- Department of Animal Science, Wollega University, Nekemte, Ethiopia
The study was conducted with the objective of determining the effects of the dietary replacement of soybean meal with graded levels of linseed meal on selected blood parameters, meat composition, fatty acid profiles, and meat quality of broiler chickens. Cobb500 broilers were fed diets containing linseed meal at 0% (T1), 6.5% (T2), 13% (T3), 19.5 (T4), and 26% (T5), replacing 0% to 100% soybean meal in compound rations for 45 days. The experiment was conducted using a completely randomized design with five treatments, each replicated three times with 12 birds. Blood hematological indices were not affected (P>0.05) by treatment diets while among the blood biochemistry triglyceride and cholesterol concentrations in T1 were higher (P<0.05) than in T3, T4, and T5. Glucose was higher in T3, T4, and T5 than in T1 and T2 (P<0.01). The breast and thigh proximate composition for crude protein (CP), ether extract (EE), ash, carbohydrate, and gross energy were similar (P>0.05) among treatments. The sensory scores for breast and thigh meat samples were not different (P>0.05) among treatments. The palmitic acid content of breast and thigh meat was higher (P<0.05) in T1 and T2 than in the other treatments. Eliadic and stearic acid concentrations in thigh meat were greater (P<0.05) in T1, T2, and T3 than in T4 and T5. The oleic and linoleic acid contents of thigh meat were higher (P<0.05) in T2 and T3 than in the rest of the treatments. Breast linolenic acid was high (P<0.05) in T5 but similar in thigh meat. Total saturated fatty acid (SFA) showed a decreasing trend with an increasing level of linseed meal (LSM) in the ration. The ratio of monounsaturated fatty acid (MUFA) to SFA for breast and thigh was higher in T4 and T5 than in T1 and T2. Breast meat ratio of omega-6 (n-6) to omega-3 (n-3) showed a decreasing trend as the level of LSM replacement for soybean meal (SBM) in the ration increased. Generally, linseed meal replacement levels up to 26% in the broilers’ diet improves the essential fatty acid content of chicken meat without affecting the proximate composition and the chickens’ normal blood indices and does not alter meat sensory attributes.
Introduction
The enrichment of animal products with biologically active functional compounds has been of increasing interest in recent years because of the growing awareness among consumers about the relationship of diet with human health (Mandal et al., 2014). The greatest effort in animal feed research has been directed towards enhancing the beneficial omega-3 (n-3) fatty acid concentrations of animal products to produce healthy foods (Palmquist, 2009; Mandal et al., 2014). Chicken meat is popular across the world, attributed to its large parts of white meat containing low levels of saturated fat and high levels of long-chain polyunsaturated fatty acid (PUFAs), resulting in its being regarded as healthy meat (Haug et al., 2007; Shunthwal et al., 2017b). Chicken meat also contains low levels of collagen that reduce meat digestibility; this is another positive characteristic that makes chicken meat easier to digest than other types of meat (Marangoni et al., 2015; Kralik et al., 2018).
In monogastric species such as poultry, the fatty acid profile of the meat and fat is directly affected by the source of fat in the diet. Research results also indicate that products such as eggs and beef produced from linseed-fed animals have increased levels of omega-3 fatty acids (Scheideler et al., 1994; Maddock et al., 2003; Mridula et al., 2011). Dietary omega-3 polyunsaturated fatty acids are essential for normal development and the maintenance of optimal health, and are found to reduce the risk of cardiovascular and allergic diseases (Van Den Elsen et al., 2012) and inflammatory conditions (Honda et al., 2015), as well as reduce harmful blood lipids like cholesterol and triacylglycerides. Linseed meal is also a rich source of protein and energy and can serve as a nutritious feed supplement for livestock or be used for the production of high-protein flour (Russo and Reggiani, 2016). Linseed proteins have important biological effects and their physiological properties are mainly a result of both their amino acid composition and their interaction with other components as polysaccharides, lignans, or fatty acids (Omoni and Aluko, 2006; Russo and Reggiani, 2016). Despite its importance, linseed meal contains anti-nutritional factors such as phytate and cyanogenic glycoside compounds that need to be considered when feeding it to animals or used as additives in the food industry (Russo and Reggiani, 2016).
Hematological parameters both in humans and animals are important indices in the physiological state of individuals (Maidala et al., 2014). The determination of blood components assists the diagnosis of various poultry diseases and disorders caused by several factors such as nutritional status, season, and management (Café et al., 2012). Therefore, hematological and serum parameters have been observed as good indicators of the physiological status of animals and changes in these parameters are important in assessing the response of such animals to various physiological situations (Khan and Zafar, 2005; Tijani et al., 2015).
Changes in the dietary fatty acid composition could be reflected in the blood, which in turn would be transported to target organs such as muscles (Aghwan et al., 2014). In the process of increasing the level of ω-3 PUFA in meat, it is important to achieve positive nutritional and functional effects on meat and at the same time not diminish the sensory quality (Zivkovic et al., 2017). The major parameters considered in the assessment of meat quality are appearance, juiciness, tenderness, flavor, and chemical composition of the meat (Lawrie and Ledward, 2006; Kishawy et al., 2019). Therefore, this study aims to assess the effect of the dietary replacement of soybean meal with graded levels of linseed meal on selected blood parameters, broilers’ meat chemical composition, fatty acid profiles, and meat quality.
Materials and methods
Description of the study area
The experiments were conducted at the Haramaya University Poultry farm located at 42°3’ east longitude and 9°26’ north latitude at an altitude of 1,980 m above sea level and 505 km east of Addis Ababa (Tamasgen et al., 2021). The mean annual rainfall in the area amounts to 780 mm and the average minimum and maximum temperatures are 8°C and 24°C, respectively (Samuel, 2008).
Experiment setup
The data for this study were taken from the feeding trial conducted to determine the effects of replacing soybean meal with graded levels of linseed meal on broilers’ performance (Tamasgen et al., 2021). The ingredients used for compounding the treatment rations include noug seed cake (NSC), wheat shorts, linseed meal (LSM), soybean meal (SBM), salt, vitamin premix, and limestone. The treatment rations labeled as T1, T2, T3, T4, and T5 were formulated with T1 containing 26% SBM as an economic maximum level of inclusion, while T2, T3, T4, and T5 were formulated with linseed meal replacing the actual portion of soybean meal in the T1 ration at rates of 6.5%, 13%, 19.5%, and 26%, respectively, which correspond to 25%, 50%, 75%, and 100% replacement of SBM. The five treatment rations were further assigned to the pen of three replicates, each consisting of 12 chicks. The design of the experiment was a completely randomized design (CRD). The growth experiment lasted for 44 days.
Hematological and serum biochemical determination
At 44 days of the growth experiment, 5-ml blood samples were collected from the broiler’s wing vein. The blood was collected in two labeled sterile universal bottles. One set of the bottles contained ethylene diamine tetra acetic acid (EDTA) as an anticoagulant, while the other set did not contain an anticoagulant. Blood samples in bottles with anticoagulant was spun in a centrifuge at 3,000 rpm for 10 min and plasma was separated and stored frozen at -10°C. The red blood cell (RBC) and white blood cell (WBC) counts were done using a hemocytometer (Irizaary-Rovira, 2004). Packed cell volume (PCV) was determined by spinning blood-filled capillary tubes in a centrifuge at 1,200 rpm for 5 min and reading was done on a hematocrit reader. Hemoglobin (Hb) concentration was determined from samples in bottles with anticoagulant taken before spinning in a centrifuge by the Actin hematin method (Dacie and Lewis, 1991). The mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and mean corpuscular hemoglobin concentrations (MCHC) were calculated according to Brians et al. (2000). Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP) activities, and cholesterol, glucose, and triglyceride concentrations were measured by using enzyme/buffer and substrate kits according to standard laboratory procedures (NHANES, 2000). Total serum protein was determined using a refractometer (George, 2001).
