- 1School of Environmental and Rural Science, Faculty of Science, Agriculture, Business and Law, University of New England, Armidale, NSW, Australia
- 2Agriculture and Food, Commonwealth Scientific and Industrial Research Organization (CSIRO), Armidale, NSW, Australia
- 3Animal Welfare Science Centre, Faculty of Veterinary and Agricultural Sciences, University of Melbourne, Parkville, VIC, Australia
Range use by free-range laying hen flocks is heterogeneous. We hypothesized that ranging behaviour may be motivated by curiosity and thwarted by fearfulness. This project aimed to increase a hen’s motivation to explore by enriching the rearing environment and identify relationships between exploration, fear and ranging. Day-old Hy-Line chicks (n = 1700) were reared in environments that provided novel items, structures for perching or an industry standard floor rearing environment. Prior to range access, fear and exploratory behaviors were assessed at 18 weeks of age (cohort 1; n = 30 hens/treatment) via novel arena and novel object tests and at 22 weeks of age (cohort 2; n = 30 hens/treatment) using an 8-arm radial maze choice paradigm adapted from previous rodent research. Hens were trained to expect success in two arms (reward) and failure in two arms (mild punishment), the remaining four arms (ambiguous arms) were not available during training. After training, all hens were retested for 8 minutes with access to the four familiar arms only, then for four minutes with access to the ambiguous arms for the first time, in addition to the success and failure arms. Latency to enter the ambiguous arms and the number of ambiguous arms entered were assessed as an indicator of a hen’s willingness to forgo reward and risk punishment to explore a novel area. At 25 weeks of age, hens were provided with range access and individual range access was monitored for three weeks. Latency to access the range and the number of days the range was accessed was not related to rearing treatment (p > 0.05) and was only weakly correlated with behavior during the novel arena, novel object and 8-arm radial maze tests (r < 0.3). However, hens reared in the novelty rearing environment were more willing to forgo reward to explore the ambiguous arms than hens reared in the control environment (p = 0.004). We did not identify strong evidence that exploration or fearfulness was related to early ranging behavior. However, we show that motivation to explore increases when hens are reared in an enriched environment.
1 Introduction
Individual variation in ranging behavior by free-range laying hens has been extensively reported [for example, Hartcher et al. (2016); Larsen et al. (2017); Campbell et al. (2018a) and Kolakshyapati et al. (2020)]. Yet, the reason for the variability remains largely unknown. To date, the most prevalent hypothesis to explain ranging variation is individual differences in fearfulness. This hypothesis has been tested in a variety of controlled and experimental settings using an assortment of methods to assess fearfulness with varied, and often contradictory, conclusions.
There is some evidence that range use may reduce hen fearfulness. For example, Grigor et al. (1995) reported that hens that spent more time on an outdoor range in commercial conditions were less fearful after range access, evident by shorter righting times in a tonic immobility (TI) test and a shorter latency to emerge from cover during an emergence test. Furthermore, both Campbell et al. (2016) and Hartcher et al. (2016) showed that hens that accessed the range more frequently were more fearful than hens that rarely or never accessed the outdoor range, evident by shorter TI righting times, fewer vocalizations and less activity in an Open Field Test (OFT). Of note, all of the aforementioned studies assessed fear after the hens had access to the outdoor range suggesting that range use may reduce fearfulness but did not provide evidence that fearfulness prevents hens from accessing the outdoor range. The positive effect of range use on fearfulness has also been reported in free-range meat chickens by assessing fearfulness (OFT and physiological stress responses to an acute stressor) both before and after range access (Taylor et al., 2020). A more recent study by Campbell et al. (2021) did assess fearfulness and exploration before range access - with a cohort of hens from the same research flock as the current study - using an OFT and maze test. The authors identified some inconsistent relationships between indicators of fearfulness (latency to vocalize in the OFT test and the time to leave start box when a maze contained a novel object) with subsequent ranging behavior (the number of visits and time spent on the range) but these relationships were present only with hens that were raised in standard conditions and not wtih hens that were reared in enriched environments. The Campbell et al. (2021) study does provide some evidence that fearfulness may be a predictor of range use in laying hens.
Assessments of range use and hen fearfulness on Australian commercial farms did not show similar results as those previously reported in controlled commercial conditions. Despite reporting significant variance in ranging behavior between individual layer hens in commercial flocks, Larsen et al. (2018) and Kolakshyapati et al. (2020) found no relationships between ranging behavior and fearfulness using TI, OFT and Human-Approach and Avoidance methods to assess fearfulness of hens on commercial egg farms. However, Kolakshyapati et al. (2020) did find that birds that accessed the range more frequently were more likely to approach a novel object during a novel object test (NOT) suggesting that inquisitive exploration may be associated with range use. It is possible that the reported discrepancy in research outcomes regarding fearfulness and range use by laying hens, could rather reflect relationships between range use and motivation to explore, which can be thwarted by fear. Additional evidence to suggest environmental exploration is an important factor to promote range use is provided in previous research work with free-range meat chickens. Ferreira et al. (2021) showed that meat chickens that contrafreeload (before and after range access was provided) were more likely to access an outdoor range when provided with the opportunity. It is therefore possible that range use reduces fearfulness in layer hens but accessing the range may be driven by motivations to explore.
The NOT method utilized by Kolakshyapati et al. (2020) likely reflects a hens’ motivation for inspective exploration and neophobia, such that hens that are motivated to obtain new information about a novel stimuli (inspective exploration) will approach the NO and faster than those less motivated. Of note, animals will show exploratory behavior even before new stimuli are presented, demonstrating that animals actively search for new information; referred to as inquisitive exploration (Wood-Gush and Vestergaard, 1991), curiosity (Špinka, 2019) or seeking (Wood-Gush and Vestergaard, 1991; Panksepp, 2004). Here we refer to curiosity as the motivator of inquisitive exploration, which is unrelated to survival (i.e. food or safety) (Hughes, 1997), and is in itself hedonistically rewarding (Panksepp, 2004) and competence building (Špinka, 2019). Curiosity may be the motivation for hens to access the outdoor range and may partially explain the heterogeneous ranging behavior of hen flocks.
Curiosity can be assessed with choice paradigms such as ‘willingness to sacrifice rewards’ or ‘willingness to wait for an answer’ (Wang and Hayden (2019). For example, male rhesus macaque monkeys have been shown willing to sacrifice a larger water reward to obtain information about a gambling outcome in advance of its delivery (Blanchard et al., 2015) and humans are willing to spend more scarce resources (limited tokens or waiting time) to obtain answers to trivia questions that they are more curious about (Kang et al., 2009) and even expose themselves to electrical shocks for access to the secret behind a magic trick (i.e. information) (Lau et al., 2018).
Franks et al. (2013) proposed a novel assessment of inquisitive exploration to reflect curiosity, using a trade-off paradigm providing animals with the opportunity to explore a novel area by forgoing a known reward and risking punishment. Franks et al. (2013) trained rats to expect reward in two arms and (mild) punishment in the opposite two arms of an eight-arm radial maze. Subsequently, environmental enrichment was removed from half of the population's home environment for two weeks before exposing the rats to the radial maze again. However, this time the radial maze test also provided rats with access to unfamiliar ambiguous arms for the final minute of the test. Both groups showed interest in the ambiguous arms but animals that were continuously housed with environmental enrichment were more likely to forgo reward and risk punishment to explore novel areas than rats where enrichment had been removed for two weeks prior to the test.
In this study, we investigated the relationships between hen fearfulness and curiosity prior to range access and early ranging behavior. We predicted that hens reared with environmental enrichment would be more curious, less fearful and subsequently would range more frequently during the first three weeks of range access, compared to hens reared in more barren conditions. To assess the role of fearfulness and curiosity we assessed fearfulness and inspective exploration at 18 weeks of age with the well validated OFT and NOT tests. To determine whether range use is motivated by curiosity, we used the method developed by Franks et al. (2013) at 22 weeks of age to assess inquisitive exploration. Subsequently, range access was provided at 25 weeks of age and individual range visits were monitored by radio frequency identification (RFID) technology.
2 Materials and methods
All animals and procedures used in this study were approved by the University of New England Animal Ethics Committee (Approval number 17-092).
