
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Anim. Sci., 30 May 2023
Sec. Animal Nutrition
Volume 4 - 2023 | https://doi.org/10.3389/fanim.2023.1204767
 Rosaria Arena1†
Rosaria Arena1† Simona Manuguerra1†
Simona Manuguerra1† Eleonora Curcuraci1
Eleonora Curcuraci1 Maria Cusimano2
Maria Cusimano2 Daniela Lo Monaco2
Daniela Lo Monaco2 Calogero Di Bella2
Calogero Di Bella2 Andrea Santulli1,3
Andrea Santulli1,3 Concetta Maria Messina1*
Concetta Maria Messina1*The black soldier fly (BSF) (Hermetia illucens L.) is one of the most promising species for the production of ingredients, mainly protein, useful for animal feed formulation, owing to its ability to convert organic waste into biomass with a high nutritional value. However, the low percentage of n-3 series polyunsaturated fatty acids (PUFAs) in its fatty acid profile is a limiting factor for the utilization of BSF in fish feed. Recent studies have highlighted that wastes derived from different agro-food value chains could affect the nutritional composition of BSF larvae, depending on the composition of the wastes used as larvae-growing substrate. Due to the significant amount of n-3 PUFA in marine products, both in edible produce and in by-products, in this study, different sources of fish by-products were included in experimental diets for BSF to evaluate the effect of its addition on the final nutritional profile of BSF pre-pupae. One control diet and five experimental diets were prepared to feed the BSF larvae: wheat bran as the control diet (diet B), bycatch from Mediterranean trawl fisheries (diet F), Parapenaeus longirostris processing by-products (diet S), aquaculture processing by-products (diet R), Thunnus albacares processing by-products (diet T), and Engraulis encrasicolus processing by-products (diet A). In this study, the effects of the different diets were analyzed on the growth, body composition, and fatty acid profile of BSF larvae and pre-pupae. The obtained results showed that the different experimental diets affected total lipids content and fatty acids composition, when compared with the control. A significant increase in eicosapentaenoic (EPA) and docosahexaenoic acid (DHA) in BSF larvae and pre-pupae fed with all fish by-products was observed when compared with those fed with diet B, in particular in larvae and pre-pupae fed with diet A, demonstrating that the utilization of fish processing by-products is a suitable solution for improving the nutritional value of insects as ingredients in aqua feeds. The reuse of marine by-products can contribute to the industry’s “zero waste” goal, increasing the sustainability of the fishery value chain and the formulation of new valuable products.
Global fisheries and aquaculture production increased in 2020 due to the constant expansion of aquaculture (FAO, 2022), which reached the value of 49.2% in 2020 on the global production of aquatic animals (FAO, 2022). The growth of global aquaculture production has consequently led to an increase in the demand for fishmeal (FM) and fish oil (FO), which are indispensable ingredients for satisfying fish nutritional requirements. As the production of FM and FO fluctuates according to catches, the decrease in catches reported over the past 3 years (–4.0%) (FAO, 2022) has led to a gradual decrease in the supply of FM and FO, with a consequent increase in price and availability. This situation has stimulated the need to find additional or alternative sources of these ingredients for the sustainable growth of aquaculture (Ayoola, 2010; Barroso et al., 2014; Liland et al., 2017; Belghit et al., 2018; Belghit et al., 2019; FAO, 2020; Maiolo et al., 2020; Hoc et al., 2021).
In recent years, insects have received growing attention as a food source, owing to their potential as a sustainable, alternative source of proteins in FM for commercial aqua feed formulations and to their relatively easy cultivation (Ng et al., 2001; Osimani et al., 2017; Purschke et al., 2018; Govorushko, 2019; Huang et al., 2019; Alfiko et al., 2022).
Insects are good sources of proteins, fats, minerals, vitamins, and energy (Rumpold and Schlüter, 2013; Gasco et al., 2020). Traditionally used as a food in different countries, their cultivation and commercialization has recently become of global interest as a source of protein and fat for human food and animal food (Starčević et al., 2017).
Since the 1970s, insects have been studied as a protein source in animal feed, mainly due to their ability to convert food waste (e.g., vegetable, fruit, factory, and animal tissue waste) into high-quality protein (Calvert et al., 1969; Ichhponani and Malik, 1971; Teotia and Miller, 1974; Phelps et al., 1975; Newton et al., 1977; Belghit et al., 2019; Oonincx et al., 2019). An important group of insects, able to consume different decaying organic substrates for their larval development, are known as bioconverter species. Among bioconverter insects, the most promising species for industrial production and animal feed in the world is Hermetia illucens (order Diptera, family Stratiomyidae), better known as the black soldier fly (BSF) (Surendra et al., 2016; Müller et al., 2017). The BSF life cycle, which lasts from 6 to 7 weeks (Maroušek et al., 2022), can be divided into four stages: egg, larva, pre-pupa, and adult (Li et al., 2011; Liu et al., 2017). From the pre-pupa stage, the organism stops feeding in preparation for metamorphosis and uses the nutrients stored from the larval stage (Sheppard et al., 2002).
BSF larvae under optimal growth conditions (27°C temperature and 70% humidity) are able to bioconvert different organic substrates (of both animal and vegetable origin) into larval biomass in just 14 days (Franco et al., 2021). Among the large variety of organic materials that BSF is able to feed on, agro-food waste is receiving increasing attention (Nguyen et al., 2015; Jucker et al., 2017). Through the consumption of organic waste material, the insect gains energy and nutrients useful for its growth and then converts itself into a biomass that can be used as feed for other livestock and human food (Franco et al., 2021). Thus, the BSF can be reared as a feed ingredient [approved by the Association of American Feed Control Officials and by the European Food Safety Authority (EFSA)] using approved feed-grade materials, including food waste and by-products of food production (Lähteenmäki-Uutela et al., 2021; Maroušek et al., 2022). Several EU member countries have requested a food safety evaluation by the EFSA of new substrates to be used for BSF feeding (Lähteenmäki-Uutela et al., 2021).
In recent years, the use of insect-based meal in aquaculture has grown exponentially, due to its nutritional value, including as a protein source (Ogunji et al., 2006; Belghit et al., 2018; Belghit et al., 2019; Guerreiro et al., 2020; Karapanagiotidis et al., 2023). Several studies have reported on the importance of BSF as a source of protein (Chaklader et al., 2020; Maiolo et al., 2020; Chaklader et al., 2021); however, few studies have reported on the possibility of exploiting BSF as a source of fatty acids (Barroso et al., 2019; Hossain et al., 2023). The low levels of n-3 series polyunsaturated fatty acids (PUFAs), such as the two PUFAs essentials for fish, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), in the lipids of BSF is one of the limiting factors for its utilization in diets for farmed fish (Oonincx et al., 2020). In fact, this nutritional gap could compromise the growth, health, and quality of farmed fish (Sealey et al., 2011; Kroeckel et al., 2012; Karapanagiotidis et al., 2014; Guerreiro et al., 2020).
Recent studies have shown that the fatty acid composition of BSF larvae can be modified through the use of different feeding substrates, acquiring a new value (Surendra et al., 2016; Liland et al., 2017; Spranghers et al., 2017; Barroso et al., 2019; Bava et al., 2019; Starcevic et al., 2019; Lopes et al., 2020; Franco et al., 2021; Hoc et al., 2021). In view of this, the possibility to enrich the concentration of essential PUFAs in BSF larvae with marine-based diets is an attractive option for obtaining n-3-enriched larvae (Barroso et al., 2019). Thus, the interest in insects is related not only to their intrinsic nutritional composition (Ramos-Elorduy et al., 2002; Wang et al., 2007; Hwangbo et al., 2009), but also to the possibility of enriching them with other nutrients and micronutrients (St-Hilaire et al., 2007; Mlcek et al., 2014; Hoc et al., 2021; Ferrari et al., 2022).
The process of bioconversion via BSF is a very attractive option, as it could potentially solve two problems: organic waste management and the ability to satisfy global food demand without competing with food crops for land use (Surendra et al., 2016; Salomone et al., 2017; Chavez, 2021).
Under this framework, the use of marine by-products generated from a diverse supply chain could be an important resource for high-nutritional-value components, such as n-3 PUFAs and proteins of high biological value (Caruso et al., 2020; Messina et al., 2021a; Messina et al., 2021b; Ucak et al., 2021; Messina et al., 2022). The use of these by-products and their valorization could lead to a reduction in food losses and waste, ensuring the achievement of the industry’s “zero-waste” goal (Alfio et al., 2021; Coppola et al., 2021; Kee et al., 2023).
Recently, some researchers have highlighted the possibility of rearing BSF on a diet including marine wastes and by-products such as aquaculture waste (Lopes et al., 2020), fish trimmings (Magee et al., 2021), carp mince (Chaklader et al., 2021), fish protein hydrolysates (from yellowtail kingfish, carp, and tuna) (Chaklader et al., 2020), and discarded round sardinella (Sardinella aurita) (Barroso et al., 2019). These diets improved process performance in BSF larvae composting and improved larval quality, which was characterized by high levels of fat and protein, suitable for farmed animal feeds including pig, chicken, and fish feeds (Barroso et al., 2019; Lopes et al., 2020; Chaklader et al., 2021; Magee et al., 2021).
Owing to the increasing interest in the farming of this insect for diverse purposes in the sector of food and feed, and the increase in interventions aimed at recycling by-products from marine sectors to reach zero waste, in this present work we studied the effects of different fish by-products, obtained from the fishery and aquaculture value chain, on growth, body composition, and modulation of fatty acid profile in BSF larvae and pre-pupae. Our aim was to determine if this solution could be a reasonable method for implementing n-3 components in the body composition of these insects, which are intended as new ingredients for aqua feeds, promoting circular economy pathways at a regional level in accordance with the European Green Deal.
H. illucens larvae were purchased alive from Smart Bugs s.s. (Ponzano Veneto, Italy). The experiment was performed in an acclimatized environment at 26 ± 1°C and 65 ± 5% humidity.
Larvae were kept in transparent containers (28 cm × 20 cm × 14 cm) fitted with lids, a drainage system, and a collection system for the pre-pupae.
Air circulation was enabled through holes in the lower (1 mm diameter) and upper (2–2.5 mm diameter) sections of the boxes.
The experiment was set up to administer one control wheat bran-based diet (B) and five experimental diets, including diverse sources of fish by-products (25%) collected from fisheries, aquaculture facilities, and processing plants located in the region of Trapani (western Sicily, Italy). Each treatment was performed in three replicates. Four hundred 6-day-old BSF larvae were allowed in each container (1,200 larvae for each treatment), as described in the literature (Grossule and Lavagnolo, 2020); substrates were weighed and distributed among the feeding containers in order to provide a daily ration equal to 100 mg/larva/day (Diener et al., 2009) (Table 1). The BSF larvae were fed for 26 days and were sampled at 6, 13, 21, and 26 days.
