
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Anim. Sci., 08 March 2023
Sec. Animal Physiology and Management
Volume 4 - 2023 | https://doi.org/10.3389/fanim.2023.1032527
The supplementation of fresh alfalfa into the diet of growing lambs fed with decreasing levels of a total mixed ration (TMR) was studied for its effect on in vitro ruminal fermentation activity. Twenty-four catheterized lambs [25.2 ± 3.67 kg body weight (BW)] were assigned to one of the following treatments: “TMR100”—TMR ad libitum; “TMR75’” and “TMR50”—TMR at 0.75 and 0.50 of potential intake, respectively, supplemented with alfalfa; and ‘TMR0’—only alfalfa ad libitum. In vitro gas production kinetics and true digestibility (IVTD) were evaluated using the rumen liquid as inocula. Ruminal pH values and NH3-N and volatile fatty acid concentrations were studied at the same time as inocula extraction. As the amount of alfalfa in the diet increased—by decreasing the level of TMR—in vitro gas production, ruminal pH values, NH3-N concentrations, and acetic acid proportions linearly increased (p = 0.005, 0.008, 0.004, and 0.018, respectively). IVTD tended to linearly rise (p = 0.083) and the fermentation rate (p = 0.004) and propionic acid proportion (p< 0.001) linearly decreased. We conclude that the increase in the level of fresh alfalfa resulting from the decrease in TMR levels in lambs’ diet positively impacted rumen fermentation activity and in vitro digestibility through the promotion of a suitable environment for ruminal microbiota.
Ruminant production systems in several parts of the world are based on temperate pastures, which usually have high levels of crude protein (CP) content and highly digestible content (Pearson et al., 1997; Pontes et al., 2007; Cajarville et al., 2015). These systems are facing various challenges regarding animal welfare and sustainability (Temple and Manteca, 2020; Rivero and Lee, 2022). Even so, they offer opportunities and have several advantages over feedlot systems, in terms of expressions of natural animal behavior and final product quality (De Brito et al., 2018; Rivero and Lee, 2022), sustainability of the way of life of human communities, protection of environments, and use of food inedible for humans (Wilkinson and Lee, 2018; O′Mara et al., 2021; Wang et al., 2021). One key limiting factor of grazing systems is that pasture quality and availability are weather dependent and variable. This hinders the setting of production targets and is a limiting factor for animal productivity during some periods of the year.
On the other hand, the use of total mixed rations (TMRs) is a strategic tool in intensive production system feedlots. This kind of diet contains well-known nutrient levels, allowing for maximum animal production and efficiency throughout the year.
As an alternative, the combined use of both pasture and TMRs, alternated at different points in the day, has improved animal performance in dairy cows (Bargo et al., 2001; Mendoza et al., 2016; Santana et al., 2017). Although farmers have been using this feeding system to intensify production, the information available on this system is still scarce, particularly in sheep (Fernandez-Turren et al., 2020).
In another paper derived from the present experiment (Pérez-Ruchel et al., 2017), we reported that the decrease in the level of TMRs led to an increase of alfalfa dry matter (DM) and nutrient intake in lambs. However, contrary to expectations, these differences in intake were not related to the digestibility of diets measured in vivo, as the diets that contained more alfalfa were less digested than those that contained more TMRs, without differences in digesta transit.
According to Oba and Allen (1999), in vitro approaches would be more appropriate to evaluate some aspects of digestion because they reduce the interferences that could be generated by the in vivo measurements, such as rumen retention times or exposure to different gastrointestinal tract conditions. Therefore, through an in vitro evaluation of rumen digestion, we could explain the higher intake observed with decreasing TMRs and increasing alfalfa in the diet, which was not consistent with in vivo digestibility results (Pérez-Ruchel et al., 2017). We hypothesized that increasing the proportion of alfalfa in the diet would contribute to a more suitable rumen environment for fiber digestion, which could be ascertained through the evaluation of the rumen liquor activity of sheep consuming different proportions of alfalfa and TMRs. Therefore, this study aimed to evaluate the activity of the rumen environment of lambs consuming different proportions of alfalfa and TMRs, using in vitro methods for evaluation.
The study was carried out at the Experimental Farm of the Facultad de Veterinaria, UdelaR, Uruguay (San José Department, GPS coordinates: latitude 34′40.652″ S; longitude 56′32.349″ W). All procedures involving animals were approved by the Bioethics Committee of the Facultad de Veterinaria (UdelaR, Uruguay, CNEA-CHEAUdelaR-CEUAFVet, d. 295/99).