2.1 Animals and housing
Information regarding animals, housing and rearing treatments are reported elsewhere (Bari et al., 2020) but are also briefly outlined here. Day old Hy-Line Brown laying hen chicks (n = 1700) were sourced from Specialized breeders Australia (Bagshot, Victoria, Australia) and were transported to the Rob Cumming Poultry Innovation Centre at the University of New England (Armidale, NSW, Australia). Chicks were randomly allocated to one of three treatment groups; control, novelty or structural (section 2.2). During the first 16 weeks of life (rearing), chicks were housed in pens (6.25 m x 3.26 m; 174-190/pen) each containing litter, were visually isolated from each other and provided with ad libitum water via nipple drinkers and ad libitum commercial layer mash feed appropriate for life stages. Hy-Line Brown recommended schedules were followed for both light and temperature (Hy-Line International, 2018), specifically a 22:2 LD schedule from day old up to 16:8 L:D at 30 weeks of age and temperature from 33-36°C at day old decreasing 2-3°C each week until reaching 21°C at 6 weeks of age. At 16 weeks of age, 1386 hens were transported to the Laureldale free-range poultry research facility at the University of New England (Armidale, NSW, Australia). Pullets were mixed within treatment and housed in pens (n = 154 hens in 4.8 x 3.6 pens; 3 pen replicates per treatment) and were provided with litter substrate, nest boxes, a five-tiered perch and ad libitum feed and water. The free-range shed was naturally ventilated with no temperature or humidity control. Each pen was provided with range access at 25 weeks of age at 09:00 h for approximately 8 hours. Ranges were covered in grass but contained no trees or artificial structures. Radio Frequency Identification (RFID) systems were set up at each door from the pen onto the range (hereafter referred to as ‘pop-hole’) to track individual hen range use. The RFID systems were designed and supported by Microchips Australia Pty Ltd (Keysborough, VIC, Australia) with equipment developed and manufactured by Dorset Identification B.V. (Aalten, the Netherlands) using Trovan® technology. A schematic of the RFID system is available in Campbell et al. (2018b). Each hen was banded with a microchip (Trovan® Unique ID 100 (FDX-A): operating frequency 128 kHz) glued into an adjustable leg band (Roxan Developments Ltd, Selkirk, Scotland). Daily ranging behavior was tracked for the first three weeks of range access including total time on the range and total numbers of visits.
2.2 Rearing treatments
During rearing, chicks in the novelty treatment group were provided with novel objects that differed in shape, color and texture but were not biologically relevant, for example plastic buckets, brooms, colored string and hula hoops. From one day of age, multiple novel objects were present at any one time and replaced every seven days. Chicks in the structural treatment group were provided with four purpose made box structures (60 cm x 60 cm x 60 cm) containing black metal bars suitable for perching and some solid components to visually isolate chicks from behind the structure. Chicks in the control treatment groups were housed in industry standard floor rearing conditions, with access to litter substrate and ad libitum feed and water.
2.3 Behavior testing
2.3.1 Assessing inspective exploration and fearfulness
Two weeks after transportation to the free-range facility, at 18 weeks of age, ten hens per pen (n = 90 total, n = 30 each treatment) were randomly selected to assess inspective exploration and fearfulness via novel object and novel arena tests respectively. The testing room was within the same facility in a separate room from where the hens’ home pens were located. For the novel arena and novel object tests, hens were placed in the middle of a novel arena (170 cm x 170 cm x 170 cm; Figure 1) with wood shaving flooring and left for eight minutes. Subsequently, a small trap door at the back of the arena (20 cm x 26.5 cm) was used to place a novel object into the arena with minimal human contact, the novel object test began as soon as the trap door was closed and lasted for five minutes. The novel object was a dark purple dog chew toy (13 cm x 22 cm) inside of a pink plastic box (20 cm x 62.5 cm). The novel object was not biologically relevant to chickens and was not used in the novelty treatment group during rearing. Hen behavior during the tests was recorded via a video camera mounted directly above the arena (Sony Handycams HDR-PJ410, Sony, Tokyo, Japan) and behavior was later analyzed by one trained assessor blind to treatment. Latency to move her head, step, ground peck and vocalize during the novel arena test and the total number of vocalizations during the novel arena test were determined. Additionally, the arena was theoretically split into nine equal grids and the number of lines that a hen crossed was calculated as an indicator of movement/locomotion. Latency to peck the novel object, vocalize, ground peck and the total number of pecks to the novel object and total number of vocalizations were calculated for each hen during the novel object test. Furthermore, the arena was theoretically divided into four zones during the novel object test; an interaction, approach, center and avoidance zone (Figure 1). The time spent in each zone, and the number of entries to each zone were determined. A number of hens (n = 7) successfully escaped from the novel arena test and thus were excluded from the analysis where appropriate (e.g., latencies were included, but any total count (e.g., total lines crossed or vocalizations) were excluded).
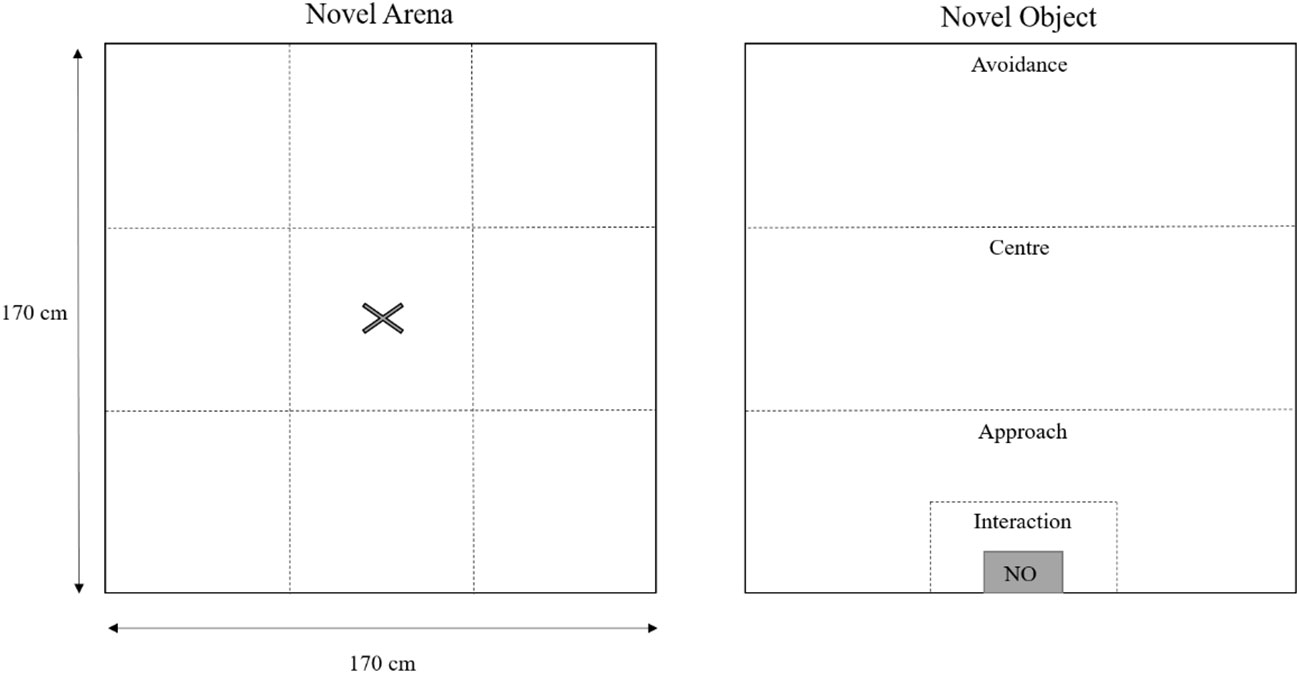
Figure 1 Novel arena and novel object test arena outlining the designated zones for each test. Zones were not marked on the ground rather were outlined over the video footage via a transparent film. The ‘×’ on the novel arena diagram indicates where each hen was placed at the start of the novel arena test. The dark grey shaded ‘NO’ box indicates where the trapdoor opened, and the novel object was placed.
2.3.2 Assessing inquisitive exploration
At 22 weeks of age, six weeks after transportation from the rearing shed to the free-range facility but three weeks prior to range access, 10 hens per pen (n = 90 total, n = 30 each treatment) were randomly chosen to assess inquisitive exploration based on Franks et al. (2013) assessment of inquisitive exploration in rodents. Hens were first habituated to the eight-arm radial maze (section 2.3.3) and conditioned to the visual cues (section 2.3.4) before inquisitive exploration was assessed. The habituation and conditioning phase was completed across eight consecutive days (all 30 hens). Assessing inquisitive exploration began the day after habituation and conditioning phase concluded and was completed within four days.
2.3.3 Habituation
Hens were placed individually in the eight-arm radial maze with only four arms exposed (no access to the ambiguous arms; Figure 2). The maze was empty except for the flooring in each arm which was slightly raised to trigger the negative stimuli during the test. Hens were left in the maze for 10 minutes before they were removed and immediately returned to their home pen. Each hen was habituated twice before testing. All tests were recorded by a video camera mounted directly above the maze for later behavioral observations and analysis.
