
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Anim. Sci., 26 May 2022
Sec. Animal Nutrition
Volume 3 - 2022 | https://doi.org/10.3389/fanim.2022.872776
This article is part of the Research TopicThe Relationship of Animal Health and Management to Food SafetyView all 6 articles
 Emily M. Davis1
Emily M. Davis1 Kayla P. Wallace2
Kayla P. Wallace2 Michael J. Cruz Penn2
Michael J. Cruz Penn2 Amy L. Petry3
Amy L. Petry3 Rand Broadway4
Rand Broadway4 Nicole C. Burdick Sanchez4
Nicole C. Burdick Sanchez4 Jeffery A. Carroll4
Jeffery A. Carroll4 Michael A. Ballou1*
Michael A. Ballou1*The objective was to investigate the effects of supplementing increasing concentrations of PowerGuard (PG), a micronized ceramic particle, to weaned pigs on health and performance following a Salmonella enterica serotype Typhimurium infection. Forty barrows were transported to the USDA facility in Liberty, TX, USA. Pigs were randomly assigned to one of five treatments (n = 8): (1) uninfected control (CON), no Salmonella typhimurium (ST) and no PG treatment; (2) infected control (ST), infected with ST but no PG treatment; (3) PG0.05, infected with ST and supplemented with 0.05% PG; (4) PG0.25, infected with ST and supplemented with 0.25% PG; and (5) PG0.50, infected with ST and supplemented with 0.5% PG. All pigs were enrolled at 21.5 ± 1.33 days of age and did not differ in initial BW (1.98 ± 0.09 kg). Pigs were anesthetized to insert temperature recording devices into the abdominal cavity. Pigs were offered feed and water ad libitum. Pigs in ST, PG0.05, PG0.25, and PG0.50 were infected orally with 1.75 × 107 colony-forming units of Salmonella typhimurium on day 7. Pig body weights and peripheral blood samples were collected on days 0, 7, 10, 14, and 21. Pigs were harvested on day 21 and ileum and liver samples were collected for histopathological analyses. There was no treatment difference for final BW (P ≥ 0.201). There was a tendency (P = 0.087) for a treatment difference in the fecal score; ST and PG0.50 had more loose fecal scores than CON and PG0.25. There was a treatment × time interaction for intraperitoneal temperature (P < 0.0001); PG0.05, PG0.25, and PG0.50 had attenuated febrile responses during the acute post-infection period compared with ST. There was a treatment × time interaction for total leukocyte counts (P = 0.007); PG treatments reduced leukocytosis post-infection compared with ST. Supplementing PG0.25 improved many health and performance variables when pigs were infected with Salmonella Typhimurium. Furthermore, supplementing PG0.05 attenuated the febrile response and many hematological variables. However, supplementing PG0.5 did not improve many aspects of health or performance. Therefore, supplementing PowerGuard between 0.05 and 0.25% of the diet may play a role in protecting weaned pigs from disease caused by Salmonella.
Many Salmonella enterica spp. are zoonotic pathogens that can have negative health and productive impacts on pigs and may also increase human exposure to food-borne pathogens. When S. enterica serotype Typhimurium colonizes the intestinal tract of a host, bacteria can invade local lymph nodes throughout the gastrointestinal tract and colonize there (Rostagno et al., 2011; Broadway et al., 2015). The invasion of internal organs, shedding via feces, and carcass contamination from digesta, all create potential reservoirs for disruption in pre-harvest food safety in the swine industry. Human infections of S. enterica serotype typhimurium ranked 3rd of total reported cases, accounting for 9.8% of total culture-confirmed Salmonella cases in the United States. From herd monitoring data collected in part by the USDA, pigs accounted for 13% of clinical Salmonella typhimurium cases in submitted animal samples (Center for Disease Control, 2013, 2016). The European Food Safety Authority (EFSA) approximates that pork is responsible for 10–20% of total Salmonella infections in humans in the European Union (EFSA, 2011). Furthermore, it was previously reported that finishing pigs can carry high concentrations of Salmonella typhimurium, between 103 and 105 CFU/g, in the gastrointestinal tract and associated lymph nodes for up to 4 weeks post-infection, while showing no clinical signs of disease (Rostagno et al., 2011). Along with the contamination potential, there are health and production losses that S. enterica infections can cause in pigs prior to harvest. In the EU, the combined cost of human and pig production losses totals 600 million Euros (FCC, 2010); more specifically, Danish reports state Salmonella causes roughly a 3 kg BW loss per infected pig (Lo Fo Wong and Hald, 2000). In the swine industry, S. enterica treatment commonly involves antibiotics. However, with the growing concern of antibiotic resistance and the increase in restrictions on antibiotic usage, the shift to nutraceutical strategies is increasing. Currently, clay is being used in the agriculture industry as a mycotoxin binder. Previously, weanling pigs fed mycotoxin contaminated diets with clay supplementation reported that the pigs fed clay either increased or maintained performance when compared to pigs not supplemented with clay (Schell et al., 1994; Thieu et al., 2008; Jiang et al., 2010; Wang et al., 2012). With a high adsorption capacity for mycotoxins, clays have been under recent investigation for the ability to adsorb to gram-negative bacteria, including S. enterica and Escherichia coli (Song et al., 2012; Pardo et al., 2020).
The nutraceutical utilized in the current study is a thermally processed montmorillonite-based micronized ceramic particle. When montmorillonite clay is thermally processed, there is the removal of the outer- and inter-layer water, which changes the physical nature of the clay to become a ceramic particle (Bala et al., 2000). Once this ceramic is created, the particle cannot be rehydrated back into a lump of clay. Along with the heat processing, there are changes in physical porosity as well. Thus, the myriad of potential adsorptive mechanisms may allow for ceramic particles to better adsorb and mediate the effects of gram-negative pathogenic bacteria such as Salmonella typhimurium. The pathogen load in pigs prior to and during harvest is the ultimate determinant of contamination along the farm-to-consumption pathway and determines the infection potential in the post-harvest and consumption stage (EFSA, 2011).
Therefore, the objective of the current study was to investigate the effects of supplementing different levels of a commercial ceramic particle to weaned pigs during a Salmonella typhimurium infection on performance and health.
Salmonella enterica serotypes Dublin and Typhimurium were utilized in a preliminary study to determine the capacity of multiple adsorbents in vitro. An overnight culture from each strain was created by inoculation of 20 ml of sterile tryptic soy broth and incubation at 37°C. The overnight culture was quantified by spread plate serial dilutions on tryptic soy agar plates. Dilutions with 30–150 colony forming units (CFU) were counted to determine the concentration of each isolate (CFU/ml). Approximate working concentrations of each serotype were performed in sterile phosphate-buffered saline (PBS) (2 × 106 CFU/ml). PowerGuard (PG; MB Nutritional Sciences, Lubbock, TX, USA), activated carbon (Sigma Aldrich, St. Louis, MO, USA), bentonite #1, and bentonite #2 were all diluted to 5 mg/ml concentrations in sterile PBS. Equal volumes of working bacterial solution and binder solutions were mixed into 50 ml conical tubes, along with negative control samples mixing an equal volume of each bacterial solution and sterile PBS. All cultures were performed in triplicate, and each tube was rocked for 2 h at 37°C. Then, each culture, including negative controls, was filtered through a series of sterile Whatman filter paper. First, a 25-micron paper (Whatman Grade 54, Whatman plc, Little Chalfont, Buckinghamshire, United Kingdom) followed by an 8-micron paper (Whatman Grade 2) to remove most of the adsorbent particles but to still allow un-adsorbed Salmonella spp. to flow through the filter paper into the filtrate. The final filtrate was clear; however, this methodology may underestimate the adsorptive capacities due to some adsorbent particle sizes being smaller than 8 microns, leading to some smaller adsorbent particles remaining in the filtrate. Each filtrate sample was serially diluted in sterile PBS and spread plated on tryptic soy agar. Plates were incubated overnight at 37°C and plates with CFU counts of 30–150 were counted. Data were reported as the log10 reduction from the control to the adsorbent solution for each isolate.
For imaging purposes utilizing scanning electron microscopy, samples were prepared by the addition of 1.75 × 109 CFU/ml of Salmonella typhimurium in 10 ml of deionized water with 25 mg of PowerGuard. The samples were incubated at 37°C for 2 h before being fixed. The samples were then chemically fixed with 2% glutaraldehyde for 1 h, then washed with deionized water three times while centrifuging between each wash. The solutions were then quickly frozen in a dry ice and ethanol bath for 10 min followed by freezing overnight. The dried samples were mounted onto sample mounts with double-sided carbon tape and coated with a thin layer of Iridium to provide conductivity. Scanning electron microscopy (SEM) images were taken by Zeiss crossbeam 540 at 5 kiloelectron volts (kV) using secondary electrospray ionization detector (Zeiss Meditec Inc., Dublin, CA, USA). Samples were observed at the Texas Tech University Microscopy Core Laboratory by SEM with the assistance of Dr. Zhao.
All procedures in this study were ap-proved by the USDA-ARS, Livestock Issues Research Unit's Institutional Animal Care and Use Committee (IACUC protocol #LIRU-2120S). The study was conducted in May 2021. Forty crossbred newly weaned barrows (L280 × Camborough, PIC, Inc., Hendersonville, TN, USA) were weighed (1.98 ± 0.09 kg) and transported 9 km from the Texas Tech Swine Unit to the swine barn at the Livestock Issues Research Unit. Pigs were transported mid-day when the temperature was 27°C. Pigs were randomly assigned to one of five treatments (n = 8): (1) Uninfected Control (CON), no Salmonella typhimurium (ST) administered and no treatment in the diet; (2) Infected Control (ST), infected with ST on day 7 but no treatment in the diet; (3) PG0.05, infected with ST on day 7 with 0.05% PG of the diet; (4) PG0.25, infected with ST on day 7 and 0.25% PG of the diet; and (5) PG0.5, infected with ST on day 7 and 0.5% PG of the diet. PowerGuard is a thermally processed ceramic particle that is micronized to a median particle size of 40 μm, with 90% of the particles being <100 μm. The barn was maintained at 32.2°C for days 0 and 1. The environmental temperature was then decreased by 0.56°C each day. The final barn temperature of 23.8°C was reached on day 16 and maintained for the duration of the study. Pigs were housed in individual stainless-steel pens (1.2 × 0.6 m) equipped with stainless steel automatic feeders and nipple waterers. All pigs had ad libitum access to feed and in order to allow ad libitum access to the feed, the automatic feeders had the gate open 7.5 cm, which likely allowed for pigs to waste some feed. Therefore, feed intake data and feed efficiency data are reported as feed disappearance. All pigs were enrolled on the same day, 21.5 ± 1.33 days of age, and immediately upon arrival to the facility were individually anesthetized briefly to insert temperature measuring loggers (Star-Offi DST micro-T; Metermall USA, Marysville, Ohio) into their abdominal cavity. This methodology is previously described (Burdick Sanchez et al., 2017). Peripheral blood (10 ml) was drawn in EDTA vacutainer tubes while each pig was anesthetized via jugular venipuncture. The entire process was completed in <10 min. Pigs had ad libitum access to feed and water. Diets were formulated to meet or exceed the NRC (2012) nutrient recommendations (NRC, 2012). They received the experimental diets over two dietary phases from day 0 to 6 (P1) and day 7 to 21 (P2), respectively. Samples of each diet were collected at each feeding and composited by period before being analyzed for dry matter, ash, crude protein, and ether extract by a commercial lab using wet chemistry methods (ServiTech, Amarillo, TX, USA). Formulated ingredients and diet nutrient compositions for both P1 and P2 are shown in Table 1.
