- Department of Agricultural & Biosystems Engineering, Iowa State University, Ames, IA, United States
This article reviews the different techniques used to monitor the respiration and sounds of livestock. Livestock respiration is commonly assessed visually by observing abdomen fluctuation; however, the traditional methods are time consuming, subjective, being therefore impractical for large-scale operations and must rely on automation. Contact and non-contact technologies are used to automatically monitor respiration rate; contact technologies (e.g., accelerometers, pressure sensors, and thermistors) utilize sensors that are physically mounted on livestock while non-contact technologies (e.g., computer vision, thermography, and sound analysis) enable a non-invasive method of monitoring respiration. This work summarizes the advantages and disadvantages of contact and non-contact technologies and discusses the emerging role of non-contact sensors in automating monitoring for large-scale farming operations. This work is the first in-depth examination of automated monitoring technologies for livestock respiratory diseases; the findings and recommendations are important for livestock researchers and practitioners who can gain a better understanding of these different technologies, especially emerging non-contact sensing.
1 Introduction
This article surveys the techniques for monitoring the respiration and sounds of livestock. Respiratory diseases are multifactorial diseases driven by complex interaction of factors associated with the environment, the pathogen, the animal, and management practices, and cause significant production and economic losses in the cattle and pork industry (Edwards, 2010; Potter and Aldridge, 2010; Peel 2020; Smith et al., 2020). Some of the impacts of respiratory diseases in livestock include mortality, reduced weight gain, decline in productivity, decreased pregnancy percentage, increase in treatment and vaccination costs etc. (Peel, 2020). Modern technologies such as sensors provide an opportunity to constantly monitor livestock health (Neethirajan, 2020). Constant monitoring of livestock assists in early detection of diseases, which in turn can help improve animal welfare, prevent further spread of disease and enhance farm revenue (Nasirahmadi et al., 2017).
Livestock production and economic losses can be minimized by early detection of respiratory health issues. Early detection (or early-warning surveillance) is defined as the “surveillance of health indicators and diseases in defined populations in order to increase the likelihood of timely detection of undefined (new) or unexpected (exotic or re-emerging) threats” (Hoinville et al., 2013). Respiratory diseases are responsible for 23.9% of cattle deaths in production operations across the United States (USDA, 2017). Swine respiratory diseases were reported as the leading cause of mortality in nursery (47.3%) and grower finisher units (75.1%) (National Animal Health Monitoring system,1995). Morbidity rates associated with respiratory diseases in pigs range from 30-70% (Opriessnig et al., 2011). Respiratory diseases accounted for nearly 5.6% of all diseases in small ruminants including sheep and goats (Hindson and Winter, 2002). Most respiratory diseases are contagious and can lead to outbreak resulting in severe economic losses for producers (Christensen and Mousing, 1992). Financial losses associated with respiratory diseases are due to increased mortality, decreased weight gain, increased feed costs, increased condemnation at slaughter, and increased costs for treatments, vaccination, and labor. The total economic value of death loss in cattle and calves due to respiratory diseases was estimated at $907.8M (Peel, 2020). Annual production losses incurred by the US pork industry due to respiratory diseases were estimated at $560M (Neumann et al., 2005); a more recent study estimated the combined economic losses due to respiratory diseases at around $663.91M per year or around US $1.8 million per day (Holtkamp et al., 2013). Respiratory diseases reduce reproductive efficiency by 1.44 pigs weaned per breeding female per year; here reduced reproduction results in annual production loss of US $302.06M or around $52.19 per head of breeding female. This production loss results in 9.93 million fewer pigs per year and 1.09 billion fewer kilograms of pork marketed per year in the United States. On a per-pig basis, respiratory diseases cost the pork industry $4.67 for every pig marketed in the United States (Holtkamp et al., 2013). Moreover, death loss comes with hidden costs that are often not reported; these hidden costs include wastage of resources such as forages, grain, supplements, water, and fossil fuel (Smith et al., 2020).
Producers have traditionally relied on a method of direct observation at regular intervals to monitor animal health. Respiration rate is estimated by visual observation of fluctuation of the abdomen of the animal (Milan et al., 2016; Lowe et al., 2019; Wu et al., 2020). However, with increasing herd size, the attention received by individual animals is decreasing (Stewart et al., 2017). This reduction in available time and resources can lead producers to underestimate the signs of respiratory diseases (White and Renter, 2009; Potter and Aldridge, 2010) and often it is too late to intervene once the symptoms become evident.
Once the symptoms of disease become evident, the producers either take no action, seek help from veterinary professionals, use antibiotics or adopt a combination of these three approaches to cure their livestock (Neethirajan, 2020). Getting to this point though involves understanding the physiological issues and novel sensing technology approaches have been investigated to estimate respiratory rates or detect behavioral changes linked to respiratory diseases to identify sick livestock at an early stage (Silva et al., 2008; Weixing and Zhilei, 2010; Mutlu et al., 2018; Al-Naji et al., 2019; Barbosa and Pereira, 2019; Lowe et al., 2019; Wu et al., 2020). Respiratory rate detection technology can be broadly classified into either contact or non-contact technologies.
