoalkaloids in feed and food, in particular in potatoes and potato‐derived products. EFSA J. 18, e06222.
Chumpawadee S., Sommart K., Vongpralub T., Pattarajinda V. (2006). Effects of synchronizing the rate of dietary energy and nitrogen release on ruminal fermentation, microbial protein synthesis, blood urea nitrogen and nutrient digestibility in beef cattle. Asian-Australasian J. Anim. Sci. 19 (2), 181–188. doi: 10.5713/ajas.2006.181
CIP. (2017). “Potato Facts and Figures.” (International Potato Center). Available at: cipotato.org/potato/potato-facts-and-figures/https://cipotato.org/potato/potato-facts-and-figures/.
Costa M. R. G. F., Pereira E. S., Silva A. M. A., Paulino P. V. R., Mizubuti I. Y., Pimentel P. G., et al. (2013). Body composition and net energy and protein requirements of Morada Nova lambs. Small Ruminant Res. 114 (2–3), 206–213. doi: 10.1016/j.smallrumres.2013.06.014
Dao L., Friedman M. (1996). Comparison of glycoalkaloid content of fresh and freeze-dried potato leaves determined by HPLC and colorimetry. J. Agric. Food Chem. 44 (8), 2287–2291. doi: 10.1021/jf9502820
De Araujo R. C., Pires A. V., Susin I., Urano F. S., Mendes C. Q., Rodrigues G. H., et al. (2008). Apparent digestibility of diets with combinations of soybean hulls and coastcross (Cynodon sp.) hay offered to ram lambs. Scientia Agricola 65 (6), 581–588. doi: 10.1590/S0103-90162008000600003
Dijkstra N. D., Reestman A. J. (1943). Potato haulms as feed for cattle. Landbouwkundig Tijdschrift 55, 191–210.
EFSA Panel on Contaminants in the Food Chain (CONTAM) (2020). Risk assessment of glycoalkaloids in feed and food, inparticular in potatoes and potato-derived products. EFSA J. published by John Wiley Sons Ltd behalf of Eur. Food Saf. Authority, 80. doi: 10.2903/j.efsa.2020.6222
El – Shinnawy A., Eassawy M. (2016). Improving potato vine utilization by sheep using biological treatment. J. Anim. Poult. Prod. 7 (9), 331–338. doi: 10.21608/jappmu.2016.48722
Erwin E. S., Marco G. J., Emery E. M. (1961). Volatile fatty acid analyses of blood and rumen fluid by gas chromatography. J. Dairy Sci. 44 (9), 1768–1771. doi: 10.3168/jds.S0022-0302(61)89956-6
FAO (2018) Transforming the livestock sector through the Sustainable Development Goals. Available at: http://www.fao.org/3/CA1201EN/ca1201en.pdf.
FAO (2023). Crop Prospects and Food Situation – Quarterly Global Report No. 2, July 2023. Rome. doi: 10.4060/cc6806en
Food and Agriculture Organization Of The United Nation (2021). Systems at breaking point. Available at: cipotato.org/potato/potato-facts-and-figures/https://cipotato.org/potato/potato-factsand-figures/.
Faryabi R., Mousaie A., Bahrampour J., Barazandeh A. (2023). The effect of dietary inclusion of Artemisia sieberi leaves on growth performance, feeding behaviors, ruminal fermentation, feed digestibility, and blood hemato-biochemical profile of growing male lambs. Trop. Anim. Health Prod. 55 (1), 41–55. doi: 10.1007/s11250-023-03455-0
Fonnesbeck P. V., Christiansen M. L., Harris L. E. (1981). Linear models for calculating digestibile energy for sheep diets 1 2 . J. Anim. Sci. 52 (5), 1183–1196. doi: 10.2527/jas1981.5251183x
Friedman M., Dao L. (1992). Distribution of glycoalkaloids in potato plants and commercial potato products. J. Agric. Food Chem. 40 (3), 419–423. doi: 10.1021/jf00015a011
Friedman M., Levin C. E. (2016). “Glycoalkaloids and calystegine alkaloids in potatoes,” in Advances in Potato Chemistry and Technology, 2nd ed. (Amsterdam, The Netherland: Elsevier Inc). doi: 10.1016/B978-0-12-800002-1.00007-8
Friedman M., McDonald G. M. (1997). Potato glycoalkaloids: chemistry, analysis, safety, and plant physiology. Crit. Rev. Plant Sci. 16 (1), 55–132. doi: 10.1080/07352689709701946
Hall M. B., Arbaugh J., Binkerd K., Carlson A., Thi Doan T., Grant T., et al. (2015). Determination of dietary starch in animal feeds and pet food by an enzymatic-colorimetric method: Collaborative study. J. AOAC Int. 98 (2), 397–409. doi: 10.5740/jaoacint.15-012
Hidanah S., Nazar D. S., Supranianondo K., Sidik R., Mangkoedihardjo S. (2016). Volatile fatty acids and ammonia levels in local sheep’s rumen fluid fed with fermented rice straw. Int. J. Eng. Technol. 8 (2), 1324–1328.
