- 1Department of Animal Science, Texas A&M University, College Station, TX, United States
- 2ABS Global, DeForest, WI, United States
- 3Kleinsasser-Porter Veterinary Service, Huron, SD, United States
- 4Department of Animal and Dairy Science, University of Georgia, Athens, GA, United States
To determine the effects of pre-synchronization and delayed fixed-time artificial insemination (TAI) on pregnancy rates (PR/AI) with sexed semen, 1,844 beef heifers were enrolled in a completely randomized design at 12 locations. Within a location, the heifers were randomly assigned to one of the five treatments: (1 and 2), heifers were administered prostaglandin F2α (PGF) on Day -7, gonadotropin-releasing hormone (GnRH), and a controlled internal drug releasing (CIDR) insert on Day 0, PGF at CIDR removal on Day 7, and a second injection of GnRH at TAI 72 h later with either conventional (CTRL72-CNV) or sexed semen (CTRL72-SEX); (3 and 4), treated the same as CTRL72 but received a CIDR insert on Day -7 at PGF administration and TAI at 60 h with either conventional (CIDR60-CNV) or sexed semen (CIDR60-SEX); (5), treated the same as CIDR60 but had TAI delayed to 72 h with sexed semen (CIDR72-SEX). Estrus detection patches were applied to all heifers on Day 7 and were evaluated for activation at TAI. Estrus expression did not differ (p = 0.92) between CIDR60 and CIDR72 heifers, but was greater (p<0.001) in CIDR60 and CIDR72 heifers compared with CTRL72 heifers. Among treatments, PR/AI differed (p < 0.001) and were greater (p ≤ 0.003) in CTRL72-CNV and CIDR60-CNV heifers than CIDR60-SEX and CIDR72-SEX heifers (51.6 and 48.1 vs. 37.5 and 25.3%, respectively). In addition, PR/AI were greater (p < 0.001) in CTRL72-SEX and CIDR60-SEX heifers when compared with CIDR72-SEX (42.0 and 37.5 vs. 25.3%, respectively) heifers but only tended (p = 0.09) to differ between CTRL72-SEX and CIDR60-CNV heifers. No differences (p = 0.33) were determined between CTRL72-CNV and CIDR60-CNV or between CTRL72-SEX and CIDR60-SEX heifers (p = 0.22). In conclusion, no differences were determined between heifers pre-synchronized with only PGF and those pre-synchronized with PGF and a CIDR insert when inseminated with either conventional or sexed semen. Therefore, the use of a CIDR insert for an additional 7 days was not beneficial to PR/AI when heifers were TAI at 60 h with either conventional or sexed semen. Furthermore, delaying TAI to 72 h with sexed semen after pre-synchronization with both PGF and a CIDR insert had a negative impact on PR/AI.
Introduction
Utilization of sexed semen is becoming increasingly popular in the beef industry, yet pregnancy rates to artificial insemination (PR/AI) with sexed semen are typically between 10 and 20% points lower than those of conventional semen when used in fixed-time artificial insemination (TAI) protocols (Thomas et al., 2019; Perry et al., 2020; Oosthuizen et al., 2021). This reduction in PR/AI has been attributed to the premature onset of sperm capacitation, which leads to a reduced sperm cell lifespan in the female reproductive tract, and may require artificial insemination with sexed semen to be performed at a time that is more synchronous with ovulation (Mocé et al., 2006; Carvalho et al., 2013; Bombardelli et al., 2016). Consequently, delayed timing of insemination after estrus synchronization has been investigated as a method to deposit sexed sperm closer to the time of ovulation and has resulted in improved PR/AI in dairy cows (Bombardelli et al., 2016). Strategies to increase the expression of estrus prior to TAI also have the potential to improve PR/AI when sexed semen is utilized since PR/AI are greater in females that express estrus (Richardson et al., 2016; Perry et al., 2020; Oosthuizen et al., 2021).
Presynchronization programs, which involve additional hormone administration prior to the initiation of an estrus synchronization protocol, have been researched as potential methods to improve PR/AI with both conventional and sexed semen (Grant et al., 2011; Perry et al., 2012; Oosthuizen et al., 2018, 2020, 2021). The purpose of pre-synchronization is to increase the proportion of females at a certain stage of the estrous cycle before estrus synchronization and has the potential to improve the synchrony of follicular development and improve estrus expression, with the ultimate goal of increasing PR/AI. Previously, when beef heifers were administered prostaglandin F2α (PGF) 7 days before the 7-day CO-Synch + controlled internal drug release (CIDR) protocol with TAI delayed to 72 h, the expression of estrus was increased and, as a result, PR/AI tended to be greater with the conventional semen (Oosthuizen et al., 2020). In the same study, heifers that were pre-synchronized with both a CIDR insert and PGF had greater estrus expression prior to TAI and greater PR/AI. Further research evaluating the use of sexed semen after pre-synchronization reported a 9.2% increase in PR/AI when beef heifers were exposed to a combination of pre-synchronization with PGF and delayed TAI (Oosthuizen et al., 2021). Nevertheless, the combination of pre-synchronization with both PGF and a CIDR insert, and TAI with sexed semen has not yet been explored in replacement beef heifers. Therefore, the objectives of this experiment were: (1) to determine if pre-synchronization with both PGF and a CIDR insert would increase PR/AI after insemination with sexed semen, and (2) to compare PR/AI with conventional and sexed semen in varying pre-synchronization protocols. It was hypothesized that PR/AI with both conventional and sexed semen would be greater in heifers pre-synchronized with PGF and a CIDR insert when compared with heifers pre-synchronized with only PGF. In addition, it was hypothesized that delaying insemination with sexed semen after pre-synchronization with both PGF and a CIDR insert would increase PR/AI.