Meat chemical composition
The chemical composition of the meat samples was analyzed following the procedure of AOAC (2000). Samples of breast and thigh muscles were minced and set in folded aluminum foil. The samples were kept in an oven at 55°C for 72 h for partial drying. Partially dried meat samples were ground and the weighed sample was dried in an oven at 105°C for 12 h to determine dry matter. Meat nitrogen (N) content was measured according to the Kjeldahl procedure and the crude protein content of the sample was calculated as N*6.25. The fat (ether extract) was determined according to the Soxhlet method. The gross energy value of breast and thigh meat was calculated by Atwater specific factors (Merrill and Watt, 1973) with a conversion factor of 4.27 Kcal/g (17.9 Kj/g) for protein and 9.02 Kcal/g (37.7 Kj/g) for meat fat. The value of total carbohydrates was calculated as 100-(ash + lipid + protein + moisture) (FAO, 1998).
Meat fatty acid profiles
Meat samples from breast and thigh muscles were collected from two birds per replicate (six per treatment) and slaughtered for carcass evaluation at the end of the growth experiment. The sample was ground and given a tag with coded bands indicating the replication and treatment number. Each sample was packaged individually for fatty acid composition analysis. Approximately 100 mg of hammer-milled breast and thigh meat sample from each replication was used for the extraction of oil through direct methylation at the Adama Science and Technology University. Fatty acids were methylated according to the procedure described by Wang et al. (2000). The extracted oil sample was transferred to a microcentrifuge tube and centrifuged by Hettich EBA 3S for 5 min, and an aliquot of the top layer was placed in a 1.5-ml vial by a separator funnel. The samples were transported to the Addis Ababa University using an icebox. One microliter of sample was injected into an Agilent Gas Chromatograph (DB-1701 GC) with a 30-m column length, 0.25-µm internal diameter, and 0.2-µm phase thickness, which was connected to mass spectrometer. The GC oven program was adjusted to 220°C, then increased to 240°C at 2°C/min, and reached the maximum temperature of 280°C. Helium was used as a carrier gas at a flow rate of 1 µl/min. Fatty acid methyl esters (FAME) were separated and quantified using gas chromatography (GC-MS) at the Addis Ababa University Organic Chemistry Laboratory. The concentration of measured fatty acids in a microliter was converted to milligram per gram. Saturated fatty acid (SFA), monounsaturated fatty acid (MUFA), polyunsaturated fatty acid (PUFA), omega-3 (n-3), and omega-6 (n-6) were calculated by a summation of their respective individual fatty acids.
Meat eating quality
The samples of chicken breast and thigh muscle were taken to determine juiciness, tenderness, flavor, and flavor intensity. Skinless breast and thigh muscle samples were frozen and kept until used. The pieces of the meats were thawed at room temperature, minced, and cut into approximately 2.5-cm cubes. The meats were packed with aluminum foil and cooked for 45 min at 145°C in an oven. The cooked meats were cooled to room temperature for about 10 min. A total of 20 semi-experienced panelists selected among Haramaya University Food and Animal Sciences staff rated the samples according to Keeton (1983), using 8-point hedonic scales for tenderness (1 = extremely tough to 8 = extremely tender), juiciness (1 = dry to 8 = extremely juicy), chicken flavor intensity (1 = weak to 8 = strong), and hedonic scales for flavor liking (1 = dislike extremely to 8 = like extremely), and overall liking (1 = dislike extremely to 8 = like extremely).
Statistical analysis
Data were analyzed using the general linear model procedure of the Statistical Analysis Systems (SAS, 2009) software. Differences between treatment means were separated using Duncan’s Multiple Range Test at a 5% level of significance. P-values less than the level of significance (5%) were considered significant. The following model was used for data analysis: Yij = μ + Ti + eij, where: Yij = represents the jth observation in the ith treatment level, μ = overall mean, Ti = treatment effect, and eij = random error.
Results
Hematological and serum biochemical parameters
Blood hematological parameters were not affected (P>0.05) by levels of linseed meal as a replacement for soybean meal in the compound ration (Table 1). Among the serum biochemical parameters, only triglyceride, cholesterol, and glucose concentrations differed among the treatments. Triglyceride concentration in T1 was higher (P<0.05) than in T3, T4, and T5.Cholesterol level was significantly higher for T1 than the other treatments (P<0.05). The glucose concentrations for T3 and T5 were greater (P<0.01) than for T1 and T2.
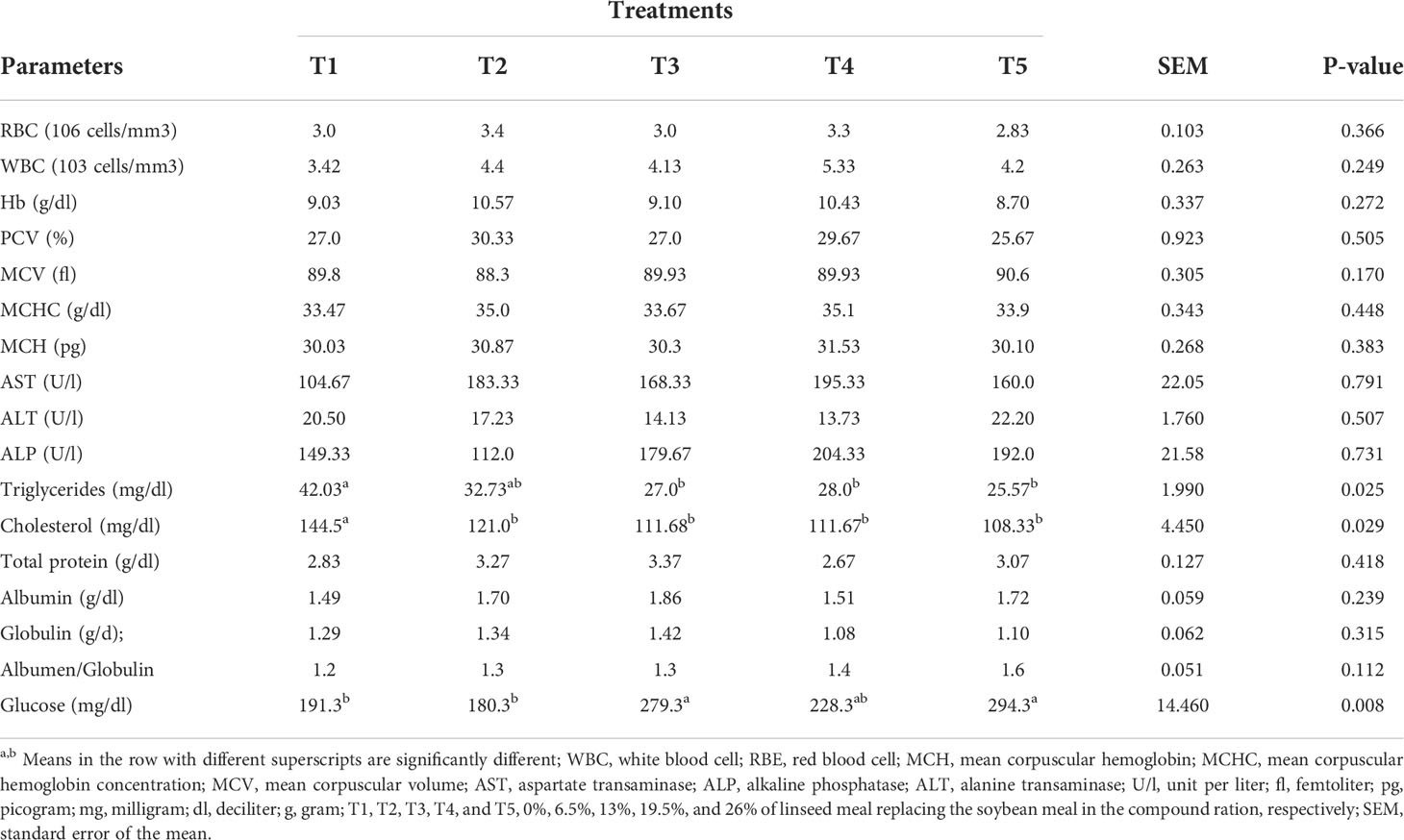
Table 1 Hematological and serum biochemical parameters of broilers fed linseed meal as a replacement for soybean meal in the compound ration.