This experiment was performed using the same animals, diets, and experimental design published by Pérez-Ruchel et al. (2017). Briefly, 24 Corriedale × Milchschaf lambs (age 4 months), with an average body weight (BW) of 25.2 ± 3.67 kg, fitted with permanent rumen catheters, were individually housed in metabolism cages and fed with decreasing levels of TMRs and fresh alfalfa (Medicago sativa) ad libitum. The experiment was a randomized complete block design, with a 27-day experimental period (21 days of adaptation and 6 days of measurements). Lambs were divided into blocks according to BW (i.e., six blocks) and randomly assigned to receive one of the following treatments: TMR100—TMRs offered ad libitum; TMR75—TMRs at a level of 0.75 of the potential intake complemented with fresh alfalfa ad libitum; TMR50—TMRs at a level of 0.50 of the potential intake complemented with fresh alfalfa ad libitum; and TMR0—fresh alfalfa ad libitum.
The TMRs were formulated to meet growing lambs’ requirements, with an estimated daily gain of 300 g according to the National Research Council (NRC, 2007) regulations (Table 1). For all treatments, TMRs were prepared daily and offered at 09:00 (hour 0), without restriction in quantity for animals receiving TMR100 treatment. The level of TMRs for the TMR75 and TMR50 treatments was fixed according to the potential intake and provided in one meal. Potential intake was individually calculated by measuring voluntary intake during a period of 15 days. Each animal was supplied with 0.75 or 0.50 of their respective individual intake, according to the treatment.
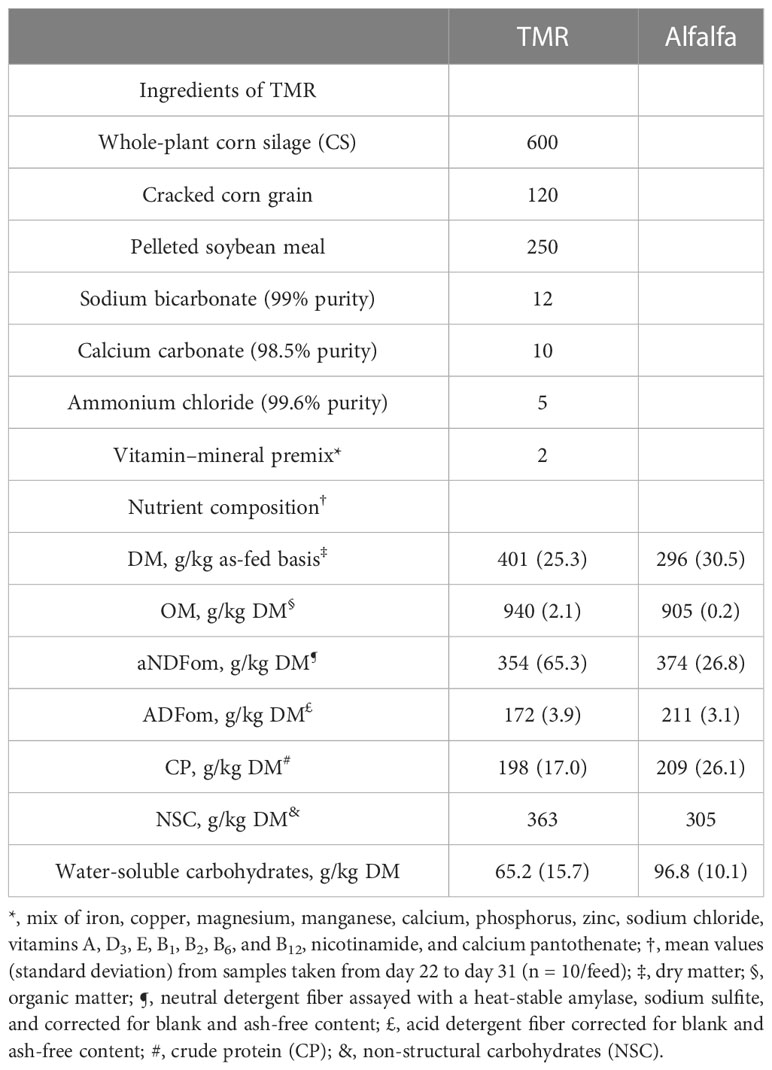
Table 1 Ingredients used and nutrient composition of total mixed rations (TMRs) and alfalfa [g/kg dry matter (DM), unless noted].