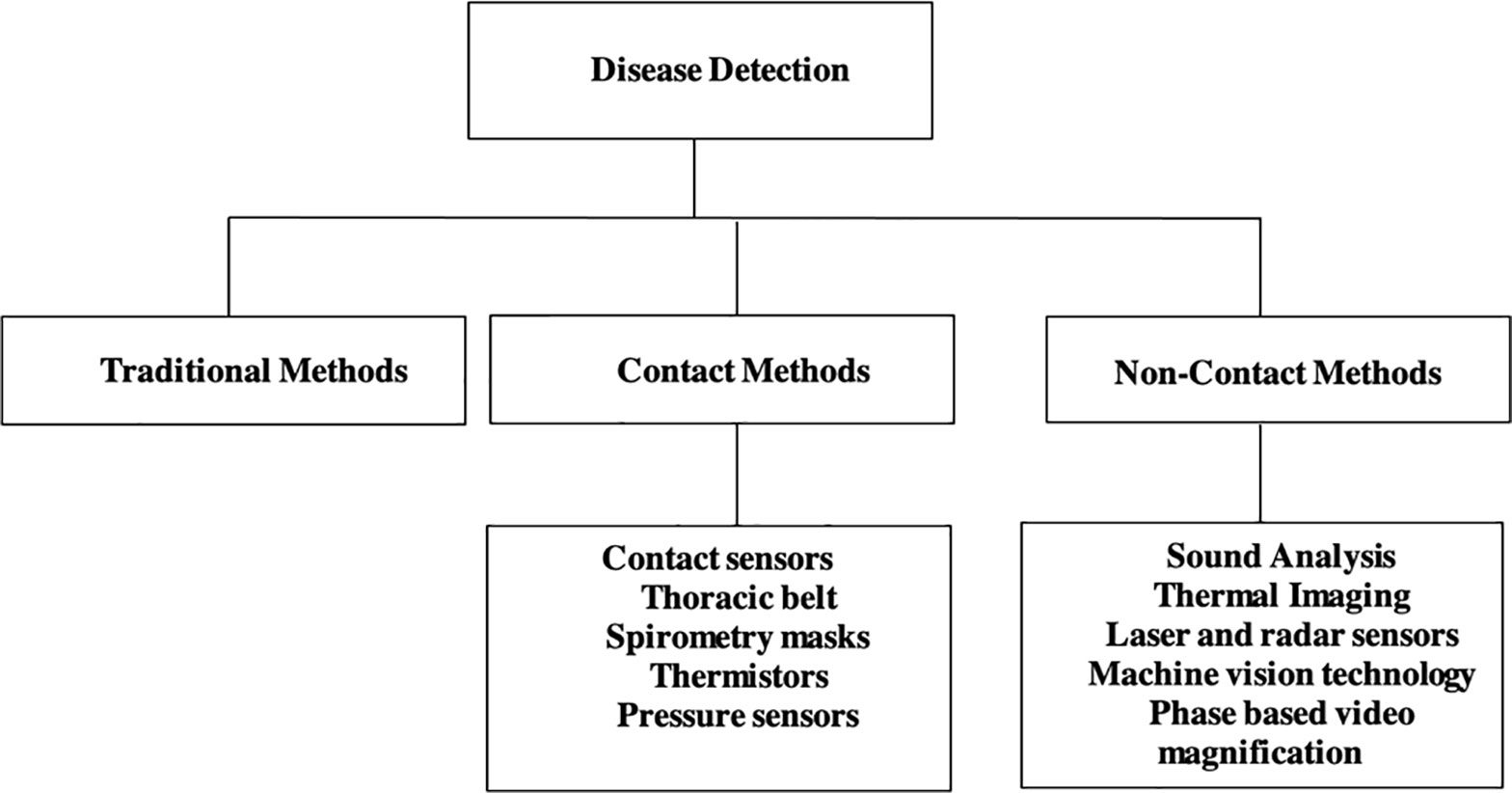
The contact methods of respiration estimation involve the use of sensors that need to be mounted or implanted in the livestock to monitor respiration rate. Domain specific examples of contact sensor technology include thoracic belts (Egenberg et al., 2000), spirometry masks (Maia et al., 2014), temperature sensors (Milan et al., 2016), and pressure sensors (Strutzke et al., 2019). Demand for automated non-invasive methods of disease detection in livestock has increased because it provides a remote and non-invasive method of respiration monitoring in livestock. Unlike contact sensors, the non-contact methods of monitoring cause no influence on livestocks’ behavior and do not induce stress. In this article, the methods of non-contact technology reviewed and summarized are: (i) sound analysis, (ii) thermal imaging, (iii) laser and radar sensors, and (iv) machine vision technology.
The rest of the article is organized as follows. Section 2 describes traditional methods of respiration rate estimation. Sections 3 and 4 describe contact and non-contact methods of respiration estimation and their advantages and limitations. Section 5 concludes this article.
2 Methods of Livestock Respiration Rate Estimation
This section describes the methods used for livestock respiration rate estimation. The methods reviewed include traditional methods, contact, and non-contact. Figure 1 summarizes the novel technologies employed under contact and non-contact of respiratory disease detection in livestock, which are described in detail in section 3 and 4.
2.1 Traditional Methods
Producers on-farm keep track of livestock respiration rates as determined by visual observation of abdominal fluctuation exhibited by an animal (Milan et al., 2016; Lowe et al., 2019; Wu et al., 2020). To visually observe respiration rate, it is recommended to start on an exhale and use the stopwatch to record the time it takes to count 10 full breaths. One full breath is defined as one inhale and one exhale. The livestock is exhaling when the flank looks sunken in and inhaling when the flank looks full of air. Once the time required to take 10 full breaths is recorded, the following equation is used to calculate breaths per minute, B (Becker et al., 2020):
where t10 is the number of seconds it took to take 10 breaths. These first-person observation methods of estimating respiration rate can be subjective and time sensitive, making them impractical for large scale production operations. The flank counting method, for example, can lead to erroneous results if the flank movements are caused by activities other than respiration, such as from stretching, swatting flies, etc. (Egenberg et al., 2000). Further, the flank counting method can be affected by climate; rapid and heavy breathing might be observed during summers, with slower and less noticeable breathing in colder weather making the observations difficult to capture with consistency (Stewart et al., 2017).
2.2 Contact Methods
Advancement in sensor technology has assisted in the replacement of traditional methods of animal monitoring in commercial farms. A sensor can be defined as a device which measures or detects a biological, chemical, physical, or mechanical property or a combination of these properties, records and collects the data for interpretation by a human or a machine (Neethirajan, 2020). The sensors can automatically monitor animals in real time for any changes in behavior (e.g., aggression, increased resting), detection of symptoms of disease (e.g., increased respiration rate, cough), identifying sick animals from the herd etc. Using sensors allows producers to improve animal welfare, prevent disease outbreak, reduce labor requirements, and improve farm returns.