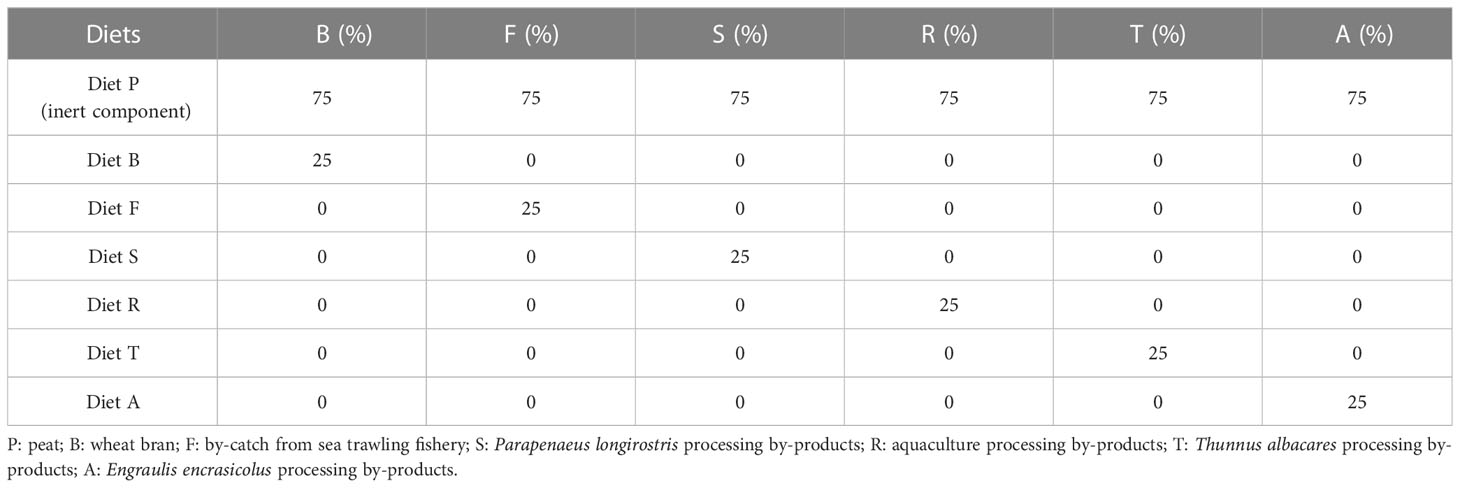
Table 1 Percentage of inclusion of fish by-products in the experimental diets provided to BSF larvae.
The five experimental diets were as follows: diet F, comprising fishery bycatch from the Mediterranean trawler fishing species Trachurus trachurus (Atlantic horse mackerel), Merluccius merluccius (European hake), Spicara flexuosa (Mediterranean picarel), Munida sp. (squat-lobsters), Helicolenus dactylopterus (blackbelly rosefish), Phycys blennoides (greater forkbeard), Galeus melastomus (blackmouth catshark), Gadiculus argenteus (silvery pout); diet S, comprising Parapenaeus longirostris (deep-water rose shrimp) processing by-products; diet R, comprising aquaculture by-products (from sea bass and sea bream filleting); diet T, made from Thunnus albacares (yellowfin tuna) processing by-products; and diet A, comprising Engraulis encrasicolus (European anchovy) by-products (heads and viscera) from specimens collected at the Trapani fish market. P. longirostris and T. albacares processing by-products from companies in the province of Trapani. All the materials were oven dried at 60°C for 48 hours and then ground. The obtained meal was stored under vacuum at −20°C. Different by-products and wheat bran were mixed into the peat in the ratios indicated in Table 1. The water content was increased to 75% (Diener et al., 2009). The choice of peat as an inert component was suggested owing to its easy availability, homogeneity of composition, and high degree of sustainability. The proximate composition and fatty acid profile of the experimental diets are shown in Tables 2, 3.
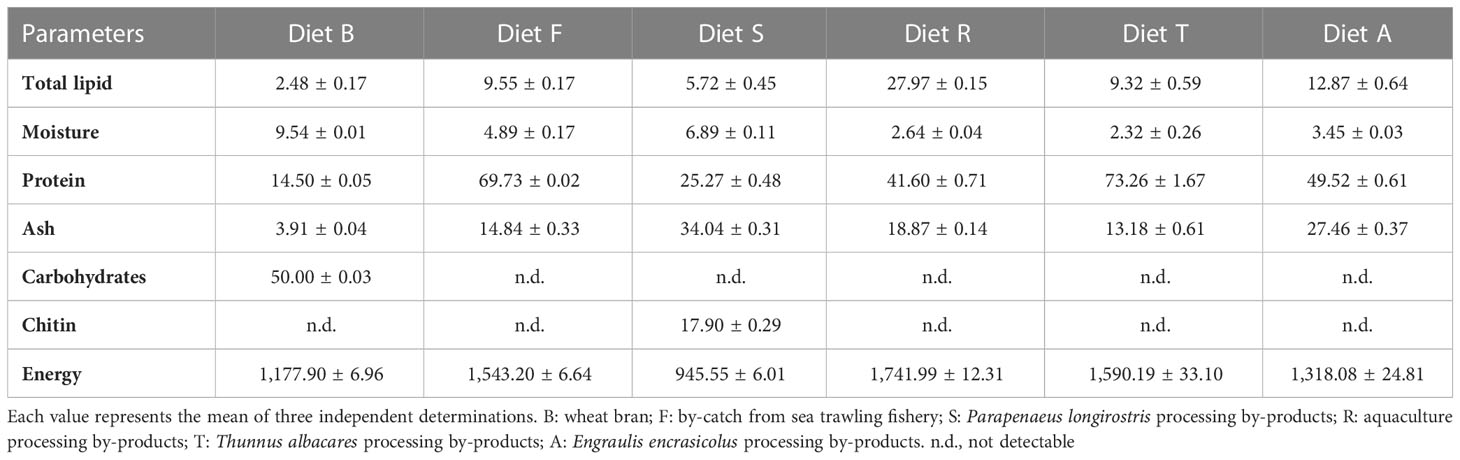
Table 2 Proximate composition (g/100 g wet), chitin (g/100 g wet), and energy content (kJ/100 g) of experimental diets provided to BSF larvae.
Diet aliquots were prepared to be added to the substrate at start-up and then every 2 days as a supplement to the diet until the end of the trial (Diener et al., 2009; Parra Paz et al., 2015).
In order to determine the growth rate, 10 larvae at 2, 6, and 13 days and 10 pre-pupae at 21 and 26 days from each replicate were randomly sampled and weighed individually. The “pre-pupae” stage was defined as the mobile, non-feeding, final larval stage of BSF, as defined in the literature (Myers et al., 2008; Tomberlin et al., 2009; Martínez-Sánchez et al., 2011; Harnden and Tomberlin, 2016). Pre-pupae were identified by a dark brown–black dorsal and ventral integument and reduced, darkened mouth parts (May, 1961; Harnden and Tomberlin, 2016). Larvae and pre-pupae were immediately frozen at –20°C for subsequent chemical analysis.
The proximate composition of both diets and of BSF was measured as follows: the total lipids (TL) were determined according to Folch et al. (1957); moisture and ash content was determined using the AOAC method (AOAC, 1990); total nitrogen was determined using the Kjeldahl method (AOAC, 1992); and crude protein (P) and chitin (Q) content were determined by applying the equations:
used by Díaz-Rojas et al. (2006), where Nt is the total nitrogen content, K is the sum of total lipid, moisture and ash, and Cp and Cq are the conversion coefficients that relate to the mass fraction of nitrogen with protein and chitin (Díaz-Rojas et al., 2006). The protein content in the literature is mainly based on nitrogen content, using the nitrogen-to-protein conversion factor (Cp) of 6.25 (Gnaiger and Bitterlich, 1984; Woyewoda et al., 1986), while the value of Cq is 14.5 (Díaz-Rojas et al., 2006; Messina et al., 2019).
Carbohydrate content was determined following the method described by Dubois et al. (1956).
Caloric content was measured as total energy content using an isoperibolic oxygen bomb calorimeter (model 6200, Parr Instrument Company, USA).
The composition of experimental diets varies considerably according to the species used and the current fishing season (Kim and Mendis, 2006).
Total fatty acid (FA) methyl esters were determined from the total lipid content, following the method described by Lepage and Roy (1984) and analyzed using the conditions described by Messina et al. (2013), employing a Perkin Elmer (Waltham, MA, USA) CLARUS 580 instrument equipped using a silica capillary column (30 m × 0.32 mm, df 0.25 μm, Omegawax 320, Supelco, Bellefonte, PA, USA). Individual FAME were measured by the comparison of known standards (a mix of PUFA 1, PUFA 2, and PUFA 3 oil, Supelco).
Statistical differences were evaluated for each parameter at each time point by diet using analysis of variance (ANOVA). The differences among the mean values were assessed using the Student–Newman–Keuls test. The degree of heterogeneity was measured by the Cochran test (Underwood, 1997). A principal component analysis (PCA) was performed to highlight the differences in fatty acid profiles in BSF larvae and pre-pupae fed different diets. The ANOVA and PCA were performed using STATISTICA (version 8.0, Statsoft Inc. USA).
The growth performance of BSF larvae during the experiment is shown in Figure 1. Larvae fed diet S died after a few days and did not develop into pre-pupae (data not shown). The best growth performance was recorded in larvae fed the control diet (diet B), which weighed significantly more than the other larvae and pre-pupae (p< 0.05) fed marine by-products. The larvae and pre-pupae fed the experimental diets (diet F, diet R, diet T, and diet A) did not differ regarding growth performance (Figure 1).
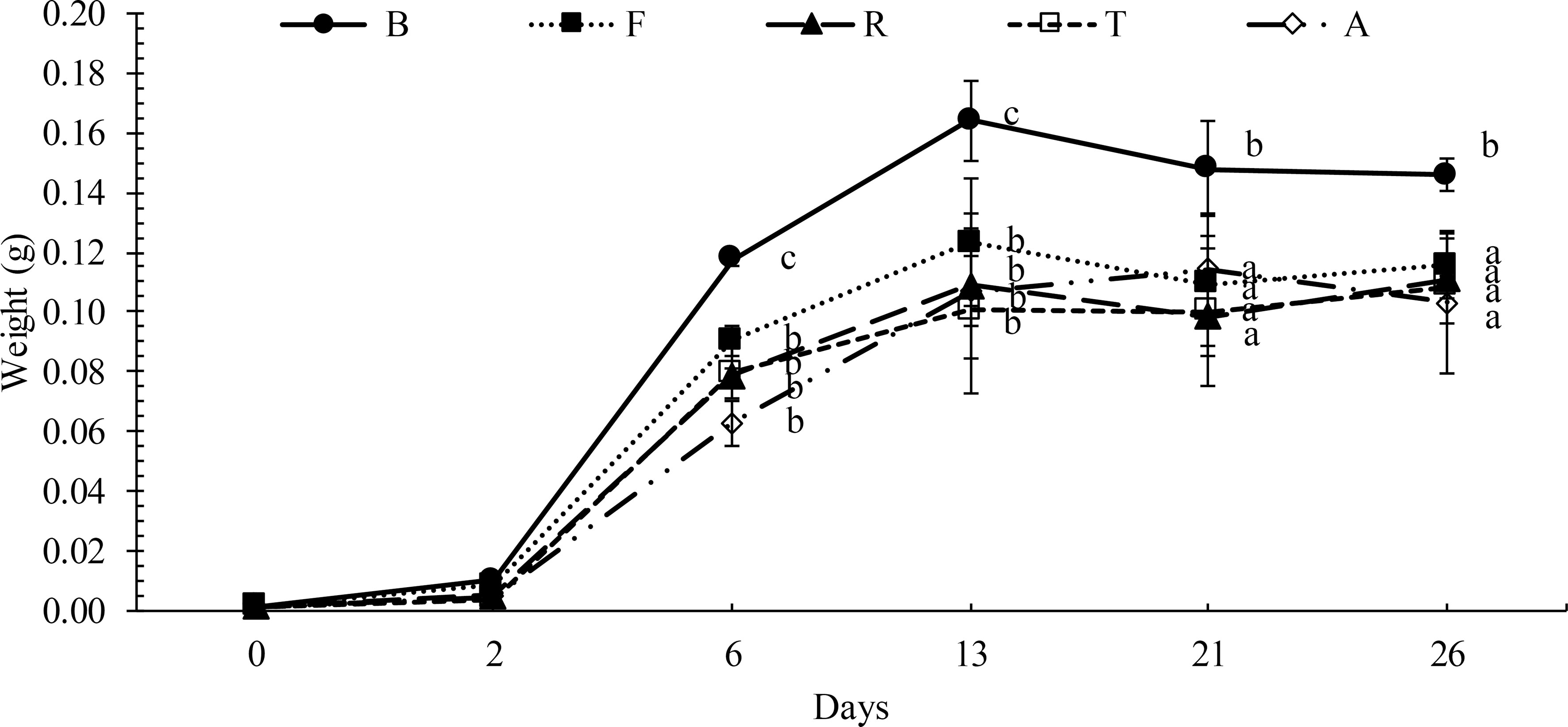
Figure 1 Growth performance of BSF fed with B: wheat bran; F: by-catch from sea trawling fishery; S: Parapenaeus longirostris processing by-products; R: aquaculture processing by-products; T: Thunnus albacares processing by-products; A: Engraulis encrasicolus processing by-products. Each value represents the mean of three independent determinations. Different letters within the same day indicate significant differences (p< 0.05) within treatments.
