
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Anim. Sci., 13 April 2022
Sec. Animal Nutrition
Volume 3 - 2022 | https://doi.org/10.3389/fanim.2022.878408
This article is part of the Research TopicThe Relationship of Animal Health and Management to Food SafetyView all 6 articles
We set out to describe the prevalence of Salmonella enterica in three large, adjacent cattle operations in the southern High Plains of the United States. Operations included two dairies (one of which routinely administers a commercially available Salmonella vaccine) and one feedlot. Samples were collected monthly for 12 months. At each sample collection, 25 freshly voided fecal pats and a sample from each of the water troughs were collected from each of five pens of cattle within an operation. Each monthly collection included a total of 375 fecal and ~32 water samples for a yearly total of 4,500 and 379 samples, respectively (note that the number of water troughs per pen varied within an operation). Salmonella was commonly recovered from fecal (71.3%) and water (28.5%) samples and tended to follow somewhat similar temporal patterns over time. However, its prevalence varied among operations despite being adjacent properties in that Salmonella was recovered from 61.3, 80.1, and 75% of fecal samples from dairy 1, dairy 2 and the feedlot, respectively. Salmonella prevalence in water samples across collection times averaged 36.1, 70.2, and 46.1% for dairy 1, dairy 2, and the feedlot, respectively. While it is uncertain why the Salmonella prevalence varied from operation to operation, the higher observed prevalence of Salmonella in water on dairy 2 and/or the use of a commercial Salmonella vaccine by dairy 1 may offer a partial explanation.
Salmonella continues to be a challenge for both livestock producers, meat processors, and public health officials. Cattle are natural reservoirs for Salmonella and while this pathogen can and does cause significant disease in some instances, most infections appear to be asymptomatic carriage (Fedorka-Cray et al., 1998; Edrington et al., 2004a) producing no readily detectible effects to the animal. Salmonella contamination of beef products for human consumption continues to occur despite significant improvements in beef processing including use of many successful post-harvest sanitation processing aids and interventions. Recently, a petition has arisen calling for the labeling of Salmonella as an adulterant as was done for E. coli O157:H7 and various other Shiga-toxin producing E. coli strains. In other work, Salmonella was readily recovered from peripheral, non-mesenteric lymph nodes of cattle, where presumably it escapes many in-plant sanitary processes and unless physically removed by trimming, may be incorporated into trim used in the production to ground beef (Arthur et al., 2008; Li et al., 2015). Hence there is considerable interest in reducing the carriage of Salmonella in both beef and dairy cattle on the farm, to reduce the opportunity for disease, as well as improve the safety of our beef supply.
Considerable research has been conducted to better understand the ecology of Salmonella within a cattle operation (dairy, feedlot), as well as transmission dynamics of this pathogen among animals within an operation (Fitzgerald et al., 2003; Edrington et al., 2004a,b,c; Gragg et al., 2013; Brown et al., 2015). In general, Salmonella is more prevalent in cattle operations in the southern vs. northern latitudes of the United States, and more prevalent in the animals in the summer and early fall compared to the winter and spring (Gragg et al., 2013; Webb et al., 2017). Not surprisingly however, exceptions to these generally accepted paradigms occur. A fuller understanding of these exceptions and their determinants may provide important insight for the design and implementation of on-farm intervention strategies.
The southern high plains region of Texas and New Mexico is home to a significant proportion of the feedlots and dairies in the United States and it is not uncommon to find large-scale operations in relatively close proximity to one another. In the current research, a longitudinal investigation was conducted on the fecal prevalence and concentrations of Salmonella on three adjacent cattle operations, two dairies and one beef feedlot. As water troughs have been implicated as a significant source of pathogen exposure (LeJeune et al., 2001), water samples were also obtained at each collection timepoint. Prior research had demonstrated that Salmonella is readily recoverable from cattle (both feedlot and lactating dairy cows) and their environments within this region of the United States. Given the unusual proximity of the enrolled operations described herein, we set out to describe within and among variation of Salmonella prevalence across operations, management practices, and cattle types.