Various methods have been developed to automatically monitor the respiration rate of livestock to attempt to provide the producers with consistent data with minimum labor requirements and least interference to the animal activities (Egenberg et al., 2000). For instance, Egenberg et al. (2000) developed a system consisting of a transducer with a thoracic belt that measured changes in thoracic and abdomen movement of the cattle. The transducer provided an electrical signal in response to pulmonary effort. The belt was used to hold the sensor in place and was designed to be unaffected by precipitation, manure, and urine. The disadvantages of using thoracic belt include frequent slipping of the belt, chewing of the belt by other cattle, behavioral changes etc. This can lead to sensor damage and inaccurate respiration rate estimation (Milan et al., 2016; Strutzke et al., 2019). Additionally, this system was found to be unsuitable for measuring respiration rate in pigs. The increased resting time and decreased definitive respiratory movement of pigs as compared to cattle made it unsuitable for use in pigs (Eigenberg et al., 2002). To overcome these challenges, the researchers developed a respiratory rate monitor that utilized auditory component of the pig’s respiration. The sensor consisted of a speaker attached to the pig’s throat using a bandaging tape, wrapped with an electric wrap. The sensor responded to air movement through larynx. Signal processing was then performed to input meaningful signal to the data logger. Although, the monitor developed performed well, still it required critical mounting of sensor. The use of bandaging tape may not be suitable for adoption in commercial swine farms. In 2018, Atkins et al. developed a continuous respiration rate sensor similar to the one designed by Eigenberg et al. (2000). The sensor was designed using a force sensitive resistor to measure respiration rate of heat stressed dairy cows. Elastic straps were used to attach the sensor around the cow’s abdomen. The sensor measured respiration by detecting changes in the pressure on inhalation and exhalation. The researchers developed an algorithm to automatically filter segments of unreliable signal. Loosening of the harness and damaging of the sensors resulted in weak or non-uniform signals. The researchers observed an increase in respiration rate and body temperature while lying and decrease in respiration rate and body temperature while standing. The increase in body temperature and respiration rate while lying was caused due to decrease in amount of cow’s surface area exposed to ambient air.
Maia et al. (2014) utilized spirometry mask to determine the respiration rate of livestock. A spirometry mask is a facial mask designed to measure the gaseous exchange of oxygen and carbon dioxide. The masks were shaped to best suit the geometry of the livestock’s face, for example, the researchers designed a cylindrical, triangular, and ellipsoidal shaped mask for sheep, goats, and cattle respectively. Using spirometry masks requires training and restraining livestock. Additionally, masks must be fitted individually on all livestock, which is not suitable in commercial scale farming (Milan et al., 2016; Lowe et al., 2019). In a study conducted by Milan et al. (2016), the authors developed a device that employed temperature sensors to monitor thermal fluctuations caused due to respiration near the nostril of the animal. This device enabled the researchers to continuously monitor the respiration rate of the cattle in their natural habitat without any need to train or restrain them. The cattle showed no sign of discomfort during mounting and wearing of the device. However, the drawback of this device was reduced accuracy of the sensor in case of similar ambient and body temperature. Strutzke et al. (2019) developed a device consisting of differential pressure sensor, a micro controller and software to measure the difference in the strength of exhaled and inhaled pressure to determine the respiration rate in cattle. One port of the pressure sensor was inserted 10 cm into the nasal cavity of the cattle, the other port was left open and exposed to the ambient pressure. The researchers reported a drop in incoming pressure at the sensor at the beginning of inhalation and an increase in pressure at the beginning of exhalation. The limitations of the differential pressure sensor include limited battery life, and difficulty in mounting the sensor on the cattle. Apart from using thoracic belts, thermistors, pressure sensors, researchers have also employed telemetry sensors to estimate respiratory rate in rats. Telemetry sensors are based on changes in cardiovascular data resulting from respiratory effort. These sensors are implanted via surgery which can lead to stress and reduced mobility in animals (Eigenberg et al., 2000; Milan et al., 2016).
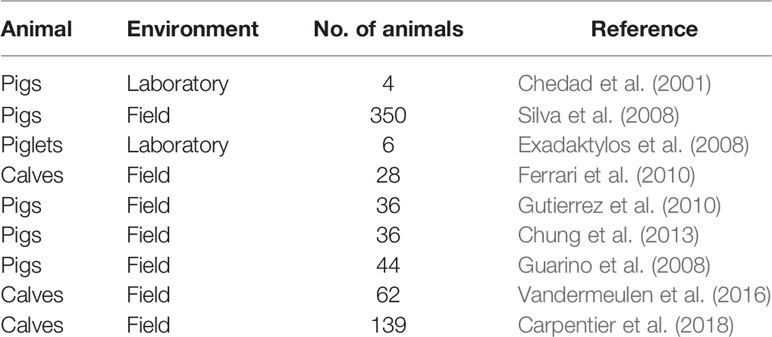
The contact sensor technology has been successfully applied for respiration rate estimation in animals. Table 1 summarizes few studies on respiration rate detection using contact sensors. Some of the disadvantages of using contact sensor technology are: dislodging of the sensor, difficulty of mounting the sensor, requirement of restraining the animals, discomfort of the animal wearing the sensor, changes in animal behavior, vulnerability to environmental and weather conditions, need for frequent changing of batteries etc.