The proximate composition of the BSF pre-pupae, analyzed at the end of the experiment, is shown in Table 4. Since larvae fed diet S died after few days into the experiment, their proximate composition is not reported among the pre-pupae results.
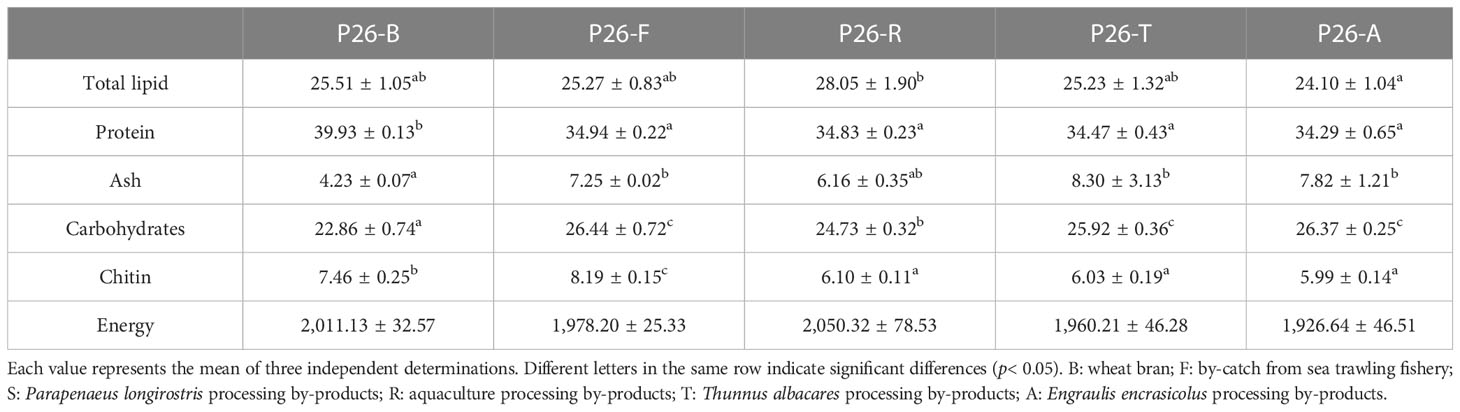
Table 4 Proximate composition (g/100 g dw), chitin (g/100 g dw), and energy content (kJ/100 g) of pre-pupae analyzed at the end of the experiment (P26).
The different experimental diets affected the proximate composition of the BSF pre-pupae (Table 4). Total lipid content was highest in pre-pupae fed diet R (P26-R, 28.05 ± 1.90 g/100 g) (p< 0.05) (Table 4), which aligns with diet R having the highest energy content of (Table 2). The pre-pupae fed diets B, F, T, and A did not differ significantly in lipid content (ranging between 24.1 and 25.51 g/100 g dw). The highest protein content was observed in pre-pupae fed diet B (P26-B, 39.93 g/100 g) (p< 0.05) (Table 4). The pre-pupae fed experimental diets showed a comparable total protein content, ranging from 34.29 to 34.98 g/100 g (Table 4). Ash content was significantly higher (p< 0.05) in pre-pupae fed diets T, A, and F than in those fed the control diet (diet B); no significant differences were observed between diet R and diet B. Carbohydrates, despite being absent in all diets except diet B (Table 2), ranged from 22.86 g/100 g (P26-B) to 26.34 g/100 g (P26-F) in the BSF (Table 4). Similarly, the levels of chitin in BSF body composition was comparable regardless of its concentration in diets, ranging between 6 and 8.1 g/100 g (Table 4). Analysis of body energy content showed no significant differences among the pre-pupae fed the experimental diets and control diet (Table 4).
The FA profiles of the BSF larvae and pre-pupae fed different diets are shown in Tables 5–10. The larvae fed the experimental diets reflected the FA composition of the diets, showing significant differences (p< 0.05) when compared with the larvae fed diet B.
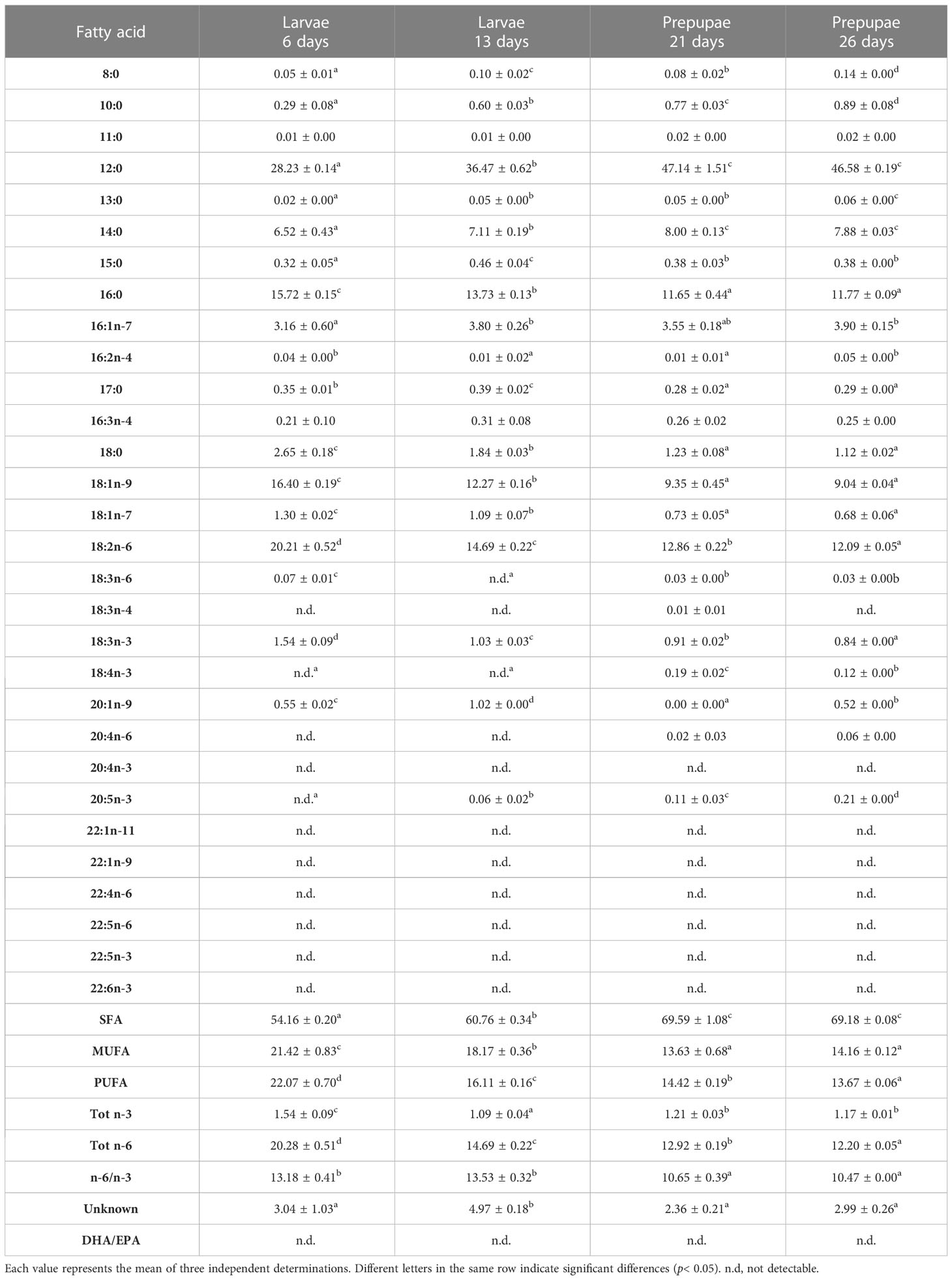
Table 5 FA profile (% of total FA) of larvae (L) and pre-pupae (P) of BSF, analyzed at 6, 13, 21, and 26 days, fed diet B.
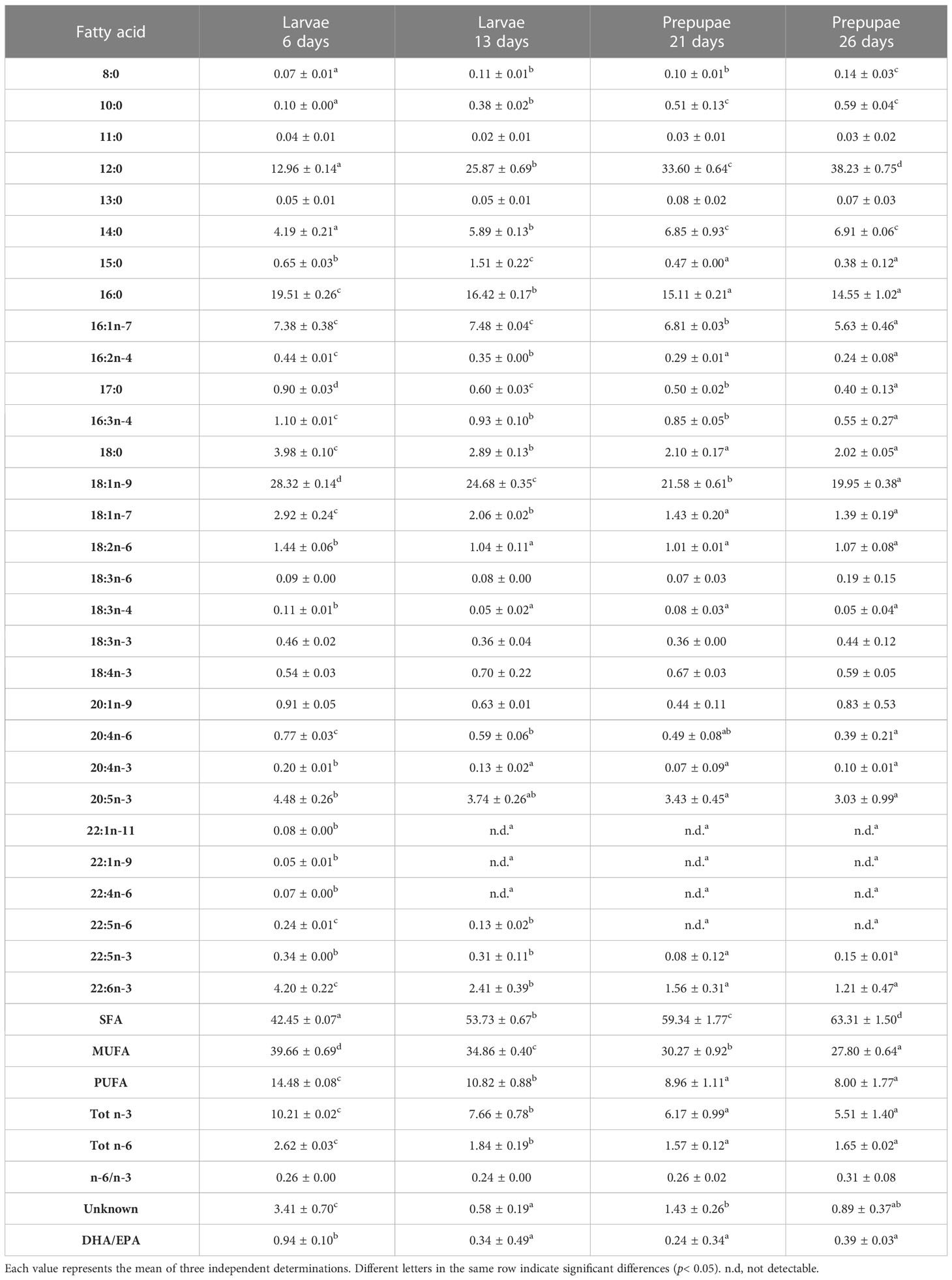
Table 6 FA profile (% of total FA) of larvae (L) and pre-pupae (P) of BSF, analyzed at 6, 13, 21, and 26 days, fed diet F.