Pigs in treatments ST, PG0.05, PG0.25, and PG0.5 were all infected on day 7 with 1.75 × 107 CFU of Salmonella typhimurium that was resistant to nalidixic acid and in the mid-logarithmic phase of growth ATCC 14028, which was originally isolated from cardiac tissue in chickens. The infection and identification methods were adapted from Broadway et al. (2015). To summarize, an individual colony from a streak plate was placed into trypticase soy broth with nalidixic acid (50 mg/L) and incubated at 37°C agitating at 200 rpm overnight. Then, 100 μl of the overnight culture was added to 20 ml of fresh trypticase soy broth with nalidixic acid (50 mg/L) incubated at 37°C agitating at 200 rpm until reaching an optical density of 0.8 at 450 nm. This was determined previously to be the mid-logarithmic phase of growth. This broth was diluted by the addition of 1 ml of the bacterial broth with 9 ml of sterile saline for a final concentration of 1.75 × 107 CFU per the 10 ml. Sodium bicarbonate was also added to buffer the bacteria at 1 g/10 ml. This buffered bacterial solution was then administered by oral gavage utilizing a 10 ml syringe with an attached 1 mm internal diameter silicone tube, 6 mm in length, administered behind the tongue slowly as the pig swallowed within 10 min of creating the working bacteria solution. Uninfected control pigs (CON) were administered 10 ml of sterile saline with 1 g/10 ml of sodium bicarbonate prior to any handling of infectious doses or pigs in any other treatment. To estimate the infection dose, the working bacteria solution was serially diluted on trypticase soy agar with nalidixic acid at 50 mg/L and incubated overnight at 37°C. Plates were counted with colony counts between 30 and 150, and the infection dose was determined to be 1.75 × 107 CFU.
Individual pig body weights and peripheral blood samples were collected on days 0, 7, 10, 14, and 21. Peripheral blood (10 ml) was collected by jugular venipuncture via a 20-gauge needle 1.5 inches in length in EDTA vacutainer tubes while the pig was restrained in a v-trough. Complete blood count analysis was performed on the samples using an IDEXX analyzer with the swine-specific algorithm (ProCyte Hematology Analyzer, IDEXX, Westbrook, ME, USA). Fecal swabs for bacteria prevalence were collected following Broadway et al. (2015). Two fecal swabs were collected from fresh feces from each crate beginning on day 7 prior to Salmonella typhimurium administration. As described by Broadway et al. (2015), one swab was placed directly into Rappaport-Vassiliadis (RV) broth (Thermo Fischer Scientific, Waltham, MA, USA) and the other swab was placed directly into Difco Tetrathionate (TT) broth (Thermo Fischer Scientific, Waltham, MA, USA). Fresh fecal swabs were collected every morning from day 7 through day 21 by two trained individuals only swabbing fresh feces. If no fresh feces were present, a fecal swab was taken directly from the rectum for that sample day. The fecal swabs in the RV broth were incubated overnight at 42°C, and the TT broth swabs were incubated overnight at 37°C. All samples were then streak plated on Xylose Lysine Deoxycholate Agar (XLD) (Thermo Fischer Scientific, Waltham, MA, USA). The plates were then incubated overnight at 37°C to determine if there was Salmonella colony growth via examination for the presence or absence of black colonies. Enrichment samples were recorded as either positive or negative. At harvest, a section of the ileum, as well as associated mesenteric lymph nodes, were collected, rinsed, and immediately dipped into boiling water for no more than 5 s to remove potential external contamination from the harvest process for later bacterial quantification and enrichment. These samples were then stomached in a 1:10 dilution of sterile 1X PBS and sampled three subsequent times. Two swabs were collected from each tissue sample and grown overnight in both TT and RV enrichment broth and subsequently streak plated on differential XLD agar as stated previously in the fecal swabs. These samples provided enrichment data on only the presence of ST in the tissue samples and not quantification. The third sample from these stomached tissues was serially plated for Salmonella typhimurium quantification purposes on XLD agar as well.
On day 21, pigs were humanely euthanized. Tissue samples were collected from both the ileum and liver, washed with sterile saline to remove blood and digesta, then placed directly into 10% buffered formalin for histological analyses after hematoxylin and eosin staining. Slides were created at the Anatomical Pathology Lab at the Texas Tech University Health Sciences Center (Texas Tech University Health Science Center, Lubbock, TX, 79415). Slides were analyzed with an anatomical histopathologist at the Texas Tech School of Veterinary Medicine and scanned via an Olympus slide scanner for imaging and count purposes (Olympus Life Sciences, Center Valley, PA, 18034). The method of scoring histology slides is as follows. Inflammation scoring was on a 0 to 3, scale with 0 = normal tissue; 1 = mild inflammation; 2 = moderate inflammation; and 3 = marked inflammation. Villi blunting: 0 = long, slender villi; 1 = 75% of normal height; 2 = 50% of normal height; and 3 = 25% of normal height. Villi epithelium score: 0 = single layer of the columnar epithelium; 1 = degeneration, vacuolation, or separation of focal areas of the superficial epithelium; 2 = more marked changes and some focal loss of epithelium; and 3 = widespread ulceration of the epithelium surface. Lacteal dilation score: 0 = central lacteal represents approximately 25% of the width of the villi; 1 = represents approximately 50% of the villi width; 2 = 75% of the villi width; and 3 = dilated to 100% of the villi width. Goblet cell count in the crypt and intraepithelial lymphocyte counts are the number of cells counted per 50 epithelial cells. Lamina propria counts were the number of each leukocyte (lymphocytes, neutrophils, and eosinophils) observed between crypts in an x40 field. Connective tissue (CT) prominence scores ranged from 0 to 3, with a score of 0 = no prominent CT, 1 = slightly prominent CT, not diffuse; 2 = more prominent CT, throughout the sample, and 3 = all CT present is severe and prominent. Lymphocyte foci scores ranged from 0 to 3, with 0 = no lymphocyte aggregates present; 1 = few lymphocyte aggregates present; 2 = multiple lymphocyte aggregates present; and 3 = severe and diffuse lymphocyte aggregates present throughout the sample. Lymphocyte foci area was measured in μm and conducted on up to 5 foci/samples and averaged per sample. The neutrophil scores ranged from 0 to 3, with 0 = no neutrophils present in lymphocyte aggregates, 1 = <5 neutrophils within lymphocyte aggregates; 2 = between 5 and 10 neutrophils within the lymphocyte aggregates; and 3 = more than 10 neutrophils within the lymphocyte aggregates.
Preliminary in vitro adsorption data were analyzed using the GLM procedure of SAS with adsorbent, Salmonella enterica serotype, and their interaction as the fixed effects. Continuous, repeatedly measured data were analyzed using the Mixed procedure in SAS (SAS 9.4, Cary, NC, USA). The model included fixed effects of treatment, time, and treatment × time. Initial body weight was included in the model as a covariate and retained in the model if it was significant. The subject of the repeated statement was pig nested within treatment. All covariance and variance structures were analyzed, and the most appropriate model was selected based on the smallest Bayesian information criterion. All continuous, non-repeated data were analyzed using a restricted maximum-likelihood ANOVA with treatment included as the fixed effect using the MIXED procedure of SAS. Count data were analyzed using the GLIMMIX procedure of SAS with treatment as the fixed effect and fit with a Poisson distribution using the log link function. Differences of P ≤ 0.05 were considered significant and a tendency was reported when 0.05 < P ≤ 0.1. Treatment × time interactions found to be significant were further evaluated using a Duncan adjustment to control for familywise error. All pairwise comparisons at each significant time point were reported.
Measures of the capacity for different adsorbents to reduce bacteria concentrations reported as log base 10 reductions, in the final filtrate are reported in Figure 1 for Salmonella enterica serotypes Dublin and Typhimurium. There was no adsorbent treatment × Salmonella enterica serotype interaction (P = 0.696); however, there was an adsorbent treatment difference for log10 reduction of both Salmonella enterica serotypes (P < 0.0001; Figure 1). Both PowerGuard and activated carbon had a greater log10 reduction of both Salmonella enterica serotypes when compared to two bentonite clays. The reported electron micrograph shows Salmonella typhimurium, highlighted in yellow, adsorbed to the surface of PowerGuard (Figure 2).
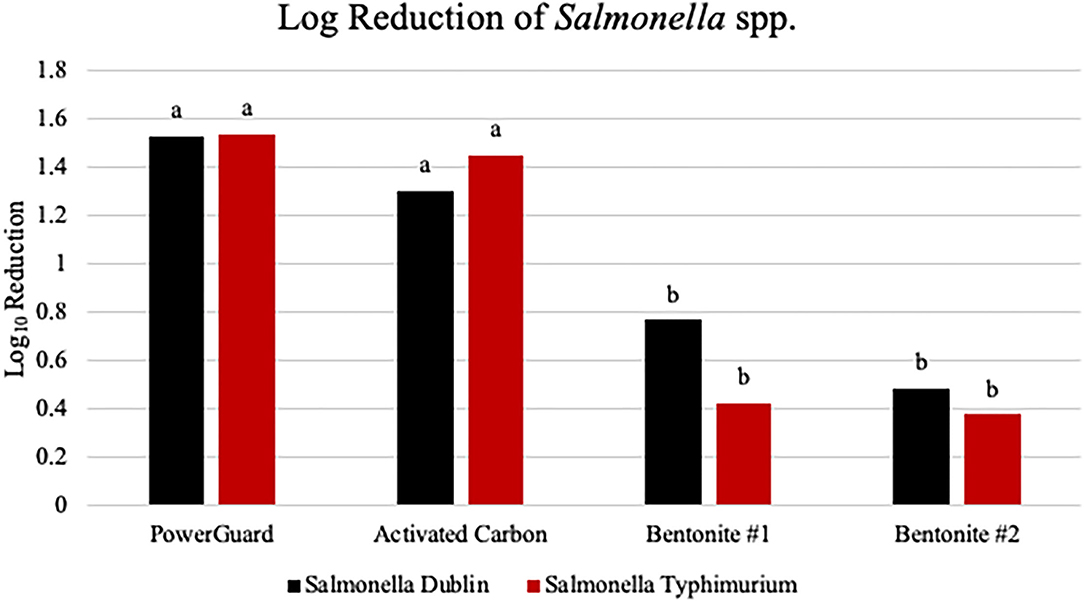
Figure 1. Measures of the capacity for different adsorbents to reduce bacteria concentrations, reported as log10 reduction, in final filtrate for Salmonella enterica serotypes Dublin and Typhimurium. There was no adsorbent treatment × S. enterica serotype interaction (P = 0.696). However, there was an adsorbent treatment difference for log10 reduction of both Salmonella enterica serotypes (P < 0.0001). The largest scanning electron microscopy (SEM) is reported as log10 reduction and is 0.2123. Bars with different superscripts are statistically different (P < 0.05).