Meat chemical composition
The breast and thigh proximate compositions, except the thigh dry matter (DM) percentage, were similar (P>0.05) among treatments (Table 2). Greater (P<0.05) thigh DM was observed for T1 as compared to T4 and T5 but T2 and T3 are similar to T1.
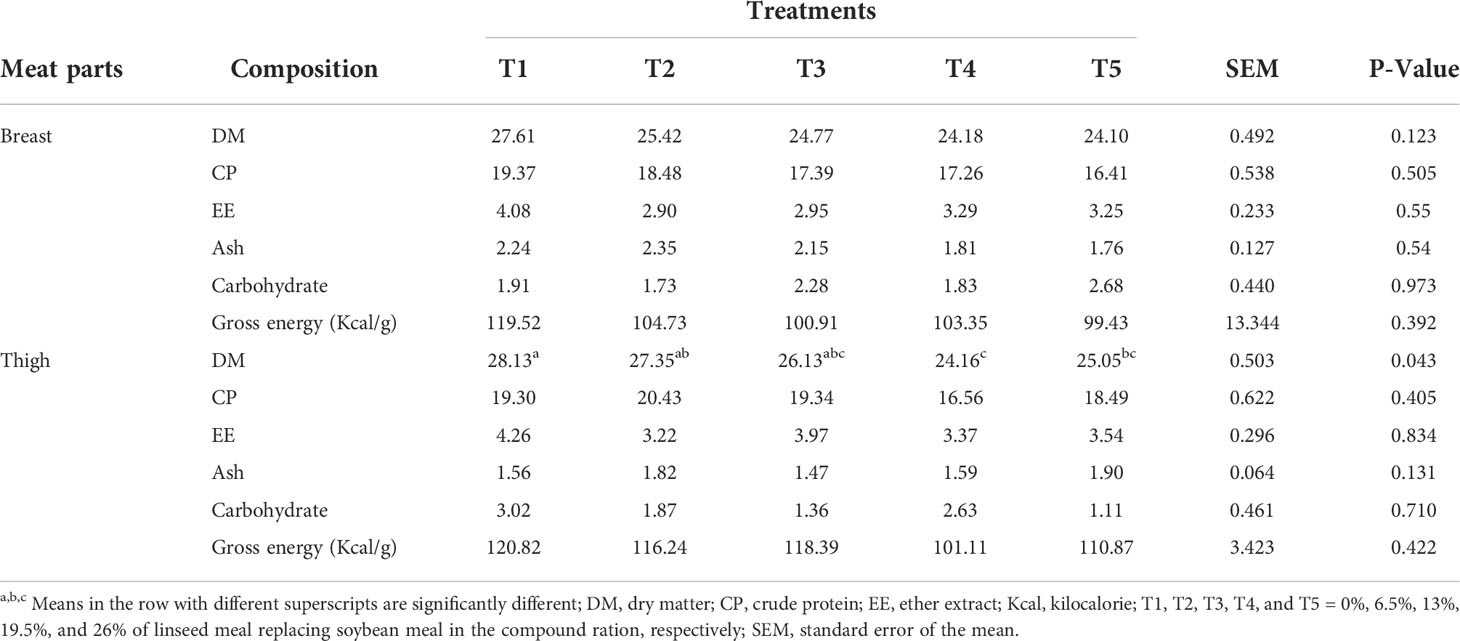
Table 2 Breast and thigh meat chemical compositions of broiler chickens fed linseed meal as a replacement for soybean meal in the compound ration (%DM basis).
Meat fatty acid compositions
The concentration of eliadic acid, stearic acid, oleic acid, and linoleic acid in breast meat did not differ among treatments, but greater (P<0.05) concentrations of these fatty acids were recorded in thigh meat for treatments (T1, T2 and T3) with a lower level of linseed substitution for soybean (Table 3). The concentration of palmitic acid in breast and thigh meat was high in T1 and T2 than in the other treatments (P<0.05). Linolenic acid concentration was generally low in both breast and thigh meat and its value in breast meat is high (P<0.001) for T5 than in the rest of the treatments, while no difference was observed in thigh meat among treatments. A high (P<0.05) concentration of total SFA was recorded for T1 while T5 contained low SFA (P<0.05). Breast meat total PUFA and MUFA/SFA concentrations were high (P<0.05) in T5 than in the rest of the treatments while T5 had low n-6 to n-3 ratio (P<0.05). Thigh palmitoleic acid, total PUFA, and the ratio of n-6 to n-3 were similar (P>0.05) among treatment groups. Low (P<0.05) MUFA for the thigh was recorded in T5 and T4 whereas high MUFA/SFA was observed in T4 and T5.
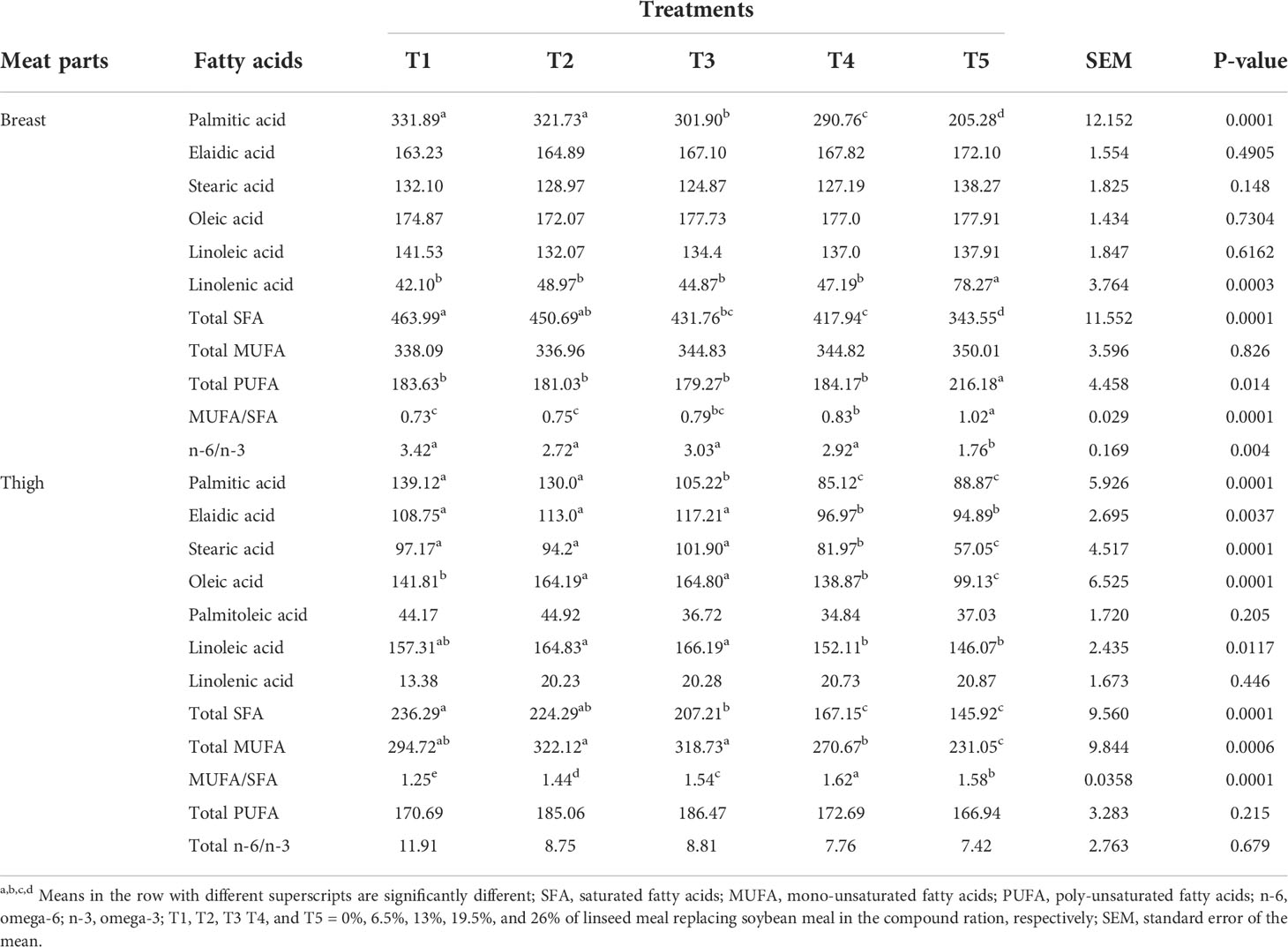
Table 3 Breast and thigh meat fatty acid composition of broiler chickens fed linseed meal as a replacement for soybean meal in the compound ration (mg/g fat).