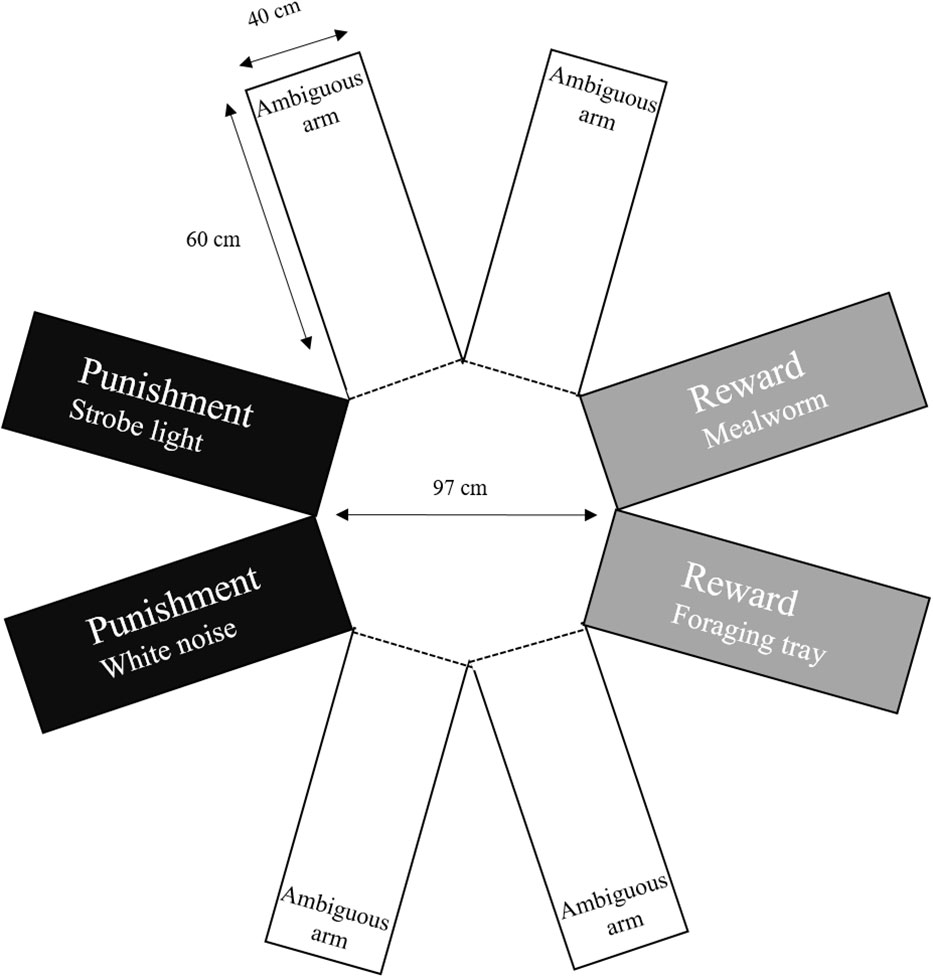
Figure 2 Eight arm radial maze used to assess inquisitive exploration based on previously published methods by Franks et al. (2013). Punishment arms contained a foraging tray with a strobe light or empty bowl with a speaker that played white noise. Negative stimuli were automatically activated when a hen entered the arm. Reward arms contained a foraging tray with wood shaving litter and a laying hen grain mix or a bowl containing mealworms. An additional mealworm was placed into the bowl each time the hen entered the mealworm reward arm. Ambiguous arms were empty.
2.3.4 Conditioning
To condition hens to the rewards and visual cues of the reward (green bowl or tray) hens were given access to the rewards during a conditioning phase, which was repeated twice before assessing inquisitive exploration. All 10 hens from each treatment pen were placed into a temporary holding pen adjacent to the home pens for two hours with access to feed, water and litter substrate. Additionally, hens were provided with access to eight green bowls each with three mealworms and eight foraging trays with wood shavings and layer grain mix (Norco, NSW, Australia). Every 30 minutes, additional mealworms and grain were provided in each bowl/tray.
2.3.5 Eight arm radial test to assess inquisitive exploration
Hens were placed into the center of the radial maze and left for 8 minutes. The maze contained a visual cue of reward in each of the four arms; either a green bowl or foraging tray. The positive arms contained one mealworm in the green bowl or a foraging tray with layer grain mix. Each time a hen entered the positive arm with the green bowl, a new mealworm was dropped into the bowl; the worm dropped from outside the maze into the bowl via a piece of plastic tubing and the experimenter was always out of sight to the hen. The hen was required to leave the positive arm and re-enter before another mealworm was released into the green bowl. The negative arms contained a strobe light inside the foraging tray or white noise under the green bowl, both out of sight to hens but triggered when a hen entered halfway into the arm and stopped once the hen moved out of the arm. The other four arms between the positive and negative designated arms were closed for the first eight minutes of the test. The positive and negative arms were swapped after every ten hens to control for any potential location effects. After 8 minutes, the lights were turned off and the ambiguous doors were opened at the same time by two experimenters, immediately after the lights were turned back on and the hens were left in the maze for an additional four minutes. It was expected that hens that were more curious would forgo the known rewards in the positive arms and risk punishment to explore the new environment. After four minutes with access to all eight arms, hens were removed from the maze and returned to their home pens. Any feces were removed before the next hen was tested. The inquisitive exploration test was recorded via two video cameras mounted directly above the maze (Sony Handycams HDR-PJ410, Sony, Tokyo, Japan) that were later analyzed by one observer blind to rearing treatment. Latency to step, vocalize and enter an arm were recorded in the habituation and inquisitive exploration test and latency to enter an ambiguous arm was recorded in the inquisitive exploration test. The number of arms entered, and time spent in each arm were recorded for each test. An arm was considered entered when more than 50% of the hen’s body had crossed the entryway.
2.3.6 Statistical analysis
All statistical analyses were performed using SPSS (v23, IBM Corp, Armonk, NY, USA). All censored data (e.g., latencies to ‘x’ and proportion of hens that did not perform ‘x’) were analyzed with a Cox regression analysis with treatment and pen included in each model. Binary data (e.g., did or did not access the range) were analyzed with a generalized linear mixed model with a binomial distribution, count data were analyzed with a generalized linear mixed model with a Poisson distribution and log link function and continuous data that met the criteria for normality were analyzed with a generalized linear mixed model with a Gaussian distribution. All models included treatment as a main factor and test cohort and pen nested within treatment as random factors.
For the inquisitive exploration analysis, five hens were excluded due to methodology issues (e.g., ambiguous doors left open) and hens that did not enter either (or both) positive or negative arms in the radial maze before the ambiguous doors were opened were excluded from the appropriate analysis. The numbers of hens used to assess whether they would forgo reward (e.g., had experienced positive arms) were 25 control hens, 26 novelty hens and 24 structural hens and the numbers of hens used for the analysis to determine if they were willing to risk punishment (e.g., had experienced negative arms) were 17 control hens, 22 novelty hens and 19 structural hens. The number of ambiguous arms entered were analyzed with a generalized linear mixed model with a Poisson distribution and log link function. Treatment was included as a fixed factor and day and pen as random factors. Post hoc analyses were corrected for multiple comparisons with the LSD method. The differences between habituation 1, 2 and the inquisitive exploration test on the latency to step, latency to enter an arm and the number of arms entered were analyzed with a generalized linear mixed model with an autoregressive covariance structure to account for repeated measures. The model included test and treatment and the interaction between test and treatment as fixed factors and pen nested within treatment as a random factor. An interaction was removed from a model when it was not significant to improve the model fit as confirmed by the Akaike’s Information Criterion (AIC).
There was no difference on any ranging variable between test cohorts (e.g., inspective or inquisitive exploration hens) and therefore data were pooled from both test cohorts to investigate the impact of rearing treatments on ranging behavior, but test cohort was included as a random factor in all ranging analysis.
Relationships between behavioral measures during the novel arena, novel object and inquisitive exploration test and early life ranging (for example latency to range or total number of range visits) were analyzed with Spearman correlations. Relationships between ranging variables (latency to access the range, number of visits to the range and the total time spent on the range) were analyzed with partial regressions controlling for treatment.
Estimated marginal means are reported unless otherwise noted.
3 Results
3.1 The impact of the rearing environment on inspective exploration (novel object test) and general fearfulness (novel arena test)
There was no impact of rearing treatment on latency to step (p = 0.425), attempt to escape (p = 0.327), vocalize (p = 0.384) or ground peck (p = 0.153) during the novel arena test (Table 1). Furthermore, there was no difference between rearing treatment groups in the number of vocalizations (p = 0.429), lines crossed (p = 0.453) or escape attempts (p = 0.376) during the novel arena test (Table 1).

Table 1 Comparisons of behavioural indicators observed during the novel arena and novel object tests for hens that were reared in control, novelty or structural environments.
During the novel object test, hens from the novelty treatment group were more likely to peck the ground and were quicker to do so than hens from the control and structural treatment group (X2 = 4.9, df = 1, ExpB = 4.46, CI 2.14, 34.05, p = 0.028; Figure 3).