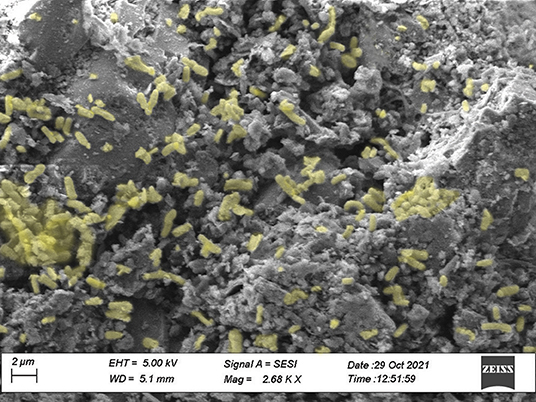
Figure 2. The reported electron micrograph shows Salmonella Typhimurium, highlighted in yellow, adsorbed to the surface of PowerGuard. SEM images were taken by Zeiss crossbeam 540 at 5 kiloelectron volts (kV) using secondary electrospray ionization detector (Zeiss Meditec Inc., Dublin, CA, USA). Samples were observed at the Texas Tech University Microscopy Core Laboratory by SEM with the help of Dr. Zhao.
Measures of performance are reported in Table 2. There was no difference among treatments for initial body weight or final body weight (P ≥ 0.201). There was a treatment × time interaction for feed disappearance (P = 0.028; Figure 3). There was no treatment or treatment × time interaction for ADG (P = 0.312). There was a tendency for a treatment × time interaction for feed disappearance to gain (P = 0.059; Figure 4); where on days 11-14 PG0.25 had decreased feed disappearance to gain when compared to both PG0.5 and CON treatments.
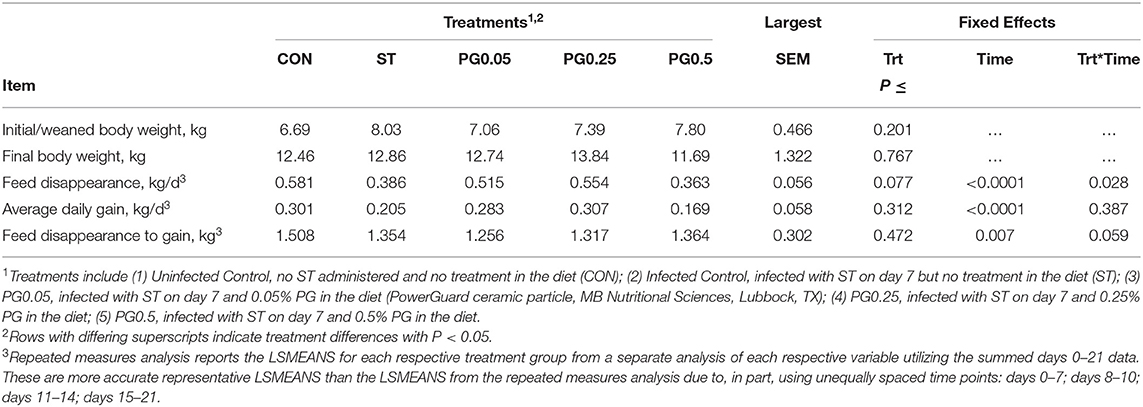
Table 2. Performance effects of a ceramic particle fed to pigs after a Salmonella enterica serotype typhimurium infection.
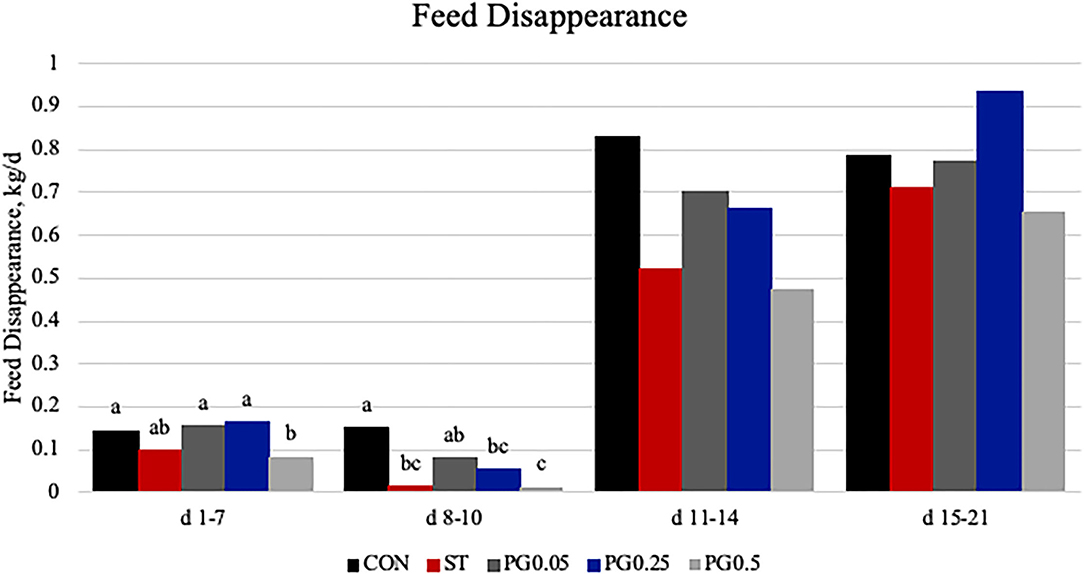
Figure 3. Feed disappearance was measured on days 7, 10, 14, and 21. There was a treatment × time interaction (P = 0.0283). There was a tendency for a difference from days 1 to 7 (P = 0.0826), where the PG0.5 treatment was decreased when compared to the CON, PG0.05, and PG0.25 treatments (P ≤ 0.065). There was a treatment difference on days 8–10 (P = 0.0023), where CON was greater than all other treatments and PG0.05 was greater than both the ST and PG0.5 treatments (P ≤ 0.0353). The largest SEM per time point is expressed as kg and includes 0.026, 0.082, 0.1586, and 0.1248 for days 1–7, 8–10, 11–14, and 15–21, respectively. Bars with different superscripts are statistically different (P < 0.05).
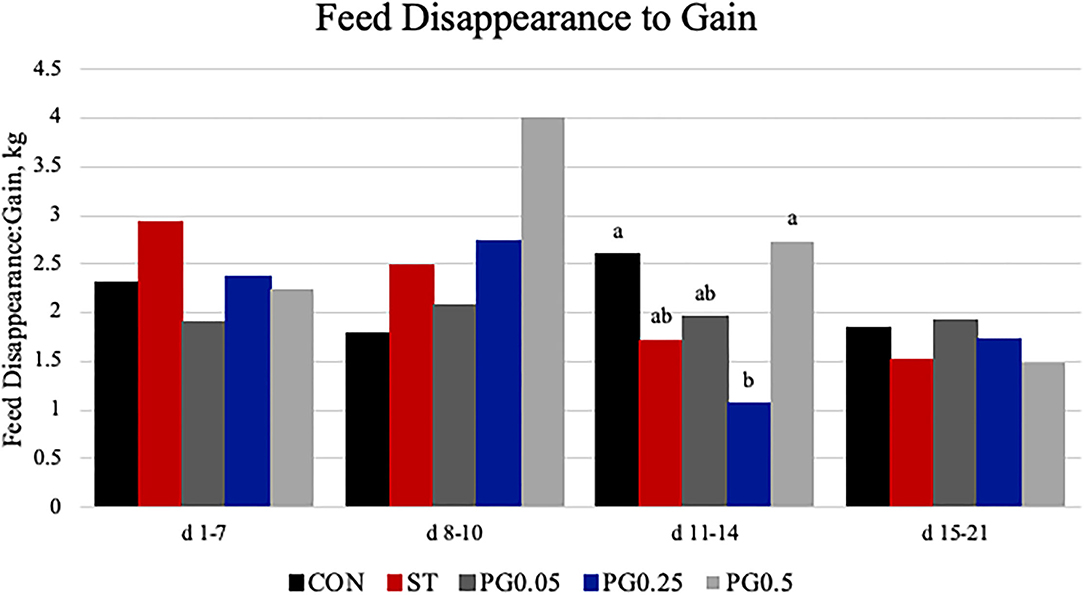
Figure 4. Feed disappearance to gain was calculated from the feed disappearance and change in weight gain from days 1 to 7, 8 to 10, 11 to 14, and 15 to 21. There was a tendency for a treatment × time interaction (P = 0.0593). On days 11–14, there was a tendency for treatment difference (P = 0.0528), where treatment PG0.25 had decreased FD:G when compared to both PG0.5 and CON treatments (P ≤ 0.0123). Largest SEM per time point is reported in kg and include 0.8118, 0.9323, 0.6647, 0.3707 for days 1–7, 8–10, 11–14, and 15–21, respectively. Bars with different superscripts are statistically different (P < 0.05).
Hematological counts are reported in Table 3. There was a treatment difference for red blood cell count (P = 0.03), where the CON treatment had increased red blood cell counts when compared to the ST and PG0.5 treatments, and PG0.05 and PG0.25 treatments were intermediate. There was a tendency for a treatment × time interaction for hematocrit (P = 0.0837; Figure 5). There was a treatment difference for hemoglobin (P = 0.0003), where the ST and PG0.5 treatments had decreased hemoglobin concentrations when compared to all other treatments. There was a tendency for a treatment × time interaction for reticulocyte counts (P = 0.1; Figure 6). There was no treatment difference for platelet count (P = 0.163). There were treatment differences in mean corpuscular volume (MCV) and mean corpuscular hemoglobin (MCH) (P ≤ 0.019), where the ST and PG0.5 had decreased MCV and MCH when compared to all other treatments. There was a treatment × time interaction for total leukocyte count (P = 0.0074; Figure 7), where leukocyte counts were increased post-infection for the ST pigs compared to all other treatments. There was a treatment × time interaction for neutrophil count (P = 0.0026; Figure 8), where neutrophil counts were increased post-infection for the ST pigs compared to all other treatments. There was a treatment × time interaction for monocyte count (P = 0.0048; Figure 9), where monocyte counts were increased post-infection for the ST pigs compared to all other treatments. There was no treatment difference for lymphocyte count (P = 0.512).