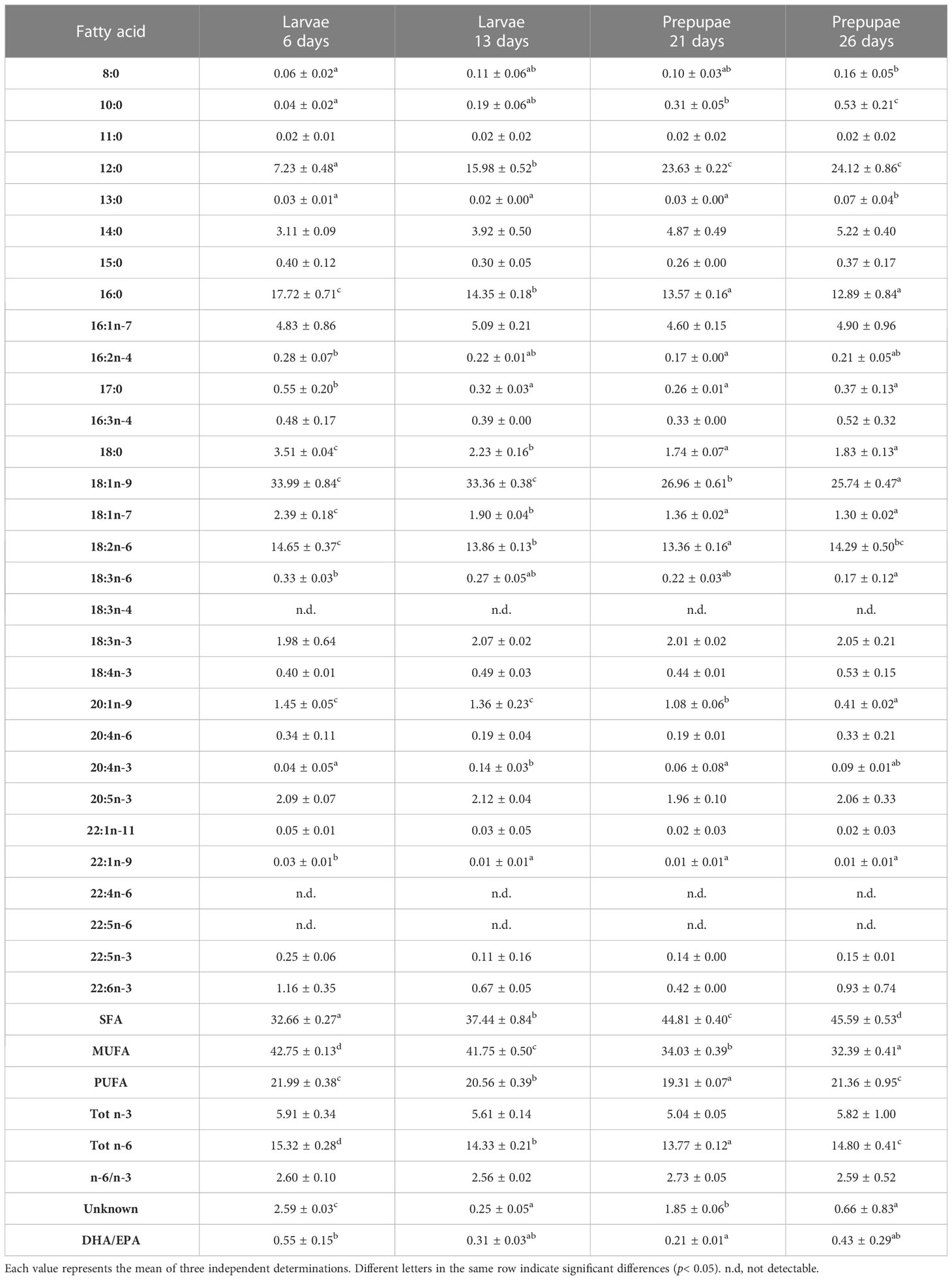
Table 7 FA profile (% of total FA) of larvae (L) and pre-pupae (P) of BSF, analyzed at 6, 13, 21, and 26 days, fed diet R.
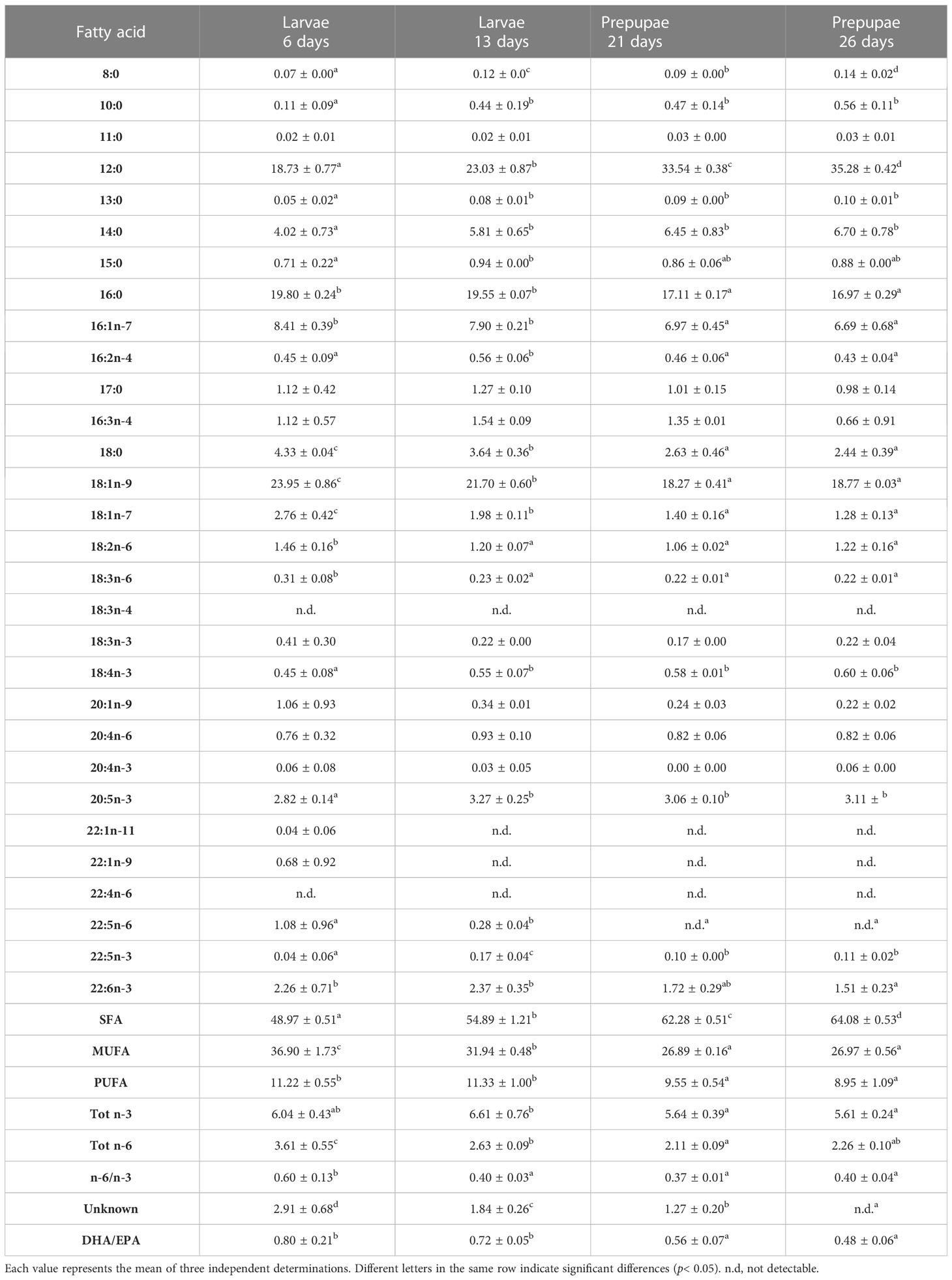
Table 8 FA profile (% of total FA) of larvae (L) and pre-pupae (P) of BSF, analyzed at 6, 13, 21, and 26 days, fed diet T.
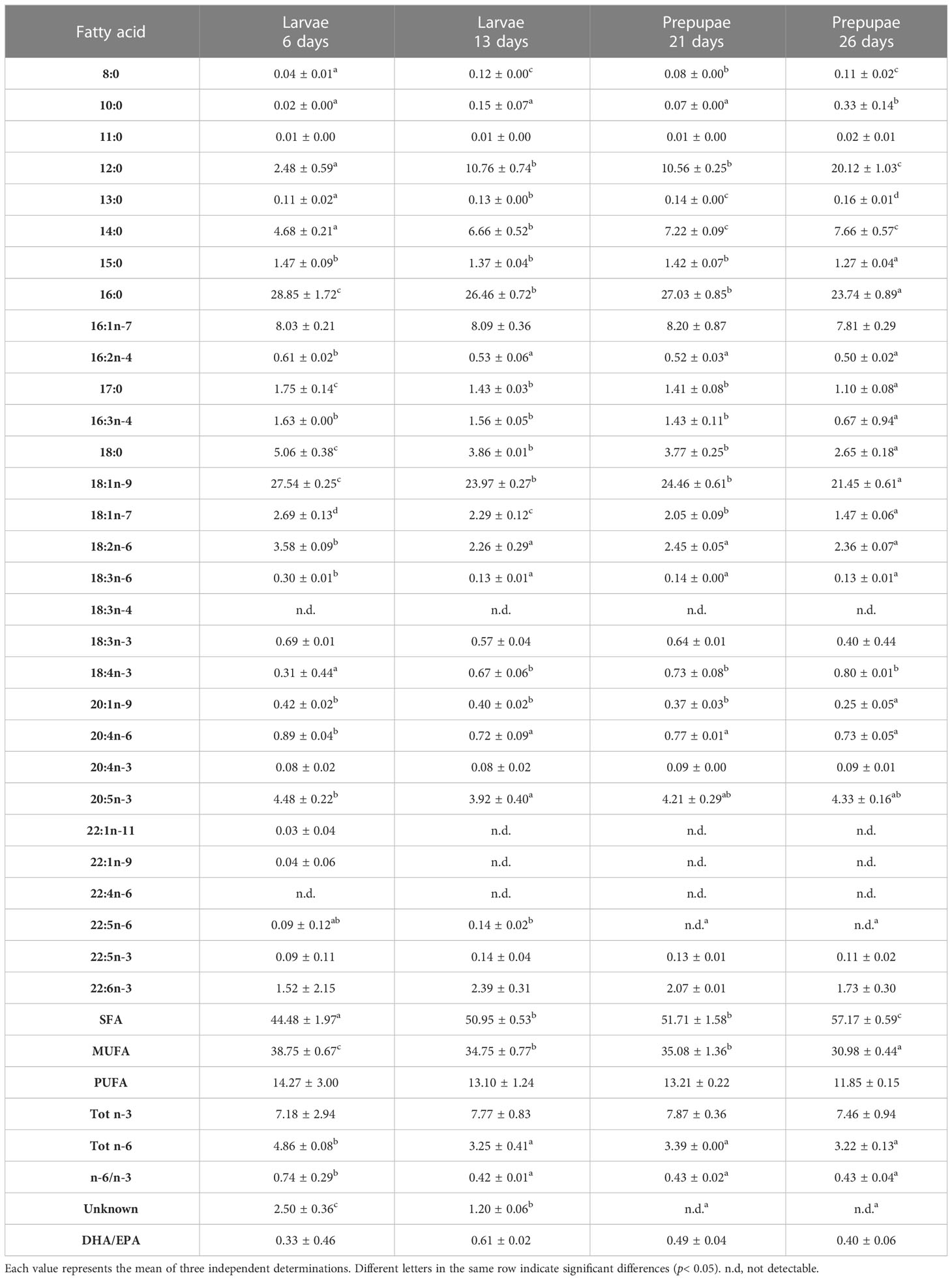
Table 9 FA profile (% of total FA) of larvae (L) and pre-pupae (P) of BSF, analyzed at 6, 13, 21, and 26 days, fed diet A.