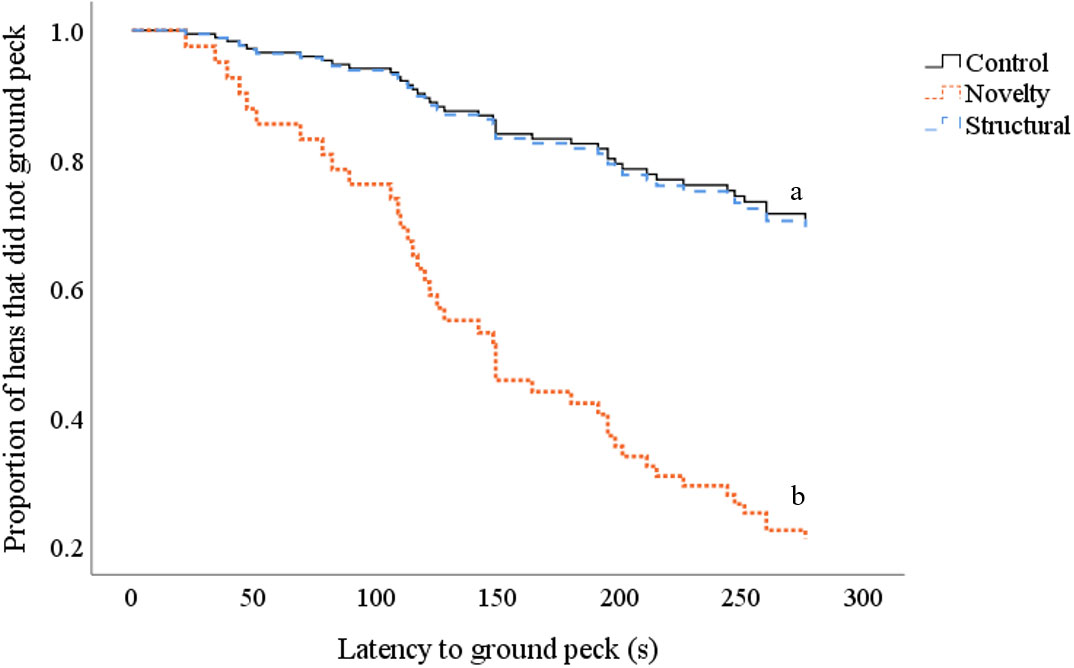
Figure 3 Survival curves indicating the proportion of control (black solid line), novelty (orange dotted line) and structural (light blue dashed line) hens that ground pecked (y-axis) over time (seconds) (x-axis) during the novel object test. Every time a hen pecked at the ground, the probability on the y-axis drops. Differing subscript is indicative of differences between treatment groups p < 0.05.
There was no difference between treatment groups in the latency to peck the novel object (p = 0.118) or vocalize (p = 0.255) during the novel object test (Table 1). Of note, the number of hens that pecked the novel object was quite low (Table 1). The number of vocalizations during the novel object test did not differ between treatment groups (p = 0.880).
There was no impact of rearing treatment on a hen’s preferred zone (i.e., the zone the hen spent the most time in during the test; p = 0.828), or the time spent in any of the zones (avoidance zone p = 0.633; center zone p = 0.622; approach zone p = 0.624; interaction zone p = 0.583; Table 1) during the novel object test.
3.2 The effect of rearing on inquisitive exploration
There was no interaction between treatment and test (habituation 1, habituation 2 or inquisitive exploration test) on the latency to step (p = 0.236), latency to enter an arm in the radial maze (p = 0.116) or the number of times an arm was entered (p = 0.919). There was an impact of test on the latency to step (F(2,159)= 6.9, p = 0.001) but no effect of treatment (p = 0.878); hens in all treatment groups were quicker to step during habituation 2 (22.1 ± 7.1 s) and the inquisitive exploration test (11.5 ± 7.1 s) compared to habituation 1 (47.3 ± 7.1). There was an impact of test on the latency to enter an arm (F(2,150)= 18.0, p < 0.0001) but no effect of treatment (p = 0.569). Hens from all treatment groups were quicker to enter an arm in habituation 2 (92.2 ± 17.5 s) compared to habitation 1 (168.6 ± 17.6 s) and during the inquisitive exploration test (34.3 ± 17.4 s) compared to habituation 1 (p < 0.0001) and habituation 2 (p = 0.003; Table 2). There was no effect of treatment or test on the number of arm entries (p > 0.05; Table 2). There was an effect of treatment on the number of hens that entered, and latency to enter, an ambiguous arm (X2(11,75)= 9.56, p = 0.004). More hens from the novelty treatment entered the ambiguous arm than hens from the control group and were quicker to do so (X2 = 9.2, df = 1, ExpB = 8.54, CI 2.14, 34.05, p = 0.002; Figure 4). There was no difference between control and structural hens (p = 0.155) or structural and novelty hens (p = 0.115).
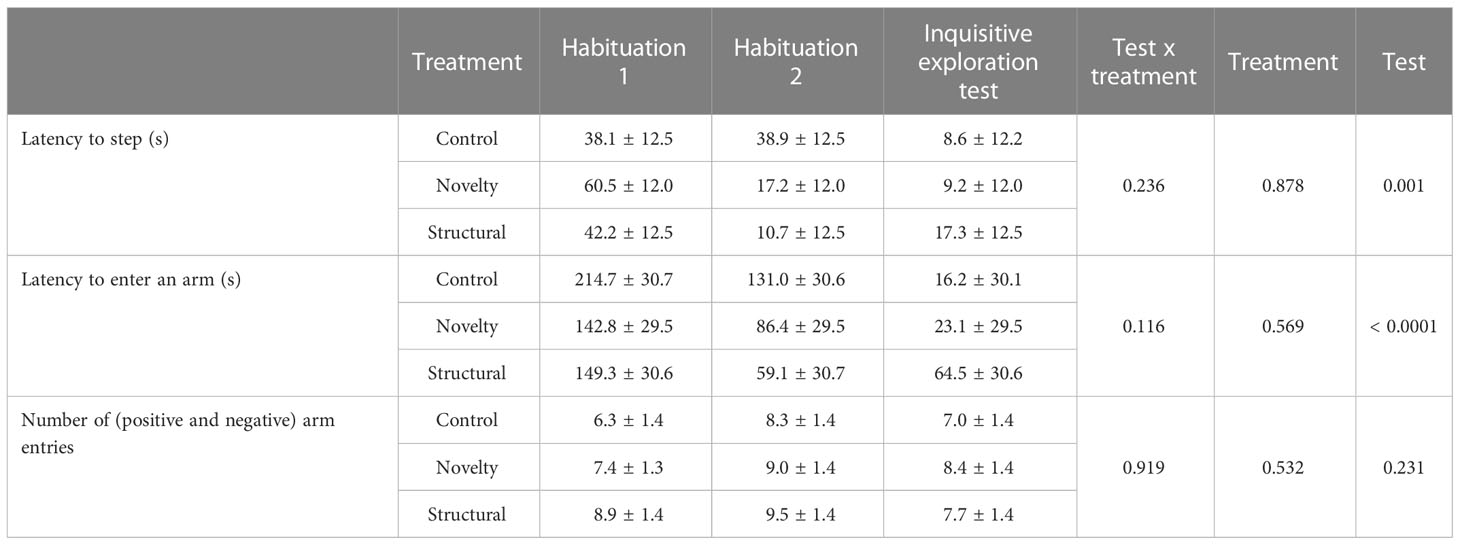
Table 2 Latency to step and enter any arm and the number of arm entries during the 8-arm radial maze test during the first and second round of habituation (when no reward was present) and the first 8 minutes of the inspective exploration test (when visual cues and reward and punishment were present, but ambiguous arms were closed) for hens that were housed in control (industry like conditions), novel environments or provided with structures during rearing (0-16 weeks of age).