Materials and Methods
Animals and Treatments
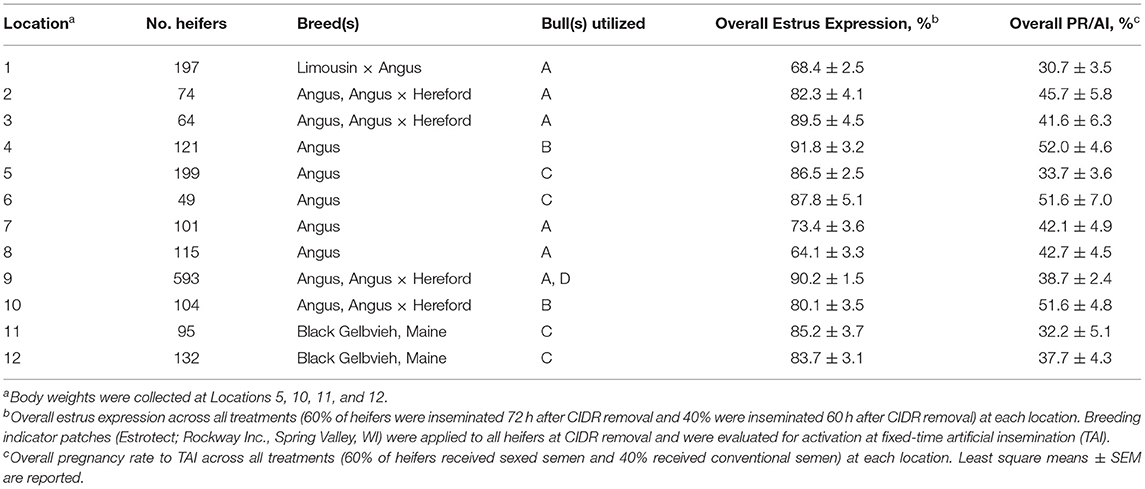
All heifers were handled in accordance with procedures approved by Texas A&M University's Animal Care and Use Committee. A total of 1,844 spring-born Bos taurus beef heifers (between 12 and 15 months of age) from 12 locations in South Dakota (Table 1) were enrolled in a completely randomized design. Within location, the heifers were randomly assigned to one of five treatments (Figure 1): treatments 1 and 2, heifers were estrus synchronized using the PG-7 7-day CO-Synch + CIDR protocol (Oosthuizen et al., 2020, 2021) as a control (CTRL) wherein they were administered 2 ml PGF (500 μg im; Estrumate; cloprostenol; Merck & Co., Kenilworth, NJ) on Day -7, 2 ml GnRH (100 μg im; Fertagyl; gonadorelin acetate; Merck & Co.), and a CIDR insert (EAZI-BREED CIDR; 1.38 g progesterone; Zoetis Animal Health) on Day 0, 2 ml PGF at CIDR removal on Day 7, and 2 ml GnRH at TAI 72 ± 2 h later with either conventional (CTRL72-CNV; n = 359) or sexed semen (CTRL72-SEX; n = 363); treatments 3 and 4, heifers were treated the same as CTRL72 but received a CIDR insert on Day -7 concurrent with PGF administration and were TAI 60 ± 2 h after CIDR removal with either conventional (CIDR60-CNV; n = 384) or sexed semen (CIDR60-SEX; n = 366); and treatment 5, treated the same as CIDR60 heifers but had TAI delayed to 72 ± 2 h with sexed semen (CIDR72-SEX; n = 372). No conventional semen was utilized for a CIDR72-CNV treatment, as previous research reported significantly reduced PR/AI in beef heifers through the use of that estrus synchronization protocol (Oosthuizen et al., 2020). Breeding indicator patches (Estrotect; Rockway Inc., Spring Valley, WI) were applied to all heifers at CIDR removal and were evaluated for activation at TAI to determine if estrus had been expressed. Breeding indicator patches were considered activated when at least 50% of the rub-off coating was removed from the patch or when the patch was missing. On Day -7, heifer body weight (BW) was recorded at 4 locations (n = 526). The time of CIDR removal and TAI were recorded for each heifer at 5 locations (n = 1,070); therefore, the time between these two points could be calculated. Each location provided their own insemination technician(s) and received both conventional and sexed semen (Sexcel™, gender-ablated semen) from their respective bull(s) of choice, which was provided by a commercial semen company (ABS Global, DeForest, WI). In total, conventional (~15 × 106 sperm cells per 0.25 ml straw before freezing) and sexed (~3 × 106 sperm cells per 0.25 ml straw before freezing) semen were used from 4 different sires. Within each location, conventional and sexed semen were derived from the same sires and were equally represented in each treatment. Clean-up bulls were introduced no less than 10 days after TAI at each location. Transrectal ultrasonography (Ibex portable ultrasound, 5.0-MHz curved linear multi-frequency transducer, Ibex, E.I. Medical Imaging, Loveland, CO) was performed at each location between 30 and 55 days after TAI to determine PR/AI. Artificial insemination and clean-up bull pregnancies were differentiated based on differences in crown-to-rump length (Riding et al., 2008; Fontes et al., 2019).
