
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Anim. Sci., 16 March 2023
Sec. Precision Livestock Farming
Volume 4 - 2023 | https://doi.org/10.3389/fanim.2023.1093851
The objective of this manuscript was to present the e-Synch system, integrating an intravaginal electronically controlled hormone delivery and sensing device with an IoT platform for remote programming and monitoring. Secondary objectives were to demonstrate system functionality and cow responses to e-Synch. External components of e-Synch include a 3D-printed case with retention wings, a flexible wideband antenna, and silicone membrane for pressure balancing. Internal components include a central control board, battery, wireless charging coil, and two silicone hormone reservoirs connected to individual peristaltic pumps. An accelerometer and a high-accuracy temperature sensor are integrated in the custom printed circuit board (PCB). The IoT platform includes a gateway consisting of Raspberry PI 3 and a CC1352 radiofrequency module that collects sensor data at 915 mHz. Data is transferred to the Google Cloud utilizing the IoT Core service through TCP/IP, and then is pulled by the Pub/Sub service. After routing to a BigQuery table by the Dataflow service, data visualization is provided by Data Studio. Drug delivery protocols are selected using an IOS device app that connects to e-Synch through Bluetooth. Experiments with lactating Holsteins cows were conducted to demonstrate proof-of-concept system functionality and evaluate cow responses. Despite unstable communication and signal discontinuity because of signal strength attenuation by body tissue, devices (n=6) communicated with the IoT platform in 89% (24/27) of use instances. Temperature and accelerometer data were received for at least one 15 min period during an 8 h insertion period from all devices that communicated with the IoT platform. Variation in accelerometer data (± 8.565 m/s2) was consistent with cow activity during experimentation and mean vaginal temperature of 39.1 °C (range 38.6 to 39.5 °C) demonstrated sensor functionality. Hormone release was confirmed in all instances of device use except for one. Cow behavior evaluated through signs of discomfort and pain, and tail raising scores was mostly unaltered by e-Synch. Vaginal integrity and mucus scores also remained unaltered during and after device insertion. In conclusion, the e-Synch device integrated with a controlling app and IoT platform might be used to automate intravaginal hormone delivery and sensing for controlling the estrous cycle of cattle.
Artificial insemination (AI) with semen of superior genetic merit bulls and embryo transfer (ET) are used to accelerate genetic gain and achieve optimal reproductive performance of dairy and beef cattle herds. Cows and heifers receive AI or ET at detected estrus or at a fixed time without detection of estrus. The latter is accomplished after manipulation of the estrous cycle with a sequence of reproductive hormone treatments known as synchronization of ovulation protocols (De Rensis and Peters, 1999; Wiltbank and Pursley, 2014). Timed AI (TAI) and timed ET (TET) are widely used because there is no need to detect estrus, which can be challenging for both dairy and beef operations. In addition, days to insemination and ET are fully controlled (Pursley et al., 1995; Pursley et al., 1997), and labor for hormonal treatments and AI or ET can be organized for specific days of the week. Moreover, compared with AI and ET at detected estrus, TAI and TET after protocols that optimize the reproductive endocrine environment and improve synchrony of ovulation can also enhance fertility (Souza et al., 2008; Giordano et al., 2012; Santos et al., 2017). A drawback of implementing synchronization of ovulation protocols is the need to inject cows with multiple hormone treatments in a specific sequence and dose over a period of days or weeks. For many farms, implementing these protocols in a systematic manner is labor intensive, repetitive, prone to errors, and disruptive of cow routines. Multiple injections can also increase the risk of damage to muscle and other tissues (Fajt et al., 2011; Pfeiffer et al., 2018).
Advances in engineering technology and Internet of Things (IoT), coupled with a better understanding of animal biology through sensing, are facilitating the development of digital tools for improving livestock monitoring and management (Berckmans, 2017; Halachmi et al., 2019; Unold et al., 2020). For example, implantable electronic devices designed to automate control of the estrous cycle could help eliminate injections with hormonal treatments for synchronization of ovulation, which would reduce cow discomfort (Cross et al., 2004; Masello et al., 2020). Providing full control of hormone release with an implantable electronic device could help reduce labor needs, disruption of cow routines, reproductive management costs, and enable ovulation synchronization protocol optimization through unrestrained hormone release. Despite the potential benefits of automated control of the estrous cycle with electronic hormone delivery and sensing devices, only a few have been developed and demonstrated. For example, the drug release and monitoring unit (DMU) consisted of a single 40 mL hormone reservoir with a drug delivery mechanism that included a piston actuated by a gas cell operated by a microcontroller (Cross et al., 2004). Temperature, activity, light, and pressure sensors were integrated for remote control and animal monitoring. Although limited data is currently available about this device, a few in vivo feasibility studies with a limited number of cows demonstrated controlled fluid delivery, external control through a wireless link, and basic sensing capabilities. Our group previously reported the development of the e-Synch device for automated control of the estrous cycle of cattle through intravaginal delivery of reproductive hormones (Masello et al., 2020). The first generation e-Synch was designed to accomplish automated hormone delivery only (Masello et al., 2020). Neither remote control nor communication for enabling device remote programming, control, and monitoring were possible. Moreover, the device had no sensing capabilities which could be used for improving functionality of the system through device and animal monitoring.
Therefore, our objective was to develop a second generation e-Synch system integrating hormone delivery and sensing with remote programming and communication through an IoT platform. Herein, this manuscript describes the second generation e-Synch system and demonstrated in vivo functionality of the hormone delivery device and associated IoT platform under farm conditions. Our secondary objective was to characterize behavioral and physiological responses after e-Synch insertion because there is limited data about effects of device insertion and retention on cow behavioral responses and integrity of the vaginal mucosa. Implementation and evaluation of in vivo automated hormone delivery was also evaluated but is presented in a companion manuscript (Ren et al., 2023).
A conceptual framework of the proposed e-Synch system is shown in Figure 1. The e-synch device with drug delivery and sensing capabilities is controlled by a smartphone or smart device application (app) through Bluetooth communication. The app allows the user to select pre-established synchronization of ovulation protocols or customize a protocol by setting the type, dose, and time of hormone release. Once programmed to schedule hormone release, activated, and inserted in the vagina, hormones are released according to the selected protocol or a customized sequence and hormone quantity selected by the user. Concomitantly, sensor data is broadcasted towards a gateway that uploads data to a cloud server. Data on the server is used by the application end for device monitoring and data visualization. The user controls and monitors the system through the app.