2.3 Non-Contact Methods
2.3.1 Sound Analysis
Rapid and effective diagnosis require early detection of symptoms of the disease in animals. Respiratory diseases in animals are associated with clinical signs of fever, nasal discharge and dyspnea. Coughing is recognized as the principle clinical symptom of respiratory diseases in animals (Opriessnig et al., 2011; Berckmans, 2014; Carpentier et al., 2018). It is body’s defense mechanism against respiratory infections and is presented by sudden expulsion of air from the airways which is characterized by a typical sound (Gutierrez et al., 2010). Previous studies have analyzed the cough sounds characterized by acoustic features like amplitude, frequency, and duration to monitor and detect respiratory diseases in animals (Table 2). The cough sound analysis technology makes use of microphones to collect/record the data. The microphones are non-contact, affordable and do not influence animal behavior. Microphones convert changes in sound pressure into electrical signals, which are then captured by specific audio equipment and processed as digital signals in standard computers (Matthews et al., 2016). The detection and recognition of cough sounds for identification of respiratory diseases has been performed successfully under both laboratory and field conditions (Chedad et al., 2001; Guarino et al., 2008; Silva et al., 2008; Vandermeulen et al., 2016). Classification and detection of cough sounds from sick animals under laboratory conditions offers better control over the quality of the sound recorded as the sound is recorded individually from each animal. In field or real-life commercial farm conditions the presence of ambient noise interferes with the recorded sound (Guarino et al., 2008). Additionally, the sounds are recorded from a group of animals and not individually, which leads to decreased clarity and increased overlapping of the sounds (Carpentier et al., 2018). Ferrari et al. (2010) demonstrated that it is possible to distinguish cough sounds from other sounds common in stables like metal rack noise etc. The researchers reported a significant difference in the duration, amplitude and fundamental frequency of both the sounds. Further studies have also developed online cough monitors (Exadaktylos et al., 2008; Guarino et al., 2008). The online cough monitors utilize algorithms for real time continuous monitoring of cough sounds. The real time monitoring aids decision making process, and helps in localization of sick livestock from healthy (Exadaktylos et al., 2008). Gutierrez et al. (2010) classified pig wasting diseases based on differences in acoustic features of the cough. Chung et al. (2013) extended existing classification of pig wasting diseases further using widely used feature of sound analysis, Mel Frequency Cepstrum Coefficient (MFCC) in combination with Support Vector Data Description (SVDD) and Sparse Representation Classifier (SRC) to detect and classify pig wasting diseases. The authors reported a 94% accuracy in detection and 91% accuracy in classification of the wasting diseases. Carpentier et al. (2018) proposed an algorithm to detect coughing events in calves under field conditions. The authors developed a novel labelling technique which took the quality of the reference data into account. The coughs were given a label between number 1 and 5, where 1 indicated very unclear cough and 5 indicated very clear cough. The coughs with label greater than or equal to 3 were considered as coughs the algorithm should be able to detect. This approach helped the researchers to eliminate the need for calibrated reference labels. The algorithm was robust and could easily be adapted into different calf compartments under different conditions.
Cough sound analysis has become an important tool to interpret the behavior, health condition and well-being of the animals (Gutierrez et al., 2010). It offers a remote, non-invasive and economic method to detect and monitor respiratory diseases in animals before they become too severe. However, some of the limitations of the cough sound analysis are: (i) most of the algorithms developed for cough identification rely on a reference dataset. The reference dataset requires manual observation and annotation of the recorded sounds. This can be time consuming and subjective to the observer’s experience (Carpentier et al., 2018). (ii) The quality of the sound recorded is influenced by environmental conditions, number of animals and the distance of the microphone from the animal.
2.3.2 Thermal Imaging
Similar to sound analysis, thermal imaging or Infrared thermography (IRT) has been used as a non-invasive, safe, and remote method to monitor respiration in animals. IRT uses thermal cameras to measure the infrared radiation emitted by the surface of the object, which is then converted into electrical signals and a map illustrating the temperature distribution is generated (Incropera, 2007). Studies have shown that IRT technology can detect thermal biometric changes in animals. These thermal biometric changes could be caused due to changes in blood flow as a response to changes in environmental or physiological conditions (McManus et al., 2016).
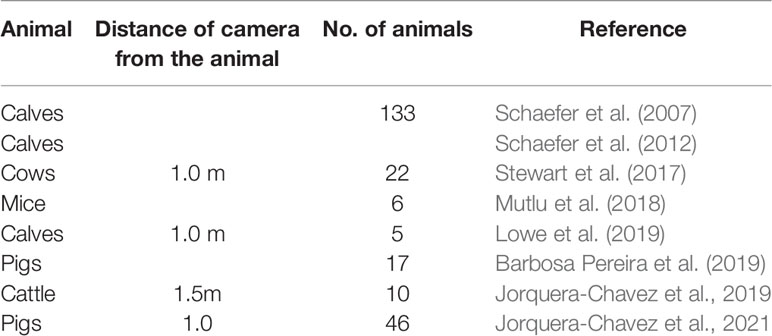
At the onset of respiratory diseases animals radiate heat to maintain a normal core body temperature which can be measured using IRT (Stewart et al., 2017). Schaefer et al. (2007) demonstrated that IRT technology could diagnose bovine respiratory disease at an earlier stage as compared to conventional methods. The authors also used IRT as a tool to detect respiratory diseases in calves (Schaefer et al., 2012). The orbital area (eye plus one centimeter surrounding the eyes) of the calves was monitored for observing radiated temperature changes. The data was collected automatically by mounting the infrared camera on a motor capable of rotating in two directions. This system was installed at the water station and IRT images were captured every time the animal visited water trough to drink water. The results indicated a significant increase in eye temperature with the onset of respiratory disease. An efficiency of 93% in detecting bovine respiratory disease onset was reported. Stewart et al. (2017), used continuous IRT imaging of nostrils of cows to detect thermal changes caused due to breathing. During breathing cycle, inhalation brings in external air and cools the nostrils, whereas exhalation expels air from the body and warms the nostrils. The authors recorded the time taken to complete 10 breaths and converted it into breaths per min (respiration rate). The results reported a high correlation between respiration rate measured using IRT and respiration rate determined by observing flank movements in both real time and video recordings. Similar methodology was adopted by Mutlu et al. (2018) and Lowe et al. (2019) to measure respiration rate in mice and calves respectively. In a study conducted by Barbosa Pereira et al. (2019), the authors developed an algorithm that could accurately estimate respiration rate of anesthetized pigs. Jorquera-Chavez et al. (2019, 2021) employed IRT to calculate respiration rate of cattle and pigs. The algorithm developed calculated respiration rate based on changes in pixel intensity values in the selected region of interest. In case of cattle, nose was selected as region of interest, and eye and ear were selected as region of interest for pigs. The results reported that in comparison to visual observation, the respiration rate of cattle calculated by IRT was underestimated. In the study involving pigs, significantly higher temperatures were observed in pigs infected with respiratory diseases as compared to healthy pigs.