Hristov A. N., Bannink A., Crompton L. A., Huhtanen P., Kreuzer M., McGee M., et al. (2019). Invited review: Nitrogen in ruminant nutrition: A review of measurement techniques. J. Dairy Sci. 102 (7), 5811–5852. doi: 10.3168/jds.2018-15829
International, A. (1980). AOAC: Official Methods of Analysis 1980 (Maryland, USA: AOAC International, Gaithersburg), Vol. 552.
Jadhav S. J., Sharma R. P., Salunkhe D. K. (1981). Naturally occurring toxic alkaloids in foods. CRC Crit. Rev. Toxicol. 9 (1), 21–104. doi: 10.3109/10408448109059562
Johansen I. E., Liu Y., Jørgensen B., Bennett E. P., Andreasson E., Nielsen K. L., et al. (2019). High efficacy full allelic CRISPR/Cas9 gene editing in tetraploid potato. Sci. Rep. 9 (1), 1–7. doi: 10.1038/s41598-019-54126-w
Kaneko J., John Harvey M. B. (2008). Clinical Biochemistry of Domestic Animals. 6th ed. Eds. Jiro Kaneko M. B., John Harvey (Elsevier press). Available at: https://www.sciencedirect.com/book/9780123704917/clinical-biochemistry-of-domestic-animals.
Kaplan M., Ulger I., Kokten K., Uzun S., Oral E. V., Ozaktan H., et al. (2018). Nutritional composition of potato (Solanum tuberosum L.) Haulms. Prog. Nutr. 20 (July), 90–95. doi: 10.23751/pn.v20i1-S.5541
Karan Y. B. (2021). The impact of haulm killing on yield and quality of potato (Solanum tuberosum L.). PloS One 16 (8 August), 1–9. doi: 10.1371/journal.pone.0255536
Kaur L., Singh J. (2016). “Novel applications of potatoes,” in Advances in Potato Chemistry and Technology, 2nd ed. (Amsterdam, The Netherland: Elsevier Inc). doi: 10.1016/B978-0-12-800002-1.00021-2
Kempenaar C., Struik P. C. (2007). The canon of potato science: 33. Haulm killing. Potato Res. 50 (3–4), 341–345. doi: 10.1007/s11540-008-9082-5
Kilama J., Yakir Y., Shaani Y., Adin G., Kaadan S., Wagali P., et al. (2023). Heliyon Chemical composition , in vitro digestibility , and storability of selected agro-industrial by-products: Alternative ruminant feed ingredients in Israel. Heliyon 9 (3), e14581. doi: 10.1016/j.heliyon.2023.e14581
King R. R., McQueen R. E. (1981). Transformations of potato glycoalkaloids by rumen microorganisms. J. Agric. Food Chem. 29 (5), 1101–1103. doi: 10.1021/jf00107a056
Knuthsen P., Jensen U., Schmidt B., Larsen I. K. (2009). Glycoalkaloids in potatoes: Content of glycoalkaloids in potatoes for consumption. J. Food Composition Anal. 22 (6), 577–581. doi: 10.1016/j.jfca.2008.10.003
Koli P., Kumar Misra A., Kunwar Singh K. (2019). Utilization of potato haulms: an alternate feed resource for livestock. Acta Sci. Agric. 3 (8), 83–85. doi: 10.31080/asag.2019.03.0568
Krits P., Fogelman E., Ginzberg I. (2007). Potato steroidal glycoalkaloid levels and the expression of key isoprenoid metabolic genes. Planta 227 (1), 143–150. doi: 10.1007/s00425-007-0602-3