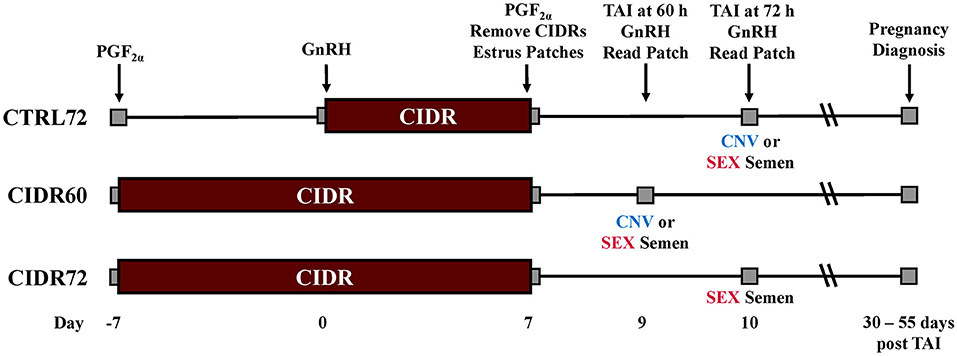
Figure 1. Schematic of treatments. GnRH, gonadotropin-releasing hormone; PGF, prostaglandin F2α; CIDR, controlled internal drug releasing insert (progesterone). CTRL72, PG-7 7-d CO-Synch + CIDR protocol; CIDR60, same as CTRL72 but CIDR inserted on Day −7 and TAI performed 60 h after CIDR removal; CIDR72, same as CIDR60 but TAI delayed to 72 h after CIDR removal; CNV, conventional semen used for TAI; and SEX, sexed semen used for TAI. Estrus patches = Breeding indicator patches (Estrotect; Rockway Inc., Spring Valley, WI) were applied to all heifers at CIDR removal and were evaluated for activation at their respective time of TAI.
Statistical Analyses
All data were analyzed as a completely randomized design using the SAS statistical package (version 9.4; SAS/STAT, SAS Inst. Inc., Cary, NC, USA). Heifer was the experimental unit in all analyses. The GLIMMIX procedure of SAS was used to analyze the binary response (estrus expression and PR/AI) and continuous descriptive (BW) variables. Heifers were simplified into estrus synchronization groups (CTRL72, CIDR60, and CIDR72) for the analysis of estrus expression as estrus expression was recorded prior to heifers receiving their respective semen types. The model for estrus expression included the fixed effect of the estrus synchronization group and the random effect of the location. The initial model for PR/AI included the fixed effects of treatment, estrus expression, and the respective interaction as well as the random effect of the location. The treatment × estrus expression interaction was determined to be non-significant (p > 0.05), and was therefore removed from the model. Denominator degrees of freedom were adjusted using the Satterthwaite adjustment for the tests of fixed effects. For all models, when significance (p ≤ 0.05) was determined for a fixed effect, least-squares means were separated by the PDIFF option of SAS. Insemination technician (s) and sire were equally distributed among treatments and, consequently, were not included in any of the models.
The probability of heifers in each treatment group of becoming pregnant to TAI was evaluated according to the time between CIDR removal and TAI. The GLM procedure of SAS was initially used to determine if each individual measurement influenced PR/AI linearly, quadratically, or cubically. The LOGISTIC procedure was used to generate a regression model and to determine the intercept and slope(s) values according to maximum likelihood estimates from each significant continuous order effect. The probability of pregnancy was determined according to the following equation: probability = (elogistic equation)/(1 + elogistic equation). Logistic curves were constructed according to the values detected for each variable. Statistical significance was declared at p ≤ 0.05, with 0.05 < p ≤ 0.10 considered a tendency. Least square means ± SEM are reported.
Results
Heifer BW (405.2 ± 52.4 kg) at 4 locations did not differ (p = 0.52) based on treatments on Day 7, but differed (p < 0.001) by location and ranged from 331.9 to 455.7 kg. The percentage of heifers expressing estrus, as determined by activated breeding indicator patches, differed (p < 0.001) among estrus synchronization groups, which is depicted in Figure 2. Estrus expression was greater (p < 0.001) in CIDR60 and CIDR72 when compared with CTRL72 heifers, yet it did not differ (p = 0.92) between CIDR60 and CIDR72 heifers. Overall, 83.5% of heifers exhibited estrus, which ranged from 64.1 to 91.8% among locations (Table 1).
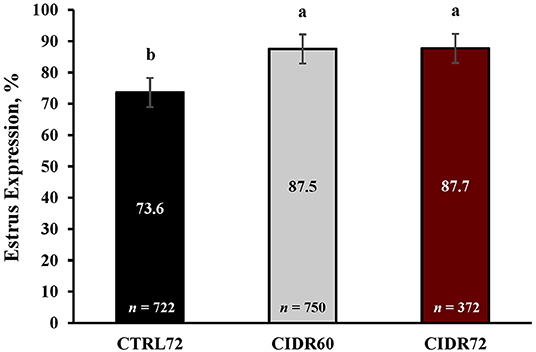
Figure 2. Estrus expression by synchronization treatment. CTRL72, PG-7 7-d CO-Synch + CIDR protocol; CIDR60, same as CTRL72 but CIDR inserted on Day −7 and TAI performed 60 h after CIDR removal; CIDR72, same as CIDR60 but TAI delayed to 72 h after CIDR removal; Estrus patches, Breeding indicator patches (Estrotect; Rockway Inc., Spring Valley, WI) were applied to all heifers at CIDR removal and were evaluated for activation at their respective time of TAI. Least square means ± SEM are reported. a, bRows with different superscripts differ (P ≤ 0.05).