IRT has been used to detect respiratory diseases in animals (Table 3). It provides a non-invasive method to estimate the respiration rate. However, IRT technology is expensive, and is impacted by sunlight, wind, humidity and dirt (McManus et al., 2016; Barbosa Pereira et al., 2019). The distance between the animal and thermal camera also affects the accuracy as temperature distribution can only be measured accurately within several meters (Minkina and Chudzik, 2004). IRT technology detects changes in temperature of the region of interest example nose, mouth etc. Thermal images can only be captured if the animal is facing towards the camera. This can be a limitation and may require the animals to be sedated or restrained before performing IRT (Mutlu et al., 2018; Barbosa Pereira et al., 2019). This is not feasible in large scale commercial farms.
2.3.3 Laser and Radar Sensors
Laser and Radar sensors have been utilized to remotely measure respiration rate in livestock animals. Pastell et al. (2007) used a laser distance sensor to measure respiration rate of dairy cows. The respiration rate was monitored by measuring the flank movement by pointing the laser sensor at the cow’s side. Doppler radars have been used for obtaining accurate respiration and pulse rate signals in dairy cows (Li et al., 2017). Two antenna beams were directed to the belly and neck of the cows. The obtained respiration and pulse rate were validated by a veterinarian with help of a stethoscope.
2.3.4 Machine Vision Technology
In recent years, machine vision technology has generated considerable interest for its application in animal industry. Machine vision technology has been used to track animals, detect abnormal behaviors, identify sick animals etc. (Kashiha et al., 2014; Nasirahmadi et al., 2017; Nasirahmadi et al., 2019). Similar to sound and IRT technology, machine vision technology is non-invasive method of monitoring. Additionally, the method is largely cheap, causes no discomfort to animals, does not rely on batteries, and is suitable for both indoor and outdoor conditions (Nasirahmadi et al., 2017). In this article, the authors review the studies using machine vision technology for respiration monitoring in animals.
Machine learning is defined as the ability of the machine to learn from experience without being explicitly programmed. Machine vision technology requires datasets to learn or gain experience. The data can be obtained using different types of cameras (e.g. depth cameras, charge couple device cameras etc.) depending on the application. The raw data is then annotated to mark certain features which are then used for training the model. Training is followed by applying algorithms developed to perform the required task. Statistical and mathematical models can be used to evaluate the performance of the model. Al-Naji et al. (2019) used machine vision to calculate the respiration rate of exotic animals (like Giant panda, African lions, Sumatran tiger, Koala, Red kangaroo, Alpaca, little blue penguin, Sumatran orangutan and Hamadryas baboon). The videos were recorded using a digital camera (Nikon D610, Nikon Inc., Tokyo, Japan) when the animals were not moving much to avoid any noise in the data. The respiration rate was calculated by detecting intensity variation of the signal from abdominal thoracic region of the animals. Wu et al. (2020) investigated the use of a combination of semantic segmentation and Phase based video magnification (PBVM) technology to estimate respiratory rate of standing resting cows. PBVM is a technology developed with aim of revealing temporal variations in videos that are not visible to the naked eye. In this method, the input video is decomposed into different spatial bands and temporal filtering is applied. The filtered bands are then amplified and added back to the original signal to get the output video (Wadhwa et al., 2013). This technique has been used to amplify human skin color variations due to blood circulation, estimate breathing rate of a baby, and to amplify low magnitude motion (Wadhwa et al., 2013). PBVM technology was used to amplify the weak respiratory movements of the standing resting cows. The data was recorded using a digital camera fixed on tripod at a distance of 3m from the cows. About 3000 images were randomly selected and manually labelled from the recorded videos to train the model to detect cows from the background. The abdomen of the cow was selected as region of interest to perform amplification. The reported accuracy of respiration rate estimation using PBVM technology ranged from 80% to 100%, with a mean accuracy of 93.04%.
Machine vision technology is an improvement over thermal imaging and sound analysis technology as this technology does not require expensive equipment, restraining of animals, and is not impacted by changing environmental conditions.
The above-mentioned studies indicate that machine vision technology can be successfully adopted to monitor respiration rate in animals. However, both Al-Naji et al. (2019) and Wu et al. (2020) made use of tripod to position the camera close to the animals (at distance of 3-40m and 3m respectively) to collect the data. This type of camera positioning setup is not feasible in commercial farms. Most of the commercial farms have camera located at the top or at the corner of the ceiling. Moreover, the recordings were captured when the movement of the animals was relatively stable. The position of the camera and movement of animals might impact the accuracy of the algorithms. The dataset collection and annotation is time consuming and laborious task. To the authors knowledge only 1 dataset containing pig images from a commercial farm is available in public domain (Psota et al., 2019). More research is needed before large scale commercial application of machine vision technology can be made feasible.
3 Findings
Finding 1: Human observation method is the most commonly used method of respiration rate estimation in commercial farms. This method requires minimum investment in terms of equipment (only a stopwatch). However, in terms of time the investment is very high. Proper training of the observer is also critical for the accurate estimation of respiration rate. This technology has also been used by researchers to validate the contact sensor technology.
Finding 2: The performance and durability of contact sensors is subject to the livestock behavior and environmental factors. The performance of contact sensors is compromised in the presence of dust, dirt, precipitation, sunlight etc. Damage to the sensors due to biting by other livestock is also a common issue. Additionally, not all practitioners are trained to properly mount the sensors on the livestock. Improving the robustness of contact sensors and decreasing the per head cost could lead to wider acceptance and usage in commercial farms.