Lammers B. P., Buckmaster D. R., Heinrichs A. J. (1996). A simple method for the analysis of particle sizes of forage and total mixed rations. J. Dairy Sci. 79 (5), 922–928. doi: 10.3168/jds.S0022-0302(96)76442-1
Lavrenčič A., Stefanon B., Susmel P. (1997). An evaluation of the Gompertz model in degradability studies of forage chemical components. Anim. Sci. 64 (3), 423–431. doi: 10.1017/S1357729800016027
Loh Z. H., Ouwerkerk D., Klieve A. V., Hungerford N. L., Fletcher M. T. (2020). Toxin degradation by rumen microorganisms: A review. Toxins 12 (10), 1–37. doi: 10.3390/toxins12100664
Luo S. F., Wang Y. C., Wang X., Dai C. P., Wang Q. Y. (2022). Dietary energy and protein levels on lactation performance and progeny growth of Hu sheep. J. Appl. Anim. Res. 50 (1), 526–533. doi: 10.1080/09712119.2022.2110501
Maga A. J. (1980). “Potato glycoalkaloids,” in C R C Critical Reviews in Food Science and Nutrition, vol. 12. . doi: 10.1080/10408398009527281
Makkar H. P. S. (2014). Sustainable increase in livestock productivity in developing countries through efficient utilisation of feed resources. Cuban J. Agric. Sci. 48 (1), 55–58.
Makkar H. P. S. (2018). Review: Feed demand landscape and implications of food-not feed strategy for food security and climate change. Animal 12 (8), 1744–1754. doi: 10.1017/S175173111700324X
McDonald P., Edwards R. A., Greenhalgh J. D. F., Morgan C. A. (2002). Animal Nutrition. Microbial digestion in ruminants and other herbivores. Sixth edition (Pretice Hall. Gosport,London: Pearson Education Limited, Harlow. United Kingdom), 187–190. Available at: https://scholar.google.co.uk/scholar?hl=en&as_sdt=0%2C5&q=McDonald%2C+P%2CR.A.%2C+Edwards%2C+and+Greenhalgh%2C+J.D.F.%2C+C.A.+Morgan.+Animal+Nutrition.+Sixth+Edition.+Pretice+Hall.+Gosport%2CLondon%2C+2002.&btnG=.
Mohapatra P. P., Batra V. K. (2017). Tissue culture of potato (Solanum tuberosum L.): A review. Int. J. Curr. Microbiol. Appl. Sci. 6 (4), 489–495. doi: 10.20546/ijcmas.2017.604.058
Morris S., Lee T. H. (1984). The toxicity and teratogenicity of Solanaceae glycoalkaloids, particularly those of the potato (Solanum tuberosum): a review. Food Technol. Aust. 36 (3), 118–124.
National Research Council (2001). Nutrient Requirements of Dairy Cattle Vol. 123 (Washington, DC, USA: The National Academies Press). doi: 10.17226/9825
Nicholson J. W. G., Young D. A., Mcqueen R. E., De Jong H., Wood F. A. (1978). the feeding value potential of potato vines. Can. J. Anim. Sci. 58 (4), 559–569. doi: 10.4141/cjas78-074
Noordar H., Malecky M., Jahanian Najafabadi H., Navidshad B. (2017). Evaluating nutritional value of processed potato vines by in vitro gas production. New Z. J. Agric. Res. 60 (2), 189–204. doi: 10.1080/00288233.2017.1296471
National Research Council (2007). Nutrient Requirements of Small Ruminants: Sheep, Goats, Cervids, and New World Camelids. Washington, DC, USA: National Academies Press.