Two commercial dairy farms and one feedlot operation located in eastern New Mexico were enrolled in the study. These commercial cattle operations were adjacent to one another, and all located within a three km radius (Figure 1). The two dairies maintained ~2,200 and 6,500 lactating cows each, while the feedlot had a one-time capacity of 35,000 cattle. All operations were representative of dairy and feedlots within this region of the United States. One dairy used a commercially available Salmonella vaccine (Salmonella Newport Bacterial Extract vaccine with SRP® Technology, Zoetis Inc., Kalamazoo, MI, USA) according to manufacturer's recommendations (dairy 1) and the feedlot incorporated a direct-fed microbial (Bovamine®, Chr Hansen, Hoersholm, Denmark) into the cattle diets for pathogen control. Dairy 2 did not use either of these products.
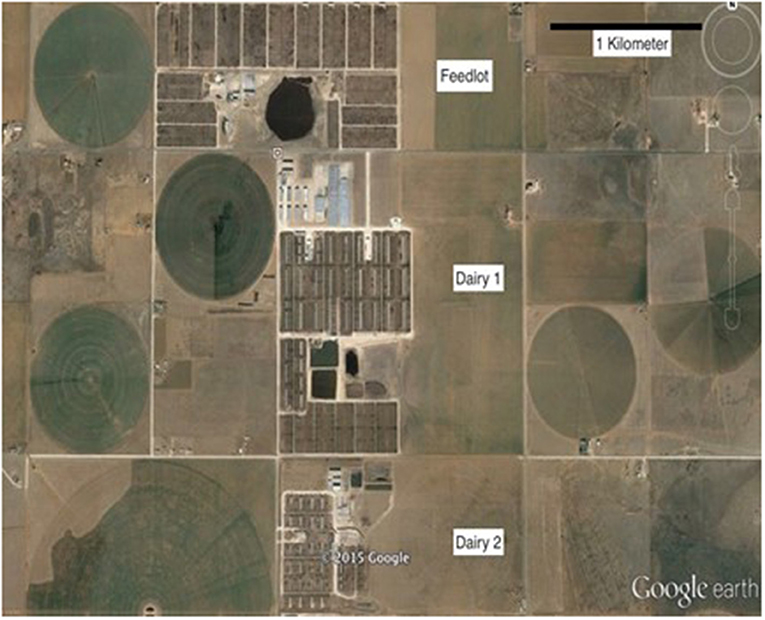
Figure 1. Google earth image displaying the proximity of the three facilities sampled in the southern high plains.
Fecal and water samples were collected monthly for 1 year from each of the cattle operations. Twenty-five fecal samples were collected from each of five pens on each operation monthly (125 fecals/farm/month). On the dairies, pens housing animals nearest peak lactation (~100 days in milk) were sampled, while on the feedlot, pens containing animals within 30 days of slaughter were collected. Freshly voided, undisturbed fecal pats were sampled utilizing clean plastic spoons (one per sample) to transfer the feces to individual specimen cups.
Water samples were collected from all water troughs in the pens in which fecal samples were obtained. Some pens shared water troughs such that with the exception of the first collection in which 32 water samples were collected, 27 water samples were collected monthly. A total of 4,500 fecal and 379 water samples were collected over the 12-month study period. All samples were placed in coolers on ice and shipped overnight to the USDA laboratory in College Station, TX for bacterial culture described below.
Fecal and water samples were quantitatively and qualitatively cultured for Salmonella within 24 h of collection as described previously (Edrington et al., 2018). For quantitative culture, 10 g of each fecal sample was mixed with 90 ml of tetrathionate broth, one ml of this mixture removed and from that 50 μl direct plated on xylose lysine deoxycholate (XLD) agar (Oxoid, Basingstoke, UK) using a commercial spiral plater (Spiral Biotech Autoplate 4000; Advanced Instruments, Inc. Norwood, MA). The XLD plates were incubated (37°C for 24 h) and morphologically typical black colonies were counted and converted to log10 CFU per gram of feces. For qualitative detection of Salmonella, the feces-tetrathionate mixture above was incubated overnight (37°C); after which, 100 μl of the enrichment was transferred to a second enrichment (5 ml Rappaport-Vassiliadis broth (RV; Remel Products, Lenexa, KS) and incubated at 42°C for 24 h. Following the second enrichment, each sample was streaked for isolation on brilliant green agar (Oxoid) containing novobiocin (25 μg/ml; BGAnov) and incubated (37°C, overnight). Presumptive Salmonella isolates were confirmed biochemically using lysine and triple sugar iron agars.