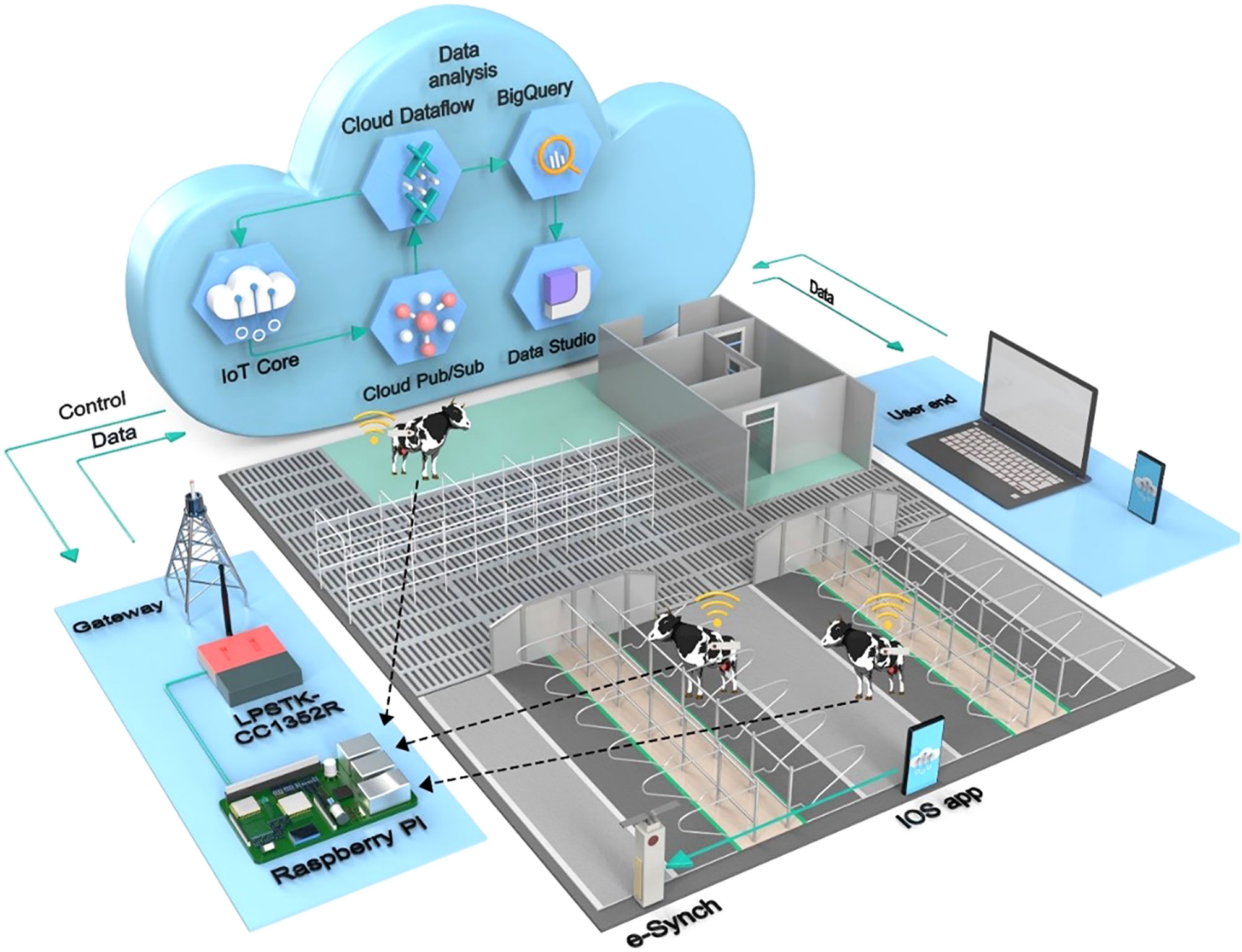
Figure 1 Conceptual framework of the proposed e-Synch system. The e-synch device with drug delivery and sensing capabilities is controlled and monitored by a smartphone or smart device IOS application (app) through Bluetooth communication. The app allows the user to select pre-established synchronization of ovulation protocols or customize a protocol. Once programmed to schedule hormone release, activated, and inserted in the cow vagina, hormones are released according to the selected protocol or customized sequence and hormone quantity selected by the user. Sensor information is sent through 915 MHz RF to the gateway connected to Raspberry PI 3 through a UART connection. The gateway uploads all information to the Google Cloud utilizing the IoT Core service through TCP/IP. Data published to the IoT core are pulled by the Pub/Sub service, which is routed to the BigQuery table by the Dataflow service. Data Studio utilizes the BigQuery table for visualization. Data on the server is used by the application end for device monitoring and data visualization.
The current e-Synch design consists of an outer case (2.8 x 3.4 x 11 cm) 3D-printed with polypropylene filament for reduced weight (122 g) and two double Nylon wings (each of 6 x 1 cm) attached to the top as a retention mechanism (Figure 2). A flexible silicone membrane (2 x 1.5 x 2.5 cm) is sealed at the bottom for pressure balancing during drug delivery. A power switch and a flexible wideband antenna (MOLEX, Lisle, IL, USA) are placed on the side. Internal components include a central control board and two silicone hormone reservoirs with ~5 mL capacity each connected to individual peristaltic pumps (Takasago Fluidic Systems, Westborough, MA, USA) through a Luer lock connection. Fluid release into the vaginal cavity is through 90 degree polyethylene elbows with 2 mm hose barb connected to each peristaltic pump and placed onto one of the laterals of the device (Figure 2). An 820 mAh Lipo battery regulated by a micro-Lipo charger (Adafruit, New York, NY, USA) is sealed inside the case to provide power to the system. A Qi wireless charging coil with a receiver module is attached to the external side of the device to serve as a power input for the micro-Lipo charger through a sealed hole.
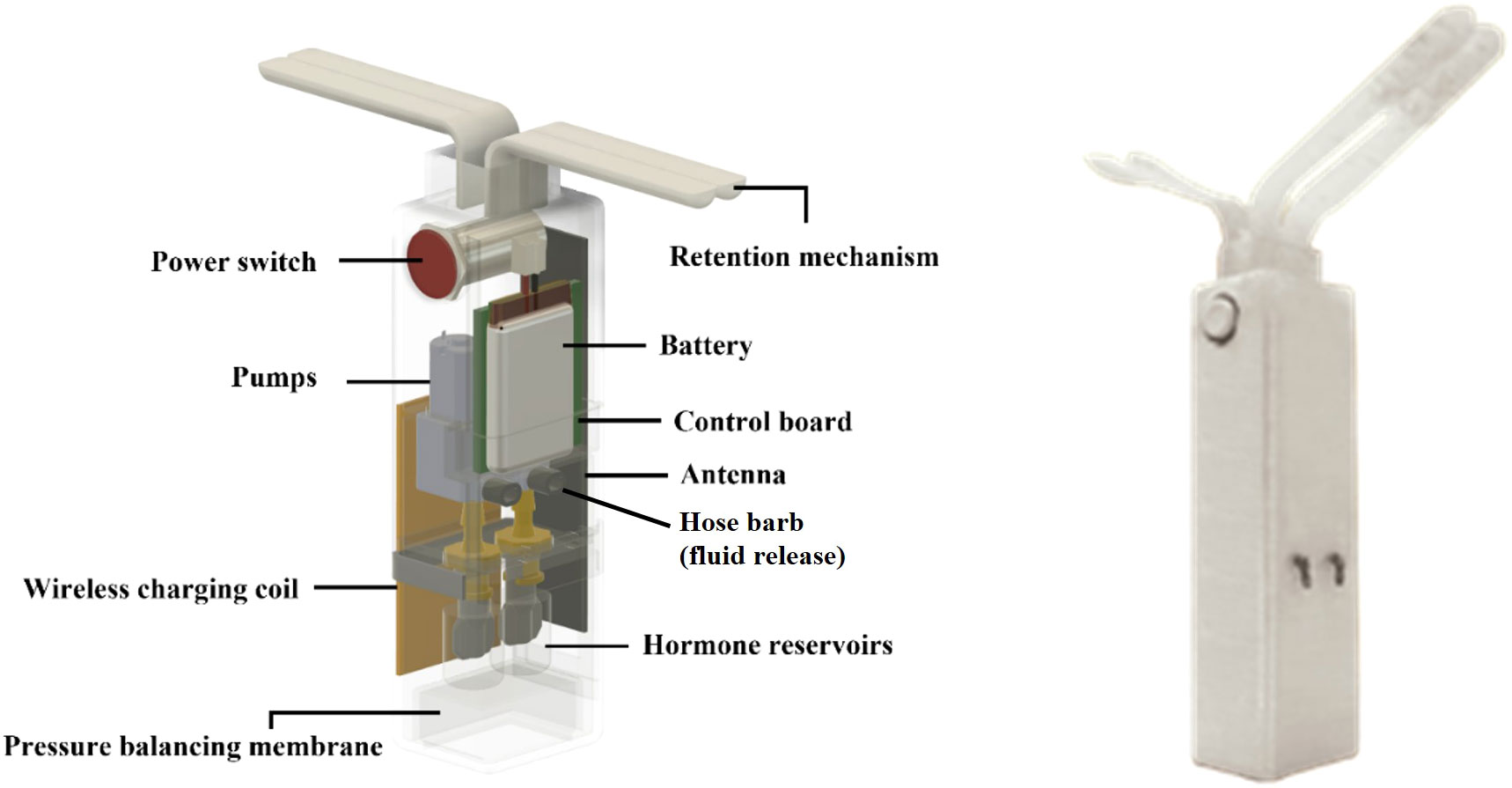
Figure 2 3D model (left) of the e-Synch device and its components and fully assembled e-Synch device (right). e-Synch consists of an outer case 3D-printed, with two double Nylon wings for retention, and a flexible silicone membrane at the bottom for pressure balancing during drug delivery. A power switch and flexible wideband antenna are placed on the side. Internal components include a central control board and two silicone hormone reservoirs with ~5 mL capacity connected to individual peristaltic pumps through a Luer lock connection. Polyethylene elbows with hose barb are connected to each peristaltic pump and placed onto one of the laterals of the device. A Lipo battery is sealed inside the case to provide power. A Qi wireless charging coil with a receiver module is attached to the external side of the device to serve as a power input for the micro-Lipo charger through a sealed hole.