The main effect (p = 0.002) of estrus was determined on PR/AI, where a greater proportion of heifers that expressed estrus became pregnant (45.9 ± 2.2%) than those which did not (35.9 ± 3.4%). The proportion of heifers pregnant to TAI are depicted in Figure 3, which differed (p < 0.001) among treatment groups. In particular, PR/AI were greater (p ≤ 0.003) in CTRL72-CNV and CIDR60-CNV heifers compared with CIDR60-SEX and CIDR 72-SEX heifers, yet it did not differ (p = 0.33) between CTRL72-CNV and CIDR60-CNV heifers. Furthermore, PR/AI were greater (p < 0.001) in CTRL72-SEX and CIDR60-SEX heifers when compared with CIDR72-SEX heifers, but did not differ (p = 0.22) between CTRL72-SEX and CIDR60-SEX heifers. Pregnancy rates to TAI were greater (p = 0.008) in CTRL72-CNV when compared with CTRL72-SEX heifers, yet PR/AI only tended (p = 0.09) to differ between CTRL72-SEX and CIDR60-CNV heifers. Overall, 42.8% of the heifers became pregnant to TAI, and PR/AI ranged from 30.7 to 52.0% among locations (Table 1).
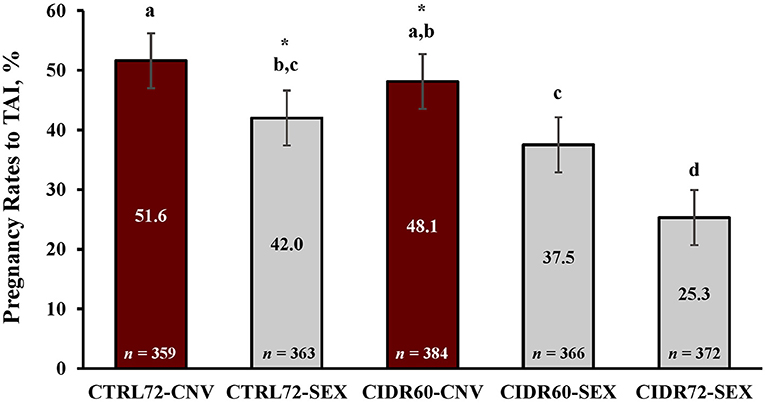
Figure 3. Pregnancy rates to fixed-time artificial insemination (TAI) among treatments. CTRL72, PG-7 7-d CO-Synch + CIDR protocol; CIDR60, same as CTRL72 but CIDR inserted on Day −7 and TAI performed 60 h after CIDR removal; CIDR72, same as CIDR60 but TAI delayed to 72 h after CIDR removal; CNV, conventional semen used for TAI; and SEX, sexed semen used for TAI. Pregnancy rates were determined via transrectal ultrasonography between 30 and 45 d after TAI. Least square means ± SEM are reported. a, b, c, dBars with different superscripts differ (P ≤ 0.05). *Indicates a tendency (0.05 < P ≤ 0.10) for treatment differences.
The time between CIDR removal and TAI ranged from 57.7 to 62.4 h in CIDR60-CNV and CIDR60-SEX heifers, with a mean of 59.9 ± 0.2 h. In addition, the time between CIDR removal and TAI ranged from 70.1 to 75.1 h in CTRL72-CNV, CTRL72-SEX, and CIDR72-SEX heifers with a mean of 72.0 ± 0.2 h. No relationships (p ≥ 0.20) between the probability of pregnancy and the time between CIDR removal and TAI were determined for CTRL72-CNV, CIDR60-CNV, CIDR60-SEX, or CIDR72-SEX heifers; however, there was a tendency (p = 0.06) for a positive linear relationship between the probability of pregnancy and the time between CIDR removal and TAI in CTRL72-SEX heifers.
When probabilities of pregnancy according to the time between CIDR removal and TAI were analyzed by whether or not estrus was expressed, no relationships (p ≥ 0.31) were determined between the probability of pregnancy and time between CIDR removal and TAI for any treatment when estrus was not expressed. When estrus was expressed, no relationships (p ≥ 0.12) were determined for CTRL72-CNV, CIDR60-CNV, CIDR60-SEX, or CIDR72-SEX heifers between the probability of pregnancy and the time between CIDR removal and TAI, yet there was a tendency (p = 0.07) for a positive linear relationship in CTRL72-SEX heifers.
Discussion
The purpose of the present experiment was to determine the effects of pre-synchronization with PGF and progesterone, as well as delayed TAI on PR/AI with sexed semen in replacement beef heifers. It was hypothesized that PR/AI would be greater in heifers pre-synchronized with both PGF and a CIDR insert (CIDR60) when compared with heifers pre-synchronized with PGF only (CTRL72) after insemination with both conventional and sexed semen. Contrary to our hypothesis, results indicate that PR/AI did not differ between CTRL72-CNV and CIDR60-CNV or between CTRL72-SEX and CIDR60-SEX heifers. It was also hypothesized that delaying insemination to 72 h with sexed semen after pre-synchronization with both PGF and progesterone (CIDR72-SEX) would increase PR/AI when compared with females who underwent TAI at 60 h (CIDR60-SEX); however, results contradict this hypothesis as PR/AI were significantly reduced in CIDR72-SEX heifers compared with all other treatments.