The forage was collected from one paddock and was predominantly alfalfa, with the following botanical composition: 792 g/kg DM of alfalfa, 156 g/kg DM of Lolium multiflorum, 10 g/kg DM of Lotus corniculatus, and 42 g/kg DM of senescent forage and herbs. Alfalfa was cut daily at 13:00 with a disk mower (5 cm in height from the ground). The pre-cut biomass was 1,475 kg DM/ha (chemical composition in Table 1). Animals receiving the TMR0 treatment were offered an unrestricted quantity of fresh alfalfa, beginning at hour 5 after the morning feeding, and continuously throughout the day. Animals receiving treatments TMR75 and TMR50 received an unrestricted amount of fresh alfalfa after they finished the TMR ingestion. Drinking water was provided in buckets connected to running water, at room temperature (i.e., at ≈ 15°C), and was freely available.
The total DM intake of lambs was 0.75, 0.92, 0.89, and 1.08 kg/day for those receiving TMR100, TMR75, TMR50, and TMR0 treatments, respectively, and linearly increased as TMRs in the diet were reduced (p< 0.001). Alfalfa represented 0%, 44%, 62%, and 100% of the diet in TMR100, TMR75, TMR50, and TMR0, respectively (Pérez-Ruchel et al., 2017).
The rumen activity was evaluated through in vitro gas production kinetics and in vitro true digestibility (IVTD). Rumen liquor was collected from each animal at hour 2 after the morning feed on day 22 of the experimental period.
The IVTD of the alfalfa used for the experiment (Table 1) was determined using DAISY equipment (DAISY® in vitro rumen fermenter, Ankom Technology Corp., Fairport, NY, USA) with 400 mL of rumen liquid for each treatment. Liquor was combined within each treatment and immediately used as inocula to evaluate IVTD. The alfalfa sample was ground to pass through a 2-mm screen and was incubated in porous bags for 48 hours (two replicates). After incubation, the bags were washed for 1 hour at 100°C in a neutral detergent solution and dried in an air-forced oven at 105°C to a constant weight. The IVTD was calculated as the percentage of material disappearing after incubation and washing procedures. The same procedure was performed two times (two batches, beginning on days 22 and 25).
For the evaluation of ruminal fermentation activity, the liquor of each animal was individually inoculated to measure gas production kinetics. Samples of whole-plant corn silage (180 g/kg DM, 344 g/kg ADFom, 81.1 g/kg CP, DM basis), corn grain (890 g/kg DM, 32 g/kg ADFom, 136 g/kg CP, DM basis), and alfalfa used for the experiment (Table 1) were used as substrates. These feedstuffs were ground to pass through a 1-mm screen, and weighed into 125-mL flasks (one flask/substrate/animal + two blanks/animal, total = 120 flasks), which were filled with 40.5 mL of a buffer solution (Williams et al., 2005) and 10 mL of strained rumen fluid, sealed with a rubber septum plus a crimp seal, and incubated for 96 hours in a water bath at 39°C, following the technique described by Mauricio et al. (1999). All incubation procedures were performed under a CO2 stream. Internal pressure in flasks was recorded at 2, 4, 6, 8, 10, 12, 18, 24, 48, 72, and 96 hours of incubation with a D1005PS manometer (Ashcroft®, Stratford, CT, USA) and a hypodermic needle. After each reading the gas was vented. Pressure readings were converted into volume using the equation obtained in a previous experiment under similar conditions:
V = 4.40×P + 0.09×P2
where V (mL) is the gas volume, and P (psi) is the observed pressure (R2 = 0.998). The accumulated volume of gas for each incubation time until 96 hours was fitted to the model:
Gas = A {1 – e [-kd(t-lag)]}
where A (mL) is the potential gas production, kd (h–1) is the rate of gas production, and lag (h) is the lag time.
Moreover, ruminal fluid samples were collected from each animal using the permanent rumen catheters at hour 2 (day 22). Ruminal pH was immediately measured using a digital pH meter (eChem Instruments Pte., Oakton, Singapore). Two samples (1 mL) were mixed with 0.02 mL of sulfuric acid (50%, v/v) and two samples were mixed with 1 mL of perchloric acid (0.1 M) and frozen for later determination of NH3-N and volatile fatty acid (VFA) concentrations, respectively.
The NH3-N concentration in ruminal samples was analyzed by spectrophotometry according to Weatherburn (1967) using a spectrophotometer (BEL Photonics®, S-2000, SP, Brazil). The VFA concentrations were analyzed in accordance with Adams et al. (1984) using high-performance liquid chromatography (Dionex Ultimate® 3000, Waltham, MA, USA) with an Acclaim Rezex Organic Acid H+ (8%) and a 7.8 mm × 300 mm column at 210 nm. The total VFA concentration was calculated as acetate + propionate + butyrate concentrations (mM) and each VFA concentration was expressed as a percentage of the total VFA concentration.