A customized e-Synch applicator for device insertion was 3D-printed with polypropylene filament and coated with Dragon Skin FX-pro (Smooth-on Inc., Macungie, PA, USA). The device is inserted into the applicator with its retention wings folded.
A central control board with a CC1352R core enables low-power concurrent multiprotocol wireless communication and includes an autonomous ultra-low power sensor controller central processing unit (CPU). Through Bluetooth 5.2 Low Energy (BLE), the control board is programmed to receive commands that establish drug delivery protocols, including number of drug release events from a reservoir, release event duration, and the interval between release events. Controlled release is accomplished with peristaltic pumps for accurate flow control and leakage avoidance. The microcontroller controls the peristaltic pumps through N-type metal oxide semiconductor (NMOS) drivers controlled by the general-purpose input/output (GPIO). In previous work from our group, we demonstrated a constant rate dispensing profile using saline solution in vitro (Masello et al., 2020), which enables controlling the amount of fluid released through the amount of time that pumps are activated. The flexible silicone membrane sealed at the bottom of the device enables drug delivery from the flexible silicone reservoirs by adjusting the pressure change caused by the collapse of the hormone reservoirs as fluid is dispensed and by the temperature change exerted by body temperature. The board also broadcasts sensor data through 915 MHz RF and Bluetooth. Sensors included are an ADXL362 accelerometer with sensitivity of 2 mg in the 4g range and less than 2 µA power consumption, and a high-accuracy (± 0.1°C) and low power consumption (3.5 µA with 1 Hz conversion cycle) TMP117 temperature sensor. Both sensors are connected and monitored through a Sensor Controller Engine, which is a power-optimized CPU to perform sensing tasks autonomously. Acceleration and temperature data generated are logged and sent to the system CPU. Once received by the system CPU, the device and sensor information are sent in a 915 MHz RF packet and received by the external gateway. BLE5 and Sub-1G communication are managed concurrently by a Dynamic Multi-protocol manager.
To protect electronic components from corrosion and avoid contamination of the vaginal cavity with chemical residuals (e.g., flux, plastics) or soluble metals (e.g., solder, battery), the unit is completely sealed and the Lipo battery is encased in epoxy potting. To avoid sealing and resealing of the PCB circuit between multiple device uses, the battery is charged through wireless power transmission using an external wireless coil. The receiver module, antenna patch, and joint parts are coated with XTC-3D™. The whole unit is encased in a skin-safe silicone rubber shell made with Dragon Skin FX-pro.
Sensor information is sent every second through 915 MHz RF (WB-DSSS 30 kbps, 2-GFSK, 195 kHz deviation, 8x spreading) to the gateway, which is made with LPSTK-CC1352R (Texas Instruments, Dallas, TX, USA) connected to Raspberry PI 3 through an UART connection (Figure 1). The gateway then uploads all the received information to the Google Cloud utilizing the IoT Core service through TCP/IP. Data published to the IoT core are pulled by the Pub/Sub service, which is routed to the BigQuery table by the Dataflow service. Thereafter, Data Studio utilizes the BigQuery table for visualization.
Data collected by the current system includes (1) device and associated animal identification number, programmed drug delivery protocol, and start time; and (2) real-time sensor information including x, y, z-axis acceleration, and intravaginal temperature in degrees Celsius.
Pre-established and customizable drug delivery protocols can be selected using a customizable smart IOS device app (Figure 3). Pre-established protocols include the sequence and timing of hormone release events for each hormone type [e.g., Prostaglandin F2α (PGF), GnRH, Progesterone] as well as the amount of hormone to dispense at each event. These pre-established protocols are commonly used ovulation synchronization protocols by commercial cattle operations or protocols published in the literature. Alternatively, users can use the control App to define a customized drug delivery protocol including the type, sequence, timing, and amount of drug to release to accomplish a biological goal such as synchronization of ovulation. After a drug delivery protocol is selected or established, the protocol is sent through BLE to the e-Synch device. The device is then inserted in the cow to execute drug delivery according to the schedule and perform sensing tasks.
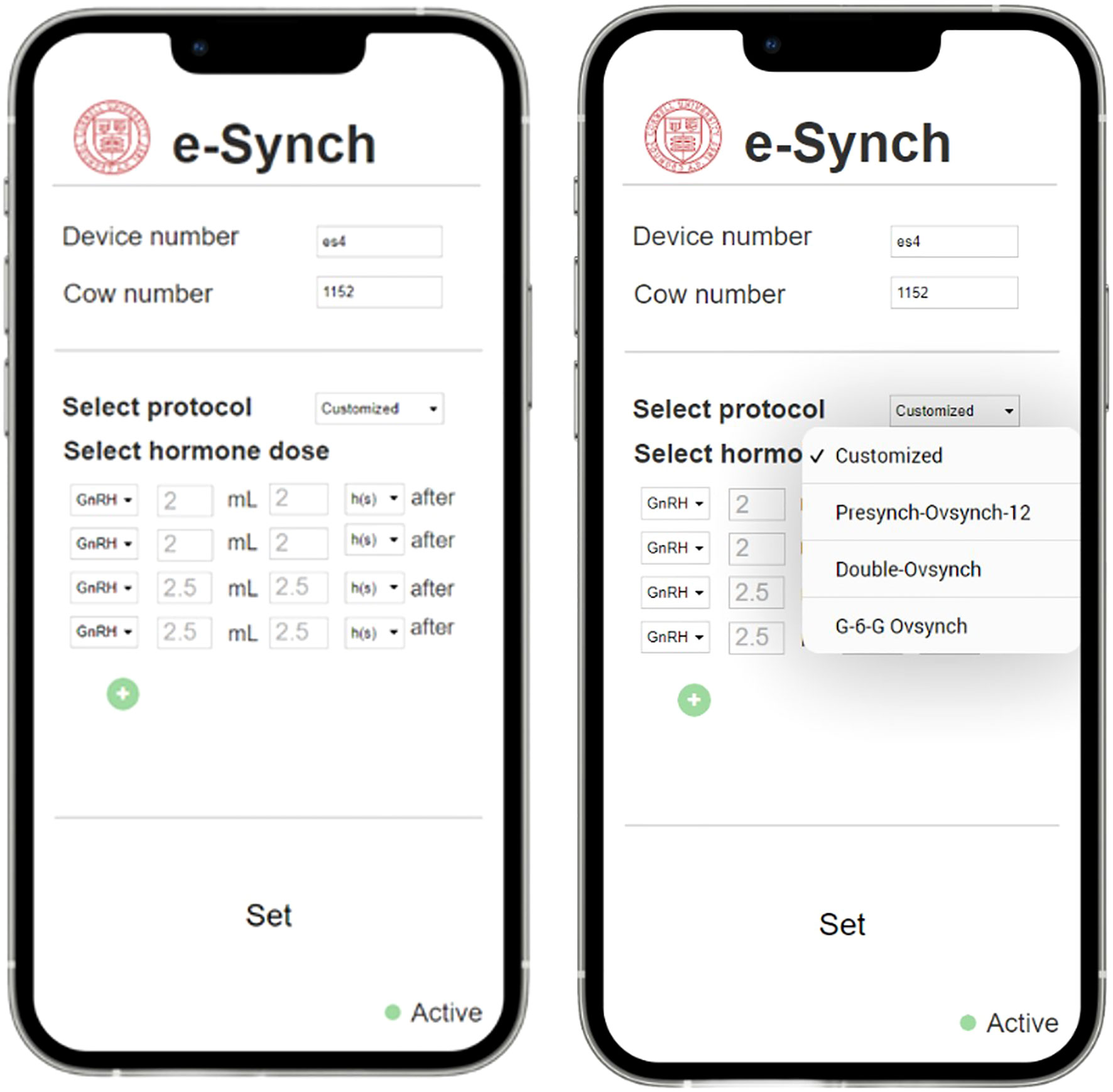
Figure 3 IOS app developed for remote control and monitoring of the e-Synch device. Pre-established and customizable drug delivery protocols can be selected. Users can use the control App to define a customized drug delivery protocol including the type, solution volume, and sequence of drug to release. The app send the protocol through Bluetooth Low Energy to the e-Synch device.