Sensory evaluation of meat
The sensory score for breast and thigh meat samples did not differ (P>0.05) among treatment groups (Table 4). The sensory value of both breast and thigh meats, as rated by the panelists, ranged from 4.67 to 7 in the hedonic scale indicating that the meat’s normal sensory attributes were not affected.
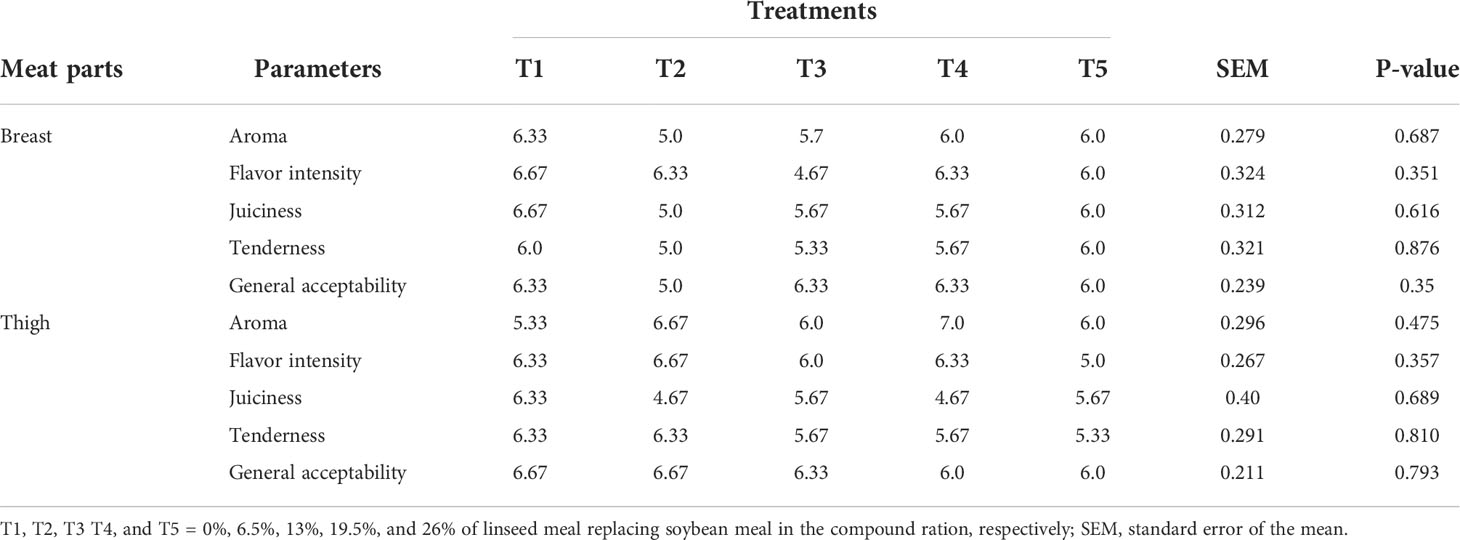
Table 4 Sensory value of breast and thigh meat of broiler chickens fed linseed meal as a replacement for soybean meal in the compound ration.
Discussion
Hematological and serum biochemical parameters
Feed enriched with PUFA can be prone to oxidation, causing oxidative stress in animals (Salami et al., 2015) and alteration of animal performance and health (Lopez-Ferrer et al., 2001). Linseed meal contains high levels of PUFA and oxidation can occur when used at high dietary levels. However, feeding of increased levels of linseed meal in the present study did not influence the hematological indices of broilers. This might be attributed to some possible antioxidant constituents found in some ingredients used in the ration like maize and vitamin supplements which, when combined with n-3 FA, might have lowered lipid oxidation levels and increased antioxidant status in the plasma of broilers (Leskovec et al., 2018).
The fact that all hematological values fall within the clinically healthy values for broilers shows that no potential toxicity of diet and content of biologically active compounds in linseed meal such as omega-3 contributed to the health of the broilers. Additionally, Negasa et al. (2020) did not observe any harmful effect of linseed-meal feeding to layers.
The lack of harmful effects of feeding increasing levels of linseed meal to broilers on aspartate aminotransferase (AST), alanine aminotransferase (ALT), and alkaline phosphatase (ALP) activities wasconsistent with the result obtained by Matusiewicz et al. (2015) who found that the addition of flaxseed cake to diets for rats did not affect the activities of alanine transaminase and aspartate aminotransferase, indicating normal liver function and the absence of a hepatotoxic effect of the diet. Similarly, Yassein et al. (2015) showed that feeding moderate levels of flaxseed (5%–10%) had no significant effect on the AST activity of laying hens. However, Al-Nawass (2015) reported that a high dose (12%–16%) of linseed in broilers increased blood AST activity, which they credited to high hydrogen cyanide in flaxseed. Plasma AST greater than 275 IU/l results from either hepatic or muscle injury leading to leakage of intracellular AST into the blood, and AST activity greater than 800 IU/l is an indication of severe hepatic disorder (Hochleithner et al., 2005; Motaghi et al., 2017). Unlike the present result, Al-Nawass (2015) and Al-Azzawi et al. (2011) reported reduced total protein in the blood due to the anti-nutrient linatin in linseed, which could be related to the effectiveness of the processing methods employed to reduce anti-nutritional factors in linseed. The total protein and globulin values obtained in the present study were within the normal range of 2.5–4.5 and 0.5–1.8 g/dl, respectively (Thrall, 2007; Café et al., 2012). The reduced serum triglycerides and cholesterol concentration in the broilers’ consumed diet containing linseed meal in the present study were consistent with that reported by Al-Hilali (2018) who found a significant decline in total serum cholesterol and triglycerides with an increased concentration of flaxseed oil supplementation in the broilers’ diet. The reduction in serum triglyceride concentration in broilers is due to the increase in the β-oxidation rate of unsaturated fatty acids resulting in the removal of triglycerides from the blood and increased transportation to the tissues (Shunthwal and Sheoran, 2017). Similarly, Osek et al. (2008) and Kishawy et al. (2019) noted that replacing soybean oil with linseed oil resulted in decreased total cholesterol levels. Shunthwal et al. (2017a) reported decreased cholesterol levels with increased replacement levels of linseed oil for sunflower oil in the broilers’ diet. According to Pavlovic et al. (2018), the positive effects of cholesterol-lowering in meat from the broiler (breast and thigh) are important for human nutrition and health since it particularly reduces cardiovascular disease problems. Glucose concentration was increased with levels of linseed meal replacement and the values obtained at 13%, 19.5%, and 26% linseed meal-containing diet were within the normal range of 200–500 mg/dl (Campbell, 2012), while the level recorded at 0% and 6.5% was greater than the critical value of 150 mg/dl (Brar et al., 2000), which indicated the absence of stress or an abnormal glucose level-related problem (Kassa et al., 2016). The increased glucose level at higher linseed meal levels could be attributed to lower omega-6 levels in those diets when compared with control, since omega-3 FA has been shown to decrease blood glucose levels (Aguilar et al., 2011). Furthermore, it is suggested that diets rich in SFA increase levels of serum insulin (Crespo and Esteve-Garcia, 2003), and unsaturated fatty acids lower blood insulin, which has a direct effect on blood glucose concentration (Bird et al., 1994; Aguilar et al., 2011).