Feed samples were analyzed for DM, OM, and N in accordance with the Association of Official Analytical Chemists (AOAC, 1990, methods ID 934.01, ID 942.05, and ID 984.13, respectively); neutral detergent fiber assayed with a heat-stable amylase, sodium sulfite, and corrected for blank and ash-free content (aNDFom) and acid detergent fiber corrected for blank and ash-free content (ADFom) were analyzed according to Robertson and Van Soest (1981), using a Tecnal fiber analyzer (TE-149, Tecnal, Piracicaba, SP, Brazil), expressed without residual ash and corrected for blanks, as recommended by Mertens (2003). The aNDFom was analyzed using sodium sulfite and amylase. Crude fat content (CF) was determined, according to Nielsen (2003), using a Goldfisch fat extractor (Goldfisch, Labconco 35001, TX, USA) under a petroleum ether reflux at 180°C for 3 hours; water-soluble carbohydrates (WSCs) (Yemm and Willis, 1954) were also analyzed. In addition, the non-structural carbohydrate (NSC) content of feeds was calculated in accordance with Sniffen et al. (1992) as:
Data were analyzed using the MIXED procedure in SAS (version 8.2; SAS Institute, Cary, NC, USA). Data related to gas production parameters were compared between treatments (inocula) as repeated measures over the flask, using the model:
where Yijk is the dependent variable, µ is the general mean, Ti is the fixed effect of the treatment, Sj is the fixed effect of the substrate, (TxS)ij is the interaction between inoculum and substrate, and eijk is the residual error.
The IVTD data were compared between treatments (inocula) as repeated measures over the batch, using the model:
where Yij is the dependent variable, µ is the general mean, Ti is the fixed effect of the treatment, and eij is the residual error.