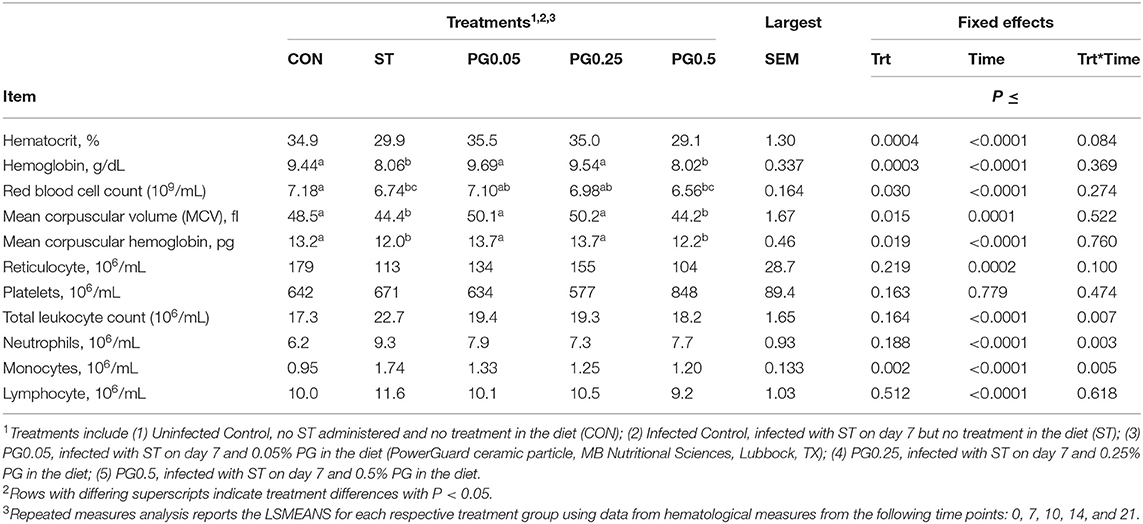
Table 3. Hematological effects of a ceramic particle fed to pigs after a S. enterica serotype typhimurium infection.
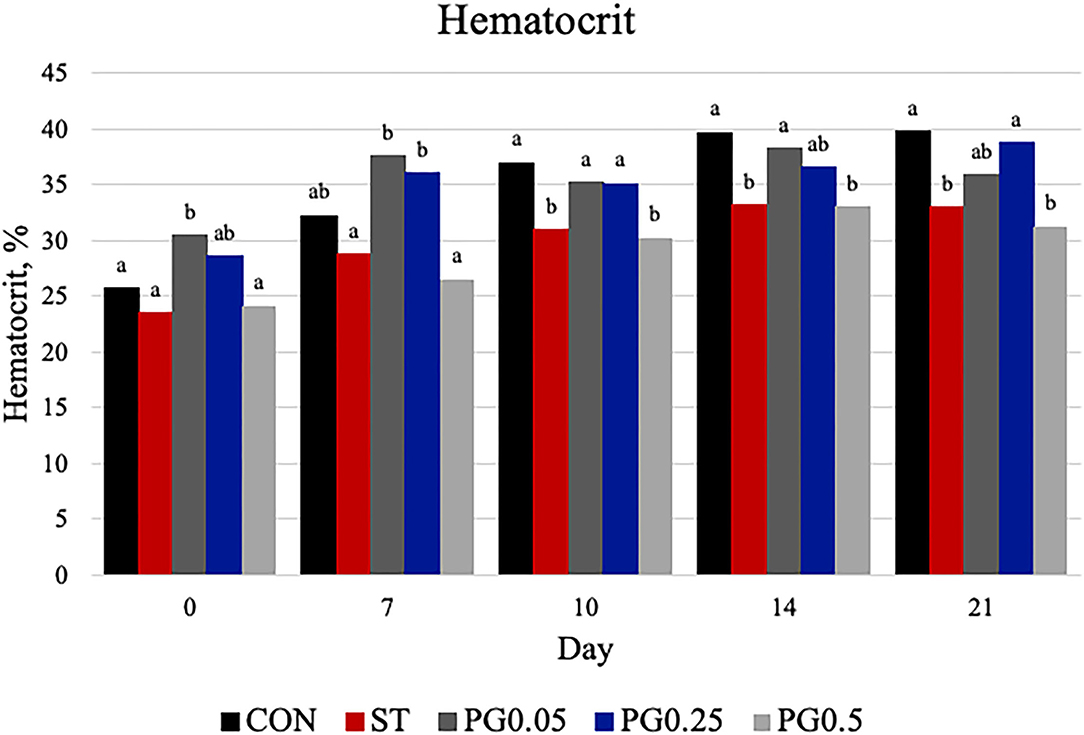
Figure 5. Hematocrit was measured on days 0, 7, 10, 14, and 21. There was a tendency for a treatment × time interaction (P = 0.0837). There were treatment differences at day 0 (P = 0.0083), day 7 (P = 0.0057), day 10 (P = 0.272), day 14 (P = 0.019), and day 21 (P = 0.0037). On day 0, treatment PG0.05 was increased when compared to ST, CON, and PG0.5 (P ≤ 0.023); PG0.25 was increased when compared to ST and PG0.5 (P ≤ 0.025). On day 7, treatment PG0.05 was increased compared to CON, ST, and PG0.5 (P ≤ 0.0135); PG0.25 was increased compared to ST and PG0.5 (P ≤ 0.0135); CON was increased when compared to ST (P = 0.063). On day 10, treatments PG0.5 and ST were decreased compared to all other treatments (P ≤ 0.496). On day 14, treatment PG0.05 was increased compared to ST and PG0.5 (P ≤ 0.0502); CON was increased when compared to ST and PG0.5 (P ≤ 0.0095). On day 21, treatment PG0.5 was decreased when compared to PG0.05, PG0.25, and CON (P ≤ 0.0695); ST was decreased when compared to CON and PG0.25 (P ≤ 0.0245). Largest SEM are reported as percentage and include 2.036, 3.192, 2.423, 2.362, and 2.442 for days 0, 7, 10, 14, and 21, respectively. Bars with different superscripts are statistically different (P < 0.05).
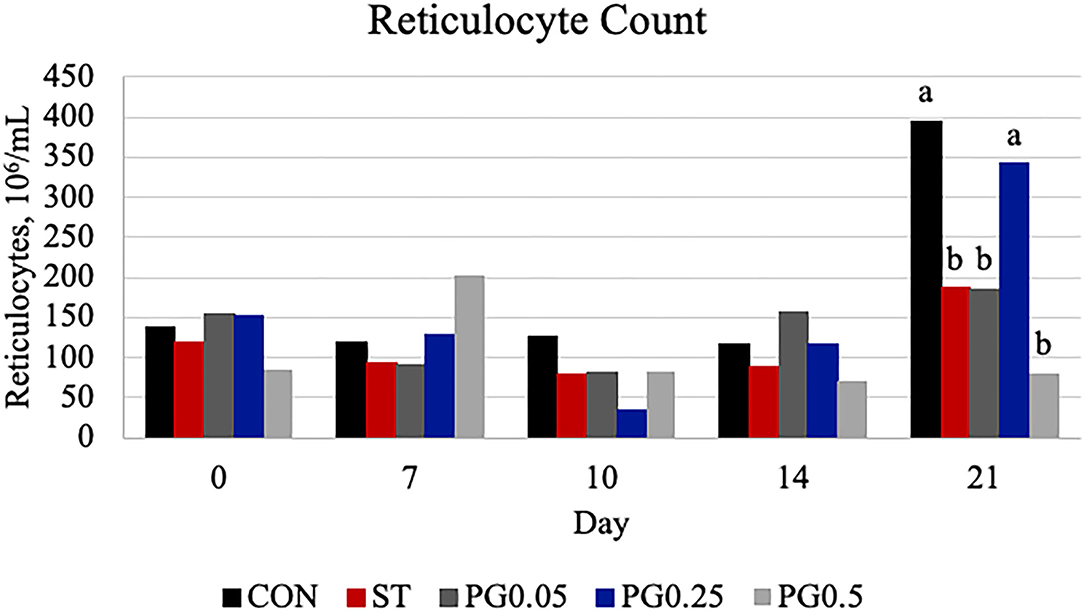
Figure 6. Reticulocyte count was measured on days 0, 7, 10, 14, and 21. There was a tendency for a treatment × time interaction (P = 0.1004). There was a treatment difference on day 21 (P = 0.0013), where CON and PG0.25 were increased when compared to all other treatments (P ≤ 0.0426). The largest SEM per time point is reported as 106/ml and include 82.72, 90.69, 65.83, 57.46, and 87.72 for days 0, 7, 10, 14, and 21, respectively. Bars with different superscripts are statistically different (P < 0.05).
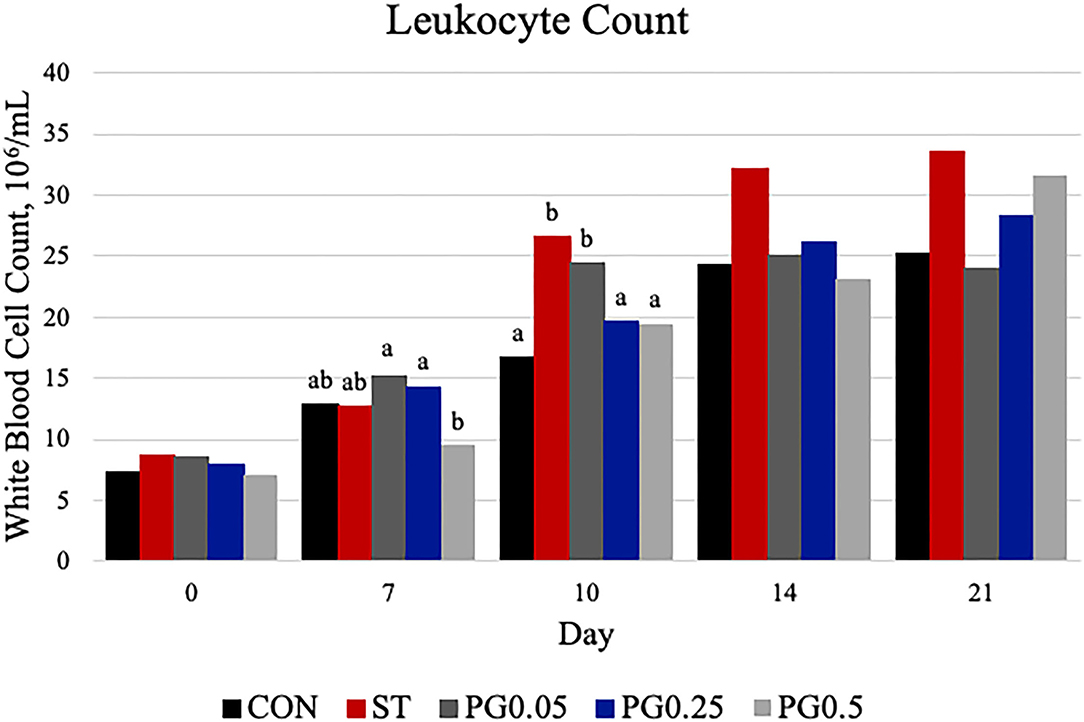
Figure 7. Leukocyte count was measured on days 0, 7, 10, 14, and 21. There was a treatment × time interaction (P = 0.0074). There were treatment differences on day 7 (P = 0.0468) and day 10 (P = 0.0148). On day 7, treatment PG0.5 was decreased when compared to the CON, PG0.05, and PG0.25 treatments (P ≤ 0.0116). On day 10, the ST and PG0.05 treatments were increased when compared to all other treatments (P ≤ 0.0247). The largest SEM per time point is expressed as 106/mL and include 1.506, 1.954, 2.959, 3.601, and 4.648 for days 0, 7, 10, 14, and 21, respectively. Bars with different superscripts are statistically different (P < 0.05).