Omar J. M. A. (2002). Effects of feeding different levels of sesame oil cake on performance and digestibility of Awassi lambs. Small Ruminant Res. 46 (2–3), 187–190. doi: 10.1016/S0921-4488(02)00173-6
Owens F. N., Basalan M. (2016). Ruminal Fermentation BT - Rumenology. Eds. Millen D. D., De Beni Arrigoni M., Lauritano Pacheco R. D. (Heidelberg, Germany: Springer International Publishing), 63–102. doi: 10.1007/978-3-319-30533-2_3
Parfitt D. E., Peloquin S. J., Jorgensen N. A. (1982). The nutritional value of pressed potato vine silage. American Potato J. 59, 415–423. doi: 10.1007/BF02874509
Parisi G., Tulli F., Fortina R., Marino R., Bani P., Dalle Zotte A., et al. (2020). Protein hunger of the feed sector: the alternatives offered by the plant world. Ital. J. Anim. Sci. 19 (1), 1205–1227. doi: 10.1080/1828051X.2020.1827993
Patra A. K. (2010). Effects of supplementing low-quality roughages with tree foliages on digestibility, nitrogen utilization and rumen characteristics in sheep: A meta-analysis. J. Anim. Physiol. Anim. Nutr. 94 (3), 338–353. doi: 10.1111/j.1439-0396.2008.00914.x
Pavlista A. (2013). Potato Vines Can Be Deadly to Cattle and Other Livestock (Nebraska, USA: IANR News, Nebraska Institute of Agriculture and Natural Resources, 0107300, 107300). Available at: ianrnews.unl.edu/0107300.html.
Pavlista A. D., Rush I. G. (2002). EC02-152-S value of potatoes for feeding livestock. Historical Mater. Univ. Nebraska-Lincoln Extension, 4754.
Penner G. B. (2014). “Mechanisms of volatile fatty acid absorption and metabolism and maintenance of a stable rumen environment,” in 25th Florida Ruminant Nutrition Symposium, 2014 - Animal.Ifas.Ufl.Edu, Vol. 306. 92–107.
Radostits O. M., Gay C. C., Hinchcliff K. W., Constable P. D. (2007). “A textbook of the disease of cattle, horses, sheep, pigs and goats,” in Veterinary Medicine (London. UK: Balliere Tindall), 1452–1461.
Ramos S., Tejido M. L., Martínez M. E., Ranilla M. J., Carro M. D. (2009). Microbial protein synthesis, ruminal digestion, microbial populations, and nitrogen balance in sheep fed diets varying in forage-to-concentrate ratio and type of forage. J. Anim. Sci. 87 (9), 2924–2934. doi: 10.2527/jas.2009-1938
Riaz M. Q., Südekum K. H., Clauss M., Jayanegara A. (2014). Voluntary feed intake and digestibility of four domestic ruminant species as influenced by dietary constituents: A meta-analysis. Livestock Sci. 162 (1), 76–85. doi: 10.1016/j.livsci.2014.01.009
Roba R. B., Letta M. U., Aychiluhim T. N., Minneeneh G. A. (2022). Intake, digestibility, growth performance and blood profile of rams fed sugarcane bagasse or rice husk treated with Trichoderma viride and effective microorganisms. Heliyon 8 (12), e11958. doi: 10.1016/j.heliyon.2022.e11958
Ryle M., Ørskov E. R. (1990). Energy nutrition in ruminants. Energy Nutr. Ruminants. doi: 10.1007/978-94-009-0751-5
Rymuza K., Gugała M., Zarzecka K., Sikorska A., Findura P., Malaga-Toboła U., et al. (2020). The effect of light exposures on the content of harmful substances in edible potato tuber. Agric. (Switzerland) 10 (5), 1–11. doi: 10.3390/agriculture10050139
Saleh M. (2014). Effect of natural total glycoalkaloids in discarded potato tubers and vines on milk yield and rumen enveronmental in dairy Zaribi goats. J. Anim. Poult. Prod. 5 (12), 737–757. doi: 10.21608/jappmu.2014.70752
Sawai S., Ohyama K., Yasumoto S., Seki H., Sakuma T., Yamamoto T., et al. (2014). Sterol side chain reductase 2 is a key enzyme in the biosynthesis of cholesterol, the common precursor of toxic steroidal glycoalkaloids in potato. Plant Cell 26 (9), 3763–3774. doi: 10.1105/tpc.114.130096