The pH values and VFA and NH3-N concentrations were analyzed according to the model:
where Yijk is the dependent variable, µ is the general mean, Ti is the fixed effect of the treatment in k animal replicates, Bj is the random effect of the block, and eijk is the residual error.
The effect of decreasing levels of TMRs in the diet (1.0, 0.75, 0.50, and 0.0) on the average values was evaluated by linear and quadratic regression.
Significant differences were declared if p ≤ 0.05 and 0.05< p< 0.10 was considered to indicate a tendency toward significance.
Total gas production (A) linearly increased (p = 0.005) and the fermentation rate (kd) linearly decreased (p = 0.004) as the levels of alfalfa increased and TMRs decreased (Table 2 and Figure 1). The three substrates incubated (corn silage, corn grain, and alfalfa) produced different gases, at different rates (effect of the substrate p< 0.001, not shown in the table), but with a similar response, with no interactions between treatment and substrates (p > 0.05, not shown in the table).
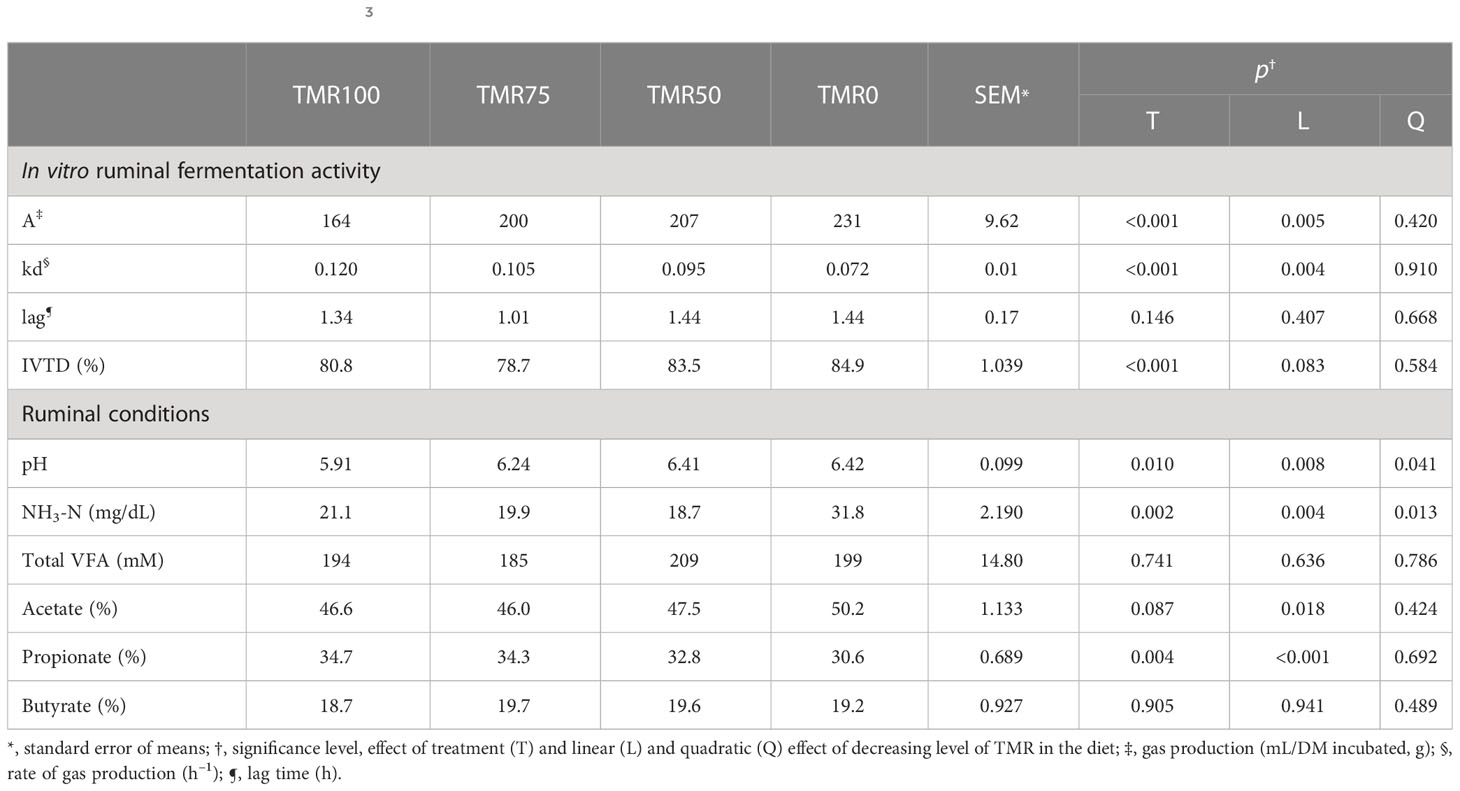
Table 2 In vitro gas production parameters and true digestibility (IVTD) using ruminal liquor of lambs fed total mixed rations (TMRs) (TMR100), 75% TMR and alfalfa (TMR75), 50% TMR and alfalfa (TMR50), or only alfalfa (TMR0) as inocula, and ruminal liquor conditions at the same hour of the inocula extraction (pH values and NH3-N and VFA concentrations).
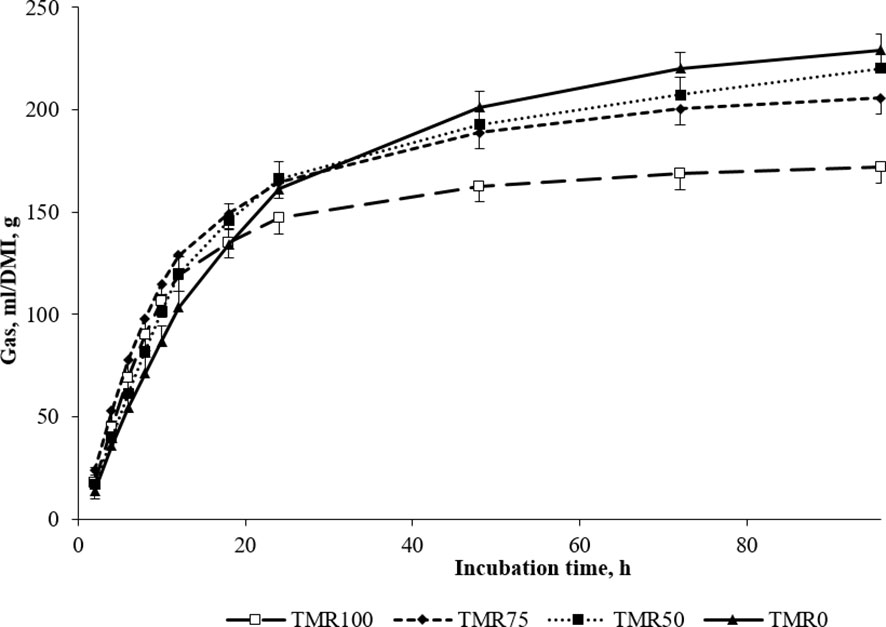
Figure 1 In vitro gas production (mL/DM incubated, g) using ruminal liquor of lambs fed TMR (TMR100), 75% TMR and alfalfa (TMR75), 50% TMR and alfalfa (TMR50), or only alfalfa (TMR0) as inocula (mean values ± standard error).
The IVTD tended to rise linearly (p = 0.083) as the levels of TMRs decreased and alfalfa increased (Table 2).