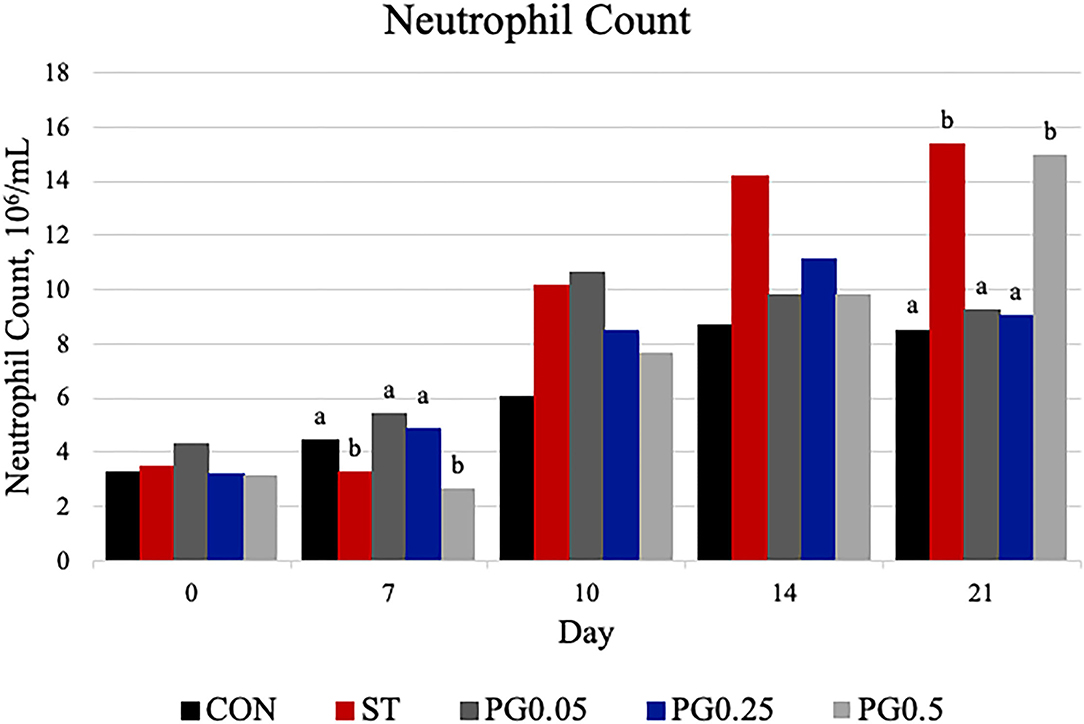
Figure 8. Neutrophil count was measured on days 0, 7, 10, 14, and 21. There was a treatment × time interaction (P = 0.0026). There were treatment differences on day 7 (P = 0.0023) and day 21 (P = 0.0116). On day 7, treatments PG0.05 and PG0.25 were decreased when compared to ST and PG0.5 (P ≤ 0.0078); CON was also increased when compared to PG0.5 (P = 0.0177). On day 21, treatments PG0.05, PG0.25, and CON were decreased when compared to the ST and PG0.5 treatments (P ≤ 0.0313). The largest SEM is reported per time point as 106/ml and include 1.066, 0.7756, 2.243, 2.278, and 2.692 for days 0, 7, 10, 14 and 21, respectively. Bars with different superscripts are statistically different (P < 0.05).
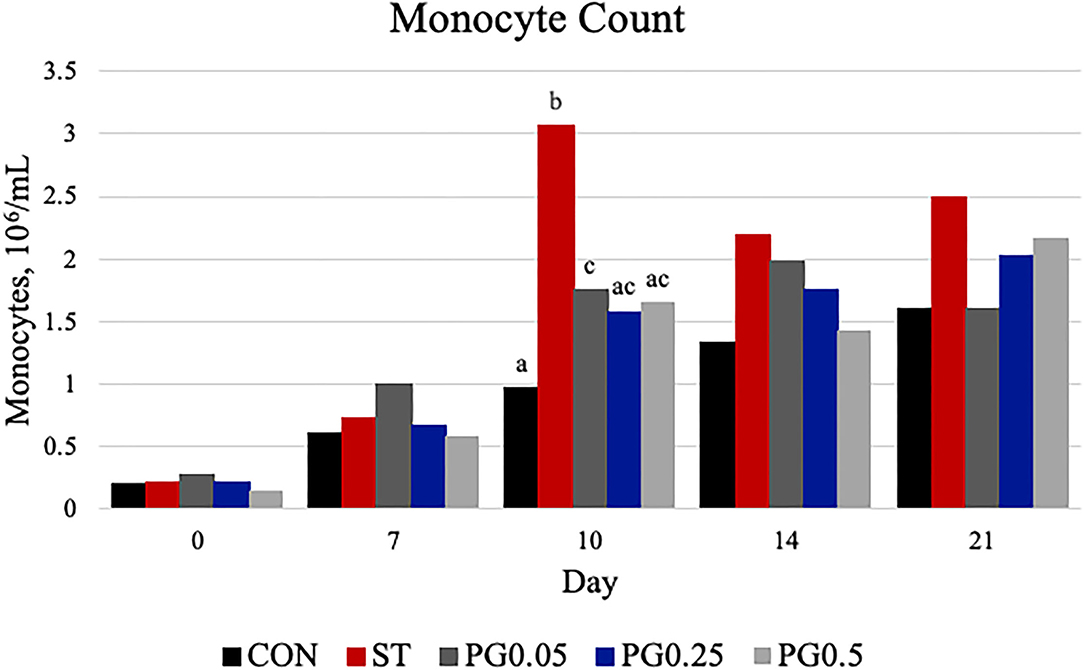
Figure 9. Monocyte count was measured on days 0, 7, 10, 14, and 21. There was a treatment × time interaction (P = 0.0048). There was a treatment difference on day 10 (P = 0.0003), where the ST treatment was increased when compared to all other treatments (P ≤ 0.0038). The largest SEM is reported as 106/ml and include 0.059, 0.2, 0.3925, 0.3714, and 0.5068 for days 0, 7, 10, 14, and 21, respectively. Bars with different superscripts are statistically different (P < 0.05).
Measures of health are reported in Table 4. There was a tendency for treatment difference in the fecal score (P = 0.087), where the ST and PG0.5 treatments tended to have increased fecal scores when compared to the CON and PG0.25 treatments. There were treatment differences for both fecal shedding in RV and TT broth (P < 0.0001), where the CON treatment shed less ST in feces than every other treatment. There was no difference among treatments for the presence of ST in either lymph node tissue or ileum tissue samples (P ≥ 0.311). There was a treatment × time interaction for intraperitoneal temperature (P < 0.0001; Figure 10), where the addition of PG to the diet attenuated the post-infection febrile response.
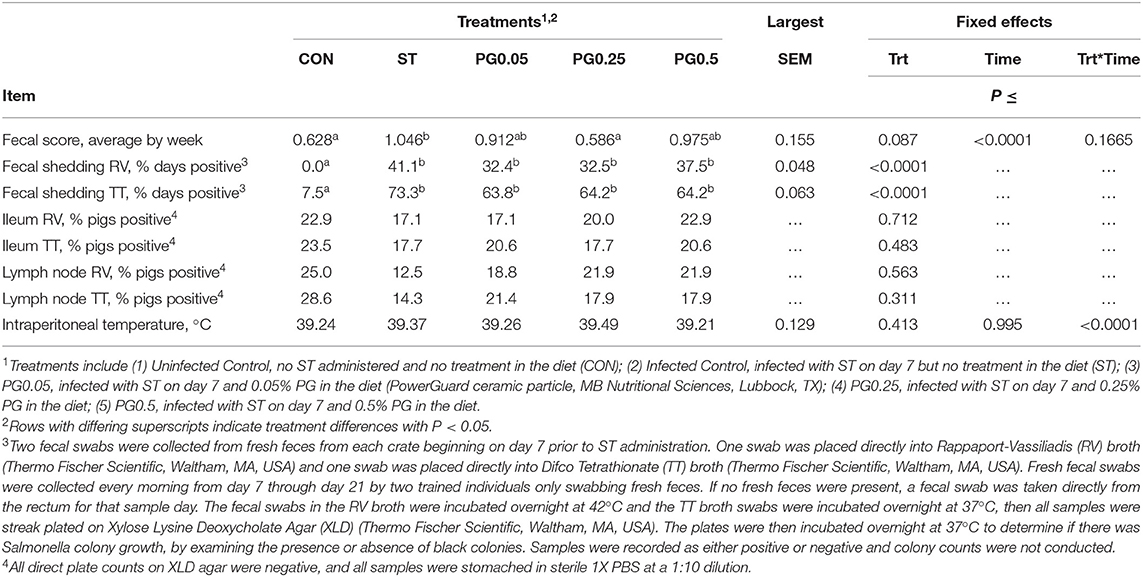
Table 4. Health effects of a ceramic particle fed to pigs after a S. enterica serotype typhimurium infection.