The FA composition of the larvae and pre-pupae was dominated mainly by saturated fatty acids (SFAs) and increased in the pre-pupae (Tables 5–10). In particular, lower values were observed in the larvae and pre-pupae fed fish by-products (diet F, diet R, diet T, and diet A), than in those fed the control diet (diet B) (p< 0.05) (Tables 5–10). The predominant fatty acid in the SFA was lauric acid (12:0), which increased significantly in content (p< 0.05) during the growth phase in all treatments. In particular, it was observed that larvae and pre-pupae fed control diet (diet B) had the highest value of lauric acid, reaching 46.58 ± 0.19% in P26 (Tables 5, 10). Significantly lower values (p< 0.05) were observed in the larvae and pre-pupae fed fish by-products, particularly in those fed diet A. However, after 26 days, the pre-pupae (P26) fed fish by-products showed a lauric acid concentration above 20%. The larvae and pre-pupae fed diet A showed a higher concentration of palmitic acid (16:0) (Tables 9, 10) due to the higher amount present in the diet (Table 2).
Monounsaturated FA (MUFA) represented the second most abundant class of FA in almost all larvae and in all analyzed pre-pupae. L6 showed the highest MUFA content (Tables 5–9). A decrease in MUFAs occurred during the growth phase due to a reduction in oleic acid (18:1n-9), while palmitoleic acid (16:1 n-7) remained almost constant over time (Tables 5–9). Pre-pupae fed the experimental diets had significantly higher MUFA content (p< 0.05) than the larvae fed diet B (Table 10).
Similarly, polyunsaturated FA (PUFA) concentrations in the BSF larvae and pre-pupae decreased during growth (Tables 5–9). Linoleic acid (LA, 18:2n6) was the most represented fatty acid in BSF larvae and pre-pupae fed diets B and R (Tables 5, 7). In contrast, pre-pupae fed diets A, T, and F showed significantly lower LA values (p< 0.05) (Table 10). These larvae and pre-pupae fed diets A, T, and F also demonstrated an improvement in the concentration of eicosapentaenoic acid (EPA, 20:5n3) and docosahexaenoic acid (DHA, 22:6n3) (Tables 6, 8, 9). The most remarkable result was obtained for EPA, which reached 4.33% in P26 fed diet A and was undetected in P26 fed the control diet (diet B) (Table 10). Regarding DHA, the highest value was observed in L6 fed diet F, and levels then decreased during the growth phase. Larvae and pre-pupae fed diet A showed an average DHA content equal to 2% in all experiments (Table 9). DHA was not detected in larvae and pre-pupae fed diet B (Table 5). Larvae and pre-pupae fed diets A, F, and T showed higher Tot n-3 content than Tot n-6 and, consequently, the n-6/n-3 ratio was significantly lower in BSF larvae and pre-pupae (p< 0.05) than in BSF larvae and pre-pupae fed the control diet (diet B) (Tables 5–10).
Due to the selection of appropriate biomarkers and quality indicators, PCA allowed us to appreciate the differences among samples. PCA was applied to fatty acid classes (Figure 2). Eight variables (12:0, SFA, MUFA, PUFA, Tot n-3, Tot n-6, DHA/EPA, and n-6/n-3) were considered for the analysis; PCA was used to examine 25 cases consisting of the average fatty acid values of the diets and of the BSF larvae and pre-pupae fed different diets. The analysis generated a small number of linear combinations on the eight variables and three principal components with an eigenvalue greater than one were identified. These three components could explain the 95.51% of the variance of the original variables.
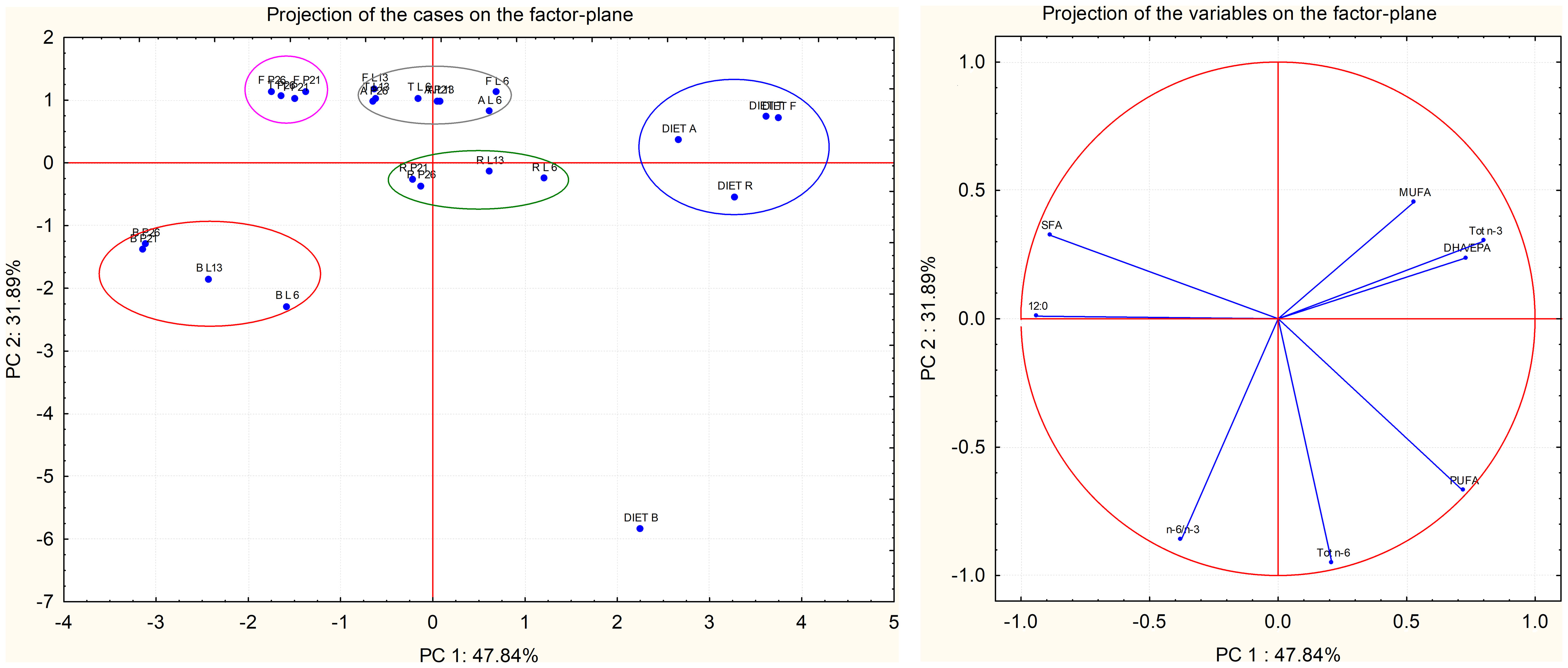
Figure 2 Principal component analysis (PCA) obtained from correlation of eight components (12:0, SFA, MUFA, PUFA, Tot n-3, Tot n-6, DHA/EPA, and n6/n-3) and 25 cases determined on larvae and pre-pupae fed with different diets. (A) Correlation circle; (B) factor-plan. Red circle: larvae and pre-pupae fed diet B; blue circle: experimental diets; green circle: larvae and pre-pupae fed diet R; purple circle: pre-pupae fed diet F and T; gray circle: larvae and pre-pupae fed diet A and larvae fed diets F and T.
PCA was performed considering the first two principal components (PCs) (Figure 2); this analysis explained 79.73% of the variability in the original data (Figure 2). The first principal component (PC1) explained 47.84% of the combined variance and the second component (PC2) explained 31.89% (Figure 2).
The correlation circle (Figure 2A) showed the correlation of the eight variables in the experimental cases. In particular, a positive correlation was observed between 12:0 and SFA; in fact, 12:0 was the predominant fatty acid in the SFA class. Figure 2B shows the factorial plane of the samples, identifying distinct groups that characterize the different larvae and pre-pupae fed different diets.
It is can be seen that larvae fed diet B were spatially distanced from the other samples (Figure 2B). As shown in the correlation circle (Figure 2A), these samples were mainly influenced by 12:0, which showed the highest values in P26 fed diet B (Table 10).
PCA allowed to discriminate how larvae are influenced by their diet (Figure 2B) since the beginning of feeding. The larvae and pre-pupae fed diet R are grouped together, distinguishing them from larvae and pre-pupae fed other diets; this is influenced by MUFA, as can be observed from the circle of correlations (Figure 2A).
This study investigated the effects of different fish by-products, characterized by a wide variability in chemical composition, on the development and nutritional composition of BSF larvae and pre-pupae at the laboratory scale. The use of bioconverting insects, such as BSF, allows for the use and valorization of by-products to produce larvae enriched with protein and fat. The obtained results, although not derived from an industrial production process, provide an important insight into the potential sustainable uses for BSF in aquaculture. The use of marine by-products as feed for BSF larvae supports a circular system, enabling the achievement of “zero-waste” goals.
Larvae fed the different experimental diets (Figure 1) showed a lower growth rate than larvae fed wheat bran (diet B). As reported by other authors (Grossule et al., 2019; Grossule and Lavagnolo, 2020), these results could reflect the different availability of carbon and degradable nutrients in the feeding substrates employed, thus confirming the predominant role of bran as a source of carbon and nutrients (Grossule et al., 2019; Grossule and Lavagnolo, 2020). Larvae fed diet S died after few days of the trial, probably due to the high level of chitin, which has negative effects on growth, food efficiency, and nutrient (particularly protein) digestibility (Shiau and Yu, 1999; Olsen et al., 2006; Marono et al., 2015; Guerreiro et al., 2020), or due to the lowest energy content of diet S among the diets (Starcevic et al., 2019) (Table 2).
Recently, interest in the utilization of BSF larvae as a raw material for animal feed has increased, mainly due to its potential as a sustainable source of high-quality protein (Kroeckel et al., 2012; Veldkamp et al., 2012; Liland et al., 2017)
BSF is a scavenger, commonly used to accelerate the composting of organic material, as it can efficiently utilize organic resources, such as fruit, vegetable, and meat waste, (Čičková et al., 2015; Surendra et al., 2016) and, depending on the growing medium, can modulate its own body composition, reaching a high concentration of lipids [(>30% of dry weight (dw)] and proteins (around 40% of dw) (St-Hilaire et al., 2007; Diener et al., 2009).
As reported by Cammack and Tomberlin (2017) diet does not have a significant impact on adult life-history traits; in fact, BSF feeds only in the larval stage. Dietary composition affects larval growth and performance (Nairuti et al., 2022).