Ruminal pH values increased linearly and quadratically (p = 0.08 and 0.041, respectively) at decreasing rates with the increase of alfalfa in the diet. NH3-N concentrations also increased linearly and quadratically (p = 0.004 and 0.013, respectively), but at increasing rates. Meanwhile, the total VFA concentrations presented no differences between treatments, but some percentages of individual fatty acids varied. The concentration of acetate linearly increased (from 46.6% to 50.2% of total VFAs, p = 0.018), and propionate linearly decreased (from 34.7% to 30.6% of total VFAs, p< 0.001) with the reduction of TMRs and increase of alfalfa (Table 2).
The higher total gas production and IVTD of lambs with diets with higher levels of alfalfa and lower levels of TMRs indicate an improvement of rumen activity, which a priori can be considered contrary to expectations, since the NSC concentration was 20% higher in TMRs than in alfalfa. In fact, Chen et al. (2022), working in vitro with different proportions of rumen-degradable starch and rumen-degradable protein, observed that gas production increased with the increase in starch provided by degradable grains.
It is interesting to note that the type of fermentation promoted by the alfalfa forage (see TMR0 in Figure 1) was slower but more extended, with greater total fermentation than the other combinations. This type of fermentation would lead to a suitable environment for ruminal microbiota, especially for fibrinolytic communities, which are sensitive to ruminal variations and drops in pH (Zhang et al., 2017) and have crucial importance in ensuring fiber digestion of feeds (Firkins, 2021). The fact that the in vitro digestibility was measured for a relatively long time (48 hours) explains the strong tendency of higher IVTD observed in animals consuming more alfalfa.
According to Dryhurst and Wood (1998), when available N is not limiting, as in this case, gas production depends on carbohydrate degradability. In this sense, TMRs and alfalfa had not only different NSC concentrations but also different NSC partitioning. In TMRs the WSC fraction represented 18% of the total NSC concentration, whereas in alfalfa WSC represented 32% of the total NSC concentrations. The positive action of WSC on microbiota activity is well known (Russell, 1993) and can be associated with the increase of in vitro digestibility and gas production, as reported by Cajarville et al. (2015).
The higher pH values, NH3-N concentrations, and acetate percentages and the lower propionate levels observed as alfalfa increased in the diet are explained by the lower fiber and CP content of TMR. The greater WSC proportion in the forage compared with TMRs was not reflected in a higher butyrate concentration as observed by Heldt et al. (1999). Although this measurement corresponds to only one sample extracted simultaneously with the liquid extraction for in vitro assays, the results are consistent, in general terms, with data of ruminal environment observed by Pérez-Ruchel et al. (2017) throughout 24 hours on another day of the same experiment. In the mentioned paper, the average pH values and NH3-N concentrations throughout the day linearly increased as TMRs were reduced and alfalfa was increased in the diets. The quadratic effect observed for these variables in this study was probably related to the time of measurement, which was 2 hours after TMR provision in treatments T0, T50, and T75, and, therefore, with NSC entering the rumen.
Overall, these results can explain the higher DM and OM intake observed in vivo as TMRs were reduced and alfalfa increased in the diets (Pérez-Ruchel et al., 2017), and indicate that digestible fibers and sugars, instead of other NSCs (e.g., starch), promote a sustained fermentation that would favor intake. It can also be hypothesized that the higher intake levels of the treatments with high levels of alfalfa observed by Pérez-Ruchel et al. (2017) were due to a preference of the animals for alfalfa, as observed in dairy cows (Buse et al., 2022), and this higher intake level led to a more active ruminal environment, as observed in this study, owing to higher rates of substrate entry and content turnover (Mitsumori et al., 2019).
Whatever the causality, this study shows the positive link between the ingestion of high-quality, fresh forage and the rumen fermentation activity of lambs.
Increasing levels of high-quality, fresh alfalfa, by decreasing levels of TMRs in the diet of lambs positively impacted rumen fermentation activity and in vitro digestibility. This result could be associated with the high-quality fiber and WSC contents of the fresh forage, which promoted a suitable environment for ruminal microbiota and fermentation activity.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The animal study was reviewed and approved by Comisión de Ética en uso de Animales (CEUA, Committee on Ethics in the Use of Animals).
AP-R participated in the design of the work, data acquisition (laboratory analysis), analysis and interpretation of results, and writing of the manuscript. JR participated in the design of the work, interpretation of results, and writing of the manuscript. CC participated in the design of the work, interpretation of results, and writing of the manuscript. All authors contributed to the article and approved the submitted version.