Chemical composition of breast and thigh meat of broiler chicken
The replacement levels of linseed meal in the present study did not cause a significant change in the proximate composition of thigh and breast meat of broilers. Zivkovic et al. (2017) also stated that the diet to which extruded flaxseed was added did not have a large influence on the chemical composition. The finding of Kouba et al. (2008) and Peiretti and Meineri (2010) also indicated that n-3 PUFA-rich extruded linseed did not show significant effects on the dry matter, protein, and lipids of the rabbit muscles. The CP contents of the breast and thigh meat used in the current study were similar to that of Mridula et al. (2015) who noted that different levels of linseed in the broilers’ diet did not affect the protein content of breast and thigh meat. The calculated carbohydrate values for breast and thigh meat were higher than the broilers’ meat carbohydrate (1.2%) reported by the USDA (2006), except for the highest linseed meal-fed birds. The variation observed is attributed to methods of carbohydrate determination in meat cuts. The calculated gross energy values of breast and thigh in this study were lower than the value for breast (140 Kcal/g) and thigh (156 Kcal/g) reported by Atyeo and Cook (2014). The low energy value of breast and thigh meat in this study could probably be attributed to the high polyunsaturated fatty acid content of linseed meal.
Fatty acid profiles of breast and thigh meat of broiler chickens
The decreased concentration of palmitic acid in breast meat in the current study agreed with the findings of Chiroque et al. (2018) who reported the same trend when linseed and pumpkin seed meals were fed to guinea fowls. Similarly, the concentration of oleic acid was not affected by the increased levels of linseed meal, which also agreed with that observed by Chiroque et al. (2018). Linseed, a valuable source of ALA in chicken diets, can be effectively incorporated from feed to the bird’s tissues (Abbasi et al., 2019), thereby significantly increasing n-3 PUFA concentrations and decreasing n-6:n-3 PUFA ratios in birds fed diets containing soybean oil (Jankowski et al., 2012). The highest concentration of linolenic acid in breast meat at 26% levels of linseed meal is also similar to that reported by Mridula et al. (2011) and Mir et al. (2018) who mentioned that the linolenic acid (ALA) content in both breast and thigh tissues increased significantly with increasing levels of flaxseed meal in the broilers’ diet. The non-significant decreasing tendency of linoleic acid concentration of breast meat among the treatment groups agreed with that observed by Mridula et al. (2015) who declared decreased linoleic acid content when linseed was fed to broiler chickens. The change in the total polyunsaturated fatty acid in broilers’ breast meat agreed with that of Mir et al. (2018) who reported a higher proportion of unsaturated fatty acids (UFA) in meat due to flaxseed meal feeding, which has a higher proportion of UFAs that undergoes faster absorption in the gut. It is mentioned that meat muscle enrichment with PUFAs, especially omega-3 and omega-6 fatty acids, improves meat quality for the consumer and enhances human health (Harris, 1989; Kishawy et al., 2019). The highest concentration of total breast SFA recorded in the control group of the present study was similar to that of Zhaleh et al. (2019) who reported a high amount of saturated fatty acids (SFA), monounsaturated fatty acids (MUFA), and a low composition of polyunsaturated fatty acid (PUFA) concentrations in the control group when up to 15% levels of both rolled and extruded flaxseed were fed to chickens. The increased ratio of MUFA : SFA with increased levels of linseed meal was similar to the findings of Abdulla et al. (2015). The total n-6:n-3 ratio of breast meat was similar to that of Kostadinovic et al. (2016) who reported a decreased ratio with increased levels of extruded linseed in broiler ration. This is related to the increase in omega3 FA of broiler muscles, which may induce reduction in a corresponding omega6 FA in breast muscles, because the two groups of FA compete for the same desaturation enzymes (Nuernberg et al., 2005). In fatty acid composition analysis, a low n-6:n-3 ratio has suppressive effects on many diseases such as cardiovascular disease, cancer, and inflammatory and autoimmune diseases (Scollan et al., 2006; Kostadinovic et al., 2016). According to the review of Alagawany et al. (2019), the ratio of n-6 to n-3 fatty acids that is considered an imbalance for humans is approximately 10 to 20:1, whereas a ratio of 1 to 4:1 is used as nutritional advice (Scollan et al., 2006).
The thigh meat palmitic acid and stearic acid concentrations reported in the current study were similar to that of Mir et al. (2017a) who found the highest palmitic acid and stearic acid concentrations in the control group when linseed was fed to broiler chickens. Moreover, the thigh meat oleic acid and linoleic acid concentrations were also in line with that of Mir et al. (2017a) who reported the lowest concentration in a control group when flaxseed was fed to chickens. The linolenic acid concentration in thigh meat tended to increase with increased levels of linseed meal, which is consistent with that of Mir et al. (2017a) who reported the same trend when a 10% flaxseed-containing diet was fed to broilers. However, the concentration of linoleic acid in the thigh cuts decreased at a high level of linseed meal feeding (19.5% and 26%), which agreed with the findings of Mridula et al. (2015) who found decreased linoleic acid content in thigh meat when linseed was fed to broilers. This might have happened because linoleic acid and linolenic acid compete for the same enzymatic system, especially delta-6 and delta-5 desaturase enzymes, which usually exhibit a higher affinity for n-3 fatty acids (Jing et al., 2013).
The decrease in the total SFA concentration and increase in MUFA was consistent with that of Mir et al. (2017a). Similarly, Abdulla et al. (2015) observed that the supplementation of the diets with linseed or linseed oil significantly increased the UFA : SFA and the PUFA : SFA ratios. Even though the thigh meat concentration of total poly-UFA and n-6:n-3 in the present study were not affected, Rahimi et al. (2011) reported that the incorporation of flaxseed and canola seed in the diet significantly increased the proportions of the n-3 PUFA in the form of ALA, along with the increase in the PUFA and PUFA : SFA ratio.
Sensory evaluation of meat
In the current study, the response of the panelists for the breast and thigh meat of broilers fed increased levels of linseed meal did not influence the sensory attributes of broiler meat. Similar to the current findings, Živković et al. (2017) concluded that the addition of extruded flaxseed to chicken feed did not lead to major changes in the sensory characteristics of broiler meat. Moreover, Mridula et al. (2015) and Mir et al. (2017b) reported no significant effect of flaxseed on the sensory characteristics of broiler meat. Additionally, Martínez et al. (2010) stated that seed meal with a high content of oleic acid maintained the sensory quality of breast meat since monounsaturated fatty acid has high stability in cell membranes (Levental et al., 2016; Chiroque et al., 2018). In contrast, Anjum et al. (2013) found a significant reduction in the aroma, flavor, taste, and overall acceptability values of nugget meat from broilers fed with increasing levels of dietary flaxseed.