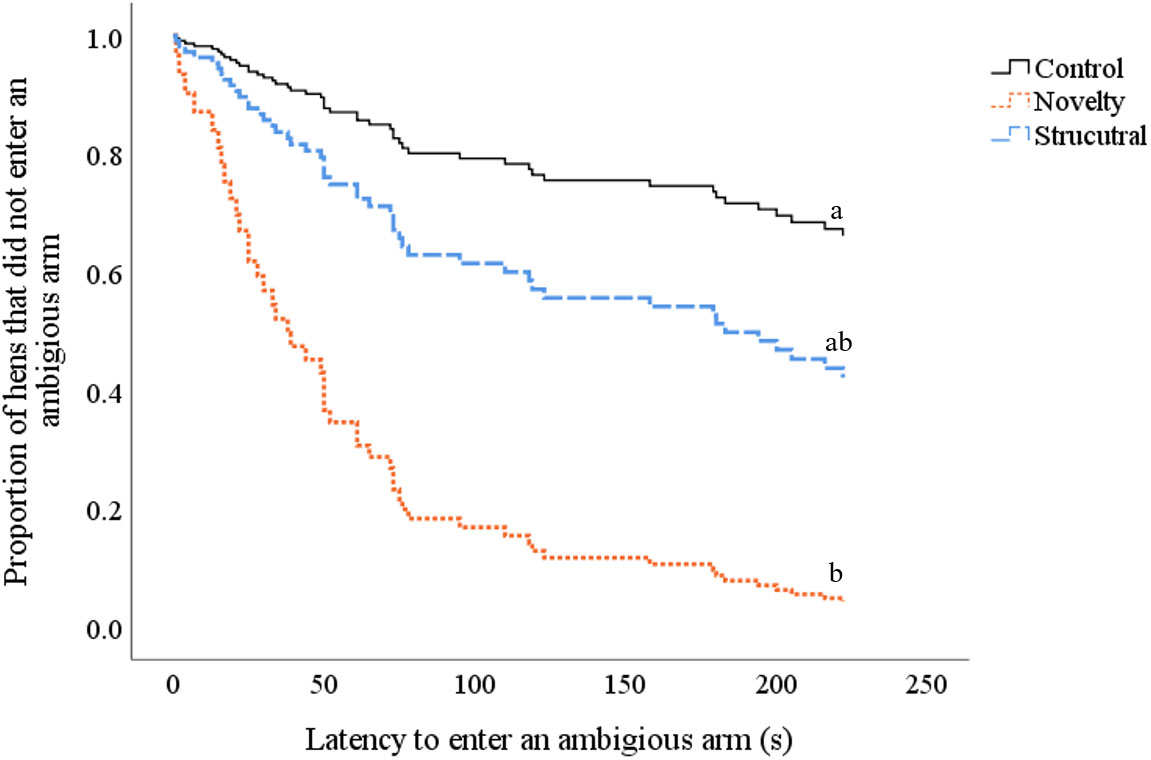
Figure 4 Survival curves indicating the proportion of control (black solid line), novelty (orange dotted line) and structural (light blue dashed line) hens that entered an ambiguous arm (y-axis) over time (seconds) (x-axis) during the inquisitive exploration test. Every time a hen entered an ambiguous arm, the probability on the y-axis drops. Differing subscript is indicative of differences between treatment groups p < 0.05.
Treatment affected the average number of ambiguous arms entered (F(2,72)=3.46, p = 0.037; estimated marginal mean number of arms entered: control hens 1.11 ± 0.28; novelty hens 2.08 ± 0.45; structural hens 1.42 ± 0.34). Hens in the novelty treatment group entered more ambiguous arms than hens in the control group (t = - 2.38, p = 0.02). There was no difference between hens from the novelty and structural treatment group (p = 0.115), or between structural and control hens (p = 0.37) in the number of arms entered.
There was no difference in the total time spent in the ambiguous arms (p = 0.965) or time spent in the positive ambiguous (p = 0.348) or negative ambiguous (p = 0.676) arms between treatment groups.
3.3 The effect of rearing on early life ranging behavior
The number of hens that accessed the range at least once did not differ between the two test cohorts (i.e., groups of hens tested for either inspective or inquisitive exploration) (p = 0.688) nor did the total number of range visits (p = 0.442), time spent on the range (p = 0.621) or latency to range (p = 0.833). Therefore, the ranging behavior and relationships with rearing treatments were pooled, analyzed and presented together. There was no impact of rearing treatments on any early ranging behavior (all p > 0.05; Table 3). There was individual variation in ranging behavior in the first three weeks of access (Table 4). Latency to access the range was negatively correlated with the number of days the range was accessed (r = - 0.876, p < 0.0001), the total number of range visits (r = - 0.696, p < 0.0001) and the total time spent on the range (r = - 0.690, p < 0.0001). Time spent on the range, frequency of visits and the number of days the range was accessed were strongly positively correlated (Time spent on the range and frequency of visits r = 0.895; p < 0.0001; Time spent on the range and number of days the range was accessed r = 0.834; p < 0.0001; Frequency of visits and number of days the range was accessed r = 0.854; p < 0.0001).
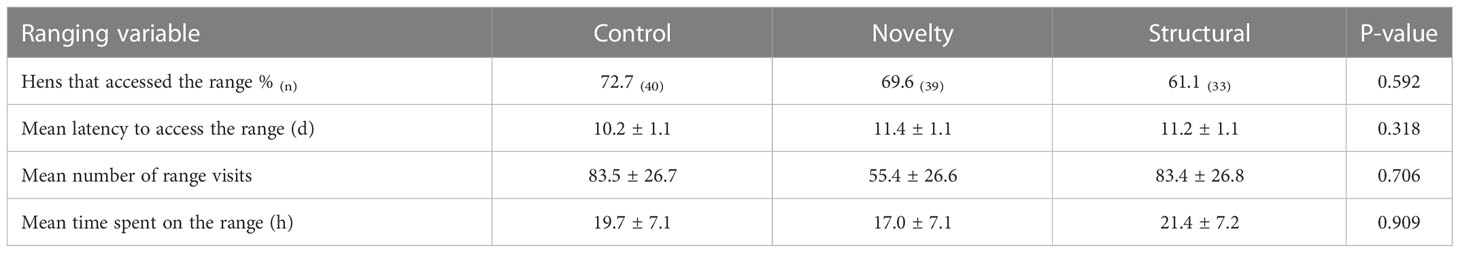
Table 3 Early life ranging behaviour of hens reared in either a control, novel or structurally complex environment.
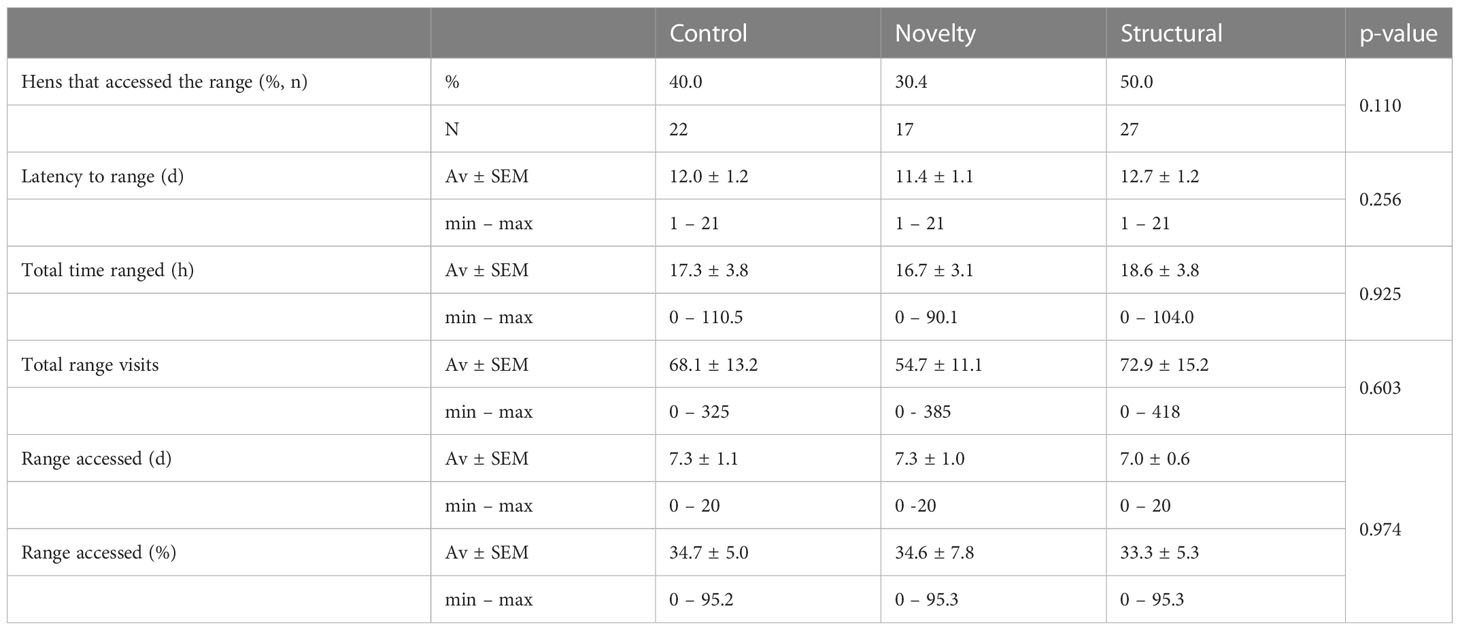
Table 4 Descriptive statistics of ranging variables collected during the first three weeks of range access, including averages ± standard error of the mean (SEM) and proportion of hens that accessed the range for hens reared in a control, novelty or structurally complex environment.
3.4 Relationships between early life ranging behavior and behavioral indicators of fearfulness and exploration
Few behavioral measures that were assessed during the novel arena, novel object, habituation or inquisitive exploration tests were related to ranging behavior and significant relationships that were identified were only weakly correlated (Table 5).