Pre-synchronization involves the administration of exogenous hormones prior to the start of an estrus synchronization protocol, and it has been utilized as a tool to increase the proportion of females at a particular stage of the estrous cycle prior to estrus synchronization, to increase estrus expression before insemination, and to increase PR/AI in both beef cows and heifers (Perry et al., 2012; Giles et al., 2013; Oosthuizen et al., 2020, 2021; Andersen et al., 2021). In the CTRL72 treatment, it is likely that a large proportion of heifers responded to the initial injection of PGF on Day -7 and would have expressed estrus before Day 0 as 62–70% of beef heifers expressed estrus when administered PGF at a random stage of their estrous cycle (Oosthuizen et al., 2018, 2020). Heifers that responded to the PGF would likely have ovulated and initiated a new follicular wave before Day 0. A proportion of heifers may have had follicles large enough to respond to the injection of GnRH on Day 0 and would have initiated a new follicular wave thereafter, of which the dominant follicle may have ovulated spontaneously or been induced to ovulate through the injection of GnRH at TAI. Alternatively, the dominant follicle that developed during this new follicular wave may have been too small to respond to GnRH administration on Day 0 since it is less likely that smaller follicles (<10.1 mm) will ovulate in response to an injection of GnRH (Perry et al., 2007). It is also likely that this dominant follicle underwent atresia between Day 0 and 7 as a result of the elevated circulating concentrations of progesterone produced by the newly formed corpus luteum in combination with that of the new CIDR insert (Adams et al., 2008). Due to the greater circulating concentrations of progesterone, the subsequent dominant follicle would likely have developed to a lesser extent by the time of CIDR removal on Day 7 and would require more time to reach a size capable of inducing behavioral estrus (Cerri et al., 2011; Mercadante et al., 2015). In the CIDR60 and CIDR72 treatments, a large proportion of heifers would have responded to the injection of PGF on Day -7 and would have undergone luteolysis; however, if a dominant follicle was present in the ovary, we speculate that instead of ovulating between Day -7 and 0, the follicle would have continued to grow due to the subluteal circulating concentrations of progesterone from the CIDR insert and would have formed a persistent follicle (Sirois and Fortune, 1990). This follicle likely responded to the injection of GnRH on Day 0, as others have shown a greater proportion of cows and heifers responding to the GnRH injection after pre-synchronization with both PGF and a CIDR insert (Bonacker et al., 2020; Mercadante et al., 2021, unpublished). Subsequently, a new follicular wave would have been initiated between Day 0 and 7 during which the dominant follicle would likely have developed under lower circulating concentrations of progesterone as a result of the reduced amount of progesterone being emitted from the CIDR insert after 7 days of use (Chacher et al., 2017). Therefore, the dominant follicles developing between Day 0 and 7 were likely older and larger at CIDR removal on Day 7 in CIDR60 and CIDR72 heifers than in CTRL72 heifers due to a greater proportion of heifers initiating a follicular wave sooner after responding to the injection of GnRH on Day 0. Furthermore, follicles in CIDR60 and CIDR72 heifers are also likely larger at CIDR removal than the dominant follicles in the CTRL72 treatment because follicles that develop under lower concentrations of progesterone develop at a faster rate (Mercadante et al., 2015). The expression of estrus did not differ between CIDR60 and CIDR72 heifers, which indicates that most heifers express estrus between CIDR removal and 60 h when pre-synchronized with both PGF and a CIDR insert, and that few heifers express estrus between 60 and 72 h. Furthermore, pre-synchronization with both PGF and a CIDR insert resulted in ~14% more heifers expressing estrus (CIDR60 and CIDR72) compared with heifers pre-synchronized with PGF only (CTRL72). These results are consistent with those from previous research, wherein beef heifers that were pre-synchronized with both PGF and a CIDR insert had between 13 and 55.5% greater expression of estrus when compared with heifers that were only pre-synchronized with PGF and where estrus expression did not differ between heifers pre-synchronized with both PGF and a CIDR insert when TAI was performed at either 54 or 72 h (Oosthuizen et al., 2020). Therefore, the differences in estrus expression between heifers pre-synchronized with both PGF and a CIDR insert and those pre-synchronized with only PGF can likely be attributed to the size of the dominant follicle at the time of CIDR removal, where follicles in CIDR60 and CIDR72 heifers are likely larger at CIDR removal than those of CTRL72 heifers.
It has been well-documented that PR/AI are greater in females that express estrus prior to insemination and can partially be attributed to improved sperm transportation and improved embryo quality (Perry et al., 2007, 2020; Larimore et al., 2015; Richardson et al., 2016). Therefore, it is unsurprising that PR/AI were greater in heifers that exhibited estrus in the present study when compared with those which did not. Yet, even though estrus expression was greater in heifers pre-synchronized with both PGF and a CIDR insert when compared with those pre-synchronized with PGF only, PR/AI did not differ between CTRL72-CNV and CIDR60-CNV or between CTRL72-SEX and CIDR60-SEX heifers. Similarly, previous research using conventional semen reported no differences in PR/AI between beef heifers pre-synchronized with both PGF and a CIDR and inseminated at 54 h or pre-synchronized with PGF only and inseminated at 72 h (Oosthuizen et al., 2020). Considering the results in the present study and those from previous research, shifting TAI from 54 to 60 h after pre-synchronization with both PGF and a CIDR insert does not appear beneficial to PR/AI when compared with pre-synchronization with only PGF. Furthermore, utilization of a CIDR insert for an additional 7 days does not seem advantageous to pregnancy success with either conventional or sexed semen when TAI is performed at 60 h.