The employed by-products in this study demonstrated an influence on the nutrient profiles of the BSF pre-pupae. Obtained results showed the highest lipid content in pre-pupae fed diet R (28.05 ± 1.90 g/100 g dw), which showed the highest lipid content (27.97 ± 0.15 g/100 g wet) of the experimental diets. The lipid content in insects is largely dependent on their diets and stage of development (Stanley-Samuelson and Dadd, 1983) and it is affected by the composition of the rearing substrate (Franco et al., 2021). In fact, the lipid content can vary by 15–49% of the total dry weight (Franco et al., 2021). This confirms that rearing BSF pre-pupae on substrates with a high lipid content resulted in higher lipid and lower protein contents in BSF pre-pupae, as reported by Nairuti et al. (2022).
The pre-pupae fed with experimental diets showed a protein content ranging from 34.29 to 34.94 g/100 g. The highest protein content was observed in pre-pupae fed diet B (P26-B, 39.93 g/100 g), in accordance with the values reported in literature (31.9%–46.3%) (Diener et al., 2009; Barroso et al., 2014; Sánchez-Muros et al., 2014; Surendra et al., 2016).
The varying proximate composition of BSF fed with various diets confirms the entirely generalist nature of this insect and therefore its plasticity in the use of food waste of varying origins and compositions, and confirms its ability to skillfully convert this food waste into nutrients (Cammack and Tomberlin, 2017). Although BSF exhibits high plasticity, some substrates appear to perform less well in the growth processes of reared larvae, as observed in larvae reared on diet S (Shiau and Yu, 1999; Olsen et al., 2006; Marono et al., 2015; Guerreiro et al., 2020). Numerous studies are in progress to define which substrates are most suitable for the optimal growth of this species in qualitative and quantitative terms (Ribeiro et al., 2022).
Our results indicate the high potential of insects as alternative sources of new and renewable animal proteins and fat (Finke, 2002; Gobbi et al., 2013; Rumpold and Schlüter, 2013; Sánchez-Muros et al., 2014; Zielińska et al., 2015).
BSF larvae have been reared mainly for their protein content (Halloran et al., 2016; Nogales-Mérida et al., 2019) and only recently have attracted interest for their lipid content (Spranghers et al., 2017; Barroso et al., 2019; Smetana et al., 2019; Rodrigues et al., 2022; Hossain et al., 2023).
Our results show that the FA profile of the different provided diets influenced the FA profiles of the BSF larvae and partially affected the fatty acid profile of the pre-pupae, as shown in the PCA analysis (Figure 2), which is in agreement with Barroso et al. (2019) and Spranghers et al. (2017). In the pre-pupae the main fatty acid was C12:0, even if this fatty acid was contained in the substrates only in trace amounts. Obtained results, in accordance with Barroso et al. (2019), highlighted the higher C12:0 content in pre-pupae fed the control diet than in pre-pupae fed with fish by-product substrates; indeed, several studies show that larvae fed on substrates containing cereals, fruit, and vegetables show the lauric acid highest content (Rodrigues et al., 2022). High lauric acid content could be due to the bioconversion of carbohydrates to lipids (Guil-Guerrero et al., 2018). Lauric acid represents the main lipid component of BSF and it is converted by animals and humans into monolaurin (antiviral, antibacterial, and antiprotozoal glyceride) (Leong et al., 2015; Ushakova et al., 2016). A high amount of lauric acid enables the larvae to survive in substrates with temperatures above 40°C, as lauric acid gives the larvae a solid consistency that is not subject to the rapid oxidation of fats (the melting point of lauric acid is 43.2°C) (Ushakova et al., 2016).
BSF pre-pupae fed different fish by-products showed higher contents of n-3 fatty acids than the control, in agreement with recent studies (Barroso et al., 2019; Ewald et al., 2020; Rodrigues et al., 2022). In particular, pre-pupae fed diet A had the highest levels of EPA, followed by pre-pupae fed diets T, F, and R (Table 10). Regarding DHA, the pre-pupae accumulated a maximum of 1.73% DHA (P26 diet A), whereas DHA constituting 17.54% of the total FA content was detected in diet A (Table 10). In addition, the n-6/n-3 values decreased in the larvae and pre-pupae fed diets R, A, T, and F (Tables 5–10) when compared with control, as was also reported by Barroso et al. (2019).
Since the essential fatty acids EPA and DHA, which belong to marine environments, are not found in BSF (Barroso et al., 2019), our results, which show an increase of EPA and DHA in BSF larvae and pre-pupae fed fish by-products, confirm that marine sources, in particular fish by-products, are capable of enriching BSF with these fatty acids (St-Hilaire et al., 2007; Sealey et al., 2011; Barroso et al., 2019; Ewald et al., 2020; Rodrigues et al., 2022).
The aim of this study was to investigate the effects of different fishery and aquaculture by-product diets on the growth, body composition, and fatty acid profile of BSF larvae and pre-pupae, with the aim of converting organic waste into high-value biomass as part of a “zero-waste” approach. Our results show that E. encrasicolus processing by-products, by-catch from a sea trawling fishery, T. albacares processing by-products, and aquaculture processing by-products could be interesting feeding substrates for the rearing of H. illucens. Using these by-products, it was possible to modulate the lipid and fatty acid profile of BSF larvae, increasing their level of n-3 fatty acids. This possibility represents added value to BSF as an organism capable of modulating its lipid and fatty acid composition for its utilization as an alternative source of ingredients for aquaculture feeds.
Furthermore, the utilization of these by-products could support circular economy pathways, contributing to the reduction of food losses and waste.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
MC and CM contributed to the conception and design of the study. CB and DM provided resources. MC performed the feeding experiment. RA, SM, and EC performed the analyses. RA and SM wrote the first draft of the manuscript. CM and AS revised, and read the submitted version. RA and SM contributed equally to the work. All authors contributed to the article and approved the submitted version.
The author RA and this work are funded by the PON Research and Innovation 2014-2020 - Action IV.5 "Doctorates on topics Green" Cod DOT1320192 CUP B73D21009130006 PhD fellowship:" From waste to profit: high biological value marine products, for the valorization and recovery of fisheries, aquaculture and fish processing by-products for the improvement of the sustainability of the supply chain".
The authors from University of Palermo would like to thanks the Project “Engage4BIO: Better understanding, intensified engagement, training and development in regional bio-based systems”- HORIZON EUROPE- Proposal number: 101059565.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Alfiko Y., Xie D., Astuti R. T., Wong J., Wang L. (2022). Insects as a feed ingredient for fish culture: status and trends. Aquac. Fish. 7, 166–178. doi: 10.1016/j.aaf.2021.10.004
Alfio V. G., Manzo C., Micillo R. (2021). From fish waste to value: an overview of the sustainable recovery of omega-3 for food supplements. Molecules 26, 1002. doi: 10.3390/molecules26041002
AOAC (1990). Official methods of analysis. Assoc. Off. Anal. Chem. 1, 1230. doi: 10.1002/jps.2600650148
AOAC (1992). Association of official analytical chemists official method, 981.10 crude protein in meat block digestion method. J. AOAC Inter 65, 1339.
Barroso F. G., de Haro C., Sánchez-Muros M. J., Venegas E., Martínez-Sánchez A., Pérez-Bañón C. (2014). The potential of various insect species for use as food for fish. Aquaculture 422–423, 193–201. doi: 10.1016/j.aquaculture.2013.12.024
Barroso F. G., Sánchez-Muros M. J., Rincón M. Á., Rodriguez-Rodriguez M., Fabrikov D., Morote E., et al. (2019). Production of n-3-rich insects by bioaccumulation of fishery waste. J. Food Compos. Anal. 82, 103237. doi: 10.1016/j.jfca.2019.103237
Bava L., Jucker C., Gislon G., Lupi D., Savoldelli S., Zucali M., et al. (2019). Rearing of hermetia illucens on different organic by-products: influence on growth, waste reduction, and environmental impact. Animals 9, 289. doi: 10.3390/ani9060289
Belghit I., Liland N. S., Gjesdal P., Biancarosa I., Menchetti E., Li Y., et al. (2019). Black soldier fly larvae meal can replace fish meal in diets of sea-water phase Atlantic salmon (Salmo salar). Aquaculture 503, 609–619. doi: 10.1016/j.aquaculture.2018.12.032
Belghit I., Liland N. S., Waagbø R., Biancarosa I., Pelusio N., Li Y., et al. (2018). Potential of insect-based diets for Atlantic salmon (Salmo salar). Aquaculture 491, 72–81. doi: 10.1016/j.aquaculture.2018.03.016
Calvert C. C., Martin R. D., Morgan N. O. (1969). House fly pupae as food for poultry. J. Econ. Entomol. 62, 938–939. doi: 10.1093/jee/62.4.938
Cammack J. A., Tomberlin J. K. (2017). The impact of diet protein and carbohydrate on select life-history traits of the black soldier fly hermetia illucens (L.) (Diptera: stratiomyidae). Insects 8, 56. doi: 10.3390/insects8020056
Caruso G., Floris R., Serangeli C., Di Paola L. (2020). Fishery wastes as a yet undiscovered treasure from the Sea: biomolecules sources, extraction methods and valorization. Mar. Drugs 18 (12), 622. doi: 10.3390/md18120622
Chaklader M. R., Fotedar R., Howieson J., Siddik M. A. B., Foysal M. J. (2020). The ameliorative effects of various fish protein hydrolysates in poultry by-product meal based diets on muscle quality, serum biochemistry and immunity in juvenile barramundi, lates calcarifer. Fish Shellfish Immunol. 104, 567–578. doi: 10.1016/J.FSI.2020.06.014
Chaklader M. R., Howieson J., Foysal M. J., Fotedar R. (2021). Transformation of fish waste protein to hermetia illucens protein improves the efficacy of poultry by-products in the culture of juvenile barramundi, lates calcarifer. Sci. Total Environ. 796, 149045. doi: 10.1016/j.scitotenv.2021.149045