Conclusion
Soybean meal replacement with a graded level of linseed meal in broilers’ rations did not affect blood hematology. The majority of blood serum biochemical indices AST, ALT, ALP, total protein, globulin, and albumin were also not affected by treatments and values fell within the normal ranges. The triglyceride and cholesterol concentrations were reduced more in linseed meal-fed groups than in the control, but glucose concentration increased. The proximate compositions of breast and thigh meat were also not affected by treatments, except that high thigh DM was observed in T1. High breast palmitic acid was recorded in broilers fed with no linseed meal, indicating that linseed meal contributed to decreased palmitic acid. Total breast meat SFA and the ratio of n-6:n-3 decreased with increasing levels of linseed meal. The linolenic acid and the total PUFA of breast meat were increased at 26% total replacement for soybean meal in the ration indicating that linseed meal enriches meat with omega-3. The thigh meat palmitic acid, stearic acid, and total SFA decreased with increased levels of linseed meal, concomitantly making the meat healthy and preferable for the consumers. The sensory values of both breast and thigh meat were not influenced by levels of linseed meal in the ration of broilers. Generally, total linseed meal replacement for soybean meal, up to 26% of the actual compound broilers diet, improved the essential fatty acids of broilers’ meat without significant effects on proximate composition, blood indices, and meat sensory attributes.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the author, without undue reservation.
Ethics statement
The blood collection procedure was approved by Haramaya University Animal Welfare Ethical Committee formed from the School of Animal and Range Sciences and Research Affairs.
Author contributions
The author confirms being the sole contributor of this work and has approved it for publication.
Acknowledgments
The author gives special thanks to the Haramaya University Postgraduate directorate and Italian project ISSD for the supplementary fund to make this research achievable.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abbasi F., Samadi F., Jafari S. M., Ramezanpour S., Shargh M. S. (2019). Ultrasound-assisted preparation of flaxseed oil nanoemulsions coated with alginate-whey protein for targeted delivery of omega-3 fatty acids into the lower sections of gastrointestinal tract to enrich broiler meat. Ultrasonics Sonochem. 50, 208–217. doi: 10.1016/j.ultsonch.2018.09.014
Abdulla N. R., Loh T. C., Akit H., Sazili A. Q., Foo H. L., Mohamad R., et al. (2015). Fatty acid profile, cholesterol and oxidative status in broiler chicken breast muscle fed different dietary oil sources and calcium levels. South Afr. J. Anim. Sci. 45 (2), 153–163. doi: 10.4314/sajas.v45i2.6
Aghwan Z., Alimon A., Goh Y., Nakyinsige K., Sazili A. (2014). Fatty acid profiles of supraspinatus, longissimus lumborum and semitendinosus muscles and serum in kacang goats supplemented with inorganic selenium and iodine. Asian-Australasian J. Anim. Sci. 27, 543–550. doi: 10.5713/ajas.2013.13545
Aguilar Y. M., Yero O. M., Navarro M. I. V., Hurtado C. A. B., López J. A. C., Mejía L. B. G. (2011). Effect of squash seed meal (Cucurbita moschata) on broiler performance, sensory meat quality, and blood lipid profile. Braz. J. Poult. Sci. 13 (4), 219–226. doi: 10.1590/S1516-635X2011000400001
Alagawany M., Shaaban S. E., Farag M. R., El-Hack M. E. A., Khafaga A. A., Taha A. E., et al. (2019). Omega-3 and omega-6 fatty acids in poultry nutrition: Effect on production performance and health. Animals 9, 573. doi: 10.3390/ani9080573
Al-Azzawi Y. G., AL-Fleeh R. N., AL-Shuraby M. M. (2011). Effect of flaxseed meal treated by soaked or boiling in water on some productive and physiological characteristics for two hybrid broiler. J. Tikrit Univ. Agric. Sci. 11, 355–372.
Al-Hilali A. H. (2018). Effect of dietary flaxseed oil on growth performance and serum lipid profiles in broilers. Pakistan J. Nutr. 17, 512–517. doi: 10.3923/pjn.2018.512.517
Al-Nawass K. J. (2015). Effect of different levels of golden flaxseed (Linum usitatissimumL.) powder on some blood biochemical parameters in male and female broiler. Res. Opin. Anim. Veterinary Sci. 5 (11), 425–428.
Anjum F. M., Haider M. F., Khan M. I., Sohaib M., Arshad M. S. (2013). Impact of extruded flaxseed meal supplemented diet on growth performance, oxidative stability and quality of broiler meat and meat products. Lipids Health Dis. 12, 13–24. doi: 10.1186/1476-511X-12-13
AOAC (2000). Official methods of analysis of the association of official analytical chemists (MD, USA: Gaithersburg).
Atyeo K., Cook D. (2014). Nutrient analysis report (Chicken Farmers of Canada). Available at: https://106fyz3cd4vi2lc2uq1tlyl4-wpengine.netdna-ssl.com/wp-content/uploads/2020/09/Nutrient-Analysis-Report-Chicken-Farmers-of-Canada-ENG.pdf
Bird A. R., Croom J. W., Daniel L. R., Black B. L. (1994). Age-related changes in jejunal glucose absorption in mice. Nutr. Res. 14 (3), 411–422. doi: 10.1016/S0271-5317(05)80179-4
Brar R. S., Sandhu H. S., Singh A. (2000). Veterinary clinical diagnosis by laboratory methods (New Delhi: Kalyaril Publishers).
Brians S. B., John A. K., Elkin S., Onno W. A. (2000). Procedure for determining packed cell volume by the microhematocrit method; approved standard, 3rd ed., 20. 18. Available at: https://clsi.org/media/2392/h07a3e_sample.pdf
Café M. B., Rinaldi F. P., Morais H. R., de Mattos Nascimento M. R. B., Mundim A. V., Marchini C. F. P. (2012) Biochemical blood parameters of broilers at different ages under thermoneutral environment. world poultry congress at Salvador bahia-Brazil. Available at: https://www.researchgate.net/publication/283215287.
Campbell T. W. (2012). “Clinical chemistry of birds,” in Veterinary hematology and clinical chemistry, 2nded. Eds. Thrall M. A., Weiser G., Allison R. W., Campbell T. W. (Wiley-Blackwell), 582–598.
Chiroque G., Vásquez G., Vásquez E., Vásquez E., Más D., Betancur C., et al. (2018). Growth performance, carcass traits and breast meat fatty acids profile of helmeted Guinea fowls (Numida meleagris) fed increasing level of linseed (Linum usitatissimum) and pumpkin seed (Cucurbita moschata) meals. Braz. J. Poult. Sci. 20 (4), 665–674. doi: 10.1590/1806-9061-2018-0760
Crespo N., Esteve-Garcia E. (2003). Polyunsaturated fatty acids reduce insulin and very low density lipoprotein levels in broiler chickens. Poult. Sci. 82, 1134–1139. doi: 10.1093/ps/82.7.1134
FAO (1998). Carbohydrates in human nutrition (Rome). Report of a Joint FAO/WHO Expert Consultation. 66, 1–140.
George J. W. (2001). The usefulness and limitations of hand-held refractometers in veterinary laboratory medicine: An historical and technical review. Veterinary Clin. Pathol. 30, 201–210. doi: 10.1111/j.1939-165X.2001.tb00432.x
Harris W. S. (1989). Fish oils and plasma lipid and lipoprotein metabolism in humans: A critical review. J. Lipid Res. 30, 785–807. doi: 10.1016/S0022-2275(20)38310-3
Haug A., Eich-Greatorex S., Bernhoft A., Wold J. P., Hetland H., Christophersen O. A., et al. (2007). Effect of dietary selenium and omega-3 fatty acids on muscle composition and quality in broilers. Lipids Health Dis. 6 (1), 1–9. doi: 10.1186/1476-511X-6-29
Hochleithner M., Hochleithner C., Harrison L. D. (2005). Clinical avian medicine Vol. 2005 (Florida: Spix Publishing), 441–450.