Utilization of sexed semen in TAI protocols is known to result in reduced PR/AI, which are usually in the range of 75–85% of those with conventional semen (Crites et al., 2018; Thomas et al., 2019; Perry et al., 2020). This disparity in PR/AI has been partially attributed to acrosomal alterations that can occur during the semen sorting process, premature sperm capacitation, a reduction in the number of sperm cells with intact membranes, and lower post-thaw sperm motility (Mocé et al., 2006; Schenk et al., 2009; Carvalho et al., 2010). In the present study, PR/AI were reduced when sexed semen was utilized within each estrus synchronization treatment and were 81.4 and 78.0% (a reduction of 9.6 and 10.6%) of those with conventional semen (CTRL72-CNV vs. CTRL72-SEX and CIDR60-CNV vs. CTRL72-SEX, respectively). These results are similar to those reported previously in beef heifers wherein PR/AI with sexed semen ranged from 73.2 to 88.0% of those with conventional semen (Oosthuizen et al., 2021). Since PR/AI only tended to differ between CTRL72-SEX and CIDR60-CNV heifers, further evidence is provided that the additional 7 days of CIDR use is not required when inseminating beef heifers with sexed semen.
Due to a potentially decreased lifespan in the female reproductive tract, it has been suggested that sexed semen should be deposited at a time that is more synchronous with the timing of ovulation to maximize pregnancy success (Maxwell et al., 2004; Mocé et al., 2006; Carvalho et al., 2013; Bombardelli et al., 2016). Dairy cows inseminated with sexed semen closer to the expected time of ovulation had greater pregnancy success than those inseminated closer to the time of estrus onset (45.6 vs. 20.0%, respectively; Bombardelli et al., 2016). Furthermore, beef cows inseminated within 12 h of ovulation had greater PR/AI with sexed semen than those inseminated 12–24 h, or more than 24 h from ovulation (37.9 vs. 19.4 and 5.8%, respectively, Sales et al., 2011). In the present study, although estrus expression did not differ between CIDR60 and CIDR72 heifers, PR/AI were reduced in CIDR72-SEX heifers when compared with both CIDR60-CNV and CIDR72-SEX heifers. Previous research in beef heifers also reported a reduction in PR/AI after presynchronization with both PGF and a CIDR insert and insemination at 72 h with conventional semen when compared with those inseminated at 54 h (38.4 vs. 50.4%, respectively; Oosthuizen et al., 2020). The reduction in PR/AI after pre-synchronization with both PGF and a CIDR insert is likely due to heifers ovulating too far in advance for insemination at this time point, which is supported by the fact that only a small proportion of heifers expressed estrus between 60 and 72 h. Therefore, insemination performed 72 h after CIDR removal in heifers exposed to pre-synchronization with both PGF and progesterone is too delayed for acceptable PR/AI with both conventional and sexed semen.
To further explore the potential influence of the interval between CIDR removal and TAI on PR/AI, the present study evaluated the relationships between the time of insemination and the probability of pregnancy; however, no significant relationships were determined. Previous research also reported no significant relationships between the time of insemination and the probability of pregnancy in beef heifers pre-synchronized with PGF and TAI at 72 h with conventional semen and in heifers pre-synchronized with both PGF and a CIDR insert and TAI at either 54 or 72 h with conventional semen (Oosthuizen et al., 2020). In the present study, it is plausible that data was collected from too few heifers within each treatment or that heifers were inseminated in a time range too narrow to adequately detect differences in the probabilities of pregnancy. Nevertheless, according to these results, TAI can be performed ~2 h earlier or later than the specified TAI time (54 or 72 h) in each treatment without any negative effects on PR/AI.
In conclusion, PR/AI were greater in heifers artificially inseminated with conventional semen compared with sexed semen within pre-synchronization treatments. No differences were determined between heifers exposed to the PG-7 7-day CO-Synch + CIDR protocol and those pre-synchronized with both PGF and a CIDR insert when inseminated with either conventional or sexed semen. Subsequently, the utilization of a CIDR insert for an additional 7 days does not appear beneficial to fertility in replacement beef heifers when TAI is performed at 60 h with either conventional or sexed semen. Moreover, delaying TAI to 72 h with sexed semen after pre-synchronization with both PGF and a CIDR insert has a negative impact on fertility and is therefore, not recommended as a pre-synchronization strategy in beef heifers. Further research is required to overcome the reduction in PR/AI with sexed semen and to determine the optimal timing of insemination for conventional and sexed semen after pre-synchronization with both PGF and a CIDR insert in replacement beef heifers.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The animal study was reviewed and approved by Texas A&M University's Animal Care and Use Committee. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author Contributions
GL and NO: conceptualization. GL, NO, and KGP: methodology. PF and KGP: resources. NO, KP, SB, LG, and PF: data collection. NO: formal analysis and writing—original draft preparation. NO, PF, and GL: writing—review and editing. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by a grant from ABS Global Inc. (M2100931).
Conflict of Interest
NO was employed by ABS Global, DeForest.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank Merck & Co. (Kenilworth, NJ) for their donation of PGF2α (Estrumate), GnRH (Fertagyl); Zoetis Animal Health (Parsippany, NJ) for their donation of CIDR inserts (EAZI-BREED CIDR); Estrotect (Rockway Inc., Spring Valley, WI) for the donation of breeding indicator patches; and ABS Global for providing the semen for this study. The authors also thank Damon Smith for his assistance in data collection and Gabriela Melo for her assistance in product shipment. Lastly, the authors thank all of the cattle producers who allowed them to utilize their heifers in this study.