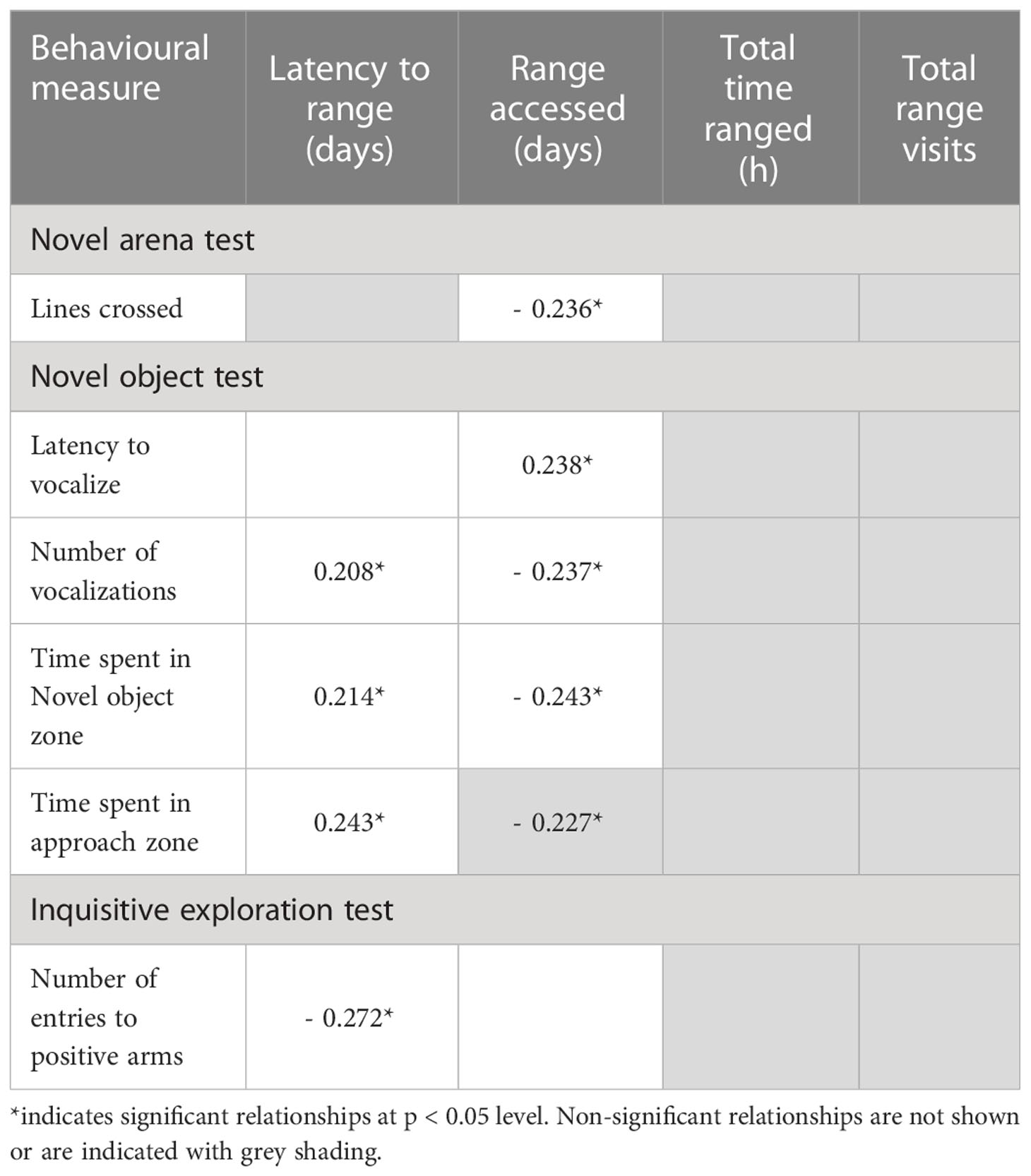
Table 5 Spearman correlations indicating weak relationships (r) between behavioural measures during the novel arena, novel object and inquisitive exploration tests and ranging behaviour.
The latency to range and the number of days that the range was accessed were weakly related to indicators of general fearfulness, social isolation and inspective exploration. Such that, the faster a hen accessed the range the less time she spent in the novel object and approach zones (NO zone r(89)= 0.214, p = 0.044; approach zone r(89)= 0.243, p = 0.022) and vocalized fewer times (r(90)= 0.208, p = 0.05) during the novel object test. Additionally, the more days a hen visited the range the fewer lines she crossed (r(83) = - 0.236, p = 0.032) and vocalized quicker (r(90)= 0.238, p = 0.024) during the novel arena test (r(90)= - 0.237, p = 0.024) and the less time she spent in the novel object zone (r(89)= - 0.243, p = 0.022) and the approach zone (r(89)= - 0.227, p = 0.032) during the novel object test.
4 Discussion
We aimed to better understand the mechanisms behind the well-known variability in ranging behavior of free-range laying hens. Specifically, we hypothesized that hens that were curious and less fearful of novelty would access the outdoor range sooner after range access was first provided and would range more frequently during the first three weeks of range access relative to hens that were less curious and more fearful of novelty. However, we found few (weak) correlations between range use in the first three weeks of range access and behavioral indicators of inspective exploration and fearfulness. Yet, by utilizing a novel 8-arm radial test adapted from Franks et al. (2013), we found evidence that providing novelty during rearing increased a hen’s willingness to forgo reward and risk punishment to explore a novel area. Therefore, we provide evidence that a more novel and/or complex environment during rearing increases hen curiosity but is not related to ranging behavior.
Hens reared with novelty were quicker to enter the ambiguous arms of the 8-arm radial maze and entered the ambiguous arms more frequently than control hens, although there was no difference between hens provided with structures during rearing and hens provided with novelty. To our knowledge, this is the first time this choice paradigm has been reported in laying hens. Novelty in an environment (especially the rearing environment) has been shown to have an impact on various traits such as exploration and seeking novelty, learning and memory and anxiety and stress reactivity (De Jong et al., 2000; Altan et al., 2013). Although fearfulness has been shown to impact a hen’s ability to learn (De Haas et al., 2017), we found little evidence of this effect related to treatment in the 8-arm radial maze assessment and therefore we provide evidence that our novelty rearing treatment likely influenced inquisitive exploration. For example, it is unlikely that rearing treatment had an impact on learning as there was no difference between treatment groups on latency to enter a positive or negative arm (i.e. no differences in conditioning between treatment groups). Similarly, in a different group of hens from the same larger research flock, there were no effects of rearing treatment on learning during a maze test (Campbell et al., 2021). Additionally, although we saw habituation to the test arena [i.e. a reduction in the latency to step from habituation 1 to habituation 2, which is a validated indicator of fearfulness in chickens (Forkman et al., 2007)] this was not related to treatment suggesting that any differences in the motivation to explore the ambiguous arms were unlikely related to fearfulness or learning. It is possible that the differences observed may be related to reward expectation rather than curiosity per se.
Our results support those of Franks et al. (2013) that showed rodents forgo reward and risk punishment to explore a novel environment and therefore provide evidence that inquisitive exploration is valued. But the relationship between inquisitive exploration identified in the current study and hen welfare requires further investigation. Franks et al. (2013) removed environmental enrichment from rodents with the aim to reduce welfare (Bateson and Matheson, 2007; Latham and Mason, 2010) and subsequently showed that inquisitive exploration was reduced. Furthermore, Franks et al. (2013) showed that inquisitive exploration was positively related to sucrose consumption; sucrose was provided in the home pen as a reward to assess the rodent’s willingness to approach reward as well as an indicator of anhedonia and poor welfare. Sucrose consumption reduced when animals were exposed to chronic unpredictable stress and restored after treatment with an antidepressant (Willner et al., 1987). As such, Franks et al. (2013) suggest that the willingness to forgo reward and risk punishment to explore a novel environment may provide evidence as to an animal’s state of welfare. Further investigation is required to extrapolate these results to hens. However, much of the neurobiological research suggests that obtaining information that resolves uncertainty is rewarding (Panksepp, 2004; Bromberg-Martin and Hikosaka, 2009; Špinka, 2019). Therefore, enhancing curiosity in animals can help to ensure captive animals are permitted positive experiences throughout life, which is a critical component of animal welfare (Mellor, 2015a; Rault et al., 2020). It must also be noted that thwarting opportunities for inquisitive exploration for curious animals will compromise animal welfare [see Wood-Gush and Vestergaard (1989); Appleby et al. (2011); Mellor (2015b)]. Therefore, care must be taken to ensure that such curious animals are provided with an appropriate environment to seek information. Such opportunities may include problem solving tasks, as the success of completing such tasks itself is shown to be intrinsically rewarding in cattle (Hagen and Broom, 2004) and canines (Mcgowan et al., 2014). For example, physiological indicators of arousal were greater when cattle were rewarded with a food treat after completing a problem solving task compared to simply being provided with the food reward alone (Hagen and Broom, 2004).