To demonstrate the functionality of the e-Synch IoT platform and the ability of the e-Synch device to release hormones and collect sensor data in vivo, two experiments with lactating Holstein cows were conducted. These experiments were also designed to evaluate cow behavioral and vaginal tissue responses to short-term exposure (i.e., < 1 d) to functional e-Synch devices. A detailed description of experimental procedures, hormone release, and effects of hormone release are presented in a companion manuscript (Ren et al., 2023).
All procedures performed with cows were approved by the Animal Care and Use Committee of Cornell University (Ithaca, NY, USA) under protocols 2016-0093 and 2021-0010.
Briefly, experiments 1 (Exp 1) and 2 (Exp 2) were conducted using lactating Holstein cows (n = 20 in Exp 1 and n = 37 in Exp 2) from the Dairy Unit of the Cornell University Ruminant Center (Harford, NY, USA). Experiment 1 was conducted from June to July of 2020 and Exp 2 from January to March of 2022. In both experiments, the estrous cycle of cows was synchronized using the sequence of hormonal treatments of a Double-Ovsynch protocol (Souza et al., 2008; Wiltbank et al., 2015) up to the time of induction of luteolysis of the Breeding-Ovsynch portion of the protocol [Pre-Ovsynch: Gonadotropin releasing hormone (GnRH), 7 d later PGF, 3 d later GnRH, 7 d later Breeding-Ovsynch: GnRH, 7 d later PGF, 1 d later PGF]. Cows received all hormonal treatments of the Double-Ovsynch protocol through intramuscular (i.m.) injections. All GnRH treatments were 100 µg of Gonadorelin diacetate tetrahydrate (Cystorelin, Merial Ltd., Duluth, GA, USA) and all PGF treatments were 25 mg of Dinoprost tromethamine (Lutalyse HighCon, Zoetis, Parsippany, NJ, USA). The estrous cycle was synchronized to replicate the hormonal environment observed at the time of induction of ovulation with GnRH before TAI, which is characterized by low circulating concentrations of progesterone and elevated circulating concentrations of estradiol (Giordano et al., 2012; Motta et al., 2020). At 48 h after induction of luteolysis with the first PGF treatment of the Breeding-Ovsynch portion of the protocol, cows were randomized to the experimental treatments.
In Exp 1, cows were randomly assigned to a positive control group (GnRH-IM; n = 6), in which cows received 100 µg of GnRH through i.m. injection, a negative control group (Placebo-eS; n = 5), in which cows received an empty e-Synch device as a placebo, or an e-Synch GnRH group (GnRH-eS; n = 6) in which cows received an e-Synch device loaded with 2 mL of solution with 100 µg of GnRH and 10% Citric Acid (CA). Two replicates, with 9 cows in replicate 1 and 8 cows in replicate 2, were required to complete this experiment, due to the number of devices available. A total of 4 functional and 4 non-functional devices (Dev) were assembled and used to complete the experiment (number of times used for functional and non-functional devices: Dev1 = 2, Dev2 = 1, Dev3 = 1, Dev4 = 1).
In Exp 2, cows were randomly assigned to a positive control group (GnRH-IM; n = 7), in which cows received 100 µg of GnRH through i.m. injection, or one of four treatments in which cows received GnRH intravaginally via an e-Synch device. Treatments administered with e-Synch were 100 µg of GnRH diluted in 2 mL of solution (LoD-LoV; n = 6), 100 µg of GnRH in 10 mL of solution (LoD-HiV; n = 7), 1,000 µg of GnRH in 2 mL of solution (HiD-LoV; n = 7), and 1,000 µg of GnRH in 10 mL of solution (HiD-HiV; n = 7). All solutions for intravaginal instillation contained 10% CA. Six replicates, with 5 to 6 cows per replicate, were required to complete this experiment, due to the number of devices available. Six fully functional devices were assembled and used to complete this experiment. All devices were used more than once (number of times used: Dev1 = 4, Dev2 = 4, Dev3 = 5, Dev4 = 6, Dev5 = 5, Dev6 = 3).
One day before application of treatments in both experiments, cows were moved to a tie-stall barn to facilitate device insertion, cow monitoring, and sampling. Immediately before device insertion, the vulva and perineal area of cows was cleaned and disinfected using 2% Chlorhexidine solution (Nolvasan, Fort Dodge Animal Health, Fort Dodge, IA, USA) diluted in water. The perineal area was then dried off with paper towels. Individual devices were turned on and functionality evaluated. Devices were mounted in the front portion of the custom-built applicator, which was rubbed with a thin film of sterile lubricant (Priority Care, First Priority Inc, Elgin, IL, USA). The applicator containing the device was inserted into the vagina until the vaginal fornix. At this point, the applicator was pulled backwards 5 to 10 cm to enable release of the e-Synch device through pressure on the applicator rod. Once the device was released, the applicator was removed. In both experiments, devices remained in the cow for up to 8 h after insertion.
Each device was programmed to begin releasing the GnRH solution within five minutes of insertion through a command set with an IOS app using Bluetooth. At the same time, the gateway was initiated to initiate sensor data logging. During insertion, the gateway was placed within the barn approximately 10 m away from cows to enable communication with devices. To evaluate signal attenuation by body tissue, impedance of the wideband antenna was measured (MOLEX, Lisle, IL, USA) before and after insertion into the vagina of the cow using a portable vector network analyzer (NanoVNA). Impedance data before and after insertion was analyzed using a t-test.
Two days prior to application of treatments, immediately after device removal, and 7 or 2 d after device removal in Exp 1 and 2, respectively, vaginoscopy was performed utilizing a speculum and a source of light to evaluate vaginal integrity as described in Walsh et al. (2008). A scoring system ranging from 0 to 3 was used whereby 0 = no visible lesion, 1 = only superficial lesions, and 2 = visible erosion of the vaginal mucosa. In addition, vaginoscopy was used to evaluate the presence and appearance of vaginal mucus using a scale from 0 to 3 as described in Sheldon (2004), whereby 0 = clear, 1 = clear mucus with <50% of white flecks, 2 = >50% of white flecks and 3 = presence of cream or bloody and fetid pus. In Exp 2, within approximately 4 h of device insertion, a video of the vaginal cavity was recorded using a vaginal endoscope (Alpha Vision, IMV Technologies, France) to visualize the position of e-Synch devices.
To evaluate behavioral changes associated with device insertion, cow behavior was evaluated and recorded in both experiments. General behavior and noticeable signs of pain on visual inspection were evaluated at 0, 2, 4, 6 and 8 h after device insertion. Recorded behaviors were general attitude, position of ears, facial expression, standing posture, limb posture, lying position, miscellaneous abnormal behavior, and clinical signs as described in de Boyer des Roches et al. (2017). In Exp 2, a tail position score using a three point scale (0 = normal tail base position, 1 = tail base inclined upward but not in straight line, 2 = tail base in a straight line) was recorded at 0, 2, 2.5, 3, 4, 6 and 8 h after device insertion. The position of the tail was evaluated when cows were not defecating or urinating and at least 30 seconds after an episode of defecation or urination. Data for tail position scores were analyzed with a linear mixed model fitting a normal distribution using the MIXED procedure of SAS (version 9.4, SAS Institute Inc., Cary, NC, USA). The model included time after e-Synch insertion as fixed effect.
Blood samples for determination of circulating concentrations of hormones were collected by puncture of caudal blood vessels using heparinized evacuated tubes (Vacutainer; BD, Franklin Lakes, NJ) as described in detail in the companion manuscript (Ren et al., 2023). In both experiments blood was collected at the same timepoints. Briefly, samples were collected on d -9, -2, 0, and 7 relative to treatment day for estimation of circulating concentrations of progesterone (P4) and on the day of treatment (d 0) at 0, 1, 2, 2.5, 3, 4, 6, and 8 h after application of treatment to estimate circulating concentrations of LH.