References
Adams, G. P., Jaiswal, R., Singh, J., and Malhi, P. (2008). Progress in understanding ovarian follicular dynamics in cattle. Theriogenology 69, 72–80. doi: 10.1016/j.theriogenology.2007.09.026
Andersen, C. M., Bonacker, R. C., Smith, E. G., Spinka, C. M., Poock, S. E., and Thomas, J. M. (2021). Evaluation of the 7 & 7 Synch and 7-day CO-Synch + CIDR treatment regimens for control of the estrous cycle among beef cows prior to fixed-time artificial insemination with conventional or sex-sorted semen. Anim. Reprod. Sci. 235, 106892. doi: 10.1016/j.anireprosci.2021.106892
Bombardelli, G. D., Soares, H. F., and Chebel, R. C. (2016). Time of insemination relative to reaching activity threshold is associated with pregnancy risk when using sex-sorted semen for lactating Jersey cows. Theriogenology 85, 533–539. doi: 10.1016/j.theriogenology.2015.09.042
Bonacker, R. C., Stoecklein, K. S., Locke, J. W. C., Ketchum, J. N., Knickmeyer, E. R., Spinka, C. M., et al. (2020). Treatment with prostaglandin F2α and an intravaginal progesterone insert promotes follicular maturity in advance of gonadotropin-releasing hormone among postpartum beef cows. Theriogenology 157, 350–359. doi: 10.1016/j.theriogenology.2020.08.018
Carvalho, J. O., Sartori, R., Machado, G. M., Mourão, G. B., and Dode, M. A. N. (2010). Quality assessment of bovine cryopreserved sperm after sexing by flow cytometry and their use in in vitro embryo production. Theriogenology 74, 1521–1530. doi: 10.1016/j.theriogenology.2010.06.030
Carvalho, J. O., Silva, L. P., Sartori, R., and Dode, M. A. N. (2013). Nanoscale differences in the shape and size of X and Y chromosome-bearing bovine sperm heads assessed by atomic force microscopy. PLoS ONE 8, e59387. doi: 10.1371/journal.pone.0059387
Cerri, R. L. A., Chebel, R. C., Rivera, F., Narciso, C. D., Oliveira, R. A., and Thatcher, W. W. (2011). Concentration of progesterone during the development of the ovulatory follicle : I. Ovarian and embryonic responses. J. Dairy Sci. 94, 3342–3351. doi: 10.3168/jds.2010-3734
Chacher, M. F. A., Çolak, A., and Hayirli, A. (2017). Efficacy of repeatedly used CIDR device in cattle reproduction :a metaanalysis review of progesterone concentration and conception rate. Turk. J. Vet. Anim. Sci. 41, 692–697. doi: 10.3906/vet-1706-75
Crites, B. R., Vishwanath, R., Arnett, A. M., Bridges, P. J., Burris, W. R., McLeod, K. R., et al. (2018). Conception risk of beef cattle after fixed-time artificial insemination using either SexedUltraTM 4M sex-sorted semen or conventional semen. Theriogenology 118, 126–129. doi: 10.1016/j.theriogenology.2018.05.003
Fontes, P. L. P., Oosthuizen, N., Ciriaco, F. M., Sanford, C. D., Canal, L. B., Pohler, K. G., et al. (2019). Impact of fetal vs. maternal contributions of Bos indicus and Bos taurus genetics on embryonic and fetal development. J. Anim. Sci. 97, 1645–1655. doi: 10.1093/jas/skz044
Giles, R. L., Ahola, J. K., Whittier, J. C., French, J. T., Repenning, P. E., Kruse, S. G., et al. (2013). Administration of a GnRH analog on day 9 of a 14-day controlled internal drug release insert with timed artificial insemination in lactating beef cows. J. Anim. Sci. 91, 1866–1873. doi: 10.2527/jas.2012-5497
Grant, J. K., Abreu, F. M., Hojer, N. L., Fields, S. D., Perry, B. L., and Perry, G. A. (2011). Influence of inducing luteal regression before a modified controlled internal drug-releasing device treatment on control of follicular development. J. Anim. Sci. 89, 3531–3541. doi: 10.2527/jas.2011-3852
Larimore, E. L., Amundson, O. L., Bird, S. L., Funnell, B. J., Kruse, S. G., Bridges, G. A., et al. (2015). Influence of estrus at fixed-time artificial insemination on early embryonic development in beef cattle. J. Anim. Sci. 93, 2806–2812. doi: 10.2527/jas.2015-8892
Maxwell, W. M. C., Evans, G., Hollinshead, F. K., Bathgate, R., de Graaf, S. P., Eriksson, B. M., et al. (2004). Integration of sperm sexing technology into the ART toolbox. Anim. Reprod. Sci. 82–83, 79–95. doi: 10.1016/j.anireprosci.2004.04.013
Mercadante, V. R. G., Kozicki, L. E., Ciriaco, F. M., Henry, D. D., Dahlen, C. R., Crosswhite, M. R., et al. (2015). Effects of administration of prostaglandin F at initiation of the seven-day CO-Synch+controlled internal drug release ovulation synchronization protocol for suckled beef cows and replacement beef heifers. J. Anim. Sci. 93, 5204–5213. doi: 10.2527/jas.2015-8967
Mercadante, V. R. G., Lamb, G. C., Oosthuizen, N., Dias, N. W., Pancini, S., Haines, H., et al. (2021). Estrus response and pregnancy rates of beef replacement heifers enrolled in two fixed-time artificial insemination protocols, with or without pre-synchronization. J. Anim. Sci. 99, 125–126. doi: 10.1093/jas/skab235.228