Water samples were vortexed and 1 ml of each sample removed and plated onto XLD agar using the spiral plater, incubated and counted as described above. For qualitative analysis, 25 ml of each water sample were enriched with the addition of 25 ml tryptic soy broth (TSB) and incubated overnight at 37°C. A secondary enrichment was conducted as above by transfer of 100 μl of the TSB enrichment to 5 ml RV broth and incubated at 42°C for 24 h. Presumptive Salmonella isolates confirmed as above.
Salmonella prevalence and concentrations were analyzed using quassibinomial and gaussian generalized linear models, respectively (R version 3.1.2, The R Foundation for Statistical Computing Platform). The quassibinomial model incorporated dispersion parameters to account for either under- or over-dispersion of data due to clustering within each cohort. Prevalence estimates were calculated for each operation individually by dividing the number of culture positive samples by the total number of samples collected monthly. Model-adjusted estimates of prevalence and imprecision were computed. For quantitative data, a missing value was recorded in instances where Salmonella concentration was below the limit of quantification and for those samples from which Salmonella was recovered (i.e., Salmonella was present at a concentration above the limit of detection) but below the limit of quantification (i.e., no morphologically typical colonies were observed using direct plating).
Of the 4,500 fecal and 379 water samples examined, Salmonella was recovered (following enrichment of samples) from 71.3% [n = 3,208; 95% confidence limit (CL) = 69.9, 72.6%] and 46.7% (n = 177; CL = 41.6, 51.7%) of fecal and water samples, respectively (Table 1). Of those with quantifiable concentrations of Salmonella (Table 1), the mean concentration of Salmonella in fecal samples was 3.27 log10 CFU/g with a range of 2.08–5.68 log10 CFU/g and 2.42 log10 CFU/ml of water (range: 2.3–3.38 log10 CFU/ml). For the fecal and water samples, 28.5 (n = 1,284; CL = 27.2, 29.8%) and 4.5% (n = 17; CL = 2.39, 6.6%) of the fecal and water samples contained concentrations above the limit of quantification, respectively (Table 1). The distribution of concentrations in fecal samples is presented in Figure 2 across operation and month of collection. The majority of samples were negative (by quantitative and qualitative culture) or negative following direct plating, but positive after enrichment. Of those samples quantitatively positive following direct plating, most samples fell in the range of 2.0–4.99 log10 CFU/g feces, with only a small percentage of samples containing concentrations above 5.0 log10/g feces.
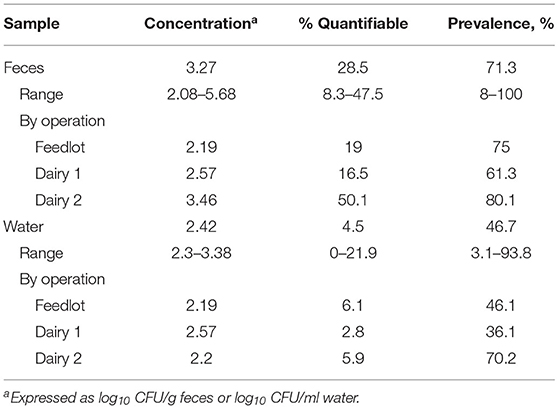
Table 1. Prevalence and quantification of Salmonella in fecal and water samples collected from commercial dairy and feedlot operations within close proximity.
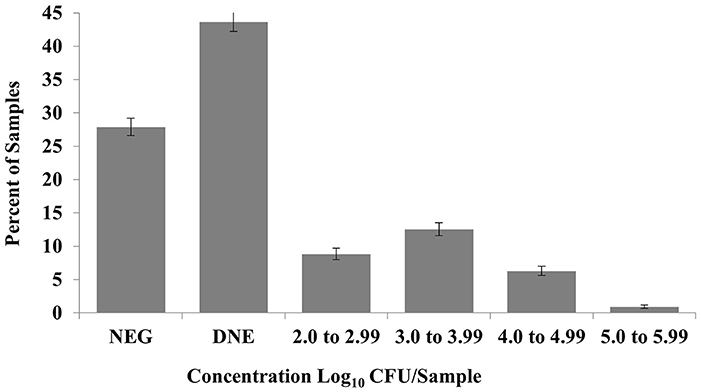
Figure 2. Concentration of Salmonella positive fecal samples by log. NEG, negative following quantitative and qualitative culture; DNE, negative on quantitative culture, positive on qualitative culture.