All devices were successfully initiated on-site in Exp 1 and using the IOS app through Bluetooth in Exp 2. Release time was set (1,000 or 2,000 seconds depending on volume to release) to ensure that the content of preloaded reservoirs was completely emptied. The on-status of all devices was confirmed before insertion, through an LED indicator, and after insertion, by gateway data logging.
During the insertion period for Exp 2, devices connected successfully to the IoT platform at least once in 89% (24/27) of device use instances. The average (± std dev) signal strength before insertion was -36.9 ± 6.8 dBm, whereas after insertion, the signal strength was -87.4 ± 5.7 dBm. This signal strength reduction indicated a significant (P < 0.0001) attenuation of the signal by body tissue equivalent to 50.5 dB (Figure 4A). During insertion, signal strength fluctuated substantially while the devices were exposed to the changes of the intravaginal environment and cow motion (Figure 4B). This resulted in an unstable communication link and signal discontinuity. A shift of impedance in the operation from 900 MHz to 930 MHz was observed (Supplementary Figure 1A) with an average 60 Ω shift from before to after insertion (Supplementary Figure 1B) coupled with a substantial return loss (Supplementary Figure 1C).
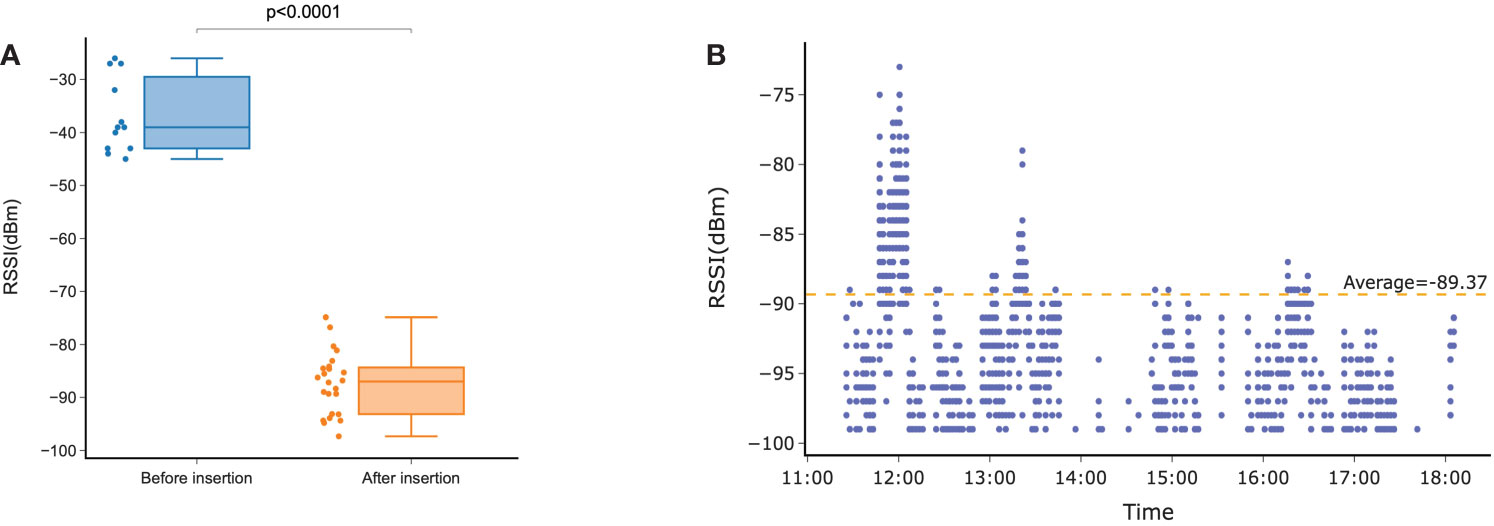
Figure 4 (A) Signal strength received at the gateway before and after insertion of the device in the vagina. (B) Received signal strength indication (RSSI) during the insertion period.
The proportion of instances in which a Dev that communicated with the IoT platform generated at least 10 datapoints every 15 min ranged from 0 to 100% [Dev1 = 0% (0/3), Dev2 = 50% (2/4), Dev3 = 75% (3/4), Dev4 = 83% (5/6), Dev5 = 80% (4/5), Dev6 = 100% (2/2)]. Out of the devices that connected and generated at least 10 datapoints every 15 min, in 67% (16/24) of instances, the device generated data every 15 min for at least 10 times (i.e., at least ~150 min after insertion). For these instances, the average (± std dev) number of data points generated per device was 17.1 ± 7.1. Conversely, for instances in which the device generated fewer than 10 data points every 15 min, the average (± std dev) number of data points generated per device was 7.1 ± 1.9. Although the pattern of missing data points was random, a trend for more missing data points during the second half of the insertion period was noticeable on visual inspection of the data. The pattern of vaginal temperature from 0.5 to 7 h after device insertion for cows (n = 16) with at least 10 datapoints is presented in Figure 5A. The mean and standard deviation for temperature was 39.1 ± 0.2 °C and fluctuated between 38.9 °C observed within 30 min of device insertion to 39.3 °C observed from 345 to 375 min after insertion. An example of temperature and accelerometer data collected from an individual cow from the time before insertion until 420 min after insertion is presented in Figure 5B. As expected, temperature rose from ambient temperature levels of 23.6 °C before insertion to body temperature levels at the 30 min timepoint. Thereafter, mean temperature was 39.1 °C and ranged from 38.6 to 39.5 °C. Accelerations fluctuated in all three dimensions within ± 8.565 m/s2.
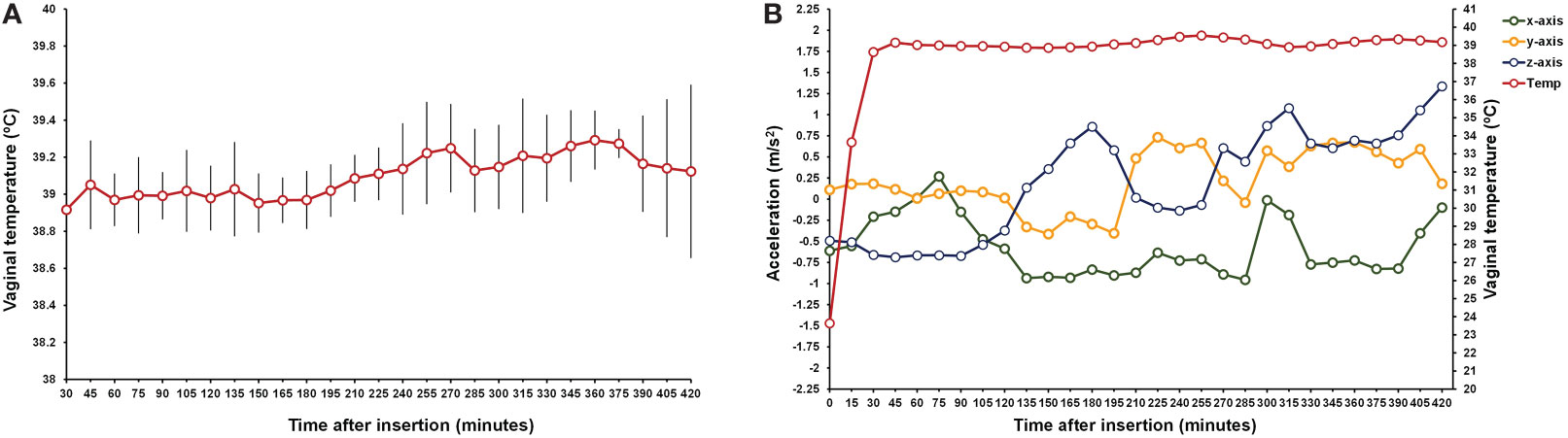
Figure 5 (A) Mean and SD for vaginal temperature recorded by the e-Synch device from 30 to 420 min after insertion in the vagina for cows (n = 16) in experiment 2 with at least 10 datapoints collected every 15 min. The mean temperature and SD was 39.1 ± 0.2 °C and fluctuated between 38.9 °C observed within 30 min of device insertion to 39.3 °C observed from 345 to 375 min after insertion. (B) Temperature and raw accelerometer data collected from an individual cow from before until 420 min after e-Synch insertion. Vaginal temperature rose from 23.6 °C before insertion to 38.6 at the 30 min timepoint. Thereafter, temperature ranged from 38.6 to 39.5 °C. Accelerations in the x, y, and z axis fluctuated in all three dimensions within ± 8.565 m/s2.