Finding 3: The non-contact sensor technology is increasingly becoming popular as it does not interfere with farm activities. However, non-contact sensor technology requires the use of cameras for recording the livestock behavior. The video recordings are affected by factors such as lighting conditions in a pen, dirt, occlusion etc. Moreover, processing the video data is computationally intensive and requires access to storage capacity, high processing systems (such as GPUs), and high-speed internet which may not be feasible at all commercial farms. The complexity of non-contact sensors poses a challenge to their widespread adoption. Developing a mobile phone-based application capable of real time monitoring would significantly improve acceptance by the animal practitioners, especially in rural areas.
4 Conclusions
To summarize, three techniques to monitor respiration and sounds for livestock animals were synthesized from the research literature: traditional (human observation), contact methods (physical interaction), and non-contact methods (non-physical interaction). The current state of each of these three technique categories was surveyed for 23 different studies. The most significant finding from this work is the inconsistency in reporting of important study data (e.g., distance of camera from animals in camera-based studies) and the comparison of new methods against traditional or other established approaches. This suggests there might be suboptimal understanding and an inherent inability to make comparisons when new methods for monitoring respiration and sounds are proposed.
Observation of respiration rate and sounds in commercial livestock farms occurred most commonly through direct human observation. Additional equipment usage was minimal but this also requires an opportunity cost of time expended by the observer as well intermittent and transient observation points when compared against continuous monitoring techniques. Additional training requirements were also found to be necessary for human observers which might lead to errors through either misinterpretations or inconsistencies among observations.
The deployment of both contact and non-contact sensor technologies can be impacted by the environment in which they are placed. Common occurrences in livestock production facilities are dust and moisture, both of which can impact electronic and optics of these sensors. Similar to the considerations for livestock size and housing practices, so too much the type of animals and how they are housed be considered due to the different wants an animal might behaviorally interact with a sensor (e.g., chewing or biting). But in this comparison, when considering contact and non-contact technologies, non-contact sensors (cameras) would help resolve many of these issues since they could be more remotely located from the animals.
One area of concern in comparing techniques across multiple types of livestock is reconciling differences in animal physiology and the layout of production facilities. For example, cattle and swine are different in size and might be housed in different numbers in a production facility; this might result in the need for different techniques in the observation approaches. However, the main responsibility of the observer, human or artificial, for all livestock and facilities includes data acquisition for decision-making, providing a commonality for comparisons.
This survey suggests that more work is needed to realize sensor-based techniques for respiration and sounds, specifically in terms of integration and flexibility, for decision-making opportunities in livestock production systems. A set of data capture design recommendations should be developed and tested to support optimal levels of integration and flexibility. In the future, it is anticipated that both contact and non-contact technologies, across the entire scale of livestock production systems, will permit more customization for automation and human decision-making; therefore, understanding how to optimize respiration and sound data capture will be important for increasing productivity and reducing manpower in livestock operations.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This work was supported by the Iowa Pork Producers Association under award 19-214.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Al-Naji A., Tao Y., Smith I., Chahl J. (2019). A Pilot Study for Estimating the Cardiopulmonary Signals of Diverse Exotic Animals Using a Digital Camera. Sensors 19 (24), 5445. doi: 10.3390/s19245445
Atkins I. K., Cook N. B., Mondaca M. R., Choi C. Y. (2018). Continuous Respiration Rate Measurement of Heat-Stressed Dairy Cows and Relation to Environment, Body Temperature, and Lying Time. Trans. ASABE 61 (5), 1475–1485. doi: 10.13031/trans.12451
Barbosa Pereira C., Dohmeier H., Kunczik J., Hochhausen N., Tolba R., Czaplik M. (2019). Contactless Monitoring of Heart and Respiratory Rate in Anesthetized Pigs Using Infrared Thermography. PLos One 14 (11), e0224747. doi: 10.1371/journal.pone.0224747
Becker C. A., Woolums A. R., Stone A. E. (2020). Recognizing Heat Stress in Dairy Cattle: How to Visually Record Respiration Rate. Mississip. State. Univ.—Exten.
Berckmans D. (2014). Precision Livestock Farming Technologies for Welfare Management in Intensive Livestock Systems. Rev. Sci. Tech. 33 (1), 189–196. doi: 10.20506/rst.33.1.2273
Carpentier L., Berckmans D., Youssef A., Berckmans D., van Waterschoot T., Johnston D., et al. (2018). Automatic Cough Detection for Bovine Respiratory Disease in a Calf House. Biosyst. Eng. 173, 45–56. doi: 10.1016/j.biosystemseng.2018.06.018
Chedad A., Moshou D., Aerts J. M., Van Hirtum A., Ramon H., Berckmans D. (2001). AP—animal Production Technology: Recognition System for Pig Cough Based on Probabilistic Neural Networks. J. Agric. Eng. Res. 79 (4), 449–457. doi: 10.1006/jaer.2001.0719
Christensen G., Mousing J. (1992). “Respiratory System,” in Diseases of Swine, 7th edn. Eds. Leman A. D., Straw B. E., Mengeling W. L., Dallaire S., Taylor D. J. (Ames, IA: Iowa State University Press), 138–162.