When examined across month of collection by operation, fecal prevalence estimates in enriched samples were 75 (n = 1,125; CL = 72.7, 77.2%), 61.3 (n = 919; CL = 58.7, 63.7%), and 80.1% (n = 1,202; CL = 78.0, 82.1%) for samples from the feedlot, dairy 1, and dairy 2, respectively (Table 1). The percentage of fecal samples with Salmonella concentrations above the limit of detection (% quantifiable) were 19 (n = 285; CL = 17.0, 20.9%), 16.5 (n = 248; CL = 14.6, 18.4%), and 50.1% (n = 751; CL = 47.5, 52.6%) for the feedlot, dairy 1, and dairy 2, respectively (Table 1). Fecal concentrations averaged 2.19, 2.57, and 3.46 log10 CFU/g feces for the feedlot, dairy 1 and dairy 2, respectively (Table 1).
Salmonella prevalence estimates of enriched water samples (Table 1) were 46.1% (feedlot; n = 53/115; CL = 36.9, 55.2%), 36.1% (dairy 1; n = 65/180; CL = 29.1, 43.1%), and 70.2% (dairy 2; n = 59/84; CL = 60.4, 79.9%). The prevalence of Salmonella in water trough samples ranged from 3.1 (n = 1; CL = 0.1, 16.2%) to 93.8 % (n = 30; CL = 79.2, 99.2%) across all collections and operations. The percentage of water samples with quantifiable concentrations averaged 6.1 (n = 7; CL = 1.7, 10.4%), 2.8 (n = 5; CL = 0.3, 5.2%), and 5.9% (n = 5; CL = 0.9, 11.1%) for the feedlot, dairy 1, and dairy 2, respectively. Of these water samples, the mean concentration of Salmonella by operation was 2.19, 2.57, and 2.20 log10 CFU/ml of water for the feedlot, dairy 1 and dairy 2, respectively (Table 1).
Fecal data is presented by month of collection and operation in Table 2. Variation in fecal Salmonella prevalence among the different operations was most pronounced from March through May and again in October through March, whereas the June through September prevalence was relatively consistent among operations and the greatest (74–100%). Dairy 2 had the greatest prevalence of Salmonella in fecal samples for 9 of the 12 collections. The percentage of samples with quantifiable populations showed considerable variation throughout the study, and like prevalence, was most frequently on dairy 2 (10 or 12 collections). Concentration of Salmonella in the feces in comparison was similar among operations in each of the collection months with one exception, April, in which dairy 2 did not have any fecal samples with quantifiable Salmonella concentrations. Otherwise, concentrations were relatively consistent month to month among operations, ranging from 2.67 to 4.06 log10 CFU/g feces.
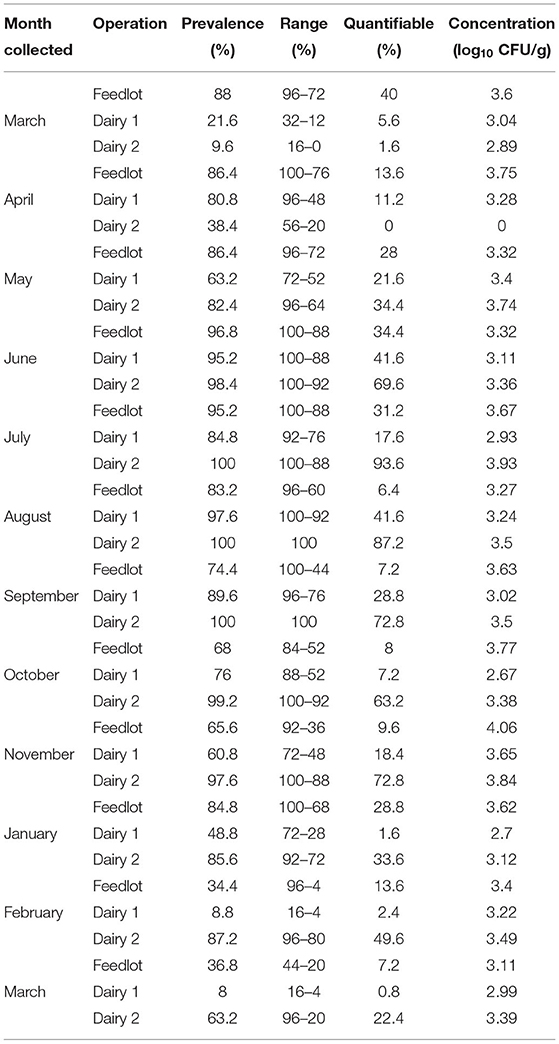
Table 2. Comparison of prevalence, range, and pathogen load within and across operations by month of sample collection.