After removal from the vagina, hormone release was confirmed by evaluating the presence of solution residue in fluid reservoirs. One device used in Exp 2 (Dev3) did not release any of the hormone solution. Conversely, all hormone reservoirs in all other devices in both experiments were completely empty suggesting that all fluid was released during insertion. Hormone solution release was also confirmed based on the physiological response observed in both experiments as described in the companion manuscript (Ren et al., 2023). This was more evident for cows that received a GnRH dose and volume of solution that caused an increase in circulating concentrations of LH above baseline.
All devices in both experiments remained in situ for the duration of the observation period. An example of the position of e-Synch in the vaginal cavity is provided under Supplementary Videos 1. One device was removed from 1 cow approximately 5 h after insertion because the device became visible when the cow was in a laying position.
In Exp 1, all cows had vaginal integrity and vaginal mucus scores of 0 at all timepoints, except one cow on the GnRH-eS treatment which had an e-Synch device inserted and had a vaginal mucus score of 1 on the day of application of treatment. In Exp 2, all cows had vaginal integrity scores of 0 at 2 d before and after application of treatments. Three cows which had an e-Synch device inserted had a vaginal integrity score of 1 on the day of treatment. The superficial lesion consisted of mild redness of the vaginal mucosal floor covering an area of less than 2 by 2 cm. These lesions were attributed to friction caused by the e-Synch applicator during device insertion. Similarly, all cows had vaginal mucus score of 0 at 2 d before and after application of treatments. Only two cows had a vaginal mucus score of 1 on the day of treatment. One of these cows was also a cow with a vaginal integrity score of 1.
In both experiments, there were no noticeable abnormal behaviors at any timepoint. None of the cows presented noticeable changes for general attitude, ear position, facial expression, standing posture, limb posture, lying position, miscellaneous abnormal behaviors, or developed clinical signs for the duration of the observation period. All behavioral scores were 0 at all timepoints.
In Exp 2, tail position was unaltered throughout the entire observation period in all cows that received the i.m. injection of GnRH. Only one cow in this group had a tail position score of 1 at 6 h after treatment. Conversely, all cows except for one that had an e-Synch device inserted, had a tail position score of 1 for at least one timepoint. Because for the control group the tail position score was 0 in all cows at all timepoints (except for 1 cow at 1 timepoint), the association between tail position score and time was evaluated only for cows that received an e-Synch device. The dynamic of tail position score (LSM ± SEM) followed a U shape with the highest score of ~0.8 ± 0.2 arbitrary units (AU) immediately after insertion at time 0, the lowest score of ~0.1 ± 0.7 AU at 6 h after insertion, and finally, an increase to ~0.6 ± 0.2 AU at 8 h after insertion (Figure 6).
Automating control of the bovine estrous cycle could facilitate and increase adoption of reproductive technologies such as TAI and TET in dairy and beef cattle operations. To this end, the e-Synch system including an electronically-controlled fluid delivery and sensing device integrated with an IoT platform was developed. e-Synch was designed to enable automated control of the bovine estrous cycle through delivery of arbitrary amounts of reproductive hormones at intervals that synchronize estrus and ovulation. An integrated phone or smart device app and IoT platform maximizes ease of use and minimizes operation needs through remote programming, control, and monitoring. Our in vivo experiments with dairy cows demonstrated that the current e-Synch system could be successfully programmed to deliver hormone solutions in a timely manner. Programmed hormone release was evidenced by the absence of fluid residue after device disassemble and the temporal dynamic of LH concentrations for cows that received GnRH in Exp 2 (Ren et al., 2023). Cows that received the high dose of GnRH had an LH surge of at least similar magnitude and timing than that of cows that received GnRH by i.m. injection. Unlike our previous experiment (Masello et al., 2020) in which the e-Synch device was programmed by a wired connection, it was now demonstrated for the first time, that e-Synch can be programmed using the associated IOS app. A caveat of the current in vivo experiments was that all devices were loaded with the exact amount of fluid to be released and all the fluid in the hormone reservoirs was released at once. Thus, it was not possible to test the ability of the device to release different amounts of fluid at more than one timepoint. This capability of the e-Synch system will be tested in future experiments because most synchronization of ovulation protocols require at least two treatments with a single hormone.
Wearable sensors attached to body parts or inserted in body cavities are used widely in the cattle industry to monitor behavioral and physiological parameters (Stangaferro et al., 2016; Berckmans, 2017; Halachmi et al., 2019). Using one or more of these sensor parameters, the health, reproductive status, feeding, productivity, welfare, and location of animals can be monitored automatically and in real-time. Some systems also offer actionable alerts for identifying cows in estrus, cows with health disorders, abnormal behavior, and low productivity (Stangaferro et al., 2016; Schilkowsky et al., 2021). In Exp 2, it was demonstrated through proof-of-concept the functionality of the accelerometer and temperature sensor of e-Synch and the ability to transfer and log sensor data in the IoT platform. Although accelerometer data was not transformed into physical activity or cow position data, changes in acceleration for the x, y, and z axis were consistent with the minimal activity and changes in cow position during the short duration of the experiment. As cows were housed in a tie-stall barn with limited mobility, changes in acceleration were expected to be minimal. On the other hand, the change in temperature observed for individual cows after device insertion (example in Figure 5) and the mean temperature recorded when the device was inserted provided proof-of-concept evidence that the temperature sensor was functional. Indeed, the mean and variation for vaginal temperature recorded in our experiment agrees with the pattern of vaginal temperature reported for lactating dairy cows under thermoneutral conditions (Kendall et al., 2006; Vickers et al., 2010). A limitation of our current sensor data was lack of validation as no reference method was used. We only focused on testing and providing proof-of-concept that the sensors functioned while inserted in the cow and generated data for logging. In future experiments, measurements will be validated using established reference tests.
Sensors were added to the e-Synch system for allowing remote animal and device monitoring and management. Sensing behavioral (i.e., activity) and physiological (i.e., temperature) parameters indicative of device location and cow position could enhance system functionality and enable tailoring treatments to match individual animal physiological status and needs (Cross et al., 2004). For example, used alone or in combination, activity and vaginal temperature data could verify the presence of e-Synch in the vagina and detect when a device falls to the ground. Prolonged periods of inactivity combined with a change in temperature larger than the expected biological variation in body temperature was proposed as a method for detection of expulsion of intravaginal devices during calving in cattle (Pearson et al., 2020). Cow position data from the accelerometer could be used to prevent backflow from the vagina by releasing fluid only when cows are standing. Monitoring physical activity could also be used alone or in combination with vaginal temperature for detection of estrus because cows present a substantial increase in physical activity, mounting behavior, and a rise in vaginal temperature (Zartman et al., 1983; At-Taras and Spahr, 2001; Schilkowsky et al., 2021) when in estrus. Information about the timing, duration, and intensity of estrus could be used to tailor synchronization of ovulation protocols and other hormonal interventions for individual cows (Cross et al., 2004). For example, cows that express estrus during a synchronization of ovulation protocol could be detected with e-Synch and immediately inseminated before the protocol is completed. Alternatively, cows detected in estrus could receive hormonal treatments known to increase fertility. Recently, it has been shown that cows with different estrous intensity, as determined by accelerometer-based automated systems for detection of estrus, have different fertility potential (Tippenhauer et al., 2021; Madureira et al., 2022), and cows with low estrous intensity treated with GnRH at the time of insemination had improved fertility (Burnett et al., 2022).
Communication for programming, control, and monitoring is necessary for optimizing functionality and maximizing the value of devices for automating animal management and monitoring tasks (Cross et al., 2004; Berckmans, 2017). We demonstrated the ability of the e-Synch device to communicate with the controlling app and IoT platform. Except for the one device in one instance in Exp 2 that failed to release hormone solution, all other devices were successfully programmed to release fluid after vaginal insertion. It is unclear what caused the device failure in Exp 2. Receiving a signal at least once from all devices inserted in all instances was also evidence of successful communication and functioning of device and IoT platform components required for communication. Nevertheless, there was large device-to-device variation, communication was unstable at times, and the signal from several devices was interrupted in many instances. Communication over long distances remains a challenge for implantable devices. Body tissue tends to dissipate and absorb the energy of the signal carrying the data and the natural conductivity of the body changes the electrical performance of an antenna (Wotherspoon and Higgins, 2013). The impedance shift caused by the body tissue environment also leads to a return loss and poor communication signal. To enhance communication and covering distance, a more sensitive antenna at the receiver side is needed in conjunction with different antenna designs for the e-Synch device to match the vaginal environment and increase power output for RF transmission.