Chavez M. (2021). The sustainability of industrial insect mass rearing for food and feed production: zero waste goals through by-product utilization. Curr. Opin. Insect Sci. 48, 44–49. doi: 10.1016/j.cois.2021.09.003
Čičková H., Newton G. L., Lacy R. C., Kozánek M. (2015). The use of fly larvae for organic waste treatment. Waste Manage. 35, 68–80. doi: 10.1016/j.wasman.2014.09.026
Coppola D., Lauritano C., Esposito F. P., Riccio G., Rizzo C., De Pascale D., et al. (2021). Fish waste: from problem to valuable resource. Mar. Drugs 19, 1–39. doi: 10.3390/md19020116
Díaz-Rojas E. I., Argüelles-Monal W. M., Higuera-Ciapara I., Hernández J., Lizardi-Mendoza J., Goycoolea F. M. (2006). Determination of chitin and protein contents during the isolation of chitin from shrimp waste. Macromol. Biosci. 6, 340–347. doi: 10.1002/mabi.200500233
Diener S., Zurbrügg C., Tockner K. (2009). Conversion of organic material by black soldier fly larvae: establishing optimal feeding rates. Waste Manage. Res. 27, 603–610. doi: 10.1177/0734242X09103838
Dubois M., Gilles K. A., Hamilton J. K., Rebers P. A., Smith F. (1956). Colorimetric method for determination of sugars and related substances. Anal. Chem. 28, 350–356. doi: 10.1021/ac60111a017
Ewald N., Vidakovic A., Langeland M., Kiessling A., Sampels S., Lalander C. (2020). Fatty acid composition of black soldier fly larvae (Hermetia illucens) – possibilities and limitations for modification through diet. Waste Manage. 102, 40–47. doi: 10.1016/j.wasman.2019.10.014
FAO (2020). The state of world fisheries and aquaculture 2020. sustainability in action. State World Fish. Aquac. 2020, 1–224. doi: 10.4060/ca9229en
FAO (2022). The state of world fisheries and aquaculture 2022. towards blue transformation (Rome: FAO). doi: 10.4060/cc0461en
Ferrari L., Sele V., Silva M., Bonilauri P., De Filippo F., Selmin F., et al. (2022). Biofortification of selenium in black soldier fly (Hermetia illucens) prepupae reared on seaweed or selenium enriched substrates. J. Insects as Food Feed 8, 887–899. doi: 10.3920/JIFF2021.0153
Finke M. D. (2002). Complete nutrient composition of commercially raised invertebrates used as food for insectivores. Zoo Biol. 21, 269–285. doi: 10.1002/zoo.10031
Folch J., Lees M., Stanley G. H. S. (1957). A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 226, 497–509. doi: 10.1007/s10858-011-9570-9
Franco A., Scieuzo C., Salvia R., Petrone A. M., Tafi E., Moretta A., et al. (2021). Lipids from hermetia illucens, an innovative and sustainable source. Sustainability 13 (18), 10198. doi: 10.3390/su131810198
Gasco L., Acuti G., Bani P., Dalle Zotte A., Danieli P. P., De Angelis A., et al. (2020). Insect and fish by-products as sustainable alternatives to conventional animal proteins in animal nutrition. Ital. J. Anim. Sci. 19, 360–372. doi: 10.1080/1828051X.2020.1743209
Gnaiger E., Bitterlich G. (1984). Proximate biochemical composition and caloric content calculated from elemental CHN analysis: a stoichiometric concept. Oecologia 62, 289–298. doi: 10.1007/BF00384259
Gobbi P., Martínez-Sánchez A., Rojo S. (2013). The effects of larval diet on adult life-history traits of the black soldier fly, hermetia illucens (Diptera: stratiomyidae). Eur. J. Entomol. 110, 461–468. doi: 10.14411/eje.2013.061
Govorushko S. (2019). Global status of insects as food and feed source: a review. Trends Food Sci. Technol. 91, 436–445. doi: 10.1016/j.tifs.2019.07.032
Grossule V., Lavagnolo M. C. (2020). The treatment of leachate using black soldier fly (BSF) larvae: adaptability and resource recovery testing. J. Environ. Manage. 253, 109707J. doi: 10.1016/j.jenvman.2019.109707
Grossule V., Vanin S., Lavagnolo M. C. (2019). Potential treatment of leachate by hermetia illucens (Diptera, stratyomyidae) larvae: performance under different feeding conditions. Waste Manag. Res. 38, 537–545. doi: 10.1177/0734242X19894625
Guerreiro I., Castro C., Antunes B., Coutinho F., Rangel F., Couto A., et al. (2020). Catching black soldier fly for meagre: growth, whole-body fatty acid profile and metabolic responses. Aquaculture 516, 734613. doi: 10.1016/j.aquaculture.2019.734613
Guil-Guerrero J. L., Ramos-Bueno R. P., González-Fernández M. J., Fabrikov D., Sánchez-Muros M. J., Barroso F. G. (2018). Insects as food: fatty acid profiles, lipid classes, and sn-2 fatty acid distribution of Lepidoptera larvae. Eur. J. Lipid Sci. Technol. 120, 1700391. doi: 10.1002/ejlt.201700391
Halloran A., Roos N., Eilenberg J., Cerutti A., Bruun S. (2016). Life cycle assessment of edible insects for food protein: a review. Agron. Sustain. Dev. 36, 1–13. doi: 10.1007/s13593-016-0392-8
Harnden L. M., Tomberlin J. K. (2016). Effects of temperature and diet on black soldier fly, hermetia illucens (L.) (Diptera: stratiomyidae), development. Forensic Sci. Int. 266, 109–116. doi: 10.1016/j.forsciint.2016.05.007
Hoc B., Francis F., Carpentier J., Mostade L., Blecker C., Purcaro G., et al. (2021). ω3-enrichment of hermetia illucens (L. 1758) prepupae from oilseed byproducts. J. Saudi Soc Agric. Sci. 20, 155–163. doi: 10.1016/j.jssas.2021.01.001
Hossain S., Small B. C., Hardy R. (2023). Insect lipid in fish nutrition : recent knowledge and future application in aquaculture. Rev. Aquac. 1–22. doi: 10.1111/raq.12810
Huang C., Feng W., Xiong J., Wang T., Wang W., Wang C., et al. (2019). Impact of drying method on the nutritional value of the edible insect protein from black soldier fly (Hermetia illucens l.) larvae: amino acid composition, nutritional value evaluation, in vitro digestibility, and thermal properties. Eur. Food Res. Technol. 245, 11–21. doi: 10.1007/s00217-018-3136-y
Hwangbo J., Hong E. C., Jang A., Kang H. K., Oh J. S., Kim B. W., et al. (2009). Utilization of house fly-maggots, a feed supplement in the production of broiler chickens. J. Environ. Biol. 30, 609–614.
Ichhponani J. S., Malik N. S. (1971). Evaluation of de-oiled silkworm pupae meal and corn-steep fluid as protein sources in chick rations. Br. Poult. Sci. 12, 231–234. doi: 10.1080/00071667108415874
Jucker C., Erba D., Leonardi M. G., Lupi D., Savoldelli S. (2017). Assessment of vegetable and fruit substrates as potential rearing media for hermetia illucens (Diptera: stratiomyidae) larvae. Environ. Entomol. 46, 1415–1423. doi: 10.1093/ee/nvx154
Karapanagiotidis I. T., Daskalopoulou E., Vogiatzis I., Rumbos C., Mente E., Athanassiou C. G. (2014). Substitution of fishmeal by fly hermetia illucens prepupae meal in the diet of gilthead seabream (Sparus aurata). HydroMedit 2014, 110–114.
Karapanagiotidis I. T., Neofytou M. C., Asimaki A., Daskalopoulou E., Psofakis P., Mente E., et al. (2023). Fishmeal replacement by full-fat and defatted hermetia illucens prepupae meal in the diet of gilthead seabream (Sparus aurata). Sustainability 15, 786. doi: 10.3390/su15010786
Kee P. E., Cheng Y.-S., Chang J.-S., Yim H. S., Tan J. C. Y., Lam S. S., et al. (2023). Insect biorefinery: a circular economy concept for biowaste conversion to value-added products. Environ. Res. 221, 115284. doi: 10.1016/J.ENVRES.2023.115284
Kim S. K., Mendis E. (2006). Bioactive compounds from marine processing byproducts - a review. Food Res. Int. 39, 383–393. doi: 10.1016/j.foodres.2005.10.010
Kroeckel S., Harjes A. G. E., Roth I., Katz H., Wuertz S., Susenbeth A., et al. (2012). When a turbot catches a fly: evaluation of a pre-pupae meal of the black soldier fly (Hermetia illucens) as fish meal substitute - growth performance and chitin degradation in juvenile turbot (Psetta maxima). Aquaculture 364–365, 345–352. doi: 10.1016/j.aquaculture.2012.08.041
Lähteenmäki-Uutela A., Marimuthu S. B., Meijer N. (2021). Regulations on insects as food and feed: a global comparison. J. Insects as Food Feed 7, 849–856. doi: 10.3920/JIFF2020.0066
Leong S. Y., Kutty S. R. M., Tan C. K., Tey L. H. (2015). Comparative study on the effect of organic waste on lauric acid produced by hermetia illucens larvae via bioconversion. J. Eng. Sci. Technol. 10, 52–63.
Lepage G., Roy C. C. (1984). Improved recovery of fatty acid through direct transesterification without prior extraction or purification. J. Lipid Res. 25, 1391–1396. doi: 10.1016/S0022-2275(20)34457-6
Li Q., Zheng L., Cai H., Garza E., Yu Z., Zhou S. (2011). From organic waste to biodiesel: black soldier fly, hermetia illucens, makes it feasible. Fuel 90, 1545–1548. doi: 10.1016/j.fuel.2010.11.016
Liland N. S., Biancarosa I., Araujo P., Biemans D., Bruckner C. G., Waagbø R., et al. (2017). Modulation of nutrient composition of black soldier fly (Hermetia illucens) larvae by feeding seaweed-enriched media. PloS One 12 (8), e0183188. doi: 10.1371/journal.pone.0183188
Liu X., Chen X., Wang H., Yang Q., Ur Rehman K., Li W., et al. (2017). Dynamic changes of nutrient composition throughout the entire life cycle of black soldier fly. PloS One 12 (8), e018601. doi: 10.1371/journal.pone.0182601
Lopes I. G., Lalander C., Vidotti R. M., Vinnerås B. (2020). Using hermetia illucens larvae to process biowaste from aquaculture production. J. Clean. Prod. 251, 119753. doi: 10.1016/j.jclepro.2019.119753
Magee K., Halstead J., Small R., Young I. (2021). Valorisation of organicwaste by-products using black soldier fly (Hermetia illucens) as a bio-convertor. Sustain 13, 8345. doi: 10.3390/su13158345
Maiolo S., Parisi G., Biondi N., Lunelli F., Tibaldi E., Pastres R. (2020). Fishmeal partial substitution within aquafeed formulations: life cycle assessment of four alternative protein sources. Int. J. Life Cycle Assess. 25, 1455–1471. doi: 10.1007/s11367-020-01759-z
Marono S., Piccolo G., Loponte R., Di Meo C., Attia Y. A., Nizza A., et al. (2015). In vitro crude protein digestibility of tenebrio molitor and hermetia illucens insect meals and its correlation with chemical composition traits. Ital. J. Anim. Sci. 14, 338–343. doi: 10.4081/ijas.2015.3889
Maroušek J., Strunecký O., Maroušková A. (2022). Insect rearing on biowaste represents a competitive advantage for fish farming. Rev. Aquac. 1–11. doi: 10.1111/raq.12772
Martínez-Sánchez A., Magaña C., Saloña M., Rojo S. (2011). First record of hermetia illucens (Diptera: stratiomyidae) on human corpses in Iberian peninsula. Forensic Sci. Int. 206, e76–e78. doi: 10.1016/j.forsciint.2010.10.021
May B. M. (1961). The occurrence in new Zealand and the life-history of the soldier fly hermetia illucens (L.) (Diptera: stratiomyidae). New Zeal. J. Sci. 4, 55–65.