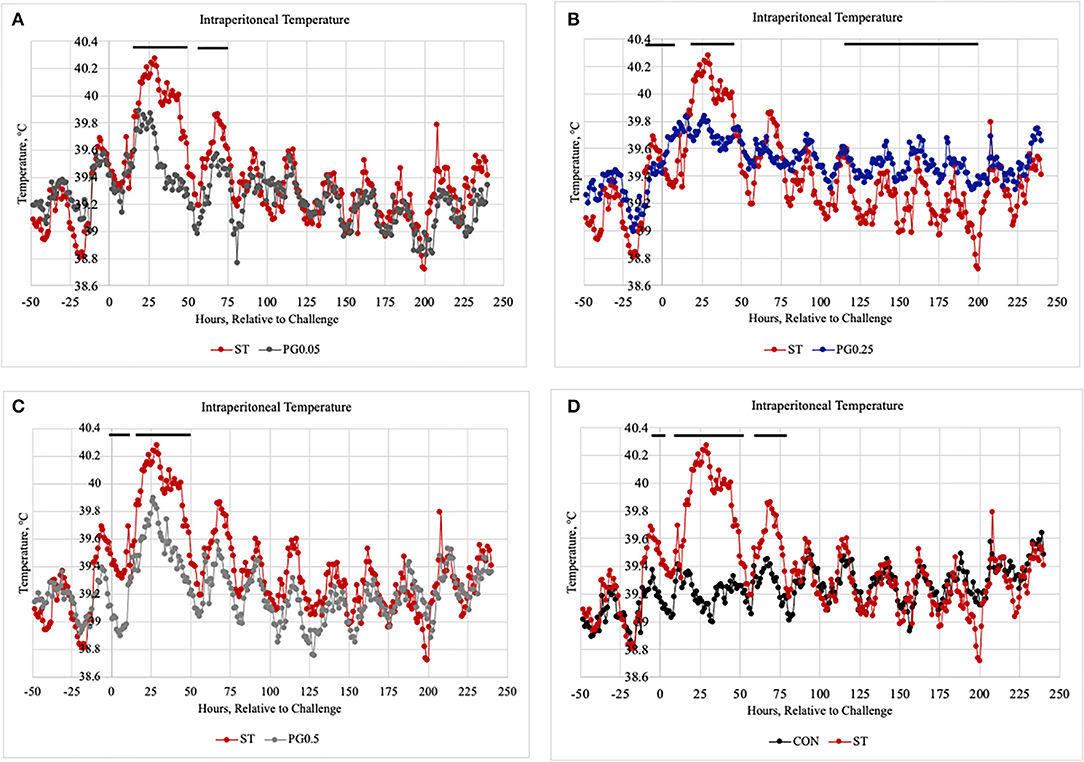
Figure 10. The intraperitoneal temperature was measured every 5min and averaged to give an hourly temperature from 48 h prior to infection until 240 h post-infection when pigs were harvested, and temperature probes removed. (A–D) Separate treatments and make the figures clearer for the reader. (A) comparison of ST vs PG0.05, (B) comparison of ST vs PG0.25, (C) comparison of ST vs PG0.5, and (D) comparison of ST to CON. There was a treatment × time interaction (P < 0.0001). There were treatment differences at hours 4–10, 20–45, and 81 (P ≤ 0.0461) and treatment tendencies at hours 17, 19, 47, 48, 83, 181, 199, and 200 (P ≤ 0.0894).
Ileal histology data are reported in Table 5. There was a treatment difference for villi length (P = 0.0482) where the PG0.05 treatment had the shortest villi compared to all other treatments. There was no treatment difference in crypt depth (P = 0.213). There was a treatment difference in villi length:crypt depth (P = 0.0115) where the PG0.05 treatment had a decreased ratio compared to all other treatments. There was a treatment difference for villus blunting score (P = 0.0169) where the CON and PG0.25 treatments had decreased blunting scores, ST and PG0.5 had intermediate scores, and the PG0.05 treatment had an increased villus blunting score. There was no treatment difference in villus epithelium score (P = 0.7053). There was a treatment difference for lacteal dilation score (P = 0.0383), where the ST treatment had an increased score when compared to all other treatments. There was no treatment difference for goblet cell count (P = 0.7856). There was a treatment difference for intraepithelial lymphocyte count (P = 0.0017), where the ST treatment had increased cell counts when compared to all other treatments. There were no treatment differences for lamina propria lymphocyte, neutrophil, or eosinophil cell counts (P ≥ 0.1425).
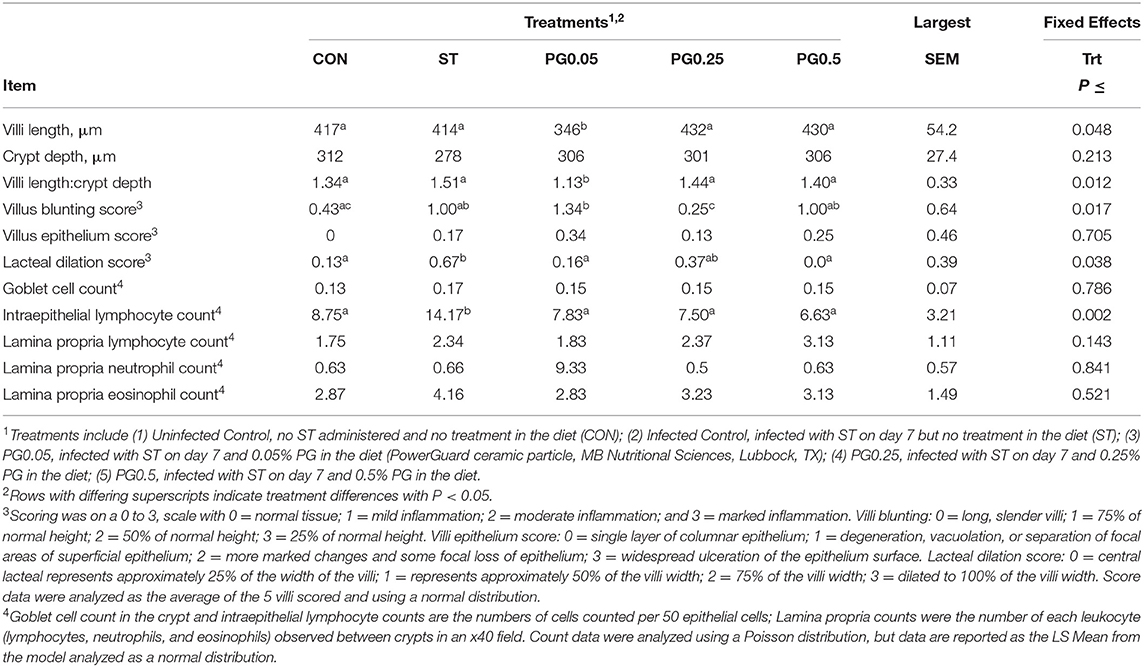
Table 5. Ileum histopathological effects of a ceramic particle fed to pigs after a S. enterica serotype typhimurium infection.
Liver histology data are presented in Table 6. There was no treatment difference in the hazy vacuolization score (P = 0.159). There was a treatment difference in connective tissue prominence score (P = 0.009), where the CON treatment had a decreased score and PG0.5 had an intermediate score compared to all other treatments. There was a treatment difference for lymphocyte foci score (P = 0.02), where the CON treatment had a decreased score, PG0.25 had an increased score, and all other treatments were intermediate. There was no treatment difference for the lymphocyte foci area (P = 0.199). There was a treatment tendency for neutrophil score (P = 0.052), where the CON treatment had a decreased score and PG0.25 had an increased score, with all other treatments intermediary.
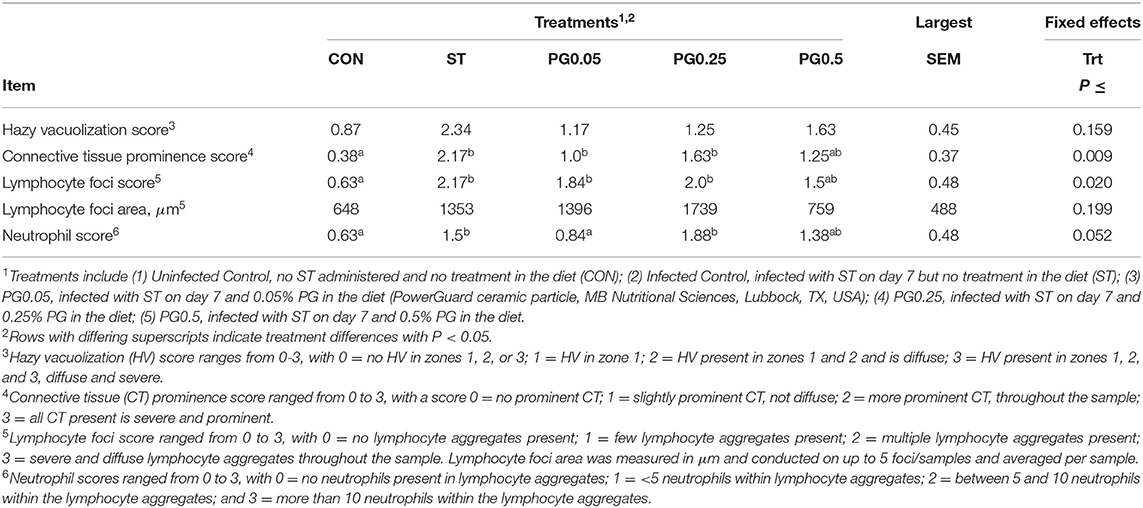
Table 6. Liver histopathological effects of a ceramic particle fed to pigs after a S. enterica serotype typhimurium infection.
The current study investigated the in vitro capacity for adsorption of Salmonella enterica serotypes Dublin and Typhimurium, and the in vivo dose-response of supplementation of a porous ceramic particle, PG, on the health, recovery, and histopathology of weanling pigs infected with Salmonella typhimurium. The microporosity and extensive available surface area of ceramic particles are contributing factors for effective adsorption to bacteria, particularly pathogenic species. Unprocessed montmorillonite was shown to adsorb, per gram, a total of 4 × 109 CFU of S. enterica. Similar to the current study, adsorption of S. enterica occurred exclusively on the external surface of the clay (Pardo et al., 2020). Clay adsorption of S. enterica can depend on the interlayer cations present, which can differ among and within mineral deposits. The lipopolysaccharide molecules present in gram-negative bacteria, like S. enterica, have a strong negative charge which can preferentially bind to the Ca2+ and Na+ cations present in the interlayer space in the structure of montmorillonite clays (Unuabonah et al., 2018). When clays are thermally processed into ceramics the surface micropores increase in size and number, thus the adsorption surface area potential increases (Schell et al., 1993; Diaz et al., 2002).
The adsorption capacities for processed clay, like PG, to remove potential toxins and bacteria from the environment and the gastrointestinal tract are still being determined. A chemically processed montmorillonite clay was utilized as a soil amendment to determine its capacity to adsorb the hydrophobic pesticide, Dieldrin. When treatment was applied, it achieved a 70% reduction in the amount of Dieldrin recovered in soil. The adsorption to Dieldrin was theorized to occur via the interlayer surfaces of the clay (Hearon et al., 2020). An in vitro S. typhimurium adsorption study was conducted utilizing a Cu-montmorillonite clay. Clay at 0.05 g/mL adsorbed the best to S. typhimurium included at 4 × 108 CFU and was the most effective dose of clay for sterilization (Ting et al., 2020). The capacity for clay or ceramic particles to reduce bacteria load is critically important when considering the farm-to-consumption food safety pathway. Results from a quantitative microbiological risk assessment (QMRA) in the EU indicate a reduction in one to two log base 10 of Salmonella on the pre-chill carcass can potentially prevent up to 90% of human Salmonella infections of pork product origin (EFSA, 2011). In the current study, the in vitro Salmonella adsorption analyses determined that PG and activated carbon could reduce Salmonella typhimurium concentration by approximately 1.5 log10 when initial bacteria concentrations began at 2 × 106 CFU/mL. This reduction is biologically relevant when considering the impacts of bacteria reduction on pre-harvest carcasses. Similarly, PG and activated carbon were also capable of reducing the final concentration of Salmonella dublin by 1.5 and 1.3 log10, respectively, when initial Salmonella dublin concentrations began at 2 × 106 CFU/mL. Both adsorption assays included two unprocessed commercial bentonite clays as comparative controls, and the adsorption capacity was less than one log10 for both bacteria strains. Data from the literature suggests the mechanisms for adsorption are similar between processed clays and activated carbon products and were shown to have similar capacities in the current data (van der Mei et al., 2008; Chu et al., 2013; Annan et al., 2018; Schmidt et al., 2019; Ting et al., 2020). This increase in adsorption capacity for biotoxins and bacteria make ceramic particles a viable option for feed additives to lower the exposure load of infectious or zoonotic pathogens, such as Salmonella sp., that food animals encounter.