Wearable devices for animals must either avoid or minimize discomfort and alterations of body parts or tissues. In the case of intravaginal devices, it is critical to avoid causing vaginal discomfort, disrupting the integrity of the vaginal mucosa, and causing infection or excessive production of vaginal mucus. In the in vivo experiments, we did not observe significant cow behavior and tail position changes during insertion of the e-Synch device. Cow behavior was unaltered by the presence of the device in the vaginal cavity, as determined by a detailed cow behavior scoring system including observations of eight possible signs of discomfort and pain. Although the e-Synch device has a different shape and dimensions than other electronic IVG devices for cattle, our observations agree with the lack of changes in cow behavior or pain observed after insertion of IVG devices (Crociati et al., 2021; Stephen and Norman, 2021). The tail raising scores dynamic for cows that received an i.m. injection of GnRH versus those which received an e-Synch device, indicated that device insertion and removal was accompanied by a minor cow behavioral change. Average tail raising score values did not reach 1 AU at any timepoint, and during most of the insertion period remained below 0.5 AU. Of note, the dynamic of tail raising scores for cows that received a device suggested that the effect might not have been necessarily associated with discomfort and pain due to the presence of the device, but rather because of the procedures used for inserting and removing the device. These procedures included washing of the perineal area, manual tail rising, insertion of the applicator, and rectal manipulation of the device at the time of removal. Similarly, a short period of tail raising has been reported for heifers that received an electronic IVG device for calving monitoring (Crociati et al., 2021).
Vaginal integrity and mucus scores also remained unaltered, which demonstrated that insertion of the current version of e-Synch for short periods of time (~8 h) did not cause macroscopic damage to the vaginal mucosa or changes to the vaginal mucus indicative of irritation or infection. Although difficult to quantify, in the current and previous experiments (Masello et al., 2020) some cows presented increased mucus production immediately after e-Synch insertion; however, the mucus produced was clear and not different from that observed in cows during estrus. Our current observations contrast with our previous results for an experiment in which approximately half the cows that received a similar version of the e-Synch device presented vaginal mucus with small flecks of pus (Masello et al., 2020). Because in our previous experiment devices remained in the vaginal cavity for two full days, the most likely reason for the discrepancy between studies was the duration of device insertion. Collectively, data for cow behavior, tail raising, and vaginal mucosa integrity and mucus production suggested that our current e-Synch device does not cause noticeable cow discomfort and pain and does not cause major alterations to the vaginal mucosa integrity and function.
Our current in vivo experiments with the e-Synch system had limitations including short duration, testing the release of a fixed amount of a single hormone all at once, and no validation and full integration of sensor data. In addition, the sample size was limited for evaluation of some outcomes related to cow behavior and vaginal integrity. This is not unusual in the process of developing biomedical devices including several components which have the potential to affect several biological and behavioral aspects of the target individual and when proof-of-concept is necessary before device redesign and further refinement. Thus, future research must be conducted to demonstrate the ability of the e-Synch system to fully automate control of the estrous cycle and generate actionable sensor information for animal and device monitoring and management. Specifically, efforts must focus on evaluating the effect of longer-term e-Synch insertion (e.g., ≥10 d) on cow behavior and vaginal integrity and demonstrate the ability to deliver different types and amounts of hormones to resemble the sequence of treatments of synchronization of estrus or ovulation protocols. Moreover, communication and sensing must be improved to ensure seamless control and monitoring of e-Synch devices while inserted and enable use of sensor data for monitoring and tailoring treatments to individual animals.
A system for automated control and monitoring of the estrous cycle in cattle was developed and demonstrated. The electronically-controlled intravaginal delivery and sensing device was fully integrated with a phone or smart device app and IoT platform. In vivo studies with dairy cows demonstrated that the e-Synch system can be programed through the accompanying app to automatically dispense hormone solution in the vaginal cavity. Communication capabilities enabled transfer of accelerometer and temperature data to the IoT platform, which could be used for monitoring device functionality and optimizing hormone delivery protocols by tracking animal behavioral and physiological parameters.
This article is the first part of a set of companion articles. The companion article is Ren Y., Duhatschek D., Bartolomeu C.C., Laplacette A.L., Perez M.M., Rial C., Erickson D., and Giordano J.O. (2023). An automated system for cattle reproductive management under the IoT framework. Part II: induction of Luteinizing Hormone release after Gonadotropin Releasing Hormone delivery with e-Synch. Front. Anim. Sci. 4:1093857. doi: 10.3389/fanim.2023.1093857.
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author/s.
The animal study was reviewed and approved by the Animal Care and Use Committee of Cornell University (Ithaca, NY, USA) under protocols 2016-0093 and 2021-0010.
YR, DE, and JG designed the e-Synch system. YR, DD, DE, and JG contributed to the design of the experiments conducted. YR, DD, CB, and JG carried out the in vivo experiments. YR and DD wrote a first draft of the manuscript and DE and JG revised and edited the manuscript. All authors contributed to the article and approved the submitted version.
This project was supported by Agriculture and Food Research Initiative Competitive Grant no. 2021-67015-34503 from the USDA National Institute of Food and Agriculture (Washington, DC). Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the National Institute of Food and Agriculture (NIFA) or the USDA. Partial support was also provided by project numbers 1014955 and 1007421 from the USDA National Institute of Food and Agriculture and the Research Innovation Fund of the Cornell Initiative for Digital Agriculture (Cornell University, Ithaca, NY, USA).
The authors thank Sheridan Tompkins from the Department of Animal Science at Cornell University (Ithaca, NY, USA) for her help with making the GnRH solutions. Our gratitude is also extended to personnel from the Dairy Unit of the Cornell University Ruminant Center (Harford, NY, USA) for helping with cow management.
The authors have filed a patent application US 63/016,235 for the system(s), device(s) and 514 method(s) published in this paper.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fanim.2023.1093851/full#supplementary-material
Supplementary Figure 1 | Smith Chart with impedance shift of wideband antenna before and after insertion: (A) Plotted in smith chart; (B) Plotted in impedance magnitude (Ω); (C) Plotted in S11 return loss.
SUPPLEMENTARY VIDEO 1 | Smith Example of the position of e-Synch in the vaginal cavity.