Chung Y., Oh S., Lee J., Park D., Chang H. H., Kim S. (2013). Automatic Detection and Recognition of Pig Wasting Diseases Using Sound Data in Audio Surveillance Systems. Sensors 13 (10), 12929–12942. doi: 10.3390/s131012929
Edwards T. A. (2010). Control Methods for Bovine Respiratory Disease for Feedlot Cattle. Veterin. Clinic.: Food Anim. Pract. 26 (2), 273–284. doi: 10.1016/j.cvfa.2010.03.005
Eigenberg R. A., Brown–Brandl T., Nienaber J. A. (2002). Development of a Respiration Rate Monitor for Swine. Trans. ASAE 45 (5), 1599. doi: 10.13031/2013.11066
Eigenberg R. A., Hahn G. L., Nienaber J. A., Brown-Brandl T. M., Spiers D. E. (2000). Development of a New Respiration Rate Monitor for Cattle. Trans. ASAE 43 (3), 723. doi: 10.13031/2013.2755
Exadaktylos V., Silva M., Aerts J. M., Taylor C. J., Berckmans D. (2008). Real-Time Recognition of Sick Pig Cough Sounds. Comput. Electron. Agric. 63 (2), 207–214. doi: 10.1016/j.compag.2008.02.010
Ferrari S., Piccinini R., Silva M., Exadaktylos V., Berckmans D., Guarino M. (2010). Cough Sound Description in Relation to Respiratory Diseases in Dairy Calves. Prev. Veterin. Med. 96 (3-4), 276–280. doi: 10.1016/j.prevetmed.2010.06.013
Guarino M., Jans P., Costa A., Aerts J. M., Berckmans D. (2008). Field Test of Algorithm for Automatic Cough Detection in Pig Houses. Comput. Electron. Agric. 62 (1), 22–28. doi: 10.1016/j.compag.2007.08.016
Gutierrez W. M., Kim S., Kim D. H., Yeon S. C., Chang H. H. (2010). Classification of Porcine Wasting Diseases Using Sound Analysis. Asian-Australas. J. Anim. Sci. 23 (8), 1096–1104. doi: 10.5713/ajas.2010.90483
Hindson J. C., Winter A. C. (2002). “Respiratory Disease,” in Manual of Sheep Diseases, 2nd Edition (Oxford, UK: Blackwell Science), 196–209.
Hoinville L. J., Alban L., Drewe J. A., Gibbens J. C., Gustafson L., Häsler B., et al. (2013). Proposed Terms and Concepts for Describing and Evaluating Animal-Health Surveillance Systems. Prev. Veterin. Med. 112 (1-2), 1–12. doi: 10.1016/j.prevetmed.2013.06.006
Holtkamp D. J., Kliebenstein J. B., Neumann E., Zimmerman J. J., Rotto H., Yoder T. K., et al. (2013). Assessment of the Economic Impact of Porcine Reproductive and Respiratory Syndrome Virus on United States Pork Producers. J. Swin. Health Prod. 21 (2), 72.
Incropera D. (2007). Bergman, and Lavine. Fundamentals of Heat and Mass Transfer, Vol. 6. (New York: Wiley).
Jorquera-Chavez M., Fuentes S., Dunshea F. R., Jongman E. C., Warner R. D. (2019). Computer Vision and Remote Sensing to Assess Physiological Responses of Cattle to Pre-Slaughter Stress, and Its Impact on Beef Quality: A Review. Meat Sci. 2019 (156), 11–22.
Jorquera-Chavez M., Fuentes S., Dunshea F. R., Warner R. D., Poblete T., Unnithan R. R., et al. (2021). Using Imagery and Computer Vision as Remote Monitoring Methods for Early Detection of Respiratory Disease in Pigs. Computers and Electronics in Agriculture 187, 106283.
Kashiha M. A., Bahr C., Ott S., Moons C. P., Niewold T. A., Tuyttens F., et al. (2014). Automatic Monitoring of Pig Locomotion Using Image Analysis. Livestock Science 159, 141–148. doi: 10.1016/j.livsci.2013.11.007
Li C., Peng Z., Huang T. Y., Fan T., Wang F. K., Horng T. S., et al. (2017). A Review on Recent Progress of Portable Short-Range Noncontact Microwave Radar Systems. IEEE Trans. Microw. Theory Tech. 65 (5), 1692–1706. doi: 10.1109/TMTT.2017.2650911
Lowe G., Sutherland M., Waas J., Schaefer A., Cox N., Stewart M. (2019). Infrared Thermography—A non-Invasive Method of Measuring Respiration Rate in Calves. Animals 9 (8), 535. doi: 10.3390/ani9080535
Maia A. S., Gebremedhin K. G., Nascimento S. T., Carvalho M. D., Simão B. R., Camerro L. Z., et al. (2014). “Development of Facial Masks for Indirect Calorimetric Studies for Livestock,” (Montreal, Quebec Canada: American Society of Agricultural and Biological Engineers), (p. 1). doi: 10.13031/aim.20141897355
Matthews S. G., Miller A. L., Clapp J., Plötz T., Kyriazakis I. (2016). Early Detection of Health and Welfare Compromises Through Automated Detection of Behavioural Changes in Pigs. Veterin. J. 217, 43–51. doi: 10.1016/j.tvjl.2016.09.005
McManus C., Tanure C. B., Peripolli V., Seixas L., Fischer V., Gabbi A. M., et al. (2016). Infrared Thermography in Animal Production: An Overview. Comput. Electron. Agric. 123, 10–16. doi: 10.1016/j.compag.2016.01.027
Milan H. F. M., Maia A. S. C., Gebremedhin K. G. (2016). Device for Measuring Respiration Rate of Cattle Under Field Conditions. J. Anim. Sci. 94 (12), 5434–5438. doi: 10.2527/jas.2016-0904
Minkina W., Chudzik S. (2004). Pomiary Parametrów Cieplnych Materiałów Termoizolacyjnych: Przyrządy I Metody (Wydawn: Politechniki Częstochowskiej).