Rearing treatment was related to the number of birds that entered the ambiguous arm and how quickly they entered the ambiguous arm. Hens reared with novelty were more likely to enter an ambiguous arm and were quicker to do so than hens raised in an industry standard environment. Although the difference was observed in the hen’s willingness to forgo reward but not risk punishment, hens from the novelty rearing group were more likely to enter ambiguous arms closer to the reward rather than the ambiguous arms located next to the punishment arms. This may suggest that hens that were reared with novelty were more curious than hens raised in more stable and barren conditions. Furthermore, the judgement of ambiguous cues has been shown to be related to the expectation of outcomes for human and non-human animals and is thought to reflect the underlying mood/state of the animal [see Roelofs et al. (2016) and Lagisz et al. (2020)]. For example, it has been shown that ambiguous cues are perceived more pessimistically if animals are anxious (evident by anxiogenic and anxiolytic pharmacological interventions) or more optimistically when animals are in a positive emotional state [see Neville et al. (2020)].
We defined our rearing treatment as a ‘novelty treatment’ as the environmental enrichment items were not biologically relevant and were changed every 7 days. However, we cannot determine if the effects here are reflective of a more complex rearing environment relative to the control group or the novelty provided every few days or a specific enrichment item(s) provided at one point in time during rearing, which may or may not have coincided with an important sensitive period for neurodevelopment (Jones and Waddington, 1993; Kempermann, 2019). Fox and Millam (2007) provide evidence that it is indeed novelty that is the important characteristic of environmental enrichment programs to reduce neophobia of orange-winged Amazon parrots. However, Abou-Ismail and Mendl (2016) found that the provision of novelty to rodents in the absence of diversity of objects at one time is less beneficial. A comparison of complexity and diversity compared to novelty in the rearing environment of laying hens is required to fully understand the relationships identified in the current study.
The lack of evidence that treatment affected a hen’s behavior during the novel arena and novel object tests suggests that the eight-arm radial maze is assessing a different component of the bird’s temperament, such as inquisitive exploration (rather than inspective exploration), reward expectation or even as Franks et al. (2013) suggests, a broader reflection of well-being. As evidence suggests that emotional reactivity impacts exploration but the propensity for novelty seeking is thought to be independent (Grossman et al., 2007), the 8-arm radial assessment utilized in the current study may be a useful tool to identify behavioral indicators specific to inquisitive exploration and may prove a useful tool to assess hen welfare that warrants further investigation.
We expected that a hen’s latency to first access the range would be related to fear or curiosity, such that fearful hens would take longer to access the range and curious hens would access the range shortly after access was provided. We did identify a few weak correlations between behavioral indicators in the novel arena and novel object tests and ranging behavior. Specifically, a shorter latency to first access the range and more range visits in the first three weeks of range access was associated with fewer vocalizations during the novel arena test and less time spent in the areas close to the novel object during the novel object test. These findings were unexpected and contrast findings from Kolakshyapati et al. (2020) who found that time spent interacting with a novel object was associated with more frequent range use of laying hens on a commercial farm. As Kolakshyapati et al. (2020) assessed hen behavior in the novel object test after range access in contrast to our findings which assessed hens prior to range access, the relationships identified with inspective exploration and range use in the Kolakshyapati et al. (2020) study may reflect an effect of ranging on laying hens as a consequence of the complex range environment rather than a hen’s motivation to range. Of note, Armstrong et al. (2020) found relationships between indicators of adult neurogenesis (proliferating cell nuclear antigen and doublecortin) in the rostral and caudal subregion of the hippocampus of laying hens that visited the range more frequently which further supports brain and behavioral changes associated with range use, perhaps from the more complex environment or simply an increase in activity associated with range use. However, Armstrong et al. (2020) did not find any differences in measures of TI between birds that accessed the range frequently and those that did not, suggesting that the associated brain changes were not associated with a bird’s response to unfamiliar stimuli. Pre- and post-ranging assessments of hen temperament may help unpack these complex relationships and explain some of the factors that influence ranging behavior of laying hens.
Whilst we identified some statistically significant relationships between ranging behavior during the first three weeks of access and behavioral responses during all three behavioral tests, results were very weak and inconsistent. This may indicate that no such relationships exist, or the presence of a type I error may be present due to the methodological constraints, i.e. small sample sizes (n = 30 hens/treatment each test) or the fact that treatments were only replicated across three pens during rearing and prior to testing. However, we did observe a willingness to forgo reward to explore a novel environment in hens that were reared with novel items. Further research utilizing this novel test is required to examine the effects of novelty during rearing on hen curiosity and ranging behavior.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://figshare.com/articles/dataset/Range_use_fearfulness_and_curiosity_test_data/21762485.
Ethics statement
The animal study was reviewed and approved by University of New England Animal Ethics Committee.
Author contributions
PT wrote the manuscript. PT, PH, DC and CL developed the concepts and methodologies. PT, EJ and ND conducted all data collection and analysis. All authors contributed to the article and approved the submitted version.
Funding
This work was partially funded by Poultry Hub Australia (grant number 2017-20).
Acknowledgments
The authors wish to acknowledge the following people for their skillful involvement, Andrew Gasbarri and UNE Science Engineering Workshop, Dean Astrella, Lara Fanning, Lachlan Thurtell, Andrew Cohen-Barnhouse, Tim Dyall and Jim Lea.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abou-Ismail U. A., Mendl M. T. (2016). The effects of enrichment novelty versus complexity in cages of group-housed rats (Rattus norvegicus). Appl. Anim. Behav. Sci. 180, 130–139. doi: 10.1016/j.applanim.2016.04.014
Altan O., Seremet C., Bayraktar H. (2013). The effects of early environmental enrichment on performance, fear and physiological responses to acute stress of broiler. Archiv Fur Geflugelkunde 77, 23–28. doi: 10.1590/1806-9061-2016-0402
Appleby M. C., Hughes B. O., Mench J. A., Olson S. L. (2011). Animal welfare (Wallingford: UK International Publishing).
Armstrong E. A., Voelkl B., Voegeli S., Gebhardt-Henrich S. G., Guy J. H., Sandilands V., et al. (2020). Cell proliferation in the adult chicken hippocampus correlates with individual differences in time spent in outdoor areas and tonic immobility. Front. Vet. Sci. 7. doi: 10.3389/fvets.2020.00587
Bari M. S., Downing J. A., Dyall T. R., Lee C., Campbell D. L. M. (2020). Relationships between rearing enrichments, range use, and an environmental stressor for free-range laying hen welfare. Front. Vet. Sci. 7, 480. doi: 10.3389/fvets.2020.00480
Bateson M., Matheson S. (2007). Performance on a categorisation task suggests that removal of environmental enrichment induces 'pessimism' in captive European starlings (Sturnus vulgaris). Anim. Welfare 16 (S1), 33–36. doi: 10.1017/S0962728600031705
Blanchard T. C., Hayden B. Y., Bromberg-Martin E. S. (2015). Orbitofrontal cortex uses distinct codes for different choice attributes in decisions motivated by curiosity. Neuron 85, 602–614. doi: 10.1016/j.neuron.2014.12.050
Bromberg-Martin E. S., Hikosaka O. (2009). Midbrain dopamine neurons signal preference for advance information about upcoming rewards. Neuron 63, 119–126. doi: 10.1016/j.neuron.2009.06.009