The authors thank Jacinta Ubilla, Matías Morales, Elizabeth Artigas, Pia Arrieche, Santiago Gonnet, Marcos Padilla, Daniela Silva, Matías Muniz, Martín Estramil, Santiago Ultra, and Germán Gonçalvez (veterinary students) for their assistance with animal management and sampling.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Adams R. F., Jones R. L., Conway P. L. (1984). High performance liquid chromatography of microbial acid metabolites. J. Chromatogr. 336, 125–137. doi: 10.1016/S0378-4347(00)85136-1
Association of Official Analytical Chemist (1990). Official methods of analysis. 15th (Arlington (VA: AOAC).
Bargo F., Rearte D. H., Santini F. J., Muller L. D. (2001). Ruminal digestion by dairy cows grazing winter oats pasture supplemented with different levels and sources of protein. J. Dairy Sci. 84, 2260–2272. doi: 10.3168/jds.S0022-0302(01)74673-5
Buse K., Carroll A., Kononof P. (2022). Testing palatability of alfalfa hay of different relative feed value compared to brome hay in lactating Jersey cows. J. Dairy Sci. 105 (Suppl. 1), 358.
Cajarville C., Britos A., Errandonea N., Gutiérrez L., Cozzolino D., Repetto J. L. (2015). Diurnal changes in water-soluble carbohydrate concentration in lucerne and tall fescue in autumn and the effects on in vitro fermentation. New Zeal. J. Agr. Res. 58, 281–291. doi: 10.1080/00288233.2015.1018391
Chen P., Li Y., Shen Y., Cao Y., Li Q., Wang M., et al. (2022). Effect of dietary rumen-degradable starch to rumen-degradable protein ratio on In vitro rumen fermentation characteristics and microbial protein synthesis. Animals 12, 2633. doi: 10.3390/ani12192633
De Brito G. F., Ponnampalam E. N., Hopkins D. L. (2018). The effect of extensive feeding systems on growth rate, carcass traits, and meat quality of finishing lambs. Compr. Rev. Food Sci. Food Saf. 16, 23–38. doi: 10.1111/1541-4337.12230
Dryhurst N., Wood C. D. (1998). The effect of nitrogen source and concentration on in vitro gas production using rumen micro-organisms. Anim. Feed Sci. Technol. 71, 131–143. doi: 10.1016/S0377-8401(97)00124-7
Fernandez-Turren G., Repetto J. L., Arroyo J. M., Pérez-Ruchel A., Cajarville C. (2020). Lamb fattening under intensive pasture-based systems: A review. Animals 10, 1–22. doi: 10.3390/ani10030382
Firkins J. L. (2021). Invited review: Advances in rumen efficiency. Appl. Anim. Sci. 37, 388–403. doi: 10.15232/aas.2021-02163
Heldt J. S., Cachran R. C., Mathis C. P., Woods B. C., Olson K. C., Titgemeyer E. C., et al. (1999). Effects of level and source of carbohydrate and level of degradable protein on intake and digestion of low-quality tall-grass-praire hay by beef steer. J. Anim. Sci. 77, 2846–2854. doi: 10.2527/1999.77102846x
Mauricio R. M., Mould F. L., Dhanoa M. S., Owen E., Channa K. S., Theodorou M. K. (1999). A semi-automated in vitro gas production technique for ruminant feedstuff evaluation. Anim. Feed Sci. Technol. 79, 321–330. doi: 10.1016/S0377-8401(99)00033-4
Mendoza A., Cajarville C., Repetto J. L. (2016). Intake, milk production and milk fatty acid profile of dairy cows fed diets combining fresh forage with a total mixed ration. J. Dairy Sci. 99, 1938–1944. doi: 10.3168/jds.2015-10257
Mertens D. R. (2003). Challenges in measuring insoluble dietary fiber. J. Anim. Sci. 81, 3233–3249. doi: 10.2527/2003.81123233x
Mitsumori M., Hasunuma T., Okimura T., Takumi Shinkai T., Kobayashi Y., Hirako M., et al. (2019). Theoretical turnover rate of the rumen liquid fraction in dairy cows and its relationship to feed intake, rumen fermentation, and milk production. Anim. Sci. J. 90, 1556–1566. doi: 10.1111/asj.13305
National Research Council (2007). Nutrient requirements in small ruminants (Washington (DC: National Academies Press).
Nielsen S. S. (2003). Food analysis laboratory manual (New York (NY: Kluwer Academic/ Plenum Publisherd).