Mutlu K., Rabell J. E., Del Olmo P. M., Haesler S. (2018). IR Thermography-Based Monitoring of Respiration Phase Without Image Segmentation. J. Neurosci. Methods 301, 1–8. doi: 10.1016/j.jneumeth.2018.02.017
Nasirahmadi A., Edwards S. A., Sturm B. (2017). Implementation of Machine Vision for Detecting Behaviour of Cattle and Pigs. Livest. Sci. 202, 25–38. doi: 10.1016/j.livsci.2017.05.014
Nasirahmadi A., Sturm B., Edwards S., Jeppsson K. H., Olsson A. C., Müller S., et al. (2019). Deep Learning and Machine Vision Approaches for Posture Detection of Individual Pigs. Sensors 19 (17), 3738. doi: 10.3390/s19173738
Neethirajan S. (2020). The Role of Sensors, Big Data and Machine Learning in Modern Animal Farming. Sens. Bio-Sens. Res. 29, 100367. doi: 10.1016/j.sbsr.2020.100367
Neumann E. J., Kliebenstein J. B., Johnson C. D., Mabry J. W., Bush E. J., Seitzinger A. H., et al. (2005). Assessment of the Economic Impact Ofporcine Reproductive and Respiratory Syndrome on Swine Production in Theunited States. J. Am. Vet. Med. Assoc. 227, 385–392. doi: 10.2460/javma.2005.227.385
Opriessnig T., Giménez-Lirola L. G., Halbur P. G. (2011). Polymicrobial Respiratory Disease in Pigs. Anim. Health Res. Rev. 12 (2), 133. doi: 10.1017/S1466252311000120
Pastell M., Kaihilahti J., Aisla A. M., Hautala M., Poikalainen V., Ahokas J. (2007). A System for Contact-Free Measurement of Respiration Rate of Dairy Cows. Journal of Precision Livestock Farming 7, 105–109. doi: 10.3920/978-90-8686-604-5
Peel D. S. (2020). The Effect of Market Forces on Bovine Respiratory Disease. Veterin. Clinic.: Food Anim. Pract. 36 (2), 497–508. doi: 10.1016/j.cvfa.2020.03.008
Potter T., Aldridge B. (2010). Systematic Approach to Calf Pneumonia. UK Vet. Livest. 15 (6), 31–34. doi: 10.1111/j.2044-3870.2010.tb00317.x
Psota E. T., Mittek M., Pérez L. C., Schmidt T., Mote B. (2019). Multi-Pig Part Detection and Association With a Fully-Convolutional Network. Sensors 19 (4), 852. doi: 10.3390/s19040852
Schaefer A. L., Cook N. J., Bench C., Chabot J. B., Colyn J., Liu T., et al. (2012). The non-Invasive and Automated Detection of Bovine Respiratory Disease Onset in Receiver Calves Using Infrared Thermography. Res. Veterin. Sci. 93 (2), 928–935. doi: 10.1016/j.rvsc.2011.09.021
Schaefer A. L., Cook N. J., Church J. S., Basarab J., Perry B., Miller C., et al. (2007). The Use of Infrared Thermography as an Early Indicator of Bovine Respiratory Disease Complex in Calves. Res. Veterin. Sci. 83 (3), 376–384. doi: 10.1016/j.rvsc.2007.01.008
Silva M., Ferrari S., Costa A., Aerts J. M., Guarino M., Berckmans D. (2008). Cough Localization for the Detection of Respiratory Diseases in Pig Houses. Comput. Electron. Agric. 64 (2), 286–292. doi: 10.1016/j.compag.2008.05.024
Smith R. A., Step D. L., Woolums A. R. (2020). Bovine Respiratory Disease: Looking Back and Looking Forward, What Do We See? Veterin. Clinic.: Food Anim. Pract. 36 (2), 239–251. doi: 10.1016/j.cvfa.2020.03.009
Stewart M., Wilson M. T., Schaefer A. L., Huddart F., Sutherland M. A. (2017). The Use of Infrared Thermography and Accelerometers for Remote Monitoring of Dairy Cow Health and Welfare. J. Dair. Sci. 100 (5), 3893–3901. doi: 10.3168/jds.2016-12055
Strutzke S., Fiske D., Hoffmann G., Ammon C., Heuwieser W., Amon T. (2019). Technical Note: Development of a Noninvasive Respiration Rate Sensor for Cattle. Journal of Dairy Science 102, 1, 690–695. doi: 10.3168/jds,14999
USDA (2017) Death Loss in U.S. Cattle and Calves Due to Predator and Nonpredator Causes. Available at: https://www.aphis.usda.gov/animal_health/nahms/general/downloads/cattle_calves_deathloss_2015.pdf.
Vandermeulen J., Bahr C., Johnston D., Earley B., Tullo E., Fontana I., et al. (2016). Early Recognition of Bovine Respiratory Disease in Calves Using Automated Continuous Monitoring of Cough Sounds. Comput. Electron. Agric. 129, 15–26. doi: 10.1016/j.compag.2016.07.014
Wadhwa N., Rubinstein M., Durand F., Freeman W. T. (2013). Phase-Based Video Motion Processing. ACM Trans. Graphics (TOG) 32 (4), 1–10. doi: 10.1145/2461912.2461966
Weixing Z., Zhilei W. (2010) Detection of Porcine Respiration Based on Machine Vision. in 2010 Third International Symposium on Knowledge Acquisition and Modeling. Wuhan, China. 398–401 (Wuhan, China: IEEE). doi: 10.1109/KAM.2010.5646284
White B. J., Renter D. G. (2009). Bayesian Estimation of the Performance of Using Clinical Observations and Harvest Lung Lesions for Diagnosing Bovine Respiratory Disease in Post-Weaned Beef Calves. J. Veterin. Diagn. Invest. 21 (4), 446–453. doi: 10.1177/104063870902100405
Keywords: respiration, livestock, sensors, precision livestock farming, animal production and health
Citation: Handa D and Peschel JM (2022) A Review of Monitoring Techniques for Livestock Respiration and Sounds. Front. Anim. Sci. 3:904834. doi: 10.3389/fanim.2022.904834
Received: 25 March 2022; Accepted: 18 May 2022;
Published: 29 June 2022.
Edited by:
Yang Zhao, The University of Tennessee, Knoxville, United StatesReviewed by:
Takemi Matsui, Tokyo Metropolitan University, JapanJacquelyn Boerman, Purdue University, United States
Copyright © 2022 Handa and Peschel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joshua M. Peschel, cGVzY2hlbEBpYXN0YXRlLmVkdQ==
 Divya Handa
Divya Handa Joshua M. Peschel
Joshua M. Peschel