Due to the relatively few water samples with detectable Salmonella concentrations, Salmonella prevalence is presented in Figure 3 by month of collection and operation. Not surprisingly, the summer months found the greatest prevalence of Salmonella in the water troughs. Dairy 2 demonstrated the greatest Salmonella water prevalence, with 100% of the water samples positive in June through November and over 50% incidence in February and March. Dairy 1 and the feedlot followed the same general month to month trends as dairy 2, with mostly lower prevalence at each collection.
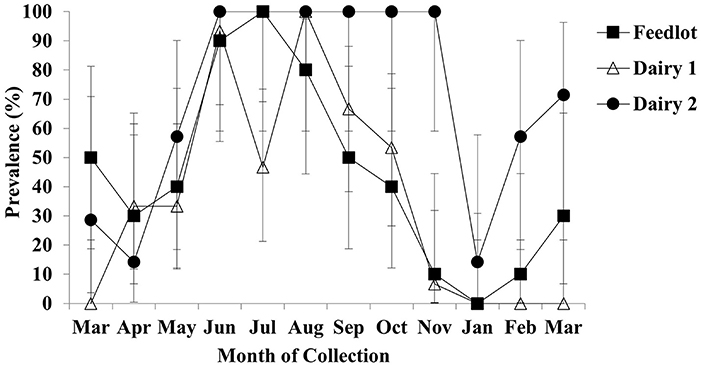
Figure 3. Prevalence of Salmonella in post-enriched water samples by operation and month. Error bars represent 95% confidence intervals.
To the best of our knowledge, this is the first descriptive study to explore Salmonella prevalence in different commercial cattle operations type (feedlot and two dairies) that are adjacent to one another, in that they share common property boundaries. Within animals, fecal prevalence when examined across month of collection, was relatively similar as were Salmonella concentrations, however, the likelihood of finding a sample with concentrations above the LOD was greater for dairy 2, as compared to dairy 1 and the feedlot. Salmonella prevalence within the water troughs, was likewise similar for the feedlot and dairy 1, but greater for dairy 2. The number of water samples with detectable concentrations was low across all operations as were the actual concentrations. The variation in Salmonella prevalence between the two dairies is somewhat surprising, as they are immediately adjacent each other, have similar pen and housing design, and utilize similar feedstuffs. Given the geographic co-location and similarity of housing and cattle, other unmeasured factors such as general management, herd health programs and hygiene practices, could account for the variation in the prevalence of Salmonella across these operations. Breed could be a factor, as dairy 1 was a Holstein herd, whereas dairy 2 was Jerseys. To our knowledge there are no reports citing dairy breed accounting for Salmonella shedding differences. Another potential is the utilization of a commercially available Salmonella vaccine by dairy 1. Previous research with Salmonella vaccines and the effectiveness on Salmonella control in dairy cattle has yielded mixed results. In one study, fecal prevalence of Salmonella in animals culled from dairies utilizing a whole herd vaccination program was significantly lower than in animals culled from herds that were unvaccinated (Loneragan et al., 2012). Others reported no difference in fecal shedding of Salmonella in sub-clinically infected dairy cows (Heider et al., 2008; Hermesch et al., 2008) although in one study an increase in milk production was noted for vaccinated cows (Hermesch et al., 2008). Similarly, a vaccine effect was not observed in the prevalence of Salmonella in feedlot cattle (Dodd et al., 2011). Hence while the differences in prevalence between the two dairies could be attributed, at least in part, to the vaccine, it cannot be conclusively verified from this data.
There was a much greater prevalence of Salmonella in the water troughs on dairy 2 as compared to dairy 1 and the feedlot, which could explain the greater fecal incidence in that dairy. The frequency of water trough cleaning could partially explain these results as it was conducted every 2 weeks on the feedlot and dairy 1, compared to an as-needed basis for dairy 2. Chlorination of drinking water is deployed by some dairies in this region of the United States, but was not employed on any of the operations enrolled, to include the feedlot, in this study.