The S. typhimurium infection in the current study decreased feed disappearance and numerically decreased ADG immediately following the infection. Supplementing PG at 0.05% of the diet prevented the decrease in feed disappearance when compared to the uninfected controls from days 8 to 10. The PG0.25 had improved feed disappearance:gain when compared to the CON and PG0.5 pigs from days 11 to 14. The PG0.5 treatment did not improve any aspect of performance. It was reported that the inclusion of clay at increased feed rates from 0.5 to 3% of the diet can decrease performance and may adsorb micronutrients or interfere with nutrient absorption (Elliot et al., 2020). Similar data supplementing montmorillonite clay were reported in both healthy and infected pigs (Song et al., 2012; Liu et al., 2020). However, other data reported no difference in production performance when healthy, uninfected pigs were supplemented with a montmorillonite clay (Thacker, 2003; Kim et al., 2006; Almeida et al., 2013; Holanda and Kim, 2020). This suggests clay supplementation may be the most beneficial when animals are under specific conditions, including stressors like weaning, transport, or comingling, and times of natural increased exposure to more pathogens and their biotoxins.
Diarrhea is common in pigs infected with Salmonella enterica, and the ST infection in the current study caused diarrhea. Supplementing PG at 0.25% of the diet prevented diarrhea, whereas the lower or greater doses of PG at 0.05 and 0.5% of the diet, respectively, did not prevent the pigs from developing diarrhea. The diarrheal response of a host to infection with S. typhimurium is due to the influx of neutrophils into the gastrointestinal tract resulting in lumen edema (Lou et al., 2019). These data suggest that supplementing the diet with PG at 0.25% of the diet reduced the local inflammatory response in the intestinal tract. The exact reason why the PG0.05 or PG0.5 pigs did not have improved fecal consistency compared to the ST-infected pigs is not known especially since both of those treatments did reduce the early neutrophilia that was observed in the ST pigs after infection.
Hematological changes expected during an S. typhimurium infection include an increase in total leukocytes, which is primarily driven by neutrophilia. Additionally, the infection commonly causes a decrease in hemoglobin and red blood cell counts and morphology. In the current study, the S. typhimurium infection caused an increase in total leukocytes and neutrophil counts over the duration of the study in ST infected pigs as expected; however, the increase in total leukocytes and neutrophil counts was more moderate and transient when PG was supplemented at 0.05, 0.25, or 0.5% of the diet. In agreement, supplementing clay to pigs without any signs of infection or disease also resulted in a decrease in total leukocyte counts at both 25 and 70 days of age (Bederska-Lojewska and Pieszka, 2019). There was a delayed increase in neutrophil counts in the PG0.5 treatment on day 21. Furthermore, the RBC profile, hemoglobin concentrations, red blood cell counts, and red blood cell morphology in the PG0.05 and PG0.25 treatments were similar to the CON pigs, whereas the ST group was similar to the PG0.5 pigs and indicative of infection and inflammation (Barba-Vidal et al., 2017). These data further support that PG at 0.05 and 0.25 attenuated the infection and inflammatory response associated with the ST infection. The exact mechanisms for the delayed increase in neutrophil counts and that the red blood cell data were not different than the ST in the PG0.5 are not known.
The intraperitoneal temperature began to increase in ST-infected pigs around 12 h post-infection, which was previously documented (Balaji et al., 2000; Jenkins et al., 2004; Price et al., 2010). The addition of PG at any dose resulted in an intermediary reduction in febrile response for the first 50 h post-infection, whereas all PG treatment groups had reduced febrile responses when compared to the ST pigs but increased temperatures when compared to the CON pigs. Similar responses in temperature were observed in prior S. typhimurium infection models when other nutraceuticals were supplemented (Spiehs et al., 2008; Price et al., 2010; Liang et al., 2020). Taken together, the improved performance, reduced diarrhea, attenuated changes in hematology, and reduced febrile responses in pigs supplemented with PG at 0.25% of the diet indicate that PG adsorbed some of the ST and reduced the local and systemic inflammatory responses to the ST infection. Furthermore, supplementing PG at a lower inclusion, 0.05% of the diet, also likely adsorbed some of the ST and reduced many of the systemic inflammatory responses.
Shedding data in the current study was collected by swabbing fresh feces each day and enriching the swabs overnight, revealing only positive or negative results, and there were no differences among the ST-infected treatment groups. No quantification was done on fecal ST swabs in the current study, but data from another pig S. typhimurium infection revealed only 8% of pigs had quantifiable levels of S. typhimurium in feces for 8 days post-infection (Barba-Vidal et al., 2017). It is important to note that fecal shedding results from the enrichment media show that the CON group did not shed any ST from the RV enrichment and shed statistically less ST in the TT enrichment. These results suggest that the biosecurity measures used in the current study were reasonably successful in preventing horizontal transmission of the ST infection to the negative control group. The presence of ST in the ileum and lymph nodes of CON pig at harvest may be explained by exposure to environmental Salmonella spp. prior to initiation of the study. In fact, QMRA on Salmonella spp. indicate that interventions in sow farms and farrowing crates may have a greater impact on reducing the risk for Salmonella spp. infections than during the grow to finish phase (EFSA, 2011). The propensity for S. typhimurium to survive within tissues, such as these is what makes it such an important food safety risk. The prevalence of S. typhimurium in the lymph nodes and the active shedding during transport are both risk factors for the harvest facility to contaminate food. A QMRA on Salmonella in the harvest plant and in breeder pigs was conducted in the EU, where they determined a strong linear relationship between the lymph node prevalence of Salmonella at slaughter and human infection from pork products (EFSA, 2011). The ability of PG at lower inclusion levels, 0.05–0.25% of the diet, to adsorb Salmonella spp. in the gastrointestinal tract without any detrimental effects on performance may reduce the total pathogen load that can infect the host, thereby lessening the exposure load that pigs face during the entire pre-harvest stage. This reduction in exposure may have even greater impacts post-harvest by ultimately lowering the contamination of pork products pre-consumption. Future research should investigate the use of PowerGuard throughout the entire production system to reduce pre-harvest colonization of lymph nodes and fecal shedding of Salmonella spp. at harvest.
Supplementing PowerGuard at 0.25% of the diet during a Salmonella typhimurium infection prevented some of the negative effects on production performance, reduced diarrhea, and improved pathophysiological measures in post-weaned pigs. Further, supplementing PowerGuard at a lower inclusion rate of 0.05% of the diet was intermediately effective at reducing inflammation post Salmonella typhimurium infection. Interestingly, supplementing PowerGuard at the greater inclusion rate of 0.5% of the diet did not consistently improve the response of pigs to the Salmonella typhimurium challenge. More research needs to be completed to understand this response, although this inclusion rate is greater than what this study seemed to determine as more of the optimum dose of 0.25% of the diet. If pathogen load pre-harvest can be reduced between just 0.5 and 1 log10, the post-harvest contamination potential and the human disease outcomes can be greatly reduced. PowerGuard, a porous commercial ceramic particle, adsorbed S. enterica serotypes in vitro and reduced disease in vivo. Therefore, supplementing PowerGuard may be one intervention strategy along the farm-to-consumption pathway to help mitigate pathogen load and improve food safety.
Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. The U.S. Department of Agriculture (USDA) prohibits discrimination in all its programs and activities based on race, color, national origin, age, disability, and where applicable, sex, marital status, familial status, parental status, religion, sexual orientation, genetic information, political beliefs, reprisal, or because all or part of an individual's income is derived from any public assistance program. (Not all prohibited bases apply to all programs.) Persons with disabilities who require alternative means for communication of program information (Braille, large print, audiotape, etc.) should contact USDA's TARGET Center at (202) 720-2600 (voice and TDD). To file a complaint of discrimination, write to USDA, Director, Office of Civil Rights, 1400 Independence Avenue, S.W., Washington, D.C. 20250-9410, or call (800) 795-3272 (voice) or (202) 720-6382 (TDD). USDA is an equal opportunity provider and employer.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The animal study was reviewed and approved by the USDA-ARS, Livestock Issues Research Unit's Institutional Animal Care and Use Committee (IACUC protocol #LIRU-2120S).
ED, KW, NB, RB, JC, and MB contributed to conception and design of the study and performed the animal work. MB performed the statistical analysis, involved in the design of the experiment, consulted on data analyses, and edited the final version of the manuscript. MC assisted on sample analysis and aided in manuscript writing. AP assisted in animal work and formulation of diets. All authors contributed to manuscript revision, read, and approved the submitted version.
MB has equity ownership in MB Nutritional Sciences LLC.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors would like to dedicate this manuscript to and thank the late J. W. Dailey (USDA-ARS) for his outstanding animal care and facilities support throughout the study. The authors would also like to thank Dr. Bo Zhao with the Texas Tech University Microscopy Core Laboratory for her assistance with electron microscopy.
Almeida, J. A. S., Liu, Y., Song, M., Lee, J. H., Gaskins, H. R., Maddox, C. W., et al. (2013). Escherichia coli challenge and one type of smectite alter intestinal barrier of pigs. J. Anim. Sci. Biotechnol. 4, 52. doi: 10.1186/2049-1891-4-52
Annan, E., Agyei-Tuffour, B., Bansah, Y. D., Konadu, D. S., Yaya, A., Onwona-Agyeman, B., et al. (2018). Application of clay ceramics and nanotechnology in water treatment: a review. Cogent. Eng. 5, 1–35. doi: 10.1080/23311916.2018.1476017
Bala, P., Samantaray, B. K., and Srivastava, S. K. (2000). Dehydration transformation in Ca-montmorillonite. Bull. Mater. Sci. 23, 61–67. doi: 10.1007/BF02708614
Balaji, R., Wright, K. J., Hill, C. M., Dritz, S. S., Knoppel, E. L., and Menton, J. E. (2000). Acute phase responses of pigs challenged orally with Salmonella typhimurium. J. Anim. Sci. 78, 1885–1891. doi: 10.2527/2000.7871885x
Barba-Vidal, R., Roll, V. F. B., Castillejos, L., Guerra-Ordaz, A. A., Manteca, X., Mallo, J. J., et al. (2017). Response to a Salmonella Typhimurium challenge in piglets supplemented with protected sodium butyrate or Bacillus licheniformis: effects on performance, intestinal health and behavior. Transl. Anim. Sci. 1, 186–200. doi: 10.2527/tas2017.0021
Bederska-Lojewska, D., and Pieszka, M. (2019). Dietary Kaolin clay in pre- and post-weaned piglets and its influence on haematological and biochemical parameters, and intensional microflora status. Ann. Anim. Sci. 19, 1021–1034. doi: 10.2478/aoas-2019-0031
Broadway, P. R., Carroll, J. A., Brooks, J. C., Donaldson, J. R., Burdick Sanchez, N. C., Schmidt, T. B., et al. (2015). Salmonella prevalence of lymph nodes and synovial fluid of orally inoculated swine. Ag. Food. Analy. Bacter. 5, 6–14.