Campbell D. L. M., Hinch G. N., Downing J. A., Lee C. M. (2016). Fear and coping styles of outdoor-preferring, moderate-outdoor and indoor-preferring free-range laying hens. Appl. Anim. Behav. Sci. 185, 73–77. doi: 10.1016/j.applanim.2016.09.004
Campbell D. L. M., Hinch G. N. A., Downing J., Lee C. N. (2018a). Early enrichment in free-range laying hens: Effects on ranging behaviour, welfare and response to stressors. Animal 12, 575–584. doi: 10.1017/S1751731117001859
Campbell D. L. M., Talk A. C., Loh Z. A., Dyall T. R., Lee C. (2018b). Spatial cognition and range use in free-range laying hens. Animals 8, 26. doi: 10.3390/ani8020026
Campbell D. L. M., Whitten J. M., Slater E., Lee C. (2021). Rearing enrichments differentially modified hen personality traits and reduced prediction of range use. Anim. Behav. 179, 97–109. doi: 10.1016/j.anbehav.2021.06.024
De Haas E. N., Lee C., Rodenburg T. B. (2017). Learning and judgment can be affected by predisposed fearfulness in laying hens. Front. Vet. Sci. 4, 113. doi: 10.3389/fvets.2017.00113
De Jong I. C., Prelle I. T., Van De Burgwal J. A., Lambooij E., Korte S. M., Blokhuis H. J., et al. (2000). Effects of environmental enrichment on behavioral responses to novelty, learning, and memory, and the circadian rhythm in cortisol in growing pigs. Physiol. Behav. 68, 571–578. doi: 10.1016/S0031-9384(99)00212-7
Ferreira V. H. B., Simoni A., Germain K., Leterrier C., Lansade L., Collin A., et al. (2021). Working for food is related to range use in free-range broiler chickens. Sci. Rep. 11, 1–11. doi: 10.1038/s41598-021-85867-2
Forkman B., Boissy A., Meunier-Salaün M.-C., Canali E., Jones R. (2007). A critical review of fear tests used on cattle, pigs, sheep, poultry and horses. Physiol. Behav. 92, 340–374. doi: 10.1016/j.physbeh.2007.03.016
Fox R. A., Millam J. R. (2007). Novelty and individual differences influence neophobia in orange-winged Amazon parrots (Amazona amazonica). Appl. Anim. Behav. Sci. 104, 107–115. doi: 10.1016/j.applanim.2006.04.033
Franks B., Champagne F. A., Higgins E. T. (2013). How enrichment affects exploration trade-offs in rats: Implications for welfare and well-being. PLoS One 8, e83578. doi: 10.1371/journal.pone.0083578
Grigor P. P., Hughes B. N., Appleby M. O. (1995). Effects of regular handling and exposure to an outside area on subsequent fearfulness and dispersal in domestic hens. Appl. Anim. Behav. Sci. 44, 47–55. doi: 10.1016/0168-1591(95)00576-E
Grossman H. C., Hale G., Light K., Kolata S., Townsend D. A., Goldfarb Y., et al. (2007). Pharmacological modulation of stress reactivity dissociates general learning ability from the propensity for exploration. Behav. Neurosci. 121, 949. doi: 10.1037/0735-7044.121.5.949
Hagen K., Broom D. M. (2004). Emotional reactions to learning in cattle. Appl. Anim. Behav. Sci. 85, 203–213. doi: 10.1016/j.applanim.2003.11.007
Hartcher K. M., Hickey K. A., Hemsworth P. H., Cronin G. M., Wilkinson S. J., Singh M. (2016). Relationships between range access as monitored by radio frequency identification technology, fearfulness, and plumage damage in free-range laying hens. Animal 10, 847–853. doi: 10.1017/S1751731115002463
Hughes R. N. (1997). Intrinsic exploration in animals: Motives and measurement. Behav. Processes 41, 213–226. doi: 10.1016/S0376-6357(97)00055-7
Hy-Line International (2018) Ly-line management guide. Available at: https://www.hyline.com/UserDocs/Pages/BRN_COM_ENG.pdf (Accessed November 2018).
Jones R. B., Waddington D. (1993). Attenuation of the domestic chick’s fear of human beings via regular handling: In search of a sensitive period. Appl. Anim. Behav. Sci. 36, 185–195. doi: 10.1016/0168-1591(93)90009-E
Kang M. J., Hsu M., Krajbich I. M., Loewenstein G., Mcclure S. M., Wang J. T.-Y., et al. (2009). The wick in the candle of learning: Epistemic curiosity activates reward circuitry and enhances memory. Psychol. Sci. 20, 963–973. doi: 10.1111/j.1467-9280.2009.02402.x
Kempermann G. (2019). Environmental enrichment, new neurons and the neurobiology of individuality. Nat. Rev. Neurosci. 20, 235–245. doi: 10.1038/s41583-019-0120-x
Kolakshyapati M., Taylor P. S., Hamlin A., Sibanda T. Z., Vilela J. D. S., Ruhnke I. (2020). Frequent visits to an outdoor range and lower areas of an aviary system is related to curiosity in commercial free-range laying hens. Animals 10, 1706. doi: 10.3390/ani10091706
Lagisz M., Zidar J., Nakagawa S., Neville V., Sorato E., Paul E. S., et al. (2020). Optimism, pessimism and judgement bias in animals: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 118, 3–17. doi: 10.1016/j.neubiorev.2020.07.012
Larsen H., Cronin G. M., Gebhardt-Henrich S. G., Smith C. L., Hemsworth P. H., Rault J.-L. (2017). Individual ranging behaviour patterns in commercial free-range layers as observed through RFID tracking. Animals 7, 21. doi: 10.3390/ani7030021
Larsen H., Hemsworth P., Cronin G., Gebhardt-Henrich S., Smith C., Rault J.-L. (2018). Relationship between welfare and individual ranging behaviour in commercial free-range laying hens. Animal 12, 2356–2364. doi: 10.1017/S1751731118000022
Latham N., Mason G. (2010). Frustration and perseveration in stereotypic captive animals: Is a taste of enrichment worse than none at all? Behav. Brain Res. 211, 96–104. doi: 10.1016/j.bbr.2010.03.018
Lau J. K. L., Ozono H., Kuratomi K., Komiya A., Murayama K. (2018). Hunger for knowledge: how the irresistible lure of curiosity is generated in the brain. bioRxiv, 473975. doi: 10.1101/473975
Mcgowan R. T., Rehn T., Norling Y., Keeling L. J. (2014). Positive affect and learning: Exploring the “Eureka effect” in dogs. Anim. Cogn. 17, 577–587. doi: 10.1007/s10071-013-0688-x
Mellor D. (2015a). Enhancing animal welfare by creating opportunities for positive affective engagement. N. Z. Vet. 63, 3–8. doi: 10.1080/00480169.2014.926799
Mellor D. (2015b). Positive animal welfare states and encouraging environment-focused and animal-to-animal interactive behaviours. N. Z. Vet. 63, 9–16. doi: 10.1080/00480169.2014.926800
Neville V., Nakagawa S., Zidar J., Paul E. S., Lagisz M., Bateson M., et al. (2020). Pharmacological manipulations of judgement bias: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 108, 269–286. doi: 10.1016/j.neubiorev.2019.11.008
Panksepp J. (2004). Affective neuroscience: The foundations of human and animal emotions (Oxford, UK: Oxford University Press).
Rault J. L., Hintze S., Camerlink I., Yee J. R. (2020). Positive welfare and the like: Distinct views and a proposed framework. Front. Vet. Sci. 7, 370. doi: 10.3389/fvets.2020.00370
Roelofs S., Boleij H., Nordquist R. E., van der Staay F. J. (2016). Making decisions under ambiguity: Judgment bias tasks for assessing emotional state in animals. Front. Behav. Neurosci. 10, 119. doi: 10.3389/fnbeh.2016.00119
Špinka M. (2019). Animal agency, animal awareness and animal welfare. Anim. Welfare 28, 11–20. doi: 10.7120/09627286.28.1.011
Taylor P. S., Hemsworth P. H., Groves P. J., Gebhardt-Henrich S. G., Rault J.-L. (2020). Frequent range visits further from the shed relate positively to free-range broiler chicken welfare. Animal 14, 138–149. doi: 10.1017/S1751731119001514
Wang M. Z., Hayden B. Y. (2019). Monkeys are curious about counterfactual outcomes. Cognition 189, 1–10. doi: 10.1016/j.cognition.2019.03.009
Willner P., Towell A., Sampson D., Sophokleous S., Muscat R. A. (1987). Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology 93, 358–364. doi: 10.1007/BF00187257
Wood-Gush D. G., Vestergaard K. (1989). Exploratory behavior and the welfare of intensively kept animals. J. Agric. Ethics 2, 161–169. doi: 10.1007/BF01826929
Keywords: curiosity, free-range, inspective, welfare, affect, environmental enrichment
Citation: Taylor PS, Campbell DLM, Jurecky E, Devine N, Lee C and Hemsworth PH (2023) Novelty during rearing increased inquisitive exploration but was not related to early ranging behavior of laying hens. Front. Anim. Sci. 4:1128792. doi: 10.3389/fanim.2023.1128792
Received: 21 December 2022; Accepted: 27 January 2023;
Published: 10 February 2023.
Edited by:
Fabio Abeni, Consiglio per la Ricerca in Agricoltura e l’Analisi dell’Economia Agraria (CREA), ItalyReviewed by:
Cécile Bienboire-Frosini, Institut de Recherche en Sémiochimie et Ethologie Appliquée (IRSEA), FranceTakashi Bungo, Okayama University of Science, Japan
Copyright © 2023 Taylor, Campbell, Jurecky, Devine, Lee and Hemsworth. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peta S. Taylor, cGV0YS50YXlsb3JAdW5pbWVsYi5lZHUuYXU=
 Peta S. Taylor
Peta S. Taylor Dana L. M. Campbell
Dana L. M. Campbell Emma Jurecky1
Emma Jurecky1 Natalie Devine
Natalie Devine Caroline Lee
Caroline Lee Paul H. Hemsworth
Paul H. Hemsworth