Scott G. J., Suarez V. (2012). From Mao to McDonald’s: emerging markets for potatoes and potato products in China 1961-2007. Am. J. Potato Res. 89 (3), 216–231. doi: 10.1007/s12230-012-9246-3
Setyono W., Kustantinah K., Yusiati L. M., Suwignyo B., Umami N. (2022). “Nitrogen Balance of Thin Tailed Sheep with the Addition of Soybean Meal and Artocarpus heterophyllus in Pennisetum purpureum cv. Mott as Basal Feed,” in Proceedings of the 9th International Seminar on Tropical Animal Production (ISTAP 2021), Vol. 18. 108–111. doi: 10.2991/absr.k.220207.022
Silanikove N., Gilboa N., Nitsan Z. (1997). Interactions among tannins, supplementation and polyethylene glycol in goats given oak leaves: Effects on digestion and food intake. Anim. Sci. 64 (3), 479–483. doi: 10.1017/S135772980001609X
Soto R. C., Muhammed S. A., Newbold C. J., Stewart C. S., Wallace R. J. (1994). Influence of peptides, amino acids and urea on microbial activity in the rumen of sheep receiving grass hay and on the growth of rumen bacteria in vitro. Anim. Feed Sci. Technol. 49 (1–2), 151–161. doi: 10.1016/0377-8401(94)90088-4
Tripathi M. K., Chaturvedi O. H., Karim S. A., Singh V. K., Sisodiya S. L. (2007). Effect of different levels of concentrate allowances on rumen fluid pH, nutrient digestion, nitrogen retention and growth performance of weaner lambs. Small Ruminant Res. 72 (2–3), 178–186. doi: 10.1016/j.smallrumres.2006.10.008
Van Soest P. J., Robertson J. B., Lewis B. A. (1991). Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74 (10), 3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2
Waldo D. R., Smith L. W., Cox E. L. (1972). Model of cellulose disappearance from the rumen. J. Dairy Sci. 55 (1), 125–129. doi: 10.3168/jds.S0022-0302(72)85442-0
Wang S., Panter K. E., Gaffield W., Evans R. C., Bunch T. D. (2005). Effects of steroidal glycoalkaloids from potatoes ( Solanum tuberosum ) on in vitro bovine embryo development ଝ. Anim. Reprod. Sci. 1, 243–250. doi: 10.1016/j.anireprosci.2004.06.002
Yang W. Z., Beauchemin K. A., Rode L. M. (1999). Effects of an enzyme feed additive on extent of digestion and milk production of lactating dairy cows. J. Dairy Sci. 82 (2), 391–403. doi: 10.3168/jds.S0022-0302(99)75245-8
Yang C. T., Wang C. M., Zhao Y. G., Chen T. B., Aubry A., Gordon A. W., et al. (2020). Updating maintenance energy requirement for the current sheep flocks and the associated effect of nutritional and animal factors. Animal 14 (2), 295–302. doi: 10.1017/S1751731119002064
Zarzecka K., Gugala M., Mystkowska I. (2013). Glycoalkaloid contents in potato leaves and tubers as influenced by insecticide application. Plant Soil Environ. 59 (4), 183–188. doi: 10.17221/763/2012-pse
Keywords: digestibility, feed, low-glycoalkaloid potato haulm (LGPH), metabolizable energy, nutrition
Citation: Kilama J, Izhiman B, Wagali P, Sabastian C, Ngomuo G, Rabinowitch H and Mabjeesh SJ (2024) Novel quality feed from a wasted resource: measuring the nutritional value of low-glycoalkaloids potato haulm in sheep. Front. Anim. Sci. 4:1242989. doi: 10.3389/fanim.2023.1242989
Received: 20 June 2023; Accepted: 08 December 2023;
Published: 04 January 2024.
Edited by:
Americo Froes Garcez Neto, Federal University of Bahia (UFBA), BrazilReviewed by:
Obert Chenjerayi Chikwanha, Stellenbosch University, South AfricaDaniel Menezes, Federal University of São Francisco Valley, Brazil
Copyright © 2024 Kilama, Izhiman, Wagali, Sabastian, Ngomuo, Rabinowitch and Mabjeesh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sameer J. Mabjeesh, c2FtZWVyLm1hYmplZXNoQG1haWwuaHVqaS5hYy5pbA==
 Justine Kilama
Justine Kilama Batool Izhiman1
Batool Izhiman1 Philip Wagali
Philip Wagali Godliver Ngomuo
Godliver Ngomuo Sameer J. Mabjeesh
Sameer J. Mabjeesh