Messina C. M., Arena R., Manuguerra S., Barbera L., Curcuraci E., Renda G., et al. (2022). Valorization of side stream products from Sea cage fattened bluefin tuna (Thunnus thynnus): production and In vitro bioactivity evaluation of enriched ω-3 polyunsaturated fatty acids. Mar. Drugs 20, 309. doi: 10.3390/MD20050309
Messina C. M., Arena R., Manuguerra S., Renda G., Laudicella V. A., Ficano G., et al. (2021a). Farmed gilthead Sea bream (Sparus aurata) by-products valorization: viscera oil ω-3 enrichment by short-path distillation and In vitro bioactivity evaluation. Mar. Drugs 19, 160. doi: 10.3390/md19030160
Messina C. M., Gaglio R., Morghese M., Tolone M., Arena R., Moschetti G., et al. (2019). Microbiological profile and bioactive properties of insect powders used in food and feed formulations. Foods 8, 400. doi: 10.3390/foods8090400
Messina C. M., Manuguerra S., Arena R., Renda G., Ficano G., Randazzo M., et al. (2021b). In vitro bioactivity of astaxanthin and peptides from hydrolisates of shrimp (Parapenaeus longirostris) by-products: from the extraction process to biological effect evaluation, as pilot actions for the strategy “From waste to profit”. Mar. Drugs 19, 216. doi: 10.3390/md19040216
Messina C. M., Renda G., La Barbera L., Santulli A. (2013). By-products of farmed European sea bass (Dicentrarchus labrax l.) as a potential source of n-3 PUFA. Biol. (Bratisl). 68, 288–293. doi: 10.2478/s11756-013-0148-8
Mlcek J., Borkovcova M., Bednarova M. (2014). Biologically active substances of edible insects and their use in agriculture, veterinary and human medicine-a review. J. Cent. Eur. Agric. 15, 225–237. doi: 10.5513/JCEA01/15.4.1533
Müller A., Wolf D., Gutzeit H. O. (2017). The black soldier fly, hermetia illucens - a promising source for sustainable production of proteins, lipids and bioactive substances. Z. fur Naturforsch. - Sect. C J. Biosci. 72, 351–363. doi: 10.1515/znc-2017-0030
Myers H. M., Tomberlin J. K., Lambert B. D., Kattes D. (2008). Development of black soldier fly (Diptera: stratiomyidae) larvae fed dairy manure. Environ. Entomol. 37, 11–15. doi: 10.1093/ee/37.1.11
Nairuti R. N., Musyoka S. N., Yegon M. J., Opiyo M. A. (2022). Utilization of black soldier fly (Hermetia illucens linnaeus) larvae as a protein source for fish feed: a review. Aquac. Stud. 22 (2), AQUAST697. doi: 10.4194/AQUAST697
Newton G. L., Booram C. V., Barker R. W., Hale O. M. (1977). Dried hermetia illucens larvae meal as a supplement for swine. J. Anim. Sci. 44, 395–400. doi: 10.2527/jas1977.443395x
Ng W.-K., Liew F., Ang L., Wong K. (2001). Potential of mealworm (Tenebrio molitor) as an alternative protein source in practical diets for African catfish, clarias gariepinus. Aquac. Res. 32, 273–280. doi: 10.1046/j.1355-557x.2001.00024.x
Nguyen T. T. X., Tomberlin J. K., Vanlaerhoven S. (2015). Ability of black soldier fly (Diptera: stratiomyidae) larvae to recycle food waste. Environ. Entomol. 44, 406–410. doi: 10.1093/ee/nvv002
Nogales-Mérida S., Gobbi P., Józefiak D., Mazurkiewicz J., Dudek K., Rawski M., et al. (2019). Insect meals in fish nutrition. Rev. Aquac. 11, 1080–1103. doi: 10.1111/raq.12281
Ogunji J. O., Kloas W., Wirth M., Schulz C., Rennert B. (2006). Housefly maggot meal (magmeal): an emerging substitute of fishmeal in tilapia diets. Conf. Int. Agric. Res. Dev., 7.
Olsen R. E., Suontama J., Langmyhr E., Mundheim H., Ringø E., Melle W., et al. (2006). The replacement of fish meal with Antarctic krill, euphausia superba in diets for Atlantic salmon, salmo salar. Aquac. Nutr. 12, 280–290. doi: 10.1111/j.1365-2095.2006.00400.x
Oonincx D. G. A. B., Laurent S., Veenenbos M. E., van Loon J. J. A. (2020). Dietary enrichment of edible insects with omega 3 fatty acids. Insect Sci. 27, 500–509. doi: 10.1111/1744-7917.12669
Oonincx D. G. A. B., van Broekhoven S., van Huis A., van Loon J. J. A. (2019). Correction: feed conversion, survival and development, and composition of four insect species on diets composed of food byproducts (PLoS one, (2015) 10:12 (e0144601) DOI: 10.1371/journal.pone.0144601). PloS One 14, 1–7. doi: 10.1371/journal.pone.0222043
Osimani A., Garofalo C., Milanović V., Taccari M., Cardinali F., Aquilanti L., et al. (2017). Insight into the proximate composition and microbial diversity of edible insects marketed in the European union. Eur. Food Res. Technol. 243, 1157–1171. doi: 10.1007/s00217-016-2828-4
Parra Paz A. S., Carrejo N. S., Gómez Rodríguez C. H. (2015). Effects of larval density and feeding rates on the bioconversion of vegetable waste using black soldier fly larvae hermetia illucens (L.), (Diptera: stratiomyidae). Waste Biomass Valorization 6, 1059–1065. doi: 10.1007/s12649-015-9418-8
Phelps R. J., Struthers J. K., Moyo S. J. L. (1975). Investigations into the nutritive value of macrotermes falciger (Isoptera: termitidae). Zool. Africana 10, 123–132. doi: 10.1080/00445096.1975.11447501
Purschke B., Brüggen H., Scheibelberger R., Jäger H. (2018). Effect of pre-treatment and drying method on physico-chemical properties and dry fractionation behaviour of mealworm larvae (Tenebrio molitor l.). Eur. Food Res. Technol. 244, 269–280. doi: 10.1007/s00217-017-2953-8
Ramos-Elorduy J., González E. A., Hernández A. R., Pino J. M. (2002). Use of tenebrio molitor (Coleoptera: tenebrionidae) to recycle organic wastes and as feed for broiler chickens. J. Econ. Entomol. 95, 214–220. doi: 10.1016/B978-0-12-374144-8.00270-8
Ribeiro N., Costa R., Ameixa O. M. C. C. (2022). The influence of non-optimal rearing conditions and substrates on the performance of the black soldier fly (Hermetia illucens). Insects 13 (7), 639. doi: 10.3390/insects13070639
Rodrigues D. P., Ameixa O. M. C. C., Vázquez J. A., Calado R. (2022). Improving the lipid profile of black soldier fly (Hermetia illucens) larvae for marine aquafeeds: current state of knowledge. Sustain 14 (11), 6472. doi: 10.3390/su14116472
Rumpold B. A., Schlüter O. K. (2013). Nutritional composition and safety aspects of edible insects. Mol. Nutr. Food Res. 57, 802–823. doi: 10.1002/mnfr.201200735
Salomone R., Saija G., Mondello G., Giannetto A., Fasulo S., Savastano D. (2017). Environmental impact of food waste bioconversion by insects: application of life cycle assessment to process using hermetia illucens. J. Clean. Prod. 140, 890–905. doi: 10.1016/j.jclepro.2016.06.154
Sánchez-Muros M. J., Barroso F. G., Manzano-Agugliaro F. (2014). Insect meal as renewable source of food for animal feeding: a review. J. Clean. Prod. 65, 16–27. doi: 10.1016/j.jclepro.2013.11.068
Sealey W. M., Gaylord T. G., Barrows F. T., Tomberlin J. K., McGuire M. A., Ross C., et al. (2011). Sensory analysis of rainbow trout, oncorhynchus mykiss, fed enriched black soldier fly prepupae, hermetia illucens. J. World Aquac. Soc 42, 34–45. doi: 10.1111/j.1749-7345.2010.00441.x
Sheppard D. C., Tomberlin J. K., Joyce J. A., Kiser B. C., Sumner S. M. (2002). Rearing methods for the black soldier fly (diptera: stratiomyidae). J. Med. Entomol. 39, 695–698. doi: 10.1603/0022-2585-39.4.695
Shiau S. Y., Yu Y. P. (1999). Dietary supplementation of chitin and chitosan depresses growth in tilapia, oreochromis niloticus X o. aureus. Aquaculture 179, 439–446. doi: 10.1016/S0044-8486(99)00177-5
Smetana S., Schmitt E., Mathys A. (2019). Sustainable use of hermetia illucens insect biomass for feed and food: attributional and consequential life cycle assessment. Resour. Conserv. Recycl. 144, 285–296. doi: 10.1016/j.resconrec.2019.01.042
Spranghers T., Ottoboni M., Klootwijk C., Ovyn A., Deboosere S., De Meulenaer B., et al. (2017). Nutritional composition of black soldier fly (Hermetia illucens) prepupae reared on different organic waste substrates. J. Sci. Food Agric. 97, 2594–2600. doi: 10.1002/jsfa.8081
Stanley-Samuelson D. W., Dadd R. H. (1983). Long-chain polyunsaturated fatty acids: patterns of occurrence in insects. Insect Biochem. 13, 549–558. doi: 10.1016/0020-1790(83)90014-8
Starčević K., Gavrilović A., Gottstein Ž., Mašek T. (2017). Influence of substitution of sunflower oil by different oils on the growth, survival rate and fatty acid composition of Jamaican field cricket (Gryllus assimilis). Anim. Feed Sci. Technol. 228, 66–71. doi: 10.1016/j.anifeedsci.2017.04.007
Starcevic K., Lozica L., Gavrilovic A., Heruc Z., Masek T. (2019). Fatty acid plasticity of black soldier fly (Hermetia illucens) larvae reared on alternative feeding media: crude olive cake and processed animal protein. J. Anim. Feed Sci. 28, 374–382. doi: 10.22358/jafs/114434/2019
St-Hilaire S., Sheppard C., Tomberlin J. K., Irving S., Newton L., McGuire M. A., et al. (2007). Fly prepupae as a feedstuff for rainbow trout, oncorhynchus mykiss. J. World Aquac. Soc 38, 59–67. doi: 10.1111/j.1749-7345.2006.00073.x
Surendra K. C., Olivier R., Tomberlin J. K., Jha R., Khanal S. K. (2016). Bioconversion of organic wastes into biodiesel and animal feed via insect farming. Renew. Energy 98, 197–202. doi: 10.1016/j.renene.2016.03.022
Teotia J. S., Miller B. F. (1974). Nutritive content of house fly pupae and manure residue1. Br. Poult. Sci. 15, 177–182. doi: 10.1080/00071667408416093
Tomberlin J. K., Adler P. H., Myers H. M. (2009). Development of the black soldier fly (Diptera: stratiomyidae) in relation to temperature. Environ. Entomol. 38, 930–934. doi: 10.1603/022.038.0347
Ucak I., Afreen M., Montesano D., Carrillo C., Tomasevic I., Simal-Gandara J., et al. (2021). Functional and bioactive properties of peptides derived from marine side streams. Mar. Drugs 19, 71. doi: 10.3390/md19020071
Underwood A. J. (1997). Experiments in ecology: Their logical design and interpretation using analysis of variance (Cambridge: Cambridge university press).
Ushakova N. A., Brodskii E. S., Kovalenko A. A., Bastrakov A. I., Kozlova A. A., Pavlov D. S. (2016). Characteristics of lipid fractions of larvae of the black soldier fly hermetia illucens. Dokl. Biochem. Biophys. 468, 209–212. doi: 10.1134/S1607672916030145
Veldkamp T., van Duinkerken G., van Huis A., Lakemond C., Ottevanger E., et al. (2012)Insects as a sustainable feed ingredient in pig and poultry diets - a feasibility study. In: Wageningen UR livest. res (Accessed November 17, 2022).
Wang D., Zhai S. W., Zhang C. X., Zhang Q., Chen H. (2007). Nutrition value of the Chinese grasshopper acrida cinerea (Thunberg) for broilers. Anim. Feed Sci. Technol. 135, 66–74. doi: 10.1016/j.anifeedsci.2006.05.013
Woyewoda A. D., Shaw S. J., Ke P. J., Burns B. G. (1986). Recommended laboratory methods for assessment of fish quality (Halifax, Canada), 143.
Keywords: Hermetia illucens, black soldier fly, fatty acids, marine by-products, PUFA, EPA, DHA
Citation: Arena R, Manuguerra S, Curcuraci E, Cusimano M, Lo Monaco D, Di Bella C, Santulli A and Messina CM (2023) Fisheries and aquaculture by-products modulate growth, body composition, and omega-3 polyunsaturated fatty acid content in black soldier fly (Hermetia illucens) larvae. Front. Anim. Sci. 4:1204767. doi: 10.3389/fanim.2023.1204767
Received: 12 April 2023; Accepted: 09 May 2023;
Published: 30 May 2023.
Edited by:
Federica Cheli, University of Milan, ItalyCopyright © 2023 Arena, Manuguerra, Curcuraci, Cusimano, Lo Monaco, Di Bella, Santulli and Messina. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Concetta Maria Messina, Y29uY2V0dGEubWVzc2luYUB1bmlwYS5pdA==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.