O′Mara F., Richards K. G., Shalloo L., Donnellan T., Finn J. A., Lanigan G. (2021). Sustainability of ruminant livestock production in Ireland. Anim. Front. 11, 32–43. doi: 10.1093/af/vfab037
Oba M., Allen M. S. (1999). Evaluation of the importance of the digestibility of neutral detergent fiber from forage: Effects on dry matter intake and milk yield of dairy cows. J. Dairy Sci. 82, 589–596. doi: 10.3168/jds.S0022-0302(99)75271-9
Pearson C. J., Brown R., Collins W. J., Archer K. A., Wood M. S., Petersen C., et al. (1997). An Australian temperate pastures database aust. J. Agric. Res. 48, 453–466. doi: 10.1071/A96095
Pérez-Ruchel A., Repetto J. L., Cajarville C. (2017). Supplementing high quality fresh forage to growing lambs fed a total mixed ration diet led to higher intake without altering nutrient utilization. Animal 11, 2175–2183. doi: 10.1017/S1751731117000933
Pontes L. S., Carrère P., Andueza D., Louault F., Soussana J. F. (2007). Seasonal productivity and nutritive value of temperate grasses found in semi-natural pastures in Europe: responses to cutting frequency and n supply. Grass Forage Sci. 62, 485–496. doi: 10.1111/j.1365-2494.2007.00604.x
Rivero M. J., Lee M. R. F. (2022). A perspective on animal welfare of grazing ruminants and its relationship with sustainability. Anim. Prod. Sci. 62, 1739–1748. doi: 10.1071/AN21516
Robertson J. B., Van Soest P. J. (1981). “The detergent system of analysis and its application to human foods,” in The analysis of dietary fiber in food. Eds. James W. P. T., Theander O. (New York (NY: Marcel Dekker), 123.
Russell J. B. (1993). Glucose toxicity in prevotella ruminicola: methylglyoxal accumulation and its effect on membrane physiology. Appl. Environ. Microbiol. 59, 2844–2850. doi: 10.1128/aem.59.9.2844-2850.1993
Santana A., Cajarville C., Mendoza A., Repetto J. L. (2017). Combination of legume-based herbage and total mixed ration (TMR) maintains intake and nutrient utilization of TMR and improves nitrogen utilization of herbage in heifers. Animal 11, 616–624. doi: 10.1017/S1751731116001956
Sniffen C. J., O’Connor J. D., Van Soest P. J., Fox D. G., Russell J. B. (1992). A net carbohydrate and protein system for evaluating cattle diets: II. Carbohydr. Protein availability J. Anim. Sci. 70, 3562–3577. doi: 10.2527/1992.70113562x
Temple D., Manteca X. (2020). Animal welfare in extensive production systems is still an area of concern. Front. Sust. Food Syst. 4. doi: 10.3389/fsufs.2020.545902
Wang B., Luo Y., Wang Y., Wang D., Hou Y., Yao D., et al. (2021). Rumen bacteria and meat fatty acid composition of sunit sheep reared under different feeding regimens in China. J. Sci. Food Agric. 101, 1100–1110. doi: 10.1002/jsfa.10720
Weatherburn M. W. (1967). Phenol-hypochlorite reaction for determination of ammonia. Anal. Chem. 39, 971–974. doi: 10.1021/ac60252a045
Wilkinson J. M., Lee M. R. F. (2018). Review: Use of human-edible animal feeds by ruminant livestock. Animal 12, 1735–1743. doi: 10.1017/S175173111700218X
Williams B., Bosch M., Boer H., Verstegen M., Tamminga S. (2005). An in vitro batch culture method to assess potential fermentability of feed ingredients for monogastric diets. Anim. Feed Sci. Technol. 123–124, 445–462. doi: 10.1016/j.anifeedsci.2005.04.031
Yemm E. W., Willis A. J. (1954). The estimation of carbohydrates in plant extract by anthrone. Biochem. J. 57, 508–514. doi: 10.1042/bj0570508
Keywords: alfalfa, growing lambs, ruminal fermentation, true digestibility, microbial activity
Citation: Pérez-Ruchel A, Repetto JL and Cajarville C (2023) Supplementation of high-quality fresh forage to lambs fed a total mixed ration increased in vitro ruminal fermentation and digestibility. Front. Anim. Sci. 4:1032527. doi: 10.3389/fanim.2023.1032527
Received: 30 August 2022; Accepted: 14 February 2023;
Published: 08 March 2023.
Edited by:
Pasquale De Palo, University of Bari Aldo Moro, ItalyReviewed by:
Emanuela Valle, University of Turin, ItalyCopyright © 2023 Pérez-Ruchel, Repetto and Cajarville. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Analía Pérez-Ruchel, YW5hcGV2ZXRAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.