Burdick Sanchez, N. C., Broadway, P. R., Carroll, J. A., Gart, E. V., Bryan, L. K., and Lawhon, S. D. (2017). Weaned pigs experimentally infected with Salmonella display sexually dimorphic innate immune responses without affecting pathogen colonization patterns. Trans. Anim. Sci. 1, 69–76. doi: 10.2527/tas2016.0008
Center for Disease Control (2013). An Atlas of Salmonella in the United States, 1968-2011, Salmonella typhimurium. National Center for Emerging and Zoonotic Infectious Diseases, Division of Foodborne, Waterborne, and Environmental Diseases, Department of Health and Human Services, Atlanta, GA, United States.
Center for Disease Control (2016). National Enteric Disease Surveillance: Salmonella Annual Report, 2016. National Center for Emerging and Zoonotic Infectious Diseases, Division of Foodborne, Waterborne, and Environmental Diseases, Department of Health and Human Services, Atlanta, GA, United States.
Chu, G. M., Kim, J. H., Kim, H. Y., Ha, J. H., Jung, M. S., Song, Y., et al. (2013). Effects of bamboo charcoal on the growth performance, blood characteristics and noxious gas emission in fattening pigs. J. Appl. Anim. Res. 41, 48–55. doi: 10.1080/09712119.2012.738219
Diaz, D. E., Hagler, W. M. Jr, Hopkins, B. A., and Whitlow, L. W. (2002). Aflatoxin binders I: in vitro binding assay for aflatoxin B1 by several potential sequestering agents. Mycopathologia 156, 223–226. doi: 10.1023/a:1023388321713
EFSA (2011). Quantitative Microbiological Risk Assessment on Salmonella in Slaughter and Breeder Pigs: Final Report. Available online at: Available online at: https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/sp.efsa.2010.EN-46
Elliot, C. T., Connolly, L., and Kolawole, O. (2020). Potential adverse effects on animal health and performance caused by the addition of mineral adsorbents to feeds to reduce mycotoxin exposure. Mycotoxin Res. 36, 115–126. doi: 10.1007/s12550-019-00375-7
FCC (2010). Analysis of the Costs and Benefits of Setting a Target for the Reduction of Salmonella in Slaughter Pigs for European Commission Health and Consumers Directorate-General SANCO/2008/E2/036 Final Report. p. 1–198.
Hearon, S. E., Wang, M., and Phillips, T. D. (2020). Strong adsorption of Dieldrin by parent and processed montmorillonite clays. Environ. Toxicol. Chem. 39, 517–525. doi: 10.1002/etc.4642
Holanda, D. M., and Kim, S. W. (2020). Efficacy of mycotoxin detoxifiers on health and growth of newly-weaned pigs under chronic dietary challenge of Deoxynivalenol. Toxins 12, 1–19. doi: 10.3390/toxins12050311
Jenkins, N. L., Turner, J. L., Dritz, S. S., Durham, S. K., and Minton, J. E. (2004). Changes in circulating insulin-like growth factor-I, insulin-like growth factor binding proteins, and leptin in weaned pigs infected with Salmonella enterica serovar Typhimurium. Domest. Anim. Endocrinol. 26, 49–60. doi: 10.1016/j.domaniend.2003.09.001
Jiang, S., Zaibin, Y., Yang, W., Gao, J., Liu, F., Chen, C., et al. (2010). Physiopathological effects of zearalenone in post-weaning female piglets with or without montmorillonite clay adsorbent. Livestock Sci. 131, 130–136. doi: 10.1016/j.livsci.2010.02.022
Kim, Y. H., Wang, Y., Cho, J. H., Chen, Y. J., Kim, H. J., Yoo, J. S., et al. (2006). Effects of dietary supplemental Megazone on growth performance, nutrient digestibility, blood characteristics, meat quality, and carcass traits in weanling-to-finishing pigs. Korean J. Food Sci. Res. 26, 447–453. Available online at: https://www.koreascience.or.kr/article/JAKO200606142042060.view?orgId=anpor&hide=breadcrumb,journalinfo
Liang, Y., Hudson, R. E., and Ballou, M. A. (2020). Supplementing neonatal Jersey calves with a blend of probiotic bacteria improves the pathophysiological response to an oral Salmonella enterica serotype Typhimurium challenge. J. Dairy Sci. 103, 7351–7363. doi: 10.3168/jds.2019-17480
Liu, H., Wang, C., Gu, X., Zhao, J., Nie, C., Zhang, W., et al. (2020). Dietary montmorillonite improves the intestinal mucosal barrier and optimizes the intestinal microbial community of weaned piglets. Front. Microbiol. 11, 593056. doi: 10.3389/fmicb.2020.593056
Lo Fo Wong, D. M. A., and Hald, T. (2000). Salmonella in Pork (SALINPORK): Preharvest and Harvest Control Options Based on Epidemiological, Diagnostic and Economic Research. Final Report. Contract No: FAIR1 CT95-0400.
Lou, L., Zhang, P., Piao, R., and Wang, Y. (2019). Salmonella pathogenicity island 1 (SPI-1) and its complex regulatory network. Front. Cell. Infect. Microbiol. 9, 270. doi: 10.3389/fcimb.2019.00270
Pardo, L., Domínguez-Maqueda, M., Cecilia, J. A., Rodríguez, M. P., Osajima, J., Moriñigo, M. A., et al. (2020). Adsorption of Salmonella in clay minerals and clay-based materials. Minerals 10, 130. doi: 10.3390/min10020130
Price, K. L., Totty., H. R, Lee, H. B., Utt., M. D, Fitzner, G. E., Yoon, I., et al. (2010). Use of Saccharomyces cerevisiae fermentation product on growth performance and microbiota of weaned pigs during Salmonella infection. J. Anim. Sci. 88, 3896–3908. doi: 10.2527/jas.2009-2728
Rostagno, M. H., Eicher, S. D., and Lay, D. C. Jr. (2011). Immunological, physiological, and behavioral effects of Salmonella enterica carriage and shedding in experimentally infected finishing pigs. Foodborne Path. Dis. 8, 623–630. doi: 10.1089/fpd.2010.0735
Schell, T. C., Lindemann, M. D., Kornegay, E. T., and Blodgett, D. J. (1994). Effects of feeding Aflatoxin-contaminated diets with and without clay to weanling and growing pigs on performance, liver function, mineral metabolism. J. Anim. Sci. 71, 1209–1218. doi: 10.2527/1993.7151209x
Schell, T. C., Lindemann, M. D., Kornegay, E. T., Blodgett, D. J., and Doerr, J. A. (1993). Effectiveness of different types of clay for reducing the detrimental effects of aflatoxin-contaminated diets on performance and serum profiles of weanling pigs. J. Anim. Sci. 71, 1226–1231. doi: 10.2527/1993.7151226x
Schmidt, H. P., Hagemann, N., Draper, K., and Kammann, C. (2019). The use of biochar in animal feeding. PeerJ. 7, e7373. doi: 10.7717/peerj.7373
Song, M., Liu, Y., Soares, J. A., Che, T. M., Osuna, O., Maddox, C. W., et al. (2012). Dietary clays alleviate diarrhea of weaned pigs. J. Anim. Sci. 90, 345–360. doi: 10.2527/jas.2010-3662
Spiehs, M. J., Shurson, G. C., and Johnston, L. J. (2008). Effects of two direct fed microbials on the ability of pigs to resist an infection with Salmonella enterica serovar Typhimurium. J. Swine Health Prod. 16, 27–36. Available online at: https://www.aasv.org/jshap/issues/v16n1/v16n1p27.pdf
Thacker, P. A. (2003). Performance of growing-finishing pigs fed diets containing graded levels of Biotite, an aluminosilicate clay. Asian-Australian J. Anim. Sci. 16, 1666–1672. doi: 10.5713/ajas.2003.1666
Thieu, N. Q., Ogle, B., and Pettersson, H. (2008). Efficacy of bentonite clay in ameliorating aflatoxicosis in piglets fed aflatoxin contaminated diets. Trop. Anim. Health Prod. 40, 649–656. doi: 10.1007/s11250-008-9144-3
Ting, C. H., Chen, S. C., Chang, C. D., Shih, W. L., Shen, P. C., and Wu, H. Y. (2020). Effects of copper- bearing montmorillonite on Salmonella spp. in vitro study. Thai J. Vet. Med. 50, 97–102. Available online at: https://he01.tci-thaijo.org/index.php/tjvm/article/view/243262
Unuabonah, E. I., Ugwuja, C. G., Omorogie, M. O., Adewuyi, A., and Oladoja, N. A. (2018). Clays for efficient disinfection of bacteria in water. Appl. Clay Sci. 151, 211–223. doi: 10.1016/j.clay.2017.10.005
van der Mei, H. C., Atema-Smit, J., Jager, D., Langworthy, D. E., Collias, D. I., Mitchell, M. D., et al. (2008). Influence of adhesion to activated carbon particles on viability of waterborne pathogenic bacteria under flow. Biotechnol. Bioeng. 100, 810–813. doi: 10.1002/bit.21820
Keywords: biotoxin binder, clay, infection, montmorillonite, nutraceutical
Citation: Davis EM, Wallace KP, Cruz Penn MJ, Petry AL, Broadway R, Burdick Sanchez NC, Carroll JA and Ballou MA (2022) A Dose-Response Investigation of a Micronized Porous Ceramic Particle to Improve the Health and Performance of Post-weaned Pigs Infected With Salmonella enterica Serotype Typhimurium. Front. Anim. Sci. 3:872776. doi: 10.3389/fanim.2022.872776
Received: 09 February 2022; Accepted: 22 April 2022;
Published: 26 May 2022.
Edited by:
Amira Leila Dib, Université Frères Mentouri Constantine 1, AlgeriaReviewed by:
Tsungcheng Tsai, University of Arkansas, United StatesCopyright © 2022 Davis, Wallace, Cruz Penn, Petry, Broadway, Burdick Sanchez, Carroll and Ballou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael A. Ballou, bWljaGFlbC5iYWxsb3VAdHR1LmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.