As cattle in feedlots are typically younger, fed different diets typically containing more concentrates, arrive from multiple sources and management practices, and are managed for very different outcomes than animals on dairies, fecal shedding of Salmonella as compared to the dairies was similar. The use of Bovamine was employed by the feedlot and not either dairy. While typically used for the mitigation of E. coli O157:H7, there is little evidence that Bovamine affects Salmonella to the same degree. That said, the current study design was not adequate to draw any conclusions around the use of Bovamine. In that sense, one ought to limit conclusions between the dairies and the feedlot included in this study to infer that the prevalence differed between these two production systems without generalizing more broadly to a wider population of feedlots and dairies. Obviously, a broader sample of enrolled, co-located feedlots and dairies would be needed for such inferential discussion. However, we are not aware of other situations where feedlots and dairies share such close (i.e., adjacent) proximity.
Further work may be warranted to explore and describe the unmeasured factors that account for operational-level variation in Salmonella prevalence. If such factors can be identified, an assessment of their practicality (i.e., their broad adoptability) and efficacy would be logical next steps (Wheeler et al., 2014). That said, this work highlights the significant variation that occurs in fecal shedding of cattle among operations within a small geographic region, as well as within an operation, hence research efforts may be better spent designing and evaluating interventions strategies than attempting to decipher the multitude of factors that may account for this variability. Regardless of management practices implemented on an operation, the environmental conditions that exist in this region of the United States, particularly wind, have the potential to affect pathogen dispersion both within and across operations. Such that efforts, such as vaccination, may have limited efficacy due to the infection pressure resulting from the potential spread of Salmonella from one operation to the other. This could explain the elevated Salmonella incidence and the seemingly continual exposure to Salmonella, particularly in densely populated cattle producing regions such as examined in the current research.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethical review and approval was not required for the animal study because fecal samples were collected from pen floors with no distress to the animals.
DH and GL conceived the research and conducted sample collection. TE and TB cultured all the samples. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Arthur, T. M., Brichta-Harhay, D. M., Bosilevac, J. M., Guerini, M. N., Kalchayanand, N., Wells, J. E., et al. (2008). Prevalence and characterization of Salmonella in bovine lymph nodes potentially destined for use in ground beef. J. Food Prot. 71, 1685–1688. doi: 10.4315/0362-028X-71.8.1685
Brown, T. R., Edrington, T. S., Loneragan, G. H., Hanson, D. L., Malin, K., Ison, J. J., et al. (2015). Investigation into possible differences in Salmonella prevalence in the peripheral lymph nodes of cattle derived from distinct production systems and of different breed types. J. Food Prot. 78, 2081–2084. doi: 10.4315/0362-028X.JFP-15-198
Dodd, C. C., Renter, D. G., Thompson, D. U., and Nagaraja, T. G. (2011). Evaluation of the effects of a commercially available Salmonella Newport siderophore receptor and porin protein vaccine on fecal shedding of Salmonella bacteria and health and performance of feedlot cattle. Am. J. Vet. Res. 2, 239–247. doi: 10.2460/ajvr.72.2.239
Edrington, T. S., Garcia Buitrago, J. A., Hagevoort, G. R., Loneragan, G. H., Bricta-Harhay, D. M., Callaway, T. R., et al. (2018). Effect of waste milk pasteurization on fecal shedding of Salmonella in preweaned calves. J. Dairy Sci. 101, 9266–9274. doi: 10.3168/jds.2018-14668
Edrington, T. S., Hume, M. E., Looper, M. L., Schultz, C. L., Fitzgerald, A. C., Callaway, T. R., et al. (2004c). Variation in the faecal shedding of Salmonella and E. coli O157, H7. in lactating dairy cattle and examination of Salmonella genotypes using pulsed-field gel electrophoresis. Lett. Appl. Microbiol. 38, 366–372. doi: 10.1111/j.1472-765X.2004.01495.x