Honda K. L., Lamon-Fava S., Matthan N. R., Wu D., Lichtenstein A. H. (2015). EPA And DHA exposure alters the inflammatory response but not the surface expression of toll-like receptor 4 in macrophages. Lipids 50, 121–129. doi: 10.1007/s11745-014-3971-y
Irizaary-Rovira A. R. (2004). “Avian and reptilian clinical pathology (Avian hematology & biochemical analysis). section XI,” in Veterinary clinical pathology secrets. Ed. Cowell R. L. (St. Louis, MO, USA: Elsevier Inc), 282–313.
Jankowski J., Zdunczyk Z., Mikulski D., Juskiewicz J., Naczmanski J., Pomianowski J. F. (2012). Fatty acid profile, oxidative stability, and sensory properties of breast meat from turkeys fed diets with a different n-6/n-3 PUFA ratio. Eur. J. Lipid Sci. Technol. 114, 1025–1035. doi: 10.1002/ejlt.201200003
Jing M., Gakhar N., Gibson R. A., House J. D. (2013). Dietary and ontogenic regulation of fatty acid desaturase and elongase expression in broiler chickens. Prostaglandins Leukotrienes Essential Fatty Acids 89, 107–113. doi: 10.1016/j.plefa.2013.05.006
Kassa S., Mengistu U., Getachew A. (2016). Effect of different levels of Lepidium sativum l. @ on growth performance, carcass characteristics, hematology and serum biochemical parameters of broilers. Springer plus 5 (1), 1441.
Keeton J. T. (1983). Effect of fat, nacl and phosphate levels on the chemical and sensory properties of pork patties. J. Food Sci. 48, 878–881. doi: 10.1111/j.1365-2621.1983.tb14921.x
Khan T. A., Zafar F. (2005). Haematological study in response to varying doses of estrogen in broiler chicken. Int. J. Poult. Sci. 10, 748–751.
Kishawy A. T. Y., Amer S. A., Abd El-Hack M. E., Saadeldin I. M., Swelum A. A. (2019). The impact of dietary linseed oil and pomegranate peel extract on broiler growth, carcass traits, serum lipid profile, and meat fatty acid, phenol, and flavonoid contents. Asian-Australasian J. Anim. Sci. 32 (8), 1161–1171. doi: 10.5713/ajas.18.0522
Kostadinovic L., Popović S., Colovic D., Vukmirović Đ., Tasić T., Puvačan N., et al. (2016). Effect of extruded flaxseed in broiler diets on blood oxidative stability and meat fatty acid composition. Eur. poult. Sci. 80, 1–14. doi: 10.1399/eps.2016.140
Kouba M., Benatmane F., Blochet J. E., Mourot J. (2008). Effect of a linseed diet on lipid oxidation, fatty acid composition of muscle, perirenal fat, and raw and cooked rabbit meat. Meat Sci. 80, 829–834. doi: 10.1016/j.meatsci.2008.03.029
Lawrie R., Ledward D. (2006). Lawrie's meat science. Seventh English, edition (Cambridge England: Woodhead Publishing Limited).
Leskovec J., Levart A., Nemec Svete A., Peric L., Dukic Stojcic M., Zikic D. (2018). Effects of supplementation with α-tocopherol, ascorbic acid, selenium, or their combination in linseed oilenriched diets on the oxidative status in broilers. Poult. Sci. 97, 1641–1650. doi: 10.3382/ps/pey004
Levental K. R., Lorent J. H., Lin X., Skinkle A. D., Surma M. A., Stockenbojer E. A., et al. (2016). Polyunsaturated lipids regulate membrane domain stability by tuning membrane order. Biophys. J. 110 (8), 1800–1810. doi: 10.1016/j.bpj.2016.03.012
Lopez-Ferrer S., Baucells M. D., Barroeta A. C., Grashorn M. A. (2001). N-3 enrichment of chicken meat. 1. use of very long-chain fatty acids in chicken diets and their influences on meat quality: Fish oil. Poult. Sci. 80, 741–752. doi: 10.1093/ps/80.6.741
Maddock T. D., Anderson V. L., Berg P. T., Maddock R. J., Marchello M. J. (2003). “Influence of level of flaxseed addition and time fed flaxseed on carcass characteristics, sensory panel evaluation and fatty acid content of fresh beef,” in Proc. 56th Reciprocal Meats Conference, Am. Meat Sci. Assoc, Columbia, Mo.
Maidala A., Doma U. D., Egbo L. M. (2014). “Heamatological and serum biochemical indices of broiler chickens fed differently processed African locust bean seeds (Parkia biglobosa),” in Nigerian Society for animal production (Ilishan-Remo, Ogun State, Nigeria: Babcock University).
Mandal G. P., Ghosh T. K., Patra A. K. (2014). Effect of different dietary n-6 to n-3 fatty acid ratios on the performance and fatty acid composition in muscles of broiler chickens. Asian Australas. J. Anim. Sci. 27 (11), 1608–1614. doi: 10.5713/ajas.2014.14013
Marangoni F., Corsello G., Cricelli C., Ferrara N., Ghiselli A., Lucchin L., et al. (2015). Role of poultry meat in a balanced diet aimed at maintaining health and wellbeing: An Italian consensus document. Food Nutr. Res. 59 (1), 1–11. doi: 10.3402/fnr.v59.27606
Martínez Y., Valdivié M., Martínez O., Estarrón M., Córdova J. (2010). Utilization of pumpkin (Cucurbita moschata) seed in broiler chicken diets. Cuban J. Agric. Sci. 44 (4), 387–392.
Matusiewicz M., Kosieradzka I., Zuk M., Szopa J. (2015). Effect of dose and administration period of seed cake of genetically modified and non-modified flax on selected antioxidative activities in rats. Int. J. Mol. Sci. 16, 14259–14275. doi: 10.3390/ijms160614259
Merrill A. L., Watt B. K. (1973). Energy value of foods: Basis and derivation (Washington DC: ARS United States Department of Agriculture).
Mir N. A., Praveen K. T., Biswas A. K., Pramod K. T., Mandal A. B., Kumar F., et al. (2017b). Effect of feeding broken rice and distillers dried grains with solubles in a flaxseed-based diet on the growth performance, production efficiency, carcass characteristics, sensory evaluation of meat, and serum biochemistry of broiler chickens. Turkish J. Veterinary Anim. Sci. 41 (5), 583–589. doi: 10.3906/vet-1701-51
Mir N. A., Tyagi P. K., Biswas A. K., Tyagi P. K., Mandal A. B., Sheikh S. A., et al. (2017a). Impact of feeding chromium supplemented flaxseed based diet on fatty acid profile, oxidative stability and other functional properties of broiler chicken meat. J. Food Sci. Technol. 54 (12), 3899–3907. doi: 10.1007/s13197-017-2846-7
Mir N. A., Tyagi P. K., Biswas A. K., Tyagi P. K., Mandal A. B., Wani M. A., et al. (2018). Performance and meat quality of broiler chicken fed a ration containing flaxseed meal and higher dietary lysine levels. J. Agric. Sci. 156, 291–299. doi: 10.1017/S0021859618000242
Motaghi S., Laji A. A., Sepehri G., Salehi M., Khodadadi H. (2017). The effect of fosbac on liver and kidney function parameters in broilers. J. Coast. Life Med. 5 (11), 492–495. doi: 10.12980/jclm.5.2017J7-138
Mridula D., Daljeet K., Nagra S. S., Barnwal P., Sushma G., Singh K. K. (2011). Growth performance, carcass traits and meat quality in broilers fed flaxseed meal. Asian-Australasian J. Anim. Sci. 24 (12), 1729–1735. doi: 10.5713/ajas.2011.11141
Mridula D., Kaurb D., Nagrab S. S., Barnwala P., Sushma G., Singh K. K. (2015). Growth performance and quality characteristics of flaxseed-fed broiler chicks. J. Appl. Anim. Res. 43 (3), 345–351. doi: 10.1080/09712119.2014.978773
Negasa T., Mengistu U., Meseret G., Ajebu N. (2020) Effect of replacing soybean meal with linseed meal on production and quality of eggs from white leghorn hens. livestock research for rural development. Available at: http://www.lrrd.cipav.org.co/lrrd32/6/cont3206.html.