At-Taras E., Spahr S.J.J.o. (2001). Detection and characterization of estrus in dairy cattle with an electronic heatmount detector and an electronic activity tag. J. Dairy Sci. 84, 792–798. doi: 10.3168/jds.S0022-0302(01)74535-3
Berckmans D. (2017). General introduction to precision livestock farming. Anim. Front. 7, 6–11. doi: 10.2527/af.2017.0102%JAnimalFrontiers
Burnett T. A., Madureira A. M. L., Bauer J. W., Cerri R. L. A. (2022). Impact of gonadotropin-releasing hormone administration at the time of artificial insemination on conception risk and its association with estrous expression. J. Dairy Sci. 105, 1743–1753. doi: 10.3168/jds.2021-20156
Crociati M., Sylla L., Stradaioli G., Monaci M., Zecconi A. (2021). Assessment of sensitivity and profitability of an intravaginal sensor for remote calving prediction in dairy cattle Vol. 21 (Basel, Switzerland: Sensors). doi: 10.3390/s21248348
Cross P. S., Künnemeyer R., Bunt C. R., Carnegie D. A., Rathbone M. J. (2004). Control, communication and monitoring of intravaginal drug delivery in dairy cows. Int. J. pharmaceutics 282, 35–44. doi: 10.1016/j.ijpharm.2004.05.023
de Boyer des Roches A., Faure M., Lussert A., Herry V., Rainard P., Durand D., et al. (2017). Behavioral and patho-physiological response as possible signs of pain in dairy cows during escherichia coli mastitis: A pilot study. J. Dairy Sci. 100, 8385–8397. doi: 10.3168/jds.2017-12796
De Rensis F., Peters A. R. (1999). The control of follicular dynamics by PGF2α, gnrh, hCG and oestrus synchronization in cattle. Reproduction in Domestic Animals 34, 49–59. doi: 10.1111/j.1439-0531.1999.tb01383.x
Fajt V. R., Wagner S. A., Pederson L. L., Norby B. (2011). The effect of intramuscular injection of dinoprost or gonadotropin-releasing hormone in dairy cows on beef quality. J. Anim. Sci. 89, 1939–1943. doi: 10.2527/jas.2010-2923
Giordano J. O., Fricke P. M., Guenther J. N., Lopes G. Jr., Herlihy M. M., Nascimento A. B., et al. (2012). Effect of progesterone on magnitude of the luteinizing hormone surge induced by two different doses of gonadotropin-releasing hormone in lactating dairy cows. J. Dairy Sci. 95, 3781–3793. doi: 10.3168/jds.2011-5155
Halachmi I., Guarino M., Bewley J., Pastell M. J. A. R. A. B. (2019). Smart animal agriculture: Application of real-time sensors to improve animal well-being and production. Annu Rev Anim Biosci. 7, 403–425. doi: 10.1146/annurev-animal-020518-114851
Kendall P. E., Nielsen P. P., Webster J. R., Verkerk G. A., Littlejohn R. P., Matthews L. R. (2006). The effects of providing shade to lactating dairy cows in a temperate climate. Livestock Sci. 103, 148–157. doi: 10.1016/j.livsci.2006.02.004
Madureira A. M. L., Burnett T. A., Marques J. C. S., Moore A. L., Borchardt S., Heuwieser W., et al. (2022). Occurrence and greater intensity of estrus in recipient lactating dairy cows improve pregnancy per embryo transfer. J. Dairy Sci. 105, 877–888. doi: 10.3168/jds.2021-20437
Masello M., Ren Y., Erickson D., Giordano J. O. (2020). An automated controlled-release device for intravaginal hormone delivery. JDS Commun. 1, 15–20. doi: 10.3168/jdsc.2020-18816
Motta J. C. L., Madureira G., Silva L. O., Alves R., Silvestri M., Drum J. N., et al. (2020). Interactions of circulating estradiol and progesterone on changes in endometrial area and pituitary responsiveness to GnRH†. Biol. Reprod. 103, 643–653. doi: 10.1093/biolre/ioaa065
Pearson C., Lush L., González L. A. J. R. S. (2020). Intravaginal devices and gnss collars with satellite communication to detect calving events in extensive beef production in northern australia. Remote Sensing 12, 3963. doi: 10.3390/rs12233963
Pfeiffer M. M., Mafi G. G., Ramanathan R., Neilson T. M., VanOverbeke D. L. (2018). Frequencies and severity of injection-site lesions in muscles from rounds of cow carcasses. Trans. Anim. Sci. 3, 130–134. doi: 10.1093/tas/txy094%JTranslationalAnimalScience
Pursley J. R., Kosorok M. R., Wiltbank M. C. (1997). Reproductive management of lactating dairy cows using synchronization of ovulation. J. Dairy Sci. 80, 301–306. doi: 10.3168/jds.S0022-0302(97)75938-1
Pursley J. R., Mee M. O., Wiltbank M. C. (1995). Synchronization of ovulation in dairy cows using PGF2alpha and GnRH. Theriogenology 44, 915–923. doi: 10.1016/0093-691x(95)00279-h
Ren Y., Duhatschek D., Bartolomeu C. C., Laplacette A. L., Perez M. M., Rial C., et al. (2023). An automated system for cattle reproductive management under the IoT framework. Part II: Induction of luteinizing hormone release after gonadotropin releasing hormone analogue delivery with e-synch frontiers in animal science. 4. doi: 10.3389/fanim.2023.1093857
Santos V. G., Carvalho P. D., Maia C., Carneiro B., Valenza A., Fricke P. M. (2017). Fertility of lactating Holstein cows submitted to a double-ovsynch protocol and timed artificial insemination versus artificial insemination after synchronization of estrus at a similar day in milk range. J. Dairy Sci. 100, 8507–8517. doi: 10.3168/jds.2017-13210
Schilkowsky E. M., Granados G. E., Sitko E. M., Masello M., Perez M. M., Giordano J. O. (2021). Evaluation and characterization of estrus alerts and behavioral parameters generated by an ear-attached accelerometer-based system for automated detection of estrus. J. Dairy Sci. 104, 6222–6237. doi: 10.3168/jds.2020-19667
Sheldon I. M. (2004). The postpartum uterus. the veterinary clinics of north America. Food Anim. Pract. 20, 569–591. doi: 10.1016/j.cvfa.2004.06.008
Souza A. H., Ayres H., Ferreira R. M., Wiltbank M. C. (2008). A new presynchronization system (Double-ovsynch) increases fertility at first postpartum timed AI in lactating dairy cows. Theriogenology 70, 208–215. doi: 10.1016/j.theriogenology.2008.03.014
Stangaferro M. L., Wijma R., Caixeta L. S., Al-Abri M. A., Giordano J. O. (2016). Use of rumination and activity monitoring for the identification of dairy cows with health disorders: Part I. Metab. digestive Disord. J. Dairy Sci. 99, 7395–7410. doi: 10.3168/jds.2016-10907
Stephen C., Norman S. (2021). Design and development of a biological implant for long term intravaginal retention in cattle. Aust. Vet. J. 99 (8), 326–333. doi: 10.1111/avj.13076
Tippenhauer C. M., Plenio J. L., Madureira A. M. L., Cerri R. L. A., Heuwieser W., Borchardt S. (2021). Timing of artificial insemination using fresh or frozen semen after automated activity monitoring of estrus in lactating dairy cows. J. Dairy Sci. 104, 3585–3595. doi: 10.3168/jds.2020-19278
Unold O., Nikodem M., Piasecki M., Szyc K., Maciejewski H., Bawiec M., et al. (2020). IoT-based cow health monitoring system (Cham: Springer International Publishing), Pages 344–356.
Vickers L. A., Burfeind O., von Keyserlingk M. A. G., Veira D. M., Weary D. M., Heuwieser W. (2010). Technical note: Comparison of rectal and vaginal temperatures in lactating dairy cows. J. Dairy Sci. 93, 5246–5251. doi: 10.3168/jds.2010-3388
Walsh R. B., LeBlanc S. J., Vernooy E., Leslie K. E. (2008). Safety of a progesterone-releasing intravaginal device as assessed from vaginal mucosal integrity and indicators of systemic inflammation in postpartum dairy cows. Can. J. veterinary Res. = Rev. Can. Recherche veterinaire 72, 43–49.
Wiltbank M. C., Baez G. M., Cochrane F., Barletta R. V., Trayford C. R., Joseph R. T. (2015). Effect of a second treatment with prostaglandin F2α during the ovsynch protocol on luteolysis and pregnancy in dairy cows. J. Dairy Sci. 98, 8644–8654. doi: 10.3168/jds.2015-9353
Wiltbank M. C., Pursley J. R. (2014). The cow as an induced ovulator: timed AI after synchronization of ovulation. Theriogenology 81, 170–185. doi: 10.1016/j.theriogenology.2013.09.017
Wotherspoon T. K., Higgins M. (2013). “14 - implantable wireless body area networks,” in Implantable sensor systems for medical applications. Eds. Inmann A., Hodgins D. (Swaston, United Kingdom; Woodhead Publishing), Pages 437–468.
Keywords: estrous cycle control, sensors, internet of things, cow, device
Citation: Ren Y, Duhatschek D, Bartolomeu CC, Erickson D and Giordano JO (2023) An automated system for cattle reproductive management under the IoT framework. Part I: the e-Synch system and cow responses. Front. Anim. Sci. 4:1093851. doi: 10.3389/fanim.2023.1093851
Received: 09 November 2022; Accepted: 23 February 2023;
Published: 16 March 2023.
Edited by:
Guilherme J.M. Rosa, University of Wisconsin-Madison, United StatesReviewed by:
Vishal Suthar, Kamdhenu University, IndiaCopyright © 2023 Ren, Duhatschek, Bartolomeu, Erickson and Giordano. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Julio O. Giordano, am9nMjVAY29ybmVsbC5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.