Edrington, T. S., Schultz, C. L., Bischoff, K. M., Callaway, T. R., Looper, M. L., Genovese, K. J., et al. (2004b). Antimicrobial resistance and serotype prevalence of Salmonella isolated from dairy cattle in the southwestern United States. Microbial Drug Res. 10, 51–56. doi: 10.1089/107662904323047808
Edrington, T. S., Schultz, C. L., Genovese, K. J., Callaway, T. R., Looper, M. L., Bischoff, K. M., et al. (2004a). Examination of heat stress and stage of lactation (early versus late) on fecal shedding of E. coli O157, H7. and Salmonella in dairy cattle. Foodborne Path. Dis. 1, 114–119. doi: 10.1089/153531404323143639
Fedorka-Cray, P. J., Dargatz, D. A., Thomas, L. A., and Gray, J. T. (1998). Survey of Salmonella serotypes in feedlot cattle. J. Food Prot. 61, 525–530. doi: 10.4315/0362-028X-61.5.525
Fitzgerald, A. C., Edrington, T. S., Looper, M. L., Callaway, T. R., Genovese, K. J., Bischoff, K. M., et al. (2003). Antimicrobial susceptibility and factors affecting the shedding of E. coli O157, H7. and Salmonella in dairy cattle. Lett. Appl. Microbiol. 37, 392–398. doi: 10.1046/j.1472-765X.2003.01417.x
Gragg, S. E., Loneragan, G. H., Brashears, M. M., Arthur, T. M., Bosilevac, J. M., Kalchayanand, N., et al. (2013). Cross-sectional study examining Salmonella enterica carriage in subiliac lymph nodes of cull and feedlot cattle at harvest. Foodborne Path. Dis. 10, 368–374. doi: 10.1089/fpd.2012.1275
Heider, L. C., Meiring, R. W., Hoet, A. E., Gebreyes, W. A., Funk, J. A., and Wittum, T. E. (2008). Evaluation of vaccination with a commercial subunit vaccine on shedding of Salmonella enterica in subclinically infected dairy cows. J. Am. Vet. Med. Assoc. 233, 466–469. doi: 10.2460/javma.233.3.466
Hermesch, D. R., Thompson, D. U., Loneragan, G. H., Renter, D. R., and White, B. J. (2008). Effects of a commercially available vaccine against Salmonella enterica serotype Newport on milk production, somatic cell count, and shedding of Salmonella organisms in female dairy cattle with no clinical signs of salmonellosis. Am. J. Vet. Res. 9, 1229–1234. doi: 10.2460/ajvr.69.9.1229
LeJeune, J. T., Besser, T. E., Merrill, N. L., Rice, D. H., and Hancock, D. D. (2001). Livestock drinking water microbiology and the factors influencing the quality of drinking water offered to cattle. J. Dairy Sci. 84, 1856–1862. doi: 10.3168/jds.S0022-0302(01)74626-7
Li, M., Malladi, S., Hurd, H. S., Goldsmith, T. J., Brichta-Harhay, D. M., and Loneragan, G. H. (2015). Salmonella spp. in lymph nodes of fed and cull cattle: relative assessment of risk to ground beef. Food Control 50, 423–434. doi: 10.1016/j.foodcont.2014.09.011
Loneragan, G. H., Thompson, D. U., McCarthy, R. M., Webb, H. E., Daniels, A. E., Edrington, T. S., et al. (2012). Salmonella diversity and burden in cows on and culled from dairy farms in the Texas high plains. Foodborne Path. Dis. 9, 549–555. doi: 10.1089/fpd.2011.1069
Webb, H. E., Brichta-Harhay, D. M., Brashears, M. M., Nightingale, K. K., Arthur, T. M., Bosilevac, J. M., et al. (2017). Salmonella in peripheral lymph nodes of healthy cattle at slaughter. Front. Microbiol. 8, 2214. doi: 10.3389/fmicb.2017.02214
Keywords: Salmonella, feedlot cattle, dairy cattle, feces, water
Citation: Hanson DL, Loneragan GH, Brown TR and Edrington TS (2022) Salmonella Prevalence Varies Over Time and Space in Three Large, Adjacent Cattle Operations in the Southwestern United States. Front. Anim. Sci. 3:878408. doi: 10.3389/fanim.2022.878408
Received: 18 February 2022; Accepted: 04 March 2022;
Published: 13 April 2022.
Edited by:
Todd Riley Callaway, University of Georgia, United StatesReviewed by:
Lisa Gorski, Agricultural Research Service, United StatesCopyright © 2022 Hanson, Loneragan, Brown and Edrington. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tom S. Edrington, dGVkcmluZ3RvbkBkaWFtb25kdi5jb20=
†Present address: Devin L. Hanson and Tom S. Edrington, Diamond V Mills, Cedar Rapids, IA, United States
Tyson R. Brown, Cargill Inc., Wichita, KS, United States
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.