Mocé, E., Graham, J. K., and Schenk, J. L. (2006). Effect of sex-sorting on the ability of fresh and cryopreserved bull sperm to undergo an acrosome reaction. Theriogenology 66, 929–936. doi: 10.1016/j.theriogenology.2006.01.063
Oosthuizen, N., Canal, L. B., Fontes, P. L. P., Sanford, C. D., Dilorenzo, N., Dahlen, C. R., et al. (2018). Prostaglandin F2α 7 d prior to initiation of the 7-d CO-synch + CIDR protocol failed to enhance estrus response and pregnancy rates in beef heifers. J. Anim. Sci. 96, 1466–1473. doi: 10.1093/jas/sky058
Oosthuizen, N., Fontes, P. L. P., Oliveira Filho, R. V., Dahlen, C. R., Grieger, D. M., Hall, J. B., et al. (2021). Pre-synchronization of ovulation timing and delayed fixed-time artificial insemination increases pregnancy rates when sex-sorted semen is used for insemination of heifers. Anim. Reprod. Sci. 226, 106699. doi: 10.1016/j.anireprosci.2021.106699
Oosthuizen, N., Fontes, P. L. P., Porter, K., and Lamb, G. C. (2020). Presynchronization with prostaglandin F2a and prolonged exposure to exogenous progesterone impacts estrus expression and fertility in beef heifers. Theriogenology 146, 88–93. doi: 10.1016/j.theriogenology.2020.02.010
Perry, G. A., Perry, B. L., Krantz, J. H., and Rodgers, J. (2012). Influence of inducing luteal regression before a modified fixed-time artificial insemination protocol in postpartum beef cows on pregnancy success. J. Anim. Sci. 90, 489–494. doi: 10.2527/jas.2011-4319
Perry, G. A., Smith, M. F., Roberts, A. J., MacNeil, M. D., and Geary, T. W. (2007). Relationship between size of the ovulatory follicle and pregnancy success in beef heifers. J. Anim. Sci. 85, 684–689. doi: 10.2527/jas.2006-519
Perry, G. A., Walker, J. A., Rich, J. J. J., Northrop, E. J., Perkins, S. D., Beck, E. E., et al. (2020). Influence of SexcelTM (gender ablation technology) gender-ablated semen in fixed-time artificial insemination of beef cows and heifers. Theriogenology 146, 140–144. doi: 10.1016/j.theriogenology.2019.11.030
Richardson, B. N., Hill, S. L., Stevenson, J. S., Djira, G. D., and Perry, G. A. (2016). Expression of estrus before fixed-time AI affects conception rates and factors that impact expression of estrus and the repeatability of expression of estrus in sequential breeding seasons. Anim. Reprod. Sci. 166, 133–140. doi: 10.1016/j.anireprosci.2016.01.013
Riding, G. A., Lehnert, S. A., French, A. J., and Hill, J. R. (2008). Conceptus-related measurements during the first trimester of bovine pregnancy. Vet. J. 175, 266–272. doi: 10.1016/j.tvjl.2007.01.022
Sales, J. N. S., Neves, K. A. L., Souza, A. H., Crepaldi, G. A., Sala, R. V., Fosado, M., et al. (2011). Timing of insemination and fertility in dairy and beef cattle receiving timed artificial insemination using sex-sorted sperm. Theriogenology 76, 427–435. doi: 10.1016/j.theriogenology.2011.02.019
Schenk, J. L., Cran, D. G., Everett, R. W., and Seidel, G. E. (2009). Pregnancy rates in heifers and cows with cryopreserved sexed sperm: Effects of sperm numbers per inseminate, sorting pressure and sperm storage before sorting. Theriogenology 71, 717–728. doi: 10.1016/j.theriogenology.2008.08.016
Sirois, J., and Fortune, J. E. (1990). Lengthening the bovine estrous-cycle with low-levels of exogenous progesterone - a model for studying ovarian follicular dominance. Endocrinology 127, 916–925. doi: 10.1210/endo-127-2-916
Keywords: beef heifers, fixed-time artificial insemination, pre-synchronization, progesterone, prostaglandin F2α, sexed semen
Citation: Oosthuizen N, Porter K, Burato S, Goncalves LM, Pohler KG, Fontes PLP and Lamb GC (2022) Effects of Pre-Synchronization With Prostaglandin F2α and a Progestin, and Delayed Insemination on Pregnancy Rates With Sexed Semen in Replacement Beef Heifers. Front. Anim. Sci. 3:870978. doi: 10.3389/fanim.2022.870978
Received: 07 February 2022; Accepted: 14 March 2022;
Published: 13 April 2022.
Edited by:
Mohan Mondal, ICAR-National Dairy Research Institute, IndiaReviewed by:
Santosh Sahu, Ministry for Primary Industries, New ZealandWeinan Zhou, University of Illinois at Urbana-Champaign, United States
Nishant Kumar, Indian Council of Agricultural Research (ICAR), India
Jack Whittier, University of Nebraska-Lincoln, United States
Halima Sultana, University of Florida, United States
Rick Funston, University of Nebraska-Lincoln, United States
Copyright © 2022 Oosthuizen, Porter, Burato, Goncalves, Pohler, Fontes and Lamb. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: G. Cliff Lamb, Z2NsYW1iQHRhbXUuZWR1; Nicola Oosthuizen, bm9vc3RodWl6ZW5AdGFtdS5lZHU=
 Nicola Oosthuizen
Nicola Oosthuizen Kristina Porter3
Kristina Porter3 Lucas M. Goncalves
Lucas M. Goncalves Ky G. Pohler
Ky G. Pohler Pedro L. P. Fontes
Pedro L. P. Fontes G. Cliff Lamb
G. Cliff Lamb