Nuernberg K., Fischer K., Nuernberg G., Kuechenmeister U., Klosowska D., Eliminowska-Wenda G. (2005). Effects of dietaryolive and linseed oil on lipid composition, meat quality, sensorycharacteristics and muscle structure in pigs. Meat Sci. 70, 63–74. doi: 10.1016/j.meatsci.2004.12.001
Omoni A. O., Aluko R. E. (2006). Mechanism of the inhibition of calmodulin-dependent neuronal nitric oxide synthase by flaxseed protein hydrolysates. J. Am. Oil Chemists’ Soc. 83, 335–340. doi: 10.1007/s11746-006-1209-8
Osek M., Górska A., Milczarek A., Świnarska R. (2008). Effect of soybean and linseed oil contents in mixtures for broiler chickens on the contents of triglycerides and cholesterol in serum and on meat quality. Poult. Production 61, 307–312.
Palmquist D. L. (2009). Omega-3 fatty acids in metabolism, health, and nutrition and for modified animal product foods. Prof. AnimalScientist 25, 207–249. doi: 10.15232/S1080-7446(15)30713-0
Pavlovic J., Greenland P., Deckers J. W., Kavousi M., Hofman A., Ikram M. A. (2018). Assessing gaps in cholesterol treatment guidelines for primary prevention of cardiovascular disease based on available randomised clinical trial evidence: The rotterdam study. Eur. J. Prev. Cardiol. 25 (4), 420–431. doi: 10.1177/2047487317743352
Peiretti P. G., Meineri G. (2010). Effects of diets with increasing levels of goldenflaxseed on carcass characteristics, meat qualityand lipid traits of growing rabbits. Ital. J. Anim. Sci. 9 (4), 373–377.
Rahimi S. S., Azad K., Torshizi M. A. K. (2011). Omega-3 enrichment of broiler meat by using two oil seeds. J. Agric. Sci. Technol. 13, 353–365.
Russo R., Reggiani R. (2016). Evaluation of protein and anti-nutritional compounds content in meal from seven flax varieties. J. Global Agric. Ecol. 6 (3), 182–188.
Salami S. A., Majokaa M. A., Saha S., Garbera A., Gabarroua J. F. (2015). Efficacy of dietary antioxidants on broiler oxidative stress, performance and meat quality: Science and market. Avian Biol. Res. 8, 65–78. doi: 10.3184/175815515X14291701859483
Samuel S. (2008). The epidemiology and management options of chocolate spot disease (Botrytis fabae sard) on faba bean (Vicia faba l.) in northern Ethiopia Vol. pp (Ethiopia: Haramaya University), 175. Ph.D Dissertation.
SAS (Statistical Software System) (2009). “SAS User’s Guide, Statistics.,” (Cary, NC. USA: SAS Institute, Inc.).
Scheideler S. E., Cuppett S., Froning G. (1994). “Dietary flaxseed for poultry: Production effects, dietary vitamin levels, fatty acid incorporation into eggs and sensory analysis,” in Proc. 55th Flax Institute, Fargo, N.D, Jan. 26-28. 86–95.
Scollan N., Hocquette J. F., Nuernberg K., Dannenberger D., Richardson I., Moloney A. (2006). Innovations in beef production systems that enhance the nutritional and health value ofbeeflipids and their relationship with meat quality. Meat Sci. 74, 17–33. doi: 10.1016/j.meatsci.2006.05.002
Shunthwal J., Sheoran N. (2017). Influence of linseed oil feeding on performance and fatty acid composition of muscles in broiler chicks. Pharma Innovation J. 6 (11), 268–273.
Shunthwal J., Sheoran N., Promila, Vinus, Sihag S. (2017a). Effect of linseed oil supplementation on hematological parameters and economics of feeding in broiler chicks. Int. J. Pure Appl. Bioscience 5 (5), 1258–1265. doi: 10.18782/2320-7051.5943
Shunthwal J., Sihag S., Promila, Sheoran N. (2017b). Effect of feeding linseed oil on growth performance and nutrients utilization efficiency in broiler chicks. Pharma Innovation J. 6 (11), 120–124.
Tamasgen N., Urge M., Girma M., Nurfeta A. (2021). Effect of dietary replacement of soybean meal with linseed meal on feed intake, growth performance and carcass quality of broilers. Heliyon 7(2021), e08297. doi: 10.1016/j.heliyon.2021.e08297
Thrall M. A. (2007). Hematology and clinical veterinary biochemistry (Sao Paulo: Philadelphia, Lippincott, Williams & Wilkins), 582p. Roca.
Tijani L. A., Akanji A. M., Agbalaya K., Onigemo M. (2015). Haematological and serum biochemical profiles of broiler chickens fed diets containing moringa leaf meals. J. Trop. Agricult. Food Environ. Extension 14:3, 7–11.
USDA (2006) National nutrient database for standard reference, release 19. Available at: http://www.nalusde.gov/fnic/foodcomp/cgi-bin/list_nut_edit.pl.
Van Den Elsen L., Garssen J., Willemsen L. (2012). Long chain n-3 polyunsaturated fatty acids in the prevention of allergic and cardiovascular disease. Curr. Pharm. Design 18, 2375–2392. doi: 10.2174/138161212800165960
Wang Y., Sunwoo H., Cherian G., Sim. J. S. (2000). Fatty acid determination in chicken egg yolk: A comparison of different methods. Poult. Sci. 79, 1168–1171. doi: 10.1093/ps/79.8.1168
Yassein S. A., El-Mallah G. M., Ahmed S. M., El-Ghamry A. A., Abdel-Fattah M. M., El-Hariry D. M. (2015). Response of laying hens to dietary flaxseed levels on performance, egg quality criteria, fatty acid composition of egg and some blood parameters. Int. J. Res. Stud. Biosci. 3, 27–34.
Zhaleh S., Golian A., Zerehdaran S. (2019). Effect of rolled or extruded flaxseeds in finisher diet on pellet quality, performance, and n-3 fatty acids in breast and thigh muscles of broiler chickens. Poult. Sci. J. 7 (1), 63–75.
Keywords: broiler, chemical composition, fatty acids, linseed meal, sensory characteristic
Citation: Tamasgen N (2022) Effects of replacing soybean meal with linseed meal in broiler diet on selected broilers’ blood parameters, meat chemical composition, fatty acid profiles, and sensory characteristics. Front. Anim. Sci. 3:945685. doi: 10.3389/fanim.2022.945685
Received: 16 May 2022; Accepted: 09 September 2022;
Published: 29 September 2022.
Edited by:
Ahmet Yavuz Pekel, Istanbul University Cerrahpasa, TurkeyReviewed by:
Veronica Lolli, University of Parma, ItalyP Al Vlaicu, National Research Development Institute for Animal Biology and Nutrition, Romania
Copyright © 2022 Tamasgen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Negasa Tamasgen, c2VlbmFhc2hlZUBnbWFpbC5jb20=
 Negasa Tamasgen
Negasa Tamasgen